Abstract
Proboscideans (Mammalia: Proboscidea) originated during the Eocene (perhaps already during the Paleocene) in Africa. Their fossil record narrates an amazing evolutionary history, ranging from the Paleogene to the Quaternary. Proboscideans experienced in the past a great diversification and wide distribution in Africa, Europe, Asia, and the Americas. They persist until today with only two genera, Loxodonta and Elephas, geographically confined in regions of Africa and Asia, respectively. The review of the fossil record of the Neogene proboscideans (excluding the members of Elephantidae that are treated elsewhere) in Greece revealed the presence of deinotheres (Deinotheriidae), mammutids (Mammutidae), choerolophodonts (Choerolophodontidae), amebelodonts (Amebelodontidae), tetralophodont gomphotheres (Gomphotheriidae), and stegodonts (Stegodontidae) in more than fifty localities, ranging from the early Miocene to the Early Pleistocene. Fourteen taxa are here considered valid, three of them (Choerolophodon chioticus, C. pentelici, and Konobelodon atticus) erected from type localities in Greece. The most diverse localities are Pikermi and Samos, where at least four proboscidean species have been recorded. The peak in taxonomic diversity occurred during the Turolian (late Miocene). The Greek proboscidean fossil record contains several highlights. The earliest appearance of the family Deinotheriidae in Europe is documented in Gavathas of Lesvos Island, and it is the proboscidean family with the widest temporal distribution in Greece. A deinotheriid skull from Samos Island is so far the most complete juvenile one known from Eurasia and Africa. Choerolophodon presents the widest temporal distribution among the genera in Greece, and where present, it is the dominant genus in terms of abundance. The rich choerolophodont sample allows the distinction into evolutionary stages and renders the genus as biostatigraphically important for Southeastern Europe. The late Miocene Anancus from Chomateri represents the first appearance of the genus in Greece and one of the earliest occurences in Europe. The sample of the late Pliocene Mammut borsoni from Milia, Grevena, is the richest one of this species, including partial skeletons, the longest upper tusks ever recorded in the world and the most complete mandible in Europe. During the Pliocene–Early Pleistocene, the most frequent and widespread proboscidean is the last European gomphothere Anancus arvernensis. Finally, the Siatista Stegodon is the first evidence of the presence of stegodontids in Europe.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The order Proboscidea is represented today by the largest living terrestrial mammals, the African savanna and forest elephants Loxodonta africana and Loxodonta cyclotis , respectively, and the Asian elephant Elephas maximus , which are all locally restricted and considered as threatened to extinction. The extant elephants are relics of a group that was once extremely diversified and widely distributed across Africa, Europe, Asia, and the Americas, especially during the Miocene (Shoshani and Tassy 1996a). Most proboscideans are characterized by the presence of tusks and molars of specialized morphology, making them easily recognizable in the fossil record and placing them among the most iconic vertebrates that have ever lived on this planet.
The earliest known proboscideans are Phosphatherium and Daouitherium from the Εarly Eocene of Morocco, at ~55.0 Ma (Gheerbrant et al. 1996, 2002). The cladistic position of Eritherium from the Late Paleocene (~60.0 Ma) of Morocco (Gheerbrant 2009) remains unresolved; it may be a stem proboscidean, or otherwise a sister group either to both Proboscidea and Sirenia or to all tethytherians (Gheerbrant et al. 2018). If Eritherium belongs to Proboscidea, then this species will represent the earliest proboscidean and will indicate a Paleocene emergence for the order. Since their appearance, the evolutionary history of proboscideans is marked by three major radiation events: (1) the Eocene radiation of primitive lophodont taxa, endemic in the, at that time, Afro-Arabian continent; (2) the Miocene radiation of gomphotheres sensu lato and stegodonts; (3) the Mio–Pliocene emergence of the family Elephantidae (Shoshani and Tassy 1996b). Not only the emergence and the subsequent first radiation of proboscideans took place in Africa, but also the other two radiations appear to have been triggered also in this continent (Sanders et al. 2010).
Proboscidean remains are common in the Neogene faunas of Greece, and especially during the late Miocene, including several taxa. They are present in almost all the large mammal fossiliferous sites of the country, with relatively abundant material. The Greek fossil record includes proboscideans from all three radiations. The first two radiations are presented in this chapter and comprise members of the families Deinotheriidae, Mammutidae, Amebelodontidae, Choerolophodontidae, Gomphotheriidae, and Stegodontidae; the third radiation of Elephantidae is the subject of the next chapter (Athanassiou this volume). For the sake of simplification, we use the informal name “Neogene proboscideans of Greece” for the taxa described in the present study, acknowledging however that the elephantid Mammuthus (presented in the following chapter) appeared in Greece during the late Pliocene and the fact that Anancus’ biostratigraphic range extends to the Early Pleistocene.
2 Historical Overview
The presence of proboscideans in the Greek fossil record was recognized already from the first excavations that were carried out in the nineteenth century in the rich late Miocene vertebrate localities of Pikermi (Attica, discovered in 1836) and Samos [Aegean Sea; Forsyth-Major (1888, 1894)] and the beginning of the twentieth century in Axios valley (Central Macedonia, discovered in 1915–1916; Arambourg and Piveteau (1929)]. The first systematic studies on Miocene proboscideans included important material from these localities and were conducted, among others, by Wagner (1848, 1857), Gaudry (1862–1867), and Vacek (1877) for Pikermi; Forsyth-Major (1894), Schlesinger (1917, 1922), and Lehmann (1950) for Samos; and Arambourg and Piveteau (1929) for Axios valley (“Salonique”). These publications served (and still do) as a reference point for future research on Neogene proboscideans. In all of the abovementioned localities, the fossils occur in different stratigraphic levels from multiple quarries and unfortunately—as is the case with several historical collections—most of the fossils lack precise stratigraphic information. Subsequent important Miocene proboscidean specimens were studied in Paraskevaidis (1940) and Tobien (1980) from the middle Miocene of Chios Island, Melentis (1967, 1969) from the late Miocene of Halmyropotamos, Koufos (1980) from the late Miocene of Axios valley, Tassy (1985) from the late Miocene of Pikermi, Tsoukala and Melentis (1994) from Kassandra Peninsula in Chalkidiki, Koufos et al. (2003) from the early Miocene of Gavathas on Lesvos Island, Theodorou et al. (2003) from the late Miocene of Kerassia, Athanassiou (2004), Poulakakis et al. (2005) and Iliopoulos et al. (2014) from the late Miocene of Crete Island, and Lazaridis and Tsoukala (2014a) from the late Miocene of Kryopigi. Furthermore, the new series of excavations in Axios valley (started in 1972; Koufos 2013), Samos (started in 1993; Koufos and Nagel 2009), and Pikermi (started in 2009; Theodorou et al. 2010), as well as in Nikiti (started in 1990; Koufos and Kostopoulos 2016), which aimed in the discovery of new stratified and dated material, provided a wealth of specimens, including several proboscideans. Therefore, in the recent years the need has arisen for a complete revision and study of the taxonomy, biostratigraphy, and paleoecology of the Miocene proboscideans. This was part of the investigations of Konidaris (2013) and of subsequent studies (Konidaris and Koufos 2009, 2013a, 2016, 2019; Konidaris et al. 2014, 2016, 2017; Konidaris and Roussiakis 2019).
The Pliocene–Early Pleistocene sediments of Greece have been also intensively explored during the last decades. From this period, mammutids and gomphotheres are well-known from the recent excavations in Milia (started in 1996), which provided a wealth of proboscidean specimens (Tsoukala 2000; Tsoukala and Mol 2016) and in Sesklo (Symeonidis and Tataris 1983; Athanassiou 2016), but also from Apolakkia (Theodorou et al. 2000), Vatera (de Vos et al. 2002), and Gephyra (Crégut-Bonnoure and Tsoukala 2017).
The extensive and systematic fieldwork during the last decades in Greece has greatly increased the number of proboscidean specimens, which together with the specimens from the old collections, constitute a rich proboscidean sample. Recent advances in proboscidean taxonomy, along with biostratigraphic correlations and magnetostratigraphic calibrations, allow a more comprehensive classification and biostratigraphy of the Neogene proboscideans from Greece, which are presented in this chapter.
3 Phylogenetic Relationships
The order Proboscidea consists predominantly of fossil taxa, which complicates their classification and phylogeny, in particular of the early representatives. Based on recent findings and new cladistic analyses, Proboscidea together with the orders Sirenia (sea cows) and the Hyracoidea (hyraxes), as well as with the extinct Embrithopoda (e.g., Arsinoitherium zitelli from the Early Oligocene of Fayum in Egypt), belong to the clade of Paenungulata; in turn, proboscideans, sirenians, and Embrithopoda constitute the clade of Tethytheria (Gheerbrant et al. 2018) (Fig. 1). Anthracobunidae and Desmostylia, which were usually classified within tethytheres, are in fact related to Laurasian Euungulata and Perissodactyla (Cooper et al. 2014). Proboscidean synapomorphies include the well-developed zygomatic process of the maxillary, which contributes significantly to the ventral border of the orbit and to the zygomatic arch, the relatively large size of the pars mastoidea of the periotic, the hypoconulid in a labial position, and possibly also the loss of i3 and (d)p1 (Gheerbrant et al. 2005). Well-recognized proboscidean features, such as the large size, the presence of a trunk (proboscis), the huge-sized and pneumatized crania with retracted nasal apertures , the hypertrophy of the second incisors to form upper tusks, the graviportal stance, the shortening of the tooth row, the horizontal tooth displacement, the reduction in the number of teeth with regard to the complete eutherian dentition, the enlargement and specialization of the cheek teeth, and the increase in the value of the encephalization quotient, constitute evolutionary trends within Proboscidea that evolved progressively since the Eocene (Shoshani and Tassy 1996a; Shoshani 1998, 2002; Gheerbrant and Tassy 2009; Sanders et al. 2010).
In this chapter, we mainly follow the classification proposed by Shoshani and Tassy (2005). However, we include choerolophodonts and amebelodonts in the families Choerolophodontidae and Amebelodontidae, respectively (Gheerbrant and Tassy 2009), and the tetralophodont gomphotheres Tetralophodon and Anancus of the family Gomphotheriidae in the subfamilies Tetralophodontinae and Anancinae, respectively (Sanders et al. 2010).
Focusing on the proboscidean taxa that are present during the Neogene of Greece, Deinotheriidae are more closely related to Elephantiformes, than to the more basal proboscideans (Fig. 1) based on dental features (e.g., presence of hypocone in P3 and P4 and the higher hypolophid than the protolophid in the lower molars) and postcranial traits (e.g., astragalus with long neck, enlarged lateral calcaneal facet, and reduced fibular facet) (Tassy 1994, 1996a; Gheerbrant et al. 2005). Deinotheres are unique within Proboscidea in lacking upper tusks and possessing strong and downward curved lower tusks (Gheerbrant and Tassy 2009). Apart from Deinotheriidae, all other proboscideans of the Greek fossil record belong to the monophyletic clade Elephantimorpha (Fig. 1). The monophyly of Elephantimorpha (Eritreum, Hemimastodon, Mammutida, Elephantida) is based among others on the “horizontal tooth displacement”, i.e., a pattern of dental eruption , in which the cheek teeth during their use move anteriorly along the jaws and when they are completely worn, they are succeeded by teeth that emerge more distally, in a manner that is reminiscent of a “conveyor belt” (Tassy 1994, 1996a; Shoshani et al. 2006; Sanders 2018; Tassy 2018). Within Elephantimorpha, the family Mammutidae is defined by the zygolophodont molars, with sharper loph(id)s due to anteroposterior compression, and with presence of pretrite and posttrite zygodont crests instead of central conules (Tassy 1996a; Shoshani 1996).
The clade Elephantida includes taxa with bunodont molars, which are transformed into molars with lamellae in the families Stegodontidae and Elephantidae (Gheerbrant and Tassy 2009). The family Choerolophodontidae is distinguished by the fused mesoconelets and central conules and by the mesially pointed chevrons of the intermediate and third molars (“V,” chevroning) formed by the more mesial position of the conelets in regard to the main cusps, especially in the distal lophids (Tassy 1996a; Sanders 2003; Konidaris et al. 2016). The straight medial border of the lower tusks and the posttrite conules on the molars are the distinguishing traits of the family Amebelodontidae; their most distinctive feature, the flattened lower tusks, is a plesiomorphic character present in Phiomia (Tassy 1996a; Shoshani 1996). The paraphyletic family Gomphotheriidae includes the trilophodont gomphotheres [with three loph(id)s in the intermediate molars] represented in Europe by the genus Gomphotherium and the tetralophodont gomphotheres [with four loph(id)s in the intermediate molars], which in Europe include the longirostrine Tetralophodon (with rounded to pyriform lower tusks) and the brevirostrine Anancus (lacking lower tusks) (Tassy 1996a; Shoshani 1996).
4 Distribution
Neogene proboscidean remains are present in almost all the large mammal fossiliferous sites of Greece, from the northernmost regions of the country (e.g., Thermopigi, Dikaia) to the southern-most ones (Crete) and from the western ones (e.g., Milia, western Peloponnese) to the easternmost ones (Samos, Kos, and Rhodes Islands); they are present in both today’s continental and insular Greece (Figs. 2 and 3). The earliest occurrence of Neogene proboscideans in the Greek fossil record is documented in the early Miocene of Gavathas (Lesvos Island) with the primitive deinothere Prodeinotherium, whereas their last occurrence is recorded in the Early Pleistocene of Vatera (Lesvos Island) with the gomphothere Anancus (Fig. 2). The taxonomic diversity reached its peak during the Turolian (late Miocene), when at least four proboscidean species have been recorded in Pikermi and Samos. Deinotheres present the widest biostratigraphic range, covering almost the whole Miocene (late MN3 until at least MN12), followed by choerolophodonts, which were present from the middle Miocene until the end of the late Miocene (MN5–13). Choerolophodon presents the widest temporal distribution among the genera in Greece, and where present, it is the dominant genus in terms of abundance.
Biostratigraphic distribution of the Neogene proboscideans in Greece. Solid lines indicate the biostratigraphic range of the species based on their occurrences in Greece; dots indicate single occurrences in Greece; dashed lines indicate the known biostratigraphic range based on data outside Greece; the top of MN12 is controversial and appears in grey. Selected localities with radiometric, biostratigraphical, or magnetostratigraphical data are also shown with letters: A, Gavathas; B, Thymiana; C, Pentalophos-1; D, Xirochori-1, Ravin de la Pluie; E, Nikiti-2, Ravin des Zouaves-5; F, Prochoma-1, Vathylakkos-2, Perivolaki, Pikermi, Pikermi Valley-1, 3, Mytilinii-1A, B, ?Halmyropotamos, ?Kerassia, ?Kryopigi, Chomateri; G, Dytiko-2, 3; H, Maramena; I, Milia, Sesklon, Gephyra; J, Vatera. Further details are given in the Appendix
5 Systematic Paleontology
Comments
Deinotheres were specialized browsing proboscideans with low-crowned, tapir-like lophodont and bilophodont cheek teeth—apart from the trilophodont dp4/DP4 and m1/M1—and with a dental formulae 0.0.3/1.0.3 for the deciduous and 0.0.2.3/1.0.2.3 for the permanent teeth (Harris 1973; Sanders et al. 2010) (Figs. 3, 4 and 5). Deciduous dentition is replaced by the permanent one in a vertical manner, and all permanent cheek teeth are in function simultaneously. In this feature, as well as in other cranial, dental, and postcranial traits, deinotheres differ markedly from elephantimorphs. The most distinctive feature of deinotheres is the downcurved mandibular symphysis bearing the lower tusks, the latter emerging almost vertically, whereas they lack upper tusks (Harris 1973). Known from the Late Oligocene of Africa, where they persisted until 1.0 Ma, deinotheres have an evolutionary history of ~25.0 myr (Sanders et al. 2010). In Greece, deinotheres are known from several localities ranging from the early to the late Miocene and belong to the genera Prodeinotherium and Deinotherium. The original generic name Deinotherium Kaup 1829 (consisting of the Greek words δεινός = fearfully great, and θηρίον = wild animal/beast) is valid and not Dinotherium used subsequently by several authors (see also comment in Huttunen 2002). Accordingly, Harris (1973) emended the original name Prodinotherium Éhik 1930 to Prodeinotherium , which is justified and valid.
