Abstract
Dynamic evaluation mechanisms of the human upper limb are of great value for research and applications in upper limb rehabilitation, especially for the development of robotic upper limb rehabilitation systems. This paper proposes a muscle force prediction method based on the Hill muscle model. The proposed approach, which combines sEMG signals and kinematic data, provides a deep understanding of the dynamic motion mechanisms and parameters that characterize the upper limbs of the human body. The study provides a theoretical benchmark for the evaluation of rehabilitation training practices and for improved designs of upper limb rehabilitation robots that are used for upper limb neuro-rehabilitation. Specifically, the system collected motion data and sEMG signals from the upper limbs of the human body through a high-speed infrared motion capture system and skin sEMG sensors. By applying human kinematics and dynamics theories, real-time joint angle and torque information was obtained and imported into OpenSim. This platform can simulate the real-time muscle force values produced by the upper limbs during movements. The myoelectric signals were first filtered to remove noise, and an exponential model was then used to obtain the muscle activation. These data were then entered into the Hill-type prediction model to determine an individual’s muscle forces. In this paper, grasping movements commonly used in everyday situations were taken as a testing case. The results of the experiments showed that an individual’s muscle forces can be predicted using a Hill-type model. The results are consistent with those from simulated muscle force models and can reflect the real forces experienced during upper limb exercises.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cardio-cerebrovascular disease, more commonly referred to as a stroke or heart attack, is the second most common cause of death and the eighth most common cause of severe disability in the elderly population worldwide [1, 2]. One of the most serious symptoms of stroke is hemiparesis. It usually leads to the loss of motor function in the upper limb, which is important for activities of daily living, such as eating, bathing, and getting dressed independently. Studies have shown that rehabilitation robots can effectively improve the motor function of stroke patients because the device is designed to have multiple DOFs to mimic various limb movements and accommodate all types of exercises [3]. Rehabilitation machines are becoming increasingly important for stroke rehabilitation, and they have many potential advantages over traditional rehabilitation therapies in treating motor dysfunction in stroke patients [4].
At present, upper limb rehabilitation robots can be divided into three main types based on their structure [5,6,7,8]. The first type of robots is called rehabilitation robots with end-guided structures. The subject’s body is placed on a separate structure, while a separate robotic mechanism guides the movement of the forearm or the hand to train and rehabilitate the affected limb [9,10,11]. The second type of robots is called exoskeleton rehabilitation robots, where the robotic arms closely follow the form and function of the affected limb and can consequently achieve a variety of rehabilitation training actions [12,13,14,15]. The third type of robots is called compound rehabilitation training robots, which have a combination of features from the first two types of rehabilitation robots and are used to complete rehabilitation training [16,17,18,19,20,21]. Regardless of the type of upper limb rehabilitation robot used, it is necessary to conduct a quantitative evaluation of the patient’s upper limb impairment and provide personalized rehabilitation treatment. Since the mechanism of human upper limb movement is very complicated and the range of functional movements achievable is wide, the sports medicine field has not developed a standard for assessing upper limb movement patterns. Currently, the most immediate challenge is to develop objective indicators that are related to upper limb motion and can be applied to assess upper limb function under everyday conditions.
To address the challenges mentioned above, a model simulating the output force from muscle fibers was established, and predictive models of muscle forces for individuals with different demographics were obtained in this paper. We combined the musculoskeletal model of an upper extremity with the movement and EMG-assisted method to estimate the individual muscle forces of the musculoskeletal structure. The EMG signals were considered inputs to the musculoskeletal model to estimate the muscle activation information. A simulation tool commonly used in biomechanical analyses, OpenSim, was used to analyze the agreement in muscle force estimations. Through our method, therapists can more accurately describe the power of stroke patients’ upper limbs during daily activities and can design better rehabilitation techniques. In addition, the research can also be useful for controlling an upper limb exoskeleton.
