Abstract
Human IgA is comprised of two subclasses, IgA1 and IgA2. Monomeric IgA (mIgA), polymeric IgA (pIgA), and secretory IgA (SIgA) are the main molecular forms of IgA. The production of IgA rivals all other immunoglobulin isotypes. The large quantities of IgA reflect the fundamental roles it plays in immune defense, protecting vulnerable mucosal surfaces against invading pathogens. SIgA dominates mucosal surfaces, whereas IgA in circulation is predominately monomeric. All forms of IgA are glycosylated, and the glycans significantly influence its various roles, including antigen binding and the antibody effector functions, mediated by the Fab and Fc portions, respectively. In contrast to its protective role, the aberrant glycosylation of IgA1 has been implicated in the pathogenesis of autoimmune diseases, such as IgA nephropathy (IgAN) and IgA vasculitis with nephritis (IgAVN). Furthermore, detailed characterization of IgA glycosylation, including its diverse range of heterogeneity, is of emerging interest. We provide an overview of the glycosylation observed for each subclass and molecular form of IgA as well as the range of heterogeneity for each site of glycosylation. In many ways, the role of IgA glycosylation is in its early stages of being elucidated. This chapter provides an overview of the current knowledge and research directions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
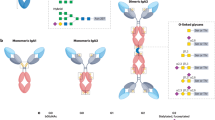
Immunoglobulin A (IgA) is one of the five primary immunoglobulins and its production in humans is greater than that of all other immunoglobulins. Humans expend a considerable amount of energy to produce IgA, as ∼70 mg of IgA per kilogram of body weight is synthesized daily (Mestecky et al. 1986; Conley and Delacroix 1987). IgA is secreted by IgA-producing cells in two main molecular forms: monomeric IgA (mIgA) and dimeric IgA (dIgA), the latter having a joining chain (J chain) to bind two mIgA molecules. Furthermore, secretory IgA (SIgA) is the main form of IgA found on mucosal surfaces. SIgA has an additional protein chain attached, a secretory component, which is derived from polymeric immunoglobulin (Ig) receptor during transcytosis of dIgA through mucosal epithelial cells. IgA is the second most abundant Ig in the circulation, predominantly as mIgA, (~2 mg/mL), after IgG (~12 mg/mL); however, IgA is the most abundant antibody in external secretions (tears, saliva, colostrum, milk, nasal fluid, gallbladder bile, and intestinal fluid) of mucosal surfaces, secreted locally as SIgA (Jackson et al. 2005). The abundance of IgA highlights the important roles it plays in immune defense processes. Specifically, mucosal surfaces (i.e., respiratory, gastrointestinal, and genitourinary tracts) are often exposed to invading pathogens and IgA acts to protect such vulnerable areas from infection. Equipped with unique structural attributes of its heavy chain and its ability to form monomeric, dimeric, and higher polymeric forms (i.e., three or more mIgA molecules associated with J chain), both circulatory IgA and secretory forms of IgA are capable of neutralizing and removing pathogens by activating innate and acquired immune functions—blocking pathogens by antigen-specific and nonspecific binding (Russell 2007; Renegar and Small 1994; Phalipon et al. 2002).
Serum IgA is produced predominantly by plasma cells in the bone marrow. It is primarily a monomeric protein with a quaternary structure consisting of two heavy chains (HC) and two light chains (LC) linked by disulfide bonds. In contrast, mucosal IgA, specifically dIgA linked together by the J chain, is produced close to the epithelium by plasma cells in the lamina propria of mucosal surfaces (Koshland 1985). This IgA becomes SIgA when, during transcytosis, the secretory component (SC) is attached. Although most IgA-producing cells are localized in the gastrointestinal system, SIgA is also one of the major antibodies in human tears, saliva, milk, and colostrum (Underdown and Mestecky 1994). Maternal milk is essential for the passive immunity of infants (Newburg et al. 2005; Hanson 1998; Peterson et al. 2013), protecting babies from a variety of infections that otherwise may lead to sepsis and meningitis (Schroten et al. 1998). The diversity of IgA post-translational modifications, due to variable glycosylation, contributes to the effector functions of IgA.
Regardless of the source of IgA, heavy chains of all molecular forms of IgA, as well as the J chain and SC, are glycosylated. In general, glycosylation is the result of a non-template, multi-enzymatic multistep process that ultimately results in a range of glycan heterogeneity. Thus, the post-translationally modified IgA proteoforms are cell type- and lineage-specific. Overall, glycans associated with Igs have been shown to influence antibody functions. Specifically, glycosylation patterns differentially affect the effector functions of Igs (Lin et al. 2015; Li et al. 2017) depending on the type, branching, and modifications of N-glycans and/or the terminal sugars of N- and/or O-glycans, which include galactose and sialic acid (Steffen et al. 2020). In fact, Ig glycosylation can determine whether an antibody glycoform is pro-inflammatory or anti-inflammatory (Li et al. 2017; Pagan et al. 2018). The multiple biological roles of IgA glycans include glycan-mediated antigen-nonspecific binding to bacteria and viruses. The complexity is enhanced by the diverse glycan structures that create a range of heterogeneity.
Herein, we will focus on the glycosylation of human IgA. Specifically, we discuss the location and observed structures at individual amino-acid sites and/or individual clusters of sites. We include a summary of the assessment of native populations of IgA glycans, the range of glycans observed at each site, and how changes in the glycans at a given site have been connected to biological activity and/or diseases.
2 IgA Subtypes and Overall Structure
Human IgA exists in two subclasses: IgA1 and IgA2 (occurring in two allotypes—IgA2m(1) and IgA2m(2)) (Fig. 14.1). Each Ig comprises two HCs and two LCs, each with one variable (V) and one (LC) or three (HC) constant domains. Each domain is approximately 110–130 amino acids, averaging 12–13 kDa. Functionally, the variable regions of HC and LC are responsible for antigen binding, while the constant domains are important for structure/function and defining/modifying the effector functions. Each of the two identical Fab regions of IgA consists of VH and VL and CH1 and CL, with CL being either kappa or lambda. HCs differ within their constant regions encoded by the Cα genes, Cα1 and 2. Each chain begins at its N-terminus with the variable region (VH), followed by the constant region, CH1, 2, and 3. CH1 is connected by the hinge region (HR) to the Fc region, consisting of CH2 and 3. Interchain disulfide bridges connect HC and LC of the Fab region and the two HC CH2 domains of the Fc region. Pairings between the collapsed β-barrel domains of adjacent chains (VH with VL, CH1 with CL, and both CH3 domains) are mediated through non-covalent interactions, primarily hydrogen bonds and van der Waals contacts. A conserved 18-amino-acid extension of the CH3 heavy chain C-terminus is known as the tailpiece. This feature is unique for IgA and IgM and plays a critical role in dimer and pentamer formation, respectively, though binding with the J chain (Atkin et al. 1996; Yoo et al. 1999).
Human IgA. Schematic diagram of IgA1 (top) and IgA2 (bottom) in several molecular forms (monomeric, dimeric, and secretory IgA). Although secretory IgA is depicted as a dimer, larger polymers such as trimers and tetramers can be formed. Heavy chains (VH, CH1, CH2, and CH3) are shown in orange (IgA1) and blue (IgA2), and light chains (VL and CL) are shown in light orange (IgA1) and light blue (IgA2). The tailpiece (TP) is portion of the CH3 domain that is depicted as an extension. The J chain (green) is present in dimeric and secretory IgA, the secretory component (pink) is a component of secretory IgA
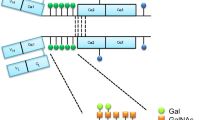
The HR between the Fab and Fc portions is another feature distinguishing each IgA isotype. IgA1, unlike IgA2, has an HR segment consisting of two octapeptide repeats rich in Ser, Thr, and Pro residues with nine potential O-glycosylation sites (Fig. 14.2). Usually, three to six of these sites are O-glycosylated (Novak et al. 2012, 2018). In contrast, the IgA2 HR lacks potential O-glycosylation sites. Unlike in IgG, IgA HR amino-acid sequence allows for greater flexibility, particularly for IgA1 with its extended sequence. Although this structural characteristic may enhance antigen binding by IgA, it also provides a target for bacterial IgA-specific proteases.
IgA1 and IgA2 hinge regions. Comparison of amino-acid sequences of human IgA1 (top) and IgA2 (bottom) hinge regions. Human IgA1 has nine Ser (S) and Thr (T) amino-acid residues (underlined) in the hinge region segment (between constant regions CH1 and CH2 of the heavy chains). Usually, three to six clustered O-glycans are attached per hinge region (shown in red). IgA2 hinge region is shorter compared to that of IgA1, does not have Ser and Thr residues and, thus, IgA2 does not have O-glycans
3 IgA Glycosylation Sites
The N-glycans at individual sites create a range of heterogeneity for both IgA isotypes, as they do for most Igs. Both IgA subclasses carry N-linked oligosaccharides. IgA1 and IgA2 have two and four conserved sites of N-glycosylation, respectively, all located in constant regions of the heavy chains. N-glycosylation significantly contributes to the total molecular mass of human IgA, accounting for 6–7% of the mass of IgA1, and 8–10% of the mass of IgA2 (Tomana et al. 1976). The IgA subclasses differ from other Igs in both the attachment sites and the overall positions of the N-linked glycans and the proximal disulfide bridges. IgA1 and IgA2 possess two similarly located conserved sites of N-linked glycosylation. The first site is at Asn144 (IgA1) and Asn131 (IgA2) in the CH2 domain of the heavy chain (the N-glycosylation sites herein are indicated by the residue number based on UniProt numbering, IgA1: P01876; IgA2: P01877) (UniProt Consortium 2017). The glycopeptide resulting from trypsin proteolytic digestion, as is conventional for analysis by mass spectrometry (MS), has an identical amino-acid sequence for both isotypes. Thus, if the two isotypes are not separated initially, these two N-glycosylation sites are observed as a mixture in standard MS glycosylation analysis (Steffen et al. 2020; Plomp et al. 2018). This is also the case for the second conserved site at Asn340 (IgA1) and Asn327 (IgA2) in the tailpiece. IgA2 has two additional sites of N-glycosylation, Asn47 located in the CH1 domain and in the CH2 domain at N205. All adjacent domains (VH with VL, CH1 with CL, and CH3 with CH3) are paired in close proximity to each other, except for the neighboring chains of the CH2 domains, which are not closely aligned. Such non-pairing is a feature also observed in IgG (CH2) and IgE (CH3). The potential solvent exposure, a consequence of the distance between the two CH2 domains, seems to be limited by the presence of N-glycans (located at Asn144 in IgA1 and Asn131 and Asn205 in IgA2) which shield the outer surface of the domain. The CH2 domain of IgA1 and IgA2 is stabilized by inter-chain disulfide bonds formed between three or four cysteine residues (Cys241, Cys242, Cys299, and Cys301) in the upper region of the domain. This feature is in contrast to IgG which instead has several disulfide connections located in the HR (2 HR disulfide bonds for IgG1 and IgG4, 4 for IgG2, and 11 for IgG3) (Liu and May 2012).
The tailpiece extension of the CH3 domain, of both IgA1 and IgA2, has a conserved N-glycan located at Asn340 and Asn327, respectively. The tailpiece, similar to the one found in IgM, contains a cysteine residue responsible for polymerization with the J chain. Previous studies have indicated that the tailpiece N-glycan of IgA plays an important role in J chain incorporation (Atkin et al. 1996; Sørensen et al. 2000). In plant-produced IgA proteins, the tailpiece is incompletely glycosylated and this deficiency may be the reason for the observed inefficient dimer formation (Göritzer et al. 2017, 2020; Westerhof et al. 2015; Castilho et al. 2018). Recently, it has been demonstrated that the tailpiece of IgA provides an innate line of defense against viruses, with the N-glycan mediating such activity (Maurer et al. 2018).
For IgA1, further glycosylation diversity arises through the clustered O-glycans attached to the HR. The glycosylated HR is a unique feature to Igs, shared by IgA1 and IgD, and in some forms of IgG3. In IgA1, these clustered O-linked glycans are composed of core 1 glycans or terminal or sialylated N-acetylgalactosamine (GalNAc), often attached to Thr225, Thr228, Ser230, Ser232, Thr233, and/or Thr236 (amino-acid numbering is based on conventionally used nomenclature for IgA1 HR). Each Ser/Thr residue can be modified by a GalNAc residue that can be further extended by the addition of galactose (Gal) through a β1,3 glycosidic bond. Up to two sialic acid (SA) residues can be added, one attached to the GalNAc through an α2,6-linkage and the other to Gal by an α2,3-linkage.
4 Molecular Forms of IgA and Their Distributions (Monomeric and Polymeric IgA, SIgA, J Chain, Secretory Component)
Unlike most other Ig isotypes, IgA exists in multiple molecular forms (Fig. 14.1). IgA in the circulation is produced mainly in the bone marrow and to a lesser extent in the spleen and lymph nodes. The predominant form of serum IgA is monomeric. IgA destined for mucosal surfaces is produced locally by plasma cells in a polymeric form (pIgA), predominantly dimeric. The ~16-kDa J chain is an additional polypeptide that connects together two or more monomers of IgA via the Fc region tailpiece through disulfide bonds, forming pIgA (Fig. 14.1). Two of the J chain’s eight cysteine residues are involved in covalent binding to IgAs tailpiece and the additional six cysteine residues form intramolecular disulfide bridges (Cys12-Cys100, Cys17-Cys91, and Cys108-Cys133) (Bastian et al. 1992). The J chain has a single N-linked glycan attached at Asn48. pIgA is formed after glycosylation occurs. Glycosylation is required for dimer assembly, as drastically reduced dimer formation was observed when Asn340 was substituted with alanine to prevent the attachment of N-glycans to the tailpiece (Atkin et al. 1996).
SIgA is a multi-polypeptide complex produced in mucosal tissues from pIgA, produced by local plasma cells, undergoing transcytosis through mucosal epithelial cells (Norderhaug et al. 1999). SIgA consists of an SC covalently attached to pIgA (i.e., IgA plus J chain). SIgA is mainly dimeric, although higher oligomers, including trimers and tetramers, are also present. The ~80-kDa SC is comprised of five immunoglobulin-like domains. To form SIgA, a polymeric immunoglobulin receptor-mediated pathway transports pIgA by transcytosis into the mucosal secretions. On reaching the mucosal surface, the polymeric immunoglobulin receptor (pIgR) that binds IgA is cleaved to release SIgA with bound SC which is a proteolytic cleavage product of the pIgR. SC is highly glycosylated by seven N-glycans, which contribute up to 25% of its molecular mass (Norderhaug et al. 1999). The SC glycans have several functions, in addition to protecting the SC and the SIgA from proteases (Crottet and Corthésy 1998), they can interact with adhesins and lectins. SC has been shown to bind to a range of bacteria via its glycans (Schroten et al. 1998; Borén et al. 1993; Wold et al. 1990), thereby inhibiting attachment and the subsequent infection of epithelial surfaces. The SC glycans are also involved in the localization of SIgA by anchoring the SIgA to the mucus lining the epithelial surface through its carbohydrate residues.
Circulating IgA is predominantly monomeric and predominantly of IgA1 subclass (~84% IgA1 and ~16% IgA2) (Mestecky et al. 1986), with a small proportion of pIgA (~5–8%) (Delacroix et al. 1982). Dimeric SIgA represents the dominant IgA at almost all mucosal surfaces, although the levels and subclass distributions among the different compartments of the mucosal immune system can vary considerably. For example, in saliva, 90–95% of total IgA is SIgA with a subclass distribution of ~65% IgA1 and ~35% IgA2. This is different for hepatic bile which has a much lower abundance of SIgA (~65%) and a subclass distribution of ~75% IgA1 and ~25% IgA2 (Delacroix et al. 1982). However, even the same compartment, sampled via different methods or times, can provide varying levels of isotype distribution and molecular forms. Furthermore, these distinct differences reflect the dominant Ig source (local production in mucosal tissue versus plasma), the expression of isotype-specific receptors that transport Igs, and the effects of specific regulatory mechanisms (e.g., cytokines and hormones) that influence Ig distribution.
