Abstract
The aim of the diagnostic work-up of cholangiocarcinoma (CCA) is diagnosis and staging of the disease as (borderline) resectable, locally advanced (i.e., unresectable), or metastatic. The focus of this chapter is on the various challenges often encountered in this diagnostic work-up. For instance, one major challenge is that CCA involves three disease entities that differ in genomic alterations, signs, and symptoms: intrahepatic (iCCA), perihilar (pCCA), and distal cholangiocarcinoma (dCCA). A second challenge is that it is frequently difficult to obtain adequate tissue for a definitive diagnosis. A third challenge is that several nonmalignant diseases can masquerade as CCA. These and other challenges are illustrated herein with a series of case presentations which also help highlight the importance of a multidisciplinary effort of gastroenterologists, surgeons, medical oncologists, radiologists, and pathologists. In some cases, despite a meticulous patient history, physical examination, and evaluation of imaging and laboratory tests by multidisciplinary experts, a diagnosis cannot be established with absolute certainty, and treatment may sometimes be started based on CCA being the most likely diagnosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cholangiocarcinoma
- Unknown primary tumor
- Adenocarcinoma
- Fasciola hepatica
- Echinococcus
- Liver cirrhosis
- Cholelithiasis
- IgG4-related sclerosing cholangitis
- Cholangitis
Introduction
The previous chapters in this book have focused on one of the three main diagnostic modalities for CCA: biochemical (Chap. 6), imaging (Chap. 7), and pathological (Chap. 8). In the present chapter, we discuss how these three modalities come together in the diagnostic work-up of patients with a suspicion of CCA, with a focus on important pitfalls.
The aim of the diagnostic work-up of CCA is efficient diagnosis and staging of the disease to guide subsequent management. This work-up presents several challenges. The first diagnostic challenge is that CCA involves three disease entities that differ in genomic alterations, signs, and symptoms; intrahepatic (iCCA), perihilar (pCCA), and distal cholangiocarcinoma (dCCA). Therefore, they each have their own diagnostic approaches and AJCC staging system (Chap. 8). Moreover, distinguishing these different entities is important to guide proper management. The main aspect they share is that they all arise from the epithelial lining of the biliary tree, albeit in different anatomical locations: iCCA arises upstream from the second-order biliary ducts, then pCCA until the origin of the cystic duct, and then dCCA until the ampulla. These boundaries are ambiguous, and at the interface the diagnoses cannot be discerned with certainty.
The second challenge is that a definitive diagnosis can only be made with pathological evaluation. This, in itself, comprises two challenges. Firstly, for most other cancers, it is straightforward to obtain tissue, because they are mostly large and have easy access for tissue acquisition. pCCA and dCCA, however, are mostly small lesions with periductal growth (rather than mass-forming) and are difficult to visualize and biopsy. Despite the effort to obtain enough tissue by means of brushing, intraductal biopsies, and/or endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA), sensitivity does not exceed 74% (Chap. 8). Secondly, even when enough tissue has been obtained, the pathological evaluation can be challenging. For example, iCCA is the only CCA that is typically large and mass-forming, but at pathological evaluation, iCCA may be difficult to distinguish from hepatocellular carcinoma (HCC) or metastatic disease (e.g., pancreatic cancer).
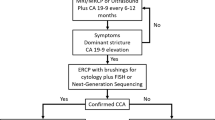
The third challenge is that several nonmalignant diseases can masquerade as CCA. For example, a dominant stricture in primary sclerosing cholangitis (PSC) can be benign or due to pCCA (or dCCA) [1,2,3,4,5,6]. After repeated failed attempts of pathological confirmation, the suspicion of CCA can remain high enough to justify resection. Therefore, approximately 5–10% of patients undergo a major hepatic or pancreatic resection for a disease that may turn out to be benign at pathological assessment of the resected specimen [7].
In this chapter we first discuss the current standard diagnostic work-up of iCCA, pCCA, and dCCA. The focus of the chapter is thereafter on the challenges of the diagnostic work-up. These challenges are illustrated with case presentations.
Standard Diagnostic Work-Up
The aim of the diagnostic work-up is to confidently diagnose and stage the disease in order to guide subsequent treatment. Staging should distinguish patients with (borderline) resectable, locally advanced (i.e., unresectable), and metastatic disease. Patients with (borderline) resectable CCA are considered for curative-intent resection, patients with locally advanced disease for systemic treatment (palliative and sometimes for induction) or locoregional treatments, and patients with metastatic disease for palliative systemic treatment.
