Abstract
Stents have been used in congenital heart disease since the late 1980s with increasing experience and application in more diverse lesions at all ages over the past few years. The earliest balloon-expandable stents were mounted onto a balloon catheter with a crimper device and carefully passed through a previously placed long delivery sheath that crossed the lesion. Stents slipping off the balloon during this passage or at the time of inflation were the most significant problems. More reliable mounting techniques and the development of premounted stents have virtually eliminated this problem. While a long sheath is still commonly used, the premounted stent can also be safely placed without using a long sheath. Meticulous attention to detail is the key to both avoiding complications and being able to deal with them, and a stable guide wire position is essential. Vessel rupture from overexpansion of a stent can occur and covered stents are now electively placed in very tight stenoses. They are also able to seal aneurysms or dissections from previous surgery, balloon dilation or bare-metal stents. Open-cell stents allow access to side branches “jailed” by the stent and dilating and stenting through the side of a stent is increasingly performed. Stents that have reached their limit of expansion can be disrupted with high-pressure balloons to allow placement of a stent with a larger diameter inside the original to avoid surgical resection.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Stent implantation in congenital heart disease became available in the late 1980s with a rapid uptake in the 1990s. While standard balloon dilatation was a successful approach to the treatment of stenotic lesions, limitations were apparent. Fibrotic stenotic lesions allowed controlled dissection with remoulding of the vessel wall during the healing phase but more elastic lesions, long-segment stenoses, hypoplastic vessels, stenoses related to kinking or tension on a vessel rarely responded well often with immediate vessel recoil. Balloon oversizing in this setting could lead to vessel tearing with dissection flaps, vessel rupture with haemodynamic collapse and late aneurysm formation.
Stent implantation prevented the immediate elastic recoil, allowed the vessel to be dilated only to the required diameter and sealed small intimal flaps to the vessel wall. Stenosis relief was superior both acutely and in the long term with a lower risk of acute vessel complications. By not overdilating the vessel, stent implantation could be used early after cardiac surgery.
Issues unique to the paediatric population include small patients’ size limiting vessel access and difficulty in advancing the rigid stent through a tortuous vascular route. After somatic growth, stent redilation is needed until the patient is adult size. Stents unable to be dilated to adult size result in a fixed stenosis after growth.
2 Indications
Initially limited to patients big enough to accommodate an appropriate sheath and stent that would not need redilatation, the encouraging early results and improvements in stent, balloon and delivery sheath design widened the indications (Table 8.1) [1,2,3]. Stents are implanted as a bridge in neonates and infants with elective surgical removal during the next stage of treatment. Indeed, stents that are eventually dilatable to adult size can now be introduced through 6 F sheaths. A hybrid surgical approach further expanded the benefits with cooperation between surgeons and interventionalists. Covered stents can seal native aneurysms and fistulae and those resulting from surgery, balloon dilation or bare-metal stents (Fig. 8.1). Coronary artery interventions are increasing.
Covered stent (Premounted Cheatham Platinum on a balloon-in-balloon) implantation into a lateral tunnel of an 8-year-old boy with exercise-induced cyanosis and protein-losing enteropathy. Angiogram in (a) shows a tunnel stenosis before the branch pulmonary arteries and a patent fenestration. After passing a long sheath over a stiff guide wire placed in the SVC, the stent is advanced (b). Angiography via the long sheath is used to position the stent (c, d). The inner balloon is inflated with angiography confirming position in the stenosis and continued flow across the fenestration (e) followed by the outer balloon (f). Final angiogram shows the fenestration to be occluded (g) and the stenosis dilated. Note the position and shortening of the stent between (d) and (f) so that the final position does not obstruct the right pulmonary artery. RSVC right superior vena cava, RPA right pulmonary artery, LPA left pulmonary artery, FEN fenestration, IVC inferior vena cava
3 Stent Features
Given the diversity of lesions and patient size range, a single type of stent does not suit all situations. Stent implantation is an art of choosing the best device for a specific patient and condition. It is better to be experienced with a limited range of stents (Table 8.2) rather than trying to master all.
Attributes of an ideal stent include:
-
Safe delivery to the target lesion.
-
Low profile allowing use of a small sheath and crossing of tight stenoses.
-
Flexibility and easy trackability through tortuous pathways.
-
Premounting to ease introduction and passage through the sheath and vascular system.
