Abstract
Low serum concentration of testosterone is the biochemical hallmark of male hypogonadism, but to assess the pathogenesis of the condition, it is important to measure serum concentrations of the pituitary gonadotropins, luteinizing hormone, and follicle-stimulating hormone. If gonadotropin levels are elevated, the patient has primary hypogonadism, caused by failure of testicular function. If the gonadotropin concentrations are low or inappropriately normal, the condition is secondary hypogonadism, caused by malfunction at the hypothalamic-pituitary level. Historically, hypogonadism starting in childhood was usually felt to be secondary to a genetic, structural, or anatomical fault in function of the hypothalamic-pituitary-testicular axis. This was termed organic hypogonadism and was felt to be irreversible. In contrast, hypogonadism starting in adulthood was historically felt to represent a milder condition, with less pronounced testosterone deficiency, no organic cause, and represented a reversible state. This condition was termed functional hypogonadism and was further subdivided into primary and secondary forms. A third category of functional hypogonadism, hallmarked by elevated gonadotropin levels in the face of normal testosterone, was termed compensated or subclinical hypogonadism. In this chapter, we will emphasize the role of gonadotropins and gonadotropin measurements in its pathogenesis, differential diagnosis, and treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hypogonadism
- Compensated (subclinical) hypogonadism
- Primary or hypergonadotropic hypogonadism
- Secondary or hypogonadotropic hypogonadism
- Functional hypogonadism
- Gonadotropins
- Luteinizing hormone
- Follicle-stimulating hormone
- Testosterone
3.1 Introduction
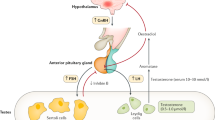
The two main functions of the testis are the production of testosterone (T) and spermatogenesis. The endocrine regulation of these activities occurs along the hypothalamic-pituitary-testicular (HPT) axis (Fig. 3.1), where the key role is played by the two gonadotropic hormones produced by the anterior pituitary gland – luteinizing hormone (LH) and follicle-stimulating hormone (FSH) [23, 43]. The secretion of LH and FSH is stimulated by the hypothalamic gonadotropin-releasing hormone (GnRH), which is under negative feedback control via sex steroids [19]. GnRH neurons do not respond directly to androgen (T) and estrogen (estradiol) feedback, but mainly regulate GnRH release by inhibiting the production of kisspeptin, a proximal hypothalamic peptide hormone with stimulatory action on GnRH synthesis [42]. The negative feedback regulation of FSH additionally occurs at the level of the pituitary gland through action of the testicular peptide hormone inhibin B.
Balanced function (homeostasis) of the HPT axis, whereby gonadotropins maintain the physiological levels of testicular androgen production and spermatogenesis, and testicular hormones feed back to the hypothalamic-pituitary level to maintain the required gonadotropin stimulus, are the hallmark of male eugonadism. T deficiency, the pathognomonic finding in male hypogonadism, can occur at any age from the fetal period until old age. Hypogonadism can be primary or hypergonadotropic (PH) , when the testis tissue is unable to produce sufficient amounts of T and/or sperm despite sufficient gonadotropin simulation. In this case, the lack of gonadal negative feedback brings about elevated gonadotropin levels. The other form of hypogonadism, termed secondary or hypogonadotropic (SH) , is caused by low or inappropriately normal (i.e., insufficient) gonadotropin secretion that is unable to support testicular function. It can be caused by deficiency of the pituitary gonadotrope cells to produce gonadotropins or by the lack of their hypothalamic stimulation by GnRH and/or kisspeptin. Gonadotropin measurements therefore form an essential part in the differential diagnosis between PH and SH.
