Abstract
Textile industries play a crucial role in Indian economy and also cause serious issues on environmental pollution. The demand of colors, fade-resistant garments, and other hue products are increasing in day-to-day lifestyle, triggering the blooming of dyeing industries. These factories utilize colossal volume of water and various chemical substances for dyeing process and release toxic organic pollutants such as organic azo-dyes, surfactants, and phenolic compounds with effluent to the environment which severely disturb the natural balance and affect all forms of living beings. Higher concentration of colored compounds in the effluent mixed with surface water reduces the flow of sunlight and prevents the photosynthetic process of aquatic vegetation. Also, it affects the fertility of agricultural land and health impacts in human and animals. The changes in physicochemical nature of surface and underground water quality due to textile effluent lead to water crisis. Conventional treatment methods are expensive and not sufficient in efficient removal of organic pollutants from the wastewater. Thus, economically and ecologically safe approaches are needed for the treatment of textile industry effluent to prevent and conserve natural resources without affecting the growth of this sector. In this chapter, eco-friendly techniques such as bioremediation, phytoremediation, coagulation process by natural coagulants, and biogenic nanomaterials applied for the removal of organic pollutants from the textile effluent are elaborately discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Globally, environmental pollution is considered as a serious issue due to the rapid escalation of diverse industries as well as human population explosion (Ali et al., 2020). Textile dyeing industries are economically important, and it holds the second place after agriculture sector in India. Among all the type of industries, textile industries are contributing 14% overall production in India (Raichurkar & Ramachandran, 2015). These industries consume huge quantity of water for dyeing and finishing process; generate wastewater with toxic organic contaminants such as unfixed dye stuffs (e.g., xanthenes, Congo red, anthraquinones, phthalocyanines), phenolic compounds, and surfactants (Chanwala et al., 2019; Yang et al., 2019; Fernandez et al., 2010; Mahajan et al., 2019).
Annually, 2,80,000 tons of unfixed dyestuffs waste water from textile dyeing industry is released into the environment with or without partial treatment (Gita et al., 2019). In India, around 10,000 textile factories, 2100 bleaching as well as finishing units are running to produce textile products such as readymade cloth, handlooms, silk, wool, jute, nylon, and polyester and these factories are exporting their produce to various countries (Sachin et al., 2010).
2 Textile Dyeing Process
Textile dyeing industries widely implement a conventional approach known as “wet processing” method for dyeing the cotton yarn and fabric materials. In this process, various complex sequential steps such as sizing, desizing, scouring, bleaching, dyeing, and finishing processes are being employed to manufacture a complete furnished colorful garment (Punzi, 2015; Bisschops & Spanjers, 2003; Mattioli et al., 2002; Verma et al., 2012). Wet dyeing process of cotton fabric materials is summarized in Fig. 1. Before weaving and spinning steps, the yarn or cotton fabrics are subjected to sizing process with diverse kinds of sizing materials (starch, carboxymethylcellulose, polyvinyl alcohol, polyacrylase). The desizing process removes impurities and chemical substances attached to the sized fabrics. Diluted H2SO4 (0.5%) is generally used as a desizing agent along with alkali, surfactants, and enzymes.
In the scouring process, the organic impurities such as oil, grease, fats, wax, and other dirt present in the fabric material are removed before dyeing process. This process is carried out by boiling the raw materials with alkaline solution and scouring agents (soaps, detergents, wetting agents, alkalis, lubricants, defoaming agents) to improve the dye fixation ability. After this treatment, the materials are washed thoroughly with caustic soda, sodium silicate, detergents, and water. The bleaching step is a major step which eradicates any natural tint found in the fabric and bleaches it into white by bleaching substances such as hydrogen peroxide, sodium hypochlorite, and optical brightness for efficient dyeing attachment. Subsequently, the mercerizing process, which is a continuous chemical process, enhances the strength, appearance, luster, and dye binding ability of fabrics toward the dye substances. Sodium hydroxide is commonly used as a mercerizing agent. After this process, alkaline pH of the processed material has to be neutralized by sequential washing with water. Figure 2 shows the wet dyeing process of cotton fabric material.
Dyeing process is an essential step of wet processing, which makes use of various kinds of organic and inorganic dye stuffs (Imtiazuddin et al., 2012) that belong to azo, reactive, disperse, direct, and vat dye classes. The dye fixation with the fabrics takes place under alkaline condition with higher temperature. This method utilizes various organic, inorganic, and polymeric compounds for processing (Robinson et al., 2001). In the printing process, processed materials are headed to print required designs with multi-colored dyes and pigments. The printing paste comprises water, dyes, thickening agents, solvents, surfactants, and urea. Various methods such as flatbed screen, heat transfer, resist style, direct style, and rotary screen are widely employed for printing process (Bisschops & Spanjers, 2003; Wadje, 2009). The finishing process improves and enhances the quality, appearance, durability, and acceptability of the printed fabrics for commercialization. Generally, formaldehyde mixed with dye fixing agents, resins, softeners are used to increase water and flame resistant, antimicrobial, antistatic, antiwrinkling and insect repellent properties of the printed fibers. The finished garments are subjected to quality analysis, folding, packing, and exporting to national as well as international level.
3 Water Consumption for Wet Processing
Wet processing requires large quantity of water for dyeing, washing, chemical processing, bleaching, and finishing process. USEPA reported that a typical dyeing factory utilizes approximately 36,000 L of water for producing 20,000 Ib/day for a fully furnished fabric garment. Nigam et al. (2000) have reported that the textile dyeing units use 200–500 L of water to manufacture 1 kg of processed products. Also, 1 kg of cotton materials dyeing with reactive dyes requires 70–150 L of water, 30–60 g of dye substances, and 0.6–0.8 kg of sodium chloride for dye fixation process.
Marcucci et al. (2002) stated that a classic dyeing industry needs 200–500 L of water to produce 1 kg of processed garments. Mainly, the water consumption level depends on the employing process, raw materials, dye types, and machines used in the industries. Table 1 illustrates the liters of water required for wet process (http://www.fibre2fashion.com). Figure 3 shows the percentage of water consumption in overall process.
4 Organic Dyes in Textile Dyeing Processes
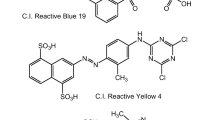
Mankind has used dyes for thousands of years and the earliest known use of a colorant is believed to be with a Neanderthal man about 1,80,000 years ago. Though, the first known use of an organic colorant was much later, being nearly 4000 years ago, when the blue dye indigo was found in the wrappings of mummies in Egyptian tombs (Gupta & Suhas, 2009). Dye molecules generally comprise chromophore groups, which are responsible for color formation and auxochrome and act as electron acceptor and assist in water solubility nature. In addition to this, it enhances the binding affinity of dye toward the fibers, and it increases the color intensity. Thus, the auxochromes are also represented as “color enhancer.” Chromophore groups are usually found in the organic dyes as carbonyl group (=C=O), =C=C=, C=NH, or C=S sulphur bonding (Hunger, 2003; Pereira & Alves, 2012). Most of the dyes are of synthetic origin with complex aromatic structural property. The dye structures are based on the presence of hydrocarbons such as naphthalene, benzene, toluene, xylene, and anthracene (Rivera et al., 2011). Dye classes and few examples of organic dyes used in dyeing industries are given in Table 2. The structure of some widely used organic textile dyes is shown in Fig. 4.
5 Characteristics and Effects of Textile Industry Effluent
The characteristics and composition of waste water generated from dyeing industries depend on the utilization of organic, inorganic chemicals and dye substances as well as processing methods. The physicochemical parameters, such as color, pH, temperature, total solids, total dissolved solids, chemical oxygen demand, biological oxygen demand, chloride, carbonate’s heavy metal content in the effluent, show extreme fluctuation in the concentration, which indicates the quality of dyeing wastewater (Phalakornkule et al., 2010; Carmen & Daniela, 2012). The persistent organic dyes are difficult to degrade due to its complex structural feature and higher molecular mass (Verma et al., 2011). The toxic dyeing effluent chiefly contributes to the deterioration of terrestrial as well as aquatic environment (Tehrani-Bagha & Mahmoodi, 2010).
In Tamilnadu, the quality of Noyyal (Erode), Amaravathy (Karur), Cauvery (Trichy), and Bhavani (Coimbatore) river water is drastically polluted by the direct release of treated or untreated dyeing effluent (Sathiyaraj et al., 2017). The wastewater with higher concentration of color and TDS impedes the re-oxygenation potential, prevents sunlight penetration, affects the biological activities as well as photosynthetic process, and leads to destroy aquatic flora and fauna (Zaharia et al., 2009). In addition to this, phosphorous and nitrogen from the industrial effluent mixed into the river cause eutrophication (Qasim & Mane, 2013), reduction in yielding crop in agriculture land, pollution due to the organic contaminants like carboxylate, azo compounds, naphthalene (Annamalai et al., 2014), and cause human health issues like infertility, cancer, skin allergy, and respiratory problems. Fernandes et al. (2018) and Bonet et al. (2018) reported liver damage, inflammatory diseases, and bladder cancer because of the toxic effects of organic dye substances.
The reservoir bed of Orathapalayam dam increased several meters with settling of toxic sludge from textile dyeing industries released with higher TDS content. Due to this harmful effect, about 400 tons of fishes died. In Coimbatore, the Bhavani River has been polluted with dyeing effluent, and the farmers utilize polluted water for the irrigation to cultivate vegetables, paddy, banana, and turmeric. The toxic compounds which enter into the food chain may cause severe health impacts in all trophic levels.
6 Organic Pollutants in Dyeing Wastewater
The processed wastewater discharge during the dyeing as well as finishing process is characterized with higher concentration of organic contaminants, unfixed toxic organic dye stuffs, heavy metals, and various kinds of chemical impurities (Wu et al., 2016; Arslan-Alaton & Alaton, 2007). The recalcitrant organic dyes, surfactants, salts, and chlorinated chemicals used for dyeing purpose are considered as the most important organic pollutants in dyeing effluent (Ben Mansour et al., 2012). Most of the organic toxicants contain different characteristics and include aliphatic or aromatic cyclic rings or halogenated substitution compounds especially chlorine that enhances their persistent nature, which are difficult to remove from the environment; also its carcinogenic properties cause severe health impacts in humans and other animals (Huang et al., 2017). The foremost ingress of organic pollutants to the textile effluent is from the raw materials used for garments production. Natural (cotton, wool) and chemical-based fibers (nylon, polyester) are widely used as a raw material for the production textile products. Natural fibers usually contain organic pollutants like oil, grease, fats, wax which also contributes in the increase of COD and BOD of textile industry wastewater (Le Marechal et al., 2012). In addition to this, the organic chemical compounds of pesticides and herbicides such as pentachlorophenol, diazinon, fenvalerate, cypermethrin, and cyromazine used during the cultivation period remain attached with the natural fibers thereby increasing the organic pollutant load in the effluent. The lipophilic properties of certain pesticides also aided to strong affinity with cotton fibers. Various kinds of pollutants are released from each step of wet processing due to the usage of different organic chemical substances.
