Abstract
Autologous skin graft are the current standard of care for deep burn lesion. The major limitation of this treatment are the necessity of remaining area of healthy skin to replace the damaged skin and the poor quality of the scar obtained. Mesenchymal stem cells derived from adipose tissue have trophic, anti-inflammatory, and anti-fibrotic properties making them a potential therapeutic tool for the management of acute burns. Animal model studies reporting their use are increasing. They are used both in regenerative medicine to improve healing of intermediate-depth burns, split thickness skin graft (STSG) donor sites, and expanded autografts, and in tissue engineering in the creation of reconstructed skin models. The translation of stem cell therapy from the laboratory to the bedside over the next few decades is promising to have a significant impact on standards of burn care.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keyword
- Acute burn treatment
- Burn wound
- Mesenchymal stem cells
- Adipose-derived stem cells
- Stromal vascular fraction
- Regenerative medicine
- Stem cell transplantation
- Skin regeneration
-
1.
There is a potential interest for the use of mesenchymal stem cells from adipose tissue for the management and the prevention of burn deepening in acute phase.
-
2.
There is a potential interest for the use of mesenchymal stem cells from adipose tissue for the management of major burns by potentiating the spontaneous regeneration capacities of intermediate burns, split thickness skin graft donor sites, and expanded grafts.
-
3.
There is a potential interest for the use of mesenchymal stem cells from adipose tissue in tissue engineering for the creation of complex reconstructed skin.
-
4.
The clinical efficacy of mesenchymal stem cells from adipose tissue in burn induced lesion has not yet been demonstrated.
-
5.
The comparison of the efficacy of treatment with autologous or allogeneic cells has not been yet reported.
-
6.
The optimal delivery modalities are to be defined.
-
7.
The use of SVF as a source of stem cell in 3D bioprinting could be promising and avoid medico-legal issue.
-
8.
The tangential excision procedure operative residue can be used as a source of ADSC.
1 Introduction
The main factors limiting the treatment of major burns are the limited capacity for spontaneous healing of burned skin tissue and the lack of the availability of the healthy autologous skin graft for surgical replacement of burned tissue. Cell therapy and tissue engineering work are aimed for improving spontaneous healing of burned skin or creating in vitro autologous reconstructed skin to overcome this lack. Those work are mainly based on the use of epidermal stem cells or fibroblasts. The discovery of the major regenerative potential of mesenchymal stem cells (MSCs) is now leading to their use in this field. The immunomodulatory and anti-inflammatory properties of MSC could also have a therapeutic impact on the systemic inflammatory response syndrome secondary to burns and are being studied in this indication. Adipose tissue is the ideal reservoir of mesenchymal stem cells due to the easy access to these cells and their high potential for in vitro multiplication. The use of adipose tissue fractions (vascular stromal fraction (SVF) or mesenchymal stem cells derived from adipose tissue (ADSC)) for the treatment of acute burn injuries are explored in numerous pre-clinical studies but has not yet been implemented in clinical practice.
1.1 Burn-Induced Skin Lesions
Acute burns cause localized destruction of exposed skin, varying in extent and depth, determined by temperature and time exposure: Cellular necrosis occurs after 1 s exposure at 156 °F (69 °C), or after 1 h at 113 °F (45 °C). The zone of coagulation in the center of the wound and at the point of application of the thermal agent has no viable cells: the applied heat causes protein denaturation and loss of plasma membrane integrity (Fig. 40.1). The zone of stasis, which surrounds the zone of coagulation, shows progressive lesions over the first 24 h. Approximately half of the cells in the stasis zone undergo apoptosis or necrosis due to oxidative stress, continued inflammation, and decreased blood flow due to microthrombosis. Depending on local conditions (quantity of inflammatory and free radical mediators and progression of microthrombosis) and general conditions (quality of perfusion), the tissues will evolve towards complete necrosis or recovery from the few remaining viable cells. At the periphery of the burned tissue, the zone of hyperemia is the site of vasodilatation mediated by local inflammatory mediators without cell destruction. Local and general care immediately after the burn aim at limiting the spread of lesions within the zone of stasis and are based on cooling, application of antiseptic dressings, and maintenance of optimal perfusion pressure through adequate fluid resuscitation.
Once the burn occurred, the depth of the burns is the main prognostic factor. In the absence of surgical treatment, burns first go through a detersion phase: burned tissue gradually delimits and detaches from the underlying healthy tissue. Then, depending on the depth of the injury, three types of evolution are described. In case of superficial damage, reaching at most the papillary dermis, and thus preserving a large number of keratinocyte stem cells, particularly in the epidermal appendages, complete healing is possible in 10–15 days (Fig. 40.2). When the burn destroys many keratinocyte stem cells, healing is possible, but is delayed for several weeks and will lead to a retractile and hypertrophic scar. In case of deep damage that does not leave any keratinocyte stem cells in place, only a centripetal re-epithelialization can take place. If the burn is extensive, the healing may take several months, and combines centripetal re-epithelialization and major retraction.