Another way to classify hypogonadism is to divide it into organic (OH) and functional (FH) hypogondism. OH is the classical form of hypogonadism caused by a structural, functional, or genetic disturbance at one of the levels of the HPT axis, often congenital and usually irreversible. FH, also historically termed late-onset hypogonadism (LOH) or andropause, occurs usually in adulthood and is often associated with comorbidities and/or old age. LOH can be considered a misnomer because comorbidities (in particular obesity) are more important causes for the condition than advanced age. As a general rule, T levels in FH are not as profoundly reduced as they are in OH, and they may be reversible. Also, FH can be primary or secondary, but often the patient has mixed features of both. In cases of secondary FH, the etiology is more often a comorbidity (e.g., obesity), while OH is more commonly due to primary hypothalamic or testicular dysgenesis issues. Purely age-dependent hypogonadism is likely less common and typically is due to primary testicular insufficiency [55]. Compensated or subclinical hypogonadism (CH) , wherein gonadotropin levels are elevated in the setting of normal T, is less well defined and may occur with either OH or FH and may represent a precursor condition of hypogonadism in some cases [12, 45].
The purpose of this review is to discuss the role of gonadotropins in the pathogenesis, diagnosis, and treatment of different types of male FH.
3.2 Gonadotropin Measurements
3.2.1 Gonadotropin Assays
The sandwich-type immunometric methods currently used in clinical laboratories have high specificity and sensitivity (down to 0.05–0.1 IU/L) for LH and FSH, allowing reliable detection of the concentrations of male patients in most physiological and pathophysiological situations [53]. The reference range for LH in adult men is commonly reported between 1.5 and 8.5 IU/L, while FSH is reported to be 1.5–12.5 IU/L. However, in the setting of infertility, lower thresholds have been reported to indicate subfertility, including maximal FSH levels of 4.6–7.6 IU/L. Individual results should be interpreted against a reference range established by regional laboratories in healthy men from a representative local general population. Due to the pulsatile secretion and short half-life (20 min) of circulating LH, multiple measurements, or pooling of 2–3 individual samples, can be used to reduce sample-to-sample variability. In contrast, the half-life of FSH is longer (3–4 h), and a single measurement of this hormone is usually sufficient.
Gonadotropin assays used today in clinical laboratories are reliable with rare technical problems. Heterophilic antibodies in patient serum can be a rare cause of erroneously high gonadotropin levels [54]. There is a common genetic polymorphism of the LHB gene (W8R/I15T), with 10–20% allelic frequency in various populations, giving rise to an immunological variant of LH that is not detected by some immunometric assays, especially those using antibodies against the LH α/β dimer [29]. An inappropriately low level of LH is found in carriers of the polymorphic gene, even though the bioactivity of the aberrant hormone is roughly normal. Such an unexpected finding should be verified by another assay using a different combination of antibodies. Inactivating mutations of gonadotropin subunit genes and inactivating and activating mutation of gonadotropin receptor genes are extremely rare causes of inappropriate gonadotropin levels, unlikely to be found in men with FH.
3.2.2 Gonadotropin Bioactivity, Polymorphisms, and Microheterogeneity
Older studies have reported that low gonadotropin levels (such as in hypogonadotropic hypogonadism) have a low ratio of bioactivity to immunoreactivity, as measured by in vitro bioassay and competitive immunoassay [44, 49]. It has more recently been established that such apparent bioimmuno ratio differences were due to overestimation of low concentrations by older immunoassays [22, 27]. The understanding now is that the quality of gonadotropins throughout the concentration range occurring in men does not change, suggesting that the current immunometric methods are functionally relevant. Admittedly, circulating gonadotropins display microheterogeneity due to variations in the degree of glycosylation of individual molecules [4], but the clinical relevance of this phenomenon is likely marginal.
There are several common polymorphisms in gonadotropin subunit and receptor genes [7, 29, 37], which have been shown to influence the basal circulating levels of gonadotropins. Determination of the polymorphisms may, therefore, help identify one reason for an unexpected gonadotropin finding in hypogonadism. Alternatively, a GnRH stimulation test may be performed to evaluate responsiveness of the HPT axis [2].
3.2.3 Interpretation of the Findings
The first suspicion of hypogonadism is based on symptoms (and signs) and low concentration of peripheral serum T. Due to significant day-to-day and diurnal variability in T levels, at least two tests should be obtained from fasting blood samples within several hours of waking. If T levels are low, basal gonadotropin secretion should be measured in the peripheral serum to differentiate between PH and SH. Defining PH vs SH is clinically important, as it provides prognostic information (i.e., reversible vs non-reversible), may lead to further diagnostic testing (i.e., prolactin, pituitary MRI), and influences treatment options. In cases where fertility is not relevant, LH measurement is usually sufficient without the need for FSH. However, as FSH and LH are typically concordant, FSH may be ordered in cases where the diagnosis of PH vs SH is unclear to provide further information about pituitary activity.
