Abstract
Twenty-seven water samples including precipitation (3), streams (6) and springs (18) from Bringi watershed, southeast Kashmir were bimonthly collected for 1 year and analysed for ionic concentrations, stable isotopes and tritium. The objectives of the study were to recognize the site of recharge for Karst springs, components and mechanism of groundwater recharge. The local meteoric water line (LMWL) is δD = 7.094 × δ18O + 9.791 (r2 = 0.82) on the basis of monthly averages weighted amount. The winter precipitation isotopic composition (average = −10.4‰ for δ18O and −58.2‰ for δD) is reflected in streams (average = −8.5‰ for δ18O and −47.3‰ for δD) and spring water (average = −8.8‰ for δ18O and −51.7‰ for δD) during summer and late spring, which is representative of winter snow melting. Mean elevation of recharge was estimated between 2500 and 2900 m above the mean sea level (amsl) using altitude effect (δ18O = −0.27‰ per 100 m). Based on the isotopic mass balance equations, the average surface to groundwater contribution in peak flow time was 337.35 m3/s, approximately 75% of total discharge from the stream and 7.5 m3/s during lean period, which is approximately 18.6% of total runoff. In addition, average residence time of springs is very short (less than 1 year) and hence responds very quickly to the hydrological events. The quality of surface and groundwater is good for drinking, domestic and agricultural purposes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
20.1 Introduction
Kashmir Valley is bestowed with adequate assets of water in variety of glaciers, snow, groundwater and surface water. Several springs of freshwater occur in southeast Kashmir, in Anantnag District (‘Ananta’ means infinite and ‘Naga’ means water springs) controlled by Karst terrain (Lawrence 1967; Bhat et al. 2014, 2019a; Alam et al. 2017). For decades, the springs are used for various purposes (i.e. drinking, agriculture, aquaculture, floriculture, tourism, etc.). For the flourishing of any socioeconomic culture, water resources which includes glaciers, lakes, groundwater are of immense importance (Singh et al. 2017; Kumar et al. 2020; Taloor et al. 2020a, b)
Among the upper land catchments of River Jhelum, Bringi catchment is a karst terrain with replacement of water between Karst springs and streams (Coward et al. 1972; Jeelani et al. 2011, 2014). To reduce contamination of water resources of the area, it is important to demarcate the potential sites of springs recharge and their recharging mechanism (Jeelani et al. 2014; Gat 1971; Ford and Williams 1989). Environmental isotopes (δ2H, δ18O and 3H) along with hydrogeochemistry and hydrogeology have been used by several workers (Ford and Williams 1989; Eyankware et al. 2018). The isotopic signature of meteoric water at a particular location serves as a basis for demarcating ground water recharge area (Gat 1971; Lee et al. 1999; Gonfiantini et al. 1976; Jeelani et al. 2010; McConville et al. 2001). On the other hand, as a result of interaction between rock and water, the chemistry of groundwater changes until a quasi-chemical equilibrium is reached especially HCO3, Ca and Mg (Goldscheider and Drew 2007; Adimalla and Taloor 2020a; Freeze and Cherry 1979; Jasrotia and Kumar 2014; Fetter 1980; Jeelani et al. 2010; Sah et al. 2017; Bisht et al. 2018; Jasrotia et al. 2018, 2019; Adimalla and Taloor 2020b; Adimalla et al. 2020; Sarkar et al. 2020).
20.2 Area of Study
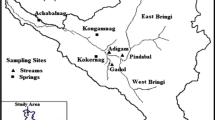
Bringi catchment, an upland watershed of River Jhelum in Kashmir Valley, lies between 33°20′ to 33°45′N latitudes and 75°10′ to 75°30′E longitudes (Fig. 20.1), with an area of approximately 595 km2 (Bhat and Jeelani 2018). The elevation of watershed ranges 1650 m amsl at Achabal to >4000 m amsl at Sinthan top. Bringi Stream and its tributaries especially east Bringi and west Bringi which joins with the Jhelum River near Anantnag are drained in this watershed (Bhat et al. 2014).
The area is characterized by temperate climate with four well-developed seasons (Jeelani et al. 2014) and monthly variation in the average temperature and precipitation from 1990 to 2009 is shown in Fig. 20.2 (Bhat and Jeelani 2018).