iCCA
Patients with iCCA mostly present with nonspecific abdominal complaints and weight loss. Approximately 15% of iCCA patients present with jaundice, because the tumor is growing toward the liver hilum. Carbohydrate antigen (CA) 19.9 is elevated in most patients, and about 5% of patients have elevated alpha-fetoprotein (AFP) above 200 ng/mL [8]. At imaging, one or multiple lesions are seen in the liver. Multiple lesions can involve a single large lesion with smaller nearby satellites or several lesions spread across the liver. Computed tomography (CT) imaging demonstrates peripheral enhancement of the lesions with subsequent central filling (Chap. 7). CT of the abdomen and chest can detect nodal and distant metastases (e.g., lung and peritoneum). Enlarged locoregional lymph nodes can be present in the hepatoduodenal and gastrohepatic ligament. More distant lymph nodes (e.g., aortocaval) represent metastatic disease (stage IV) [9]. Magnetic resonance imaging (MRI) is unlikely to change management based on CT. A positron emission tomography (PET) scan is not part of the standard work-up but may detect metastatic disease in some patients [10]. Imaging can generally distinguish iCCA from HCC. The other differential diagnosis of iCCA is metastatic disease with primary tumors in the colon, esophagus, stomach, pancreas, and breast. Upper and lower endoscopy and mammography are recommended if imaging is not consistent with iCCA [11, 12].
A solitary lesion of iCCA is resectable if a margin-negative resection is anticipated with an adequate liver remnant volume (Chap. 14). Selected patients with two or three lesions can also be considered for resection. A biopsy is unlikely to change management if presentation and imaging are consistent with iCCA. Most patients have locally advanced or metastatic disease. These patients should undergo biopsy if they are eligible for locoregional or systemic treatment. Pathological examination of iCCA is difficult, as no pathognomonic immunohistochemistry markers are available (Chap. 8). The diagnosis is mainly made by ruling out HCC and metastatic disease. Historically, iCCA has often been mislabeled as adenocarcinoma of unknown primary (ACUP). Sometime iCCA is detected incidentally in patients with liver cirrhosis who undergo surveillance for early detection of HCC. These lesions tend to be small and can be difficult to distinguish from HCC.
pCCA
Patients with pCCA mostly present with painless jaundice and often weight loss. Blood tests generally show elevated bilirubin, except in the earliest of stages. Ultrasound shows dilated intrahepatic bile ducts, and sometimes a mass can be visualized in the liver hilum. The extrahepatic bile duct and gallbladder are not distended, contrary to distal bile duct obstruction in dCCA and pancreatic cancer. CT of the abdomen may show a small mass (typically less than 3 cm) at the confluence of the left and right bile duct, with proximal biliary dilatation. Masses larger than 3 cm mostly represent iCCA or metastases that have grown toward the liver hilum. CT of the chest is performed to rule out metastatic disease. Patients with metastatic disease may have metastases in the liver, lung, peritoneum, or lymph nodes beyond the hepatoduodenal ligament (e.g., aortocaval). PET scans may detect otherwise occult metastatic disease in a small percentage of patients but are not routinely recommended.
Analogous to iCCA, pCCA is resectable if a complete resection with adequate future liver remnant appears feasible. Resectability of pCCA depends on biliary extension of the tumor, as defined by the Bismuth classification [13]. Resectability also depends on vascular involvement; portal vein reconstruction may be required for a margin-negative resection. Hepatic artery reconstruction is less commonly performed as it increases surgical risk and has worse long-term survival. While CT appears more reliable to assess vascular involvement, magnetic resonance cholangiopancreatography (MRCP) provides superior delineation of the extent of biliary involvement.
Most patients require biliary drainage prior to resection or systemic treatment. An intraductal brushing or biopsy should be performed at the time of drainage but has a sensitivity below 74%, even when adding FISH or NGS (Chap. 8). Percutaneous biliary drainage should be avoided in patients eligible for liver transplantation, because it may cause seeding along the biopsy tract [14]. The differential diagnosis includes several nonmalignant diseases such as IgG4-mediated cholangitis and stone disease [7]. Presentation and imaging may provide clues for the likelihood of benign disease.
It is important to complete all imaging prior to biliary drainage, as stents distort imaging [15]. Surgical resection is sometimes performed in the absence of pathological confirmation of cancer, [7] in particular when presentation and imaging are highly suspicious for pCCA. Metastatic disease (e.g., intrahepatic metastases) should be confirmed by biopsy to avoid withholding surgery for distant lesions that may appear malignant but are in fact benign.
dCCA
Patients with dCCA typically present with painless jaundice and weight loss. Serum bilirubin level is elevated. Ultrasound and CT demonstrate dilated intra- and extrahepatic bile ducts and a distended gallbladder, without an obvious mass in the head of the pancreas. CT may also demonstrate an intraductal mass, stricture, or enhancing wall thickening. CT of the abdomen and chest can detect distant metastatic disease (i.e., in the liver, lungs, or peritoneum). Most patients, however, do not have metastatic disease at presentation, because a small dCCA quickly causes jaundice, thereby prompting clinical evaluation. MRI and PET have no substantial impact on diagnosis and staging of dCCA.