-
Highly radio-opaque for precise positioning.
-
-
Performance at the site of implantation.
-
Expansion without shortening.
-
High radial force.
-
Conformation to vessel curvature.
-
Smooth edges that do not damage the balloon or vessel wall.
-
Side-branch flow that is not compromised.
-
Minimal neointimal proliferation and non-thrombogenic.
-
Capacity to redilate to adult size.
-
Retrievability if malpositioned.
-
-
Additional features.
-
Covering for aneurysms and fistulae (not compatible with side-branch patency—unless perforated and stented through side branch struts!)
-
MRI compatibility for follow-up.
-
Drug delivery to prevent restenosis.
-
Types of stent (Fig. 8.2):
-
Closed-cell design: The original traditional closed-cell design consists of regular cells that do not have direct communication with each other. With expansion the cell changes configuration but all have the same shape—becoming shorter but wider with a high radial force at all diameters. They are inflexible and straighten a vessel rather than conform to its shape.
-
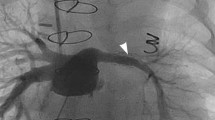
Open-cell design: A lack of a bridging connection between some adjacent cells allows them to merge into larger areas during stent expansion. This gives greater access to side branches allowing balloon dilation through the cells to improve flow (Fig. 8.3). They are more flexible, can pass around tighter curves and conform to the vessel shape. They do not crimp as well onto a balloon but the irregular outer surface anchors it to the target lesion reducing the risk of stent migration. They shorten less especially when expanded sequentially but lack radial strength at large diameters. Restenosis may occur due to neointimal hyperplasia through the larger open cells.
-
Hybrid design: Some stents are designed with an association of open and closed elements in order to keep together radial force, flexibility and anchoring properties.
-
Premouted stents: Open or closed cells are available in a range of diameters and lengths and can be manufactured in custom lengths and larger diameters. They are quick to prepare and can be advanced safely without a long sheath as they adhere firmly to the balloon catheter.
-
Self-expanding stents: These are not used as often in congenital heart disease as they have a much lower radial force than balloon-expandable stents but conform well to the vessel shape.
-
Covered stents: Increasing role in native coarctation and pulmonary conduits allowing full dilation with a reduced risk of vessel damage compared to bare stents. More recently for closure of sinus venosus ASDs.
-
Coronary stents: A huge range is available for use in coronary arteries as well as other lesions in neonates and infants.
-
Growth and biodegradable stents: Metals or polymers that are absorbed by the body or stents with weakened joints that allow easy balloon disruption and a new larger stent to be implanted have been tested but are yet to reach commercial release.
-
Stent grafts: These are used for aneurysms and dissections of the aorta and beyond the scope of this review.
Large balloon-expandable stent in (a) expanded closed-cell (Cheatham Platinum) stent and (b) expanded and unexpanded hybrid cell (Andramed) stent. Medium-sized balloon-expandable stents in (c) closed cell (Palmaz) above and open cell (Valeo) below. Medium-sized self-expanding stents in (d) Zilver upper and Sinus-Superflex lower
Premounted Visi-Pro stent implantation into a native common left pulmonary vein stenosis after two previous cutting balloon dilations in a 15-month-old boy. Tight stenosis in (a) shown by pulmonary artery wedge injection. Long sheath advanced over two guide wires into upper lobe branch and stent uncovered guided by pulmonary artery wedge injection (b, c). After stent implantation the inferior pulmonary vein is jailed (d) and the origin easily dilated with a coronary balloon (e) due to the open-cell design with opening of the ostium (circle in f). CT angiogram 18 months later confirms patency of upper and lower veins into the stent (g)
4 Stent Implantation
The basic principles of stent implantation are common to most lesions (Table. 8.1). Meticulous attention to detail and a structured approach are critical to success without complications:
-
Pre-procedure imaging: Echocardiography, MRI and CT scanning allow the lesion (length, diameter, side branches, adjacent vessel diameters, extrinsic structures (bronchus, coronary artery), aneurysms) and access vessels to be evaluated which when put into the clinical context ensure that:
-
Appropriate stents, sheaths, guide wires, etc. are available.
-
Vascular access is tailored to the lesion (jugular, brachial, carotid, transhepatic, trans-septal, double access, hybrid).
-
Angiographic planes are chosen to reduce contrast and radiation during the procedure.
