Abstract
The potato tuber induction and subsequent developmental process are complex phenomenon involving several stages which includes the stolons formation, initiation of tuber by sub-apical swelling, longitudinal expanding of tissues and tuber growth. Potato tubers are known to serve as food storage organ and vegetative propagation for next generation. The potato tuberization process has been reported to be regulated via various extrinsic and extrinsic factors including light, temperature, photoperiod, phytohormones and balanced nutrition. The phytohormones including auxins, cytokinins, gibberellins, abscisic acid, ethylene, brassinosteroids, jasmonic acid, salicylic acid and strigolactones have been reported to play an important role in potato tuberization and development. Studies on endogenous hormone content and their synergestic relationship are of special interest in potato tuber formation. The gibberellins stimulate the tuberizationin potato tactfully via normalizing the shoot growth, while the cytokinins, auxins and abscisic acid evidently regulate the sink activity of the tubers via controlling cell division and cell expansion. The salicylic acid and associated compounds have also been reported to influence the tuber inducing activities and tolerance to biotic and abiotic stresses in potato, however, the tuber inducing activities of salicylic acid is unexplored. Molecular and proteomic studies have greatly extended the knowledge on the mechanism of phytohormone regulating the tuberization in potato. This chapter provides biochemical, genetic and molecular mechanism regulating the potato tuberization and further development mediated by different phytohormones with special emphasis on role of salicylic acid in tuber development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Potato being the third most important food crop after wheat and rice are valuable for the diets and livelihoods of millions of people worldwide (FOASTAT, 2017). The potato tubers have been considered as a good source of dietary energy, micronutrients, vitamins, and protein in comparison with other roots and tubers (http://www.fao.org/potato-2008/en/potato/index.html). Potatoes are an important source of income for the farmers playing a major role in food security and nutrition with thrust on social welfare of the people. The potato is vegetatively propagated, herbaceous, dicotyledonous and annual plant, however, can also grow as a perennial in select environments (Sarkar, 2008). In addition to the known difficulties associated with pests and diseases, growers continuously face various abiotic stresses hindering the overall potato yield . The increasing frequency of extreme weather events is generally responsible for decline of this important cash crop. According to an estimate, the world’s population will approach 10 billion by 2050, and in order to meet the adequate food requirement, the agricultural production should increase by over 60%a as of the present. The International Potato Centre as estimated that 1 °C increase in temperature causes approximately 5% decline in the agricultural productivity; therefore, the global potato yields are estimated to decrease to 32% by 2060 (https://cipotato.org/annualreport2019/stories/next-generation-breeding/). The new verity of potato developed through the breeding improvement program has been considered to withstand the climate change and global warming. A potato plant tuberizes under short days and cool nights. The potato tuberization takes under short days and cool nights, well synchronized morpho-physiological process occurring on the underground stolons under the control of both extrinsic and intrinsic factors. The tuberization in potato has been considered as most sensitive stage of various developmental phases which limits its climate-associated geographical distribution as well as overall potato yield . Therefore, understanding the potato tuberization process becomes even more importantin view of the changing global climate which shall augment in development of new varieties. Development of potato tuber is the morphological transformation of underground stem into stolon which leads to the formation of tubers with an axillary dormant buds or an eye which help in the vegetative propagation under the favourable conditions. Moreover, the nutritional value of potato is conserved because of its vegetative mode of propagation (Navarre et al., 2016). The inherent plasticity of each tuber bud of vegetative organ to form a complete potato plants is certainly a biological impediment towards considering the precise in-planta mechanism regulating the tuberization process (Strunik et al., 1999). The most prominent environmental factor includes the lower temperature and day-length which are reported to be perceived by the above-ground parts of the plants. The hormones are major signalling pathways which operate between areal and below-ground parts playing major role in the regulation of tuber formation which are extensively reviewed in several literature (Vreugdenhil & Struik, 1989; Ewing & Struik, 1992; Ewing, 1995; Aksenova et al., 2012; Dutt et al., 2017).
Phytochromes are major photoreceptor of photoperiodic signalling resulting in changes in various morphological phenomenons including the tuberizationin potato. Currently, several forms of phytochrome are known with apoproteins encoded by different genes. Phytochrome B has been recognized to play a vital role controlling the photoperiod mediated regulation of tuberization. This is also reported to be involved in the inhibition of tuber initiation by long day (LD) photoperiodic. One of the ways for LD action on potato tuberization is the control of hormone content by a photoperiod (Chailakhyan, 1984). Several phytohormones, gibberellins (GAs) and cytokinins (CKs) primarily, affect substantially tuber formation (Ewing, 1995). It has been reported that the molecular mechanisms of phytochrome B action are tightly related to both GA and CK activities (Jackson et al., 2000; Fankhauser, 2002). Role of the phytohormone , the GA has also been recognized in different dimension of potato tuberization which includes stolon formation, elongation and branching (Vreugdenhil & Struik, 1989).
