Abstract
Astroglia are key regulators of synaptic function, playing central roles in homeostatic ion buffering, energy dynamics, transmitter uptake, maintenance of neurotransmitter pools, and regulation of synaptic plasticity through release of neuroactive chemicals. Given the myriad of crucial homeostatic and signaling functions attributed to astrocytes and the variety of neurotransmitter receptors expressed by astroglia, they serve as prime cellular candidates for establishing maladaptive synaptic plasticity following drug exposure. Initial studies on astroglia and addiction have placed drug-mediated disruptions in the homeostatic regulation of glutamate as a central aspect of relapse vulnerability. However, the generation of sophisticated tools to study and manipulate astroglia have proven that the interaction between addictive substances, astroglia, and relapse-relevant synaptic plasticity extends far beyond the homeostatic regulation of glutamate. Here we present astroglial systems impacted by drug exposure and discuss how changes in astroglial biology contribute to addiction biology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Overview of Addiction Biology
Circuitry and Transmitters
The elevation of extracellular dopamine in the ventral striatum in response to salient and rewarding stimuli underlies several reinforcement and addiction-related behaviors extensively studied in preclinical models of substance use and relapse (Scofield et al. 2016a). Anatomically, the ventral striatum is divided into two subregions, the nucleus accumbens core (NAcore) and shell (NAshell), which both receive dopaminergic innervation from the midbrain nucleus the ventral tegmental area (VTA) (Ikemoto 2007) and that are both involved in encoding reward and motivation. Increased levels of extracellular dopamine in the ventral striatum are evoked by administration of addictive drugs (reviewed in (Willuhn et al. 2010)) including alcohol (Howard et al. 2008), as well as by sucrose intake (Bassareo et al. 2017), and a substantial body of lesion, microdialysis, voltammetry, and pharmacology studies has led to the general conception that the NAshell is involved in encoding the rewarding effects of addictive drugs, while the NAcore coordinates behavioral outputs in response to salient stimuli (Scofield et al. 2016a; Sellings and Clarke 2003; Salgado and Kaplitt 2015).
Though natural (i.e., sucrose) as well as pathological (i.e., drugs of abuse) rewards elevate dopamine in the ventral striatum, dopaminergic signaling within this circuit is modulated by glutamatergic transmission, and glutamatergic signaling is uniquely disrupted following repeated intake of addictive substances (Kalivas et al. 1989, 2009; Kalivas 2009). In keeping with important but separable roles for these two transmitters in addiction-related behaviors are recent studies demonstrating that subpopulations of dopaminergic VTA neurons that project to the NAshell co-release glutamate along with dopamine (Stuber et al. 2010; Mongia et al. 2019; Mingote et al. 2019). Glutamate release from dopamine neurons in the NAshell is linked to intake of sucrose and psychostimulants and locomotor responses to acute and repeated psychostimulant administration (Hnasko et al. 2010; Alsio et al. 2011; Birgner et al. 2010; Bimpisidis and Wallen-Mackenzie 2019). Glutamate modulates dopamine neurotransmission through its action on glutamate receptors expressed on dopaminergic terminals (Floresco et al. 1998; Howland et al. 2002) and through facilitation of dopamine loading in synaptic vesicles (Hnasko et al. 2010; Hnasko and Edwards 2012). Though VTA inputs to the NAcore contribute to drug seeking induced by drug prime (Shen et al. 2014a), the impact of dopamine transmission on seeking behavior can be blocked by glutamate receptor antagonists even in the presence of dopamine (Cornish and Kalivas 2000), consistent with a critical role for glutamatergic transmission in drug seeking behavior.
The ventral striatum receives excitatory input from a number of brain regions, in addition to VTA. Cortical regions provide glutamatergic input to the NAcore and NAshell, with prelimbic (PL) cortical afferents in the NAcore or infralimbic (IL) afferents in the NAshell contributing to drug seeking or refraining behavior, respectively. Glutamate transmission in the NAcore is necessary for initial learning of drug-reward associations (Kelley et al. 1997; Smith-Roe and Kelley 2000) and later for retrieval of drug-contingency information during reinstatement of drug seeking following extinction of behavioral responding (Kalivas 2009; Kalivas and Volkow 2005; Koob and Volkow 2010). PL afferents in the NAcore are engaged during cue-, drug-, context- and stress-reinstated seeking of different classes of addictive substances, including opioids, psychostimulants, and alcohol (Rogers et al. 2008; Ball and Slane 2012; Rocha and Kalivas 2010; Doncheck et al. 2020; McFarland et al. 2003; Willcocks and McNally 2013; Chaudhri et al. 2008). The IL input to the NAshell, on the other hand, is recruited during extinction training, whereby operant responding for drug reinforcers is gradually reduced over time when the drug is no longer available (Peters et al. 2008).
The NAcore also receives significant glutamatergic input from basolateral amygdala (BLA) that contributes to both acquisition of self-administration and reinstatement initiated by cues (Carelli et al. 2003; Whitelaw et al. 1996; Di Ciano and Everitt 2004; Puaud et al. 2020), through direct glutamatergic projections to the NAcore and/or projections to the PL (Stefanik and Kalivas 2013). BLA glutamate may also be engaged to a certain extent by operant training for natural reinforcers, since optogenetically stimulating BLA projections to the NAcore can initiate responding for sucrose (Stuber et al. 2011). A number of other brain regions have been strongly implicated in promoting addiction-related behaviors, including dorsal and ventral hippocampus (Fuchs et al. 2005; Lasseter et al. 2010; Atkins et al. 2008), ventral subiculum (Bossert and Stern 2014), and lateral septum (McGlinchey and Aston-Jones 2018), though not necessarily via direct NAcore projections. Interestingly, excitatory projections to the NAcore can also suppress seeking behavior, since glutamate release from the paraventricular thalamus in the NAcore serves to suppress seeking behavior in the absence of a reinforcer (Do-Monte et al. 2017), arguing that excitatory transmission in the NAcore is necessary, but not sufficient for reward seeking, and that unique innervation of postsynaptic targets may be an important factor in ultimate behavioral outputs.
Astrocytes are likely mediators of the long-lasting excitatory plasticity that arises within the corticostriatal circuitry in response to chronic drug intake. Astroglial processes are positioned proximal to excitatory synapses, and their expression of neurotransmitter transporters regulates stimulation of pre- and postsynaptic receptors. Moreover, astroglia are capable of signaling directly to synapses through transmitter release and play critical roles in homeostatic regulation of synapses. Astroglial adaptations following exposure to addictive drugs are being actively investigated within the circuits outlined above. Given that vast brain region-dependent heterogeneity in astroglial structure and transcriptomic profiles has recently been described (Batiuk et al. 2020; Matias et al. 2019; Cuevas-Diaz Duran et al. 2019; Kohler et al. 2019), astrocyte function as it pertains to addiction is likely unique in each region. Below we explore what is known regarding astroglial contributions to synaptic physiology at baseline and after use of addictive substances.
Transmitter Uptake
Glutamate
The glutamate transporter GLT-1 (EAAT2 in humans) conducts the majority of glutamate uptake and is mostly expressed by astroglia in the adult brain (Danbolt 2001), despite reports of low-level expression in some neurons (Rimmele and Rosenberg 2016). GLT-1 has long been thought to contribute to addiction-related glutamatergic dysregulation in the ventral striatum since its expression and/or function is disrupted after long-term use of psychostimulants, opioids, and alcohol (Roberts-Wolfe and Kalivas 2015). GLT-1 knockdown in astroglia during the early postnatal period leads to repetitive behaviors in drug-naïve animals and enhanced neuronal excitability in the dorsal striatum (Aida et al. 2015). In rats trained to self-administer cocaine, GLT-1 downregulation in the NAcore is dependent upon withdrawal duration as well as cocaine intake, with increasing amounts of either exacerbating the severity of GLT-1 downregulation (Fischer-Smith et al. 2012). Further, extended withdrawal from long-access cocaine self-administration (i.e., daily 6-hour sessions) reduces mRNA levels of the predominant of three GLT-1 isoforms, GLT-1a (Holmseth et al. 2009), in the NAcore, coincident with enhanced methylation of Slc1a2, the gene that encodes GLT-1 (Kim et al. 2018). Since the authors of this study found no changes in GLT-1a or b mRNA levels after withdrawal or extinction from short access cocaine self-administration, but many other studies show GLT-1 downregulation in these paradigms (Roberts-Wolfe and Kalivas 2015), it may be that posttranslational rather than transcriptional mechanisms are responsible for its reduced expression and/or function in short-access models. Interestingly, up- or downregulation of GLT-1 in the striatum disrupts spike-timing dependent plasticity, a type of long-term potentiation that requires a tight temporal relationship between pre- and postsynaptic activity (Valtcheva and Venance 2016). When the action of GLT-1 is blocked, long-term potentiation requires signaling through extrasynaptic GluN2B-containing NMDA receptors. Similarly, signaling through GluN2B is required for cue- or heroin prime-induced reinstatement of heroin seeking (Shen et al. 2011). These data are consistent with diminished function of GLT-1 after extinction from heroin self-administration (Shen et al. 2014b). Further, disrupted forms of synaptic plasticity involving downregulated GLT-1 after drug use and/or withdrawal may conceivably impair mechanisms required for extinction learning, which is somewhat impaired in drug-trained compared with sucrose-trained animals (Martin-Fardon and Weiss 2017). Notably, operant sucrose training does not produce downregulation of GLT-1 (Kruyer and Kalivas 2020a), which may account for these behavioral differences.
