Abstract
The binomial nervous system involves the central nervous system (CNS), comprising the brain and the spinal cord, and the peripheral nervous system (PNS). Both divisions of the nervous system contain electrically excitable neurons as well as a number of supporting neuroglial cells, which include oligodendrocytes, astrocytes, microglia, choroid plexus ependymal cells in the CNS, and satellite and Schwann cells in the PNS. Connective tissues rich in fibrillar collagens form the outermost cover for the nervous system proper. Moreover, there are rich basement membranes (BM) surrounding all nervous system tissues and vessels within these structures. BMs compartmentalize nervous tissues and contribute to selective barrier and filtration functions essential for brain homeostasis. While BMs are absent from the brain parenchyma, there are extracellular matrices (ECM) in these regions that remain under-explored. The composition and types of matrices differ substantially in different parts of the nervous system. ECMs are significantly abundant during development, guiding cellular migration and differentiation as well as axon navigation and synaptogenesis. Additionally, ECMs promote neuronal health, contribute to synaptic homeostasis and plasticity, and are upregulated in response to disease and trauma. It is for these reasons that the mutation and malfunction of collagens have been linked to neurodevelopmental, degenerative, and psychiatric disorders as well as motor and sensory dysfunction. In recent years, it has become clear that collagens, constituting a major family of ECM proteins, and other extracellular components are generated not only by glial cells but also by neurons. The functions and expression patterns of the collagen superfamily members in the nervous system are summarized in this chapter, where we focus on their roles in vitro and in vivo in a number of animal models, and in human diseases of the nervous system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Collagen
- Central nervous system
- Peripheral nerve
- Nervous system disease
- Extracellular matrix
- Basement membrane
- Blood–brain barrier
- Neural circuit
- Synaptogenesis
- Neuromuscular junction
8.1 Extracellular Matrix of the Nervous System
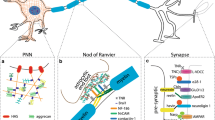
Animals sense changes in their environment through a variety of sensory organs or receptor cells, which then send signals into the brain through afferent sensory neurons of either the PNS or CNS. For example, in the case of the somatosensory and pain systems, the somas of these first-order neurons lie outside the CNS in the dorsal root ganglia (DRG), where they are embedded and shielded by peripheral glial satellite cells and extracellular matrices (ECM). In the case of the visual system, however, the detection of light photons occurs in the retina, a portion of the CNS that resides in the back of the eye. Signals from the retina are sent to the brain along the optic nerve, which is also a component of the CNS. Regardless of whether sensory signals propagate along components of the PNS or CNS, once they reach the brain they are processed by a complex network of central excitatory and inhibitory interneurons. The neurons of the brain are supported by astrocytes and ensheathed by oligodendrocytes. The astrocytes further function in limiting other structures such as the blood–brain barrier (BBB) and meninges, and they also guide neuronal migration. The astrocytes also contribute to neurotransmission by maintaining the homeostasis of the brain’s extracellular compounds. In response to internal and external stimuli, central neurons conduct decisions to command efferent neurons of the PNS. Efferent axons of motor nerves run from the spinal cord nuclei, where their somas reside, to innervate their peripheral targets, muscles and glands. In the peripheral nerve, Schwann cells generate support and myelin around axons to promote neural actions. In this chapter, we go through various ECMs of the PNS and CNS with an established link with collagens. Despite collagens being relatively broadly expressed in the retina, retinas are not discussed here as we chose to mainly focus on the brain.
8.1.1 ECMs of the Central Nervous System
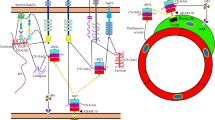
In adults, the CNS is encapsulated by the BM, a specialized ECM chiefly built of laminin and collagen IV networks interconnected by nidogens and perlecan, providing comprehensive boundaries to tissue structures. The BM is further covered by ECM that is especially rich in fibrillar collagens, and the two are interconnected by a complexity of molecules (Hubert et al. 2009; Gordon and Hahn 2010; Ricard-Blum 2011). Together with cellular components, these ECMs form the outermost, protecting layer of the CNS, the meninges (Fig. 8.1a). All three layers of the meninges, the pia mater, arachnoid mater, and dura mater, derive from the meningeal mesenchyme and are of neural crest origin. Meningeal cells produce many ECM constituents, including BM components and interstitial fibrillar collagens, and together these extracellular cues promote the development of radial glial cells, a key component of the developing brain that plays critical and necessary roles in directing neuronal migration (Sievers et al. 1994; Hartmann et al. 1998). At the edge of brain tissues, astrocyte end-feet produce components that contribute to the pial BM and establish the cortical glial limitans (Sievers et al. 1994; Heck et al. 2003). The pia mater separates the brain from the cerebrospinal fluid circulating within the subarachnoid space. Choroid plexuses filter and secrete the cerebrospinal fluid that both protects and nourishes the brain and the spinal cord. The BMs of both ependymal epithelia and capillaries participate in this filtration process from blood to ventricles (Fig. 8.1b) and compromise in this function may result in hydrocephalus (Utriainen et al. 2004). In addition, the BM plays a relevant role in the CNS blood vessels where it contributes to the BBB, a selective semipermeable segregator between blood and brain. Endothelial cells, pericytes, and astrocyte foot projections, together with the ECM produced by these cell types, all constitute the four layers of the BBB (Fig. 8.1c), which is critical for brain homeostasis. Different from peripheral pericytes, which originate from the mesoderm, BBB pericytes are of neural crest origin. Compromises in the barrier function may result in hemorrhage as well as a wide range of other neurological disorders (Sharif et al. 2018; Xu et al. 2018).
The CNS parenchyma has low amounts of BMs and fibrillar collagens and therefore it relies on the bony skull and vertebra for structural support. Long ago, electron microscopy on brain tissue, which requires highly fixed and dehydrated samples before embedding and sectioning, gave the impression that there is very little extracellular space in the brain. However, this may be an artifact of these imaging techniques (Korogod et al. 2015), and increasing evidence indicates that the brain is in fact richer in ECMs than previously thought. Specialized brain ECMs exist in several distinct forms and several precise locations. Unlike outside of the nervous system, where BMs are largely composed of collagens, laminins, nidogens, and proteoglycans, the primary ECM constituents in the brain include proteoglycans and glycosaminoglycans (Novak and Kaye 2000; Zimmermann and Dours-Zimmermann 2008; Krishnaswamy et al. 2019). One specialized brain ECM rich in these components is the fractone (Fig. 8.1d), which is associated with neural stem cell niches adjacent to the walls of the lateral ventricles (Kerever et al. 2007). A second, specialized brain ECM is the perineuronal net (PNN), a robust structure that ensheaths the soma and proximal processes of selective interneurons dendrites and the initial segment of axons (Fig. 8.1e). Not only do PNNs provide support and promote neuronal health, they contribute to synaptic plasticity as they emerge at the end of the critical period of brain development. In addition to these well-defined ECMs, it is becoming abundantly clear that astroglial processes that contact pre- and postsynaptic elements (and therefore contribute to the tripartite synapse) generate and deposit ECM into and around the synaptic cleft, which is critical for synapse formation and function (Rohrbough et al. 2007; Ferrer-Ferrer and Dityatev 2018; Song and Dityatev 2018). In fact, the role of the ECM is so important at brain synapses (like those of the neuromuscular junction in the PNS) that it may be better to re-term the central synapse as a tetrapartite synapse. In the developing brain, the extracellular space is even more substantial and during this period the brain ECM plays important roles in axon guidance, synaptogenesis, neurotransmission, and plasticity. Reduced brain ECM is connected with complex neurological diseases, but on the other hand, accumulation of ECM aggregates also promotes cellular dysfunction and neurodegeneration (Frischknecht and Gundelfinger 2012; Mouw et al. 2014). As we learn more about the roles of ECM in brain development, function, and disease, there is growing evidence that non-fibrillar collagens, such as collagens VI, XVII, XVIII, XIX, and XXV (Hashimoto et al. 2002; Seppänen et al. 2006; Su et al. 2010; Cescon et al. 2016) are not only generated by neural cells but also that brain development and function requires them and/or their proteolytically released, functionally active fragments, collectively termed as matricryptins (Fox 2008; Su et al. 2010, 2012, 2016, 2017; Cescon et al. 2016).
8.1.2 ECMs of the Peripheral Nervous System
While the ECM is relatively sparse in the CNS proper, the PNS is rich in ECMs. In a peripheral nerve, all motor axons, as well as a portion of sensory and autonomic axons, are individually encapsulated by myelinating Schwann cells. Small caliber, unmyelinated sensory axons, called C-fibers, are enwrapped by non-myelinating Schwann cells. The BM surrounding axons, and its adjacent ECM containing thin collagen fibrils (Osawa and Ide 1986), are both produced by the Schwann cells and constitute together the endoneurium which provides insulation. Axons, vessels, supporting cells, and the interstitial ECM together form a nerve fascicle that is covered by a tight perineurium, which is produced by perineural fibroblasts and serves as a diffusion barrier. Several fascicles constitute the peripheral nerve, covered by epineurial BM which is further ensheathed by ECM that is rich in fibrillar collagens, like the CNS (Fig. 8.1f). The epineurium constructs the nerve trunk and provides mechanical strength. The epineurial collagen fibrils are as thick as those in the dermis of the skin, while the endoneurial collagen fibrils generated by the Schwann cells are thin (Osawa and Ide 1986). Although collagen IV is a typical and essential component of endo-, peri-, and epineural BMs, several other, nontypical collagens associate with the peripheral nerve BMs, and these are discussed here. Schwann cells, for their part, express critical receptors on their cell membrane, through which collagen-rich/dependent ECMs transmit signals to affect cell behavior such as adhesion and migration (Chernousov et al. 2008). As described above for the brain, during PNS development the ECM plays essential roles in neural migration, axon outgrowth, and guidance, and in the formation of peripheral synapses, such as the neuromuscular junction (NMJ) (Fox et al. 2007). In the PNS, the ECM surrounding nerve fibers is so robust that it is preserved following nerve injury (such as nerve crushing or cutting), and in these cases, the preserved endoneurium and perineurium serve as a hollow tube, studded with extrinsic guidance factors, that not only provide a path for regenerating axons to regrow, but also facilitate regrowth (Sanes and Lichtman 1999; Nguyen et al. 2002).
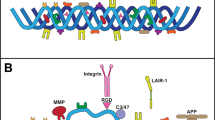
At innervation, the neural axon forms synapses with the muscle to form the NMJ (Fig. 8.1g). Non-myelinating terminal Schwann cells enshield the axon terminus and are themselves encapsulated by BM. The postnatal motor synapse is further covered by mesenchymal cells, kranocytes, which are suggested to contribute to synapse regeneration (Court et al. 2008). The BM that passes through the synapse, between the nerve and muscle, is unique in its ECM composition and contains specific synaptic isoforms different from the muscle BM counterparts (Fig. 8.1g). The synaptic BM is primarily and prior to innervation secreted by the developing postsynaptic muscle, but later also other cellular parties of the NMJ contribute to the synaptic BM. These molecules maintain the homeostasis and enable plasticity by both retro- and anterograde signaling (Sanes and Lichtman 1999, 2001; Kummer et al. 2006; Heikkinen et al. 2020). Moreover, the ECMs embed “hidden” potential in regulating cell behavior; for example, many ECM components are capable of releasing matricryptins which, once liberated from the ECM, can contribute to distinct bio-activities such as acting as guidance or synaptogenic cues (Ackley et al. 2001; Fox et al. 2007; Meyer and Moussian 2009; Ricard-Blum and Ballut 2011).
8.2 Distribution of Collagen Subtypes in the Nervous System
The collagen superfamily comprises 28 distinct collagen types that are divided into subfamilies according to the three-dimensional assemblies they form. Due to their trimeric characteristics, each distinct collagen type can be encoded by a single gene or by multiple genes. The distribution of collagens in the CNS and PNS is summarized in Tables 8.1 and 8.2, respectively.
8.2.1 Fibrillar Collagens and Other Fibril-Forming Collagens
Fibrillar collagens, comprised of collagen types I, II, III, V, XI, XXIV, and XXVII, form the best-known collagen subfamily as they are assembled into structural fibers that densely populate connective tissues throughout the body. Fibrillar collagens form heterotypic fibers where collagens III/V and XI preferentially incorporate collagen I and II fibers, respectively. The former collagen types nucleate the fibers, but they also limit the fiber diameter by occasional N-peptides retained. Through the latter property, fibers achieve unique features that are important for tissue-specific functions (Myllyharju and Kivirikko 2004; Gordon and Hahn 2010; Ricard-Blum 2011). As mentioned, fibrillar collagens form the outermost layer of both the CNS and PNS. Pia Mater is rich in collagens I and III while collagen V colocalizes with collagen IV at the pial BM (Sievers et al. 1994), and is expressed already in the neuroepithelium (Roulet et al. 2007). Collagen V is present in the ECM of small CNS capillaries, but collagen I/III fibrils are absent (Maxwell et al. 1984; Munji et al. 2019). Collagen II, typically found in cartilaginous tissues, is transiently expressed in the developing choroid plexus and meninges, temporally coinciding with active tissue remodeling (Cheah et al. 1991; Andrikopoulos et al. 1992; Sandberg et al. 1993; Lui et al. 1995a; Yoshioka et al. 1995). Collagen XI coexists with collagen II and is similarly transiently present in developing meninges and the brain cortex (Nah et al. 1992; Sandberg et al. 1993; Lui et al. 1995b; Yoshioka et al. 1995). Collagens XXIV and XXVII are either absent or present at very low levels in the brain (Boot-Handford et al. 2003; Matsuo et al. 2008). Although collagen VII is definitively not a fibrillar collagen, it forms anchoring fibers that are especially critical in anchoring of the epidermis to the dermis in the skin (see Chap. 7 for further details). In the brain, collagen VII underlies choroid plexus epithelia and surrounds pineal gland and pituitary gland cell nests (Paulus et al. 1995).
In the PNS, collagen I is generally abundant in endo-, peri-, and epineurium, while collagens III and V are rather enriched in endo- and perineurium (Shellswell et al. 1979; Osawa and Ide 1986; Wälchli et al. 1994). Different from collagen III, in that it exclusively encompasses delicate endoneurial collagen fibrils, collagen V is additionally present at the Schwann cell BM (Shellswell et al. 1979; Chernousov et al. 2006). The incorporation of collagen V limits the diameter of collagen fibers (Birk et al. 1990) and speculatively may therefore motivate the presence of small-caliber fibers in the endoneurium (Osawa and Ide 1986). Collagen II is expressed during development by Schwann cell precursors, later by both non-myelinating and myelinating Schwann cells, and in adulthood, it accumulates at the nodes of Ranvier (D’Antonio et al. 2006). The expression of collagen II is regulated by nerve-derived signals (D’Antonio et al. 2006). Since deficiency of major fibril-forming collagens results in devastating connective tissue diseases, such as osteogenesis imperfecta and Ehlers–Danlos syndromes (see Chaps. 2 and 3), assessing their roles specifically in the nervous system is challenging.
8.2.2 FACIT Collagens
As the name implies, FACIT collagens (fibril-associated collagens with interrupted triple helices) associate with fibrils with a preference for a major collagen type, I or II. FACITs contribute to fibrillogenesis by limiting the fibril diameter and participate in forming supramolecular aggregates within the ECM. Collagen types IX, XII, XIV, XVI, XIX, XX, XXI, and XXII constitute the FACIT subfamily (Wälchli et al. 1994; Gordon and Hahn 2010). The abundance of some of these FACITs varies within the nervous tissue, with some being present broadly in the nervous system and some being restricted to very localized brain regions.
Collagen IX is bound to collagen II fibrils and in developing chicken it is found to be present in the meninges (Ring et al. 1995). Collagen XII stabilizes collagen I fibers and by antibody staining on mouse tissues, it is defined in the dense connective tissue of meninges, but also in the endoneurium and perineurium, especially during embryonic development (Oh et al. 1993). Collagen XIV limits lateral fibril fusion, and in situ hybridizations and immunofluorescence staining of chicken embryos show that collagen XIV colocalizes with collagen I in the epineurium and perineurium (Wälchli et al. 1994). Collagen XVI modulates ECM aggregates and is present both in the CNS and PNS. In situ hybridizations reveal adult mouse hippocampus positive for Col16a1 signal and in cultured hippocampal neurons, collagen XVI locates in the soma, along neurites and at the axonal growth cone (Hubert et al. 2009), resembling findings on DRG neurons in the PNS (Hubert et al. 2007). Studies in mice indicate that collagen XVI is an ECM component of the DRG where it surrounds neuronal cell bodies (Lai and Chu 1996). Its expression peaks at around birth and reverts again if exposed to nerve injury (Lai and Chu 1996; Hubert et al. 2007). Increasing Col16a1 gene expression in the DRG in the latter half of gestation suggests that it may participate in the final steps of DRG maturation. In culture, besides the soma, axotomized DRG neurons, preconditioned for regenerative growth, exhibit collagen XVI along neurites but also at their tips, representing the growth cone. Besides neurons, collagen XVI is highly induced in Schwann/fibroblastic cells growing out from axotomized DRG explants (Hubert et al. 2007). A combination of neuronal and glial cell deposition of collagen XVI in vivo is thus highly probable.
