Abstract
Changes in immunoglobulin G (IgG) glycosylation pattern have been observed in a vast array of auto- and alloimmune, infectious, cardiometabolic, malignant, and other diseases. This chapter contains an updated catalog of over 140 studies within which IgG glycosylation analysis was performed in a disease setting. Since the composition of IgG glycans is known to modulate its effector functions, it is suggested that a changed IgG glycosylation pattern in patients might be involved in disease development and progression, representing a predisposition and/or a functional effector in disease pathology. In contrast to the glycopattern of bulk serum IgG, which likely relates to the systemic inflammatory background, the glycosylation profile of antigen-specific IgG probably plays a direct role in disease pathology in several infectious and allo- and autoimmune antibody-dependent diseases. Depending on the specifics of any given disease, IgG glycosylation read-out might therefore in the future be developed into a useful clinical biomarker or a supplementary to currently used biomarkers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Since the first reports on glycans attached to immunoglobulin G (IgG) in the 1970s (Ciccimarra et al. 1976; Williams et al. 1973; Koide et al. 1977; Hymes et al. 1979) and the seminal papers by Parekh and al. on the association of a changed IgG glycome composition with a diseased status and aging, (Parekh et al. 1985, 1988) IgG glycans are today universally recognized as modulators of IgG activity (Yamaguchi and Barb 2020). The importance of IgG glycome composition is implied in various physiological and pathological states. IgG glycans are discussed as potential contributors to disease development and progression, as well as a diagnostic, prognostic, and follow-up biomarker. This chapter is a brief update and extension of our comprehensive review on IgG glycosylation in aging and diseases published 3 years ago (Gudelj et al. 2018a), with a focus on the potential functionality of the skewed IgG glycosylation pattern. The table presents the updated list of publications that examined IgG glycosylation in various diseased states.
2 IgG Glycans are an Integral Structural and Functional Part of the Molecule
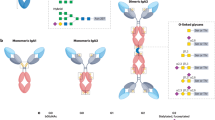
IgG glycans represent about 15% of the molecule’s weight (Arnold et al. 2007). Each IgG molecule contains an N-glycan covalently attached to the conserved asparagine (Asn) at position 297 within the Fc region on each of the two heavy chains (Shade and Anthony 2013). In addition, 15–20% IgG molecules contain an N-glycan within the Fab region, attached to the asparagine within an N-glycosylation sequon formed by somatic hypermutation during affinity maturation (Dunn-Walters et al. 2000; van de Bovenkamp et al. 2016).
Fc N-glycans are placed in the cavity between the CH2 domains of the two opposing heavy chains (Pincetic et al. 2014; Deisenhofer et al. 1976) and are important for the molecule’s structural integrity, stability, and serum-half life (Boune et al. 2020; Cymer et al. 2018). They are also involved in the modulation of IgG effector functions, by affecting the molecule’s affinity toward its ligands and receptors: type I and type II Fc receptors, C1q complement component, mannan-binding lectin, etc. (Pincetic et al. 2014; Peschke et al. 2017; Malhotra et al. 1995; Dekkers et al. 2017). Although markedly less explored than Fc glycans, Fab glycans are also reported to affect IgG’s biological properties and effector functions, such as half-life, stability, solubility, and antigen-binding (van de Bovenkamp et al. 2016, 2018a; Wu et al. 2010; Wright et al. 1991; Higel et al. 2016; Liu 2015, 2018).
3 IgG Glycans Affect IgG Functions
The composition of both Fab and Fc glycans has been confirmed to influence IgG functionality and activity. Since this has been described in detail in Chap. 12, the main findings are only briefly summarized here as a reminder for the reader.
3.1 Fc Glycans
Due to the positioning of the Fc N-glycan at the Asn-297, structural differences of the N-glycans attached to the Fc region influence the affinity to the IgG ligands and receptors that interact with IgG at the CH2 domain and the CH2-CH3 domain interface (Dekkers et al. 2017; Reusch and Tejada 2015; Li et al. 2017; Wada et al. 2019; Vidarsson et al. 2014).
Core-Fucosylation
Contrary to most other plasma proteins, over 90% of all Fc glycans are core-fucosylated (fucosylated glycans, F) (van de Bovenkamp et al. 2016; Štambuk et al. 2020; Baković et al. 2013; Clerc et al. 2016). The lack of core fucose significantly increases the IgG’s affinity for the Fcγ receptor III (FcγRIII), both A and B, enhancing the FcγRIII-mediated effector functions, particularly the antibody-dependent cell-mediated cytotoxicity (ADCC) (Dekkers et al. 2017; Shields et al. 2002; Shinkawa et al. 2003). This prominent effect of alternative Fc glycosylation on the IgG function found its application in the industrial production of therapeutic monoclonal antibodies (Garber 2018).
Bisection
Up to 10% of all IgG Fc glycans are bisected, i.e., contain a bisecting N-acetylglucosamine (GlcNAc) (bisected glycans, B) (van de Bovenkamp et al. 2016). Since the presence of GlcNAc and core fucose, to a degree, preclude each other during glycan synthesis (Benedetti et al. 2017; Schuster et al. 2005; Ferrara et al. 2006), the increase in binding affinity fo FcγRIII sometimes associated with bisected glycans (Umaña et al. 1999; Davies et al. 2001; Lifely et al. 1995) cannot be easily uncoupled from the same effect observed for core-fucosylated IgG glycans (Shinkawa et al. 2003).
Galactosylation
Galactosylation is the IgG glycosylation trait with the most pronounced inter-individual variation (Huhn et al. 2009; Gornik et al. 2012). On average, about 35% of IgG Fc glycans contain no terminal galactose residues (agalactosylated glycans, G0), about 35% contain one (monogalactosylated glycans, G1), and about 15% contain two terminal galactoses (digalactosylated glycans, G2) (Baković et al. 2013; Huffman et al. 2014). Terminal galactoses modulate IgG inflammatory potential by affecting binding affinities to complement components and FcγRs. Agalactosylated Fc glycans are considered to act pro-inflammatory by activating the complement through the alternative pathway (Banda et al. 2008), and the lectin pathway after binding to the mannose-binding lectin (Malhotra et al. 1995; Ji et al. 2002; Arnold et al. 2006). Galactosylation was also held responsible for the anti-inflammatory activity of immune complexes by binding to the inhibitory FcγRIIB (Karsten et al. 2012). However, Fc galactosylation has also been reported to enhance complement-dependent cytotoxicity (CDC) through the classical complement pathway by increasing the IgG’s affinity for the C1q complement component (Peschke et al. 2017; Boyd et al. 1995; Hodoniczky et al. 2005). Likewise, by increasing the affinity of IgG for FcyRs, it enhances the downstream processes mediated by FcyRs, in particular ADCC (Dekkers et al. 2017; Kumpel et al. 1994, 1995; Houde et al. 2010; Subedi and Barb 2016). We should therefore not rush to proclaim terminal IgG galactosylation simply “anti-inflammatory,” before considering the entire context and the nature and extent of IgG involvement in the process we are investigating.
Sialylation
On average, 10–15% of IgG Fc glycans carry a single terminal sialic acid (monosialylated glycans, S1) or two sialic acids (disialylated glycans, S2) (Baković et al. 2013; Huffman et al. 2014). Similar to terminal galactosylation, sialylation is most often discussed as a modulator of IgG functions regarding inflammation (Böhm et al. 2014).
The importance of sialylation became evident when the presence of the sialylated Fc fraction was discovered indispensable for the anti-inflammatory activity of the intravenous immunoglobulin (IVIg) preparation in a K/BxN serum-transfer mouse model of RA (Kaneko et al. 2006). Mouse studies on several antibody-dependent autoimmune disease models helped elucidate the mechanistic pathway for its activity, starting with the binding of the sialylated Fc fraction to specific ICAM-3 grabbing non-integrin-related 1 (SIGN-R1) on the surface of splenic macrophages and ending in enhanced FcγRIIB expression on the effector macrophages (Kaneko et al. 2006; Schwab and Nimmerjahn 2013; Anthony et al. 2008, 2011; Schwab et al. 2012, 2014; Washburn et al. 2015; Galeotti et al. 2017; Fiebiger et al. 2015). However, this finding did not hold in several other in vitro and in vivo models, nor human studies (Galeotti et al. 2017; Guhr et al. 2011; Leontyev et al. 2012; Campbell et al. 2014; Temming et al. 2019). This confirms the well-established notion that the IVIg mode of action is complex and tightly connected with the corresponding immune context.
Depending on the sialylation status of the Fc glycan, the Fc domain is suggested to adopt either an “open” or a “closed” conformation, for sialylated and asialylated glycans, respectively. The “open” conformation favors binding to the type I FcγRs, whereas the “closed” conformation favors the binding of type II FcRs (Pincetic et al. 2014; Sondermann et al. 2013). Terminal sialylation is thus proposed to act as a switch between two distinct immunological effector functions.
To summarize—agalactosylated, asialylated, and bisected IgG molecules are often simply described as “pro-inflammatory,” and terminally galactosylated and sialylated IgG molecules as “anti-inflammatory,” while afucosylated IgG has an augmented capacity to trigger ADCC through enhanced FcγRIIIA binding. We should, however, always bear in mind that this generalization is a simplification, and exercise caution when considering its implications.
3.2 Fab Glycans
As expected, Fab glycans are mostly reported to affect antigen-binding (Wright et al. 1991; Coloma et al. 1999; Schneider et al. 2015; Wallick et al. 1988; Tachibana et al. 1997; Leibiger et al. 1999; Khurana et al. 1997; Man Sung Co et al. 1993; Fujimura et al. 2000; Van De Bovenkamp et al. 2018b). Besides the obvious, they are also suggested to influence IgG aggregation and precipitation (Courtois et al. 2016), immune complex formation (Gutierrez et al. 2006), and have a role in the IVIg mode of action (Käsermann et al. 2012; Wiedeman et al. 2013; Massoud et al. 2014; Séïté et al. 2010, 2014).
4 Regulation of IgG Glycosylation
IgG glycosylation is a complex trait, influenced by both, genetics (Menni et al. 2013; Pučić et al. 2011; Klarić et al. 2020) and the environment (Štambuk et al. 2020; Yu et al. 2016; Krištić et al. 2014; De Jong et al. 2016). More precisely, the compound IgG glycosylation pattern seems to be, to different degrees, modulated by IgG aminoacid sequence (Lund et al. 1996; Zaitseva et al. 2018; Yu et al. 2013), the intra- and extracellular milieu affecting the glycosylation machinery (Ohtsubo and Marth 2006; Oefner et al. 2012; Bartsch et al. 2020; Hess et al. 2013; Canellada et al. 2002; Gutiérrez et al. 2001; Miranda et al. 2005; Wang et al. 2011; Pfeifle et al. 2017; Liu et al. 2014; Fan et al. 2015), and environmental factors (Novokmet et al. 2014; Greto et al. 2020; Ercan et al. 2017; Engdahl et al. 2018; Klasić et al. 2018; Tijardović et al. 2019; Sarin et al. 2019; Peng et al. 2019). Solving the outstanding question of IgG glycosylation regulation would likely bring us one step closer to understanding the possible functionality of changes in IgG glycan composition in different physiological and pathological states.
5 Common IgG Glycosylation Pattern in Inflammatory Diseases and Aging
Advances in the development of high-throughput glycomic and glycoproteomic analyses (Huhn et al. 2009; Mariño et al. 2010; Trbojević-Akmačić et al. 2016, 2017) have enabled a significant number of large-scale epidemiological studies examining total IgG glycosylation pattern in diseased vs. healthy control subjects (Štambuk et al. 2020; Singh et al. 2020; Lemmers et al. 2017; Menni et al. 2018; Šimurina et al. 2018; Theodoratou et al. 2016; Wahl et al. 2018). In many of the diseases that were studied, a similar pattern emerged: diseased subjects often exhibited a decreased abundance of galactosylated, sialylated, and—occasionally—an increased abundance of bisected bulk IgG glycans when compared to healthy controls (Fig. 13.1). In addition, the trend was often associated with disease severity and reverted to baseline values upon successful application of therapy. Interestingly, the same pattern that was observed in diseases with an inflammatory component was also evident in aging subjects (Fig. 13.1) (Gudelj et al. 2018a; Lauc 2016). This “pro-inflammatory IgG glycome composition” is likely associated with the common background inflammatory component of the studied diseases. In some cases, it might reflect a predisposition toward disease development, or/and even be involved as an effector of inflammation. Additionally, it might represent a consequence of environmental exposure to antigens through a lifetime or unhealthy lifestyle choices.
General patterns of IgG glycosylation changes are similar in several diseases and aging. The effect (shown on the y-axis) is shown as the difference between means of case and control populations (for aging, population over vs. population under 50 years of age), expressed in standard deviations. The whiskers represent the 95% confidence interval. The reference populations for disease cohorts are age- and sex-matched healthy controls. SLE systemic lupus erythematosus, RA rheumatoid arthritis, CD Crohn’s disease, UC ulcerous colitis, T2D type 2 diabetes mellitus, CRC colorectal carcinoma. G0 agalactosylated glycans, G2 digalactosylated glycans, B bisected glycans, S sialylated glycans, F core-fucosylated glycans. Reused with permission from Lauc (2016)
Indeed, the composition of IgG glycome was reported to associate with many physiological and biochemical traits, as well as with traits correlated to inflammation and poor metabolic health (Gudelj et al. 2018a) and the expected lifespan (Štambuk et al. 2020). IgG glycome was thus suggested to be a biomarker of general immune activation (De Jong et al. 2016), while we propose total IgG glycoprofile can be positioned as a read-out of a general state of health, i.e. biological age (Vilaj et al. 2019).
6 Role of Skewed IgG Glycosylation in Diseases
When we take into account the complexity of the IgG’s multiple roles in our immune system, it is no wonder there is no single common interpretation of the altered IgG glycopattern across the wide spectrum of diseases (Table 13.1). The multiple pleiotropic loci, i.e. shared associations of IgG glycome composition and autoimmune, inflammatory, and other diseases (Klarić et al. 2020; Lauc et al. 2013), as well as the changes in IgG glycopattern preceding disease development—such as in the case of RA (Gudelj et al. 2018b) and cardiovascular diseases (Menni et al. 2018)—suggest that a skewed bulk serum IgG glycoprofile might reflect a disease risk or predisposition. This predisposition can manifest through an inherited (Klarić et al. 2020; Lauc et al. 2013) or acquired propensity for inflammation modulation (Franceschi et al. 2018).
In most other cases, when it comes to glycosylation of the bulk serum IgG, the role of a shifted glycosylation pattern is not clear. As already mentioned, decreased galactosylation and sialylation often accompany diseases that involve an inflammatory immune response. The evidence that would enable us to unambiguously determine whether the “pro-inflammatory” IgG glycoforms represent one of many drivers of disease pathology or merely byproducts of the inflamed immune system is still lacking. The current consensus is that total IgG glycopattern is likely relevant in the general modulation of the immune activation threshold.