Occasionally, GnRH stimulation testing may be useful to clarify indeterminate findings, such as in men with normal LH and low T [2]. Following GnRH stimulation, a low gonadotropin response suggests malfunction at the pituitary level, whereas increases in gonadotropins and testosterone suggest impairment at the hypothalamic level. Similarly, human chorionic gonadotropin (hCG) stimulation can be used to assess the testicular steroidogenic capacity when primary hypogonadism is suspected [2, 13]. Bang et al. [2] recently reported reference ranges for gonadotropin and T responses following GnRH and hGG stimulation tests in healthy men as well as in men with various forms of hypogonadism. Results obtained in obese men with suspected FH demonstrated normal LH responses to GnRH stimulation, suggesting that the low basal gonadotropin levels were more commonly secondary to inadequate hormonal release at the hypothalamic level [46]. A similar finding of maintained pituitary responsiveness to GnRH has been reported in aging men [39]. In contrast, hCG stimulation in obese men resulted in diminished T response at the level of the testicle [2, 26, 40], possibly due to the negative influence of adipose tissue-derived hormones on Leydig cell function. Interestingly, common polymorphisms of gonadotropin subunit and receptor genes have marginal effects on gonadotropin concentrations and receptor activity, and thus do not influence the results of GnRH or hCG stimulation tests [2].
3.3 Pathogenesis of Functional Hypogonadism (FH)
FH has been used to describe a milder form of hypogonadism which results from chronic disease, stress, inadequate nutrition, obesity, and/or old age [17]. Unlike OH, FH can often be transient and/or reversible, depending on the nature of the etiology. FH should only be diagnosed after exclusion of OH and should not be assumed based on age alone. For example, Klinefelter syndrome represents a common etiology for OH but may not be diagnosed until later in life [16].
FH can, in principle, occur at any age, although it may occur more commonly in older men. In both children and adults, it may be caused by multiple systemic diseases (see [3, 57]), with obesity, arguably, representing the most common etiology. Although most studies have focused on low T in men with obesity, lesser attention has been directed to the other part of testicular function, spermatogenesis. As will be discussed below, suppressed spermatogenesis may also occur in a distinct subgroup of men with FH. This condition may often go unnoticed in older men, as fertility is often a lesser concern in this population.
Because of differing etiologies, clinical management and prognosis of primary and secondary FH are often different. It is helpful to differentiate men into different diagnostic categories according to their T and gonadotropin levels. Based on results from 3119 community-dwelling men aged 40–79 years (EMAS study) [45], four distinct categories were defined (Fig. 3.2): (1) normal T and LH (eugonadism – 76.7%), (2) low T and high LH (PH – 2.0%), (3) low T and low-to-normal LH (SH – 11.8%), and (4) normal T and high LH (CH – 9.5%). It is noteworthy that when the definition for hypogonadism included both abnormal levels and symptoms (i.e., sexual symptoms), the rates of hypogonadism declined significantly. It is important to emphasize the need of gonadotropin measurements to define the diagnosis (see also below), because, at least in the United States, only 12% of men with FH have their LH and/or FSH measured before the initiation of T replacement therapy [33]. The distinction may also help contribute to the selection of therapy for men, particularly among those desiring to maintain fertility.
Correlation of testosterone and LH levels in the EMAS population [45], a cohort of 3016 community-dwelling men in aged 40–79 years in eight European centers (UK, Sweden, Belgium, Estonia, Poland, Hungary, Italy, and Spain). The vertical line corresponds to lower limit of normal total T (10.5 nmol/l) and the horizontal line corresponds to upper limit of normal LH (9.4 U/l). The population can be divided into eugonadal (T > 10.5 nmol/L; LH > 9.4 IU/L); primary hypogonadal (T < 10.5 nmol/L; LH > 9.4 IU/L), secondary hypogonadal (T < 10.5 nmol/L; LH > 9.4 IU/L), and men with compensatory hypogonadism (T > 10.5 nmol/L; LH > 9.4 IU/L). (Modified from Tajar et al. [45])
3.3.1 Primary (Hypergonadotropic) Hypogonadism
Primary hypogonadism is diagnosed through both low T and usually elevated LH. In most cases, FSH is more elevated than LH, and as such, it may be used as an adjunctive test in cases where the diagnosis is in question [32]. Although PH is a common etiology among the classical early-onset hypogonadism (OH), it may also occur in older men, particularly among those with comorbid conditions [56].
