Abstract
Intraventricular meningiomas are very rare and generally do not differ from extraventricular meningiomas in terms of histopathology and imaging features. Trigonal meningiomas grow silently for a long period of time due to the relatively ample ventricular space available. GTR is the treatment of choice; recurrences are possible in WHO grade II tumours, therefore requiring more frequent MRI follow-ups in the first 2 years.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
A 70-year-old man was examined in the ER department because of a sudden loss of consciousness followed by confusion. During the neurological examination and work-up, he experienced a seizure. He also demonstrated left-sided weakness. His medical history included surgery and irradiation for prostatic carcinoma and hypertension.
A CT exam of the brain was requested by a neurologist, particularly to check for intracranial space-occupying lesions (Fig. 16.1).
Non-contrast CT exam of the brain. Axial (a), coronal (b) and sagittal (c) reformats. A large, mostly hyperdense, partially calcified mass lesion emerging from the trigone of the right-sided lateral ventricle. Central hypodensity may suggest necrosis. Most likely diagnosis—an intraventricular meningioma
The work-up continued with an MRI of the brain (Fig. 16.2).
MRI of the brain. Axial (a) and coronal (b) T2WI, sagittal T1WI (c), axial T2-FLAIR (d), axial ADC map (e) and DWI (f), axial post-contrast T1WI (g, h). A large mass lesion centred at the choroid plexus of the trigone of the right-sided lateral ventricle, mostly hypointense in T2WI and well enhanced in post-contrast T1WI, except centrally where signs of hypovascularity are seen. Note mildly decreased diffusion (e, f) in solid enhanced portions, a feature found in hypercellular tumours such as this meningioma
After the diagnosis of an intraventricular meningioma had been confirmed by MRI, the patient underwent surgery which was uneventful. Histopathology findings reported a WHO grade II meningioma.
Another patient, a 60-year-old man, had been lethargic and depressed, with cognitive impairment, forgetfulness and drowsiness in the month prior to being admitted to hospital. A few days prior to arrival to our facility, he became disoriented, agitated during the night, ataxic, did not recognize family members nor household items.
After a psychiatrical and neurological evaluation, he underwent a brain CT exam (Fig. 16.3a, b), followed by a brain MRI exam (Fig. 16.3c–f):
Brain CT (a, b). Brain MRI (c–f). Non-contrast (a) and contrast-enhanced brain CT. T2-FLAIR (c), ADC map (d), coronal T2WI (e) and axial post-contrast T1WI MR images of the brain. An intraventricular, homogenously enhancing mass lesion in the medial aspect of the right trigone , with marked enlargement of the right-sided trigone and occipital horn. In MR images, the trigone is already decompressed by a shunt (note a linear hypodensity posterolateral to the right ventricular trigone in image f). Evidence of vasogenic oedema in the parenchyma adjacent to the tumour. Enhancement, hypointensity in T2WI and mild diffusion impairment suggested a meningioma
The patient underwent surgery which resulted in complete removal of the tumour.
Two days later, a postoperative MRI exam was performed (Fig. 16.4):
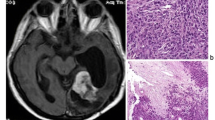
The histopathology confirmed the tumour was an atypical meningioma (WHO grade II).
1 Intraventricular Meningioma (IVM)
Primary intraventricular meningiomas make approximately 0.5–3% of all intracranial meningiomas [1] and therefore are very rare lesions. Still, they remain a neurosurgical challenge due to their size at the time of diagnosis, proximity to vital intraventricular structures and vascular supply which can be evaluated only after significant debulking [2]. Most are sited in the trigones of the lateral ventricles, left more often than right. They are more common in women (2.1:1 female-to-male ratio) [1] and peak at 30–60 years of age. Usually they are quite large at the time of diagnosis. Most commonly they present with raised intracranial pressure (70%), visual impairment (27%), dizziness (24%), motor deficits, sensory disturbances, cognitive and personality disturbances (10%) and gait ataxia [1]. Seizures, both motor and sensory, have also been noted [1, 2]. The majority of symptoms are caused by the mass effect causing hydrocephalus or direct compression of the brain.