5 IgA1 O-Glycosylation Pathways
The IgA1 HR with clustered sites of mucin-like O-glycosylation represents a unique acceptor site for a post-translational modification process that differs from most other Igs (only IgD has clustered O-glycans in its HR). Of the nine potential O-glycosylation sites in the HR of human IgA1, usually three to six are O-glycosylated (Baenziger and Kornfeld 1974; Mattu et al. 1998; Novak et al. 2000; Renfrow et al. 2007; Takahashi et al. 2010; Hiki et al. 1998; Iwase et al. 1996a; Royle et al. 2003; Franc et al. 2013; Wada et al. 2010a), although glycoforms with up to seven O-glycans have been observed in serum IgA1(Pouria et al. 2004). These clustered O-glycans of serum IgA1 are usually core 1 glycan (Field et al. 1989; Iwase et al. 1996b). The GalNAc-Gal disaccharide may be sialylated on GalNAc, Gal, or both sugars (Field et al. 1989; Takahashi et al. 2012). N-acetylneuraminic acid can be attached to GalNAc through an α2,6-linkage or to Gal by an α2,3-linkage. In addition to the core 1 glycans, some glycans may remain without Gal, i.e., as terminal GalNAc or sialylated GalNAc (Ohyama et al. 2020a, b). Unlike in the process of N-glycan biosynthesis, there is no control system for O-glycan biosynthesis, and O-glycosylation is also not required for folding or export of IgA1 (Gala and Morrison 2002).
Biosynthesis of IgA1 O-glycans occurs in the Golgi apparatus of IgA1-producing cells. As for other proteins with clustered O-glycans, it is a stepwise process that is not template-driven but rather controlled by expression, activity, and localization of different glycosyltransferases catalyzing the sequential addition of monosaccharides to the acceptor (glyco)protein during its transition through the Golgi apparatus (Reily et al. 2019). The clustered nature of the potential sites of O-glycosylation in the IgA1 HR creates a unique amino-acid synthesis template where each step of monosaccharide addition changes the template and has implications for the subsequent steps of monosaccharide addition at adjacent sites. This effect occurs due to some of the glycosyltransferases having glycan-recognizing lectin domains that strongly influence their activity (Stewart et al. 2019, 2021). Also, as more O-glycan chains are added and extended in the confined space of the HR, there is a steric hindrance that precludes or inhibits further addition or extension of the clustered O-glycans. Still, these physical constraints on the HR create a consistent fidelity of the range and distribution of O-glycans that are attached. This is similar to the clustered O-glycosylation sites of mucins that have multiple amino-acid tandem repeats as opposed to the two found in IgA1.
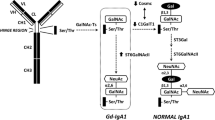
IgA1 O-glycosylation begins with the attachment of GalNAc to some of the Ser/Thr residues in the HR. In humans, some members of a family of 20 enzymes, GalNAc-transferases (GalNAc-Ts), are involved in this process (Daniel et al. 2020) (Fig. 14.3). It has been proposed that GalNAc-T2 is the main GalNAc-T enzyme responsible for the initiation of O-glycosylation of IgA1 (Iwasaki et al. 2003), although other GalNAc-Ts may contribute to the process, including GalNAc-T1, -T11, and -T14 (Stewart et al. 2021; Daniel et al. 2020; Wandall et al. 2007).
IgA1 O-glycosylation pathways. Biosynthesis of IgA1 O-glycans occurs in the Golgi apparatus of IgA1-producing cells. The stepwise process begins with the attachment of GalNAc to some of the Ser/Thr residues in the hinge region catalyzed by GalNAc-transferases (GalNAc-Ts). The attached GalNAc residues can be then modified by addition of Gal, mediated by core 1 β1,3-galactosyltransferase (C1GalT1). Production of the active C1GalT1 enzyme depends on its chaperone (C1GalT1C1, Cosmc). The core 1 structures (GalNAc-Gal) of IgA1 can be further modified by sialyltransferases that attach sialic acid to Gal (mediated by an ST3Gal enzyme, e.g., ST3Gal-I) and/or GalNAc residues. Sialylation of GalNAc is mediated by ST6GalNAc-II, as the usual ST6GalNAc-I is not expressed in IgA1-producing cells. Conversely, if terminal GalNAc is sialylated by ST6GalNAc-II, this structure cannot be further modified
GalNAc-Ts determine not only the sites of GalNAc attachment but also the final glycan density, as there are three to six sites per IgA1 HR that are typically glycosylated. Most GalNAc-T enzymes have two domains, a catalytic domain and lectin domain. Much of our understanding of GalNAc-T enzyme activities comes from studies of purified enzymes, synthetic peptides, glycopeptides, and glycoprotein substrates (Stewart et al. 2019, 2021; de Las et al. 2017). Data from multiple studies point to distinct and complementary roles of catalytic and lectin domains of GalNAc-Ts in the biosynthesis of clustered O-glycans of IgA1. Each monosaccharide addition can generate multiple isomers due to the selection of a specific attachment site. Whereas the selection of the first site is driven by the catalytic domain of the GalNAc-T enzyme, the subsequent site selection is substantially impacted by the lectin domain. The glycan density, i.e., the number of GalNAc residues per HR glycopeptide, depends on the expression and activity of GalNAc-Ts isoenzymes that can act on IgA1 and on the selection of the initiation and follow-up sites in the HR. Despite the variability of this step of O-glycan biosynthesis, most IgA1 HR glycoforms have three to six O-glycans.
The attached GalNAc residues can be modified by addition of Gal, mediated by core 1 β1,3-galactosyltransferase (C1GalT1) (Stewart et al. 2021; Ju et al. 2002, 2006; Aryal et al. 2012). The production of the active enzyme depends on its chaperone (C1GalT1C1, Cosmc) (Ju and Cummings 2002) (Fig. 14.3).
The core 1 structures of IgA1 may be further modified by sialyltransferases that attach sialic acid to Gal (mediated by a ST3Gal enzyme) and/or GalNAc residues (Stewart et al. 2021; Takahashi et al. 2014). Sialylation of GalNAc is mediated by ST6GalNAc-II, as the usual ST6GalNAc-I is not expressed in IgA1-producing cells (Takahashi et al. 2014; Raska et al. 2007; Stuchlova Horynova et al. 2015; Suzuki et al. 2008) (Fig. 14.3). Notably, sialylation of GalNAc on IgA1 by ST6GalNAc-II prevents subsequent galactosylation (Stewart et al. 2021; Stuchlova Horynova et al. 2015; Suzuki et al. 2014). Furthermore, studies with purified enzymes ST6GalNAc-II and ST3Gal-I and partially Gal-deficient IgA1 substrate revealed that prior sialylation by either enzyme influences the activity of the second enzyme (Stewart et al. 2021). These data suggest that the extent of sialylation of the clustered IgA1 HR segment is not only a net result of the enzyme activity but also involves steric hindrances of the clustered O-glycans (Novak et al. 2018; Stewart et al. 2021).
Additional knowledge about IgA1 O-glycan biosynthesis was obtained from genetic and genomic studies as well as studies of Epstein–Barr virus (EBV)-immortalized IgA1-secreting cells derived from the cells in human peripheral blood (Suzuki et al. 2008). EBV-immortalized IgA1-secreting cell lines derived from healthy individuals and patients with IgA nephropathy (IgAN) provided a new tool for comparative studies of IgA1 O-glycosylation in health and disease states. These cell lines produce IgA1 that mimics glycosylation of serum IgA1 of the respective donors (Suzuki et al. 2008). As detailed elsewhere, patients with IgAN have IgA1 in the glomerular immunodeposits and in the circulation enriched for glycoforms with some O-glycans deficient in Gal (Novak et al. 2018). Studies of EBV-immortalized IgA1-secreting cell lines from healthy individuals and patients IgAN revealed that dysregulation of expression and activity of several key enzymes is associated with elevated production of Gal-deficient IgA1 (Suzuki et al. 2008, 2014). Specifically, reduced expression/activity of C1GalT1 and its chaperone Cosmc and elevated expression/activity of ST6GalNAc-II in the cells from IgAN patients are associated with reduced Gal content in the secreted IgA1 (Suzuki et al. 2008).
Studies of familial and sporadic IgAN cohorts revealed heritability of serum levels of Gal-deficient IgA1 (Gharavi et al. 2008; Hastings et al. 2010; Kiryluk et al. 2011). Genome-wide association studies (GWAS) revealed single nucleotide polymorphisms (SNPs) in the noncoding region of C1GALT1, the gene encoding the C1GalT1 galactosyltransferase, associated with serum levels of Gal-deficient IgA1 (Kiryluk et al. 2017; Gale et al. 2017). One of these studies also found associations between SNPs in the noncoding region of C1GALT1C1 (Cosmc), the gene encoding the C1GalT1-specific chaperone, and serum levels of Gal-deficient IgA1 (Kiryluk et al. 2017). siRNA knock-down experiments using immortalized IgA1-secreting cell lines further validated these findings (Kiryluk et al. 2017).
Furthermore, the genetically co-determined Gal content can be further influenced by some cytokines and growth factors, such as interleukin 6 (IL-6), IL-4, and leukemia inhibitory factor (LIF) (Suzuki et al. 2014; Yamada et al. 2010, 2020). This cytokine-mediated overproduction of Gal-deficient IgA1 is due to further dysregulation of expression/activity of specific enzymes (C1GalT1, Cosmc, ST6GalNAc-II) (Suzuki et al. 2014). This process, uniquely enhanced in the IgA1-producing cells from patients with IgAN, is associated with the enhanced and prolonged cytokine signaling, likely due to an aberrant regulation of cellular signaling in JAK-STAT pathways that are engaged by IL-6 or LIF in the cells derived from IgAN patients (Yamada et al. 2017, 2020).
These observed genetic and genomic differences provide points of investigation as to how the final fidelity of IgA1 clustered O-glycans can be altered and lead to differences in observed distributions of IgA1 glycosylated proteoforms (IgA1 O-glycoforms).
6 Assessing Heterogeneity: Analysis of Glycan-Attachment Sites and Range of Heterogeneity
Analysis of IgA1 and IgA2 glycosylation has proven technically challenging due to the multiple molecular forms of IgA, the overall heterogeneity of N- and O-glycosylation, and the nature of the IgA1 HR where O-glycans are attached. Historically, several strategies have been used, including monosaccharide compositional analysis by gas–liquid chromatography (Renfrow et al. 2005) or high-performance anion-exchange chromatography with pulsed-amperometric detection (HPEAC-PAD), Edman sequencing (Baenziger and Kornfeld 1974; Mattu et al. 1998), glycan-specific lectin blotting and ELISA (Moldoveanu et al. 2007), and more recently liquid chromatography-mass spectrometry (LC-MS) (Takahashi et al. 2010). For IgA1 O-glycosylation, lectin ELISA has become a standard means to allow high-throughput analyses in a quantitative manner for serum-based studies (Moldoveanu et al. 2007; Gomes et al. 2010; Moore et al. 2007); however, limitations for several of these methodologies include lack of individual glycoform specificity and the inability to provide information on the sites of attachment and heterogeneity in the context of amino-acid sequence. As a result, much of the standard analysis of IgA glycosylation has moved toward LC-MS.
MS-based techniques utilized to profile glycans and address glycan heterogeneity have become the standard tool for the structural characterization of carbohydrates. Over the last two decades, our understanding of the structures and diversity of glycans in biological samples has increased dramatically. This is the result of the significant improvements in the sensitivity and variety of mass spectrometry approaches. With the help of chromatographic separation, glycan derivatization to improve ionization, and tandem MS fragmentation techniques, oligosaccharide structures and sites of attachment can be elucidated (de Haan et al. 2019). Due to the increased use of IgG as a therapeutic, the majority of analytical development regarding glycosylation has focused on exploring N-glycan structures and heterogeneity. While the enzymatic release of N-glycans is still a common tool for global N-glycan analysis (glycomics), for Ig N-glycosylation, amino-acid site-specific analysis has become standard as well as MS analysis of intact proteins to assess the composition and distribution of N-glycan heterogeneity (de Haan et al. 2019). Such methodologies that have become standard for the analysis of IgG N-glycans are easily applicable to IgA N-glycans. As we will discuss more below, that trend has occurred but comparatively there are considerably more studies of N-glycans of IgG than IgA. For analysis of IgA N-glycans, as discussed above, nearly all sources of IgA in the body have some level of mixture of isotypes and molecular forms. Thus, many of the currently reported analyses of IgA glycosylation are mixtures of IgA produced from multiple sources, however, some groups have begun to make distinctions between various molecular forms.
The features of IgA1 clustered O-glycans create a unique analytical challenge. The O-glycans are often referred to as “mucin-like” given they mimic O-glycans found on the heavily O-glycosylated mucin family of proteins that line most epithelial surfaces. Mucins have a large number of tandem repeats that are O-glycosylated. The IgA1 HR has only two tandem repeats making it somewhat more amenable to heterogeneity analysis. The O-glycans of IgA1 have been implicated in the pathogenesis of IgAN (Tomana et al. 1997, 1999; Novak et al. 2005), and the closely related IgA vasculitis with nephritis (IgAVN) (Allen et al. 1998; Levinsky and Barratt 1979). The goal of identifying differences in IgA1 HR O-glycan heterogeneity and composition between patients and normal healthy individuals has driven the development of methodologies. This includes determining the range of IgA1 O-glycoforms present and the sites of O-glycan attachment defined in the context of adjacent sites as well as the heterogeneity at each individual site (Novak et al. 2018).
As stated, investigators in the IgAN field drove the initial progress in assessing IgA1 O-glycosylation. In 1996, Iwase et al. identified two IgA1 HR glycopeptides containing four or five O-glycan chains by use of MALDI-TOF mass spectrometry (Iwase et al. 1996a). These trypsin-released O-glycopeptides were confirmed as the IgA1 HR by sequential glycosidase treatment (neuraminidase, galactosidase, and N-acetylgalactosaminidase) and the spectra showed a shift in the peak distribution toward lower masses as carbohydrate residues of the O-glycan chains were removed. This promising beginning led to several reports of IgA1 O-glycopeptides isolated from serum (Iwase et al. 1998, 1999) and pooled sera of patients with IgAN (Odani et al. 2000), IgA1 isolated from pooled renal biopsies (Hiki et al. 2001), and tonsillar IgA1 (Horie et al. 2003). In 2000, Novak et al. reported the use of IgA-specific proteases that released distinct IgA1 HR fragments (Fig. 14.4) and provided novel means of generating IgA1 HR O-glycopeptides for analysis by mass spectrometry and lectins (Novak et al. 2000). This preparation was unique because one of the IgA-specific proteases (Haemophilus HK50) cleaved between two sites of O-glycan attachment (cleavage after Pro231 between Ser230 and Ser232) and therefore provided a general localization of specific O-glycan chains N- or C-terminal to the cleavage site.
Heterogeneity of IgA1 hinge-region O-glycosylation. (a) Amino-acid sequence of the hinge region of human IgA1 showing fragments cleaved by IgA-specific proteases (see Fig. 14.6 legend). The six commonly utilized O-glycosylation sites are highlighted in red and marked by the numbers below Ser/Thr residues for the AK183-HF48 fragment. The two tandem repeats are underlined in the trypsin fragment (top). (b) Examples of two identified IgA1 HR O-glycopeptide positional isomers, GalNAc4Gal3 and GalNAc4Gal4 produced by trypsin + AK183 IgA-specific protease. (c) Illustration of the potential for sialic acid to be attached to Gal and/or GalNAc residues creating isomers at a single site. (d) Four initial sites of GalNAc addition observed by in vitro GalNAc-T2 reactions (Stewart et al. 2019)
In terms of IgA1 O-glycan site localization, two groups initially identified the predominant sites of O-glycan attachment to include Thr225, Thr228, Ser230, Ser232, and Thr236 by use of modified N-terminal sequencing methods (Baenziger and Kornfeld 1974; Mattu et al. 1998). However, these reports analyzed the IgA1 population as a whole with no separation of individual IgA1 O-glycopeptides. Later, the introduction of electron radical fragmentation (electron capture dissociation, ECD, and electron transfer dissociation, ETD) in tandem MS lead to the direct assessment of individual IgA1 HR O-glycopeptide ions released by trypsin and other proteases, including IgA-specific proteases (Fig. 14.4a). A series of reports by the Renfrow group demonstrated the direct assessment of sites of O-glycan attachment for several IgA1 O-glycopeptide forms ranging from three to five glycans and having one or two sites comprised of only terminal GalNAc (i.e., galactose-deficient sites-containing IgA1; Gd-IgA1). In the 2012 paper, Takahashi et al. also reported the presence of several IgA1 O-glycopeptides that were comprised of isomeric mixtures of equal O-glycan chain compositions that were attached at alternate amino-acid sites in the IgA1 HR (Fig. 14.4b) (Takahashi et al. 2012). They also identified a predominant sixth site of O-glycan attachment at Thr233. The three attachment sites at Ser230, Thr236, and Thr233 were identified as the predominant sites with galactose-deficient IgA1 (Gd-IgA1) glycans in the IgA1 myeloma protein that was studied as well as two samples of serum IgA1 isolated from healthy individuals. Interestingly, it was the combination of the IgA-specific proteases (Fig. 14.4) plus the novel tandem MS fragmentation that allowed elucidation of many of the details of O-glycan attachment that are now considered standard in our understanding of what the heterogeneity of IgA1 O-glycans includes. Concurrent with these studies, a study comparing methodologies for assessing O-glycans was reported in 2010 using an IgA1 myeloma protein as the reference standard analyzed by fifteen different laboratories (Wada et al. 2010a). While none of the participating laboratories included amino-acid site-specific analysis of IgA1 myeloma reference standard, the study demonstrated that the range of IgA1 O-glycopeptides observed was consistent across MS platforms and that there was also a level of consistency in the relative abundance of individual IgA1 O-glycopeptides. Since these studies, there has been a steady increase in the use of MS and LC-MS methodologies to assess the distribution of IgA1 hinge-region O-glycosylated forms.