Superficial burn of the back of right breast in a 42-year-old patient. (a) D7 of the burn, detersion phase. (b) D12, epidermization from epidermal appendage can be seen. (c) D20, start of compressive garment and silicone dressing use. (d) 7-month post-burn, a slight scar remains visible as complete epidermization by spontaneous healing take more than 10 days, hypertrophy is well prevented by compressive therapy
1.2 Systemic and Metabolic Disturbances
In case of major burns, which exceeding 20–30% of the total body surface area, the destruction of skin will lead to major systemic repercussions. The local inflammatory reaction induce the release of a large quantities of inflammatory mediators, causing a systemic inflammatory response syndrome with hypovolemic shock and multiple organs failure. The loss of barrier function of the vascular endothelium results in increased fluid loss and electrolytes imbalance. The patient’s survival depends on the early implementation of initial resuscitation measures, foremost of which is massive vascular filling. After the initial shock phase, the prolonged inflammatory state will lead to lasting metabolic disturbances with hypercatabolism leading to muscle wasting and immunosuppression. The patient's prognosis depends on the early excision of burned skin and restoration of the skin barrier.
1.3 Current Surgical Treatment of Burn Injuries
The surgical treatment of burn lesions is conditioned by the depth of burns and the capacity for spontaneous healing. Superficial burns, respecting the keratinocyte stem cell foci located in the epidermal appendages, thus have a spontaneous healing and favorable results. Their treatment is based on dressing care and optimal medical management. Lesions of intermediate depth are of variable progression. While spontaneous healing can be generally obtained, it is of poor quality, leaving hypertrophic and retractile scars, and it may be preferable to perform excision and split thickness skin grafting of these lesions (Figs. 40.3 and 40.4). As it is difficult to distinguish between superficial and intermediate deep lesions in the initial phase, we are considering the burn that has not healed spontaneously in 10 days requires surgical management. Deep lesions, destroying the epidermis and dermis over their entire height, require excision of the damaged tissues, and their replacement by the so-called autologous split thickness skin graft (STSG) 2–3—10th of a millimeter thick taken from healthy skin (Fig. 40.4). The donor site latter will heals spontaneously in 15–21 days from keratinocyte stem cells located in the epidermal appendages left in place deep down.
Intermediate-depth burn of the dorsal hand and fingers in a 68-year-old patient, (a) preoperative aspect at D17 of the burn, persistence of non-epidermized areas on the dorsum of the hand, (b) intraoperative aspect, split thickness skin graft, (c) aspect at 6 months postoperatively, the grafted areas are less inflammatory and hypertrophic than the areas of controlled wound healing at the fingers and the dorsum of the hand despite the use of compressive garment
Intermediate-depth burn of face in a 6-year-old patient, (a) intraoperative aspect after eschar removal, (b) intraoperative aspect after split thickness skin graft harvest from the scalp at D10 of the burns c. aspect at 14 months postoperatively, the scars are still quite inflammatory and hypertrophic despite the use of continuous facial pressure mask. Notice the hypertrophy of controlled wound healing area of the lateral aspect of the nose and the chin despite the use of compressive garment
In case of major burns, there is a mismatch between the healthy skin surface and the burned skin surface. Allografts (donor dermal-epidermal grafts) can be used as a temporary dressing to cover wounds after the excision of involved areas in the absence of immediately available healthy skin. Initially integrated and revascularized, they will then be rejected in about 3 weeks, following the onset of adaptive immunity. To obtain definitive coverage, it is then necessary to harvest split thickness skin graft several times from the same donor area during different surgical procedures, and to use STSG expansion techniques (multiplication of its surface by fragmentation in the form of a net or multiple small grafts). Complete restoration of the skin barrier from expanded skin graft is obtained by healing from the healthy cells brought by the transferred skin net. When the expansion factor is important (up to ×9 or ×12) meshed split thickness skin graft are covered by allografts or xenograft (pig skin mainly) for mechanical protection a procedure called Alexander technique (Fig. 40.5).
Two tissue-engineered products are also used in routine practice for the management of patients with severe major burns these are: keratinocyte culture sheets and dermal regeneration matrices.
Keratinocyte culture sheets, which can be allogeneic or autologous, are used to promote healing of graft donor sites, superficial burns, or expanded skin grafts (Fig. 40.6). Applied to these different types of wounds, they secrete numerous growth factors that accelerate local healing. Autologous keratinocyte culture sheets can also be used to replace split thickness skin grafts as a definitive covering solution according to the CUENO method. However, their cost and the fragility of the skin cover obtained limits their use.
The dermal regeneration matrices are acellular scaffolds, that can be biologic, made of collagen elastin and glycoaminoglycane of bovine origin (Integra® and Matriderm®) or derived from decellularized human dermis (Glyaderm®), or synthetic (BTM, Novosorb®). Applied to the wound after excision, they will be recolonized by fibroblasts and endothelial cells from the bottom and edges of the wound, and therefore progressively revascularized and replaced by the extra-cellular matrix secreted by the fibroblasts. A dermis with an almost physiological appearance is formed. They must be secondarily covered with a split thickness skin graft. They make it possible to improve the result of the latter by limiting the phenomena of hypertrophy and retraction. The restoration of the dermis allows to obtain a more supple and elastic scarred skin.