An intraductal brushing or biopsy to confirm malignancy can be performed with endoscopic retrograde cholangiopancreatography (ERCP) (or percutaneously when ERCP is not feasible). However, sensitivity of a brushing is below 50% and of an intraductal biopsy no more than 75% (Chap. 8). Results of such specimens may repeatedly come back as negative or inconclusive. Moreover, ERCP is an invasive procedure with a 3–8% risk of pancreatitis, in addition to other potential adverse events [16]. EUS with FNA is a good alternative for pathological confirmation in dCCA, with a comparably high sensitivity and specificity (82% and 88%) reported in some studies [17, 18]. Importantly, if cancer is highly likely based on presentation and imaging, it will remain (highly) likely even after an inconclusive brush or biopsy; therefore, an ERCP with brush or biopsy is only justified if biliary drainage is needed or if the multidisciplinary team agrees to withhold surgical resection if the pathology result comes back negative or inconclusive. With regard to the need for biliary drainage, most but not all dCCA patients will undergo ERCP and stent placement to relieve biliary obstruction, in particular if bilirubin is too high for immediate surgery (e.g., >15 mg/dL) or if surgery is infeasible within 1 or 2 weeks. However, fit patients with a very high suspicion of resectable dCCA have fewer complications if they undergo resection without prior biliary drainage [19]. For patients who are nonoperative candidates, pathological confirmation is generally considered a prerequisite prior to palliative systemic treatment.
The differential diagnosis of dCCA includes several nonmalignant diseases such as IgG4-mediated cholangitis and stone disease. Presentation and imaging may provide clues for the likelihood of benign disease. The distal bile duct is surrounded by the pancreas; pancreatic ductal adenocarcinoma arising close to the distal bile duct may thus be indistinguishable from dCCA. Even pathological examination cannot distinguish dCCA from pancreatic ductal adenocarcinoma (PDAC) with certainty. Both cancers, however, require the same surgical procedure (i.e., pancreatoduodenectomy). Preoperative certainty about the diagnosis is only relevant for patients with (borderline) resectable PDAC who may benefit from neoadjuvant chemotherapy.
EUS may play a role in lymph node staging [20]. EUS-guided biopsy of enlarged aortocaval lymph nodes, for example, may confirm distant metastatic disease. This will be further discussed in Chap. 13.
Case Presentations
Case 1: IgG4-Related Sclerosing Cholangitis
A 72-year-old male patient presented with pain, itching, and jaundice for 2 weeks. Blood tests revealed fluctuating but generally abnormal liver enzyme tests and a high bilirubin level (Table 9.1). CT and MRCP showed a mass at the biliary confluence and an enlarged hilar lymph node (Fig. 9.1a). The diagnosis of pCCA was discussed with the patient. The patient was referred to a tertiary referral center. Upon presentation to the referral center, jaundice had decreased. His past medical history included a skin rash, lymphadenopathy, infections of the feet, and diffuse skeletal hyperostosis. No clear diagnosis had been found for all these conditions. The patient was discussed in a multidisciplinary meeting. MRI and CT scan reassessment were suspicious for pCCA showing a perihilar mass with bilateral involvement of the second-order bile ducts (i.e., Bismuth IV) without vascular involvement (Fig. 9.1a). An extended right hemihepatectomy to remove the mass was technically feasible.
Case 1: IgG4-related sclerosing cholangitis. (a) CT at first presentation: centrally obstructing tumor with dilatation of the intrahepatic bile ducts. (b) Pathology: lymph node biopsy with normal architecture (follicle formation) and sinus histiocytosis. Immunohistochemistry shows IgG- and IgG4-positive plasma cells. (c) CT after prednisone treatment: decrease in both size of tumor and dilatation of the intrahepatic bile ducts
No ERCP had been performed given serum bilirubin had normalized without treatment (Table 9.1). Moreover, CA19.9 was normal, and serum IgG4 was 12 g/L (i.e., five times the upper limit of normal). Thus, IgG4-related sclerosing cholangitis appeared more likely than pCCA, because of highly elevated IgG4, spontaneous normalization of bilirubin, and previously unexplained systemic symptoms. A few years prior, a biopsy of mediastinal lymph nodes had been performed; this biopsy was reassessed, and IgG4 staining was positive (Fig. 9.1b). Treatment with 40 mg prednisone daily was commenced. Within a few days, the patient experienced improvement of his symptoms. Not only did his jaundice disappear, but he also described improvement of all other symptoms. Repeat imaging (Fig. 9.1c) showed near normalization of the bile duct dilatation and disappearance of the mass. Repeat blood tests (Table 9.1) showed normalization of cholestasis. Treatment response confirmed the diagnosis of IgG4-related sclerosing cholangitis, and surgical intervention was appropriately averted.
