Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enveloped, positive-sense RNA coronavirus responsible for the COVID-19 pandemic. Since December 2019, coronavirus disease 2019 (COVID-19) has affected more than 127 million people, 2.7 million deaths globally (as per WHO dashboard, dated 31 March, 2020), the virus is capable of transmitting from human to human via inhalation of infected respiratory droplets or aerosols or contact with infected fomites. Clinically, patients with COVID-19 present with severe respiratory distress syndrome, which is very similar to the presentation of other respiratory viral infections. A huge variation in the host response exists, with the resulting symptoms varying from mild to moderate. Comorbidities such as cardiovascular disease, hypertension, diabetes, coagulation dysfunction, stroke, malignant tumor and multiple organ dysfunction syndrome, as well as age and sex, are associated with severe COVID-19 cases. So far, no targeted therapies have been developed to treat this disease and existing drugs are being investigated for repurposing. This chapter discusses the epidemiology, clinical features of COVID-19, pathogenesis and the innate and adaptive immune response mounted by the host to the SARS-CoV-2 infection. A deeper understanding of the host-pathogen interaction is fundamental to the development of a vaccine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 was first reported in Wuhan and related regions in Hubei province, People’s Republic of China in December 2019 and subsequently spreading to most countries across the world. The infection clinically presents as atypical pneumonia which can progress to acute lung injury and acute respiratory distress syndrome (ARDS). SARS-CoV-2 was found highly homologous to the coronavirus (CoV) that caused the SARS (Severe acute respiratory syndrome) outbreak in 2003 in China (Zhu et al. 2020; National Health Commission of People’s Republic of China, 2020). On 11 February 2020, The International Committee on Taxonomy of Viruses (ICTV) named it Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Simultaneously, the WHO named the disease caused by this virus COVID-19 (WHO report, 2020). This is the seventh coronavirus known to infect humans: SARS-CoV, Middle east respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV-2 can cause severe disease. The other four, HKU1, NL63, OC43 and 229E, are endemic in the population accounting for up to 30% of annual respiratory infections and are associated with mild symptoms that are typically self-limiting. These cause seasonal infections in temperate climate during winter months (Charlton et al. 2018; Monto et al. 2020; Chan et al. 2020). CoV are associated with an increased risk of lower respiratory tract infections that are particularly debilitating in neonates, the elderly and in individuals with comorbidities (van der Hoek et al. 2005). Major symptoms of CoV infection include fever, sore throat and swollen adenoids (Liu et al. 2017) as well as viral or bacterial pneumonia or bronchitis (Forgie and Marrie 2009). NL-63 has been associated with onset of acute laryngotracheitis (van der Hoek et al. 2005). SARS-CoV-2 disseminates via asymptomatically infected individuals (Rothe et al. 2020; Ling et al. 2020; Pan et al. 2020b; Ghinai et al. 2020; Mazumder et al. 2020). The overall mortality rate is 0.5–3.5% (Guan et al. 2020; Wolfel et al. 2020; Wu and McGoogan 2020).
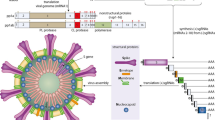
2 Structural Organization of SARS-CoV-2
SARS-CoV-2, a β-coronavirus, is a non-segmented positive single-stranded RNA virus with a genome size of 29.9 kb (Wu et al. 2020a, b). The one-third of the viral genome encodes for structural proteins: Spike glycoprotein (S), Envelope protein (E), Membrane glycoprotein (M) and Nucleocapsid Protein (N). The remaining two-third of the genome is comprised of two open reading frames ORF1a and ORF1b that encode for non-structural proteins (nsps), which form the replication transcription complex (RTC); this controls the viral multiplication within host (Kim et al. 2020). The non-structural proteins that are translated include papain-like protease (PLpro), 3C-like protease (3CLpro), RNA dependent RNA polymerase (RdRp), helicase (Hel), and exonuclease (ExoN) (Tang et al. 2020a, b). The S genes of 2019-nCoV and RaTG13 (BatCoV) are longer than other SARS-CoV mainly found in bats (Zhou et al. 2020).
The most prominent viral envelope protein is the S-protein (Cavanagh 1995). It is heavily glycosylated to form large transmembrane homotrimeric spikes; this bulbous crown-like structure is what gives the name coronavirus. The S-protein is cleaved during viral internalization in endocytic vesicles to form two sub-units, S1 and S2, by host furin-like protease and assists viral integration into the host (Coutard et al. 2020; Walls et al. 2020a, b; Wrapp et al. 2020). The S1 sub-unit accommodating the receptor binding domain (RBD) determines the cellular tropism, while the S2 subunit containing the membrane binding domain (MBD) mediates fusion between cell and viral membranes for cell entry. The S1 subunit contains a signal peptide and two subdomains, the N-terminal domain (NTD) and the C-terminal domain (CTD), both domains can serve as the RBD (Tang et al. 2020a, b). RBD is a twisted five-stranded anti-parallel β-sheet (β1, β2, β3, β4 and β7) structure with an extended insertion region containing β5 and β6 strands, α4 and α5 helices and loops forming the receptor binding motif (RBM) (Lan et al. 2020). SARS-CoV-2 utilizes the CTD to bind angiotensin converting enzyme-2 (ACE2) for entry into the host cell (Zhou et al. 2020). The S2 subunit contains other regions; the fusion peptide (FP), HR1 (heptad repeat 1), HR2 (heptad repeat 2), transmembrane (TM) and cytoplasmic region (CP).
The E-protein and M-protein are conserved across the β-coronavirus (Bianchi et al. 2020). The E-protein is a small integral membrane polypeptide which can oligomerize and form ion channels-fundamental in the release of viral particles (Verdia-Baguena et al. 2012). The M-protein is prevalent within the viral membrane and maintains structural integrity of the virion envelope. It is important for budding process (Bianchi et al. 2020). It is a multi-spanning membrane protein with three trans-membrane segments with the major domain of the molecule being a large carboxy terminus situated in the interior of the virion (Rottier 1995). The M-protein is capable of interacting with other M, N, E and S proteins during the process of viral assembly (Alsaadi and Jones 2019; Neuman et al. 2011). The N-protein binds the RNA genome, continuously packaging it into the viral particle during assembly and also providing stability to the viral RNA. Moreover, it can antagonize antiviral RNAi and inhibit the activity of cyclin-dependent kinase (cyclin-CDK) complex, which results in the hypophosphorylation of retinoblastoma protein (pRB), inhibiting the genome replication (S-Phase) of the cell.
RNA dependent RNA polymerase (RdRp) is paramount in viral genome replication. It is a highly conserved protein between RNA viruses, hence a promising candidate for an antiviral drug development. Targeting the RdRp active site may inhibit viral replication (Aftab et al. 2020).
3 Transmission of SARS-CoV-2
Statistically, by the beginning of September 2020, there were an estimated 22,602,665 positive cases of COVID-19, with 852,758 confirmed deaths across 190 countries. This number increased to 127 million affected people with 2.7 million deaths globally by 31 March 2020. SARS-CoV was the causative agent of the 2002–2003 SARS outbreak that originated in Guandong province, China and resulted in approximately 8098 cases and 774 deaths during a nine-month period, with an average mortality rate of 9%. In the elderly population, mortality peaked at almost 50% (Drosten et al. 2003). In 2012, a novel β-coronavirus emerged in Saudi Arabia, MERS-CoV, the causative agent in a number of highly virulent respiratory tract infections across the Middle East (Zaki et al. 2012). From 2012 to January 2020, there were 2506 cases with a 35% fatality rate, approximately four times higher than SARS-CoV (Killerby et al. 2020). The number of patients infected with SARS-CoV-2 is notably higher than SARS-CoV and MERS-CoV, suggesting a higher rate of infection per exposure for SARS CoV-2.