Map of Greece showing the geographic distribution of the most important localities with Neogene and Early Pleistocene proboscideans. 1, Vatera localities; 2, Sani; 3, Kalliphytos; 4, Antimachia; 5, Kardamaena; 6, Pylos; 7, Skoura; 8, Nigrita; 9, Gephyra-1; 10, Sesklon; 11, Milia localities; 12, Apolakkia I; 13, Apolakkia II; 14, Agia Triada; 15, Maramena; 16, Dikaia; 17, Pyrgos Vassilissis; 18, Servia; 19, Fourka; 20, Chelona beach; 21, sea bed of Kryopigi; 22, Neokaisareia; 23, Dytiko localities; 24, Kryopigi; 25, Samos localities; 26, Chomateri; 27, Perivolaki; 28, Pikermi localities; 29, Halmyropotamos; 30, Prochoma-1; 31, Vathylakkos localities; 32, Kerassia localities; 33, Ravin des Zouaves-5; 34, Maronia; 35, Gela; 36, Nikiti-2; 37, Ravin X; 38, Platania; 39, Ravin de la Pluie; 40, Xirochori 1; 41, Ravin des Zouaves 1; 42, Agia Paraskevi; 43, Pentalophos 1; 44, Thymiana; 45, Psara; 46, Gavathas; 47, Thermopigi; 48, Antonios; 49, Siatista. See Appendix for more information. Image exported from Google Earth Pro © 2019, map data from US Dept. of State Geographer, SIO, NOAA, U.S. Navy, NGA, GEBCO, image from Landsat/Copernicus. Scale bar equals 80 km, North faces upward
Right mandible with the lower tusk and p3–m3 (NHMC 21.4.2.27) of Deinotherium proavum from the late Miocene of Gela, Aghia Photia (under permission from C. Fassoulas, copyright Natural History Museum of Crete). Maximum anteroposterior diameter of the mandible: 1040 mm (Iliopoulos et al. 2014)
-
Prodeinotherium Éhik, 1930
Type Species
Prodinotherium hungaricum Éhik, 1930.
Other Included Taxa
P. cuvieri (Kaup, 1832a); P. hobleyi (Andrews, 1911); P. pentapotamiae (Falconer, 1868); P. sinense Qiu et al., 2007.
Distribution
Early–early late Miocene of Africa, Europe and Asia.
Comments
Based on the conservativeness and the minor evolutionary changes of deinotheres (apart from a gradual increase in size through time) several researchers recognize only Deinotherium as the valid European genus (e.g., Gräf 1957; Ginsburg and Chevrier 2001; Pickford and Pourabrishami 2013). Here we follow Harris (1973), Huttunen (2002), and Aiglstorfer et al. (2014) and regard Prodeinotherium as a distinct genus from Deinotherium, based on several cranial, dental, and postcranial features. In Europe, Prodeinotherium includes the small and primitive deinotheres from the early until the middle Miocene (early Orleanian–early Astaracian, MN3–6). In Greece this genus is recognized with two species, Prodeinotherium cuvieri and Prodeinotherium bavaricum .
-
Prodeinotherium cuvieri ( Kaup, 1832a )
Nomenclatural and Taxonomical History
The species was erected by Kaup (1832a), who distinguished it from Deinotherium giganteum , based mainly on the smaller dimensions of the teeth from Chevilly (France) and Eppelsheim Formation (Germany). During the subsequent years and until today, the validity of this species is accepted by several authors (e.g., Ginsburg and Chevrier 2001; Pickford and Pourabrishami 2013), whereas others synonymize it with Prodeinotherium bavaricum (e.g., Gräf 1957; Huttunen 2002). The taxonomical status of the early Miocene deinotheres of Europe is not yet clarified and could be briefly summarized into the question whether P. cuvieri, P. hungaricum, or P. petenyii Vörös 1989 are conspecific or not, although the small dental dimensions of all three of them supports the synonymy; in this case, the type species of Prodeinotherium would be P. cuvieri (Markov 2008a: p. 144, footnote). Regardless of the specific name, dental dimensions and mandibular morphology (and perhaps also cranial and postcranial features) are in favor of a specific distinction from P. bavaricum (Markov 2008a).
Type Material
MNHN-CHE 13 (lectotype), right and left mandibular fragments (Mayet 1908: pl. 8, figs. 3, 4).
Type Locality
Chevilly, France, early Miocene, middle Orleanian, MN4.
Distribution
Εarly Miocene (end of early Orleanian, late MN3) of Greece (Gavathas on Lesvos Island); early Miocene, middle Orleanian (MN4) of Hungary, Germany, France, and Spain.
Remarks
This species comprises the smallest, earliest, and most basal deinotheres in Europe, known in Greece from a single occurrence of great importance. In particular, Koufos et al. (2003) studied tooth rows with permanent dentition from the locality Gavathas on Lesvos Island (Fig. 4), well-known for the Petrified Forest of Sigri. The authors noted that the dental morphology and the small dimensions of the Gavathas specimen indicate that it belongs to a primitive form of P. bavaricum (following the two European deinothere species concept of P. bavaricum and D. giganteum, accepted at that time). According to these observations and accepting here the validity of a distinct species from P. bavaricum—the valid name for which seems to be P. cuvieri—the Gavathas deinothere is provisionally attributed to P. cuvieri, pending complete revision of the primitive European deinotheres and new findings. The Gavathas deinothere, dated to as older than 18.4 ± 0.5 Ma (upper part of the early Miocene, late MN3), documents the earliest occurrence of deinotheres in Europe (Koufos et al. 2003). As part of the complex “Proboscidean Datum Event,” it marks the penetration of deinotheres into Europe (roughly together with the mammutid Zygolophodon, the gomphotheriid Gomphotherium, and the amebelodontid Archaeobelodon), after the establishment of the so-called Gomphotherium landbridge in the middle Burdigalian (~19.0–18.0 Ma, early MN3) (Tassy 1990; Rögl 1999; Antoine et al. 2003; Koufos et al. 2003).
Nomenclatural and Taxonomical History
The species was originally coined and described as a member of Deinotherium by von Meyer (1831, 1833). Almost a century after, it was transferred to Prodeinotherium by Éhik (1930). By being the oldest available name for the small and basal deinotheres, it has been considered by several authors (e.g., Gräf 1957; Bergounioux and Crouzel 1962; Huttunen 2002) as the senior synonym of the species Prodeinotherium cuvieri, P. hungaricum, and P. petenyii (but see above).
Type Material
SNSB-BSPG-AS I 220 (lectotype), right p3 (von Meyer 1833: pl. 34, figs. 12–15).
Type Locality
Georgensmünd?, Germany, middle Miocene, early Astaracian, MN6.
Distribution
Middle Miocene (late Orleanian, MN5) of Greece (Thymiana); middle Miocene (late Orleanian–early Astaracian, MN5–6) of Germany, Austria, France, Hungary, Slovakia, Spain, Bulgaria, Czech Republic.
Remarks
This species is well-known with abundant (mainly dental) material from several European localities, including at least two partial skeletons (Huttunen and Göhlich 2002; Huttunen 2004). In Greece its presence is documented with dental material from Thymiana (Paraskevaidis 1940), a vertebrate locality on Chios Island, bio- and magnetostratigraphically dated to ~15.5 Ma (middle Miocene, late Orleanian, MN5; Koufos 2006). Originally attributed to a new subspecies under the original spelling “Dinotherium bavaricum var. Aegäum,” the dimensions and morphology of the teeth indicate that are within the intraspecific variability of P. bavaricum without the need for subspecific distinction (Besenecker and Symeonidis 1974). The species is also known from the nearby Psara Island with an isolated M1 (Besenecker and Symeonidis 1974).
-
Deinotherium Kaup, 1829
Type Species
Deinotherium giganteum Kaup, 1829.
Other Included Taxa
D. bozasi Arambourg, 1934; D. indicum Falconer, 1845; D. levius Jourdan, 1861; D. proavum (Eichwald, 1831).
Distribution
Middle Miocene–Early Pleistocene of Africa, Europe, and Asia.
Comments
The genus Deinotherium includes the large-sized deinotheres, and in Europe it is known from the middle until the late Miocene (late Astaracian–Turolian, MN7/8–13).
-
Deinotherium giganteum Kaup, 1829
Nomenclatural and Taxonomical History
The species was erected and originally described by Kaup (1829, 1832a). Because it was the oldest available name for the large and derived deinotheres, it has been considered by several authors (e.g., Bergouniouz and Crouzel 1962; Huttunen 2002) as the senior synonym of the species Deinotherium levius and D. proavum (but for D. levius see: Gräf 1957; Ginsburg and Chevrier 2001; Böhme et al. 2012; Konidaris and Koufos 2019; and below for D. proavum).
Type Material
HLMD-Din 466 (holotype), left hemimandible with tusk, m2–m3 and right mandibular fragment with symphysis and tusk fragment (Kaup 1832a: pl. 4, add. pl. 1, fig. 5).
Type Locality
Eppelsheim, Germany, late Miocene.
Distribution
Late Miocene (late Vallesian, MN 10) of Greece (Ravin de la Pluie); late Miocene, Vallesian (MN9–10) of Germany, Austria, France, Hungary, Spain, Bulgaria, and Turkey.
Remarks
This species is well-known with abundant material from several European localities (mainly Germany, Austria, France, and Spain). In Greece it is rare, and known from only few localities and with limited specimens. Konidaris (2013) and Konidaris and Koufos (2013a) described an isolated p4 from the late Vallesian of Ravin de la Pluie in Axios valley, and Tsoukala and Melentis (1994) described an upper cheek tooth row from Agia Paraskevi in Kassandra, Chalkidiki.
-
Deinotherium proavum ( Eichwald, 1831 )
Nomenclatural and Taxonomical History
The species was erected by Eichwald (1831, 1835) based mainly on the large dimensions of some teeth from Rakhny Lesovye (Ukraine). Several years later, Stefanescu (1892) coined another deinothere species with huge dimensions from Găiceana and Mânzaţi (Romania), which he named D. gigantissimum. During the subsequent years, although the species name proavum was used by several authors, it was mostly neglected in favor of gigantissimum, whereas others included these huge-sized deinotheres within Deinotherium giganteum . Nowadays, Turolian deinotheres are mostly accepted as a distinct species. Codrea (1994) pointed to the priority of Eichwald’s species, which was further supported recently by Pickford and Pourabrishami (2013). Meanwhile, however, Kovachev and Nikolov (2006) erected D. thraceiensis, for the Ezerovo (Turolian; Bulgaria) skeleton, yet its specific distinction is not supported by morphological and metrical data (Markov 2008b).
Type Material
left p4 and m1 (holotype) (Eichwald 1835: pl. 60, figs. 1–5).
Type Locality
Rakhny Lesovye in Podolia, Ukraine.
Distribution
Late Miocene (Turolian) of Greece (Pikermi, Samos Island, Andriano, Halmyropotamos, Perivolaki, Maronia, Gela, Zakros, ?Kerassia); late Miocene (Turolian) of Austria, Spain, Germany, Hungary, Slovakia, Moldova, Ukraine, Romania, North Macedonia, Bulgaria, South Russia, Turkey, Afghanistan, Iran, Iraq (Konidaris et al. 2017).
Remarks
During the Turolian, European deinotheres reached huge dimensions and are attributed to D. proavum. This species represents the terminal stage of the gradual increase in size characterizing the evolutionary history of deinotheres during the Miocene in western Eurasia. Diagnostic characters include, among others (see Tarabukin 1974; Markov 2008b; Pickford and Pourabrishami 2013; Konidaris and Koufos 2019), the large-sized cheek teeth (juvenile and permanent), the morphology of the dp2, and the strongly developed mandibular angle protruding beyond the ventral border of the horizontal ramus (Figs. 4 and 5). The species is well-known from eastern-southeastern Europe, from where several skeletons are documented (Romania, Moldova, Bulgaria, Russia, Greece). In Greece, D. proavum is known with the most abundant material among deinotheres. The most complete material is a partial skeleton from Gela in Crete (Fig. 5; Poulakakis et al. 2005; Iliopoulos et al. 2014). Pikermi, Samos, and Halmyropotamos yielded a very important collection of craniomandibular, dental, and postcranial remains, including a skull from Samos, which is the most complete juvenile deinotheriid skull so far in Eurasia and Africa (Konidaris et al. 2017; Konidaris and Koufos 2019) (Fig. 6).
-
Elephantimorpha Tassy and Shoshani in Shoshani et al., 1998
-
Mammutida Tassy and Shoshani in Shoshani et al., 1998
-
Mammutidae Hay, 1922
Comments
The family includes elephantimorphs, whose cheek teeth are characterized by zygolophodonty (presence of yoke-like transverse crests), mesiodistally compressed and sharp transverse ridges, absence of accessory conules, and presence of zygodont crests (Tassy 1996a; Shoshani 1996). The intermediate molars remain trilophodont throughout the evolutionary history of the family (Tobien 1996). In Europe, two genera are present: Zygolophodon and Mammut (subfamily Mammutinae). The more basal Zygolophodon retains a more bunodont character on its cheek teeth, whereas in the more derived Mammut, the zygodont character is strongly developed (Tobien 1996). The family originated during the Late Oligocene in Africa, and migrated to Europe in the early Miocene, where it existed until the Pliocene. Mammutids migrated via the Bering Strait to North America during the middle Miocene and persisted there until the Late Pleistocene (Saunders 1996). In Greece, although some occurrences have been previously attributed to Zygolophodon , the only so far representative of Mammutidae is Mammut. This genus is present in several localities ranging from the late Miocene to the late Pliocene.
-
Mammut Blumenbach, 1799
Type Species
Elephas americanus Kerr, 1792.
Other Included Taxa
M. borsoni (Hays, 1834); M. matthewi (Osborn, 1921); M. obliquelophus (Mucha, 1980); M. pacificus Dooley et al., 2019.
Distribution
Late Miocene–Late Pleistocene of Europe, Asia and North America.
Comments
Apart from the well-expressed zygolophodonty on the cheek teeth, the genus Mammut is characterized by its shortened mandibular symphysis with relatively small or even vestigial lower tusks and by its straight or upturned upper tusks that lack an enamel band (Fig. 6). In these features, it differs from Zygolophodon, which has a longer mandible with well-developed lower tusks, and downwardly curved upper tusks with an enamel band (Tobien 1996). In Europe, the genus is well-known from the Pliocene; however, its first representatives are recorded during the Turolian (late Miocene). It should be noted, however, that the generic name Mammut for the Eurasian representatives is in question, pending revision of the whole sample and comparison with the North American specimens (see discussion in Markov 2008b, and von Koenigswald and Göhlich 2019).
-
Mammut sp. [ M. obliquelophus ? ( Mucha, 1980 )]
Nomenclatural and Taxonomical History
The species was erected by Mucha (1980) for a mammutid mandible from Romanovka (Ukraine). Markov (2008b) validated the species and included into it the skull remains from the Balta Sands in Podolia (Ukraine), which were attributed by Kubiak (1972) to Mammut praetypicum as well as material from other late Miocene localities (see discussion in Markov 2008b).