In sizing and desizing process, the discharge of organic pollutants depends on the raw materials (cotton, linen, synthetic fibers) as well as chemical substances. Typical organic contaminants produced during these processes are enzymes, hemicelluloses, carboxymethyl cellulose, starch, fats, etc. During the scouring process, anionic, ionic, non-ionic surfactants, formate, wool waxes, and anionic detergents are liberated into the effluent. After the bleaching process, the organic pollutant load increased with the presence of bleaching agents and other chemical matter, including EDTA, polyacrylates, and gluconates. Higher organic contaminants are formed after the dyeing, finishing, and printing processes. Volatile organic compounds like benzene, xylene, and methane used in printing process are also released into the atmosphere. Major pollutants are unfixed lethal organic dyes (contribute to rise of color intensity in wastewater) along with auxiliary chemical components such as alkylphenol, EDTA, methylenephosphonic acid, fatty amine ethoxylates, ammonia, glycerin, polyvinyl alcohol, mineral oil, and halogenated compounds, which dynamically lift the concentration of organic pollutants, TDS, COD, and salts in the textile industry wastewater. Generally, these noxious contaminants present in the contaminated environment are detected qualitatively and quantitatively by spectroscopic (UV-visible spectroscopy and surface-enhanced Raman scattering) as well as chromatographic (LC-MS and GC) instrumental analytical methods (Borah et al., 2020). Dan et al. (2018) detected organic pollutants using UV spectrophotometer in the wavelength ranges from 250 to 300 nm. The persistent, recalcitrant dye components and other organic contaminants entirely alter the nature of dyeing effluent and lead to difficulty in the reduction of pollutants and also cause inefficiency in treatment process. A few examples of organic pollutants released during the wet processing method are presented in Table 3.
7 Eco-friendly Techniques for the Removal of Organic Pollutants
Traditionally, the organic contaminants present in the polluted environment are eliminated by physical (adsorption, reverse osmosis, membrane filtration, photolysis, sonication), chemical (coagulation, flocculation, precipitation, chemical oxidation and reduction), physicochemical (electrocoagulation, ion-pair extraction, electrochemical treatment), biological (aerobic and anaerobic digestion, oxidation ponds, microbial degradation, enzymatic and plant-based treatment), and combination of all these approaches (Robinson et al., 2001; Kabra et al., 2013; Jain et al., 2020; Zhou et al., 2019; Senthilkumar et al., 2018; Santhanama et al., 2019).
Schematic treatment process typically carried out in effluent treatment plant (ETP) is given in Fig. 5. Most of the abovementioned techniques involve high cost; tedious, ineffective, and difficult to degrade certain kinds of toxic dyes; and produce secondary pollutants like intermediate compounds, huge amount of sludge, and alteration in pH of the treated water. Moreover, many of these methods are harmful to the environment. To overcome these disadvantages, various researchers reported biological methods for industrial effluent treatment. In this chapter, eco-friendly treatment techniques such as adsorption, coagulation (plant and animal-based adsorbents and bio-coagulants), microbial biodegradation (bacteria and fungi), phytoremediation (terrestrial and aquatic plants), and green-synthesized nanomaterials (silver, iron, copper, zinc oxide, graphene oxide nanoparticles) adapted to remove organic contaminants from the textile dyeing industry wastewater are provided.
7.1 Adsorption
Adsorption is referred as the binding substances on the surface of adsorbent through chemical bonding. This treatment is considered as an efficient and non-toxic technique to eliminate soluble and insoluble organic pollutants from the wastewater. Granular or powdered activated carbon is a broadly used commercial adsorbent, but it is expensive. Recently, researchers focus on preparing bio-based adsorbents from various sources like plant wastes (leaf, wood, flower), algae, rice husk, seaweed, hair, mud, zeolite, clay, hydroxides, and metal oxides, as an alternative to activated carbon (Ali et al., 2012; Velmurugan et al., 2011).Importantly, an excellent adsorbent should possess various properties such as porosity, polarity, solubility, surface area, and solution of the pH to enhance the adsorption capacity (Kyzas et al., 2013). Activated carbon from biological-based raw materials is prepared through carbonization and physical as well as chemical activation method. Usually, higher temperature is applied for carbonization process, which eliminates non-carbon compounds like hydrogen, oxygen, and nitrogen (Ali et al., 2012).
Miyah et al. (2018) proved the decolorizing efficiency of walnut powder as a natural low-cost adsorbent and exhibited higher adsorption capability of methylene blue dye from aqueous solution than commercially available expensive adsorbents.
Yang and Hong (2018) reported the adsorption potential of Glyssogyne tenuifolia leaves treated with lauric acid toward organic toxic dyes. In another investigation, shells of almond, apricot, and walnut treated with levulinic acid efficiently adsorbed methylene blue dye from aqueous solution (Kocaman, 2020). Likewise, Saeed et al. (2010) reduced the concentration of crystal violet dye concentration in aqueous solution by grape fruit peel powder as an effectual adsorbent through adsorption mechanism. Various researchers prepared bio-based adsorbents for the removal of textile organic dye decolorization (Table 4).
7.2 Coagulation
Coagulation is a widely used conventional treatment technique employed in various industries to reduce organic pollutants and suspended solids and achieve complete decolorization of toxic organic dyes from the dyeing wastewater with the aid of chemical and biological coagulating agents. In addition to this, it evades the formation of toxic intermediate compounds and secondary pollutants during the degradation process (Golob et al., 2005; Verma et al., 2012). Aluminum hydroxide (alum), iron-based salts (ferric chloride, ferrous sulphate), calcium hydroxide, and polyaluminum chloride are broadly used for the pre-treatment of dyeing effluent (Kang et al., 2003; Huang et al., 2009; Patel & Vashi, 2010). The basic mechanism of coagulation is charge neutralization, inter-particle bridging, and destabilization of colloidal particles like soluble dye molecules as well as suspended solids leads to the formation of microflakes or agglomerates. The aggregation of microflocs forms large-sized aggregates and settle down. The separation of settled sludge can be carried out by sedimentation as well as filtration process (Verma et al., 2012; Wang et al., 2018; Manholer et al., 2019). The performance of coagulants crucially depends on the optimum dosage of coagulant, pH of the solution, and characteristics of the effluents or pollutants (Patel & Vashi, 2010).
The application of synthetic polymeric and metal-based coagulants in effluent treatment has some limitations including the production of settled solid sludge after coagulation process, alteration in pH and nature of treated water, inefficient in the decolorization of insoluble dye stuffs, and complications in the disposal as well as limited to recycling of synthetic coagulants (Anjaneyulu et al., 2005; Ramavandi, 2014; Anastasakis et al., 2009). Additionally, researchers reported harmful effect of chemical coagulants such as alum, which leads to Alzheimer’s disease in humans (Vijayaraghavan et al., 2011; Flaten, 2001). Natural polymers and coagulants are an alternative source for toxic chemical coagulants because of low cost, eco-friendly nature, biodegradability, easily available, and not causing any detrimental effects on humans or other animals. Furthermore, solid sludge obtained after treatment of effluent using natural coagulants can be used as a soil conditioning agent and manure (Zahrim et al., 2011). In general, polysaccharides, proteins, tannin, and mucilage are extensively used plant-based coagulants (Yin, 2010).
The phytocompounds, namely, polysaccharides such as galactomannan, galactan, galacturonic acid, and mucilage are capable coagulants aided for the removal of suspended solids, color, COD, turbidity, and toxic organic pollutants from the textile industry wastewater. Many researchers validated the coagulation property of mucilage extracted from Abelmoschus esculentus, fenugreek, dragon fruit peel, cactus, etc. (Freitas et al., 2015; Anastasakis et al., 2009; de Souza et al., 2014; Le et al., 2020).
Moringa oleifera Lam seed powder is a well-known natural coagulant efficiently used for the treatment of dye related wastewater (Fig. 6). It eliminated 95% of azo dyes from the aqueous solution and shown better performance in the removal of textile dyes such as Blue 71, Acid Yellow 23, and Reactive Red from the effluent (Altamirano-Corona et al., 2015). Cationic protein molecules found in the seed extract possess positive charge and are actively participating in the diminution of anionic dye contaminants by means of charge neutralization as well as adsorption mechanism (Sapna et al., 2012).
In another investigation, M. oleifera Lam seed powder effectively decreased 74% of color, 84% of TDS, 39% of COD, and 65% of turbidity from the dyeing-related wastewater (Kalaicelvi et al., 2016). Sanghi et al. (2006) achieved 87% decolorization of acid dye form the aqueous solution using coagulant prepared using seed gum of Ipomeoa dasysperma. Similarly, Mahmoudabadi et al. (2019a) confirmed the coagulant property of Plantago major extract and successfully decolorized Reactive Blue 19 dye.
In contrast to plant coagulants, animal-based materials also encompass effective coagulation property. Chitosan is a major coagulant derived from deacetylated chitin, which is composed of D-glucosamine and N-acetyl D-glucosamine (Yin, 2010; Kurita, 2006; Verma et al., 2012). These natural polymers are capable in the reduction of suspended solids, color causing organic dye substances, COD, and turbidity from the dyeing wastewater (Guibal & Roussy, 2007). Mahmoodi et al. (2011) successfully removed 75% of Acid green 25 dye and 95% of Direct red 23 dye from textile wastewater using chitosan. Likewise, Abdullah and Jaeel (2019) studied the coagulation potential of chitosan and reduced acid dye concentration from textile dyeing wastewater. Some natural coagulants used for the remediation of textile dyeing effluent and organic dye decolorization are given in Table 5.