Finally, some surgical teams have access to in vitro reconstructed skins, associating autologous epidermis and dermis. These seem to offer a real alternative to STSG, with functionally satisfactory results apart from the absence of pigmentation. Their long production time, generally 6–8 weeks, and their cost severely limit their accessibility.
1.4 Potential of ADSC
The transfer of non-purified fat tissue is used in the reconstructive surgeries of burn sequelae either to correct contour anomalies (volumizing effect), sliding planes or to improve skin texture trophicity. The use of adipose tissue as a volumizer has no indication in the management of acute burn injuries. Conversely, the use of the same fat tissue as a potential source of stem cells, mainly mesenchymal, seems promising. The regenerative potential of these stem cells has been demonstrated in numerous wound healing models.
In the more specific case of burn lesions, three properties seem to be of major therapeutic interest:
-
The recruitment of the cell of the wound edges by paracrine effect could allow to accelerate the healing of the superficial burns, the STSG donor sites and the expanded skin graft.
-
The anti-fibrotic effect could improve the quality of the scar’s texture.
-
The local and general anti-inflammatory effect could potentially limit the local extension of burn lesions in the initial phase and reduces the systemic inflammatory response syndrome associated with major burns.
It has also been shown that MSCs can participate in skin regeneration by differentiating into fibroblasts, but also into keratinocytes or endothelial type cells [1, 2]. Thus, several authors propose their use in tissue engineering for the in vitro creation of complex reconstructed skin.
2 Use of Fat Tissue in Cell Therapy for the Treatment of Acute Burn Injuries
Mesenchymal stem cells derived from bone marrow, or umbilical cord, have been widely used in pre-clinical protocol in the management of acute burn injuries. The discovery of the presence of these same stem cells in fat tissue, which could be easily harvest in large quantities, with less morbidity, and exhibit a greater capacity to multiply in vitro, has led the authors to focus more and more on this stem cell reservoir. While mesenchymal cells from different sources are similar in many aspects and all appear to be therapeutically effective in the management of burns, MSC have also been shown to retain epigenetic modifications specific to their tissue of origin even after extensive in vitro expansion. Their ability to differentiate also appears to vary according to the in vitro studies carried out on the subject. The ideal source of MSC for the management of burn lesions has not yet been determined. The results concerning efficacy on wound healing are inconsistent across studies, in favor of either ADSC or Umbilical cord MSC [3]. ADSC appear to be the population most stimulating angiogenesis when there some concern about a pro-fibrotic action of bone marrow-derived MSC [4, 5].
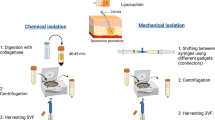
Here, we report the main potential benefits of adipose-derived mesenchymal stem cells in the management of acute burn wounds, based on the studies of their use or in the absence, of MSC of another origin. These are used either after In Vitro expansion in specific media and selection based on their plastic adhesion, or unpurified within the stromal vascular fraction (SVF). The use of SVF could allow the fast development of the clinical applications since In Vitro manipulation are avoid, but the lack of reproducibility and the multiplicity of the SVF isolation protocols raises the question of the reproducibility of the results.
2.1 Improvement of the Skin Healing
2.1.1 Prevention of Deepening of Burns in the Acute Phase
In line with the works carried out in myocardial infarction or cerebral ischemia models, the use of MSCs has been proposed to prevent the extension of microthromboses following an acute burn injury and therefore limit the evolution of the zone of stasis towards necrosis. MSCs are expected to interact with the ischemic microenvironment by decreasing the release of pro-inflammatory mediators and preserving the surrounding cells from apoptosis. The cellular mechanism at play is yet not clearly identifyed. In 2013, Oksuz et al. demonstrated a decrease in the size of the cutaneous zone of necrosis at D3 after thermal burns in rats after subcutaneous injection of allogeneic bone marrow MSC [6]. Four years later, Eyboglu et al. found similar results by performing subcutaneous injections of allogeneic SVF (obtained by collagenase digestion and centrifugation of fat tissue) 30 min after contact burns in rats [7]. They objectify a decrease in microthromboses visualized at D7 by angiography (vessel counts of 10.28 +/− 1.28 for untreated wounds and 19.43 +/− 1.72 in the treated group), and a lower percentage of tissue necrosis in the zone of stasis (viable tissue at 32% +/− 3.28% in the control group and 57 +/− 4.28% for the treated zone). The results of these two studies cannot be compared due to the disparity of the protocols used and the different evaluation time points.
2.1.2 Healing of the Intermediate Burns
The administration of SVF and ADSC or untreated fat has been proposed for the treatment of the superficial burns. Atalay et al. showed in 2014 that an intradermal injection of autologous SVF after scald burns in rats allowed the decrease in local inflammation at D10 post-burn, an acceleration of neo-vascularization with an increase in local VEGF expression, as well as fibroblastic proliferation observable as early as D3 in the treated group versus D7 in the control group [8]. The speed of healing was not evaluated.
Foubert et al. demonstrated, after intravenous injection of autologous stromal vascular fraction obtained after purification by the solution 800 IV System, a significant acceleration of the epithelialization rate of intermediate burn wounds in pigs (81.8 +/− 7.1% of the epidermis surface in the treated group at D21 vs. 59.8 +/− 7.1% for the control group; p = 0.03). The restoration of the barrier function of the epidermis was objectified by the decrease in water losses at its surface from D11 in the treated group [9].
