Abstract
Acne is a prototypic chronic inflammatory disease of the pilosebaceous units. Even though the disease is common, clinically well-characterized, the initiator of the pathogenic processes and the first steps are still not clear. According to our current knowledge, innate and adaptive immune activation and inflammation are critical events in all stages of acne lesion development, even in the very early steps, known as comedone formation. Microbial contribution, the etiopathogenic role of Cutibacterium acnes (C. acnes, formerly known as Propionibacterium acnes, P. acnes), has long been suggested, but how exactly an important member of the cutaneous microbiota turns into an opportunistic pathogen during puberty remains to be an open question. In this chapter, we aim to summarize our current knowledge of acne pathogenesis and highlight the role of the skin immune system and inflammation in the disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acne vulgaris
- Cutaneous immune system
- Pathogenesis
- Innate and adaptive immune system
- Inflammation
- Cutibacterium acnes (C. acnes)
- Microbiota
Introduction
Acne vulgaris is a prevalent and clinically well-characterized skin disease. In the last three decades, the rapid advancement of experimental dermatology significantly improved our view, but there are still open questions regarding its exact molecular pathogenesis. This chapter aims to highlight the role of the skin immune system in the pathogenesis of acne.
Immune Events Are Crucial in All Stages of Acne Pathogenesis
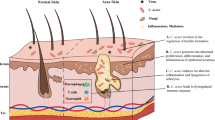
According to the classical view, the most important pathogenic factors in the development of this skin condition includes a hormonal trigger, follicular epidermal hyperproliferation of the ductal keratinocytes within the pilosebaceous unit (PSU), excess and altered sebum production, presence and activity of the skin commensal Cutibacterium acnes (C. acnes, formerly known as Propionibacterium acnes, P. acnes), and inflammation [1,2,3]. Although the exact sequence of events and the primary cause is still not known, initial steps of acne pathogenesis may include microcomedo formations. These are clinically not yet visible precursor lesions that later often develop into comedones (open or closed), papules, pustules, nodules, or cysts [1].
Earlier it was thought that androgen imbalance, follicular hyperkeratinization, and reduced desquamation lead to the formation of a keratin plug at the infundibulum part of the PSU [4]. Below the obstruction, increasingly anaerobic conditions favor the growth of C. acnes, resulting in enhanced immune activation and more pronounced inflammation and the formation of inflammatory acne lesions. The severity of inflammation is also enhanced by the frequent rupturing of the follicle wall, the leakage of bacterial antigens, cellular debris, and immunogenic sebum components into the surrounding tissues, where these greatly enhance inflammation [5].
Results, however, of detailed clinical investigations started to challenge this concept (reviewed by Kircik et al.,) [6]. It became accepted that hyperproliferation and increased retention of infundibular keratinocytes were among the main initiators of microcomedo formation [7], and interleukin (IL)-1 appeared as an important molecule inducing keratinocyte hypercornification [8, 9]. More than one study described signs of inflammation and immune activation, which preceded or occurred parallel with the keratinization process during microcomedo formation, and researchers identified leukocytes, mostly CD4+ T cells, polymorphonuclear cells, and CD68+ macrophages in the immune infiltrate around these early structures [5, 10]. Later studies also detected a higher number of CD3+ and CD4+ T cells already in the clinically uninvolved skin of acne patients, where the levels of different molecules related to inflammation (e.g., IL-1, E-selectin, vascular adhesion molecule 1 – VCAM1) were also elevated [9]. Early in lesion formation, CD1+ dendritic cells were also identified around the PSUs, while neutrophils only appeared in increasing numbers in the more advanced states, around the forming pustules. Finally, CD8+ cells have also been recognized in the early infiltrate around the affected follicles [5, 10]. These data strongly argue that inflammation, and parallel with that immune activation, is already present even before lesion formation and throughout all the subsequent steps during lesion development [11]. What are the initial driving forces, however, is still a question that remains uncertain. Altogether, these results strongly argue that acne is a prototypic chronic inflammatory, rather than a hyperproliferative disorder [6, 12], and the classical distinction of non-inflammatory (microcomedos, comedones) and inflammatory (papules, pustules, nodules, cysts) lesions theory needs to be revised.
Discovery of the Immune Properties of Keratinocytes
As opposed to vertebrates, where immune recognition is provided by the organized efforts of the two arms of their immune system, the innate and the acquired ones, in less evolved organisms, their protection relies only on the former type. Research efforts toward the end of the twentieth century led to the discovery of Toll, a protein in fruitfly (Drosophila melanogaster), which plays a role not only early in development in a process called embryonic segmentation [13] but also in antifungal responses in adults [14]. Soon after that, members of this protein family were also discovered in humans (Toll-like receptors, TLRs), and their importance in vertebrate immune recognition was proposed [15].