SARS CoV-2 is highly contagious and efficient at spreading. In nature, the lipid bilayer of the virion protects the virus from denaturation for a short time during which it can bind to a suitable target receptor. Transmission can occur from an infected individual through respiratory droplets in direct transmission, where they are expelled as aerosols while coughing, sneezing or talking in close contact, or saliva during intimate contact. Indirect transmission following deposition of the virus on fomites (surfaces) has also been observed (Chan et al. 2020; Li et al. 2020a, b, c; Ghinai et al. 2020). It has been suggested that airway secretions may protect the virus, enhancing its persistence and transmission via contaminated fomites (Pastorino et al. 2020). Aerosol suspension studies suggest that SARS-CoV-2 can persist for long periods in the aerosol form, with viral bioaerosols retaining infectivity and virion integrity for up to 16 h (Fears et al. 2020). Airborne transmission potentially occurs by inhaling aerosols containing a critical titre of the virus sufficient enough to cause infection, though the optimum and basal infectious doses of SARS-CoV-2 are yet to be ascertained. Droplets containing the coronavirus are heavy due to their large diameter, and therefore, are incapable of travelling long distances through air. Van Doremalen et al. (2020) have studied the stability of SARS CoV-2 in aerosols and various surfaces; it can remain viable in aerosols for 3 h, being more stable on plastic (for up to 72 h) and stainless steel (for up to 48 h) compared to copper (no viable virus after 4 h) and cardboard (no viable virus after 8 h). SARS CoV-2 RNA can also be detected in the urine and feces of some patients; however, due to low titres in plasma and serum, the potential of bloodborne transmission remains uncertain.
Hao et al. (2020) analyzed the transmission dynamics of the COVID-19 outbreak in Wuhan, and highlighted two key features: high covertness and high transmissibility. These features synergistically propelled the COVID-19 pandemic (Hao et al. 2020). In 40% of cases, the virus has been reported to spread via asymptomatically-infected individuals worldwide (Rothe et al. 2020; Ling et al. 2020; Pan et al. 2020b; Ghinai et al. 2020; Mazumder et al. 2020). Various statistical analyses were undertaken to ascertain the role of asymptomatic individuals in transmitting SARS-CoV-2. In a study involving cruise ship passengers off the coast of Japan carrying 3711 passengers and crew members, there were 634 confirmed infection cases: 306 symptomatic and 328 asymptomatic (Mizumoto et al. 2020). Similarly, a study on passengers flying from Wuhan to Japan up to the 6 February 2020, suggested half of the infected individuals were asymptomatic (Nishiura et al. 2020). Tong et al. (2020) identified two symptomatic COVID-19 cases after their exposure to a pre-symptomatic individual who was later diagnosed with laboratory-confirmed COVID-19. These two individuals later transmitted SARS-CoV-2 to three other family members, who also remained asymptomatic (Tong et al. 2020).
At the New York–Presbyterian Allen Hospital and Columbia University Irving Medical Center between March 22 and April 4, 2020, a total of 215 pregnant women who delivered infants were screened on admission for symptoms of COVID-19. Four women had SARS-CoV-2 related symptoms on admission while the remaining 211 women were asymptomatic and afebrile. Nasopharyngeal swabs indicated 33 patients were positive for SARS-CoV-2 at admission, 29 had no symptoms of COVID-19 (Sutton et al. 2020). Similarly, in another study, 55 asymptomatic cases were identified with SARS-CoV-2 infection, their ages ranged from 30 to 49 years asymptomatic cases occurred more often in middle aged people in Shenzhen, China (Wang et al. 2020a, b, c, d, e, f, g). Although COVID-19 was found to have lower severity and mortality than SARS, it is highly contagious and affects comparatively more men than women (Jin et al. 2020; Huang et al. 2020; Mazumder et al. 2020).
A quantitative RT-PCR study showed that the viral load of SARS-CoV-2 in throat samples peaked around 5–6 days after the onset of symptoms (Pan et al. 2020a). SARS-CoV-2 can also be detected in deep throat saliva samples for 20 days or longer (To et al. 2020). In fecal samples, SARS-CoV-2 can be traced after 28 days from the first onset while the respiratory samples remained positive for around 17 days (Wu et al. 2020a, b). This possibly suggests that the virus may be actively replicating in the gastrointestinal tract, even when it is absent in the respiratory tract (Wu et al. 2020a, b). Viral RNA has also been detected in urine on 42 days post infection in very low quantities (Sun et al. 2020a, b). In a study involving 71 COVID-19 patients (68 cases were above 18 years) who were in the convalescence period, 32.5% patients were positive for viral RNA (results turned from negative to positive) and the longest RNA reversal phase time was 7 days (Liu et al. 2020a, b). In the same study, 52.9% of adults showed no obvious clinical symptoms, whereas the remainder exhibited mild and non-specific clinical symptoms (Liu et al. 2020a, b).
4 Epidemiology of SARS-CoV-2
Epidemiological studies have revealed that CoV are epizootic to bats; in particular, Chinese horseshoe bats harbor viral genomic sequences and serological evidence of prior infection with SARS-related CoV (Lau et al. 2005; Li et al. 2005). The coronavirus subfamily is genotypically and serologically divided into four genera; α, β, γ, and δ coronaviruses. The α- and β-coronaviruses both originate from bats and are mainly found in mammals such as bats, rodents, civets, and humans. Several exotic animals have tested positive for antibodies to SARS-related CoV, including hog badgers and raccoon dogs in Chinese wet wildlife markets. Moreover, masked palm civets inoculated with SARS-CoV develop lung pathology (Wu et al. 2005). Repetitive viral genome sequencing of SARS patients and suspected intermediary hosts produced a dendrogram suggesting that the first human SARS-CoV was related to a civet-derived virus; after several transmissions between human hosts, the virus had acquired point mutations augmenting its pathogenicity in humans (Song et al. 2005).
