Abstract
Daratumumab is a monoclonal antibody approved for the treatment of multiple myeloma (MM). Daratumumab exerts its anti-myeloma effects by targeting the CD38 (a transmembrane glycoprotein highly expressed on myeloma cells) and inducing antibody-dependent cytotoxicity, complement-dependent cytotoxicity, and antibody-dependent cellular phagocytosis. Despite well-established efficacy in both newly diagnosed and relapsed/refractory MM patient populations, a large proportion of patients fail to respond to daratumumab and thus may be daratumumab-resistant. This chapter will review the efficacy of daratumumab in notable clinical trials and discuss its mechanisms of action and the potential mechanisms behind daratumumab-resistance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Daratumumab was first introduced into the clinical setting in 2008. This was driven by the poor prognosis of multiple myeloma (MM) patients who were double refractory to immunomodulatory imide drugs (IMiDs) and proteasome inhibitors (PI) triggering a demand for new treatment options with unique mechanisms of action.
Daratumumab is a high-affinity monoclonal antibody (MoAb) targeting CD38 with unique cytotoxic abilities, shown to effectively kill myeloma cells from patients by antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). It targets a unique epitope on CD38, a transmembrane glycoprotein with differential high expression on malignant myeloma cells [1]. It was developed by Genmab, a Danish-Dutch biotech company in collaboration with the scientists at the University of Utrecht [2].
Emerging clinical trials have demonstrated the efficacy and tolerability of daratumumab when used alone and in combination with standard anti-myeloma therapies in both the newly diagnosed and relapsed and refractory setting.
This chapter will focus on daratumumab’s mechanism of action, mechanisms behind drug resistance, and the efficacy data from emerging clinical trials in both the newly diagnosed and relapsed refractory setting for myeloma.
5.2 Mechanism of Action
Daratumumab is an immunoglobulin G1 kappa (IgG1k) human MoAb binding to a unique CD38 epitope on CD38 expressing cells with high affinity and was developed by immunization of human immunoglobulin transgenic mice with recombinant CD38 protein [1]. CD38 is a 46-kDa type II transmembrane glycoprotein with physiological roles in receptor-mediated adhesion, signaling events and has a bifunctional ecto-enzymatic activity that contributes to intracellular calcium mobilization [3]. CD38 is highly expressed in myeloma cells and represents a promising target for MoAb-based immunotherapy. It is also expressed in relatively lower levels on lymphoid, myeloid, and non-hematopoietic tissue [4].
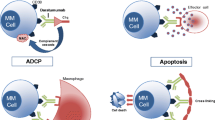
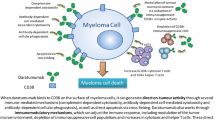
Daratumumab targets CD38-positive myeloma cells via several mechanisms. The immune-mediated mechanisms include CDC, ADCC, and antibody-dependent cellular phagocytosis (ADCP) . It also exerts its effect via apoptosis and crosslinking [2, 5]. The unique epitope of daratumumab on CD38 clusters and positions the Fc region of the antibody in a way that facilitates optimal binding and activation of complement proteins [6].
In vitro experiments have shown that daratumumab induces ADCC in many different tumor cell lines with varying CD38 expression. The ADCC activity was preserved despite testing on myeloma patients who had undergone a variety of previous chemotherapeutic schedules [7]. This demonstrates the ability of daratumumab to circumvent the observation that Fc gamma receptor polymorphisms in cancer patients may have a negative impact on the therapeutic responses to antibodies [8].
Yu, Qiao et al demonstrated that daratumumab induced effective lysis via ADCC and CDC in the presence of both peripheral immune effector cells and bone marrow stem cells. This observation is suggestive of daratumumab activity in the bone marrow microenvironment, an advantage from a drug resistance perspective [1]. Daratumumab has also demonstrated high efficacy in interrupting tumor growth in mouse xenograft models [1]. Nijhof et al. showed that there was no difference in daratumumab induced ADCC or CDC between newly diagnosed, relapsed/refractory, or IMID refractory myeloma patients, suggesting that resistance to prior therapies does not affect the efficacy of daratumumab [9, 10].