During the 1980s, epidermal keratinocytes have been considered passive building blocks of the human skin, which serves as the very first line of defense and an essential delimiter between the outside world and our body. However, studies beginning in these years started to question this static view and suggested for the first time that our epithelial cells can actively identify sources of danger in the external environment and initiate active defense processes. The discovery that human keratinocytes not only recognize the presence of Candida albicans (C. albicans) in their culture but also actively kill this fungus opened a new path for subsequent research studies. These results suggested that keratinocytes, although does not belong to professional immune cells, still possess some functional properties allowing them to identify and respond to the presence of harmful foreign invaders [16,17,18,19].
Around the turning of the century, different research laboratories reported the presence of TLRs in epidermal keratinocytes, and it was also proved that these receptors are functional in this epithelial cell type. Challenge with microbial ligand resulted in the activation of the known, downstream signaling cascades in these cells, too, resulting in innate immune and inflammation activation due to the organized expression changes of pro-inflammatory cytokines, chemokines, antimicrobial peptides, and other factors [20,21,22]. It also became clear that not only pathogenic microbes but different members of the commensal microflora or their conserved structural molecules (e.g., C. acnes, C. albicans, lipopolysaccharide (LPS), lipoteichoic acid (LTA)) are recognized by these pattern recognition receptors (PRRs); thus it may contribute to the molecular pathogenesis of different chronic inflammatory diseases [22].
Innate Immune Events
The Role of Keratinocytes in Acne Pathogenesis
Immune activation and inflammation are central events in acne pathogenesis. Nevertheless, what are the exact driving forces of these reactions during the different phases in lesion development is still not clear. Etiopathogenic role of C. acnes in these processes was suggested for the first time more than 100 years ago, but since its first mention, a long-standing scientific debate formed about the exact role the bacterium plays in the disease [23, 24], reviewed by Dessinioti and Katsambas [25]. In the early 2000s, studies, investigating the keratinocyte – C. acnes interaction identified TLR2 as the major receptor playing indispensable roles in bacterial recognition. TLR4 has also been implicated in these processes [20,21,22], and the expression of both receptors was found to be increased in acne lesions [26]. Studies showed that receptor activation led to innate immune and inflammation activation, and the central mediator of these events was MAPK, NF-kB, and AP-1 transcription factor-dependent [21, 27]. As a result, coordinated expression changes of different pro-inflammatory mediators, among them cytokines (IL-1α, IL-6, IL-10, IL-12, GM-CSF, TNFα), chemokines (CXCL8), antimicrobial peptides (hBD2, CAMP(LL37)), matrix remodeling proteins (MMP-1, MMP-3, MMP-9), and other factors were detected [9, 20,21,22, 28,29,30,31,32]. Apart from these molecules, PRR activation also leads to the generation and elevated expression of factors (e.g., TNIP1) exhibiting negative regulatory effects on innate immune activation [33].
PRR activation is not restricted to keratinocytes, but monocytes and freshly isolated peripheral blood mononuclear cells from healthy controls and acne patients also reacted very similarly to the bacterium [20, 34,35,36].
The question that remains is how and exactly at which steps are these processes play a role in acne pathogenesis? The presence of IL-1α-like activity in the comedonal extracts [37], together with the fact that the same cytokine may cause hyperkeratinization of infundibular keratinocytes, suggests that IL-1α may be one of the initiators or early factors in microcomedo formation [8, 38]. The source of the cytokine is not clear, but in vitro data suggests the role of keratinocytes, which are close to C. acnes in the still intact PSU [36, 39].
Parallel with TLR activation, another system capable of inflammation activation may also play a role in acne pathogenesis, the inflammasomes. Immunohistochemical studies around the turning of the century revealed the presence of lymphocytes and macrophages around the healthy-looking follicles of acne patients and in early acne lesions [9, 10]. Later, Qin and colleagues detected mature caspase-1 and NLRP3 molecules in the proteasome of macrophages. These data suggest a role of the NLRP3 inflammasome pathway and IL-1β in disease pathogenesis, particularly in shaping the innate receptors-induced immune and inflammatory reactions [35]. At this point, essential sources of secreted IL-1β are the monocytes, which may also play a role in the induction of neutrophilic inflammatory responses [36, 40]. Different inflammasomes (NLRP1, NLRP3, and AIM2) are also present in keratinocytes [41, 42], but whether and how they contribute to acne pathogenesis and if they react to the presence of C. acnes are not clear. It is a rather interesting fact that in various autoinflammatory diseases (e.g., PAPA, SAPHO syndrome), skin involvement often includes severe acne [42].