Several studies have also reported that higher level of GA content in stolon under non-inductive LDs (long days), whereas GA activity decreases when leaves are exposed to inductive SD (short days) condition (Machackova et al., 1998; Xu et al., 1998a). An inhibitory effect of the GA under the In-vitro conditions is also reported; however, the degradation of active GAs at the stolon tip during tuber formation have been recognized as an important condition for tuberization to advance in general (Ewing & Struik, 1992; Carrera et al., 2000; Kloosterman et al., 2007). The involvement of CKs in the conception of metabolic sinks gave an indication of its major role in the potato tuber initiation and development. Studies reported that the exogenous application of CK under the In-vitro conditions increased the total tuber yield . Similarly, the overexpression of the gene encoding regulatory enzyme of the CK biosynthesis pathway, the isopentenyltransferase (IPT) also induces tuber induction and development and overall tuber yield (Mauk & Langille, 1978. Romanov et al., 2000). The phytohormone , abscisic acid (ABA) has also been shown promoting effect on tuberization when applied exogenously and acting antagonistically to GA (Xu et al., 1998a). The salicylic acid (SA) and its related compounds have also been reported to influence the tuber inducing activities in potato. The current studies showed the role of auxins phytohormone in tuberization with an appropriate ratio of CK (Sergeeva et al., 2000). Expression analysis of the auxin biosynthetic pathway gene have also shown an association of auxin response factor 6 (ARF6) protein in tuberization and tuber dormancy release (Faivre-Rampant et al., 2004).
2 Hormonal Cascade Regulating Potato Tuberization
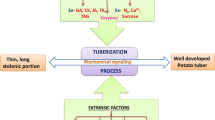
The tuberization is an important survival mechanism for the potato plant which has been considered as an intricate plant developmental phenomenon involving several (van den Berg et al., 1996). The potato tuber formation starts with the swelling of the modified stem referred as stolons which further elongates longitudinally via storage of carbohydrates subsequently initiating the tuber formation. During this development, the tissues of the stolons undergoes a series of cell division and cell enlargement followed by an adjustment in the orientation of cell growth at the subapical region of the tip of the stolon (Ewing & Struik, 2010; Vreugdenhil et al., 1999; Xu et al., 1998b). The principal biochemical process in potato tuberization involves synthesis and accumulation of storage carbohydrates and proteins which has been reported to be synchronized by precise expression patterns of different genes (Taylor et al., 1998; Bachem et al., 2000; Tauberger et al., 2000; Verhees et al., 2002). Studies on the cDNA microarray analysis, quantitative trait loci (QTLs) have revealed differential gene expression pattern and role of various endogenous phytohormones involving potato tuberization (Ewing et al., 2004; Kloosterman et al., 2005). Role of various phytohormones in tuberization has been studies extensively with an established fact that the coordinated levels of hormones regulate the potato tuberization under the natural conditions. Role of various hormones affecting the steps of the potato tuberization is represented in Table 13.1.
3 Role of Gibberelic Acid (GA) in Tuberization
The GA has been reported to play a key protagonist in the potato tuber development, hence studied extensively in literature. The biochemical characterizations have identified over 120 GAs in the plants species; however, only GA1 and GA4 are reported to be naturally vigorous participating in the tuber development (Jackson & Prat, 1996; Vreugdenhil & Struik, 1989). The GA has been reported as a prevailing regulator for potato development, and its endogenous level are controlled by the cellular content of the sucrose and abscisic acid (ABA). The GA also induces stolon elongation and branching through longitudinal cell extension and transverse direction of microtubules and microfibrils to the cell axis (Vreugdenhil & Struik, 1989). Moreover, the GA also participates in the photoperiodic control of tuberization (Xu et al., 1998a). GA play an important role in the stolons formation and analysis of diverse GA biosynthetic gene revealed enhanced expression of the gene during early stages of tuber induction; however a swift decline in the gene expression of biosynthetic gene confirmed decrees in the active GA during stolon tip swelling and initiation of the tubers. Analysis of the endogenous GAs content also exhibited lowering of active GAs content in the swelling stolon tips upon tuber induction (Koda & Okazawa, 1983). It was reported that the expression of the potato GA reducing gene (StGA2ox), induced prior to stolon swelling immediately after the initiation of the stolons formation rendering their importance in the stolon formation (Kloosterman et al., 2007). Thereafter, Carrera et al. (2000) showed that the overexpression of an active GAs biosynthetic gene (StGA20ox1 gene) in potato delayed the tuberization while or antisense expression of the gene advanced the time of tuberization. The antisense transgenic lines showed an increase in the active GA content, inferior stolon growth and consequent tuberization. In another experiment, the overexpression of StGA2ox1 gene overdue the tuberization and change the tuber morphology (Kloosterman et al., 2007). Furthermore, another gene referred as StGA3ox2 has been used for tissue specific or constitutive overexpression in the plants. The overexpression of the gene driven by the constitutive 35S promoter resulted in early tuberization and overall tuber yield ; however, the tuber specific expression slightly delayed tuberization (Bou-Torrent et al., 2011). Further biochemical analysis revealed that the overexpression of gene in the leaves dynamically converted GA20 to GA1 in the leaves, thereby; a lower GA20 transported to the stolon tips causing a lower accessibility of GA20 in the stolon tips resulting in reduced GA1 content leading to quicker tuber formation. A line diagram representing the role of GA and other phytohormone regulating various stages of the potato tuberization is represented in Fig. 13.1.
Schematic representation of regulation of tuberization via various hormonal cascades
StSP6A and GAs act as mobile signals that are transported through phloem to stolon. Translocation of mobiles signals are regulated by a complex regulatory network. Phy-B and StCO repress tuberization in response to LDs. GAs seem to act as repressors, whereas StSP6A (GA biosynthetic regulator) help to induce tuber induction under SD conditions (Dutt et al., 2017). Under LDs, Phy-B represses the expression of StSP6A and StGA20ox1, which encodes an enzyme that catalyzes the synthesis of GA20. Under LDs, StCO represses StSP6A. StGA3ox2 catalyzes the conversion of GA20 to GA1, an active GA. Other phytohormones like Auxins, CKs, ABA and JA act as a inducer of tuber formation in stolon
Photograph of plant captured from Plant Molecular Biology Lab, Dept. of Biotechnology, Dr. Harisingh Gour Vishwavidyalaya, Sagar, M.P., India.