(Source Bhat and Jeelani 2018)
Mean monthly precipitation and temperature from 1990 to 2009
20.3 Geology and Hydrogeology
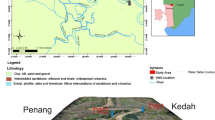
Geologically, the area is covered by permo-Triassic rocks especially Triassic Limestone and Panjal Traps. Recent alluvium and Karewa deposits occurs towards low-lying area (Fig. 20.3) (Alam et al. 2017, 2018). Panjal Volcanics and rocks of Upper Palaeozoic are present along the marginal parts of study area. Triassic Limestone is >1000 m high deposit with different layers of sandstone and shale (Bhat et al. 2019c, d). The Karewa deposits are fluviatile and lacustrine sediments that lie on top of Triassic limestone, with unconsolidated coarse to fine-grained sand, dark grey clays, light grey sands, varved clays, brown loam, marl, gravel, silt, lignite, etc. (Bhat et al. 2019c, d). The fluvial deposits contain boulders, gravel, sand, silt, clay, which represent active flood plain sediments (Jeelani et al. 2010; Taloor et al. 2019).

(Source Bhat et al. 2014)
Geological map of study site
Three main springs, namely, Achabalnag, Kokernag and Kongamnag hosted by the Triassic Limestone and Karewa deposits were studied (Fig. 20.4a–c) during the present work (Jeelani et al. 2014; Bhat and Jeelani 2018). Achabalnag is a Karst spring at the base of Sosanwar Hills, where the water gushes out from two sites about 150 m apart, with one major outlet carrying about 80–90% of the total spring discharge (Fig. 20.4a). The water is channelled through the Achabal Garden and Villages downstream. Kokernag, a set of seven springs, is another major Karst spring where the water gushes out at various places (Fig. 20.4b). The water is channelled through the Kokernag garden and the villages downstream. At Kongamnag, water forms a pool, at bottom of the hill of limestone (Fig. 20.4c) and the water is drained downstream through villages. Kokernag and Achabal springs are cold (8 °C–14 °C) as compared to Kongamnag with temperature ranging from 14 °C to 19 °C. Higher temperature of Kongamnag spring might be due to deep motion of infiltrating water. Kokernag discharge varies 600 L/s during winters to 8000 L/s during summers and Achabal discharge varies from 160 L/s in winter to 3000 L/s in summers. The variability in discharge is mainly due to fast response of spring to hydrological events. Discharge of Kongamnag varies 12L/s during winters to 20 L/s during spring and summer seasons.
20.4 Methodology
Water samples from Bringi Stream (two sites), three sites for precipitation (Pindabal, Kokernag and Achabal) and springs (Kokernag, Achabalnag, Kongamnag) were collected bimonthly for one hydrological cycle March 2008 to January 2009 (Fig. 20.1). The samples were analysed for ions including Na+, Mg2+, Ca2+, K+, Cl−, HCO3−, SO −24 , SiO2, F−, NO3− and isotopes (δ18O, δ2H and 3H) by using the techniques (APHA 2006; Epstein and Mayeda 1953). Precipitation collectors were installed for collection of precipitation samples for stable isotopes. About 2 ml glycerine was added into the containers, which form a thin film above water to avoid evaporation. For 3H analysis, the samples were collected in 1 litre sampling bottles during January to September 2008. Master parameters including pH, electric conductivity (EC) and temperature were measured in the field. Major ion analysis was carried out in Hydrogeology Laboratory, Department of Earth Science, Kashmir University, Srinagar. To separate the suspended sediments, waters were filtered through <0.45 μm nucleopore filter paper. All the cations and anions were calculated using the titration methods, flame photometer and spectrophotometer. The tritium and oxygen isotope analysis was carried out in Bhaba Atomic Research Centre (BARC) Mumbai whereas hydrogen isotope in NIH Roorkee (Epstein and Mayeda 1953). In addition, precipitation and temperature data were collected from IMD, Srinagar, Kokernag Station (Table 20.1).
20.5 Results
The statistical results of physicochemical parameters are given in Table 20.2 and isotope (δ18O, δD and 3H) details are presented in Table 20.3.
20.5.1 Precipitation
The precipitation samples are moderately alkaline with pH varying from 8.6 to 8.9 with mean of 8.75. EC ranges from 64 to 78 µS/cm with average of 70 µS/cm. Total dissolved solids show a narrow range of salinity (41–50 mg/l) with mean of 46 mg/l. Ca (66%) is higher cation followed by Mg (21%) > Na (12%) > K (1%) and anions as HCO3(83%) > SO4 (10%) > Cl (7%). The concentration of different ions may be due to dissolution of various gases and atmospheric pollutants (Bhat and Jeelani 2015).