IVMs arise from the arachnoid cap (outer lining) cells which migrate along with choroid plexus at the time of ventricular system invagination, by the 25th week of gestation. The bulkiest choroid plexuses are sited in the trigones of the lateral ventricles—that is why most of the IVMs are found there. The relatively large size of the trigones allows for significant asymptomatic growth. In terms of histology, IVMs do not differ from other meningiomas. The majority are WHO grade I—meningothelial, fibrous (fibroblastic), transitional or lymphoplasmacyte rich, while only a small number are grade II (atypical) [3]. There have also been sporadic cases of malignant IVMs with distal metastases.
Imaging features of IVMs do not differ from meningiomas in other locations—these are well-defined, highly cellular tumours isodense or hyperdense on CT, isointense to the cerebral grey matter in T1 and T2 weighted images on MR, although some variants demonstrate lower signal intensity in T1- and higher in T2-weighted images. There is vivid contrast enhancement which can be inhomogeneous in cases of tumour infarctions or necrosis. The diffusion is slightly impaired. MR spectroscopy reveals a high choline level and low to non-existent NAA signal, with an alanine doublet (1.3–1.5 ppm). They appear to harbour calcifications more frequently than extraventricular meningiomas. There is often hydrocephalus or a trapped occipital or temporal horn and some vasogenic oedema of the adjacent brain parenchyma. The arterial blood supply of an IVM mainly comes from the posterior choroidal arteries (branches of the P2 segment of the PCA) and in some cases also from branches of the anterior choroidal artery (originating from the ICA). Catheter angiography is not essential as these tumours are usually not amenable to preoperative embolization [4]—there is a considerable risk of stroke, affecting posterior choroidal arteries territory, causing visual field and sensory loss, although there are case reports of such a procedure [5]. However, catheter angiography may yield information on IVM blood supply and position of the draining veins which is useful during surgery [1]. MR angiography can usually provide information on displacement of the anterior choroidal artery by the tumour. Neuronavigation helps to identify and dissect the supplying vessels during surgery [3].
Differential diagnoses include choroid plexus papilloma, glial tumours (astrocytoma, ependymoma), choroid plexus metastases (renal cell carcinoma, melanoma) and CNS lymphoma.
Gross total resection remains the treatment of choice for the IVMs. Non-adherent margins and benign nature of the IVMs make for a feasible complete microsurgical resection. The challenges are deep location, anatomy of the blood supply and proximity of eloquent brain areas. Superior parietal lobule approach allows for trigonal IVM resection, while temporal horn and inferior trigone may be reached by inferior or middle temporal gyrus approach. Anterior corpus callosum approach is suitable for tumours in frontal horn and body of the lateral ventricle, as well as in the third ventricle, while posterior corpus callosum approach opens a path to a tumour in trigone area. Median suboccipital craniotomy is the preferred route to the fourth ventricle tumour [1].
Tumour recurrence is possible, less likely in low WHO grade tumours. The recurrence of WHO grade II IVMs is most likely in the first 2 years after resection, so MRI evaluation should be performed every 6 months for the first 2 years and annually afterwards.
References
Chen C, et al. Clinical features, surgical management, and long-term prognosis of intraventricular meningiomas: a large series of 89 patients at a single institution. Medicine. 2019;98(16):e15334. https://doi.org/10.1097/MD.0000000000015334.
Lyngdoh BT, et al. Intraventricular meningiomas: a surgical challenge. J Clin Neurophysiol. 2007;14(5):442–8. https://doi.org/10.1016/j.jocn.2006.01.005.
Bertalanffy A, et al. Intraventricular meningiomas: a report of 16 cases. Neurosurg Rev. 2006;29(1):30–5. https://doi.org/10.1007/s10143-005-0414-5.
Fusco DJ, Spetzler RF. Surgical considerations for intraventricular meningiomas. World Neurosurg. 2015;83(4):460–1. https://doi.org/10.1016/j.wneu.2014.08.042.
Jack AS, et al. Pre-operative embolization of an intraventricular meningioma using onyx. Can J Neurol Sci. 2016;43(1):206–9.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vavro, H. (2021). Intraventricular Meningioma. In: Neuroradiology - Images vs Symptoms. Springer, Cham. https://doi.org/10.1007/978-3-030-69213-1_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-69213-1_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69212-4
Online ISBN: 978-3-030-69213-1
eBook Packages: MedicineMedicine (R0)