The ADCC mechanism of daratumumab by natural killer (NK) cells has been shown to be enhanced by drugs that increase NK cell activity such as lenalidomide. Van de Veer et al. demonstrated in vitro that the pretreatment of peripheral blood mononuclear cells with lenalidomide enhanced daratumumab-induced ADCC against myeloma cell lines derived from patient bone marrow myeloma cells. The combination of daratumumab and lenalidomide was synergistic, increasing tumor lysis by 20% [11]. Other studies support the notion that it is the NK cell activation of lenalidomide that contributed to the synergistic effect of daratumumab [9, 10, 12].
A significant association was observed between CD38 expression and daratumumab-induced ADCC and CDC. They observed all-trans retinoic acid (ATRA) induced upregulation of CD38 expression and reduced expression of complement inhibitory proteins CD55 and CD59 in myeloma cells. This resulted in a significant increase in daratumumab activity in vitro, and enhanced activity in mouse models, providing rationale for further evaluation of daratumumab in combination with ATRA [9, 10].
In addition to ADCC and CDC, ADCP was another mechanism induced by daratumumab. Overijk and colleagues demonstrated daratumumab-induced ADCP in vitro and in vivo in leukemic xenograft mouse models. It also triggered macrophage-mediated phagocytosis ex vivo in patient-derived MM cell samples [13]. ADCP may have an important function in the bone marrow microenvironment, as tumor-associated macrophages in the marrow have been shown to have a Fc-dependent antitumor function [14].
The off-target immunomodulatory effects of daratumumab were studied in two earlier daratumumab monotherapy trials analyzing peripheral blood and bone marrow samples before, during, and at relapse. These studies found that depletion of CD38 immunosuppressive cells was associated with an increase in T helper cells, cytotoxic T cells, and improvement in T cell functionality [5]. These findings may explain the significant prolongation in overall survival (OS) in these early clinical trials conducted with daratumumab monotherapy [15].
Elimination of immunosuppressive cells belonging to T cell, B cell, and monocyte-macrophage system expressing CD38 are observed with daratumumab. These immunosuppressive cells inhibit cytotoxic T cells from exerting antitumor control on myeloma patients. In addition, antibody-mediated inhibition of the enzymatic activity of CD38 on cytotoxic T cells may directly boost the antitumor activity of these cells [16].
Preclinical studies have demonstrated significant additive and synergistic effects of daratumumab in combination with other anti-myeloma therapies. This has been confirmed in multiple clinical trials, highlighting daratumumab’s unique mechanism of action without overlapping toxicity.
5.3 Mechanisms Behind Daratumumab-Resistance
Despite the well-established efficacy of daratumumab, 60% of patients do not achieve partial response (PR) and the majority who initially respond will eventually experience disease progression [17]. Insights into the mechanisms of daratumumab-resistance have been highlighted in several studies.
It has been shown that the CD38 expression on myeloma cells correlates with in vitro daratumumab-mediated ADCC and CDC [9, 10]. However, it has also been shown that the variability in daratumumab-mediated killing in vitro is not completely explained by CD38 expression alone and that there are CD38 independent mechanisms at play. The overexpression of complement inhibitory proteins is known to play a role in tumor immune evasion and resistance against therapeutic antibodies. Resistance towards daratumumab was associated with the upregulation of CD55 and CD59 on myeloma cells. A reduced expression of CD38 on myeloma cells was also found to confer protection against daratumumab. All-trans retinoic acid was found to increase CD38 expression and reduce CD55 and CD59 expression, increasing CDC against myeloma cells [18].