The efficacy of the different fractions of the adipose tissue were compared in this indication. The results of the studies are inconsistent. For Loder et al. the injection of lipoaspirate seemed less effective than the administration of purified ADSC, both in terms of reduction of the surface area of the wound and secretion of early healing markers, while Chen et al. demonstrated that a better efficacy of lipoaspirate or allogeneic differentiated adipocytes subdermal injection than ADSC injection in rats [10, 11].
2.1.3 Deep Burns with the Secondary Healings
The results concerning the secondary healing of the deep burns diverge among the studies, but the results are overall not really encouraging. Bliley et al. evaluated the benefit of subcutaneous injection of human ADSC in rats after the deep burns. They did not revealed any change in the speed of epithelialization but showed an increase in neo-vascularization (CD31+ labeling) and an increase in type I and III collagen synthesis [12]. In a full thickness burn model in rats, Karimi et al. failed to demonstrate significant improvement after subcutaneous injection of ADSC or allogeneic lipoaspirate. Matomed et al. propose the use of ADSC seeded into amniotic membranes in rats. No significant effects were observed onre-epithelialization, local inflammation, or fibrosis within the scar [13].
2.1.4 Acceleration of the Healing of the Expanded Skin Grafts
In 2017, Burmeister et al. sought to highlight the value of the use of ADSC in conjunction with the use of mesh skin grafts for the treatment of deep burns in pigs (N = 6) [14]. The cells were incorporated into a polyethylene glycol and fibrin hydrogel and applied in a 2 mm layer after the autografts were performed. It appears that the hydrogel drastically reduces the retraction of skin grafts, with a result equivalent to the unmeshed skin grafts. A possible benefit of adding ADSCs is not highlighted, but it appears that their addition modifies the production of collagen and the development of neo-vascularization.
One year later, Foubert et al. demonstrated an acceleration in the re-epithelialization of the meshed split thickness skin graft after intravascular injection of autologous stromal vascular fraction obtained after purification by the solution1 800 IV System in pigs: at D5 an epithelialization of 27.1 +/− 11.8% of the interstices was observed compared to 1.1 +/− 1.1% in the control group [9]. A decrease in trans-epidermal water loss was also observed, and histological analyses performed at D18 revealed the presence of a thicker epidermis and an improvement in the dermal elasticity.
2.1.5 Donor Site of the Split Thickness Skin Graft
Autologous or allogeneic keratinocyte cultured sheets can be used to accelerate the healings of STSG donor site in the patients with major burns (more than 50–60% TSC), so that repeated harvesting of STSG could be performed with shorter time interval (about 10 days versus 21 days). In the previously cited study, Foubert et al. found an acceleration of the re-epithelialization rate of donor sites in pigs after intravenous injection of SVF with an epithelialization at D7 of 91.7 +/− 3.8% for the treated group vs. 62.5 +/− 8.8%, for the untreated group; (p = 0.001) [9]. There is no study to date comparing keratinocyte culture sheets and ADSC in this indication.
2.1.6 Use of ADSC in Conjunction with the Dermal Regeneration Matrix
The inoculation of dermal regeneration matrices with cells allows an acceleration of their revascularization speed and maturation in the different animal models studied. Doornaert et al. inoculated a dermal regeneration matrix obtained from decellularized human skin (Glyaderm®) with ADSC of human origin and implanted it in immunodeficient mice. They demonstrate a significant acceleration of wound re-epithelialization rate on the side treated with ADSC and survival of the transplanted ADSC within the dermal matrix up to 12 days post-implantation. At D7, the thickness of granulation tissue was increased (134 ± 49.3 vs. 76 ± 26 mm; P = 0.043) and the number of neo-formed capillaries was significantly higher (21.1 ± 25.3 vs. 7.3 ± 0.29 per field of study P = 0.043). Foubert et al. found similar results in pigs by inoculating a collagen-based matrix (Integra®) with the stromal vascular fraction obtained after purification by the solution1 800 IV System of fatty tissue, with a concentration of 0.3 × 106 cells/cm2 [15]. The maturation of the wound bed tissue was accelerated in wounds treated with a dermal matrix seeded with ADSC compared to those treated with dermal matrix alone: the dermis obtained was of quasi-physiological morphology, with significantly higher collagen synthesis at D14 (p < 0.001), and higher microvascular density at D21 (p = 0.05).
2.1.7 Use in Conjunction with Allografts
The immunomodulatory properties of ADSC could be used to delay the rejection of allografts used as a temporary coverage solution. In a study in baboons, Bartholomew et al. demonstrated prolonged survival of allogenic skin graft after intravenous injection of MSC, whether or not the allograft and MSC were from the same donor (control group: 7. 0 ± 0 days, group that received total skin graft and MSC from the same donor: 11.3 ± 0.3 days, group that received MSC and total skin graft from different donors: 11.8 ± 1.4 days) [16]. Although there are no published clinical or pre-clinical studies to our knowledge reporting the use of ADSC to limit the rejection of skin allografts in a burn model, numerous studies have demonstrated the immunotolerance induced by the administration of ADSC in composite allotransplant models. Plock et al. compared MSC from bone marrow and adipose tissue in this indication and found similar activity of both cell types with prolonged survival beyond 27 days of 47% of grafts in the treated groups and total lysis of all grafts in the control group in rats [17].