Abbreviation: GAs Gibberellins, GA20OX1 GA 20-oxidases-1, GA3OX2 GA 3-oxidases-2, GA2OX1 GA 20-oxidases-1, LD Long Day photoperiod, SD Short day photoperiod, Phy-B Phytochrome-B, StCO Constans, StSP6A Solanum tuberosum SELF PRUNING 6A, PUFAs Poly unsaturated fatty acids, HPOT hydroperoxides, JA Jasmonic acid, TA Tuberonic acid, TAG Tuberonic acid glucoside, LOX Lypoxigenase, ABA Abscisic acid, CKs Cytokinins, IAA Indole 3-acitic acid
4 Role of Cytokinin in Tuberization
Several other plant hormones have also been reported to play an important role in the tuber induction and development in potato. Plant phytohormone, the Cytokinins (CKs) have also been recognized to function as a common mechanism of storage-organ formation in the plants (Roitsch & Ehneß, 2000; Abelenda & Prat, 2013). Several reports existed in literature on the role of CKs in potato tuberization and development (Krauss & Marschner, 1976; Galis et al., 1995; Banfalvi et al., 1997; Miyazawa et al., 1999; Romanov et al., 2000; Guivarch et al., 2002). The CKs is known to promote potato tuber initiation and advance the commencement of tuber formation (Gális et al., 1995; Kefi et al., 2000; Romanov et al., 2000). Application of CKs in in-vitro tuberization medium could explicitly advance the yield of microtubers, tuber number and biomass in the potato (Levy et al., 1993; Romanov et al., 2000). Field application of CK prior to tuber formation has also been reported to induce the overall tuber yield in potato (Pavlista, 2001). The initial stage of tuber formation require the presence of CK in combination of GA which stimulate cell proliferation, cellular division, radial cell growth and starch accumulation via source–sink movement directing the development of initial tubers (Aksenova et al., 2009; Abelenda & Prat, 2013). Metabolic pathway analysis also showed that CK also activates the ADP-glucose pyrophosphorylase and starch synthase enzyme activity resulting in starch accumulation and enhancing the sink capacity of developing potato tubers (Ronzhina & Mokronosov, 1994; Aksenova et al., 2012). In non-tuberizing plants, an increase of active CK levels can stimulate morphogenesis, resembling tuber formation (Guivarc’h et al., 2002; Eviatar-Ribak et al., 2013). Further studies on the development of transgenic potato altering the CK biosynthetic pathways also showed importance of this plant hormone in the tuber development. The transformation of potato with the isopentenyl transferase gene (IPT) which catalyzes the rate-limiting step of CK biosynthesis, exerted a stimulating consequence in the tuber initiation process and thereby an increased tuber yield , fresh tuber weight per plant (Gális et al., 1995; Hirose et al., 2008). Hartmann et al. (2011) overexpressed Arabidopsis cytokinin oxidase/dehydrogenase1 gene (CKX1) in potato under the constitutive promoter. Biochemical analysis revealed a lowered endogenous CK levels which resulted in deformed tuber with reduced tuber yield .
5 Role of Auxin in Tuberization
Another important plant hormone, the auxin has also been shown to play an important role in several aspects of plant architecture including embryogenesis, lateral root formation and flower development (Marchant et al., 2002; Luo et al., 2011; Krizek, 2011). The potato tuberization include three stages which include tuber induction, tuber initiation, and tuber growth; however, during the initial stage of tuber initiation the longitudinal cell division at the stolon and horizontal cell elongation provides the space for the cell expansion resulting in development of younger tuber through swelling (Xu et al., 1998b). Therefore, changes in the orientation of cell division that result in swelling of the stolon tip in tuber development are due to the activity of auxin . The plain of cell division remains transversal during this phase. Auxin content remains relatively low and the role of auxin is to maintain stolon apical dominance (Roumeliotis et al., 2012). The In-vitro studies revealed the importance of an important auxin , the indoleacetic acid (IAA) in tuber initiation. Experiments showed that the tuber initiation in the presence of IAA depends upon the content of sucrose in the culture medium. Presence of IAA in the in-vitro medium with lower sucrose content enhanced tuber number, whereas higher sucrose content (up to 5–8%) in the medium, the IAA exerted negative impact on the number of tubers (Aksenova et al., 2000). In addition, removal of stolon tip (auxin source) from explants taken for In-vitro tuberization experiments also showed increased tuber formation from axillary buds (Roumeliotis et al., 2012).
Differential gene expression analysis showed expression of several auxin-responsive genes during early tuberization including auxin biosynthesis, auxin transport (PIN gene family) gene, auxin response (ARF) factors (Faivre-Rampant et al., 2004; Kloosterman et al., 2007). A few transgenic studies overexpressing the auxin pathway gene also prompted the auxin response in the potato tuberization. Kolachevskaya et al. (2015) reported development of transgenic potato generated potato by overexpressing Agrobacterium auxin biosynthetic genes tms-1. The gene was cloned in binary vector, pBinB33-tms1 fused to tuber-specific promoter of the class I patatin gene (B33-promoter) of potato. Molecular analysis revealed tms1 gene expression in the tuber tissues with enhanced auxin content in the tuber tissues and plants with increased auxin levels were predominantly in tubers which probably accelerated and intensified the process of tuber formation. Overall, progressive relationships were witnessed among tms1 expression, the IAA content in tubers and stimulation of tuber formation.