Based on its primary structure, collagen XIX resembles FACIT collagens (Khaleduzzaman et al. 1997) but immunohistochemistry suggests it associates in PNS with BMs (Myers et al. 1997; Sumiyoshi et al. 2001, 2004). Collagen XIX expression peaks transiently during embryonic muscle development, following myogenic regulatory factor Myf-5 expression and declines as myogenin emerges in differentiating skeletal muscle progenitors (Sumiyoshi et al. 2001). Studies in zebrafish have revealed its expression at intermediate targets of navigating motor axons (Beattie et al. 2000; Hilario et al. 2010). Moreover, collagen XIX is expressed by developing and postnatal smooth muscle cells of the gastroesophageal junction prior to their transdifferentiation into skeletal muscle cells (Sumiyoshi et al. 2001). In the brain, collagen XIX expression increases postnatally, peaks during periods of synaptogenesis, and in the adult brain it is largely confined to telencephalic interneurons (Sumiyoshi et al. 2001; Su et al. 2010, 2016).
8.2.3 Network-Forming Collagens
Collagens IV, VI, VIII, and X form networks and sheath-like structures. Collagen X acts primarily outside the nervous system. With the aid of amino-terminal 7S domain and carboxyterminal NC1 (non-collagenous) domain, collagen IV self-assembles into a network to constitute BMs together with laminins, nidogens, and perlecan (Xu et al. 2018; Gatseva et al. 2019). Collagen IV presents in three heterotrimeric isoforms; [α1(IV)]2α2(IV), α3(IV)α4(IV)α5(IV), and [α5(IV)]2α6(IV), giving rise to chain-dependent matricryptins. The classical [α1]2α2(IV) isoform is broadly expressed and it is the main isoform in BMs of both CNS and PNS (Miner and Sanes 1994; Khoshnoodi et al. 2008). Due to its abundance, a broad clinical spectrum of human diseases arises from COL4A1 and COL4A2 mutations (see also Chap. 5). Various immunohistochemical and transcript-level studies on human and animal tissues have shown that [α1(IV)]2α2(IV) colonizes all CNS BMs. Collagen IV appears in the developing BMs very early on in embryonic development (Dziadek and Timpl 1985). At mid-gestation in mice, collagen IV locates beneath the neuroepithelium (Sund et al. 2001a). In the adult brain, it shields the glia limitans at pial BM, ependymal, and vascular BMs of the choroid plexus and BMs of meningeal and intraparenchymal vessels, as well as gray and white matter BBB capillaries (Shellswell et al. 1979; Roggendorf et al. 1988; Halfter et al. 1998; Kleppel et al. 1989; Sievers et al. 1994). Together with some other BM proteins, [α1(IV)]2α2(IV) is a component of fractones (Kerever et al. 2007). Collagen IV is synthesized at the glia limitans by both meningeal cells and astrocytes (Urabe et al. 2002), and by both endothelial cells and pericytes at the BBB (Jeanne et al. 2015). Besides the major [α1(IV)]2α2(IV) isotype, α3(IV)α4(IV)α5(IV) is found at the choroid plexus and [α5(IV)]2α6(IV) at the glia limitans (Urabe et al. 2002), reflecting context-dependent functions of these collagens IV.
In the peripheral nerve, [α1(IV)]2α2(IV) heterotrimers inhabit the endoneurial and perineural BMs, and collagen IV also occurs in the capsule surrounding the DRG (Shellswell et al. 1979; Miner and Sanes 1994; Halfter et al. 1998; Sund et al. 2001a). Schwann cells are capable of synthesizing and secreting collagen IV (Carey et al. 1983). While [α1(IV)]2α2(IV) heterotrimers inhabit all muscle BMs, α3-α6 chains are present exclusively in the synaptic BM at the NMJ where they appear in the third postnatal week in mice (Miner and Sanes 1994; Fox et al. 2007).
Although six distinct collagen VI alpha chains exist in mammals, typically collagen VI molecules are composed of α1, α2, and α3 chains, with the secretion of collagen VI molecules being regulated by the latter (Lamande et al. 1998). Collagen VI molecules form antiparallel dimers and further tetramers, and in the ECM they are deposited as end-to-end beaded microfilaments (Gregorio et al. 2018). Collagen VI was originally reported to be present in the brain, both in the BMs and nervous tissue proper (Roggendorf et al. 1988). Collagen VI expression is defined within the superficial glia and the sheath of cranial nerves, the adventitia of meningeal and larger intraparenchymal vessels and choroid plexus, starting at the embryonic stage and persisting into adulthood (Roggendorf et al. 1988; Kamei et al. 1992; Dziadek et al. 1996; Cescon et al. 2016). Moreover, nerve cell-related transcripts for α1-α3 chains are identified in the brain (Karkheiran et al. 2013; Zech et al. 2015; Cescon et al. 2016) and collagen VI protein is detected in the hippocampus and corpus callosum (Cescon et al. 2016). Cultured cortical and hippocampal neurons produce collagen VI, which retains primarily intracellular (Cescon et al. 2016). Transcripts for α4-α6 chains are also present in both developing and adult mouse brain (Gara et al. 2008), but their distribution remains to be resolved.
In the adult peripheral nerve, collagen VI inhabits the BMs at the endo-, peri-, and epineurium (Keene et al. 1988; Peltonen et al. 1990; Allen et al. 2009; Chen et al. 2014) where it is deposited by Schwann cells, perineural cells, and fibroblasts (Peltonen et al. 1990; Muona et al. 1993; Chen et al. 2014). During development, collagen VI expression is induced in Schwann cells by nerve-derived neuregulin at the time of differentiation toward a myelinating phenotype and is downregulated in mature myelinating Schwann cells (Vitale et al. 2001). Furthermore, resident macrophages in the peripheral nerve are capable of producing collagen VI (Chen et al. 2014), similar to cultured human macrophages (Schnoor et al. 2008). Moreover, collagen VI inhabits the synaptic BM at the NMJ (Cescon et al. 2018). Primarily COL6A1/COL6A2/COL6A3 mutations affect the muscle, causing severe Ullrich congenital muscular dystrophy and milder Bethlem myopathy. Mouse models have allowed to segregate disease phenotypes and helped in defining a wide range of collagen VI functions (see also Chap. 6). Collagen VI in the nervous system was thoroughly reviewed recently (Gregorio et al. 2018).
Structurally, collagen XXVIII, the latest member of the collagen superfamily, resembles collagen VI, although it does not form networks. Its expression is highly restricted to special structures in the PNS. Collagen XXVIII surrounds non-myelinating glial cells of the PNS; satellite cells of the DRG, non-myelinating Schwann cells of peripheral nerves, and terminal Schwann cells of sensory end organs and NMJs, but also nodes of Ranvier at myelinated axons (Veit et al. 2006; Grimal et al. 2010).
Yet another network-forming collagen, collagen VIII, is expressed by several ectoderm-derived ECMs, although its overall distribution is limited. In the CNS it appears in fibrillar arrays at the meninges surrounding the brain, spinal cord, and optic nerve, and in the white matter of the spinal cord (Kapoor et al. 1988; Muragaki et al. 1992). Proteolytic processing of collagen VIII gives rise to matricryptin vastatin (Ricard-Blum and Ballut 2011).
8.2.4 Multiplexin Collagens
Multiplexin (multiple triple-helix domains with interruptions) collagens XV and XVIII share structural similarities, owing to their common evolutional origin. Collagen XVIII presents in three amino terminally different isoforms; the short, medium, and long, which share common collagenous portions and a carboxyterminal endostatin domain. This proteolytically releasable matricryptin conveys distinct biological activities, and a corresponding domain also exists in collagen XV. Collagen XVIII is a heparan sulfate proteoglycan, while collagen XV bears both heparan and chondroitin sulfate side chains (Bretaud et al. 2020). Collagen XVIII forms are differentially deposited in various BMs where they orient in a polarized manner, while collagen XV bridges the rear face of BM with surroundings (Seppinen and Pihlajaniemi 2011; Heljasvaara et al. 2017; Gatseva et al. 2019). Collagen XV exists both in the CNS and PNS. It is transiently expressed in the developing CNS capillaries, while in adults collagen XV is downregulated in BBB vessels but confined primarily to meningeal and choroid plexus blood vessels (Muona et al. 2002). In the PNS in mice, collagen XV appears in the skeletal muscle upon primary myotube formation. It is deposited to the developing BM, delineating individual muscle fibers and persisting into adulthood. Moreover, collagen XV is enriched at the NMJ, but not in the synaptic BM but instead in the terminal Schwann cell-capping BM. Collagen XV is a component of the developing endoneurial and perineural BM zones and it is also transiently expressed in the ECM of developing DRG (Muona et al. 2002). In vivo mouse studies emphasize the significance of collagen XV in Schwann cell differentiation.
Early studies with chicken embryos show that collagen XVIII delineates the pial BM, DRG, and peripheral nerve, and it is also detected in post-hatch chicken peripheral nerves and muscle fibers (Halfter et al. 1998). Further immunolabelling studies on mouse and human tissues confined collagen XVIII at the developing and adult BMs of pia (Miosge et al. 2003; Utriainen et al. 2004; Caglayan et al. 2014). Besides pial BM, collagen XVIII is present at the BMs of most brain blood vessels and those associated with the choroid plexus (Miosge et al. 2003; Utriainen et al. 2004; Caglayan et al. 2014). In the brain parenchyma, all three Col18a1 transcripts are detected exclusively in the cerebellum, expressed by Purkinje neurons and temporally coinciding with synaptogenesis. The presence of endostatin in extracted synaptosomes suggests its involvement in synaptogenesis (Su et al. 2012). The polarized orientation of collagen XVIII in BMs was originally identified in the Bruch’s membrane beneath the retinal pigment epithelium (Marneros et al. 2004). In the developing mouse PNS, the ganglial BM and endoneurium stain positively for collagen XVIII (Miosge et al. 2003). Similar to collagen XV, collagen XVIII is a component of the terminal Schwann cell BM at the NMJ (Su et al. 2012) and it also inhabits extrasynaptic muscle BM (Saarela et al. 1998).
8.2.5 Transmembrane Collagens
Types XIII, XVII, XXIII, and XXV are type II transmembrane collagens, composed largely of collagenous sequences. All four membrane-spanning collagens are shed by proteases to release pericellular ectodomains (Franzke et al. 2005; Heikkinen et al. 2012). Collagen XVII is unique in primary structure while collagens XIII, XXIII, and XXV share structural similarities due to their evolution through gene duplication of a common ancestor and are collectively called membrane-associated collagens with interrupted triple helices (MACIT) (Tu et al. 2015).
An early in situ hybridization study did not detect COL13A1 transcripts in the fetal human brain (Sandberg et al. 1989). Immunostaining on mouse embryos, however, suggests a transient expression of collagen XIII during mouse development in neurons of the CNS as well as in the PNS. In the late embryonic period meninges also become positive for collagen XIII (Sund et al. 2001a). Postnatally, collagen XIII expression in the brain becomes radically reduced and Col13a1 transcripts are hardly detectable in distinct brain regions (Monavarfeshani et al. 2017). Instead, meninges as well as blood vessels in the brain (Fig. 8.2) and peripheral nerve exhibit collagen XIII protein in adult mice (Zainul et al. 2018). Furthermore, collagen XIII colonizes the NMJ (Hägg et al. 2001; Latvanlehto et al. 2010; Logan et al. 2015; Härönen et al. 2017, 2019; Zainul et al. 2018) where it is expressed postsynaptically, shed, and incorporated into the synaptic cleft BM (Latvanlehto et al. 2010). Collagen XIII deficiency causes congenital myasthenia, highlighting its importance at the NMJ.
Expression of collagen XIII in the cerebral cortex. Adult mouse brain stained (a) for collagen XIII with an anti-human collagen XIII/NC3 antibody (Hägg et al. 1998), (b) for perlecan, and (c) merged. Arrows point to pia mater
In situ hybridization confines collagen XXIII expression in the developing dura mater of the brain and spinal cord in the mouse embryo (Koch et al. 2006). A detailed examination of different CNS regions reveals Col23a1 transcripts selectively in the accessory olfactory bulb (Monavarfeshani et al. 2017). According to Western blotting, in the adult mouse brain collagen XXIII protein is primarily in proteolytically processed forms (Koch et al. 2006). To date, no human disease or study on mutant animals has been published on collagen XXIII.
The shed form of collagen XXV (originally named collagen-like Alzheimer amyloid plaque component, CLAC) was originally found to associate with senile plaques in the Alzheimer’s disease (AD) brain and its precursor, collagen XXV (CLAC-P), expressed by CNS neurons (Hashimoto et al. 2002). In the mouse brain transcripts for Col25a1 are present both during embryonic development and in adulthood (Tanaka et al. 2014; Monavarfeshani et al. 2017). The spinal cord demonstrates higher collagen XXV expression during development compared to the adult situation (Tanaka et al. 2014).
In the brain, collagen XXV becomes increasingly expressed postnatally, being far more prevalent than the other MACITs (Monavarfeshani et al. 2017). Collagen XXV is found broadly in distinct subsets of both inhibitory and excitatory CNS neurons, with the hippocampus exhibiting the highest expression (Monavarfeshani et al. 2017). Collagen XXV is enriched in regions of the brain innervated by axons of retinal ganglion cells, and it can also be found in the retina per se (Kay et al. 2011; Monavarfeshani et al. 2017).
Besides being present both in the developing and adult CNS, collagen XXV is transiently expressed early in the developing muscle, coinciding with myogenic differentiation and becoming depressed by nerve-induced excitation (Tanaka et al. 2014; Goncalves et al. 2019; Munezane et al. 2019). In addition, developing spinal motor neurons and DRG neurons are positive for Col25a1 transcripts, as demonstrated at the time of myoblast fusion (Tanaka et al. 2014). Muscle-derived collagen XXV is important in the development of the neuromuscular system.
Collagen XVII is the fourth transmembrane collagen with a unique structure and is different from the MACIT collagens XIII/XXIII/XXV. Similar to the latter, its ectodomain can also become proteolytically shed (Franzke et al. 2005). Collagen XVII is a component of dermal hemidesmosomes and genetic mutations lead to junctional epidermolysis bullosa while autoantibodies targeting shed collagen XVII cause a blistering disease bullous pemphigoid (BP), whereby collagen XVII is also known as BP180 (see Chap. 7 for further details). Collagen XVII is also broadly although variably present among different neuroanatomical regions of the CNS which share a common developmental origin with the ectoderm (Seppänen et al. 2007). Therefore, it is not surprising that BP patients exhibit variable neurological symptoms. Collagen XVII is expressed in human cortical, hippocampal and amygdaloid neurons, most intensively in motor nuclei and Betz cells, and pyramidal neurons (Seppänen et al. 2006, 2007, 2009). Collagen XVII colonizes the soma and proximal axons and it accumulates intracellularly in neuronal lipofuscin granules (Seppänen et al. 2007, 2010) but does not cumulate over time (Seppänen et al. 2007).
8.3 Collagen Functions in the Nervous System
Collagens are critical constituents of the nervous system ECMs. Since many of the different collagen types often occupy ECMs in several tissues, collagen-related diseases typically involve several organ systems. Collagens and their proteolytic products are involved in multiple signaling pathways and provide extracellular cues that are important in guiding developmental processes. Such actions are reutilized in tissue repair in response to injury, and consequently, several collagens typically become upregulated. Further in adulthood, collagen assemblies provide support and flexibility in stabilizing cellular structures such as synapses. Aging impairs cellular metabolism, which also affects the extracellular environment and results in protein accumulation, neurotoxicity, and neurodegeneration. Mutations in genes coding for collagens result in a broad range of symptoms affecting nervous tissues and thereby have a major impact on patients’ life. Neuropathologies entailing collagens are reviewed in the following paragraphs. In addition, the involvement of collagens in inherited CNS and PNS diseases and gene-specific animal models are summarized in Tables 8.3 and 8.4, respectively.