A significant body of work has established that compounds that restore GLT-1 expression reduce drug craving and relapse, both in animal models and in human clinical trials (Roberts-Wolfe and Kalivas 2015). For example, the antioxidant N-acetylcysteine, the ß-lactam antibiotic ceftriaxone, and the xanthine derivative propentofylline have all demonstrated promise in restoring GLT-1 expression after drug withdrawal and in blunting reinstated drug seeking in preclinical models (summarized in (Scofield et al. 2016a)). N-acetylcysteine is extremely safe for use in humans but has proven only marginally efficacious in reducing craving and drug use in patients (summarized in (Roberts-Wolfe and Kalivas 2015)), potentially due to limited bioavailability. Given that recent studies indicate that GLT-1 upregulation alone is not sufficient to blunt reinstated cocaine seeking (Logan et al. 2018), it may very well be that additional manipulations are required to adequately suppress relapse vulnerability. Moreover, it may be the case that upregulation rather than homeostatic restoration of GLT-1 expression may not entirely restore mechanisms of synaptic plasticity, as both GLT-1 blockade and overexpression do not properly permit spike timing-dependent plasticity at striatal synapses (Valtcheva and Venance 2016).
Although drug-induced changes in GLT-1 expression after chronic use of addictive drugs have been less well documented in the midbrain when compared to the ventral striatum, astroglial glutamate transporters in the VTA also play an important role in modulating avoidance behaviors (Gomez et al. 2019). As mentioned above, the VTA sends dopaminergic, glutamatergic, as well as GABAergic projections to the nucleus accumbens, and these projections encode the reinforcing and aversive aspects of drug use (Morales and Margolis 2017). Additionally, GABAergic interneurons within the VTA regulate dopaminergic projection neurons that signal avoidance vs. motivated approach behaviors (McCullough et al. 1993; Tan et al. 2012). This was demonstrated with optogenetic stimulation of VTA astrocytes, which permits excitation of GABAergic interneurons that inhibit dopaminergic projections and promote avoidance behavior (Gomez et al. 2019). It was subsequently shown that astrocyte-dependent facilitation of GABAergic activation depends on GLT-1, as conditional knockout of GLT-1 in VTA astrocytes impairs the optogenetic stimulation-mediated GABAergic excitation (Gomez et al. 2019). While the authors do not clarify the mechanism by which GLT-1 contributes to GABAergic excitation, they illustrate that manipulation of astroglial GLT-1 interferes with conditioned place avoidance, but does not impact conditioned place preference (Gomez et al. 2019), suggesting divergence of these circuits and a contribution of astrocytes through GLT-1 to avoidance, but not approach behavior (Gomez et al. 2019). Although changes in GLT-1 expression have not been as clearly demonstrated in the VTA after drug use and withdrawal compared with the NAcore (Knackstedt et al. 2009), analysis of GLT-1 function may be warranted given these surprising findings. These data also highlight the importance of analyzing transporter function in a pathway-specific manner, rather than in whole tissue, since functional changes that impact discrete subcircuits may not be discernable when protein levels are assessed in tissue extracts.
GABA
While uptake of glutamate release from cortical terminals in the striatum is thought to directly regulate relapse-like behaviors in animal models (Kalivas 2008), cortical stimulation triggers uptake of both GABA and glutamate by striatal astrocytes and clearance of both transmitters contributes to postsynaptic excitation of MSNs (Goubard et al. 2011). These findings illustrate the relevance of local GABAergic signaling on corticostriatal synaptic transmission and the contribution of transporter uptake in regulating striatal outputs. In support of this concept, studies where Ca2+ signaling in astrocytes is inhibited in vivo through viral delivery of a genetically encoded plasma membrane Ca2+ pump that expels cytosolic Ca2+ from astrocytes (CalEx) (Yu et al. 2018) demonstrate the behavioral involvement of GABA transporters expressed on striatal astrocytes in compulsive-like behaviors. Importantly, the CalEx vector reduces astroglial intracellular Ca2+ levels at baseline and also reduces the amplitude and duration of Ca2+ elevations. When delivered to astroglia in the dorsolateral striatum, CalEx expression results in upregulation of the largely astroglial GABA transporter GAT-3 and produces excessive self-grooming behavior, reminiscent of features of obsessive-compulsive disorder (Yu et al. 2018). Further, GAT-3 upregulation reduces tonic inhibition, a known role for GAT-3 that is expressed most densely in extrasynaptic zones (Melone et al. 2015), selectively at D1 receptor expressing MSNs (D1-MSNs) (Yu et al. 2018). Interestingly, when a different strategy was used to upregulate astroglial GAT-3 in a pathway-nonspecific manner, the authors found no impact on grooming behavior, suggesting that perhaps the cell selectivity of the effects were necessary for the disrupted behavior observed by the authors.
The dorsal striatum is involved in habit learning (Yin et al. 2004) and is uniquely recruited in cases of substance use disorder characterized by compulsive seeking. For example, in humans who drink socially, but not compulsively, alcohol-associated cues stimulate activation of the ventral striatum. Instead, the same cues stimulate activation of the dorsal rather than the ventral striatum in heavy drinkers, and ventral striatal activity in response to alcohol-associated cues is negatively associated with measures of compulsive craving in these individuals (Vollstadt-Klein et al. 2010). Interestingly, compounds effective in suppressing compulsive behaviors, like N-acetylcysteine, which reduces symptoms of trichotillomania (Farhat et al. 2020) and pathological gambling (Grant et al. 2007), also reduce reinstated drug seeking in animal models (Kalivas and Kalivas 2016). As mentioned above, N-acetylcysteine was most studied in the context of addiction for its ability to upregulate GLT-1 in the ventral striatum, but its impact on astroglial expression of GABA transporters like GAT-3 in the ventral or dorsal striatum has not been assessed.
Dopamine
Although there is in vitro evidence that astrocytes can recover extracellular dopamine through expression of the dopamine transporter (DAT), norepinephrine transporter (NET), and/or plasma membrane monoamine transporter (PMAT) (Pelton 2nd et al. 1981; Takeda et al. 2002; Naganuma et al. 2014), evidence is less clear-cut in vivo. The organic cation transporter 3 (OCT3), a low-affinity monoamine transporter, has been reported on neurons and astroglia in the rodent substantia nigra and striatum (Cui et al. 2009), and electron microscopy studies revealed OCT3 on the plasma membrane of perisynaptic astroglial processes in the rodent amygdala, consistent with its role in monoamine uptake near synaptic sites (Gasser et al. 2017). OCT3 was recently found to function in reverse in the presence of amphetamine, releasing dopamine into the extracellular space (Mayer et al. 2018). The authors also confirmed that while OCT3 was expressed by dopaminergic neurons, the majority of OCT3-expressing cells were likely glia as well as non-dopaminergic neurons (Mayer et al. 2018). Importantly, while astroglia express OCT3 and therefore may also theoretically employ this mechanism, releasing dopamine in the presence of amphetamine, amphetamine enters into dopaminergic neurons through DAT and not through OCT3 (Mayer et al. 2018). Thus, in the absence of a mechanism for amphetamine uptake by astroglia, astroglial OCT3 is not likely to be involved in this process. Because studies on the contribution of OCT3 to dopamine transport in the striatum and elsewhere have emerged relatively recently (Holleran et al. 2020), there are no studies investigating changes in OCT3 expression in astrocytes following drug use and withdrawal in brain regions that contribute to addiction-related behaviors, though it remains a fundamental research question.
Gliotransmission
Glutamate
Gliotransmission by astrocytes is most often described as involving intracellular Ca2+ flux and vesicular exocytosis of transmitters, which signal to nearby neurons (Parpura et al. 1994; Araque et al. 2014; Papouin et al. 2017; Scofield 2018). Though electron microscopy and expression studies support the existence of vesicular release machinery in astrocytes, the relevance of this modality for gliotransmission has been contested by studies indicating a lack of glutamate receptor expression in astrocytes in the adult brain in vivo (Sun et al. 2013) as well as studies where deletion of key mediators of intracellular Ca2+ flux in astrocytes had little impact on neuronal function (Petravicz et al. 2014; Bazargani and Attwell 2016). Despite this, there are a number of well-established mechanisms by which astroglia signal to neurons through non-exocytotic mechanisms and without associated changes in intracellular Ca2+ (Agulhon et al. 2010; Gomez-Gonzalo et al. 2018). Here we will discuss evidence for exocytotic and non-exocytotic mechanisms of transmitter release given the important role for excitatory signaling in formation of drug-cue associations and in reinstatement of drug seeking.