The mechanisms of resistance to daratumumab can be summarized into the following categories:
5.3.1 Reduced Cell Surface Expression of Target Antigen CD38
A reduction in myeloma surface CD38 expression is a mechanism involved in primary and/or acquired resistance [18]. CD38 reduction was postulated to occur via clonal selection [19]. Another mechanism is the downstream effects of daratumumab, triggering CD38 internalization leading to cytoskeletal reorganization and redistribution of CD38 into polar aggregates in myeloma cells. These are then released into the bone marrow microenvironment as microvesicles, leading to the modulation of inflammatory cytokines and the abrogation of anti-myeloma immune responses [20].
The IMiDs lenalidomide and pomalidomide can increase the expression of CD38 on myeloma cells and synergize its activity with daratumumab in vitro and in vivo [21, 22].
5.3.2 Antibody-Dependent Cell Cytotoxicity Resistance
Daratumumab induces fratricide of NK cells via its CD38 expression which can then in turn affect NK mediated ADCC, influencing its own efficacy [23]. Interestingly, ex vivo experiments have shown an enhanced proliferative and anti-myeloma activity in the remaining NK cells with low CD38 expression, lending to the hypothesis that daratumumab-resistance may be overcome by infusion of ex vivo expanded autologous NK cells [24].
The concept of bone marrow stromal cells conferring resistance to daratumumab mediated ADCC was demonstrated by de Haart et al. showing overexpression of the anti-apoptotic protein survivin in myeloma cells upon its interaction with BMSCs [25].
5.3.3 Antibody-Dependent Cellular Phagocytosis Resistance
The overexpression of CD47 on myeloma cells aids its immune escape from ADCP via its binding to signal regulatory protein-alpha (SIRPa) and tumor-associated macrophages (TAMS), effectively inhibiting phagocytosis [26].
5.3.4 Complement-Dependent Cytotoxicity Resistance
Overexpression of complement inhibitory proteins is known to play a role in tumor immune evasion and resistance against therapeutic antibodies. Cells are protected from complement activation by fluid phase regulators and by membrane-associated inhibitory proteins such as CD46 and glycosyl-phosphatidyl-inositol anchored proteins such as CD55 and CD59 [27].
Samples in the GEN501 study showed increased expression of CD55 and CD59 in myeloma cells during disease progression, confirming that overexpression of these complementary inhibitory proteins can be postulated to be a mechanism of resistance for daratumumab.
5.3.5 Immune Modulated Resistance
There is the intriguing hypothesis that the immune system itself is contributory to the resistance to daratumumab. This is postulated to be via several mechanisms, including the downregulation of intracellular pathways in the bone marrow stromal cells, a decrease in effector memory T cells and M1 macrophages, and the CD28 expression in T cells [28, 29].
5.4 Clinical Efficacy of Daratumumab
The initial clinical testing of daratumumab in the GEN501 phase I/II clinical trials enrolled 23 patients over three and a half years due to limited preclinical data, resulting from the lack of cross reactivity of daratumumab with the CD38 molecule in other species. The tested doses of antibody were extremely low, starting at 0.005 mg/kg to a maximum of 24 mg/kg [30]. Clinical efficacy was observed when the dosage was between 2 and 4 mg/kg, translating to a decrease in M protein with no major side effects observed. Target saturation was seen at doses of 16 mg/kg with eight weekly dosing, followed by eight bi-weekly dosing then dosing every 4 weeks, with a maximum tolerated dose not reached at even 24 mg/kg. Another clinical trial has observed superior efficacy at 16 mg/kg over 8 mg/kg [31].
Preclinical studies demonstrate significant synergistic and additive effects in combination with other anti-myeloma therapies. This has been confirmed in multiple clinical trials, supporting the unique mechanism of action of daratumumab without overlapping toxicity.
5.4.1 Daratumumab in the Relapsed and Refractory Setting
The GEN501 and SIRIUS study led to US Food and Drug Administration (FDA) approval of daratumumab for the treatment of multiple myeloma patients who had received at least three prior lines of therapy including a proteasome inhibitor (PI) and an IMiD or who are double refractory to a PI and an IMiD [31, 32].