Type Material
Paleontological Museum of Odessa State University, Nr. 3347, mandible with m2–m3 (holotype) (Mucha 1980: pl. 1).
Type Locality
Romanovka, Ukraine.
Distribution
Late Miocene (Turolian) of Greece (Pikermi, Mytilinii-1A-Samos, Ravin des Zouaves-5, Halmyropotamos, Neokaisareia, Palaio Keramidi), and from the late Miocene (Turolian) of Bulgaria, Hungary, Spain, North Macedonia, Moldova, Ukraine, Southern Russia, Spain, and China.
Remarks
The late Miocene representative of European (and possibly also Asian) mammutids, M. obliquelophus , shows similar cheek tooth morphology as Mammut borsoni; however, the length of the symphysis and the size of the lower tusks are distinguishing features between the species: M. obliquelophus is characterized by a symphysis that is longer than the tooth row and equipped with well-developed tusks, whereas in M. borsoni the symphysis is much reduced and bears small tusks (Markov 2008b). Apart from the Neokaisareia partial skeleton (including an upper tusk) and the Palaio Keramidi molar of this taxon (Konidaris and Tsoukala 2020), the Greek material includes predominantly juvenile specimens (Pikermi, Ravin des Zouaves-5, Mytilinii-1A, Halmyropotamos), but it is very important, including two crania, two maxillae, two mandibles, and isolated teeth (Melentis 1967; Koufos 1980; Tassy 1985; Konidaris and Koufos 2009, 2013a; Konidaris 2013). Their complete study is in progress, and we tentatively attribute here this material to Mammut sp. (M. obliquelophus?).
-
Mammut borsoni ( Hays, 1834 )
Nomenclatural and Taxonomical History
Borson (1823) described and figured a tooth from Villanova d’Asti (Italy). Later on, Hays (1834), who had in his possession a cast of this tooth, erected the new species Mastodon borsoni . Osborn (1926, 1936) refers to the species as Zygolophodon borsoni, a binomen already used by Pohlig (1988). Meanwhile, however, Schlesinger (1922) already included the species within subgenus Mastodon (Mammut), currently elevated to the genus level.
Type Material
MGPT-PU 14896 (holotype), right M3 (Borson 1823: pl. 2).
Type Locality
Villanova d’Asti, Piedmont, Italy.
Distribution
Late Pliocene (early Villafranchian, MN16) of Greece (Milia, Grevena) and various Pliocene localities (Ruscinian–early Villafranchian, MN14–MN16) of Europe and Asia; its presence during the middle Villafranchian (MNQ 17; Early Pleistocene) of Europe is insecure based on the current evidence.
Remarks
The Borson’s mastodon is best known from Milia, a locality which has yielded the richest and most important material of this species. The material consists of several partial skeletons and isolated skeletal elements, including two complete pairs of the longest upper tusks ever recorded in the world and the most complete mandible in Europe (Tsoukala 2000; Tsoukala and Mol 2016). The upper tusks are almost straight with a slight upward curvature and torsion, long but slender, they have almost circular cross-section, and they lack enamel (Fig. 7). The brevirostrine mandible bears rudimentary lower tusks of oval section and the molars show well-expressed zygolophodonty (Fig. 7a). Mammut borsoni is mentioned also from other localities in Greece, albeit with no precise stratigraphic information (see Appendix).
Mammut borsoni remains from the late Pliocene of Milia. (a), Mandible with lower tusks and m2–m3 of both sides (MIL 143), left ulna (MIL 141) and right tibia (MIL 142) from Milia 1; all belong to the same individual; (b), partial skeleton from Milia 5; (c), close view of the left (MIL 561) and right (MIL 560) upper tusks from Milia 5
Comments
The family Choerolophodontidae includes bunodont trilophodont elephantimorphs, whose most distinctive dental features are the chevroning of the half-loph(id)s (mesially pointing chevrons, “V”), the choerodonty (multiplication of accessory conules), the ptychodonty (corrugated enamel), and the cementodonty (cement cover), with all these traits more-expressed in the later and more derived species. Other choerolophodont features include the upward curvature of the upper tusks that lack enamel, the long mandibular symphysis without tusks, and the strong development of the facial region of the cranium, which is elongated in comparison with the cerebral region. Choerolophodonts originated during the early Miocene, a period when they were distributed in northern/eastern Africa and South Asia. Subsequently, during the end of the early Miocene–middle Miocene, they dispersed to Southeastern Europe and China, and possibly to North America. The last appearances of choerolophodonts are traced at the latest Miocene. In Greece, they are known from several localities ranging from the middle to the late Miocene.
-
★Choerolophodon Schlesinger, 1917
Type Species
Mastodon pentelicus Gaudry and Lartet, 1856.
Other Included Taxa
C. anatolicus (Ozansoy, 1965); C. chioticus Tobien, 1980; C. corrugatus (Pilgrim, 1913); C. guangheensis Wang and Deng, 2011; C. kisumuensis (MacInnes, 1942); C. ngorora (Maglio, 1974); C. palaeindicus (Lydekker, 1884); C. zaltaniensis Gaziry, 1987a.
Distribution
Miocene of Europe, Africa, and Asia.
-
★Choerolophodon chioticus Tobien, 1980
Nomenclatural and Taxonomical History
A complete skull from the middle Miocene of Thymiana on Chios Island (Greece), originally attributed to Gomphotherium angustidens (Tobien 1973), constitutes the holotype of Choerolophodon chioticus, erected by Tobien (1980). Pickford (2001), who studied cranial material from Kenya, erected the genus Afrochoerodon, in which he included also the Thymiana specimen. According to Shoshani and Tassy (2005) Afrochoerodon constitutes a paraphyletic taxon placed within Choerolophodon.
Type Material
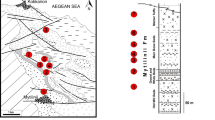
AMPG-937 (holotype), cranium with I2, M1–M2, and erupting M3 of both sides, and associated mandible with m1–m2 and erupting m3 of both sides (Fig. 8).
Type Locality
Thymiana, Chios Island, Greece, middle Miocene, late Orleanian, MN5.
Distribution
Middle Miocene (late Orleanian, MN5) of Greece (Thymiana), and possibly from the middle Miocene of Turkey and Bulgaria.
Remarks
Choerolophodon chioticus from Thymiana represents the earliest known choerolophodont in Europe. The skull is characterized, among others, by: moderate elongation, steeply inclined facial region and high cerebral region of the cranium; upper tusks that emerge downwards, then curve upward and at the tip inward; long and downwards deflected mandibular symphysis (Fig. 8); and, weak to moderate expressed choerodonty, ptychodonty, and cementodonty on the molars. The Thymiana cranium is less derived than the late Miocene choerolophodonts and closer to C. kisumuensis (Tobien 1980; Tassy et al. 1989; Pickford 2001; Konidaris et al. 2016). The distal fragment of an m3 from Thymiana, attributed by Paraskevaidis (1940: pl. 14, fig. 6) to Trilophodon (Mastodon) angustidens , possibly also belongs to Choerolophodon. Study of additional material from Thymiana is in progress. Thymiana is bio- and magnetostratigraphically correlated to the middle Miocene (late Orleanian, MN 5), at ~15.5 Ma (Koufos 2006).
-
Choerolophodon anatolicus ( Ozansoy, 1965 )
Nomenclatural and Taxonomical History
Trilophodon (Choerolophodon) anatolicus was erected as a new choerolophodont species by Ozansoy (1965) based on material from Yassiören (Middle Sinap, Turkey). However, in the subsequent years, the species was regarded as synonymous with C. pentelici, until Sanders (2003), who studied new material from the Sinap localities, re-established its validity. Sanders attributed to this species specimens from several other Turkish localities, including some of the material that had been attributed by Gaziry (1976) to C. pentelici and a mandible from Eşme Akçaköy assigned to C. pentelici lydiensis by Tassy et al. (1989) (see below). Recently, Konidaris et al. (2016) revised C. anatolicus and marked its presence for the first time outside Turkey in the Greek locality Pentalophos-1 of Axios valley.
Type Material
MNHN-TRQ-1000 (lectotype), maxilla with right DP2–DP4 and left DP3–DP4.
Type Locality
Yassiören, Middle Sinap, Turkey, early Vallesian, MN9.
Distribution
Late Miocene (early Vallesian, MN9) of Greece (Pentalophos-1), and the late Miocene (early Vallesian–?late Vallesian, MN9–?10) of Turkey, and possibly of Moldova and Romania.
Remarks
Choerolophodon anatolicus is diagnosed and differentiated from C. pentelici, among other characters, by the moderate retracted perinasal area in the cranium, the ventrally deflected mandibular symphysis in adult individuals, the small size of the deciduous teeth and the weak development of the distal cingulum in the DP3/dp3, which is connected to the second loph(id) (Sanders 2003; Konidaris and Koufos 2013a; Konidaris et al. 2016). The first and so far only record of C. anatolicus in Greece is traced in the early Vallesian locality Pentalophos-1 of Axios valley (Konidaris and Koufos 2013a; Konidaris et al. 2016). The material consists of juvenile and adult specimens (Fig. 9), which present more primitive morphology and smaller dimensions than the rest of the choerolophodont material from Axios valley, as well as from Pikermi and Samos, and are similar to those of C. anatolicus from Yassiören, Sinap 12 and Eşme Akçaköy (Turkey).
-
★Choerolophodon pentelici (Gaudry and Lartet, 1856)
Nomenclatural and Taxonomical History
Gaudry and Lartet (1856) erected the species Mastodon pentelicus based on proboscidean remains from the Turolian locality Pikermi. This material was subsequently studied in detail by Gaudry (1862–1867) (specimens of Konobelodon atticus were included in this study as well; see below), who emended also the species name Mastodon pentelici , although such a correction was not necessary (Tassy 1985: p. 617). Later on, Schlesinger (1917, 1922) studied choerolophodonts from Samos Island and Maragheh (Iran) and erected the subgenus Mastodon (Choerolophodon) , while Arambourg and Piveteau (1929) studied specimens from Axios valley. Since then, all the late Miocene choerolophodont material has been referred to this species without any distinction. Gaziry (1976) was the first, who recognized different evolutionary dental features within the late Miocene choerolophodonts of Turkey. Subsequently, Tassy et al. (1989) erected the subspecies C. p. lydiensis for an adult mandible from Eşme Akçaköy, a specimen, which was later transferred by Sanders (2003) to C. anatolicus (see above). From the fossiliferous sites of Kemiklitepe, Tassy (1994) identified two different evolutionary morphs, a primitive one in Kemiklitepe-D and a more advanced one in Kemiklitepe A-B. Recently, Konidaris (2013) examined all the choerolophodont material from Greece (old and new collections) and revised specimens from western Eurasia, and recognized the presence of two evolutionary stages within C. pentelici. In particular for Greece, “C. pentelici primitive” is correlated to the late Vallesian until possibly the early Turolian (MN10–?11; Xirochori-1, Ravin de la Pluie, Ravin des Zouaves-1, and from an unknown locality from the Turolian of Samos Island) and “C. pentelici advanced” from the Turolian (MN11–13; Pikermi, Samos Island, Mytilinii-1B-Samos, Andriano-Samos, Nikiti-2, Ravin des Zouaves-5, Prochoma-1, Vathylakkos-2, Ravin X and Dytiko-2, 3 (Konidaris and Koufos 2016, 2019; Konidaris et al. 2016).
Type Material
MNHN-PIK-3665 (lectotype), juvenile cranium with right DP2–DP4, left I2 and left DP4, and associated mandible with right dp3–dp4 and left dp4 (Fig. 10a–d).
(a–d), Juvenile cranium with right DP2–DP4, left upper tusk and left DP4, and associated mandible with right dp3–dp4 and left dp4 (lectotype; MNHN-PIK-3665) of Choerolophodon pentelici from the late Miocene (Turolian) of Pikermi; (a), left lateral view of the cranium; (b), ventral view of the cranium; (c), lateral view of the right hemimandible; (d), dorsal view of the mandible (copyright G. Konidaris); (e), right upper tusk (LGPUT-NIK-1776) in lateral view of C. pentelici from the late Miocene (Turolian) of Nikiti-2 (copyright G. Konidaris). Scale bar equals 10 cm
Type Locality
Pikermi , Attica, Greece, late Miocene, middle Turolian, MN12.
Distribution
Late Miocene (late Vallesian–late Turolian, MN10–13) of Greece (Xirochori-1, Ravin de la Pluie, Ravin des Zouaves-1, Ravin des Zouaves-5, Nikiti-2, Prochoma-1, Vathylakkos-2, Pikermi, Mytilinii-1B, Samos Island-old collections, Andriano, Kerassia, Ravin X, Kryopigi, Dytiko-2, 3, Dikaia, Sani, ?Maramena, ?Pyrgos Vassilissis, ?Servia). Outside Greece it is known from the late Miocene (?late Vallesian–middle Turolian, ?MN10, MN11–12) of Turkey, Bulgaria, North Macedonia, Romania, Moldova, Iran, Iraq, and possibly in Ukraine and Azerbaijan.
Remarks
The skull of C. pentelici is characterized, among other traits, by an elongated cranium with moderately inclined facial region and low cerebral region, orbits situated at the top of the cranium and far behind the last molar in function, retracted perinasal area and narrow nasal aperture, and mandibular symphysis situated ventrally at the extension of the corpus in both juvenile and adult specimens (Fig. 10a–d). The upper tusks emerge sub-horizontally and outwards and then curve upward (Fig. 10e). The deciduous teeth are large, the distal cingulum of the dp3/DP3 is well developed, and it is separated from the second loph(id) in the more advanced morphs. Choerodonty, ptychodonty, and cementodonty are well-expressed in the cheek teeth. The last appearance of C. pentelici is traced in the late Turolian (MN 13) localities of Dytiko in Axios valley (Konidaris et al. 2016). Schmidt-Kittler et al. (1995) note the occurrence of this species in the faunal list of Maramena (Serres basin), dated to the Miocene/Pliocene (MN13/14) boundary. If this occurrence is indeed C. pentelici, then the species survived until the uppermost Miocene.
-
Amebelodontidae Barbour, 1927
Comments
The family includes predominantly bunodont trilophodont elephantimorphs (tetralophodonty in all intermediate molars has reached only the genus Konobelodon), commonly called “shovel-tuskers” due to the broadening and flattening of their lower tusks. However, the monophyly of the family is based on the straight medial border of the lower tusks and the posttrite conules on the molars (Tassy 1996a; see also Shoshani 1996). Based on the internal structure of the lower tusks, two groups of amebelodontids are distinguished: one with only concentric laminated dentine (Archaeobelodon, Protanancus, Amebelodon, Serbelodon, Aphanobelodon) and another with presence of tubular dentine or otherwise called dentinal rods (Platybelodon, Torynobelodon, Konobelodon) (Tassy 1986, 1996a; Konidaris et al. 2014; Wang et al. 2017a). The family originated during the early Miocene in Africa, but rapidly dispersed into Europe and Asia, and subsequently into North America; by the beginning of the Pliocene amebelodontids had vanished (Lambert and Shoshani 1998; Sanders et al. 2010). In Greece, amebelodontids are represented so far only by the late Miocene Konobelodon .
-
Konobelodon Lambert, 1990
Type Species
Amebelodon (Konobelodon) britti Lambert, 1990.
Other Included Species
K. atticus (Wagner, 1857); K. cyrenaicus (Gaziry, 1987b); K. robustus Wang et al., 2016.
Distribution
Late Miocene of Europe, Africa, Asia, and North America; in Europe is known from the late Vallesian until the late Turolian.