In some cases, however, a clear link/evidence for the functionality of IgG glycans is provided. A mouse study investigating the link between obesity and the development of hypertension resulted in a very intriguing observation. Hyposialylated IgG from mice in which obesity was induced by a high-fat diet (HFD) induced an elevated blood pressure when transferred to IgG-deficient mice. Moreover, supplementing HFD-feed mice with a sialic acid precursor, N-acetyl-d-mannosamine (ManNAc), resulted in the restoration of the baseline level of IgG sialylation and protected them from obesity-induced hypertension development (Peng et al. 2019). This finding thus demonstrated the functional role of IgG glycans in the development of hypertension. Interestingly, the same treatment restored IgG sialylation and reduced tumor load and bone loss in a mouse model of myeloma (Westhrin et al. 2020).
On the level of total serum IgG, increased level of glycosylation of the Fab region observed in some malignant diseases (Zhu et al. 2002, 2003; Radcliffe et al. 2007; Coelho et al. 2010; McCann et al. 2008) is proposed to contribute to disease development and progression by enhancing tumor cell persistence and expansion (Coelho et al. 2010; Amin et al. 2015).
Glycosylation changes on antigen-specific IgG are more likely to be directly involved in disease pathology in case of antibody-mediated auto- or alloimmune diseases or defense from pathogens in case of infectious diseases. The role of differential IgG glycosylation in these cases corresponds to the specifics of a particular disease and the molecular mechanisms underlying its pathology.
In addition to the change in total IgG, multiple infectious diseases are characterized by a distinct glycosylation pattern of relevant antigen-specific IgG in comparison to total IgG (Table 13.1). This implies a distinct regulation of IgG glycosylation, depending on both the disease and the antigen (Ackerman et al. 2013), even within a single individual (Mahan et al. 2016). This supports the notion that IgG glycome relevance should be interpreted in the disease-specific functional context.
One of the rare instances where the role of IgG glycosylation is mechanistically explained is once more linked to the enhanced affinity of afucosylated IgG molecules for FcγRIIIA. In the case of dengue fever, occasionally a secondary, heterologous dengue infection results in severe dengue hemorrhagic fever and dengue shock syndrome. This is attributed to antibody-dependent enhancement (ADE) of the disease by cross-reactivity of afucosylated anti-dengue IgG with platelet antigens, resulting in platelet depletion (Wang et al. 2017). Additionally, the enhanced binding of afucosylated IgG to FcγRIIA and FcγRIIIA promotes the FcγR-mediated viral entry and signaling in cells bearing these receptors on their surface, primarily monocytes and macrophages, resulting in infection progression (Thulin et al. 2020).
A similar relevance for afucosylated antigen-specific IgG is observed in COVID-19 patients. Anti-SARS-CoV-2 IgG with a higher core-fucosylation level is associated with unaided clearance of the infection (Larsen et al. 2020). By contrast, critically ill patients display lower levels of fucosylated anti-SARS-CoV-2 IgG (Larsen et al. 2020; Chakraborty et al. 2021). Furthermore, in in vitro studies afucosylated anti-S/-RBD antibodies were shown to induce enhanced natural killer (NK) cell degranulation (Chakraborty et al. 2021) and elevated production of pro-inflammatory cytokines by primary monocytes and alveolar macrophages, which is likely the background of the severe disease phenotype associated with this glycoprofile in vivo (Larsen et al. 2020; Chakraborty et al. 2021; Hoepel et al. 2020).
Similarly, afucosylated antigen-specific IgG in fetal and neonatal alloimmune thrombocytopenia (FNAIT) and hemolytic disease of the fetus and newborn (HDFN) are thought to contribute, again through enhanced FcγRIIIA-mediated mechanisms, namely phagocytosis and ADCC, to the more severe disease phenotype (Kapur et al. 2014a, b; Sonneveld et al. 2016, 2017a).
In lupus nephritis, a serious complication of SLE, the presence of core fucose was shown to induce upregulated calcium/calmodulin kinase IV expression in podocytes, leading to podocyte injury and limited nephrin synthesis. In the same experimental setting, the presence of terminal galactoses acted protectively (Bhargava et al. 2021).
An interesting recent finding on the importance of Fab glycans emerged in the most explored disease in the context of IgG glycosylation. In RA, a high percentage of anti-citrullinated protein antibody (ACPA) is additionally glycosylated at the Fab region (Rombouts et al. 2016; Hafkenscheid et al. 2017), a feature distinguishing RA patients from ACPA+ but healthy subjects (Kissel et al. 2019; Hafkenscheid et al. 2019). This suggests Fab glycosylation of ACPA might be mechanistically involved in RA development (Rombouts et al. 2016).
7 Perspectives for IgG Glycosylation in Precision Medicine
A skewed IgG glycoprofile in comparison to the personal baseline value (requiring longitudinal monitoring) or in comparison to ethnicity-, age-, and sex-matched subjects (requiring a population baseline cohort) in a cross-sectional experimental design, might indicate an increased risk for disease development (Gudelj et al. 2018b), or disease progression (Gudelj et al. 2018a). However, since the alterations in bulk serum IgG glycome composition are not disease-specific, they cannot be used as a stand-alone diagnostic marker. A total IgG glycoprofile of the composition significantly removed from the baseline can instead be used as an indication of a necessity for an examination by an expert clinician.
In case of an established diagnosis, bulk IgG glycome might serve as a predictor of disease progression—e.g., decreased IgG2/3 galactosylation in patients progressing from undifferentiated to rheumatoid arthritis (Sénard et al. 2021). Similarly, IgG glycome is proposed to bear potential for a useful add-on tool for monitoring functional disease progression and response to therapy (Parekh et al. 1988; Kanoh et al. 2004a, 2008; Váradi et al. 2015; Collins et al. 2013; Van Zeben et al. 1994; Rook et al. 1994; Pasek et al. 2006; Gindzienska-Sieskiewicz et al. 2007; Croce et al. 2007; Ercan et al. 2010).
The relevance and biomarker potential of IgG glycome analysis is more evident in some cases of antigen-specific IgG. For instance, due to the increased level of ACPA Fab glycosylation in individuals at risk for RA development, IgG glycome analysis might in the future provide the currently missing understanding (and biomarker) for the first determining pathogenic event leading to disease development (Rombouts et al. 2015; Scherer et al. 2010). Furthermore, as already mentioned, in several diseases a particular antigen-specific IgG glycopattern is associated with a risk for the severe phenotype (Kapur et al. 2014a, b; Sonneveld et al. 2016; Sonneveld et al. 2017a). Similarly, following the mechanistical explanation for the role of afucosylated anti-dengue IgG described in the previous section, afucosylated maternal anti-dengue IgG is proposed to denote a susceptibility to symptomatic dengue infection in infants (Thulin et al. 2020). The knowledge that a particular glycan profile of antigen-specific IgG, including post-vaccination status for some infectious diseases, is related to the risk of developing (the severe form of) a disease might in the future enable or aid the stratification of patients at risk and timely preventive action.
Another sought-after biomarker type is the one enabling patient stratification aiming at improved differential (sub-)diagnosis and subsequent selection of appropriate therapeutic measures. Differential IgG glycosylation was also suggested as a possibility for such applications. Indeed, the IgG sialylation level predicted response to therapy in Kawasaki disease (Ogata et al. 2013), and the galactosylation level response to anti-tumor necrosis factor (TNF) therapy in RA and Crohn’s disease (Váradi et al. 2015), and response to methotrexate therapy in RA (Lundström et al. 2017). Having the means to distinguish non-responders before the very initiation of long and expensive therapeutic treatments is truly an exciting prospect.
In summary, there are multiple possibilities for IgG glycosylation to enter the arena of clinical disease management. Currently, all of the possible applications mentioned here are still at the level of basic research and further studies are necessary to validate the initial findings and propel the IgG glycome analysis to the status of a full-fledged clinical biomarker.
8 Conclusions
IgG glycans can modulate virtually all of its numerous effector roles, the specifics depending on the disease and immune context. The associations of multiple IgG glycosylation traits with an immense array of heterogeneous diseases and their different stages imply that there is no single pathway connecting IgG glycome composition and disease development and progression.
Many inflammatory, autoimmune, infectious, cardiometabolic, and neoplastic diseases share a common IgG glycosylation profile of bulk (total) serum IgG, also characteristic for aging and often described as “pro-inflammatory”: a decreased level of galactosylated and sialylated glycans, and (sometimes) an increased level of bisected IgG glycans. This pattern is presumably associated with an inflammatory disease component as a part or consequence of disease pathology, or environmental events, such as antigen exposure. It might be mechanistically involved in disease advancement through modulation of inflammation, and, in some cases, manifest before the occurrence of symptoms, thus representing disease predisposition or mark the risk for disease development or progression.
When it comes to a distinct glycosylation profile of antigen-specific versus total serum IgG, IgG glycans are more likely to be directly involved in disease pathogenesis and progression through disease-specific effector mechanisms. This is often the case with afucosylated IgG glycans enhancing the affinity of IgG toward FcγRIIIA.
The read-out of IgG glycosylation has a potential for an (add-on) biomarker helping improve current algorithms for disease prediction and diagnosis, patient stratification, monitoring of disease progression, and response to therapy.
Abbreviations
- ACPA:
-
Anti-citrullinated protein antibody
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- Asn:
-
Asparagine
- CH2:
-
Constant heavy 2
- Fab:
-
Fragment antigen binding
- Fc:
-
Fragment crystallizable
- FcγRs:
-
Fcγ receptors
- FNAIT:
-
Fetal and neonatal alloimmune thrombocytopenia
- GlcNac:
-
N-acetylglucosamine
- HFD:
-
High-fat diet
- HDFN:
-
Haemolytic disease of the fetus and newborn
- IgG:
-
Immunoglobulin G
- IVIg:
-
Intravenous immunoglobulin
- RA:
-
Rheumatoid arthritis
References
Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, Streeck H, Nigrovic PA, Bailey-Kellogg C, Scanlan C, Alter G (2013) Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest 123:2183–2192. https://doi.org/10.1172/JCI65708
Alley WR, Vasseur JA, Goetz JA, Svoboda M, Mann BF, Matei DE, Menning N, Hussein A, Mechref Y, Novotny MV (2012) N-linked glycan structures and their expressions change in the blood sera of ovarian cancer patients. J Proteome Res 11:2282–2300. https://doi.org/10.1021/pr201070k
Amin R, Mourcin F, Uhel F, Pangault C, Ruminy P, Dupré L, Guirriec M, Marchand T, Fest T, Lamy T, Tarte K (2015) DC-SIGN-expressing macrophages trigger activation of mannosylated IgM B-cell receptor in follicular lymphoma. Blood 126:1911–1920. https://doi.org/10.1182/blood-2015-04-640912
Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV (2008) Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG fc. Science (80-) 320:373–376. https://doi.org/10.1126/science.1154315
Anthony RM, Kobayashi T, Wermeling F, Ravetch JV (2011) Intravenous gammaglobulin suppresses inflammation through a novel T H 2 pathway. Nature 475:110–114. https://doi.org/10.1038/nature10134
Arnold JN, Dwek RA, Rudd PM, Sim RB (2006) Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol Lett 106:103–110. https://doi.org/10.1016/j.imlet.2006.05.007
Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA (2007) The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol 25:21–50. https://doi.org/10.1146/annurev.immunol.25.022106.141702
Aurer I, Lauc G, Dumić J, Rendić D, Matišić D, Miloš M, Heffer-Lauc M, Flogel M, Labar B (2007) Aberrant glycosylation of IgG heavy chain in multiple myeloma. Coll Antropol 31:247–251
Axford JS, Sumar N, Alavi A, Isenberg DA, Young A, Bodman KB, Roitt IM (1992) Changes in normal glycosylation mechanisms in autoimmune rheumatic disease. J Clin Invest 89:1021–1031. https://doi.org/10.1172/JCI115643