SARS-CoV-2 caused severe respiratory pathology in hosts, its symptomatology and incubation period resemble SARS-CoV and MERS-CoV. There are two notable features of the SARS CoV-2 genome: mutations in the contact residues of SARS-CoV-2 S-protein, and the inherent polybasic cleavage site at the two subunits of the S-protein. Genetic analyses of SARS-CoV-2 patient samples confirmed an 88% sequence similarity to bat SARS-related CoV, with a 79% similarity to SARS-CoV and 50% to MERS-CoV (Liu et al. 2020a, b). The similarity exhibited between SARS-CoV-2 and bat SARS-CoV suggested bats to be the possible reservoir. Zoonotic reservoirs are well maintained due to their population structure, migration patterns and life span (Calisher et al. 2006). They are capable of transmitting CoV, which has seen the re-emergence of this infectious disease globally. These findings corroborate that SARS-CoV-2 is a novel coronavirus with significant tolerance to genetic variability and is unlike previously known CoVs.SARS-CoV-2 is a rapidly evolving RNA virus which is continually exhibiting genomic mutations as its transmits. Thus, the mutational landscape has been under constant global scrutiny to understand the infectivity and antigenicity of the new variants.United Kingdom, on December 14, 2020, reported a SARS-CoV-2 variant of concern (VOC 202012/01), B.1.1.7 lineage. This B.1.1.7 variant became the dominant circulating SARS-CoV-2 variant in England since its emergence in September 2020. It has also been detected in other 30 countries including the United States. Compared to ancestral viruses containing the D614G mutation, the B.1.1.7 variant has accumulated several other mutations where six nucleotide deletions in the S-gene resulted in the loss of two amino acids, H69 and V90 (Kemp et al. 2021; McCarthy et.al. 2020; Galloway et al. 2021). Several mathematical modelling and epidemiological studies predicted that variant can spread 56% faster than other lineages resulting in higher nasopharyngeal viral loads compared to the wild-type strain (Davies et al. 2020).On 18 December 2020, another highly transmissible variant of SARS-CoV-2 named B.1.351 was reported by the authorities from Republic of South Africa. Compared to the Wuhan reference strain, the B.1.351 variant has 12 non-synonymous mutations and one deletion (Gómez et al. 2021). This variant has three mutations in the S-protein: K417N (a lysine to asparagine substitution at amino acid position 417), E484K (a Glutamic acid to lysine substitution at amino acid position 484) and N501Y (an asparagine to tyrosine substitution at amino acid position 501). The N501Y mutation is common in both B.1.1.7 and B.1.351 variant (Gómez et al. 2021).P.1 (B.1.1.28.1) is the third variant of SARS-CoV-2 that was detected by Japan’s National Institute of Infectious Diseases on 6 January 2021, which was isolated from the four travellers who arrived in Tokyo from Brazil. Later on, P.1 variant was identified in Brazil, as the widely transmitted variant (Candido et al. 2020; Gómez et al. 2021). The patient samples collected during October 2020 form the municipal region of Reo De Janeiro State identified the first variant individual with the S-Protein mutation E484K. This 484K.V2 variant has been transmitted to various other countries such as England, Norway, Singapore, Denmark, Ireland and Canada (Gómez et al. 2021; Resende et al. 2021; Vasqueset al. 2021). The B.1.617.2 (Delta) variant of SARS-CoV-2 was identified in India in late 2020 and has subsequently been detected in around 60 countries (CDC. 2021). The B.1.617.2 variant has a potentially higher rate of transmission than other variants and currently account for approximately 95% of sequenced and 92% genotyped cases from 7 to 21 June 2021 in the UK (Public Health England, 2021) and became the dominant variant in the UK.The rapid establishment of a national sequencing collaboration by the United Kingdom, the COVID-19 Genomics UK consortium (COG-UK, 2020) facilitated the robust systematic sampling of the viral genome. A considerable attention has been drawn on the D614G mutation, becoming the dominant form worldwide as the virus spreads from Asia into Europe and USA (Volz et al. 2021). D614G mutation in SARS-CoV-2 is a non-synonymous mutation resulting in a replacement of aspartic acid with glycine at position 614 of the virus spike protein. D614G has been found to be associated with higher viral load and with younger age of patient and not with higher mortality or clinical severity of the disease (Volz et al. 2021). Different demographic events such as population growth, random genetic drift, founder effects, positive selection and several other factors can be the reason for the spread of viral mutation that need to be monitored globally.
5 Clinical Aspects of COVID-19
COVID-19 typically begins with a mild, self-limiting respiratory tract illness, progressing to severe ARDS, and then leading to multiple organ failure in some cases. Within approximately 5.2 days of incubation, SARS-CoV-2 infection presents its first symptoms (Li et al. 2020a, b, c). The period from initial symptoms to potential fatality ranges from 6 to 41 days with a median of 14 days (Wang et al. 2020a, b, c, d, e, f, g). This variable time span is contingent on a number of co-factors, sex and immune status being the main issues. At the onset of the disease, most patients exhibit common symptoms such as headache, fever and dry cough. Other symptoms include muscle pain/fatigue, chest pain, diarrhoea, nausea, vomiting, and less often haemoptysis and anosmia (Huang et al. 2020; Chen et al. 2020a, b, c, d; Wang et al. 2020a, b, c, d, e, f, g; D’Amico et al. 2020; Kerslake et al. 2020). It was also observed that diabetes, hypertension, and cholesterol levels possess an apparent relation to COVID-19 severity (Wang et al. 2020a, b, c, d, e, f, g) (Fig. 6.1); these patients also show high levels of IL-6, IL-10, TNF-α, and lactate dehydrogenase (LDH) in serum (Li et al. 2020a, b, c).
Mortality is higher in adults above the age of 65 years (approximately 6.4%) (WHO situation report, 127, 2020). Amongst the elderly population, the virus spreads rapidly into the gas exchange regions of lung possibly due to reduced muco-ciliary clearance (Ho et al. 2001). Pathological features of COVID-19 resemble those of SARS and MERS. While the virus is in the airway, it may also present symptoms such as hoarseness, ulceration and edema in the epiglottis and subglottis (Oliver et al. 2020). In the lungs, viral infection shows as multiple infrahilar airspace opacities on chest X rays (Lei et al. 2020); chest CT scans reveal ground-glass opacities, bilateral multifocal infiltrates, lymphadenopathy and invasive lung lesions with thoracic tissue injury (Ghinai et al. 2020; Ren et al. 2020) and may even lead to fibrosis (Mason 2020). Elevated D-dimers that are associated with inflammation suggest high risk of ARDS as observed in COVID-19 patients (Tang et al. 2020a, b). The risk of developing a lethal form of COVID-19 increases in the elderly, amongst adults with underlying health conditions and in individuals with compromised immunity (Gralinski and Menachery 2020).
The neutrophil-to-lymphocyte ratio (NLR) can be a predictive factor for identifying those at risk of critical illness following COVID-19, patients aged ≥ 50 and with NLR ≥ 3.13 being at high risk (Liu et al. 2020a, b). Out of the first 41 patients diagnosed with COVID-19 in Wuhan, 5 had myocardial injury, which mainly manifested as an increase in high-sensitivity cardiac troponin I (Wang et al. 2020a, b, c, d, e, f, g). Laboratory tests showed elevated C-reactive protein (CRP), transaminases and LDH, and lymphopenia (Bonomi et al. 2020). The myocardial zymogram showed high levels of creatine kinase in several patients (Wang et al. 2020a, b, c, d, e, f, g). COVID-19 patients may predispose to thromboembolic disease due to excessive inflammation, hypoxia and diffuse intravascular coagulation (Wang et al. 2020a, b, c, d, e, f, g; Chen et al. 2020a, b, c, d; Guan et al. 2020). The majority of the ICU patients admitted with COVID-19 exhibited thrombotic complications, such as symptomatic acute pulmonary embolism, deep vein thrombosis, ischemic stroke and myocardial infarction (concomitant with high plasma levels of IL-2, IL-7, IL-10, GSCF, IP-10, MCP-1, MIP-1A, and TNF-α) (Klok et al. 2020). Kidney damage in COVID-19 patients was observed mainly due to sepsis, hypovolaemia, and nephrotoxins. Cardiorenal syndrome may also lead to acute kidney injury in COVID-19 patients (Wang et al. 2020a, b, c, d, e, f, g). Abnormal liver function was further documented in COVID-19 patients with alanine aminotransferase (ALT) or aspartate aminotransferase (AST) above the normal range (Wang et al. 2020a, b, c, d, e, f, g). Symptoms such as olfactory and gustatory dysfunctions were also found (Vaira et al. 2020). Moderate conjunctivitis could be the first sign of severe respiratory distress in COVID-19 patients (Daruich et al. 2020). A case of brain damage by SARS-CoV-2 in Beijing Ditan Hospital (Xiang et al. 2020) and a another case of SARS-CoV-2 infection-related encephalitis were also reported (Ye et al. 2020).