Comments
Konobelodon was originally erected as a subgenus of Amebelodon, which included the shovel-tuskers with tetralophodont first and second molars, and flattened lower tusks bearing internal dentinal rods from the late Miocene of North America (Barbour and Hibbard 1941; Gregory 1945; Lambert 1990) [we note here that the DP4, associated with fully tetralophodont M1 in the palate KUVP-3477 from the Rhino Hill site, Kansas, U.S.A. (Barbour and Hibbard 1941), albeit considerably worn shows an incipient fourth loph (GK observation on photos provided by C. Beard, University of Kansas); Mebrate (1987: p. 232) described the DP4 as trilophodont with a talon, Lambert (1990) mentioned it as trilophodont, while Lambert (pers. comm. 2020 to GK) as quasi-tetralophodont]. Lambert (1990) included also to the same genus/subgenus the Sahabi Amebelodon cyrenaicus —described by Gaziry (1987b) and considered a possible junior synonym of Mastodon grandincisivus by Tassy (1999) —whose lower tusk shows tubular dentine; trilophodont m1 and tetralophodont m2 were originally attributed to this taxon; however, it is uncertain whether these molars belong to the same taxon as the lower tusk (holotype) or each other (Markov 2008b; Sanders 2008). Konidaris et al. (2014) described juvenile mandibles from Pikermi belonging to a tetralophodont grade species bearing flat lower tusks with tubular dentine, as well as an adult large lower tusk with flattened-pyriform cross-section and internal dentinal rods. The upper and lower deciduous premolars (some of the lower ones associated with flat lower tusks) are both morphologically and metrically similar to those of Mastodon atticus (Wagner 1857; Gaudry 1862–1867; usually attributed to Tetralophodon atticus), and the adult lower tusk is morphologically and metrically similar to the holotype of Mastodon grandincisivus from Maragheh (Iran; Schlesinger 1917). Konidaris et al. (2014) revised the tetralophodont material from Pikermi (see below), noting the similarities with the lower tusks from North America, and proposed the elevation of Konobelodon to generic level, the synonymy between Mastodon atticus and Mastodon grandincisivus , and the inclusion of all the Turolian tetralophodont shovel-tuskers from western Eurasia to Konobeledon atticus . New tetralophodont amebelodontid material from China, including juvenile specimens (with striking morphological resemblance to the deciduous premolars from Pikermi, including the holotype of Mastodon atticus ) and adult mandibles preserving the flattened lower tusks with internal dentinal tubules (Wang et al. 2016), corroborates further the attribution of the Pikermi tetralophodont material to an amebelodontid. Tassy (2016) suggested that the paratype of K. britti is similar to the holotype of Torynobelodon loomisi Barbour 1929 [Torynobelodon is considered a junior synonym of Platybelodon in Shoshani and Tassy (1996b: app. A)] and therefore that Konobelodon is a junior synonym of Torynobelodon; Konobelodon atticus should thus be Torynobelodon atticus . However, the tusk of T. loomisi has different cross-sectional shape from the tusk of K. britti, as well as from those of K. robustus and K. atticus, e.g., from Pikermi, Maragheh and Pestszentlörinc (Hungary), having wide and straight medial border, roughly the same width in the middle and lateral parts, and with very shallow dorsal and ventral concavities. Moreover, it is unknown whether the tusk of T. loomisi, which is so far the single known specimen of this species, belonged to a trilophodont or tetralophodont grade species. Consequently, the current evidence does not support the synonymy between Konobelodon and Torynobelodon, and they should be considered distinct genera. Finally, the co-existence between a tetralophodont amebelodontid (here Konobelodon) and a derived tetralophodont taxon, such as that found in the Turolian of Crevillente 2 [Spain, Mazo and Montoya 2003; possibly related to Stegotetrabelodon (Tassy 2016)], or Stegotetrabelodon (present in the Turolian of Cessaniti, Italy; Ferretti et al. 2003), both having oval/sub-circular cross-sections with lamellar dentine in their lower tusks, cannot be excluded for Pikermi or other Turolian localities of the wider region, as discussed by Tassy (2016). Nonetheless, such evidence is so far not recorded in the Turolian of the Greco-Iranian-Afghan (sensu Bonis et al. 1992; Balkano-Iranian or Sub-Paratethyan) paleobiogeographic province.
-
★Konobelodon atticus (Wagner, 1857)
Nomenclatural and Taxonomical History
The type locality of the tetralophodont amebelodontid K. atticus is Pikermi, from where the richest material of this species is known. The species has a long and complicated taxonomical history. The presence of tetralophodonts in Pikermi was recognized early by Wagner (1857), who described a juvenile maxilla and erected the species “Mastodon atticus G. and L.” However, Wagner (1857) attributed Mastodon atticus to Gaudry and Lartet (1856), but as noted by Gaudry (1862: p. 142 footnote), the only bunodont proboscidean referred by Gaudry and Lartet (1856: p. 273) was the trilophodont Mastodon pentelicus (= Choerolophodon pentelici). Few years later, Lartet (1859) transferred the Pikermi tetralophodonts to Mastodon longirostris (= Tetralophodon longirostris). Gaudry (1862–1867) included all bunodont proboscideans from Pikermi (tri- and tetralophodonts) to Mastodon pentelici. Subsequently, Vacek (1877: p. 32, pl. 7) attributed a third molar from Pikermi to Mastodon atticus , which was transferred to Stegotetrabelodon grandincisivus by Tobien (1978). The tetralophodont material of Wagner (1857) and Gaudry (1862–1867), as well as that described by the latter author as Mastodon turicensis , was included in the zygodont taxon Turicius atticus by Osborn (1936). More recently, Tassy (1985) referred the tetralophodonts of Pikermi to Tetralophodon atticus, although he later questioned this generic attribution (Tassy 2005). Konidaris et al. (2014) studied all the published tetralophodont material from Pikermi, as well as previously unpublished material from the locality originating from the old excavations, and attributed them to the amebelodontid Konobelodon atticus (see discussion above).
Type Material
SNSB-BSPG-AS II 182 (holotype), left maxillary fragment with DP2–DP3 (Fig. 10a).
Type Locality
Pikermi, Attica, Greece, late Miocene, middle Turolian, MN12.
Distribution
Late Miocene (Turolian) of Greece (Pikermi, Samos-old collections, Kerassia-4, and possibly from Platania). Outside Greece, it is known from the late Miocene (Turolian, MN11–13) of Turkey, Bulgaria, Moldova, Ukraine, Hungary, North Macedonia, and Iran.
Remarks
The species is characterized, among other features, by the tetralophodont intermediate molars, the enlarged third loph in the DP3 with posttrite-pretrite connection in both transverse valleys and well-marked second ento-/ectoflexus (Fig. 11a), the large-sized and dorsoventrally flattened-pyriform adult lower tusks that have thin concentric laminated dentine layer externally and tubular dentine internally (Fig. 11b), and by the long and high mandibular symphysis (Konidaris et al. 2014). Apart from Pikermi, the species is known from Samos, Kerassia-4 and possibly from Platania (Lehmann 1950; Theodorou et al. 2003; Konidaris et al. 2014; Konidaris and Koufos 2019; Konidaris and Tsoukala 2020). It is well distributed from the early until the late Turolian of eastern Europe-western Asia and in Greece, in particular, is present possibly from the Vallesian/Turolian boundary (Platania) until the middle Turolian (Pikermi).
-
Gomphotheriidae Hay, 1922
Comments
This paraphyletic family includes the bunodont trilophodont and tetralophodont elephantimorphs, excluding the choerolophodontids and amebelodontids, and constitutes the most diverse family within Elephantimorpha. By the early Miocene, the primitive Gomphotherium “annectens group” had already a cosmopolitan distribution in Africa, Europe, and Asia (Tassy 1996b); during the middle Miocene, gomphotheriids entered North America and persisted in South America until the beginning of the Holocene (Lambert and Shoshani 1998; Mothé et al. 2017). In Europe, gomphotheriids are represented by the trilophodont Gomphotherium and the tetralophodonts Tetralophodon and Anancus. In Greece, only tetralophodont gomphotheres are known so far.
Type Species
Tetracaulodon longirostre Kaup, 1832b.
Other Included Species
T. euryrostris Wang et al., 2017b; T. xialongtanensis (Chow and Chang, 1974).
Distribution
Middle–late Miocene of Europe, Asia, and Africa.
Comments
Tetralophodon includes tetralophodont gomphotheres with long mandibular symphysis bearing pyriform to oval in cross-section lower tusks (in contrast to the brevirostrine and tuskless Anancus), consisting of concentric lamellar dentine (no dentinal rods like Konobelodon), intermediate molars, and third molars that show trefoil wear patterns (not plate like pattern such as Stegotetrabelodon) and rounded upper tusks that lack enamel bands.
-
Tetralophodon longirostris ( Kaup, 1832b )
Nomenclatural and Taxonomical History
Tetralophodont proboscidean remains from the Eppelsheim Formation (Dinotheriensande) of the Mainz Basin in Germany were originally allocated to the species Tetracaulodon longirostre by Kaup (1832b) (Tetracaulodon = Mammut), and slightly later they were assigned to Mastodon longirostris (Kaup 1835). This species was subsequently included within the subgenus Mastodon (Tetralophodon) by Falconer (1857).
Type Material
HLMD-Din 111 (holotype), mandible fragment with left m2–m3.
Type Locality
Eppelsheim, Germany, Miocene.
Distribution
?Late Miocene (?Vallesian) of Greece (Fourka area, seabed of Kryopigi, Chelona beach). Outside Greece, this species is known from the middle–late Miocene (?early Astaracian, late Astaracian–Vallesian, ?MN6, MN7/8–9, ?MN10) of Europe.
Remarks
Tetralophodon longirostris shows an evident variation in the dental morphology and the shape of the mandible (especially the curvature of the mandibular symphysis), which were attributed to polymorphism (Tassy 1985, 1999). Most of the known specimens originate from the Eppelsheim Formation, which was generally considered to be of Vallesian age. However, recent studies indicate the stratigraphic inhomogeneity due to reworking of the sediments and a chronological range of the fauna from the middle Miocene to the late Miocene (Böhme et al. 2012; Pickford and Pourabrishami 2013). In this aspect, the revision of all known material from Europe and the discovery of new specimens are necessary to clarify the taxonomy of the European Tetralophodon, and especially whether T. curvirostris Bergounioux and Crouzel 1960 and T. gigantorostris (Klähn 1922) are indeed varieties of T. longirostris.
In Greece, Lazaridis and Tsoukala (2014b) and Lazaridis (2015) report on the presence of isolated findings of T. longirostris from several collection spots (two of them were recovered from the seabed) of provisionally Vallesian age within the Kassandra sand deposits, considering that they originate from the Antonios Formation of Western Chalkidiki and date to the late Miocene. This Formation is biostratigraphically dated from the early/middle Miocene boundary (close to MN4/5) until the late Miocene (pre-middle Turolian but possibly Vallesian ) (Syrides 1990; Koufos 2013; Lazaridis et al. 2017).
Type Species
Mastodon arvernensis Croizet and Jobert, 1828.
Other Included Species
A. capensis Sanders, 2007; A kenyensis (MacInnes, 1942); A. lehmanni Gaziry, 1997; A. osiris Arambourg, 1945; A. perimensis (Falconer and Cautley, 1847); A. petrocchii Coppens, 1965; A. sinensis (Hopwood, 1935); A. sivalensis (Cautley, 1836); A. ultimus Sanders, 2011.
Distribution
Late Miocene–Early Pleistocene of Europe, Africa, and Asia.
Comments
Anancine gomphotheres, represented by the single genus Anancus, have tetralophodont (to pentalophodont) intermediate molars, whose main morphological feature is the dislocation of the pretrite and posttrite half-loph(id)s and the resultant alternate arrangement of the successive loph(id)s (anancoidy). In particular, in upper molars, the pretrite half-lophs are mesially offset, whereas in lower molars the pretrite half-lophids are distally dislocated, establishing thus an alternate contact of the successive loph(id)s (Tassy 1986). Other anancine characters include the high and short cranium with domed and elevated vault, the enlarged tympanic bullae, the brevirostrine mandible without tusks, the straight upper tusks (Anancus means “without a bend”; although slightly curved upper tusks exist) lacking enamel bands, and the absence of premolars, except in A. kenyensis (Tassy 1986; Hautier et al. 2009). The earliest occurrences of Anancus are traced during the Turolian (late Miocene). The genus flourished in the Old World during the Pliocene (in Europe commonly co-occurring with the zygolophodont Mammut borsoni) and survived until the Early Pleistocene (co-occurring also with Mammuthus), being the last gomphothere of the Old World.
-
Anancus lehmanni Gaziry, 1997
Nomenclatural and Taxonomical History
Anancine gomphotheres from the late Miocene of Europe were recognized as Mastodon cf. longirostris (Schlosser 1907), subsequently to transitional forms between Mastodon longirostris and Mastodon arvernensis (e.g., Schlesinger 1917; Zapfe 1957) and later on to Anancus sp. (Tassy 1986; Markov 2008b). Gaziry (1997) studied the elephantimorphs from Dorn-Dürkheim 1 (Germany) and described four taxa: Tetralophodon longirostris, Anancus arvernensis turoliensis, Stegotetrabelodon lehmanni, and Stegolophodon caementifer, the second one a new subspecies and the latter two new species. However, apart from the material referred by Gaziry to “A. a. turoliensis,” the holotype of “S. lehmanni” and other specimens attributed to this species are also included in Anancus, as well as specimens of T. longirostris (Konidaris and Roussiakis 2019). The species name lehmanni has nomenclatural priority over the subspecies name turoliensis due to its original higher taxonomic ranking (see also discussion in Markov 2008b). Konidaris and Roussiakis (2019) revised the known anancine specimens from the late Miocene of Europe (see below) and attributed them to Anancus lehmanni .
Type Material
SMF-DD 3151(holotype), right M3 (Gaziry 1997: pl. 3, fig. 3).
Type Locality
Dorn-Dürkheim-1, Germany, Turolian.
Distribution
Late Miocene (middle Turolian, MN12) of Greece (Chomateri), and from numerous localities from the late Miocene (middle–late Turolian, MN12–13) of Germany, Austria, Slovakia, Hungary, Spain, Romania, North Macedonia, Bulgaria, Northern Caucasus, and Turkey.
Remarks
The late Miocene anancines from Europe are well distinguished from the roughly contemporaneous, but more derived, A. kenyensis, and the Plio–Pleistocene A. arvernensis, as well as from other anancines, in the morphology of the mandible (longer symphysis, condyle only slightly higher than the coronoid process), the cranium (straight and parallel premaxillary tusk alveoli, more anterior location of the orbit), the upper tusks (downturned, running almost parallel), and the primitive molar features on the cheek teeth (weak anancoidy, simple occlusal morphology, thick and unfolded to coarsely folded enamel on the molars) (Konidaris and Roussiakis 2019). The combination of the above traits is unique among anancines and permits the distinction at the species level, the proper name of which is Anancus lehmanni [see discussions in Markov (2008b) and Konidaris and Roussiakis (2019)]. Previously known only from the Pliocene and the Pleistocene of Greece, Anancus was recently reported for the first time from the late Miocene of Greece in Chomateri (Fig. 12a), marking its earliest occurrence in the Greek fossil record (Konidaris 2013; Konidaris and Koufos 2013a, b; Konidaris and Roussiakis 2017, 2019). The appearance of Anancus in Europe at the second half of the Turolian (~7.2 Ma) coincides with a faunal turnover in both the eastern and western sector of the European Mediterranean region and, in the Southern Balkans in particular, with the decline of the “Pikermian” large mammal fauna (Kostopoulos 2009; Böhme et al. 2017).