The rest of this paper is organized as follows. Section 12.2 presents the related works and muscle estimation growing trend. Section 12.3 describes experiment details and data procession and muscle estimation model. Section 12.4 compares the estimated muscle force by our NMS (Neuromuscular Subjective) model with force simulated in OpenSim. Section 12.5 evaluates the performances of NMS model and discusses. Section 12.6 summarizes this article.
2 Related Work
Three main approaches including clinical scales, movement evaluations, and surface electromyography (sEMG) analyses, are widely applied in objective evaluations of the upper extremities [22, 23]. Clinical scales are inherently subjective due to their reliance on a physician’s visual assessment of a movement and generally. It is difficult to summarize a movement with a single score, especially when several aspects, such as the speed and amplitude of the movement, have to be taken into account in the evaluation of functional tasks [24]. Movement evaluation methods mainly include motion capture systems, which represent the gold standard in human movement analysis [25,26,27]. Motion capture systems can accurately assess the kinematics of upper limb movements during daily activities in stroke patients [28]. However, although they can overcome the limitations of clinical scales, which lack the ability to monitor a patient’s movements, motion capture systems cannot assess the internal features of a patient’s muscles.
Surface electromyography (sEMG) is a popular research tool that is used extensively in sports medicine and rehabilitation sciences. Based on sEMG analysis, researchers have attempted to draw conclusions concerning the neuro and electrophysiological mechanisms of force production and make hypotheses about potential muscular force adaptation rates and hypertrophy [29]. sEMG signal decomposition algorithms can be roughly divided into two categories. The first type of sEMG signal decomposition involves waveform detection and pattern recognition. The second type involves a blind source separation method or system identification so that the sEMG signal can be described and interpreted. Recent experiments have suggested that the central nervous system can spontaneously follow certain optimization criteria to overcome the motion uncertainty caused by kinematic mechanism positional redundancy [30, 31]. Although sEMG signal analysis has value in certain applications in upper limb function evaluations, quantitative evaluations cannot be conducted due to the lack of deep muscle activation information. Surface EMG signals can be easily obtained, but the activations of deep muscles cannot be measured by non-invasive methods [32,33,34]. The identification of force profiles of individual muscles during upper limb movements may help provide a better understanding of the functional roles of these muscles as well as the neuro-musculoskeletal impairments, leading to a better understanding of how these factors affect movement [35, 36].
Hill-type or Hill-type modified models are vastly used in muscle estimation. All the models take sEMG as the only one input parameter in order to simplify compute procession. Muscle forces are calculated just like a black box. But it involves non-linear relationships in the expressions like muscle fiber length verse muscle force curve or muscle contraction velocity verse muscle force curve, which makes the computational process very complex. In order to acquire universality, the relationship between muscle contraction velocity and fiber length verse muscle force must be scaled [35, 39, 41]. In contrast to Hill-type model compute muscle force using sEMG, there is another method called inverse dynamics. The inverse dynamics method takes position, velocity, and acceleration as input parameters to calculate the jet moment. Then static or dynamic optimization is used to obtain individual muscle force. This method greatly simplifies the calculation procession but suffers from the problem of imprecision.
3 Materials and Methods
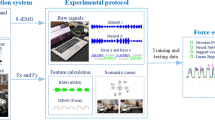
The experimental goal for this paper was to simultaneously capture data about a human subject’s upper limb motion and about his or her limb surface EMG signals. These data were then analyzed and processed using optimizing calculations to obtain accurate values predicting muscle forces during exercise. The human upper limb musculoskeletal model was established by OpenSim, and then the kinematic data gathered by motion capture were used to simulate the muscle forces during the upper limb movements. The surface EMG signal data were then imported into a Hill-type model. By adjusting the parametric coefficients to achieve model predictive values close to the simulated muscle force values, we established a method of predicting muscle force directly from the surface EMG signal data (Fig. 12.1).