C. acnes also enhances the production of reactive oxygen species (ROS), in particular, superoxide anions (O2·-) by keratinocytes, and these functions depend on the scavenger receptor, CD36. This pathway may also function as an important modulator of bacterially induced TLR signaling events in several different levels; among others, O2·- itself can induce inflammation, modulates the production of the CXCL8 chemokine, and directly inhibits C. acnes bacterial growth [30].
Bacterially secreted enzymes, including lipases, proteases, and hyaluronidases [32, 43,44,45,46], may also exert different biologic functions that contribute to acne inflammation and lesion development. C. acnes-produced proteases can generate tissue injury, by weakening and subsequently rupturing the follicular epithelium. However, the same enzymes may also be recognized by PAR-2 (protease-activated receptor-2), and these events can modulate the production of inflammatory mediators [32].
These results argue for the role of microbes, especially C. acnes itself and/or bacterially secreted metabolic products in innate immune and inflammation induction in acne vulgaris pathogenesis. Opposers question its role because this bacterium is one of the most prominent commensal microbes, especially in the sebum-rich skin regions. Thus it is rather difficult to consider the same microbe as a prototypic pathogen [47]. Ongoing research still aims to find a definite answer and explain a seemingly dual role this bacterium plays in skin physiology.
Sebocytes Are Active Players in Acne Immunity Too
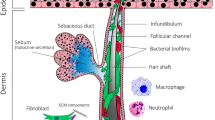
The fact that acne mostly present in skin regions (face, shoulders, upper chest, and back), which are sebaceous gland rich [31], already suggests that besides keratinocytes, another cell type that may play a key role in disease pathogenesis is the sebocytes. Hyperseborrhea and altered sebum composition have long been considered as important factors in the pathophysiology of this disease. The cause of enhanced sebum secretion, however, may be complex; hormonal and genetic factors, together with dietary habits, may influence it [48].
Earlier, sebum was considered as a substance playing important roles in the moisturization of the skin surface. It is also an important food source for the C. acnes bacterium, which uses sebaceous triglycerides for its growth [49]. It is clear now that sebum composition and secretion rate rapidly change together with the changing environment, and specific lipids may exert antimicrobial and pro- or anti-inflammatory properties. Through their sebum production, sebocytes may also act as important modifiers of the inflammatory processes [48, 50, 51].
Nevertheless, sebocyte functions are not restricted to sebum production [52, 53]. These cells are also immunocompetent, actively respond to different external signals, and produce inflammatory cytokines and other mediators, similarly to keratinocytes [20, 54,55,56]. C. acnes recognition in sebocytes, similarly to keratinocytes, takes place through the activation of, among others, TLR2, CD14 and CD1, and inflammasomes. As a result, this cell type also plays an essential role in immune and inflammation activation in the PSU [54, 57,58,59]. On the other hand, through these signaling pathways, the bacterium also influences sebocyte viability and differentiation [54] and directly enhances lipogenesis, and sebum secretion rates tend to correlate with the severity of skin symptoms [60, 61].
These data suggest that this cell type may act as an important regulator of a complex equilibrium. By regulating the amount and composition of sebum, sebocytes may promote the growth and metabolism of the skin commensals. They, on the other hand, may also limit bacterial viability during bacterial dysbiosis and pathogenic events and enhance microbial clearance by contributing to innate immune and inflammation activation.
This cell type is regulated by many factors (reviewed by Makrantonaki et al., [48]), among them, sex hormones. Pubertal hormonal changes, especially local androgen synthesis, are markedly higher in acne patients, which results in increased sebocyte activity and hyperseborrhea [48, 62, 63]. Recently, another hormone has emerged with complex roles in acne, the insulin-like growth factor 1 (IGF-1). In sebocytes, it induces increased lipid synthesis, while in keratinocytes, it also acts as a mitogen [64, 65], and in in vivo studies, IGF-1 levels were elevated in acne patients [66, 67]. The levels of this hormone are also increased in individuals following a Westernized lifestyle and diet (decreased physical activity, consuming high glycemic index food and milk products), which would explain how diet and acne may be linked [68].
One crucial point that should be mentioned is that it is still not clear whether and how sebocyte-C. acnes interaction takes place in the skin. Sebaceous glands usually are free from bacteria [69], so direct interaction in intact PSU may not happen. It is possible, though, that bacterially derived structural proteins, enzymes, and other secreted molecules and metabolic products reach the sebaceous glands and the sebocytes in the distance. In this way, the bacterium may still exert a biologic function on these cells [70].
