Abstract
Lung cancer (LC) is the most incident neoplasm with the highest lethality rates, responsible for 1.8 million (18.4%) deaths in 2018. The great majority of LC (about 80–85%) is non-small cell (NSCLC), whose 5-year survival is about 50%, falling to 1% with the progress of the disease. The majority (80%) of diagnoses are late and with locally advanced disease (22%) or metastatic disease (57%), requiring chemotherapy and/or radiotherapy. The NSCLC is a group of histologically heterogeneous tumors, a characteristic that can be explained by intratumoral heterogeneity (IHT) derived from molecular and genetic changes beyond the expression of immunological biomarkers. This knowledge enabled the development of drugs that interact with specific mutations such as tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) capable of directing the immune response to target tumor cells. To analyze the efficacy of treatment with ICIs and the somatic mutations in NSCLC, it is necessary to evaluate in real time, the changes that occur in tumor cells with the use of these therapies. However, it is difficult to perform this evaluation due to the invasive nature of conventional biopsy. Circulating tumor cells (CTCs), known as “liquid biopsy,” have the potential to monitor the response to treatments, being, in addition, a promising source of early diagnosis in the NSCLC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Non-small cell lung cancer (NSCLC)
- Tyrosine kinase inhibitors (TKIs)
- Immune checkpoint inhibitors (ICIs)
- Circulating tumor cells
5.1 Introduction
Lung cancer is the neoplasm with the highest incidence rate and mortality, affecting men and women. In 2018, the global annual incidence of lung cancer was 2.1 million cases (11.6%), in addition to being responsible for 1.8 million deaths. Based on these data, we can observe that lung cancer is a serious public health problem [25].
Non-small cell lung cancer (NSCLC) is the most incident lung cancer, accounting for about 80–85% of cases being subdivided into three main types: adenocarcinoma, squamous carcinoma, and large cell carcinoma. The overall survival rate of NSCLC is approximately 50% in 5-year but the progression from stage I to stage IV decreases this rate to 1% [50].
The main obstacles to the treatment of NSCLC are late diagnosis, metastatic behavior, and disease recurrence. A small percentage of patients with NSCLC, approximately 20%, are diagnosed in the early stages of the disease (I or II), where they could be treated by surgical resection; however, about 80% are diagnosed late and present with locally advanced disease (22%) or metastatic disease (57%), requiring chemotherapy and/or radiotherapy. Even patients eligible for surgical resection may have recurrences due to distant metastases within the first 24 months [41, 50, 65, 67].
A characteristic of NSCLC is histological heterogeneity. There are variations within the main groups, such as adenocarcinomas, with distinct subtypes, diagnostic, prognostic, therapy, and demography, being necessary for the notification of the NSCLC, the realization of an immunohistochemical profile for differentiation [52].
Histological heterogeneity can be explained by intratumoral heterogeneity (ITH), present in the NSCLC. ITH is understood to be the molecular and genetic changes that occur in this neoplasm. The origin of molecular heterogeneity can be explained by several mechanisms such as genomic or chromosomal instability, epigenetic modifications, adaptations to the microenvironment, clonal evolution due to selective pressure from the tumor microenvironment, or by chemotherapy action. In addition to these molecular changes, the NSCLC expresses biomarkers such as the PD-L1 protein whose ligand, programmed death receptor 1 (PD-1), is expressed by T cells that may be present in the composition of the tumor microenvironment. This discovery enabled the targeting of the immune response to the target tumor cells [2, 3, 52].
Currently, the tumor material used to characterize NSCLC histologically, to identify molecular alterations and protein expression, is obtained by conventional biopsy. However, this examination is invasive and locally restrictive, making it impossible to perform with the frequency necessary to understand the molecular changes that occur in tumor dynamics [33].
In search of new methods to reduce obstacles in the treatment of NSCLC, liquid biopsy, which is the ex vivo analysis of a body fluid sample for the purpose of detecting and quantifying targets of interest, has shown a diagnostic approach with the potential to reveal health changes that include the onset and development of diseases [13].
Liquid biopsy performed by blood is feasible in patients with NSCL, because, unlike tissue biopsy, is performed in minimally invasive and safely procedure. In addition, the blood presents circulating biomarkers that, if analysed, allow a whole understanding of the tumor biology, since they come from the primary tumor and metastatic site [32, 53].