3.1 Data Collection and Preprocessing
Four healthy male subjects (age: 23.5 ± 1.2 years old, height: 171.3 ± 3.5 cm, weight: 72 ± 6.5 kg) volunteered to participate in the experiment and were included in the study after they signed written informed consent forms. The research project was pre-approved by the Research Ethics Committee of Xinjiang University. While the subjects sat in a chair, they autonomously moved their arm from the natural relaxed state to grasp a raised ball that was suspended directly in front of them at head height, released the ball and put their arm down. The body motion data from the subjects were collected using a VICON optical motion capture system, and the surface electromyography data from the relevant muscles of the upper limbs of the subjects were collected using a Neuracle 16 channel electromyography signal acquisition system. The signals from 7 muscles were recorded: the short head of the biceps brachii (BICshort), long head of the biceps brachii (BIClong), brachialis (BRA), long head of the triceps brachii (TRIlong), lateral head of the triceps brachii (TRIlat), medial head of the triceps brachii (TRImed), and anconeus (ANC). The electrodes were placed longitudinally along the muscles in the direction of the muscle fibers and on the relevant part of each muscle according to the recommendations of the SENIAM (surface electromyography for the non-invasive assessment of muscles) project. A ground electrode was placed on the elbow joint (Fig. 12.2).
3.2 Joint Angle Estimation
There are specific challenges concerning the collection of point data of human motion in an experiment. Although a human’s limbs rotate around a single point within the skeletal structure, it is relatively difficult to maintain the joint at the zero-point throughout the testing process. To compensate for displacement of the joint, the space vector method was used to calculate the relative position in space and relative angular difference between two dependent point-lines. A space vector is a relative coordinate system, and the variation of the zero-point position can therefore be ignored. The human body parts were simplified to form a stick model and calculate the angle of the joint. A unique 3D coordinate positional system was established, and the value for n points for volunteer m at time t was collected. One vector segment was defined by two points in space, and the angle was obtained by measuring the relative positions of the two vector line segments (Fig. 12.3).
The angle of the left shoulder joint (SAl), the angle of the left elbow joint (EAl), and the angle of the left wrist joint (WAl) are shown in this diagram.
Suppose that a, b, c are three points in space; then, ∠abc represents the angle of joint b, and two vectors ending at a, and c are defined, which correspond to two marker points on volunteer m. The solution to the angle is as follows:
3.3 OpenSim Simulation
OpenSim [37] skeletal muscle simulation software was used to generate a dynamic simulation. An OpenSim upper limb musculoskeletal model [38] developed by Saul et al. consisting of 7 body segments and 32 muscles was used to generate a simulation relative to the kinematic data, and muscle kinematics parameters, such as the musculotendon unit (MTU) lengths and moment arms, were obtained. This upper limb musculoskeletal model is shown in Fig. 12.4. First, scaling was carried out to calibrate the model to the subject according to the subject’s anthropometric parameters. Inverse kinematics was then used to reconcile the differences in values between the actual 3D coordinates and the simulated virtual marker points. This process was achieved by a weighted least-squares method, which reduced the values to the minimum values possible. Last, dynamic optimization was carried out on the muscle forces in the main muscle group during the upper limb movement, which was performed in the simulation.
3.4 Muscle Activation Dynamics
-
(1)
Data preprocessing
First, the original sEMG signal was preprocessed. The preprocessing phase mainly included three steps: (a) 50 Hz notch filtering to remove power frequency interference; (b) 30 Hz zero-phase high-pass filtering to remove motion artefacts; and (c) full-wave rectification, which involves taking the absolute value of the signal (Fig. 12.5).
-
(2)
Low-pass filter
The low-pass filter used was a 5 Hz zero-phase low-pass filter, which is a low-pass filter commonly used to smooth muscle signals.
-
(3)
Normalization
The same method (data preprocessing \(\to\) low-pass filter) was used to process the sEMG signal at maximal voluntary contraction (MVC) and identify the maximum value of the sEMG signal at MVC, which was considered 100% of the magnitude of the muscle activation signal. The normalized signal e(t) was obtained by dividing the processed myoelectric signal (data preprocessing \(\to\) low-pass filter) recorded during normal motion by the maximum value.