2.1.8 Clinical Cases
No study to date reports the benefit of the use of ADSC in the management of human burn injuries. Several clinical trial procotol are recoded in ClinicalTrial.gov but the results are not yet being published. Rare clinical cases report the use of MSC from other origins used to manage patients after failure of conventionnal therapy. Rasulov et al. reported in 2005 the use of bone marrow-derived MSC for the management of a 45-year-old patient with major burn (40% TBSA), whom wounds deepened after every tangential excision procedure, due to a PseudomonasAeruginosa infection. They report a significant improvement in wound appearance after application of allogeneic MSC to the wound surface, with a significant analgesic effect. Healing was obtained after two more split thickness skin graft procedures. Recently, Jeschke et al. reported the use of MSC from amniotic membrane and umbilical cord in a patient with persistent wounds at 18 months after conventional surgical management of a major burn. Complete healing was achieved after local application of MSC followed by subcutaneous injection of MSC and platelet-rich plasma [18].
2.2 Extracutaneous Burns
The use of mesenchymal stem cells has also been proposed for the management of ophthalmologic burn lesions or lung lesions secondary to smoke inhalation and investigated in animal models. As with skin lesions, ongoing clinical trial are recorded on ClinicalTrial.gov. We have not found any studies reporting the use of ADSC or SVF in these indications where the use of mesenchymal stem cells derived from bone marrow or embryonic tissue were preferred.
2.3 Systemic Effects
The use of xenogeneic bone marrow-derived MSCs administered intramuscularly or subcutaneously induces an attenuation of pro-inflammatory cytokine release and an increase in TGF β, IL-10, and IL-12 anti-inflammatory cytokines in rats compared to controls with a reduction in renal, hepatic, and pulmonary damage secondary to systemic inflammatory response syndrome and a significant increase in survival rate [19, 20]. The impact of the administration of SVF or ADSC on the systemic inflammatory response syndrome secondary to burn injuries has not been evaluated in any of the previously mentioned studies.
3 Use of Fat Tissue in Tissue Engineering
3.1 Use for the Culture of Keratinocyte Sheets
Keratinocyte culture sheets are derived from the in vitro culture and expansion of keratinocyte stem cells extracted by physical or enzymatic methods from small skin grafts taken from the patient or from the donor. In vitro as well as in vivo, the multiplication of keratinocytes is dependent on the secretions of the growth factors by surrounding cells, mainly fibroblasts of the underlying dermis. The culture method initially described by Rheinwald and Green is based on the use of a feeder layer of fibroblasts, previously irradiated to prevent their multiplication, secreting these growth factors into the culture medium. This feeder layer can be replaced by a simple fibrin coating if the medium is supplemented in serum and growth factor are supplemented.
The cultured ADSC release many growth factors ADSC conditionned media can be used for the production of keratinocyte sheets as reported by Hassanzadeh et al. [21]. Paracrine secretion of ADSC is sufficient to promote keratinocyte growth and differentiation without the addition of a feeder layer, serum, or other growth factors in the culture medium. The quality of keratinocyte sheets obtained in vitro appears to be equivalent to that obtained with commercial media, but their use in vivo has not been reported to date.
3.2 Used in the Production of Reconstructed Skin
The production of reconstructed skin, 3D bilamellar structure, in vitro is currently carried out according to three principles: 1/by assembling multiple sheets of keratinocyte and fibroblast cultures (self-assembly), 2/by using a matrix seeded with fibroblasts for the reconstruction of the dermis secondarily covered with keratinocytes allowing the reconstruction of the epidermis, and3/by means of 3D bioprinting, a technique allowing the direct printing of the cells contained in a matrix then called bio-ink. ADSC can be used as a source of stem cells for the production of reconstructed skin regardless of the production method used. The advantage would be their ease of access from liposuction or tangential excisionprocedure operative residues in patients with extensive burns.
As ADSC spontaneously differentiate into fibroblasts and they can be used as a replacement for dermal fibroblasts in dermal reconstitution. Trottier et al. compared reconstructed skin made using the self-assembly method in which the dermal portion was reconstructed either by dermal fibroblasts or by ADSC more or less predifferentiated towards the adipogenic lineage. The reconstituted dermis allowed the development of an epidermis without significant difference according to the origin of the cells used for their construction. Twenty-one days after grafting on an immunocompromised mouse model, the reconstructed skin presented a well-differentiated, proliferative epidermis with a preserved and continuous dermal-epidermal junction, without significant difference according to the origin of the cells used.
It also appears that ADSC can trans differentiate into keratinocytes and could therefore be used in the reconstruction of the epidermis. Indeed, Irfan-Maqsood et al. found that ADSC co-cultured with keratinocytes started to express keratinocyte lineage markers, namely K5, K10, K14, K18, K19, and involucrine, after 14 days [1].