Adaptive Immune Regulation in Acne
Microscopic identification of different adaptive immune cells around the affected follicles suggested that this arm of our immune system is also involved in acne pathogenesis. The findings that higher number of CD3+ and CD4+ T cells are present in the uninvolved skin of acne patients supported this idea, but what is the main initiator of such T cell infiltration remains to be unknown [5, 9, 10].
Another line of evidence suggested that C. acnes exhibited a potent immunostimulatory activity and the induction of T helper 1 immune responses in animal models [71, 72]. Finally, these data led to the identification of a T cell subpopulation in early inflamed acne lesions that exhibited increased cell proliferation in response to C. acnes extract, possibly as a result of the recognition of bacterial antigens. These T cells also exhibited a characteristic Th1 cytokine pattern and expressed IFNγ in high whereas IL-4 in low quantities [73]. Further studies also identified essential roles for Th17 activation in acne pathogenesis, and IL-1β, IL-6, and TGF-β appeared as key activators of this arm of the adaptive immune responses, similar to other systems. As proof of this concept, IL-17 expressing lymphoid cells were found around inflamed follicles by immunohistochemical analysis [74, 75]. Finally, CD4+ T cells expressing IL-17 together with IFNγ were also identified, characteristic of mixed Th1/Th17 differentiation [40].
Based on these results, C. acnes appears as one factor playing important roles in the initiation of the above adaptive immune responses. Nevertheless, apart from the bacterium, sebocytes may also act as critical factors, as the supernatant of these cells induces Th17 differentiation of naïve T cells. The resulting cell population is suggested to exhibit a dual function; it not only mediates host defense but can also actively participate in disease pathogenesis [56].
These data altogether clearly indicate that Th1, Th17, and mixed Th1/Th17-type adaptive immune responses play important roles in the cutaneous response to C. acnes and through that in acne pathogenesis [40, 73, 74]. However, there are still many open questions remains. Why acne vulgaris mostly affect the adolescent population? Why is it a self-limiting condition? What happens before, during, and after puberty? According to the current view, acne may be viewed as a transient arrest of homeostatic host-microbial dialogue between two phases of microbial tolerance [76]. This is a novel and intriguing concept, which suggests the crucial role of adaptive immune regulation in the maintenance of skin homeostasis in child- and adulthood and the lack or disturbance of these events as a critical pathogenic factor in puberty.
Not All C. acnes Strains Are Created to Be Equal?
Within the C. acnes species, different subtypes have long been identified, and currently, six major phylotypes are recognized: IA1, IA2, IB, IC, II, and III [77, 78]. A hot topic of the recent investigations is whether various C. acnes strains differ in their microbiological, metabolical, genetic, pathogenic, and other properties. The origin of these investigations comes from early findings, suggesting that various strains may differ in their innate immune induction properties in keratinocytes and sebocytes [22, 54], and differences in the internalization rates were also noted [79]. Selected strains might have different growth properties. They also exhibit variations in their metabolic traits, among them the production of short-chain fatty acids (SCFA), including acetic, propionic, and butyric acid, some of which exhibiting potent immunomodulatory properties [80,81,82].
According to the current knowledge, one of the most significant, differentially expressed molecules by the C. acnes strains are the CAMP (Christie-Atkins-Munch-Peterson) factors, co-hemolytic enzymes involved in pore formation processes [83]. These are secretory virulence factors, and genomic analysis revealed five different genes belonging to this family in C. acnes [80]. Recent studies indicate that TLR2 may directly recognize CAMP-1 [84], which would suggest that strains producing more virulence factors also are more potent innate immune activators.
Recently, it was identified that selected strains (IA, IC), and within that specific ribotypes (IB-1, IB-2, IC-2, IC-3), were frequently associated with acne [77, 85, 86], and variations in the ability of selected strains to induce adaptive immune responses have been described [87].
Acne Has a Complex Pathogenesis
Inflammation is vital in all stages of acne pathogenesis, in which C. acnes plays essential roles. What is still not clear is the initiator, the first step that pushes the delicate balance between the bacterium and the cutaneous cells to microbial dysbiosis. Or in other words, why and how exactly an important member of the skin microbiota turns into a microbe exhibiting opportunistic pathogen features? One idea is that loss of microbial diversity plays essential roles. Whether this means the loss of various species that were part of the cutaneous microbiota during human evolution [33] or the loss of the diversity of various C. acnes phylotypes [88] requires further investigations. One thing is clear; acne is an intriguingly interesting and complex skin disease. By uncovering its exact pathogenesis, we will not only gain more in-depth knowledge on the pathogenesis of the most common prototypic inflammatory skin disease but may also have more excellent knowledge on how the human body and our microbes interact to generate a healthy ecosystem.
