Abstract
Amino acids are integral for human health, influencing an array of physiological processes from gene expression to vasodilation to the immune response. In accordance with this expansive range of unique functions, the tissues of the body engage in a complex interplay of amino acid exchange and metabolism to respond to the organism’s dynamic needs for a range of nitrogenous products. Interorgan amino acid metabolism is required for numerous metabolic pathways, including the synthesis of functional amino acids like arginine, glutamate, glutamine, and glycine. This physiological process requires the cooperative handling of amino acids by organs (e.g., the small intestine, skeletal muscle, kidneys, and liver), as well as the complete catabolism of nutritionally essential amino acids such as the BCAAs, with their α-ketoacids shuttled from muscle to liver. These exchanges are made possible by several mechanisms, including organ location, as well as the functional zonation of enzymes and the cell-specific expression of amino acid transporters. The cooperative handling of amino acids between the various organs does not appear to be under the control of any centralized regulation, but is instead influenced by factors such as fluctuations in nutrient availability, hormones, changes associated with development, and altered environmental factors. While the normal function of these pathways is associated with health and homeostasis, affected by physical activity, diet and body composition, dysregulation is observed in numerous disease states, including cardiovascular disease and cancer cachexia, presenting potential avenues for the manipulation of amino acid consumption as part of the therapeutic approach to these conditions in individuals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Amino acids are integral to human nutrition and health. These nitrogen containing compounds are not just the building blocks for proteins, but serve a wide range of roles in numerous physiological processes, including cell signaling, gene expression, DNA synthesis, immune response, and nutrient intake and metabolism (Manjarín et al. 2020; Wu 2009). As such, adequate intake, handling, and exchange of amino acids are critical for proper function of virtually all tissues and organ systems, with the disorders associated with their deprivation also being legion.
Although it was once thought that most cells handled amino acids by similar means, research into amino acid nutrition and biochemistry has revealed quite the opposite (Wu 2013a). Each organ within the body is responsible for carrying out specific tasks that are critical to overall homeostasis, including unique roles in the cooperative metabolism of amino acids. This variability in metabolism gives rise to a sophisticated interplay of amino acid exchange among differentiated tissues, with the characteristic synthesis or degradation of amino acids in some organs supplying the needs of others or the participation of multiple organs in a cohesive pathway to synthesize or degrade nitrogenous compounds (Brosnan 2003; Marliss et al. 1971). These processes have vast and wide-ranging implications for amino acids in health and disease, underlying processes from branched-chain amino acid catabolism to creatine biosynthesis. In light of the importance of interorgan cooperativity regarding amino acid metabolism, this review will summarize the important concepts and current knowledge of interorgan amino acid metabolism in physiology, the means by which these processes are regulated, and how the cooperative handling of amino acids by interorgan transport can be disrupted in various disease states.
8.2 Organ-Specific and Interorgan Amino Acid Metabolism
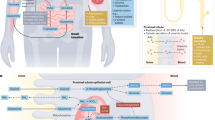
Since amino acids serve a much wider range of functions than just substrates for protein synthesis, their metabolism within the body is of paramount importance, as evidenced by the wide array of enzymes and metabolons available to handle nitrogenous products (Wu 2013a). Far from the previous view that amino acids were passively taken up by the digestive system and solely metabolized by the liver, it is now well-appreciated that amino acid metabolism varies widely across organs, with tissues degrading, metabolizing, and synthesizing amino acids in varied ways based on characteristic expression of cellular enzymes. This heterogeneous expression of enzymes and distinct pattern of amino acid release gives rise to the coordinated interorgan amino metabolism that is crucial for health and homeostasis (Fig. 1).
-
(a)
Small Intestine
As the principal site of nutrient absorption and a major site of amino acid metabolism, the small intestine plays a critical role that ultimately allows dietary amino acids into the bloodstream, providing the only route (short of intravenous infusion) by which the internal organs are supplied with exogenous amino acids (Beaumont and Blachier 2020). However, it should be noted that the first important exchange of nutrient molecules between two unique cell types is not, in fact, between two different organs, but an inter-species metabolic exchange of numerous compounds between the gut microbiome and the enterocytes of the small intestine (Nicholson et al. 2012). These microorganisms generate a number of compounds as a result of their processing of nutrients that can influence host metabolism; among which are the short-chain and branched-chain fatty acids, choline, phenols, polyamines, and some vitamins, including several of the B-complex vitamins, such as riboflavin (vitamin B2), biotin (vitamin B7), folate (vitamin B9), pyridoxine (vitamin B6), and cobalamin (vitamin B12) (Nicholson et al. 2012; LeBlanc et al. 2013). While the microbiota in the digestive tract catabolize certain dietary amino acids, thereby affecting the composition of nitrogenous substances absorbed by the gut mucosa, their capacity to supply amino acids to the enterocyte and ultimately to meet the nutritional requirements of the host remains controversial, with some evidence suggesting that the gut microbiota at least contribute lysine to the portal circulation (Dai et al. 2011). However, because non-ruminant species still exhibit deficiencies of amino acid metabolism when fed a protein-free diet, the microbiome is clearly not capable of meeting all of the amino acid demands of the host (Dai et al. 2011). In light of these findings, the contribution of the gut flora to endogenous amino acid concentrations remains an area of active study.
It has been demonstrated, however, that the microbiome influences nitrogen metabolism within the host organism through a less direct means, namely through the production of metabolites from digested amino acids. All of the aforementioned B vitamins play a role in nitrogenous metabolism within the host, including the metabolism of sulfur-containing amino acids, the catabolism of the branched-chain amino acids, and reduction of glutathione, a tripeptide involved in protection against oxidative stress (Stipanuk 2004a; Tong 2013; Ashoori and Saedisomeolia 2014). In this way, it appears that the microbiome, while not directly involved in supplying amino acids to the host, still plays a role in the host’s handling of amino acids by supplying the vitamins required for amino acid metabolism.
Although it was once thought that amino acids absorbed by the small intestine entered the portal circulation largely intact, it has now been conclusively demonstrated that this is not the case. Indeed, the enterocytes catabolize nearly all of the dietary glutamate and aspartate taken up from the intestinal lumen, along with roughly two-thirds of the dietary glutamine (Wu 1998). Further, as much as 30–50% of the arginine, methionine, lysine, threonine, glycine, serine, leucine, isoleucine, and valine absorbed in the small intestine are catabolized and unavailable to extraintestinal tissues (Wu 1998). The extensive use of glutamine and glutamate fulfills several functions in the small intestine. Glutamate is the principal energetic substrate for the enterocyte, sparing glucose for use by the rest of the body’s tissues and playing a role in gut-brain signaling (Reeds et al. 2000; Brosnan and Brosnan 2013). Glutamine is crucial for many separate processes in the small intestinal mucosa, including maintenance of intestinal integrity via tight junctions and regulation of mucosal cell growth (Krishna Rao 2012; Wang et al. 2015). A key role that both of these amino acids share, however, is serving as a precursor for the de novo synthesis of several critical amino acids produced in the small intestine.
A prime example of the small intestine’s role in interorgan amino acid metabolism involves the synthesis of citrulline. The work of Windmueller and Spaeth demonstrated that enterocytes can synthesize citrulline from glutamine, serving as the major source of de novo citrulline synthesis (Windmueller and Spaeth 1981). This citrulline is then transported out of the small intestine into the portal circulation, where it bypasses the liver, before being largely taken up by the kidneys to be used for arginine biosynthesis (Windmueller and Spaeth 1981; Curis et al. 2005). Since this seminal discovery, further investigations have revealed that other amino acids that are catabolized by the small intestine can be used for citrulline synthesis, including proline and glutamate (Wu 1997; Reeds et al. 2000). Because the small intestine is almost the exclusive source of endogenous citrulline in humans, pathologies of the small intestine such as atrophy or resection lead to decrements in citrulline synthesis, ultimately with systemic consequences (Wakabayashi et al. 1994; Crenn et al. 2008).