Cancer patients are particularly susceptible to severe form of the disease (Xia et al. 2020; Onder et al. 2020; Wang and Zhang 2020) and are significantly at higher risk of death from COVID-19 (Deng et al. 2020). An Italian population-wide study showed that out of 430 cancer patients, 118 had prostate cancer in a total of 4532 COVID-19 patients; the study also highlighted that male cancer patients were 79% more likely to test positive for SARS-CoV-2 (Montopoli et al. 2020). Studies on COVID-19 patients from a New York Health System revealed that the mortality rates were 55% for lung cancer, 14% for breast cancer, 20% for prostate cancer, and 38% for colorectal cancer (Mehta et al. 2020). Thus, cancer patients accompanying COVID-19 infection were recommended to avoid treatments causing immunosuppression (Zhang et al. 2020a, b). What is interesting is that the new data from UK and Italy seems to show that chemotherapy is not particularly a risk factor. It is suspected that there is a metabolic issue involved, consistent with susceptibility in cancer patients, elderly and male sex.
6 Pathogenesis of COVID-19
6.1 SARS-CoV-2 Attachment and Entry
SARS-CoV-2 infection ensues when the S-protein binds to ACE2 for cellular entry into the target host cell. The internalization of the virus is facilitated by TMPRSS2 protease activity and cathepsin B/L (cat B/L) activity which may substitute for TMPRSS2 (Hoffmann et al. 2020). ACE2 receptors contain two lobes at their N-terminal peptidase domain which is the peptide substrate binding site. The extended receptor binding motif (RBM) in the RBD of S1 attaches with the lower side on the small lobe of ACE2 accommodating its N-terminal helix. RBM contains most of the contact residues of SARS-CoV-2 that bind with ACE2, an estimated 17 residues of RBD interact with 20 residues of ACE2. The upper side of the RBM is capable of forming salt-bridge interactions with ACE2, which is unique to SARS-CoV-2 (Lan et al. 2020). S-protein of SARS- CoV-2 is capable of binding ACE2 with 10–20 times greater affinity than SARS-CoV (Wrapp et al. 2020). The ACE2-binding ridge in SARS-CoV-2 RBD has a more compact conformation with two virus-binding hotspots at the RBD–ACE2 interface compared to SARS-CoV RBD (Shang et al. 2020a).
SARS-CoV-2 uses two different pathways for its entry, depending on the protease availability; it can either fuse with the plasma membrane (early pathway), or with the endosomal membrane (late pathway). Upon binding with the S-protein of SARS-CoV-2, the S2 subunit is primed by type 2 transmembrane protease TMPRSS2 that expedites coalescence enabling entry at the plasma membrane surface (Hoffmann et al. 2020; Matsuyama et al. 2010). This leads to cleavage of the ACE2 receptor, thereby facilitating viral entry into the target cell. A recent study on gene expression of ACE2 in multiple scRNA-seq datasets suggested that it is expressed in multiple tissues, such as the airways, oesophagus, ileum, colon, liver, cornea, heart, kidney and testis (Sungnak et al. 2020). A study of single cell gene expression matrices revealed that ACE2 is mainly expressed in alveolar lung type II cells (AT2), oesophagal keratinocytes, liver cholangiocytes, colon colonocytes, ileum endothelial cells (EC), rectum EC, stomach epithelial cells and renal proximal tubules (Qi et al. 2020). Across the airway, ACE2 was expressed in multiple epithelial cells, including alveolar epithelial type II cells in the parenchyma, where nasal epithelium clusters of goblet cells and ciliated cells indicated the highest expression. TMPRSS2 was also highly expressed in nasal goblet and ciliated cells which suggests that these cells may act as loci of original infection and possible reservoirs for dissemination within and between individuals (Sungnak et al. 2020) (Fig. 6.2). In single-cell RNA-sequence datasets of adult human testis, ACE2 was found to be expressed in both germ cells and somatic cells; Sertoli cells, spermatogenic stem cells, and Leydig cells showed ACE abundance (Shen et al. 2020). Co-expression of ACE2 and TMPRSS2 in superficial conjunctival cells suggests the possibility of the spread of SARS-CoV-2 through the nasolacrimal duct (Sungnak et al. 2020) (Fig. 6.2). Other type II transmembrane serine proteases (TTSP) have also been found to play a role in CoV infection, such as TMPRSS11a that can cleave and activate SARS-CoV S-protein for fusion (Kam et al. 2009) and TMPRSS11d, also known as a human airway trypsin-like protease (HAT) that can activate MERS-CoV infection (Bertram et al. 2011; Zmora et al. 2018).
Cells co-expressing ACE2 and TMPRSS2. Cells present in respiratory as well as non-respiratory systems can bind SARS-CoV-2 through its ACE2 and TMPRSS2. In the respiratory system, their co-expression is observed in ciliated and secretory cells of nasal and bronchial airways; in the distal lungs, they are co-expressed in alveolar type-2 cells (AT2). Different cell types present in the cornea, oesophagus, Ileum, colon, liver, gall bladder, prostate, testis and fetal thymus have been found to co-express ACE2 and TMPRSS2 necessary for SARS-CoV-2 infection
The S1/S2 cleavage site of SARS-CoV-2 S-protein possesses several arginine residues rendering it susceptible to cleavage (Hoffmann et al. 2020). The S-protein trimer is cleaved into S1, containing the RBD and S2 subunit, S2 is further cleaved into S2′ to form the viral membrane fusion peptide which is inserted into the host cell membrane (Walls et al. 2020a, b). Heptad repeat (HR1 and HR2) of the S2 unit adopts a hydrophobic interface to drive membrane fusion and the TM region located next to HR2 anchors the S-protein in the viral membrane (Tang et al. 2020a, b). In TMPRSS2− cells, the low pH environment activates cathepsin L cleavage of the S2′ site, thus triggering the fusion pathway and is responsible for viral egress from endosomes in SARS-CoV-2 (Tang et al. 2020a, b). Therefore, protease activity possibly encourages virus infiltration by one of the two pathways. The first mode is direct fusion of the S-protein through proteolytic cleavage by the host cell surface TMPRSS2 serine protease. The second route of entry is endocytosis; cleavage results in a conformational change and promotes fusion of the viral envelope with the endosome.
In cells with low expression of TMPRSS2 and pH-dependent CatB/L proteases, furin pre-activation can facilitate SARS-CoV-2 entry by acting on furin-like cleavage sites at the S2 domain proximal to the fusion peptide site (Shang et al. 2020b; Coutard et al. 2020) (Fig. 6.3). The proprotein convertase (PPC) motif is also present at the S1/S2 boundary which is critical for SARS-CoV-2 entry into the host cell (as shown in Hela, Calu-3 and MRC-5 cells). Both TMPRSS2 and cathepsin have cumulative effects with furin favoring SARS-CoV-2 entry (Shang et al. 2020a, b) (Fig. 6.3).