Abbreviations
- AIM2:
-
Absent in melanoma 2
- AP-1:
-
Activator protein 1
- C. acnes :
-
Cutibacterium acnes
- C. albicans :
-
Candida albicans
- CAMP (LL37):
-
Cathelicidin antimicrobial peptide
- CAMP factor:
-
Christie-Atkins-Munch Peterson factor
- CXCL8:
-
C-X-C motif chemokine ligand 8
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- hBD2:
-
Human beta-defensin 2
- IFNγ:
-
Interferon-gamma
- IGF-1:
-
Insulin-like growth factor 1
- IL-1, 6, 10, 12:
-
Interleukin-1,6, 10, 12
- LPS:
-
Lipopolysaccharide
- LTA:
-
Lipoteichoic acid
- MAPK:
-
Mitogen-activated protein kinase
- MMP1, 3, 9:
-
Matrix metalloproteinase-1, 3, 9
- NF-κB:
-
Nuclear factor kappa B
- NLRP1, 3:
-
NLR Family Pyrin Domain Containing 1, 3
- P. acnes :
-
Propionibacterium acnes
- PAPA syndrome:
-
Pyogenic arthritis, pyoderma gangrenosum, and acne syndrome
- PAR2:
-
Proteinase-activated receptor 2
- PRR:
-
Pattern recognition receptor
- PSU:
-
Pilosebaceous unit
- ROS:
-
Reactive oxygen species
- SAPHO syndrome:
-
Synovitis, acne, pustulosis, hyperostosis, and osteitis
- SCFA:
-
Short-chain fatty acids
- TLR2, 4:
-
Toll-like receptor 2, 4
- TNFα:
-
Tumor necrosis factor-alpha
- TNIP1:
-
TNFAIP3-interacting protein 1
- VCAM1:
-
Vascular cell adhesion molecule 1
Literature
Leyden JJ, McGinley KJ, Vowels B. Propionibacterium acnes colonization in acne and nonacne. Dermatology. 1998;196(1):55–8.
Leeming JP, Holland KT, Cuncliffe WJ. The microbial colonization of inflamed acne vulgaris lesions. Br J Dermatol. 1988;118(2):203–8.
Koreck A, Pivarcsi A, Dobozy A, Kemény L. The role of innate immunity in the pathogenesis of acne. Dermatology. 2003;206(2):96–105.
Kurokawa I, Danby FW, Ju Q, Wang X, Xiang LF, Xia L, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18(10):821–32.
Norris JF, Cunliffe WJ. A histological and immunocytochemical study of early acne lesions. Br J Dermatol. 1988;118(5):651–9.
Kircik LH. Advances in the understanding of the pathogenesis of inflammatory acne. J Drugs Dermatol. 2016;15(1 Suppl 1):s7–10.
Knutson DD. Ultrastructural observations in acne vulgaris: the normal sebaceous follicle and acne lesions. J Invest Dermatol. 1974;62(3):288–307.
Guy R, Kealey T. The effects of inflammatory cytokines on the isolated human sebaceous infundibulum. J Invest Dermatol. 1998;110(4):410–5.
Jeremy AHT, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121(1):20–7.
Layton AM, Morris C, Cunliffe WJ, Ingham E. Immunohistochemical investigation of evolving inflammation in lesions of acne vulgaris. Exp Dermatol. 1998;7(4):191–7.
Tanghetti EA. The role of inflammation in the pathology of acne. J Clin Aesthet Dermatol. 2013;6(9):27–35.
Zouboulis CC. Is acne vulgaris a genuine inflammatory disease? Dermatology. 2001;203(4):277–9.
Nüsslein-volhard C, Wieschaus E. Mutations affecting segment number and polarity in drosophila. Nature. 1980;287(5785):795–801.
Rosetto M, Engström Y, Baldari CT, Telford JL, Hultmark D. Signals from the IL-1 receptor homolog, Toll, can activate an immune response in a Drosophila hemocyte cell line. Biochem Biophys Res Commun. 1995;209(1):111–6.
Szabó K, Bata-Csörgő Z, Dallos A, Bebes A, Francziszti L, Dobozy A, et al. Regulatory networks contributing to psoriasis susceptibility. Acta Derm Venereol. 2014;94(4):380–5.