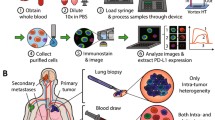
Among these biomarkers, circulating tumor cells (CTCs) are present, which are fragments of the primary tumor that circulate spontaneously individually or in groups of three or more CTCs (clusters), exclusively by lymphatic vessels and blood and precedes the metastatic behavior of neoplasms. CTCs have several components that can be analyzed, such as intact tumor DNA for mutation analysis, tumor RNA for gene expression and profile identification, and several biomarkers for proteomic analysis [33, 46].
Although not yet approved by the Food and Drug Administration (FDA) for use in clinics, CTCs have the potential to complement testing in patients with NSCLC and, in this review, we will focus on the contribution of CTCs to the comprehension of this neoplasm.
5.2 Expression of biomarkers in CTCs of patients with NSCLC
Immunotherapy revolutionized the treatment of patients with NSCLC, as it enabled the targeting of the immune response to tumor cells, allowing patients affected by different types of NSCLC to have a longer survival due to its ability to increase or restore antitumor immune function [2].
PD-L1 protein is expressed in several cell types, among them cancer cells and antigen-presenting cells (B lymphocytes, dendritic cells, macrophages) after being exposed to cytokines. Binding PD-1 to PD-L1 results in a signal that inhibits the full activity of T cells. However, in cancer patients, this inhibition mechanism causes tumor cells to pass unharmed to the immune system [3].
The most clinically advanced ICIs are directed to PD-1/PD-L1, performing immunosuppressive function in patients, obtaining authorization from the Food and Drug Administration (FDA) as a treatment option for NSCLC. Among the ICIs, there are nivolumab and pembrolizumab whose target is the PD-1 receptor and atezolizumab and durvalumab that target the PD-L1 protein [2, 11, 47].
To analyze the efficacy of treatment with ICIs, it is necessary to evaluate in real time the status of PD-1/PD-L1 expression; however, in clinical practice, it is difficult to perform this evaluation due to the invasive nature of conventional biopsy. However, through CTCs, there is the potential to monitor, via liquid biopsy, the dynamics of PD-1/PD-L1 expression of patients treated with ICIs over time.
Some groups have studied PD-1/PD-L1 expression in CTCs. The study by Kallergi et al. [26] demonstrated that CTCs PD-1+ and PD-L1+ can be detected before and after first-line chemotherapy in patients with metastatic NSCLC. For this, CTCs were isolated from 30 patients with NSCLC before chemotherapy and from 11 patients after the third treatment cycle, using the ISET Technology® (Rarecells Diagnostics, France) methodology. To identify the CTCs, Giemsa staining and immunofluorescence staining (IF) were used.
Using Giemsa staining, CTCs were identified in 28 out of 30 patients (93.3%) at baseline and in 9 out of 11 patients (81.8%) after the third chemotherapy cycle. On the other hand, with immunofluorescence staining (FI), CTCs were detected in 17 out of 30 patients (56.7%) at baseline and in 8 out of 11 patients (72.7%) after the third chemotherapy cycle. At the beginning of the study, the expression of PD-1 and PD-L1 was observed in 53% and 47% of patients, respectively. After the third treatment cycle, the corresponding numbers were 13% and 63%, respectively. Median progression-free survival (PFS) was significantly lower in patients with >3 PD-1 CTCs (+) at baseline compared to those with 3 < PD-1 CTCs (+) (p = 0.022) [26].
The pilot study conducted by Dhar et al. [7] also aimed to evaluate the expression of PD-L1 in CTCs. Twenty-two patients with metastatic NSCLC treated with pembrolizumab, nivolumab and avelumab were recruited, of whom 31 samples were collected before and after chemotherapy. Using the Vortex Chip HT device, CTCs were isolated in 30 of the 31 samples (96.8%), and samples with CTCs had 1 or more PD-L1+ CTCs. The PD-L1+ CTCs fraction ranged from 2.2 to 100%. It was possible to verify the agreement of PD-L1 expression of CTCs with tissue biopsy in only 4 patients of 22. This group demonstrated that quantification of PD-L1 CTCs levels when combined with tissue biopsy results can help identify patients with a higher probability of responding to therapy or, by monitoring throughout treatment, the patients most likely to become resistant to treatment.