This case illustrates how cross-sectional imaging that is very suspicious for pCCA may mimic IgG4-related sclerosing cholangitis (and vice versa). In particular, if IgG4 is highly elevated (i.e., at least twice the upper limit of normal), the possibility of IgG4-related sclerosing cholangitis should be carefully considered. Other clues of IgG4 disease in this case were the spontaneous improvement of symptoms, fluctuating or normalizing serum bilirubin without biliary drainage, and (previously) unexplained symptoms involving other organ systems, highlighting the importance of a thorough patient review. Of note is that although the presence of compatible histology and immunohistochemistry is essential, it is not considered sufficient because appropriate clinical findings and laboratory tests are required [15].
Case 2: IgG4-Related Sclerosing Cholangitis II
A 65-year-old female patient presented with jaundice, nausea, and malaise. Her past medical history included hypertension and mild asthma for which she used inhalation medication. Her family history revealed a brother with autoimmune pancreatitis and no family members with cancer. On physical examination no abnormalities were seen besides jaundice and mild abdominal tenderness. Lab results showed elevated bilirubin, AST, and ALT (Table 9.2). Abdominal ultrasound, CT, and MRI showed dilated intrahepatic bile ducts (Fig. 9.2a, b). On MRI a small mass was seen at the biliary confluence. The patient was referred to a tertiary center.
Case 2: IgG4-related sclerosing cholangitis. (a) CT at first presentation: centrally obstructing liver tumor with dilatation of the intrahepatic bile ducts. (b) CT at first presentation: pancreas showing mild swelling (“sausage”-like appearance), compatible with autoimmune pancreatitis. (c) CT after prednisone treatment: decrease in both size of liver tumor and dilatation of the intrahepatic bile ducts. (d) CT after prednisone treatment: pancreas showing less swelling
Upon presentation to the referral center, the patient reported spontaneous improvement of symptoms. Lab results showed that serum bilirubin had decreased without biliary drainage and serum IgG4 level was normal. Imaging was reviewed at the multidisciplinary meeting and showed thickening of the tail of the pancreas suggestive of autoimmune pancreatitis. A treatment trial of prednisone 40 mg daily was started. On repeat CT 2 weeks later, the small mass, dilated intrahepatic bile ducts, and pancreatic tail enlargement largely resolved (Fig. 9.2c, d).
This case illustrates that not all patients with IgG4-related sclerosing cholangitis have elevated serum IgG4 levels. In a large retrospective study by Tanaka et al., 84% of the patients with IgG4-related sclerosing cholangitis had elevated IgG4 levels at first presentation [21]. Fluctuating symptoms and spontaneous improving cholestasis require consideration of IgG4-related sclerosing cholangitis [21]. Moreover, patients with IgG4-related sclerosing cholangitis may have concomitant autoimmune pancreatitis, as illustrated herein [22,23,24,25].
Case 3: Stone Disease
A 58-year-old female patient presented with sudden onset abdominal pain and lab results consistent with cholestasis. On MRCP she had gallstones and isolated left intrahepatic bile duct dilatation (Fig. 9.3a) suggestive of Mirizzi syndrome. She underwent an ERCP, at which time a stenosis was seen in the left hepatic duct, and a plastic stent was placed. She subsequently underwent a laparoscopic subtotal cholecystectomy for acute cholecystitis. A necrotic gallbladder with multiple stones and pus was removed. A postoperative type “A” bile leak was treated with an ERCP to replace the migrated plastic stent. There was a persistent stenosis in the common hepatic and left intrahepatic duct. Brush cytology of this stenosis was diagnosed as adenocarcinoma. The patient was referred to a tertiary referral center.
Upon presentation to the referral center, the patient endorsed improvement in appetite and resolution of abdominal pain. On physical examination no abnormalities were found. Lab results showed improved AST/ALT but persistently elevated ALP (Table 9.3). CT and MRCP showed persistent dilatation of the intrahepatic bile ducts with thickening of the hepatic duct (Fig. 9.3a, b) suspicious for pCCA. However, reassessment of the brush cytology at the referral center did not confirm the diagnosis of adenocarcinoma but showed only atypical cells [26]. ERCP in combination with cholangioscopy was then performed for better evaluation of the stricture. Surprisingly, it showed that the left hepatic duct was obstructed by a large uncalcified stone, which was not seen on previous cross-sectional imaging and mistaken for Mirizzi syndrome. With lithotripsy the stone was fragmented and then removed with balloon sweeping. Bilateral stents were placed to optimize bile flow. She underwent progressive stenting to treat the associated benign ductal stenosis. Twelve months later, she was asymptomatic with no signs of cholestasis.
This case illustrates the difficulty for pathologists to differentiate severe inflammation from adenocarcinoma (see also Chap. 8). Brushings and biopsies should be reviewed independently by an expert pathologist. Moreover, inflammation (and consequent benign stricturing) due to stone disease can mimic pCCA on imaging. In addition, chronic hepaticolithiasis is in itself a risk factor for CCA, which further complicates the differential diagnosis and work-up. Finally, both CT and MRI may not show uncalcified intrahepatic stones (i.e., false negative findings) that are found upon cholangioscopy.