Baković MP, Selman MHJ, Hoffmann M, Rudan I, Campbell H, Deelder AM, Lauc G, Wuhrer M (2013) High-throughput IgG Fc N-glycosylation profiling by mass spectrometry of glycopeptides. J Proteome Res 12:821–831. https://doi.org/10.1021/pr300887z
Banda NK, Wood AK, Takahashi K, Levitt B, Rudd PM, Royle L, Abrahams JL, Stahl GL, Holers VM, Arend WP (2008) Initiation of the alternative pathway of murine complement by immune complexes is dependent on N-glycans in IgG antibodies. Arthritis Rheum 58:3081–3089. https://doi.org/10.1002/art.23865
Barrios C, Zierer J, Gudelj I, Stambuk J, Ugrina I, Rodríguez E, Soler MJ, Pavic T, Simurina M, Keser T, Pucic-Bakovic M, Mangino M, Pascual J, Spector TD, Lauc G, Menni C (2016) Glycosylation profile of IgG in moderate kidney dysfunction. J Am Soc Nephrol 27:933–941. https://doi.org/10.1681/ASN.2015010109
Bartsch YC, Eschweiler S, Leliavski A, Lunding HB, Wagt S, Petry J, Lilienthal GM, Rahmöller J, de Haan N, Hölscher A, Erapaneedi R, Giannou AD, Aly L, Sato R, de Neef LA, Winkler A, Braumann D, Hobusch J, Kuhnigk K, Krémer V, Steinhaus M, Blanchard V, Gemoll T, Habermann JK, Collin M, Salinas G, Manz RA, Fukuyama H, Korn T, Waisman A, Yogev N, Huber S, Rabe B, Rose-John S, Busch H, Berberich-Siebelt F, Hölscher C, Wuhrer M, Ehlers M (2020) IgG Fc sialylation is regulated during the germinal center reaction following immunization with different adjuvants. J Allergy Clin Immunol 146:652–666.e11. https://doi.org/10.1016/j.jaci.2020.04.059
Benedetti E, Pučić-Baković M, Keser T, Wahl A, Hassinen A, Yang JY, Liu L, Trbojević-Akmačić I, Razdorov G, Štambuk J, Klarić L, Ugrina I, Selman MHJ, Wuhrer M, Rudan I, Polasek O, Hayward C, Grallert H, Strauch K, Peters A, Meitinger T, Gieger C, Vilaj M, Boons GJ, Moremen KW, Ovchinnikova T, Bovin N, Kellokumpu S, Theis FJ, Lauc G, Krumsiek J (2017) Network inference from glycoproteomics data reveals new reactions in the IgG glycosylation pathway. Nat Commun 8:1483. https://doi.org/10.1038/s41467-017-01525-0
Bermingham ML, Colombo M, McGurnaghan SJ, Blackbourn LAK, Vučković F, Baković MP, Trbojević-Akmačić I, Lauc G, Agakov F, Agakova AS, Hayward C, Klarić L, Palmer CNA, Petrie JR, Chalmers J, Collier A, Green F, Lindsay RS, Macrury S, McKnight JA, Patrick AW, Thekkepat S, Gornik O, McKeigue PM, Colhoun HM (2018) N-glycan profile and kidney disease in type 1 diabetes. Diabetes Care 41:79–87. https://doi.org/10.2337/dc17-1042
Bhargava R, Lehoux S, Maeda K, Tsokos MG, Krishfield S, Ellezian LY, Pollak M, Stillman IE, Cummings RD, Tsokos GC (2021) Aberrantly glycosylated IgG elicits pathogenic signaling in podocytes and signifies lupus nephritis. JCI Insight. https://doi.org/10.1172/jci.insight.147789
Bodman-Smith K, Sumar N, Sinclair H, Roitt I, Isenberg D, Young A (1996) Agalactosyl IgG [gal(o)]-an analysis of its clinical utility in the long-term follow-up of patients with rheumatoid arthritis. Br J Rheumatol 35:1063–1066. https://doi.org/10.1093/rheumatology/35.11.1063
Böhm S, Kao D, Nimmerjahn F (2014) Sweet and sour: the role of glycosylation for the anti-inflammatory activity of immunoglobulin G. Curr Top Microbiol Immunol 382:393–417. https://doi.org/10.1007/978-3-319-07911-0-18
Bond A, Alavi A, Axford JS, Youinou P, Hay FC (1996) The relationship between exposed galactose and N-acetylglucosamine residues on IgG in rheumatoid arthritis (RA), juvenile chronic arthritis (JCA) and Sjogren’s syndrome (SS). Clin Exp Immunol 105:99–103. https://doi.org/10.1046/j.1365-2249.1996.d01-741.x
Bond A, Alavi A, Axford JS, Bourke BE, Bruckner FE, Kerr MA, Maxwell JD, Tweed KJ, Weldon MJ, Youinou P, Hay FC (1997) A detailed lectin analysis of IgG glycosylation, demonstrating disease specific changes in terminal galactose and N-acetylglucosamine. J Autoimmun 10:77–85. https://doi.org/10.1006/jaut.1996.0104
Bondt A, Hafkenscheid L, Falck D, Kuijper TM, Rombouts Y, Hazes JMW, Wuhrer M, Dolhain RJEM (2018) ACPA IgG galactosylation associates with disease activity in pregnant patients with rheumatoid arthritis. Ann Rheum Dis 77:1130–1136. https://doi.org/10.1136/annrheumdis-2018-212946
Bones J, Mittermayr S, O’Donoghue N, Guttman A, Rudd PM (2010) Ultra performance liquid chromatographic profiling of serum N-glycans for fast and efficient identification of cancer associated alterations in glycosylation. Anal Chem 82:10208–10215. https://doi.org/10.1021/ac102860w
Bones J, Byrne JC, Odonoghue N, McManus C, Scaife C, Boissin H, Nastase A, Rudd PM (2011) Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. J Proteome Res 10:1246–1265. https://doi.org/10.1021/pr101036b
Bosseboeuf A, Allain-Maillet S, Mennesson N, Tallet A, Rossi C, Garderet L, Caillot D, Moreau P, Piver E, Girodon F, Perreault H, Brouard S, Nicot A, Bigot-Corbe E, Hermouet S, Harb J (2017) Pro-inflammatory state in monoclonal gammopathy of undetermined significance and in multiple myeloma is characterized by low sialylation of pathogen-specific and other monoclonal immunoglobulins. Front Immunol 8. https://doi.org/10.3389/fimmu.2017.01347
Boune S, Hu P, Epstein AL, Khawli LA (2020) Principles of N-linked glycosylation variations of IgG-based therapeutics: pharmacokinetic and functional considerations. Antibodies 9:22. https://doi.org/10.3390/antib9020022
Boyd PN, Lines AC, Patel AK (1995) The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol Immunol 32:1311–1318. https://doi.org/10.1016/0161-5890(95)00118-2
Campbell IK, Miescher S, Branch DR, Mott PJ, Lazarus AH, Han D, Maraskovsky E, Zuercher AW, Neschadim A, Leontyev D, McKenzie BS, Käsermann F (2014) Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc portion and independent of sialylation or basophils. J Immunol 192:5031–5038. https://doi.org/10.4049/jimmunol.1301611
Canellada A, Blois S, Gentile T, Margni Idehu RA (2002) In vitro modulation of protective antibody responses by estrogen, progesterone and interleukin-6. Am J Reprod Immunol 48:334–343. https://doi.org/10.1034/j.1600-0897.2002.01141.x
Chakraborty S, Gonzalez J, Edwards K, Mallajosyula V, Buzzanco AS, Sherwood R, Buffone C, Kathale N, Providenza S, Xie MM, Andrews JR, Blish CA, Singh U, Dugan H, Wilson PC, Pham TD, Boyd SD, Nadeau KC, Pinsky BA, Zhang S, Memoli MJ, Taubenberger JK, Morales T, Schapiro JM, Tan GS, Jagannathan P, Wang TT (2021) Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat Immunol 22:67–73. https://doi.org/10.1038/s41590-020-00828-7
Chen G, Wang Y, Qiu L, Qin X, Liu H, Wang X, Wang Y, Song G, Li F, Guo Y, Li F, Guo S, Li Z (2012) Human IgG Fc-glycosylation profiling reveals associations with age, sex, female sex hormones and thyroid cancer. J Proteome 75:2824–2834. https://doi.org/10.1016/j.jprot.2012.02.001
Chen G, Wang Y, Qin X, Li H, Guo Y, Wang Y, Liu H, Wang X, Song G, Li F, Li F, Guo S, Qiu L, Li Z (2013) Change in IgG1 Fc N-linked glycosylation in human lung cancer: age- and sex-related diagnostic potential. Electrophoresis 34:2407–2416. https://doi.org/10.1002/elps.201200455
Chen XX, Chen YQ, Ye S (2015) Measuring decreased serum IgG sialylation: a novel clinical biomarker of lupus. Lupus 24:948–954. https://doi.org/10.1177/0961203315570686
Cheng HD, Stöckmann H, Adamczyk B, McManus CA, Ercan A, Holm IA, Rudd PM, Ackerman ME, Nigrovic PA (2017) High-throughput characterization of the functional impact of IgG Fc glycan aberrancy in juvenile idiopathic arthritis. Glycobiology 27:1099–1108. https://doi.org/10.1093/glycob/cwx082
Cheng HD, Tirosh I, de Haan N, Stöckmann H, Adamczyk B, McManus CA, O’Flaherty R, Greville G, Saldova R, Bonilla FA, Notarangelo LD, Driessen GJ, Holm IA, Rudd PM, Wuhrer M, Ackerman ME, Nigrovic PA (2020) IgG Fc glycosylation as an axis of humoral immunity in childhood. J Allergy Clin Immunol 145:710–713.e9. https://doi.org/10.1016/j.jaci.2019.10.012
Ciccimarra F, Rosen FS, Schneeberger E, Merler E (1976) Failure of heavy chain glycosylation of IgG in some patients with common, variable agammaglobulinemia. J Clin Invest 57:1386–1390. https://doi.org/10.1172/JCI108407
Clerc F, Reiding KR, Jansen BC, Kammeijer GSM, Bondt A, Wuhrer M (2016) Human plasma protein N-glycosylation. Glycoconj J 33:309–343. https://doi.org/10.1007/s10719-015-9626-2
Coelho V, Krysov S, Ghaemmaghami AM, Emara M, Potter KN, Johnson P, Packham G, Martinez-Pomares L, Stevenson FK (2010) Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins. Proc Natl Acad Sci USA 107:18587–18592. https://doi.org/10.1073/pnas.1009388107
Collins ES, Galligan MC, Saldova R, Adamczyk B, Abrahams JL, Campbell MP, Ng CT, Veale DJ, Murphy TB, Rudd PM, FitzGerald O (2013) Glycosylation status of serum in inflammatory arthritis in response to anti-TNF treatment. Rheumatol (United Kingdom) 52:1572–1582. https://doi.org/10.1093/rheumatology/ket189
Coloma MJ, Trinh RK, Martinez AR, Morrison SL (1999) Position effects of variable region carbohydrate on the affinity and in vivo behavior of an anti-(1-->6) dextran antibody. J Immunol 162:2162–2170. http://www.ncbi.nlm.nih.gov/pubmed/9973491
Coman DJ, Murray DW, Byrne JC, Rudd PM, Bagaglia PM, Doran PD, Treacy EP (2010) Galactosemia, a single gene disorder with epigenetic consequences. Pediatr Res 67:286–292. https://doi.org/10.1203/PDR.0b013e3181cbd542
Comunale MA, Lowman M, Long RE, Krakover J, Philip R, Seeholzer S, Evans AA, Hann HWL, Block TM, Mehta AS (2006) Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. J Proteome Res 5:308–315. https://doi.org/10.1021/pr050328x
Coss KP, Byrne JC, Coman DJ, Adamczyk B, Abrahams JL, Saldova R, Brown AY, Walsh O, Hendroff U, Carolan C, Rudd PM, Treacy EP (2012) IgG N-glycans as potential biomarkers for determining galactose tolerance in classical galactosaemia. Mol Genet Metab 105:212–220. https://doi.org/10.1016/j.ymgme.2011.10.018
Coss KP, Hawkes CP, Adamczyk B, Stöckmann H, Crushell E, Saldova R, Knerr I, Rubio-Gozalbo ME, Monavari AA, Rudd PM, Treacy EP (2014) N-glycan abnormalities in children with galactosemia. J Proteome Res 13:385–394. https://doi.org/10.1021/pr4008305
Courtois F, Agrawal NJ, Lauer TM, Trout BL (2016) Rational design of therapeutic mAbs against aggregation through protein engineering and incorporation of glycosylation motifs applied to bevacizumab. MAbs 8:99–112. https://doi.org/10.1080/19420862.2015.1112477
Cremata JA, Sorell L, Montesino R, García R, Mata M, Cabrera G, Galvan JA, García G, Valdés R, Garrote JA (2003) Hypogalactosylation of serum IgG in patients with coeliac disease. Clin Exp Immunol 133:422–429. https://doi.org/10.1046/j.1365-2249.2003.02220.x
Croce A, Firuzi O, Altieri F, Eufemi M, Agostino R, Priori R, Bombardieri M, Alessandri C, Valesini G, Saso L (2007) Effect of infliximab on the glycosylation of IgG of patients with rheumatoid arthritis. J Clin Lab Anal 21:303–314. https://doi.org/10.1002/jcla.20191
Culver EL, van de Bovenkamp FS, Derksen NIL, Koers J, Cargill T, Barnes E, de Neef LA, Koeleman CAM, Aalberse RC, Wuhrer M, Rispens T (2019) Unique patterns of glycosylation in immunoglobulin subclass G4-related disease and primary sclerosing cholangitis. J Gastroenterol Hepatol 34:1878–1886. https://doi.org/10.1111/jgh.14512
Cvetko A, Kifer D, Gornik O, Klarić L, Visser E, Lauc G, Wilson JF, Štambuk T (2020) Glycosylation alterations in multiple sclerosis show increased proinflammatory potential. Biomedicine 8:410. https://doi.org/10.3390/biomedicines8100410
Cymer F, Beck H, Rohde A, Reusch D (2018) Therapeutic monoclonal antibody N-glycosylation – structure, function and therapeutic potential. Biologicals 52:1–11. https://doi.org/10.1016/j.biologicals.2017.11.001
Davies J, Jiang L, Pan LZ, Labarre MJ, Anderson D, Reff M (2001) Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FcγRIII. Biotechnol Bioeng 74:288–294. https://doi.org/10.1002/bit.1119
de Haan N, van Tol MJD, Driessen GJ, Wuhrer M, Lankester AC (2018a) Immunoglobulin G fragment crystallizable glycosylation after hematopoietic stem cell transplantation is dissimilar to donor profiles. Front Immunol 9:1. https://doi.org/10.3389/fimmu.2018.01238
de Haan N, Boeddha NP, Ekinci E, Reiding KR, Emonts M, Hazelzet JA, Wuhrer M, Driessen GJ (2018b) Differences in IgG Fc glycosylation are associated with outcome of pediatric meningococcal sepsis. MBio 9. https://doi.org/10.1128/mBio.00546-18
De Jong SE, Selman MHJ, Adegnika AA, Amoah AS, Van Riet E, Kruize YCM, Raynes JG, Rodriguez A, Boakye D, Von Mutius E, Knulst AC, Genuneit J, Cooper PJ, Hokke CH, Wuhrer M, Yazdanbakhsh M (2016) IgG1 Fc N-glycan galactosylation as a biomarker for immune activation. Sci Rep 6:28207. https://doi.org/10.1038/srep28207
Deisenhofer J, Colman PM, Epp O, Huber R (1976) Kristallographische Studien an einem Fc-fragment, II. Ein vollständiges Modell nach einer Fourier-Synthese bei 3.5Å Auflösung, Hoppe. Seylers Z Physiol Chem 357:1421–1434. https://doi.org/10.1515/bchm2.1976.357.2.1421
Dekkers G, Treffers L, Plomp R, Bentlage AEH, de Boer M, Koeleman CAM, Lissenberg-Thunnissen SN, Visser R, Brouwer M, Mok JY, Matlung H, van den Berg TK, van Esch WJE, Kuijpers TW, Wouters D, Rispens T, Wuhrer M, Vidarsson G (2017) Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of Fc-receptor- and complement-mediated-effector activities. Front Immunol 8:877. https://doi.org/10.3389/fimmu.2017.00877