6 Role of Abscisic Acid (ABA) in Tuberization
Abscisic acid (ABA) is regarded as a general regulator that reduces GA-promoted processes in plant development. It has been reported to retard the plant growth, however, exerts a positive impact on the induction of tuberization in combination with the GA. Several studies suggested that ABA content increases under inductive conditions which indicate that ABA is a factor counteracting the GA effect on tuberization (El-Antably et al., 1967; Krauss & Marschner, 1976; Ewing, 1995). The exogenous applications of ABA to potato as foliar spray land to cultured plant sections (aqueous nutrient media) have been inconsistent in stimulating the tuberization. However, in contrast, no effect of ABA has been observed on sprout or stolon sections cultured In-vitro (Wareing & Jennings, 1979; Menzel, 1980; Stallknecht & Farnsworth, 1982). Another experiment with droopy mutant of S. phueja potato deficient in ABA synthesis showed that despite the blockage of ABA synthesis, this mutant transits to tuber formation normally under SD conditions, which indicated that ABA does not play a key role in tuber induction and that its stimulatory effect is due to its antagonism to GA signalling (Quarrie, 1982; Chailakhyan, 1984; Xu et al., 1998a).
7 Phytochrome Mediated Hormonal Regulation of Potato Tuberization
The phytochrome A (Phy-A) and B (Phy-B) as photoreceptors present at the aerial parts have been recognized to sense the photoperiod of which Phy-B arbitrate the photoperiodic regulation of tuberization in potato. Jackson et al. (1996) for the first time down regulated the Phy-B protein in potato via transgenic expression of the biosynthetic pathway gene which finally revealed that the plant lost the capability to react to an LD (Long day) photoperiod which directly reaffirmed their role as photoreceptor. Jackson et al. (1998) also showed that when the wild type potato was grafted to the Phy-B antisense plants, its regulated the grafting experiments with Phy-B antisense plants and wild-type potato plants showed that Phy-B regulates the transmission of Phy-B signal thus affecting the tuber induction in the under the LD photoperiod. The Phy-B has also been reported to controls the synthesis of an inhibitory signal that has a role in GA signal transduction. In the GA signalling cascade, a photoperiod responsive-1 (PHOR1) gene encoding a novel arm-repeat protein is a vital constituent of the GA-signalling pathway. The transgenic overexpression of this gene in potato resulted in the induced tuberization with overall tuber yield , however, the down regulation of the gene showed reduction in the PHOR1 protein with reduced stem length, earlier tuberization under SDs and enhanced insensitivity to applied Gas under the In-vitro conditions. Martinez-Garcia et al. (2002) reported the overexpression of the Arabidopsis CONSTANS (CO) transcription factor (TF) in potato under the control of the constitutive promoter which resulted in delayed tuberization in potato. This also suggested that the AtCO (a flowering response gene of Arabidopsis) also had a role in the tuberization, therefore, the reconciliation of some unknown pathway regulating the flowering and the tuberization. Thereafter, González-Schain et al. (2003) reported the isolation and transcriptomic expression of the AtCO orthologue exists in potato regulating the photoperiodic control of tuberization.
8 Role of Jasmonic Acid, Salicylic Acid on Tuberization
Role of plant hormone, the Jasmonic acid (JA) is also recognized to modulate the plant development pathways. Another orthologue of JA regulating the potato tuberization is recognized as tuberonic acid (TA) and its aglycone the tuberonic acid glucoside (TAG) (Koda et al., 1988; Yoshihara et al., 1989; Koda et al., 1991; Matsuura et al., 1993). Several studies have already identified jasmonate-related compounds as potent tuber-inducing factors in potato (Koda et al., 1991). The lipoxygenase (LOX), a dioxygenases enzyme activity has been recognized as key enzyme regulating the JA biosynthesis. The LOX oxygenates the two polyunsaturated fatty acids, the linoleic and linolenic acid resulting in the synthesis of 9(S)-hydroperoxylinolenic acid (9(S)-HPOT) and 13(S)-hydroperoxylinolenic acid (13(S)-HPOT). The JA is then synthesized from 13(S)-HPOT and then catabolozed to tuberonic acid (TA) and tuberonic acid glucoside (TAG) which has been recognized to induce tuberization in case of the potato (Koda, 1997). The cellular activation of LOX requires lower temperature, which is also an important factor for the tuber induction. The activation of LOX also induces the increase in HPOT, JA , TA and TAG content in the aerial parts of a potato plant and then transported to the underground stolons (Yoshihara et al., 1996; Nam et al., 2008). The gene expression studies have also revealed that analogous to outcome of ABA , the LOX originated metabolites might support tuberization by antagonizing the effects of GA, (Jackson & Willmitzer, 1994; Jackson, 1999; Xu et al., 1998a). Various molecular analysis also exhibited that accumulation of Lox1 class mRNA occurs at the tips of stolons, and LOX activity was highest at nascent vigorously growing tubers, thereby, controlling the advance of tubers in potato (Kolomiets et al., 2001). Sarkar et al. (2006) showed for the first time that the derivatives of 13(S)-HPOT, the JA and methyl jasmonate, are mainly occupied in tuber development fairly than in the initial step of the tuber formation recognized as tuber initiation.