Unlike other organs, the small intestine generally does not take up amino acids from the circulation (through basolateral transporters) as flux in this direction would be rather at odds with the digestive tract’s role in providing the organism with dietary acquired substrate. One exception, however, is glutamine, which is the only amino acid that is both taken up and oxidized by the small intestine in the post-absorptive state (Windmueller and Spaeth 1974; Wu et al. 1994a). This glutamine uptake is made possible through several sodium-dependent cotransporters, the most important being SNAT1, SNAT2, SNAT3, SNAT4, SNAT5, and ATB0/ASCT2 (Rhoads and Wu 2009). Due in large part to its size, under healthy conditions, the small intestine is one of the principle organs responsible for utilizing plasma glutamine in the post-absorptive state, relying on this amino acid as a fuel source and substrate for continued citrulline synthesis (Watford 2008). The importance of glutamine to intestinal health is further evidenced by studies showing the role of both oral and intravenous infusion of this amino acid in modulating commensal microbiota, innate intestinal immunity, and intestinal integrity (Peng et al. 2004; Ren et al. 2014).
-
(b)
Liver
It has been long-established that the liver functions in regulating the circulating levels of substrates such as glucose and amino acids (Hers 1976; Hou et al. 2020), but it should also be noted that it is the first organ that receives blood from the intestinal tract. Therefore, the liver is the first organ exposed to the compounds that the enterocytes release into the circulation, including dietary and synthesized amino acids. With the exception of the branched-chain amino acids (BCAAs), hepatic tissue can catabolize virtually all of the amino acids and is critical for regulating the plasma levels of a number of so-called nutritionally non-essential AA, including aspartate, asparagine, glutamate, glutamine, cysteine, glycine, serine, and tyrosine from their respective precursors, along with non-proteogenic products such as taurine (Shimomura et al. 2001; Brosnan 2003; Stipanuk 2004b). Taurine provides an illustration of the involvement of the liver in interorgan amino acid metabolism; cysteine, a sulfur-containing amino acid, is critical to protein synthesis in a wide variety of tissues, but is cytotoxic in high concentrations. While cysteine dehydrogenase is expressed in the brain and kidney, its expression (positively regulated by dietary cysteine intake) is the highest in the liver, where it catalyzes the first step of taurine production (Stipanuk 2004b). Thus, the liver serves a two-fold role in cysteine metabolism, both purifying the blood of a potential toxic compound and synthesizing a metabolite with a wide range of functions in health, including immunity and oxidant defense.
There are some notable exceptions to the liver’s wide-ranging ability to process amino acids. First are the three aforementioned BCAAs (leucine, isoleucine, and valine), which are largely spared by liver metabolism due to a low level of BCAA transaminase activity (Harper et al. 1984; Brosnan and Brosnan 2006). However, the activity of the branched-chain α-keto acid (BCKA) dehydrogenase complex, the enzyme responsible for handling the product of BCAA transamination and the rate limiting step in overall BCAA catabolism, is much higher in the liver mitochondria than other tissues, making it an important site of oxidation for BCKAs produced elsewhere in the body (Brosnan and Brosnan 2006; Holeček 2018). The non-proteogenic amino acid citrulline also largely bypasses liver metabolism to be converted to arginine in the kidney (Papadia et al. 2018) This is a crucial process, as any arginine taken up by the liver is rapidly catabolized by hepatic type-1 arginase as part of the urea cycle (Curis et al. 2005; Papadia et al. 2018). Production of citrulline from proline, glutamine, or even dietary arginine thus provides a mechanism to spare arginine from liver metabolism, allowing the kidney to resynthesize and supply the rest of the organs with this critical amino acid by resynthesizing it from citrulline.
One of the most critical tasks of the liver is to detoxify the blood of ammonia, the metabolic product of amino acid catabolism. This is achieved via the hepatic urea cycle, characterized by Hans Krebs in the 1930s, where sequential action of several enzymes in the liver mitochondria and cytosol produce urea from ammonia and bicarbonate, utilizing ornithine, citrulline, and arginine as carriers at various stages (Morris 2002). Not only is the urea (or ornithine) cycle the first metabolic cycle discovered in physiology, it also provides a fundamental example of interorgan amino acid metabolism. While intracellular concentrations of ammonia can be relatively high, measurable in the micromolar range, plasma ammonia is quite low in comparison. In humans, elevation of circulating ammonia level from outside the normal range of 20–30 μM is enough to cause vomiting, nausea, and coma, with death occurring at higher concentrations (Wright et al. 2011; Jover-Cobos et al. 2014). In order to keep the plasma levels of ammonia at non-toxic levels, glutamine synthetase in organs such as the brain, kidney, and skeletal muscle can synthesize glutamine from ammonia and glutamate, with the glutamate serving as a carrier of the ammonia molecule (Hakvoort et al. 2017). In this way, extrahepatic tissues can use interorgan cooperativity to shuttle glutamine to the liver mitochondria for oxidation into NH3 and ultimately into urea, removing a potentially toxic by-product of amino acid metabolism from the body.
In its position at the center of organism-wide metabolism, one of the principal functions of the liver is to generate glucose (gluconeogenesis) and ketones (ketogenesis) from a number of precursors, including the glucogenic and ketogenic amino acids. These macromolecules are then used as energetic substrates by a wide array of extrahepatic tissues. As the liver largely plays the role of effector in controlling plasma glucose concentration, it obtains a great deal of energy from other sources, so as to “spare” glucose for other organs in the body. This includes amino acids, as up to 50% of the liver’s ATP requirement is met by the partial catabolism of amino acids (Brosnan 2000). This catabolism is only partial as the carbon skeletons procured from breakdown of gluconeogenic amino acids are utilized to produce glucose, thereby providing the liver with necessary ATP while also allowing it to supply other tissues with substrate for ATP production, maintaining glucose availability in the periphery.
The liver also provides an interesting example of functional zonation of amino acid metabolism within an organ. Hepatocytes are divided into two heterogenous groupings: periportal cells, which are associated with the portal vein (90–95% of hepatocytes), and perivenous cells, which are located near the hepatic vein (5–10%). At concentrations lower than 1 mM, extracellular glutamine is converted into glutamate and ammonia by glutaminase in the periportal cells, while the perivenous cells resynthesize glutamine from glutamate and ammonia by the action of glutamine synthase, in an intercellular glutamine–glutamate cycle (Watford 2000). At the cost of 1 mol of ATP per mol of glutamine turnover, this cycle allows the liver to scavenge NH3 from the plasma, maintaining a low circulating level of ammonia. Further, it allows for NH3 flux into either urea (under normal pH balance) or glutamine, a neutral amino acid, in order to regulate acid–base balance during conditions that would otherwise lead to acidosis.