Case 4: Infectious Disease
A 20-year-old Caucasian male patient presented with acute abdominal pain in the right upper quadrant and jaundice. The symptoms started nearly 3 weeks prior to presentation, and he had noticed jaundice for about a week. Two weeks later his symptoms spontaneously improved. He had traveled to Croatia and France in the past 5 years. On physical examination of the abdomen, only slight tenderness in the right upper quadrant was noted. Lab results showed mildly elevated ALP and GGT, and serology was negative for hepatitis A, B, C, and E (Table 9.4). Serology for Echinococcus granulosus was positive (titer 1:160). An MRI showed cystic dilatation of the intrahepatic bile ducts, predominantly in the left liver, with possible stone formation (Fig. 9.4a). No masses or lymphadenopathy were seen. Since these findings were atypical for echinococcal infection and the differential diagnosis included iCCA, the patient was referred to a tertiary center.
At a multidisciplinary meeting, a differential diagnosis was established including focal Caroli syndrome, iCCA, HCC, and infectious diseases like echinococcal cysts. However, imaging was atypical for each of the differential diagnoses. A repeat CT scan showed several new hypodensities with eggshell calcifications in liver segments 2, 3, 4, and 8 (Fig. 9.4b). Serology came back highly positive for both Echinococcus granulosus (ELISA 58.4 U/mL) and Echinococcus multilocularis (ELISA IgG: 6400). All things considered, Echinococcus multilocularis infection was considered most likely. A PET scan was performed, which did not show extrahepatic manifestation of the disease. The patient was started on albendazole treatment 400 mg twice daily, and periodic repeat imaging will be performed to monitor the treatment effect. Surgery is the only curative option but is reserved for after albendazole has decreased the extent of disease in order to preserve as much liver tissue as possible.
This case illustrates how infection in the liver, in this instance Echinococcus multilocularis , can mimic iCCA. Echinococcosis is a zoonotic infection caused by tapeworms of the Echinococcus genus. It is a rare condition, though it has shown progressive spread to non-endemic areas in Northern Europe. Imaging features are variable, and initial misdiagnosis is common. Imaging findings include infiltrating lesions with irregular non-enhancing margins, small cystic components, scattered calcifications, and irregular septa within necrotic cavities [27]. Serology is often positive, and cross-reaction with Echinococcus granulosus is possible [28].
Case 5: Infectious Disease II
A 44-year-old healthy female patient presented with complaints of itching. No abnormalities were detected on physical examination. Lab results showed no abnormalities besides elevated total bilirubin of 1.75 mg/dL (Table 9.5). An abdominal CT scan showed a 5-centimeter hypodense liver mass with multiple satellites (Fig. 9.5). Subsequently, a PET scan was performed that showed uptake in the liver lesion (SUV of 7). The patient was diagnosed with unresectable iCCA and scheduled for palliative chemotherapy. She then came to a tertiary referral center for a second opinion.
Case 5: Infectious diseases II (a) CT at first presentation: irregular central hypodense liver lesion. (b) CT after a month: multiple irregular peripheral hypodense masses, resolution of central lesions. (c) Pathology: HE liver biopsy with preserved architecture, necrosis (yellow circle), and prominent eosinophilic infiltration. Immunohistochemistry: Keratin 7 highlights the original bile ducts along with some ductular reaction
Detailed patient history at referral found that she had traveled to Thailand 3 months prior. An MRI was performed which showed an irregular lesion, atypical for iCCA (Fig. 9.5a). A new CT showed that one lesion had a major decrease in size and change in shape without treatment, and new lesions had developed in segments 6 and 7 (Fig. 9.5b). Blood tests showed that she had severe eosinophilia. A percutaneous liver biopsy showed necrosis and a histiocytic reaction without malignancy (Fig. 9.5c). An eosinophilic infiltrate and a slight increase in IgG4-positive cells were seen. The blood tests and biopsy were consistent with hypereosinophilic syndrome (HES).
The work-up continued with excluding autoimmune diseases and parasitic infections. Autoimmune serology was negative. However, Fasciola hepatica serology was positive on indirect immunofluorescent-antibody test and ELISA. The patient was treated with triclabendazole, her symptoms resolved, and imaging showed marked improvement of the liver lesions.
This case, similar to the previous one, illustrates how infectious diseases can mimic CCA. In this case, the patient had infiltrative liver lesions resembling iCCA, but the liver lesions were caused by a liver fluke (Fasciola hepatica) that she most likely contracted during her travels in Thailand. Detailed information regarding liver flukes can be found in Chap. 11.