In some cases of PH, a man may have normal T and LH but elevated FSH. This situation may occur following radio- or chemotherapy, where spermatogenesis but not steroidogenesis may be impaired, while in other cases, it may be idiopathic [15].
Multiple mechanisms are associated with elevated LH in aging independent of comorbid states. They include age-related decreases of Leydig cell mass [34], testicular blood perfusion [41], and biochemical function [50], which are further exacerbated in conditions of poor health. Impaired testicular response to LH can also be caused by elevated cytokine levels [20, 48] associated with low-grade inflammation common in aging [18]. Primary testicular failure can also occur in connection with alcohol abuse [8], renal disease [25], COPD [28], and malignancy [14].
3.3.2 Secondary (Hypogonadotropic) Hypogonadism
It is important to identify men with SH, because it may be caused by significant pathologies, including pituitary or hypothalamic tumors, panhypopituitarism, or be due to acute illness, medications (e.g., glucocorticoids or opioids), or obesity. Secondary hypogonadism is often reversible through treating comorbidities, changing offending medications, and optimizing lifestyle factors [6].
Obesity-related suppression of HPT function has several possible mechanisms, including the pleiotropic inhibitory effects of adipocyte-produced adipokines, cytokines, and chemokines on GnRH and gonadotropin secretion [47], as well as obesity-related central insulin resistance [5, 36], which may negate the stimulatory effect of insulin on gonadotropin secretion. One candidate peptide associated with obesity-related HPT suppression is the fat-cell-produced leptin, which is reduced in men receiving T therapy [30, 31]. Additionally, proinflammatory fat tissue cytokines (e.g., tumor necrosis factor, IL-2, and IL-6) may further suppress gonadotropin secretion [51]. Other substances have shown similar suppressive effects, including endocannabinoids [35] and adiponectin [9]. Finally, lower SHBG in obesity may lower the set point of HPT feedback inhibition. As total T is predominantly suppressed in obesity, free T remains relatively higher and able to inhibit gonadotropins at lower levels of circulating total T. Interestingly, the long-held hypothesis that increases in adipose tissue result in reduced T levels due to increased estradiol feedback has been challenged by more recent findings [45].
3.3.3 Mixed Primary/Secondary Hypogonadism
The combination of both PH and SH is relatively common, especially among aged obese men, where the primary age-dependent derangement of testicular tissues and comorbidity-associated suppression of HPT function overlap. Similarly, adipokines produced by fat tissue have inhibitory effects on both hypothalamic GnRH secretion on Leydig cell steroidogenesis [40].
3.3.4 Compensated Hypogonadism
Elevated gonadotropins with normal T (LH >9.4 IU/L; T ≥ 10.5 nmol/L) is a common finding in aging men, representing 9.5% in the previously described EMAS cohort overall (Fig. 3.2) and 21% of men aged 70–79 [45]. A different cohort of >4000 men presenting to a sexual dysfunction clinic reported a lower prevalence rate of CH (4.1%), which likely relates to differences in study populations and suggests that men with CH are less likely to potentially exhibit low T-related symptoms [11]. Given these observations, the clinical significance of the isolated LH elevation is unclear. However, several clinically relevant issues related to CH include identifying associated or underlying etiologies and documenting hypogonadal symptoms. Additionally, further research is warranted to determine if CH is able to predict future health conditions, including uncompensated hypogonadism.