Stable isotopes in precipitation show marked spatial and temporal variations (Table 20.3). δD and δ18O ranged from −12.59 to −60.6‰ with a mean value of −31.1‰ and −2.1 to −10.6‰ with a mean of –5.8‰. Samples were enriched during summer and low altitudes and depleted during winter and at higher elevation. Higher values were observed in July (δD = –12.6‰; δ18O = −2.1‰) and lower in January (mean δD = −58.2‰, δ18O = −10.4‰). Stable isotopes in precipitation showed good correlation (Fig. 20.5) with precipitation amount and temperature (r2 = 0.97).

(Source Bhat and Jeelani 2018)
Variation of δ18O, temperature, precipitation and stream discharge
20.5.2 Streams
Temperature of the stream water samples varied from 9.6°C to 17.4°C with an average of 12.48 °C. pH between 7.8 and 9.8 with mean of 8.2 indicate stream water is alkaline. Electrical conductivity varied from 140 to 235 µS/cm, with mean value of 175 µS/cm. Similarly, TDS varied from 89 to 193 mg/l with average 122 mg/l. About 50% of samples show cation order as Ca (62%) > Na (21%) > Mg (13%) > K (4%) and anion order as HCO3 (88%) > SO4 (8%) > Cl (4%). Remaining 50% showed cation as Ca (61%) > Mg (26%) > Na (12%) > K (1%) and anion as HCO3 (89%) > Cl (7%) > SO4 (4%).
Stable isotopes of water collected from stream showed small variation (Table 20.3), with δD from −36.4 to −47.3‰ with average −44.1‰ and δ18O ranging from −6.8 to −8.5‰ with average −7.8‰. Water is enriched in early autumn (September) season (δD = −36.43‰ and δ18O = −6.8‰) and depleted in late spring (May) season (δ2H = −47.33‰ and δ18O = −8.5‰). The isotopic characteristic of summer precipitation (July) with enriched isotopes is not fully reflected in the streams (Fig. 20.5). However, the enriched signals of summer precipitation are clearly reflected in streams during autumn season. During winter, base flow is the main contributor to surface runoff due to low temperatures and negligible melting.
20.5.3 Springs
The water of springs is odourless and colourless. Water temperature varied from 8.2 °C to 14.4 °C with an average of 12.2 °C. The Karst springs including Kokernag and Achabalnag showed variability of annual temperature (~5.4 °C and ~3.8 °C) as compared to Kongamnag with annual inconsistency of 1.7 °C. The TDS varied between 90 and 339.2 mg/l, with average of 206 mg/l. Most of the samples show high Ca concentration than Mg while few show Na is higher than the Mg. While the anions showed high HCO3 concentration.
The temporal and spatial variability of springs is given in Table 20.3, with δD ranging from −40.7 to −57.6‰ with average value of −48.5‰ and δ18O ranging from −6.6 to −9.2‰ with a mean of −8.3‰. Achabalnag was enriched (average δD = −44.0‰; δ18O = −7.8‰) and Kongamnag was depleted in heavy isotopes (average δD = −56.0‰; δ18O = −8.8‰). The annual amplitude of δ18O variations is 1.16 to 1.97, quite similar to the Karst springs of Meramec River Basin and Kapuz Karst springs (Kattan 1997; Fredrickson and Criss 1999) with amplitude varying from 1 to 2.
20.5.4 Tritium (3H)
Tritium concentration of precipitation water varied from 13.3 to 16.9 TU and average 15.1 TU (Table 20.3). There is higher value of tritium in snow samples of winter season while less in summer season indicating different sources of precipitation (Jeelani et al. 2010).
20.6 Discussion
20.6.1 Geochemical Processes Controlling Chemistry of Water
To determine the evolution of water, ions are powerful tools (Eyankware et al. 2018; Bhat et al. 2016, 2019b, c, d). The hydrochemical data was plotted in Gibbs diagram (Fig. 20.6) to find out the source of sample which shows the rock dominance environment (Gibbs 1970).
Four hydrochemical water types were observed following the order of Ca–Mg–HCO3 > Ca–HCO3 > Ca–Mg–Na– HCO3 > Ca–Na–HCO3 in Piper trilinear diagram (Piper 1994) (Fig. 20.7). The water types have resulted due to carbonate dissolution. The evolution of stream water is Ca–Mg–HCO3 water type which is due to limited time for interaction between water and rock as well as easily carbonate mineral dissolution.
The spring water and stream water are characterized by Ca–Mg–HCO3 trend in Langelier–Ludwig diagram (Langelier and Ludwig 1942) which shows the clear dominance by carbonate dissolution (Fig. 20.8).