8.3.1 Neuronal Migration
Cell migration and differentiation lay the foundations for the development of specialized organs in multicellular organisms. ECM provides qualitative, temporal, and spatial information for migrating and differentiating cells during development for them to properly form a functional tissue. ECMs of the developing meninges promote radial glia organization and together these deposit cues and routes for migrating neural cells, respectively (Fig. 8.3a). Several members of the collagen superfamily, most importantly fibrillar and BM collagens, support morphogenesis during development, applying in the nervous system. Collagen I further promotes neural maturation. Collagen I actions are exemplified by a broad range of neuropathological findings in Osteogenesis imperfecta patients (see also Chap. 2). Those include brainstem compression but also macrocephaly and ventriculomegaly, impaired neuroblast migration, hippocampal malrotation, agyria, and abnormal neuronal lamination, among others (Charnas and Marini 1995; Emery et al. 1999). Mice deficient in collagen II display a partially penetrant phenotype showing loss of head tissue and holoprosencephaly (Leung et al. 2010). Furthermore, pial BM constituent collagen IV also contributes to brain development. Due to BBB breakage and resultant hemorrhage, collagen IV mutations (detailed later) promote the development of secondary porencephalic lesions. Alternatively, this may directly result from impaired neuronal ventricular-to-cortex migration that is dependent on radial glial support (Fig. 8.3a). Collagen IV mutations may compromise the interaction of glial end feet projections with the pial BM. In fact, Col4a1/a2-deficient embryos demonstrate focal disturbances in pial BM and concurrent misplacement of migrating neurons (Pöschl et al. 2004). Additionally, collagen XIII may affect neural migration since transgenic overexpression of truncated molecules delays brain development. Ectopic collagen XIII enhances axon outgrowth in rat hippocampal neuron cultures, suggesting a means by which collagen XIII may influence brain formation. These collagen XIII mutant mouse embryos die due to defects in angiogenesis, which provides an alternative explanation (Sund et al. 2001b).
Pathophysiological conditions of the nervous system involving collagens. Defects (a) in neural migration may result in malformations, (b) in integrity of barrier and filtration functions result in hemorrhage and hydrocephalus, respectively, (c) in neural circuit development, synaptogenesis and synaptic homeostasis lead to malfunctioning, (d) in myelination compromises peripheral nerve structure and function, (e) in the nervous system ECM integrity may result in neurodevelopmental/psychiatric/degenerative conditions, and other processes involving collagens include (f) regeneration from disease or traumatic injury
Loss-of-function mutations in COL18A1 resulting in deficiency of one or all collagen XVIII isoforms cause Knobloch syndrome (OMIM #267750), a growingly heterogeneous rare disorder defined by high myopia, vitreoretinal degeneration associating with retinal detachment and often associating with occipital scalp defect causing encephalocele (Sertie et al. 2000; Suzuki et al. 2002, 2009; Kliemann et al. 2003; Passos-Bueno et al. 2006; Keren et al. 2007; Mahajan et al. 2010; Caglayan et al. 2014; Haghighi et al. 2014; Zhang et al. 2018). Immunolabeling studies on mouse and human tissues indicate that collagen XVIII delineates the developing and adult BMs of pia (Utriainen et al. 2004; Caglayan et al. 2014). Deposition of collagen XVIII provokes novel interactions in the ECM which subsequently contribute to guiding the neuroectodermal morphogenesis and cell migration in the closure of the neural tube. Moreover, the existence of neuronal migratory and circuit formation defects in Knobloch syndrome are evident and exemplified by various CNS malformations, such as pachygyria and polymicrogyria associated with epilepsy (Suzuki et al. 2002; Kliemann et al. 2003; Keren et al. 2007; Caglayan et al. 2014; Charsar and Goldberg 2017; Corbett et al. 2017; White et al. 2017). In collagen XVIII-deficient mice, the pial BM appears outwardly normal, and obvious CNS malformations are not observed (Utriainen et al. 2004), evidencing species specificity in this respect. Studies in zebrafish have shown that collagen XVIII is involved in neural migration in the PNS. In this model, collagen XVIII transcripts are transiently expressed in a subset of muscle cell precursors (adaxial cells) in close contact with the path taken by motor axon and neural crest cells around the onset of motor axon outgrowth and neural crest cell migration (Schneider and Granato 2006). Morpholino knockdown of col18a1 in zebrafish results in both motor axon extension and neural crest cell migration defects which both normally follow the same path through the muscle territory in the somites (Schneider and Granato 2006; Banerjee et al. 2013). Interestingly, this literature hypothesizes that collagen XVIII affects motor axon extension and neural crest cell migration via its glycosylated side chains, added by the glycosyltransferase Lhr3, as the col18a1 morphants and the lhr3 mutant diwanka display similar motor axon and neural crest cell phenotypes. However, this still remains an untested hypothesis which will be among the next upcoming technical challenges in the field.
8.3.2 Barrier and Filtration Functions
The formation and maintenance of a fully functional BBB requires seamless cooperation of all four constituent layers; astrocytes, pericytes, endothelial cells, and the extracellular BM. Collagen IV is a critical component of BMs and in mice Col4a1/a2 deficiency results in embryonic disruption of BM structures, causing lethality at embryonic day (E) 10.5–11.5 (Pöschl et al. 2004). Owing to dominant mutations in COL4A1/A2 genes, collagen IV integrates with cerebrovascular disease and intracerebral hemorrhage (Fig. 8.3b) that may result in porencephaly (OMIM #614519, #175780, #614483). Mutations often represent missense mutations of glycines and cause impaired secretion of collagen IV, proposedly due to a dominant-negative effect (Gould et al. 2005; Breedveld et al. 2006; Volonghi et al. 2010; Jeanne et al. 2012). Similarly, in mutant mice, heterozygous Col4a1/a2 mutations compromise vascular BM development, cause local disruption of vascular BM, and result in cerebral hemorrhage at birth, which in turn leads to the development of porencephaly in survivors (Gould et al. 2005; Favor et al. 2007). In preclinical studies, postnatal treatment with chemical chaperones diminished intracellular accumulation of mutant collagen IV in endothelial cells and pericytes, and resulted in reduced disease severity (Jeanne et al. 2015; Hayashi et al. 2018), suggesting a potential frame to develop a therapy to treat human patients (Detailed in Chap. 5).
Interestingly, Dhungana and colleagues showed that collagen XV deficiency is beneficial in ischemic stroke generated in the middle cerebral artery in knock-out mice. Collagen XV is dispensable for cerebrovascular development and integrity in physiology, but changes in the molecular composition of the vascular BM prior to stoke proposedly contribute to beneficial events, such as an increase in VEGF-A. The effect is similar to recombinant tissue plasminogen activator treatment, indeed increasing circulating collagen XV levels in wild-type mice and therefore suggestively acting at least partly through a proteolysis of collagen XV-dependent matrix (Dhungana et al. 2017).
Additionally, some other collagens have been genetically linked to the integrity of BBB. In line with the expression of collagen XIII in intracerebral vessels (Fig. 8.2) and collagen VI at meningeal vessels, genetic polymorphisms of COL13A1 and COL6A3 genes in the Japanese population were found to associate with intracerebral and subarachnoid hemorrhage, respectively (Yoshida et al. 2010). Post-irreversible ischemic damage, collagen XVII immunoreactivity depletes which indicates a dynamic injury response (Seppänen et al. 2007). More recently, genetic polymorphisms in the COL17A1 gene have also been linked to subarachnoid hemorrhage (Yamada et al. 2017).
As collagen XVIII normally occupies both the ependymal and capillary BMs, phenotypical findings in the choroid plexus in collagen XVIII deficiency is not a surprise. Collagen XVIII knock-out mice exhibit broadened BMs, the thickness associating with deteriorated epithelial cells and tight junctions in choroid plexuses. This results in hydrocephalus in collagen XVIII deficient mice, but only within a certain mixed genetic C57 background, indicating that the composition of other factors is critical for the choroid plexus function and cerebrospinal fluid homeostasis within the collagen XVIII context (Utriainen et al. 2004). In support of this, the dilation of ventricles is also reported in Knobloch syndrome patients (Kliemann et al. 2003). BMs are critical actors of biological barriers and filters. This is also evidenced by the involvement of BM and BM-associated collagens, specifically, in these functions.
8.3.3 Neural Circuit Assembly, Maturation, and Maintenance
Studies on animal models have primarily revealed the importance of several collagens in neural circuit formation, synaptogenesis, and synaptic homeostasis both in the CNS and PNS (Fig. 8.3c). For example, CNS malformations in Knobloch syndrome predominate in the forebrain, but collagen XVIII is also expressed by cerebellar Purkinje neurons concomitantly with synaptogenesis, and endostatin is found in synaptic-rich extracts. Although the cerebellum of collagen XVIII-deficient mice is morphologically normal, the assembly of climbing fiber synapses (which are the synapses formed between presynaptic axons from the inferior olivary nucleus and postsynaptic dendrites of Purkinje cells) was impaired in the absence of collagen XVIII. In mice, lack of target-derived endostatin reduces synaptic contacts and compromises presynaptic differentiation. This concomitantly influences the motor performance in a rotarod test, which was confirmed to derive from cerebral defects, as NMJs appear morphologically normal in these mice (Su et al. 2012). In vitro, endostatin induces accumulation of synaptic vesicle proteins, indicative of presynaptic differentiation, and such an effect is reversed by the administration of anti-α3β1 integrin function-blocking antibodies (Su et al. 2012). α3β1 integrin has been identified at presynaptic active zones (Carlson et al. 2010) and further binding experiments confirm this integrin as a presynaptic ligand for the target-derived endostatin on Purkinje cells (Su et al. 2012). Collagen XVIII also acts in the PNS circuit assembly, a function that is also attributed to the closely related collagen XV. Mutants of the single orthologue in worms and flies, as well as for individual multiplexin collagens in fish, all exhibit peripheral axon guidance defects, although such associations are sparse in higher animals (reviewed in Bretaud et al. 2020). Mutants of the collagen XV/XVIII orthologs, cle-1 in C. elegans (Ackley et al. 2001) and dmp (multiplexin) in Drosophila (Meyer and Moussian 2009), fail to establish a proper innervation. CLE-1 accumulates at nerve cords and often at the anterior–posterior axon tracks in a punctate manner in regions rich in synapses. The deletion of the restin/endostatin-embedding NC1 domain results in low penetrance migration and axon guidance defects that are rescued by trimeric NC1 (Ackley et al. 2001), shown to induce cell motility (Kuo et al. 2001). On the contrary, the monomeric restin/endostatin domain is incapable of doing the same, but instead, it exaggerates the navigation defect, which suggests a role as a feedback inhibitor (Ackley et al. 2001). The vertebrate zebrafish genome harbors distinct genes for collagens XV and XVIII and evidence suggests that both proteins (isoforms Col15a1b and Col18a1a) contribute to neural circuit formation. In zebrafish diwanka mutants the myotomal glycosyltransferase activity was found to be essential for axon growth cone migration and motor innervation (Schneider and Granato 2006). Presumably its modifications on collagen XVIII-carbohydrates at muscle precursor ECM may be a prerequisite for interaction with neuronal receptor protein tyrosine phosphatases (PTP) and/or kinases, known to contribute to axon pathfinding and also to bind collagens/heparan sulfate proteoglycans (Vogel et al. 1997; Aricescu et al. 2002; Unsoeld et al. 2013; Munezane et al. 2019).
In a study with mutant zebrafish models, the deposition of collagen XV-B, one of the two collagen XV forms present in zebrafish, in the slow muscle ECM constitutes the path and branching milestones for growing axons in order for them to establish an intact neuromuscular system (Guillon et al. 2016). In the developing slow muscle, adaxial cells respond to Hedgehog/Gli and MuSK signaling by expressing col15a1b and depositing specifically the collagen XV-B protein in the motor path (Bretaud et al. 2011; Guillon et al. 2016). Reminiscent of what was suggested for collagen XVIII, collagen XV-B might also interact with discoidin domain receptors (DDR) to guide motor axon navigation as such a direct interaction between collagen XV and DDR1 is identified on tumor cells (Clementz et al. 2013). Both vertebrate multiplexin homologs, collagens XV and XVIII, are also enriched at the NMJ in the BM capping terminal Schwann cells (Muona et al. 2002; Su et al. 2012). In worms, CLE-1 concentrates at the NMJ near synaptic zones and serves as a synaptic organizer (Ackley et al. 2003). Mutant worms exhibit a high penetrance phenotype of reduced number of synapses and compromised integrity of both postsynaptic and presynaptic structures, such as sprouting (Ackley et al. 2003; Qin et al. 2014). Functional analyses indicate that synaptic transmission in cholinergic synapses is impaired (Ackley et al. 2003). In mice, neonatal examination shows that the motor nerve innervation is correctly patterned in the absence of collagen XVIII. Developmental establishment and postnatal maturation of the NMJ are also normal (Su et al. 2012). Assembly and maintenance of the NMJ are thus far not explored in collagen XV knock-out mice. This would, however, be of interest since collagen XVIII appears not to convey such functions in mammals. Still, clear defects, especially in axon navigation but also in synaptogenesis, exist in mutants of other species. Moreover, studies on collagen XV/XVIII double mutant mice confirm a lack of major compensation between the two multiplexins (Ylikärppä et al. 2003). Yet, collagen XV-deficient mice develop myopathy, and potential impairment in circuit formation could in part contribute to the evolvement of such phenotypes (Eklund et al. 2001).
Collagen IV and its NC1 domain extracted from the electric organ of Torpedo californica was initially identified to promote synaptogenesis on cultured motor neurons, and the recombinant NC1 domains of human α2 (canstatin), α3 (tumstatin), and α6 (hexastatin) chains were found to share this property (Fox et al. 2007). C. elegans genome contains only two collagen IV genes, emb-9 and let-2, encoding respectively the α1(IV) and α2(IV) chains. Mutations in emb-9 result in intracellular persistence of synaptic collagen IV, which promotes ectopic presynaptic boutons at the NMJ (Qin et al. 2014). Col4a1 mutant mice lacking [α1(IV)]2α2(IV) heterotrimers exhibit transient delay in presynaptic differentiation neonatally. The α3(IV)α4(IV)α5(IV) and [α5(IV)]2α6(IV) heterotrimers synergistically maintain synaptic integrity when it is compromised in Col5a4 mutants, while loss of either of the two heterotrimers alone has no detectable effect on the NMJ architecture (Fox et al. 2007).
At the moment, at least 30 distinct genes have been identified to cause congenital myasthenic syndrome (CMS). One of these, CMS19 (OMIM #616720), is caused by loss-of-function mutations in COL13A1, and 16 different mutations have been reported since the first discoveries in 2015 (Logan et al. 2015; Dusl et al. 2019; Marquardt and Li 2019; Rodriguez Cruz et al. 2019). Intriguingly, anti-collagen XIII autoimmune antibodies were recently identified in sera of myasthenia gravis patients, although their disease-causing potential remains to be proven (Tu et al. 2018). Typically, muscle weakness in CMS19 predominates in facial, bulbar, respiratory, and axial muscles and consequently patients suffer from neonatal respiratory distress and severe dysphagia. The critical symptoms often resolve early in infancy, and the disease continues to gradually improve, suggesting a relevant role for collagen XIII specifically in early phases of NMJ maturation postnatally. Along this line, defects in acetylcholine receptor (AChR) clustering and maturation thereafter are obvious in collagen XIII knock-out mice at 2 weeks of age and onward (Latvanlehto et al. 2010). Defects at the NMJ include sprouting axons, retracting terminal Schwann cells, and erroneous spreading of synaptic vesicles in the preterminal axon (Latvanlehto et al. 2010; Härönen et al. 2017). Occasionally, the nerve terminus is detached, allowing Schwann cell invagination (Latvanlehto et al. 2010). On the other hand, knock-in mice incapable of collagen XIII shedding exhibit enhanced transsynaptic adhesion and overwhelming neurotransmission capacity, suggestive of collagen XIII motivating transsynaptic adhesion and/or acting as a receptor for nerve-derived ligand(s). However, NMJs of the knock-in mice are not completely normal (Härönen et al. 2017), implying a relevant role also for the shed form, which was indeed shown to enhance AChR cluster maturation in cultured myotubes (Latvanlehto et al. 2010). Moreover, collagen XIII was found to bind ColQ, the collagenous tail of acetylcholinesterase, and contribute to the fine-localization of them both at the NMJ (Härönen et al. 2017). Interestingly, CMS19 patients do not respond well to anti-cholinesterase treatment in general but do respond to 3,4-Diaminopyridine, a blocker of presynaptic potassium channels and salbutamol, β2-adrenergic receptor agonist (Logan et al. 2015; Dusl et al. 2019; Rodriguez Cruz et al. 2019). Likewise, 3,4-Diaminopyridine improved muscle performance of knock-out mice in the short term (Härönen et al. 2017). Mouse models have demonstrated their value in revealing details of the disease that would not have been achievable by studying human patients solely. For example, muscle histology is relatively normal both in patients and knock-out mice, but mouse studies reveal fiber-type change at the expense of slow muscle fibers (Härönen et al. 2017), especially those dependent on neurotrophic support. Regeneration after injury is delayed in knock-out mice, further pointing to its functions in NMJ maturation. Proposedly, due to preexisting defects at the NMJ, structural and functional recovery of the NMJ in knock-out mice never reaches the pre-injury status (Zainul et al. 2018).