ADSC could also be useful in the regeneration of reconstructed skin models that are more complex than bilaminar models. Trottier et al. showed that it was possible to produce a trilamellar skin substitute incorporating an epidermis, dermis, and near-hypodermal structure by adding to bilamellar reconstructed skin models produced by self-assembly sheets of ADSC and mature adipocytes obtained by differentiation [1]. ADSCs could also be used in the reconstruction of dermal vascular networks, thanks to their paracrine action but also to their ability to differentiate into endothelial cells. In the study carried out by Sanchez-Munoz et al., it is the paracrine action of ADSC which is used to promote the development of microvascular networks from umbilical cord endothelial cells. Addition of ADSC to their models of endothelialized reconstructed skin allowed the development of tubular structure in 15 days within the reconstructed dermis whereas similar structures were absent in the classic model. Auxenfans et al. showed that ADSC can be predifferentiated into microvascular cells by culture in VEGF-rich media [2]. After the inoculation in collagenic matrices with human fibroblasts, these cells formed tubular structures similar to a dermal microvascular network (Figs. 40.7 and 40.8). This predifferentiation step in culture in VEGF-rich media appears to be essential for the formation of microvascular networks in their models, indeed, the inoculation of non-predifferentiated ADSCs does not allowed the formation of these microvascular networks.
Histological and immunoflurorescence analysis of native human skin, and human skin equivalent (SE) prepared with or without predifferentiated endothelial cells derived from adipose tissue (ASC-ECs) Top row: The Hematoxyline, Phloxine, Saffron (HPS) staining allow the visualisation of the cell nuclei in blue, cytoplasm in pink and the ECM of connective tissue in orange/yellow second, third and fourth row:Immunofluorescence labeling of endothelial markers (EN4 and vWF) and basement membrane protein laminin 5. Immunolabeling of endothelial markers (EN4 and vWF) and basement membrane protein laminin 5 is shown in green, with cell nuclei (DAPI) either in blue (vWF), or red (EN4, laminin 5). Scale bars = (HPS) 50 μm; (EN4, vWF, laminin) 25 μm
Up to date, no clinical application in humans with reconstructed skin containing ADSC has been reported, but we could imagine their future use in reconstructed skin models previously matured in vitro, as previously reported during in vitro studies, or as a source of stem cells for in vivo 3D bioprinting. In vivo 3D bioprinting consist in the in situ printing on the wound bed of a bio-ink loaded with stem cells, followed by In Vivo maturation of the construct. The patient's body acts as a bio-reactor delivering the nutrients, the hormonal environment, and the oxygen necessary for the multiplication and differentiation of the cells to obtain the formation of a new skin. In this latter application, the use of SVF could be particularly favorable since it would allow to be free from the constraints of prior In Vitro culture expansion, and therefore from the related medico-legal constraints.
4 Discussion: Administration Modalities
4.1 Autologous versus Allogeneic ADSC
After major burns, adipose tissue and burned tissue (dermis) appear to be the main sources of autologous MSC cells from the cord umbilical are by definition of allogeneic origin, as long as no systematic conservation of the umbilical cords specific to each individual is carried out and the use of bone marrow-derived MSC obtained by spinal cord puncture could lead to significant infectious and hemorrhagic complications in the burn patients. In addition, the amount of bone marrow harvestable is very limited, with a significant reduction in the number of harvestable cells with age.
The autologous or allogeneic nature of the cells to be used is a matter of debate. It has been shown that ADSCs weakly express HLA markers and secrete immunosuppressive factors inducing an immune tolerance [22]. However, animal model studies report low and rapidly decreasing survival rates of the cells after implantation (27% at 7 days, 7.6% at 14 days, and 2.5% at 28 days in mice [23]. None of these studies specified the tolerance of ADSC-derived cells nor study the induction of alloimmunization. Until proven otherwise, exploitation of the differentiation potential and long lasting engrafment of ADSC in skin wound repair or tissue engineering requires the use of autologous cells. Therapeutic efficacy of allogeneic cells are linked to their paracrine activity and limited in time.
The use of autologous ADSC, which will survive permanently after implantation, raises the problem of possible tumorigenesis, which is favored by successive passages in culture. Futhermore, after extensive burns, the adipose tissue undergoes notable alterations with a change in its macroscopic appearance known as “browning” accompanied by cellular modifications of the mature adipocytes (reduction in the size of the adipocytes, multiocular aspect, increase in the number of mitochondria and in the expression of UCP1), the mechanism of which is still being studied [24]. These alterations appear to be involved in the long-lasting metabolic disorders observed after extensive burning. Therefore, the potential impact of the burn on the ADSC need to be asses.
The absence of alterations in dermal MSCextracted from operative residues of burned tissue was verified in a previous study by Jeschke et al. [25]. With respect to MSC derived from adipose tissue, although severe burns have been shown to induce a prolonged inflammatory response in subcutaneous adipose tissue, these stem cells do not appear to be affected by this phenomenon [24, 26]. In animal model burn lesions dit not alter the expression of surface markers, nether increase inflammatory cytokine production, or alter the alter proliferation or the differentiation capabilities of the ADSC. No significant chromosomal alterations were found as a result of the burn or the different passages in culture. The use of autologous cells therefore seems possible. Prasai's work in small animals seems to indicate that it will be more favorable to take a sample at 48 h from the initial burn, at which time the ADSC presents an anti-inflammatory profile with a decrease in the expression of the pro-inflammatory cytokines IL-6, MCP-1 (or CCP-1) [26].