Ilie et al. [20] isolated CTCs, using the ISET Technology® (Rarecells Diagnostics, France) platform, in samples of 106 patients, as a non-invasive method to evaluate the status of PD-L1 in patients with advanced NSCLC and compared them with the status of PD-L1 in tumor tissue. CTCs were detected in 80 (75%) patients. In 71 samples, it was possible to compare the tissue and CTCs; 6 patients (8%) presented 1 PD-L1(+) CTCs and 11 patients (15%) presented 1% of PD-L1(+) tumor cell in the tumor tissue, with 93% agreement between tissue and CTCs, demonstrating that the status of both tissues correlate, revealing the potential of CTCs to assess real-time PD-L1 expression in patients with NCSLC.
In view of the results presented here, it is observed that CTCs can contribute to the analysis of expression levels PD-1/PD-L1 before the start of treatment and progressively over this course.
5.3 Circulating Tumor Cells: Source of Early Detection and Recurrence of NSCLC
On average, 80% of the patients are diagnosed late, that is, with the disease in advanced stages, where surgical treatment is not an option. Even with the advancement of therapies, a large portion of the patients do not survive the 5 years after diagnosis. Reducing tobacco consumption is a very important factor in controlling the number of NSCLC cases, but in addition, there is an imminent need to diagnose patients in the early stages of the disease.
The American College of Radiology Imaging Network conducted The National Lung Screening Trial (NLST) which aimed to compare two forms of early detection of lung cancer: computed helical low-dose Tomography (CT) – often referred to as spiral CT – and standard Chest X-ray [40]. The study was conducted with 53.454 smokers and ex-smokers aged between 55 and 74 years, who smoked at least 30 packs-a-year, who had no previous symptoms or history of lung cancer. The results of this study showed that low-dose CT screening was 24.2% while X-ray was 6.9%. However, among the positive results, 96.4% in the low-dose CT group and 94.5% in the X-ray group were false-positive results.
The amount of false-positive results raised the question about expanding this type of screening, which could increase the rate of consultations based on indeterminate cause nodules, generating concerns and high costs. On the basis of this study and given the imminent need for new methods for the early detection of lung cancer (LC), Ilie et al. [21] analyzed patients with chronic obstructive pulmonary disease (COPD), which, regardless of stage of development, is a risk factor for NSCLC. In addition, based on the invasive behavior of the NSCLC and data from experimental models where tumors measuring less than 1 mm can release CTCs in the bloodstream, the group proposed to investigate whether patients with COPD had CTCs, which could be an early marker of NSCLC.
For this, they analyzed the peripheral blood of 168 patients with COPD, who did not present any lung cancer detectable by imaging tests. Using ISET Technology (Rarecells Diagnostics, France), researchers detected CTCs in 3% (5 patients). The patients were followed-up and after an average of 3.2 years, all presented nodules in the lung detected by computed tomography. The 5 patients underwent surgery and analysis showed that the cancer was stage I, which means that they had not spread to lymph nodes or developed metastases. This study demonstrated, for the first time, the potential of CTCs as an early marker of invasive CL in patients at high risk [21].
CTCs are considered the primary metastatic source of cancer due to their ability to colonize organs and tissues. To this end, CTCs undergo several molecular and cellular changes, through the epithelium-mesenchymal transition process (EMT), granting a mesenchymal phenotype to epithelial cells making them more effective in their mobility due to the weakening of cell-cell adhesion and fusiform shape gain fundamental for metastatic behavior to be effective [31, 36].
The study by Xie et al. [62] investigated the possible correlations between CTCs and pathological types and staging of NSCLC during the early postoperative period. Sixty-nine patients with NSCLC were recruited. CTCs were analyzed by multiple mRNA in situ after enrichment by nanotechnology for lysis of red blood cells.
The presence of epithelial or mixed CTCs had no significant correlation with tumor size, lymph node metastasis, and distant metastasis TMN in patients with NSCLC (P > 0.05), but higher TNM levels were related to the presence of mesenchymal CTCs (P < 0.05). After surgery, the patients were divided into pathological types: 48 patients had adenocarcinoma of which 40 were positive for CTCs. Of the 16 cases of squamous cell carcinoma, only 2 were negative for CTCs and among the 5 patients with large cell carcinoma only 1 had CTCs (P < 0.5) [62].