Studies have shown that application of JA and its derivatives induce tuber initiation in potato stolons (Pelacho & Mingo-Castel, 1991). While seeking inhibitors of JA biosynthesis, found that salicylic acid (SA) and acetylsalicylic acid (ASA ) induce tuberization in stolons under the in-vitro. During the early steps of tuber initiation, the level of JA in stolons increased (Abdala et al., 2002). In-vitro studies have shown that application of the inhibitor of GA biosynthesis agent changes cell division in the stolon as opposite to that of the natural GA and the analogous changes in the cortical microtubule orientation were observed through the application of the JA and TA (Matsuki et al., 1992; Abdala et al., 2002). Treatment with JA induced complete reversion of GA3 inhibitory action on tuber formation by In-vitro cultivated potato explants (Castro et al., 2000). Involvement of JA and enzymes of their biosynthesis in processes of cell division and growth at the tuber initiation have been recognized in several studies. The apparent inductive effect of JA on tuberization might be due to the fact that a sub- optimal concentration of sucrose, one of the most important tuber-inducing signal molecules, was used in the in-vitro assay medium (Pelacho & Mingo-Castel, 1991). Although SA and related compounds affects a range of developmental and disease-response phenomena in plants, there is still little information on their tuber-inducing effects (Raskin, 1992; Pierpoint, 1994).
9 Conclusions
The regulation of potato tuberization and role of various extrinsic and intrinsic factors involved in tuberization have been studied extensively. Biochemical and molecular mechanism controlling in-plants tuberization and elucidation of specific role of phytohormones in tuberization still remains elusive. The photoperiod and temperature have been considered as most crucial environmental factor influencing the tuberization in potato. Evidence on the role the involvement of hormonal signals, numerous enzymes, transcription factors and genes in these processes have been reported, however, mechanisms of interaction between various phytohormones and their signalling pathways have been refined. Major hormones positively modulating the tuber induction are the GA, CKs, ABA , jasmonates and auxins. Studies suggest that SA also influences tuberization process positively, however, the salicylates have been recognized an important role in biotic and abiotic stress tolerance. Investigations on the composite hormonal, biosynthesis, metabolism genome modification might develop an opportunity to describe the influence of hormonal signalling in this important phenomenon.
References
Abdala, G., Castro, G., Miersch, O., & Pearce, D. (2002). Changes in jasmonate and gibberellin levels during development of potato plants (Solanum tuberosum). Plant Growth Regulation, 36(2), 121–126.
Abelenda, J. A., & Prat, S. (2013). Cytokinins: Determinants of sink storage ability. Current Biology, 23(13), R561–R563.
Aksenova, N. P., Konstantinova, T. N., Golyanovskaya, S. A., Kossmann, J., Willmitzer, L., & Romanov, G. A. (2000). Transformed potato plants as a model for studying the hormonal and carbohydrate regulation of tuberization. Russian Journal of Plant Physiology, 47(3), 370–379.
Aksenova, N. P., Konstantinova, T. N., Golyanovskaya, S. A., Sergeeva, L. I., & Romanov, G. A. (2012). Hormonal regulation of tuber formation in potato plants. Russian Journal of Plant Physiology, 59(4), 451–466.
Aksenova, N. P., Konstantinova, T. N., Lozhnikova, V. N., Golyanovskaya, S. A., & Sergeeva, L. I. (2009). Interaction between day length and phytohormones in the control of potato tuberization in the in vitro culture. Russian Journal of Plant Physiology, 56(4), 454–461.
Bachem, C., van der Hoeven, R., Lucker, J., Oomen, R., Casarini, E., Jacobsen, E., & Visser, R. (2000). Functional genomic analysis of potato tuber life-cycle. Potato Research, 43(4), 297–312.
Bánfalvi, Z., Molnar, A., Kostyál, Z., Lakatos, L., & Molnar, G. (1997). Comparative studies on potato tuber development using an in vitro tuber induction system. Acta Biologica Hungarica, 48(1), 77–86.
Bou-Torrent, J., Martınez-Garcıa, J. F., Garcıa-Martınez, J. L., & Prat, S. (2011). Gibberellin A1 metabolism contributes to the control of photoperiod-mediated. PLoS One, 6(9), e24458.
Carrera, E., Bou, J., García-Martínez, J. L., & Prat, S. (2000). Changes in GA 20-oxidase gene expression strongly affect stem length, tuber induction and tuber yield of potato plants. The Plant Journal, 22(3), 247–256.
Castro, G., Abdala, G., Agüero, C., & Tizio, R. (2000). Interaction between jasmonic and gibberellic acids on in vitro tuberization of potato plantlets. Potato Research, 43(1), 83–88.
Chailakhyan, M. K. (1984). Fotoperiodicheskaya i gormonal’naya regulyatsiya tuberizatsii u rastenii (photoperiodic and hormonal regulation of Tuberization in plants). Russian Journal of Plant Physiology, 52, 623–628.
Dutt, S., Manjul, A. S., Raigond, P., Singh, B., Siddappa, S., Bhardwaj, V., Kawar, P. G., Patil, V. U., & Kardile, H. B. (2017). Key players associated with tuberization in potato: Potential candidates for genetic engineering. Critical Reviews in Biotechnology, 37(7), 942–957.
El-Antably, H. M. M., Wareing, P. F., & Hillman, J. (1967). Some physiological responses to d, l abscisin (dormin). Planta, 73(1), 74–90.