Various binary diagrams were plotted for identifying lithological source in water. In (Ca + Mg) versus HCO3 (Fig. 20.9a), most water samples of stream lie on to the trend line, which shows carbonate weathering as the main source of solute acquisition. However, some spring water samples fall away from trendline indicating other source for HCO3 in addition to carbonate weathering. In scatter graph between (Na + K) and (Ca + Mg) (Fig. 20.9b), the stream samples fall close to the trendline which might be because of silicate weathering. However, the spring samples especially Achabalnag and Kokernag fall away from trendline suggesting less contribution of ions from silicate weathering. In some spring samples (Kongamnag), (Na + K) exceeds (Ca + Mg) due to the impact of silicate weathering. In Cl– and (Na + K) plot (Fig. 20.9c), all samples present above the trendline show different source of Na (Eyankware et al. 2018). The plots given in Fig. 20.9b, c favour the higher contribution of silicate weathering for ionic concentration, mainly Na.

(Source Bhat et al. 2014)
Binary plots depicting ionic sources in streams and springs. a (Ca + Mg) versus HCO3. b (Ca + Mg) versus Na + K. c (Na + K) versus Cl. d Ca/Mg versus Mg. e Ca/(Ca + Mg) versus SO4/(SO4 + HCO3)
The binary plot of Mg and Ca/Mg (Fig. 20.9d) represents decrease in molar ratio with increase in Mg concentration, indicating weathering of carbonate rocks especially dolomite is main source for Mg and Ca. In Ca/(Ca + Mg) versus SO4/(SO4 + HCO3) binary plot (Fig. 20.9e), high Ca/(Ca + Mg) molar ratio is due to interaction of water with calcite. The Ca/(Ca + Mg) molar ratios of 0.5 and 1 correspond to dissolution of pure calcite and stoichiometric dolomite (Frondini 2008).
20.6.2 Delineation Area of Recharge for Karst Springs Using Hydrogeological and Hydrogeochemical Approach
The analytical results suggest that Bringi Stream and the Karst springs have similar chemical composition. However, a subtle difference between the major ion chemistry of the springs is observed. Kokernag and Achabalnag springs have low concentration of ions as compared to Kongamnag. This is mainly due to greater high contact time of water with base lithology in Kongamnag as compared to Kokernag and Achabalnag springs. Both springs and streams show a different summer maximum and winter minimum temperatures (Jeelani 2010; Jeelani et al. 2014). Similarly, discharge of springs and streams is low during winter and high during summer (Fig. 20.10).

(Source Bhat et al. 2014)
Bimonthly variation of discharge, precipitation amount, stream and spring temperatures
The temporal chemographs of Ca2+, TDS and HCO3– of streams showed high concentration in January and March while low in remaining months (Fig. 20.11). In summer season, there is significant stream discharge and less interaction between water and rock, which decreases dissolved ion concentration in spring (dilution effect).

(Scource Bhat et al. 2014)
Correlation of Ca, TDS, HCO3 and chloride between Karst springs and Bringi Stream
However, the Karst springs especially Achabalnag and Kokernag confirm high concentration in July, which is mainly because of piston effect. The temporal plot of spring discharge and TDS (Fig. 20.12) represents both TDS and discharge raise concurrently in July.

(Source Bhat et al. 2014)
Relationship of TDS with discharge of Achabalnag and Kokernag showing piston effect
20.6.3 Isotopic Approach
The relationship among δ18O and δD in global precipitation is known as global meteoric water line (GMWL) (Craig 1961) and is given in Eq. 20.1.
Rozanski et al. (1993) modified the GMWL, using more available data (Eq. 20.2)
Based on the amount weighed mean monthly samples, the regression equation between δD and δ18O (Fig. 12.3) known as local meteoric water line (LMWL) is given in Eq. 20.3.
The meteoric water line of study site is almost same as western Himalayas, δD = 7.95 × δ18O + 11.51 (Kumar et al. 2010). Shallower slope and low intercept than LMWL of western Himalayas and a shallow slope and high intercept to GMWL may be due to the different sources of moisture effect and/or effect of evaporation. Effect of temperature on the precipitation isotopic composition is observed in δ18O versus δD plot (Fig. 20.13).

(Source Bhat and Jeelani 2015)
δ18O vs δD relationship of stream and spring water over GMWL and LMWL of Bringi watershed
With increasing altitude the precipitation isotopic composition decreases, known as altitude effect (Ingraham and Taylor 1991), which is a significant tool to delineate the spring recharge areas (Jeelani et al. 2010). In the study area, altitude effect of −0.27‰ per 100 m was observed. The average elevation of area of spring recharge varies from 2500 to 2900 m amsl (Fig. 20.14).