Frick et al. [12] analyzed CTCs as a prognostic marker to measure the risk of NSCLC recurrence after stereotactic body radiotherapy (SBRT) treatment. The treatment is effective in early stage of NSCLC; however, failures occur at the primary tumor site in about 10–15% and 20–25% in distant locations. For the study, 92 patients with stage I NSCLC treated with SBRT were recruited. The samples for analysis of CTCs were obtained before, during, and in series up to 24 months after treatment with SBRT. CTCs were quantified by a trial using adenoviral-based probe that expresses green fluorescent protein (GFP) that detects high telomerase activity in cancer cells.
The CTC test was positive before SBRT treatment in 38 of 92 (48%) patients. During treatment, CTCs were observed in 35 patients with a count of 0.5 CTC/mL. In the 3-month period after SBRT treatment, CTCs continued to be detected in 10 out of 35 patients (29%). The persistence of CTCs was associated with increased risk of treatment failures in distant locations and (P = 0,04) tended to increase the regional failure (P = 0,08) and local failure (P = 0,16). This study suggests that CTCs before treatment and its post-treatment maintenance are associated with the risk of recurrence outside the target treatment site, suggesting that CTCs have the potential to identify patients at higher risk of recurrence [12].
In order to identify the prognostic value of the presence and characterization of CTCs in the peripheral blood of NSCLC patients undergoing radical resection, Bayarri-Lara et al. [1] analyzed samples of 56 patients with pathological stage between IA and IIIA, obtained before and 1 month after surgery, the mean follow-up of these patients was from 3 to 16 months (variation 3–23).
In the samples prior to surgery, CTCS were detected in 29 of 56 patients (51.8%) and after 1 month of surgery, 18 patients (32.1%) presented CTCs. During follow-up, 16 patients (28.6%) presented signs of cancer recurrence in an average of 8 months; 50% of the patients who had CTCs after surgery developed recurrence, compared to 18.4% of the patients who did not have post-surgery CTCs, thus correlating the presence of CTCs after surgery to a higher risk of early recurrence.
The results of these studies demonstrated the potential of CTCs as an early marker of diagnosis and recurrence in the NSCLC, which would enable more rigorous and early decision-making, in addition to the individualization of treatment.
5.4 Identification of the NSCLC Molecular Profile in CTCs
Knowing the molecular heterogeneity of NSCLC was an important factor for the development of new precision therapies, because some of these tumors are dependent on oncogenes, that is, depend on key point mutations of signaling pathways to grow and survive.
Among NSCLC subtypes, adenocarcinoma is the most incident and may present at least one driver mutation. The main changes identified were in the epidermal growth factor receptor (EGFR) and in the anaplastic lymphoma kinase (ALK), both protein tyrosine kinases (PTKs) receptors, proteins responsible for gene expression, acting in cell growth, survival, migration, and apoptosis, these being, until now, the main targets for the treatment of NSCLC.
The discovery of these molecular changes changed the course of the treatment of patients with NSCLC, as it enabled the development of tyrosine kinase inhibitors (TKIs), whose function is to prevent the enzymatic activity of these oncogenes. EGFR TKIs are gefitinib, erlotinib, afatinib, and osimertinib, and ALK inhibitors are crizotinib, ceritinibe, and alectinib. The response to the use of TKIs has been promising, with very significant clinical benefits. Objective response rates of 60–70% are reported with the use of these different TKIs and a disease control rate of up to 80–90%. However, patients tend to develop drug resistance within 1 to 2 years due to somatic mutations [24, 27, 38, 55].
Mutations in EGFR occur mainly at sites where EGFR binds to TKIs and are detected in exons 18 to 21 of the tyrosine kinase coding gene. More than 85% of adenocarcinomas present exon 19 deletions or L858R point mutation in exon 21, targets that are clinically actionable. At exon 18, point nucleotide substitutions occur at codon 719. In the exon 20, there are point mutations and insertions including T790M, and this mutation is responsible for about 50% of all acquired resistance mutations. In ALK rearrangements, EML4-ALK is the dominant rearrangement. This mutation is found in 3–7% of NSCLC [5, 10, 51].