Eviatar-Ribak, T., Shalit-Kaneh, A., Chappell-Maor, L., Amsellem, Z., Eshed, Y., & Lifschitz, E. (2013). A cytokinin-activating enzyme promotes tuber formation in tomato. Current Biology, 23(12), 1057–1064.
Ewing, E. E. (1995). The role of hormones in potato (Solanum tuberosum L.) tuberization. In Plant hormones (pp. 698–724). Springer.
Ewing, E. E., Simko, I., Omer, E. A., & Davies, P. J. (2004). Polygene mapping as a tool to study the physiology of potato tuberization and dormancy. American Journal of Potato Research, 81(4), 281–289.
Ewing, E. E., & Struik, P. C. (1992). Tuber formation in potato: Induction, initiation and growth. Horticultural Reviews, 14(89), 197.
Ewing, E. E., & Struik, P. C. (2010). Tuber formation in potato: Induction, initiation and growth, horticultural reviews, 14(3), J. Janik (Eds). Willey.
Faivre-Rampant, O., Cardle, L., Marshall, D., Viola, R., & Taylor, M. A. (2004). Changes in gene expression during meristem activation processes in Solanum tuberosum with a focus on the regulation of an auxin response factor gene. Journal of Experimental Botany, 55(397), 613–622.
Fankhauser, C. (2002). Light perception in plants: Cytokinins and red light join forces to keep phytochrome B active. Trends in Plant Science, 7(4), 143–145.
FAOSTAT. (2017). Food and Agriculture Organization of the United Nations, Food and Agriculture Data. http://www.fao.org/faostat/en/#home
Gális, I., Macas, J., Vlasák, J., Ondřej, M., & Van Onckelen, H. A. (1995). The effect of an elevated cytokinin level using the ipt gene and N 6-benzyladenine on single node and intact potato plant tuberization in vitro. Journal of Plant Growth Regulation, 14(3), 143–150.
González-Schain, N., Prat, S., & Suárez-López, P. (2003). Isolation of a putative CONSTANS orthologue from potato. In Abstract from Proc Int Soc Plant Mol Meeting, June (24).
Guivarc'h, A., Rembur, J., Goetz, M., Roitsch, T., Noin, M., Schmülling, T., & Chriqui, D. (2002). Local expression of the IPT gene in transgenic tobacco (Nicotiana tabacum L. cv. SR1) axillary buds establishes a role for cytokinins in tuberization and sink formation. Journal of Experimental Botany, 53(369), 621–629.
Hartmann, A., Senning, M., Hedden, P., Sonnewald, U., & Sonnewald, S. (2011). Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiology, 155(2), 776–796.
Hirose, N., Takei, K., Kuroha, T., Kamada-Nobusada, T., Hayashi, H., & Sakakibara, H. (2008). Regulation of cytokinin biosynthesis, compartmentalization and translocation. Journal of Experimental Botany, 59(1), 75–83.
https://cipotato.org/annualreport2019/stories/next-generation-breeding/
Jackson, S. D. (1999). Multiple signaling pathways control tuber induction in potato. Plant Physiology, 119(1), 1–8.
Jackson, S. D., Heyer, A., Dietze, J., & Prat, S. (1996). Phytochrome B mediates the photoperiodic control of tuber formation in potato. The Plant Journal, 9(2), 159–166.
Jackson, S. D., James, P., Prat, S., & Thomas, B. (1998). Phytochrome B affects the levels of a graft-transmissible signal involved in tuberization. Plant Physiology, 117(1), 29–32.
Jackson, S. D., James, P. E., Carrera, E., Prat, S., & Thomas, B. (2000). Regulation of transcript levels of a potato gibberellin 20-oxidase gene by light and phytochrome B. Plant Physiology, 124(1), 423–430.
Jackson, S. D., & Prat, S. (1996). Control of tuberisation in potato by gibberellins and phytochrome B. Physiologia Plantarum, 98(2), 407–412.
Jackson, S. D., & Willmitzer, L. (1994). Jasmonic acid spraying does not induce tuberisation in short-day-requiring potato species kept in non-inducing conditions. Planta, 194(2), 155–159.
Kefi, S., Pavlista, A. D., Read, P. E., & Kachman, S. D. (2000). Comparison of Thidiazuron and two Nitroguanidines to kinetin on potato Microtuberization in vitro under short and long days. Journal of Plant Growth Regulation, 19(4), 429–436.
Kloosterman, B., Navarro, C., Bijsterbosch, G., Lange, T., Prat, S., Visser, R. G., & Bachem, C. W. (2007). StGA2ox1 is induced prior to stolon swelling and controls GA levels during potato tuber development. The Plant Journal, 52(2), 362–373.
Kloosterman, B., Vorst, O., Hall, R. D., Visser, R. G., & Bachem, C. W. (2005). Tuber on a chip: Differential gene expression during potato tuber development. Plant Biotechnology Journal, 3(5), 505–519.
Koda, Y. (1997). Possible involvement of jasmonates in various morphogenic events. Physiologia Plantarum, 100(3), 639–646.
Koda, Y., Kikuta, Y., Tazaki, H., Tsujino, Y., Sakamura, S., & Yoshihara, T. (1991). Potato tuber-inducing activities of jasmonic acid and related compounds. Phytochemistry, 30(5), 1435–1438.
Koda, Y., & Okazawa, Y. (1983). Characteristic changes in the levels of endogenous plant hormones in relation to the onset of potato tuberization. Japanese Journal of Crop Science, 52(4), 592–597.