Csato M, Kenderessy AS, Dobozy A. Enhancement of Candida albicans killing activity of separated human epidermal cells by ultraviolet radiation. Br J Dermatol. 1987;116(4):469–75.
Csato M, Kenderessy AS, Dobozy A. Enhancement of Candida albicans killing activity of separated human epidermal cells by α-melanocyte stimulating hormone. Br J Dermatol. 1989;121(1):145–7.
Csato M, Kenderessy AS, Judák R, Dobozy A. Inflammatory mediators are involved in the Candida albicans killing activity of human epidermal cells. Arch Dermatol Res. 1990;282(5):348–50.
Szolnoky G, Bata-Csörgö Z, Kenderessy AS, Kiss M, Pivarcsi A, Novák Z, et al. A mannose-binding receptor is expressed on human keratinocytes and mediates killing of Candida albicans. J Invest Dermatol. 2001;117(2):205–13.
Kim J, Ochoa M-T, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169(3):1535–41.
Pivarcsi A, Bodai L, Réthi B, Kenderessy-Szabó A, Koreck A, Széll M, et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003;15(6):721–30.
Nagy I, Pivarcsi A, Koreck A, Széll M, Urbán E, Kemény L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124(5):931–8.
Unna PG. The histological pathology of diseases of the skin. Br J Dermatol. 1895;7(3):83–5.
Fleming A. On the etiology of acne vulgaris and its treatment by vaccines. Lancet. 1909;173(4467):1035–8.
Dessinioti C, Katsambas AD. The role of Propionibacterium acnes in acne pathogenesis: facts and controversies. Clin Dermatol. 2010;28(1):2–7.
Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153(6):1105–13.
Kang S, Cho S, Chung JH, Hammerberg C, Fisher GJ, Voorhees JJ. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-κB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166(6):1691–9.
Graham GM, Farrar MD, Cruse-Sawyer JE, Holland KT, Ingham E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol. 2004;150(3):421–8.
Schaller M, Loewenstein M, Borelli C, Jacob K, Vogeser M, Burgdorf WHC, et al. Induction of a chemoattractive proinflammatory cytokine response after stimulation of keratinocytes with Propionibacterium acnes and coproporphyrin III. Br J Dermatol. 2005;153(1):66–71.
Grange PA, Raingeaud J, Calvez V, Dupin N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. J Dermatol Sci. 2009;56(2):106–12.
Trivedi NR, Gilliland KL, Zhao W, Liu W, Thiboutot DM. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J Invest Dermatol. 2006;126(5):1071–9.
Lee SE, Kim J-M, Jeong SK, Jeon JE, Yoon H-J, Jeong M-K, et al. Protease-activated receptor-2 mediates the expression of inflammatory cytokines, antimicrobial peptides, and matrix metalloproteinases in keratinocytes in response to Propionibacterium acnes. Arch Dermatol Res. 2010;302(10):745–56.
Szabó K, Erdei L, Bolla BS, Tax G, Bíró T, Kemény L. Factors shaping the composition of the cutaneous microbiota. Br J Dermatol. 2017;176(2):344–51.
Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63(8):3158–65.
Qin M, Pirouz A, Kim M-H, Krutzik SR, Garbán HJ, Kim J. Propionibacterium acnes induces IL-1β secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134(2):381–8.
Thiboutot DM. Inflammasome activation by Propionibacterium acnes: the story of IL-1 in acne continues to unfold. J Invest Dermatol. 2014;134(3):595–7.
Ingham E, Eady EA, Goodwin CE, Cove JH, Cunliffe WJ. Pro-inflammatory levels of interleukin-1 alpha-like bioactivity are present in the majority of open comedones in acne vulgaris. J Invest Dermatol. 1992;98(6):895–901.
Guy R, Kealey T. Modelling the infundibulum in acne. Dermatology. 1998;196(1):32–7.
Selway JL, Kurczab T, Kealey T, Langlands K. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10.
Kistowska M, Gehrke S, Jankovic D, Kerl K, Fettelschoss A, Feldmeyer L, et al. IL-1β drives inflammatory responses to propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134(3):677–85.
Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer H-D. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17(13):1140–5.
Beer HD, Contassot E, French LE. The inflammasomes in autoinflammatory diseases with skin involvement. J Invest Dermatol. 2014;134(7):1805–10.
Puhvel SM, Reisner RM. The production of hyaluronidase (hyaluronate lyase) by Corynebacterium acnes. J Invest Dermatol. 1972;58(2):66–70.
Hoeffler U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J Clin Microbiol. 1977;6(6):555–8.