(Source Bhat and Jeelani 2015)
The altitude versus δ18O, showing average elevation of spring recharge area
There is a very good correlation (r2 = 0.97) in seasonal δ18O composition of streams and springs (Fig. 20.15), which indicates that the streams recharge these springs at different heights and share similar catchments.

(Source Bhat and Jeelani 2018)
Positive temporal variations of δ18O of springs and streams of Bringi watershed
20.7 Components and Mechanism of Groundwater Recharge
Various methods are present for defining the contribution of surface to groundwater. Chloride mass balance equation (CMBE) is used by a number of researchers to calculate the contribution of precipitation to groundwater (Bhat and Jeelani 2018).
where ‘C’ is the concentration of chloride present in the precipitation and groundwater. The mean concentration of chloride of precipitation and springs is 0.57 mg/l and 3.55 mg/L. The estimated recharge through precipitation averages at 18.5%, with highest during July (about 22%) and lowest during November (<1%). Isotopic mass balance studies (IBME) defined that studies related to the isotopic mass balance indicate a mixture of two components (i.e. faction of groundwater (YG) and surface water (YS)
where YS and YG are the contribution of surface water and groundwater percentage to the mixture YM. δS, δG and δM are the isotopic composition of surface water, groundwater and admixture, respectively. Substituting Eqs. (20.5) in (20.6) for YG gives contribution of surface water component YS to the groundwater mixture.
The mean δ18O composition of surface water was −8.077‰ in high flow time (May to July 2008) and for groundwater before the high flow period (March 2008) is −8.19‰. The mean δ18O composition for mixture of groundwater and surface water in September was −7.3‰. Therefore, the components of surface recharge during high flow period, ‘YS’, average at 337.35 m3/s, about 75% of total stream discharge.
20.8 Residence Time of Groundwater
As determined by (Clark and Fritz 1997), the groundwater residence time by decay equation (Eq. 20.8) is
where a 3o H is the concentration in precipitation or initial tritium activity (expressed in TU) and a 3t H concentration of tritium in groundwater or residual activity remains after decay over time t. As mean residence time of ground water is quite short (<1 year) in the study, it is short for Achabalnag and longer for Kongamnag. During the present investigation, dye testing was carried out near Adigam (Fig. 20.16a) and Gadol (Fig. 20.16b), which confirmed connection between recharge sites and Karst springs.
20.9 Quality of Water for Drinking, Agricultural and Livestock Purposes
The water quality was carried out as per Bureau of Indian standards (BIS 2012) and World Health Organization (WHO 2011) for drinking (Table 20.1). The TDS of water samples is within the prescribed limits for the livestocks (Hamill and Bell 1986; Ravindra and Garg 2007). Based on the classification of hardness (Sawyer and McCarthy 1967), 45% samples are categorized under soft, 41.6% under moderately hard and 20.8% under very hard.
A number of plots and formulas are available for determining the suitability of water for purpose of irrigation. Wilcox diagram with specific conductance plotted against percentage Na is used for evaluating water for irrigation purposes (Wilcox 1955). The diagram shows that water is good for irrigation (Fig. 20.17).

(Source Wilcox 1955)
Plot of electrical conductivity (EC) vs percent sodium (% Na) for water classification
Appropriateness of water for the purpose of irrigation can also be determined by plotting electrical conductivity (EC) against sodium absorption ratio (SAR) (Fig. 20.18) on the US Salinity Laboratory (USSL) diagram (Richards 1954). About 62.5% (15 samples) fall in C1S1 field of the diagram indicates low sodium/low salinity-type water and 37.5% (9 samples) belong to C2S1 category indicating good condition of water for irrigation for most soils and crops.

(Source Richards 1954)
Classification of water for irrigation purpose by using USSL Salinity Hazard Diagram
20.10 Conclusions
The results concluded t with following points:
-
Ca2+ was the dominant cation and HCO3– was the dominant anion whereas four hydrochemical types of water have been identified as Ca–Mg–HCO3 > Ca–HCO3 > Ca–Mg–Na–HCO3 > Ca–Na–HCO3.
-
Carbonate weathering is mainly responsible for ions in groundwater as inferred from scatterplots and hydrogeochemical.
-
Hydrographs and chemographs for both springs and streams showed high Ca, TDS, EC and HCO3 during winter and low during summer. The positive correlation of chemographs of springs and streams indicates that Bringi Stream fed all the springs at various elevations.