Koda, Y., Omer, E. S. A., Yoshihara, T., Shibata, H., Sakamura, S., & Okazawa, Y. (1988). Isolation of a specific potato tuber-inducing substance from potato leaves. Plant and Cell Physiology, 29(6), 1047–1051.
Kolachevskaya, O. O., Alekseeva, V. V., Sergeeva, L. I., Rukavtsova, E. B., Getman, I. A., Vreugdenhil, D., Buryanov, Y. I., & Romanov, G. A. (2015). Expression of auxin synthesis gene tms1 under control of tuber-specific promoter enhances potato tuberization in vitro. Journal of Integrative Plant Biology, 57(9), 734–744.
Kolomiets, M. V., Hannapel, D. J., Chen, H., Tymeson, M., & Gladon, R. J. (2001). Lipoxygenase is involved in the control of potato tuber development. The Plant Cell, 13(3), 613–626.
Krauss, A., & Marschner, H. (1976). Influence of nitrogen nutrition and application of growth regulators on tuber initiation in potato plants. Zeitschrift fuer Pflanzenernaehrung und Bodenkunde (Germany, FR), 139, 143–155.
Krizek, B. A. (2011). Auxin regulation of Arabidopsis flower development involves members of the AINTEGUMENTA-LIKE/PLETHORA (AIL/PLT) family. Journal of Experimental Botany, 62(10), 3311–3319.
Levy, D., Seabrook, J. E., & Coleman, S. (1993). Enhancement of Tuberization of axillary shoot buds of potato (Solarium tuberosum L.) cultivars cultured in vitro. Journal of Experimental Botany, 44(2), 381–386.
Luo, Y., Qin, G., Zhang, J., Liang, Y., Song, Y., Zhao, M., Tsuge, T., Aoyama, T., Liu, J., Gu, H., & Qu, L. J. (2011). D-myo-inositol-3-phosphate affects phosphatidylinositol-mediated endomembrane function in Arabidopsis and is essential for auxin-regulated embryogenesis. The Plant Cell, 23(4), 1352–1372.
Macháčková, I., Konstantinova, T. N., Sergeeva, L. I., Lozhnikova, V. N., Golyanovskaya, S. A., Dudko, N. D., Eder, J., & Aksenova, N. P. (1998). Photoperiodic control of growth, development and phytohormone balance in Solanum tuberosum. Physiologia Plantarum, 102(2), 272–278.
Malik, S. D. A. S. A., & Madhi, A. S. Z. J. (2017). The effect of salicylic acid on the growth and Microtuberization of potato (Solanum tuberosum L.) cv. Arizona propagated in vitro. American Journal of Potato Research, 95, 395–412.
Marchant, A., Bhalerao, R., Casimiro, I., Eklöf, J., Casero, P. J., Bennett, M., & Sandberg, G. (2002). AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. The Plant Cell, 14(3), 589–597.
Martínez-García, J. F., Virgós-Soler, A., & Prat, S. (2002). Control of photoperiod-regulated tuberization in potato by the Arabidopsis flowering-time gene CONSTANS. Proceedings of the National Academy of Sciences, 99(23), 15211–15216.
Matsuki, T., Tazaki, H., Fujimori, T., & Hogetsu, T. (1992). The influences of jasmonic acid methyl ester on microtubules in potato cells and formation of potato tubers. Bioscience, Biotechnology, and Biochemistry, 56(8), 1329–1330.
Matsuura, H., Yoshihara, T., Ichihara, A., Kikuta, Y., & Koda, Y. (1993). Tuber-forming substances in Jerusalem artichoke (Helianthus tuberosus L.). Bioscience, Biotechnology, and Biochemistry, 57(8), 1253–1256.
Mauk, C. S., & Langille, A. R. (1978). Physiology of tuberization in Solanum tuberosum L: Cis-zeatin riboside in the potato plant: Its identification and changes in endogenous levels as influenced by temperature and photoperiod. Plant Physiology, 62(3), 438–442.
Menzel, C. M. (1980). Tuberization in potato at high temperatures: Responses to gibberellin and growth inhibitors. Annals of Botany, 46(3), 259–265.
Miyazawa, Y., Sakai, A., Miyagishima, S. Y., Takano, H., Kawano, S., & Kuroiwa, T. (1999). Auxin and cytokinin have opposite effects on amyloplast development and the expression of starch synthesis genes in cultured bright yellow-2 tobacco cells. Plant Physiology, 121(2), 461–470.
Nam, K. H., Kong, F., Matsuura, H., Takahashi, K., Nabeta, K., & Yoshihara, T. (2008). Temperature regulates tuber-inducing lipoxygenase-derived metabolites in potato (Solanum tuberosum). Journal of Plant Physiology, 165(2), 233–238.
Navarre, D. A., Shakya, R., & Hellmann, H. (2016). Vitamins, phytonutrients, and minerals in potato. In Advances in potato chemistry and technology (pp. 117–166). Academic.
Pavlista, A. D. (2001). Thidiazuron increased yield of potato cultivars. PGRSA Quarterly, 29(3), 72–80.
Pelacho, A. M., & Mingo-Castel, A. M. (1991). Jasmonic acid induces tuberization of potato stolons cultured in vitro. Plant Physiology, 97(3), 1253–1255.
Pierpoint, W. S. (1994). Salicylic acid and its derivatives in plants: Medicines, metabolites and messenger molecules. Advances in Botanical Research, 20, 163–235.