In mice, collagen XXV deficiency interferes with the development of the neuromuscular system far earlier than collagen XIII deficiency. Based on in vitro studies, shed collagen XXV is capable of inducing myoblast fusion into myotubes during primary myogenesis. This process does not involve transcriptional regulation, but collagen XXV rather aids in myoblast adhesion and/or recognition (Goncalves et al. 2019). In line with such a suggested role, muscle development is compromised in collagen XXV knock-out mice (Tanaka et al. 2014; Goncalves et al. 2019). This leads to a total block of motor axon invasion into the muscle, innervation and NMJ formation, and subsequent motor nerve degeneration. Loss of muscle innervation causes neonatal lethality due to respiratory failure (Tanaka et al. 2014). Tissue-specific depletion confirms that muscle-derived collagen XXV is essential for innervation, while nerve-derived collagen XXV appears dispensable (Munezane et al. 2019). Similarly, COL25A1 mutations also affect peripheral innervation in humans, but in a milder way. One form of congenital cranial dysinnervation disorder, congenital fibrosis of extra-ocular muscles-5 (CFEOM5; OMIM #616219), is caused by recessive mutations in COL25A1 influencing the structure of the collagen XXV protein (Shinwari et al. 2015; Munezane et al. 2019). Shinwari and colleagues demonstrated collagen XXV expression in human extra-ocular muscles and decreased levels of axon guidance molecules in an affected patient, suggestive of the disease evolvement from defected innervation of extra-ocular muscles during development (Shinwari et al. 2015). Collagen XXV directly binds with PTP σ and δ on invading axons, thereby inducing cell–cell interaction and axon elongation whereas mutant collagen XXV molecules are incapable of doing the same (Munezane et al. 2019). Interestingly, mutations in the C. elegans MACIT orthologue, col-99 (Tu et al. 2015), compromise peripheral axon guidance suggesting a role for COL-99 as a target-derived cue for axon outgrowth in worms (Taylor et al. 2018). Mouse models in which either collagen XIII and XVII shedding is prevented show that the increased ratio of the transmembrane form over the shed one improves selected physiological events (Jacków et al. 2016a, b; Härönen et al. 2017). Therefore, it would be of interest to evaluate to what extent preventing shedding of collagen XXV would rescue mice from dysinnervation.
Moreover, collagen VI is found to be important for motor synapse integrity and function (see also Chap. 6). It regulates various critical synaptic components through ETS and/or bHLH transcription factor family-dependent pathways and thereby affects AChR cluster homeostasis. In line, collagen VI deficiency in mice results in compromised neurotransmission, due to AChR cluster fragmentation and subunit switch, and altered expression of several synaptic and postsynaptic proteins. Similar alterations were observed in Ullrich congenital muscular dystrophy patients (Cescon et al. 2018). Furthermore, zebrafish morphants for col6a2 and col6a4–6 mammalian orthologue present altered axonal growth and a muscular disease (Ramanoudjame et al. 2015).
Animal studies and in vitro experiments both reveal that collagen XIX self-aggregates into oligomers (Myers et al. 2003). It acts as a pericellular matrix/BM zone organizer by affecting the composition of signaling cues that contribute to intercellular communication. Collagen XIX knock-out mice die by 3 weeks of age from malnourishment due to compromised swallowing and failing relaxation of the sphincter muscle. Lack of collagen XIX prevents the smooth-to-skeletal muscle transdifferentiation in the abdominal segment of the esophagus because of failed myogenin transcription induction and expression in the region that normally expresses collagen XIX. The sphincter muscle innervation is normal, but electron microscopy indicates irregular muscle ECM and thick BM, which compromises NO-dependent relaxation of the sphincter muscle. Related to its association with BM, collagen XIX may act as a BM organizer at the motor synapse as well and contribute to the function of the neuromuscular junction in sphincter muscle (Sumiyoshi et al. 2004). In zebrafish, Stumpy, an orthologue for mammalian collagen XIX, guides motor axon pathfinding and branching exemplified by a halt in trunk motor axons at their intermediate targets in stumpy mutants (Beattie et al. 2000; Hilario et al. 2010). Co-immunoprecipitation and the analysis of the mutant fish that phenocopy incomplete motor axon synaptogenesis observed in stumpy mutants identified the axonal chondrolectin as an interaction partner for muscle-derived collagen XIX through their C-type lectin domain and collagenous repeats, respectively (Oprisoreanu et al. 2019). Collectively, several non-fibrillar collagens act as target-derived organizers in neural circuit development. This is pronounced in the PNS, implying the importance of ECM cues for axon guidance and synaptogenesis.
8.3.4 Myelination
The formation of endoneurium starts at E15 in mice when BM and thin collagen fibrils appear around Schwann cells (Osawa and Ide 1986). Developing nerves at this stage are devoid of fibroblasts (Osawa and Ide 1986) and it has been shown that Schwann cells themselves are capable of producing BM and delicate collagen-fibril matrix (Bunge et al. 1980; Eldridge et al. 1987, 1989; Chernousov et al. 1998, 2006). Specifically, the production of the BM is critical for complete lay-down of the endoneurial ECM and subsequent steps in Schwann cell differentiation and myelination (Eldridge et al. 1989; Chernousov et al. 1998) (Fig. 8.3d). Oligodendrocyte myelination does not require collagen-dependent ECM and therefore collagens are dispensable for the CNS myelination (Eldridge et al. 1989). Altered Schwann cell function and myelination in the PNS is associated with a variety of neuropathies affecting both motor and sensory functions.
In in vitro cultures, the presence of neurons significantly induces Schwann cells to deposit BM (Bunge et al. 1980; Chernousov et al. 1998), indicating that interactions with both axons and ECM are critical for myelination. Early on, the essence of collagen IV in myelination is demonstrated in vitro (Eldridge et al. 1989). The multisystem disorder caused by COL4A1/A2 mutations involves myopathy in a proportion of patients (Labelle-Dumais et al. 2011; Mao et al. 2015; Jeanne and Gould 2017). Symptoms are partially explained by vascular defects but since collagen IV is such an abundant BM component, myopathy obviously evolves from various locations and levels of the BM. Collagen IV mutant mice recapitulate general and progressive human myopathy (Labelle-Dumais et al. 2011; Kuo et al. 2014). During postnatal maturation, sciatic nerves exhibit thickened myelin and impaired radial sorting. This results in reduced nerve conduction velocities and peripheral neuropathy in collagen IV mutant mice. Resembling favorable effects on vasculature, administration of the chemical chaperone 4PBA increases collagen IV secretion and consequently prevents peripheral nerve hypomyelination in mutant mice (Labelle-Dumais et al. 2019).
The significance of collagens in the peripheral nerve myelination is further highlighted by the study of sodium-dependent vitamin C transporter 2 (SVCT2)-deprived mice (Gess et al. 2011). As vitamin C is crucial for collagen synthesis (Booth and Uitto 1981; Murad et al. 1981), the deposition of collagen types IV, V, and XXVIII were found to be noticeably reduced in the peripheral nerve ECM and the peripheral nerve showed decrease myelin thickness in SVCT2+/− mice. Surprisingly, the authors showed that collagen synthesis is regulated at the transcriptional level and not at the posttranslational level revealing a specific mechanism of action of Vitamin C in the PNS. Interestingly, SVCT2 knockdown in Schwann cell cultures resulted in a significant reduction in collagen V synthesis (Gess et al. 2011). In vitro, collagen V promotes axon fasciculation and affiliation of axons in Schwann cells (Chernousov et al. 2001). Atypical for mature fibrillar collagen trimers, collagen V retains N-terminal non-collagenous domains (Chernousov et al. 2000), mediating an autocrine interaction with Schwann cell heparan sulfate receptor glypican-1 and not with collagen-binding integrins α1β1 or α2β1, to regulate myelination (Chernousov et al. 2001, 2006).
Collagen VI also promotes Schwann cell differentiation and regulates myelination and studies on animal models confirm its involvement in peripheral nerve integrity and function. Chen and colleagues showed that the reduction in collagen VI in Col6a1−/− mice does not alter the number of axons or axon fiber type distribution, but it results in hypermyelination of all fiber types later in life. Lack of collagen VI in the ECM deteriorates the regulation of signaling pathways controlling myelination in Schwann cells. This compromises nerve conduction properties and motor coordination of mice. Moreover, C-fibers are thickened and concomitantly the withdrawal response of mutant mice to both thermal and mechanical stimuli is compromised, indicating alteration of nociception (Chen et al. 2014). Collagen VI is thus proven important in Schwann cell homeostasis and function.
Axon segregation and myelination is not affected by the lack of collagen XV in mice, although the endoneurial BM occasionally exhibits mild disorganization. However, this phenomenon is associated with loosely packed axons of C-fibers and compromised radial sorting, resulting in polyaxonal myelination (Rasi et al. 2010). Double deficiency for collagen XV and laminin α4 generates additional phenotypes, further witnessing the importance of Schwann cell BM for proper peripheral nerve maturation (Rasi et al. 2010).
8.3.5 Neurodevelopmental/Psychiatric/Degenerative Diseases
An increasing body of evidence supports the involvement of ECM molecules in the development of neural systems and neurodevelopmental diseases (Jovanov Milosevic et al. 2014) (Fig. 8.3e). COL13A1 mutations cause congenital myasthenia, but in line with the collagen XIII expression pattern, patients also suffer from skeletal defects and occasionally from mild mental retardation (Rodriguez Cruz et al. 2019). Collagen XIX is defined to regions rich in PNNs, and in line, in knock-out mice the number of PNNs and levels of their constituents in distinct locations of the cerebral cortex and hippocampus normally positive for collagen XIX are reduced. This is associated with an increase in the transcripts from several protease-encoding genes involved in PNN homeostasis, exemplified by an ectopic neuronal ADAMTS4 expression in the collagen XIX-deficient cortex (Su et al. 2017). Studies of knock-out mice further show that collagen XIX is required for the establishment of axosomatic synapses in a subset of hippocampal and cortical inhibitory interneurons (Su et al. 2010, 2016). PNNs and inhibitory interneurons are involved in the emergence of schizophrenia and interestingly, collagen XIX-deficient mice express seizures and schizophrenia-like behaviors (Su et al. 2016). In humans, the deletion of a chromosomal region containing COL19A1, but also several other genes, has been associated with schizophrenia (Liao et al. 2012). Intracellularly collagen XIX-dependent cues affect transcriptional regulation and consecutive protein levels (Sumiyoshi et al. 2004; Su et al. 2017). Collagen XIX contributes to the formation of inhibitory nerve terminals in a paracrine fashion through α5β1 integrin signaling (Su et al. 2016). Correct collagen XIX expression appears to be critical for proper neuronal pericellular matrix homeostasis, since its increase associates with faster progression and higher mortality among patients suffering from Amyotrophic lateral sclerosis (ALS) (Calvo et al. 2019).
Later in life, ECM molecules provide survival signals and support cellular health. Studies of mouse models and human patients both suggest a neuroprotective role for collagen VI. In cultured primary neurons Col6a1 deficiency induces spontaneous apoptosis through defective autophagy and higher susceptibility to oxidative stress. Collagen VI expression is induced in the brain of aged mice and concordantly reactive oxygen species (ROS) and apoptosis was found to increase in aged Col6a1−/− brains, suggesting that collagen VI protects against age-induced oxidative damage. The performance of knock-out mice in motor coordination and spatial memory tests was compromised (Cescon et al. 2016) although no such cognitive impairment has thus far been reported in patients with collagen VI-related skeletal muscle diseases. Interestingly, compound heterozygous COL6A3 mutation carriers however demonstrate early-onset segmental isolated dystonia without muscular involvement (Zech et al. 2015) and COL6A2 mutations expose to progressive myoclonus epilepsy syndrome (Karkheiran et al. 2013). An additional line of evidence comes from AD mouse models and human patients. Transcripts coding for collagen α1-α3(VI) chains are particularly elevated in the brain of mice and patients with AD (Cheng et al. 2009). Neurodegenerative diseases typically involve neurotoxic intracellular accumulation and extracellular protein aggregation with aging. Senile plaques in AD are formed by extracellular accumulation of amyloid-beta (Aβ) peptides (Aβ40 and Aβ42) that are γ-secretase-cleaved proteolytic products of amyloid precursor protein (APP) (O’Brien and Wong 2011). Incubation of primary neuron cultures with Aβ42 dose-dependently promotes collagen VI protein expression through TβRII and Smad3 signaling. Wild-type neurons beat Col6a1−/− counterparts in resistance to Aβ42-mediated neurotoxicity and ectopic collagen VI augments protection against Aβ42-induced cell death. Experiments with primary neuronal cultures suggest that neuroprotection is mediated, at least partly, by preventing Aβ42 oligomer interaction with neurons (Cheng et al. 2009). Combined, these results make collagen VI a considerable instrument when designing novel therapies to treat neurodegenerative diseases.
Collagen XVIII accumulates in amyloid-positive vasculature and senile plaques in AD brains (van Horssen et al. 2002). Collagen XVIII is a collagen/proteoglycan hybrid and it may promote and stabilize senile plagues through its heparan sulfate proteoglycans. Moreover, the collagen XVIII matricryptin endostatin is increased in the cerebrospinal fluid of AD patients, providing a potential diagnostic tool in AD (Salza et al. 2015). Additionally, collagen XXV is associated with senile plaques in AD disease (Hashimoto et al. 2002; Kowa et al. 2004) and its involvement in the dynamics of β-amyloidogenesis has been demonstrated. In vitro studies are, however, even conflicting to some extent, most likely due to the utilization of variable Aβ peptides, Aβ40 or Aβ42, and different methods. Hashimoto and colleagues showed that both transmembrane and shed furin-mediated proteolytic product CLAC are capable of binding fibrillized Aβ (Hashimoto et al. 2002) whereas Söderberg et al. further showed that shed collagen XXV also interacts with the non-Aβ components of the AD amyloid plaques (Söderberg et al. 2005). Two different studies identified distinct positively charged sequences, locating either at the COL1 (collagenous) or NC2 domains of collagen XXV, important for Aβ42 and Aβ40 binding, respectively, through possible ionic interaction with the negatively charged amino acids in Aβ peptides (Kakuyama et al. 2005; Osada et al. 2005; Söderberg et al. 2005). In immunohistochemical staining of AD brains, however, Aβ40-positive plaques remain negative for collagen XXV and its accumulation to loose, rather than typical, Aβ42-positive fibrils implies early involvement in plaque formation and rather suggests an inhibitory role for collagen XXV in senile plaque maturation (Kowa et al. 2004). Indeed, shed collagen XXV inhibits Aβ fibril elongation in vitro (Kakuyama et al. 2005; Osada et al. 2005), while on the other hand, it enhances Aβ fibril aggregation and resistance against proteases (Söderberg et al. 2005). Collagen XXV may thus contribute to regulating fibril stabilization into aggregates and disease progression.
In line with in vitro results, overexpression of human collagen XXV in mouse CNS neurons exposes transgenic mice to increased Aβ accumulation, although not its fibrillization. With an unknown mechanism, excess collagen XXV appears to elevate p35/p25 expression levels, which in turn results in Cdk5 activation and subsequent increase of BACE1, a β-secretase involved in APP processing and AD pathology (Tong et al. 2010). In these transgenic mice, induced AD pathogenesis involves a decline in the level of the synaptic vesicle protein synaptophysin and an activation of astrocytes, associated with behavioral disabilities typical for AD (Tong et al. 2010). Besides a clear involvement of collagen XXV in AD pathogenesis in the transgenic mouse model and AD patients, only one study supports such a relationship at the genetic level, as Forsell and colleagues confirmed that certain COL25A1 gene polymorphisms neighboring exon 9 expose patients to AD development (Forsell et al. 2010). Such SNPs could speculatively influence AD evolvement by in/out-splicing of the NC2 Aβ binding site or affect collagen XXV protein levels. In addition to AD, collagen XXV aggregates exist in Down syndrome (Kowa et al. 2004) but not in cognitive impairment at senescence (Gal et al. 2018). Increased levels of shed collagen XIII and XXIII ectodomains are detected in cancer patients’ blood or urea due to increased expression and following proteolytic processing (Spivey et al. 2010; Miyake et al. 2017). It can be speculated whether circulating collagen XXV ectodomain/matricryptin is similarly increased in certain neurodegenerative diseases, and if so, could it be utilized as a diagnostic or prognostic marker?
Collagen XVII is one of the two major auto-antigens in BP, a subepidermal blistering skin disease (see Chap. 7 for further details). An exponentially growing body of evidence implies that BP, which typically raises in later life, predisposes to neurological comorbidities such as dementia, Parkinson’s disease, cerebrovascular disorders, multiple sclerosis, and epilepsy, and to psychiatric diseases such as schizophrenia, uni- and bipolar disorder, schizotypal and delusional disorders, and personality disorders (Foureur et al. 2001; Parker et al. 2008; Marazza et al. 2009; Brick et al. 2014; Cai et al. 2014; Ren et al. 2017). On the other hand, neuropsychiatric disorders increase the risk of development of anti-collagen XVII autoantibodies and BP (Chosidow et al. 2000; Bastuji-Garin et al. 2011; Teixeira et al. 2014; Kibsgaard et al. 2017; Kokkonen et al. 2017; Langan et al. 2011; Yu Phuan et al. 2017; Katisko et al. 2018). Due to its existence in both skin and CNS, both of which are of ectodermal origin, collagen XVII may act as a shared autoantigen, but also neuroinflammation or neurodegeneration could trigger the autoimmunization (Amber et al. 2017). Since knock-out mice die soon after birth because of dermal and glomerular defects, it is not possible to utilize these mice to investigate the role of collagen XVII in neurological diseases (Hurskainen et al. 2012). Taken together, several non-fibrillar collagens, many of which are of neural origin, contribute to the homeostasis of CNS neurons, and thereby to brain physiology and function.