Dubé R, Rook GAW, Steele J, Brealey R, Dwek R, Rademacher T, Lennard-Jones J (1990) Agalactosyl IgG in inflammatory bowel disease: correlation with C-reactive protein. Gut 31:431–434. https://doi.org/10.1136/gut.31.4.431
Dunn-Walters D, Boursier L, Spencer J (2000) Effect of somatic hypermutation on potential N-glycosylation sites in human immunoglobulin heavy chain variable regions. Mol Immunol 37:107–113. https://doi.org/10.1016/S0161-5890(00)00038-9
Engdahl C, Bondt A, Harre U, Raufer J, Pfeifle R, Camponeschi A, Wuhrer M, Seeling M, Mårtensson IL, Nimmerjahn F, Krönke G, Scherer HU, Forsblad-d’Elia H, Schett G (2018) Estrogen induces St6gal1 expression and increases IgG sialylation in mice and patients with rheumatoid arthritis: a potential explanation for the increased risk of rheumatoid arthritis in postmenopausal women. Arthritis Res Ther 20. https://doi.org/10.1186/s13075-018-1586-z
Ercan A, Cui J, Chatterton DEW, Deane KD, Hazen MM, Brintnell W, O’Donnell CI, Derber LA, Weinblatt ME, Shadick NA, Bell DA, Cairns E, Solomon DH, Holers VM, Rudd PM, Lee DM (2010) Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum 62:2239–2248. https://doi.org/10.1002/art.27533
Ercan A, Barnes MG, Hazen M, Tory H, Henderson L, Dedeoglu F, Fuhlbrigge RC, Grom A, Holm IA, Kellogg M, Kim S, Adamczyk B, Rudd PM, Son MB, Sundel RP, Foell D, Glass DN, Thompson SD, Nigrovic PA (2012) Multiple juvenile idiopathic arthritis subtypes demonstrate proinflammatory IgG glycosylation. Arthritis Rheum 64:3025–3033. https://doi.org/10.1002/art.34507
Ercan A, Kohrt WM, Cui J, Deane KD, Pezer M, Yu EW, Hausmann JS, Campbell H, Kaiser UB, Rudd PM, Lauc G, Wilson JF, Finkelstein JS, Nigrovic PA (2017) Estrogens regulate glycosylation of IgG in women and men. JCI Insight 2:e89703. https://doi.org/10.1172/jci.insight.89703
Espy C, Morelle W, Kavian N, Grange P, Goulvestre C, Viallon V, Chéreau C, Pagnoux C, Michalski JC, Guillevin L, Weill B, Batteux F, Guilpain P (2011) Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s). Arthritis Rheum 63:2105–2115. https://doi.org/10.1002/art.30362
Fan Y, Jimenez Del Val I, Müller C, Wagtberg Sen J, Rasmussen SK, Kontoravdi C, Weilguny D, Andersen MR (2015) Amino acid and glucose metabolism in fed-batch CHO cell culture affects antibody production and glycosylation. Biotechnol Bioeng 112:521–535. https://doi.org/10.1002/bit.25450
Ferrara C, Brünker P, Suter T, Moser S, Püntener U, Umaña P (2006) Modulation of therapeutic antibody effector functions by glycosylation engineering: influence of golgi enzyme localization domain and co-expression of heterologous β1, 4-N-acetylglucosaminyltransferase III and Golgi α-mannosidase II. Biotechnol Bioeng 93:851–861. https://doi.org/10.1002/bit.20777
Fickentscher C, Magorivska I, Janko C, Biermann M, Bilyy R, Nalli C, Tincani A, Medeghini V, Meini A, Nimmerjahn F, Schett G, Muñoz LE, Andreoli L, Herrmann M (2015) The pathogenicity of anti-β 2GP1-IgG autoantibodies depends on Fc glycosylation. J Immunol Res 2015:638129. https://doi.org/10.1155/2015/638129
Fiebiger BM, Maamary J, Pincetic A, Ravetch JV (2015) Protection in antibody- and T cell-mediated autoimmune diseases by antiinflammatory IgG Fcs requires type II FcRs. Proc Natl Acad Sci U S A 112:E2385–E2394. https://doi.org/10.1073/pnas.1505292112
Filley E, Andreoli A, Steele J, Waters M, Wagner D, Nelson D, Tung K, Rademacher T, Dwek R, Rook GAW (1989) A transient rise in agalactosyl IgG correlating with free interleukin 2 receptors, during episodes of erythema nodosum leprosum. Clin Exp Immunol 76:343–347. http://www.ncbi.nlm.nih.gov/pubmed/2787714
Fleming SC, Smith S, Knowles D, Skillen A, Self CH (1998) Increased sialylation of oligosaccharides on IgG paraproteins – a potential new tumour marker in multiple myeloma. J Clin Pathol 51:825–830. https://doi.org/10.1136/jcp.51.11.825
Flögel M, Lauc G, Gornik I, Maček B (1998) Fucosylation and galactosylation of IgG heavy chains differ between acute and remission phases of juvenile chronic arthritis. Clin Chem Lab Med 36:99–102. https://doi.org/10.1515/CCLM.1998.018
Fokkink WJR, Selman MHJ, Dortland JR, Durmuş B, Kuitwaard K, Huizinga R, Van Rijs W, Tio-Gillen AP, Van Doorn PA, Deelder AM, Wuhrer M, Jacobs BC (2014a) IgG Fc N-glycosylation in Guillain-Barré syndrome treated with immunoglobulins. J Proteome Res 13:1722–1730. https://doi.org/10.1021/pr401213z
Fokkink WJR, Selman MHC, Wuhrer M, Jacobs BC (2014b) Immunoglobulin G Fc N-glycosylation in Guillain-Barré syndrome treated with intravenous immunoglobulin. Clin Exp Immunol 178:105–107. https://doi.org/10.1111/cei.12530
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A (2018) Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14:576–590. https://doi.org/10.1038/s41574-018-0059-4
Freidin MB, Keser T, Gudelj I, Stambuk J, Vucenovic D, Allegri M, Pavic T, Simurina M, Fabiane SM, Lauc G, Williams FMK (2016) The association between low Back pain and composition of IgG glycome. Sci Rep 6:26815. https://doi.org/10.1038/srep26815
Fujimura Y, Tachibana H, Eto N, Yamada K (2000) Antigen binding of an ovomucoid-specific antibody is affected by a carbohydrate chain located on the light chain variable region. Biosci Biotechnol Biochem 64:2298–2305. https://doi.org/10.1271/bbb.64.2298
Galeotti C, Kaveri SV, Bayry J (2017) IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol 29:491–498. https://doi.org/10.1093/intimm/dxx039
Gao Q, Dolikun M, Štambuk J, Wang H, Zhao F, Yiliham N, Wang Y, Trbojević-Akmačić I, Zhang J, Fang H, Sun Y, Peng H, Zhao Z, Liu D, Liu J, Li Q, Sun Q, Wu L, Lauc G, Wang W, Song M (2017) Immunoglobulin G N-glycans as potential postgenomic biomarkers for hypertension in the Kazakh population. Omi A J Integr Biol 21:380–389. https://doi.org/10.1089/omi.2017.0044
Garber K (2018) No added sugar: antibody makers find an upside to ‘no fucose’. Nat Biotechnol 36:1025–1027. https://doi.org/10.1038/nbt1118-1025
Gardinassi LG, Dotz V, Ederveen AH, De Almeida RP, Costa CHN, Costa DL, De Jesus AR, Mayboroda OA, Garcia GR, Wuhrer M, de Miranda Santos IKF (2014) Clinical severity of visceral leishmaniasis is associated with changes in immunoglobulin G Fc N-glycosylation. MBio 5:e01844. https://doi.org/10.1128/mBio.01844-14
Gerçel-Taylor Ç, Bazzett LB, Taylor DD (2001) Presence of aberrant tumor-reactive immunoglobulins in the circulation of patients with ovarian cancer. Gynecol Oncol 81:71–76. https://doi.org/10.1006/gyno.2000.6102
Gindzienska-Sieskiewicz E, Klimiuk PA, Kisiel DG, Gindzienski A, Sierakowski S (2007) The changes in monosaccharide composition of immunoglobulin G in the course of rheumatoid arthritis. Clin Rheumatol 26:685–690. https://doi.org/10.1007/s10067-006-0370-7
Gińdzieńska-Sieśkiewicz E, Radziejewska I, Domysławska I, Klimiuk PA, Sulik A, Rojewska J, Gabryel-Porowska H, Sierakowski S (2016) Changes of glycosylation of IgG in rheumatoid arthritis patients treated with methotrexate. Adv Med Sci 61:193–197. https://doi.org/10.1016/j.advms.2015.12.009
Go MF, Schrohenloher RE, Tomana M (1994) Deficient galactosylation of serum IgG in inflammatory bowel disease: correlation with disease activity. J Clin Gastroenterol 18:86–87. https://doi.org/10.1097/00004836-199401000-00021
Gornik I, Maravić G, Dumić J, Flögel M, Lauc G (1999) Fucosylation of IgG heavy chains is increased in rheumatoid arthritis. Clin Biochem 32:605–608. https://doi.org/10.1016/S0009-9120(99)00060-0
Gornik O, Pavić T, Lauc G (2012) Alternative glycosylation modulates function of IgG and other proteins - implications on evolution and disease. Biochim Biophys Acta - Gen Subj 1820:1318–1326. https://doi.org/10.1016/j.bbagen.2011.12.004
Greto V, Cvetko A, Štambuk T, Dempster N, Kifer D, Deriš H, Cindrić A, Vučković F, Falchi M, Gillies R, Tomlinson J, Gornik O, Sgromo B, Spector T, Menni C, Geremia A, Arancibia-Cárcamo C, Lauc G (2020) Extensive weight loss can reduce immune age by altering IgG N-glycosylation. MedRxiv. 2020.04.24.20077867. https://doi.org/10.1101/2020.04.24.20077867
Gudelj I, Lauc G, Pezer M (2018a) Immunoglobulin G glycosylation in aging and diseases. Cell Immunol 333:65–79. https://doi.org/10.1016/j.cellimm.2018.07.009
Gudelj I, Salo PP, Trbojević-Akmačić I, Albers M, Primorac D, Perola M, Lauc G (2018b) Low galactosylation of IgG associates with higher risk for future diagnosis of rheumatoid arthritis during 10 years of follow-up. Biochim Biophys Acta Mol basis Dis 1864:2034–2039. https://doi.org/10.1016/j.bbadis.2018.03.018
Guhr T, Bloem J, Derksen NIL, Wuhrer M, Koenderman AHL, Aalberse RC, Rispens T (2011) Enrichment of sialylated IgG by lectin fractionation does not enhance the efficacy of immunoglobulin G in a murine model of immune thrombocytopenia. PLoS One 6:e21246. https://doi.org/10.1371/journal.pone.0021246
Gutiérrez G, Malan Borel I, Margni RA (2001) The placental regulatory factor involved in the asymmetric IgG antibody synthesis responds to IL-6 features. J Reprod Immunol 49:21–32. https://doi.org/10.1016/S0165-0378(00)00074-7
Gutierrez G, Gentile T, Miranda S, Margni RA (2006) Asymmetric antibodies: a protective arm in pregnancy. Chem Immunol Allergy 89:158–168. https://doi.org/10.1159/000087964
Haddad G, Lorenzen JM, Ma H, de Haan N, Seeger H, Zaghrini C, Brandt S, Kölling M, Wegmann U, Kiss B, Pál G, Gál P, Wüthrich RP, Wuhrer M, Beck LH, Salant DJ, Lambeau G, Kistler AD (2021) Altered glycosylation of IgG4 promotes lectin complement pathway activation in anti-PLA2R1-associated membranous nephropathy. J Clin Invest 131. https://doi.org/10.1172/JCI140453
Hafkenscheid L, Bondt A, Scherer HU, Huizinga TWJ, Wuhrer M, Toes REM, Rombouts Y (2017) Structural analysis of variable domain glycosylation of anti-citrullinated protein antibodies in rheumatoid arthritis reveals the presence of highly sialylated glycans. Mol Cell Proteomics 16:278–287. https://doi.org/10.1074/mcp.M116.062919
Hafkenscheid L, de Moel E, Smolik I, Tanner S, Meng X, Jansen BC, Bondt A, Wuhrer M, Huizinga TWJ, Toes REM, El-Gabalawy H, Scherer HU (2019) N-linked glycans in the variable domain of IgG anti–citrullinated protein antibodies predict the development of rheumatoid arthritis. Arthritis Rheumatol 71:1626–1633. https://doi.org/10.1002/art.40920
Hernandez-Pando R, Reyes P, Espitia C, Wang Y, Rook G, Mancilla R (1994) Raised agalactosyl IgG and antimycobacterial humoral immunity in Takayasu’s arteritis. J Rheumatol 21:1870–1876. http://www.ncbi.nlm.nih.gov/pubmed/7837153
Hess C, Winkler A, Lorenz AK, Holecska V, Blanchard V, Eiglmeier S, Schoen AL, Bitterling J, Stoehr AD, Petzold D, Schommartz T, Mertes MMM, Schoen CT, Tiburzy B, Herrmann A, Köhl J, Manz RA, Madaio MP, Berger M, Wardemann H, Ehlers M (2013) T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J Clin Invest 123:3788–3796. https://doi.org/10.1172/JCI65938
Higel F, Seidl A, Sörgel F, Friess W (2016) N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur J Pharm Biopharm 100:94–100. https://doi.org/10.1016/j.ejpb.2016.01.005
Ho CH, Chien RN, Cheng PN, Liu JH, Liu CK, Su CS, Wu IC, Li IC, Tsai HW, Wu SL, Liu WC, Chen SH, Chang TT (2015) Aberrant serum immunoglobulin G glycosylation in chronic hepatitis B is associated with histological liver damage and reversible by antiviral therapy. J Infect Dis 211:115–124. https://doi.org/10.1093/infdis/jiu388
Hodoniczky J, Yuan ZZ, James DC (2005) Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog 21:1644–1652. https://doi.org/10.1021/bp050228w
Hoepel W, Chen H-J, Allahverdiyeva S, Manz X, Aman J, Bonta P, Brouwer P, de Taeye S, Caniels T, van der Straten K, Golebski K, Griffith G, Jonkers R, Larsen M, Linty F, Neele A, Nouta J, van Baarle F, van Drunen C, Vlaar A, de Bree G, Sanders R, Willemsen L, Wuhrer M, Bogaard HJ, van Gils M, Vidarsson G, de Winther M, den Dunnen J (2020) Anti-SARS-CoV-2 IgG from severely ill COVID-19 patients promotes macrophage hyper-inflammatory responses. BioRxiv. 2020.07.13.190140. https://doi.org/10.1101/2020.07.13.190140
Holland M, Takada K, Okumoto T, Takahashi N, Kato K, Adu D, Ben-Smith A, Harper L, Savage COS, Jefferis R (2002) Hypogalactosylation of serum IgG in patients with ANCA-associated systemic vasculitis. Clin Exp Immunol 129:183–190. https://doi.org/10.1046/j.1365-2249.2002.01864.x
Holland M, Yagi H, Takahashi N, Kato K, Savage COS, Goodall DM, Jefferis R (2006) Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim Biophys Acta - Gen Subj 1760:669–677. https://doi.org/10.1016/j.bbagen.2005.11.021
Hou H, Xu X, Sun F, Zhang X, Dong H, Wang L, Ge S, An K, Sun Q, Li Y, Cao W, Song M, Hu S, Xing W, Wang W, Li D, Wang Y (2019) Hyperuricemia is associated with immunoglobulin G N-glycosylation: a community-based study of glycan biomarkers. Omi A J Integr Biol 23:660–667. https://doi.org/10.1089/omi.2019.0004