7.3 Phytoremediation of Organic Dye Pollutants
Phytoremediation or green remediation is an ecologically benign, aesthetically pleasant approach, which uses live plants (aquatic and terrestrial) for the treatment of industrial effluent, polluted soil, and restoration of contaminated sites (Sinha et al., 2007; Ekambaram et al., 2018). Naturally, plants contain defense mechanism against toxic compounds or environmental stresses. Through the enzymatic pathways, plants convert toxic compounds to non-toxic compounds, and this property assists in the degradation of organic contaminants (Govindwar & Kagalkar, 2010). Classically, plants remediate contaminants through various modes including phytoextraction, phytotransformation, phytostabilization, rhizofilteration, phytoaccumulation, rhizodegradation, and phytovolatilization (Ali et al., 2013; Muthusamy et al., 2018; Ali et al., 2020). The remediation capability of the plants mainly depends on the plant species, interaction with root region, bioavailability, nature of the growth medium, characteristics of the pollutants or effluent, selection of chelating agents, and environmental conditions such as pH, temperature, and salt concentration (Tangahu et al., 2011). Chara vulgaris, Eichhornia crassipes, Lemna minor, Pistia stratiotes, Hydrocotyle vulgaris roots, Nasturtium officinale, Typha angustifolia, Hydrilla verticillata, Azolla pinnata, Azolla filiculoides, Medicago sativa L., Bacopa monnieri L, and Sesbania cannabina Pers are some plants having remediation potential and used for wastewater and polluted soil treatment.
Kabra et al. (2013) examined the effluent treatment as well as dye decolorization potential of Glandularia pulchella (Sweet) Tronc. The toxic dye stuffs in the effluent stimulate the production of several enzymes such as tyrosinase, lignin peroxidase, alcohol oxidase, and dichlorophenolindophenol reductase which enhances the degradation of contaminants from the dyeing wastewater. Vafaei et al. (2012) obtained 99% removal of Basic Red 46 dye from the aqueous solution using Azolla filiculoides under optimized pH and temperature. The results concluded that the synthesis of antioxidant enzymes induced due to the chemical stress involved in the decolourization process. Vasanthy et al. (2011) successfully reduced 95% of Red RB and 99.5% of Black B from aqueous solution using the whole plant of E. crassipes. The chromatographic (GCMS) analysis of plant extract obtained after treatment confirmed the increased level of hexadecanoic acid and decreased level of chlorophyll and phytol content. In another analysis, E. crassipes effectively eliminated 50.64% of total solids, 55.71% of BOD, 40–70% of COD, 94.78% of chromium, 94.44% of copper, and 96.88% of zinc from the dyeing wastewater. The alkaline pH of the effluent was also neutralized by water hyacinth (Mahmood et al., 2005).
The combination of algae (Nostoc) with aquatic plants (Eichornia crassipes and P. stratiotes L) is used for the elimination of pollutants from the mixture of textile industry effluent, and it removed 65% of COD from the effluent through synergistic mechanism (Roy et al., 2010). Salvinia molesta is a submerged aquatic flora which has the dye degradation potential. Chandanshive et al. (2016) effectively removed 97% of Rubine GFL azo dye from the aqueous solution using Salvinia molesta by triggering the production of plant enzymes such as laccase, lignin peroxidase, tyrosinase, alcohol oxidase, DCIPR reductase, catalase, and supermutase. The degradation and intermediate compounds were identified using GC-MS, FTIR, and HPLC analysis. The microscopic analysis confirmed the accumulation of dye substances in the stem region after 48 hours. In addition to this, S. molesta plant reduced 82% of BOD and 76% of COD after 192 hours from real dyeing effluent.
Mahajan and Kaushal (2016) removed pH, EC, total dissolved solids, COD, and BOD from the different dilution (100%, 75%, 50%, 25%, and 10%) of textile dyeing industry effluent within 7 days through phytoextraction mechanism of H. verticillata (L.f) Royle. In the same way, Patel and Adhvaryu (2016) studied the dyeing effluent treatment potential of E. crassipes and P. stratiotes L. The treatment process was performed for 7 days, and the parameters such as pH, total solids, COD, and dissolved oxygen of the treated effluent was checked. After treatment, the COD level got decreased and the dissolved oxygen got increased. E. crassipes showed maximum removal of color than P. stratiotes L. The aquatic floating plant Azolla pinnata potentially eliminated 90% of methylene blue dye from the aqueous solution which confirmed its phytoremediation potential (Al-Baldawi et al., 2018). As well, Khataee et al. (2012) achieved 80% reduction of Acid Blue dye using Lemna minor after 6 days of treatment period. The decolorization process can be assisted by various antioxidant enzymes including ascorbate, peroxidases, and superoxide dismutase produced by Lemna minor due the stress caused by toxic effect of dye molecules (Paczkowska et al., 2007). Similarly, Imron et al. (2019) removed 80.56% of methylene dye using Lemna minor within 24 hours of duration. Bacopa monnieri (L.) Pennell plant potentially degraded 90–100% several reactive and azo dyes after 2 weeks of treatment duration under hydroponic culture (Shanmugam et al., 2019).
Most of the aquatic plants uptake the contaminants through the root region and degrade the pollutants via rhizodegradation mechanism. Moreover, the microorganisms present in the rhizosphere region get stimulated by the production exudates by plants and the degradation of pollutants occurs by synergistic effect. The absorbed toxic contaminants are easily converted into non-toxic compounds. And also, the carbonic compounds present in the organic pollutants are utilized by the plants for growth and metabolic activities (Tangahu et al., 2011; Al-Baldawi et al., 2015; Geoffroy et al., 2004). Typhonium flagelliforme significantly removed dye from the solution prepared by distilled water within 4 days duration (Kagalkar et al., 2010). In another investigation, Rai et al. (2014) analyzed the dye decolorization capability of Aloe barbadensis plant extracts and decolorized 27.33% of Congo red dye. Kamat (2014) reported the dye decolorizing potential of two terrestrial plants such as Arabidopsis thaliana and Helianthus annuus.
Sureshvarr et al. (2010) reported the azo dye degradation potential of Eucalyptus plant using GCMS analysis carried out for treated and untreated azo dye–contaminated soil samples. Tetradecanoic acid is a type of carboxylic acid, which has antioxidant property effectively involved in the degradation of organic pollutants from the dyeing effluent (Bodoprost & Rosemeyer, 2007). Chandanshive et al. (2018) treated the dye containing textile wastewater using various plants like Gaillardia grandiflora, Tagetes patula, Portulaca grandiflora, and Aster amellus through constructed wetland system. The outcome revealed the dye accumulation capability of the ornamental plants and improved the effluent contaminated soil quality. Microscopic analysis showed the accumulation of dye molecules on the plant cells. And also, the enzymatic characterization of plant tissues revealed the contribution of laccases, peroxidases, and thyrosinases enzyme in the dye decolorization as well as degradation of organic contaminants from the contaminated soil.
Phytoremediation approach is gaining several advantages over conventional treatment techniques such as eco-friendly, less expensive, require solar energy, no need for the requirement of high cost machines as well as technical experts, and efficient to degrade both organic and inorganic pollutants. Alternatively, this method has some limitations including its time-consuming nature, rooted to harmful effects in plants due to the formation of noxious intermediate by-products during the degradation, and lack of proper understanding on the phytoremediation mechanism occur in the plants (Muthusamy et al., 2018). This method can be used with the combination of other physical methods for efficient removal of organic contaminants from the textile industry wastewater. Few plants used for the degradation of organic pollutants and treatment of textile effluent are given in Table 6.
7.4 Bioremediation of Organic Pollutants
Bioremediation is a biological technique widely adapted for the treatment of various industrial effluents. It refers to the remediation of wastewater or contaminated soil using microorganisms such as bacteria, yeasts, fungi, microalgae, and biocatalysts. This method is an ecologically benign, low cost, and easier approach for the decolorization and degradation of toxic organic dyes as well as contaminants from dye-related wastewater. Common effluent treatment plants of textile industries typically carried out aerobic and anaerobic treatment using microbial inoculants (Saratale et al., 2011; Vikranta et al., 2018; Patil et al., 2020). Diverse types of microbial strains are capable to absorbing, degrading, or neutralizing organic contaminants by the activation of various enzymes like phenoloxidases, reductases, peroxidases, and monooxygenases are synthesized due to the stress caused by the toxic compounds (Vikranta et al., 2018; Kalme et al., 2007). The dye decolorization mechanism mainly occurs through breaking the bonds such as hydroxyl group, carboxylic acid, amino group found in the chromophore region of dye molecules (Ghosh et al., 2017). Many researchers reported the bioremediation potential of various bacterial strains such as Bacillus sp., Pseudomonas sp., Micrococcus, Acinetobacter, Aeromonas, Neisseria sp., and Rhodococcus (Pandey et al., 2019). Buthelezi et al. (2012) demonstrated the treatment efficiency of indigenous bacterial strains isolated from activated sludge have been effectively decolorized whale blue, mediblue, and mixed dyes. The decolorization highly influenced by the characteristics of dye, temperature, pH, and concentration of bio-flocculants.
Sethi et al. (2012) isolated indigenous bacterial strains such as Klebsiella sp., Pseudomonas sp., and Proteus sp. from textile dyeing wastewater and Klebsiella sp., Shiegella sp., and Marganella sp. from textile industry sludge waste and utilized the same for decolorization process under optimized pH, temperature, carbon, and nitrogen sources to enhance the dye decolorization. The effluent isolates reduced 80% of color, and sludge isolates reduced 40% of color. Bafana et al. (2008) separated azoreductase enzyme with 60 kDa of molecular weight through electrophoretic method, which responsible for the decolorization of azo Direct red 2 and converted into benzidine and 4-aminobiphenyl. In another evaluation, Pseudomonas putida, Lysinibacillus fusiformis, Comamonas testosteroni, Aeromonas hydrophila, P. plecoglossicida, and P. monteilii were isolated from the soil sample collected near dyeing industrial area and used for the decolorization of acid orange, methyl orange, methylene blue, malachite green, and rhodamine B dyes. P. putida removed 91% of malachite green after 5 days and decolorized 85% of acid orange and 69% of methylene blue after 7 days of incubation. Comamonas testosteroni removed 85% of methyl orange, and P. monteilii removed 56% of Rhodamine B dye after 3 days of incubation period (Fulekar et al., 2013). Similarly, Telke et al. (2009) potentially decolorized 100 mg/L of Congo red from aqueous solution and removed 50% of COD from dyeing wastewater within 12 hours optimum pH (8) and temperature (40 °C) using Pseudomonas sp. isolated from textile effluent.
Likewise, Kalyani et al. (2008) also isolated Pseudomonas sp. SUK1 from effluent and successfully degraded 99.28% of Red BLI under aerobic condition with controlled pH (6.5–7.0) and temperature (30 °C). The significant role of aminopyrine N-demethylase and NADH-DCIP reductase enzyme contributed in the degradation process was determined by UV, FTIR, and TLC techniques. Mahmood et al. (2012) screened green and red dye decolorization efficiency of six indigenous bacterial cultures such as Bacillus cereus, Bacillus mycoides, Bacillus subtilus, Micrococcus sp., Bacillus sp., and Pseudomonas sp. The bacterial consortium BMP1/SDSC-01 removed 84% of green as well as red dye and 85% reduction observed in yellow and black dye solution. In the same way, Desai (2017) studied the decolorizing capacity of native bacterial isolates such as Klebsiella sp. and Staphylococcus strains by culturing in the media enriched with Direct Red 2B dye and the strains reduced 98.83% and 98.83% of dye, respectively, under specific concentration of carbon and nitrogen sources with optimized pH and temperature. Some of the microbial strains used for the degradation of organic dyes from textile dyeing effluent are summarized in Table 7.