One area that remains a challenge is the assessment of IgA1 O-glycan sialylation. IgA1 O-glycans can be α2,6 sialylated or α2,3 sialylated at any given position. With anywhere from three to six O-glycans per hinge region, sialylation significantly increases the number of isomeric mixtures in a population of IgA1 O-glycosylated forms (Fig. 14.4c). Takahashi et al. demonstrated the potential for sialic acid isomers at a single site (Thr232). As such, many of the LC-MS analyses have used sialidases to remove the sialic acid residues and reduce the complexity of the IgA1 O-glycopeptide mixtures (Takahashi et al. 2010, 2012; Moldoveanu et al. 2007).
The goal of understanding the difference in IgA O-glycans in patients with IgAN vs. healthy individuals has led to the development of lectin-based quantitative ELISA tests (Moldoveanu et al. 2007; Zhao et al. 2012). Lectins recognize specific glycan structures and in the case of IgAN, several lectins specific for terminal GalNAc have been utilized in ELISA to provide an IgA1-specific assessment of the extent of Gal-deficient IgA1 in different cohorts. This is done by using IgA from serum or tissue samples and then probing for lectin reactivity (using lectins from Helix pomatia agglutinin, HPA or Helix aspersa agglutinin, HAA) compared to a reference standard. Through this analysis, investigators have been able to establish the range of Gd-IgA1 levels (based on standardized lectin reactivity) that exists in patients with IgAN, even to the point of utilizing Gd-IgA1 as a marker for IgAN disease progression (Zhao et al. 2012; Maixnerova et al. 2019; Reily et al. 2018). It is possible that LC-MS methodologies may at some point be able to provide a similar output, but currently, these lectin-based ELISA tests have been successfully implemented for larger-cohort studies.
7 Observed IgA N-Glycosylation Heterogeneity
The characterization and analysis of IgA N-glycosylation is a systematic process of localizing the composition and range of glycans linked to asparagine at the glycosylation sites. Assessing Ig N-glycosylation has become a common methodology in recent years due to the advent of therapeutic antibodies. This is more the case for IgG, but the principles of systematic mapping and assessment of the range of N-glycans at a given site by the use of mass spectrometry have become a tool utilized in the analysis of Igs. For example, these techniques can assess how glycosylation of IgG is affected in different forms, sources, and in responses to specific antigens/vaccines. In the case of IgA, there is the added complexity of assessing the J chain and SC sites of N-glycosylation as well as the O-glycosylated IgA1 HR (discussed below). To date, comprehensive studies have been performed for mIgA and SIgA from plasma, saliva, and colostrum samples and, in a few cases, these studies have included separation of IgA isotypes. Several different structures of N-glycans have been observed, contributing to heterogeneity for both IgA subclasses (Fig. 14.5). Glycan heterogeneity for mIgA and all potential SIgA N-glycosylation sites, including the seven on the SC, two on IgA1, four on IgA2, and one on the J chain, are detailed below. Overall, several sources identified IgA N-glycans to be mainly of the biantennary complex type, with various levels of galactosylation, sialylation, bisecting GlcNAc, and fucosylation (Steffen et al. 2020; Plomp et al. 2018; Goonatilleke et al. 2019; Bondt et al. 2016, 2017). The following text provides details of what has been observed to date, but continued experimental analysis of IgA glycan heterogeneity will ultimately aid in understanding the complex biological roles of IgA and SIgA (including SC and J chain) in health and disease.
Sites of IgA glycosylation. Illustration of IgA1 and IgA2 and their respective glycosylation sites. IgA1 has three to six observed sites of O-glycosylation (shown in cyan) and two IgA1 N-glycosylation sites (Asn144 and Asn340, shown in purple). IgA2 has four N-glycosylation sites (Asn47 and Asn205, shown in red; and Asn131 and Asn327, shown in purple). The sites in purple are conserved in both IgA subclasses. The outlined area contain representations of the possible O- and N-glycan structures reported at each site (Steffen et al. 2020; Plomp et al. 2018; Goonatilleke et al. 2019; Bondt et al. 2016, 2017; Deshpande et al. 2010; Huang et al. 2015; Gomes et al. 2008). The ± symbol indicates the variable presence of each monosaccharide. The relative abundance values (%) are as reported by Steffen et al., who performed site-specific MS quantification of glycans from isolated serum IgA1 and IgA2 (Steffen et al. 2020). Values in orange correspond to IgA1 and values in blue correspond to IgA2
7.1 IgA1/IgA2 Asn340/327 N-Glycosylation
The N-glycosylation sites Asn340 and Asn327 on the tailpiece of IgA1 and IgA2, respectively, are located on tryptic peptides common to both IgA subclasses. Therefore, unless IgA1 and IgA2 are separated (often via jacalin agarose that binds only IgA1), MS analysis will not distinguish between subclasses and information on site-specific glycosylation is gathered for total IgA. At Asn340/327, two different tryptic peptide sequences are commonly identified, the expected LAGKPTHVNVSVVMAEVDGTCY and the truncated LAGKPTHVNVSVVMAEVDGTC. The abundance of each peptide seems to depend on the origin of IgA, specifically IgA from plasma displays a higher abundance of the truncated peptide while IgA derived from saliva includes higher amounts of the peptide with the C-terminal tyrosine (Plomp et al. 2018). The glycans at this location are primarily biantennary complex-type; however, a low abundance of triantennary glycans (~5%) on the truncated peptide has been reported (Bondt et al. 2016). Additionally, high-mannose and hybrid glycans have been detected in low amounts on the non-truncated peptide (<5% IgA1; <20% IgA2) (Steffen et al. 2020). The relative abundance of bisection, galactosylation, sialylation, and fucosylation are similar for both the non-truncated and truncated peptide. Over 89% galactosylation, sialylation, and fucosylation has been observed for the N-glycans of IgA1. Sialylation of IgA1 non-truncated peptide was slightly higher compared to the truncated peptide (89% and 95%, respectively) (Bondt et al. 2016). When observed and reported, the non-truncated peptide on IgA2 displayed slightly lower bisection (<60%) compared to IgA1 (<40%). Fucosylation was also lower in IgA2 (80% IgA2 versus 95% IgA1) (Bondt et al. 2016). In SIgA versus mIgA, Plomp et al. (2018) noted lower fucosylation (1.4-times), galactosylation (2-times), and sialylation (5-times) and higher content of bisecting GlcNac (1.7-times) in salivary compared to plasma IgA. High-mannose glycans were significantly higher in salivary IgA.
7.2 IgA1/IgA2 Asn144/131 N-Glycosylation
IgA1 Asn144 and IgA2 Asn131 also share the same tryptic amino-acid sequence LSLHRPALEDLLLGSEANLTCTLTGLR, located in the CH2 domain. Analysis has shown that this site is primarily composed of complex-type N-glycans, and Plomp et al. (2018) identified approximately 20% high mannose-type glycans and up to 5% hybrid-type/mono-antennary glycans. Steffen et al. (2020) isolated IgA1 and IgA2, specifically identifying non-complex glycan abundance of each subclass at 5% and 20%, respectively. For IgA1, complete galactosylation and approximately 60% sialylation was observed. There was variation in the reported bisecting glycans of Asn144 on IgA1. For example, Bondt et al. reported approximately 25% bisecting glycans which increased to approximately 28% during pregnancy (Bondt et al. 2016). Other studies found it to be higher—between 40 and 50% for IgA1 (Steffen et al. 2020; Plomp et al. 2018). Furthermore, it has been well documented that glycans at Asn144/131 are almost entirely afucosylated (<1%), in contrast to Asn340/327 (Plomp et al. 2018; Bondt et al. 2016). Notable differences in plasma versus salivary IgA were seen for high-mannose glycans, bisecting GlcNAc, galactose, and sialylation (18-times, 1.5-times, 3-times, and 5-times higher in plasma IgA, respectively). This is an interesting finding, as a higher abundance of unprocessed glycans usually reflects rapid processing through the cellular machinery, differential expression of glycosylation enzymes, or restricted access to a specific glycosylation site.
7.3 IgA2 N-Glycosylation
For the glycosylation sites Asn47 in the CH1 domain and Asn205 in the CH2 domain, unique to IgA2, N-glycans were observed by Plomp et al. on the peptides SESGQNVTAR and TPLTANITK, respectively. The glycans at these sites were mostly complex biantennary (>98% of total abundance) (Plomp et al. 2018). For plasma IgA2, Steffen et al. observed that both glycan sites were mainly monosialylated, had a high level of bisecting GlcNAc, and were almost always fucosylated (Steffen et al. 2020). Differences in galactosylation were observed as Asn47 was mainly monogalactosylated while the antennae of Asn205 were often fully galactosylated. Overall, two- to fivefold differences were observed in the relative abundances of the different glycan types present on plasma vs. salivary IgA2. Specifically, salivary N-glycans at both Asn47 and Asn205 showed a higher degree of bisecting GlcNAc and a lower degree of galactosylation, sialylation, and sialylation per galactose compared to plasma N-glycans (Steffen et al. 2020; Plomp et al. 2018; Bondt et al. 2016).
7.4 Joining Chain (J Chain) N-Glycosylation
The single N-glycosylation site Asn71 on the J chain has been observed as two tryptic peptides: EN71ISDPTSPLR (JC Asn71) and the miscleaved IIVPLNNREN71ISDPTSPLR (JC Asn71m). Plomp et al. (2018) determined that this site contained between 20 and 50% monoantennary and hybrid-type glycans, which is significantly higher than the IgA constant-domain N-glycosylation sites. However, differences in glycans in salivary and plasma IgA J chain are consistent with observations for the IgA1 and IgA2 heavy chain N-glycosylation sites, namely a higher bisection (3.2 times higher) and lower galactosylation (1.1 times lower) and sialylation (1.7 times lower) in the saliva-derived samples. Surprisingly, the two glycopeptides, JC Asn71 and JC Asn71m, exhibited different glycoprofiles. The miscleaved glycopeptides were more abundant in fucosylation as compared to the expected tryptic glycopeptides (3.2-times higher in plasma and 2.9-times higher in saliva) (Plomp et al. 2018). This observation was previously observed of colostrum, as 10% tryptic and 67% miscleaved glycopeptides were fucosylated (Deshpande et al. 2010). The range of glycan heterogeneity at this site is enhanced as the tryptic glycopeptide exhibits partial or full sialylation, which is uncommon for the miscleaved glycopeptide.
7.5 Secretory Component N-Glycosylation
Seven N-glycosylation sites are located on six SC tryptic glycopeptides. N-glycans at Asn135, Asn186, Asn421, and Asn469 were determined to be complex-type and biantennary with antennae fully galactosylated and partially sialylated (Plomp et al. 2018; Deshpande et al. 2010). However, other reports showed that sialylation was uncommon for Asn135, Asn186, Asn469, and Asn499 and highest for Asn421 and that Asn135 was abundantly tetraantennary (Huang et al. 2015). Mono-antennary species were identified on Asn499 and the observed glycoforms carried zero to five fucose residues, contributing to both the core and antennary fucosylation. On Asn135, Asn469, and Asn499 between 1 and 4% bisection was observed. All glycosylation sites contained at least five galactose residues and one to three fucose residues.
Glycans Asn83 and Asn90 are located on the same tryptic peptide, complicating site-specific glycosylation analysis. This can be remedied by employing additional proteases. Furthermore, the joint glycan composition H10N8F2−8S0–3 at Asn83 and Asn90, reported by Plomp et al., indicates a similar composition to other SC glycans (Plomp et al. 2018).
8 IgA Receptors and Role of Glycans
As detailed above, IgA subclasses are quite diverse in the origin, molecular form, and site-specific glycosylation profiles. The isotype, glycosylation, and molecular form of IgA can impact interactions with various types of IgA receptors. In humans, these receptors include Fc receptors (Fcα-receptor I [FcαRI; CD89] and Fcα/μ receptor [CD351]), polymeric immunoglobulin receptor, transferrin receptor (CD71), lectins (e.g., asialoglycoprotein receptor on hepatocytes), cell-surface galactosyltransferase (e.g., β1,4-galactosyltransferase), and Fc receptor-like 4 (FcRL4, CD307d) (de Sousa-Pereira and Woof 2019; Monteiro and Van De Winkel 2003; Breedveld and van Egmond 2019; Cho et al. 2006; Aleyd et al. 2015; Honda et al. 2016; Yang et al. 2013; Tomana et al. 1993). FcαRI is specific for IgA1 and IgA2 and FcRL4 binds polymeric IgA and IgA found in immune complexes, whereas other receptors can bind other ligands in addition to IgA. Below, we discuss several IgA receptors, focusing on details with respect to glycosylation and molecular forms of IgA.
FcαRI is expressed by myeloid cells, such as monocytes, neutrophils, and some subsets of macrophages and dendritic cells (van Egmond et al. 2001). Serum IgA, predominantly monomeric IgA1, can bind to FcαRI (CD89) (Monteiro et al. 1990; Herr et al. 2003). This receptor plays the main role in the IgA-mediated clearance of pathogens and cancer cells (Woof and Kerr 2006; de Tymowski et al. 2019; Brandsma et al. 2019; Hansen et al. 2018, 2019; Heemskerk and van Egmond 2018). Both the ligand (IgA1 and IgA2) and the receptor are glycosylated (Mattu et al. 1998; Royle et al. 2003; de Sousa-Pereira and Woof 2019; Aleyd et al. 2015). Whereas IgA N-glycans affect IgA thermal stability but not receptor binding (Gomes et al. 2008; Göritzer et al. 2019), N-glycans of FcαRI significantly modulate binding affinity to IgA (Göritzer et al. 2019). Furthermore, the binding of IgA1 to FcαRI induces long-range conformational changes in IgA1, propagating up to the O-glycosylated HR (Posgai et al. 2018).
FcαRI does not contain any signaling motifs in its cytoplasmic tail and FcαRI-mediated signaling depends on the associated Fc receptor γ chain and its immunoreceptor tyrosine-based activation motifs. FcαRI-mediated activation can induce degranulation, phagocytosis, chemotaxis, and antibody-dependent cellular cytotoxicity. Furthermore, when IgA-FcαRI signaling is combined with activation through pattern recognition receptors (e.g., Toll-like receptors), cytokine production can be induced in antigen-presenting cells. This process is essential for controlling inflammation and inducing both innate and adaptive immune responses. Depending on whether IgA binds to FcαRI in a soluble or aggregated form, it can induce immunosuppressive or pro-inflammatory responses. Soluble forms of IgA in the circulation, monomers and dimers, have low affinity for FcαRI and bind only transiently, thus mediating inhibitory signaling under homeostatic conditions (Hansen et al. 2019). Similar inhibitory effects can be exerted by peptidomimetics and such approaches may be useful to prevent undesirable inflammatory conditions triggered by abnormal IgA-containing immune complexes, such as in IgA-mediated blistering skin diseases (Breedveld and van Egmond 2019; Heineke et al. 2017; Ben Mkaddem et al. 2019).