-
(4)
Neural activation model
EMG is a measure of the electrical activity that spreads across the muscle, causing it to activate. This process results in the production of a muscle force. However, it takes time for the force to be generated—it does not happen instantaneously. Thus, we adopted a second-order discrete linear model [39] to model the neural activation from muscle excitation obtained through preprocessing in the form of a recursive filter:
$$u(t) = \alpha e(t - d) - (c_{1} + c_{2} )u(t - 1) - c_{1} c_{2} u(t - 2),$$(12.2)
where e(t) is the muscle excitation at time t, u (t) is the neural activation, α is the muscle gain, c1 and c2 are recursive coefficients, and d is the electromechanical delay.
-
(5)
Muscle activation model
The neural activations were then adjusted to account for either a linear or non-liasdnear EMG-force relationship [40]:
where a(t) is the muscle activation, u(t) is the neural activation, and A is the non-linear shape factor.
After obtaining the muscle activation a(t), we computed the muscle force by integrating a Hill-type muscle model consisting of two elements: a contractile element producing the active muscle force \(F_{A}^{m}\) and a parallel elastic element producing the passive force \(F_{P}^{m}\). As shown in Fig. 12.6 [41], \(l^{m}\) is the muscle fiber length, \(l^{t}\) is the total length of the tendons, and \(\varphi\) is the pennation angle. Thus, the musculotendon length \(l^{mt}\) can be expressed as follows.
The muscle-tendon force (\(F^{mt}\)) is calculated as
where \(l = l^{m} /l_{o}^{m}\),\(v = v^{m} /v_{o}^{m}\), a(t) is the muscle activation, \(F_{o}^{m}\) is the maximum isometric muscle force, \(l_{o}^{m}\) represents the optimal fiber length, \(v_{o}^{m}\) is the maximum muscle contraction velocity, l is the normalized muscle fiber length, and v is the normalized muscle fiber velocity. \(f_{A} \left( l \right)\), \(f_{V} \left( v \right)\), and \(f_{P} \left( l \right)\) define the normalized active force-length relationship, force-velocity relationship, and the normalized passive elastic force-length relationship, respectively.
4 Results
Using the above formula, we can calculate the relative values of the extension angles between the wrist, elbow, and shoulder joints when the upper limb of the human body performs the exercise. The joint angle data and point data were imported into OpenSim, the steps and parameters of the model described in the previous section were followed, and the upper limb model was run to simulate the motion and the changes in muscle forces in the short head of the biceps brachii (BICshort), long head of the biceps brachii (BIClong), brachialis (BRA), long head of the triceps brachii (TRIlong), lateral head of the triceps brachii (TRIlat), medial head of the triceps brachii (TRImed), and anconeus (ANC) (Fig. 12.7).
A surface electromyography signal acquisition device was used, and the sampling frequency was 1000 Hz. The surface electromyography signals of the seven muscles (the short head of the biceps brachii (BICshort), long head of the biceps brachii (BIClong), brachialis (BRA), long head of the triceps brachii (TRIlong), lateral head of the triceps brachii (TRIlat), medial head of the triceps brachii (TRImed), and anconeus (ANC)) involved in the movement of the upper arm of the human body were collected. The original signals were preprocessed and substituted into the muscle activation values obtained by formula (12.3), the muscle force predictive values were obtained by substituting the muscle activations into formula (12.4), and the above data were calculated with MATLAB R2014b. Figure 12.8 shows the changes in the muscle force of the brachialis.
We compared the force of the same muscles with the predicted values calculated by OpenSim and sEMG, and the curves were very close, as shown in Fig. 12.9. With a statistical analysis, the muscle force values of the other six muscles were also compared, showing a strong correlation (P < 0.05). The comparative trial in this paper also verified the feasibility of predicting muscle forces by sEMG.