Perhaps the least controversial mechanism for glutamate transmission by astroglia is through the cystine-glutamate antiporter, system xc-, which contributes to 60% of extracellular glutamate in the NAcore in drug-naïve animals (Baker et al. 2002). Extracellular glutamate derived from system xc- tonically stimulates presynaptic autoinhibitory mGluRs on glutamatergic and dopaminergic terminals, regulating transmitter release during neural firing (Baker et al. 2002). System xc-, which is expressed to the greatest extent by astroglia (Pow 2001; Sagara et al. 1993), is downregulated after chronic intake of psychostimulants in the NAcore, and this downregulation is thought to disrupt autoinhibitory tone at presynaptic mGluR2/3 on glutamatergic terminals, leading to enhanced glutamate release in response to drug-associated cues (Baker et al. 2002). It should be noted that system xc- is not similarly downregulated after chronic intake of opioids (Shen et al. 2014b), so this molecular adaptation may not generalize across all substances of abuse. However, the treatments described above that have been tested preclinically for their ability to attenuate reinstated seeking through restoration of GLT-1 , including N-acetylcysteine and ceftriaxone, also increase expression of system xc- (Knackstedt et al. 2010), and N-aceytylcysteine may even increase extracellular cystine levels, promoting cystine-glutamate exchange. The contribution of GLT-1 and system xc- upregulation to relapse suppression has been tested by pairing these pharmacological treatments with morpholino knockdown of either GLT-1 or xCT, a subunit of system xc-, in the NAcore prior to reinstatement. In these studies, the authors found that GLT-1, not xCT, had the greatest impact on reinstated drug seeking (Reissner et al. 2015).
Studies exploring the involvement of exocytotic transmitter release by astrocytes in addiction-related behaviors has made use of transgenic mice that express a dominant negative SNARE protein (dnSNARE) in astroglia. These mice lose the capacity to exocytose transmitter-containing vesicles through SNARE mechanisms in astroglia but retain normal mechanisms for neuronal exocytosis (Pascual et al. 2005). dnSNARE mice exhibit similar operant responding for food compared to wild-type animals but appear to take less cocaine during self-administration, despite similar rates of operant acquisition (Turner et al. 2013). dnSNARE mice also reinstate less robustly to cocaine-paired cues (Turner et al. 2013). Whether this reinstatement deficit derives in part from changes in cocaine intake is an important question, since dnSNARE animals appear to receive fewer cocaine infusions and consequently fewer cocaine-cue pairings during training. However, reinstatement of conditioned place preference (CPP) is also abolished in these mice, supporting a role for vesicular gliotransmission in reinstatement behaviors after exposure to psychostimulants (Turner et al. 2013). One limitation of these studies is that global dnSNARE expression in astroglia does not provide information regarding which brain region(s) orchestrate gliotransmission-dependent modulation of reinstatement. Interestingly, it was found that chemogenetic stimulation of Gq signaling in NAcore astrocytes, a manipulation that engages flux of internal Ca2+ and activates SNARE-dependent vesicular release of glutamate, reduces reinstated cocaine seeking initiated by cocaine-associated cues (Scofield et al. 2015). Similarly, astroglial Gq stimulation reduces methamphetamine (Siemsen et al. 2019) and ethanol seeking (Bull et al. 2014). It was demonstrated that the suppression of cocaine seeking using this technique was a consequence of astroglial-glutamate release that stimulated presynaptic mGluR2/3 and ultimately reduced transmitter release from presynaptic terminals (Scofield et al. 2015).
In another study, engaging Gq signaling in cultured astrocytes evoked non-vesicular glutamate release through the glutamate-permeable anion channel Bestrophin 1 (Best1) (Woo et al. 2012). Importantly, Best1 is situated near synapses in vivo and would be expected to raise extracellular glutamate levels less robustly than vesicular release. Consequently, release of glutamate through this modality is more likely to impact high-affinity NMDA receptors, compared with vesicular release which might also engage lower-affinity receptors. Accordingly, it might be expected that separate modalities of glutamate release from astrocytes engage functionally distinct intracellular signaling cascades in nearby neurons, despite both modalities being linked to Gq-coupled receptor activation. Together, the findings described above lead to important questions regarding how these cellular mechanisms are employed within the reward circuitry of drug-naïve animals and how their deployment may be impacted by chronic intake of and withdrawal from addictive substances.
One valid critique of studies where either optogenetic or chemogenetic manipulation of astrocytes is employed to understand astroglial biology is that the abundance of the exogenously expressed proteins, as well as their proximity to synapses, may not reflect normal physiological features of astroglia. Nonetheless, studies in the dorsal striatum and elsewhere clearly illustrate that astrocytes signal to local MSNs using endogenous mechanisms of glutamate release. Dorsal striatal astrocytes are uniquely tuned to respond functionally to electrical stimulation of D1- or D2-MSN subtypes in response to neuronally released endocannabinoids (eCBs), with Ca2+ flux and ultimately glutamate release (Martin et al. 2015). Astrocyte glutamate release produces slow inward currents (SICs) in adjacent neurons of the same subtype as the stimulated neuron, presumably through stimulation of extrasynaptic NMDA receptors (D’Ascenzo et al. 2007), but SICs are rare in heterotypic pairs. These findings are particularly relevant given that stimulation of striatal D1-MSN projections or inhibition of D2-MSN projections trigger reinstated seeking (Heinsbroek et al. 2017; Pardo-Garcia et al. 2019). There is not yet definitive evidence for how astrocytes distinguish between neural subcircuits. It is abundantly clear that striatal astrocytes encompass both D1- and D2-MSN somata within their cellular territories (Octeau et al. 2018), so spatial segregation does not seem a likely possibility. Further, studies using dye-filling strategies illustrate that astrocytes are extensively coupled to one another through gap junctions (Octeau et al. 2018), forming large densely interconnected syncytia. Thus, it does not appear that segregated astrocyte subpopulations are joined into networks through gap junctions, at least not in drug-naïve animals (but see “Homeostatic Functions” for a discussion of drug-induced changes in astrocyte gap junctions). Still, there are a number of hypothetical ways that astrocytes might coordinate signaling within, but not between circuits, when the same receptors and transmitters are employed in both pathways. One possibility is that additional signaling molecules accompany transmitter release and astrocytes selectively express receptors for molecules released by one or the other pathway. As an example, in the ventral pallidum, D1- and D2-MSN terminals co-release unique neuropeptides, with D1-MSNs co-releasing substance P and dynorphin and D2-MSNs co-releasing enkephalin and neurotensin (Kupchik et al. 2014). The same could occur postsynaptically, with the release of different eCBs following postsynaptic excitation of either D1- or D2-MSNs or unique expression patterns of cannabinoid receptors on astroglial subtypes. Whether release of unique pre- or postsynaptic signaling molecules selectively recruits astrocytes to signal to adjacent MSNs in a homotypic manner remains to be shown. Another possibility is that astrocytes are selective in their synaptic proximity, with each astrocyte exhibiting unique proximity to D1- or D2-MSNs in order to receive and transmit signals selectively. While it does not appear that D1 or D2-MSN-containing synapses receive different degrees of astrocyte insulation overall, there is tremendous variability in synaptic insulation by astroglia in the striatum (Octeau et al. 2018; Chai et al. 2017). Thus, it remains possible that each astrocyte may exhibit some selectivity in which synapses it approaches most closely. This concept will be discussed in more depth in the subsequent section on astrocyte morphological plasticity.
D-Serine
D-serine is the R-enantiomer of the amino acid serine and functions as an NMDA receptor co-agonist, with selective affinity for the glycine binding site (Mothet et al. 2000; MacKay et al. 2019). D-serine has been studied for its improvement of positive and cognitive symptoms in schizophrenia patients when co-administered with antipsychotics (Tsai et al. 1998), based on evidence of hypofunctional NMDA receptor activity in the disorder (Coyle 2012). Whether D-serine is released by astrocytes has been a somewhat controversial topic since serine racemase, the enzyme that converts L-serine to D-serine in cells, is mostly expressed by neurons (Yoshikawa et al. 2007; Benneyworth et al. 2012). It appears that instead, D-serine is produced by serine racemase in neurons and is shuttled between neurons and astroglia for vesicular release (Wolosker 2011; Martineau et al. 2013). Regardless of its cellular origin, D-serine has been shown to play an important role in signaling via NMDA receptors in the accumbens. D-serine is required for NMDA-dependent synaptic potentiation and depression in the NAcore (Curcio et al. 2013), both of which are disrupted after withdrawal from cocaine self-administration (Moussawi et al. 2009). Linking these findings, researchers found that cocaine reduces D-serine concentration in the NAcore through increased expression of D-amino acid oxidase, an enzyme that degrades D-serine (Curcio et al. 2013; D’Ascenzo et al. 2014). The reduction in D-serine levels increases the relative proportion of AMPA/NMDA receptors in neurons postsynaptically and permits locomotor sensitization and CPP (Curcio et al. 2013; D’Ascenzo et al. 2014; Yang et al. 2013). Subsequent studies found that morphine also decreases D-serine in the nucleus accumbens and does so by reducing surface expression of AMPA receptors on astrocytes that normally trigger Ca2+ flux and vesicular D-serine release (Wu et al. 2017). Despite these promising findings, additional experimentation is required to determine the precise role of D-serine gliotransmission in mediating addiction-relevant synaptic plasticity.