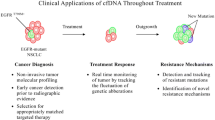
It is necessary to develop new ways of detecting somatic mutations in NSCLC. Studies have shown that CTCs have predictive, diagnostic, and prognostic value to identify mutations in NSCLC, in addition to identifying and monitoring mutations related to resistance to TKI treatments.
The study by Yang et al. [64] aimed to isolate and quantify CTCs after treatment with osimertinib, TKI) with activity against the T790M mutation in EGFR. Patients (n = 68) had samples collected at baseline and on day 28. CTCs were evaluated by the CellSearch system. CTCs were divided into favorable (<5 CTCs) and unfavorable (≥5 CTCs) groups. Patients in the favorable group at the beginning of the study showed significantly longer median progression-free survival (PFS) compared to patients in the unfavorable group (9.3 vs.6.5 months; p = 0.0002). The PFS interval for patients in the favorable group on day 28 was 9.7 months, significantly higher than the mean time of PFS of 6.2 months achieved by patients in the unfavorable group (p = 0.011). This is the first report on the presence of CTCs and its prognostic role in T790M-positive NSCLC EGFR patients after disease progression with treatment with EGFR-TKI.
The objective of the study by Pailler et al. [45] was to verify whether the sequencing of CTCs could provide information on acquired resistance to ALK inhibitors in addition to tumor heterogeneity in NSCLC mutated in ALK. Patients treated with TKI-ALK (n = 17), crizotinib (n = 14) or lorlatinib (n = 3) were recruited after progression of the disease.
The samples were filtered with ISET Technology® (Rarecells Diagnostics, France), CellSearch, and Rosettesep system. Pools of CTCs (n = 126) and 56 unique CTCs were isolated and sequenced. Hotspot regions over 48 cancer-related genes and 14 ALK mutations were examined to identify ALK-independent and ALK-dependent resistance mechanisms. Various mutations were observed in crizotinib-resistant patients in several genes on independent pathways of ALK. RTK-KRAS (EGFR, KRAS, BRAF) and TP53 pathways have been mutated recurrently. In a patient resistant to lorlatinib, two single CTCs in 12 showed mutations in the compound ALK. Mutation of the compound ALK G1202R/F1174C was observed practically similar to ALK G1202R/F1174L and ALK G1202R/T1151 mutation of the compound not detected in tumor biopsy. These results highlight the genetic heterogeneity and clinical utility of CTCs to identify TKIs-ALK resistance mutations. Therefore, CTC sequencing can be a unique tool to evaluate resistance mechanisms and assist in the personalization of treatments [45].
By means of hypermetabolic CTCs, detected by the increased uptake of glucose, Turetta et al. [58] demonstrated that it is possible to evaluate the mutational status of the NSCLC. Thirty patients with stage IV NSCLC were included in the study, of which the blood samples were incubated with 2-NBDG, a fluorescent glucose analog, and analyzed by flow cytometry. Using ddPCR, they detected mutations in EGRF and KRAS in 85% of patients, corresponding to the primary tumor in 70% of cases. Multiple mutations in KRAS were found in two patients, other two had mutations different from those detected in the primary tumor and two patients with wild primary tumor new mutations were detected: EGFR p.746_750del and KRAS p.G12V. This study demonstrated the potential of CTCs to detect distinct mutations of the primary tumor, allowing us to know the heterogeneity of the NSCLC.
Analyzing samples of 125 patients with stage IIIB-IV NSCLC, using CellSearch technology and anti-vimentin antibody to detect mesenchymal CTCs, Lindsay, et al. [34], observed that 51/125 patients (40.8%) had CTCs and 26/125 (20.8%) were CTC + vim at the beginning of the study. A multivariate analysis showed that patients with 5 CTCs (total) significantly reduced to OS but not PFS compared to patients with <5 total CTCs.
The researchers divided the patients according to the mutation of the NSCLC driver, where they observed an increase of vim + CTCs in the mutated subgroup EGFR (N = 21/94 patients), a reduction of total CTCs in the rearranged subgroup ALK (N = 13/90 patients), and a total absence of vim + CTCs in adenocarcinomas mutated with KRAS (N = 19/78 patients. This study demonstrated that EGFR mutant CTCs express epithelium-mesenchymal transition characteristics not observed in CTCs of KRAS-mutant adenocarcinoma patients [34].