-
The LMWL for Bringi watershed is δD = 7.094 × δ18O + 9.791 (r2 = 0.82) whereas the springs are the major sources of recharge. The surface recharge component using IMBE averages at 337.35 m3/s during high flow period, about 75% of total stream discharge and 7.5 m3/s flow during low flow period, about 18.6% of total stream discharge.
20.11 Recommendations
Based on the present work, certain recommendations are made for preservation of the valuable water resource of the area.
-
Fencing of the recharge sites near Adigam and Gadol is necessary to avoid contamination of water.
-
Check dams may be built across Bringi Stream at Adigam Village and Gadol Stream near Gadol Village to maintain the flow of Karst springs during lean period.
-
Continuous monitoring of water quality of streams and springs in terms of major and heavy metals. Continuous monitoring of stream and spring discharge to understand the response of springs to hydrological events.
-
Public awareness programmes need to be conducted to create awareness among the people regarding the importance of preservation of the valuable resources of water of the area.
References
Adimalla N, Dhakate R, Kasarla A, Taloor AK (2020) Appraisal of groundwater quality fordrinking and irrigation purposes in Central Telangana. India. Groundwater SustDevelop 10:P100334. https://doi.org/10.1016/j.gsd.2020.100334
Adimalla N, Taloor AK (2020a) Hydrogeochemical investigation of groundwater quality in the hard rock terrain of South India using Geographic Information System (GIS) and groundwaterquality index (GWQI) techniques. Groundwater Sust Develop 10:P100288. https://doi.org/10.1016/j.gsd.2019.100288
Adimalla N, Taloor AK (2020b) Introductory editorial for ‘Applied Water Science’special issue: “Groundwater contamination and risk assessment with an application of GIS”. Appl Water Sci 10:216. https://doi.org/10.1007/s13201-020-01291-3
Alam A, Bhat MS, Kotlia BS, Ahmad B, Ahmad S, Taloor AK, Ahmad HF (2017) Coexistent pre-existing extensional and subsequent compressional tectonic deformation in the Kashmir basin, NW Himalaya. Quat Int 444:201–208
Alam A, Bhat MS, Kotlia BS, Ahmad B, Ahmad S, Taloor AK, Ahmad HF (2018) Hybrid tectonic character of the Kashmir basin: Response to comment on “Coexistent pre-existing extensional and subsequent compressional tectonic deformation in the Kashmir basin, NW Himalaya (Alam et al. 2017)” by Shah (2017). Quat Int 468:284–289
APHA (2006) Standard methods for the examination of water and waste. American Public Health Association, Washington DC
Bhat MS, Alam A, Ahmad B, Kotlia BS, Farooq H, Taloor AK, Ahmad S (2019a) Flood frequency analysis of river Jhelum in Kashmir basin. Quat Int 507:288–294
Bhat NA, Ghosh P, Ahmed W, Naaz F, Priyadashinee A (2019b) Hydrochemical characteristics and quality assessment of stream water in parts of Gadag, Koppal and Ballery districts of Karnataka India. J Geol Soc Ind 94(6):635–640
Bhat NA, Bhat AA, Nath S, Singh BP, Guha DB (2016) Assessment of Drinking and irrigation water quality of surface water resources of South-West Kashmir India. J Civil Environ Eng 6(2):1000222. https://doi.org/10.4172/2165-784X.1000222
Bhat NA, Jeelani G (2015) Delineation of the recharge areas and distinguishing the sources of karst springs in Bringi watershed, Kashmir Himalayas using hydrochemistry and environmental isotopes. J Ear Sys Sci 124(8):1667–1676
Bhat NA, Jeelani G (2018) Quantification of Groundwater—Surface water Interactions using Environmental Isotopes; A Case Study of Bringi Watershed, Kashmir Himalayas India. J Ear Sys Sci 127(5):63. https://doi.org/10.1007/s12040-018-0964-x
Bhat NA, Jeelani G, Bhat MY (2014) Hydrogeochemical assessment of groundwater in karst environments, Bringi watershed, Kashmir Himalayas India. Curr Sci 106(7):1000–1007
Bhat NA, Singh BP, Bhat AA, Nath S, Guha DB (2019c) Application of Geochemical Mapping in Unraveling Paleoweathering and Provenance of Karewa Sediments of South Kashmir, NW Himalayas India. J Geol Soc Ind 93(1):68–74