Houde D, Peng Y, Berkowitz SA, Engen JR (2010) Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol Cell Proteomics 9:1716–1728. https://doi.org/10.1074/mcp.M900540-MCP200
Huffman JE, Pučić-Baković M, Klarić L, Hennig R, Selman MHJ, Vučković F, Novokmet M, Krištić J, Borowiak M, Muth T, Polašek O, Razdorov G, Gornik O, Plomp R, Theodoratou E, Wright AF, Rudan I, Hayward C, Campbell H, Deelder AM, Reichl U, Aulchenko YS, Rapp E, Wuhrer M, Lauc G (2014) Comparative performance of four methods for high-throughput glycosylation analysis of immunoglobulin G in genetic and epidemiological research. Mol Cell Proteomics 13:1598–1610. https://doi.org/10.1074/mcp.M113.037465
Huhn C, Selman MHJ, Ruhaak LR, Deelder AM, Wuhrer M (2009) IgG glycosylation analysis. Proteomics 9:882–913. https://doi.org/10.1002/pmic.200800715
Hymes AJ, Mullinax GL, Mullinax F (1979) Immunoglobulin carbohydrate requirement for formation of an IgG-IgG complex. J Biol Chem 254:3148–3151. https://doi.org/10.1016/S0021-9258(18)50733-X
Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FMA, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, Ezekowitz A, Carroll MC, Brenner M, Weissleder R, Verbeek JS, Duchatelle V, Degott C, Benoist C, Mathis D (2002) Arthritis critically dependent on innate immune system players. Immunity 16:157–168. https://doi.org/10.1016/S1074-7613(02)00275-3
Kaneko Y, Nimmerjahn F, Ravetch JV (2006) Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science (80-) 313:670–673. https://doi.org/10.1126/science.1129594
Kanoh Y, Mashiko T, Danbara M, Takayama Y, Ohtani S, Egawa S, Baba S, Akahoshi T (2004a) Changes in serum IgG oligosaccharide chains with prostate cancer progression. Anticancer Res 24:3135–3139. http://www.ncbi.nlm.nih.gov/pubmed/15510601
Kanoh Y, Mashiko T, Danbara M, Takayama Y, Ohtani S, Imasaki T, Abe T, Akahoshi T (2004b) Analysis of the oligosaccharide chain of human serum immunoglobulin G in patients with localized or metastatic cancer. Oncology 66:365–370. https://doi.org/10.1159/000079484
Kanoh Y, Ohara T, Mashiko T, Abe T, Masuda N, Akahoshi T (2006) Relationship between N-linked oligosaccharide chains of human serum immunoglobulin G and serum tumor markers with non-small cell lung cancer progression. Anticancer Res 26:4293–4297
Kanoh Y, Ohara T, Tadano T, Kanoh M, Akahoshi T (2008) Changes to N-linked oligosaccharide chains of human serum immunoglobulin G and matrix metalloproteinase-2 with cancer progression. Anticancer Res 28:715–720. http://www.ncbi.nlm.nih.gov/pubmed/18507012
Kanoh Y, Egawa S, Baba S, Akahoshi T (2009) Associations of IgG N-linked oligosaccharide chains and proteases in sera of prostate cancer patients with and without α2-macroglobulin deficiency. J Clin Lab Anal 23:125–131. https://doi.org/10.1002/jcla.20302
Kapur R, Della Valle L, Sonneveld M, Hipgrave Ederveen A, Visser R, Ligthart P, de Haas M, Wuhrer M, van der Schoot CE, Vidarsson G (2014a) Low anti-RhD IgG-Fc-fucosylation in pregnancy: a new variable predicting severity in haemolytic disease of the fetus and newborn. Br J Haematol 166:936–945. https://doi.org/10.1111/bjh.12965
Kapur R, Kustiawan I, Vestrheim A, Koeleman CAM, Visser R, Einarsdottir HK, Porcelijn L, Jackson D, Kumpel B, Deelder AM, Blank D, Skogen B, Killie MK, Michaelsen TE, De Haas M, Rispens T, Van Der Schoot CE, Wuhrer M, Vidarsson G (2014b) A prominent lack of IgG1-fc fucosylation of platelet alloantibodies in pregnancy. Blood 123:471–480. https://doi.org/10.1182/blood-2013-09-527978
Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D, Blanchard V, Winkler A, Hess C, Reid DM, Majoul IV, Strait RT, Harris NL, Köhl G, Wex E, Ludwig R, Zillikens D, Nimmerjahn F, Finkelman FD, Brown GD, Ehlers M, Köhl J (2012) Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat Med 18:1401–1406. https://doi.org/10.1038/nm.2862
Käsermann F, Boerema DJ, Rüegsegger M, Hofmann A, Wymann S, Zuercher AW, Miescher S (2012) Analysis and functional consequences of increased fab-sialylation of intravenous immunoglobulin (IVIG) after lectin fractionation. PLoS One 7:37243. https://doi.org/10.1371/journal.pone.0037243
Kawaguchi-Sakita N, Kaneshiro-Nakagawa K, Kawashima M, Sugimoto M, Tokiwa M, Suzuki E, Kajihara S, Fujita Y, Iwamoto S, Tanaka K, Toi M (2016) Serum immunoglobulin G fc region N-glycosylation profiling by matrix-assisted laser desorption/ionization mass spectrometry can distinguish breast cancer patients from cancer-free controls. Biochem Biophys Res Commun 469:1140–1145. https://doi.org/10.1016/j.bbrc.2015.12.114
Kazuno S, Furukawa JI, Shinohara Y, Murayama K, Fujime M, Ueno T, Fujimura T (2016) Glycosylation status of serum immunoglobulin G in patients with prostate diseases. Cancer Med 5:1137–1146. https://doi.org/10.1002/cam4.662
Kemna MJ, Plomp R, van Paassen P, Koeleman CAM, Jansen BC, Damoiseaux JGMC, Cohen Tervaert JW, Wuhrer M (2017) Galactosylation and sialylation levels of IgG predict relapse in patients with PR3-ANCA associated vasculitis. EBioMedicine 17:108–118. https://doi.org/10.1016/j.ebiom.2017.01.033
Khurana S, Raghunathan V, Salunke DM (1997) The variable domain glycosylation in a monoclonal antibody specific to GnRH modulates antigen binding. Biochem Biophys Res Commun 234:465–469. https://doi.org/10.1006/bbrc.1997.5929
Kissel T, Van Schie KA, Hafkenscheid L, Lundquist A, Kokkonen H, Wuhrer M, Huizinga TWJ, Scherer HU, Toes R, Rantapää-Dahlqvist S (2019) On the presence of HLA-SE alleles and ACPA-IgG variable domain glycosylation in the phase preceding the development of rheumatoid arthritis. Ann Rheum Dis 78:1616–1620. https://doi.org/10.1136/annrheumdis-2019-215698
Klarić L, Tsepilov YA, Stanton CM, Mangino M, Sikka TT, Esko T, Pakhomov E, Salo P, Deelen J, McGurnaghan SJ, Keser T, Vučković F, Ugrina I, Krištić J, Gudelj I, Štambuk J, Plomp R, Pučić-Baković M, Pavić T, Vilaj M, Trbojević-Akmačić I, Drake C, Dobrinić P, Mlinarec J, Jelušić B, Richmond A, Timofeeva M, Grishchenko AK, Dmitrieva J, Bermingham ML, Sharapov SZ, Farrington SM, Theodoratou E, Uh HW, Beekman M, Slagboom EP, Louis E, Georges M, Wuhrer M, Colhoun HM, Dunlop MG, Perola M, Fischer K, Polasek O, Campbell H, Rudan I, Wilson JF, Zoldoš V, Vitart V, Spector T, Aulchenko YS, Lauc G, Hayward C (2020) Glycosylation of immunoglobulin G is regulated by a large network of genes pleiotropic with inflammatory diseases. Sci Adv 6:eaax0301. https://doi.org/10.1126/sciadv.aax0301
Klasić M, Markulin D, Vojta A, Samaržija I, Biruš I, Dobrinić P, Ventham NT, Trbojević-Akmačić I, Šimurina M, Štambuk J, Razdorov G, Kennedy NA, Satsangi J, Dias AM, Pinho S, Annese V, Latiano A, D’Inca R, Lauc G, Zoldoš V (2018) Promoter methylation of the MGAT3 and BACH2 genes correlates with the composition of the immunoglobulin G glycome in inflammatory bowel disease. Clin Epigenetics 10:75. https://doi.org/10.1186/s13148-018-0507-y
Knerr I, Coss KP, Kratzsch J, Crushell E, Clark A, Doran P, Shin Y, Stöckmann H, Rudd PM, Treacy E (2015) Effects of temporary low-dose galactose supplements in children aged 5-12 y with classical galactosemia: a pilot study. Pediatr Res 78:272–279. https://doi.org/10.1038/pr.2015.107
Kodar K, Stadlmann J, Klaamas K, Sergeyev B, Kurtenkov O (2012) Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconj J 29:57–66. https://doi.org/10.1007/s10719-011-9364-z
Koide N, Nose M, Muramatsu T (1977) Recognition of IgG by Fc receptor and complement: effects of glycosidase digestion. Biochem Biophys Res Commun 75:838–844. https://doi.org/10.1016/0006-291X(77)91458-9
Krištić J, Vučković F, Menni C, Klarić L, Keser T, Beceheli I, Pučić-Baković M, Novokmet M, Mangino M, Thaqi K, Rudan P, Novokmet N, Šarac J, Missoni S, Kolčić I, Polašek O, Rudan I, Campbell H, Hayward C, Aulchenko Y, Valdes A, Wilson JF, Gornik O, Primorac D, Zoldoš V, Spector T, Lauc G (2014) Glycans are a novel biomarker of chronological and biological ages. Journals Gerontol - Ser A Biol Sci Med Sci 69:779–789. https://doi.org/10.1093/gerona/glt190
Kumpel BM, Rademacher TW, Rook GAW, Williams PJ, Wilson IBH (1994) Galactosylation of human IgG monoclonal anti-D produced by EBV-transformed B-lympboblastoid cell lines is dependent on culture method and affects Fc receptor-mediated functional activity. Hum Antibodies Hybridomas 5:143–151
Kumpel BM, Wang Y, Griffiths HL, Hadley AG, Rook GAW (1995) The biological activity of human monoclonal IgG anti-D is reduced by β-galactosidase treatment. Hum Antibodies 6:82–88. https://doi.org/10.3233/HAB-1995-6301
Larsen MD, de Graaf EL, Sonneveld ME, Plomp HR, Nouta J, Hoepel W, Chen H-J, Linty F, Visser R, Brinkhaus M, Šuštić T, de Taeye SW, Bentlage AEH, Toivonen S, Koeleman CAM, Sainio S, Kootstra NA, Brouwer PJM, Geyer CE, Derksen NIL, Wolbink G, de Winther M, Sanders RW, van Gils MJ, de Bruin S, Vlaar APJ, Rispens T, den Dunnen J, Zaaijer HL, Wuhrer M, van der Schoot CE, Vidarsson G (2020) Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science 80. https://doi.org/10.1126/science.abc8378
Lauc G (2016) Precision medicine that transcends genomics: glycans as integrators of genes and environment. Biochim Biophys Acta - Gen Subj 1860:1571–1573. https://doi.org/10.1016/j.bbagen.2016.05.001
Lauc G, Huffman JE, Pučić M, Zgaga L, Adamczyk B, Mužinić A, Novokmet M, Polašek O, Gornik O, Krištić J, Keser T, Vitart V, Scheijen B, Uh HW, Molokhia M, Patrick AL, McKeigue P, Kolčić I, Lukić IK, Swann O, van Leeuwen FN, Ruhaak LR, Houwing-Duistermaat JJ, Slagboom PE, Beekman M, de Craen AJM, Deelder AM, Zeng Q, Wang W, Hastie ND, Gyllensten U, Wilson JF, Wuhrer M, Wright AF, Rudd PM, Hayward C, Aulchenko Y, Campbell H, Rudan I (2013) Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet 9:e1003225. https://doi.org/10.1371/journal.pgen.1003225
Leibiger H, Wüstner D, Stigler RD, Marx U (1999) Variable domain-linked oligosaccharides of a human monoclonal IgG: structure and influence on antigen binding. Biochem J 338:529–538. https://doi.org/10.1042/0264-6021:3380529
Leirisalo-Repo M, Hernandez-Munoz HE, Rook GAW (1999) Agalactosyl IgG is elevated in patients with active spondyloarthropathy. Rheumatol Int 18:171–176. https://doi.org/10.1007/s002960050080
Lemmers RFH, Vilaj M, Urda D, Agakov F, Šimurina M, Klaric L, Rudan I, Campbell H, Hayward C, Wilson JF, Lieverse AG, Gornik O, Sijbrands EJG, Lauc G, van Hoek M (2017) IgG glycan patterns are associated with type 2 diabetes in independent European populations. Biochim Biophys Acta - Gen Subj 1861:2240–2249. https://doi.org/10.1016/j.bbagen.2017.06.020
Leontyev D, Katsman Y, Ma XZ, Miescher S, Käsermann F, Branch DR (2012) Sialylation-independent mechanism involved in the amelioration of murine immune thrombocytopenia using intravenous gammaglobulin. Transfusion 52:1799–1805. https://doi.org/10.1111/j.1537-2995.2011.03517.x
Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang LX (2017) Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci USA 114:3485–3490. https://doi.org/10.1073/pnas.1702173114
Li X, Wang H, Russell A, Cao W, Wang X, Ge S, Zheng Y, Guo Z, Hou H, Song M, Yu X, Wang Y, Hunter M, Roberts P, Lauc G, Wang W (2019) Type 2 diabetes mellitus is associated with the immunoglobulin G N-glycome through putative proinflammatory mechanisms in an Australian population. Omi A J Integr Biol 23:631–639. https://doi.org/10.1089/omi.2019.0075
Lifely MR, Hale C, Boyce S, Keen MJ, Phillips J (1995) Glycosylation and biological activity of CAMPATH-1H expressed in different cell lines and grown under different culture conditions. Glycobiology 5:813–822. https://doi.org/10.1093/glycob/5.8.813
Liu L (2015) Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J Pharm Sci 104:1866–1884. https://doi.org/10.1002/jps.24444
Liu L (2018) Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell 9:15–32. https://doi.org/10.1007/s13238-017-0408-4
Liu B, Spearman M, Doering J, Lattová E, Perreault H, Butler M (2014) The availability of glucose to CHO cells affects the intracellular lipid-linked oligosaccharide distribution, site occupancy and the N-glycosylation profile of a monoclonal antibody. J Biotechnol 170:17–27. https://doi.org/10.1016/j.jbiotec.2013.11.007
Liu D, Zhao Z, Wang A, Ge S, Wang H, Zhang X, Sun Q, Cao W, Sun M, Wu L, Song M, Zhou Y, Wang W, Wang Y (2018) Ischemic stroke is associated with the pro-inflammatory potential of N-glycosylated immunoglobulin G. J Neuroinflammation 15. https://doi.org/10.1186/s12974-018-1161-1
Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, Schoen MK, Tafesse F, Martin C, Leung V, Mahan AE, Sips M, Kumar MP, Tedesco J, Robinson H, Tkachenko E, Draghi M, Freedberg KJ, Streeck H, Suscovich TJ, Lauffenburger DA, Restrepo BI, Day C, Fortune SM, Alter G (2016) A functional role for antibodies in tuberculosis. Cell 167:433–443.e14. https://doi.org/10.1016/j.cell.2016.08.072
Lu LL, Das J, Grace PS, Fortune SM, Restrepo BI, Alter G (2020) Antibody Fc glycosylation discriminates between latent and active tuberculosis. J Infect Dis 222:2093–2102. https://doi.org/10.1093/infdis/jiz643