Aside from the amino acids, the liver is critical in the interorgan metabolism of several nitrogenous products as well, including glutathione (GSH) and creatine. GSH is a tripeptide composed of glutamate, cysteine, and glycine and serves as the principle intracellular antioxidant in mammalian cells (Lu 2009). While all cell types are capable of synthesizing GSH, the liver is its chief producer and exporter. Plasma content of GSH is rather low when compared to intracellular concentration, largely due to the rapid action of γ-glutamyl transpeptidase on the exterior surface of the liver plasma membrane, which degrades GSH into γ-Glu-(Cys)2. The action of this enzyme plays a key role in interorgan GSH metabolism in two ways. First, degradation of GSH into γ-Glu-(Cys)2 circumvents the extremely unfavorable concentration gradient that exists between extracellular (5–50 μM) and intracellular (0.5–10 mM) GSH, allowing extrahepatic tissues including skeletal and cardiac muscle, the kidneys, and brain, to take up γ-Glu-(Cys)2 and Cys-Gly (derived from (Cys)2) and utilize both these compounds for GSH synthesis. Second, it allows for the transport of cysteine, which at high concentrations can be toxic, by converting into a safe form as γ-Glu-(Cys)2 (Wu et al. 2004). The role of the liver in interorgan creatine metabolism is different, where hepatic cells take up guanidinoacetate released by the kidneys and convert it to creatine via methylation by guanidinoacetate N-methyltransferase (Wyss and Kaddurah-Daouk 2000). The newly synthesized creatine is then exported into the plasma, where it is taken up by skeletal muscle (primarily) and other metabolically active tissues such as the brain. Thus, the liver can either serve as the origin for amino acids and related compounds or as part of a more complex network of interorgan cooperation to synthesize or degrade nitrogenous products.
-
(c)
Kidney
As mentioned previously, the kidney is not only the primary route for excretion of waste from the body, but is key in the interorgan handling of several amino acids and nitrogenous compounds (Griffith and Meister 1979; Li et al. 2020). Perhaps the best-studied interorgan kidney function is the aforementioned renal-intestinal arginine axis, whereby citrulline released by the small intestine is converted into arginine by the kidney (Dhanakoti et al. 1990; Brosnan and Brosnan 2004). While it may seem circuitous at first glance, the intestinal-renal arginine axis is critical in arginine metabolism, namely by sparing arginine from degradation by hepatic arginase. This mechanism allows for up to 85% of the citrulline released by the intestines to be taken up by the kidney and used for the synthesis of arginine via the sequential action of renal arginosuccinate synthetase and arginosuccinate lyase. This process is critical to whole-body arginine metabolism as arginine production from the kidney amounts to roughly 1.75 g/day of synthesized amino acid in healthy people, and the renal arginine output is sufficient to sustain the needs of all other tissues, provided there is an adequate supply of citrulline (Brosnan and Brosnan 2004).
One of the main fates of renal arginine is to be used in the synthesis of creatine, which participates in energy metabolism in the brain and skeletal muscle, and also serves as an antioxidant in cells (Wu 2020a). Creatine is synthesized from three amino acids, namely arginine, glycine, and methionine. Approximately 1.7% of the creatine in the body is converted to creatinine per day, a balance that must be replaced by both dietary replenishment of the creatine molecule or its precursors, and by de novo synthesis (Brosnan et al. 2011). Creatine synthesis is initiated in the kidney, where arginine: glycine amidotransferase catalyzes the production of guanidinoacetate and ornithine by transferring the amido group of arginine to the amine group of glycine. Guanidinoacetate is then exported from the kidney, after which it is taken up by the liver, converted into a functional creatine molecule, and released back into the circulation for use by highly metabolically active tissue such as the brain and skeletal muscle. Creatine synthesis, then, adds another layer of complexity upon the interorgan handling of arginine. A representative example of this complexity is citrulline, which is potentially acquired via several distinct catabolic pathways in the small intestine, is released into the portal vein, then bypasses the liver, and is taken up by the kidney. Virtually all the citrulline absorbed by the kidney is then converted to arginine, a significant portion of which is utilized in the synthesis of creatine, a process which must be finished in the liver before the final product can be exported to and utilized by the brain and peripheral tissues (Brosnan et al. 2011). Thus, the integrated cooperation of four distinct organs is required for the de novo production and use of creatine.
-
(d)
Skeletal Muscle
The discovery that human skeletal muscles releases a large amount of glutamine (Marliss et al. 1971) led to active research on interorgan metabolism of amino acids over the past 50 years. Although a great deal of the amino acids taken up by the skeletal muscle is incorporated into myofibrillar and other proteins, several enzymatic reactions take place in muscle cells that have important implications for whole-body amino acid metabolism (Wu 2013a). The handling of the BCAAs is a prime example; while they can be catabolized by most other tissues, with the liver being a notable exception, muscle is the major site for transamination of these amino acid in the body, largely due to its size [~40% and 45% of the body mass in young and adult humans, respectively (Sandoval et al. 2020)]. As such, skeletal muscle is responsible for roughly half of the BCAA catabolism in the organism. A.L. Goldberg and his colleagues were among the first to demonstrate that leucine, isoleucine, and valine can either undergo transamination into their respective branched-chain keto acids (α-ketoisocaproate, α-keto-β-methylvalerate, and α-ketoisovalerate, respectively), with their amino groups being used as a donor to form glutamate from α-ketoglutarate. This glutamate is then either transaminated with pyruvate to produce alanine or amidated to yield glutamine, making muscle a primary source of these amino acids in the post-absorptive state (Odessey and Goldberg 1972; Chang and Goldberg 1978a).
Although the mitochondria and cytoplasm are the primary sites of BCAA transamination in skeletal muscle, the BCKAs produced by this process are not degraded in the muscle due to low levels of BCKA dehydrogenase. Instead, these ketoacids are shuttled to the liver, where hepatic mitochondrial BCKA dehydrogenase decarboxylates the BCKAs to form acyl-CoA, or in the case of 5–10% of the α-ketoisocaproate, β-hydroxy-β-methylbutyrate (HMB). HMB can be used in the liver as a precursor of acetoacetate for cholesterol synthesis or released from the liver where it has a number of effects in other tissues, including skeletal muscle, where it stimulates protein synthesis and muscle growth (Nissen and Abumrad 1997; Zanchi et al. 2011). Thus, the cooperative action of the muscle and liver is critical not only to catabolize the BCAAs, but to the metabolism and function of both these organs (Fig. 2).
Cooperative AA Metabolism Between Liver and Muscle. The liver and skeletal muscle engage in multiple interorgan amino acid exchanges, including: the cooperative metabolism of the branched chain amino acids (BCAA) into branched chain keto acids (BCKA) and ultimately β-hydroxy-β-methylbutyrate (HMB) and the glucose-alanine (Cahill) cycle, which allows for clearance of muscle pyruvate and transport of ammonia to the liver using alanine as a carrier
Beyond BCAA oxidation, the skeletal muscle and liver engage in other forms of interorgan amino acid metabolism. Based on its metabolic activity and fiber type, skeletal muscle can either catabolize glucose into pyruvate for oxidation in the Krebs cycle in the mitochondria or into lactate, which can then be released into the circulation and taken up and converted to pyruvate by more oxidative muscle fibers, or circulated to the liver where it is utilized for gluconeogenesis in the Cori cycle. A similar phenomenon occurs in the glucose-alanine (the Cahill cycle), where the muscle uses blood glucose to produce ATP and pyruvate, with the pyruvate being transaminated with glutamate to form alanine (Felig 1973; Chang and Goldberg 1978b). This alanine is then converted to glucose and urea in the liver. Although there is no net production of glucose, the glucose-alanine cycle performs several important functions, including transporting ammonia to the liver in a non-toxic form, removing pyruvate from active skeletal muscle to allow continued flux through the mitochondrial electron transport chain and effectively converting muscle glycogen (which cannot be directly exported from muscle) to glucose in the liver via the recycling of end-products of glycolysis (Sarabhai and Roden 2019). This is physiologically significant for converting muscle glycogen into glucose in the body, because skeletal muscle lacks glucose-6-phosphatase.