Proposed model for SARS-CoV-2 entry and release from the host cell. (a) SARS-CoV-2 spike protein binds ACE2 by the amino terminal region (S1 portion) for cellular entry; (b1) Upon binding with the S-protein of SARS CoV-2, it is primed by type 2 transmembrane protease TMPRSS2 that enables entry at the plasma membrane surface, the S2-portion of the S-protein fuses with the TMPRSS2; (b2) The fusion peptide (FP) is inserted into the host cell membrane to trigger the fusion event with the host cell. The HR (HR1 and HR2) of the S2 unit adopts a hydrophobic interface to drive membrane fusion and the TM region next to HR2 anchors the S-protein in the viral membrane, (c1) For TMPRSS2− cells, SARS-CoV-2 enters the host cell via CatB/L endosomal pathway. In case of cells with lower expression of TMPRSS2 and CatB/L proteases, furin pre-activation can facilitate SARS-CoV-2 entry. (c2) A low pH environment activates CatB/L cleaving S2′ site, thus triggering the fusion pathway, (d) SARS-CoV-2 genome is released inside the host cytoplasm, (e) Once the genomic RNA, which is a positive sense strand enters the cell, its two ORFs (ORF1a and ORF1b) translate into several nsps, (f) Coronavirus replication and transcription are mediated by a replication-transcription complex (RTC) which is virus-encoded, (g) RNA positive strand generates negative RNA intermediates that act as a template for the synthesis of a new positive sense RNA (gRNA) and sub-genomic RNAs (sgRNA). (h) The S glycoprotein oligomerizes in the endoplasmic reticulum and is incorporated into budding virions in a pre-Golgi compartment. The structural protein helps in packing the gRNA during virion assembly. (i) Eventually, the vesicles containing the virion fuse with the plasma membrane releasing them to infect other cells
6.2 SARS-CoV-2 Genome Translation, Replication, Assembly and Release
Following entry into the host, the SARS-CoV-2 genome is released into the cytoplasm of the host cell. The 5′ methylated cap and 3′ polyadenylated tail in the coronavirus RNA genome aid attachment of the viral replicase gene to host cell ribosomes where two-thirds are translated. ORF1a and ORF1b employ papain-like protease (PLpro) and 3C-like protease (3CLpro) to act on the polyprotein structures and cleave them at specific sites to produce several non-structural proteins (nsps). ORF1a translates into a 440–500 kDa polypeptide which gets cleaved into 11 nsps, whereas ORF1b translates into a large 740–810 kDa polypeptide which is cleaved into 15 nsps (Kim et al. 2020). The nsps assemble into the replication transcription complex (RTC) to upregulate RNA synthesis (Fig. 6.3). The viral RTC stimulates RNA synthesis of genomic and sub-genomic RNAs, which are required for accessory genes of the replicase polypeptides. RNA-dependent RNA polymerase (RdRp), also known as nsp12, is the main protein facilitating replication and transcription of viral RNA (Gao et al. 2020a, b, c, d). Papain-like protease (PLpro) and 3C-like protease (3CLpro) perform the proteolytic cleavage. RNA positive strand generates negative RNA intermediates that act as a template for synthesis of new positive sense RNA (gRNA) and subgenomic RNAs (sgRNA). RdRp, also known as nsp12, catalyzes the synthesis of viral RNA, possibly with the involvement of nsp7 and nsp8 as cofactors (Gao et al. 2020a, b, c, d). SARS-CoV-2 expresses nine canonical sgRNA (S, 3a, E, M, 6, 7a, 7b, 8 and N) along with gRNA. The structural protein helps in packing the gRNA during virion assembly. Like other coronavirus, SARS-CoV-2 RNAs also carry poly (A) tails (Kim et al. 2020). The S glycoprotein oligomerizes in the endoplasmic reticulum and is incorporated into budding virions in a pre-golgi compartment (Tooze et al. 1984). Eventually, the vesicles containing the virions fuse with the plasma membrane releasing them to infect other cells (Fig. 6.3).
7 Host Immune Response Against SARS-CoV-2
The time between exposure to SARS-CoV-2 and appearance of noticeable symptoms is the incubation period, which ranges between 2 and 14 days (median 4–5 days) (Guan et al. 2020; Li et al. 2020a, b, c; Backer et al. 2020; Wang et al. 2020a, b, c, d, e, f, g). The inhaled SARS-CoV-2 virus most likely first binds to the epithelial cells (ciliated and goblet cells) of the nasal airway through ACE2 receptors that are primed by TMPRSS2 protease. At this time, the virus can be found in nasal samples. Once inside the cell, the virus starts replicating and activates the innate immune arm of the host.
7.1 Innate Immune Response
The innate immune response is most likely initiated when the virus reaches the airways where it is detected by toll-like receptors (TLRs), this induces expression of type I interferon (IFN). ACE2 is known to regulate the Renin-Angiotensin System (RAS), thus, a reduction in ACE2 expression due to viral infection results in RAS dysfunction. This potentially modulates blood pressure and induces inflammation and vascular permeability in respiratory airways. In approximately 80% of infected patients, the virus remains restricted to the upper and conducting airways exhibiting mild symptoms (Wu and McGoogan 2020).
The virus goes on to reach the alveoli, infecting alveolar type II cells (AT2) in the lungs. The viral infected AT2 cells undergo apoptosis and/or pyroptosis leading to vascular leakage and alveolar damage releasing the virus (Huang et al. 2020; Yang 2020). IL-1β is elevated during SARS-CoV-2 which is a pivotal cytokine released during pyroptosis (Huang et al. 2020). Pyroptosis leads to the release of damage associated molecular patterns (DAMPs), which are recognized by nearby epithelial cells, endothelial cells and alveolar macrophages, triggering production of pro-inflammatory cytokines and chemokines. This sudden increase in the local and circulating levels of pro-inflammatory cytokines leads to a cytokine storm. A severe inflammatory response can result in mass macrophage death within the lungs (accounting for more than 95% of the leukocytes) due to pyroptosis, necroptosis and necrosis leading to advanced lung damage (Vincent et al. 2005; Huang et al. 2020) (Fig. 6.4).
Innate Immune response (proposed). (a) SARS-CoV-2 reaches the alveolar airways and infects the alveolar type II cells (AT2). (b) The infected AT2 cells undergo apoptosis and/or pyroptosis leading to alveolar damage releasing the virus. Damage Associated Molecular Patterns (DAMPs) released from the damaged cells are recognized by nearby epithelial cells, endothelial cells and alveolar macrophages triggering production of pro-inflammatory cytokines and chemokines, (c) This sudden acute increase in the levels of pro-inflammatory cytokines and chemokines leads to a cytokine storm and is the main cause of ARDS. Several cytokines and chemokines such as IL-1β, IL-7, IL-8, IL-9, IL-10, FGF, GCSF, GMCSF, IFN-γ, IP-10, MCP-1, MIP1A, MIP1B, PDGF, TNFα, VEGF, IL-6 and IFN-γ contribute to the cytokine storm, (d) This uncontrolled systemic inflammatory response leads to vascular permeability, pneumocyte desquamation, plasma leakage into interstitial and alveolar spaces, and pulmonary infiltration of leukocytes such as macrophages, neutrophils and lymphocytes. Neutrophils and macrophages release enormous amounts of reactive oxygen species (ROS), (e) Excessive inflammatory reactions lead to several pathological changes, such as coagulation pathway activation as well as disseminated intravascular coagulation (DIC), (f) Activation of platelets is often linked with elevation of complement activation products, leading to systemic inflammatory response syndrome (SIRS), (g) Cellular apoptosis/pyroptosis of virus infected cells leads to endothelial destruction and enables plasma flooding into alveoli; (h) Neutrophil extracellular traps (NETs) are produced in response to infection where extracellular DNA fibers are extruded by neutrophils allowing them to trap and kill extracellular microorganisms
7.2 Cytokine Storm