Chromosomal rearrangements of ROS1 in CTCs of patients with NSCLC mutated in ROS1 and treated with crizotinib were evaluated by Pailler et al. [43]. A sample of four patients was analyzed using ISET Technology® (Rarecells Diagnostics, France), and the ROS1 rearrangement was detected by filter-adapted-fluorescence in situ hybridization (FA-FISH). In CTCs of all patients, ROS1 rearrangement was detected, initially confirmed by conventional biopsy. The mean number of CTCs at the beginning of the study was 34.5/3 ml of blood. Tumor heterogeneity, assessed by the number of copies of ROS1, was significantly higher in baseline CTCs compared to tumor biopsies. The number of CTCs increased significantly in two patients who progressed during crizonitinibe treatment. This study showed for the first time the ability of CTCs to detect mutated NSCLC in ROS1.
The combination of the studies exposed in this chapter (Table 5.1) demonstrates the potential of CTCs as an auxiliary and/or independent source for mutation analysis, a tool for prognosis in treatments with TKIs and ICIs, as also for early diagnosis of NSCLC. It is essential to develop more research in order to contribute to the validation of CTCs in clinical practice, composing the biomarkers used in liquid biopsies.
References
Bayarri-Lara C, Ortega FG, Guevara ACL, et al. Circulating tumor cells identify early recurrence in patients with non-small cell lung cancer undergoing radical resection. PLoS One. 2016;11(2):e0148659.
Bianco A, Perrotta F, Barra G, et al. Prognostic factors and biomarkers of responses to immune checkpoint inhibitors in lung cancer. Int J Mol Sci. 2019;20(19):4931.
Borst J, Ahrends T, Babala N, et al. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635–47.
Castello A, Carbone FG, Rossi S, et al. Circulating tumor cells and metabolic parameters in NSCLC patients treated with checkpoint inhibitors. Cancers (Basel). 2020;12(2):487.
Chiba R, Morikawa N, Sera K, et al. Elemental and mutational analysis of lung tissue in lung adenocarcinoma patients. Transl Lung Cancer Res. 2019;8(3):224–34.
Chudasama D, Barr J, Beeson J, et al. Detection of circulating tumour cells and survival of patients with non-small cell lung cancer. Anticancer Res. 2017;37(1):169–73.
Dhar M, Wong J, Che J, et al. Evaluation of PD-L1 expression on vortex-isolated circulating tumor cells in metastatic lung cancer. Sci Rep. 2018;8(1):2592.
Dong J, Zhu D, Tang X, et al. Circulating tumor cells in pulmonary vein and peripheral arterial provide a metric for PD-L1 diagnosis and prognosis of patients with non-small cell lung cancer. PLoS One. 2019;14(7):e0220306.
Duan GC, Zhang XP, Wang HE, et al. Circulating tumor cells as a screening and diagnostic marker for early-stage non-small cell lung cancer. Onco Targets Ther. 2020;13:1931–9.
Ellison G, Zhu G, Moulis A, et al. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66(2):79–89.
Fiorelli A, Perrotta F, Mollica M, et al. Endoscopic central airway recanalization to enable first line pembrolizumab treatment in a PD-L1 strongly positive non-small cell lung cancer: a case report. J Cardiothorac Surg. 2019;14(1):50.
Frick MA, Feigenberg SJ, Jean-Baptiste SR, et al. Circulating tumor cells are associated with recurrent disease in patients with early-stage non–small cell lung cancer treated with stereotactic body radiotherapy. Clin Cancer Res. 2020;26:2372.
Gerner C, Costigliola V, Golubnitschaja O. MULTIOMIC patterns in body fluids: technological challenge with a great potential to implement the advanced paradigm of 3P medicine. Mass Spectrom Rev. 2019;1:1–10.
Guibert N, Delaunay M, Lusque A, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer. 2018;120:108–12.
Hanssen A, Wagner J, Gorges TM, et al. Characterization of different CTC subpopulations in non-small cell lung cancer. Sci Rep. 2016;6:28010.
He Y, Shi J, Schmidt B, et al. Circulating tumor cells as a biomarker to assist molecular diagnosis for early stage non-small cell lung cancer. Cancer Manag Res. 2020;12:841–54.
Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2011a;17(4):827–35.
Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer. 2011b;129(7):1651–60.