A recent study underlined differences in the effector functions of human serum IgA1 and IgA2 on myeloid cells. IgA proteins were heat-aggregated or immobilized to mimic immune complexes. Under these conditions, IgA2 was more effective than IgA1 in the induction of pro-inflammatory responses in neutrophils and macrophages (Steffen et al. 2020). However, these differences disappeared after the enzymatic removal of sialic acid or all N-glycans from IgA1. Thus, IgA effector functions depend on the subclass and glycosylation, as IgA1 and IgA2 have similar but different glycosylation profiles (IgA2 has 2 additional sites of N-glycosylation). Notably, IgA1 contains more sialic acid than IgA2, thus explaining the nature of distinct IgA subclass activities.
Asialoglycoprotein receptor on hepatocytes mediates clearance of IgA (and other asialoglycoproteins) from the circulation (Stockert et al. 1982; Baenziger and Maynard 1980; Baenziger and Fiete 1980; Rifai et al. 2000; Tomana et al. 1988). The binding of IgA is based on recognition of terminal Gal or GalNAc residues by the lectin receptor whereas sialic acid prevents this binding. The IgA molecules internalized by the asialoglycoprotein receptor are degraded and excreted into the bile. This catabolic pathway is thought to explain the relatively short half-life of IgA in the circulation (~5 days).
Polymeric immunoglobulin receptor mediates the specific transport of polymeric immunoglobulins (J-chain-containing polymeric IgA and IgM) across the mucosal epithelium into the secretions. The transport of dimeric IgA across the epithelium (transcytosis) begins with its binding to the polymeric immunoglobulin receptor at the basolateral surface of the epithelial cell, which is followed by internalization and transport via vesicular compartments to the apical surface of the cell. During the process, the polymeric immunoglobulin receptor is cleaved to release the SC part of the receptor. Secretory component is attached through a disulfide bridge to dimeric IgA, forming secretory IgA released at the apical surface of mucosal epithelium. Notably, SIgA1 exhibits different O-glycosylation compared to circulatory IgA1. Circulatory IgA1 contains core 1 O-glycans (i.e., GalNAc-Gal that may be sialylated) whereas secretory IgA1 has also core 2 O-glycans with extended branches (Royle et al. 2003).
The transferrin receptor (CD71) binds IgA1, but not IgA2, and the IgA1 binding is inhibitable by transferrin (Moura et al. 2001). CD71 binds polymeric, but not monomeric IgA1, and the binding is dependent on glycosylation, namely O-glycosylation (Moura et al. 2004). CD71 is thought to participate in the binding of pathogenic IgA1-containing immune complexes by mesangial cells in IgAN (Moura et al. 2004).
FcRL4, expressed by a subset of memory B cells in the epithelia, recognizes polymeric IgA with J chain and heat-aggregated IgA but not secretory IgA (Wilson et al. 2012; Liu et al. 2020). FcRL4 is an inhibitory receptor for IgA. In addition to the four extracellular C2-type Ig domains and a transmembrane domain, it has a cytoplasmic domain that contains three immune-receptor tyrosine-based inhibitory motifs. FcRL4 is thought to regulate B cell responses to mucosal commensal antigens (Liu et al. 2018) and FcRL4-positive B cells have significantly increased usage of the IgA isotype (Amara et al. 2017). FcRL4-positive B cells are also enriched in the joints of patients with rheumatoid arthritis and it is thought that the production of cytokines with bone remodeling activity contributes to the disease pathology (Amara et al. 2017). It is currently not known whether glycosylation of IgA impacts recognition by FcRL4.
These several examples illustrate how glycosylation can impact interactions of different molecular forms of IgA with various types of IgA receptors. Consequently, these glycan-dependent interactions impact the effector function of specific IgA glycoproteoforms.
9 Observed IgA1 O-Glycosylation Heterogeneity
Based on the involvement of IgA1 in the pathogenesis of IgAN and other similar diseases, considerable progress has been made in the analysis of O-glycosylation of serum/plasma IgA1. Much of the current knowledge of IgA1 heterogeneity is based on the analysis of IgA1 myeloma proteins that have been extensively used for the development and comparisons of methodologies discussed above (Renfrow et al. 2005, 2007; Takahashi et al. 2010, 2012). These proteins have also served as model proteins for in vitro and in vivo experiments to understand the pathogenesis of IgAN (Takahashi et al. 2014; Novak et al. 2007, 2011a, b, 2015; Yanagihara et al. 2012; Knoppova et al. 2016; Suzuki et al. 2009, 2019; Rizk et al. 2019; Moldoveanu et al. 2021). Additionally, as the IgA1 HR has only two amino-acid tandem repeats rather than multiple repeats found in mucins, it has been used in many studies as a template for the understanding of clustered O-glycan synthesis by glycosyltransferases (Wandall et al. 2007; Ten Hagen et al. 2003; Fritz et al. 2004). This has also contributed to the understanding of what the final IgA1 O-glycan heterogeneity is and how it could be altered in a disease such as IgAN.
Nomenclature for IgA1 O-glycosylation forms core 1 O-glycans is based on glycan composition starting with the first monosaccharide attached, GalNAc, followed by Gal and then sialic acid (SA). A designation of GalNAc4Gal4 implies four O-glycan chains per heavy-chain HR comprised of GalNAc + Gal disaccharides. A designation of GalNAc5Gal4 implies five O-glycan chains comprised of four disaccharides and a single GalNAc monosaccharide. When sialic acid residues are assigned, such as GalNAc4Gal3SA1 or GalNAc5Gal5SA2, there is no assumption of where or how the SA is linked. IgA1 with at least one GalNAc without Gal in the HR O-glycans is often referred to as Gd-IgA1. Among the observed Gd-IgA1 glycoforms, there are variants with one to three Gal-deficient sites per HR. However, it should be noted that these forms are seen in IgA1 from healthy individuals as well as patients with IgAN. Still, most patients with IgAN exhibit elevated circulatory levels of Gd-IgA1.
Early studies of IgA1 from patients with IgAN and healthy controls observed the HR with three to six O-glycans (Baenziger and Kornfeld 1974; Mattu et al. 1998; Takahashi et al. 2010, 2012). This distribution has been consistent across many analyses by MS of serum and IgA1 myeloma proteins. There have been some examples of IgA1 myeloma proteins with as few as one to three O-glycans per HR, but these appear to be outside the norm (Renfrow et al. 2007). Consistently, the distribution of O-glycosylated forms appears to center around those with four (GalNAc4Gal4) and five (GalNAc5Gal4) O-glycans (Novak et al. 2000; Takahashi et al. 2010; Iwase et al. 1996a; Wada et al. 2010a; Renfrow et al. 2005). The 2010 comparison study (Wada et al. 2010a) across fifteen different labs confirmed that there was a clear distinction in the abundance of glycoforms no matter what MS technology was used. MS studies of site-specific protein and peptide glycosylation heterogeneity have made use of relative quantitation to reflect the consistent pattern and abundance of glycoform distributions that are observed. Experimentally manipulated changes in abundance are consistent and reflect that the ionization is driven by the peptide portion of the molecule (Stewart et al. 2019). Several groups have demonstrated this for both N- and O-glycosylation (Steffen et al. 2020; Plomp et al. 2018; Goonatilleke et al. 2019; Deshpande et al. 2010; Huang et al. 2015) as well as in the analysis of therapeutic glycoproteins (Yang et al. 2016). When IgA1 populations are analyzed with sialidase pretreatment, the distribution is dominated by GalNAc4Gal4 and GalNAc5Gal4 (usually >50% of total combined) followed by a second tier including GalNac4Gal3, GalNAc5Gal3, GalNAc5Gal5, and GalNAc3Gal3 (usually 30–40% of total combined). The remaining distribution is comprised of GalNAc6Gal4, GalNAc6Gal3, GalNac6Gal5, GalNAc3Gal2, GalNAc4Gal2, and GalNAc5Gal2 (each representing <5% of total) (Takahashi et al. 2012; Ohyama et al. 2020b). As discussed more below, most of these identified IgA1 O-glycopeptides are mixtures of amino-acid positional isomers (i.e., glycans attached at variable sites) further complicating the complete assignment of the total distribution of clustered O-glycan heterogeneity (Fig. 14.4b).
When viewed in the context of the IgA1 structure, the distribution of O-glycosylated forms is a result of the number of available Ser and Thr in the two tandem repeat amino-acid sequence, the enzymatic activity of the glycosyltransferases, and the three-dimensional constraints of the clustered amino-acid sites and flanking cysteines that are involved in disulfide bonds as part of the overall IgA1 quaternary structure. A recent analysis of in vitro reactions of GalNAc-T2 enzyme with IgA1 HR acceptor peptide identified a fast-rate phase of clustered GalNAc addition. For the GalNAc-T2 clustered activity on the native IgA1 HR, the fast phase ends after three GalNAc residues are added, matching the lower end of the predominant IgA1 O-glycosylated forms observed in serum IgA1 (Stewart et al. 2019). Interestingly, the natural occurrence of proteoforms with up to six O-glycan chains observed for serum IgA1 clustered O-glycans is less than what is observed for in vitro synthesis reactions on IgA1 HR peptide substrates where seven and eight additions of GalNAc have been reported (Stewart et al. 2019, 2021). This is where the overall structure of the IgA1 heavy chain and the flanking cysteine residues likely play a role in limiting the final O-glycan density. It is also possible that the concurrent addition of Gal and SA to the existing GalNAc residues limits the final heterogeneity as well.
As mentioned above, within this range of three to six O-glycans per HR, it is also the presence of amino-acid positional isomers that were initially identified in serum IgA1 (Takahashi et al. 2012). These isomeric mixtures occur from the outset of the synthesis process as demonstrated by in vitro GalNAc-T2 reactions (Stewart et al. 2019). Instead of a strictly ordered process of preferred amino-acid site addition, Stewart et al. demonstrated that there were four alternative initial sites of GalNAc addition at Thr228, Thr236, Ser230, and Ser232 with Thr228 and Thr236 being favored over the other two (Fig. 14.4d) (Stewart et al. 2019). Each initial site of GalNAc addition led to a unique combination of possible second sites of GalNAc addition. While the sites of GalNAc addition were all consistent with those seen with serum IgA1, these experiments revealed multiple GalNAc-addition pathways and explained the biosynthetic origin of isomeric mixtures of IgA1 O-glycosylated forms. Additional types of isomers can then occur due to variable sites of the addition of Gal and SA residues. Despite these variable O-glycosylation pathways, there is a consistent fidelity of the final IgA1 O-glycosylation distribution. For example, Ser230, Thr236, and Thr233 are the sites that show the predominant mixtures of isomers at the disaccharide and monosaccharide levels (Takahashi et al. 2012). These same sites are the predominant sites of Gal deficiency in serum IgA1. A recent study of serum IgA1 used enzymatic removal of GalNAc-Gal disaccharides (Ohyama et al. 2020b). This process resulted in consolidated mixtures of Gd-IgA1 forms with one, two, or three Gal-deficient sites (Ohyama et al. 2020b). These results corroborated Ser230, Thr236, and Thr233 as the predominant sites with Gal-deficient glycans but also indicated low levels of Thr228 and Thr232 with a single GalNAc residue as well. This study thus correlates with the data from in vitro studies of GalNAc-T2 that revealed multiple isomer possibilities. Since there are other GalNAc-Ts that have demonstrated activity on the IgA1 HR, it is possible that the distribution of IgA1 glyco-isomers is affected by expression patterns of GalNAc-Ts in specific IgA1-producing cells.
O-glycan heterogeneity of IgA1 can be impacted by additional constraints for the addition of Gal and SA. Based on the predominance of GalNAc4Gal4 and GalNAc5Gal4 HR glycoforms, it is logical to conclude the addition of up to four Gal residues is feasible for the C1GalT1 enzyme. The abundance of GalNAc5Gal5 HR glycoforms varies across some reports (~5–10%). This could reflect steric hindrances affecting the addition of a fifth Gal addition and/or a competition with SA addition occurring at the same time. The presence of SA on adjacent O-glycan chains has been demonstrated to inhibit C1GalT1 addition of Gal (Stewart et al. 2021; Takahashi et al. 2014; Suzuki et al. 2014). A similar inhibition has been demonstrated for SA addition by ST6GalNAc2 with prior addition of SA by ST3Gal1 and vice versa (Stewart et al. 2021; Suzuki et al. 2014). Interestingly, reports on O-glycan heterogeneity of serum IgA1 and IgA1 myeloma proteins have not found more than five SA residues for single IgA1 HR O-glycoforms despite there being two potential sites of addition on each glycan of core 1 disaccharide. This could be either the result of the steric hindrance of the clustered O-glycans or an artifact of analyzing IgA1 HR by positive-ion mode MS where SA residues add a negative charge. To date, it is unclear if a full accounting of the range of SA in IgA1 O-glycans has been accomplished.
In summary, while the generalized IgA1 O-glycan heterogeneity is consistent with three to six O-glycans per HR, there are underlying isomer mixtures that are likely cell-specific. Additionally, there is ample evidence from lectin ELISA studies that this complex heterogeneity is altered or shifted, reflecting elevated serum levels of Gd-IgA1 in patients with IgAN. The origin of these differences in IgA1 O-glycan heterogeneity is an active area of research.
10 IgA-Related Diseases
10.1 IgA Nephropathy
IgA nephropathy (IgAN) is an autoimmune disease characterized by the glomerular deposition of immune complexes containing galactose-deficient IgA1 (Gd-IgA1) (Knoppova et al. 2016; Rizk et al. 2019). This altered glycosylation results in the increased presence of terminal GalNAc or sialylated-GalNAc in the IgA1 HR. Gd-IgA1 in the glomerular immunodeposits is usually co-deposited with complement C3 and IgG autoantibodies specific to the abnormal glycosylation on the HR of Gd-IgA1. IgAN patients often have elevated levels of Gd-IgA1 and the corresponding IgG autoantibodies in the circulation, leading to the formation of Gd-IgA1-IgG circulating immune complexes. Circulatory levels of Gd-IgA1 are predictive of both disease progression and recurrence after transplantation (Berthoux et al. 2017). The current hypothesis on the pathobiology of IgAN highlights a four-hit mechanism, where elevated levels of Gd-IgA1 in the circulation (1) coupled with the production of anti-Gd-IgA1 IgG autoantibodies (2) leads to the formation of circulating immune complexes (3) some of which deposit in the glomeruli (4) to induce renal injury (Novak et al. 2008).
The galactose deficiency in IgAN affects HR glycans of the IgA1 and, thus, represents a change in O-glycosylation (Fig. 14.3). Gd-IgA1 in the circulation is predominantly in the polymeric (dimeric) form, although monomeric IgA1 is the predominant molecular form in the circulation; mechanisms leading to circulation of this molecular-form-specific effect are not known. In addition to genetically determined serum levels of Gd-IgA1, several studies show that some cytokines can elicit increased Gd-IgA1 production in IgA1-producing cells from IgAN patients. This effect is mediated by reduced expression of the galactosyltransferase encoded by the C1GALT1 gene, C1GalT1, an enzyme that is responsible for addition of galactose to the GalNAc residues in the HR of IgA1 (Suzuki et al. 2014; Yamada et al. 2017, 2020). Two GWAS studies have found association between IgAN and SNPs in the C1GALT1 locus, as well as its chaperone protein Cosmc encoded by C1GALT1C1 (Kiryluk et al. 2017).
The glycosylation of Gd-IgA1 in IgAN exhibits microheterogeneity patterns, both at the level of site attachments and specific glycan composition at each site (Novak et al. 2018; Franc et al. 2013; Ohyama et al. 2020a; Moore et al. 2007). The implication for this variability in glycosylation motifs suggests a semi-stochastic process based on enzyme activities and locations in the Golgi apparatus. Further research is needed to ascertain details of the mechanisms affecting the levels of Gd-IgA1 in the circulation of IgAN patients.