Cytokine storm is an aggravated inflammatory response, which causes significant immunopathology involving widespread tissue damage. Cytokine storm has been reported in several viral infections including influenza (Kalaiyarasu et al. 2016), SARS-CoV and MERS-CoV (Channappanavar and Perlman 2017). In alveoli, the cytokine storm leads to acute lung injury and may set up ARDS, a major cause of morbidity in SARS-CoV-2 infection. Along with IL-1β, several other cytokines and chemokines such as IL-7, IL-8, IL-9, IL-10, basic fibroblast growth factor (FGF), granulocyte colony-stimulating factor (GCSF), GM-CSF, IFN-γ, interferon-γ-inducible protein-10 (IP-10) (also known as CXCL10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1A and 1B (MIP-1A, MIP-1B), platelet derived growth factor (PDGF), TNF-α, and VEGF, have been identified as constituents of this rogue response. IL-6 and IFN-γ levels are significantly higher in both ICU and non-ICU cases of COVID-19 patients compared to healthy adults (Huang et al. 2020). Plasma concentrations of IL-2, IL-7, IL-10, G-CSF, IP-10, MCP-1, MIP-1A, and TNF-α are higher in ICU patients than non-ICU patients, suggesting the severity of COVID-19 and its associated morbidity is possibly due to virally driven hyperinflammation. IL-6 is one of the frequently reported cytokines elevated in COVID-19 patientswhose level is significantly higher in severe cases than in mild cases (Ruan et al. 2020; Gao et al. 2020a, b, c, d; Chen et al. 2020a, b, c, d). In addition to IL-6, IL-10 and TNF-α are also linked with severe COVID-19 cases (Chen et al. 2020a, b, c, d). This uncontrolled and overwhelming systemic inflammatory response leads to vascular permeability, pneumocyte desquamation, plasma leakage into interstitial and alveolar spaces, and pulmonary infiltration of leukocytes such as macrophages and neutrophils (Martines et al. 2020). At this stage, chest CT scans exhibit bilateral glass opacities with multifocal infiltrates due to alveolar collapse and edema (Ghinai et al. 2020). Alveolar collapse causing hypoxemia and dyspnea is initiated potentially due to increase in alveoli surface tension as the level of surfactant protein in lungs drops. Lung autopsies in severe cases of COVID-19 show bilateral alveolar damage with cellular fibromyxoid exudates and mononuclear inflammatory lymphocytes (Xu et al. 2020a, b, c; Bonomi et al. 2020) (Fig. 6.4).Surfactant proteins, SP-A and SP-D, are involved in innate immune responses at the mucosal surfaces, especially in the lungs, against various pathogens including viruses (Yasmin and Kishore 2021). In case of SARS-type pneumonia, SP-D levels were significantly elevated (Leth-Larsen et al. 2007; Wu et al. 2009). HCoV-229E, a common non-SARS human CoV binds with SP-A and SP-D; pre-treatment of HCoV-229E with SP-A or SP-D inhibits viral infection. SP-D is more effective in inhibiting 16HBE cells infection whereas SP-A is more in inhibiting infection of alveolar macrophages (Funk et al. 2012). A recent work showed recombinant SP-D (rfhSP-D) was capable of competing with ACE-2 for binding to the S1 spike protein of SARS-CoV-2. rfhSP-D treatment inhibited viral replication by ~5.5 fold and a 2-fold reduction in viral infectivity was also observed in SARS-CoV-2 positive clinical samples (Madan et al. 2021). In an another study, rfhSP-D showed a dose-responsive binding to S1 spike protein and its receptor binding domain of SARS-CoV-2. rfhSP-D was capable in inhibiting interaction of S1 protein with the HEK293T cells overexpressing ACE-2 (Hsieh et al. 2021). These results highlight the possible therapeutic potential of rfhSP-D in SARS-CoV-2 infection.
Severe COVID-19 patients show symptoms related to secondary haemophagocytic lymphohistiocytosis (SHLH), which is typically characterized by sudden fatal hypercytokinaemia with multiorgan failure (Ramos-Casals et al. 2014). Approximately 50% of SHLH patients show clinical features similar to ARDS (Seguin et al. 2016). The cytokine profile (IL-2, IL-7, MIP-1A, G-CSF, TNF-α) elevated in severe COVID-19 also draws parallels with SHLH (Huang et al. 2020). The host repair system in many cases restores normal function but excessive tissue damage can often trigger wound healing through fibrosis that can eventually result in persistent organ dysfunction. The pulmonary cytokine storm circulates to other organs (systemic inflammatory response syndrome) causing increased capillary permeability in systemic circulation leading to decreased blood pressure (hypotension). This hypotension reduces the organ perfusion pressure causing multi-organ failure. SARS-CoV-2 infection often becomes life-threatening by inducing multi-organ injury involving the heart, liver, kidney, brain, intestine, and eyes (Li et al. 2020a, b, c; Klok et al. 2020).
7.3 Complement Associated Pathogenesis
Excessive inflammation precipitates several pathological changes, such as coagulation pathway activation, disseminated intravascular coagulation (DIC), cellular apoptosis/pyroptosis, increased vasopermeability and hypermetabolism, which finally proceeds into a septic pro-inflammatory microenvironment. Cardiovascular complications in COVID-19 patients such as acute thrombosis of the abdominal aorta and pulmonary embolism have been observed (Le Berre et al. 2020). Coagulation and complement, though being two distinct systems, are similar in how they are controlled and interact with each other. These controls occur at two basic levels, either by inhibiting the enzyme activity or by blocking the binding of a cascade component (Oikonomopoulou 2012). In critical COVID-19 cases, there is an increasing recognition of a hypercoagulable condition with possible complement activation noted in some patients (Magro et al. 2020).
Activation of the complement cascade is correlated with thrombosis and the development of multiple organ failure. Both C3 and C5 can be proteolytically activated by several components of the coagulation cascade in addition to thrombin. C5a, exhibiting chemotactic activity towards neutrophils, is produced by the enzymatic action of thrombin. Activation of complement components both upstream and downstream of C3 and C5 convertases can also be initiated by the coagulation cascade (Ghebrehiwet et al. 1981). For example, coagulation factor XIIa can activate C1 initiating the classical pathway; C1q as well as C1 inhibitors, C4b-binding protein and factor H can bind to platelet surfaces (Ghebrehiwet et al. 1983; Hamad et al. 2010). Further hyper-activation of complement can also be linked to excessive septic inflammation leading to systemic inflammatory response syndrome (SIRS). Activation of platelets is a common event during sepsis along with elevation of complement activation products, such as C3a, C4a, and C5a (Younger et al. 2010; Hack et al. 1989). C5a is a major player in the pathogenesis of several diseases and is capable of activating the coagulation and TLR pathways (Hajishengallis and Lambris 2010; Rittirsch et al. 2008; Hawlisch et al. 2005). Activated platelets can release a serine/ threonine protein kinase that is able to phosphorylate C3 (Ekdahl and Nilsson 1995; Gulla et al. 2010). This modification can result in the generation of a phosphorylated C3b fragment that is resistant to further proteolytic processing into iC3b by factor I. Complement Factor H has an inhibitory effect on the Hageman FXII contact plasma activation by acidic phospholipids (Ferluga et al. 2014). Factor H can downregulate the complement classical pathway, by competing with C1q binding to anionic phospholipid surface (Tan et al. 2010; Kishore and Sim 2012). Endothelial cell injury in tissue factor-dependent thrombosis, where activated platelets were found to secrete granular polyphosphates can further activate FXII inducing occlusive thrombosis (Muller et al. 2009; Renne et al. 2012).
Severe COVID-19 patients show a pro-coagulant profile characterized by increased clot strength, elevated D-dimer levels, hyper-fibrinogenemia, and increase in CRP, factor-VIII and von Willebrand factor (Panigada et al. 2020; Ranucci et al. 2020). AMY-101, a C3 inhibitor, has been evaluated for its anti-inflammatory response in severe cases of COVID-19 infection. Intravenous administration of AMY-101 showed a dramatic improvement with CRP and LDH getting normalized progressively, while leukocytosis and lymphocytopenia improved more gradually. A significant improvement in the respiratory performance was also observed. Treatment with AMY-101 was found to be safe with no side effects and with no further worsening of renal and hepatic function (Mastaglio et al. 2020). High fibrinogen levels are also associated with IL-6. With increased thromboprophylaxis, the pro-coagulant profile attained normalization and depreciated the D-dimer levels in COVID-19 patients (Ranucci et al. 2020).