Although the exact cause of isolated LH elevation in these men is unknown, it may relate to T suppression that has occurred within the defined “normal” reference range and therefore goes essentially unnoticed. With this hypothesis, these men would normally have T levels in the mid to upper eugonadal range (e.g., 30 nmol/L), and even with 70% suppression (11 nmol/L), they would still be considered normal. In cases such as this, hypogonadal symptoms may be more likely to occur in CH. Interestingly, in the EMAS cohort, men with CH had no sexual symptoms after adjustment for age, BMI, smoking status, alcohol intake, and comorbidity [45]. However, their inability to do vigorous activity after adjustments persisted. This may suggest mild T deficiency, since when the T thresholds of the different hypogonadal symptoms were compared, the thresholds of suppressed physical activity were reported at 13 nmol/L, while those of various sexual domains ranged between 8 and 11 nmol/L [55]. Hence, although many men with CH are not overtly hypogondal, they may exhibit borderline symptoms of T deficiency. Whether they truly exhibit high baseline T levels when eugonadal remains an unproven hypothesis.
CH may also represent an intermediary stage in the transition from eugonadism to PH. When the testicular capacity to produce T starts waning as a result of various factors, the negative feedback of T on gonadotropin secretion decreases and serum LH increases. Initially, elevations in LH are able to maintain normal T levels (CH). When testicular function deteriorates further, the pituitary is unable to further compensate and PH ensues. As the latter is commonly associated with chronological aging [56], we would similarly expect aging to be associated with CH. In reviewing findings from EMAS, this finding was indeed observed, with the overall cohort having a mean age of 58.5, CH of 67.3, and PH of 70.0 [45].
The suggestion of CH being a harbinger of impending PH was also supported by findings from the 4.3-year prospective data obtained during the EMAS study [12]. In the study cohort, 5.2% of the men experienced incident CH (i.e., developed CH during the follow-up period), while 6.6% had persistent CH and 2.4% reverted to eugonadism during follow-up. Men with CH at baseline, indeed, had a 15-fold higher risk to subsequently develop PH at follow-up than men with baseline normal LH and T [12]. The development of CH was associated with several factors, including age > 70 years, diabetes, chronic pain, pre-degree education, and low physical activity. These men also developed erectile dysfunction, poor health, CVD, and cancer more frequently, and their cognitive and physical function deteriorated more than in men with persistently normal LH.
Other supporting findings of the concept of CH include lower hemoglobin levels, erectile dysfunction, poor health, cardiovascular disease, cognitive and physical deterioration, and cancer compared to eugonadal men [12]. It could therefore be concluded that elevated LH in the presence of normal T is not an incidental finding. Although it may revert to normal levels spontaneously, it remains associated with multiple signs and symptoms of deteriorating health. However, at the present time, isolated elevations in LH are not an unequivocal biomarker of hypogonadism.
Several other studies support the finding that elevated LH in the setting of normal T may be associated with other medical conditions. Hyde and colleagues reported that increased LH was a risk factor for ischemic heart disease in older men [24]. The same conclusion was made by another study on CH where elevated LH was associated with psychiatric and cardiovascular symptoms, but not specifically with sexual symptoms [11]. These men were also more likely to develop PH compared to men with normal LH and T. A study from Denmark [21] found a positive association with elevated LH but not T and all-cause mortality, suggesting that CH may be a risk factor for early death. These preliminary findings underpin the potential clinical importance of detecting CH in the diagnostic work-up of men with suspected hypogonadism.
Another cohort study by Ventimiglia et al. [52] provided additional and somewhat contrasting information on CH in relation to other forms of hypogonadism. In their analysis of infertile, hypogonadal men, the authors stratified groups into PH, SH, and CH according to the EMAS criteria [45]. Results showed that CH represented a mild form of PH, while no differences in age were noted between groupings. Similar to the EMAS study, obesity was common among all men, with SH displaying the highest overall risk. Testicular volume was low, FSH high, and inhibin B low in both CH and PH. Impaired spermatogenesis was also identified in men with CH, which is not commonly evaluated in aging male studies.