5 Discussion
Calculating joint angles and muscle forces from motion capture data is a simple process. There are many formulas and simulation software available, but the shortcomings of motion capture experiments are that the space required for experiments is too large and the experimental process is cumbersome. Motion capture data can be used for offline scientific experiments but are unreliable when used to generate real-time or online active control signals, such as those applied to an upper limb robotic exoskeleton. The advantages of surface EMG include the facts that the acquisition process is simple, the associated experimental equipment is small and portable, and real-time signals can be acquired and generated for control, but EMG signals are weak, and the process of calculating and processing the signals is complicated. Therefore, an experiment for the synchronous acquisition of motion capture data and sEMG signals was carried out to verify the sEMG signal calculation results obtained by using the calculated motion capture data. The final verification results also suggest the feasibility of using EMG signals to calculate muscle forces.
As upper extremity robotic exoskeletons and rehabilitation robots continue to develop, pattern recognition, which is an offline control method, does not meet the needs of practical applications. A control source signal requires real-time acquisition control, which requires the acquisition process to be simple and easy to perform and the signal to be stable and continuous. sEMG signals can meet these demands. This paper studies the dynamic evaluation mechanism of human upper limb movement, which was designed to convert offline motion capture calculations to online electromyography calculations. Real-time calculations of muscle force can provide more accurate control of robotic exoskeletons and real-time evaluations of upper limb motion states. In the future, sEMG signal changes and upper limb joint angles should be analyzed more deeply to detect changes in the joint through the surface EMG signal; then, robotic exoskeletons or rehabilitation robots can be controlled in real time by surface EMG signals.
Several limitations should be noted. First, uncertain noises in EMG signals still existed, even if we tried to avoid it, such as cross-talk from other muscles, baseline noise, and artifact. Due to difference of individual physiologic and electrode positions, the outputs will be a little bit different. But it can still serve as a reference in rehabilitation.
6 Conclusions
Multi-parameter human–computer interaction technology is an important new development in the field of human physics and neuro-rehabilitation. This study proposes a set of upper limb kinematic analysis methods, which include muscle force prediction methods. In addition, this work can provide reference values for the evaluation of upper limb motor function and the auxiliary control of upper limb rehabilitation robots. Accurate muscle force prediction methods can be used to assess an individual’s ability to generate limb movements, which can promote a deeper understanding of the condition of the patient’s nervous system. This knowledge can be used to guide the selection of rehabilitative treatments and to design better rehabilitation robots that can assist people with upper extremity dyskinesia during upper limb tasks.
References
World Health Organization. World health statistics 2018, Available online. http://www.who.org/
Perez R, Costa U, Torrent M, Solana J, Opisso E, Caceres C, Tormos JM, Medina J, Gomez EJ (2017) Upper limb portable motion analysis system based on inertial technology for neurorehabilitation purposes. Sensors 10:10733–10751
Rahman MH, Rahman MJ, Cristobal OL, Saad M, Kenné JP, Archambault PS (2015) Development of a whole arm wearable robotic exoskeleton for rehabilitation and to assist upper limb movements. Robotica 33:19–39