10.2 IgA Vasculitis with Nephritis (Formerly Known as Henoch–Schönlein Purpura Nephritis)
IgA vasculitis (IgAV) with nephritis (IgAVN) exhibits kidney-pathology features similar to IgAN, including IgA1 immunodeposits in the mesangium (Selewski et al. 2018). Notable, some patients with IgAVN progress to IgAN. As in patients with IgAN, IgAVN patients have elevated circulating levels of Gd-IgA1 (Kiryluk et al. 2011; Suzuki et al. 2019; Lau et al. 2007; Nakazawa et al. 2019; Pillebout et al. 2017) that form pathogenic immune complexes (Suzuki et al. 2019; Novak et al. 2007). The Gd-IgA1 immune complexes in IgAV typically deposit in the small vessels, leading to systemic vasculitis. In patients with IgAV, only a small fraction develops nephritis, 4–6 weeks from the disease onset, with mesangial proliferation found upon renal biopsy (Lau et al. 2010; Boyd and Barratt 2011). These biopsies show deposition of Gd-IgA1 in a fashion similar to IgAN (Zhao et al. 2020; Sugiyama et al. 2020). This, along with genetic studies showing heritability of circulatory Gd-IgA1 levels in patients with IgAN and IgAVN, indicates a close relationship between IgAVN and IgAN (Kiryluk et al. 2011; Suzuki et al. 2018; Hastings et al. 2021).
10.3 Crohn’s Disease
Crohn’s disease is a subset of inflammatory bowel diseases, which affects approximately 1% of the US population, and is characterized by inflammation of the gastrointestinal tract (Hanauer 2006). Serological predictors of this disease have been difficult to determine, but recent work on IgA1 HR glycoforms found that decreased number of GalNAc residues per HR was associated with progression vs. recovery in Crohn’s patients. Additionally, IgA1 with reduced content of O-glycans was found in the inflamed sections of intestinal biopsies from Crohn’s patients (Inoue et al. 2012).
10.4 Sjögren’s Syndrome
Sjögren’s syndrome is an autoimmune disease that can affect the tear and salivary glands, with a minority of patients developing other rheumatoid complications, such as systemic lupus erythematosus and rheumatoid arthritis. Patients with Sjögren’s syndrome have increased sialic acid content and decreased galactose content on both IgA1 and IgA2 N-glycans (Dueymes 1995). Additionally, Sjögren’s syndrome patients also have elevated serum levels of IgA1, and follow-up analysis showed that IgA1 was predominantly oversialylated in N-glycans (Basset et al. 2000; Levy et al. 1994).
10.5 Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune disease where the immune system targets multiple tissues and organs, such as the brain, lung, kidneys, joints, and vasculature. Most SLE patients have a positive anti-nuclear antibody test, but additional tests for anti-ds DNA antibodies, anti-Smith antibodies, and anti-U1RP antibodies can be more specific (Mummert et al. 2018; Olsen et al. 2017). Elevated circulating levels of IgA, 4–6-times higher than in healthy individuals, and abnormal glycosylation of IgA were observed in SLE patients. IgA isolated from female SLE patients showed decreased levels of unbisected biantennary, and tri- and tetraantennary oligosaccharides. Additionally, decreased galactosylation of the HR of IgA1 was found in the same female SLE patients (Matei and Matei 2000).
10.6 Rheumatoid Arthritis
The clinical manifestations of rheumatoid arthritis (RA) are swelling and pain in the joints, bone, and cartilage due to inflammation from inappropriate targeting by the immune system. The standard test for RA is a blood analysis of IgG/IgA/IgM antibodies against IgG (RF; rheumatoid factor) and ACPA (anti-citrullinated protein antibodies) (Westra et al. 2021; Kurowska et al. 2017). Mass spectrometry analysis of IgA1 isolated from the circulation of RA patients showed decreased content of GalNAc in the HR of IgA1 (Wada et al. 2010b). Additionally, both IgG and IgA from synovial fluid showed differential galactosylation and sialylation at early and late stages of RA. Terminal α2,6 sialic acid on IgA was lower in early RA compared to advanced RA, while terminal Gal on IgA was lower at early stages of RA and normalized later in the disease (Kratz et al. 2010).
10.7 IgA Myeloma
IgA myeloma is a clonal expansion of some IgA-producing cells that undergo genetic mutations leading to uncontrolled proliferation and increased serum levels of IgA (ranging from <30 g/L to >30 g/L) as the disease develops and progresses. Patients with IgA myeloma can develop multiple complications affecting the kidneys and bone and can exhibit various hematological pathologies. Production of IgA from clonal expansion in myeloma was found to include IgA glycoforms that were hyposialylated, which can affect FcαR1 binding (Basset et al. 1999; Bosseboeuf et al. 2020). Studies of the HR O-glycans from different IgA1 myeloma proteins have shown ranges of O-glycans outside the norm of three to six glycans observed in normal human serum (Renfrow et al. 2007). IgA1 deposition in the kidneys of some patients with IgA myeloma has been found concomitantly with under-galactosylated HRs, similar to the abnormality common to IgAN (Zickerman et al. 2000).
10.8 Celiac Disease
Celiac disease is an inflammatory intestinal condition brought on by antibodies targeting the transglutaminase 2 (TG2) protein and gluten-derived TG2-deamidated gliadin peptides. In patients with celiac disease, circulating IgA that targets TG2 has been found to be under-galactosylated, in addition to nonTG2 targeting IgA in circulation (Lindfors et al. 2011; Abbad et al. 2020). CD89 may be involved (Papista et al. 2015) and the interaction of multiple proteins, including CD71, was proposed (Lebreton et al. 2012; Papista et al. 2012).
10.9 Complement and IgA
IgA has been considered an anti-inflammatory immunoglobulin (Monteiro 2014; Diana et al. 2013; Ben Mkaddem et al. 2013). Unlike IgG, IgA-mediated activation of complement is not well understood, but it is generally accepted that IgA does not activate the classical complement pathway. However, IgA is thought to be able to activate alternative and lectin pathways of complement, under some circumstances. For example, the less abundant monomeric IgA2 was found to bind mannose-binding lectin (MBL) in ELISA. Additionally, when IgA1 or IgA2, both monomeric and polymeric, were treated with a galactosidase to remove galactose, their binding to MBL increased by over 10-fold (Terai et al. 2006). This would presumably be due to reducing the heterogeneity complexity of N-glycans in favor of high mannose glycans such as those observed at Asn340/327 sites. Additionally, IgA1 N-glycosylation is critical for binding C3, as evidenced by using point-mutation deletions in critical Asn sites in the Fc region (Chuang and Morrison 1997). This observation was further validated by inhibiting N-glycosylation of IgA using tunicamycin and testing for C3 binding (Zhang and Lachmann 1994). Interestingly, sialic acid content was also found to be critical for both IgA1 and IgA2 to fix C3b (Nikolova et al. 1994). In IgAN, where Gd-IgA1 is found in the polymeric form, the polymeric IgA1 is found to be able to bind MBL, resulting in C4 mesangial deposition (Oortwijn et al. 2006; Roos et al. 2001). Of note, complement activation resulting from abnormal IgA1 glycosylation can be found indirectly via anti-Gd-IgA1 IgG autoantibodies in IgAN, where the IgG presumably activates C3 when bound in an immune-complex with Gd-IgA1 (Novak et al. 2015; Knoppova et al. 2016; Rizk et al. 2019; Maillard et al. 2015).
11 Infectious Diseases
Some bacteria that can cause human infections utilize tools that negatively affect the defense mechanisms of the host. These tools include IgA-specific proteases and various glycosidases. Whereas the former act exclusively on IgA, the latter affect multiple glycoproteins at the mucosal surfaces. Below, we focus on several aspects of these bacterial virulence factors most relevant to IgA structural integrity and function.
11.1 Bacterial IgA-Specific Proteases
Multiple bacterial species that colonize human mucosal surfaces in the oral cavity, digestive tract, respiratory tract, and genital tract produce IgA-specific proteases. These bacteria include streptococci (e.g., Streptococcus pneumoniae, S. sanguinis, S. mitis, and S. oralis), Prevotella and Capnocytophaga species and several Neisseria, Clostridium, and Haemophilus species (e.g., Clostridium ramosum, Neisseria gonorrhoeae, N. meningitidis, Haemophilus influenzae) (Kilian and Holmgren 1981; Kilian et al. 1979, 1983; Senior et al. 2000; Frandsen et al. 1987, 1995, 1997; Reinholdt et al. 1990; Poulsen et al. 1989, 1996; Lomholt et al. 1992; Lomholt and Kilian 1994; Reinholdt and Kilian 1997; Kosowska et al. 2002; Wang et al. 2020; Chi et al. 2017; Murphy et al. 2015; Janoff et al. 2014; Johnson et al. 2009; Wani et al. 1996).
The bacterial IgA-specific proteases are post-proline endopeptidases that include metalloproteases, serine proteases, and cysteine proteases. These proteases recognize and cleave HR of IgA1, except the protease from Clostridium ramosum that can cleave both IgA1 and IgA2, with variable site-specific preferences (Senior et al. 2000; Kosowska et al. 2002; Batten et al. 2003; Mistry and Stockley 2011; Senior and Woof 2005). Figure 14.6 shows examples of commonly recognized sites in IgA1 HR by several representative IgA-specific proteases.
Bacterial IgA-specific proteases target the hinge region of IgA1. Amino-acid sequence of the hinge region of human IgA1. Six commonly utilized O-glycosylation sites are marked by the numbers below Ser/Thr residues. Numbers 1–5 with arrows above the amino-acid sequence mark peptide bonds that are cleaved by IgA-specific proteases produced by the following species and strains shown as examples: 1, Clostridium ramosum AK183; 2, Streptococcus pneumoniae TIGR4; 3, Haemophilus influenzae HK50; 4, Neisseria gonorrhoeae HF13; and 5, Neisseria gonorrhoeae HF48
It is thought that bacterial IgA-specific proteases represent a virulence factor for many pathogenic bacteria. Cleavage of IgA1 breaks the Fc fragment from its antigen-binding Fab fragments. This process thus reduces the protective effects of IgA1, such as inhibition of bacterial adherence and antibody complement-dependent killing of bacteria by phagocytic cells. In vitro studies of these proteases have demonstrated that the clustered IgA1 HR O-glycans can inhibit cleavage of some proteases indicating a shielding effect of this susceptible region of IgA1 by the O-glycans. Various studies examined and confirmed these concepts and searched for IgA-specific protease inhibitors (Wang et al. 2020; Janoff et al. 2014; Weiser et al. 2003; Garner et al. 2013).
11.2 Bacterial Glycosidases and Metabolic Utilization of IgA Glycans
Various bacteria occupying mucosal surfaces produce hydrolytic enzymes, such as endo- and exo-glycosidases, able to degrade O- and N-glycans of mucosal glycoproteins, including IgA. For example, in bacterial vaginosis, a common polymicrobial imbalance of the vaginal flora, IgA in the secretions is subjected to stepwise deglycosylation and subsequently enhanced proteolysis. These modifications are thought to compromise the ability of IgA to neutralize and eliminate pathogens (Lewis et al. 2012; Robinson et al. 2019; Govinden et al. 2018; Moncla et al. 2016; Smith and Ravel 2017). Streptococcus pneumoniae is a major airways pathogen that can produce multiple exo- and endoglycosidases to degrade O- and N-glycans of IgA and other glycoproteins (King et al. 2006; Marion et al. 2009; Syed et al. 2019; Robb et al. 2017; Blanchette et al. 2016; Kahya et al. 2017). For S. pneumoniae colonization of the upper airways, neuraminidase NanA is essential (Brittan et al. 2012). In some instances, a synergy between S. pneumoniae and influenza A sialidases can impact nasal colonization and middle ear infection by pneumococci (Wren et al. 2017). Conversely, IgA glycans, specifically sialic acid of the tail N-glycans, can inhibit influenza A and other enveloped viruses that use sialic acid as a receptor (Maurer et al. 2018). Another example of pathogenic bacteria utilizing glycosidases includes periodontitis and the associated bacteria, such as Porphyromonas gingivalis known to target host sialylated glycoproteins for immune dysregulation (Sudhakara et al. 2019). In the gastrointestinal system, IgA has multiple functions and glycans of secretory IgA1 (SIgA) are known to mediate the binding of SIgA to the microbiota. In fact, it has been proposed that IgA1 N- and O-glycans provide a link between innate and adaptive immune systems that sustain intestinal homeostasis (Royle et al. 2003; Reily et al. 2019; Mathias and Corthésy 2011a; Pabst and Slack 2020; Gupta et al. 2019; Nakajima et al. 2018; Corthésy 2013; Mathias and Corthésy 2011b). The net result of releasing the glycans is not only immune dysregulation and immune evasion but also a metabolic advantage. Some bacteria can metabolize the released glycans to support their growth (Perman and Modler 1982; Garbe and Collin 2012; Sjögren and Collin 2014).
11.3 IgA as a Potential Therapeutic Antibody
As outlined earlier, functions of human IgA subclasses, IgA1 and IgA2, are impacted by multiple aspects, including glycosylation, that directly and indirectly regulate IgA effector functions. For example, IgA1, but not IgA2, has O-glycans in addition to N-glycans and is more sialylated at the N-glycans. Consequently, IgA2 can induce pro-inflammatory responses in neutrophils and macrophages more effectively than IgA1 (Steffen et al. 2020). Conversely, polymeric IgA1, but not polymeric IgA2 or monomeric versions of either subclass, interacts with transferrin receptor CD71, apparently through IgA1 HR O-glycans (Moura et al. 2004). Furthermore, IgA glycans, specifically Gal and GalNAc, impact catabolism of IgA in the liver, a process that depends on the asialoglycoprotein receptor on hepatocytes and contributes to the short half-life of IgA in the circulation.
These aspects are at the forefront of recent research due to a growing interest in using IgA as a therapeutic antibody. The therapeutic applications of IgA may include systemic and mucosal delivery. In either setting, a better understanding is needed about the impact of producing-cell-specific glycosylation on IgA effector functions, receptor interactions, and pharmacokinetics. Biotechnological systems for the production of recombinant IgA, as well as other recombinant Igs, can include plants (Göritzer et al. 2017, 2019, 2020; Strasser et al. 2021; Kallolimath et al. 2016, 2020; Montero-Morales et al. 2019; Montero-Morales and Steinkellner 2018; Kallolimath and Steinkellner 2015). A better understanding of the differential glycosylation of various molecular forms and subclasses of IgA is needed to inform design and production of recombinant therapeutic IgA for future preclinical and clinical trials.
12 Therapeutic IgA Antibodies
Monoclonal antibody therapeutics have gained popularity in the medical field to treat a large set of pathologies due to their intrinsic specificities to target antigens. These therapies have largely been using IgG, although some research has been performed in vivo and in vitro to assess the feasibility of IgA therapeutics. IgA can be both pro-inflammatory and anti-inflammatory depending on the structure of its glycans which can regulate its binding to the FcαR1 and FcαR2 receptors. For example, treatment with IgA monoclonal antibodies against human epidermal growth factor receptor 2 (HER2) and epidermal growth factor receptor (EGFR) enhanced antitumor activity in FcαR1 transgenic mice (Boross et al. 2013; Meyer et al. 2016). This is thought to be done via activation of neutrophils and macrophages, which can be activated through IgA binding to FcαR1 and activating the immunoreceptor tyrosine-based activation motifs (ITAMs) pathway (Heineke and van Egmond 2017).
Targeting FcαR1 could prove to be an important clinical modality for IgA monoclonal treatments. However, a number of hurdles need to be overcome for IgA therapies to work. IgA is cleared more rapidly than IgG from the circulation in part due to asialoglycoprotein receptor expression in the liver. This receptor can bind multiple glycosylation sites on the IgA molecule, which can be inhibited by glycoengineering IgA to have increased terminal sialic acids (Rifai et al. 2000; Boross et al. 2013; Meyer et al. 2016). Interestingly, decreasing sialic acid content of IgA increases its pro-inflammatory capabilities (Steffen et al. 2020), leading to a trade-off between circulatory residence time (bioavailability) and the desired effector function of monoclonal IgA generated.
Production of properly folded and glycosylated IgA proteins can be difficult in mammalian systems, especially tuning the glycoengineering component to create the needed structures. This is in part due to the many different forms of N- and O-glycans that can be produced by mammalian cells, which makes it difficult to generate consistent glycoforms on a complex background (Montero-Morales and Steinkellner 2018). A more understudied way of generating consistent glycosylated proteins is to use modified plant cells that have undergone genetic modifications to specific glycosyltransferases involved in N- and O-glycosylation pathways. This approach was recently used successfully to generate IgG monoclonal therapies against the Ebola virus outbreak in Africa (Qiu et al. 2014; Davey et al. 2016). The generation of mammalian-like N-glycosylation-competent plants for IgG antibodies was first done in the Nicotiana benthamiana species, using genetic manipulations to inactivate the endogenous β1,2-xylosyltransferase (XylT) and α1,3-fucosyltransferase (FucT) genes (ΔXT/FT). This produced a biologically competent anti-HIV monoclonal antibody, with the same glycosylation as those expressed in Chinese hamster ovary (CHO) cell lines (Strasser et al. 2008). These ΔXT/FT plants have now been deployed to produce normally N- and O-glycosylated IgA1. While the whole IgA1 protein was not reproduced in the ΔXT/FT variant, the researchers were able to overcome hydroxyproline formation at the HR by overexpressing GalNAc-T2. This produced a variant of the core-1 motif-containing galactose, and only modest sialylation (Dicker et al. 2016). These studies that generate the technology for robust manufacturing of glycosylation competent IgA will be able to open up a larger sector for IgA therapeutics.