Lund J, Takahashi N, Pound JD, Goodall M, Jefferis R (1996) Multiple interactions of IgG with its core oligosaccharide can modulate recognition by complement and human Fc gamma receptor I and influence the synthesis of its oligosaccharide chains. J Immunol 157:4963–4969. http://www.ncbi.nlm.nih.gov/pubmed/8943402
Lundström SL, Yang H, Lyutvinskiy Y, Rutishauser D, Herukka SK, Soininen H, Zubarev RA (2014) Blood plasma IgG Fc glycans are significantly altered in Alzheimer’s disease and progressive mild cognitive impairment. J Alzheimers Dis 38:567–579. https://doi.org/10.3233/JAD-131088
Lundström SL, Hensvold AH, Rutishauser D, Klareskog L, Ytterberg AJ, Zubarev RA, Catrina AI (2017) IgG fc galactosylation predicts response to methotrexate in early rheumatoid arthritis. Arthritis Res Ther 19:182. https://doi.org/10.1186/s13075-017-1389-7
Magorivska I, Muñoz LE, Janko C, Dumych T, Rech J, Schett G, Nimmerjahn F, Bilyy R, Herrmann M (2016) Sialylation of anti-histone immunoglobulin G autoantibodies determines their capabilities to participate in the clearance of late apoptotic cells. Clin Exp Immunol 184:110–117. https://doi.org/10.1111/cei.12744
Mahan AE, Jennewein MF, Suscovich T, Dionne K, Tedesco J, Chung AW, Streeck H, Pau M, Schuitemaker H, Francis D, Fast P, Laufer D, Walker BD, Baden L, Barouch DH, Alter G (2016) Antigen-specific antibody glycosylation is regulated via vaccination. PLoS Pathog 12:e1005456. https://doi.org/10.1371/journal.ppat.1005456
Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB (1995) Glycosylation changes of IgG associated with rheumatooid arthritis can activate complement via the mannose-binding protein. Nat Med 1:237–243. https://doi.org/10.1038/nm0395-237
Man Sung Co, Scheinberg DA, Avdalovic NM, Mcgraw K, Vasquez M, Caron PC, Queen C (1993) Genetically engineered deglycosylation of the variable domain increases the affinity of an anti-CD33 monoclonal antibody. Mol Immunol 30:1361–1367. https://doi.org/10.1016/0161-5890(93)90097-U
Maratha A, Stockmann H, Coss KP, Estela Rubio-Gozalbo M, Knerr I, Fitzgibbon M, McVeigh TP, Foley P, Moss C, Colhoun HO, Van Erven B, Stephens K, Doran P, Rudd P, Treacy E (2016) Classical galactosaemia: novel insights in IgG N-glycosylation and N-glycan biosynthesis. Eur J Hum Genet 24:976–984. https://doi.org/10.1038/ejhg.2015.254
Mariño K, Bones J, Kattla JJ, Rudd PM (2010) A systematic approach to protein glycosylation analysis: a path through the maze. Nat Chem Biol 6:713–723. https://doi.org/10.1038/nchembio.437
Martin TC, Šimurina M, Zabczynska M, Kavur M, Rydlewska M, Pezer M, Kozłowska K, Burri A, Vilaj M, Turek-Jabrocka R, Krnjajić-Tadijanović M, Trofimiuk-Müldner M, Ugrina I, Lityn A, Hubalewska-Dydejczyk A, Trbojevic-Akmacic I, Lim EM, Walsh JP, Pocheć E, Spector TD, Wilson SG, Lauc G (2020) Decreased immunoglobulin G core fucosylation, a player in antibody-dependent cell-mediated cytotoxicity, is associated with autoimmune thyroid diseases. Mol Cell Proteomics 19:774–792. https://doi.org/10.1074/mcp.RA119.001860
Massoud AH, Yona M, Xue D, Chouiali F, Alturaihi H, Ablona A, Mourad W, Piccirillo CA, Mazer BD (2014) Dendritic cell immunoreceptor: a novel receptor for intravenous immunoglobulin mediates induction of regulatory T cells. J Allergy Clin Immunol 133:853–863. e5. https://doi.org/10.1016/j.jaci.2013.09.029
Matsumoto A, Shikata K, Takeuchi F, Kojima N, Mizuochi T (2000) Autoantibody activity of IgG rheumatoid factor increases with decreasing levels of galactosylation and sialylation. J Biochem 128:621–628. https://doi.org/10.1093/oxfordjournals.jbchem.a022794
McCann KJ, Ottensmeier CH, Callard A, Radcliffe CM, Harvey DJ, Dwek RA, Rudd PM, Sutton BJ, Hobby P, Stevenson FK (2008) Remarkable selective glycosylation of the immunoglobulin variable region in follicular lymphoma. Mol Immunol 45:1567–1572. https://doi.org/10.1016/j.molimm.2007.10.009
Mehta AS, Long RE, Comunale MA, Wang M, Rodemich L, Krakover J, Philip R, Marrero JA, Dwek RA, Block TM (2008) Increased levels of galactose-deficient anti-gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis. J Virol 82:1259–1270. https://doi.org/10.1128/jvi.01600-07
Meng Z, Li C, Ding G, Cao W, Xu X, Heng Y, Deng Y, Li Y, Zhang X, Li D, Wang W, Wang Y, Xing W, Hou H (2020) Glycomics: immunoglobulin GN-glycosylation associated with mammary gland hyperplasia in women. Omi A J Integr Biol 24:551–558. https://doi.org/10.1089/omi.2020.0091
Menni C, Keser T, Mangino M, Bell JT, Erte I, Akmačić I, Vučković F, Baković MP, Gornik O, McCarthy MI, Zoldoš V, Spector TD, Lauc G, Valdes AM (2013) Glycosylation of immunoglobulin G: role of genetic and epigenetic influences. PLoS One 8:e82558. https://doi.org/10.1371/journal.pone.0082558
Menni C, Gudelj I, MacDonald-Dunlop E, Mangino M, Zierer J, Bešić E, Joshi PK, Trbojević-Akmačić I, Chowienczyk PJ, Spector TD, Wilson JF, Lauc G, Valdes AM (2018) Glycosylation profile of immunoglobulin G is cross-sectionally associated with cardiovascular disease risk score and subclinical atherosclerosis in two independent cohorts. Circ Res 122:1555–1564. https://doi.org/10.1161/CIRCRESAHA.117.312174
Miranda S, Canellada A, Gentile T, Margni R (2005) Interleukin-6 and dexamethasone modulate in vitro asymmetric antibody synthesis and UDP-Glc glycoprotein glycosyltransferase activity. J Reprod Immunol 66:141–150. https://doi.org/10.1016/j.jri.2005.04.001
Mittermayr S, Lê GN, Clarke C, Millán Martín S, Larkin AM, O’Gorman P, Bones J (2017) Polyclonal immunoglobulin G N-glycosylation in the pathogenesis of plasma cell disorders. J Proteome Res 16:748–762. https://doi.org/10.1021/acs.jproteome.6b00768
Miyoshi E, Shinzaki S, Fujii H, Iijima H, Kamada Y, Takehara T (2016) Role of aberrant IgG glycosylation in the pathogenesis of inflammatory bowel disease. Proteomics – Clin Appl 10:384–390. https://doi.org/10.1002/prca.201500089
Moore JS, Wu X, Kulhavy R, Tomana M, Novak J, Moldoveanu Z, Brown R, Goepfert PA, Mestecky J (2005) Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS 19:381–389. https://doi.org/10.1097/01.aids.0000161767.21405.68
Muenchhoff M, Chung AW, Roider J, Dugast A-S, Richardson S, Kløverpris H, Leslie A, Ndung’u T, Moore P, Alter G, Goulder PJR (2020) Distinct immunoglobulin Fc glycosylation patterns are associated with disease nonprogression and broadly neutralizing antibody responses in children with HIV infection. MSphere 5. https://doi.org/10.1128/msphere.00880-20
Nakajima S, Iijima H, Shinzaki S, Egawa S, Inoue T, Mukai A, Hayashi Y, Kondo J, Akasaka T, Nishida T, Kanto T, Morii E, Mizushima T, Miyoshi E, Tsujii M, Hayashi N (2011) Functional analysis of agalactosyl IgG in inflammatory bowel disease patients. Inflamm Bowel Dis 17:927–936. https://doi.org/10.1002/ibd.21459
Nakao H, Nishikawa A, Nishiura T, Kanayama Y, Tarui S, Taniguchi N (1991) Hypogalactosylation of immunoglobulin G sugar chains and elevated serum interleukin 6 in Castleman’s disease. Clin Chim Acta 197:221–228. https://doi.org/10.1016/0009-8981(91)90142-Y
Nishiura T, Kanayama Y, Tomiyama Y, Iida M, Karasuno T, Nakao H, Yonezawa T, Tarui S, Fujii S, Nishikawa A, Taniguchi N (1990) Carbohydrate analysis of immunoglobulin G myeloma proteins by lectin and high performance liquid chromatography: role of glycosyltransferases in the structures. Cancer Res 50:5345–5350. http://www.ncbi.nlm.nih.gov/pubmed/2386941
Novak J, Tomana M, Shah GR, Brown R, Mestecky J (2005) Heterogeneity of IgG glycosylation in adult periodontal disease. J Dent Res 84:897–901. https://doi.org/10.1177/154405910508401005
Novokmet M, Lukić E, Vučković F, Durić Ž, Keser T, Rajšl K, Remondini D, Castellani G, Gašparović H, Gornik O, Lauc G (2014) Changes in IgG and total plasma protein glycomes in acute systemic inflammation. Sci Rep 4. https://doi.org/10.1038/srep04347
Oefner CM, Winkler A, Hess C, Lorenz AK, Holecska V, Huxdorf M, Schommartz T, Petzold D, Bitterling J, Schoen AL, Stoehr AD, Vu Van D, Darcan-Nikolaisen Y, Blanchard V, Schmudde I, Laumonnier Y, Ströver HA, Hegazy AN, Eiglmeier S, Schoen CT, Mertes MMM, Loddenkemper C, Löhning M, König P, Petersen A, Luger EO, Collin M, Köhl J, Hutloff A, Hamelmann E, Berger M, Wardemann H, Ehlers M (2012) Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs. J Allergy Clin Immunol 129:1647–1655.e13. https://doi.org/10.1016/j.jaci.2012.02.037
Ogata S, Shimizu C, Franco A, Touma R, Kanegaye JT, Choudhury BP, Naidu NN, Kanda Y, Hoang LT, Hibberd ML, Tremoulet AH, Varki A, Burns JC (2013) Treatment response in Kawasaki disease is associated with sialylation levels of endogenous but not therapeutic intravenous immunoglobulin G. PLoS One 8:e81448. https://doi.org/10.1371/journal.pone.0081448
Ohtsubo K, Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126:855–867. https://doi.org/10.1016/j.cell.2006.08.019
Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K, Takeuchi F, Nagano Y, Miyamoto T, Kobata A (1985) Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316:452–457. https://doi.org/10.1038/316452a0
Parekh RB, Isenberg DA, Ansell BM, Roitt IM, Dwek RA, Rademacher TW (1988) Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet 331:966–969. https://doi.org/10.1016/S0140-6736(88)91781-3
Parekh R, Isenberg D, Rook G, Roitt I, Dwek R, Rademacher T (1989) A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J Autoimmun 2:101–114. https://doi.org/10.1016/0896-8411(89)90148-0
Pasek M, Duk M, Podbielska M, Sokolik R, Szechiński J, Lisowska E, Krotkiewski H (2006) Galactosylation of IgG from rheumatoid arthritis (RA) patients – changes during therapy. Glycoconj J 23:463–471. https://doi.org/10.1007/s10719-006-5409-0
Pavić T, Dilber D, Kifer D, Selak N, Keser T, Ljubičić CDS, Vukić Dugac A, Lauc G, Rumora L, Gornik O (2018) N-glycosylation patterns of plasma proteins and immunoglobulin G in chronic obstructive pulmonary disease 11 medical and health sciences 1102 cardiorespiratory medicine and haematology. J Transl Med 16:323. https://doi.org/10.1186/s12967-018-1695-0
Pekelharing JM, Hepp E, Kamerling JP, Gerwig GJ, Leijnse B (1988) Alterations in carbohydrate composition of serum IgG from patients with rheumatoid arthritis and from pregnant women. Ann Rheum Dis 47:91–95. https://doi.org/10.1136/ard.47.2.91
Peng J, Vongpatanasin W, Sacharidou A, Kifer D, Yuhanna IS, Banerjee S, Tanigaki K, Polasek O, Chu H, Sundgren NC, Rohatgi A, Chambliss KL, Lauc G, Mineo C, Shaul PW (2019) Supplementation with the sialic acid precursor N-acetyl-D-Mannosamine breaks the link between obesity and hypertension. Circulation 140:2005–2018. https://doi.org/10.1161/CIRCULATIONAHA.119.043490
Perdivara I, Peddada SD, Miller FW, Tomer KB, Deterding LJ (2011) Mass spectrometric determination of IgG subclass-specific glycosylation profiles in siblings discordant for myositis syndromes. J Proteome Res 10:2969–2978. https://doi.org/10.1021/pr200397h
Peschke B, Keller CW, Weber P, Quast I, Lünemann JD (2017) Fc-galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front Immunol 8:646. https://doi.org/10.3389/fimmu.2017.00646
Petrović T, Alves I, Bugada D, Pascual J, Vučković F, Skelin A, Gaifem J, Villar-Garcia J, Vicente MM, Fernandes Â, Dias AM, Kurolt I-C, Markotić A, Primorac D, Soares A, Malheiro L, Trbojević-Akmačić I, Abreu M, Castro RSE, Bettinelli S, Callegaro A, Arosio M, Sangiorgio L, Lorini LF, Castells X, Horcajada JP, Pinho SS, Allegri M, Barrios C, Lauc G (2020) Composition of the immunoglobulin G glycome associates with the severity of COVID-19. Glycobiology. https://doi.org/10.1093/glycob/cwaa102
Pezer M, Stambuk J, Perica M, Razdorov G, Banic I, Vuckovic F, Gospic AM, Ugrina I, Vecenaj A, Bakovic MP, Lokas SB, Zivkovic J, Plavec D, Devereux G, Turkalj M, Lauc G (2016) Effects of allergic diseases and age on the composition of serum IgG glycome in children. Sci Rep 6:33198. https://doi.org/10.1038/srep33198
Pfeifle R, Rothe T, Ipseiz N, Scherer HU, Culemann S, Harre U, Ackermann JA, Seefried M, Kleyer A, Uderhardt S, Haugg B, Hueber AJ, Daum P, Heidkamp GF, Ge C, Böhm S, Lux A, Schuh W, Magorivska I, Nandakumar KS, Lönnblom E, Becker C, Dudziak D, Wuhrer M, Rombouts Y, Koeleman CA, Toes R, Winkler TH, Holmdahl R, Herrmann M, Blüml S, Nimmerjahn F, Schett G, Krönke G (2017) Regulation of autoantibody activity by the IL-23-T H 17 axis determines the onset of autoimmune disease. Nat Immunol 18:104–113. https://doi.org/10.1038/ni.3579
Pilkington C, Yeung E, Isenberg D, Lefvert AK, Rook GAW (1995) Agalactosyl IgG and antibody specificity in rheumatoid arthritis, tuberculosis, systemic lupus erythematosus and myasthenia gravis. Autoimmunity 22:107–111. https://doi.org/10.3109/08916939508995306
Pilkington C, Taylor PV, Silverman E, Isenberg DA, Costello AMDL, Rook GAW (1996a) Agalactosyl IgG and materno-fetal transmission of autoimmune neonatal lupus. Rheumatol Int 16:89–94. https://doi.org/10.1007/BF01409979