Ichimura H, Nawa T, Yamamoto Y, et al. Detection of circulating tumor cells in patients with lung cancer using metallic micro-cavity array filter: a pilot study. Mol Clin Oncol. 2020;12(3):278–83.
Ilié M, Szafer-Glusman E, Hofman V, et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol. 2018;29(1):193–9.
Ilie M, Hofman V, Mira EL, et al. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One. 2014;9(10):4–10.
Ilie M, Long E, Butori C, et al. ALK-gene rearrangement: a comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann Oncol. 2012;23(11):2907–13.
Ilie M, Szafer-Glusman E, Hofman V, et al. Expression of MET in circulating tumor cells correlates with expression in tumor tissue from advanced-stage lung cancer patients. Oncotarget. 2017;8(16):26112–21.
Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150(6):1107–20.
International Agency For Research On Cancer. Lung. GLOBOCAN. 2018. Disponível em (https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf). Acesso em 13/01/2020.
Kallergi G, Vetsika EK, Aggouraki D, et al. Evaluation of PD-L1/PD-1 on circulating tumor cells in patients with advanced non-small cell lung cancer. Ther Adv Med Oncol. 2018;10:1758834017750121.
Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with Crizotinib-refractory anaplastic lymphoma kinase–positive non–small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490–8.
Koh Y, Yagi S, Akamatsu H, et al. Heterogeneous expression of programmed death receptor-ligand 1 on circulating tumor cells in patients with lung cancer. Clin Lung Cancer. 2019;20(4):207–77.
Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung Cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7(2):306–15.
Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non–small-cell lung cancer. Clin Oncol. 2011;29(12):1556–63.
Ksiazkiewicz M, Markiewicz A, Zaczek AJ. Epithelial-mesenchymal transition: a hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology. 2012;79(4):195–208.
Lichan A, Zigang C, Bode D. Circulating tumor cells: moving biological insights into detection. Theranostics. 2017;7(10):2606–19.
Lim M, Kim CJ, Sunkara V, et al. Liquid biopsy in lung cancer: clinical applications of circulating biomarkers (CTCs and ctDNA). Micromachines (Basel). 2018;9(3):100.
Lindsay CR, Faugeroux V, Michiels S, et al. A prospective examination of circulating tumor cell profiles in non-small-cell lung cancer molecular subgroups. Ann Oncol. 2017;28(7):1523–31.
Liu DG, Xue L, Li J, et al. Epithelial-mesenchymal transition and GALC expression of circulating tumor cells indicate metastasis and poor prognosis in non-small cell lung cancer. Cancer Biomark. 2018;22(3):417–26.
Lozar T, Gersak K, Cemazar M, et al. The biology and clinical potential of circulating tumor cells. Radiol Oncol. 2019;53(2):131–47.
Marchetti A, Del Grammastro M, Felicioni L, et al. Assessment of EGFR mutations in circulating tumor cell preparations from NSCLC patients by next generation sequencing: toward a real-time liquid biopsy for treatment. PLoS One. 2014;9(8):e103883.
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57.
Monterisi S, Castello A, Toschi L, et al. Preliminary data on circulating tumor cells in metastatic NSCLC patients candidate to immunotherapy. Am J Nucl Med Mol Imaging. 2019;9(6):282–95.
National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409.
Osmani L, Askin F, Gabrielson E, et al. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(1):103–9.
Pailler E, Adam J, Barthélémy A, et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non–small-cell lung cancer. J Clin Oncol. 2013;31(18):2273–81.
Pailler E, Auger N, Lindsay CR, et al. High level of chromosomal instability in circulating tumor cells of ROS1-rearranged non-small-cell lung cancer. Ann Oncol. 2015;26(7):1408–15.
Pailler E, Oulhen M, Borget I, et al. Circulating tumor cells with aberrant ALK copy number predict progression-free survival during Crizotinib treatment in ALK-rearranged non–small cell lung cancer patients. Cancer Res. 2017;77(9):2222–30.
Pailler E, Faugeroux V, Oulhen M, et al. Acquired resistance mutations to ALK inhibitors identified by single circulating tumor cell sequencing in ALK-rearranged non–small-cell lung cancer. Clin Cancer Res. 2019;25(22):6671–82.
Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC): detection clinical impact and future directions. Cancer Lett. 2007;253:180–204.
