Abstract
Colorectal cancer (CCR) is the second most diagnosed cancer in women and third most in men, accounting for approximately 10% of annually diagnosed cancers. It is a prevalent disease in older patients, but the incidence is rising in younger ones. The systemic therapy generally most used in metastatic CCR includes the basis of chemotherapy paired with a biological treatment. Usually, chemotherapy is composed of fluoropyrimidines combined with oxaliplatin and irinotecan. At the beginning of the formation and growth of a primary tumor, the cells are eliminated from the primary tumor and then circulate through the bloodstream, called circulating tumor cells (CTCs). In CCR, CTCs can be used for screening, in localized and metastatic cancer, which is explicated in this chapter.
Figures separated by Ludmilla T.D. Chinen and revised by Mauro Saieg (Cytopathologist from AC Camargo Cancer Center, São Paulo, SP – Brasil)
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Colorectal cancer (CCR) is the second most common cancer diagnosed in women and third most in men, accounting for approximately 10% of all annually diagnosed cancers and cancer-related deaths worldwide [8]. These rates also vary geographically, with the highest rates seen in the most developed countries. It is a prevalent disease in older patients, but the incidence is rising in younger ones, especially rectal cancer and left-sided colon cancer [19].
CCR is largely an asymptomatic disease until it reaches an advanced stage; in these cases, symptoms such as rectal bleeding, change in bowel habits, anemia, or abdominal pain should alert patients to look for a doctor. In asymptomatic patients, screening methods are important. Colonoscopy, occult blood in feces, and sigmoidoscopy are the most common used methods, but each one has its own limitations [12]. Thus, new and less invasive methods need to be investigated.
For metastatic CCR, systemic therapy typically includes chemotherapy backbone paired with a biological treatment. Fluoropyrimidines combined with oxaliplatin (FOLFOX) and irinotecan (FOLFIRI) chemotherapies are the most commonly used regimens [12]. In terms of response rate and survival, the addiction of a biologic (anti-VEGF or anti-EGFR) antibody in the chemotherapy regimen, depending on the tumor-specific factor, must be considered.
It is known that genetic intratumor heterogeneity contributes to treatment failure and drug resistance [14]. Several studies comparing mutational profiles of primary tumors and associated metastatic lesions [13, 36] and local recurrences [29] have provided evidence of intratumor heterogeneity.
Early during the formation and growth of a primary tumor, cells are shed from the primary tumor and then circulate through the bloodstream. These circulating tumor cells (CTCs) can be enriched and detected by different technologies, which take advantage of their physical and biological properties. CTC analysis is considered a real-time “liquid biopsy” for patients with cancer [3].
Compared with conventional biopsy, the “liquid biopsy” has some advantages: requires only a small amount of blood [23], is minimally invasive [24], allows early detection of cancer [17] and-real time monitoring for treatment responses and resistance, by repeated analysis [6]. Some disadvantages are the lack of standardization techniques [9] and insufficient clinical and technical validation [4].
In CCR, CTCs can be used for screening (early detection of invasive cancers), in localized cancer (risk stratification) , prognosis and monitoring after treatment, and metastatic cancer (selection of therapy, monitoring of response, and resistance mechanisms).
4.2 CTCs for Colorectal Cancer Screening
Although the prognostic value of CTCs in the early stages of CCR has already been evaluated in several clinical studies, its role in screening and early detection remains controversial, but it is a very promising topic [22, 30].
The main study on CTCs with the screening approach was recently presented at ASCO 2018 with 620 participants (182 healthy controls, 111 participants with precancerous lesions, and 327 patients with stage I-IV CRC). The results were compared to a standard clinical protocol, including colonoscopy and biopsy results, revealing an overall accuracy of 88% for all stages of the disease, including precancerous lesions. It is the first study to show high sensitivity in the detection of precancerous colorectal lesions [33].
The simple collection of blood for liquid biopsy can be easily integrated into the routine physical examination of the patient, increasing adherence to the test and, thus, allowing an increase in early diagnosis without the need for invasive tests; however, we still need more studies to support this tracking strategy in colorectal cancer.