7.5 Nanoremediation of Organic Pollutants
Nanotechnology is one of the most fascinating fields of science which deals with the synthesis of nanoscale material size ranging from 1 to 100 nm. These nanomaterials are having vast applications in medicine (drug delivery), environmental cleanup, textile, electronics, biosensors, optics pace industries, etc. (Christian et al., 2008; Saif et al., 2016). Moreover, the application of metal and metal oxide nanoparticles in the degradation of organic/inorganic contaminants are gaining crucial part because of their unique properties in terms of larger volume of surface area, reusability, stability, recyclability, and less/non-toxic and facile synthesizing approach (Gautam et al., 2019; Beshkar et al., 2017).
Generally, nanoparticles are synthesized by two strategies such as such as “top-down” and “bottom-up” approaches. The nanoparticles produced from the larger materials by reducing its size is referred as top-down, while in bottom-up, assembling of smaller molecules to hefty structure takes place (Rotello, 2004) (Fig. 7). Synthesis of nanoparticles is carried out by various physical and chemical methods such as co-precipitation, micro emulsion, flame synthesis, laser ablation, and chemical vapor condensation. Most of these methods are having demerits such as higher cost, noxious and are corrosive in nature, explosive properties of precursors, require high temperature, and need of surfactants (Hamed, 2005; Ghaedi et al., 2006; Huang et al., 2008; Mamania et al., 2013; Ghorbani et al., 2017). Due to these limitations, the environmentally benign, facile, and reliable biological materials such as plant, bacteria, fungi, algae, and yeast are applied as precursors and reducing agents to synthesis of nanoparticles (Mandal et al., 2006; Jebali et al., 2011).
The green or biogenic route of nanoparticle fabrication can be aided by various bioactive compounds present in plant and microbial extracts in terms of enzymes, polyphenol, amino acids, vitamins, polysaccharide, and terphenoids which act as stabilizing as well as capping agents through bioreduction or bio-precipitation mechanism (Park et al., 2011). Green nanomaterials facilitate solutions to technological and environmental challenges in the field of solar energy conversion, catalysis, medicine, and drinking water and wastewater treatment. Various types of metal nanoparticles like silver, gold, iron, zinc oxide, copper, selenium, tin oxide, and titanium oxide are widely synthesized using plant and microbial biocomponents as reducing agent which are having the potential to degrade organic pollutants from the contaminated site and convert into non-toxic compounds through oxidation or reduction mechanism. Furthermore, biogenic nanoparticles are effective in varied temperature as well as pH and recyclable without the loss of notable reduction on catalytic property. These nanomaterials are used as an efficient nano-adsorbents or nanocatalyst for clean-up purposes (Gautam et al., 2019).
Among metallic nanomaterials, silver nanoparticle is an important one that has been extensively studied by many researchers because of its unique potential as antibacterial activity. Diverse types of plant and microbial extract-based reducing agents are used for the synthesis of biogenic silver nanoparticles and are actively participating in the degradation of toxic organic contaminants. AgNP synthesized using C. pyrenoidosa extract acts as a nanocatalyst and efficiently degraded methylene blue dye from aqueous solution (Aziz et al., 2015). Khan et al. (2016) produced C. japonicum plant extract–mediated AgNP and applied for the removal of organic dye bromophenyl blue. Similarly, Duran et al. (2007) revealed the textile treatment capability of silver nanoparticles synthesized using extract obtained from the fungi F. oxysporum. Bhakya et al. (2015) exposed organic dye degradation potential of silver nanoparticles synthesized with the extract obtained from leaf, bark, and root extracts of Helicteres isora plant by means of catalytic activity. Interestingly, the dye degradation process by silver nanoparticles occurred through the adsorption toward the surface of contaminants due to the higher surface volume ratio of nanoparticles and enhancement of catalytic property by the exposure of UV/visible light or sunlight (Ansari et al., 2013).
Gold is a well-known precious metal chiefly used for ornamental purposes. Unlike bulk gold material, gold nanoparticle is tremendously recognized as a competent nanocatalyst because of its unique optical absorption property in the presence of UV as well as visible light. The photocatalytic property of gold nanoparticles can be proficiently utilized for the elimination of noxious organic compounds (Laoufi et al., 2011). As a green route, the leaf extract of Lagersteroemia speciosa rapidly reduced precursor gold ions to 41-91 nm nanosized gold nanoparticles, and it hastily decolorized several organic dyes namely Bromophenol blue, bromocresol, methylene blue, and methyl orange via photocatalytic reaction (Choudhary et al., 2016). In another analysis, fungal (C. oxysporum extract) mediated gold nanoparticle easily decolorized the toxic dye Rhodamine B within 7 minutes of reaction time (Bhargava et al., 2016). Basically, biogenic gold nanoparticles detoxify or degrade the azo dyes by cleaving the azo bond present in the dye molecules and convert lethal nitroaromatic compounds to non-toxic carboxylic acid by oxidation process (Sharma & Deswal, 2018).
Zinc oxide is an effective superconducting metal, and it is broadly noted for optical, electrical, and photocatalytic properties. Golmohammadi et al. (2019) proved the photocatalytic activity of zinc oxide nanoparticles fabricated using the extract of jujube fruit by degrading organic dyes including methylene blue and Eriochrome black-T from model wastewater. Lemon juice promptly reduced the precursor chemical zinc acetate to zinc oxide nanoparticles through reduction process, and it successfully decolorized certain organic dyes like reactive blue 21, methylene blue, methyl red, and orange via photocatalytic activity (Davar et al., 2015).
Iron is considered as a versatile metal widely used for various applications. Iron nanoparticles are gaining more attention from researchers than bulk iron material because of its distinctive features specifically magnetic property, higher surface volume, less toxicity, low cost, and facile preparation methods (McHenry & Laughlin, 2000; Chin et al., 2011). Iron oxide magnetic nanoparticles include zero-valent iron, magnetite, and maghemite nanoparticles which are extensively used for water and wastewater treatment. The unique properties such as their small particle size, large surface area to volume ratio, magnetism, rapid kinetics, and high reactivity with the pollutants aid efficient purging of contaminants from the water and wastewater. The magnetic property of the iron nanoparticles easily removes the minute contaminants than the well-defined membrane filtration system. The recovery of magnetic iron nanoparticles can be carried out by low-gradient magnetic field or hand-held magnet (Hu et al., 2007; Yantasee et al., 2007; Shen et al., 2009).
Huang et al. (2014) developed 40–50 nm nanosized iron nanoparticles assisted with oolong tea extract as an eco-friendly reducing agent and revealed its decolorizing efficiency by removing 75.5% of malachite green dye from aqueous solution through adsorption mechanism. Bishnoi et al. (2018) degraded methylene blue dye through photocatalytic activity of magnetic iron nanoparticles prepared using fruit extract of Cynometra ramiflora plant. In another analysis, iron oxide nanoparticles removed 99.09% of dye from the effluent through adsorption mechanism (Arifin et al., 2017). Cheera et al. (2018) effectively decolorized Congo red dye with the aid of green-synthesized iron nanoparticles using Acacia nelotica plant extract. The utilization of biological-based reducing agents facilitates to evade the usage of toxic chemicals and paved toward the preparation of eco-friendly nanoparticles and assist in multipurpose applications including effective removal of organic pollutants from the textile dyeing effluent. Green-synthesized metal nanoparticles used for the removal of organic pollutants are given in Table 8.
8 Conclusion
Textile industries are vitally significant areas which produce more beautiful colored products as well as most dangerous creator of environmental pollution that lead to the deterioration of natural resources. In the current scenario, dyeing industries need to conserve huge volume of water by recycling and reusing remediated water. These dyeing effluents are characterized with higher concentration of harmful organic pollutants that cause lethal effect in flora and fauna of the ecosystem. To eradicate these organic contaminants, industries need less-expensive and eco-friendly treatment approaches. In this overview, promising biological techniques in terms of adsorption, coagulation using natural coagulants, microbial remediation, plant-based remediation, and green-synthesized nanomaterials employed for the treatment of textile effluent were elaborately discussed. However, these biological approaches are having certain limitations that need to be conquered for the proficient reduction of organic pollutants from the contaminated environment.
References
Abdel-Khalek, M. A., Abdel Rahman, M. K., & Francis, A. A. (2017). Exploring the adsorption behavior of cationic and anionic dyes on industrial waste shells of egg. Journal of Environmental Chemical Engineering, 5(1), 319–327.
Abdullah, H. A., & Jaeel, A. J. (2019). Chitosan as a widely used coagulant to reduce turbidity and color of model textile wastewater containing an anionic dye (acid blue). IOP Conference Series: Materials Science and Engineering, 584, 012036.
Ahila, K. G., Vasanthy, M., & Thamaraiselvi, C. (2018a). Green synthesis of magnetic iron nanoparticle using Moringa oleifera Lam seeds and its application in textile effluent treatment. In S. K. Ghosh (Ed.), Utilization and management of bioresources. Springer Nature Singapore Pte Ltd.
Ahila, K. G., Vasanthy, M., & Thamaraiselvi, C. (2018b). Preparation of iron magnetic nanoparticles using aquatic macrophyte Salvinia adnata Desv and its application in dye decolorization. International Journal of Advanced Research and Development, 3(2), 175–179.
Al-Baldawi, I. A., Abdullah, S. R. S., Anuar, N., & Hasan, H. A. (2018). Phytotransformation of methylene blue from water using aquatic plant (Azolla pinnata). Environmental Technology and Innovation, 11, 15–22.
Al-Baldawi, I. A., Abdullah, S. R. S., Anuar, N., Suja, F., & Mushrifah, I. (2015). Phytodegradation of total petroleum hydrocarbon (TPH) in diesel-contaminated water using Scirpus grossus. Ecological Engineering, 74, 463–473.
Ali, H., Khan, E., & Sajad, M. A. (2013). Phytoremediation of heavy metals: Concepts and applications. Chemosphere, 91, 869–881.
Ali, I., Asim, M., & Khan, T. A. (2012). Low cost adsorbents for the removal of organic pollutants from wastewater. Journal of Environmental Management, 113, 170–183.