4.2 How to Collect the Cells to be Used in the Burned Patient
SVF and ADSC are usually obtained by purification of lipoaspirate. In the patient with major burns, obtaining autologous cells by liposuccion seems difficult due to the septic risk and blood loss and could only be considered for very early management as long as the burn wounds are considered sterile. The sampling modality that seems the most suitable for clinical practice is the use of tangential excision procedure operative residues. It has been showned that MSC can be extracted from these operative residues, and incorporated into dermal regeneration matrix after characterization and In Vitro expansion and retained their therapeutic potential to treat burn lesion in mouse or pig models [25].
4.3 Administration Modalities
Several routes of administration are proposed for the administration of ADSC in the management of burn wounds: intravenous injection, local injection, or application to the wound bed in suspension or within various types of scaffold (collagen-based dermal regeneration matrix, fibrin sealant, complex bio-ink or amniotic membrane). Only one study compared different routes of administration and found similar benefits between local injection and topical application [27]. The studies conducted using intravenous injection of these cells showed that the ADSC migrated spontaneously to the injured site in aso-called homing phenomenon within 4 days. To date no study compared this mode of administration to the previous ones [9].
4.4 Medico-Legal Issue of Using ADSC Pour Burn Treatment
Medico-legal issue linked to ADSC use for burn treatment could be important if a laboratory step of culture for isolation or expansion, or maturation is needed as in ADSC cell therapy or tissue engineering. Since 2007 in Europe, these treatments are considered to be advanced therapy medicinal products (which include somatic cell therapy medicinal product, tissue-engineered medicinal product, gene therapy medicinal product). They should therefore be subject to good manufacturing practice for medicinal products (guidelines edited by EU) which include a dedicated manufacturing facility, with a strictly aseptic environment, a qualified and certificated personal, and strict production and validation procedures.
The use of SVF could be more favorable, as it does not contain cells that have been subject to substantial manipulation and contain cells that are intended to be used for the same essential functions in the recipient and the donor and therefore does not fall within the definition of an advanced therapy medicinal product. The use of SVF in a procedure of in vivo 3D bioprinting, may allow to avoid major medico-legal constraint as no laboratory handling step will be necessary to product the reconstructed skin.
5 Conclusion
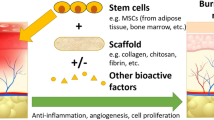
Fat stem cell research promises to improve our ability to treat and care for the burn patients. As studies progress, stem cells are demonstrating their potential in promoting the local regenerative capabilities of superficial burns, STSG donor sites, and expanded STSG, and their value in the production of better-quality skin substitutes (Fig. 40.9). Their involvement and effects in the regulation of the inflammatory syndrome and metabolic alterations that accompany extensive burns could also make them a major therapeutic tool. The translation of stem cell therapy from the laboratory to the bedside over the next few decades promises to have a significant impact on standards of burn care.
Potential therapeutic indication for SVF and ADSC in the management of acute burns. **SVF or ADSC have proven their potential efficiency for these different application through animal studies, *MSC have proven their potential efficiency for these different application through animal studies, and potential efficiency of SVF or ADSC is suspected but not yet proven, ¤ only in vitro study have been realized, § use of ASC or SVF in this indication have been reported in clinical cases
References
Trottier V, Marceau-Fortier G, Germain L, Vincent C, Fradette J. IFATS collection: Using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells Dayt Ohio. 2008;26(10):2713–23.
Auxenfans C, Lequeux C, Perrusel E, Mojallal A, Kinikoglu B, Damour O. Adipose-derived stem cells (ASCs) as a source of endothelial cells in the reconstruction of endothelialized skin equivalents. J Tissue Eng Regen Med. 2012;6(7):512–8.
Lee DE, Ayoub N, Agrawal DK. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther. 2016;7:37.
Hsiao ST-F, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, et al. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012;21(12):2189–203.
Ding J, Ma Z, Shankowsky HA, Medina A, Tredget EE. Deep dermal fibroblast profibrotic characteristics are enhanced by bone marrow-derived mesenchymal stem cells. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2013;21(3):448–55.
Öksüz S, Ülkür E, Öncül O, Köse GT, Küçükodaci Z, Urhan M. The effect of subcutaneous mesenchymal stem cell injection on statis zone and apoptosis in an experimental burn model. Plast Reconstr Surg. 2013;131(3):463–71.
Eyuboglu AA, Uysal CA, Ozgun G, Coskun E, Markal Ertas N, Haberal M. The effect of adipose derived stromal vascular fraction on stasis zone in an experimental burn model. Burns J Int Soc Burn Inj. 2018;44(2):386–96.
Atalay S, Coruh A, Deniz K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns J Int Soc Burn Inj. 2014;40(7):1375–83.