13 Conclusions
Understanding IgA glycosylation and its role in disease is still a developing field of study. However, with the help of MS, and recent technological advances, substantial progress has been made. Although still in its early stages, it is becoming more feasible to evaluate and decipher glycan structures and range of heterogeneities, especially when the different IgA isotypes and molecular forms are separated. Our knowledge of the role of IgA glycosylation in various diseases is currently based on observational studies. The one exception is the role that IgA1 O-glycans have been shown to play in the pathogenesis of IgA nephropathy. As the role of IgA in the immune response is further delineated from other immunoglobulins, the role of the glycans, especially those unique to the various IgA isotypes and molecular forms will be better understood. This will likely lead to IgA being more frequently used as a therapeutic to target specific disease states and/or locations within the human body.
Abbreviations
- C1GalT1:
-
Core 1 β1,3-galactosyltransferase
- dIgA:
-
Dimeric IgA
- EBV:
-
Epstein–Barr virus
- ECD:
-
Electron capture dissociation
- ETD:
-
Electron transfer dissociation
- FcRL4:
-
Fc receptor-like 4
- Gal:
-
Galactose
- GalNAc:
-
N-acetylgalactosamine
- GalNAc-T:
-
GalNAc-transferase
- Gd-IgA1:
-
Galactose-deficient IgA1
- HC:
-
Heavy chain
- HR:
-
Hinge region
- Ig:
-
Immunoglobulin
- IgAN:
-
IgA nephropathy
- IgAV:
-
IgA vasculitis
- IgAVN:
-
IgA vasculitis with nephritis
- LC:
-
Light chain
- MBL:
-
Mannose-binding lectin
- mIgA:
-
Monomeric IgA
- MS:
-
Mass spectrometry
- pIgA:
-
Polymeric IgA
- RA:
-
Rheumatoid arthritis
- SA:
-
Sialic acid
- SC:
-
Secretory component
- SIgA:
-
Secretory IgA
- SLE:
-
Systemic lupus erythematosus
- TG2:
-
Transglutaminase 2
- V:
-
Variable
References
Abbad L, Monteiro RC, Berthelot L (2020) Food antigens and Transglutaminase 2 in IgA nephropathy: molecular links between gut and kidney. Mol Immunol 121:1–6
Aleyd E, Heineke MH, van Egmond M (2015) The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease. Immunol Rev 268(1):123–138
Allen AC, Willis FR, Beattie TJ, Feehally J (1998) Abnormal IgA glycosylation in Henoch-Schönlein purpura restricted to patients with clinical nephritis. Nephrol Dial Transplant 13(4):930–934
Amara K, Clay E, Yeo L et al (2017) B cells expressing the IgA receptor FcRL4 participate in the autoimmune response in patients with rheumatoid arthritis. J Autoimmun 81:34–43
Aryal RP, Ju T, Cummings RD (2012) Tight complex formation between Cosmc chaperone and its specific client non-native T-synthase leads to enzyme activity and client-driven dissociation. J Biol Chem 287(19):15317–15329
Atkin JD, Pleass RJ, Owens RJ, Woof JM (1996) Mutagenesis of the human IgA1 heavy chain tailpiece that prevents dimer assembly. J Immunol 157(1):156–159
Baenziger JU, Fiete D (1980) Galactose and N-acetylgalactosamine-specific endocytosis of glycopeptides by isolated rat hepatocytes. Cell 22(2 Pt 2):611–620
Baenziger J, Kornfeld S (1974) Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem 249(22):7270–7281
Baenziger JU, Maynard Y (1980) Human hepatic lectin. Physiochemical properties and specificity. J Biol Chem 255(10):4607–4613
Basset C, Devauchelle V, Durand V et al (1999) Glycosylation of immunoglobulin A influences its receptor binding. Scand J Immunol 50(6):572–579
Basset C, Durand V, Jamin C et al (2000) Increased N-linked glycosylation leading to oversialylation of monomeric immunoglobulin A1 from patients with Sjögren’s syndrome. Scand J Immunol 51(3):300–306
Bastian A, Kratzin H, Eckart K, Hilschmann N (1992) Intra- and interchain disulfide bridges of the human J chain in secretory immunoglobulin A. Biol Chem Hoppe Seyler 373(12):1255–1263
Batten MR, Senior BW, Kilian M, Woof JM (2003) Amino acid sequence requirements in the hinge of human immunoglobulin A1 (IgA1) for cleavage by streptococcal IgA1 proteases. Infect Immun 71(3):1462–1469
Ben Mkaddem S, Rossato E, Heming N, Monteiro RC (2013) Anti-inflammatory role of the IgA Fc receptor (CD89): from autoimmunity to therapeutic perspectives. Autoimmun Rev 12(6):666–669
Ben Mkaddem S, Benhamou M, Monteiro RC (2019) Understanding Fc receptor involvement in inflammatory diseases: from mechanisms to new therapeutic tools. Front Immunol 10:811
Berthoux F, Suzuki H, Mohey H et al (2017) Prognostic value of serum biomarkers of autoimmunity for recurrence of IgA nephropathy after kidney transplantation. J Am Soc Nephrol 28(6):1943–1950
Blanchette KA, Shenoy AT, Milner J 2nd et al (2016) Neuraminidase A-exposed galactose promotes Streptococcus pneumoniae biofilm formation during colonization. Infect Immun 84(10):2922–2932
Bondt A, Nicolardi S, Jansen BC et al (2016) Longitudinal monitoring of immunoglobulin A glycosylation during pregnancy by simultaneous MALDI-FTICR-MS analysis of N- and O-glycopeptides. Sci Rep 6:27955
Bondt A, Nicolardi S, Jansen BC et al (2017) IgA N- and O-glycosylation profiling reveals no association with the pregnancy-related improvement in rheumatoid arthritis. Arthritis Res Ther 19(1):160
Borén T, Falk P, Roth KA, Larson G, Normark S (1993) Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262(5141):1892–1895
Boross P, Lohse S, Nederend M et al (2013) IgA EGFR antibodies mediate tumour killing in vivo. EMBO Mol Med 5(8):1213–1226
Bosseboeuf A, Seillier C, Mennesson N et al (2020) Analysis of the targets and glycosylation of monoclonal IgAs from MGUS and myeloma patients. Front Immunol 11:854
Boyd JK, Barratt J (2011) Inherited IgA glycosylation pattern in IgA nephropathy and HSP nephritis: where do we go next? Kidney Int 80(1):8–10
Brandsma AM, Bondza S, Evers M et al (2019) Potent Fc receptor signaling by IgA leads to superior killing of cancer cells by neutrophils compared to IgG. Front Immunol 10:704
Breedveld A, van Egmond M (2019) IgA and FcαRI: pathological roles and therapeutic opportunities. Front Immunol 10:553
Brittan JL, Buckeridge TJ, Finn A, Kadioglu A, Jenkinson HF (2012) Pneumococcal neuraminidase A: an essential upper airway colonization factor for Streptococcus pneumoniae. Mol Oral Microbiol 27(4):270–283
Castilho A, Beihammer G, Pfeiffer C et al (2018) An oligosaccharyltransferase from Leishmania major increases the N-glycan occupancy on recombinant glycoproteins produced in Nicotiana benthamiana. Plant Biotechnol J 16(10):1700–1709
Chi YC, Rahkola JT, Kendrick AA et al (2017) Streptococcus pneumoniae IgA1 protease: a metalloprotease that can catalyze in a split manner in vitro. Protein Sci 26(3):600–610
Cho Y, Usui K, Honda S, Tahara-Hanaoka S, Shibuya K, Shibuya A (2006) Molecular characteristics of IgA and IgM Fc binding to the Fcalpha/muR. Biochem Biophys Res Commun 345(1):474–478
Chuang PD, Morrison SL (1997) Elimination of N-linked glycosylation sites from the human IgA1 constant region: effects on structure and function. J Immunol 158(2):724–732
Conley ME, Delacroix DL (1987) Intravascular and mucosal immunoglobulin A: two separate but related systems of immune defense? Ann Intern Med 106(6):892–899
Corthésy B (2013) Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol 4:185
Crottet P, Corthésy B (1998) Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab’)2: a possible implication for mucosal defense. J Immunol 161(10):5445–5453
Daniel EJP, Las Rivas M, Lira-Navarrete E et al (2020) Ser and Thr acceptor preferences of the GalNAc-Ts vary among isoenzymes to modulate mucin-type O-glycosylation. Glycobiology 30(11):910–922
Davey RT Jr, Dodd L, Proschan MA et al (2016) A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med 375(15):1448–1456
de Haan N, Falck D, Wuhrer M (2019) Monitoring of immunoglobulin N- and O-glycosylation in health and disease. Glycobiology 30(4):226–240
de Las RM, Lira-Navarrete E, Daniel EJP et al (2017) The interdomain flexible linker of the polypeptide GalNAc transferases dictates their long-range glycosylation preferences. Nat Commun 8(1):1959
de Sousa-Pereira P, Woof JM (2019) IgA: structure, function, and developability. Antibodies (Basel) 8(4)
de Tymowski C, Heming N, Correia MDT et al (2019) CD89 is a potent innate receptor for bacteria and mediates host protection from sepsis. Cell Rep 27(3):762–775.e765
Delacroix DL, Dive C, Rambaud JC, Vaerman JP (1982) IgA subclasses in various secretions and in serum. Immunology 47(2):383–385
Deshpande N, Jensen PH, Packer NH, Kolarich D (2010) GlycoSpectrumScan: fishing glycopeptides from MS spectra of protease digests of human colostrum sIgA. J Proteome Res 9(2):1063–1075
Diana J, Moura IC, Vaugier C et al (2013) Secretory IgA induces tolerogenic dendritic cells through SIGNR1 dampening autoimmunity in mice. J Immunol 191(5):2335–2343
Dicker M, Tschofen M, Maresch D et al (2016) Transient glyco-engineering to produce recombinant IgA1 with defined N- and O-glycans in plants. Front Plant Sci 7:18
Dueymes M, Bendaoud B, Pennec YL, Youinou P (1995) IgA glycosylation abnormalities in the serum of patients with primary Sjögren’s syndrome. Clin Exp Rheumatol 13(2):247–250
Field MC, Dwek RA, Edge CJ, Rademacher TW (1989) O-linked oligosaccharides from human serum immunoglobulin A1. Biochem Soc Trans 17(6):1034–1035
Franc V, Řehulka P, Raus M et al (2013) Elucidating heterogeneity of IgA1 hinge-region O-glycosylation by use of MALDI-TOF/TOF mass spectrometry: role of cysteine alkylation during sample processing. J Proteome 92:299–312
Frandsen EV, Reinholdt J, Kilian M (1987) Enzymatic and antigenic characterization of immunoglobulin A1 proteases from Bacteroides and Capnocytophaga spp. Infect Immun 55(3):631–638
Frandsen EV, Reinholdt J, Kjeldsen M, Kilian M (1995) In vivo cleavage of immunoglobulin A1 by immunoglobulin A1 proteases from Prevotella and Capnocytophaga species. Oral Microbiol Immunol 10(5):291–296
Frandsen EV, Kjeldsen M, Kilian M (1997) Inhibition of Prevotella and Capnocytophaga immunoglobulin A1 proteases by human serum. Clin Diagn Lab Immunol 4(4):458–464
Fritz TA, Hurley JH, Trinh LB, Shiloach J, Tabak LA (2004) The beginnings of mucin biosynthesis: the crystal structure of UDP-GalNAc:polypeptide alpha-N-acetylgalactosaminyltransferase-T1. Proc Natl Acad Sci USA 101(43):15307–15312
Gala FA, Morrison SL (2002) The role of constant region carbohydrate in the assembly and secretion of human IgD and IgA1. J Biol Chem 277(32):29005–29011
Gale DP, Molyneux K, Wimbury D et al (2017) Galactosylation of IgA1 is associated with common variation in C1GALT1. J Am Soc Nephrol 28(7):2158–2166
Garbe J, Collin M (2012) Bacterial hydrolysis of host glycoproteins – powerful protein modification and efficient nutrient acquisition. J Innate Immun 4(2):121–131
Garner AL, Fullagar JL, Day JA, Cohen SM, Janda KD (2013) Development of a high-throughput screen and its use in the discovery of Streptococcus pneumoniae immunoglobulin A1 protease inhibitors. J Am Chem Soc 135(27):10014–10017
Gharavi AG, Moldoveanu Z, Wyatt RJ et al (2008) Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol 19(5):1008–1014
Gomes MM, Wall SB, Takahashi K, Novak J, Renfrow MB, Herr AB (2008) Analysis of IgA1 N-glycosylation and its contribution to FcαRI binding. Biochemistry 47(43):11285–11299
Gomes MM, Suzuki H, Brooks MT et al (2010) Recognition of galactose-deficient O-glycans in the hinge region of IgA1 by N-acetylgalactosamine-specific snail lectins: a comparative binding study. Biochemistry 49(27):5671–5682
Goonatilleke E, Smilowitz JT, Mariño KV, German BJ, Lebrilla CB, Barboza M (2019) Immunoglobulin A N-glycosylation presents important body fluid-specific variations in lactating mothers. Mol Cell Proteomics 18(11):2165–2177
Göritzer K, Maresch D, Altmann F, Obinger C, Strasser R (2017) Exploring site-specific N-glycosylation of HEK293 and plant-produced human IgA isotypes. J Proteome Res 16(7):2560–2570
Göritzer K, Turupcu A, Maresch D et al (2019) Distinct Fcα receptor N-glycans modulate the binding affinity to immunoglobulin A (IgA) antibodies. J Biol Chem 294(38):13995–14008
Göritzer K, Goet I, Duric S et al (2020) Efficient N-glycosylation of the heavy chain tailpiece promotes the formation of plant-produced dimeric IgA. Front Chem 8:346
Govinden G, Parker JL, Naylor KL, Frey AM, Anumba DOC, Stafford GP (2018) Inhibition of sialidase activity and cellular invasion by the bacterial vaginosis pathogen Gardnerella vaginalis. Arch Microbiol 200(7):1129–1133
Gupta S, Basu S, Bal V, Rath S, George A (2019) Gut IgA abundance in adult life is a major determinant of resistance to dextran sodium sulfate-colitis and can compensate for the effects of inadequate maternal IgA received by neonates. Immunology 158(1):19–34
Hanauer SB (2006) Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 12(Suppl 1):S3–S9
Hansen IS, Krabbendam L, Bernink JH et al (2018) FcαRI co-stimulation converts human intestinal CD103(+) dendritic cells into pro-inflammatory cells through glycolytic reprogramming. Nat Commun 9(1):863
Hansen IS, Baeten DLP, den Dunnen J (2019) The inflammatory function of human IgA. Cell Mol Life Sci 76(6):1041–1055
Hanson LA (1998) Breastfeeding provides passive and likely long-lasting active immunity. Ann Allergy Asthma Immunol 81(6):523–533; quiz 533–524, 537
Hastings MC, Moldoveanu Z, Julian BA et al (2010) Galactose-deficient IgA1 in African Americans with IgA nephropathy: serum levels and heritability. Clin J Am Soc Nephrol 5(11):2069–2074
Hastings MC, Rizk DV, Kiryluk K et al (2021) IgA vasculitis with nephritis: update of pathogenesis with clinical implications. Pediatr Nephrol
Heemskerk N, van Egmond M (2018) Monoclonal antibody-mediated killing of tumour cells by neutrophils. Eur J Clin Invest 48(Suppl 2):e12962
Heineke MH, van Egmond M (2017) Immunoglobulin A: magic bullet or Trojan horse? Eur J Clin Investig 47(2):184–192
Heineke MH, van der Steen LPE, Korthouwer RM et al (2017) Peptide mimetics of immunoglobulin A (IgA) and FcαRI block IgA-induced human neutrophil activation and migration. Eur J Immunol 47(10):1835–1845
Herr AB, Ballister ER, Bjorkman PJ (2003) Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc. Nature 423(6940):614–620
Hiki Y, Tanaka A, Kokubo T et al (1998) Analyses of IgA1 hinge glycopeptides in IgA nephropathy by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Am Soc Nephrol 9(4):577–582
Hiki Y, Odani H, Takahashi M et al (2001) Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int 59(3):1077–1085
Honda S, Sato K, Totsuka N et al (2016) Marginal zone B cells exacerbate endotoxic shock via interleukin-6 secretion induced by Fcα/μR-coupled TLR4 signalling. Nat Commun 7:11498
Horie A, Hiki Y, Odani H et al (2003) IgA1 molecules produced by tonsillar lymphocytes are under-O-glycosylated in IgA nephropathy. Am J Kidney Dis 42(3):486–496