Perrotta F, Rocco D, Vitiello F, et al. Immune checkpoint blockade for advanced NSCLC: a new landscape for elderly patients. Int J Mol Sci. 2019;20(9):2258.
Reddy RM, Murlidhar V, Zhao L, et al. Pulmonary venous blood sampling significantly increases the yield of circulating tumor cells in early-stage lung cancer. J Thorac Cardiovasc Surg. 2016;151(3):852–8.
Schehr JL, Schultz ZD, Warrick JW, et al. High specificity in circulating tumor cell identification is required for accurate evaluation of programmed death-ligand 1. PLoS One. 2016;11(7):e0159397.
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2015;65(1):5–29.
Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6.
Sousa VML, Carvalho L. Heterogeneity in lung cancer. Pathobiology. 2018;85:96–107.
Sumanasuriya S, Lambros MB, Bono JS. Application of liquid biopsies in cancer targeted therapy. Clin Pharmacol Ther. 2017;102(5):745–7.
Tamminga M, De Wit S, Hiltermann TJN, et al. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J Immunother Cancer. 2019;7(1):173.
THE CANCER GENOME ATLAS RESEARCH NETWORK. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–25.
Tong B, Xu Y, Zhao J, et al. Prognostic role of circulating tumor cells in patients with EGFR-mutated or ALK-rearranged non-small cell lung cancer. Thorac Cancer. 2018;9(5):640–5.
Tong B, Xu Y, Zhao J, et al. Prognostic significance of circulating tumor cells in non-small cell lung cancer patients undergoing chemotherapy. Oncotarget. 2017;8(49):86615–24.
Turetta M, Bulfoni M, Brisotto G, et al. Assessment of the mutational status of NSCLC using Hypermetabolic circulating tumor cells. Cancers (Basel). 2018;10(8):270.
Watanabe M, Kenmotsu H, Ko R, et al. Isolation and molecular analysis of circulating tumor cells from lung cancer patients using a microfluidic chip type cell sorter. Cancer Sci. 2018;109(8):2539–48.
Wei T, Zhu D, Yang Y, et al. The application of nano-enrichment in CTC detection and the clinical significance of CTCs in non-small cell lung cancer (NSCLC) treatment. PLoS One. 2019;14(7):e0219129.
Wu CY, Lee CL, Wu CF, et al. Circulating tumor cells as a tool of minimal residual disease can predict lung cancer recurrence: a longitudinal, prospective trial. Diagnostics (Basel). 2020;10(3):144.
Xie Z, Gao X, Cheng K, et al. Correlation between the presence of circulating tumor cells and the pathologic type and staging of non-small cell lung cancer during the early postoperative period. Oncol Lett. 2017;14(5):5825–30.
Yang B, Qin A, Zhang K, et al. Circulating tumor cells predict prognosis following tyrosine kinase inhibitor treatment in EGFR-mutant non-small cell lung Cancer patients. Oncol Res. 2017;25(9):1601–6.
Yang B, Zheng D, Zeng Y, et al. Circulating tumor cells predict prognosis following secondline AZD 9291 treatment in EGFR-T790M mutant non small cell lung cancer patients. J BUON. 2018;23(4):1077–81.
Yu H, Xu L, Liu Z, et al. Circ_MDM2_000139, Circ_ATF2_001418, Circ_CDC25C_002079, and Circ_BIRC6_001271 are involved in the functions of XAV939 in non-small cell lung cancer. Can Respir J. 2019;2019:9107806.
Yu XM, Wu YC, Liu X, et al. Cell-free RNA content in peripheral blood as potential biomarkers for detecting circulating tumor cells in non-small cell lung carcinoma. Int J Mol Sci. 2016;17(11):1845.
Zhang Y, Yang X, Liu H, et al. Inhibition of tumor Lymphangiogenesis is an important part that EGFR-TKIs play in the treatment of NSCLC. J Cancer. 2020;11(1):241–50.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Torres, J.A. (2021). Circulating Tumor Cells in the context Non-small Cell Lung Cancer. In: Chinen, L.T.D. (eds) Atlas of Liquid Biopsy. Springer, Cham. https://doi.org/10.1007/978-3-030-69879-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-69879-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69878-2
Online ISBN: 978-3-030-69879-9
eBook Packages: MedicineMedicine (R0)