Case 6: Adenocarcinoma of Unknown Primary
A 45-year-old female patient with a past medical history of stage III Hodgkin lymphoma, stage II papillary thyroid carcinoma, and stage I breast carcinoma presented with fatigue. On physical examination of the abdomen, tenderness in the upper abdomen was noted. Lab results showed abnormal liver tests (Table 9.6). A CT was performed and showed multiple bilobar liver lesions suspicious for liver metastases. A PET scan found no extrahepatic disease. A liver biopsy was performed, and pathological examination showed a poorly differentiated adenocarcinoma. Immunohistochemical analyses ruled out her previous malignancies as well as colorectal cancer. Upper endoscopy ruled out esophageal and gastric cancer. She was diagnosed with ACUP and started systemic chemotherapy with carboplatin and paclitaxel. She came to a tertiary referral center for a second opinion.
A new CT was performed that showed a large liver tumor with peripheral enhancement and capsular retraction (Fig. 9.6a, b). Outside pathology was reviewed and showed that the tumor cells were of epithelial origin, and therefore a lymphoma was excluded. Immunohistochemical profile (negative staining for thyroglobulin, TTF-1, and PAX-8) ruled out a thyroid (papillary) carcinoma. Moreover, negative staining for GATA-3, ER, and BAP loss (which were all positive in her breast carcinoma) together with the inconsistent histomorphology excluded metastasis from her breast carcinoma. Both histomorphology and immunohistochemical profile were consistent with iCCA (Fig. 9.6c). Based on imaging and pathology, she was diagnosed with iCCA. She is currently undergoing treatment with systemic chemotherapy (gemcitabine and cisplatin) and concomitant hepatic arterial infusion pump chemotherapy with floxuridine. Several studies have found 5-year OS of about 20% with combined systemic and intra-arterial chemotherapy for unresectable iCCA [29].
This case illustrates that radiologists and pathologists are sometimes unfamiliar with the imaging and immunohistochemical profile of iCCA, thus resulting in an incorrect diagnosis of ACUP by medical oncologists. ACUP in the liver should only be diagnosed after ruling out iCCA by a multidisciplinary team experienced with iCCA.
Case 7: Cirrhotic Liver
A 62-year-old male patient with a past medical history of sarcoidosis treated with steroids, obesity, and alcohol abuse disorder was diagnosed with a Child-Pugh B7 liver cirrhosis. Due to continued alcohol use, a liver transplantation was contraindicated. Biannual abdominal ultrasound was performed for HCC surveillance.
After 3 years of follow-up, a hypodense lesion was seen on ultrasound. Lab results showed normal liver tests and tumor markers (Table 9.7). An MRI scan showed a lesion with a diameter of 14 mm in segment 7 (Fig. 9.7a). The lesion showed rim hyperenhancement, without decrease in intensity from earlier to later phase (i.e., non-peripheral washout). The lesion was classified as LI-RADS M (i.e., malignant but not HCC) (Fig. 9.7b).
Case 7: Cirrhotic liver. (a) MRI (portal venous phase, Primovist contrast): cirrhotic liver, with a peripherally enhancing spheroid lesion in segment 7. (b) MRI (late phase, Primovist contrast): in this phase, after contrast injection, the lesion can still be seen. (c) Pathology: HE liver biopsy with adenocarcinoma, with relatively small gland formation. Immunohistochemistry: cytokeratin 19 positivity in tumor cells. HepPar-1 highlights the preexistent hepatocytes (tumor cells are negative)
A CT scan of the chest and a PET scan were performed to rule out metastatic disease. A liver biopsy was performed, and pathology reported adenocarcinoma with an immunohistochemical profile inconsistent with hepatocellular differentiation (no HepPar-1 or AFP expression and no canalicular pattern of expression with CD10 and polyclonal CEA). The tumor cells, however, did stain positive for markers of biliary differentiation (such as keratin 7 and keratin 19), and together with BerEp-4 expression and histomorphology, this was consistent with iCCA (Fig. 9.7c). Radiofrequency ablation was performed without complications.
This case demonstrates that cirrhosis is a risk factor for iCCA [30]. Indeed, iCCA is more common in cirrhosis than previously recognized. Most new hepatic lesions represent HCC, but small lesions may be difficult to distinguish from iCCA. Treatment of the new liver lesion in this patient would have been ablation regardless of a diagnosis of HCC or iCCA. However, distinguishing iCCA from HCC is relevant in patients that may be eligible for liver transplantation, because survival outcomes are worse for iCCA after transplantation (Chap. 15). Moreover, systemic and locoregional treatments differ between iCCA and HCC (discussed further in Chaps 17 and 18).
Case 8: Primary Sclerosing Cholangitis
A 54-year-old male patient presented with jaundice, itching, and malaise. His past medical history included mild ulcerative proctitis and PSC. Blood tests revealed elevated liver enzyme tests and bilirubin level (Table 9.8). An abdominal CT scan showed a liver with irregular surface contour with minimal dilatation of the intrahepatic bile ducts, discontinuous narrowing of the bile ducts, and slight arterial enhancement of the common hepatic duct and the cystic duct junction (Fig. 9.8a). A well-defined mass was not identifiable.