(a), Right maxilla fragment with DP2–DP4 (AMPG-13Π/1972) of Anancus lehmanni from the late Miocene (Turolian) of Chomateri (copyright G. Konidaris); (b–c), mandible with the m3s (AMPG-1918) of Anancus arvernensis from the late Pliocene of Sesklon in b dorsal view and c right lateral view (copyright A. Athanassiou). Scale bars equal 5 cm
-
Anancus arvernensis ( Croizet and Jobert, 1828 )
Nomenclatural and Taxonomical History
Croizet and Jobert (1828) erected Mastodon arvernensis based on juvenile dental specimens from Perrier in Puy-de- Dôme (Auvergne-Rhône-Alpes, France). In 1855 Aymard coined the genus Anancus and the species A. macroplus, which was slightly later synonymized with Mastodon arvernensis (Lartet 1859), although the new combination was not utilized. Osborn (1936) recognized the binomen Anancus arvernensis. Meanwhile, Schlesinger (1917, 1922) attributed material from Austria and Hungary to Mastodon (Dibunodon) arvernense , a subgenus which is though a junior synonym of Anancus (Matsumoto 1927; Gaziry 1976).
Type Material
MNHN-A.C. 1830 (lectotype), right maxilla fragment with DP2–DP3 (Croizet and Jobert 1828: pl. 2, fig. 7).
Type Locality
Perrier-les-Étouaires, France, late Pliocene, late Ruscinian, MN16.
Distribution
Several Pliocene–Early Pleistocene localities of Greece, (e.g., Milia, Gephyra, Sesklon, Vatera, Apolakkia, Nigrita) and in the Pliocene–Early Pleistocene (Ruscinian–middle Villafranchian, MN14–MNQ17) of Europe.
Remarks
This species represents the Pliocene–Pleistocene representative of the European anancines and the last gomphothere to have survived in Europe. The richest material originates from Dorkovo (early Pliocene; Bulgaria), but important material is known also from Chilhac (France) and Valdarno (Italy) (Weithofer 1890; Boeuf 1992; Metz-Muller 2000; Rook et al. 2013). The research of Metz-Muller (2000) proved the high intraspecific variability (including the complexity of the cheek teeth and the occasional occurrence of pentalophodont m2) and included the biometrical study of the mandibles resulting in the definition of dental ages; moreover, the study showed that A. arvernensis presents a tendency towards shortening of the molars, increase of the hypsodonty, and simplification of the crown.
In Greece, although several A. arvernensis specimens are known, most of them are isolated dental findings with uncertain stratigraphic position. However, important and stratified material is known from Sesklon (including a partial cranium, a mandible and an upper tusk, Fig. 12b–c; Symeonidis and Tataris 1983; Athsanassiou 2016, 2018), Nigrita (a partial cranium with the upper tusks and the molars; Athanassiou 2017), Apolakkia (a partial cranium with the upper tusks and the molars; Theodorou et al. 2000), Milia (mandible and teeth; Tsoukala and Mol 2016), Gephyra (mandibles and postcranials; Crégut-Bonnoure and Tsoukala 2017), Kalliphytos (mandible; Athanassiou 2016), and Vatera (teeth; de Vos et al. 2002).
Type Species
Mastodon elephantoides Clift, 1828.
Other Included Species
See Saegusa et al. (2005) and Aiba et al. (2010) for a complete list.
Distribution
Late Miocene–Late Pleistocene of Asia, Africa, and Europe.
Comments
The genus Stegodon includes the stegodontids, which are characterized and differentiated from the more archaic Stegolophodon by their intermediate molars with five or more loph(id)s, third molars with no distinct central conule, absence of lower tusks, and two lophids above the mesial root of the lower third molar (Saegusa et al. 2005).
-
Stegodon sp.
Remarks
A fragment of a third molar of Stegodon sp. (Fig. 13) from an unknown locality (and therefore no information about geological age), but possibly from the wider area of Siatista (Kozani), constitutes the first evidence of the presence of stegodontids in Europe, extending their previously known geographical distribution (Mol et al. 2010).
6 Concluding Remarks
Proboscideans are common in the Neogene faunas of Greece with relatively abundant material, and they are present in most of the Neogene fossiliferous sites, both in today’s continental and insular Greece. They have been the largest terrestrial mammals in the Neogene faunas of the country, dominating the ecosystems of that time. Recent investigations on the Miocene and Pliocene proboscideans, including the revision and study of old collections, and the study of new specimens from excavations carried out in Greece during the last years, have resulted in the update of their taxonomy and biostratigraphy. Based on our current knowledge, we can reach several conclusions.
During the early Miocene, the only proboscidean that is known in Greece is the deinothere Prodeinotherium cuvieri , documented at Gavathas (Lesvos Island), where the find-bearing locality is dated as older than 18.4 Ma (late MN3). The Gavathas deinothere marks the first appearance of deinotheres in Europe and documents their penetration into the continent as part of the complex “Proboscidean Datum Event,” after the establishment of the so-called Gomphotherium landbridge in the middle Burdigalian (~19.0–18.0 Ma, early MN3).
In the middle Miocene, the deinothere Prodeinotherium bavaricum and the trilophodont choerolophodontid Choerolophodon chioticus are recognized in Thymiana (Chios Island), dated to ~15.5 Ma (ΜΝ5). Choerolophodon chioticus is the most primitive choerolophodontid in Europe and marks their first penetration into the continent, being part of the third European phase of the “Proboscidean Datum Event” (Tassy 1990). This phase is probably part of the first middle Miocene migrational event that took place in the late Orleanian (17.0–15.0 Ma, MN5), involving also the arrival in Europe of the amebelodontid Protanancus (Rögl 1999; Koufos et al. 2005; Markov and Vergiev 2010).
During the late Miocene, proboscideans are more diverse and well documented in the Greek fossil record due to a high number of fossiliferous localities; however, Choerolophodon is in most of the localities the dominant proboscidean in terms of abundance. In the early Vallesian (MN9), Choerolophodon anatolicus is identified in Pentalophos-1 (Axios valley). During the late Vallesian (MN 10), the deinothere Deinotherium giganteum and the primitive morph of Choerolophodon pentelici co-existed in Ravin de la Pluie (Axios valley). In the early Turolian (MN11) appeared the zygodont Mammut sp. (M. obliquelophus?), which co-existed with the advanced morph of C. pentelici in Ravin des Zouaves-5 (Axios valley). In the middle Turolian C. pentelici, the tetralophodont amebelodontid Konobelodon atticus, Mammut sp. (M. obliquelophus?), and the huge-sized deinothere D. proavum are recorded, the co-occurrence of which is documented in Pikermi. These four species are recognized also in the Mytilini Formation of Samos Island. During the middle Turolian, but postdating the classical Pikermi, appeared the tetralophodont gomphothere Anancus lehmanni , the most primitive anancine of Europe. In the late Turolian (MN13) are traced the last occurrences of C. pentelici, present with certainty in the Dytiko localities (Axios valley) and perhaps in Maramena.
During the Pliocene, Anancus arvernensis and Mammut borsoni are recognized. The fossiliferous localities of this period are few and the lack of stratigraphic information for several findings makes the determination of their first appearances problematic. However, M. borsoni survived until the end of the Pliocene (MN16) and A. arvernensis until the beginning of the Early Pleistocene (MNQ17). The co-occurrence of these species is documented in Milia (early late Pliocene, MN16a). Finally, the first recorded occurrence of Stegodon in Greece represents also the first evidence of the presence of stegodontids in Europe.
References
Aiba H, Baba K, Matsukawa M (2010) A new species of Stegodon (Mammalia, Proboscidea) from the Kazusa Group (lower Pleistocene), Hachioji City, Tokyo, Japan and its evolutionary morphodynamics. Palaeontology 53:471–490
Aiglstorfer M, Göhlich UB, Böhme M, Gross M (2014) A partial skeleton of Deinotherium (Proboscidea, Mammalia) from the late Middle Miocene Gratkorn locality (Austria). Palaeobiodivers Palaeoenviron 94:49–70
Andrews CW (1911) On a new species of Dinotherium (Dinotherium hobleyi) from British East Africa. Proc Zool Soc London 81:943–945
Antoine PO, Welcomme JL, Marivaux L, Baloch I, Benammi M, Tassy P (2003) First record of Paleogene Elephantoidea (Mammalia, Proboscidea) from the Bugti Hills of Pakistan. J Vertebr Paleontol 23:977–980
Arambourg C (1934) Le Dinotherium des gisements de l’Omo. C R Soc Géol Fr 1934:86–87
Arambourg C (1945) Anancus osiris, un mastodonte nouveau du Pliocène inférieur d’Egypte. Bull Soc Géol Fr 15:479–495
Arambourg C, Piveteau J (1929) Les Vertébrés du Pontien de Salonique. Ann Paléontol 18:59–138
Athanassiou A (2004) On a Deinotherium (Proboscidea) finding in the Neogene of Crete. Carnets Géol 2004(05):1–7
Athanassiou A (2016) Craniomandibular remains of Anancus arvernensis (Proboscidea, Mammalia) from Greece: The samples from Kallíphytos (E. Macedonia) and Sésklo (Thessaly). Quat Int 406:25–34
Athanassiou A (2017) A cranial specimen of Anancus arvernensis from Nigríta, Northern Greece. 7th International Conference on Mammoths and their Relatives, Taichung
Athanassiou A (2018) A Villafranchian Hipparion-bearing mammal fauna from Sésklo (E. Thessaly, Greece): implications for the question of Hipparion–Equus sympatry in Europe. Quaternary 1:1–24
Athanassiou A (this volume) The fossil record of non-endemic elephants and mammoths (Mammalia: Proboscidea: Elephantidae) in Greece. In: Vlachos E (ed) The fossil vertebrates of Greece vol. 1 – basal vertebrates, amphibians, reptiles, afrotherians, glires, and primates. Springer, Cham
Barbour EH (1927) Preliminary notice of a new proboscidean Amebelodon fricki, gen. et sp. nov. Bull Nebr State Museum 13:131–134
Barbour EH (1929) Torynobelodon loomisi, gen. et sp. nov. Bull Nebr State Museum 16:147–153
Barbour EH, Hibbard CW (1941) A shovel-tusked mastodon, Amebelodon fricki, from Kansas. Bull Univ Nebr State Museum 2:37–46
Bergounioux FM, Crouzel F (1960) Tetralophodon curvirostris n. sp. (Mamm., Proboscidea) aus dem Unterpliozän (Pontien) von Esselborn (Rheinhessen). Jahresber Mitt Oberrhein Geol Ver 42:109–121
Bergounioux FM, Crouzel F (1962) Les Déinothéridés d’Europe. Ann Paléontol 48:1–56
Besenecker H, Symeonidis NK (1974) Der erste Säugetierfund aus dem Neogen der griechischen Insel Psara (Ostägäis). Ann Géol Pays Hellén 26:109–117
Blumenbach JF (1799) Handbuch der Naturgeschichte, 6th edn. Dieterich, Göttingen
Boeuf O (1992) Anancus arvernensis chilhiacensis nov. subsp. (Proboscidea, Mammalia), un Mastodonte du Plio-Pléistocène de Haute-Loire, France. Geobios 14:179–188
Böhme M, Aiglstorfer M, Uhl D, Kullmer O (2012) The antiquity of the Rhine River: stratigraphic coverage of the Dinotheriensande (Eppelsheim Formation) of the Mainz Basin (Germany). PLoS One 7:e36817
Böhme M, Spassov N, Ebner M, Geraads D, Hristova L, Kirscher U, Kötter S, Linnemann U, Prieto J, Roussiakis S, Theodorou G, Uhlig G, Winklhofer M (2017) Messinian age and savannah environment of the possible hominin Graecopithecus from Europe. PLoS One 12:e0177347
Bonaparte CL (1845) Catalogo Metodico dei Mammiferi Europei. Luigi di Giacomo Pirola, Milan
Borson E (1823) Note sur des dents du grand mastodonte trouvées en Piémont et sur des machoires et dents fossiles prises dans la mine de houille de Cadibona proche Savone. Mem Reale Acad Sci Torino 27:31–42
Brunn JH (1956) Contribution à l’étude géologique du Pinde septentrional et d’une partie de la Macédoine occidentale. Ann Géol Pays Hellén 7:1–358
Cautley PT (1836) Note on the teeth of the mastodon à dents etroites of the Siwálik Hills. J Asiat Soc Bengal 5:294–296
Charrier G, Giglio A (1969) Primi risultati di una campagna di rilevamento geologico nell’isola di Coo (Sporadi meridionali – Mare Egeo). Boll Assoc Min Subalp 6:482–516
Chow MC, Chang YP (1974) Chinese fossil elephantoids (in Chinese). Science Press, Beijing
Clift W (1828) On the fossil remains of two new species of Mastodon, and of other vertebrated animals, found on the left bank of the Irawadi. Trans Geol Soc Lond 2:369–375
Codrea V (1994) A priority issue: Deinotherium proavum Eichwald or Deinotherium gigantissimum Ştefănescu? In: Nicorici E (ed) The Miocene from the Transylvanian Basin-Romania, Cluj-Napoca, pp 105–110
Cooper LN, Seiffert ER, Clementz M, Madar SI, Bajpai S, Hussain ST, Thewissen JGM (2014) Anthracobunids from the Middle Eocene of India and Pakistan are stem perissodactyls. PLoS One 9:e109232
Coppens Y (1965) Les proboscidiens du Tchad. In: Actes du Ve Congrés Panafricain de Préhistoire et de l’Étude du Quaternaire, Santa Cruz de Tenerife, pp 331–387
Crégut-Bonnoure E, Tsoukala E (2017) The Pliocene Artiodactyla and Proboscidea (Mammalia) from Gephyra (lower Axios valley, Macedonia, Greece). Discovery of a new boselaphine. Quat Int 445:200–214
Croizet JB, Jobert ACG (1828) Recherches sur les ossemens fossiles du département du Puy-de-Dôme. Principaux Libraries, Paris
de Bonis L, Brunet M, Heintz E, Sen S (1992) La province greco-irano-afghane et la réparition des faunes mammaliennes au Miocène supérieur. Paleontol i Evol 24–25:103–112
de Vos J, van der Made J, Athanassiou A, Lyras G, Sondaar PY, Dermitzakis MD (2002) Preliminary note on the Late Pliocene fauna from Vatera (Lesvos, Greece). Ann Géol Pays Hellén 39:37–70