Daratumumab monotherapy demonstrated approximately 30% response in patients with relapsed refractory multiple myeloma (RRMM) [15, 31]. Half of the patients in the trials demonstrated a significant prolongation of survival due to the immunomodulatory effect of daratumumab [5].
The enhanced efficacy and tolerability of several daratumumab-based combinations in both transplant ineligible and eligible patients have been demonstrated without compromising transplant ability [33].
A deeper response and increase in progression free survival (PFS) has been seen with the addition of daratumumab to a PI and an IMiD. Phase III studies (POLLUX and CASTOR) have demonstrated a higher response rate, depth of response, and PFS in MM patients who have received more than one line of therapy. As a result, daratumumab is now placed in second- and first-line treatment in MM [35,36,37,38,38].
5.4.2 Daratumumab in Newly Diagnosed, Transplant Ineligible Patients
The phase III ALCYONE trial evaluated the efficacy of daratumumab in combination with bortezomib, melphalan, and dexamethasone (D-VMP) compared with bortezomib, melphalan, and dexamethasone alone (VMP). The addition of daratumumab demonstrated significant improvement in PFS among 706 transplant ineligible, newly diagnosed myeloma patients. The benefit in overall responses in the daratumumab group was also translated to other patient groups including older age (>75 years), higher ISS stage, and poorer performance status with impaired organ function. As expected, there was less benefit in the high-risk cytogenetic groups compared to the standard risk group [36].
The phase III MAIA trial demonstrated the benefit of daratumumab in addition to lenalidomide and dexamethasone in newly diagnosed, transplant ineligible myeloma patients. A superior PFS was demonstrated in interim analysis in the older age group of greater than 75, but not in the high-risk cytogenetic subgroup [39]. Interestingly, the POLLUX study evaluating the addition of daratumumab to lenalidomide and dexamethasone in RRMM patients demonstrated a longer PFS in the high-risk cytogenetics subgroup compared to the standard risk group [40].
Due to these promising results, to date daratumumab in combination with either VMP or lenalidomide and dexamethasone is being approved in Europe and the USA. Maturation of data will hopefully result in widespread global approval.
There is currently little evidence to guide treatment choice between the various standard regimens. Cao et al. recently published a meta-analysis comparing the efficacy of currently used regimens compared to lenalidomide and dexamethasone. In general, three drug combinations with daratumumab (with either lenalidomide and dexamethasone or VMP) showed superiority to two-drug combinations (lenalidomide and dexamethasone) [41]. Proteasome inhibitor-based doublet regimens in combination with daratumumab are being evaluated. The phase II HOVON 143 study demonstrated manageable side effects in its first planned safety analysis with promising overall response rates (ORR) [42]. Ongoing trials of daratumumab-based combinations in transplant ineligible, newly diagnosed myeloma patients are currently being conducted.
5.4.3 Daratumumab in Newly Diagnosed, Transplant Eligible Patients
The promising results of daratumumab in combination with transplant ineligible myeloma patients led to its evaluation in the transplant eligible group. There are several studies being conducted to evaluate its efficacy. The phase III CASSIOPEIA trial evaluated the efficacy of daratumumab combined with bortezomib, thalidomide, and dexamethasone during induction and consolidation. The daratumumab treatment group showed favorable results with increased rates of stringent complete response (CR) at 100 days posttransplant and higher ORR including CR, very good partial response (VGPR) and minimal residual disease (MRD) negativity (64% versus 44%, p < 0.0001). This benefit was demonstrated in many patient groups including those over the age of 50, those with poorer performance status, and those with renal or hepatic dysfunction. However, a benefit was not seen in the ISS stage 3 subgroup. Less benefit was seen in the higher cytogenetic risk group. There was a higher incidence of grade 3 and 4 cytopenia and a lower yield of stem cells requiring plerixafor during mobilization in the daratumumab group. These results led to the approval of daratumumab in transplant eligible myeloma patients [43]. The phase II GRIFFIN study where patients received daratumumab with bortezomib, lenalidomide, and dexamethasone induction and posttransplant consolidation, followed by maintenance with daratumumab and lenalidomide, demonstrated promising results in safety profile and response rates after consolidation [44]. The subcutaneous formulation of daratumumab is currently being utilized to minimize toxicity in the phase III PERSUES trial comparing the efficacy of bortezomib, lenalidomide, and dexamethasone with or without daratumumab in transplant eligible populations.