Pilkington C, Basaran M, Barlan I, Costello AMDL, Rook GAW (1996b) Raised levels of agalactosyl IgG in childhood tuberculosis. Trans R Soc Trop Med Hyg 90:167–168. https://doi.org/10.1016/S0035-9203(96)90124-8
Pincetic A, Bournazos S, Dilillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger BM, Ravetch JV (2014) Type i and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol 15:707–716. https://doi.org/10.1038/ni.2939
Pučić M, Knežević A, Vidič J, Adamczyk B, Novokmet M, Polašek O, Gornik O, Šupraha-Goreta S, Wormald MR, Redžic I, Campbell H, Wright A, Hastie ND, Wilson JF, Rudan I, Wuhrer M, Rudd PM, Josić D, Lauc G (2011) High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics 10:M111.010090–M111.010090. https://doi.org/10.1074/mcp.M111.010090
Qian Y, Wang Y, Zhang X, Zhou L, Zhang Z, Xu J, Ruan Y, Ren S, Xu C, Gu J (2013) Quantitative analysis of serum IgG galactosylation assists differential diagnosis of ovarian cancer. J Proteome Res 12:4046–4055. https://doi.org/10.1021/pr4003992
Radcliffe CM, Arnold JN, Suter DM, Wormald MR, Harvey DJ, Royle L, Mimura Y, Kimura Y, Sim RB, Inogès S, Rodriguez-Calvillo M, Zabalegui N, De Cerio ALD, Potter KN, Mockridge CI, Dwek RA, Bendandi M, Rudd PM, Stevenson FK (2007) Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B-cell receptor. J Biol Chem 282:7405–7415. https://doi.org/10.1074/jbc.M602690200
Rademacher TW, Parekh RB, Dwek RA, Isenberg D, Rook G, Axford JS, Roitt I (1988) The role of IgG glycoforms in the pathogenesis of rheumatoid arthritis. Springer Semin Immunopathol 10:231–249. https://doi.org/10.1007/BF01857227
Reusch D, Tejada ML (2015) Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology 25:1325–1334. https://doi.org/10.1093/glycob/cwv065
Rombouts Y, Ewing E, Van De Stadt LA, Selman MHJ, Trouw LA, Deelder AM, Huizinga TWJ, Wuhrer M, Van Schaardenburg D, Toes REM, Scherer HU (2015) Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis 74:234–241. https://doi.org/10.1136/annrheumdis-2013-203565
Rombouts Y, Willemze A, Van Beers JJBC, Shi J, Kerkman PF, Van Toorn L, Janssen GMC, Zaldumbide A, Hoeben RC, Pruijn GJM, Deelder AM, Wolbink G, Rispens T, Van Veelen PA, Huizinga TWJ, Wuhrer M, Trouw LA, Scherer HU, Toes REM (2016) Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann Rheum Dis 75:578–585. https://doi.org/10.1136/annrheumdis-2014-206598
Rook GAW, Onyebujoh P, Wilkins E, Ly HM, Al Attiyah R, Bahr G, Corrah T, Hernandez H, Stanford JL (1994) A longitudinal study of per cent agalactosyl IgG in tuberculosis patients receiving chemotherapy, with or without immunotherapy. Immunology 81:149–154
Williams RC, Osterland CK, Margherita S, Tokuda S, Messner RP (1973) Studies of biologic and serologic activities of rabbit IgG antibody depleted of carbohydrate residues. J Immunol 111:1690–1698
Yamaguchi Y, Barb AW (2020) A synopsis of recent developments defining how N-glycosylation impacts immunoglobulin G structure and function. Glycobiology 30:214–225. https://doi.org/10.1093/glycob/cwz068
Shade K-T, Anthony R (2013) Antibody glycosylation and inflammation. Antibodies 2:392–414. https://doi.org/10.3390/antib2030392
van de Bovenkamp FS, Hafkenscheid L, Rispens T, Rombouts Y (2016) The emerging importance of IgG Fab glycosylation in immunity. J Immunol 196:1435–1441. https://doi.org/10.4049/jimmunol.1502136
van de Bovenkamp FS, Derksen NIL, van Breemen MJ, de Taeye SW, Ooijevaar-de Heer P, Sanders RW, Rispens T (2018a) Variable domain N-linked glycans acquired during antigen-specific immune responses can contribute to immunoglobulin G antibody stability. Front Immunol 9:740. https://doi.org/10.3389/fimmu.2018.00740
Wu SJ, Luo J, O’Neil KT, Kang J, Lacy ER, Canziani G, Baker A, Huang M, Tang QM, Raju TS, Jacobs SA, Teplyakov A, Gilliland GL, Feng Y (2010) Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng Des Sel 23:643–651. https://doi.org/10.1093/protein/gzq037
Wright A, Tao MH, Kabat EA, Morrison SL (1991) Antibody variable region glycosylation: position effects on antigen binding and carbohydrate structure. EMBO J 10:2717–2723. https://doi.org/10.1002/j.1460-2075.1991.tb07819.x
Wada R, Matsui M, Kawasaki N (2019) Influence of N-glycosylation on effector functions and thermal stability of glycoengineered IgG1 monoclonal antibody with homogeneous glycoforms. MAbs 11:350–372. https://doi.org/10.1080/19420862.2018.1551044
Vidarsson G, Dekkers G, Rispens T (2014) IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5:520. https://doi.org/10.3389/fimmu.2014.00520
Štambuk J, Nakić N, Vučković F, Pučić-Baković M, Razdorov G, Trbojević-Akmačić I, Novokmet M, Keser T, Vilaj M, Štambuk T, Gudelj I, Šimurina M, Song M, Wang H, Salihović MP, Campbell H, Rudan I, Kolčić I, Eller LA, McKeigue P, Robb ML, Halfvarson J, Kurtoglu M, Annese V, Škarić-Jurić T, Molokhia M, Polašek O, Hayward C, Kibuuka H, Thaqi K, Primorac D, Gieger C, Nitayaphan S, Spector T, Wang Y, Tillin T, Chaturvedi N, Wilson JF, Schanfield M, Filipenko M, Wang W, Lauc G (2020) Global variability of the human IgG glycome. Aging (Albany NY) 12:1–13. https://doi.org/10.18632/AGING.103884
Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Gloria Meng Y, Weikert SHA, Presta LG (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem 277:26733–26740. https://doi.org/10.1074/jbc.M202069200
Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K (2003) The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 278:3466–3473. https://doi.org/10.1074/jbc.M210665200
Schuster M, Umana P, Ferrara C, Brünker P, Gerdes C, Waxenecker G, Wiederkum S, Schwager C, Loibner H, Himmler G, Mudde GC (2005) Improved effector functions of a therapeutic monoclonal Lewis Y-specific antibody by glycoform engineering. Cancer Res 65:7934–7941. https://doi.org/10.1158/0008-5472.CAN-04-4212
Umaña P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE (1999) Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol 17:176–180. https://doi.org/10.1038/6179
Subedi GP, Barb AW (2016) The immunoglobulin G1 N-glycan composition affects binding to each low affinity Fc γ receptor. MAbs 8:1512–1524. https://doi.org/10.1080/19420862.2016.1218586
Schwab I, Nimmerjahn F (2013) Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol 13:176–189. https://doi.org/10.1038/nri3401
Schwab I, Biburger M, Krönke G, Schett G, Nimmerjahn F (2012) IVIg-mediated amelioration of ITP in mice is dependent on sialic acid and SIGNR1. Eur J Immunol 42:826–830. https://doi.org/10.1002/eji.201142260
Washburn N, Schwabb I, Ortiz D, Bhatnagar N, Lansing JC, Medeiros A, Tyler S, Mekala D, Cochran E, Sarvaiya H, Garofalo K, Meccariello R, Meador JW, Rutitzky L, Schultes BC, Ling L, Avery W, Nimmerjahn F, Manning AM, Kaundinya GV, Bosques CJ (2015) Controlled tetra-fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc Natl Acad Sci USA 112:E1297–E1306. https://doi.org/10.1073/pnas.1422481112
Schwab I, Mihai S, Seeling M, Kasperkiewicz M, Ludwig RJ, Nimmerjahn F (2014) Broad requirement for terminal sialic acid residues and FcγRIIB for the preventive and therapeutic activity of intravenous immunoglobulins in vivo. Eur J Immunol 44:1444–1453. https://doi.org/10.1002/eji.201344230
Temming AR, Dekkers G, van de Bovenkamp FS, Plomp HR, Bentlage AEH, Szittner Z, Derksen NIL, Wuhrer M, Rispens T, Vidarsson G (2019) Human DC-SIGN and CD23 do not interact with human IgG. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-46484-2
Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV (2013) General mechanism for modulating immunoglobulin effector function. Proc Natl Acad Sci USA 110:9868–9872. https://doi.org/10.1073/pnas.1307864110
Schneider D, Von Minden MD, Alkhatib A, Setz C, Van Bergen CAM, Benkißer-Petersen M, Wilhelm I, Villringer S, Krysov S, Packham G, Zirlik K, Römer W, Buske C, Stevenson FK, Veelken H, Jumaa H (2015) Lectins from opportunistic bacteria interact with acquired variable-region glycans of surface immunoglobulin in follicular lymphoma. Blood 125:3287–3296. https://doi.org/10.1182/blood-2014-11-609404
Wallick SC, Kabat EA, Morrison SL (1988) Glycosylation of a VH residue of a monoclonal antibody against α(1→6) dextran increases its affinity for antigen. J Exp Med 168:1099–1109. https://doi.org/10.1084/jem.168.3.1099
Tachibana H, Kim JY, Shirahata S (1997) Building high affinity human antibodies by altering the glycosylation on the light chain variable region in N-acetylglucosamine-supplemented hybridoma cultures. Cytotechnology 23:151–159. https://doi.org/10.1023/a:1007980032042
Van De Bovenkamp FS, Derksen NIL, Ooijevaar-de Heer P, Van Schie KA, Kruithof S, Berkowska MA, Ellen van der Schoot C, IJspeert H, Van Der Burg M, Gils A, Hafkenscheid L, Toes REM, Rombouts Y, Plomp R, Wuhrer M, Marieke van Ham S, Vidarsson G, Rispens T (2018b) Adaptive antibody diversification through N-linked glycosylation of the immunoglobulin variable region. Proc Natl Acad Sci U S A 115:1901–1906. https://doi.org/10.1073/pnas.1711720115
Wiedeman AE, Santer DM, Yan W, Miescher S, Käsermann F, Elkon KB (2013) Contrasting mechanisms of interferon-α inhibition by intravenous immunoglobulin after induction by immune complexes versus toll-like receptor agonists. Arthritis Rheum 65:2713–2723. https://doi.org/10.1002/art.38082
Séïté JF, Cornec D, Renaudineau Y, Youinou P, Mageed RA, Hillion S (2010) IVIg modulates BCR signaling through CD22 and promotes apoptosis in mature human B lymphocytes. Blood 116:1698–1704. https://doi.org/10.1182/blood-2009-12-261461
Séïté JF, Goutsmedt C, Youinou P, Pers JO, Hillion S (2014) Intravenous immunoglobulin induces a functional silencing program similar to anergy in human B cells. J Allergy Clin Immunol 133. https://doi.org/10.1016/j.jaci.2013.08.042
Yu X, Wang Y, Kristic J, Dong J, Chu X, Ge S, Wang H, Fang H, Gao Q, Liu D, Zhao Z, Peng H, Bakovic MP, Wu L, Song M, Rudan I, Campbell H, Lauc G, Wang W (2016) Profiling IgG N-glycans as potential biomarker of chronological and biological ages: a community-based study in a Han Chinese population. Med (United States) 95:e4112. https://doi.org/10.1097/MD.0000000000004112
Zaitseva O, Krištić J, Jansen B, Stojković R, Pezer M, Morahan G, Lauc G (2018) Fc-linked N-glycosylation of murine IgG1 variants. In: 2nd GlycoCom 1st Hum. Glycome Project Meeting, pp 62–63
Yu X, Baruah K, Harvey DJ, Vasiljevic S, Alonzi DS, Song BD, Higgins MK, Bowden TA, Scanlan CN, Crispin M (2013) Engineering hydrophobic protein-carbohydrate interactions to fine-tune monoclonal antibodies. J Am Chem Soc 135:9723–9732. https://doi.org/10.1021/ja4014375
Wang J, Balog CIA, Stavenhagen K, Koeleman CAM, Scherer HU, Selman MHJ, Deelder AM, Huizinga TWJ, Toes REM, Wuhrer M (2011) Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol Cell Proteomics 10:M110 004655. https://doi.org/10.1074/mcp.M110.004655
Tijardović M, Marijančević D, Bok D, Kifer D, Lauc G, Gornik O, Keser T (2019) Intense physical exercise induces an anti-inflammatory change in IgG N-glycosylation profile. Front Physiol 10:1522. https://doi.org/10.3389/fphys.2019.01522
Sarin HV, Gudelj I, Honkanen J, Ihalainen JK, Vuorela A, Lee JH, Jin Z, Terwilliger JD, Isola V, Ahtiainen JP, Häkkinen K, Jurić J, Lauc G, Kristiansson K, Hulmi JJ, Perola M (2019) Molecular pathways mediating immunosuppression in response to prolonged intensive physical training, low-energy availability, and intensive weight loss. Front Immunol 10:907. https://doi.org/10.3389/fimmu.2019.00907
Trbojević-Akmačić I, Vilaj M, Lauc G (2016) High-throughput analysis of immunoglobulin G glycosylation. Expert Rev Proteomics 13:523–534. https://doi.org/10.1080/14789450.2016.1174584
Trbojević-Akmačić I, Ugrina I, Lauc G (2017) Comparative analysis and validation of different steps in glycomics studies. Methods Enzymol 586:37–55. https://doi.org/10.1016/bs.mie.2016.09.027
Singh SS, Heijmans R, Meulen CKE, Lieverse AG, Gornik O, Sijbrands EJG, Lauc G, Van Hoek M (2020) Association of the IgG N-glycome with the course of kidney function in type 2 diabetes. BMJ Open Diabetes Res Care 8:e001026. https://doi.org/10.1136/bmjdrc-2019-001026
Šimurina M, de Haan N, Vučković F, Kennedy NA, Štambuk J, Falck D, Trbojević-Akmačić I, Clerc F, Razdorov G, Khon A, Latiano A, D’Incà R, Danese S, Targan S, Landers C, Dubinsky M, Campbell H, Zoldoš V, Permberton IK, Kolarich D, Fernandes DL, Theorodorou E, Merrick V, Spencer DI, Gardner RA, Doran R, Shubhakar A, Boyapati R, Rudan I, Lionetti P, Krištić J, Novokmet M, Pučić-Baković M, Gornik O, Andriulli A, Cantoro L, Sturniolo G, Fiorino G, Manetti N, Arnott ID, Noble CL, Lees CW, Shand AG, Ho GT, Dunlop MG, Murphy L, Gibson J, Evenden L, Wrobel N, Gilchrist T, Fawkes A, Kammeijer GSM, Vojta A, Samaržija I, Markulin D, Klasić M, Dobrinić P, Aulchenko Y, van den Heuve T, Jonkers D, Pierik M, McGovern DPB, Annese V, Wuhrer M, Lauc G (2018) Glycosylation of immunoglobulin G associates with clinical features of inflammatory bowel diseases. Gastroenterology 154:1320–1333.e10. https://doi.org/10.1053/j.gastro.2018.01.002