Dermitzakis MD, Symeonidis NK, De Boer LEM, Sondaar PY (1982) The evolution of the elephants (in Greek). Edit Lab Geol Palaeont, Univ Athens
Desio A (1931) Le isole Italiane dell’Egeo. Mem Descrittive Carta Geol Ital 24:1–534
Dietrich WO (1916) Über die Hand und den Fuss von Dinotherium. Zeitschr D Geol Ges 68:44–53
Dooley ACJ, Scott E, Green J, Springer KB, Dooley BS, Smith GJ (2019) Mammut pacificus sp. nov., a newly recognized species of mastodon from the Pleistocene of western North America. Peer J 7:e6614
Dorlhac MJ (1855) Notice géologique sur le cratère de Coupet et sur son gisement de gemmes et d’ossements fossiles. Ann Soc Agric Sci Arts Commer Puy 19:497–517
Éhik J (1930) Prodinotherium hungaricum n. g., sp., with an appendix by Szalay, T.: on the geological occurrence of Prodinotheirum hungaricum ÉHIK. Geol Hung, Ser Palaeontol 6:1–24
Eichwald E (1831) Zoologia specialis quam expositis animalibus tum vivis, tum fossilibus potissimum Rossiae in universum, et Poloniae in specie. Josephi Zawadzki, Vilnae
Eichwald E (1835) De pecorum et pachydermorum reliquiis fossilibus in Lithuania, Volhynia et Podolia repertis. Nova Acta Acad Caesareae Leopoldino-Carolinae Ger Nat Curiosorum 17:677–780
Falconer H (1845) Description of some fossil remains of Dinotherium, Giraffe, and other Mammalia from the gulf of Cambay, western Coast of India. Q J Geol Soc Lond 1:356–372
Falconer H (1857) On the species of mastodon and elephant occurring in the fossil state in Great Britain. Part I. Mastodon. Q J Geol Soc Lond 13:307–360
Falconer H (1868) Description of some fossil remains of Dinotherium, Giraffe, and other Mammalia, from Perim Island, Gulf of Cambay, western coast of India. In: Murchison C (ed) Palaeontological memoirs and notes of the late Hugh Falconer, A.M., M.D, London, pp 391–411
Falconer H, Cautley PT (1847) Fauna antiqua sivalensis, being the fossil zoology of the Sewalik Hills, in the North of India. Smith, Elder and Co., London
Fassoulas C, Iliopoulos G (2011) The excavations of Deinotherium giganteum from Siteia: life and environment in Crete during the Miocene. In: Proceedings of the 10th international cretological congress, Chania, pp 11–25
Ferretti MP, Rook L, Torre D (2003) Stegotetrabelodon (Proboscidea, Elephantidae) from the late Miocene of southern Italy. J Vertebr Paleontol 23:659–666
Forsyth Major CJ (1887) Faune mammalogische delle isole di Cos et di Samos. Atti Soc Tosc Sci Nat 5:272–275
Forsyth Major CJ (1888) Sur un gisement d’ossements fossiles dans l’île de Samos, contemporains de l’âge de Pikermi. C R Hebd Séances Acad Sci 107:1178–1181
Forsyth Major CJ (1894) Le gisement ossifère de Mytilinii et catalogue d’ossements fossiles recueillis à Mitylini, île de Samos, et déposés au Collège Galliard à Lausanne. Georges Bridel and Cie, Lausanne
Freyberg BV (1951) Die Pikermifauna von Tour la Reine. Ann Géol Pays Hellén 3:7–10
Gaudry A (1862) Animaux fossiles et géologie de l’Attique. Savy, Paris
Gaudry A (1867) Animaux fossiles et géologie de l’Attique. Atlas, Savy, Paris
Gaudry A, Lartet E (1856) Résultats des recherches paléontologiques entreprises dans l’Attique sous les auspices de l’Académie. C R Acad Sci Paris 43:271–274
Gaziry AW (1976) Jungtertiäre Mastodonten aus Anatolien (Türkei). Geol Jb 22:3–143
Gaziry AW (1987a) New mammals from the Jabal Zaltan site, Libya. Senckenb Lethaea 68:69–89
Gaziry AW (1987b) Remains of Proboscidea from the early Pliocene of Sahabi, Libya. In: Boaz NT, El-Arnauti A, Gaziry AW, Heinzelin JD, Boaz DD (eds) Neogene paleontology and geology of Sahabi. A. R. Liss, New York, pp 183–203
Gaziry AW (1997) Die Mastodonten (Proboscidea, Mammalia) aus Dorn-Dürkheim 1 (Rheinhessen). Cour Forsch-Inst Senck 197:73–115
Georgalas CG (1941) Über das Vorkommen von Anancus (Mastodon) arvernensis Croiz. und Job. in der Umgebung von Skoura (SÖ von Sparta). Prakt Akad Ath 16:94–100
Gheerbrant E (2009) Paleocene emergence of elephant relatives and the rapid radiation of African ungulates. PNAS 106:10717–10721
Gheerbrant E, Tassy P (2009) L’origine et l’évolution des éléphants. C R Palevol 8:281–294
Gheerbrant E, Sudre J, Cappetta H (1996) A Palaeocene proboscidean from Morocco. Nature 383:68–70
Gheerbrant E, Sudre J, Cappetta H, Iarochéne M, Amaghzaz M, Bouya B (2002) A new large mammal from the Ypresian of Morocco: evidence of surprising diversity of early proboscideans. Acta Palaeontol Pol 47:493–506
Gheerbrant E, Sudre J, Tassy P, Amaghzaz M, Bouya B, Iarochène M (2005) Nouvelles données sur Phosphatherium escuilliei (Mammalia, Proboscidea) de l’Éocène inférieur du Maroc, apports à la phylogénie des Proboscidea et des ongulés lophodontes. Geodiversitas 27:239–333
Gheerbrant E, Schmitt A, Kocsis L (2018) Early African fossils elucidate the origin of embrithopod mammals. Curr Biol 28:2167–2173
Gidarakos D (1938) Trouvailles paléontologiques des mammifères en Béotie. Prakt Akad Ath 13:418–422
Ginsburg L, Chevrier F (2001) Les Dinothères du bassin de la Loire et l’évolution du genre Deinotherium en France. Symbioses 5:9–24
Gräf IE (1957) Die Prinzipien der Artbestimmung bei Dinotherium. Palaeontographica 108:131–185
Gregory JT (1945) An Amebelodon jaw from the Texas Panhandle. Univ Texas Publ 4401:477–484
Harris JM (1973) Prodeinotherium from Gebel Zelten, Libya. Bull Brit Mus (Nat Hist) (Geol) 23:285–348
Hautier L, Mackaye HT, Lihoreau F, Tassy P, Vignaud P, Brunet M (2009) New material of Anancus kenyensis (Proboscidea, Mammalia) from Toros-Menalla (Late Miocene, Chad): contribution to the systematics of African anancines. J Afr Earth Sci 53:171–176
Hay OP (1922) Further observations on some extinct elephants. Proc Biol Soc Wash 35:97–101
Hays I (1834) Descriptions of the specimens of inferior maxillary bones of mastodons in the cabinet of the American Philosophical Society, with remarks on the genus Tetracaulodon (Godman), etc. Trans Am Philos Soc 4:317–339
Hopwood AT (1935) Fossil Proboscidea from China. Palæont Sin 9:1–108
Huttunen K (2002) Systematics and taxonomy of the European Deinotheriidae (Proboscidea, Mammalia). Ann Naturhist Mus Wien 103(A):237–250
Huttunen K (2004) On a Prodeinotherium bavaricum (Proboscidea, Mammalia) skeleton from Franzensbad, Czech Republic. Ann Nat Mus Wien 105(A):333–361
Huttunen K, Göhlich UB (2002) A partial skeleton of Prodeinotherium bavaricum (Proboscidea, Mammalia) from the Middle Miocene of Unterzolling (Upper Freshwater Molasse, Germany). Geobios 35:489–514
Iliopoulos G, Fassoulas C, Tzortzi M (2014) An almost complete skeleton of a large Deinotherium (Proboscidea, Mammalia) from the Late Miocene of Aghia Photia, Siteia (Crete Island, Greece). In: Kostopoulos DS, Vlachos E, Tsoukala E (eds) 6th International Conference on Mammoths and their Relatives, Grevena-Siatista. Sci Annals, School Geol, Aristotle Univ Thessaloniki, Greece, sp vol 102: 72–73
Illiger C (1811) Prodromus systematis mammalium et avium additis terminis zoographicis utriusque classis. C. Salfield, Berlin
Jourdan M (1861) Des terrains sidérolitiques. C R Hebd Séances Acad Sci 53:1009–1014
Kaup JJ (1829) Deinotherium giganteum. Eine Gattung der Vorwelt aus der Ordnung der Pachydermen. Isis 22:401–404
Kaup JJ (1832a) Description d’ossements fossiles de mammifères inconnus jusqu’à présent, qui se trouvent au Muséum grand-ducal de Darmstadt. J.G. Heyer, Darmstadt
Kaup J (1832b) Ueber zwei Fragmente eines Unterkiefers von Mastodon angustidens Cuv., nach welchen diese Art in die Gattung Tetracaulodon Godmann gehört. Isis 25:628–631
Kaup JJ (1835) Description d’ossements fossiles de mammifères inconnus jusqu’à-présent qui se trouvent au Muséum grand-ducal de Darmstadt. J.P. Diehl, Darmstadt
Kerr R (1792) The animal kingdom, or zoological system, of the celebrated Sir Charles Linnaeus, Edinburgh
Klähn H (1922) Die badischen Mastodonten und ihre süddeutschen Verwandten. Borntraeger, Berlin
Konidaris GE (2013) Palaeontological and biostratigraphical study of the Neogene Proboscidea from Greece. Ph.D. thesis, Sci Annals, School Geol, Aristotle Univ Thessaloniki, Greece 153, 1–326
Konidaris GE, Koufos GD (2009) The late Miocene mammal faunas of the Mytilinii Basin, Samos Island, Greece: new collection. 8. Proboscidea. In: Koufos GD, Nagel D (eds) The late Miocene Mammal Faunas of Samos, vol 31. Beit Paläontol Wien, pp 139–155
Konidaris GE, Koufos GD (2013a) Late Miocene Proboscidea (Mammalia) from Macedonia and Samos Island, Greece: preliminary results. Paläont Z 87:121–140
Konidaris GE, Koufos GD (2013b) The late Miocene proboscideans of Greece: taxonomy, biochronology, palaeoecology. 14th Congress of the Regional Committee on Mediterranean Stratigraphy (RCMNS), Istanbul
Konidaris GE, Koufos GD (2016) Proboscidea. In: Koufos GD, Kostopoulos DS (eds) Paleontology of the upper Miocene vertebrate localities of Nikiti (Chalkidiki Peninsula, Macedonia, Greece). Geobios 49:37–44
Konidaris GE, Koufos GD (2019) Late Miocene proboscideans from Samos Island (Greece) revisited: new specimens from old collections. Paläont Z 93:115–134
Konidaris GE, Roussiakis SJ (2017) Anancus (Proboscidea, Mammalia) from Chomateri – the first record of the genus in the late Miocene of Greece. 15th Congress of the Regional Committee on Mediterranean Neogene Stratigraphy (RCMNS), Athens
Konidaris GE, Roussiakis SJ (2019) The first record of Anancus (Mammalia, Proboscidea) in the late Miocene of Greece and reappraisal of the primitive anancines from Europe. J Vertebr Palaeontol:e1534118
Konidaris GE, Tsoukala E (2020) Proboscideans from the upper Miocene localities of Thermopigi, Neokaisareia and Platania (Northern Greece). Ann Paléontol 106:102380
Konidaris GE, Roussiakis SJ, Theodorou GE, Koufos GD (2014) The Eurasian occurrence of the shovel-tusker Konobelodon (Mammalia, Proboscidea) as illuminated by its presence in the late Miocene of Pikermi (Greece). J Vertebr Paleontol 34:1437–1453
Konidaris GE, Koufos GD, Kostopoulos DS, Merceron G (2016) Taxonomy, biostratigraphy and palaeoecology of Choerolophodon (Proboscidea, Mammalia) in the Miocene of SE Europe-SW Asia: implications for phylogeny and biogeography. J Syst Palaeontol 14:1–27
Konidaris GE, Roussiakis SJ, Athanassiou A, Theodorou GE (2017) The huge-sized deinothere Deinotherium proavum (Proboscidea, Mammalia) from the Late Miocene localities Pikermi and Halmyropotamos (Greece). Quat Int 430:5–21
Kostopoulos DS (2009) The Pikermian Event: temporal and spatial resolution of the Turolian large mammal fauna in SE Europe. Palaeogeogr Palaeoclimatol Palaeoecol 274:82–95
Koufos GD (1977) New finds of mastodonts from Macedonia (Greece). Sci Ann Fac Phys Mathem Univ Thessaloniki 17:97–115
Koufos GD (1980) Palaeontological and stratigraphical study of the continental Neogene deposits of Axios Basin. Ph.D. thesis, Sci Annals, Fac Phys & Mathem, Univ Thessaloniki, Greece 19:1–322
Koufos GD (2006) The Neogene mammal localities of Greece: Faunas, chronology and biostratigraphy. Hellenic J Geosci 41:183–214
Koufos GD (2013) Neogene mammal biostratigraphy and chronology of Greece. In: Wang X, Flynn LJ, Fortelius M (eds) Fossil mammals of Asia. Neogene biostratigraphy and chronology. Columbia University Press, New York, pp 596–621
Koufos GD, Kostopoulos DS (eds) (2016) Palaeontology of the upper Miocene vertebrate localities of Nikiti (Chalkidiki Peninsula, Macedonia, Greece). Geobios 49(1–2):1–154
Koufos GD, Nagel D (eds) (2009) The Late Miocene mammal faunas of Samos, vol 31. Beit Paläontol Wien
Koufos GD, Syrides GE (1997) A new Early/Middle Miocene mammal locality from Macedonia, Greece. C R Acad Sci Paris 325:511–516
Koufos GD, Zouros N, Mourouzidou O (2003) Prodeinotherium bavaricum (Proboscidea, Mammalia) from Lesvos island, Greece; the appearance of deinotheres in the eastern Mediterranean. Geobios 36:305–315
Koufos GD, Kostopoulos DS, Vlachou TD (2005) Neogene/Quaternary mammalian migrations in Eastern Mediterranean. Belgian J Zool 135:181–190
Kovachev D, Nikolov I (2006) Deinotherium thraceiensis sp. nov. from the Miocene near Ezerovo, Plovdiv District. Geol Balc 35:5–40
Kubiak H (1972) The skull of Mammut praetypicum (Proboscidea, Mammalia) from the collection of the Jagiellonian University in Cracow. Poland Acta Zool Cracoviensia 17:305–324
Lambert WD (1990) Rediagnosis of the genus Amebelodon (Mammalia, Proboscidea, Gomphotheriidae), with a new subgenus and species, Amebelodon (Konobelodon) britti. J Paleontol 64:1032–1040
Lambert WD, Shoshani J (1998) Proboscidea. In: Janis CM, Scott KM, Jacobs LL (eds) Evolution of tertiary mammals of North America. Cambridge University Press, Cambridge, pp 606–621
Lartet E (1859) Sur la dentition des proboscidiens fossiles (Dinotherium, Mastodontes et Éléphants) et sur la distribution géographique et stratigraphique de leurs débris en Europe. Bull Soc Géol Fr 16:469–515
Lazaridis G (2015) Study of the Late Miocene vertebrate locality of Kryopigi and other localities of Kassandra Peninsula, Chalkidiki (Greece). Systematics, taphonomy, paleoecology, biochronology. Ph.D. thesis, Sci Annals, School Geol, Aristotle Univ Thessaloniki, Greece 174, 1–355
Lazaridis G, Tsoukala E (2014a) Choerolophodon pentelici (Gaudry and Lartet, 1856) from the Turolian locality of Kryopigi (Kassandra, Chalkidiki, Greece). In: Kostopoulos DS, Vlachos E, Tsoukala E (eds) 6th international conference on mammoths and their relatives, Grevena-Siatista. Sci Annals, School Geol, Aristotle Univ Thessaloniki, Greece, sp vol 102:100