Huang J, Guerrero A, Parker E et al (2015) Site-specific glycosylation of secretory immunoglobulin A from human colostrum. J Proteome Res 14(3):1335–1349
Inoue T, Iijima H, Tajiri M et al (2012) Deficiency of N-acetylgalactosamine in O-linked oligosaccharides of IgA is a novel biologic marker for Crohn’s disease. Inflamm Bowel Dis 18(9):1723–1734
Iwasaki H, Zhang Y, Tachibana K et al (2003) Initiation of O-glycan synthesis in IgA1 hinge region is determined by a single enzyme, UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 2. J Biol Chem 278(8):5613–5621
Iwase H, Tanaka A, Hiki Y et al (1996a) Estimation of the number of O-linked oligosaccharides per heavy chain of human serum IgA1 by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOFMS) analysis of the hinge glycopeptide. J Biochem 120(2):393–397
Iwase H, Tanaka A, Hiki Y et al (1996b) Abundance of Gal beta 1,3GalNAc in O-linked oligosaccharide on hinge region of polymerized IgA1 and heat-aggregated IgA1 from normal human serum. J Biochem 120(1):92–97
Iwase H, Tanaka A, Hiki Y et al (1998) Application of matrix-assisted laser desorption ionization time-of-flight mass spectrometry to the analysis of glycopeptide-containing multiple O-linked oligosaccharides. J Chromatogr B Biomed Sci Appl 709(1):145–149
Iwase H, Tanaka A, Hiki Y et al (1999) Aggregated human serum immunoglobulin A1 induced by neuraminidase treatment had a lower number of O-linked sugar chains on the hinge portion. J Chromatogr B Biomed Sci Appl 724(1):1–7
Jackson S, Mestecky J, Moldoveanu Z, Spearman PW (2005) Appendix I – Collection and processing of human mucosal secretions. In: Mestecky J, Lamm ME, McGhee JR, Bienenstock J, Mayer L, Strober W (eds) Mucosal immunology, 3rd edn. Academic, Burlington, pp 1829–1839
Janoff EN, Rubins JB, Fasching C et al (2014) Pneumococcal IgA1 protease subverts specific protection by human IgA1. Mucosal Immunol 7(2):249–256
Johnson TA, Qiu J, Plaut AG, Holyoak T (2009) Active-site gating regulates substrate selectivity in a chymotrypsin-like serine protease the structure of Haemophilus influenzae immunoglobulin A1 protease. J Mol Biol 389(3):559–574
Ju T, Cummings RD (2002) A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci U S A 99(26):16613–16618
Ju T, Brewer K, D’Souza A, Cummings RD, Canfield WM (2002) Cloning and expression of human core 1 beta1,3-galactosyltransferase. J Biol Chem 277(1):178–186
Ju T, Zheng Q, Cummings RD (2006) Identification of core 1 O-glycan T-synthase from Caenorhabditis elegans. Glycobiology 16(10):947–958
Kahya HF, Andrew PW, Yesilkaya H (2017) Deacetylation of sialic acid by esterases potentiates pneumococcal neuraminidase activity for mucin utilization, colonization and virulence. PLoS Pathog 13(3):e1006263
Kallolimath S, Steinkellner H (2015) Glycosylation of plant produced human antibodies. Hum Antibodies 23(3–4):45–48
Kallolimath S, Castilho A, Strasser R et al (2016) Engineering of complex protein sialylation in plants. Proc Natl Acad Sci USA 113(34):9498–9503
Kallolimath S, Hackl T, Gahn R et al (2020) Expression profiling and glycan engineering of IgG subclass 1-4 in Nicotiana benthamiana. Front Bioeng Biotechnol 8:825
Kilian M, Holmgren K (1981) Ecology and nature of immunoglobulin A1 protease-producing streptococci in the human oral cavity and pharynx. Infect Immun 31(3):868–873
Kilian M, Mestecky J, Schrohenloher RE (1979) Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect Immun 26(1):143–149
Kilian M, Thomsen B, Petersen TE, Bleeg HS (1983) Occurrence and nature of bacterial IgA proteases. Ann N Y Acad Sci 409:612–624
King SJ, Hippe KR, Weiser JN (2006) Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol Microbiol 59(3):961–974
Kiryluk K, Moldoveanu Z, Sanders JT et al (2011) Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int 80(1):79–87
Kiryluk K, Li Y, Moldoveanu Z et al (2017) GWAS for serum galactose-deficient IgA1 implicates critical genes of the O-glycosylation pathway. PLoS Genet 13(2):e1006609
Knoppova B, Reily C, Maillard N et al (2016) The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front Immunol 7:117
Koshland ME (1985) The coming of age of the immunoglobulin J chain. Annu Rev Immunol 3:425–453
Kosowska K, Reinholdt J, Rasmussen LK et al (2002) The Clostridium ramosum IgA proteinase represents a novel type of metalloendopeptidase. J Biol Chem 277(14):11987–11994
Kratz EM, Borysewicz K, Katnik-Prastowska I (2010) Terminal monosaccharide screening of synovial immunoglobulins G and A for the early detection of rheumatoid arthritis. Rheumatol Int 30(10):1285–1292
Kurowska W, Kuca-Warnawin EH, Radzikowska A, Maśliński W (2017) The role of anti-citrullinated protein antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Cent Eur J Immunol 42(4):390–398
Lau KK, Wyatt RJ, Moldoveanu Z et al (2007) Serum levels of galactose-deficient IgA in children with IgA nephropathy and Henoch-Schönlein purpura. Pediatr Nephrol 22(12):2067–2072
Lau KK, Suzuki H, Novak J, Wyatt RJ (2010) Pathogenesis of Henoch-Schönlein purpura nephritis. Pediatr Nephrol 25(1):19–26
Lebreton C, Ménard S, Abed J et al (2012) Interactions among secretory immunoglobulin A, CD71, and transglutaminase-2 affect permeability of intestinal epithelial cells to gliadin peptides. Gastroenterology 143(3):698–707.e694
Levinsky RJ, Barratt TM (1979) IgA immune complexes in Henoch-Schönlein purpura. Lancet 2(8152):1100–1103
Levy Y, Dueymes M, Pennec YL, Shoenfeld Y, Youinou P (1994) IgA in Sjögren’s syndrome. Clin Exp Rheumatol 12(5):543–551
Lewis WG, Robinson LS, Perry J et al (2012) Hydrolysis of secreted sialoglycoprotein immunoglobulin A (IgA) in ex vivo and biochemical models of bacterial vaginosis. J Biol Chem 287(3):2079–2089
Li W, Zhu Z, Chen W, Feng Y, Dimitrov DS (2017) Crystallizable fragment glycoengineering for therapeutic antibodies development. Front Immunol 8:1554–1554
Lin C-W, Tsai M-H, Li S-T et al (2015) A common glycan structure on immunoglobulin G for enhancement of effector functions. Proc Natl Acad Sci USA 112(34):10611–10616
Lindfors K, Suzuki H, Novak J et al (2011) Galactosylation of serum IgA1 O-glycans in celiac disease. J Clin Immunol 31(1):74–79
Liu H, May K (2012) Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. mAbs 4(1):17–23
Liu Y, McDaniel JR, Khan S et al (2018) Antibodies encoded by FCRL4-bearing memory B cells preferentially recognize commensal microbial antigens. J Immunol 200(12):3962–3969
Liu Y, Goroshko S, Leung LYT et al (2020) FCRL4 is an Fc receptor for systemic IgA, but not mucosal secretory IgA. J Immunol 205(2):533–538
Lomholt H, Kilian M (1994) Antigenic relationships among immunoglobulin A1 proteases from Haemophilus, Neisseria, and Streptococcus species. Infect Immun 62(8):3178–3183
Lomholt H, Poulsen K, Caugant DA, Kilian M (1992) Molecular polymorphism and epidemiology of Neisseria meningitidis immunoglobulin A1 proteases. Proc Natl Acad Sci USA 89(6):2120–2124
Maillard N, Wyatt RJ, Julian BA et al (2015) Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol 26(7):1503–1512
Maixnerova D, Ling C, Hall S et al (2019) Galactose-deficient IgA1 and the corresponding IgG autoantibodies predict IgA nephropathy progression. PLoS One 14(2):e0212254
Marion C, Limoli DH, Bobulsky GS, Abraham JL, Burnaugh AM, King SJ (2009) Identification of a pneumococcal glycosidase that modifies O-linked glycans. Infect Immun 77(4):1389–1396
Matei L, Matei I (2000) Lectin-binding profile of serum IgA in women suffering from systemic autoimmune rheumatic disorders. Rom J Intern Med 38–39:73–82
Mathias A, Corthésy B (2011a) N-Glycans on secretory component: mediators of the interaction between secretory IgA and gram-positive commensals sustaining intestinal homeostasis. Gut Microbes 2(5):287–293
Mathias A, Corthésy B (2011b) Recognition of gram-positive intestinal bacteria by hybridoma- and colostrum-derived secretory immunoglobulin A is mediated by carbohydrates. J Biol Chem 286(19):17239–17247
Mattu TS, Pleass RJ, Willis AC et al (1998) The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem 273(4):2260–2272
Maurer MA, Meyer L, Bianchi M et al (2018) Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 23(1):90–99
Mestecky J, Russell MW, Jackson S, Brown TA (1986) The human IgA system: a reassessment. Clin Immunol Immunopathol 40(1):105–114
Meyer S, Nederend M, Jansen JH et al (2016) Improved in vivo anti-tumor effects of IgA-Her2 antibodies through half-life extension and serum exposure enhancement by FcRn targeting. MAbs 8(1):87–98
Mistry DV, Stockley RA (2011) The cleavage specificity of an IgA1 protease from Haemophilus influenzae. Virulence 2(2):103–110
Moldoveanu Z, Wyatt RJ, Lee JY et al (2007) Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 71(11):1148–1154
Moldoveanu Z, Suzuki H, Reily C et al (2021) Experimental evidence of pathogenic role of IgG autoantibodies in IgA nephropathy. J Autoimmun 118:102593
Moncla BJ, Chappell CA, Debo BM, Meyn LA (2016) The effects of hormones and vaginal microflora on the glycome of the female genital tract: cervical-vaginal fluid. PLoS One 11(7):e0158687
Monteiro RC (2014) Immunoglobulin A as an anti-inflammatory agent. Clin Exp Immunol 178(Suppl 1):108–110
Monteiro RC, Van De Winkel JG (2003) IgA Fc receptors. Annu Rev Immunol 21:177–204
Monteiro RC, Kubagawa H, Cooper MD (1990) Cellular distribution, regulation, and biochemical nature of an Fc alpha receptor in humans. J Exp Med 171(3):597–613
Montero-Morales L, Steinkellner H (2018) Advanced plant-based glycan engineering. Front Bioeng Biotechnol 6:81
Montero-Morales L, Maresch D, Crescioli S et al (2019) In planta glycan engineering and functional activities of IgE antibodies. Front Bioeng Biotechnol 7:242
Moore JS, Kulhavy R, Tomana M et al (2007) Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol 44(10):2598–2604
Moura IC, Centelles MN, Arcos-Fajardo M et al (2001) Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J Exp Med 194(4):417–425
Moura IC, Arcos-Fajardo M, Sadaka C et al (2004) Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol 15(3):622–634
Mummert E, Fritzler MJ, Sjöwall C, Bentow C, Mahler M (2018) The clinical utility of anti-double-stranded DNA antibodies and the challenges of their determination. J Immunol Methods 459:11–19
Murphy TF, Kirkham C, Jones MM, Sethi S, Kong Y, Pettigrew MM (2015) Expression of IgA proteases by Haemophilus influenzae in the respiratory tract of adults with chronic obstructive pulmonary disease. J Infect Dis 212(11):1798–1805
Nakajima A, Vogelzang A, Maruya M et al (2018) IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J Exp Med 215(8):2019–2034
Nakazawa S, Imamura R, Kawamura M et al (2019) Evaluation of IgA1 O-glycosylation in Henoch-Schönlein purpura nephritis using mass spectrometry. Transplant Proc 51(5):1481–1487
Newburg DS, Ruiz-Palacios GM, Morrow AL (2005) Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 25:37–58
Nikolova EB, Tomana M, Russell MW (1994) The role of the carbohydrate chains in complement (C3) fixation by solid-phase-bound human IgA. Immunology 82(2):321–327
Norderhaug IN, Johansen FE, Schjerven H, Brandtzaeg P (1999) Regulation of the formation and external transport of secretory immunoglobulins. Crit Rev Immunol 19(5–6):481–508
Novak J, Tomana M, Kilian M et al (2000) Heterogeneity of O-glycosylation in the hinge region of human IgA1. Mol Immunol 37(17):1047–1056
Novak J, Tomana M, Matousovic K et al (2005) IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int 67(2):504–513
Novak J, Moldoveanu Z, Renfrow MB et al (2007) IgA nephropathy and Henoch-Schoenlein purpura nephritis: aberrant glycosylation of IgA1, formation of IgA1-containing immune complexes, and activation of mesangial cells. Contrib Nephrol 157:134–138
Novak J, Julian BA, Tomana M, Mestecky J (2008) IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol 28(1):78–87
Novak J, Raskova Kafkova L, Suzuki H et al (2011a) IgA1 immune complexes from pediatric patients with IgA nephropathy activate cultured human mesangial cells. Nephrol Dial Transplant 26(11):3451–3457
Novak J, Moldoveanu Z, Julian BA et al (2011b) Aberrant glycosylation of IgA1 and anti-glycan antibodies in IgA nephropathy: role of mucosal immune system. Adv Otorhinolaryngol 72:60–63
Novak J, Julian BA, Mestecky J, Renfrow MB (2012) Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin Immunopathol 34(3):365–382
Novak J, Rizk D, Takahashi K et al (2015) New insights into the pathogenesis of IgA nephropathy. Kidney Dis 1(1):8–18
Novak J, Barratt J, Julian BA, Renfrow MB (2018) Aberrant glycosylation of the IgA1 molecule in IgA nephropathy. Semin Nephrol 38(5):461–476
Odani H, Hiki Y, Takahashi M et al (2000) Direct evidence for decreased sialylation and galactosylation of human serum IgA1 Fc O-glycosylated hinge peptides in IgA nephropathy by mass spectrometry. Biochem Biophys Res Commun 271(1):268–274
Ohyama Y, Nakajima K, Renfrow MB, Novak J, Takahashi K (2020a) Mass spectrometry for the identification and analysis of highly complex glycosylation of therapeutic or pathogenic proteins. Expert Rev Proteomics 17(4):275–296
Ohyama Y, Yamaguchi H, Nakajima K et al (2020b) Analysis of O-glycoforms of the IgA1 hinge region by sequential deglycosylation. Sci Rep 10(1):671
Olsen NJ, Choi MY, Fritzler MJ (2017) Emerging technologies in autoantibody testing for rheumatic diseases. Arthritis Res Ther 19(1):172
Oortwijn BD, Roos A, Royle L et al (2006) Differential glycosylation of polymeric and monomeric IgA: a possible role in glomerular inflammation in IgA nephropathy. J Am Soc Nephrol 17(12):3529–3539
Pabst O, Slack E (2020) IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol 13(1):12–21
Pagan JD, Kitaoka M, Anthony RM (2018) Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell 172(3):564–577.e513
Papista C, Gerakopoulos V, Kourelis A et al (2012) Gluten induces coeliac-like disease in sensitised mice involving IgA, CD71 and transglutaminase 2 interactions that are prevented by probiotics. Lab Investig 92(4):625–635
Papista C, Lechner S, Ben Mkaddem S et al (2015) Gluten exacerbates IgA nephropathy in humanized mice through gliadin-CD89 interaction. Kidney Int 88(2):276–285
Perman JA, Modler S (1982) Glycoproteins as substrates for production of hydrogen and methane by colonic bacterial flora. Gastroenterology 83(2):388–393
Peterson R, Cheah WY, Grinyer J, Packer N (2013) Glycoconjugates in human milk: protecting infants from disease. Glycobiology 23(12):1425–1438
Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthésy B (2002) Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17(1):107–115
Pillebout E, Jamin A, Ayari H et al (2017) Biomarkers of IgA vasculitis nephritis in children. PLoS One 12(11):e0188718
Plomp R, de Haan N, Bondt A, Murli J, Dotz V, Wuhrer M (2018) Comparative glycomics of immunoglobulin A and G from saliva and plasma reveals biomarker potential. Front Immunol 9:2436