Bhat NA, Singh BP, Guha DB, Bhat AA, Nath S (2019d) Geochemistry of stream sediments and soil samples from Karewa deposits of South Kashmir, NW Himalaya, India. Environ Ear Sci 78(9):278. https://doi.org/10.1007/s12665-019-8212-5
Bisht H, Arya PC, Kumar K (2018) Hydro-chemical analysis and ionic flux of meltwater runoff from Khangri Glacier, West Kameng, Arunachal Himalaya, India. Environ Earth Sci. 77:1–16. https://doi.org/10.1007/s12665-018-7779-6
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, Boca Raton
Coward JMH, Waltham AC, Bowser RJ (1972) Karst springs in the Vale of Kashmir. J Hydrol 16:213–223
Craig H (1961) Isotopic variation in meteoric waters. Science 133:1702–1703
Epstein S, Mayeda T (1953) Variation of δ18O content in waters from natural sources. Geochim Cosmochim Act 4:213–224
Eyankware MO, Nnajieze VS, Aleke CG (2018) Geochemical assessment of water quality for irrigation in abandoned limestone quarry pit at Nkalagu area, southern Benue Trough. Nigeria. Environ Earth Sci 77:66. https://doi.org/10.1007/s12665-018-7232-x
Fetter CW (1980) Applied hydrology, 2nd edn. Merrill, Columbus
Ford D, Williams P (1989) Karst Geomorphology and Hydrology. Chapman and Hall, London
Fredrickson GC, Criss RE (1999) Isotope hydrology and time constants of the unimpounded Meramec River basin, Missouri. Chem Geol 157:303–317
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, N.J
Frondini F (2008) Geochemistry of regional aquifer systems hosted by carbonate–evaporite formations in Umbria and southern Tuscany (central Italy). App Geochem 23(8):2091–2104
Gat JR (1971) Comments on the stable isotope method in regional groundwater investigations. Wat Res Res 7:980–993
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Goldscheider N, Drew D (2007) Methods in Karst Hydrogeology. Taylor and Francis, London
Gonfiantini R, Gallo G, Payne BR, Taylor CB (1976) Environmental isotopes and hydrochemistry in groundwater of Gran Canaria. Interpretation of environmental isotope and hydrochemical data in groundwater hydrology. IAEA, Vienna, pp 159–170
Hamill L, Bell FG (1986) Groundwater resource development and management. The University Press, Cambridge, Great Britain, p 34
Ingraham NL, Taylor BE (1991) Light stable isotope systematic of large-scale hydrologic regimes in California and Nevada. Wat Res Res 27:77–90
Jasrotia AS, Kumar A (2014) Estimation of replenishable groundwater resources and their status of utilization in Jammu Himalaya, J&K, India. Eur Water 48:17–27
Jasrotia AS, Taloor AK, Andotra U, Bhagat BD (2018) Geoinformatics based groundwater quality assessment for domestic and irrigation uses of the Western Doon valley, Uttarakhand, India. Groundwater Sust Develop 6:200–212
Jasrotia AS, Taloor AK, Andotra U, Kumar R (2019) Monitoring and assessment of groundwaterquality and its suitability for domestic and agricultural use in the Cenozoic rocks ofJammu Himalaya, India: A geospatial technology based approach. Groundwater Sust Develop 8:554–566
Jeelani G, Bhat NA, Shivanna K (2010) Use of δ18O tracer to identify stream and spring origins of a mountainous catchment: A case study from Liddar watershed, western Himalaya, India. J Hydrol 393:257–264
Jeelani G, Bhat NA, Shivanna K, Bhat MY (2011) Geochemical characterization of surface water and spring water in SE Kashmir valley, western Himalayas: Implication to water–rock interaction. J Ear Sys Sci 120(5):921–932
Jeelani G, Kumar SU, Bhat NA, Sharma S, Kumar B (2014) Variation of δ18O, δD and 3H in karst springs of south Kashmir, western Himalayas (India). Hydrol Process 29:522–530. https://doi.org/10.1002/hyp.10162
Kattan Z (1997) Environmental isotope study of the major karst springs in Damuscus limestone aquifer system: case of the Figeh and Barada springs. J Hydrol 193:161–182
Kumar B, Rai SP, Kumar US, Verma SK, Garg P, Kumar SVV, Jaiswal R, Purendra BK, Kumar SR, Pande NG (2010) Isotopic characteristics of Indian precipitation. Wat Res Res 46:1–15
Kumar D, Singh AK, Taloor AK, Singh DS (2020) Recessional pattern of Thelu andSwetvarn glaciers between 1968 and 2019, Bhagirathi basin, Garhwal Himalaya, India. Quat Int. https://doi.org/10.1016/j.quaint.2020.05.017