3.4 Gonadotropins in the Treatment of Functional Hypogonadism
The first line of treatment for FH is lifestyle modifications, including weight reduction, dietary optimization, medication review, and treatment of comorbidities, after which T therapy can be considered in men with no contraindications [10]. If maintained fertility is desired, exogenous T is contraindicated, except in certain scenarios, as it reduces gonadotropins and intratesticular T below the threshold required to maintain spermatogenesis. In this setting, gonadotropin treatment may be useful in men with SH but not PH. One meta-analysis of men with organic SH reported outcomes of men who received gonadotropins and demonstrated successful results (mean sperm count 6 mil/mL and with at least one spermatozoon in ejaculate) in 75% of patients [38]. Improved success rates were noted among men who received both human chorionic gonadotropin (hCG) and FSH compared to hCG alone. Similar beneficial results were also observed among men who receive GnRH therapy.
Other fertility-preserving alternatives to treat hypogonadism include aromatase inhibitors (e.g., Ietrozole and anastrozole) or selective estrogen receptor modulators (SERMs ; e.g., clomiphene citrate and enclomiphene citrate), both of which increase gonadotropin levels by reducing the negative feedback at the hypothalamic level, thus potentiating the stimulation of intratesticular T and spermatogenesis. Aromatase inhibitors are generally not recommended for extended use because of their variable efficacy and deleterious effects on bone mineral density. SERMs might be a better alternative to achieve the same goal; however, more research is required to better evaluate their efficacy and long-term safety profile [1].
References
Awouters M, Vanderschueren D, Antonio L. Aromatase inhibitors and selective estrogen receptor modulators: unconventional therapies for functional hypogonadism? Andrology. 2019. https://doi.org/10.1111/andr.12725.
Bang AK, Nordkap L, Almstrup K, Priskorn L, Petersen JH, Rajpert-De Meyts E, et al. Dynamic GnRH and hCG testing: establishment of new diagnostic reference levels. Eur J Endocrinol. 2017;176:379–91.
Boehm U, Bouloux PM, Dattani MT, de Roux N, Dodé C, Dunkel L, et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism – pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2015;11:547–64.
Bousfield GR, Dias JA. Synthesis and secretion of gonadotropins including structure-function correlates. Rev Endocr Metab Disord. 2011;12:289–302.
Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control on body weight and reproduction. Science. 2000;289:2122–5.
Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, et al. Age-associated changes in hypothalamic–pituitary– testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European male ageing study. Eur J Endocrinol. 2013;168:445–55.
Casarini L, Pignatti E, Simoni M. Effects of polymorphisms in gonadotropin and gonadotropin receptor genes on reproductive function. Rev Endocr Metab Disord. 2011;12:303–21.
Castilla-García A, Santolaria-Fernández FJ, González-Reimers CE, Batista-López N, González-García C, Jorge-Hernández JA, Hernández-Nieto L. Alcohol induced hypogonadism: reversal after ethanol withdrawal. Drug Alcohol Depend. 1987;20:255–60.
Cheng XB, Wen JP, Yang J, Yang Y, Ning G, Li XY. GnRH secretion is inhibited by adiponectin through activation of AMP-activated protein kinase and extracellular signal-regulated kinase. Endocrine. 2011;39:6–12.
Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males. Andrology. 2020. https://doi.org/10.1111/andr.12770.
Corona G, Maseroli E, Rastrelli G, Sforza A, Forti G, Mannucci E, et al. Characteristics of compensated hypogonadism in patients with sexual dysfunction. J Sex Med. 2014;11:1823–34.
Eendebak RJAH, Ahern T, Swiecicka A, Pye SR, O’Neill TW, Bartfai G, et al. Elevated luteinizing hormone despite normal testosterone levels in older men-natural history, risk factors and clinical features. Clin Endocrinol. 2018;88:479–90.
Forest MG. How should we perform the human chorionic gonadotrophin (hCG) stimulation test? Int J Androl. 1983;6:1–4.
Garcia JM, Li H, Mann D, Epner D, Hayes TG, Marcelli M, Cunningham GR. Hypogonadism in male patients with cancer. Cancer. 2006;106:2583–91.
Giannetta E, Gianfrilli D, Barbagallo F, Isidori AM, Lenzi A. Subclinical male hypogonadism. Best Pract Res Clin Endocrinol Metab. 2010;26:539–50.
Gravholt CH, Chang S, Wallentin M, Fedder J, Moore P, Skakkebæk A. Klinefelter syndrome: integrating genetics, neuropsychology, and endocrinology. Endocr Rev. 2018;39:389–423.