8.3.6 Reactive Collagen Production/Distribution Following Disease or Trauma
Tissue repair typically involves matrix remodeling to mediate signaling and support regrowth. Genes active during development are often reactivated during repair due to similarities shared by developmental and repair processes. Dysfunction of the BBB is linked to various neurological and neurodegenerative diseases and several collagens are induced in injured BBB vessels (Christov et al. 2008; Munji et al. 2019). For example, levels of collagens I and III are elevated at BBB in experimental mouse models for seizure, multiple sclerosis, stroke, and traumatic brain injury (Munji et al. 2019). It is suggested that post-hemorrhagic meningeal fibrosis is the cause for following hydrocephalus both in humans and in a rat model (Sajanti et al. 1999). In a cerebrovascular disease, stroke-like episodes induce accumulation of the fibrillar collagens I, III, and V in the wall of intracerebral arterioles (Zhang et al. 1994). The accumulation of fibrillar collagens and subsequent vascular fibrosis is a response to prolonged repair signaling. In hypertensive patients, vascular BMs generally become thickened but additionally, collagen VI is atypically deposited in the broadened vascular wall of larger arteries and cortical vessels, indicative of ECM remodeling in response to hypertension (Roggendorf et al. 1988). In the brain vessels of AD patients, the levels of collagens I and III increase while collagen IV level is reduced, suggestive of BM disruption and postinjury response (Christov et al. 2008). Expression of some additional collagens, such as type VI, VIII, and XVIII either in neurons or in reactive microglia/astrocytes is reported to be induced after brain injuries (Hirano et al. 2004; Zhang et al. 2007; Cheng et al. 2011).
Unlike in CNS, injured nerves in the PNS do regenerate (Perrin et al. 2005). Recruited macrophages, exhibiting M2 regenerative phenotype, engulf debris at the injured site and secrete signals responsible for the induction of axonal regrowth (Mokarram et al. 2012). Thereafter, new projections grow along the preexisting path to reestablish innervation and synapses (Fig. 8.3f). Laminin α2β1γ1 is the most important BM player of nerve regeneration (Chen and Strickland 2003) but fibrillar collagens also support axon growth. Also, collagen VI appears important in several reactive processes in the nervous system. Its expression is induced in injured nerves and collagen VI is shown to promote monocyte adhesion (Schnoor et al. 2008) and macrophage migration (Chen et al. 2014). In line, in collagen VI-deficient injured nerves myelin clearance is delayed due to defective recruitment of macrophages (Chen et al. 2014). Regenerative M2 macrophages produce more collagen VI over proinflammatory M1 macrophages (Schnoor et al. 2008) and collagen VI was found to be important for macrophage M2 polarization. Collagen VI regulates macrophage migration and polarization through the regulation of AKT and PKA pathways. Concomitantly, collagen VI deficiency in mice compromises peripheral nerve regeneration (Chen et al. 2014). In diabetic neuropathy patients, collagen VI accumulates at the epineurium and perineurium, and in in vitro experiments its expression is induced by glucose (Muona et al. 1993; Hill 2009). As collagen VI expression normally ceases following the completion of myelination (Vitale et al. 2001), a surplus of collagen VI may thus become a pathogenic signal. Besides collagen VI, collagen V is also found at increased levels in the endoneurium of diabetic neuropathy but collagen IV remains unaltered (Hill 2009). Moreover, collagen types I, III, IV, V, and VI are upregulated, especially in Charcot–Marie–Tooth type 1 disease but also in some other inherited demyelinating neuropathies caused by mutations in genes expressed by myelinating Schwann cells (Palumbo et al. 2002). Collagen XXVIII is also induced in the dysmyelinated nerves in a Charcot–Marie–Tooth disease mouse model (Grimal et al. 2010).
Tumors that develop in both the peripheral and central nervous systems contain distinct ECM from those described throughout this chapter. Collagens as part of the tumor stroma may support tumor growth and invasion or counteractingly limit tumor growth. Several collagens are differentially, typically increasingly, expressed between the normal nervous tissue cells and the tumoral counterparts. Abnormal levels of at least on collagens I, III, IV, VI, VII, VIII, XI, XVI, XVII, and XXVIII have been reported in brain tumors or cancer cells (Sage et al. 1984; Paulus et al. 1991; Fujita et al. 2008; Senner et al. 2008; An et al. 2009; Di Rosa et al. 2015; Ishihara et al. 2019; Yang et al. 2019). Abnormal expression of collagens can be utilized as prognostic markers or therapeutic targets in neuro-oncology. One of those collagens differentially expressed in brain cancer is collagen XVI since COL16A1 expression is induced in glioblastoma (Senner et al. 2008). Suggestively, collagen XVI affects cell adhesion through its recognized interaction with α1β1 integrin (Eble et al. 2006) and promotes tumor invasiveness (Bauer et al. 2011). In vitro COL16A1 knockdown results in a reduction of focal adhesion number and β1-integrin activation (Senner et al. 2008; Bauer et al. 2011). Collagen XVI promotes the invasion of oral cancer through the induction of MMP9 expression (Bedal et al. 2014) and this may also apply to brain tumors, since MMP9 is a recognized organizer of the PNN (Ethell and Ethell 2007). Although collagen XVII is confined to neurons, transcriptome sequencing identifies a novel PTEN-COL17A1 fusion gene transcribed in glioblastoma, that results in a significant increase in collagen XVII expression and association with recurrence (Yan et al. 2017). In glioma cell lines, collagen XVII induction promotes invasiveness while its knockdown concomitantly decreases MMP9 expression and suppresses invasiveness (Yan et al. 2017), suggesting a regulatory role for collagen XVII in glioblastoma malignance. Angiogenesis is a prerequisite for tumor growth. Collagen VII becomes neo-expressed in scattered abnormal vessels in astrocytic and ependymal tumors (Paulus et al. 1995). Similarly, collagen VIII is not expressed in the normal brain parenchyma or nonneoplastic cerebral disorders, but it is highly induced in blood vessels of distinct brain tumors (Paulus et al. 1991). Interestingly, ectopic expression of the collagen α1(VIII) matricryptin vastatin results in reduced angiogenesis and glioblastoma inhibition in an orthotopic model in mice (Li et al. 2017), suggesting a role reminiscent of endostatin (O’Reilly et al. 1997). Injuries result in changes in cellular signaling and responding alteration in ECMs to support regeneration. Variable collagens are either neo- or re-deposited in injured or cancerous nervous tissue stroma. The accumulation of fibrillar collagens concomitant with the appearance of fibrotic tissues is indicative of adverse repair response, while the assembly of variable collagens in the tumorous stroma may promote or limit cancer growth on a case-by-case basis.
8.4 Conclusion
While the presence of fibrillar and BM collagens in the nervous tissue was established decades ago, exciting emerging data suggest the existence of previously unknown types of ECMs in the CNS. Novel roles for matrix molecules in the nervous tissues have been identified and, for example, it is now known that neuronal cells themselves express several types of collagens. An increasing number of distinct collagens are involved in the assembly of neural circuits, signifying the importance of the collagen-dependent ECMs, especially in founding platforms and in providing target-derived signals for navigating axons, thus enabling proper synaptogenesis. In recent years with the aid of next-generation sequencing, several novel collagens have been linked to human nervous system diseases and their number is expected to still increase. Moreover, animal models have provided valuable information on the functional mechanisms of collagens. The expansion of “omics” analyses will certainly lead to the identification of more alterations in collagens regarding their structure or expression levels in various animal models and cell types, and in patients. Confidently this chapter will not only serve as an introduction to the diverse roles of collagens in the nervous system but will also inspire researchers not to overlook collagens as mere artifacts in their datasets. The field of collagens in the brain is only in its infancy, and it will be interesting to see how the field evolves in the next decade or even sooner.
Abbreviations
- Aβ:
-
Amyloid beta
- AChR:
-
Acetylcholine receptor
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- APP:
-
Amyloid precursor protein
- BBB:
-
Blood–brain barrier
- BM:
-
Basement membrane
- BP:
-
Bullous pemphigoid
- COL:
-
Collagenous domain
- CLAC:
-
Collagen-like Alzheimer amyloid plaque component
- CLAC-P:
-
CLAC precursor
- CNS:
-
Central nervous system
- CMS:
-
Congenital myasthenic syndrome
- DDR:
-
Discoidin domain receptor
- DRG:
-
Dorsal root ganglion
- ECM:
-
Extracellular matrix
- FACIT:
-
Fibril-associated collagens with interrupted triple helices
- MACIT:
-
Membrane-associated collagens with interrupted triple helices
- Multiplexin:
-
Multiple triple-helix domains with interruptions
- NC:
-
Non-collagenous domain
- NMJ:
-
Neuromuscular junction
- PNN:
-
Perineuronal net
- PNS:
-
Peripheral nervous system
- PTP:
-
Protein tyrosine phosphatase
- ROS:
-
Reactive oxygen species
- SVCT2:
-
Sodium-dependent vitamin C transporter 2
References
Ackley BD, Crew JR, Elamaa H, Pihlajaniemi T, Kuo CJ, Kramer JM (2001) The NC1/endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance. J Cell Biol 152:1219–1232
Ackley BD, Kang SH, Crew JR, Suh C, Jin Y, Kramer JM (2003) The basement membrane components nidogen and type XVIII collagen regulate organization of neuromuscular junctions in Caenorhabditis elegans. J Neurosci 23:3577–3587
Allen JM, Zamurs L, Brachvogel B, Schlotzer-Schrehardt U, Hansen U, Lamande SR, Rowley L, Fitzgerald J, Bateman JF (2009) Mice lacking the extracellular matrix protein WARP develop normally but have compromised peripheral nerve structure and function. J Biol Chem 284:12020–12030
Amber KT, Zikry J, Hertl M (2017) A multi-hit hypothesis of bullous pemphigoid and associated neurological disease: is HLA-DQB1*03:01, a potential link between immune privileged antigen exposure and epitope spreading? HLA 89:127–134
An JH, Lee SY, Jeon JY, Cho KG, Kim SU, Lee MA (2009) Identification of gliotropic factors that induce human stem cell migration to malignant tumor. J Proteome Res 8:2873–2881
Andrikopoulos K, Suzuki HR, Solursh M, Ramirez F (1992) Localization of pro-alpha 2(V) collagen transcripts in the tissues of the developing mouse embryo. Dev Dyn 195:113–120
Aricescu AR, McKinnell IW, Halfter W, Stoker AW (2002) Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol Cell Biol 22:1881–1892
Banerjee S, Isaacman-Beck J, Schneider VA, Granato M (2013) A novel role for Lh3 dependent ECM modifications during neural crest cell migration in zebrafish. PLoS One 8:e54609
Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, Roujeau JC, Bernard P, Guillaume JC, Ingen-Housz-Oro S, Maillard H, Pauwels C, Picard-Dahan C, Dutronc Y, Richard MA, French Study Group for Bullous Diseases (2011) Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol 131:637–643
Bauer R, Ratzinger S, Wales L, Bosserhoff A, Senner V, Grifka J, Grassel S (2011) Inhibition of collagen XVI expression reduces glioma cell invasiveness. Cell Physiol Biochem 27:217–226
Beattie CE, Melancon E, Eisen JS (2000) Mutations in the stumpy gene reveal intermediate targets for zebrafish motor axons. Development 127:2653–2662
Bedal KB, Grassel S, Oefner PJ, Reinders J, Reichert TE, Bauer R (2014) Collagen XVI induces expression of MMP9 via modulation of AP-1 transcription factors and facilitates invasion of oral squamous cell carcinoma. PLoS One 9:e86777
Birk DE, Fitch JM, Babiarz JP, Doane KJ, Linsenmayer TF (1990) Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci 95(Pt 4):649–657
Booth BA, Uitto J (1981) Collagen biosynthesis by human skin fibroblasts. III. The effects of ascorbic acid on procollagen production and prolyl hydroxylase activity. Biochim Biophys Acta 675:117–122. 0304-4165(81)90076-3 [pii]
Boot-Handford RP, Tuckwell DS, Plumb DA, Rock CF, Poulsom R (2003) A novel and highly conserved collagen (pro(alpha)1(XXVII)) with a unique expression pattern and unusual molecular characteristics establishes a new clade within the vertebrate fibrillar collagen family. J Biol Chem 278:31067–31077
Breedveld G, de Coo IF, Lequin MH, Arts WF, Heutink P, Gould DB, John SW, Oostra B, Mancini GM (2006) Novel mutations in three families confirm a major role of COL4A1 in hereditary porencephaly. J Med Genet 43:490–495. jmg.2005.035584 [pii]
Bretaud S, Pagnon-Minot A, Guillon E, Ruggiero F, Le Guellec D (2011) Characterization of spatial and temporal expression pattern of Col15a1b during zebrafish development. Gene Expr Patterns 11:129–134
Bretaud S, Guillon E, Karppinen S, Pihlajaniemi T, Ruggiero F (2020) Collagen XV, a multifaceted multiplexin present across tissues and species. Matrix Biol Plus 6–7
Brick KE, Weaver CH, Savica R, Lohse CM, Pittelkow MR, Boeve BF, Gibson LE, Camilleri MJ, Wieland CN (2014) A population-based study of the association between bullous pemphigoid and neurologic disorders. J Am Acad Dermatol 71:1191–1197
Bunge MB, Williams AK, Wood PM, Uitto J, Jeffrey JJ (1980) Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation. J Cell Biol 84:184–202
Caglayan AO, Baranoski JF, Aktar F, Han W, Tuysuz B, Guzel A, Guclu B, Kaymakcalan H, Aktekin B, Akgumus GT, Murray PB, Erson-Omay EZ, Caglar C, Bakircioglu M, Sakalar YB, Guzel E, Demir N, Tuncer O, Senturk S, Ekici B, Minja FJ, Sestan N, Yasuno K, Bilguvar K, Caksen H, Gunel M (2014) Brain malformations associated with Knobloch syndrome—review of literature, expanding clinical spectrum, and identification of novel mutations. Pediatr Neurol 51:806–813.e8
Cai SC, Allen JC, Lim YL, Chua SH, Tan SH, Tang MB (2014) Mortality of bullous pemphigoid in Singapore: risk factors and causes of death in 359 patients seen at the National Skin Centre. Br J Dermatol 170:1319–1326
Calvo AC, Cibreiro GA, Merino PT, Roy JF, Galiana A, Rufian AJ, Cano JM, Martin MA, Moreno L, Larrode P, Vazquez PC, Galan L, Mora J, Munoz-Blanco JL, Munoz MJ, Zaragoza P, Pegoraro E, Soraru G, Mora M, Lunetta C, Penco S, Tarlarini C, Esteban J, Osta R, Redondo AG (2019) Collagen XIX alpha 1 improves prognosis in amyotrophic lateral sclerosis. Aging Dis 10:278–292
Carey DJ, Eldridge CF, Cornbrooks CJ, Timpl R, Bunge RP (1983) Biosynthesis of type IV collagen by cultured rat Schwann cells. J Cell Biol 97:473–479
Carlson SS, Valdez G, Sanes JR (2010) Presynaptic calcium channels and alpha3-integrins are complexed with synaptic cleft laminins, cytoskeletal elements and active zone components. J Neurochem 115:654–666