4.3 CTCs for Evaluation of Minimal Residual Disease in metastatic CCR
Treatment for patients with localized CRC consists of surgery, and in some cases, stages II and III, adjuvant treatment with chemotherapy in addition to surgery is indicated. Identifying patients at high risk of recurrence and treating them with adjuvant therapy remains an important clinical issue. In current practice, we used tumor markers such as carcinoembryonic antigen and clinical-pathological factors to define the risk of recurrence and prognosis, with limitations in identifying minimal residual disease (MRD). Therefore, the monitoring of CTCs during post-surgical follow-up evaluations may allow the patient to better stratify in relation to the risk of recurrence.
In a study with 141 patients (stages II and III), the presence of CTCs after curative surgery was associated with worse progression-free survival and overall survival. In this study, recurrence occurred in 72.5% of patients with positive CTCs after surgery, on the other hand, recurrence occurred in only 12.2% of patients with negative CTCs [20].
A research with 138 patients showed that postoperative patients with positive CTC and negative CTC before surgery is an independent indicator of poor prognosis for CRC patients treated with curative resection [38].
A study with 130 patients with stage II-III CRC demonstrated that the postoperative CTC counts were earlier than the preoperative CTCs in predicting tumor recurrence survival in patients with non-metastatic CRC undergoing surgery. In addition, the authors developed CTC-based prognostic models to predict tumor recurrence in stage II-III CRC, which can be used to identify patients at high risk for recurrence and guide aggressive treatment to improve the clinical outcomes of these patients [35]. Please see some pictures of CTCs isolated from localized colon cancer by ISET in Figs. 4.1, 4.2, 4.3, 4.4, 4.5, 4.6, 4.7, 4.8, 4.9, 4.10, 4.11, 4.12, 4.13, 4.14, 4.15, 4.16, 4.17, 4.18, 4.19, and 4.20.
Patient with 58 years old, male, with stage IIIC (1st collection, at diagnosis). CTC count was 4.60 CTCs/mL. The CTC count was 0.33 CTCs/mL after surgery and 4.33 CTCs/mL after adjuvancy. On letter C, we can better visualize nuclear irregularity and lobular nuclei. In boxes (a–d) we can oberve CTCs with different shapes
CTCs from the same patient of Fig. 4.3. Isolated CTC of 3rd collection (after adjuvancy). The CTC count was 5.66 CTCs/mL
CTCs from the same patient of Fig. 4.9. This picture is of the 2nd collection. The count was 3 CTCs/ml (cytoplasm staining for ERCC1)
Same patient of picture Fig. 4.13. Here, we can observe a proliferation of epithelial cells with three-dimensional arrangements and columnar-looking cells. Staining for TGF-βRI
Same patient of Fig. 4.16
Same patient of Fig. 4.16. Three-dimensional cluster of neoplastic epithelial cells
Finally, a study with 438 patients, with the objective to evaluate the presence of CTCs in the pre- and postoperative scenario in patients with colorectal cancer in stages I-III undergoing curative resection and, thus, identifying a subgroup of patients at high risk of relapse, suggested that the persistent presence of CTCs in the postoperative period can be a crucial prognostic factor, in addition to conventional tumor markers in patients with CRC undergoing curative resection. The identification of these high-risk patients with persistent positive CTCs is important and, therefore, can help to define patients for adjuvant therapy with this tumor entity [34].
4.4 CTCs for Prognostic Evaluation in Metastatic Disease
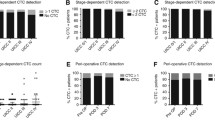
The role of CTCs in the prognostic stratification of patients with metastatic CRC has been demonstrated in several studies emphasizing that the presence of CTCs can predict future metastasis (disease progression) and unfavorable outcome as demonstrated in Table 4.1.
In a previous publication of our group, with 54 mCRC patients, we demonstrated that in addition to the initial CTC count, kinetics was also important for prognostic definition [27]. Evaluating CTC kinetics, when we compared the baseline (pretreatment) CTC level (CTC1) with the level at first follow-up (CTC2), we observed that CTC1-positive patients (CTCs above the median), who became negative (CTCs below the median) had a favorable evolution (n = 14), with a median progression-free survival (PFS) of 14.7 months. This was higher than that for patients with an unfavorable evolution (CTC1− that became CTC2+; n = 13, 6.9 months; p = 0.06). Patients with WT KRAS with favorable kinetics had higher PFS (14.7 months) in comparison to those with WT KRAS with unfavorable kinetics (9.4 months; p = 0.02). Moreover, patients whose imaging studies showed radiological progression had an increased quantification of CTCs at CTC2 compared to those without progression (p = 0.04). This study made possible the presentation of ISET as a feasible tool for evaluating CTC kinetics in patients with mCRC, which can be promising in their clinical evaluation.