Grossmann M, Matsumoto AM. A perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. J Clin Endocrinol Metab. 2017;102:1067–75.
Haring R, Baumeister SE, Völzke H, Dörr M, Kocher T, Nauck M, et al. Prospective inverse associations of sex hormone concentrations in men with biomarkers of inflammation and oxidative stress. J Androl. 2012;33:944–50.
Herbison AE. Physiology of the adult gonadotropin-releasing hormone neuronal network. In: Plant TM, Zeleznik AJ, editors. Knobil and Neil’s physiology of reproduction, vol. 1. 4th ed. Waltham: Academic Press; 2015. p. 399–467.
Hong CY, Park JH, Ahn RS, Im SY, Choi HS, Soh J, et al. Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol Cell Biol. 2004;24:2593–604.
Holmboe SA, Vradi E, Kold Jensen T, Linneberg A, Husemoen LLN, Scheike T, et al. The association of reproductive hormone levels and all-cause, cancer, and cardiovascular disease mortality in men. J Clin Endocrinol Metab. 2015;100:4472–80.
Huhtaniemi I, Ding YQ, Tähtelä R, Välimäki M. The bio/immuno ratio of plasma luteinizing hormone does not change during the endogenous secretion pulse: reanalysis of the concept using improved immunometric techniques. J Clin Endocrinol Metab. 1992;75(6):1442–5.
Huhtaniemi IT, Howard S, Dunkel L, Anderson RA. The gonadal axis: a life perspective. In: Pfaff DW, Joels M, editors. Hormones, brain, and behavior. 3rd ed. London: Elsevier; 2017. p. 3–58.
Hyde Z, Norman PE, Flicker L, et al. Elevated LH predicts ischaemic heart disease events in older men: the Health in Men Study. Eur J Endocrinol. 2011;164:569–77.
Iglesias P, Carrero JJ, Díez JJ. Gonadal dysfunction in men with chronic kidney disease: clinical features, prognostic implications and therapeutic options. J Nephrol. 2012;25:31–42.
Isidori AM, Caprio M, Strollo F, Moretti C, Frajese G, Isidori A, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab. 1999;84:3673–80.
Jaakkola T, Ding YQ, Kellokumpu-Lehtinen P, Valavaara R, Martikainen H, Tapanainen J, et al. The ratios of serum bioactive/immunoreactive luteinizing hormone and follicle-stimulating hormone in various clinical conditions with increased and decreased gonadotropin secretion: reevaluation by a highly sensitive immunometric assay. J Clin Endocrinol Metab. 1990;70:1496–505.
Kamischke A, Kemper DE, Castel MA, Lüthke M, Rolf C, Behre HM, et al. Testosterone levels in men with chronic obstructive pulmonary disease with or without glucocorticoid therapy. Eur Respir J. 1998;11:41–5.
Lamminen T, Huhtaniemi I. A common genetic variant of luteinizing hormone; relation to normal and aberrant pituitary-gonadal function. Eur J Pharmacol. 2001;414:1–7.
Landry D, Cloutier F, Martin LJ. Implications of leptin in neuroendocrine regulation of male reproduction. Reprod Biol. 2013;13:1–14.
Luukkaa V, Pesonen U, Huhtaniemi I, Lehtonen A, Tilvis R, Tuomilehto J, Koulu M, Huupponen R. Inverse correlation between serum testosterone and leptin in men. J Clin Endocrinol Metab. 1998;83:3243–6.
Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Morley PM, Stauber PM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46:410–3.
Muram D, Zhang X, Cui Z, Matsumoto AM. Use of hormone testing for the diagnosis and evaluation of male hypogonadism and monitoring of testosterone therapy: application of hormone testing guideline recommendations in clinical practice. J Sex Med. 2015;12:1886–94.
Neaves WB, Johnson L, Porter JC, Parker CR Jr, Petty CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984;59:756–63.
Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100.
Porte D Jr, Baskin DG, Schwartz MW. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes. 2005;54:1264–76.