-
Special measures arranged (transoesophageal echocardiography for atrial septal stenting; radiofrequency perforation for aortic atresia; bronchoscopy, coronary angiography, coils and plugs for hepatic access; surgical standby or ECMO for high-risk patients or lesions).
-
-
Procedure
Most stenting procedures are performed under general anaesthesia with strict aseptic technique.
-
Access
This depends on the lesion, patient’s size and the available vessels. Usually a direct course is preferred if possible. In very small children or when access is limited or the course is tortuous, a carotid or iliac cutdown or hybrid approach may be needed. For large-bore arterial access, a vascular preclosure suture may be appropriate.
-
Angiography
Good quality images profiling the stenosis (ideally two orthogonal planes) with measurements of the lesion and adjacent vasculature are essential for the final choice of stent size and length and serve as a reference for stent placement. Alternatively, three-dimensional rotational angiography (3DRA) or fusion of computed tomography (Fig. 8.4) or magnetic resonance imaging may be used for guidance of stent implantation [4]. This will be described in details in Chap. 61.
-
Predilation
Balloon dilatation of tight stenoses/subatretic is occasionally needed to introduce the sheath and balloon/stent assembly. Predilation to the planned stent diameter is generally avoided except in special situations. If balloon inflation abolished the stenosis, in distensible lesions, the stent might be insecure after placement and be displaced on balloon withdrawal. In potentially non-compliant lesions (branch pulmonary artery stenosis), predilation testing is important as the stent may obstruct or fracture (Figs. 8.5) if the lesion cannot be dilated; initial high-pressure or cutting balloon dilation may allow subsequent stenting. Balloon inflation can mimic the effects of the stent on adjacent structures (coronary arteries during RVOT stenting, left main bronchus after Norwood surgery (Fig. 8.6)).
-
Stent choice
Many factors influence the stent choice for a particular patient and lesion—not least an operator’s experience and preferences (Table 8.2). One important determinant is the current and final size of the target vessel.
-
Guide wire and sheath placement
Different catheters and guide wires are used to cross the lesion to as distal and stable a position as possible—the time spent at this stage is essential to ensure a smooth procedure. The guide wire to carry the stent balloon assembly is then passed into position. The thickness is dictated by the lumen of the balloon catheter that the stent is mounted on and is usually as stiff as possible.
In most instances a long sheath is advanced across the lesion (Figs. 8.1 and 8.3). It facilitates safe stent placement without displacing the stent when negotiating a tortuous course, tight bends and stenoses. It allows angiography for proper stent positioning and pressure monitoring. After stent implantation it allows safe balloon withdrawal and placement of a larger balloon if needed and gives control in the event of complications. In small patients, advancing a long sheath over a stiff wire can cause significant tricuspid regurgitation and hypotension. Positioning the tip of the sheath in the right atrium and using the balloon/stent assembly as the sheath “dilator” during advancement can shorten the period of haemodynamic compromise and avoid sheath kinking on dilator removal. Hydrophilic and kink-resistant sheaths also facilitate the procedure. The alternative is to use a short sheath with a premounted stent (a less rigid system)—stent positioning relying on previously acquired landmarks or a separate angiographic catheter.
-
Mounting
Unmounted stents are centred and manually crimped onto the balloon. A stiff guide wire in the balloon prevents compromise of the lumen. Gradually increasing manual force is used symmetrically around the circumference and along the length of the stent. Poor stent adherence can be overcome by application of contrast to the balloon to act as temporary “glue”; umbilical tape wrapped tightly around the stent enhances the crimping; partial balloon inflation allows the stent to grip better. It is important to match the length of the stent and the balloon. Too short a balloon results in the ends not inflating—a risk for stent displacement when the balloon is withdrawn. If too long a balloon is used, the distal end may “milk” back from a small distal vessel causing deployment too proximally. The balloon should therefore ideally be only a few mm longer than the stent itself.
-
Stent introduction
Premounted stents pass easily through the valve of the sheaths. With hand-mounted and covered stents, the stent or covering may be displaced off the balloon or stent if passed directly through the valve. A plastic or metal introducer provided in the stent packet or a short section of another sheath protects the stent during introduction through the valve. Before deploying the stent, it is important to confirm that the stent has not slipped off the balloon—else withdrawal and remounting may be necessary.