Cescon M, Chen P, Castagnaro S, Gregorio I, Bonaldo P (2016) Lack of collagen VI promotes neurodegeneration by impairing autophagy and inducing apoptosis during aging. Aging (Albany NY) 8:1083–1101
Cescon M, Gregorio I, Eiber N, Borgia D, Fusto A, Sabatelli P, Scorzeto M, Megighian A, Pegoraro E, Hashemolhosseini S, Bonaldo P (2018) Collagen VI is required for the structural and functional integrity of the neuromuscular junction. Acta Neuropathol 136:483
Charnas LR, Marini JC (1995) Neurologic profile in osteogenesis imperfecta. Connect Tissue Res 31:23
Charsar BA, Goldberg EM (2017) Polymicrogyria and intractable epilepsy in siblings with Knobloch syndrome and homozygous mutation of COL18A1. Pediatr Neurol 76:91–92. S0887-8994(17)30804-4 [pii]
Cheah KS, Lau ET, Au PK, Tam PP (1991) Expression of the mouse alpha 1(II) collagen gene is not restricted to cartilage during development. Development 111:945–953
Chen ZL, Strickland S (2003) Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol 163:889–899
Chen P, Cescon M, Megighian A, Bonaldo P (2014) Collagen VI regulates peripheral nerve myelination and function. FASEB J 28:1145–1156
Cheng JS, Dubal DB, Kim DH, Legleiter J, Cheng IH, Yu GQ, Tesseur I, Wyss-Coray T, Bonaldo P, Mucke L (2009) Collagen VI protects neurons against Abeta toxicity. Nat Neurosci 12:119–121
Cheng IH, Lin YC, Hwang E, Huang HT, Chang WH, Liu YL, Chao CY (2011) Collagen VI protects against neuronal apoptosis elicited by ultraviolet irradiation via an Akt/phosphatidylinositol 3-kinase signaling pathway. Neuroscience 183:178–188
Chernousov MA, Stahl RC, Carey DJ (1998) Schwann cells use a novel collagen-dependent mechanism for fibronectin fibril assembly. J Cell Sci 111(Pt 18):2763–2777
Chernousov MA, Rothblum K, Tyler WA, Stahl RC, Carey DJ (2000) Schwann cells synthesize type V collagen that contains a novel alpha 4 chain. Molecular cloning, biochemical characterization, and high affinity heparin binding of alpha 4(V) collagen. J Biol Chem 275:28208–28215
Chernousov MA, Stahl RC, Carey DJ (2001) Schwann cell type V collagen inhibits axonal outgrowth and promotes Schwann cell migration via distinct adhesive activities of the collagen and noncollagen domains. J Neurosci 21:6125–6135
Chernousov MA, Rothblum K, Stahl RC, Evans A, Prentiss L, Carey DJ (2006) Glypican-1 and alpha4(V) collagen are required for Schwann cell myelination. J Neurosci 26:508–517
Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S (2008) Regulation of Schwann cell function by the extracellular matrix. Glia 56:1498–1507
Chosidow O, Doppler V, Bensimon G, Joly P, Salachas F, Lacomblez L, Prost C, Camu W, Frances C, Herson S, Meininger V (2000) Bullous pemphigoid and amyotrophic lateral sclerosis: a new clue for understanding the bullous disease? Arch Dermatol 136:521–524
Christov A, Ottman J, Hamdheydari L, Grammas P (2008) Structural changes in Alzheimer’s disease brain microvessels. Curr Alzheimer Res 5:392–395
Clementz AG, Mutolo MJ, Leir SH, Morris KJ, Kucybala K, Harris H, Harris A (2013) Collagen XV inhibits epithelial to mesenchymal transition in pancreatic adenocarcinoma cells. PLoS One 8:e72250
Corbett MA, Turner SJ, Gardner A, Silver J, Stankovich J, Leventer RJ, Derry CP, Carroll R, Ha T, Scheffer IE, Bahlo M, Jackson GD, Mackey DA, Berkovic SF, Gecz J (2017) Familial epilepsy with anterior polymicrogyria as a presentation of COL18A1 mutations. Eur J Med Genet 60:437–443
Court FA, Gillingwater TH, Melrose S, Sherman DL, Greenshields KN, Morton AJ, Harris JB, Willison HJ, Ribchester RR (2008) Identity, developmental restriction and reactivity of extralaminar cells capping mammalian neuromuscular junctions. J Cell Sci 121:3901–3911
D’Antonio M, Michalovich D, Paterson M, Droggiti A, Woodhoo A, Mirsky R, Jessen KR (2006) Gene profiling and bioinformatic analysis of Schwann cell embryonic development and myelination. Glia 53:501–515
Dhungana H, Huuskonen MT, Pihlajaniemi T, Heljasvaara R, Vivien D, Kanninen KM, Malm T, Koistinaho J, Lemarchant S (2017) Lack of collagen XV is protective after ischemic stroke in mice. Cell Death Dis 8:e2541
Di Rosa M, Sanfilippo C, Libra M, Musumeci G, Malaguarnera L (2015) Different pediatric brain tumors are associated with different gene expression profiling. Acta Histochem 117:477–485
Dusl M, Moreno T, Munell F, Macaya A, Gratacos M, Abicht A, Strom TM, Lochmuller H, Senderek J (2019) Congenital myasthenic syndrome caused by novel COL13A1 mutations. J Neurol 266:1107–1112
Dziadek M, Timpl R (1985) Expression of nidogen and laminin in basement membranes during mouse embryogenesis and in teratocarcinoma cells. Dev Biol 111:372–382
Dziadek M, Darling P, Bakker M, Overall M, Zhang RZ, Pan TC, Tillet E, Timpl R, Chu ML (1996) Deposition of collagen VI in the extracellular matrix during mouse embryogenesis correlates with expression of the alpha 3(VI) subunit gene. Exp Cell Res 226:302–315
Eble JA, Kassner A, Niland S, Morgelin M, Grifka J, Grassel S (2006) Collagen XVI harbors an integrin alpha1 beta1 recognition site in its C-terminal domains. J Biol Chem 281:25745–25756
Eklund L, Piuhola J, Komulainen J, Sormunen R, Ongvarrasopone C, Fässler R, Muona A, Ilves M, Ruskoaho H, Takala TE, Pihlajaniemi T (2001) Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc Natl Acad Sci USA 98:1194–1199
Eldridge CF, Bunge MB, Bunge RP, Wood PM (1987) Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol 105:1023–1034
Eldridge CF, Bunge MB, Bunge RP (1989) Differentiation of axon-related Schwann cells in vitro: II. Control of myelin formation by basal lamina. J Neurosci 9:625–638
Emery SC, Karpinski NC, Hansen L, Masliah E (1999) Abnormalities in central nervous system development in osteogenesis imperfecta type II. Pediatr Dev Pathol 2:124–130
Ethell IM, Ethell DW (2007) Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res 85:2813–2823
Favor J, Gloeckner CJ, Janik D, Klempt M, Neuhauser-Klaus A, Pretsch W, Schmahl W, Quintanilla-Fend L (2007) Type IV procollagen missense mutations associated with defects of the eye, vascular stability, the brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: an extension of the Col4a1 allelic series and the identification of the first two Col4a2 mutant alleles. Genetics 175:725–736
Ferrer-Ferrer M, Dityatev A (2018) Shaping synapses by the neural extracellular matrix. Front Neuroanat 12:40
Forsell C, Björk BF, Lilius L, Axelman K, Fabre SF, Fratiglioni L, Winblad B, Graff C (2010) Genetic association to the amyloid plaque associated protein gene COL25A1 in Alzheimer’s disease. Neurobiol Aging 31:409–415
Foureur N, Descamps V, Lebrun-Vignes B, Picard-Dahan C, Grossin M, Belaich S, Crickx B (2001) Bullous pemphigoid in a leg affected with hemiparesia: a possible relation of neurological diseases with bullous pemphigoid? Eur J Dermatol 11:230–233
Fox MA (2008) Novel roles for collagens in wiring the vertebrate nervous system. Curr Opin Cell Biol 20:508–513
Fox MA, Sanes JR, Borza DB, Eswarakumar VP, Fässler R, Hudson BG, John SW, Ninomiya Y, Pedchenko V, Pfaff SL, Rheault MN, Sado Y, Segal Y, Werle MJ, Umemori H (2007) Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell 129:179–193
Franzke CW, Bruckner P, Bruckner-Tuderman L (2005) Collagenous transmembrane proteins: recent insights into biology and pathology. J Biol Chem 280:4005–4008
Frischknecht R, Gundelfinger ED (2012) The brain’s extracellular matrix and its role in synaptic plasticity. Adv Exp Med Biol 970:153–171
Fujita A, Sato JR, Festa F, Gomes LR, Oba-Shinjo SM, Marie SK, Ferreira CE, Sogayar MC (2008) Identification of COL6A1 as a differentially expressed gene in human astrocytomas. Genet Mol Res 7:371–378
Gal J, Chen J, Katsumata Y, Fardo DW, Wang WX, Artiushin S, Price D, Anderson S, Patel E, Zhu H, Nelson PT (2018) Detergent insoluble proteins and inclusion body-like structures immunoreactive for PRKDC/DNA-PK/DNA-PKcs, FTL, NNT, and AIFM1 in the amygdala of cognitively impaired elderly persons. J Neuropathol Exp Neurol 77:21–39
Gara SK, Grumati P, Urciuolo A, Bonaldo P, Kobbe B, Koch M, Paulsson M, Wagener R (2008) Three novel collagen VI chains with high homology to the alpha3 chain. J Biol Chem 283:10658–10670
Gatseva A, Sin YY, Brezzo G, Van Agtmael T (2019) Basement membrane collagens and disease mechanisms. Essays Biochem 63:297–312
Gess B, Rohr D, Fledrich R, Sereda MW, Kleffner I, Humberg A, Nowitzki J, Strecker JK, Halfter H, Young P (2011) Sodium-dependent vitamin C transporter 2 deficiency causes hypomyelination and extracellular matrix defects in the peripheral nervous system. J Neurosci 31:17180–17192
Goncalves TJM, Boutillon F, Lefebvre S, Goffin V, Iwatsubo T, Wakabayashi T, Oury F, Armand AS (2019) Collagen XXV promotes myoblast fusion during myogenic differentiation and muscle formation. Sci Rep 9:5878–5876
Gordon MK, Hahn RA (2010) Collagens. Cell Tissue Res 339:247–257
Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, Aguglia U, van der Knaap, M S, Heutink P, John SW (2005) Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science 308:1167–1171
Gregorio I, Braghetta P, Bonaldo P, Cescon M (2018) Collagen VI in healthy and diseased nervous system. Dis Model Mech 11
Grimal S, Puech S, Wagener R, Venteo S, Carroll P, Fichard-Carroll A (2010) Collagen XXVIII is a distinctive component of the peripheral nervous system nodes of ranvier and surrounds nonmyelinating glial cells. Glia 58:1977–1987
Guillon E, Bretaud S, Ruggiero F (2016) Slow muscle precursors lay down a collagen XV matrix fingerprint to guide motor axon navigation. J Neurosci 36:2663–2676
Hägg P, Rehn M, Huhtala P, Väisänen T, Tamminen M, Pihlajaniemi T (1998) Type XIII collagen is identified as a plasma membrane protein. J Biol Chem 273:15590–15597
Hägg P, Väisänen T, Tuomisto A, Rehn M, Tu H, Huhtala P, Eskelinen S, Pihlajaniemi T (2001) Type XIII collagen: a novel cell adhesion component present in a range of cell-matrix adhesions and in the intercalated discs between cardiac muscle cells. Matrix Biol 19:727–742
Haghighi A, Tiwari A, Piri N, Nurnberg G, Saleh-Gohari N, Haghighi A, Neidhardt J, Nurnberg P, Berger W (2014) Homozygosity mapping and whole exome sequencing reveal a novel homozygous COL18A1 mutation causing Knobloch syndrome. PLoS One 9:e112747
Halfter W, Dong S, Schurer B, Cole GJ (1998) Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J Biol Chem 273:25404–25412
Härönen H, Zainul Z, Tu H, Naumenko N, Sormunen R, Miinalainen I, Shakirzyanova A, Oikarainen T, Abdullin A, Martin P, Santoleri S, Koistinaho J, Silman I, Giniatullin R, Fox MA, Heikkinen A, Pihlajaniemi T (2017) Collagen XIII secures pre- and postsynaptic integrity of the neuromuscular synapse. Hum Mol Genet 26:2076–2090
Härönen H, Zainul Z, Naumenko N, Sormunen R, Miinalainen I, Shakirzyanova A, Santoleri S, Kemppainen AV, Giniatullin R, Pihlajaniemi T, Heikkinen A (2019) Correct expression and localization of collagen XIII are crucial for the normal formation and function of the neuromuscular system. Eur J Neurosci 49:1491–1511
Hartmann D, Ziegenhagen MW, Sievers J (1998) Meningeal cells stimulate neuronal migration and the formation of radial glial fascicles from the cerebellar external granular layer. Neurosci Lett 244:129–132
Hashimoto T, Wakabayashi T, Watanabe A, Kowa H, Hosoda R, Nakamura A, Kanazawa I, Arai T, Takio K, Mann DM, Iwatsubo T (2002) CLAC: a novel Alzheimer amyloid plaque component derived from a transmembrane precursor, CLAC-P/collagen type XXV. EMBO J 21:1524–1534
Hayashi G, Labelle-Dumais C, Gould DB (2018) Use of sodium 4-phenylbutyrate to define therapeutic parameters for reducing intracerebral hemorrhage and myopathy in Col4a1 mutant mice. Dis Model Mech 11
Heck N, Garwood J, Schutte K, Fawcett J, Faissner A (2003) Astrocytes in culture express fibrillar collagen. Glia 41:382–392
Heikkinen A, Tu H, Pihlajaniemi T (2012) Collagen XIII: a type II transmembrane protein with relevance to musculoskeletal tissues, microvessels and inflammation. Int J Biochem Cell Biol 44:714–717
Heikkinen A, Härönen H, Norman O, Pihlajaniemi T (2020) Collagen XIII and other ECM components in the assembly and disease of the neuromuscular junction. Anat Rec (Hoboken)
Heljasvaara R, Aikio M, Ruotsalainen H, Pihlajaniemi T (2017) Collagen XVIII in tissue homeostasis and dysregulation – lessons learned from model organisms and human patients. Matrix Biol 57–58:55–75
Hilario JD, Wang C, Beattie CE (2010) Collagen XIXa1 is crucial for motor axon navigation at intermediate targets. Development 137:4261–4269
Hill R (2009) Extracellular matrix remodelling in human diabetic neuropathy. J Anat 214:219–225
Hirano S, Yonezawa T, Hasegawa H, Hattori S, Greenhill NS, Davis PF, Sage EH, Ninomiya Y (2004) Astrocytes express type VIII collagen during the repair process of brain cold injury. Biochem Biophys Res Commun 317:437–443
Hubert T, Grimal S, Ratzinger S, Mechaly I, Grassel S, Fichard-Carroll A (2007) Collagen XVI is a neural component of the developing and regenerating dorsal root ganglia extracellular matrix. Matrix Biol 26:206–210
Hubert T, Grimal S, Carroll P, Fichard-Carroll A (2009) Collagens in the developing and diseased nervous system. Cell Mol Life Sci 66:1223–1238
Hurskainen T, Moilanen J, Sormunen R, Franzke CW, Soininen R, Löffek S, Huilaja L, Nuutinen M, Bruckner-Tuderman L, Autio-Harmainen H, Tasanen K (2012) Transmembrane collagen XVII is a novel component of the glomerular filtration barrier. Cell Tissue Res 348:579–588
Ishihara E, Takahashi S, Fukaya R, Ohta S, Yoshida K, Toda M (2019) Identification of KLRC2 as a candidate marker for brain tumor-initiating cells. Neurol Res 41:1043–1049
Jacków J, Löffek S, Nyström A, Bruckner-Tuderman L, Franzke CW (2016a) Collagen XVII shedding suppresses re-epithelialization by directing keratinocyte migration and dampening mTOR signaling. J Invest Dermatol 136:1031–1041
Jacków J, Schlosser A, Sormunen R, Nyström A, Sitaru C, Tasanen K, Bruckner-Tuderman L, Franzke CW (2016b) Generation of a functional non-shedding collagen XVII mouse model: relevance of collagen XVII shedding in wound healing. J Invest Dermatol 136:516–525
Jeanne M, Gould DB (2017) Genotype-phenotype correlations in pathology caused by collagen type IV alpha 1 and 2 mutations. Matrix Biol 57-58:29–44
Jeanne M, Labelle-Dumais C, Jorgensen J, Kauffman WB, Mancini GM, Favor J, Valant V, Greenberg SM, Rosand J, Gould DB (2012) COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am J Hum Genet 90:91–101
Jeanne M, Jorgensen J, Gould DB (2015) Molecular and genetic analyses of collagen type IV mutant mouse models of spontaneous intracerebral hemorrhage identify mechanisms for stroke prevention. Circulation 131:1555–1565
Jovanov Milosevic N, Judas M, Aronica E, Kostovic I (2014) Neural ECM in laminar organization and connectivity development in healthy and diseased human brain. Prog Brain Res 214:159–178
Kakuyama H, Soderberg L, Horigome K, Winblad B, Dahlqvist C, Naslund J, Tjernberg LO (2005) CLAC binds to aggregated Abeta and Abeta fragments, and attenuates fibril elongation. Biochemistry 44:15602–15609
Kamei A, Houdou S, Mito T, Konomi H, Takashima S (1992) Developmental change in type VI collagen in human cerebral vessels. Pediatr Neurol 8:183–186