ATP
Astrocyte-derived adenosine has been shown to contribute to synaptic modulation in various brain regions and in response to synaptically released glutamate as well as dopamine (Pascual et al. 2005; Zhang et al. 2003; Quon et al. 2018; Corkrum et al. 2020). In the dorsolateral striatum, high-frequency stimulation of cortical terminals stimulates astroglial mGluR5, a Gq-coupled receptor, to produce Ca2+ increases, ATP release, and adenosine receptor-mediated long-term depression of postsynaptic cells (Cavaccini et al. 2020). In a similar study in the dorsomedial striatum, the authors show that astroglial expression of the adenosine transporter equilibrative nucleoside transporter 1, ENT1 is required for astroglial Gq stimulation to impact neural activity. Interestingly, triggering this signaling cascade alters firing properties of both D1- and D2-MSNs, reducing spontaneous EPSCs in D1- and increasing them in D2-MSNs, promoting goal-directed rather than habitual behaviors (Kang et al. 2020).
Altogether, Gq-dependent signaling in striatal astrocytes has been linked to vesicular glutamate release (Scofield et al. 2015), slow and low-volume glutamate transmission through Best1 (Woo et al. 2012), and adenosine transport through ENT1 (Kang et al. 2020), and activation of astroglial mGluR5, a Gq-coupled receptor, is linked to astrocyte release of ATP (Cavaccini et al. 2020). Perhaps it is not problematic that such a diverse range of outcomes can be recruited using the same tools or by engaging the same signaling cascade in astroglia. In most cases, it cannot be ruled out that multiple forms of gliotransmission occur following Gq receptor stimulation, as exhaustive tests are often not feasible. Also, gliotransmission in vivo would be expected to be more refined, since local neural signals would be detected by receptors on astrocyte processes to tune local responses. Indeed, it has been demonstrated previously that the same astrocyte is capable of signaling in distinct ways using different gliotransmitters depending on the dynamics of incoming signals (Covelo and Araque 2018), supporting the tremendous plasticity of this cell type.
Synaptic Proximity
Morphological Plasticity of Astrocytes
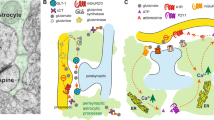
A central aspect underlying efficacy of transporter uptake and gliotransmission is the physical proximity of astroglial processes with synapses (see Fig. 1). The fidelity of this spatial relationship is crucial for normal neurobiology since perisynaptic astroglial processes (PAPs) express machinery for uptake and release of transmitters and maintenance of homeostatic neuronal function. Interestingly, PAPs display morphological plasticity more dynamic than what is observed in dendritic spines (Haber et al. 2006), and astrocyte morphological plasticity has been reported in the presence of extracellular glutamate (Genoud et al. 2006; Bernardinelli et al. 2014a; Verbich et al. 2012; Perez-Alvarez et al. 2014) and dopamine (Galloway et al. 2018). In the hippocampus, astroglial processes exhibit enhanced physical interaction with the postsynaptic compared with the presynaptic compartment (Lehre and Rusakov 2002). Further, astroglial interaction with neurons in this region tends to be biased toward dendritic spines as opposed to the larger dendritic shaft (Gavrilov et al. 2018). The general consensus regarding this conformation is that PAPs are positioned to perform glutamate transport upon transmitter release while also permitting a degree of transmitter spillover presynaptically, allowing for stimulation of presynaptic autoreceptors as a negative feedback loop that maintains elegant regulation of transmitter release probability.
Super-resolution confocal imaging of an astroglial plasma membrane, GFAP arbor and neighboring dendritic segment on an NAcore-projecting PL neuron. Top, plasma membrane labeling of an astrocyte in the PL using the AAV-GFAP-LcK-GFP viral vector from the Khakh laboratory (green) (Shigetomi et al. 2013). Immunohistochemical labeling of the cytoskeletal protein GFAP is shown in blue. Bottom, association of the astrocyte plasma membrane (green) with a retrogradely labeled dendritic segment from a neighboring PL neuron that projects to the NAcore (red). Here the neuron was labeled with retrograde delivery of Cre in the NAcore combined with Cre-dependent AAV-DIO-mCherry expression in the PL. For both the top and bottom series, panels transition to higher magnification to show detail from left to right. The dashed box depicts the inset region, scale bars are 10, 5, 2 and 0.5 microns
In the striatum, there is a tremendous degree of variability in synaptic insulation by astroglial processes, perhaps due to the different input types (dopaminergic vs. glutamatergic), with astrocytes more closely abutting glutamatergic vs. dopaminergic terminals (Octeau et al. 2018). Regarding postsynaptic selectivity, high-resolution techniques have been applied to astrocytes and different synapse types in the striatum to demonstrate that astrocytes encompass similar numbers of D1- and D2-MSNs, with a slight bias toward D1-MSNs (Octeau et al. 2018). Nevertheless, both neuronal types receive similar degrees of synaptic insulation by astrocytes (Octeau et al. 2018).
Impact of Synaptic Insulation on Synapse Function
Synaptic proximity of astrocyte processes promotes synapse stability and maturation (Bernardinelli et al. 2014b; Nishida and Okabe 2007; Blanco-Suarez et al. 2018), and it is taken for granted that transmitter uptake and release by astrocytes requires a high degree of synaptic proximity to effectively regulate synaptic physiology. Not only would loss of synaptic apposition impact efficacy of these functions, but synaptic retraction of astrocyte processes has been shown to favor synaptic recruitment at excitatory synapses, with transmitter spillover potentiating nearby synapses when astrocyte processes have retracted (Henneberger et al. 2020). Retraction of astroglial processes may also permit stimulation of extrasynaptic glutamate receptors (Pal 2018; Kruyer and Kalivas 2020b) and impact neuronal excitability and plasticity. Extrasynaptic mGluRs pertinent to excitatory signaling underlying relapse include the aforementioned presynaptic mGluR2/3 that serves as a brake on glutamate and dopamine release (Xi et al. 2002) and mGluR5 expressed on nNOS interneurons that initiate degradation of the extracellular matrix and facilitate postsynaptic potentiation (Smith et al. 2017; Kruyer et al. 2019a).
Glutamate spillover permitted by retraction of astrocyte processes also recruits high-affinity NMDA receptors, which play an important role during drug relapse. The GluN2b (NR2b or NMDAR2b) subunit is largely extrasynaptic, as opposed to the mostly synaptic GluN2a (D’Ascenzo et al. 2007). Stimulation of GluN2b-containing NMDA receptors contributes to postsynaptic potentiation during reinstated heroin seeking, and GluN2b knockdown or blockade prevents increases in AMPA/NMDA and spine head diameter induced by a priming heroin injection and reduces reinstated seeking induced by cues or heroin prime (Shen et al. 2011).
Drug-Induced Morphological Plasticity
Astrocytes exhibit profound morphological adaptations after exposure to substances of abuse. For example, astrocyte volume is reduced in the NAcore by exposure to both opioids and psychostimulants (Siemsen et al. 2019; Scofield et al. 2016b; Kruyer et al. 2019b). NAcore astrocytes are also more densely packed after alcohol and astrocyte density correlate positively with breakpoint for ethanol, a measure of motivation to acquire alcohol (Bull et al. 2014). Analysis of astrocyte plasticity is complicated given that both immunological and synaptic events can impact their morphology, and substances of abuse can trigger both types of CNS adaptations. For example, chronic cocaine or morphine exposure causes reactive astrogliosis characterized by changes in GFAP expression, a type of neuroinflammation that induces an altered functional and morphological state in astroglia (Beitner-Johnson et al. 1993; Sil et al. 2018; Bowers and Kalivas 2003). Whether changes in astrocyte proximity to synapses is a by-product of an immunoreactive state and whether synaptic functions of astroglia are interrupted by immunological processes is an ongoing question that necessitates further investigation.
Recent studies show that cues that stimulate drug seeking trigger morphological plasticity in NAcore astrocytes in animals trained to self-administer heroin, but not sucrose (Kruyer et al. 2019b). This plasticity appears homeostatic in nature, resulting from cue-induced neuronal activity and glutamate release. Moreover, interrupting the re-association of astroglial processes with synapse during reinstatement elevates responding for cues that signal heroin availability. Changes in synaptic proximity of astrocyte processes in this case are linked to phosphorylation of ezrin, an actin-blinding protein selectively expressed in astroglial processes (Derouiche and Frotscher 2001). Currently, neither the signaling cascade that drives ezrin phosphorylation nor the mechanism by which astrocyte process motility suppresses reinstated seeking has been uncovered. Additionally, whether NAcore D1- or D2-MSN synapses retain different degrees of astrocyte insulation during withdrawal from drug use or during reinstated seeking is a fundamental remaining question.