Langelier WE, Ludwig HF (1942) Graphical method for indicating the mineral character of natural water. J Am Wat Wor Assoc 34:335–352
Lawrence W (1967) The Valley of Kashmir, Kesar Publishers
Lee KS, Wenner DB, Lee I (1999) Using H- and O-isotopic data for estimating the relative contributions of rainy and dry season precipitation to groundwater: Example from Cheju Island, Korea. J Hydrol 222:65–74
McConville C, Kalin RM, Johnston H, McNeil GW (2001) Evaluation of recharge in a small temperate catchment using natural and applied δ18O profiles in the unsaturated zone. Groundwater 39:616–624
Middlemiss CS (1910) A revision of Silurian-Triassic sequence of Kashmir. Rec Geol Sur Ind 40(3):6–260
Piper AMA (1994) graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Uni 25:914–928
Ravindra K, Garg VK (2007) Hydrochemical survey of ground water of Hisar city and assessment of defluoridation methods used in India. Environ Monit Assess 132:33–43
Richards LA (1954) Diagnosis Improvement Saline Alkali Soils. US Department of Agriculture Handbook. 60
Rozanski K, Arugu´as-Arugu´as L, Ganfiantini R (1993) Isotopic patterns in modern global precipitation. Geophys Mono 78:1–36
Sah S, Bisht H, Kumar K, Tiwari A, Tewari M, Joshi H (2017) Assessment of hydrochemical properties and annual variation in meltwater of Gangotri glacier system. ENVIS Bullet Himalayan Ecol 25:17–23
Sarkar T, Kannaujiya S, Taloor AK, Ray PKC, Chauhan P (2020) Integrated study of GRACE data derived interannual groundwater storage variability over water stressed Indian regions.Groundwater Sustain Develop 10:100376. https://doi.org/10.1016/j.gsd.2020.100376
Sawyer GN, McCarthy DL (1967) Chemistry of sanitary engineers, 2nd edn. McGraw Hill, New York, p 518
Siegenthaler U, Oeschger H (1980) Correlation of 18O in precipitation with temperature and altitude. Nature 285:314–317
Singh AK, Jasrotia AS, Taloor AK, Kotlia BS, Kumar V, Roy S, Ray PKC, Singh KK, SinghAK SA (2017) Estimation of quantitative measures of total water storage variation from GRACE and GLDAS-NOAH satellites using geospatial technology. Quat Int 444:191–200
Taloor AK, Kotlia BS, Jasrotia AS, Kumar A, Alam A, Ali S, Kouser B, Garg PK, Kumar R, Singh AK, Singh B (2019) Tectono-climatic influence on landscape changes in the glaciatedDurung Drung basin, Zanskar Himalaya, India: A geospatial approach. Quat Int 507:262–273
Taloor AK, Kumar V, Singh VK, Singh AK, Kale RV, Sharma R, Khajuria V, Raina G, Kouser B, Chowdhary NH (2020a) Land Use Land Cover Dynamics Using Remote Sensing and GIS Techniques in Western Doon Valley, Uttarakhand, India. InGeoecology of Landscape Dynamics 2020 (pp. 37–51). Springer, Singapore
Taloor AK, Pir RA, Adimalla N, Ali S, Manhas DS, Roy S, Singh AK (2020b) Spring water quality and discharge assessment in the Basantar watershed of Jammu Himalaya using geographic information system (GIS) and water quality Index(WQI). Groundwater Sust Develop 10:P100364. https://doi.org/10.1016/j.gsd.2020.100364
Wilcox LV (1955) Classification and Use of Irrigation Waters. USDA. Circ 969, Washington, DC
Acknowledgements
The authors are grateful to the Editors of the Book for providing us an opportunity to publish this chapter. We are also extremely thankful to reviewers for constant support during the publication process.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bhat, N.A., Jeelani, G., Ahmad Mir, R. (2021). Application of Environmental Isotopes and Hydrogeochemistry in Groundwater Management—A Case Study of Bringi Watershed, Kashmir Himalayas, India. In: Taloor, A.K., Kotlia, B.S., Kumar, K. (eds) Water, Cryosphere, and Climate Change in the Himalayas. Geography of the Physical Environment. Springer, Cham. https://doi.org/10.1007/978-3-030-67932-3_20
Download citation
DOI: https://doi.org/10.1007/978-3-030-67932-3_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67931-6
Online ISBN: 978-3-030-67932-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)