A thin layer of endothelial-epithelial septum separates the alveolar cavity from blood. Endothelial destruction due to pyroptosis/apoptosis allows large amounts of plasma and cells to flood into alveoli causing ARDS (Fig. 6.4). Endothelial injury can also cause microvascular angiopathy and thrombosis, this damage can activate the complement lectin pathway. The lectin pathway effector enzyme, mannan-binding lectin-associated serine protease-2 (MASP-2), aids in the activation of thrombin (Krarup et al. 2007; Gulla et al. 2010). SARS-CoV-2 nucleocapsid protein can activate MASP-2; it has also been traced in the lung tissue of COVID-19 patients along with C4d and the membrane attack complex, C5b-9 (Gao et al. 2020a, b, c, d; Magro et al. 2020). Narsoplimab is a high-affinity humanised monoclonal antibody, which is capable of binding with MASP-2 and blocking the lectin pathway, was found to be effective in treating COVID-19 patients with no adverse drug reactions (Rambaldi et al. 2020).
Neutrophil infiltration in capillaries with fibrin deposition is observed in COVID-19 patients (Zuo et al. 2020). Neutrophil extracellular traps (NETs) are produced in response to infection where extracellular DNA fibers are extruded by neutrophils allowing them to trap and kill extracellular microorganisms. NETs cause platelet adhesion (often associated with deep vein thrombosis) (Costanzo et al. 2020). Sera of COVID-19 patients showed elevated levels of myeloperoxidase-DNA (MPO-DNA) and citrullinated histone H3 (Cit-H3), which are specific markers of NETs (Zuo et al. 2020). COVID-19 patient’s plasma showed spontaneous formation of NETs expressing functional tissue factor (TF) and considerable increase in plasma level of sC5b-9 (terminal complement component). Thrombin or NETosis inhibition or C5aR1 blockade could attenuate platelet-mediated NET-driven thrombogenicity in COVID-19 patients. Cp40-mediated C3 inhibition was capable of disrupting TF expression in neutrophils, thus preventing complement activation and impairing thrombogenicity (Skendros et al. 2020). Thus, complement activation during SARS-CoV-2 infection can possibly influence the platelet-NETs-TF-thrombin axis.
7.4 Adaptive Immune Response
COVID-19 patients show high level of SARS-CoV-2 specific IgM at early time points which decline over time while the IgG antibodies remain relatively stable (Sun et al. 2020a, b). However, antibody responses is not detectable in all patients, especially those with less severe forms of COVID-19 (Long et al. 2020a, b; Mallapaty 2020; Woloshin et al. 2020). COVID-19 patients have anti-viral IgG within 19 days of symptom onset, however both IgM and IgG titres reach plateau within 6 days of seroconversion (Long et al. 2020a, b). Zhou et al. (2020) reported that COVID-19 patients exhibited nucleocapsid protein (NP)-specific antibody responses, with IgM peaking at the ninth day post disease onset and then switching to IgG by week 2. Sera from COVID-19 patients were capable of inhibiting SARS-CoV-2 entry in target cells (Zhou et al. 2020). In another study, the median seroconversion time for IgM and IgG were day 11, 12 and 14 post symptom onset. Within 1 week of onset, the presence of antibodies was low but increased considerably from 15 day onwards (more IgG than IgM) (Zhao et al. 2020). COVID-19 patients mounted IgG and IgM responses to N-protein and spike-RBD proteins, and infected patients could maintain IgG levels for at least 2 weeks (Ni et al. 2020). Most COVID-19 convalescent individuals have a detectable level of neutralizing antibodies, as judged using the pseudovirus particle-based neutralization assay. The anti-S-RBD IgG might be predictive of serum neutralization capabilities in COVID-19 patients (Ni et al. 2020). In a recent study, antibody responses to 15 different SARS-CoV-2 antigens in COVID-19 patients was assessed with a luciferase immunoprecipitation system (LIPS) (Hachim et al. 2020). Antibodies representing the structural and nonstructural viral proteins [four structural proteins (S, N, M and E), three S subunits (S1, S2 and S2′), the seven available ORFs (ORF3a, ORF3b, ORF6, ORF7a, ORF7b, ORF8 and ORF10) and one relevant NSP within ORF1ab (NSP1)] were considered for the LIPS assay. Elevated antibody responses were seen against 11 antigens (the structural proteins full S, S1, S2′, N and M and the ORFs: NSP1, ORF3a, ORF3b, ORF7a, ORF7b and ORF10), with nucleocapsid, open reading frame (ORF)-8 and ORF3b eliciting the strongest specific antibody responses. ORF8 and ORF3b antibodies are therefore potential serological markers for SARS-CoV-2 infection, identified in 96.5% of COVID-19 samples at early and late time points of disease with 99.5% specificity (Hachim et al. 2020). Anti-RBD IgM and IgA were also detected in the majority of recovered COVID-19 patients (Grifoni et al. 2020).
A recent detailed study has sought to characterize humoral and circulating follicular helper T cell (cTFH) immunity against the S-protein in COVID-19 recovered patients (Juno et al. 2020). Comparatively low frequencies of B cells and cTFH specific cells for the RBD of the S-protein were found. The frequency and specificity of class-switched (CD19+IgD−) B cells were examined using an S or RBD flow cytometric probe, where populations of B cells binding spike (S+RBD−), spike and RBD (S+RBD+) or RBD alone (S−RBD+) in convalescent COVID-19 patients were compared to a healthy control. The majority of S+RBD− B cells were IgG+ with smaller proportions of IgM+ and IgA+. The activation phenotype of antigen-specific B cells was examined using CD21 and CD27. The S+RBD− or S+RBD+ B cells were found predominantly in the resting memory phenotype (CD21+CD27+), consistent with the median time since infection. A considerable population of activated memory B cells (CD21−CD27+) was observed for both S+RBD− and S+RBD+ populations. Thus, S-specific antibodies, memory B cells and cTFH are consistently elicited after SARS-CoV-2 infection, exhibiting robust humoral immunity that positively correlates with plasma neutralizing activity (Juno et al. 2020) (Fig. 6.5).
Adaptive Immune response against SARS-CoV-2. SARS-CoV-2 viruses are engulfed by phagocytic cells such as macrophages (MΦ). These phagocytic cells further express the viral peptide on their surface to present CD4+T cells. This binding activates CD4+T cells, which secrete cytokines to further activate (a) CD8+T cells which mount a cytotoxic response towards virally infected macrophages or other cells by secreting perforin and granzymes (b) B cells which undergo Ig class switching and secrete virus specific antibodies which are capable of neutralizing the virus. Antibodies secreted by these activated B cells can also mount a response through ADCC by activating NK cells and complement pathways leading to MAC formation that eventually destroys the virally infected cells. Antibodies can also enhance opsonization by binding with the Fc portion of macrophages, and/or clearance from circulation by Kupffer cells
A flow cytometry study of peripheral blood monocytic cells (PBMCs) showed an increased frequency of NK cells in discharged patients while the percentage of T cells remained unchanged. An S-RBD induced T cell immune response was identified with a higher percentage of IFN-γ-secreting S-RBD specific T cells compared with healthy donors (Ni et al. 2020). Patients with acute, moderate or severe COVID-19 showed low frequencies of CD4+ and CD8+ T cells (Liu et al. 2020a, b). A study was carried out involving unexposed individuals, exposed family members, and individuals with acute or convalescent COVID-19 to understand the functional and phenotypic landscape of SARS-CoV-2-specific T cell responses. Memory CD8+ T cells from patients with acute, moderate or severe COVID-19 were found to express CD38, HLA-DR, Ki-67, and PD-1, markers that are associated with activation and cell division. This suggests that T cells may establish a more robust early SARS-COV-2-specific adaptive immune response in COVID-19 patients (Sekine et al. 2020). The results also revealed a clear segregation between memory T cells from patients with acute, moderate or severe COVID-19 and those from convalescent individuals and healthy blood donors and more importantly T cell activation, characterized by the expression of CD38. SARS-CoV-2-specific T cells displayed a highly activated cytotoxic phenotype in the acute phase of the disease that correlated well with various clinical markers of disease severity such as age, hemoglobin concentration, platelet count, and plasma levels of alanine aminotransferase, albumin, D-dimer, fibrinogen, and myoglobin (Sekine et al. 2020).