Quarrie, S. A. (1982). Droopy: A wilty mutant of potato deficient in abscisic acid. Plant, Cell & Environment, 5(1), 23–26.
Raskin, I. (1992). Role of salicylic acid in plants. Annual Review of Plant Biology, 43(1), 439–463.
Roitsch, T., & Ehneß, R. (2000). Regulation of source/sink relations by cytokinins. Plant Growth Regulation, 32(2), 359–367.
Romanov, G. A., Aksenova, N. P., Konstantinova, T. N., Golyanovskaya, S. A., Kossmann, J., & Willmitzer, L. (2000). Effect of indole-3-acetic acid and kinetin on tuberisation parameters of different cultivars and transgenic lines of potato in vitro. Plant Growth Regulation, 32(2), 245–251.
Ronzhina, E. S., & Mokronosov, A. T. (1994). Source-sink relations and the role of cytokinins in the regulation of transport and partitioning of organic substances in plants. Russian Journal of Plant Physiology, 41(3), 396–406.
Roumeliotis, E., Kloosterman, B., Oortwijn, M., Kohlen, W., Bouwmeester, H. J., Visser, R. G., & Bachem, C. W. (2012). The effects of auxin and strigolactones on tuber initiation and stolon architecture in potato. Journal of Experimental Botany, 63(12), 4539–4547.
Sarkar, D. (2008). The signal transduction pathways controlling in planta tuberization in potato: An emerging synthesis. Plant Cell Reports, 27(1), 1–8.
Sarkar, D., Pandey, S. K., & Sharma, S. (2006). Cytokinins antagonize the jasmonates action on the regulation of potato (Solanum tuberosum) tuber formation in vitro. Plant Cell, Tissue and Organ Culture, 87(3), 285–295.
Sergeeva, L. I., De Bruijn, S. M., Koot-Gronsveld, E. A., Navratil, O., & Vreugdenhil, D. (2000). Tuber morphology and starch accumulation are independent phenomena: Evidence from ipt-transgenic potato lines. Physiologia Plantarum, 108(4), 435–443.
Stallknecht, G. F., & Farnsworth, S. (1982). The effect of the inhibitors of protein and nucleic acid synthesis on the coumarin-induced tuberization and growth of excised axillary shoots of potato sprquts (Solanum tuberosum l.) cultured in vitro. American Potato Journal, 59(2), 69–75.
Strunik, P. C., Vreugdenhil, D., van Eck, H. J., Bachem, C. W., & Visser, R. G. (1999). Physiological and genetic control of tuber formation. Potato Research, 42(2), 313–331.
Tauberger, E., Fernie, A. R., Emmermann, M., Renz, A., Kossmann, J., Willmitzer, L., & Trethewey, R. N. (2000). Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose-6-phosphate. The Plant Journal, 23, 43–53.
Taylor, M. A., George, L. A., Ross, H. A., & Davies, H. V. (1998). cDNA cloning and characterisation of an α-glucosidase gene from potato (Solanum tuberosum L.). The Plant Journal, 13(3), 419–425.
Van den Berg, J. H., Ewing, E. E., Plaisted, R. L., McMurry, S., & Bonierbale, M. W. (1996). QTL analysis of potato tuberization. Theoretical and Applied Genetics, 93(3), 307–316.
Verhees, J., van der Krol, A. R., Vreugdenhil, D., & van der Plas, L. H. (2002). Characterization of gene expression during potato tuber development in individuals and populations using the luciferase reporter system. Plant Molecular Biology, 50(4), 653–665.
Vreugdenhil, D., & Struik, P. C. (1989). An integrated view of the hormonal regulation of tuber formation in potato (Solanum tuberosum). Physiologia Plantarum, 75(4), 525–531.
Vreugdenhil, D., Xu, X., Jung, C. S., van Lammeren, A. A., & Ewing, E. E. (1999). Initial anatomical changes associated with tuber formation on single-node potato (Solanum tuberosum L.) cuttings: A re-evaluation. Annals of Botany, 84(5), 675–680.
Wareing, P. F., & Jennings, A. M. V. (1979). In plant growth substances (pp. 293–300). F. Skoog (Ed). Springer.
Xu, X., van Lammeren, A. A., Vermeer, E., & Vreugdenhil, D. (1998a). The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiology, 117(2), 575–584.
Xu, X., Vreugdenhil, D., & Lammeren, A. A. V. (1998b). Cell division and cell enlargement during potato tuber formation. Journal of Experimental Botany, 49(320), 573–582.
Yoshihara, T., Amanuma, M., Tsutsumi, T., Okumura, Y., Matsuura, H., & Ichihara, A. (1996). Metabolism and transport of [2-14C](±) jasmonic acid in the potato plant. Plant and Cell Physiology, 37(5), 586–590.
Yoshihara, T., Omir, E. S. A., Koshino, H., Sakamura, S., Kkuta, Y., & Koda, Y. (1989). Structure of a tuber-inducing stimulus from potato leaves (Solanum tuberosum L.). Agricultural and Biological Chemistry, 53(10), 2835–2837.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pathak, A., Upadhyaya, C.P. (2021). A Brief Insight on the Role of Various Phytohormones in Potato (Solanum tuberosum L) Tuber Development. In: Hayat, S., Siddiqui, H., Damalas, C.A. (eds) Salicylic Acid - A Versatile Plant Growth Regulator. Springer, Cham. https://doi.org/10.1007/978-3-030-79229-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-79229-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-79228-2
Online ISBN: 978-3-030-79229-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)