These data are reinforced by the meta-analysis with 13 studies that showed that the rate of disease control was significantly higher in patients with CRC with low CTC compared to high CTC (RR = 1354, 95% CI [1002–1830], p = 0.048). CRC patients in the CTC-high group were significantly associated with poor progression-free survival (PFS; HR = 2500, 95% CI [1746–3580], p < 0.001) and poor overall survival (OS; HR = 2856, 95% CI [1959-4164], p < 0.001). Patients who converted from low CTC to high CTC or who were persistently high CTC had a worse disease progression (OR = 27.088, 95% CI [4960–147,919], p < 0,001), PFS (HR = 2095, 95% CI [1105–3969], p = 0.023) and OS (HR = 3604, 95% CI [2096–6197], p < 0,001) than patients who converted from high CTC to low CTC. Thus, it concludes that CTCs can be used as a new marker capable of predicting the response to chemotherapy in patients with metastatic CRC [15].
Another more recent meta-analysis with 15 published studies containing 3129 patients reinforces that the presence of CTCs was significantly associated with poor mortality (overall survival: HR = 2.36, 95% CI: 1.87–2.97; P = 0.006) along with aggressive disease progression (progression-free survival: HR = 1.83, 95% CI: 1.42–2.36; P < 0.00001) (Yi Tan et al. 2017).
Another study by our group in the metastatic setting evaluated the expression of TYMS in CTCs, in 34 samples and was TYMS considered positive in 9 (26.5%). Six of these patients had tumor progression after treatment with 5-FU. An association was found between CTC TYMS staining and disease progression (PD), although without statistical significance (p = 0.07). Patients who had a CTC count above the median (2 CTCs / mL) had higher TYMS expression (p = 0.02) correlating with a worse prognosis. These results suggest that TYMS analysis may be a useful tool as a biomarker predictor of 5-FU resistance if analyzed in CTCs of patients with mCRC [1]. In addition, in another study developed by our group, we analyzed the immunocytochemical expression of MRP1 and ERCC1 in patients with metastatic CRC who had previously detectable CTCs. Among patients treated with irinotecan-based chemotherapy, 4 out of 19 cases with MRP1-positive CTCs showed a worse progression-free survival (PFS) compared to those with negative MRP1 CTCs (2.1 months vs. 9.1 months; p = 0.003). These results show MRP1 as a potential biomarker of resistance to treatment with irinotecan when found in CTCs of patients with mCRC [2].
4.5 CTCs as a Predictive Factor in the Treatment of Locally Advanced Rectal Cancer
Neoadjuvant chemoradiation (NCRT) followed by total mesorectal excision (TME) is the standard treatment for locally advanced rectal cancer (LARC). Our group developed a study aiming to explore the role of CTCs in patients undergoing NCRT followed by surgery for treatment of LARC. In addition, we evaluated the predictive values of TYMS and RAD23B expression in CTC before and after NCRT. The initial analysis of 30 patients was published and demonstrated that the complete pathological response (pCR; p = 0.02) or the partial response (p = 0.01) could correlate with CTC counts. Regarding protein expression, TYMS was absent in 100% of CTCs from patients with pCR (p = 0.001) yet was expressed in 83% of non-responders at S2 (p < 0.001). Meanwhile, RAD23B was expressed in CTCs from 75% of non-responders at S1 (p = 0.01) and in 100% of non-responders at S2 (p = 0.001); 100% of non-responders expressed TYMS mRNA at both timepoints (p = 0.001). In addition, TYMS/RAD23B was not detected in the CTCs of patients exhibiting pCR (p = 0.001). Thus, TYMS mRNA and/or TYMS/RAD23B expression in CTCs, as well as CTC kinetics, have the potential to predict non-response to NCRT and avoid unnecessary radical surgery for LARC patients with pCR [32].
References
Abdallah EA, Fanelli MF, Buim ME, et al. Thymidylate synthase expression in circulating tumor cells: a new tool to predict 5-fluorouracil resistance in metastatic colorectal cancer patients. Int J Cancer. 2015;137(6):1397–4.