-
Stent positioning
At the target site, the long sheath is withdrawn leaving the stent in place. Multiple contrast injections through the sheath (or additional angiographic catheter) are used to fine-tune the position (Fig. 8.1). Still frames with landmarks (bones, trachea, temperature probe), image overlay and roadmapping can be used to help in the final stent positioning. Reliance on these alone may be compromised by distortion of the anatomy by the stiff guide wire/stent balloon assembly. The whole balloon as well as the stent must be uncovered or the proximal part of the balloon may not inflate.
-
Stent deployment
The balloon is inflated with an indeflator up to the recommended pressure to avoid balloon rupture. The primary operator controls the stent balloon assembly and guide wire to reposition the stent if it moves, e.g. if the balloon only inflates proximally pushing the stent distally. The rate of inflation varies—some operators prefer a slow inflation; others a rapid inflation (that gives less scope for repositioning). A balloon-in-balloon results in less stent shortening and an opportunity to reposition the partially expanded stent before full inflation (Fig. 8.1). Rapid ventricular pacing-induced hypotension helps to maintain the position of coarctation and transverse arch stents.
After deployment the balloon is deflated and angiography used to confirm the stent position. It is important to fully deflate the balloon as withdrawal of a partially inflated balloon may displace the stent. A long sheath can be advanced over the deflated balloon and into the stent to reduce the risk of displacement and allow repeat angiography and placement of a larger/higher pressure balloon if necessary.
Coarctation of the aorta (CoA) stenting with fusion of computed tomography. A raw computed tomography dataset was manually segmented to expose the narrowing and highlight the nearby vessels (a). Stored fluoroscopy in anterior-posterior and left lateral projections with vertebral bodies (white stars) of the mid and lower thoracic spine served as a reference for matching the 3D reconstruction with the fluoroscopy (b). A Cheatham Platinum stent was positioned with guidance of the 3D overlay (c). A three-dimensional rotational angiography was preformed to evaluate the final outcome (d). Images with permission from Góreczny S et al. 3D image fusion for live guidance of stent implantation in aortic coarctation–magnetic resonance imaging and computed tomography image overlay enhances interventional technique. Postepy Kardiol Interwencyjnej. 2017;13:269–272
Examples of complications after stent implantation. Proximal LPA & RPA stenoses were previously treated with two stents Isthums and Valeo, respectively (a, b). The stent (black star) in the LPA embolized distally several months after the procedure. During implantation of a new stent (LD Max, arrow) into the LPA, the previously implanted stent (white star) in the RPA was compressed (white dashed circle). A guidewire placed electively across the RPA stent allowed subsequent redilation of the proximal end. A frame in antero-posterior projection from routine fluoroscopy of Melody valve (after presenting with a Cheatham Platinum stent) shows several insignificant fractures (white dashed rectangle) without fragmentation (c). An image in caudal projection reveals compression of the right anterior wall of the valve and stent (d). A Cheatham Platinum stent fracture (white dashed circle) late after re-coarctation stenting in a patient after surgical treatment of interrupted aortic arch (e, f). Due to stable position of the fractured struts and close proximity of head vessels originating from the arch through a single trunk, the stent was redilated without implantation of another stent. Left pulmonary artery Palmaz Genesis stent facture in patient after Glenn shunt (g). Fractured parts of the stent were reconnected with implantation of a Cheatham Platinum stent (h). LPA left pulmonary artery, RPA right pulmonary artery
Stent implantation into left pulmonary artery after stage III Norwood procedure in a 17-year-old boy (a). MRI scanning raised concerns of proximity of both the left main bronchus and native aorta to the stenosed segment confirmed on angiography in AP and lateral projections with the guide wire in position (b, c). Trial balloon inflation to the stent diameter was performed with simultaneous native aorta angiography and bronchoscopy without compromising either structure (d, e). A Cheatham Platinum stent was implanted (f, g)
5 Complications
The larger sheaths and stiffer guide wires used may increase the frequency and severity of complications associated with cardiac catheterisation though they are in general low. Acute stent-related complications can largely be prevented by meticulous attention to detail. When they occur, however, it is vital to maintain guide wire position for remedial action with the stent and vessel still accessible.