Ali, S., Abbas, Z., Rizwan, M., Zaheer, I. E., Yava, I., Unay, A., Abdel-Daim, M. M., Bin-Jumah, M., Hasanuzzaman, M., & Kalderis, D. (2020). Application of floating aquatic plants in phytoremediation of heavy metals polluted water: A review. Sustainability, 12, 1927.
Al-Kadhi, N. S. (2019). The kinetic and thermodynamic study of the adsorption Lissamine Green B dye by micro-particle of wild plants from aqueous solutions. Egyptian Journal of Aquatic Research, 45(3), 231–238.
Altamirano-Corona, M., Cotes-Martinez, R., Flores-Gonzalez, D. S. F., & Alfaro-Cuevas-Villanueva, R. (2015). Decolorization of an effluent from textile industry using Moringa oleifera seed extract. The International Journal of Sciences: Basic and Applied Research, 19(2), 99–111.
Anastasakis, K., Kalderis, D., & Diamadopoulos, E. (2009). Flocculation behavior of mallow and okra mucilage in treating wastewater. Desalination, 249(2), 786–791.
Anjaneyulu, Y., Chary, N. S., & Raj, D. S. S. (2005). Decolourization of industrial effluents- Available methods and emerging technologies- A review. Reviews in Environmental Science and Biotechnology, 4, 245–273.
Annamalai, S., Santhanam, M., Sundaram, M., & Curras, M. P. (2014). Electrokinetic remediation of inorganic and organic pollutants in textile effluent contaminated agricultural soil. Chemosphere, 117, 673–678.
Ansari, S. A., Khan, M. M., Ansari, M. O., Lee, J., & Cho, M. H. (2013). Biogenic synthesis, photocatalytic, and photoelectrochemical performance of Ag–ZnO nanocomposite. Journal of Physical Chemistry C, 117, 27023–27030.
Arifin, A., Ismail, I., Abdullah, A. H., Shafiee, F. N., Nazlan, R., & Ibrahim, I. R. (2017). Iron oxide nanoparticles derived from mill scale waste as potential scavenging agent in dye wastewater treatment for Batik industry. Solid State Phenomena, 268, 393–398.
Arslan-Alaton, I., & Alaton, I. (2007). Degradation of xenobiotics originating from the textile preparation, dyeing, and finishing industry using ozonation and advanced oxidation. Ecotoxicology and Environmental Safety, 68(1), 98–107.
Aziz, N., Faraz, M., Pandey, R., Shakir, M., Fatma, T., Varma, A., Barman, I., & Prasad, R. (2015). Facile algae-derived route to biogenic silver nanoparticles: Synthesis, antibacterial, and photocatalytic properties. Langmuir, 31, 11605–11612.
Bafana, A., Chakrabarti, T., & Devi, S. S. (2008). Azoreductase and dye detoxification activities of Bacillus velezensis strain AB. Applied Microbiology and Biotechnology, 77, 1139–1144.
Bayoumi, M. N., Al-Wasify, R. S., & Hamed, S. R. (2014). Bioremediation of textile wastewater dyes using local bacterial isolates. International Journal of Current Microbiology and Applied Sciences, 3(12), 962–970.
Ben Mansour, H., Houas, I., Montassar, F., Ghedira, K., Barillier, D., Mosrati, R., & Chekir-Ghedira, L. (2012). Alteration of in vitro and acute in vivo toxicity of textile dyeing wastewater after chemical and biological remediation. Environmental Science and Pollution Research International, 19, 2634–2643.
Benghazi, L., Record, E., & Suarez, A. (2014). Production of the Phanerochaete flavido-alba in Aspergillus niger for synthetic dyes decolorization and biotransformation. World Journal of Microbiology and Biotechnology, 30, 201–211.
Beshkar, F., Zinatloo-Ajabshir, S., Bagheri, S., & Salavati-Niasari, M. (2017). Novel preparation of highly photocatalytically active copper chromite nanostructured material via a simple hydrothermal route. PLoS One, 12, 0158549.
Bhakya, S., Muthukrishnan, S., Sukumaran, M., Muthukumar, M., Senthil Kumar, T., & Rao, M. V. (2015). Catalytic degradation of organic dyes using synthesized silver nanoparticles: A green approach. Journal of Bioremediation & Biodegradation, 6, 312. https://doi.org/10.4172/2155-6199.1000312
Bhargava, A., Jain, N., Khan, M. A., Pareek, V., Dilip, R. V., & Panwar, J. (2016). Utilizing metal tolerance potential of soil fungus for efficient synthesis of gold nanoparticles with superior catalytic activity for degradation of rhodamine B. Journal of Environmental Management, 183, 22–32.
Bishnoi, S., Kumar, A., & Selvaraj, R. (2018). Facile synthesis of magnetic iron oxide nanoparticles using inedible Cynometra ramiflora fruit extract waste and their photocatalytic degradation of methylene blue dye. Materials Research Bulletin, 97, 121–127.
Bisschops, I., & Spanjers, H. (2003). Literature review on textile wastewater characterization. Environmental Technology, 24, 1399–1411.
Bodoprost, H., & Rosemeyer, J. (2007). Analysis of phenacylester derivatives of fatty acids from human skin surface sebum by RP-HPLC: Chromatographic mobility as a function of physico-chemical properties. International Journal of Molecular Sciences, 8, 1111–1124.
Bonet, M., Bonfill, T., Nunez, M., De Verdonces, L., Mur, E., Gallardo, E., & Arenas, M. (2018). Curative radiation therapy for very elderly bladder cancer patients with localized disease. Clinical & Translational Oncology, 20, 899–905.
Borah, P., Kumar, M., & Devi, P. (2020). Recent trends in the detection and degradation of organic pollutants. In Abstement of environmental pollutants: Trends and strategies (pp. 67–79). Elsevier.
Buthelezi, S. P., Olaniran, A. O., & Pillay, B. (2012). Textile dye removal from wastewater using bioflocculants produced by indigenous bacterial isolates. Molecules, 17, 14260–14274.
Carmen, Z., & Daniela, S. (2012). Textile organic dyes—Characteristics, polluting effects and separation/elimination procedures from industrial effluents—A critical overview. In Organic pollutants ten years after the Stockholm convention—Environmental and analytical update (pp. 55–86). IntechOpen.
Chaibakhsh, N., Ahmadi, N., & Zanjanchi, M. A. (2014). Use of Plantago major L. as a natural coagulant for optimized decolorization of dye-containing wastewater. Industrial Crops and Products, 61, 169–175.
Chandanshive, V. V., Kadam, S. K., Khandare, R. V., Kurade, M. B., Jeon, V. B., Jadhav, J. P., & Govindwar, S. P. (2018). In-situ phytoremediation of dyes from textile wastewater using garden ornamental plants, effect on soil quality and plant growth. Chemosphere, 210, 968–976.
Chandanshive, V. V., Rane, N., Gholave, A., Patil, S., Jeon, B., & Givindwar, S. (2016). Efficient decolourization and detoxification of textile industry effluent by Salvinia molesta in lagoon treatment. Environmental Research, 150, 88–96.
Chang, G., Yue, B., Gao, T., Yan, W., & Pan, G. (2019). Phytoremediation of phenol by Hydrilla verticillata (L.f.) Royle and associated effects on physiological parameters. Journal of Hazardous Materials, 388, 121569.
Chanwala, J., Kaushik, G., Dar, M. A., Upadhyay, S., & Agarwal, A. (2019). Process optimization and enhanced decolorization of textile effluent by Planococcus sp. isolated from textile sludge. Environmental Technology and Innovation, 13, 122–129.
Cheera, P. K., Sreenivasulu, V., Govinda, S., Himageerish, K. K., Deepa, T., Vasantha Jyothi, N. V. V., & Venkateswarlu, P. (2018). Biosynthesis of the Fe3O4 nanoparticles using Acacia nilotica leaf extract and their effect on degradation of Congo red dye in aqueous solution. Trends in Textile Engineering and Fashion Technology, 1(3), 1–4.
Chen, S. H., & Ting, A. S. Y. (2015). Biodecolorization and biodegradation potential of recalcitrant triphenylmethane dyes by Coriolopsis sp. isolated from compost. Journal of Environmental Management, 150(1), 274–280.
Chethana, M., Sorokhaibam, L. G., Bhandari, V. M., Raja, S., & Ranade, V. V. (2016). Green approach to dye wastewater treatment using biocoagulants. ACS Sustainable Chemistry & Engineering, 4(5), 2495–2507.
Chin, S. F., Pang, S. C., & Tan, C. H. (2011). Green synthesis of magnetic nanoparticles (via thermal decomposition method) with controllable size and shape. Journal of Materials and Environmental Science, 2(3), 299–302.
Choudhary, B. C., Paul, D., Gupta, T., Tetgure, S. R., Garole, V. J., Borse, A. U., & Garole, D. J. (2016). Photocatalytic reduction of organic pollutant under visible light by green route synthesized gold nanoparticles. Journal of Environmental Sciences, 00914, 1–13.
Christian, P., Kammer, V. D., Baalousha, M., & Hofmann, T. (2008). Nanoparticles: Structure, properties, preparation and behavior in environmental media. Ecotoxicology, 17(5), 326–343.
Dan, S., Rong, Y., Feng, L., & Anna, Z. (2018). Applications of magnetic nanoparticles in surface-enhanced Raman scattering (SERS) detection of environmental pollutants. Journal of Environmental Sciences, 80, 14–34.
Davar, F., Majedi, A., & Mirzaei, A. (2015). Green synthesis of ZnO nanoparticles and its application in the degradation of some dyes. Journal of the American Ceramic Society, 98, 1739–1746.
David, L., & Moldovan, B. (2020). Green synthesis of biogenic silver nanoparticles for efficient catalytic removal of harmful organic dyes. Nanomaterials, 10(202), 1–16.
de Souza, M. T. F., Ambrosio, E., Freitas, T. K. F. S., Santos, L. B., Almeida, V. C., & Garcia, J. C. (2014). The use of a natural coagulant (Opuntia ficus-indica) in the removal for organic materials of textile effluents. Environmental Monitoring and Assessment, 186, 5261–5271.
Degermenci, G. D., Degermenci, N., Ayvaoglu, V., Durmaz, E., Çakır, D., & Akan, E. (2019). Adsorption of reactive dyes on lignocellulosic waste; characterization, equilibrium, kinetic and thermodynamic studies. Journal of Cleaner Production, 225, 1220–1229.
Deivasigamani, C., & Das, N. (2011). Biodegradation of Basic Violet 3 by Candida krusei isolated from textile wastewater. Biodegradation, 22, 1169–1180.