Case 8: Primary sclerosing cholangitis. (a) CT at first presentation (coronal view): thickening and slight enhancement of the common hepatic duct (two yellow arrows). No wall thickening is seen at the common bile duct (orange arrow). (b) ERCP: significant stenosis of the common hepatic duct and cystic duct conjunction extending to the confluence of the left and right main ducts
ERCP was performed, and the fluoroscopic images showed a significant stenosis of the common hepatic duct and cystic duct conjunction extending proximally to the confluence of the left and right main ducts (Bismuth-Corlette type II classification). Intraductal brushing and biopsy was performed, and a plastic stent was placed across the stenosis (Fig. 9.8b). Malignancy was not found in the obtained specimens.
Irregular wall thickening at the level of the dominant stenosis was seen at MRCP, and combined with high signal on diffusion-weighted imaging, the diagnosis CCA was deemed more likely than a benign stricture. Based on the clinical presentation and imaging, CCA was sufficiently likely to proceed with surgical resection. Because of the extent of the stenosis on fluoroscopy, a right hemihepatectomy with extrahepatic bile duct resection was performed, and pathological examination showed a well-differentiated perihilar adenocarcinoma.
This case illustrates the difficulty of diagnosing CCA in patients with PSC. Interpreting imaging is often challenging due to pre-existing biliary strictures and intraductal tumor growth without clear extra-ductal mass forming. Moreover, pathology is often inconclusive.
Conclusion
In this chapter, and by way of these eight illustrative cases, we have demonstrated that the diagnostic work-up of patients with (suspected) CCA is a challenging multidisciplinary effort including gastroenterologists, surgeons, medical oncologists, radiologists, and pathologists, among others. The differential diagnosis should include IgG4-related sclerosing cholangitis, stone disease, parasitic disease, and metastatic disease from extrahepatic primary cancers, and in some cases even more esoteric disorders may be included. A meticulous patient history, physical examination, and evaluation of imaging and laboratory tests by multidisciplinary experts are required to determine the correct diagnosis and identify the best course of management.
In addition, for the subset of cases which evade definitive diagnosis despite these measures, continued developments in diagnostic tools, including but not limited to molecular biological (e.g., “omics”-based approaches) and other advanced techniques, are clinically needed and anticipated.
Abbreviations
- ACUP:
-
Adenocarcinoma of unknown primary
- AFP:
-
Alpha-fetoprotein
- AJCC:
-
American Joint Committee on Cancer
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate aminotransferase
- BAP:
-
Biofilm-associated protein
- CA19.9:
-
Carbohydrate antigen 19.9
- CCA:
-
Cholangiocarcinoma
- CD10:
-
Cluster of differentiation 10
- CEA:
-
Carcinoembryonic antigen
- CT:
-
Computed tomography
- dCCA:
-
Distal cholangiocarcinoma
- ELISA:
-
Enzyme-linked immunosorbent assay
- ER:
-
Estrogen receptor
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- EUS:
-
Endoscopic ultrasound
- FISH:
-
Fluorescence in situ hybridization
- FNA:
-
Fine needle aspiration
- GATA-3:
-
GATA binding protein 3
- GGT:
-
Gamma-glutamyl transferase
- HCC:
-
Hepatocellular carcinoma
- HepPar-1:
-
Hepatocyte-specific antigen
- HES:
-
Hypereosinophilic syndrome
- iCCA:
-
Intrahepatic cholangiocarcinoma
- IgG4:
-
Immunoglobulin G4
- LI-RADS:
-
Liver Imaging Reporting and Data System
- MRCP:
-
Magnetic resonance cholangiopancreatography
- MRI:
-
Magnetic resonance imaging
- NGS:
-
Next-generation sequencing
- PAX-8:
-
Paired box gene 8
- pCCA:
-
Perihilar cholangiocarcinoma
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PET:
-
Positron emission tomography
- SUV:
-
Standardized uptake value
- TTF-1:
-
Thyroid transcription factor 1
References
Fung BM, Tabibian JH. Cholangiocarcinoma in patients with primary sclerosing cholangitis. Curr Opin Gastroenterol. 2020;36(2):77–84.
Hilscher MB, Tabibian JH, Carey EJ, Gostout CJ, Lindor KD. Dominant strictures in primary sclerosing cholangitis: a multicenter survey of clinical definitions and practices. Hepatol Commun. 2018;2(7):836–44.
Fung BM, Lindor KD, Tabibian JH. Cancer risk in primary sclerosing cholangitis: epidemiology, prevention, and surveillance strategies. World J Gastroenterol. 2019;25(6):659–71.
Ali AH, Tabibian JH, Nasser-Ghodsi N, Lennon RJ, DeLeon T, Borad MJ, et al. Surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis. Hepatology. 2018;67(6):2338–51.
Tabibian JH, Lindor KD. Challenges of cholangiocarcinoma detection in patients with primary sclerosing cholangitis. J Anal Oncol. 2012;1(1):50–5.