Albert CL, Peter DG, Lorie GR, Jodie KH, George FW, Daniel GF, Robert JR, Todd HW, Hermano IK, Bruce TV, Christopher TB, Dawn MB, Pamela WD, Barbara HC, Alysia DM, Stephen EN, Susan SC, Janet MP, Grant DH, Peter P (2010) Robot-assisted therapy for long-term upper-limb impairment after stroke. New England J Med 362:1772–1783
Farulla GA, Pianu D, Cempini M, Cortese M, Russo LO, Indaco M, Nerino R, Chimienti A, Oddo CM, Vitiello N (2016) Vision-based pose estimation for robot-mediated hand telerehabilitation. Sensors 16:208
Borboni A, Maddalena M, Rastegarpanah A, Saadat M, Aggogeri F (2016) Kinematic performance enhancement of wheelchair-mounted robotic arm by adding a linear drive. 2016 IEEE international symposium on medical measurements and applications (MeMeA)
Zhang L, Zheng Z, Li G, Sun Y, Jiang G, Kong J, Tao B, Xu S, Yu H, Liu H (2018) Tactile sensing and feedback in SEMG hand. Int J Comput Sci Math 9(4):365–376
Rzyman G, Szkopek J, Redlarski G, Palkowski A (2020) Upper limb bionic orthoses: general overview and forecasting changes. Appl Sci 10(15):5323
Meattini R, Chiaravalli D, Palli G, Melchiorri C (2020) sEMG-based human-in-the-loop control of elbow assistive robots for physical tasks and muscle strength training. IEEE Robot Autom Lett 5(4):5795–5802
Xu G, Song A, PanL, Li H, Liang Z, Zhu S, Xu B, Li J (2012) Adaptive hierarchical control for the muscle strength training of stroke survivors in robot-aided upper-limb rehabilitation. Int J Adv Robot Syst, https://doi.org/10.5772/51035
Hogan N, Krebs HI, Charnnarong J, Srikrishna P, Sharon A (1993) MIT-MANUS: a workstation for manual therapy and training II[P]. Other Conferences
Gassert R, Dietz V (2018) Rehabilitation robots for the treatment of sensorimotor deficits: a neurophysiological perspective. J NeuroEng Rehab, https://doi.org/10.1186/s12984-018-0383-x
Lenzi T, De Rossi SMM, Vitiello N, Carrozza MC (2012) Intention-based EMG control for powered exoskeletons. IEEE Trans Bio-Med Eng 59(8)
Shing LH, Quan XS (2011) Exoskeleton robots for upper-limb rehabilitation: state of the art and future prospects[J]. Med Eng Phys 34(3)
Hao L, Jun T, Pan L (2018) Human-robot cooperative control based on sEMG for the upper limb exoskeleton robot. Robot Auton Syst
He L, Xiong C, Liu K, Huang J, He C, Chen WB (2018) Mechatronic design of a synergetic upper limb exoskeletal robot and wrench-based assistive control. J Bionic Eng 15:247–259
Nelson CA, Nouaille L, Poisson G (2020) A redundant rehabilitation robot with a variable stiffness mechanism. Mech Mach Theory 150
Leiyu Z, Jianfeng L, Ying C, Mingjie D, Bin F, Pengfei Z (2020) Design and performance analysis of a parallel wrist rehabilitation robot (PWRR). Robot Auton Syst 125(C)
Nelson Carl A, Laurence N, Gérard P (2019) A redundant rehabilitation robot with a variable stiffness mechanism. Mechan Mach Theory 150
Ning Y, Xu W, Huang H, Li B, Liu F (2019) Design methodology of a novel variable stiffness actuator based on antagonistic-driven mechanism. Proc Inst Mech Eng Part C: J Mech Eng Sci 233(19–20)
Meshram DA, Patil DD (2020) 5G Enabled tactile internet for tele-robotic surgery. Procedia Comput Sci 171
Platz T, Pinkowski C, Van WF, Wijck FV, Kim IH, Bella PD, Johnson G (2005) Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer test, action research arm test and box and block test: a multicentre study. Clin Rehab 19:404–411
Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B (2018) Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev, https://doi.org/10.1002/14651858.cd006876.pub5
Proud EL, Miller KJ, Bilney B, Balachandran S, McGinley JL, Morris ME (2015) Evaluation of measures of upper limb functioning and disability in people with Parkinson disease: a systematic review. Arch Phys Med Rehab 96:540–551