Punab AM, Grigorova M, Punab M, Adler M, Kuura T, Poolamets O, et al. Carriers of variant luteinizing hormone (V-LH) among 1593 Baltic men have significantly higher serum LH. Andrology. 2015;3:512–9.
Rastrelli G, Corona G, Mannucci E, Maggi M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology. 2014;2:794–808.
Roelfsema F, Liu PY, Takahashi PY, Yang RJ, Veldhuis JD. Dynamic interactions between LH and testosterone in healthy community-dwelling men: impact of age and body composition. J Clin Endocrinol Metab. 2020;105:1–14.
Roumaud P, Martin LJ. Roles of leptin, adiponectin and resistin in the transcriptional regulation of steroidogenic genes contributing to decreased Leydig cells function in obesity. Horm Mol Biol Clin Investig. 2015;24:25–45.
Sasano N, Ichijo S. Vascular patterns of the human testis with special reference to its senile changes. Tohoku J Exp Med. 1969;99:269–80.
Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20:485–500.
Smith LB, Walker WH. Hormone signaling in the testis. In: Plant TM, Zeleznik AJ, editors. Knobil and Neil’s physiology of reproduction, vol. 1. 4th ed. Waltham: Academic Press; 2015. p. 637–90.
St-Arnaud R, Lachance R, Kelly SJ, Belanger A, Dupont A, Labrie F. Loss of luteinizing hormone bioactivity in patients with prostatic cancer treated with an LHRH agonist and a pure antiandrogen. Clin Endocrinol. 1986;24:21–30.
Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–8.
Tripathy D, Dhindsa S, Garg R, Khaishagi A, Syed T, Dandona P. Hypogonadotropic hypogonadism in erectile dysfunction associated with type 2 diabetes mellitus: a common defect? Metab Syndr Relat Disord. 2003;1:75–80.
Tsatsanis C, Dermitzaki E, Avgoustinaki P, Malliaraki N, Mytaras V, Margioris AN. The impact of adipose tissue-derived factors on the hypothalamic-pituitary-gonadal (HPG) axis. Hormones (Athens). 2015;14:549–62.
van der Poll T, Romijn JA, Endert E, Sauerwein HP. Effects of tumor necrosis factor on the hypothalamic-pituitary-testicular axis in healthy men. Metabolism. 1993;42:303–7.
Veldhuis JD, Dufau ML. Estradiol modulates the pulsatile secretion of biologically active luteinizing hormone in man. J Clin Invest. 1987;80:631–8.
Veldhuis JD, Liu PY, Keenan DM, Takahashi PY. Older men exhibit reduced efficacy of and heightened potency downregulation by intravenous pulses of recombinant human LH: a study in 92 healthy men. Am J Physiol Endocrinol Metab. 2012;302:E117–22.
Veldhuis J, Yang R, Roelfsema F, Takahashi P. Proinflammatory cytokine infusion attenuates LH’s feedforward on testosterone secretion: modulation by age. J Clin Endocrinol Metab. 2016;101:539–49.
Ventimiglia E, Ippolito S, Capogrosso P, Pederzoli F, Cazzaniga W, Boeri L, et al. Primary, secondary and compensated hypogonadism: a novel risk stratification for infertile men. Andrology. 2017;5:505–10.
Wheeler MJ. Assays for LH, FSH, and prolactin. Methods Mol Biol. 2006;324:109–24.
Witherspoon LR, Witkin M, Shuler SE, Neely H, Gilbert S. Heterophilic antibody as source of error in immunoassay. South Med J. 1986;79:836–9.
Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–35.
Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–45.
Young J, Xu C, Papadakis GE, Acierno JS, Maione L, Hietamäki J, Raivio T, et al. Clinical management of congenital hypogonadotropic hypogonadism. Endocr Rev. 2019;40:669–710.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Huhtaniemi, I. (2021). Role of Gonadotropins in Adult-Onset Functional Hypogonadism. In: Mulhall, J.P., Maggi, M., Trost, L. (eds) Controversies in Testosterone Deficiency . Springer, Cham. https://doi.org/10.1007/978-3-030-77111-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-77111-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-77110-2
Online ISBN: 978-3-030-77111-9
eBook Packages: MedicineMedicine (R0)