Homeostatic Functions
Synaptogenesis
Astrocytes play key roles in both synapse formation and elimination. Astrocytes express and release several signaling factors that contribute to formation of dendritic spines and spine density (Walker et al. 2020; Ikeda et al. 2010; Wang et al. 2020a; Chung et al. 2015), a measure that is aberrantly altered in the striatum after drug use and during reinstated drug seeking (Shen et al. 2011; Dos Santos et al. 2017; Anderson and Self 2017). During development, thrombospondins released by astrocytes promote genesis of new synapses that are postsynaptically silent (Christopherson et al. 2005). In adult animals, thrombospondin expression is elevated in response to cocaine exposure, and astrocyte thrombospondins in the NAshell contribute to generation of silent synapses (Wang et al. 2020b). In both cases, it is expected that presence of silent synapses facilitates induction of functional synapses rapidly upon further signaling that induces postsynaptic insertion of AMPA receptors. Indeed blocking thrombospondin release by astroglia during exposure to cocaine cues blunts cued reinstatement of seeking (Wang et al. 2020b), which is strongly linked to measures of postsynaptic potentiation (Gipson et al. 2013). Astrocytes also participate in synapse elimination, through phagocytosis directly, and through coordinated signaling with microglia (Chung et al. 2013, 2015; Wilton et al. 2019). While astrocytes prune synapses to refine neural circuits during development, there is evidence that astrocytes express the machinery for synapse engulfment into adulthood (Chung et al. 2015), and recruitment of this process during acquisition of drug taking behavior or extinction learning is a possibility.
BDNF, a growth factor expressed and released by both astrocytes and neurons (Zafra et al. 1991; Ohno et al. 2018; Bergami et al. 2008), confers bidirectional effects on addiction-related behaviors (McGinty et al. 2010). For instance, animals that receive BDNF infusions in the VTA 2 h after a cocaine self-administration session exhibit enhanced reinstated seeking after withdrawal (Lu et al. 2004). Instead, BDNF infusion in the PL attenuates reinstated seeking as well as reinstatement-associated increases in accumbens glutamate according to the same timeline (McGinty et al. 2010). Generally BDNF undergoes activity-dependent upregulation and contributes to synapse stability and plasticity (Gomez-Palacio-Schjetnan and Escobar 2013). Interestingly, BDNF also increases astrocyte morphological complexity (Holt et al. 2019). Whether BDNF expression is altered selectively in astrocytes within corticostriatal or other circuitries pertinent to addiction and relapse or whether neuronal BDNF exerts its effects in part through astroglial signaling has not been determined.
Network Homeostasis
The coupling of astroglia through gap junctions provides them with the unique capacity to scale network activity.
Although some connexin proteins are expressed at relatively high levels in the nucleus accumbens of naïve animals, connexin protein expression is reduced up to 21 days after cocaine self-administration (Bennett et al. 1999). Consistent with this finding, methamphetamine reduces gap junction coupling when delivered to cultured astroglia directly, indicated by lack of dye transfer to nearby astroglia that are normally extensively coupled (Castellano et al. 2016). The gap junction protein connexin 30 regulates excitatory synaptic strength broadly in the hippocampus by coordinating widespread synaptic insertion leading to efficient glutamate uptake through increased synaptic proximity, but not increased transporter expression (Pannasch et al. 2014). Perhaps relevant to these findings is the discovery that inhibiting astrocytes in the medial prefrontal cortex impairs cognitive flexibility, involving coordinated neural oscillations through astroglial transmission (Brockett et al. 2018). How this may relate with flexibility in drug-related learned behaviors, such as extinction, is a relevant question given the involvement of corticostriatal glutamate in measures of drug seeking and refraining. Interestingly, blockade of gap junction proteins in astrocytes in the PL prevents extinction and reinstatement of cocaine CPP (Fitzgerald 2016). Research momentum on the involvement of gap junction proteins in addiction-related behaviors is slowly growing, and there is enthusiasm for the concept of lateral regulation by astroglia, where given their coupling, plasticity, and the multitude of mechanisms by which they impact synaptic function, astrocytes are poised to coordinate synapses that are neither directly nor indirectly linked otherwise (Covelo and Araque 2016).
Conclusions
Emerging research highlights the critical contribution of astrocytes to synaptic function, and astroglial adaptations across a number of brain regions have been shown to contribute to the encoding and expression of motivated behaviors relevant to drug addiction. A majority of early literature on astroglial function was generated in vitro and ex vivo, providing information on general astroglial responses to neural activity or pharmacological manipulation. An accumulating body of work links these early findings with in vivo behavioral measures, highlighting new avenues for research and experimentation. Here, we have highlighted research avenues poised to promote future discovery. Generation of new tools, including CalEx and viruses for astroglial labeling and functional manipulation of gene expression, and improved imaging methodologies are facilitating studies of astroglial function in rodent models of drug addiction and relapse. We expect that advances made in the coming years using these tools will drastically expand our understanding of ways in which astrocytes impact synaptic physiology during normal motivated behavior and in psychiatric disorders such as drug addiction.
References
Agulhon C, Fiacco TA, McCarthy KD (2010) Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327:1250–1254. https://doi.org/10.1126/science.1184821
Aida T et al (2015) Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsychopharmacology 40:1569–1579. https://doi.org/10.1038/npp.2015.26
Alsio J et al (2011) Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice. J Neurosci 31:12593–12603. https://doi.org/10.1523/JNEUROSCI.2397-11.2011
Anderson EM, Self DW (2017) It's only a matter of time: longevity of cocaine-induced changes in dendritic spine density in the nucleus accumbens. Curr Opin Behav Sci 13:117–123. https://doi.org/10.1016/j.cobeha.2016.11.013
Araque A et al (2014) Gliotransmitters travel in time and space. Neuron 81:728–739. https://doi.org/10.1016/j.neuron.2014.02.007
Atkins AL, Mashhoon Y, Kantak KM (2008) Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav 90:481–491. https://doi.org/10.1016/j.pbb.2008.04.007
Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW (2002) The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci 22:9134–9141
Ball KT, Slane M (2012) Differential involvement of prelimbic and infralimbic medial prefrontal cortex in discrete cue-induced reinstatement of 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) seeking in rats. Psychopharmacology 224:377–385. https://doi.org/10.1007/s00213-012-2762-5
Bassareo V, Cucca F, Frau R, Di Chiara G (2017) Changes in dopamine transmission in the nucleus Accumbens Shell and Core during ethanol and sucrose self-administration. Front Behav Neurosci 11:71. https://doi.org/10.3389/fnbeh.2017.00071
Batiuk MY et al (2020) Identification of region-specific astrocyte subtypes at single cell resolution. Nat Commun 11:1220. https://doi.org/10.1038/s41467-019-14198-8
Bazargani N, Attwell D (2016) Astrocyte calcium signaling: the third wave. Nat Neurosci 19:182–189. https://doi.org/10.1038/nn.4201
Beitner-Johnson D, Guitart X, Nestler EJ (1993) Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J Neurochem 61:1766–1773. https://doi.org/10.1111/j.1471-4159.1993.tb09814.x
Bennett SA et al (1999) Long-term changes in connexin32 gap junction protein and mRNA expression following cocaine self-administration in rats. Eur J Neurosci 11:3329–3338. https://doi.org/10.1046/j.1460-9568.1999.00752.x
Benneyworth MA, Li Y, Basu AC, Bolshakov VY, Coyle JT (2012) Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol Neurobiol 32:613–624. https://doi.org/10.1007/s10571-012-9808-4
Bergami M et al (2008) Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J Cell Biol 183:213–221. https://doi.org/10.1083/jcb.200806137
Bernardinelli Y et al (2014a) Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol 24:1679–1688. https://doi.org/10.1016/j.cub.2014.06.025
Bernardinelli Y, Muller D, Nikonenko I (2014b) Astrocyte-synapse structural plasticity. Neural Plast 2014:232105. https://doi.org/10.1155/2014/232105
Bimpisidis Z, Wallen-Mackenzie A (2019) Neurocircuitry of reward and addiction: potential impact of dopamine-glutamate co-release as future target in substance use disorder. J Clin Med 8. https://doi.org/10.3390/jcm8111887
Birgner C et al (2010) VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci U S A 107:389–394. https://doi.org/10.1073/pnas.0910986107