Kapoor R, Sakai LY, Funk S, Roux E, Bornstein P, Sage EH (1988) Type VIII collagen has a restricted distribution in specialized extracellular matrices. J Cell Biol 107:721–730
Karkheiran S, Krebs CE, Makarov V, Nilipour Y, Hubert B, Darvish H, Frucht S, Shahidi GA, Buxbaum JD, Paisan-Ruiz C (2013) Identification of COL6A2 mutations in progressive myoclonus epilepsy syndrome. Hum Genet 132:275–283
Katisko K, Kokkonen N, Kruger J, Hartikainen P, Koivisto AM, Helisalmi S, Korhonen VE, Kokki M, Tuusa J, Herukka SK, Solje E, Haapasalo A, Tasanen K, Remes AM (2018) The association between frontotemporal lobar degeneration and bullous pemphigoid. J Alzheimers Dis 66:743–750
Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, Meister M, Sanes JR (2011) Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci 31:7753–7762
Keene DR, Engvall E, Glanville RW (1988) Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J Cell Biol 107:1995–2006
Keren B, Suzuki OT, Gerard-Blanluet M, Bremond-Gignac D, Elmaleh M, Titomanlio L, Delezoide AL, Passos-Bueno MR, Verloes A (2007) CNS malformations in Knobloch syndrome with splice mutation in COL18A1 gene. Am J Med Genet A 143A:1514–1518
Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F (2007) Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells 25:2146–2157
Khaleduzzaman M, Sumiyoshi H, Ueki Y, Inoguchi K, Ninomiya Y, Yoshioka H (1997) Structure of the human type XIX collagen (COL19A1) gene, which suggests it has arisen from an ancestor gene of the FACIT family. Genomics 45:304–312
Khoshnoodi J, Pedchenko V, Hudson BG (2008) Mammalian collagen IV. Microsc Res Tech 71:357–370
Kibsgaard L, Rasmussen M, Lamberg A, Deleuran M, Olesen AB, Vestergaard C (2017) Increased frequency of multiple sclerosis among patients with bullous pemphigoid: a population-based cohort study on comorbidities anchored around the diagnosis of bullous pemphigoid. Br J Dermatol 176:1486–1491
Kleppel MM, Santi PA, Cameron JD, Wieslander J, Michael AF (1989) Human tissue distribution of novel basement membrane collagen. Am J Pathol 134:813–825
Kliemann SE, Waetge RT, Suzuki OT, Passos-Bueno MR, Rosemberg S (2003) Evidence of neuronal migration disorders in Knobloch syndrome: clinical and molecular analysis of two novel families. Am J Med Genet A 119A:15–19
Koch M, Veit G, Stricker S, Bhatt P, Kutsch S, Zhou P, Reinders E, Hahn RA, Song R, Burgeson RE, Gerecke DR, Mundlos S, Gordon MK (2006) Expression of type XXIII collagen mRNA and protein. J Biol Chem 281:21546–21557
Kokkonen N, Herukka SK, Huilaja L, Kokki M, Koivisto AM, Hartikainen P, Remes AM, Tasanen K (2017) Increased levels of the bullous pemphigoid BP180 autoantibody are associated with more severe dementia in Alzheimer’s disease. J Invest Dermatol 137:71–76
Korogod N, Petersen CC, Knott GW (2015) Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. Elife 4
Kowa H, Sakakura T, Matsuura Y, Wakabayashi T, Mann DM, Duff K, Tsuji S, Hashimoto T, Iwatsubo T (2004) Mostly separate distributions of CLAC- versus Abeta40- or thioflavin S-reactivities in senile plaques reveal two distinct subpopulations of beta-amyloid deposits. Am J Pathol 165:273–281
Krishnaswamy VR, Benbenishty A, Blinder P, Sagi I (2019) Demystifying the extracellular matrix and its proteolytic remodeling in the brain: structural and functional insights. Cell Mol Life Sci 76:3229–3248
Kummer TT, Misgeld T, Sanes JR (2006) Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol 16:74–82
Kuo CJ, LaMontagne KR, Garcia-Cardena G, Ackley BD, Kalman D, Park S, Christofferson R, Kamihara J, Ding YH, Lo KM, Gillies S, Folkman J, Mulligan RC, Javaherian K (2001) Oligomerization-dependent regulation of motility and morphogenesis by the collagen XVIII NC1/endostatin domain. J Cell Biol 152:1233–1246
Kuo DS, Labelle-Dumais C, Mao M, Jeanne M, Kauffman WB, Allen J, Favor J, Gould DB (2014) Allelic heterogeneity contributes to variability in ocular dysgenesis, myopathy and brain malformations caused by Col4a1 and Col4a2 mutations. Hum Mol Genet 23:1709–1722
Labelle-Dumais C, Dilworth DJ, Harrington EP, de Leau M, Lyons D, Kabaeva Z, Manzini MC, Dobyns WB, Walsh CA, Michele DE, Gould DB (2011) COL4A1 mutations cause ocular dysgenesis, neuronal localization defects, and myopathy in mice and Walker-Warburg syndrome in humans. PLoS Genet 7:e1002062
Labelle-Dumais C, Schuitema V, Hayashi G, Hoff K, Gong W, Dao DQ, Ullian EM, Oishi P, Margeta M, Gould DB (2019) COL4A1 mutations cause neuromuscular disease with tissue-specific mechanistic heterogeneity. Am J Hum Genet 104:847–860
Lai CH, Chu ML (1996) Tissue distribution and developmental expression of type XVI collagen in the mouse. Tissue Cell 28:155–164
Lamande SR, Sigalas E, Pan TC, Chu ML, Dziadek M, Timpl R, Bateman JF (1998) The role of the alpha3(VI) chain in collagen VI assembly. Expression of an alpha3(VI) chain lacking N-terminal modules N10-N7 restores collagen VI assembly, secretion, and matrix deposition in an alpha3(VI)-deficient cell line. J Biol Chem 273:7423–7430
Langan SM, Groves RW, West J (2011) The relationship between neurological disease and bullous pemphigoid: a population-based case-control study. J Invest Dermatol 131:631–636
Latvanlehto A, Fox MA, Sormunen R, Tu H, Oikarainen T, Koski A, Naumenko N, Shakirzyanova A, Kallio M, Ilves M, Giniatullin R, Sanes JR, Pihlajaniemi T (2010) Muscle-derived collagen XIII regulates maturation of the skeletal neuromuscular junction. J Neurosci 30:12230–12241
Leung AW, Wong SY, Chan D, Tam PP, Cheah KS (2010) Loss of procollagen IIA from the anterior mesendoderm disrupts the development of mouse embryonic forebrain. Dev Dyn 239:2319–2329
Li Y, Li J, Woo YM, Shen Z, Yao H, Cai Y, Lin MC, Poon WS (2017) Enhanced expression of Vastatin inhibits angiogenesis and prolongs survival in murine orthotopic glioblastoma model. BMC Cancer 17:126–128
Liao HM, Chao YL, Huang AL, Cheng MC, Chen YJ, Lee KF, Fang JS, Hsu CH, Chen CH (2012) Identification and characterization of three inherited genomic copy number variations associated with familial schizophrenia. Schizophr Res 139:229–236
Logan CV, Cossins J, Rodriguez Cruz PM, Parry DA, Maxwell S, Martinez-Martinez P, Riepsaame J, Abdelhamed ZA, Lake AV, Moran M, Robb S, Chow G, Sewry C, Hopkins PM, Sheridan E, Jayawant S, Palace J, Johnson CA, Beeson D (2015) Congenital myasthenic syndrome type 19 is caused by mutations in COL13A1, encoding the atypical non-fibrillar collagen type XIII alpha1 chain. Am J Hum Genet 97:878–885
Lui VC, Ng LJ, Nicholls J, Tam PP, Cheah KS (1995a) Tissue-specific and differential expression of alternatively spliced alpha 1(II) collagen mRNAs in early human embryos. Dev Dyn 203:198–211
Lui VC, Kong RY, Nicholls J, Cheung AN, Cheah KS (1995b) The mRNAs for the three chains of human collagen type XI are widely distributed but not necessarily co-expressed: implications for homotrimeric, heterotrimeric and heterotypic collagen molecules. Biochem J 311(Pt 2):511–516
Mahajan VB, Olney AH, Garrett P, Chary A, Dragan E, Lerner G, Murray J, Bassuk AG (2010) Collagen XVIII mutation in Knobloch syndrome with acute lymphoblastic leukemia. Am J Med Genet A 152A:2875–2879
Mao M, Alavi MV, Labelle-Dumais C, Gould DB (2015) Type IV collagens and basement membrane diseases: cell biology and pathogenic mechanisms. Curr Top Membr 76:61–116
Marazza G, Pham HC, Scharer L, Pedrazzetti PP, Hunziker T, Trueb RM, Hohl D, Itin P, Lautenschlager S, Naldi L, Borradori L, Autoimmune bullous disease Swiss study group (2009) Incidence of bullous pemphigoid and pemphigus in Switzerland: a 2-year prospective study. Br J Dermatol 161:861–868
Marneros AG, Keene DR, Hansen U, Fukai N, Moulton K, Goletz PL, Moiseyev G, Pawlyk BS, Halfter W, Dong S, Shibata M, Li T, Crouch RK, Bruckner P, Olsen BR (2004) Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J 23:89–99
Marquardt RJ, Li Y (2019) Congenital myasthenic syndrome type 19 due to a novel mutation in the COL13A1 GENE. Muscle Nerve 60:E3–E4
Matsuo N, Tanaka S, Yoshioka H, Koch M, Gordon MK, Ramirez F (2008) Collagen XXIV (Col24a1) gene expression is a specific marker of osteoblast differentiation and bone formation. Connect Tissue Res 49:68–75
Maxwell WL, Duance VC, Lehto M, Ashurst DE, Berry M (1984) The distribution of types I, III, IV and V collagens in penetrant lesions of the central nervous system of the rat. Histochem J 16:1215–1229
Meyer F, Moussian B (2009) Drosophila multiplexin (Dmp) modulates motor axon pathfinding accuracy. Develop Growth Differ 51:483–498
Miner JH, Sanes JR (1994) Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J Cell Biol 127:879–891
Miosge N, Simniok T, Sprysch P, Herken R (2003) The collagen type XVIII endostatin domain is co-localized with perlecan in basement membranes in vivo. J Histochem Cytochem 51:285–296
Miyake M, Hori S, Morizawa Y, Tatsumi Y, Toritsuka M, Ohnishi S, Shimada K, Furuya H, Khadka VS, Deng Y, Ohnishi K, Iida K, Gotoh D, Nakai Y, Inoue T, Anai S, Torimoto K, Aoki K, Tanaka N, Konishi N, Fujimoto K (2017) Collagen type IV alpha 1 (COL4A1) and collagen type XIII alpha 1 (COL13A1) produced in cancer cells promote tumor budding at the invasion front in human urothelial carcinoma of the bladder. Oncotarget 8:36099–36114
Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV (2012) Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 33:8793–8801
Monavarfeshani A, Knill CN, Sabbagh U, Su J, Fox MA (2017) Region- and cell-specific expression of transmembrane collagens in mouse brain. Front Integr Neurosci 11:20
Mouw JK, Ou G, Weaver VM (2014) Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol 15:771–785
Munezane H, Oizumi H, Wakabayashi T, Nishio S, Hirasawa T, Sato T, Harada A, Yoshida T, Eguchi T, Yamanashi Y, Hashimoto T, Iwatsubo T (2019) Roles of collagen XXV and its putative receptors PTPsigma/delta in intramuscular motor innervation and congenital cranial dysinnervation disorder. Cell Rep 29:4362–4376.e6
Munji RN, Soung AL, Weiner GA, Sohet F, Semple BD, Trivedi A, Gimlin K, Kotoda M, Korai M, Aydin S, Batugal A, Cabangcala AC, Schupp PG, Oldham MC, Hashimoto T, Noble-Haeusslein LJ, Daneman R (2019) Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood-brain barrier dysfunction module. Nat Neurosci 22:1892–1902
Muona P, Jaakkola S, Zhang RZ, Pan TC, Pelliniemi L, Risteli L, Chu ML, Uitto J, Peltonen J (1993) Hyperglycemic glucose concentrations up-regulate the expression of type VI collagen in vitro. Relevance to alterations of peripheral nerves in diabetes mellitus. Am J Pathol 142:1586–1597
Muona A, Eklund L, Väisänen T, Pihlajaniemi T (2002) Developmentally regulated expression of type XV collagen correlates with abnormalities in Col15a1(−/−) mice. Matrix Biol 21:89–102
Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR (1981) Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci USA 78:2879–2882
Muragaki Y, Shiota C, Inoue M, Ooshima A, Olsen BR, Ninomiya Y (1992) alpha 1(VIII)-collagen gene transcripts encode a short-chain collagen polypeptide and are expressed by various epithelial, endothelial and mesenchymal cells in newborn mouse tissues. Eur J Biochem 207:895–902
Myers JC, Li D, Bageris A, Abraham V, Dion AS, Amenta PS (1997) Biochemical and immunohistochemical characterization of human type XIX defines a novel class of basement membrane zone collagens. Am J Pathol 151:1729–1740
Myers JC, Li D, Amenta PS, Clark CC, Nagaswami C, Weisel JW (2003) Type XIX collagen purified from human umbilical cord is characterized by multiple sharp kinks delineating collagenous subdomains and by intermolecular aggregates via globular, disulfide-linked, and heparin-binding amino termini. J Biol Chem 278:32047–32057
Myllyharju J, Kivirikko KI (2004) Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet 20:33–43
Nah HD, Barembaum M, Upholt WB (1992) The chicken alpha 1 (XI) collagen gene is widely expressed in embryonic tissues. J Biol Chem 267:22581–22586
Nguyen QT, Sanes JR, Lichtman JW (2002) Pre-existing pathways promote precise projection patterns. Nat Neurosci 5:861–867
Novak U, Kaye AH (2000) Extracellular matrix and the brain: components and function. J Clin Neurosci 7:280–290
O’Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci 34:185–204
Oh SP, Griffith CM, Hay ED, Olsen BR (1993) Tissue-specific expression of type XII collagen during mouse embryonic development. Dev Dyn 196:37–46
Oprisoreanu AM, Smith HL, Arya S, Webster R, Zhong Z, Wehner D, Cardozo MJ, Becker T, Talbot K, Becker CG (2019) Interaction of axonal chondrolectin with collagen XIXa1 is necessary for precise neuromuscular junction formation. Cell Rep 29:1082–1098.e10
O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277–285
Osada Y, Hashimoto T, Nishimura A, Matsuo Y, Wakabayashi T, Iwatsubo T (2005) CLAC binds to amyloid beta peptides through the positively charged amino acid cluster within the collagenous domain 1 and inhibits formation of amyloid fibrils. J Biol Chem 280:8596–8605
Osawa T, Ide C (1986) Changes in thickness of collagen fibrils in the endo- and epineurium of the mouse sciatic nerve during development. Acta Anat (Basel) 125:245–251
Palumbo C, Massa R, Panico MB, Di Muzio A, Sinibaldi P, Bernardi G, Modesti A (2002) Peripheral nerve extracellular matrix remodeling in Charcot-Marie-Tooth type I disease. Acta Neuropathol 104:287–296
Parker SR, Dyson S, Brisman S, Pennie M, Swerlick RA, Khan R, Manos S, Korman BD, Xia Z, Korman NJ (2008) Mortality of bullous pemphigoid: an evaluation of 223 patients and comparison with the mortality in the general population in the United States. J Am Acad Dermatol 59:582–588
Passos-Bueno MR, Suzuki OT, Armelin-Correa LM, Sertie AL, Errera FI, Bagatini K, Kok F, Leite KR (2006) Mutations in collagen 18A1 and their relevance to the human phenotype. An Acad Bras Cienc 78:123–131
Paulus W, Sage EH, Liszka U, Iruela-Arispe ML, Jellinger K (1991) Increased levels of type VIII collagen in human brain tumours compared to normal brain tissue and non-neoplastic cerebral disorders. Br J Cancer 63:367–371
Paulus W, Baur I, Liszka U, Drlicek M, Leigh I, Bruckner-Tuderman L (1995) Expression of type VII collagen, the major anchoring fibril component, in normal and neoplastic human nervous system. Virchows Arch 426:199–202
Peltonen J, Jaakkola S, Hsiao LL, Timpl R, Chu ML, Uitto J (1990) Type VI collagen. In situ hybridizations and immunohistochemistry reveal abundant mRNA and protein levels in human neurofibroma, schwannoma and normal peripheral nerve tissues. Lab Investig 62:487–492
Perrin FE, Lacroix S, Aviles-Trigueros M, David S (2005) Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain 128:854–866
Pöschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U (2004) Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131:1619–1628
Qin J, Liang J, Ding M (2014) Perlecan antagonizes collagen IV and ADAMTS9/GON-1 in restricting the growth of presynaptic boutons. J Neurosci 34:10311–10324
Ramanoudjame L, Rocancourt C, Laine J, Klein A, Joassard L, Gartioux C, Fleury M, Lyphout L, Kabashi E, Ciura S, Cousin X, Allamand V (2015) Two novel COLVI long chains in zebrafish that are essential for muscle development. Hum Mol Genet 24:6624–6639