Boser QA, Valevicius AM, Lavoie EB, Chapman CS, Pilarski PM, Hebert JS, Vette AH (2018) Cluster-based upper body marker models for three-dimensional kinematic analysis: comparison with an anatomical model and reliability analysis. J Biomech 72:228–234
Caimmi M, Guanziroli E, Malosio M, Pedrocchi N, Vicentini F, Molinari Tosatti L, Molteni F (2015) Normative data for an instrumental assessment of the upper-limb functionality. Biomed Res Int 484131
Hebert JS, Lewicke J, Williams TR, Vette AH (2014) Normative data for a modified box and blocks test measuring upper limb function via motion capture. J Rehab Res Dev 51:919–932
Sohn WJ, Sipahi R, Sanger TD, Sternad D (2019) Portable motion-analysis device for upper-limb research, assessment, and rehabilitation in non-laboratory settings. IEEE J Trans Eng Health Med 7:1–14
Vigotsky AD, Halperin I, Lehman GJ, Trajano GS, Vieira TM (2018) Interpreting signal amplitudes in surface electromyography studies in sport and rehabilitation sciences. Front Physiol 108:227–237
Goble JA, Zhang Y, Shimansky Y, Sharma S, Dounskaia NV (2007) Directional biases reveal utilization of arm’s biomechanical properties for optimization of motor behavior. J Neurophysiol 98:1240–1252
William HJ, Shivam P, Priyanshu A, Sadie HR, Bowie RL, Michael DB, Marcia KO’M (2018) Toward improved surgical training: Delivering smoothness feedback using haptic cues. 2018 IEEE haptics symposium (HAPTICS), pp 241–246
Zhang LL, Zhou J, Zhang XA, Wang CT (2011) Upper limb musculo-skeletal model for biomechanical investigation of elbow flexion movement. J Shanghai Jiaotong Univ (Science) 16:61–64
Ma R, Zhang L, Li G, Jiang D, Xu S, Chen D (2020) Grasping force prediction based on sEMG signals. Alexandria Eng J 59(3):1135–1147
Dai C, Hu X (2019) Extracting and classifying spatial muscle activation patterns in forearm flexor muscles using high-density electromyogram recordings. Int J Neural Syst, https://doi.org/10.1142/s0129065718500259
Ursula T, Hermann S, Richard B, Nathalie A (2019) Muscle force estimation in clinical gait analysis using AnyBody and OpenSim. J Biomech 86:55–63
Malesevic N, Björkman A, Andersson GS, Matran-Fernandez A, Citi L, Cipriani C, Antfolk C (2020) A database of multi-channel intramuscular electromyogram signals during isometric hand muscles contractions. Scientific Data, https://doi.org/10.1038/s41597-019-0335-8
Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG (2007) OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng 54(11):1940–1950
Saul KR, Hu X, Goehler CM, Vidt ME, Daly M, Velisar A, Murray WM (2015) Benchmarking of dynamic simulation predictions in two software platforms using an upper limb musculoskeletal model. Comput Methods Biomech Biomed Eng 18:1–14
David GL, Thor FB (2003) An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J Biomech 36:765–776
Christian F, Günter H (2008) A human–exoskeleton interface utilizing electromyography. IEEE Trans Rob 24:872–882
Jiateng H, Yingfei S, Lixin S, Bingyu P, Zhipei H, Jiankang W, Zhiqiang Z (2018) A pilot study of individual muscle force prediction during elbow flexion and extension in the neurorehabilitation field. Sensors 16:2–15
Acknowledgements
Supported by National Natural Science Foundation of China (Grant No. 51865056), and Xi’an Jiaotong University State Key Laboratory for Manufacturing Systems Engineering (Grant No. sklms2018006).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tao, Q., Li, Z., Lai, Q., Wang, S., Liu, L., Kang, J. (2021). A Dynamic Evaluation Mechanism of Human Upper Limb Muscle Forces. In: Pham, T.D., Yan, H., Ashraf, M.W., Sjöberg, F. (eds) Advances in Artificial Intelligence, Computation, and Data Science. Computational Biology, vol 31. Springer, Cham. https://doi.org/10.1007/978-3-030-69951-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-69951-2_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69950-5
Online ISBN: 978-3-030-69951-2
eBook Packages: Computer ScienceComputer Science (R0)