Fung BM, Fejleh MP, Tejaswi S, Tabibian JH. Cholangioscopy and its role in primary sclerosing cholangitis. Eur Med J Hepatol. 2020;8(1):42–53.
Corvera CU, Blumgart LH, Darvishian F, Klimstra DS, DeMatteo R, Fong Y, et al. Clinical and pathologic features of proximal biliary strictures masquerading as hilar cholangiocarcinoma. J Am Coll Surg. 2005;201(6):862–9.
Liver Cancer Study Group of J. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211(3):277–87.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9.
Corvera CU, Blumgart LH, Akhurst T, RP DM, D’Angelica M, Fong Y, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206(1):57–65.
Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–89.
Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17(8):669–80.
Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140(2):170–8.
Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143(1):88–98 e3. quiz e14.
Benson AB, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, et al. Guidelines insights: hepatobiliary cancers, version 2.2019. J Natl Compr Cancer Netw. 2019;17(4):302–10.
Kochar B, Akshintala VS, Afghani E, Elmunzer BJ, Kim KJ, Lennon AM, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81(1):143–9 e9.
Onoyama T, Matsumoto K, Takeda Y, Kawata S, Kurumi H, Koda H, et al. Endoscopic ultrasonography-guided fine needle aspiration for extrahepatic cholangiocarcinoma: a safe tissue sampling modality. J Clin Med. 2019;8(4):417.
Jo JH, Cho CM, Jun JH, Chung MJ, Kim TH, Seo DW, et al. Same-session endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography-based tissue sampling in suspected malignant biliary obstruction: a multicenter experience. J Gastroenterol Hepatol. 2019;34(4):799–805.
van der Gaag NA, de Castro SM, Rauws EA, Bruno MJ, van Eijck CH, Kuipers EJ, et al. Preoperative biliary drainage for periampullary tumors causing obstructive jaundice; DRainage vs. (direct) OPeration (DROP-trial). BMC Surg. 2007;7:3.
Malikowski T, Levy MJ, Gleeson FC, Storm AC, Vargas Valls EJ, Topazian MD, et al. EUS-FNA is effective for lymph node staging in patients with cholangiocarcinoma. Hepatology. 2019;72(3)940–48.
Tanaka A, Tazuma S, Okazaki K, Nakazawa T, Inui K, Chiba T, et al. Clinical features, response to treatment, and outcomes of igg4-related sclerosing cholangitis. Clin Gastroenterol Hepatol. 2017;15(6):920–6 e3.
Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, et al. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012;19(5):536–42.
Okazaki K, Uchida K, Miyoshi H, Ikeura T, Takaoka M, Nishio A. Recent concepts of autoimmune pancreatitis and IgG4-related disease. Clin Rev Allergy Immunol. 2011;41(2):126–38.
Montano-Loza AJ, Lalor E, Mason AL. Recognizing immunoglobulin G4 related overlap syndromes in patients with pancreatic and hepatobiliary diseases. Can J Gastroenterol. 2008;22(10):840–6.
Kamisawa T, Nakazawa T, Tazuma S, Zen Y, Tanaka A, Ohara H, et al. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci. 2019;26(1):9–42.
Layfield LJ, Pitman MB, DeMay RM, Shidham VB. Pancreaticobiliary tract cytology: journey toward “bethesda” style guidelines from the papanicolaou society of cytopathology. Cytojournal. 2014;11:18.
Chouhan MD, Wiley E, Chiodini PL, Amin Z. Hepatic alveolar hydatid disease (Echinococcus multilocularis), a mimic of liver malignancy: a review for the radiologist in non-endemic areas. Clin Radiol. 2019;74(4):247–56.
Ito A, Ma L, Schantz PM, Gottstein B, Liu YH, Chai JJ, et al. Differential serodiagnosis for cystic and alveolar echinococcosis using fractions of Echinococcus granulosus cyst fluid (antigen B) and E. multilocularis protoscolex (EM18). Am J Trop Med Hyg. 1999;60(2):188–92.
Cercek A, Boerner T, Tan BR, Chou JF, Gonen M, Boucher TM, et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2019;6(1):60–7.
Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128(3):620–6.
Acknowledgments
The authors thank Dr. Michael Doukas for providing and reassessing the pathology slides and Dr. Roy Dwarkasing for providing and reassessing the radiological images.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Franssen, S., de Jong, D.M., van Driel, L.M.J.W., Groot Koerkamp, B. (2021). Challenges in Diagnosing Cholangiocarcinoma: Pulling Together Biochemical, Radiological, and Cytopathological Data. In: Tabibian, J.H. (eds) Diagnosis and Management of Cholangiocarcinoma. Springer, Cham. https://doi.org/10.1007/978-3-030-70936-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-70936-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-70935-8
Online ISBN: 978-3-030-70936-5
eBook Packages: MedicineMedicine (R0)