5.5 Toxicity Profile
The most important side effect to note in daratumumab is the infusion-related reactions that may occur in the first infusion in approximately half of the patients with incidences subsiding thereafter. These reactions are managed with the premedications with glucocorticoids, antihistamines, montelukast, and paracetamol prior to the infusions. Patients with chronic obstructive pulmonary disease may require a prolonged course of glucocorticoids.
The expression of CD38 on erythrocytes complicates the antibody identification work-up in transfusion medicine [45]. Pan-agglutination caused by daratumumab and other anti-CD38 antibodies may mask the presence of a clinically significant RBC alloantibody in the patient’s plasma during an antibody screen and identification process, consequently putting a patient at risk of an acute or delayed transfusion reaction [46]. Methods for circumventing this include group and screening for all potential baseline alloantibodies at baseline and extended red cell phenotyping and genotyping [47].
5.6 Conclusion
Daratumumab is a high-affinity monoclonal antibody targeting a unique epitope on CD38 and exerts its therapeutic effects via CDC, ADCC, ADCP, and off-target immunomodulatory effects with overall improvement in T cell functionality observed in preclinical studies. Its unique mechanisms of action have led to its favorable tolerability profile with nonoverlapping toxicity when used in combination therapy.
Insights into Daratumumab’s evolving mechanisms of resistance provide avenues for further drug and synergy development. These include upregulation of complement inhibitory proteins, reduced CD38 expression, inhibition of NK mediated ADCC, and escape from ADCP by overexpression CD47 among other immune modulatory effects.
Daratumumab has shown promising efficacy in both the newly diagnosed and relapsed refractory setting in emerging clinical trials. Its favorable tolerability profile has extended its benefit in OS even in the older and frail population. It serves as an important armamentarium in the treatment of multiple myeloma.
Abbreviations
- ADCC:
-
Antibody-dependent cellular toxicity
- ADCP:
-
Antibody-dependent cellular phagocytosis
- ATRA:
-
All-trans retinoic acid
- CDC:
-
Complement dependent cytotoxicity
- CR:
-
Complete response
- D-VMP:
-
Daratumumab, bortezomib, melphalan, and dexamethasone
- IgG1k:
-
Immunoglobulin G1 kappa
- IMiD:
-
Immunomodulatory imide drug
- MM:
-
Multiple myeloma
- MoAb:
-
Monoclonal antibody
- MRD:
-
Minimal residual disease
- NK:
-
Natural killer
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- PI:
-
Proteasome inhibitor
- PR:
-
Partial response
- RRMM:
-
Relapsed/refractory multiple myeloma
- SIRPa:
-
Signal regulatory protein-alpha
- TAMS:
-
Tumor associated macrophages
- VGPR:
-
Very good partial response
- VMP:
-
Bortezomib, melphalan, and dexamethasone
References
de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol (Baltimore, MD: 1950). 2011;186(3):1840–8.
Plesner T, Krejcik J. Daratumumab for the treatment of multiple myeloma. Front Immunol. 2018;9:1228.
Mehta K, Shahid U, Malavasi F. Human CD38, a cell-surface protein with multiple functions. FASEB J. 1996;10(12):1408–17.
Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88(3):841–86.
Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–94.
Yu T, Qiao C, Lv M, Tang L. Novel anti-CD38 humanized mAb SG003 possessed enhanced cytotoxicity in lymphoma than Daratumumab via antibody-dependent cell-mediated cytotoxicity. BMC Biotechnol. 2019;19(1):28.