Foubert P, Liu M, Anderson S, Rajoria R, Gutierrez D, Zafra D, et al. Preclinical assessment of safety and efficacy of intravenous delivery of autologous adipose-derived regenerative cells (ADRCs) in the treatment of severe thermal burns using a porcine model. Burns J Int Soc Burn Inj. 2018;44(6):1531–42.
Loder S, Peterson JR, Agarwal S, Eboda O, Brownley C, DeLaRosa S, et al. Wound healing after thermal injury is improved by fat and adipose-derived stem cell isografts. J Burn Care Res Off Publ Am Burn Assoc. 2015;36(1):70–6.
Chen Y-W, Scutaru TT, Ghetu N, Carasevici E, Lupascu CD, Ferariu D, et al. The effects of adipose-derived stem cell-differentiated adipocytes on skin burn wound healing in rats. J Burn Care Res Off Publ Am Burn Assoc. 2017;38(1):1–10.
Bliley JM, Argenta A, Satish L, McLaughlin MM, Dees A, Tompkins-Rhoades C, et al. Administration of adipose-derived stem cells enhances vascularity, induces collagen deposition, and dermal adipogenesis in burn wounds. Burns J Int Soc Burn Inj. 2016;42(6):1212–22.
Motamed S, Taghiabadi E, Molaei H, Sodeifi N, Hassanpour SE, Shafieyan S, et al. Cell-based skin substitutes accelerate regeneration of extensive burn wounds in rats. Am J Surg. 2017;214(4):762–9.
Burmeister DM, Stone R, Wrice N, Laborde A, Becerra SC, Natesan S, et al. Delivery of allogeneic adipose stem cells in polyethylene glycol-fibrin hydrogels as an adjunct to meshed autografts after sharp debridement of deep partial thickness burns. Stem Cells Transl Med. 2018;7(4):360–72.
Foubert P, Barillas S, Gonzalez AD, Alfonso Z, Zhao S, Hakim I, et al. Uncultured adipose-derived regenerative cells (ADRCs) seeded in collagen scaffold improves dermal regeneration, enhancing early vascularization and structural organization following thermal burns. Burns J Int Soc Burn Inj. 2015;41(7):1504–16.
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–8.
Plock JA, Schnider JT, Zhang W, Schweizer R, Tsuji W, Kostereva N, et al. Adipose- and bone marrow-derived mesenchymal stem cells prolong graft survival in vascularized composite allotransplantation. Transplantation. 2015;99(9):1765–73.
Jeschke MG, Rehou S, McCann MR, Shahrokhi S. Allogeneic mesenchymal stem cells for treatment of severe burn injury. Stem Cell Res Ther. 2019;10(1):337.
Caliari-Oliveira C, Yaochite JNU, Ramalho LNZ, Palma PVB, Carlos D, de Queiróz Cunha F, et al. Xenogeneic mesenchymal stromal cells improve wound healing and modulate the immune response in an extensive burn model. Cell Transplant. 2016;25(2):201–15.
Yagi H, Soto-Gutierrez A, Kitagawa Y, Tilles AW, Tompkins RG, Yarmush ML. Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn. Cell Transplant. 2010;19(6):823–30.
Hassanzadeh H, Matin MM, Naderi-Meshkin H, Bidkhori HR, Mirahmadi M, Raeesolmohaddeseen M, et al. Using paracrine effects of Ad-MSCs on keratinocyte cultivation and fabrication of epidermal sheets for improving clinical applications. Cell Tissue Bank. 2018;19(4):531–47.
Najar M, Raicevic G, Fayyad-Kazan H, Kazan HF, De Bruyn C, Bron D, et al. Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: the expression and impact of inflammatory priming. Stem Cell Rev Rep. 2012;8(4):1188–98.
Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells Dayt Ohio. 2007;25(10):2648–59.
Patsouris D, Qi P, Abdullahi A, Stanojcic M, Chen P, Parousis A, et al. Burn induces browning of the subcutaneous white adipose tissue in mice and humans. Cell Rep. 2015;13(8):1538–44.
Amini-Nik S, Dolp R, Eylert G, Datu A-K, Parousis A, Blakeley C, et al. Stem cells derived from burned skin—the future of burn care. EBioMedicine. 2018;37:509–20.
Prasai A, El Ayadi A, Mifflin RC, Wetzel MD, Andersen CR, Redl H, et al. Characterization of adipose-derived stem cells following burn injury. Stem Cell Rev Rep. 2017;13(6):781–92.
Foubert P, Gonzalez AD, Teodosescu S, Berard F, Doyle-Eisele M, Yekkala K, et al. Adipose-derived regenerative cell therapy for burn wound healing: a comparison of two delivery methods. Adv Wound Care. 2015;5(7):288–98.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Brosset, S., Alkhotani, M., Boucher, F., Shipkov, H., Auxenfans, C., Mojallal, A.A. (2022). Fat and Stromal Cells for Acute Burn Treatment. In: Kalaaji, A. (eds) Plastic and Aesthetic Regenerative Surgery and Fat Grafting. Springer, Cham. https://doi.org/10.1007/978-3-030-77455-4_40
Download citation
DOI: https://doi.org/10.1007/978-3-030-77455-4_40
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-77454-7
Online ISBN: 978-3-030-77455-4
eBook Packages: MedicineMedicine (R0)