Blanco-Suarez E, Liu TF, Kopelevich A, Allen NJ (2018) Astrocyte-secreted chordin-like 1 drives synapse maturation and limits plasticity by increasing synaptic GluA2 AMPA receptors. Neuron 100:1116–1132.e1113. https://doi.org/10.1016/j.neuron.2018.09.043
Bossert JM, Stern AL (2014) Role of ventral subiculum in context-induced reinstatement of heroin seeking in rats. Addict Biol 19:338–342. https://doi.org/10.1111/adb.12015
Bowers MS, Kalivas PW (2003) Forebrain astroglial plasticity is induced following withdrawal from repeated cocaine administration. Eur J Neurosci 17:1273–1278. https://doi.org/10.1046/j.1460-9568.2003.02537.x
Brockett AT et al (2018) Evidence supporting a role for astrocytes in the regulation of cognitive flexibility and neuronal oscillations through the Ca2+ binding protein S100beta. PLoS One 13:e0195726. https://doi.org/10.1371/journal.pone.0195726
Bull C et al (2014) Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology 39:2835–2845. https://doi.org/10.1038/npp.2014.135
Carelli RM, Williams JG, Hollander JA (2003) Basolateral amygdala neurons encode cocaine self-administration and cocaine-associated cues. J Neurosci 23:8204–8211
Castellano P, Nwagbo C, Martinez LR, Eugenin EA (2016) Methamphetamine compromises gap junctional communication in astrocytes and neurons. J Neurochem 137:561–575. https://doi.org/10.1111/jnc.13603
Cavaccini A, Durkee C, Kofuji P, Tonini R, Araque A (2020) Astrocyte signaling gates long-term depression at Corticostriatal synapses of the direct pathway. J Neurosci 40:5757–5768. https://doi.org/10.1523/JNEUROSCI.2369-19.2020
Chai H et al (2017) Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95:531–549.e539. https://doi.org/10.1016/j.neuron.2017.06.029
Chaudhri N, Sahuque LL, Cone JJ, Janak PH (2008) Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci 28:2288–2298. https://doi.org/10.1111/j.1460-9568.2008.06517.x
Christopherson KS et al (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120:421–433. https://doi.org/10.1016/j.cell.2004.12.020
Chung WS et al (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504:394–400. https://doi.org/10.1038/nature12776
Chung WS, Allen NJ, Eroglu C (2015) Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol 7:a020370. https://doi.org/10.1101/cshperspect.a020370
Corkrum M et al (2020) Dopamine-evoked synaptic regulation in the nucleus Accumbens requires astrocyte activity. Neuron 105:1036–1047.e1035. https://doi.org/10.1016/j.neuron.2019.12.026
Cornish JL, Kalivas PW (2000) Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20:RC89
Covelo A, Araque A (2016) Lateral regulation of synaptic transmission by astrocytes. Neuroscience 323:62–66. https://doi.org/10.1016/j.neuroscience.2015.02.036
Covelo A, Araque A (2018) Neuronal activity determines distinct gliotransmitter release from a single astrocyte. elife 7. https://doi.org/10.7554/eLife.32237
Coyle JT (2012) NMDA receptor and schizophrenia: a brief history. Schizophr Bull 38:920–926. https://doi.org/10.1093/schbul/sbs076
Cuevas-Diaz Duran R, Wang CY, Zheng H, Deneen B, Wu JQ (2019) Brain region-specific gene signatures revealed by distinct astrocyte subpopulations unveil links to glioma and neurodegenerative diseases. eNeuro 6. https://doi.org/10.1523/ENEURO.0288-18.2019
Cui M et al (2009) The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci U S A 106:8043–8048. https://doi.org/10.1073/pnas.0900358106
Curcio L et al (2013) Reduced D-serine levels in the nucleus accumbens of cocaine-treated rats hinder the induction of NMDA receptor-dependent synaptic plasticity. Brain 136:1216–1230. https://doi.org/10.1093/brain/awt036
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
D’Ascenzo M et al (2007) mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A 104:1995–2000. https://doi.org/10.1073/pnas.0609408104
D’Ascenzo M, Podda MV, Grassi C (2014) The role of D-serine as co-agonist of NMDA receptors in the nucleus accumbens: relevance to cocaine addiction. Front Synaptic Neurosci 6:16. https://doi.org/10.3389/fnsyn.2014.00016
Derouiche A, Frotscher M (2001) Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia 36:330–341
Di Ciano P, Everitt BJ (2004) Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci 24:7167–7173. https://doi.org/10.1523/JNEUROSCI.1581-04.2004
Do-Monte FH, Minier-Toribio A, Quinones-Laracuente K, Medina-Colon EM, Quirk GJ (2017) Thalamic regulation of sucrose seeking during unexpected reward omission. Neuron 94:388–400.e384. https://doi.org/10.1016/j.neuron.2017.03.036
Doncheck EM et al (2020) Sex, stress, and prefrontal cortex: influence of biological sex on stress-promoted cocaine seeking. Neuropsychopharmacology. https://doi.org/10.1038/s41386-020-0674-3
Dos Santos M et al (2017) Rapid synaptogenesis in the nucleus Accumbens is induced by a single cocaine administration and stabilized by mitogen-activated protein kinase interacting Kinase-1 activity. Biol Psychiatry 82:806–818. https://doi.org/10.1016/j.biopsych.2017.03.014
Farhat LC et al (2020) Pharmacological and behavioral treatment for trichotillomania: an updated systematic review with meta-analysis. Depress Anxiety 37:715–727. https://doi.org/10.1002/da.23028
Fischer-Smith KD, Houston AC, Rebec GV (2012) Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience 210:333–339. https://doi.org/10.1016/j.neuroscience.2012.02.049
Fitzgerald M (2016) Gap junction communication in memory retrieval and extinction of cocaine seeking. Theses Dissertations 1140:39–40
Floresco SB, Yang CR, Phillips AG, Blaha CD (1998) Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci 10:1241–1251. https://doi.org/10.1046/j.1460-9568.1998.00133.x
Fuchs RA et al (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30:296–309. https://doi.org/10.1038/sj.npp.1300579
Galloway A et al (2018) Dopamine triggers CTCF-dependent morphological and genomic remodeling of astrocytes. J Neurosci 38:4846–4858. https://doi.org/10.1523/JNEUROSCI.3349-17.2018
Gasser PJ, Hurley MM, Chan J, Pickel VM (2017) Organic cation transporter 3 (OCT3) is localized to intracellular and surface membranes in select glial and neuronal cells within the basolateral amygdaloid complex of both rats and mice. Brain Struct Funct 222:1913–1928. https://doi.org/10.1007/s00429-016-1315-9
Gavrilov N et al (2018) Astrocytic coverage of dendritic spines, dendritic shafts, and axonal boutons in hippocampal neuropil. Front Cell Neurosci 12:248. https://doi.org/10.3389/fncel.2018.00248
Genoud C et al (2006) Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol 4:e343. https://doi.org/10.1371/journal.pbio.0040343
Gipson CD et al (2013) Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron 77:867–872. https://doi.org/10.1016/j.neuron.2013.01.005
Gomez JA et al (2019) Ventral tegmental area astrocytes orchestrate avoidance and approach behavior. Nat Commun 10:1455. https://doi.org/10.1038/s41467-019-09131-y
Gomez-Gonzalo M, Zehnder T, Requie LM, Bezzi P, Carmignoto G (2018) Insights into the release mechanism of astrocytic glutamate evoking in neurons NMDA receptor-mediated slow depolarizing inward currents. Glia 66:2188–2199. https://doi.org/10.1002/glia.23473
Gomez-Palacio-Schjetnan A, Escobar ML (2013) Neurotrophins and synaptic plasticity. Curr Top Behav Neurosci 15:117–136. https://doi.org/10.1007/7854_2012_231
Goubard V, Fino E, Venance L (2011) Contribution of astrocytic glutamate and GABA uptake to corticostriatal information processing. J Physiol 589:2301–2319. https://doi.org/10.1113/jphysiol.2010.203125
Grant JE, Kim SW, Odlaug BL (2007) N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry 62:652–657. https://doi.org/10.1016/j.biopsych.2006.11.021
Haber M, Zhou L, Murai KK (2006) Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci 26:8881–8891. https://doi.org/10.1523/JNEUROSCI.1302-06.2006
Heinsbroek JA et al (2017) Loss of plasticity in the D2-Accumbens Pallidal pathway promotes cocaine seeking. J Neurosci 37:757–767. https://doi.org/10.1523/JNEUROSCI.2659-16.2016
Henneberger C et al (2020) LTP induction boosts glutamate spillover by driving withdrawal of Perisynaptic Astroglia. Neuron. https://doi.org/10.1016/j.neuron.2020.08.030
Hnasko TS, Edwards RH (2012) Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol 74:225–243. https://doi.org/10.1146/annurev-physiol-020911-153315
Hnasko TS et al (2010) Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron 65:643–656. https://doi.org/10.1016/j.neuron.2010.02.012
Holleran KM et al (2020) Organic cation transporter 3 and the dopamine transporter differentially regulate catecholamine uptake in the basolateral amygdala and nucleus accumbens. Eur J Neurosci. https://doi.org/10.1111/ejn.14927
Holmseth S et al (2009) The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience 162:1055–1071. https://doi.org/10.1016/j.neuroscience.2009.03.048
Holt LM et al (2019) Astrocyte morphogenesis is dependent on BDNF signaling via astrocytic TrkB.T1. elife 8. https://doi.org/10.7554/eLife.44667
Howard EC, Schier CJ, Wetzel JS, Duvauchelle CL, Gonzales RA (2008) The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neuroscience 154:1042–1053. https://doi.org/10.1016/j.neuroscience.2008.04.014