Abdallah EA, Fanelli MF, Souza E, Silva V, et al. MRP1 expression in CTCs confers resistance to irinotecan-based chemotherapy in metastatic colorectal cancer. Int J Cancer. 2016;139(4):890–8.
Alix-Panabières C, Pantel K. Real-time liquid biopsy: circulating tumor cells versus circulating tumor DNA. Ann Transl Med. 2013;1(2):18.
Arneth B. Update on the types and usage of liquid biopsies in the clinical setting: a systematic review. BMC Cancer. 2018;18:527.
Barbazan J, Muinelo-Romay L, Vieito M, Candamio S, Diaz-Lopez A, Cano A, Gómez-Tato A, Casares de Cal Mde L, Abal M, López-López R. A multimarker panel for circulating tumor cells detection predicts patient outcome and therapy response in metastatic colorectal cancer. Int J Cancer. 2014;135:2633–43.
Bardelli A, Pantel K. Liquid biopsies, what we do not know (yet). Cancer Cell. 2017;31:172–9.
Bidard FC, Kiavue N, Ychou M. Circulating tumor cells and circulating tumor DNA detection in potentially resectable metastatic colorectal cancer: a prospective ancillary study to the Unicancer Prodige-14 trial. Cell. 2019;8(6):516.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre L, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394–424.
Castro-Giner F, Gkountela S, Donato C, Alborelli I, Quagliata L, Ng CKY, Piscuoglio S, Aceto N. Cancer diagnosis using a liquid biopsy: challenges and expectations. Diagnostics. 2018;8:31.
Chen CJ, Sung W-W, Chen HC, et al. Early assessment of colorectal cancer by quantifying circulating tumor cells in peripheral blood: ECT2 in diagnosis of colorectal cancer. Int J Mol Sci. 2017;18(4):743.
de Albuquerque A, Kubisch I, Stolzel U, Ernst D, Boese-Landgraf J, Breier G, Stamminger G, Fersis N, Kaul S. Prognostic and predictive value of circulating tumor cell analysis in colorectal cancer patients. J Transl Med. 2012;10:222.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467–80.
Ding L, Ellis MJ, Li S, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005.
Gerlinger M, Rowan AJ, Horswell S. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92.
Huang X, Gao P, Song Y, et al. Relationship between circulating tumor cells and tumor response in colorectal cancer patients treated with chemotherapy: a meta-analysis. BMC Cancer. 2014;14:976.
Sastre J, de la Orden V, Martínez A, et al. Association between baseline circulating tumor cells, molecular tumor profiling, and clinical characteristics in a large cohort of chemo-naïve metastatic colorectal cancer patients prospectively collected. Clin Colorectal Cancer. 2020;19:e110. S1533-0028(20)30037-2
Johann DJ, Steliga M, Shin IJ, Yoon D, Arnaoutakis K, Hutchins L, Liu M, Liem J, Walker K, Pereira A, et al. Liquid biopsy and its role in an advanced clinical trial for lung cancer. Exp Biol Med. 2018;243:262–71.
Karen Tan, Sai Mun Leong, Zizheng Kee, et al. Longitudinal monitoring reveals dynamic changes in Circulating Tumor Cells (CTCs) and CTC-associated miRNAs in response to chemotherapy in metastatic colorectal cancer patients. Cancer Lett. 2018;423:1–8.
Kasi PM, Shahjehan F, Cochuyt JJ, Li Z, Colibaseanu DT, Merchea A. Rising proportion of young individuals with rectal and colon cancer. Clin Colorectal Cancer. 2019;18(1):e87–95.
Lu CY, Uen YH, Tsai HL, Chuang SC, Hou MF, Wu DC, Juo SH, Lin SR, Wang JY. Molecular detection of persistent postoperative circulating tumour cells in stages II and III colon cancer patients via multiple blood sampling: prognostic significance of detection for early relapse. Br J Cancer. 2011;104:1178–84.
Matsusaka S, Suenaga M, Mishima Y, Kuniyoshi R, et al. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in Japanese patients with metastatic colorectal cancer. Cancer Sci. 2011;102:1188–92.
Marcuello M, Vymetalkova V, Neves RPL, et al. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol Asp Med. 2019;69:107–22.
Oxnard GR, Paweletz CP, Kuang Y, Mach SL, O’Connell A, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20:1698–705.