Rasi K, Hurskainen M, Kallio M, Staven S, Sormunen R, Heape AM, Avila RL, Kirschner D, Muona A, Tolonen U, Tanila H, Huhtala P, Soininen R, Pihlajaniemi T (2010) Lack of collagen XV impairs peripheral nerve maturation and, when combined with laminin-411 deficiency, leads to basement membrane abnormalities and sensorimotor dysfunction. J Neurosci 30:14490–14501
Ren Z, Hsu DY, Brieva J, Silverberg NB, Langan SM, Silverberg JI (2017) Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol 176:87–99
Ricard-Blum S (2011) The collagen family. Cold Spring Harb Perspect Biol 3:a004978
Ricard-Blum S, Ballut L (2011) Matricryptins derived from collagens and proteoglycans. Front Biosci (Landmark Ed) 16:674–697
Ring C, Lemmon V, Halfter W (1995) Two chondroitin sulfate proteoglycans differentially expressed in the developing chick visual system. Dev Biol 168:11–27
Rodriguez Cruz PM, Cossins J, Estephan EP, Munell F, Selby K, Hirano M, Maroofin R, Mehrjardi MYV, Chow G, Carr A, Manzur A, Robb S, Munot P, Wei Liu W, Banka S, Fraser H, De Goede C, Zanoteli E, Conti Reed U, Sage A, Gratacos M, Macaya A, Dusl M, Senderek J, Topf A, Hofer M, Knight R, Ramdas S, Jayawant S, Lochmuller H, Palace J, Beeson D (2019) The clinical spectrum of the congenital myasthenic syndrome resulting from COL13A1 mutations. Brain 142:1547–1560
Roggendorf W, Opitz H, Schuppan D (1988) Altered expression of collagen type VI in brain vessels of patients with chronic hypertension. A comparison with the distribution of collagen IV and procollagen III. Acta Neuropathol 77:55–60
Rohrbough J, Rushton E, Woodruff E, Fergestad T, Vigneswaran K, Broadie K (2007) Presynaptic establishment of the synaptic cleft extracellular matrix is required for post-synaptic differentiation. Genes Dev 21:2607–2628
Roulet M, Ruggiero F, Karsenty G, LeGuellec D (2007) A comprehensive study of the spatial and temporal expression of the col5a1 gene in mouse embryos: a clue for understanding collagen V function in developing connective tissues. Cell Tissue Res 327:323–332
Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, Pihlajaniemi T (1998) The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am J Pathol 153:611–626
Sage H, Balian G, Vogel AM, Bornstein P (1984) Type VIII collagen. Synthesis by normal and malignant cells in culture. Lab Investig 50:219–231
Sajanti J, Björkstrand AS, Finnila S, Heikkinen E, Peltonen J, Majamaa K (1999) Increase of collagen synthesis and deposition in the arachnoid and the dura following subarachnoid hemorrhage in the rat. Biochim Biophys Acta 1454:209–216
Salza R, Oudart JB, Ramont L, Maquart FX, Bakchine S, Thoannes H, Ricard-Blum S (2015) Endostatin level in cerebrospinal fluid of patients with Alzheimer’s disease. J Alzheimers Dis 44:1253–1261
Sandberg M, Tamminen M, Hirvonen H, Vuorio E, Pihlajaniemi T (1989) Expression of mRNAs coding for the alpha 1 chain of type XIII collagen in human fetal tissues: comparison with expression of mRNAs for collagen types I, II, and III. J Cell Biol 109:1371–1379
Sandberg MM, Hirvonen HE, Elima KJ, Vuorio EI (1993) Co-expression of collagens II and XI and alternative splicing of exon 2 of collagen II in several developing human tissues. Biochem J 294(Pt 2):595–602
Sandberg-Lall M, Hägg PO, Wahlström I, Pihlajaniemi T (2000) Type XIII collagen is widely expressed in the adult and developing human eye and accentuated in the ciliary muscle, the optic nerve and the neural retina. Exp Eye Res 70:401–410
Sanes JR, Lichtman JW (1999) Development of the vertebrate neuromuscular junction. Annu Rev Neurosci 22:389–442
Sanes JR, Lichtman JW (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci 2:791–805
Schneider VA, Granato M (2006) The myotomal diwanka (lh3) glycosyltransferase and type XVIII collagen are critical for motor growth cone migration. Neuron 50:683–695
Schnoor M, Cullen P, Lorkowski J, Stolle K, Robenek H, Troyer D, Rauterberg J, Lorkowski S (2008) Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. J Immunol 180:5707–5719
Senner V, Ratzinger S, Mertsch S, Grassel S, Paulus W (2008) Collagen XVI expression is upregulated in glioblastomas and promotes tumor cell adhesion. FEBS Lett 582:3293–3300
Seppänen A, Autio-Harmainen H, Alafuzoff I, Särkioja T, Veijola J, Hurskainen T, Bruckner-Tuderman L, Tasanen K, Majamaa K (2006) Collagen XVII is expressed in human CNS neurons. Matrix Biol 25:185–188
Seppänen A, Suuronen T, Hofmann SC, Majamaa K, Alafuzoff I (2007) Distribution of collagen XVII in the human brain. Brain Res 1158:50–56
Seppänen A, Pikkarainen M, Hartikainen P, Hofmann SC, Majamaa K, Alafuzoff I (2009) Expression of collagen XVII and ubiquitin-binding protein p62 in motor neuron disease. Brain Res 1247:171–177
Seppänen A, Miettinen R, Alafuzoff I (2010) Neuronal collagen XVII is localized to lipofuscin granules. Neuroreport 21:1090–1094
Seppinen L, Pihlajaniemi T (2011) The multiple functions of collagen XVIII in development and disease. Matrix Biol 30:83–92
Sertie AL, Sossi V, Camargo AA, Zatz M, Brahe C, Passos-Bueno MR (2000) Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retinal structure and in neural tube closure (Knobloch syndrome). Hum Mol Genet 9:2051–2058
Sharif Y, Jumah F, Coplan L, Krosser A, Sharif K, Tubbs RS (2018) Blood brain barrier: a review of its anatomy and physiology in health and disease. Clin Anat 31:812–823
Shellswell GB, Restall DJ, Duance VC, Bailey AJ (1979) Identification and differential distribution of collagen types in the central and peripheral nervous systems. FEBS Lett 106:305–308
Shinwari JM, Khan A, Awad S, Shinwari Z, Alaiya A, Alanazi M, Tahir A, Poizat C, Al Tassan N (2015) Recessive mutations in COL25A1 are a cause of congenital cranial dysinnervation disorder. Am J Hum Genet 96:147–152
Sievers J, Pehlemann FW, Gude S, Berry M (1994) Meningeal cells organize the superficial glia limitans of the cerebellum and produce components of both the interstitial matrix and the basement membrane. J Neurocytol 23:135–149
Söderberg L, Kakuyama H, Moller A, Ito A, Winblad B, Tjernberg LO, Naslund J (2005) Characterization of the Alzheimer’s disease-associated CLAC protein and identification of an amyloid beta-peptide-binding site. J Biol Chem 280:1007–1015
Song I, Dityatev A (2018) Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull 136:101–108
Spivey KA, Banyard J, Solis LM, Wistuba II, Barletta JA, Gandhi L, Feldman HA, Rodig SJ, Chirieac LR, Zetter BR (2010) Collagen XXIII: a potential biomarker for the detection of primary and recurrent non-small cell lung cancer. Cancer Epidemiol Biomarkers Prev 19:1362–1372
Su J, Gorse K, Ramirez F, Fox MA (2010) Collagen XIX is expressed by interneurons and contributes to the formation of hippocampal synapses. J Comp Neurol 518:229–253
Su J, Stenbjorn RS, Gorse K, Su K, Hauser KF, Ricard-Blum S, Pihlajaniemi T, Fox MA (2012) Target-derived matricryptins organize cerebellar synapse formation through alpha3beta1 integrins. Cell Rep 2:223–230
Su J, Chen J, Lippold K, Monavarfeshani A, Carrillo GL, Jenkins R, Fox MA (2016) Collagen-derived matricryptins promote inhibitory nerve terminal formation in the developing neocortex. J Cell Biol 212:721–736
Su J, Cole J, Fox MA (2017) Loss of interneuron-derived collagen XIX leads to a reduction in Perineuronal nets in the mammalian telencephalon. ASN Neuro 9:1759091416689020
Sumiyoshi H, Laub F, Yoshioka H, Ramirez F (2001) Embryonic expression of type XIX collagen is transient and confined to muscle cells. Dev Dyn 220:155–162
Sumiyoshi H, Mor N, Lee SY, Doty S, Henderson S, Tanaka S, Yoshioka H, Rattan S, Ramirez F (2004) Esophageal muscle physiology and morphogenesis require assembly of a collagen XIX-rich basement membrane zone. J Cell Biol 166:591–600
Sund M, Väisänen T, Kaukinen S, Ilves M, Tu H, Autio-Harmainen H, Rauvala H, Pihlajaniemi T (2001a) Distinct expression of type XIII collagen in neuronal structures and other tissues during mouse development. Matrix Biol 20:215–231
Sund M, Ylönen R, Tuomisto A, Sormunen R, Tahkola J, Kvist AP, Kontusaari S, Autio-Harmainen H, Pihlajaniemi T (2001b) Abnormal adherence junctions in the heart and reduced angiogenesis in transgenic mice overexpressing mutant type XIII collagen. EMBO J 20:5153–5164
Suzuki OT, Sertie AL, Der Kaloustian VM, Kok F, Carpenter M, Murray J, Czeizel AE, Kliemann SE, Rosemberg S, Monteiro M, Olsen BR, Passos-Bueno MR (2002) Molecular analysis of collagen XVIII reveals novel mutations, presence of a third isoform, and possible genetic heterogeneity in Knobloch syndrome. Am J Hum Genet 71:1320–1329
Suzuki O, Kague E, Bagatini K, Tu H, Heljasvaara R, Carvalhaes L, Gava E, de Oliveira G, Godoi P, Oliva G, Kitten G, Pihlajaniemi T, Passos-Bueno MR (2009) Novel pathogenic mutations and skin biopsy analysis in Knobloch syndrome. Mol Vis 15:801–809
Tanaka T, Wakabayashi T, Oizumi H, Nishio S, Sato T, Harada A, Fujii D, Matsuo Y, Hashimoto T, Iwatsubo T (2014) CLAC-P/collagen type XXV is required for the intramuscular innervation of motoneurons during neuromuscular development. J Neurosci 34:1370–1379
Taylor J, Unsoeld T, Hutter H (2018) The transmembrane collagen COL-99 guides longitudinally extending axons in C. elegans. Mol Cell Neurosci 89:9–19
Teixeira VB, Cabral R, Brites MM, Vieira R, Figueiredo A (2014) Bullous pemphigoid and comorbidities: a case-control study in Portuguese patients. An Bras Dermatol 89:274–278
Tong Y, Xu Y, Scearce-Levie K, Ptacek LJ, Fu YH (2010) COL25A1 triggers and promotes Alzheimer’s disease-like pathology in vivo. Neurogenetics 11:41–52
Tu H, Huhtala P, Lee HM, Adams JC, Pihlajaniemi T (2015) Membrane-associated collagens with interrupted triple-helices (MACITs): evolution from a bilaterian common ancestor and functional conservation in C. elegans. BMC Evol Biol 15:281–283
Tu H, Pirskanen-Matell R, Heikkinen A, Oikarainen T, Risteli J, Pihlajaniemi T (2018) Autoimmune antibodies to collagen XIII in myasthenia gravis patients. Muscle Nerve 57:506–510
Unsoeld T, Park JO, Hutter H (2013) Discoidin domain receptors guide axons along longitudinal tracts in C. elegans. Dev Biol 374:142–152
Urabe N, Naito I, Saito K, Yonezawa T, Sado Y, Yoshioka H, Kusachi S, Tsuji T, Ohtsuka A, Taguchi T, Murakami T, Ninomiya Y (2002) Basement membrane type IV collagen molecules in the choroid plexus, pia mater and capillaries in the mouse brain. Arch Histol Cytol 65:133–143
Utriainen A, Sormunen R, Kettunen M, Carvalhaes LS, Sajanti E, Eklund L, Kauppinen R, Kitten GT, Pihlajaniemi T (2004) Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum Mol Genet 13:2089–2099
van Horssen J, Wilhelmus MM, Heljasvaara R, Pihlajaniemi T, Wesseling P, de Waal RM, Verbeek MM (2002) Collagen XVIII: a novel heparan sulfate proteoglycan associated with vascular amyloid depositions and senile plaques in Alzheimer’s disease brains. Brain Pathol 12:456–462
Veit G, Kobbe B, Keene DR, Paulsson M, Koch M, Wagener R (2006) Collagen XXVIII, a novel von Willebrand factor A domain-containing protein with many imperfections in the collagenous domain. J Biol Chem 281:3494–3504
Vitale P, Braghetta P, Volpin D, Bonaldo P, Bressan GM (2001) Mechanisms of transcriptional activation of the col6a1 gene during Schwann cell differentiation. Mech Dev 102:145–156
Vogel W, Gish GD, Alves F, Pawson T (1997) The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell 1:13–23
Volonghi I, Pezzini A, Del Zotto E, Giossi A, Costa P, Ferrari D, Padovani A (2010) Role of COL4A1 in basement-membrane integrity and cerebral small-vessel disease. The COL4A1 stroke syndrome. Curr Med Chem 17:1317–1324
Wälchli C, Koch M, Chiquet M, Odermatt BF, Trueb B (1994) Tissue-specific expression of the fibril-associated collagens XII and XIV. J Cell Sci 107(Pt 2):669–681
White RJ, Wang Y, Tang P, Montezuma SR (2017) Knobloch syndrome associated with Polymicrogyria and early onset of retinal detachment: two case reports. BMC Ophthalmol 17:214-z
Xu L, Nirwane A, Yao Y (2018) Basement membrane and blood-brain barrier. Stroke Vasc Neurol 4:78–82
Yamada Y, Sakuma J, Takeuchi I, Yasukochi Y, Kato K, Oguri M, Fujimaki T, Horibe H, Muramatsu M, Sawabe M, Fujiwara Y, Taniguchi Y, Obuchi S, Kawai H, Shinkai S, Mori S, Arai T, Tanaka M (2017) Identification of six polymorphisms as novel susceptibility loci for ischemic or hemorrhagic stroke by exome-wide association studies. Int J Mol Med 39:1477–1491
Yan X, Zhang C, Liang T, Yang F, Wang H, Wu F, Wang W, Wang Z, Cheng W, Xu J, Jiang T, Chen J, Ding Y (2017) A PTEN-COL17A1 fusion gene and its novel regulatory role in collagen XVII expression and GBM malignance. Oncotarget 8:85794–85803
Yang H, Jin L, Sun X (2019) A thirteengene set efficiently predicts the prognosis of glioblastoma. Mol Med Rep 19:1613–1621
Ylikärppä R, Eklund L, Sormunen R, Muona A, Fukai N, Olsen BR, Pihlajaniemi T (2003) Double knockout mice reveal a lack of major functional compensation between collagens XV and XVIII. Matrix Biol 22:443–448
Yoshida T, Kato K, Yokoi K, Oguri M, Watanabe S, Metoki N, Yoshida H, Satoh K, Aoyagi Y, Nozawa Y, Yamada Y (2010) Association of genetic variants with hemorrhagic stroke in Japanese individuals. Int J Mol Med 25:649–656
Yoshioka H, Iyama K, Inoguchi K, Khaleduzzaman M, Ninomiya Y, Ramirez F (1995) Developmental pattern of expression of the mouse alpha 1 (XI) collagen gene (Col11a1). Dev Dyn 204:41–47
Yu Phuan CZ, Yew YW, Tey HL (2017) Bullous pemphigoid and antecedent neurological diseases: an association with dementia. Indian J Dermatol Venereol Leprol 83:457–461
Zainul Z, Heikkinen A, Koivisto H, Rautalahti I, Kallio M, Lin S, Härönen H, Norman O, Rüegg MA, Tanila H, Pihlajaniemi T (2018) Collagen XIII is required for neuromuscular synapse regeneration and functional recovery after peripheral nerve injury. J Neurosci 38:4243–4258
Zech M, Lam DD, Francescatto L, Schormair B, Salminen AV, Jochim A, Wieland T, Lichtner P, Peters A, Gieger C, Lochmuller H, Strom TM, Haslinger B, Katsanis N, Winkelmann J (2015) Recessive mutations in the alpha3 (VI) collagen gene COL6A3 cause early-onset isolated dystonia. Am J Hum Genet 96:883–893
Zhang WW, Ma KC, Andersen O, Sourander P, Tollesson PO, Olsson Y (1994) The microvascular changes in cases of hereditary multi-infarct disease of the brain. Acta Neuropathol 87:317–324
Zhang ZY, Zhang Z, Fauser U, Artelt M, Burnet M, Schluesener HJ (2007) Dexamethasone transiently attenuates up-regulation of endostatin/collagen XVIII following traumatic brain injury. Neuroscience 147:720–726
Zhang LS, Li HB, Zeng J, Yang Y, Ding C (2018) Knobloch syndrome caused by homozygous frameshift mutation of the COL18A1 gene in a Chinese pedigree. Int J Ophthalmol 11:918–922
Zimmermann DR, Dours-Zimmermann MT (2008) Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol 130:635–653
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Heikkinen, A., Fox, M.A., Pihlajaniemi, T. (2021). Collagens as New Players in Nervous System Diseases. In: Ruggiero, F. (eds) The Collagen Superfamily and Collagenopathies. Biology of Extracellular Matrix, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-030-67592-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-67592-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67591-2
Online ISBN: 978-3-030-67592-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)