Posgai MT, Tonddast-Navaei S, Jayasinghe M, Ibrahim GM, Stan G, Herr AB (2018) FcαRI binding at the IgA1 C(H)2-C(H)3 interface induces long-range conformational changes that are transmitted to the hinge region. Proc Natl Acad Sci USA 115(38):E8882–e8891
Poulsen K, Brandt J, Hjorth JP, Thøgersen HC, Kilian M (1989) Cloning and sequencing of the immunoglobulin A1 protease gene (iga) of Haemophilus influenzae serotype b. Infect Immun 57(10):3097–3105
Poulsen K, Reinholdt J, Kilian M (1996) Characterization of the Streptococcus pneumoniae immunoglobulin A1 protease gene (IgA) and its translation product. Infect Immun 64(10):3957–3966
Pouria S, Corran PH, Smith AC et al (2004) Glycoform composition profiling of O-glycopeptides derived from human serum IgA1 by matrix-assisted laser desorption ionization-time of flight-mass spectrometry. Anal Biochem 330(2):257–263
Qiu X, Wong G, Audet J et al (2014) Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514(7520):47–53
Raska M, Moldoveanu Z, Suzuki H et al (2007) Identification and characterization of CMP-NeuAc:GalNAc-IgA1 alpha2,6-sialyltransferase in IgA1-producing cells. J Mol Biol 369(1):69–78
Reily C, Rizk DV, Julian BA, Novak J (2018) Assay for galactose-deficient IgA1 enables mechanistic studies with primary cells from IgA nephropathy patients. BioTechniques 65(2):71–77
Reily C, Stewart TJ, Renfrow MB, Novak J (2019) Glycosylation in health and disease. Nat Rev Nephrol 15(6):346–366
Reinholdt J, Kilian M (1997) Comparative analysis of immunoglobulin A1 protease activity among bacteria representing different genera, species, and strains. Infect Immun 65(11):4452–4459
Reinholdt J, Tomana M, Mortensen SB, Kilian M (1990) Molecular aspects of immunoglobulin A1 degradation by oral streptococci. Infect Immun 58(5):1186–1194
Renegar KB, Small PA Jr (1994) Passive immunization: systemic and mucosal. Handb Mucosal Immunol:347–356
Renfrow MB, Cooper HJ, Tomana M et al (2005) Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation fourier transform-ion cyclotron resonance mass spectrometry. J Biol Chem 280(19):19136–19145
Renfrow MB, Mackay CL, Chalmers MJ et al (2007) Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: implications for IgA nephropathy. Anal Bioanal Chem 389(5):1397–1407
Rifai A, Fadden K, Morrison SL, Chintalacharuvu KR (2000) The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (Ig)A1 and IgA2 isotypes. J Exp Med 191(12):2171–2182
Rizk DV, Saha MK, Hall S et al (2019) Glomerular immunodeposits of patients with IgA nephropathy are enriched for IgG autoantibodies specific for galactose-deficient IgA1. J Am Soc Nephrol 30(10):2017–2026
Robb M, Hobbs JK, Woodiga SA et al (2017) Molecular characterization of N-glycan degradation and transport in Streptococcus pneumoniae and its contribution to virulence. PLoS Pathog 13(1):e1006090
Robinson LS, Schwebke J, Lewis WG, Lewis AL (2019) Identification and characterization of NanH2 and NanH3, enzymes responsible for sialidase activity in the vaginal bacterium Gardnerella vaginalis. J Biol Chem 294(14):5230–5245
Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR (2001) Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol 167(5):2861–2868
Royle L, Roos A, Harvey DJ et al (2003) Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems*. J Biol Chem 278(22):20140–20153
Russell MW (2007) Biological functions of IgA. Mucosal Immune Defense: Immunoglobulin A:144–172
Schroten H, Stapper C, Plogmann R, Köhler H, Hacker J, Hanisch FG (1998) Fab-independent antiadhesion effects of secretory immunoglobulin A on S-fimbriated Escherichia coli are mediated by sialyloligosaccharides. Infect Immun 66(8):3971–3973
Selewski DT, Ambruzs JM, Appel GB et al (2018) Clinical characteristics and treatment patterns of children and adults with IgA nephropathy or IgA vasculitis: findings from the CureGN study. Kidney Int Rep 3(6):1373–1384
Senior BW, Woof JM (2005) Effect of mutations in the human immunoglobulin A1 (IgA1) hinge on its susceptibility to cleavage by diverse bacterial IgA1 proteases. Infect Immun 73(3):1515–1522
Senior BW, Dunlop JI, Batten MR, Kilian M, Woof JM (2000) Cleavage of a recombinant human immunoglobulin A2 (IgA2)-IgA1 hybrid antibody by certain bacterial IgA1 proteases. Infect Immun 68(2):463–469
Sjögren J, Collin M (2014) Bacterial glycosidases in pathogenesis and glycoengineering. Future Microbiol 9(9):1039–1051
Smith SB, Ravel J (2017) The vaginal microbiota, host defence and reproductive physiology. J Physiol 595(2):451–463
Sørensen V, Rasmussen IB, Sundvold V, Michaelsen TE, Sandlie I (2000) Structural requirements for incorporation of J chain into human IgM and IgA. Int Immunol 12(1):19–27
Steffen U, Koeleman CA, Sokolova MV et al (2020) IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat Commun 11(1):120
Stewart TJ, Takahashi K, Whitaker RH et al (2019) IgA1 hinge-region clustered glycan fidelity is established early during semi-ordered glycosylation by GalNAc-T2. Glycobiology 29(7):543–556
Stewart TJ, Takahashi K, Xu N et al (2021) Quantitative assessment of successive carbohydrate additions to the clustered O-glycosylation sites of IgA1 by glycosyltransferases. Glycobiology 31(5):540–556
Stockert RJ, Kressner MS, Collins JC, Sternlieb I, Morell AG (1982) IgA interaction with the asialoglycoprotein receptor. Proc Natl Acad Sci USA 79(20):6229–6231
Strasser R, Stadlmann J, Schähs M et al (2008) Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J 6(4):392–402
Strasser R, Seifert G, Doblin MS et al (2021) Cracking the “Sugar Code”: a snapshot of N- and O-glycosylation pathways and functions in plants cells. Front Plant Sci 12:640919
Stuchlova Horynova M, Vrablikova A, Stewart TJ et al (2015) N-acetylgalactosaminide α2,6-sialyltransferase II is a candidate enzyme for sialylation of galactose-deficient IgA1, the key autoantigen in IgA nephropathy. Nephrol Dial Transplant 30(2):234–238
Sudhakara P, Sellamuthu I, Aruni AW (2019) Bacterial sialoglycosidases in virulence and pathogenesis. Pathogens 8(1)
Sugiyama M, Wada Y, Kanazawa N et al (2020) A cross-sectional analysis of clinicopathologic similarities and differences between Henoch-Schönlein purpura nephritis and IgA nephropathy. PLoS One 15(4):e0232194
Suzuki H, Moldoveanu Z, Hall S et al (2008) IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest 118(2):629–639
Suzuki H, Fan R, Zhang Z et al (2009) Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119(6):1668–1677
Suzuki H, Raska M, Yamada K et al (2014) Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem 289(8):5330–5339
Suzuki H, Yasutake J, Makita Y et al (2018) IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int 93(3):700–705
Suzuki H, Moldoveanu Z, Julian BA, Wyatt RJ, Novak J (2019) Autoantibodies specific for galactose-deficient IgA1 in IgA vasculitis with nephritis. Kidney Int Rep 4(12):1717–1724
Syed S, Hakala P, Singh AK et al (2019) Role of pneumococcal NanA neuraminidase activity in peripheral blood. Front Cell Infect Microbiol 9:218
Takahashi K, Wall SB, Suzuki H et al (2010) Clustered O-glycans of IgA1: defining macro- and microheterogeneity by use of electron capture/transfer dissociation. Mol Cell Proteomics 9(11):2545–2557
Takahashi K, Smith AD, Poulsen K et al (2012) Naturally occurring structural isomers in serum IgA1 o-glycosylation. J Proteome Res 11(2):692–702
Takahashi K, Raska M, Stuchlova Horynova M et al (2014) Enzymatic sialylation of IgA1 O-glycans: implications for studies of IgA nephropathy. PLoS One 9(2):e99026
Ten Hagen KG, Fritz TA, Tabak LA (2003) All in the family: the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology 13(1):1r-16r
Terai I, Kobayashi K, Vaerman JP, Mafune N (2006) Degalactosylated and/or denatured IgA, but not native IgA in any form, bind to mannose-binding lectin. J Immunol 177(3):1737–1745
Tomana M, Niedermeier W, Mestecky J, Skvaril F (1976) The differences in carbohydrate composition between the subclasses of IgA immunoglobulins. Immunochemistry 13(4):325–328
Tomana M, Kulhavy R, Mestecky J (1988) Receptor-mediated binding and uptake of immunoglobulin A by human liver. Gastroenterology 94(3):762–770
Tomana M, Zikan J, Moldoveanu Z, Kulhavy R, Bennett JC, Mestecky J (1993) Interactions of cell-surface galactosyltransferase with immunoglobulins. Mol Immunol 30(3):265–275
Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J (1997) Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int 52(2):509–516
Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J (1999) Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 104(1):73–81
Underdown BJ, Mestecky J (1994) Mucosal immunoglobulins. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGhee JR, Bienenstock J (eds) Handbook of mucosal immunology. Academic, Boston, pp 79–97
UniProt Consortium (2017) UniProt: the universal protein knowledgebase. Nucleic Acids Res 45(D1):D158–d169
van Egmond M, Damen CA, van Spriel AB, Vidarsson G, van Garderen E, van de Winkel JG (2001) IgA and the IgA Fc receptor. Trends Immunol 22(4):205–211
Wada Y, Dell A, Haslam SM et al (2010a) Comparison of methods for profiling O-glycosylation: human proteome organisation human disease glycomics/proteome initiative multi-institutional study of IgA1. Mol Cell Proteomics 9(4):719–727
Wada Y, Tajiri M, Ohshima S (2010b) Quantitation of saccharide compositions of O-glycans by mass spectrometry of glycopeptides and its application to rheumatoid arthritis. J Proteome Res 9(3):1367–1373
Wandall HH, Irazoqui F, Tarp MA et al (2007) The lectin domains of polypeptide GalNAc-transferases exhibit carbohydrate-binding specificity for GalNAc: lectin binding to GalNAc-glycopeptide substrates is required for high density GalNAc-O-glycosylation. Glycobiology 17(4):374–387
Wang Z, Rahkola J, Redzic JS et al (2020) Mechanism and inhibition of Streptococcus pneumoniae IgA1 protease. Nat Commun 11(1):6063
Yoo EM, Coloma MJ, Trinh KR et al (1999) Structural requirements for polymeric immunoglobulin assembly and association with J chain. J Biol Chem 274(47):33771–33777
Westerhof LB, Wilbers RH, van Raaij DR et al (2015) Transient expression of secretory IgA in planta is optimal using a multi-gene vector and may be further enhanced by improving joining chain incorporation. Front Plant Sci 6:1200
Wold AE, Mestecky J, Tomana M et al (1990) Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun 58(9):3073–3077
Yamada K, Kobayashi N, Ikeda T et al (2010) Down-regulation of core 1 beta1,3-galactosyltransferase and Cosmc by Th2 cytokine alters O-glycosylation of IgA1. Nephrol Dial Transplant 25(12):3890–3897
Yamada K, Huang ZQ, Raska M et al (2020) Leukemia inhibitory factor signaling enhances production of galactose-deficient IgA1 in IgA nephropathy. Kidney Dis (Basel) 6(3):168–180
Yamada K, Huang ZQ, Raska M et al (2017) Inhibition of STAT3 signaling reduces IgA1 autoantigen production in IgA nephropathy. Kidney Int Rep 2(6):1194–1207
Zhao N, Hou P, Lv J et al (2012) The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int 82(7):790–796
Yang X, Zhao Q, Zhu L, Zhang W (2013) The three complementarity-determining region-like loops in the second extracellular domain of human Fc alpha/mu receptor contribute to its binding of IgA and IgM. Immunobiology 218(5):798–809
Woof JM, Kerr MA (2006) The function of immunoglobulin A in immunity. J Pathol 208(2):270–282
Wilson TJ, Fuchs A, Colonna M (2012) Cutting edge: human FcRL4 and FcRL5 are receptors for IgA and IgG. J Immunol 188(10):4741–4745
Yanagihara T, Brown R, Hall S et al (2012) In vitro-generated immune complexes containing galactose-deficient IgA1 stimulate proliferation of mesangial cells. Results Immunol 2:166–172
Yang Y, Liu F, Franc V, Halim LA, Schellekens H, Heck AJR (2016) Hybrid mass spectrometry approaches in glycoprotein analysis and their usage in scoring biosimilarity. Nat Commun 7(1):13397
Zhao L, Peng L, Yang D et al (2020) Immunostaining of galactose-deficient IgA1 by KM55 is not specific for immunoglobulin A nephropathy. Clin Immunol 217:108483
Westra J, Brouwer E, Raveling-Eelsing E et al (2021) Arthritis autoantibodies in individuals without rheumatoid arthritis: follow-up data from a Dutch population-based cohort (Lifelines). Rheumatology (Oxford) 60(2):658–666
Zickerman AM, Allen AC, Talwar V et al (2000) IgA myeloma presenting as Henoch-Schönlein purpura with nephritis. Am J Kidney Dis 36(3):E19
Zhang W, Lachmann PJ (1994) Glycosylation of IgA is required for optimal activation of the alternative complement pathway by immune complexes. Immunology 81(1):137–141
Wani JH, Gilbert JV, Plaut AG, Weiser JN (1996) Identification, cloning, and sequencing of the immunoglobulin A1 protease gene of Streptococcus pneumoniae. Infect Immun 64(10):3967–3974
Weiser JN, Bae D, Fasching C, Scamurra RW, Ratner AJ, Janoff EN (2003) Antibody-enhanced pneumococcal adherence requires IgA1 protease. Proc Natl Acad Sci USA 100(7):4215–4220
Wren JT, Blevins LK, Pang B et al (2017) Pneumococcal Neuraminidase A (NanA) promotes biofilm formation and synergizes with influenza A virus in nasal colonization and middle ear infection. Infect Immun 85(4)
Acknowledgments
The authors express their appreciation to the colleagues and collaborators as well as to all volunteers participating in the cited studies. The authors apologize to their colleagues in the field whose work is not adequately discussed or cited in this review due to the space limitations.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Ethics declarations
Funding
The authors of this study were supported in part by NIH grants AR069516, DK106341, CD122194, GM098539, DK078244, and DK082753 and by a gift from the IGA Nephropathy Foundation of America.
Conflict of Interest
MBR and JN are co-founders and co-owners of and consultants for Reliant Glycosciences, LLC. MBR and JN are co-inventors on US patent application 14/318,082 (assigned to UAB Research Foundation). ALH and CR declare no conflict of interest.
Ethical Approval
Not applicable, as this is a review article and, thus, there are no human subjects recruited for this study or any animals used.
Informed Consent
Not applicable, as this is a review article and, thus, there are no human subjects recruited for this study.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hansen, A.L., Reily, C., Novak, J., Renfrow, M.B. (2021). Immunoglobulin A Glycosylation and Its Role in Disease. In: Pezer, M. (eds) Antibody Glycosylation. Experientia Supplementum, vol 112. Springer, Cham. https://doi.org/10.1007/978-3-030-76912-3_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-76912-3_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-76911-6
Online ISBN: 978-3-030-76912-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)