Rolfo C, Castiglia M, Hong D, Alessandro R, Mertens I, Baggerman G, Zwaenepoel K, Gil-Bazo I, Passiglia F, Carreca AP, et al. Liquid biopsies in lung cancer: the new ambrosia of researchers. Biochim Biophys Acta (BBA) Rev Cancer. 2014;1846:539–46.
Sastre J, Maestro ML, Gomez-Espana A, Rivera F, Valladares M, et al. Circulating tumor cell count is a prognostic factor in metastatic colorectal cancer patients receiving first-line chemotherapy plus bevacizumab: a Spanish Cooperative Group for the Treatment of Digestive Tumors study. Oncologist. 2012;17:947–55.
Souza E, Silva V, Chinen LTD, Abdallah EA, et al. Early detection of poor outcome in patients with metastatic colorectal cancer: tumor kinetics evaluated by circulating tumor cells. Onco Targets Ther. 2016;9:7503–13.
Souza E Silva V, Chinen LT, Abdallah EA, ET AL. Early detection of poor outcome in patients with metastatic colorectal cancer: tumor kinetics evaluated by circulating tumor cells. Onco Targets Ther. 2016;9:7503–13.
Cohen SJ, Punt CJA, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213–21.
Tao Y, Ruan J, Yeh SH, et al. Rapid growth of a hepatocellular carcinoma and the driving mutations revealed by cell-population genetic analysis of whole-genome data. Proc Natl Acad Sci U S A. 2011;108:12042–7.
Tan Y, Wu H. The significant prognostic value of circulating tumor cells in colorectal cancer: a systematic review and meta-analysis. Curr Probl Cancer. 2018;42:95–106.
Tol J, Koopman M, Miller MC, Tibbe A, Cats A, Creemers GJ, Vos AH, Nagtegaal ID, Terstappen LW, Punt CJ. Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol. 2010;21:1006–12.
Troncarelli Flores BC, Souza E, Silva V, Ali Abdallah E, Mello CAL, Gobo Silva ML, Gomes Mendes G, Camila Braun A, Aguiar Junior S, Thomé Domingos Chinen L. Molecular and kinetic analyses of circulating tumor cells as predictive markers of treatment response in locally advanced rectal cancer patients. Cell. 2019;8(7):641. https://doi.org/10.3390/cells8070641. PMID: 31247977; PMCID: PMC6679115
Tsai W-S, Nimgaonkar A, Segurado O, Chang Y, Hsieh B, Shao H-J, Wu J, Lai J-M, Javey M, Watson D, Mei R. Prospective clinical study of circulatingtumor cells for colorectal cancer screening. J Clin Oncol. 2018;36:556.
Uen YH, Lu CY, Tsai HL, Yu FJ, Huang MY, Cheng TL, Lin SR, Wang JY. Persistent presence of postoperative circulating tumor cells is a poor prognostic factor for patients with stage I-III colorectal cancer after curative resection. Ann Surg Oncol. 2008;15(8):2120–8.
Wang D, Yang Y, Jin L, Wang J, Zhao X, Wu G, Zhang J, Kou T, Yao H, Zhang Z. Prognostic models based on postoperative circulating tumor cells can predict poor tumor recurrence-free survival in patients with stage II-III colorectal cancer. J Cancer. 2019;10(19):4552–63.
Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7.
Yang C, Zou K, Zheng L, et al. Prognostic and clinicopathological significance of circulating tumor cells detected by RT-PCR in non-metastatic colorectal cancer: a meta-analysis and systematic review. BMC Cancer. 2017;17:725.
Yang C, Shi D, Wang S, Wei C, Zhang C, Xiong B. Prognostic value of pre- and post-operative circulating tumor cells detection in colorectal cancer patients treated with curative resection: a prospective cohort study based on ISET device. Cancer Manag Res. 2018;10:4135–44.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Souza e Silva, V., de Brito, A.B.C., Costa, D. (2021). Circulating Tumor Cells in Colorectal Cancer. In: Chinen, L.T.D. (eds) Atlas of Liquid Biopsy. Springer, Cham. https://doi.org/10.1007/978-3-030-69879-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-69879-9_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69878-2
Online ISBN: 978-3-030-69879-9
eBook Packages: MedicineMedicine (R0)