Desai, S. A. (2017). Isolation and characterization dye degrading bacteria for detoxification of dark red 2B. Bioscience Discovery, 8(3), 426–431.
Duran, N., Marcato, P. D., De Souza, G. I. H., Alves, O. L., & Esposito, E. (2007). Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. Journal of Biomedical Nanotechnology, 3, 203–208.
Ekambaram, S. P., Perumal, S. S., Rajendran, D., Samivel, D., & Khan, M. N. (2018). New approach of dye removal in textile effluent: A cost effective management for cleanup of toxic dyes in textile effluent by water hyacinth. In E. Bidoia & R. Montagnolli (Eds.), Toxicity and biodegradation testing (pp. 241–267). New York: Humana Press.
Fernandes, F. H., Botasso-Nasciutti, M. O., Svio, A. L. V., Souza, L. C. M., Fernandes Cal, J. R., Cardoso, F. F., Fontes, M. R. M., Albuquerque, A. F., Munari, C. C., & Kummrow, F. (2018). In vivo genotoxicity of a commercial C.I. Disperse Red 1 dye. Environmental and Molecular Mutagenesis, 59, 822–828.
Fernandez, C., Larrechi, M. S., & Callao, M. P. (2010). An analytical overview of processes for removing organic dyes from wastewater effluents. Trends in Analytical Chemistry, 29(10), 1202–1211.
Flaten, T. P. (2001). Aluminium as a risk factor in Alzheimer’s disease with emphasis on drinking water. Brain Research Bulletin, 55, 187–196.
Freitas, T. K. F. S., Oliveira, V. M., de Souza, M. T. F., Geraldino, H. C. L., Almeida, V. C., Favaro, S. L., & Garcia, J. C. (2015). Optimization of coagulation-flocculation process for treatment of industrial textile wastewater using okra (A.esculentus) mucilage as natural coagulant. Industrial Crops and Products, 76, 538–544.
Fulekar, M. H., Wadgaonkar, S. L., & Singh, A. (2013). Decolourization of dye compounds by selected bacterial strains isolated from dyestuff industrial area. International Journal of Advanced Research and Technology, 2(7), 182–192.
Gautam, P. K., Singh, A., Misra, K., Sahoo, A. K., & Samanta, S. K. (2019). Synthesis and applications of biogenic nanomaterials in drinking and wastewater treatment. Journal of Environmental Management, 231, 734–748.
Gelebo, G. G., & Ahmed, F. E. (2019). Removal of direct and reactive dyes from textile wastewater using Moringa stenopetala seed extract. Journal of Textile Engineering & Fashion Technology, 5(4), 184–191.
Geoffroy, L., Frankart, C., & Eullaffroy, P. (2004). Comparison of different physiological parameter responses in Lemna minor and Scenedesmus obliquus exposed to herbicide flumioxazin. Environmental Pollution, 131(2), 233–241.
Ghaedi, M., Fathi, M. R., Shokrollahi, A., & Shajarat, F. (2006). Highly selective and sensitive pre-concentration of mercury ion and determination by cold vapor atomic absorption spectroscopy. Analytical Letters, 39, 1171–1185.
Ghorbani, H. R., Pazoki, H., & Rad, A. S. (2017). Synthesis of magnetite nanoparticles by biological technique. Biosciences, Biotechnology Research Asia, 14(2), 631–633.
Ghosh, A., Dastidar, M. G., & Sreekrishnan, T. R. (2017). Bioremediation of chromium complex dyes and treatment of sludge generated during the process. International Biodeterioration and Biodegradation, 119, 448–460.
Gita, S., Shukla, S. P., Saharan, N., Prakash, C., & Deshmukhe, G. (2019). Toxic effects of selected textile dyes on elemental composition, photosynthetic pigments, protein content and growth of a freshwater Chlorophycean alga Chlorella vulgaris. Bulletin of Environmental Contamination and Toxicology, 102, 795–801.
Golmohammadi, M., Honarmand, M., & Ghanbari, S. (2019). A green approach to synthesis of ZnO nanoparticles using jujube fruit extract and their application in photocatalytic degradation of organic dyes. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 131(2), 233–241.
Golob, V., Vinder, A., & Simonic, A. (2005). Efficiency of the coagulation/flocculation method for the treatment of dye bath effluents. Dyes and Pigments, 67, 93–97.
Govindwar, S. P., & Kagalkar, A. N. (2010). Phytoremediation technologies for the removal of textile dyes: An overview and future prospectus (pp. 472–494). New York: Nova Science Publisher.
Guibal, E., & Roussy, J. (2007). Coagulation and flocculation of dye containing solutions using a biopolymer (chitosan). Reactive and Functional Polymers, 67, 33–42.
Gupta, V. K., & Suhas. (2009). Application of low-cost adsorbents for dye removal- A review. Journal of Environmental Management, 90, 2313–2342.
Hamed, M. A. (2005). Pre-concentration and separation of Fe (III), Co (II), Ni (II) and Zn (II) by solid phase extraction using silica modified with N-propylsalicylaldimine. Egyptian Journal of Aquatic Research, 31(1), 31–41.
Hilal, N. M., Ahmed, I. A., & Badr, E. E. (2012). Removal of acid dye (AR37) by adsorption onto potatoes and egg husk: A comparative study. Journal of American Science, 8, 341–348.
Hu, J., Lo, I. M. C., & Chen, G. (2007). Comparative study of various magnetic nanoparticles for Cr (VI) removal. Separation and Purification Technology, 56, 249–256.
Huang, H., Schwab, K., & Jacangelo, J. G. (2009). Pretreatment for low pressure membranes in water treatment: A review. Environmental Science & Technology, 43, 3011–3019.
Huang, L., Weng, X., Chen, Z., Megharaj, M., & Naidu, R. (2014). Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 117, 801–804.
Huang, X., Chang, X., He, Q., Cui, Y., Zhai, Y., & Jiang, N. (2008). Tris (2-aminiethyl) amine funtionalized silica gel for solid-phase extraction and pre-concentration of Cr (III), Cd (II) and Pb (II) from water. Journal of Hazardous Materials, 157, 154–160.
Huang, Z., Li, Y., Chen, W., Shi, J., Zhang, N., Wang, X., Li, Z., Gao, L., & Zhang, Y. (2017). Modified bentonite adsorption of organic pollutants of dye wastewater. Materials Chemistry and Physics, 202, 266–276.
Hunger, K. (2003). Industrial dyes: Chemistry, properties, application. Weinheim: Willey-VCH Verlag GmbH and Co.KGaA.
Imron, M. F., Kurniawan, S. B., Soegianto, A., & Wahyudianto, F. E. (2019). Phytoremediation of methylene blue using duckweed (Lemna minor). Heliyon, 5, 02206.
Imtiazuddin, S. M., Mumtaz, M., & Mallick, K. A. (2012). Pollutants of wastewater characteristics in textile industries. Journal of Basic and Applied Sciences, 8, 554–556.
Jain, K., Johnson, J., Devpura, N., Rathour, R., Desai, C., Tiwari, O., & Madamwar, D. (2020). Emerging bioremediation technologies for the treatment of wastewater containing synthetic organic compounds. In Emerging technologies in environmental bioremediation (pp. 131–150). Elsevier.
Jebali, A., Ramezani, F., & Kazemi, B. (2011). Biosynthesis of silver nanoparticles by Geotricum sp. Journal of Cluster Science, 22, 225–232.
Jyoti, K., & Singh, A. (2016). A Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. Journal, Genetic Engineering & Biotechnology, 14(311), 317.
Kabra, A., Khandare, R., & Govindwar, S. (2013). Development of a bioreactor for remediation of textile effluent and dye mixture: A plant-bacterial synergistic strategy. Water Research, 47, 1035–1048.
Kagalkar, A. N., Jagtap, U., Jadhav, J. P., & Bapat, V. A. (2009). Biotechnological strategies for phytoremediation of the sulfonated azo dye Direct Red 5B using Blumea malcolmii Hook. Bioresource Technology, 100(18), 4104–4110.
Kagalkar, A. N., Jagtap, U. B., Jadhav, J. P., Govindwar, S. P., & Bapat, S. A. (2010). Studies on phytoremediation potentiality of Typhonium flagelliforme for the degradation of Brilliant Blue R. Planta, 232(1), 271–285.
Kalaicelvi, A., Sivasathya, B., & Kavitha, K. K. (2016). Bioremediation of colour removal in dye effluent by Moringa oleifera seed powder. International Journal of Agricultural and Life Sciences, 2(4), 101–105.
Kalme, S., Ghodake, G., & Govindwar, S. (2007). Red HE7B degradation using desulfonation by Pseudomonas desmolyticum NCIM 2112. International Biodeterioration & Biodegradation, 60, 327–333.
Kalyani, D. C., Patil, P. S., Jadhav, J. P., & Govindwar, S. P. (2008). Biodegradation of reactive textile dye Red BLI by an isolated bacterium Pseudomonas sp. SUK1. Bioresource Technology, 99, 4635–4641.
Kamat, R. B. (2014). Phytoremediation for dye decolorization (Ph.D. thesis). Kansas State University, Manhattan.
Kang, M., Kamei, T., & Magara, Y. (2003). Comparing polyaluminium chloride and ferric chloride for antimony removal. Water Research, 37, 4171–4179.
Khan, Z. U. H., Khan, A., Shah, A., Wan, P., Chen, Y., Khan, G. M., Khan, A. U., Tahir, K., Muhammad, N., & Khan, H. U. (2016). Enhanced photocatalytic and electrocatalytic applications of green synthesized silver nanoparticles. Journal of Molecular Liquids, 220, 248–257.
Khataee, A. R., Movafeghi, A., Torbati, S., Lisar, S. S. Y., & Zarei, M. (2012). Phytoremediation potential of duckweed (Lemna minor L.) in degradation of C.I. Acid Blue 92: Artificial neural network modeling. Ecotoxicology and Environmental Safety, 80, 291–298.
Khatoon, N., & Sardar, M. (2017). Efficient removal of toxic textile dyes using silver nanocomposites. Journal of Nanosciences: Current Research, 2, 2.
Kiran, S., Rafique, M. A., & Iqbal, S. (2020). Synthesis of nickel nanoparticles using Citrullus colocynthis stem extract for remediation of Reactive Yellow 160 dye. Environmental Science and Pollution Research, 27, 32998–33007.
Kocaman, S. (2020). Removal of methylene blue dye from aqueous solutions by adsorption on levulinic acid-modified natural shells. International Journal of Phytoremediation, 22, 885–895.