Ingram E, Holland KT, Gowland G, Cunliffe WJ. Studies of the extracellular proteolytic activity produced by Propionibacterium acnes. J Appl Bacteriol. 1983;54(2):263–71.
Brüggemann H. Insights in the pathogenic potential of Propionibacterium acnes from its complete genome. Semin Cutan Med Surg. 2005;24(2):67–72.
Dreno B, Gollnick HPM, Kang S, Thiboutot D, Bettoli V, Torres V, et al. Understanding innate immunity and inflammation in acne: implications for management. J Eur Acad Dermatol Venereol. 2015;29(S4):3–11.
Makrantonaki E, Ganceviciene R, Zouboulis C. An update on the role of the sebaceous gland in the pathogenesis of acne. Dermato-Endocrinol. 2011;3(1):41–9.
Webster GF. Acne vulgaris. BMJ. 2002;325(7362):475–9.
Zouboulis CC, Baron JM, Böhm M, Kippenberger S, Kurzen H, Reichrath J, et al. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;7(6):542–51.
Picardo M, Ottaviani M, Camera E, Mastrofrancesco A. Sebaceous gland lipids. Dermatoendocrinol. 2009;1(2):68–71.
Zouboulis CC. The human skin as a hormone target and an endocrine gland. Hormones (Athens). 2004;3(1):9–26.
Ottaviani M, Camera E, Picardo M. Lipid mediators in acne. Mediators Inflamm. 2010;2010:pii: 858176.
Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8(8):2195–205.
Alestas T, Ganceviciene R, Fimmel S, Müller-Decker K, Zouboulis CC. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med (Berl). 2006;84(1):75–87.
Mattii M, Lovászi M, Garzorz N, Atenhan A, Quaranta M, Lauffer F, et al. Sebocytes contribute to skin inflammation by promoting the differentiation of T helper 17 cells. Br J Dermatol. 2018;178(3):722–30.
Kim J. Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211(3):193–8.
Georgel P, Crozat K, Lauth X, Makrantonaki E, Seltmann H, Sovath S, et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect Immun. 2005;73(8):4512–21.
Li ZJ, Choi DK, Sohn KC, Seo MS, Lee HE, Lee Y, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134(11):2747–56.
Cunliffe WJ. Benzoyl peroxide in acne. J Am Acad Dermatol. 1989;20(3):518–20.
Iwata C, Akimoto N, Sato T, Morokuma Y, Ito A. Augmentation of lipogenesis by 15-deoxy-Delta12,14-prostaglandin J2 in hamster sebaceous glands: identification of cytochrome P-450-mediated 15-deoxy-Delta12,14-prostaglandin J2 production. J Invest Dermatol. 2005;125(5):865–72.
Pochi PE, Strauss JS. Sebaceous gland response in man to the administration of testosterone, delta-4-androstenedione, and dehydroisoandrosterone. J Invest Dermatol. 1969;52(1):32–6.
Sansone G, Reisner RM. Differential rates of conversion of testosterone to dihydrotestosterone in acne and in normal human skin--a possible pathogenic factor in acne. J Invest Dermatol. 1971;56(5):366–72.
Tavakkol A, Varani J, Elder JT, Zouboulis CC. Maintenance of human skin in organ culture: role for insulin-like growth factor-1 receptor and epidermal growth factor receptor. Arch Dermatol Res. 1999;291(12):643–51.
Smith TM, Cong Z, Gilliland KL, Clawson GA, Thiboutot DM. Insulin-like growth factor-1 induces lipid production in human SEB-1 sebocytes via sterol response element-binding protein-1. J Invest Dermatol. 2006;126(6):1226–32.
Aizawa H, Niimura M. Elevated serum insulin-like growth factor-1 (IGF-1) levels in women with postadolescent acne. J Dermatol. 1995;22(4):249–52.
Cappel M, Mauger D, Thiboutot D. Correlation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women. Arch Dermatol. 2005;141(3):333–8.
Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18(10):833–41.
Jahns AC, Lundskog B, Ganceviciene R, Palmer RH, Golovleva I, Zouboulis CC, et al. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case-control study. Br J Dermatol. 2012;167(1):50–8.
Iinuma K, Sato T, Akimoto N, Noguchi N, Sasatsu M, Nishijima S, et al. Involvement of Propionibacterium acnes in the augmentation of lipogenesis in hamster sebaceous glands in vivo and in vitro. J Invest Dermatol. 2009;129(9):2113–9.
Matsui K, Yoshimoto T, Tsutsui H, Hyodo Y, Hayashi N, Hiroishi K, et al. Propionibacterium acnes treatment diminishes CD4+ NK1.1+ T cells but induces type I T cells in the liver by induction of IL-12 and IL-18 production from Kupffer cells. J Immunol. 1997;159(1):97–106.