-
(e)
Other Organs
While the tissues listed above are the principal components of interorgan cooperativity in amino acid handling, other cell types [e.g., endothelial cells (Durante 2020), as well as cells in the brain (He and Wu 2020), lungs (Chen et al. 2020), endocrine organs (Flynn et al. 2020), the reproductive tract (Gao 2020), the immune system (Ren et al. 2020), the skin (Solano 2020), and other sense organs (Wu 2020b)] are involved in specialized patterns of amino acid metabolism as well. The glutamine–glutamate shuttle in the brain is one interesting examples of interorgan amino acid handling by different cell types within the same tissue. Glutamate is a well-documented excitatory neurotransmitter in the brain, as well as a potential source for anaplerotic replenishment of tricarboxylic acid cycle intermediates (Meldrum 2000; Schousboe et al. 2014). After being released into the synaptic cleft by neurons, it can be rapidly taken up by neighboring astrocytes, where cell-specific glutamine synthetase converts the glutamate into glutamine. This glutamine is then transported back to the neuron, where neuronal phosphate-activated glutaminase converts it back to glutamate, where it can again be utilized as a neurotransmitter (Bélanger et al. 2011). The glutamine–glutamate shuttle, then, serves multiple functions, namely, to clear the synaptic cleft of an excitatory glutamate, preventing excitotoxicity, while also replenishing a critical neurotransmitter.
As the negative consequences of protein deprivation on the immune system have been well-documented, it should come as no surprise that a wide array of amino acids are used in immune cell function. Of these, the interorgan fluxes of glutamine and arginine are perhaps the most important (Li et al. 2007; Grohmann and Bronte 2010). As mentioned previously, skeletal muscle is the major source of the glutamine in the body, the bulk of which is produced through BCAA transamination. Lymphocytes, macrophages, and neutrophils oxidize glutamine in a process termed glutaminolysis to yield glutamate, and lesser amounts of aspartate, alanine, lactate, and pyruvate, providing these cells with substrate for nucleotide synthesis, GSH production, and nitric oxide (NO) generation (Ganeshan and Chawla 2014; Arts et al. 2016). The production of NO is reliant on the degradation of arginine to citrulline by NO synthase and important for immunity. As such, there is evidence to suggest that high concentrations of arginine (2 mM) may be needed for the maximum synthesis of NO by lymphocytes, macrophages, and monocytes (Wu et al. 2009). The demand of immune cells for glutamine and arginine, therefore, can put added stress on skeletal muscle and the kidneys, which are the main endogenous sources of these amino acids, respectively. While under conditions of eustress, muscle and renal output of these products is sufficient to meet interorgan demand, but under more stressful conditions, the supplementation of glutamine and arginine in the diet may be required to sustain a maximal immune response (Wu et al. 2009; Tan et al. 2009).
Finally, lactating tissue provides an interesting perspective on amino acid metabolism (Kim and Wu 2009; Wu et al. 2018). While virtually every other organ in the body is dedicated to preserving the health and homeostasis of the organism, the mammary glands are unique in that, and their role is to support the development of the offspring utilizing the resources of the mother. Thus, the role of the lactating tissue in supplying glutamine, proline, glycine, 4-hydroxyproline, and many more amino acids and proteins may be more accurately termed an “inter-organismal” amino acid metabolism, as this milk is the only source of nutrients for early neonatal growth (Li and Wu 2018; Wu et al. 2019). In order to support both milk production and to ensure an adequate amount of nutritive support to the neonate, the mammary glands actively degrade an extensive number of amino acids, including arginine and BCAAs (Kim and Wu 2009). This arginine serves multiple purposes promoting increased blood flow to the mammary tissue via NO production and providing polyamines that the neonate will use for growth, and proline that can later be converted back to arginine in the neonate’s small intestine, in an arginine–proline cycle (Wu et al. 2011).
8.3 Regulation and Control of Interorgan Amino Acid Metabolism
Despite decades of investigation into the cooperative handling of amino acids between organs, a centralized control mechanism for interorgan amino acid availability has yet to be identified. Instead, the handling of amino acids in various tissues is affected by a range of inputs, including nutrient availability, changes across the developmental span, hormones, and environmental factors. Although they are not under any apparent central control, the integrated handling of amino acids among the various systems of the body in response to these various perturbations serves to reinforce the importance of interorgan amino acid metabolism.
Nutrient Availability
Perhaps the only constant affecting all organs involved in amino acid trafficking is substrate availability. Both animal- and plant-source foods contain large amounts of BCAAs, glutamate, and glutamine, whereas the content of proline and glycine is much lower in plant- than in animal-sourced foods (Hou et al. 2019; Li and Wu 2020). Although many amino acids can be synthesized within the animal (the so-called nutritionally non-essential amino acids, NEAA), a number of them cannot, and so must be supplied exogenously (the nutritionally essential amino acids, EAA). For instance, the three BCAAs cannot be synthesized de novo by any organ in the human body, and so must be replenished from the diet or risk perturbations in the downstream signaling pathways they participate in, including skeletal muscle and liver metabolism (Brosnan and Brosnan 2006). On the other hand, while arginine can be produced de novo, the kidney is entirely dependent upon the release of citrulline from the small intestine for arginine synthesis; if this supply of citrulline is disrupted, renal arginine production will be severely reduced (Brosnan and Brosnan 2004). In this instance, the categorization of amino acids as either essential or non-essential can be somewhat a misnomer. For example, because arginine is synthesized from citrulline in the kidney and some other tissues, it has largely been categorized as an NEAA. However, under several pathological conditions, such as metabolic disease, endothelial dysfunction, and wound healing, endogenous arginine synthesis is insufficient to match physiological demand, and as such must be provided through the diet (Wu et al. 2009). This has led to the development of the category of “functional amino acids,” those that the organism is capable of producing, but are needed in greater quantities in times of stress or altered growth in order to support interorgan amino acid metabolism (Wu 2013b).
Development
As discussed previously, the characteristic expression of enzymes in a tissue is what gives rise to diverging abilities to process and metabolize amino acids. However, even within a cell type, this expression can change based on developmental status or external stressors. This phenomenon is readily apparent in shifts in porcine enterocyte enzyme expression after birth. Neonates are entirely dependent upon their mother’s milk for nutrients, which, as discussed previously, is rich in proline and glutamine, but remarkably deficient in arginine (Wu et al. 1994b; Wu 1995). As such, the enterocytes of baby pigs express an armamentarium of enzymes capable of synthesizing arginine from proline and glutamine, including P5C synthase, carbamoyl phosphate synthase, and arginosuccinate lyase. This allows the intestine of the pig to sustain its need for arginine during rapid post-partum growth, at least until renal arginosuccinate lyase can be expressed and the renal arginine-citrulline axis can be established. After early growth, the expression of these enzymes in the enterocyte wanes, and arginine production from citrulline is largely taken over by the kidney.