Lazaridis G, Tsoukala E (2014b) Tetralophodon longirostris (Kaup, 1832) from Late Miocene of the Kassandra peninsula (Chalkidiki, Greece). In: Kostopoulos DS, Vlachos E, Tsoukala E (eds) 6th international conference on mammoths and their relatives, Grevena-Siatista. Sci Annals, School Geol, Aristotle Univ Thessaloniki, Greece, sp vol 102:101
Lazaridis G, Kostopoulos DS, Lyras G, Roussiakis S (2017) A new Late Miocene ovibovine-like bovid (Bovidae, Mammalia) from the Kassandra Peninsula (Chalkidiki, Northern Greece) and implications to the phylogeography of the group. Paläontol Z 91:427–437
Lehmann U (1950) Über Mastodontenreste in der Bayerischen Staatssammlung in München. Palaeontogr Abt A 99:121–228
Lydekker R (1884) Additional Siwalik Perissodactyla and Proboscidea Memoirs of the Geological Survey of India. Palaeontol Indica 10:1–34
MacInnes DG (1942) Miocene and Post-Miocene Proboscidea from East Africa. Trans Zool Soc Lond 25:33–106
Maglio VJ (1974) A new proboscidean from the late Miocene of Kenya. Palaeontology 17:699–705
Markov GN (2008a) Fossil proboscideans (Mammalia) from the vicinities of Varna: a rare indication of middle Miocene vertebrate fauna in Bulgaria. Hist Nat Bulg 19:137–152
Markov GN (2008b) The Turolian proboscideans (Mammalia) of Europe: preliminary observations. Hist Nat Bulg 19:153–178
Markov GN, Vergiev S (2010) First report of cf. Protanancus (Mammalia, Proboscidea, Amebelodontidae) from Europe. Geodiversitas 32:493–500
Matsumoto H (1927) On two new mastodonts and an archetypal stegodont of Japan. Sci Rep Tohoku Imperial Univ 10:1–11
Mayet L (1908) Étude des mammifères miocènes des Sables de l’Orléanais et des Faluns de la Touraine. Ann Univ Lyon 24:1–336
Mazo AV, Montoya P (2003) Proboscidea (Mammalia) from the Upper Miocene of Crevillente (Alicante, Spain). Scr Geol 126:79–109
Mebrate A (1987) The long-jawed gomphotheres. Ph.D. thesis, University of Kansas
Melentis JK (1967) Die Pikermi-Fauna von Halmyropotamos (Euböa, Griechenland), 1. Teil: Odontologie und Kraniologie. Ann Géol Pays Hellén 19:283–411
Melentis JK (1969) Die Pikermi-Fauna von Halmyropotamos (Euböa, Griechenland), 2. Teil: Osteologie. Ann Géol Pays Hellén 28:217–306
Metz-Muller F (2000) La population d’Anancus arvernensis (Proboscidea, Mammalia) du Pliocène de Dorkovo (Bulgarie); étude des modalités évolutives d’Anancus arvernensis et phylogénie du genre Anancus. Ph.D. thesis, Muséum National d’Histoire Naturelle
Mitzopoulos MK (1967) Zygolophodon borsoni und Anancus (Bunolophodon) arvernensis aus dem Oberpliozän von Griechenland. Ann Géol Pays Hellén 18:436–446
Mol D, Tsoukala E, Batsi A (2010) A Stegodon from the area of Siatista (Kozani, Macedonia, Greece), the first evidence of the presence of Stegodon in Europe. In: 5th International conference on mammoths and their relatives Le Puy-en-Velay. Quaternaire, sp vol 102:85–87
Mothé D, Avilla LS, Asevedo L, Borges-Silva L, Rosas M, Labarca-Encina R, Souberlich R, Soibelzon E, Roman-Carrion JL, Ríos SD, Rincon AD, de Oliveira GC, Lopes RP (2017) Sixty years after ‘The mastodonts of Brazil’: The state of the art of South American proboscideans (Proboscidea, Gomphotheriidae). Quat Int 443:52–64
Mucha BB (1980) A new species of yoke-toothed mastodont from the Pliocene of Southwest USSR. In: Negadaev-Nikonov KN (ed) Quaternary and Neogene faunas and floras of Moldavskaya SSR. Shtiintsa, Kishinev, pp 19–26
Osborn HF (1918) A long-jawed mastodon skeleton from South Dakota and phylogeny of the Proboscidea. Bull Geol Soc Am Bull 29:133–137
Osborn HF (1921) First appearance of the true mastodon in America. Am Mus Novit 10:1–6
Osborn HF (1926) Additional new genera and species of the mastodontoid Proboscidea. Am Mus Novit 238:1–16
Osborn HF (1936) Proboscidea. A monograph of the discovery, evolution, migration and extinction of the mastodonts and elephants of the world, Volume I: Moeritherioidea, Deinotherioidea, Mastodontoidea. American Museum Press, New York
Ozansoy F (1965) Étude des gisements continentaux et des mammifères du Cénozoïque de Turquie. Mem Soc Géol Fr 102:1–92
Paraskevaidis I (1940) Eine obermiozäne Fauna von Chios. N Jb Min Geol Paläont 83:363–442
Paraskevaidis I (1977) Säugetierreste aus Griechenland. Proceedings of the 6th Colloquium on the Geology of the Aegean Region 3:1143–1154
Pickford M (2001) Afrochoerodon nov. gen. kisumuensis (MacInnes) (Proboscidea, Mammalia) from Cheparawa, Middle Miocene, Kenya. Ann Paléontol 87:99–113
Pickford M, Pourabrishami Z (2013) Deciphering Dinotheriensande deinotheriid diversity. Palaeobiodivers Palaeoenviron 93:121–150
Pilgrim GE (1913) The correlation of the Siwaliks with mammal horizons of Europe. Rec Geol Surv India 43:264–326
Pohlig H (1988) Dentition und Kraniologie des Elephas antiquus Falc. mit Beiträgen über Elephas primigenius Blum. und Elephas meridionalis Nesti. Nova Acta Ksl Leop-Carol Deutsch Akad Naturf 53:1–280
Poulakakis N, Lymberakis P, Fassoulas C (2005) Deinotherium giganteum (Proboscidea, Deinotheriidae) from the late Miocene of Crete. J Vertebr Paleontol 25:732–736
Qiu ZX, Wang BJ, Li H, Deng T, Sun Y (2007) First discovery of deinothere in China. Vertebr Palas 45:261–277
Rögl F (1999) Circum-Mediterranean Miocene paleogeography. In: Rössner GE, Heissig K (eds) The Miocene land mammals of Europe. Verlag Dr. Friedrich Pfeil, München, pp 39–48
Rook L, Croitor R, Delfino M, Ferretti MP, Gallai G, Pavia M (2013) The Upper Valdarno Plio-Pleistocene vertebrate record: an historical overview, with notes on palaeobiology and stratigraphic significance of some important taxa. Ital J Geosci 132:104–125
Roussiakis S, Athanassiou A, Michailidis D, Mitsopoulou V, Solomos C, Theodorou G (2014) Remarks on new proboscidean remains from the classical Late Miocene locality of Pikermi and their associated fauna. In: Kostopoulos DS, Vlachos E, Tsoukala E (eds) 6th International Conference on Mammoths and their Relatives. Grevena-Siatista. Sci Annals, School Geol, Aristotle Univ Thessaloniki, Greece, sp vol 102:171–172
Saegusa H, Thasod Y, Ratanasthien B (2005) Notes on Asian stegodontids. Quat Int 126–128:31–48
Sakellariou-Mane H (1972) Presence d’Anancus arvernensis dans les couches supérieures du Pliocène de la Valée du Vardar. Folia Biochim Biol Graeca 9:31–36
Sanders WJ (2003) Proboscidea. In: Fortelius M, Kappelman J, Sen S, Bernor RL (eds) Geology and paleontology of the Miocene Sinap formation. Turkey. Columbia University Press, New York, pp 202–219
Sanders WJ (2007) Taxonomic review of fossil Proboscidea (Mammalia) from Langebaanweg, South Africa. Trans Royal Soc S Afr 62:1–16
Sanders WJ (2008) Review of fossil Proboscidea from the Late Miocene-Early Pliocene site of As Sahabi, Libya. In: Boaz NT, El-Arnauti A, Pavlakis P, Salem MJ (eds) Circum-Mediterranean geology and biotic evolution during the Neogene period: the perspective from Libya. University of Garyounis, Benghazi, pp 241–256
Sanders WJ (2011) Proboscidea. In: Harisson T (ed) Paleontology and geology of laetoli: human evolution in context. Volume 2: fossil hominins and the associated fauna, Vertebrate paleobiology and paleoanthropology. Springer, pp 233–262
Sanders WJ (2018) Horizontal tooth displacement and premolar occurrence in elephants and other elephantiform proboscideans. Hist Biol 30:137–156
Sanders WJ, Gheerbrant E, Harris JM, Saegusa H, Delmer C (2010) Proboscidea. In: Werdelin L, Sanders WJ (eds) Cenozoic mammals of Africa. University of California Press, Berkeley, pp 161–251
Saunders J (1996) North American Mammutidae. In: Shoshani J, Tassy P (eds) The proboscidea: evolution and palaeoecology of elephants and their relatives. Oxford University Press, New York, pp 271–279
Schlesinger G (1917) Die Mastodonten des K. K. Naturhistorischen Hofmuseums. Denkschr K K Nat Hofmus 1:1–230
Schlesinger G (1922) Die Mastodonten der Budapester. Sammlungen Geol Hung 2:1–284
Schlosser M (1907) Ueber Säugetiere und Süsswassergastropoden aus Pliocänablagerungen Spaniens und über die natürliche Grenze von Miocän und Pliocän. N Jb Min Geol Paläont 2:1–41
Schmidt-Kittler N, de Bruijn H, Doukas C (1995) 1. General introduction. In: Schmidt-Kittler N (ed) The vertebrate locality of Maramena (Macedonia, Greece) at the Turolian-Ruscinian boundary (Neogene), vol 28. Münchner Geowiss Abh, München, pp 9–18
Seiffert ER, Nasir S, Al-Harthy A, Groenke J, Kraatz B, Stevens N, Al-Sayigh A (2012) Diversity in the later Paleogene proboscidean radiation: a small barytheriid from the Oligocene of Dhofar Governorate, Sultanate of Oman. Naturwiss 99:133–141
Shoshani J (1996) Para- or monophyly of the gomphotheres and their position within Proboscidea. In: Shoshani J, Tassy P (eds) The Proboscidea: evolution and palaeoecology of elephants and their relatives. Oxford University Press, New York, pp 149–177
Shoshani J (1998) Understanding proboscidean evolution: a formidable task. Trends Ecol Evol 13:480–487
Shoshani J (2002) Proboscidea (Elephants). eLS. Wiley
Shoshani J, Tassy P (1996a) Summary, conclusions, and a glimpse into the future. In: Shoshani J, Tassy P (eds) The proboscidea: evolution and palaeoecology of elephants and their relatives. Oxford University Press, New York, pp 335–348
Shoshani J, Tassy P (eds) (1996b) The proboscidea: evolution and palaeoecology of elephants and their relatives. Oxford University Press, New York
Shoshani J, Tassy P (2005) Advances in proboscidean taxonomy and classification, anatomy and physiology, and ecology and behavior. Quat Int 126–128:5–20
Shoshani J, Golenberg EM, Yang H (1998) Elephantidae phylogeny: morphological versus molecular results. Acta Theriologica 5:89–122
Shoshani J, Walter RC, Abraha M, Berhe S, Tassy P, Sanders WJ, Marchant GH, Libsekal Y, Ghirmai T, Zinner D (2006) A proboscidean from the late Oligocene of Eritrea, a “missing link” between early Elephantiformes and Elephantimorpha, and biogeographic implications. Proc Natl Acad Sci 103:17296–17301
Steensma KJ (1988) Plio-/Pleistozäne Großsäugetiere (Mammalia) aus dem Beckem von Kastoria/Grevena, südlich von Neapolis - NW-Griechenland. Ph.D. thesis, Technische Universität Clausthal
Stefanescu G (1892) On the existence of the Dinotherium in Roumania. Bull Geol Soc Am 3:81–83
Symeonidis NK, Tataris A (1983) The first results of the geological and palaeontological study of the Sesklo basin and its broader environment (Eastern Thessaly, Greece) (in Greek). Ann Géol Pays Hellén 31:146–190
Syrides GE (1990) Lithostratigraphic, biostratigraphic and palaeogeographic study of the Neogene-Quaternary sedimentary deposits of Chalkidiki peninsula, Macedonia, Greece (in Greek). Ph.D. thesis, Sci Annals, School Geol, Aristotle Univ Thessaloniki, Greece 1:1–243
Tarabukin BA (1974) New data on the systematics, phylogeny and ecology of Suborder Deinotherioidea Osborn (1921). In: Mammals of the late Cenozoic from Southwestern USSR, Kishinev, pp 77–90
Tassy P (1985) La place des mastodontes Miocènes de l’Ancien Monde dans la phylogénie des Proboscidea (Mammalia): Hypothèses et conjectures. Ph.D. thesis, Université Pierre et Marie Curie
Tassy P (1986) Nouveaux Elephantoidea (Mammalia) dans le Miocène du Kenya. CNRS, Paris
Tassy P (1990) The “Proboscidean Datum Event”: how many proboscideans and how many events? In: Linsday EH, Mein P, Fahlbusch V (eds) European Neogene mammal chronology. Plenum Press, New York, pp 237–252
Tassy P (1994) Origin and differentiation of the Elephantiformes (Mammalia, Proboscidea). Verh Nat Ver Hamb 34:73–94
Tassy P (1996a) Who is who among the Proboscidea? In: Shoshani J, Tassy P (eds) The Proboscidea: evolution and palaeoecology of elephants and their relatives. Oxford University Press, New York, pp 39–48
Tassy P (1996b) The earliest gomphotheres. In: Shoshani J, Tassy P (eds) The Proboscidea: evolution and palaeoecology of elephants and their relatives. Oxford University Press, New York, pp 89–91
Tassy P (1999) Miocene Elephantids (Mammalia) from the Emirate of Abu Dhabi, United Arab Emirates: palaeobiogeographic implications. In: Whybrow PJ, Hill A (eds) Fossil vertebrates of Arabia. Yale University Press, New Haven/London, pp 209–233
Tassy P (2005) Proboscideans (Mammalia) from the late Miocene of Akkaşdağı, Turkey. In: Sen S (ed) Geology, mammals and environments at Akkaşdağı, late Miocene of Central Anatolia, vol 27. Geodiversitas, Paris, pp 707–714
Tassy P (2016) Proboscidea. In: Sen S (ed) Late Miocene mammal locality of Küçükçekmece, European Turkey, vol 38. Geodiversitas, Paris, pp 261–271
Tassy P (2018) Remarks on the cranium of Eozygodon morotoensis (Proboscidea, Mammalia) from the early Miocene of Africa, and the question of the monophyly of Elephantimorpha. Rev Paléobiol 37:593–607
Tassy P, Sen S, Jaeger JJ, Mazin JM, Dalfes N (1989) Une sous-espèce nouvelle de Choerolophodon pentelici (Proboscidea, Mammalia) à Esme Akçaköy, Miocène supérieur d’Anatolie occidentale. C R Acad Sci Paris 309:2143–2146
Theodorou G, Spjeldnaes N, Hanken NM, Lauritzen SE, Velitzelos E, Athanassiou A, Roussiakis S (2000) Description and taphonomic investigations of Neogene Proboscidea from Rhodos, Greece. Ann Géol Pays Hellén 38:133–156
Theodorou G, Athanassiou A, Roussiakis S, Iliopoulos G (2003) Preliminary remarks on the Late Miocene herbivores of Kerassiá (Northern Euboea, Greece). In: Reumer JWF, Wessels W (eds) Distribution and migration of Tertiary Mammals in Eurasia. A volume in honour of Hans de Bruijn, vol 10. Deinsea, Rotterdam, pp 519–530