Kumara, N., Sinhaa, S., Mehrotraa, T., Singha, R., Tandonb, S., & Thakur, I. S. (2019). Biodecolorization of azo dye Acid Black 24 by Bacillus pseudomycoides: Process optimization using Box Behnken design model and toxicity assessment. Bioresource Technology Reports, 8, 1–10.
Kurita, K. (2006). Chitin and chitosan: Funtional bioploymers from marine crustaceans. Marine Biotechnology, 8, 203–226.
Kyzas, G. Z., Fu, J., & Matis, K. A. (2013). The change from past to future for adsorbent materials in treatment of dyeing wastewaters. Materials, 6, 5131–5158.
Laoufi, I., Saint Lager, M. C., Lazzari, R., Jupille, J., Robach, O., & Garaudee, S. (2011). Size and catalytic activity of supported gold nanoparticles: An in operand study during CO oxidation. Journal of Physical Chemistry C, 115, 4679–4679.
Le Marechal, A. M., Križanec, B., Vajnhandl, S., & Valh, J. V. (2012). Textile finishing industry as an important source of organic pollutants. In T. Puzyn (Ed.), Organic pollutants ten years after the stockholm convention – Environmental and analytical update. InTech.
Le, O. T. H., Tran, L. N., Doan, V. T., Pham, Q. V., Ngo, A. V., & Nguyen, H. H. (2020). Mucilage extracted from dragon fruit peel (Hylocereus undatus) as flocculant for treatment of dye wastewater by coagulation and flocculation process. International Journal of Polymer Science, 4, 1–9.
Mahajan, P., & Kaushal, J. (2016). Phytoremediation potential of Hydrilla verticillata for the degradation of contaminants from textile effluent. Research Journal, 2(2), 69–74.
Mahajan, P., Kaushal, J., Upmanyu, A., & Bhatti, J. (2019). Assessment of phytoremediation potential of Chara vulgaris to treat toxic pollutants of textile effluent. Journal of Toxicology, 2019, 1–11.
Mahmood, Q., Zheng, P., Islam, E., Hayat, Y., Hassan, M. J., Jilani, G., & Jin, R. C. (2005). Lab scale studies on Water Hyacinth (Eichhornia crassipes Marts Solms) for biotreatment of textile wastewater. Caspian Journal of Environmental Sciences, 3(2), 83–88.
Mahmood, R., Sharif, F., Ali, S., Hayyat, M. U., & Cheema, T. A. (2012). Isolation of indigenous bacteria and consortia development for decolorization of textile dyes. Biologia (Pakistan), 58(1–2), 53–60.
Mahmoodi, N. M., Salehi, R., Arami, M., & Bahrami, H. (2011). Dye removal from coloured textile wastewater using chitosan in binary systems. Desalination, 267, 64–72.
Mahmoudabadi, T. Z., Abbasi, F., & Talebi, P. J. M. (2019a). Effectiveness of Plantago major extract as a natural coagulant in removal of Reactive Blue 19 dye from wastewater. International journal of Environmental Science and Technology, 16, 1–8.
Mahmoudabadi, T. Z., Talebi, P., & Jalili, M. (2019b). Removing Disperse red 60 and Reactive blue 19 dyes removal by using Alcea rosea root mucilage as a natural coagulant. AMB Express, 9, 113.
Mamania, J. B., Costa-Filhob, A. J., Cornejoc, D. R., Vieirad, E. D., & Gamarraa, L. F. (2013). Synthesis and characterization of magnetite nanoparticles coated with lauric acid. Materials Characterization, 81, 28–36.
Mandal, D., Bolander, M. E., Mukhopahyay, D., Sarkar, G., & Mukherjee, P. (2006). The use of microorganisms for the formation of metal nanoparticles and their application. Applied Microbiology and Biotechnology, 69, 485–492.
Manholer, D. D., de Souza, M. T. F., Ambrosio, E., de Souza Freitas, T. K. F., Geraldino, H. C. L., & Garcia, J. C. (2019). Coagulation/flocculation of textile effluent using a natural coagulant extracted from Dillwnia indica. Water Science and Technology, 80(5), 979–988.
Marcucci, M., Ciardelli, G., Matteucci, A., Ranieri, L., & Russo, M. (2002). Experimental campaigns on textile wastewater for reuse by means of different membrane processes. Desalination, 149(1–3), 137–143.
Mattioli, D., Malpei, F., Borone, G., & Rozzi, A. (2002). Water minimisation and reuse in the textile industry. In P. Lens, L. H. Pol, P. Wilderer, & T. Asano (Eds.), Water recycling and resource recovery in industry. Cornwall: IWA Publishing.
McHenry, M. E., & Laughlin, D. E. (2000). Nano-scale materials development for future magnetic applications. Acta Materialia, 48(1), 223–238.
Miyah, Y., Lahrichi, A., Idrissi, M., Khalil, A., & Zerrouq, F. (2018). Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: Equilibrium and kinetic studies. Surfaces and Interfaces, 11, 74–81.
Munagapati, V. S., Wen, J. C., Pan, C. L., Gutha, Y., Wen, J. H., & Reddy, G. M. (2019). Adsorptive removal of anionic dye (Reactive Black 5) from aqueous solution using chemically modified banana peel powder: Kinetic, isotherm, thermodynamic, and reusability studies. International Journal of Phytoremediation, 22(3), 267–278.
Muthusamy, S., Govindaraj, D., & Rajendran, D. (2018). Phytoremediation of textile dye effluents. In S. J. Varjani et al. (Eds.), Bioremediation: Applications for environmental protection and managements, energy, environment and sustainability (pp. 359–373). Springer Nature Singapore Pt Ltd., Springer.
Nigam, P., Armour, G., Banat, I. M., Singh, D., & Marchan, R. (2000). Physical removal of textile dyes and solid state fermentation of dye-adsorbed agricultural residues. Bioresource Technology, 72, 219–226.
Ozkan, Z. Y., Cakirgoz, M., Kaymak, E. S., & Erdim, E. (2017). Rapid decolorization of textile wastewater by green synthesized iron nanoparticles. Water Science and Technology, 77, 511–517.
Paczkowska, M., Kozłowska, M., & Golinski, P. (2007). Oxidative stress enzyme activity in Lemna minor L. exposed to cadmium and lead. Acta Biologica Cracoviensia Series Botanica, 49(2), 33–37.
Pandey, B. V., Dubey, M. K., & Upadhyay, R. S. (2019). Bioremediation of textile dye procion red yellow by using Pseudomonas fluorescens. Proceedings of the National Academy of Sciences / B. Section B, Biological Sciences, 90, 135–141.
Park, Y., Hong, Y., Weyers, A., Kim, Y., & Linhardt, R. (2011). Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnology, 5, 69–78.
Patel, H., & Vashi, R. T. (2010). Treatment of textile wastewater by adsorption and coagulation. European Journal of Chemistry, 7(4), 1468–1476.
Patel, S. V., & Adhvaryu, R. M. (2016). Removal of textile dye by using Eichhornia spp. and Pistia spp. by Aquatic Macrophytes Treatment Systems (AMTS) – An eco-friendly technique. International Journal of Scientific and Engineering Research, 4(7), 62–66.
Patil, S. M., Suryavanshi, M. V., Chandanshive, V. V., Kurade, M. B., Govindwar, S. P., & Jeon, B. H. (2020). Regeneration of textile wastewater deteriorated microbial diversity of soil microcosm through bioaugmentation. Chemical Engineering Journal, 380, 1–12.
Pereira, L., & Alves, M. (2012). Dyes-environmental impact and remediation. In A. Malik & E. Grohmann (Eds.), Environmental protection strategies for sustainable development, strategies for sustainability (pp. 111–162). Springer.
Phalakornkule, C., Polgumhaug, S., Tongdaung, W., Karakat, B., & Nuyut, T. (2010). Electrocoagulation of blue reactive, red disperse and mixed dyes and application in treating textile effluent. Journal of Environmental Management, 91, 918–926.
Phugare, S. S., Kalyani, D. C., Patiol, A. V., & Jadhav, J. P. (2011). Textile dye degradation by bacterial consortium and subsequent toxicological analysis of dye and dye metabolites using cytotoxicity, genotoxicity and oxidative stress studies. Journal of Hazardous Materials, 186, 713–723.
Ponnusamy, S. K. (2014). Adsorption of methylene blue dye onto surface modified cashew nut shell. Environmental Engineering and Management Journal, 13, 545–556.
Punzi, M. (2015). Treatment of textile wastewater by combining biological process and advanced oxidation (Ph.D. thesis).
Qasim, W., & Mane, A. V. (2013). Characterization and treatment of selected food industrial effluents by coagulation and adsorption techniques. Water Resources and Industry, 4, 1–12.
Rafique, M., Shafiq, F., Gillani, S. S. A., Shakil, M., Tahir, M. B., & Sadaf, I. (2019). Eco-friendly green and biosynthesis of copper oxide nanoparticles using Citrofortunella microcarpa leaves extract for efficient photocatalytic degradation of Rhodamin B dye form textile wastewater. Optik, 99, 1313–1324.
Rai, M. S., Bhat, P. R., Prajna, P. S., Jayadev, K., & Rao, P. S. V. (2014). Degradation of malachite green and congo red using Aloe barabadensis mill extract. International Journal of Current Microbiology and Applied Sciences, 3(4), 330–340.
Raichurkar, P., & Ramachandran, N. (2015). Recent trends and development in textile industry in India. International Journal on Textile Engineering and Processes, 1(4), 47–50.
Ramavandi, B. (2014). Treatment of water turbidity and bacteria by using a coagulant extracted from Plantago ovate. Water Resources and Industry, 6, 36–50.
Rane, N., Chandanshive, V., Khandare, R., Gholave, A., Yadav, S., & Govindwar, S. (2014). Green remediation of textile dyes containing wastewater by Ipomoea hederifolia L. RSC Advances, 4, 36623–36632.
Rane, N., Chandanshive, V., Watharkar, A., Khandare, R., Patil, T., Pawar, P., & Govindwar, S. (2015). Phytoremediation of sulfonated Remazol Red dye and textile effluents by Alternanthera philoxeroides: An anatomical, enzymatic and pilot scale study. Water Research, 83, 271–281.
Rivera, M., Pazos, M., & Sanroman, M. A. (2011). Development of an electrochemical cell for the removal of Reactive Black 5. Desalination, 274(1), 39–43.
Robinson, T., McMullan, G., Marchant, R., & Nigam, P. (2001). Remediation of dyes in textile effluents: A critical review on current treatment technologies with a proposed alternative. Bioresource Technology, 77, 247–255.
Rotello, V. M. (2004). Nanoparticles: Building blocks for nanotechnology. New York: Springer Science and Business Media.