Endocrine
There should be little surprise that the array of hormones involved in controlling the body’s physiological processes will also have an effect on amino acid metabolism. As hormones serve to integrate and coordinate the response of tissues, their effects on interorgan amino acid metabolism are generally in line with their overall physiological functions. For example, insulin governs a wide range of cellular reactions to feeding, including the regulation of protein synthesis, and system A amino acid uptake (Pause et al. 1994; Hyde et al. 2002), in addition to its role in facilitating glucose disposal. Growth hormone has similar effects (Møller and Jørgensen 2009). In contrast, stress hormones such as glucocorticoids attenuate protein synthesis in peripheral tissues, increasing the availability of amino acids for release to the circulation and use for gluconeogenesis in the liver, while also increasing BCKA dehydrogenase expression to facilitate the use of the BCAAs as energetic substrates (Shah et al. 2000; Rose and Herzig 2013). Glucagon acts in a similar fashion, promoting hepatic uptake of amino acids. At first glance, it may seem odd that insulin and glucagon have the same effective effect on amino acid availability, namely the uptake of plasma amino acids by tissues (Flakoll et al. 1994). However, an organ-specific action of these hormones explains their functional difference; insulin is responsible for the deposition of amino acids as proteins in the periphery, namely the skeletal muscle, while glucagon promotes hepatic uptake of amino acids for use in gluconeogenesis. In this way, these two hormones balance the flow of amino acids in response to nutrient and endocrine signals.
Environment
Changes in an organism’s environment can also lead to alterations and compensations in interorgan amino acid metabolism. For example, under conditions of chronic acidosis, plasma glutamine is increased by up to two-fold, primarily due to skeletal muscle protein breakdown and consequent glutamine release (Taylor and Curthoys 2004). While the kidney does not normally filter large amounts of glutamine, under prolonged acidosis, up to 20% of the glutamine in the plasma is filtered into the lumen of the nephron, where much of it is absorbed into the proximal tubule. This glutamine is then utilized by the tubule epithelial cells to carry out gluconeogenesis via mitochondrial glutaminase and glutamine dehydrogenase, and cytosolic phosphoenolpyruvate carboxylase, the expression of which, is induced by acidosis (Welbourne 1987). This gluconeogenesis has the benefit of producing two molecules of bicarbonate, which are then released back into the plasma, restoring pH balance.
Another perturbation that alters amino acid metabolism is physical exercise. Along with the changes in energetics that come with muscular contraction (resulting in increased pyruvate availability for alanine synthesis), vasodilation of the muscle vasculature (mediated largely by NO derived from arginine) results in increased blood flow and therefore amino acid delivery to muscle (Wagenmakers 1998). As a consequence, muscular transamination of BCAAs, as well as alanine and glutamine release, increases during exercise. Further, exercise results in reductions in liver amino acid output, increased glucogenesis and ketogenesis (at higher intensities and durations), and increases in the expression of the hepatic BCKA dehydrogenase enzyme (Shimomura et al. 2004). These changes have been observed to be independent of the increased concentration of amino acids from the muscle, indicating that physical exercise may play a role in regulating interorgan amino acid metabolism independently of changes in amino acid flux (Argilés et al. 2016).
8.4 Health and Disease
The contribution of each organ to its role in interorgan amino acid metabolism is crucial to homeostatic metabolism and physiology. As may be expected, disruption in these and other carefully balanced processes carries severe implications for health which manifest with a number of pathological conditions.
-
a.
Cachexia
Dysregulation in metabolic activity and accelerated, unchecked cell growth are at the core of cancer pathology, so it is not surprising that amino acids plays a large role in cancer cell metabolism. As cancer cells are undergoing rapid growth and division, they require a proportionate increase in substrates to sustain these processes. Glutamine serves this purpose by serving as a substrate for protein and polyamine synthesis, as well as conversion into other amino acids, while also being used as an important energetic and anaplerotic substrates (DeBerardinis and Cheng 2010). Cancers in later stages of growth also have a high demand for arginine as a precursor for protein and nucleotide synthesis, although in earlier stages of development, arginine can be useful for halting tumor growth (Wu et al. 2009).
Glutamine metabolism in cancer cachexia. Rapidly-dividing tumor cells have drastic consequences for interorgan amino acid metabolism. Chief among these is glutamine, which is required both by the cancer cell for energy and growth, and by the kidney, which must balance the reduced pH brought about by metabolically active cancers. As the skeletal muscle is one of the chief suppliers of glutamine, these demands can lead to severe protein breakdown in the muscle, resulting in the loss of mass and strength that is characteristic of cancer cachexia
This demand for glutamine has consequences for tissues distinct from the tumor and its tissue of origin, namely the skeletal muscle (Fig. 3). One of the most devastating consequences of cancer pathology is skeletal muscle cachexia, a wasting disease characterized by severe reductions in skeletal muscle mass, resulting in severe decrements in quality of life, and ultimately serving as the cause of death in 1/3rd of cancer cases (Tisdale 2009). As the muscle is the major source for endogenously produced glutamine, the high demand for this amino acid is primarily met by protein degradation in striated tissue, leading to extensive proteolysis and atrophy as the tumor continues to grow and demand more energetic substrates (Parry-Billings et al. 1991). Experiments in rats have demonstrated that glutamine supplementation can halt muscle atrophy and improve markers of health in cancer models (Yoshida et al. 2001). Another demand upon skeletal muscle may in part be due to the nature of cancer metabolism: As described by Otto Warburg, cancer cells rely extensively on non-oxidative glycolysis, producing a large amount of lactic acid as a consequence of their elevated metabolic rate. In order to adjust to this chronic acidosis, the kidney requires glutamine to produce bicarbonate and restore pH balance (Drochioiu 2008; Tisdale 2009). In support of this idea, it should be noted that cachexia is not exclusive to cancer, but can also be seen in conditions such as diabetes and chronic kidney disease, both of which result in metabolic acidosis. There are reports that dietary supplementation with arginine, glutamine, BCAAs, or a mixture of casein and whey protein is beneficial for well-being in cancer patients (Table 8.1).
-
b.
Obesity and Diabetes
Also not surprising, interorgan amino acid handling can be disrupted in metabolic disease. As hyperinsulinemia is concomitant with obesity and metabolic syndrome, alterations in the processes that insulin regulates occur as well, such as irregularities in BCAA metabolism. Because insulin suppresses the activity of BCKA dehydrogenase in the liver, which is the rate limiting step in BCAA oxidation, chronically high levels of insulin will suppress this activity further, leading to elevated plasma levels of the BCAAs and their metabolites as their degradation cannot be completed (Adams 2011; Lynch and Adams 2014). It is currently not known whether this increase in BCAA concentrations is causal of insulin resistance and metabolic disturbance or a consequence, as BCAAs have also been demonstrated to have beneficial effects on liver and skeletal muscle metabolism. In contrast, plasma levels of other amino acids, particularly arginine and glucogenic amino acids (such as glutamine and aspartate) are decreased in obese and diabetic animals (Wijekoon et al. 2004; Gancheva et al. 2018). The decrease in the content of these amino acids likely arises from several mechanisms, including increased glutamine uptake by the small intestine and kidneys. Interestingly, both supplementation with glutamine and arginine show promise for the treatment of type-2 diabetes (Table 8.2), obesity (Table 8.3), and other related metabolic problems (Table 8.4).
-
c.