Howland JG, Taepavarapruk P, Phillips AG (2002) Glutamate receptor-dependent modulation of dopamine efflux in the nucleus accumbens by basolateral, but not central, nucleus of the amygdala in rats. J Neurosci 22:1137–1145
Ikeda H et al (2010) Morphine modulation of thrombospondin levels in astrocytes and its implications for neurite outgrowth and synapse formation. J Biol Chem 285:38415–38427. https://doi.org/10.1074/jbc.M110.109827
Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56:27–78. https://doi.org/10.1016/j.brainresrev.2007.05.004
Kalivas PW (2008) Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res 14:185–189. https://doi.org/10.1007/BF03033809
Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572. https://doi.org/10.1038/nrn2515
Kalivas BC, Kalivas PW (2016) Corticostriatal circuitry in regulating diseases characterized by intrusive thinking. Dialogues Clin Neurosci 18:65–76
Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413. https://doi.org/10.1176/appi.ajp.162.8.1403
Kalivas PW, Duffy P, Barrow J (1989) Regulation of the mesocorticolimbic dopamine system by glutamic acid receptor subtypes. J Pharmacol Exp Ther 251:378–387
Kalivas PW, Lalumiere RT, Knackstedt L, Shen H (2009) Glutamate transmission in addiction. Neuropharmacology 56(Suppl 1):169–173. https://doi.org/10.1016/j.neuropharm.2008.07.011
Kang S et al (2020) Activation of astrocytes in the dorsomedial striatum facilitates transition from habitual to goal-directed reward-seeking behavior. Biol Psychiatry. https://doi.org/10.1016/j.biopsych.2020.04.023
Kelley AE, Smith-Roe SL, Holahan MR (1997) Response-reinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci U S A 94:12174–12179. https://doi.org/10.1073/pnas.94.22.12174
Kim R, Sepulveda-Orengo MT, Healey KL, Williams EA, Reissner KJ (2018) Regulation of glutamate transporter 1 (GLT-1) gene expression by cocaine self-administration and withdrawal. Neuropharmacology 128:1–10. https://doi.org/10.1016/j.neuropharm.2017.09.019
Knackstedt LA et al (2009) The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry 65:841–845. https://doi.org/10.1016/j.biopsych.2008.10.040
Knackstedt LA, Melendez RI, Kalivas PW (2010) Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry 67:81–84. https://doi.org/10.1016/j.biopsych.2009.07.018
Kohler S, Winkler U, Hirrlinger J (2019) Heterogeneity of astrocytes in Grey and white matter. Neurochem Res. https://doi.org/10.1007/s11064-019-02926-x
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. https://doi.org/10.1038/npp.2009.110
Kruyer AK, Kalivas PW (2020a) Heroin cues reveal astroglial heterogeneity in the nucleus Accumbens core. bioRxiv. https://doi.org/10.1101/2020.07.22.216036
Kruyer A, Kalivas PW (2020b) Astrocytes as cellular mediators of cue reactivity in addiction. Curr Opin Pharmacol 56:1–6. https://doi.org/10.1016/j.coph.2020.07.009
Kruyer A, Chioma VC, Kalivas PW (2019a) The opioid-addicted Tetrapartite synapse. Biol Psychiatry. https://doi.org/10.1016/j.biopsych.2019.05.025
Kruyer A, Scofield MD, Wood D, Reissner KJ, Kalivas PW (2019b) Heroin Cue-evoked astrocytic structural plasticity at nucleus Accumbens synapses inhibits heroin seeking. Biol Psychiatry. https://doi.org/10.1016/j.biopsych.2019.06.026
Kupchik YM et al (2014) Cocaine dysregulates opioid gating of GABA neurotransmission in the ventral pallidum. J Neurosci 34:1057–1066. https://doi.org/10.1523/JNEUROSCI.4336-13.2014
Lasseter HC, Xie X, Ramirez DR, Fuchs RA (2010) Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience 171:830–839. https://doi.org/10.1016/j.neuroscience.2010.09.032
Lehre KP, Rusakov DA (2002) Asymmetry of glia near central synapses favors presynaptically directed glutamate escape. Biophys J 83:125–134. https://doi.org/10.1016/S0006-3495(02)75154-0
Logan CN, LaCrosse AL, Knackstedt LA (2018) Nucleus accumbens GLT-1a overexpression reduces glutamate efflux during reinstatement of cocaine-seeking but is not sufficient to attenuate reinstatement. Neuropharmacology 135:297–307. https://doi.org/10.1016/j.neuropharm.2018.03.022
Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y (2004) A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci 24:1604–1611. https://doi.org/10.1523/JNEUROSCI.5124-03.2004
MacKay MB et al (2019) D-serine: potential therapeutic agent and/or biomarker in schizophrenia and depression? Front Psych 10:25. https://doi.org/10.3389/fpsyt.2019.00025
Martin R, Bajo-Graneras R, Moratalla R, Perea G, Araque A (2015) Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349:730–734. https://doi.org/10.1126/science.aaa7945
Martineau M et al (2013) Storage and uptake of D-serine into astrocytic synaptic-like vesicles specify gliotransmission. J Neurosci 33:3413–3423. https://doi.org/10.1523/JNEUROSCI.3497-12.2013
Martin-Fardon R, Weiss F (2017) Perseveration of craving: effects of stimuli conditioned to drugs of abuse versus conventional reinforcers differing in demand. Addict Biol 22:923–932. https://doi.org/10.1111/adb.12374
Matias I, Morgado J, Gomes FCA (2019) Astrocyte heterogeneity: impact to brain aging and disease. Front Aging Neurosci 11:59. https://doi.org/10.3389/fnagi.2019.00059
Mayer FP et al (2018) An unsuspected role for organic cation transporter 3 in the actions of amphetamine. Neuropsychopharmacology 43:2408–2417. https://doi.org/10.1038/s41386-018-0053-5
McCullough LD, Sokolowski JD, Salamone JD (1993) A neurochemical and behavioral investigation of the involvement of nucleus accumbens dopamine in instrumental avoidance. Neuroscience 52:919–925. https://doi.org/10.1016/0306-4522(93)90538-q
McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23:3531–3537
McGinty JF, Whitfield TW Jr, Berglind WJ (2010) Brain-derived neurotrophic factor and cocaine addiction. Brain Res 1314:183–193. https://doi.org/10.1016/j.brainres.2009.08.078
McGlinchey EM, Aston-Jones G (2018) Dorsal Hippocampus drives context-induced cocaine seeking via inputs to lateral septum. Neuropsychopharmacology 43:987–1000. https://doi.org/10.1038/npp.2017.144
Melone M, Ciappelloni S, Conti F (2015) A quantitative analysis of cellular and synaptic localization of GAT-1 and GAT-3 in rat neocortex. Brain Struct Funct 220:885–897. https://doi.org/10.1007/s00429-013-0690-8
Mingote S, Amsellem A, Kempf A, Rayport S, Chuhma N (2019) Dopamine-glutamate neuron projections to the nucleus accumbens medial shell and behavioral switching. Neurochem Int 129:104482. https://doi.org/10.1016/j.neuint.2019.104482
Mongia S et al (2019) The ventral tegmental area has calbindin neurons with the capability to co-release glutamate and dopamine into the nucleus accumbens. Eur J Neurosci 50:3968–3984. https://doi.org/10.1111/ejn.14493
Morales M, Margolis EB (2017) Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 18:73–85. https://doi.org/10.1038/nrn.2016.165
Mothet JP et al (2000) D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A 97:4926–4931. https://doi.org/10.1073/pnas.97.9.4926
Moussawi K et al (2009) N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12:182–189. https://doi.org/10.1038/nn.2250
Naganuma F et al (2014) Predominant role of plasma membrane monoamine transporters in monoamine transport in 1321N1, a human astrocytoma-derived cell line. J Neurochem 129:591–601. https://doi.org/10.1111/jnc.12665
Nishida H, Okabe S (2007) Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci 27:331–340. https://doi.org/10.1523/JNEUROSCI.4466-06.2007
Octeau JC et al (2018) An optical neuron-astrocyte proximity assay at synaptic distance scales. Neuron 98:49–66 e49. https://doi.org/10.1016/j.neuron.2018.03.003
Ohno Y, Kinboshi M, Shimizu S (2018) Inwardly rectifying Potassium Channel Kir4.1 as a novel modulator of BDNF expression in astrocytes. Int J Mol Sci 19. https://doi.org/10.3390/ijms19113313
Pal B (2018) **Involvement of extrasynaptic glutamate in physiological and pathophysiological changes of neuronal excitability. Cell Mol Life Sci 75:2917–2949. https://doi.org/10.1007/s00018-018-2837-5
Pannasch U et al (2014) Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci 17:549–558. https://doi.org/10.1038/nn.3662
Papouin T, Dunphy J, Tolman M, Foley JC, Haydon PG (2017) Astrocytic control of synaptic function. Philos Trans R Soc Lond Ser B Biol Sci 372. https://doi.org/10.1098/rstb.2016.0154
Pardo-Garcia TR et al (2019) Ventral pallidum is the primary target for Accumbens D1 projections driving cocaine seeking. J Neurosci 39:2041–2051. https://doi.org/10.1523/JNEUROSCI.2822-18.2018
Parpura V et al (1994) Glutamate-mediated astrocyte-neuron signalling. Nature 369:744–747. https://doi.org/10.1038/369744a0
Pascual O et al (2005) Astrocytic purinergic signaling coordinates synaptic networks. Science 310:113–116. https://doi.org/10.1126/science.1116916
Pelton EW 2nd, Kimelberg HK, Shipherd SV, Bourke RS (1981) Dopamine and norepinephrine uptake and metabolism by astroglial cells in culture. Life Sci 28:1655–1663. https://doi.org/10.1016/0024-3205(81)90322-2
Perez-Alvarez A, Navarrete M, Covelo A, Martin ED, Araque A (2014) Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J Neurosci 34:12738–12744. https://doi.org/10.1523/JNEUROSCI.2401-14.2014
Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28:6046–6053. https://doi.org/10.1523/JNEUROSCI.1045-08.2008