Theodorou GE, Roussiakis SJ, Athanassiou A, Filippidi A (2010) Mammalian remains from a new site near the classical locality of Pikermi (Attica, Greece). In: Christofides G, Kantiranis N, Kostopoulos DS, Chatzipetros AA (eds) 19th CBGA Congress, Thessaloniki. Sci Annals, School Geol, Aristotle Univ Thessaloniki, Greece sp vol 99:109–119
Tobien H (1973) On the evolution of mastodonts (Proboscidea, Mammalia). Part I: The bunodont trilophodont groups. Notizbl hess Landesamt Bodenforsch 101:202–276
Tobien H (1978) On the evolution of mastodonts (Proboscidea, Mammalia). Part 2: The bunodont tetralophodont groups. Geol Jb Hessen 106:159–208
Tobien H (1980) A note on the skull and mandible of a new choerolophodont mastodont (Proboscidea, Mammalia) from the middle Miocene of Chios (Aegean Sea, Greece). In: Jacobs LL (ed) Aspects of vertebrate history: essays in honor of Edwin Harris Colbert. Museum of Northern Arizona Press, Flagstaff, pp 299–307
Tobien H (1996) Evolution of zygodons with emphasis on dentition. In: Shoshani J, Tassy P (eds) The Proboscidea: evolution and palaeoecology of elephants and their relatives. Oxford University Press, New York, pp 76–85
Tsoukala E (2000) Remains of a Pliocene Mammut borsoni (Hays, 1834) (Proboscidea, Mammalia), from Milia (Grevena, W. Macedonia, Greece). Ann Paléontol 86:165–191
Tsoukala E (2018) Rhinocerotidae from the Late Miocene and Late Pliocene of Macedonia, Greece. A revision of the Neogene-Quaternary Rhinocerotidae of Greece. Rev Paléobiol 37:609–630
Tsoukala ES, Melentis JK (1994) Deinotherium giganteum Kaup (Proboscidea) from Kassandra peninsula (Chalkidiki, Macedonia, Greece). Geobios 27:633–640
Tsoukala E, Mol D (2016) The Proboscidea of the Early Villafranchian site of Milia (Grevena, Macedonia, Greece). Quat Int 406:4–24
Tsoukala E, Vaxevanopoulos M, Lazaridis G (2007) Neogene mammalian remains from Dikaia (Evros, Thrace, Greece). The Archaeological Work in Macedonia and Thrace 2005, 19:1–11
Vacek M (1877) Über österreichische Mastodonten und ihre Beziehungen zu den Mastodon-Arten Europas. Abh Kais-Königlichen geol Reichenstalt 7:1–45
van der Maarel FH (1932) Contributions to the knowledge of the fossil mammalian fauna of Java. Wetenschappelijke Mededelingen Dienst van den Mijnbouw in Nederlandsch-Indië 15:1–208
van der Made J, Moyà-Solà S (1989) European Suinae (Artiodactyla) from the Late Miocene onwards. Boll Soc Paleontol Ital 28:329–339
von Koenigswald W, Göhlich UB (2019) Biogeography of Mammutidae (Proboscidea) or What’s your name again? Mammut?, 90th Annual Meeting of the Paläontologische Gesellschaft. Staatliche Naturwissenschaftliche Sammlungen Bayerns - Bayerische Staatssammlung für Paläontologie und Geologie, Munich, p 83
von Meyer H (1831) Mittheilung an geheimen Rath von Leonhard. Jb Min Geogn Geol Petrefaktenkunde 2:296–297
von Meyer H (1833) Das Dinotherium bavaricum, mit Rückshicht auf die riesenmäßige fossile Thiergattung der Dinotherien überhaupt, und auf die Struktur der Mahlzähne in den Tapiren. Verh Kais Leopoldinisch-Carolinischen Akad Naturforsch 16:487–516
Vörös I (1989) Prodeinotherium petenyii sp. n. from the Lower Miocene at Putnok (North Hungary). Fragm Min Palaeont 14:101–110
Wagner A (1848) Urweltliche Säugthier-Ueberreste aus Griechenland. Abh bayerisch Akad Wiss 5:335–378
Wagner A (1857) Neue Beiträge zur Kenntnis der fossilen Säugthier-Ueberreste von Pikermi. Abh bayerisch Akad Wiss 8:109–158
Wang SQ, Deng T (2011) The first Choerolophodon (Proboscidea, Gomphotheriidae) skull from China Science. China Earth Sci 54:1326–1337
Wang SQ, Shi QQ, He W, Chen SQ, Yang XW (2016) A new species of the tetralophodont amebelodontine Konobelodon Lambert, 1990 (Proboscidea, Mammalia) from the Late Miocene of China. Geodiversitas 38:65–97
Wang SQ, Deng T, Ye J, He W, Chen SQ (2017a) Morphological and ecological diversity of Amebelodontidae (Proboscidea, Mammalia) revealed by a Miocene fossil accumulation of an upper-tuskless proboscidean. J Syst Palaeontol 15:601–615
Wang SQ, Saegusa H, Duangkrayom J, He W, Chen SQ (2017b) A new species of Tetralophodon from the Linxia Basin and the biostratigraphic significance of tetralophodont gomphotheres from the Upper Miocene of Northern China. Palaeoworld 26:703–717
Weinsheimer O (1883) Über Dinotherium giganteum Kaup. Palaeontol Abh 1:207–281
Weithofer KA (1888) Beiträge zur Kenntnis der Fauna von Pikermi bei Athen. Beitr Paläontol Österr-Ung 6:225–292
Weithofer KA (1890) Die fossilen Proboscidier des Arnothales in Toskana. Beit Palaeontol Oesterr – Ungarns und des Orients 8:107–240
Zapfe H (1957) Ein bedeutender Mastodon-Fund aus dem Unterpliozän von Niederösterreich. N Jb Geol Palaeont Abh 104:382–406
Acknowledgments
We thank all persons that allowed us access to the several proboscidean collections under their disposal in Greece and the rest of Europe. We are grateful for providing us photos of specimens and permission to use them to N. Zouros, K. Vasileiadou (Natural History Museum of the Lesvos Petrified Forest) and to G. Koufos (University of Thessaloniki) for the Lesvos specimen, A. Athanassiou (Hellenic Ministry of Culture) for the Sesklon one, and C. Fassoulas (Natural History Museum of Crete) and G. Iliopoulos (University of Patras) for the Gela one. We greatly thank G. Markov (National Museum of Natural History, Sofia) and A. Athanassiou for their comments and suggestions that improved the manuscript. GK thanks for providing important data V. Titov (Southern Scientific Centre RAS, Rostov-on-Don) about the holotype of Mammut obliquelophus, M. Pavia (University of Torino) about the holotype of M. borsoni, and D. Lambert (Holy Innocents’ Episcopal School, Atlanta), C. Beard, and M. Sims (University of Kansas) about Konobelodon britti. GK thanks G. Koufos and D. Kostopoulos (University of Thessaloniki) for all the scientific support during his research on Neogene proboscideans. GK is supported by the ERC CoG no. 724703 (CROSSROADS).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix
Appendix
List of Greek localities containing Neogene and Early Pleistocene proboscidean fossils. Type localities are marked with bold. Locality numbers refer to the collection numbers of the PaleoBiology Database (PBDB)
LocalitiesPBDB No | Age (MN; GPTS in Ma) | Taxon |
|---|---|---|
Vatera F183341 | Early Pleistocene (MNQ17) | Anancus cf. arvernensis1 |
Vatera H183343 | Early Pleistocene (MNQ17) | Anancus arvernensis 1 |
Chorigos | Pliocene–Early Pleistocene | Mammut borsoni 2 |
Neapolis-Grevena basin | Pliocene–Early Pleistocene | Mammut borsoni 3 |
Sani202529 | Pliocene–Early Pleistocene | Anancus arvernensis 4 |
Axios valley (“Dormislou”, perhaps close to Gephyra) | Pliocene–Early Pleistocene | Anancus arvernensis 5 |
Kalliphytos202532 | Pliocene–Early Pleistocene | Anancus arvernensis 6 |
Klima | Pliocene–Early Pleistocene | Anancus arvernensis 7 |
Antimachia207130 | Pliocene–Early Pleistocene | Anancus arvernensis 8 |
R. Almyri | Pliocene–Early Pleistocene | Anancus arvernensis 9 |
Kardamaena204662 | Pliocene–Early Pleistocene | Anancus arvernensis 10 |
Pylos | Pliocene–Early Pleistocene | Anancus arvernensis 11 |
Skoura | Pliocene–Early Pleistocene | Anancus arvernensis 12 |
Spaides (Eleonas) | Pliocene–Early Pleistocene | Anancus arvernensis 13,14 |
Nigrita202533 | Pliocene–Early Pleistocene | Anancus arvernensis 15 |
Chilia Dendra, Koufalia | Pliocene–Early Pleistocene | Anancus arvernensis b |
Gephyra-1182685 | Late Pliocene (MN16) | Anancus arvernensis 16 |
Sesklon34614 | Late Pliocene (MN16) | Anancus arvernensis 6,17–18 |
Milia-1187636 | Late Pliocene (MN 16a) | Mammut borsoni 19–20 |
Milia-2185862 | Late Pliocene (MN 16a) | Mammut borsoni20, Anancus arvernensis 20 |
Milia-3195291 | Late Pliocene (MN 16a) | Mammut borsoni 20 |
Milia-4182686 | Late Pliocene (MN 16a) | Mammut borsoni 20 |
Milia-5185859 | Late Pliocene (MN 16a) | Mammut borsoni20, Anancus arvernensis 20 |
Milia-6195292 | Late Pliocene (MN 16a) | Mammut borsoni 20 |
Milia-7195294 | Late Pliocene (MN 16a) | Mammut borsoni 20 |
Milia-8195295 | Late Pliocene (MN 16a) | Mammut borsoni20, Anancus arvernensis 20 |
Milia-9195296 | Late Pliocene (MN 16a) | Mammut borsoni 20 |
Milia-10185860 | Late Pliocene (MN 16a) | Mammut borsoni 20 |
Milia-11195299 | Late Pliocene (MN 16a) | Mammut borsoni 20 |
Milia202530 | Late Pliocene | Anancus arvernensis 4,a |
Apolakkia I184242 | Pliocene | Anancus arvernensis 14 |
Apolakkia II202499 | Pliocene | Proboscidea indet.14 |
Agia Triada202531 | Pliocene | Anancus arvernensis 4 |
Angelochori, Angelon beach | Pliocene | Anancus arvernensis c |
Maramena32189 | Miocene/Pliocene (MN13/14) | Choerolophodon pentelici 21 |
Dikaia210641 | Late Miocene | Choerolophodon pentelici 22 |
Pyrgos Vassilissis195555 | Late Miocene | Choerolophodon pentelici 23 |
Sani202528 | Late Miocene | Choerolophodon pentelici 4 |
Servia202257 | Late Miocene | Choerolophodon pentelici24, Deinotherium sp.24 |
Fourka202330 | Late Miocene | Tetralophodon longirostris 25 |
Chelona beach202500 | Late Miocene | Tetralophodon longirostris 25 |
seabed of Kryopigi202500 | Late Miocene | Tetralophodon longirostris 25 |
Neokaisareia206458 | Turolian | Mammut sp. (M. obliquelophus?)26 |
Palaio Keramidi | Turolian | Mammut sp. (M. obliquelophus?)26 |
Dytiko 232375 | Late Turolian (MN13) | Choerolophodon pentelici 27 |
Dytiko 332376 | Late Turolian (MN13) | Choerolophodon pentelici 27 |
Kryopigi157582 | Late Miocene (MN12–13) | Choerolophodon pentelici 28 |
Mytilinii 1A-Samos202215 | Middle Turolian (MN12; ~7.1) | Mammut sp. (M. obliquelophus?) 29–30 |
Mytilinii 1B-Samos202216 | Middle Turolian (MN12; ~7.1) | Choerolophodon pentelici 27,29,30 |
Andriano-Samos | Middle Turolian (MN12; ~7.1) | Choerolophodon pentelici30,32, Deinotherium proavum 30,32 |
Chomateri195562 | Middle Turolian (MN12; ~7.16) | Anancus lehmanni 33–34 |
Perivolaki194879 | Middle Turolian (MN12; 7.3–7.1) | Deinotherium proavum 33,35 |
Pikermi 182754 | Middle Turolian (MN12; ~7.3) | Choerolophodon pentelici27,30,33,36–38, Deinotherium proavum33,36–37,39–44, Mammut sp. (M. obliquelophus?)33,36–38, Konobelodon atticus 33,36–38,40,45 |
Pikermi Valley-1202630 | Middle Turolian (MN12) | Mammut sp. (M. obliquelophus?)46 |
Pikermi Valley-3202631 | Middle Turolian (MN12) | Deinotherium proavum 44 |
Halmyropotamos202213 | ?middle Turolian (?MN12) | Deinotherium proavum44,47, Mammut sp. (M. obliquelophus?)33,48 |
Prochoma-1202222 | Middle Turolian (MN12) | Choerolophodon pentelici 31,33 |
Vathylakkos-2202703 | Middle Turolian (MN12) | Choerolophodon pentelici 31,33 |
Kerassia-1 | ?middle Turolian (?MN 12) | Choerolophodon? sp.49 |
Kerassia-4195435 | ?middle Turolian (?MN 12) | Konobelodon atticus 45,49 |
Kerassia-6195437 | ?middle Turolian (?MN 12) | Choerolophodon sp.49 |
Kerassia195431 | ?middle Turolian (?MN 12) | Deinotherium sp.50 |
Ravin des Zouaves 5195489 | Early Turolian (MN11; ~8.2) | Mammut sp. (M. obliquelophus?) 30,33,51, Choerolophodon pentelici 30,33,51 |
Maronia, Crete202722 | Turolian (MN11–13) | Deinotherium proavum 52 |
Gela, Crete202723 | Turolian (MN11–13) | Deinotherium proavum 53–54 |
Zakros, Crete | Turolian (MN11–13) | Deinotherium proavum 54–55 |
Samos Island, NHMW collection182751 MGL collection202120 HGI collection HLMD collection202724 SMF collection202725 | Turolian (MN11–13) | Choerolophodon pentelici29–33,56–58, Konobelodon atticus30,32,33,45,58, Deinotherium proavum 30,32,33 |
Nikiti-273869 | Early Turolian (MN11; 8.7–8.2) | Choerolophodon pentelici 30,31,33,59 |
Ravin X182745 | ?early Turolian (?MN11) | Choerolophodon pentelici 30–31 |
Platania, Drama182682 | Late Vallesian/early Turolian (MN10/MN11) | Konobelodon cf. atticus26 |
Ravin de la Pluie191070 | Late Vallesian (MN10; ~9.3) | Deinotherium giganteum30,33, Choerolophodon pentelici30–31,33 |
Xirochori 1195490 | Late Vallesian (MN10; ~9.6) | Choerolophodon pentelici 30–31,33 |
Ravin des Zouaves 1182746 | Late Vallesian (MN10) | Choerolophodon pentelici 30–31,33 |
Agia Paraskevi, Kassandra202726 | Late Miocene | Deinotherium giganteum 60 |
Pentalophos 1202119 | Early Vallesian (MN9) | Choerolophodon anatolicus 30–31,33 |
Thymiana 182752 | Middle Miocene (MN5; >15.5) | Choerolophodon chioticus27,61–62, Prodeinotherium bavaricum61 |
Psara Island | ?middle Miocene | Prodeinotherium bavaricum 63 |
Gavathas, Lesvos Island195540 | Early Miocene (MN3; >18.4) | Prodeinotherium cuvieri 64,a |
Thermopigi73553 | Turolian (MN11–13) | Deinotherium sp.26, Elephantimorpha indet.26 |
Nikiti-1202729 | Late Vallesian (MN10) | Proboscidea indet.59 |
Antonios73861 | Early/middle Miocene (MN4/5) | Proboscidea indet.65 |
Central Macedonia | ? | Deinotherium giganteum 66 |
Vathylakkos area | ? | Mammut borsoni 11 |
possibly from the wider area of Siatista (Kozani)202732 | ? | Stegodon sp.67 |
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Konidaris, G.E., Tsoukala, E. (2022). The Fossil Record of the Neogene Proboscidea (Mammalia) in Greece. In: Vlachos, E. (eds) Fossil Vertebrates of Greece Vol. 1. Springer, Cham. https://doi.org/10.1007/978-3-030-68398-6_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-68398-6_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-68397-9
Online ISBN: 978-3-030-68398-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)