Okazaki T, Ozaki S, Nagaoka T, Kozuki M, Sumita S, Tanaka M, et al. Antigen-specific Th1 cells as direct effectors of Propionibacterium acnes-primed lipopolysaccharide-induced hepatic injury. Int Immunol. 2001;13(5):607–13.
Mouser PE, Baker BS, Seaton ED, Chu AC. Propionibacterium acnes-reactive T helper-1 cells in the skin of patients with acne vulgaris. J Invest Dermatol. 2003;121(5):1226–8.
Agak GW, Qin M, Nobe J, Kim M-H, Krutzik SR, Tristan GR, et al. Propionibacterium acnes induces an IL-17 response in acne vulgaris that is regulated by vitamin a and vitamin D. J Invest Dermatol. 2014;134(2):366–73.
Kelhälä H-L, Palatsi R, Fyhrquist N, Lehtimäki S, Väyrynen JP, Kallioinen M, et al. IL-17/Th17 pathway is activated in acne lesions. PLoS One. 2014;9(8):e105238.
Szegedi A, Dajnoki Z, Bíró T, Kemény L, Törőcsik D. Acne: transient arrest in the homeostatic host-microbiota dialog? Trends Immunol. 2019;40(10):873–6.
Lomholt HB, Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS One. 2010;5(8):e12277.
Barnard E, Nagy I, Hunyadkürti J, Patrick S, McDowell A. Multiplex touchdown PCR for rapid typing of the opportunistic pathogen Propionibacterium acnes. J Clin Microbiol. 2015;53(4):1149–55.
Furukawa A, Uchida K, Ishige Y, Ishige I, Kobayashi I, Takemura T, et al. Characterization of Propionibacterium acnes isolates from sarcoid and non-sarcoid tissues with special reference to cell invasiveness, serotype, and trigger factor gene polymorphism. Microb Pathog. 2009;46(2):80–7.
Valanne S, McDowell A, Ramage G, Tunney MM, Einarsson GG, O’Hagan S, et al. CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiology. 2005;151(Pt 5):1369–79.
Nakatsuji T, Tang DC, Zhang L, Gallo RL, Huang C-M. Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: potential targets for inflammatory acne treatment. PLoS One. 2011;6(4):e14797.
Tax G, Urbán E, Palotás Z, Puskás R, Kónya Z, Bíró T, et al. Propionic acid produced by Propionibacterium acnes strains contributes to their pathogenicity. Acta Derm Venereol. 2016;96(1):43–9.
Holland C, Mak TN, Zimny-Arndt U, Schmid M, Meyer TF, Jungblut PR, et al. Proteomic identification of secreted proteins of Propionibacterium acnes. BMC Microbiol. 2010;10:230.
Lheure C, Grange PA, Ollagnier G, Morand P, Désiré N, Sayon S, et al. TLR-2 recognizes Propionibacterium acnes CAMP factor 1 from highly inflammatory strains. PLoS One. 2016;11(11):e0167237.
McDowell A, Gao A, Barnard E, Fink C, Murray PI, Dowson CG, et al. A novel multilocus sequence typing scheme for the opportunistic pathogen Propionibacterium acnes and characterization of type I cell surface-associated antigens. Microbiology. 2011;157(Pt 7):1990–2003.
Fitz-Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133(9):2152–60.
Yu Y, Champer J, Agak GW, Kao S, Modlin RL, Kim J. Different Propionibacterium acnes Phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J Invest Dermatol. 2016;136(11):2221–8.
Dagnelie M-A, Corvec S, Saint-Jean M, Bourdès V, Nguyen J-M, Khammari A, et al. Decrease in diversity of Propionibacterium acnes Phylotypes in patients with severe acne on the Back. Acta Derm Venereol. 2018;98(2):262–7.
Acknowledgments
This work was supported by the GINOP-2.3.2-15-2016-00015 research grant. KSZ is a recipient of the János Bolyai Research Scholarship from the Hungarian Academy of Sciences and also supported by the UNKP-19-4 New National Excellence Program of the Ministry for Innovation and Technology.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kemény, L., Szabó, K. (2021). Innate and Adaptive Immunity in Acne Vulgaris. In: Suh, D.H. (eds) Acne. Updates in Clinical Dermatology. Springer, Cham. https://doi.org/10.1007/978-3-030-68996-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-68996-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-68995-7
Online ISBN: 978-3-030-68996-4
eBook Packages: MedicineMedicine (R0)