Petravicz J, Boyt KM, McCarthy KD (2014) Astrocyte IP3R2-dependent Ca(2+) signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci 8:384. https://doi.org/10.3389/fnbeh.2014.00384
Pow DV (2001) Visualising the activity of the cystine-glutamate antiporter in glial cells using antibodies to aminoadipic acid, a selectively transported substrate. Glia 34:27–38. https://doi.org/10.1002/glia.1037
Puaud M, Higuera-Matas A, Brunault P, Everitt BJ, Belin D (2020) The basolateral amygdala to nucleus Accumbens core circuit mediates the conditioned reinforcing effects of cocaine-paired cues on cocaine seeking. Biol Psychiatry. https://doi.org/10.1016/j.biopsych.2020.07.022
Quon EF, Wotton CA, Bekar LK (2018) Evidence for astrocyte purinergic signaling in cortical sensory adaptation and serotonin-mediated neuromodulation. Mol Cell Neurosci 88:53–61. https://doi.org/10.1016/j.mcn.2017.12.008
Reissner KJ et al (2015) Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol 20:316–323. https://doi.org/10.1111/adb.12127
Rimmele TS, Rosenberg PA (2016) GLT-1: the elusive presynaptic glutamate transporter. Neurochem Int 98:19–28. https://doi.org/10.1016/j.neuint.2016.04.010
Roberts-Wolfe DJ, Kalivas PW (2015) Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets 14:745–756
Rocha A, Kalivas PW (2010) Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci 31:903–909. https://doi.org/10.1111/j.1460-9568.2010.07134.x
Rogers JL, Ghee S, See RE (2008) The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience 151:579–588. https://doi.org/10.1016/j.neuroscience.2007.10.012
Sagara JI, Miura K, Bannai S (1993) Maintenance of neuronal glutathione by glial cells. J Neurochem 61:1672–1676. https://doi.org/10.1111/j.1471-4159.1993.tb09802.x
Salgado S, Kaplitt MG (2015) The nucleus Accumbens: a comprehensive review. Stereotact Funct Neurosurg 93:75–93. https://doi.org/10.1159/000368279
Scofield MD (2018) Exploring the role of Astroglial glutamate release and association with synapses in neuronal function and behavior. Biol Psychiatry 84:778–786. https://doi.org/10.1016/j.biopsych.2017.10.029
Scofield MD et al (2015) Gq-DREADD selectively initiates glial glutamate release and inhibits Cue-induced cocaine seeking. Biol Psychiatry 78:441–451. https://doi.org/10.1016/j.biopsych.2015.02.016
Scofield MD et al (2016a) The nucleus Accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev 68:816–871. https://doi.org/10.1124/pr.116.012484
Scofield MD et al (2016b) Cocaine Self-administration and extinction leads to reduced glial fibrillary acidic protein expression and morphometric features of astrocytes in the nucleus Accumbens Core. Biol Psychiatry 80:207–215. https://doi.org/10.1016/j.biopsych.2015.12.022
Sellings LH, Clarke PB (2003) Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci 23:6295–6303
Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW (2011) Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci U S A 108:19407–19412. https://doi.org/10.1073/pnas.1112052108
Shen HW, Gipson CD, Huits M, Kalivas PW (2014a) Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology 39:1169–1177. https://doi.org/10.1038/npp.2013.318
Shen HW, Scofield MD, Boger H, Hensley M, Kalivas PW (2014b) Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci 34:5649–5657. https://doi.org/10.1523/JNEUROSCI.4564-13.2014
Shigetomi E et al (2013) Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol 141:633–647. https://doi.org/10.1085/jgp.201210949
Siemsen BM et al (2019) Effects of methamphetamine Self-administration and extinction on astrocyte structure and function in the nucleus Accumbens Core. Neuroscience 406:528–541. https://doi.org/10.1016/j.neuroscience.2019.03.040
Sil S, Periyasamy P, Guo ML, Callen S, Buch S (2018) Morphine-mediated brain region-specific Astrocytosis involves the ER stress-autophagy Axis. Mol Neurobiol 55:6713–6733. https://doi.org/10.1007/s12035-018-0878-2
Smith ACW et al (2017) Accumbens nNOS interneurons regulate cocaine relapse. J Neurosci 37:742–756. https://doi.org/10.1523/JNEUROSCI.2673-16.2016
Smith-Roe SL, Kelley AE (2000) Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci 20:7737–7742
Stefanik MT, Kalivas PW (2013) Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci 7:213. https://doi.org/10.3389/fnbeh.2013.00213
Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A (2010) Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci 30:8229–8233. https://doi.org/10.1523/JNEUROSCI.1754-10.2010
Stuber GD et al (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475:377–380. https://doi.org/10.1038/nature10194
Sun W et al (2013) Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339:197–200. https://doi.org/10.1126/science.1226740
Takeda H, Inazu M, Matsumiya T (2002) Astroglial dopamine transport is mediated by norepinephrine transporter. Naunyn Schmiedeberg’s Arch Pharmacol 366:620–623. https://doi.org/10.1007/s00210-002-0640-0
Tan KR et al (2012) GABA neurons of the VTA drive conditioned place aversion. Neuron 73:1173–1183. https://doi.org/10.1016/j.neuron.2012.02.015
Tsai G, Yang P, Chung LC, Lange N, Coyle JT (1998) D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 44:1081–1089. https://doi.org/10.1016/s0006-3223(98)00279-0
Turner JR, Ecke LE, Briand LA, Haydon PG, Blendy JA (2013) Cocaine-related behaviors in mice with deficient gliotransmission. Psychopharmacology 226:167–176. https://doi.org/10.1007/s00213-012-2897-4
Valtcheva S, Venance L (2016) Astrocytes gate Hebbian synaptic plasticity in the striatum. Nat Commun 7:13845. https://doi.org/10.1038/ncomms13845
Verbich D, Prenosil GA, Chang PK, Murai KK, McKinney RA (2012) Glial glutamate transport modulates dendritic spine head protrusions in the hippocampus. Glia 60:1067–1077. https://doi.org/10.1002/glia.22335
Vollstadt-Klein S et al (2010) Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction 105:1741–1749. https://doi.org/10.1111/j.1360-0443.2010.03022.x
Walker CD, Risher WC, Risher ML (2020) Regulation of synaptic development by astrocyte signaling factors and their emerging roles in substance abuse. Cell 9. https://doi.org/10.3390/cells9020297
Wang W et al (2020a) TREK-1 null impairs neuronal excitability, synaptic plasticity, and cognitive function. Mol Neurobiol 57:1332–1346. https://doi.org/10.1007/s12035-019-01828-x
Wang J et al (2020b) Cocaine triggers astrocyte-mediated synaptogenesis. Biol Psychiatry. https://doi.org/10.1016/j.biopsych.2020.08.012
Whitelaw RB, Markou A, Robbins TW, Everitt BJ (1996) Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology 127:213–224
Willcocks AL, McNally GP (2013) The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci 37:259–268. https://doi.org/10.1111/ejn.12031
Willuhn I, Wanat MJ, Clark JJ, Phillips PE (2010) Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci 3:29–71. https://doi.org/10.1007/7854_2009_27
Wilton DK, Dissing-Olesen L, Stevens B (2019) Neuron-glia signaling in synapse elimination. Annu Rev Neurosci 42:107–127. https://doi.org/10.1146/annurev-neuro-070918-050306
Wolosker H (2011) Serine racemase and the serine shuttle between neurons and astrocytes. Biochim Biophys Acta 1814:1558–1566. https://doi.org/10.1016/j.bbapap.2011.01.001
Woo DH et al (2012) TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151:25–40. https://doi.org/10.1016/j.cell.2012.09.005
Wu J, Zhao R, Guo L, Zhen X (2017) Morphine-induced inhibition of Ca(2+) -dependent d-serine release from astrocytes suppresses excitability of GABAergic neurons in the nucleus accumbens. Addict Biol 22:1289–1303. https://doi.org/10.1111/adb.12417
Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW (2002) Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther 300:162–171. https://doi.org/10.1124/jpet.300.1.162
Yang FY et al (2013) D-cycloserine, sarcosine and D-serine diminish the expression of cocaine-induced conditioned place preference. J Psychopharmacol 27:550–558. https://doi.org/10.1177/0269881110388333
Yin HH, Knowlton BJ, Balleine BW (2004) Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci 19:181–189. https://doi.org/10.1111/j.1460-9568.2004.03095.x
Yoshikawa M et al (2007) The serine racemase mRNA is predominantly expressed in rat brain neurons. Arch Histol Cytol 70:127–134. https://doi.org/10.1679/aohc.70.127
Yu X et al (2018) Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron 99:1170–1187.e1179. https://doi.org/10.1016/j.neuron.2018.08.015
Zafra F, Castren E, Thoenen H, Lindholm D (1991) Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci U S A 88:10037–10041. https://doi.org/10.1073/pnas.88.22.10037
Zhang JM et al (2003) ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40:971–982. https://doi.org/10.1016/s0896-6273(03)00717-7
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kruyer, A., Scofield, M.D. (2021). Astrocytes in Addictive Disorders. In: Li, B., Parpura, V., Verkhratsky, A., Scuderi, C. (eds) Astrocytes in Psychiatric Disorders. Advances in Neurobiology, vol 26. Springer, Cham. https://doi.org/10.1007/978-3-030-77375-5_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-77375-5_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-77374-8
Online ISBN: 978-3-030-77375-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)