Liver Failure
As it lies at the center of so many metabolic processes, disorders of the liver, whether due to cirrhosis or steatosis, pose major problems for amino acid metabolism. In contrast to complications from obesity, liver disease results in reduced levels of circulating BCAAs, while the concentration of circulating aromatic amino acids increases (Morgan et al. 1982). In this case, the perturbation to BCAA metabolism does not occur in the liver, although it does arise there. Because damage to liver tissue reduces the amount of functional tissue capable of urea synthesis, other tissues must compensate to detoxify circulating ammonia. Based on its prevalence throughout the body and ability to detoxify ammonia through glutamine synthesis, skeletal muscle is key in adapting to altered ammonia metabolism (Wright et al. 2011). One of the consequences of liver failure is hyperammonemia due to the reduction in ureagenesis to remove ammonia from the blood. As a result, the skeletal muscle responds by increasing ammonia uptake and transamination of BCAAs and ammonia into glutamine, a strategy which can help stabilize plasma ammonia levels in patients with adequate muscle mass, but becomes untenable with muscle wasting, and further deprives other tissues of BCAAs. The kidney also attempts to respond by reducing glutamine release into the blood and increasing ammonia excretion in urine (Wright et al. 2011).
-
d.
Traumatic Injuries
A large body of literature supports the idea that amino acids are crucial to recovery from various forms of exogenous trauma. The main pattern of altered amino acid metabolism after a major injury is similar across various types of trauma, whether blunt trauma or burns: skeletal muscle proteolysis and increased BCAA catabolism, followed by the release of glutamine and alanine (Wilmore 2001; Herndon and Tompkins 2004). Evidence also suggests that arginine is critical to the wound healing process. These amino acids must again largely be supplied by the skeletal muscle and kidney, although exogenous supplementation may also be necessary to meet metabolic demands (Wu et al. 2009).
8.5 Conclusions and Future Directions
Based on the significant cellular infrastructure that has evolved to regulate them, amino acids, their metabolites, and the pathways that regulate them are a clearly critical component of human physiology and health. As our understanding of the importance of amino acids to cellular processes has evolved, it has become clear that these nitrogenous compounds serve as more than being substrates for the synthesis of proteins. Rather, amino acids participate in virtually every process in the cell and organism, from energy metabolism and cell growth to the regulation of blood flow. As this knowledge of their ubiquity in cell physiology has progressed, so too has the appreciation for how amino acids are handled in the body. To date, it is clear that interorgan cooperation is both crucial for maintaining the availability and metabolism of amino acids and the processes that utilize them. This realization has proven beneficial not only in the basic science of nutrition, but has led to advances in clinical medicine and therapeutics for a variety of diseases and disorders. The future of research in interorgan amino acid metabolism looks to be just as exciting, with developments and discoveries of new knowledge about how amino acids flow between organs in health and disease, opening new avenues in the sciences of nutrition, supplementation, and medicine.
Abbreviations
- BCAA:
-
Branched-chain amino acid
- BCKA:
-
Branched-chain α-keto acid
- EAA:
-
Nutritionally essential amino acid
- GSH:
-
Glutathione
- NEAA:
-
Nutritionally non-essential amino acid
- NO:
-
Nitric oxide
- SNAT:
-
Sodium-dependent neutral amino acid transporter
References
Adams SH (2011) Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr 2:445–456. https://doi.org/10.3945/an.111.000737
Argilés JM, Campos N, Lopez-Pedrosa JM et al (2016) Skeletal muscle regulates metabolism via interorgan crosstalk: roles in health and disease. J Am Med Dir Assoc 17:789–796
Arts RJW, Novakovic B, ter Horst R et al (2016) Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab 24:807–819
Ashoori M, Saedisomeolia A (2014) Riboflavin (vitamin B2) and oxidative stress: a review. Br J Nutr 111:1985–1991
Baer DJ, Stote KS, Paul DR et al (2011) Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J Nutr 141:1489–1494
Baye E, Ukropec J, De Courten MPJ et al (2018) Carnosine supplementation improves serum resistin concentrations in overweight or obese otherwise healthy adults: a pilot randomized trial. Nutrients 10:1258
Beaumont M, Blachier F (2020) Amino acids in intestinal physiology and health. Adv Exp Med Biol 1265:1–20
Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14:724–738
Brosnan JT (2000) Glutamate, at the Interface between amino acid and carbohydrate metabolism. J Nutr 130:988S-990S
Brosnan JT (2003) Interorgan amino acid transport and its regulation. J Nutr 133:2068S-2072S
Brosnan ME, Brosnan JT (2004) Renal arginine metabolism. J Nutr 134:2791S-2795S
Brosnan JT, Brosnan ME (2006) Branched-chain amino acids: enzyme and substrate regulation. J Nutr 136:207S-211S
Brosnan JT, da Silva RP, Brosnan ME (2011) The metabolic burden of creatine synthesis. Amino Acids 40:1325–1331
Brosnan JT, Brosnan ME (2013) Glutamate: a truly functional amino acid. Amino Acids 45:413–418
Brown BE, Kim CHJ, Torpy FR et al (2014) Supplementation with carnosine decreases plasma triglycerides and modulates atherosclerotic plaque composition in diabetic apo E−/−mice. Atherosclerosis 232:403–409
Chang TW, Goldberg AL (1978a) The origin of alanine produced in skeletal muscle. J Biol Chem 253:3677–3684
Chang T, Goldberg A (1978b) The metabolic fates of amino acids and the formation of glutamine in skeletal muscle. J Biol Chem 253:3685–3693
Cereda E, Turri A, Klersy C et al (2019) Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med 8:6923–6932
Chen JQ, Jin Y, Yang Y, Wu ZL, Wu G (2020) Epithelial dysfunction in lung diseases: effects of amino acids and potential mechanisms. Adv Exp Med Biol 1265:57–70
Crenn P, Messing B, Cynober L (2008) Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr 27:328–339
Curis E, Nicolis I, Moinard C et al (2005) Almost all about citrulline in mammals. Amino Acids 29:177–205
D’Antona G, Ragni M, Cardile A et al (2010) Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab 12:362–372
Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME (1990) Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol-Endocrinol Metab 259:E437-E442
Di Leo MAS, Santini SA, Gentiloni Silveri N et al (2004) Long-term taurine supplementation reduces mortality rate in streptozotocin-induced diabetic rats. Amino Acids 27:187–191
Drochioiu G (2008) Chronic metabolic acidosis may be the cause of cachexia: body fluid pH correction may be an effective therapy. Med Hypotheses 70:1167–1173
Dai Z-L, Wu G, Zhu W (2011) Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci 16:1768–1786
Dashtabi A, Mazloom Z, Fararouei M, Hejazi N (2015) Oral L-arginine administration improves anthropometric and biochemical indices associated with cardiovascular diseases in obese patients: a randomized, single blind placebo controlled clinical trial. Res Cardiovasc Med 5:e29419
DeBerardinis RJ, Cheng T (2010) Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29:313–324
Deutz NEP, Safar A, Schutzler S et al (2011) Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr Edinb Scotl 30:759–768
Dong J-Y, Qin L-Q, Zhang Z et al (2011) Effect of oral l-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J 162:959–965