Stevenson FK, Bell AJ, Cusack R, Hamblin TJ, Slade CJ, Spellerberg MB, et al. Preliminary studies for an immunotherapeutic approach to the treatment of human myeloma using chimeric anti-CD38 antibody. Blood. 1991;77(5):1071–9.
Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–8.
Nijhof IS, Groen RW, Lokhorst HM, van Kessel B, Bloem AC, van Velzen J, et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia. 2015a;29(10):2039–49.
Nijhof IS, Groen RWJ, Noort WA, van Kessel B, de Jong-Korlaar R, Bakker J, et al. Preclinical evidence for the therapeutic potential of CD38-targeted immuno-chemotherapy in multiple myeloma patients refractory to Lenalidomide and Bortezomib. Clin Cancer Res. 2015b;21(12):2802–10.
van der Veer MS, de Weers M, van Kessel B, Bakker JM, Wittebol S, Parren PW, et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica. 2011;96(2):284–90.
Nijhof IS, van Bueren JJL, van Kessel B, Andre P, Morel Y, Lokhorst HM, et al. Daratumumab-mediated lysis of primary multiple myeloma cells is enhanced in combination with the human anti-KIR antibody IPH2102 and lenalidomide. Haematologica. 2015c;100(2):263–8.
Overdijk MB, Verploegen S, Bogels M, van Egmond M, van Bueren JJL, Mutis T, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7(2):311–21.
Grugan KD, McCabe FL, Kinder M, Greenplate AR, Harman BC, Ekert JE, et al. Tumor-associated macrophages promote invasion while retaining Fc-dependent anti-tumor function. J Immunol (Baltimore, MD: 1950). 2012;189(11):5457–66.
Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128(1):37–44.
Chatterjee S, Daenthanasanmak A, Chakraborty P, Wyatt MW, Dhar P, Selvam SP, et al. CD38-NAD(+)axis regulates immunotherapeutic anti-tumor T cell response. Cell Metab. 2018;27(1):85–100.e8.
Nooka AK, Joseph NS, Kaufman JL, Heffner LT, Gupta VA, Gleason C, et al. Clinical efficacy of daratumumab, pomalidomide, and dexamethasone in patients with relapsed or refractory myeloma: utility of re-treatment with daratumumab among refractory patients. Cancer. 2019;125(17):2991–3000.
Nijhof IS, Casneuf T, van Velzen J, van Kessel B, Axel AE, Syed K, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128(7):959–70.
van de Donk NWCJ, Usmani SZ. CD38 antibodies in multiple myeloma: mechanisms of action and modes of resistance. Front Immunol. 2018;9:2134.
Saltarella I, Desantis V, Melaccio A, Solimando AG, Lamanuzzi A, Ria R, et al. Mechanisms of resistance to anti-CD38 daratumumab in multiple myeloma. Cell. 2020;9(1):167.
Boxhammer R, Steidl S, Endell J. Effect of IMiD compounds on CD38 expression on multiple myeloma cells: MOR202, a human CD38 antibody in combination with pomalidomide. J Clin Oncol. 2015;33(15_suppl):8588.
Endell J, Boxhammer R, Wurzenberger C, Ness D, Steidl S. The activity of MOR202, a fully human anti-CD38 antibody, is complemented by ADCP and is synergistically enhanced by lenalidomide in vitro and in vivo. Blood. 2012;120(21):4018.
Casneuf T, Xu XS, Adams HC III, Axel AE, Chiu C, Khan I, et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv. 2017;1(23):2105–14.
Wang Y, Zhang Y, Hughes T, Zhang J, Caligiuri MA, Benson DM, et al. Fratricide of NK cells in daratumumab therapy for multiple myeloma overcome by ex vivo−expanded autologous NK cells. Clin Cancer Res. 2018;24(16):4006–17.
de Haart SJ, Holthof L, Noort WA, Minnema MC, Emmelot ME, Aarts-Riemens T, et al. Sepantronium bromide (YM155) improves daratumumab-mediated cellular lysis of multiple myeloma cells by abrogation of bone marrow stromal cell-induced resistance. Haematologica. 2016;101(8):e339–e42.
Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47–SIRPα signalling pathway. Trends Cell Biol. 2009;19(2):72–80.
Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–40.
Neri P, Maity R, Tagoug I, Duggan P, McCulloch S, Jimenez-Zepeda V, et al. Single cell resolution profiling defines the innate and adaptive immune repertoires modulated by daratumumab and IMiDs treatment in multiple myeloma (MM). Blood. 2017;130(Supplement 1):123.
Viola D, Dona A, Gunes EG, Troadec E, Wu X, Branciamore S, et al. Immune mediated mechanisms of resistance to daratumumab. Blood. 2018;132(Supplement 1):3201.
Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207–19.
Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet (London, England). 2016;387(10,027):1551–60.
Lokhorst HM, Laubach J, Nahi H, Plesner T, Gimsing P, Hansson M, et al. Dose-dependent efficacy of daratumumab (DARA) as monotherapy in patients with relapsed or refractory multiple myeloma (RR MM). J Clin Oncol. 2014;32(15_suppl):8513.
Syed YY. Daratumumab: a review in combination therapy for transplant-ineligible newly diagnosed multiple myeloma. Drugs. 2019;79(4):447–54.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–31.
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–66.
Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518–28.
Harousseau JL, Attal M. How I treat first relapse of myeloma. Blood. 2017;130(8):963–73.
Plesner T, Arkenau HT, Gimsing P, Krejcik J, Lemech C, Minnema MC, et al. Phase 1/2 study of daratumumab, lenalidomide, and dexamethasone for relapsed multiple myeloma. Blood. 2016;128(14):1821–8.
Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab plus Lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104–15.
Dimopoulos MA, San-Miguel J, Belch A, White D, Benboubker L, Cook G, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica. 2018;103(12):2088–96.
Cao Y, Wan N, Liang Z, Xie J, Wang S, Lin T, et al. Treatment outcomes in patients with newly diagnosed multiple myeloma who are ineligible for stem-cell transplantation: systematic review and network meta-analysis. Clin Lymphoma Myeloma Leuk. 2019;19(8):e478–e88.
Stege CAM, Nasserinejad K, Levin M-D, Thielen N, Klein SK, Ludwig I, et al. Efficacy and tolerability of ixazomib, daratumumab and low dose dexamethasone (IDd) in unfit and frail newly diagnosed multiple myeloma (NDMM) patients; First Interim Safety Analysis of the Phase II HOVON 143 Study. Blood. 2018;132(Supplement 1):596.
Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet (London, England). 2019;394(10,192):29–38.
Abdallah N, Kumar SK. Daratumumab in untreated newly diagnosed multiple myeloma. Ther Adv Hematol. 2019;10:2040620719894871.
Dizon MF. The challenges of daratumumab in transfusion medicine. Lab Med. 2017;48(1):6–9.
Quach H, Benson S, Haysom H, Wilkes AM, Zacher N, Cole-Sinclair M, et al. Considerations for pre-transfusion immunohaematology testing in patients receiving the anti-CD38 monoclonal antibody daratumumab for the treatment of multiple myeloma. Intern Med J. 2018;48(2):210–20.
Lancman G, Arinsburg S, Jhang J, Cho HJ, Jagannath S, Madduri D, et al. Blood transfusion management for patients treated with anti-CD38 monoclonal antibodies. Front Immunol. 2018;9:2616.
Acknowledgment
I would like to acknowledge and thank the reviewers, editors, and my colleagues for their support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hua, V.M. (2021). Daratumumab. In: Ling, S.C., Trieu, S. (eds) Resistance to Targeted Therapies in Multiple Myeloma. Resistance to Targeted Anti-Cancer Therapeutics, vol 22. Springer, Cham. https://doi.org/10.1007/978-3-030-73440-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-73440-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-73439-8
Online ISBN: 978-3-030-73440-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)