Roy, R., Fakhruddin, A. N. M., Khatuni, R., & Islam, M. S. (2010). Reduction of COD and pH of textile industrial effluents by aquatic macrophytes and algae. Journal of Bangladesh Academy of Sciences, 34(1), 9–14.
Sachin, M. K., Gaikwad, R. W., & Misal, S. A. (2010). Low cost sugarcane bagasse ash as an adsorbent for dye removal from dye effluent. International Journal of Chemical Engineering and Applications, 1(4), 309–318.
Saeed, A., Sharif, M., & Iqbal, M. (2010). Application potential of grapefruit peel as dye sorbent: Kinetics, equilibrium and mechanism of crystal violet adsorption. Journal of Hazardous Materials, 179, 564–572.
Saif, S., Tahir, A., & Chen, Y. (2016). Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials, 6(209), 1–26.
Sanghi, R., Bhatttacharya, B., Dixit, A., & Singh, V. (2006). Ipomoea dasysperma seed gum: An effective natural coagulant for the decolorisation of textile dye solutions. Journal of Environmental Management, 81, 36–41.
Santhanama, M., Selvaraj, R., Veerasubbian, V., & Sundaram, M. (2019). Bacterial degradation of electrochemically oxidized textile effluent: Performance of oxic, anoxic and hybrid oxic-anoxic consortium. Chemical Engineer, 355, 186–195.
Sapna, M. M., Sonal, G. C., & Raut, P. D. (2012). Use of Moringa oleifera (drumstick) seed as natural absorbent and an antimicrobial agent for ground water treatment. Research Journal of Recent Sciences, 1, 31–40.
Saratale, R. G., Saratale, G. D., Chang, J. S., & Govindwar, S. P. (2011). Bacterial decolourization and degradation of azo dyes: A review. Journal of the Taiwan Institute of Chemical Engineers, 42, 138–157.
Sathiyaraj, G., Ravindran, C. K., & Malik, Z. H. (2017). Physico-chemical characteristics of textile effluent collected from Erode, Pallipalayam and Bhavani polluted regions, Tamil Nadu, India. Journal of Ecobiotechnology, 9, 1–4.
Senthilkumar, P., Varjani, S. J., & Suganya, S. (2018). Treatment of dye wastewater using an ultrasonic aided nanoparticle stacked activated carbon: Kinetic and isotherm modeling. Bioresource Technology, 250, 716–722.
Sethi, S., Shubhum, Malviya, M. M., Sharma, N., & Gupta, S. (2012). Biodecolorization of Azo dye by microbial isolates from textile effluent and sludge. Universal Journal of Environmental Research and Technology, 2(6), 582–590.
Shamsnejati, S., Chaibakhsh, N., Pendashteh, A. R., & Hayeripour, S. (2015). Mucilaginous seed of Ocimum basilicum as a natural coagulant for textile wastewater treatment. Industrial Crops and Products, 69, 40–47.
Shanmugam, L., Ahire, M., & Nikam, T. (2019). Bacopa monnieri (L.) Pennell, a potential plant species for degradation of textile azo dyes. Environmental Science and Pollution Research, 27, 9349–9363.
Sharma, B., & Deswal, R. (2018). Single pot synthesized gold nanoparticles using Hippophae rhamnoides leaf and berry extract showed shape-dependent differential nanobiotechnological applications. Artificial Cells, Nanomedicine, and Biotechnology, 46(2), 408–410.
Shen, Y. F., Tang, J., Nie, Z. H., Wang, Y. D., Ren, Y., & Zuo, L. (2009). Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Separation and Purification Technology, 68, 312–319.
Sinha, R. K., Herat, S., & Tandon, P. K. (2007). Phytoremediation: role of plants in contaminated site management. In S. N. Singh & R. D. Tripathi (Eds.), Environmental bioremediation technologies (pp. 315–330). Berlin, Heidelberg: Springer.
Sureshvarr, K., Bharathiraja, B., Jayakumar, M., Jayamuthunagai, J., & Balaji, L. (2010). Removal of azo dye compounds from paper industries waste using phytoremediation methodology. International Journal of Chemical Sciences, 8(1), 687–700.
Taher, A., Mohsin, M., Farooqui, M., & Farooqui, M. (2010). Studies on the isotherms, kinetics and thermodynamics of adsorption of crystal violet on low cost materials. Journal of Advanced Scientific Research, 3, 36–44.
Tangahu, B. V., Abdullah, S. R. S., Basri, H., Idris, M., Anuar, N., & Mukhlisin, M. (2011). A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. International Journal of Chemical Engineering, 2011, 1–31.
Tehrani-Bagha, N., & Mahmoodi, F. (2010). Degradation of a persistent organic dyes form colored textile wastewater by ozonation. Desalination, 260, 34–38.
Telke, A. A., Joshi, S. M., Jadav, S. U., Tamboli, D. P., & Govindwar, S. P. (2009). Decolorization and detoxification of Congo red and textile industry effluent by an isolated bacterium Pseudomonas sp. SU-EBT. Biodegradation, 21, 283–296.
Torok, A., Buta, E., Indolean, C., Tonk, S., Dumitrescu, L. S., & Majdik, C. (2015). Biological removal of Triphenylmethane dyes from aqueous solution by Lemna minor. Acta Chimica Slovenica, 62, 452–461.
Ugya, A. Y., Hua, X., & Ma, J. (2017). Phytoremediation as a tool for the remediation of wastewater resulting from dyeing activities. Applied Ecology and Environmental Research, 17, 3723–3735.
Vafaei, F., Khataee, A. R., Movafeghi, A., Lisar, S. Y. S., & Zarei, M. (2012). Bioremoval of an azo dye by Azolla filiculoides: Study of growth, photosynthetic pigments and antioxidant enzymes status. International Biodeterioration & Biodegradation, 75, 194–200.
Vasanthy, M., Santhiya, M., Swabna, V., & Geetha, A. (2011). Phytodegradation of textile dyes by water Hyacinth (Eichhornia crassipes) from aqueous dye solutions. International Journal of Environmental Sciences, 1, 1702–1717.
Velmurugan, P., Kumar, V. R., & Dhinakaran, G. (2011). Dye removal from aqueous solution using low cost adsorbent. International Journal of Environmental Sciences, 1(7), 1492.
Verma, A. K., Dash, R. R., & Bhunia, P. (2012). A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. Journal of Environmental Management, 93(1), 154–168.
Verma, A. K., Raghukumar, C., & Naik, C. G. (2011). A novel hybrid technology for remediation of molasses-based effluents. Bioresource Technology, 102, 2411–2418.
Vieira, A. P., Santana, S. A. A., Bezerra, C. W. B., Silva, H. A. S., Chaves, J. A. P., Melo, J. C. P., Filho, E. C. S., & Airoldi, C. (2011). Removal of textile dyes from aqueous solution by babassu coconut epicarp, Orbignya speciosa. Chemical Engineering Journal, 173, 334–340.
Vijayaraghavan, T., Sivakumar, A., & Kumar, V. (2011). Application of plant based coagulants for wastewater treatment. International Journal of Advanced Engineering Research and Studies, 1(1), 88–92.
Vikranta, K., Giria, B. S., Razab, N., Roya, K., Kimd, K. H., Raia, B. N., & Singha, R. S. (2018). Recent advancements in bioremediation of dye: Current status and challenges. Bioresource Technology, 253, 355–367.
Wadje, P. R. (2009). Textile-fiber to fabric processing. Journal of The Institution of Engineers (India): Series D, 90, 28–36.
Wang, W., Yue, Q., Li, R., Song, W., Gao, B., & Shen, X. (2018). Investigating coagulation behavior of chitosan with different Al species dual-coagulants in dye wastewater treatment. Journal of the Taiwan Institute of Chemical Engineers, 78, 423–430.
Watharkar, A. D., Kadam, S. K., Khandare, R. V., Kolekar, P. D., Jeon, B. H., Jadhav, J. P., & Govindwar, S. P. (2018). Asparagus densiflorus in a vertical subsurface flow phytoreactor for treatment of real textile effluent: A lab to land approach for in situ soil remediation. Ecotoxicology and Environmental Safety, 161, 70–77.
Wu, J., Ma, L., Chen, Y., Cheng, Y., Liu, Y., & Zha, X. (2016). Catalytic ozonation of organic pollutants from bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst: Removal and pathways. Water Research, 92, 140–148.
Yang, C. F., Liu, S. H., Su, Y. M., Lin, Y. R., & Lin, K. L. (2019). Bioremediation capability evaluation of benzene and sulfolane contaminated groundwater: Determination of bioremediation parameters. The Science of the Total Environment, 648, 811–818.
Yang, J. X., & Hong, G. B. (2018). Adsorption behavior of modified Glossogyne tenuifolia leaves as a potential biosorbent for the removal of dyes. Journal of Molecular Liquids, 252, 289–295.
Yantasee, W., Warner, C. L., Sangvanich, T., Addleman, R. S., Carter, T. G., Wiacek, R. J., Fryxell, G. E., Timchalk, C., & Warner, M. G. (2007). Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles. Environmental Science & Technology, 41(14), 5114–5119.
Yaseen, D. A., & Scholz, M. (2018). Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. International journal of Environmental Science and Technology, 16, 1193–1226.
Yin, C. Y. (2010). Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochemistry, 45, 1437–1444.
Zaharia, C., Suteu, D., Muresan, A., Muresan, R., & Popescu, A. (2009). Textile wastewater treatment by homogenous oxidation with hydrogen peroxide. Environmental Engineering and Management Journal, 8(6), 1359–1369.
Zahrim, A. Y., Tizaoui, C., & Hilal, N. (2011). Coagulation with polymers for nanofiltration pre-treatment of highly concentrated dyes: A review. Desalination, 266, 1–16.
Zhou, L., Zhou, H., & Yang, X. (2019). Preparation and performance of a novel starch-based inorganic/organic composite coagulant for textile wastewater treatment. Separation and Purification Technology, 210, 93–99.
Acknowledgment
The authors are grateful to Dr. M. Vasanthy, Associate Professor, Department of Environmental Biotechnology, Bharathidasan University, Trichy, for precious guidance.
Conflict of Interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ahila, K.G., Vinodini, S.K., Ancy Jenifer, A., Thamaraiselvi, C. (2022). An Overview on Eco-friendly Remediation of Toxic Organic Contaminants from Textile Dyeing Industry Wastewater. In: Vasanthy, M., Sivasankar, V., Sunitha, T.G. (eds) Organic Pollutants. Emerging Contaminants and Associated Treatment Technologies. Springer, Cham. https://doi.org/10.1007/978-3-030-72441-2_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-72441-2_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-72440-5
Online ISBN: 978-3-030-72441-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)