Theodoratou E, Thaçi K, Agakov F, Timofeeva MN, Stambuk J, Pueìc-Bakovic M, Vuèkovic F, Orchard P, Agakova A, Din FVN, Brown E, Rudd PM, Farrington SM, Dunlop MG, Campbell H, Lauc G (2016) Glycosylation of plasma IgG in colorectal cancer prognosis. Sci Rep 6:28098. https://doi.org/10.1038/srep28098
Wahl A, van den Akker E, Klaric L, Štambuk J, Benedetti E, Plomp R, Razdorov G, Trbojević-Akmačić I, Deelen J, van Heemst D, Eline Slagboom P, Vučković F, Grallert H, Krumsiek J, Strauch K, Peters A, Meitinger T, Hayward C, Wuhrer M, Beekman M, Lauc G, Gieger C (2018) Genome-wide association study on immunoglobulin G glycosylation patterns. Front Immunol 9:277. https://doi.org/10.3389/fimmu.2018.00277
Vilaj M, Gudelj I, Trbojević-Akmačić I, Lauc G, Pezer M (2019) IgG glycans as a biomarker of biological age. In: Moskalev A (ed) Biomarkers hum aging. Springer, New York, pp 81–99. https://doi.org/10.1007/978-3-030-24970-0_7
Westhrin M, Kovcic V, Zhang Z, Moen SH, Vikene Nedal TM, Bondt A, Holst S, Misund K, Buene G, Sundan A, Waage A, Slørdahl TS, Wuhrer M, Standal T (2020) Monoclonal immunoglobulins promote bone loss in multiple myeloma. Blood 136:2656–2666. https://doi.org/10.1182/BLOOD.2020006045
Zhu D, McCarthy H, Ottensmeier CH, Johnson P, Hamblin TJ, Stevenson FK (2002) Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood 99:2562–2568. https://doi.org/10.1182/blood.V99.7.2562
Zhu D, Ottensmeier CH, Du MQ, McCarthy H, Stevenson FK (2003) Incidence of potential glycosylation sites in immunoglobulin variable regions distinguishes between subsets of Burkitt’s lymphoma and mucosa-associated lymphoid tissue lymphoma. Br J Haematol 120:217–222. https://doi.org/10.1046/j.1365-2141.2003.04064.x
Wang TT, Sewatanon J, Memoli MJ, Wrammert J, Bournazos S, Bhaumik SK, Pinsky BA, Chokephaibulkit K, Onlamoon N, Pattanapanyasat K, Taubenberger JK, Ahmed R, Ravetch JV (2017) IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 355:395–398. https://doi.org/10.1126/science.aai8128
Thulin NK, Brewer RC, Sherwood R, Bournazos S, Edwards KG, Ramadoss NS, Taubenberger JK, Memoli M, Gentles AJ, Jagannathan P, Zhang S, Libraty DH, Wang TT (2020) Maternal anti-dengue igg fucosylation predicts susceptibility to dengue disease in infants. Cell Rep 31. https://doi.org/10.1101/565259. Accessed 24 Apr 2021
Sonneveld ME, Natunen S, Sainio S, Koeleman CAM, Holst S, Dekkers G, Koelewijn J, Partanen J, van der Schoot CE, Wuhrer M, Vidarsson G (2016) Glycosylation pattern of anti-platelet IgG is stable during pregnancy and predicts clinical outcome in alloimmune thrombocytopenia. Br J Haematol 174:310–320. https://doi.org/10.1111/bjh.14053
Sonneveld ME, Koelewijn J, de Haas M, Admiraal J, Plomp R, Koeleman CAM, Hipgrave Ederveen AL, Ligthart P, Wuhrer M, van der Schoot CE, Vidarsson G (2017a) Antigen specificity determines anti-red blood cell IgG-Fc alloantibody glycosylation and thereby severity of haemolytic disease of the fetus and newborn. Br J Haematol 176:651–660. https://doi.org/10.1111/bjh.14438
Sénard T, Flouri I, Vučković F, Papadaki G, Goutakoli P, Pučić Baković M, Pezer M, Bertsias G, Lauc G, Sidiropoulos P (2021) Baseline IgG-Fc N-glycosylation profile is associated with long-term outcome in a cohort of early inflammatory arthritis patients (Submitted)
Van Zeben D, Rook GAW, Hazes JMW, Zwinderman AH, Zhang Y, Ghelani S, Rademacher TW, Breedveld FC (1994) Early agalactosylation of IGG is associated with a more progressive disease course in patients with rheumatoid arthritis: results of a follow-up study. Rheumatology 33:36–43. https://doi.org/10.1093/rheumatology/33.1.36
Váradi C, Holló Z, Póliska S, Nagy L, Szekanecz Z, Váncsa A, Palatka K, Guttman A (2015) Combination of IgG N-glycomics and corresponding transcriptomics data to identify anti-TNF-α treatment responders in inflammatory diseases. Electrophoresis 36:1330–1335. https://doi.org/10.1002/elps.201400575
Scherer HU, Van Der Woude D, Ioan-Facsinay A, El Bannoudi H, Trouw LA, Wang J, Häupl T, Burmester GR, Deelder AM, Huizinga TWJ, Wuhrer M, Toes REM (2010) Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum 62:1620–1629. https://doi.org/10.1002/art.27414
Vanderschaeghe D, Meuris L, Raes T, Grootaert H, Van Hecke A, Verhelst X, Van de Velde F, Lapauw B, Van Vlierberghe H, Callewaert N (2018) Endoglycosidase s enables a highly simplified clinical chemistry procedure for direct assessment of serum IgG u. Mol Cell Proteomics 17:2508–2517. https://doi.org/10.1074/mcp.TIR118.000740
Tomana M, Schrohenloher RE, Koopman WJ, Alarcän GS, Paul WA (1988) Abnormal glycosylation of serum IgG from patients with chronic inflammatory diseases. Arthritis Rheum 31:333–338. https://doi.org/10.1002/art.1780310304
Van de Geijn FE, Wuhrer M, Selman MHJ, Willemsen SP, de Man YA, Deelder AM, Hazes JMW, Dolhain RJEM (2009) Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther 11. https://doi.org/10.1186/ar2892
Young A, Sumar N, Bodman K, Goyal S, Sinclair H, Roitt I, Isenberg D (1991) Agalactosyl IgG: An aid to differential diagnosis in early synovitis. Arthritis Rheum 34:1425–1429. https://doi.org/10.1002/art.1780341113
Sumar N, Isenberg DA, Bodman KB, Soltys A, Young A, Leak AM, Round J, Hay FC, Roitt IM (1991) Reduction in IgG galactose in juvenile and adult onset rheumatoid arthritis measured by a lectin binding method and its relation to rheumatoid factor. Ann Rheum Dis 50:607–610. https://doi.org/10.1136/ard.50.9.607
Tomana M, Schrohenloher RE, Reveille JD, Arnett FC, Koopman WJ (1992) Abnormal galactosylation of serum IgG in patients with systemic lupus erythematosus and members of families with high frequency of autoimmune diseases. Rheumatol Int 12:191–194. https://doi.org/10.1007/BF00302151
Vučković F, Krištić J, Gudelj I, Teruel M, Keser T, Pezer M, Pučić-Baković M, Štambuk J, Trbojević-Akmačić I, Barrios C, Pavïc T, Menni C, Wang Y, Zhou Y, Cui L, Song H, Zeng Q, Guo X, Pons-Estel BA, McKeigue P, Patrick AL, Gornik O, Spector TD, Harjaček M, Alarcon-Riquelme M, Molokhia M, Wang W, Lauc G (2015) Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol 67:2978–2989. https://doi.org/10.1002/art.39273
Shinzaki S, Iijima H, Nakagawa T, Egawa S, Nakajima S, Ishii S, Irie T, Kakiuchi Y, Nishida T, Yasumaru M, Kanto T, Tsujii M, Tsuji S, Mizushima T, Yoshihara H, Kondo A, Miyoshi E, Hayashi N (2008) IgG oligosaccharide alterations are a novel diagnostic marker for disease activity and the clinical course of inflammatory bowel disease. Am J Gastroenterol 103:1173–1181. https://doi.org/10.1111/j.1572-0241.2007.01699.x
Trbojevic Akmacic I, Ventham NT, Theodoratou E, Vučković F, Kennedy NA, Krištić J, Nimmo ER, Kalla R, Drummond H, Štambuk J, Dunlop MG, Novokmet M, Aulchenko Y, Gornik O, Campbell H, Pučić Baković M, Satsangi J, Lauc G (2015) Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm Bowel Dis 21:1237–1247. https://doi.org/10.1097/MIB.0000000000000372
Wuhrer M, Stavenhagen K, Koeleman CAM, Selman MHJ, Harper L, Jacobs BC, Savage COS, Jefferis R, Deelder AM, Morgan M (2015) Skewed Fc glycosylation profiles of anti-proteinase 3 immunoglobulin G1 autoantibodies from granulomatosis with polyangiitis patients show low levels of bisection, galactosylation, and sialylation. J Proteome Res 14:1657–1665. https://doi.org/10.1021/pr500780a
Selman MHJ, Niks EH, Titulaer MJ, Verschuuren JJGM, Wuhrer M, Deelder AM (2011) IgG Fc N-glycosylation changes in Lambert-Eaton myasthenic syndrome and myasthenia gravis. J Proteome Res 10:143–152. https://doi.org/10.1021/pr1004373
Sonneveld ME, De Haas M, Koeleman C, De Haan N, Zeerleder SS, Ligthart PC, Wuhrer M, Van Der Schoot CE, Vidarsson G (2017b) Patients with IgG1-anti-red blood cell autoantibodies show aberrant Fc-glycosylation. Sci Rep 7:8187. https://doi.org/10.1038/s41598-017-08654-y
Sonneveld ME, Koeleman CAM, Plomp HR, Wuhrer M, van der Schoot CE, Vidarsson G (2018) Fc-glycosylation in human IgG1 and IgG3 is similar for both total and anti-red-blood cell anti-k antibodies. Front Immunol 9. https://doi.org/10.3389/fimmu.2018.00129
Saldova R, Royle L, Radcliffe CM, Hamid UMA, Evans R, Arnold JN, Banks RE, Hutson R, Harvey DJ, Antrobus R, Petrescu SM, Dwek RA, Rudd PM (2007) Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology 17:1344–1356. https://doi.org/10.1093/glycob/cwm100
Ruhaak LR, Kim K, Stroble C, Taylor SL, Hong Q, Miyamoto S, Lebrilla CB, Leiserowitz G (2016) Protein-specific differential glycosylation of immunoglobulins in serum of ovarian cancer patients. J Proteome Res 15:1002–1010. https://doi.org/10.1021/acs.jproteome.5b01071
Vučković F, Theodoratou E, Thaçi K, Timofeeva M, Vojta A, Štambuk J, Pučić-Baković M, Rudd PM, Derek L, Servis D, Wennerström A, Farrington SM, Perola M, Aulchenko Y, Dunlop MG, Campbell H, Lauc G (2016) IgG glycome in colorectal cancer. Clin Cancer Res 22:3078–3086. https://doi.org/10.1158/1078-0432.CCR-15-1867
Stockmann H, Coss KP, Rubio-Gozalbo ME, Knerr I, Fitzgibbon M, Maratha A, Wilson J, Rudd P, Treacy EP (2016) IgG N-glycosylation galactose incorporation ratios for the monitoring of classical galactosaemia. JIMD Rep 27:47–53. https://doi.org/10.1007/8904_2015_490
Wang Y, Klarić L, Yu X, Thaqi K, Dong J, Novokmet M, Wilson J, Polasek O, Liu Y, Krištić J, Ge S, Pučić-Baković M, Wu L, Zhou Y, Ugrina I, Song M, Zhang J, Guo X, Zeng Q, Rudan I, Campbell H, Aulchenko Y, Lauc G, Wang W (2016) The association between glycosylation of immunoglobulin G and hypertension. Med (United States) 95:e3379. https://doi.org/10.1097/MD.0000000000003379
Zhao ZY, Liu D, Cao WJ, Sun M, Song MS, Wang W, Wang YX (2018) Association between IgG N-glycans and nonalcoholic fatty liver disease in Han Chinese. Biomed Environ Sci 31:454–458. https://doi.org/10.3967/bes2018.059
Wu Z, Pan H, Liu D, Zhou D, Tao L, Zhang J, Wang X, Li X, Wang Y, Wang W, Guo X (2021) Variation of IgG N-linked glycosylation profile in diabetic retinopathy. J Diabetes. https://doi.org/10.1111/1753-0407.13160
Wuhrer M, Porcelijn L, Kapur R, Koeleman CAM, Deelder AM, De Haas M, Vidarsson G (2009) Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens. J Proteome Res 8:450–456. https://doi.org/10.1021/pr800651j
van Erp EA, Lakerveld AJ, de Graaf E, Larsen MD, Schepp RM, Hipgrave Ederveen AL, Ahout IML, de Haan CAM, Wuhrer M, Luytjes W, Ferwerda G, Vidarsson G, van Kasteren PB (2020) Natural killer cell activation by respiratory syncytial virus-specific antibodies is decreased in infants with severe respiratory infections and correlates with Fc-glycosylation. Clin Transl Immunol 9. https://doi.org/10.1002/cti2.1112
Russell AC, Šimurina M, Garcia MT, Novokmet M, Wang Y, Rudan I, Campbell H, Lauc G, Thomas MG, Wang W (2017) The N-glycosylation of immunoglobulin G as a novel biomarker of Parkinson’s disease. Glycobiology 27:501–510. https://doi.org/10.1093/glycob/cwx022
Vadrevu SK, Trbojevic-Akmacic I, Kossenkov AV, Colomb F, Giron LB, Anzurez A, Lynn K, Mounzer K, Landay AL, Kaplan RC, Papasavvas E, Montaner LJ, Lauc G, Abdel-Mohsen M (2018) Frontline science: plasma and immunoglobulin G galactosylation associate with HIV persistence during antiretroviral therapy. J Leukoc Biol 104:461–471. https://doi.org/10.1002/JLB.3HI1217-500R
Zhang X, Yuan H, Lyu J, Meng X, Tian Q, Li Y, Zhang J, Xu X, Su J, Hou H, Li D, Sun B, Wang W, Wang Y (2021) Association of dementia with immunoglobulin G N-glycans in a Chinese Han population. Npj Aging Mech Dis 7. https://doi.org/10.1038/s41514-021-00055-w
Ruhaak LR, Nguyen UT, Stroble C, Taylor SL, Taguchi A, Hanash SM, Lebrilla CB, Kim K, Miyamoto S (2013) Enrichment strategies in glycomics-based lung cancer biomarker development. Proteomics – Clin Appl 7:664–676. https://doi.org/10.1002/prca.201200131
Sjöwall C, Zapf J, Von Löhneysen S, Magorivska I, Biermann M, Janko C, Winkler S, Bilyy R, Schett G, Herrmann M, Muñoz LE (2015) Altered glycosylation of complexed native IgG molecules is associated with disease activity of systemic lupus erythematosus. Lupus 24:569–581. https://doi.org/10.1177/0961203314558861
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Funding
This work was supported by the European Structural and Investment Funds CEKOM (Grant# KK.01.2.2.03.0006).
Conflict of Interest
MP is an employee of Genos Ltd.—a private company that specializes in high-throughput glycomic analysis and has several patents in the field, and of Genos Glycoscience Ltd.—a spin-off of Genos Ltd. that commercializes its scientific discoveries.
Ethical Approval
This article does not contain any studies with human participants.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pezer, M. (2021). Immunoglobulin G Glycosylation in Diseases. In: Pezer, M. (eds) Antibody Glycosylation. Experientia Supplementum, vol 112. Springer, Cham. https://doi.org/10.1007/978-3-030-76912-3_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-76912-3_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-76911-6
Online ISBN: 978-3-030-76912-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)