-
Stent malposition or migration
Minor malposition is dealt with by recapturing the stent with the same / larger balloon and “repositioning” it. If this is not possible, then an overlapping stent is placed to complete treatment of the lesion. If the stent is free floating, recapturing and repositioning may be possible if the stent is still on the guide wire—an alternative is to deploy it in a “safe” position that does not compromise other vessels or will not become stenotic with growth (IVC or descending aorta). If the stent cannot be repositioned with other vascular tools (snares, bioptomes, tip deflectors) and its position causes haemodynamic compromise or is free floating, then surgery is required. Withdrawal of a partially deployed stent to the access site may allow a minor surgical cutdown to remove it.
-
Stent embolization
In rare occasion a stent may embolize after initially successful implantation. For free floating stent a similar approach as described for stent malposition and migration might be used. If the stent is stable in a distal branch another stent might be implanted proximally to cover the stenosis (Fig. 8.5). If blood flow to the side branche(s) is compromised balloon dilation of struts might be necessary.
-
Balloon rupture
Balloon rupture before the stent is fully expanded is dealt with by rapid contrast injections either by hand or a power injector. If this fails it may be possible to withdraw the balloon from the stent (stabilising the stent with the long sheath or snaring the stent from another access may help) and replace with a new one.
-
Side-branch compromise
Uncovered stents only rarely obstruct a side branch to a hemodynamically significant degree, though late endothelialisation may further compromise flow. Compression of a side branch that exits acutely close to the stenosis may also occur. Open-cell stents can be opened into the side branch to improve flow (Fig. 8.3), but if compression is a concern, then a second guide wire +/− balloon into the side branch can help preserve it during stent deployment. Covered stents over a major side branch need perforating if there is insufficient collateral flow.
-
Vessel dissection and rupture
Minor dissections tend to heal. Excessive dilation of very tight stenoses, dilation much above the diameter of the normal adjacent vessel and sharp edges of some stents can lead to acute dissection and even rupture. Management involves balloon tamponade followed by covered stent implantation across the area. If this is not possible, emergency surgery may be needed. While it is tempting to implant oversized stents to eliminate or reduce the need for further redilatation, significant overdilation that is tolerated initially may lead to aneurysm formation.
-
Stent fracture
Stent fracture may occur immediately after implantation but more frequently is detected weeks later. Fracture of a single or a few struts usually has no clinical significance, whereas complete fracture with stent separation leads to recurrence of the stenosis. Very tight stenoses, sharply angled lesions, muscular structures and external compression (e.g. the sternum) all increase the risk. Balloon inflation to redilate the stent is rarely successful and risks the free-standing wires puncturing the balloon. Usually a second stronger stent (+/− covered) is implanted to bridge the gap and relieve the stenosis (Fig. 8.5).
-
Restenosis
Restenosis caused by the patient’s growth relative to the fixed diameter of the stent is managed by stent redilation. Neointimal proliferation to a degree that compromises the lumen occurs infrequently and unpredictably. It too responds to further dilation of the stent +/− addition of a further stent inside the first (bare or covered). Dilatation beyond the manufacturer’s maximum has been described with many stents although each has a limit and may cause a fixed stenosis after growth. Surgical removal or incision and patching the lesion may be needed unless an ultra-high-pressure balloon is able to disrupt the stent—simultaneously deploying a new larger (covered) stent inside the first.
References
Ing F. Stents: what’s available to the pediatric interventional cardiologist? Catheter Cardiovasc Interv. 2002;57(3):374–86.
Mullins CE. Intravascular stents in congenital heart disease—general considerations, equipment. In: Mullins CE, editor. Cardiac catheterization in congenital heart disease: pediatric and adult. Malden, MA: Blackwell Futura; 2006. p. 537–96.
Peters B, Ewert P, Berger F. The role of stents in the treatment of congenital heart disease: current status and future perspectives. Ann Pediatr Cardiol. 2009;2(1):3–23.
Goreczny S, Moszura T, Lukaszewski M, Podgorski M, Moll JA, Dryzek P. Three-dimensional image fusion of precatheter CT and MRI facilitates stent implantation in congenital heart defects. Rev Esp Cardiol (Engl Ed). 2019;72(6):512–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Góreczny, S., Rosenthal, E. (2021). Stents. In: Butera, G., Chessa, M., Eicken, A., Thomson, J. (eds) Cardiac Catheterization for Congenital Heart Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-69856-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-69856-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69855-3
Online ISBN: 978-3-030-69856-0
eBook Packages: MedicineMedicine (R0)