Durante W (2020) Amino acids in circulatory function and health. Adv Exp Med Biol 1265:39–56
Fekete ÁA, Giromini C, Chatzidiakou Y et al (2016) Whey protein lowers blood pressure and improves endothelial function and lipid biomarkers in adults with prehypertension and mild hypertension: results from the chronic Whey2Go randomized controlled trial. Am J Clin Nutr 104:1534–1544
Felig P (1973) The glucose-alanine cycle. Metabolism 22:179–207
Feng RN, Niu YC, Sun XW et al (2013) Histidine supplementation improves insulin resistance through suppressed inflammation in obese women with the metabolic syndrome: a randomised controlled trial. Diabetologia 56:985–994
Flakoll PJ, Borel MJ, Wentzel LS et al (1994) The role of glucagon in the control of protein and amino acid metabolism in vivo. Metabolism 43:1509–1516
Flynn NE, Shaw MH, Becker JT (2020) Amino acids in health and endocrine function. Adv Exp Med Biol 1265:97–109
Fu WJ, Haynes TE, Kohli R et al (2005) Dietary L-arginine supplementation reduces fat mass in zucker diabetic fatty rats. J Nutr 135:714–721
Gancheva S, Jelenik T, Álvarez-Hernández E, Roden M (2018) Interorgan metabolic crosstalk in human insulin resistance. Physiol Rev 98:1371–1415
Ganeshan K, Chawla A (2014) Metabolic regulation of immune responses. Annu Rev Immunol 32:609–634
Gao H (2020) Amino acids in reproductive nutrition and health. Adv Exp Med Biol 1265:111–131
Griffith OW, Meister A (1979) Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci USA 76:5606–5610
Grohmann U, Bronte V (2010) Control of immune response by amino acid metabolism: metabolic regulation of immune responses. Immunol Rev 236:243–264
Guo K, Yu Y-H, Hou J, Zhang Y (2010) Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr Metab 7:57
Hakvoort TBM, He Y, Kulik W et al (2017) Pivotal role of glutamine synthetase in ammonia detoxification. Hepatology 65:281–293
Harper AE, Miller RH, Block KP (1984) Branched-chain amino acid metabolism. Annu Rev Nutr 4:409–454
He J, Wofford MR, Kristi R et al (2011) Effect of dietary protein supplementation on blood pressure. Circulation 124:589–595
He W, Wu G (2020) Metabolism of amino acids in the brain and their roles in regulating food intake. Adv Exp Med Biol 1265:167–185
Herndon DN, Tompkins RG (2004) Support of the metabolic response to burn injury. The Lancet 363:1895–1902
Hers HG (1976) The control of glycogen metabolism in the liver. Annu Rev Biochem 45:167–190
Holeček M (2018) Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr Metab 15:33
Hou YQ, He WL, Hu SD, Wu G (2019) Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids 51:1153–1165
Hou YQ, Hu SD, Li XY, He WL, Wu G (2020) Amino acid metabolism in the liver: nutritional and physiological significance. Adv Exp Med Biol 1265:21–37
Hurt RT, Ebbert JO, Schroeder DR et al (2014) L-Arginine for the treatment of centrally obese subjects: a pilot study. J Diet Suppl 11:40–52
Hyde R, Peyrollier K, Hundal HS (2002) Insulin promotes the cell surface recruitment of the SAT2/ATA2 system a amino acid transporter from an endosomal compartment in skeletal muscle cells. J Biol Chem 277:13628–13634
Jobgen W, Meininger CJ, Jobgen SC et al (2009) Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr 139:230–237
Jover-Cobos M, Khetan V, Jalan R (2014) Treatment of hyperammonemia in liver failure. Curr Opin Clin Nutr Metab Care 17:105–110
Kanarek N, Keys HR, Cantor JR et al (2018) Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 559:632–636
Kim SW, Wu G (2009) Regulatory role for amino acids in mammary gland growth and milk synthesis. Amino Acids 37:89–95
Kraft M, Kraft K, Gärtner S et al (2012) L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)—a randomized multicentre trial. Nutr J 11:52
Krishna Rao R (2012) Role of glutamine in protection of intestinal epithelial tight junctions. J Epithel Biol Pharmacol 5:47–54
Lucotti P, Setola E, Monti LD et al (2006) Beneficial effects of a long-term oral l-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol-Endocrinol Metab 291:E906–E912
Laviano A, Molfino A, Lacaria MT et al (2014) Glutamine supplementation favors weight loss in nondieting obese female patients. A pilot study. Eur J Clin Nutr 68:1264–1266
LeBlanc JG, Milani C, de Giori GS et al (2013) Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol 24:160–168
Li P, Wu G (2018) Roles of dietary glycine, proline and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50:29–38
Li P, Wu G (2020) Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids 52:523–542
Li P, Yin Y-L, Li D et al (2007) Amino acids and immune function. Br J Nutr 98:237–252
Li XY, Zheng SX, Wu G (2020) Amino acid metabolism in the kidneys: nutritional and physiological significance. Adv Exp Med Biol 1265:71–95
Lu SC (2009) Regulation of glutathione synthesis. Mol Aspects Med 30:42–59
Lynch CJ, Adams SH (2014) Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10:723–736
Manjarín R, Boutry-Regard C, Suryawan A, Canovas A, Piccolo BD, Magdalena Maj M, Abo-Ismail M, Nguyen HV, Fiorotto ML, Davis TA (2020) Intermittent leucine pulses during continuous feeding alters novel components involved in skeletal muscle growth of neonatal pigs. Amino Acids 52:1319–1335
Mansour A, Mohajeri- Tehrani MR, Qorbani M et al (2015) Effect of glutamine supplementation on cardiovascular risk factors in patients with type 2 diabetes. Nutrition 31:119–126
Marliss EB, Aoki TT, Pozefsky T, Most AS, Cahill GF (1971) Muscle and splanchnic glutamine and glutamate metabolism in postabsorptive and starved man. J Clin Invest 50:814–817
May PE, Barber A, D’Olimpio JT et al (2002) Reversal of cancer-related wasting using oral supplementation with a combination of β-hydroxy-β-methylbutyrate, arginine, and glutamine. Am J Surg 183:471–479
Meldrum BS (2000) Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 130:1007S-1015S
Møller N, Jørgensen JOL (2009) Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30:152–177
Monti LD, Setola E, Lucotti PCG et al (2012) Effect of a long-term oral l-arginine supplementation on glucose metabolism: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 14:893–900
Morgan MY, Marshall AW, Milsom JP, Sherlock S (1982) Plasma amino-acid patterns in liver disease. Gut 23:362–370
Morris SM (2002) Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr 22:87–105
Muto Y, Sato S, Watanabe A et al (2005) Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol 3:705–713
Nicholson JK, Holmes E, Kinross J et al (2012) Host-gut microbiota metabolic interactions. Science 336:1262–1267
Nissen SL, Abumrad NN (1997) Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J Nutr Biochem 8:300–311
Odessey R, Goldberg A (1972) Oxidation of leucine by rat skeletal muscle. Am J Physiol-Leg Content 223:1376–1383
Oguz M, Kerem M, Bedirli A et al (2007) l-Alanin-l-glutamine supplementation improves the outcome after colorectal surgery for cancer. Colorectal Dis 9:515–520
Opara EC, Petro A, Tevrizian A et al (1996) L-Glutamine supplementation of a high fat diet reduces body weight and attenuates hyperglycemia and hyperinsulinemia in C57BL/6J mice. J Nutr 126:273–279
Pal S, Ellis V, Dhaliwal S (2010) Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br J Nutr 104:716–723
Papadia C, Osowska S, Cynober L, Forbes A (2018) Citrulline in health and disease. Review on Human Studies. Clin Nutr 37:1823–1828
Parry-Billings M, Leighton B, Dimitriadis GD et al (1991) The effect of tumour bearing on skeletal muscle glutamine metabolism. Int J Biochem 23:933–937
Pause A, Belsham GJ, Gingras A-C et al (1994) Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5’-cap function. Nature 371:762–767
Peng X, Yan H, You Z et al (2004) Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns 30:135–139
Reeds PJ, Burrin DG, Stoll B, Jahoor F (2000) Intestinal glutamate metabolism. J Nutr 130:978S-982S
Ren W, Duan J, Yin J et al (2014) Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids 46:2403–2413
Ren WK, Bin P, Yin YL, Wu G (2020) Impacts of amino acids on the intestinal defensive system. Adv Exp Med Biol 1265:133–151
Rhoads JM, Wu G (2009) Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37:111–122
Rosa FT, Freitas EC, Deminice R et al (2014) Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind, placebo-controlled study. Eur J Nutr 53:823–830
Rose AJ, Herzig S (2013) Metabolic control through glucocorticoid hormones: an update. Mol Cell Endocrinol 380:65–78
Samocha-Bonet D, Chisholm DJ, Gribble FM et al (2014) Glycemic effects and safety of L-glutamine supplementation with or without sitagliptin in type 2 diabetes patients—a randomized study. PLoS ONE 9:e113366
Sarabhai T, Roden M (2019) Hungry for your alanine: when liver depends on muscle proteolysis. J Clin Invest 129:4563–4566
Sandoval C, Wu G, Smith SB, Dunlap KA, Satterfield MC (2020) Maternal nutrient restriction and skeletal muscle development: consequences for postnatal health. Adv Exp Med Biol 1265:153–165
Schousboe A, Scafidi S, Bak LK et al (2014) Glutamate metabolism in the brain focusing on astrocytes. In: Parpura V, Schousboe A, Verkhratsky A (eds) Glutamate and ATP at the interface of metabolism and signaling in the brain. Springer International Publishing, Cham, pp 13–30
Sekhar RV, McKay SV, Patel SG et al (2011) Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 34:162–167
Shah OJ, Kimball SR, Jefferson LS (2000) Acute attenuation of translation initiation and protein synthesis by glucocorticoids in skeletal muscle. Am J Physiol-Endocrinol Metab 278:E76–E82
Shimomura Y, Obayashi M, Murakami T, Harris RA (2001) Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain alpha-keto acid dehydrogenase kinase. Curr Opin Clin Nutr Metab Care 4:419–423
Shimomura Y, Murakami T, Nakai N et al (2004) Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise. J Nutr 134:1583S-1587S
Sipahi S, Gungor O, Gunduz M et al (2013) The effect of oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine and glutamine on wound healing: a retrospective analysis of diabetic haemodialysis patients. BMC Nephrol 14:8
Stefani GP, Capalonga L, da Silva LR, Lago PD (2020) β-Alanine and l-histidine supplementation associated with combined training increased functional capacity and maximum strength in heart failure rats. Exp Physiol 105:831–841
Stipanuk MH (2004a) Role of the liver in regulation of body cysteine and taurine levels: a brief review. Neurochem Res 29:105–110
Stipanuk MH (2004b) Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr 24:539–577
Solano F (2020) Metabolism and functions of amino acids in the skin. Adv Exp Med Biol 1265:187–199
Sun X, Feng R, Li Y et al (2014) Histidine supplementation alleviates inflammation in the adipose tissue of high-fat diet-induced obese rats via the NF-κB- and PPARγ-involved pathways. Br J Nutr 112:477–485
Tan B, Li XG, Kong X et al (2009) Dietary L-arginine supplementation enhances the immune status in early-weaned piglets. Amino Acids 37:323–331
Taylor L, Curthoys NP (2004) Glutamine metabolism: role in acid-base balance. Biochem Mol Biol Educ 32:291–304
Tisdale MJ (2009) Mechanisms of cancer cachexia. Physiol Rev 89:30
Tong L (2013) Structure and function of biotin-dependent carboxylases. Cell Mol Life Sci 70:863–891
Wagenmakers AJ (1998) Muscle amino acid metabolism at rest and during exercise: role in human physiology and metabolism. Exerc Sport Sci Rev 26:287–314
Wakabayashi Y, Yamada E, Yoshida T, Takahashi H (1994) Arginine becomes an essential amino acid after massive resection of rat small intestine. J Biol Chem 269:32667–32671
Wang B, Wu G, Zhou Z et al (2015) Glutamine and intestinal barrier function. Amino Acids 47:2143–2154
Watford M (2000) Glutamine and glutamate metabolism across the liver sinusoid. J Nutr 130:983S-987S
Watford M (2008) Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J Nutr 138:2003S-2007S
Welbourne TC (1987) Interorgan glutamine flow in metabolic acidosis. Am J Physiol-Ren Physiol 253:F1069–F1076
Wijekoon EP, Skinner C, Brosnan ME, Brosnan JT (2004) Amino acid metabolism in the zucker diabetic fatty rat: effects of insulin resistance and of type 2 diabetes. Can J Physiol Pharmacol 82:506–514
Wilmore DW (2001) The effect of glutamine supplementation in patients following elective surgery and accidental injury. J Nutr 131:2543S-2549S
Windmueller HG, Spaeth AE (1974) Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem 249:5070–5079
Windmueller HG, Spaeth AE (1981) Source and fate of circulating citrulline. Am J Physiol-Endocrinol Metab 241:E473–E480
Wright G, Noiret L, Olde Damink SWM, Jalan R (2011) Interorgan ammonia metabolism in liver failure: the basis of current and future therapies: interorgan ammonia metabolism in liver failure. Liver Int 31:163–175
Wu G (1995) Urea synthesis in enterocytes of developing pigs. Biochem J 312:717–723
Wu G (1997) Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol-Gastrointest Liver Physiol 272:G1382–G1390
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2013a) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton
Wu G (2013b) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu G (2020a) Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 52:329–360
Wu G (2020b) Metabolism and functions of amino acids in sense organs. Adv Exp Med Biol 1265:201–217
Wu G, Borbolla AG, Knabe DA (1994a) The uptake of glutamine and release of arginine, citruline and proline by the small intestine of developing pigs. J Nutr 124:2437–2444
Wu G, Knabe DA, Flynn NE (1994b) Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 299:115–121
Wu G, Fang Y-Z, Yang S et al (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492
Wu G, Bazer FW, Davis TA et al (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Wu G, Bazer FW, Burghardt RC et al (2011) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40:1053–1063
Wu G, Bazer FW, Johnson GA, Hou YQ (2018) Arginine nutrition and metabolism in growing, gestating and lactating swine. J Anim Sci 96:5035–5051
Wu ZL, Hou YQ, Dai ZL, Hu CA, Wu G (2019) Metabolism, nutrition and redox signaling of hydroxyproline. Antioxid. Redox Signal. 30:674–682
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80:1107–1213
Yoshida S, Kaibara A, Ishibashi N, Shirouzu K (2001) Glutamine supplementation in cancer patients. Nutrition 17:766–768
Yu X, Xu Z, Mi M et al (2008) Dietary taurine supplementation ameliorates diabetic retinopathy via anti-excitotoxicity of glutamate in streptozotocin-induced sprague-dawley rats. Neurochem Res 33:500–507
Zanchi NE, Gerlinger-Romero F, Guimarães-Ferreira L et al (2011) HMB supplementation: clinical and athletic performance-related effects and mechanisms of action. Amino Acids 40:1015–1025
Acknowledgement
All figures made with Biorender.com.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
J. Ryan, P., Riechman, S.E., Fluckey, J.D., Wu, G. (2021). Interorgan Metabolism of Amino Acids in Human Health and Disease. In: Wu, G. (eds) Amino Acids in Nutrition and Health. Advances in Experimental Medicine and Biology, vol 1332. Springer, Cham. https://doi.org/10.1007/978-3-030-74180-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-74180-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-74179-2
Online ISBN: 978-3-030-74180-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)