CD4 and CD8 T cell responses were recognized against multiple regions of the N proteins of SARS-CoV-2 in patients convalescing from COVID-19 (Le Bert et al. 2020) (Fig. 6.5). In a group of individuals who recovered from SARS (2003), the data illustrated long-lasting memory T cells and displayed robust cross-reactivity towards the N-protein of SARS-CoV-2. Notably, ORF1-specific T cells were traced in a few individuals who were not exposed to SARS-CoV-2, at the same time T cells from individuals who recovered from COVID-19 could preferentially recognize structural proteins. ORF1-encoded proteins are produced as soon as viral RNA enters the host cells and are essential for formation of the RTC, thus, it can be assumed that ORF1-specific T cells can potentially mount a cytotoxic response towards SARS-CoV-2 infected cells prior to the formation of mature virions (Le Bert et al. 2020). The presence of SARS-CoV-2 cross reactive CD4+ T cells specific to S-protein were also observed in unexposed healthy individuals, suggesting some degree of cross-reactive pre-existing immunity to SARS-CoV-2 in the human population (Grifoni et al. 2020).
8 Increased Susceptibility to SARS-COV-2 in Men
Recent studies with single-cell RNA-sequence datasets of adult human testes appear to suggest that SARS-CoV-2 may also infect the testis. ACE2 receptors are expressed in both germ cells and somatic cells, among which major clusters are found in Sertoli cells, spermatogenic stem cells, and Leydig cells. The difference in fatality rate between males and females is underscoredby the fact that ACE2 is located on the X chromosome; oestrogen and testosterone sex hormones have different immunoregulatory functions that may contribute to protection or severity of the disease (Taneja 2018; Tay et al. 2020). In a study with 9280 SARS-CoV-2-positive patients, males developed more severe complications and had a worse clinical outcome than females (Montopoli et al. 2020). It is known that androgen receptor (AR) mediates the effects of male sex steroids and simultaneously AR regulates TMPRSS2 expression in non-prostatic tissues (Mikkonen et al. 2010), which is a vital component for SARS-CoV-2 entry in host cells and possibly explains the increased susceptibility of men to developing severe infections.
Innate immune recognition markers are encoded by genes belonging to a family of TLRs located on the X-chromosome. SARS-CoV-2 contains a host of proteins/peptides that can be recognized by TLR7/8 (Moreno-Eutimio et al. 2020). Several other immune regulatory genes located on the X chromosome include TLR8, FOXP3, CXCR3, and CD40L that usually contribute to a stronger immune response against viruses in women (Kritas et al. 2020; Flanagan et al. 2017; Klein 2012; Klein and Flanagan 2016). Detection by TLRs leads to the expression of Type I IFN (Heil et al. 2004) which are expressed in high levels by females (Klein 2012, Klein and Flanagan 2016). Female COVID-19 patients clear SARS-CoV-2 significantly earlier compared to infected male patients (Xu et al. 2020a, b, c). A meta-analysis of COVID-19 patients demonstrated a prevalence of immune mediators that are associated with adverse outcomes of SARS-CoV-2 in men, including TNFSF13B, CCL14, CCL23, IL-7, IL-16, and IL-18 (Wei et al. 2020). Males with moderate COVID-19 disease demonstrated higher level of IL-8 and IL-18 compared to female counterparts. At the same time, more robust activation of non-classical monocytes was observed in males whereas female patients mounted significantly more robust T cell activation during SARS-CoV-2 infection, suggesting the possible explanation of worse disease outcome in males. This sex bias in COVID-19 can possibly be considered as a vital factor for remedial approaches in future (Takahashi et al. 2020).
9 Repurposing Drugs in COVID-19
Developing a vaccine is time consuming and may take a substantial amount of time to become available globally. Thus, repurposing an existing drug is a more viable route, which can expedite COVID-19 treatment. Several pre-existing drugs have now been tested for COVID-19 (Table 6.1). Further details of drug repurposing have been recently reviewed (Varghese et al. 2020).
10 Neutralizing Antibodies and Passive Immunization (Convalescent Plasma Therapy)
In patients with SARS-CoV-2 infections, severe respiratory symptoms may develop after a week of symptom onset; this is associated with the release of several pro-inflammatory cytokines. This can often be related to low avidity and poor neutralizing antibodies and can be compensated by passive administration of antibodies to the patient. Antibodies show their anti-viral activity by inhibiting entry of infectious viral particles into host through neutralization. Antibodies function by triggering simultaneous binding of its Fab portion with the viral epitope or with the infected cells and Fc portion with immunocompetent cells such as macrophages and NK cells. The complement pathway is also activated by the Fc region binding to C1q, resulting in opsonization of viruses or infected cells.
The passive transfer of neutralizing antibody has been shown to confer protection in hamsters against a high-dose of SARS-CoV-2 (Rogers et al. 2020). Hamsters immunized with recombinant SARS-CoV S-protein trimer could also induce the development of neutralizing antibodies and were protected against a viral challenge (Kam et al. 2007). In an experiment with rhesus macaques, SARS-CoV-2 immunity associated with neutralizing antibodies and antibody-mediated effector functions and provided protection upon viral re-challenge at 35 days (Chandrashekar et al. 2020).
For several infectious diseases involving SARS, MERS and H1N1, convalescent plasma (CP) therapy, a classic passive immunotherapy, has been applied for its prevention and treatment. Patients recovered from COVID-19 with a high neutralizing antibody titer may become valuable donors for CP. In a study with ten severe SARS-CoV-2 patients, plasma (200 mL) at a median of 16.5 days after onset was administered, the presence of viruses in the blood was no longer detected and clinical parameters improved within 3 days (Duan et al. 2020). Several clinical trials are currently being undertaken to understand the potential clinical benefit and risk of convalescent blood products in COVID-19 (clinicaltrials.gov). In other studies, patients with severe or life-threatening COVID-19 who had undergone CP therapy did not result in a statistically significant improvement (Li et al. 2020a, b, c).
11 Type 1 Interferon Treatment Against COVID-19
Type I IFNs constitute a group of low-molecular glycoproteins and are among the first cytokines produced during a viral infection. This group of cytokines is recognized by the IFN-α receptor present at the plasma membrane in most cell types (Samuel 2001). Due to its immunomodulatory properties, it has been used for the treatment of numerous diseases including MERS-CoV and SARS-CoV, often in combination with lopinavir/ritonavir (Chan et al. 2015; Sheahan et al. 2020), ribavirin (Chen et al. 2004; Morgenstern et al. 2005; Omrani et al. 2014), and/or remdesivir, or corticosteroids (Loutfy et al. 2003).
Clinical studies in children from China revealed that IFN-α is capable of reducing viral load and shortening the disease duration for viral pneumonia, bronchiolitis and acute respiratory tract infections (Chen et al. 2005; Shang et al. 2014; Shen et al. 2018; 2020). Recombinant human IFN-α2b spray prevents SARS-CoV-2 infection by inhibiting viral replication in rhesus monkeys (Gao et al. 2005). A clinical study has suggested that IFN-α can be used as a prophylaxis against SARS-CoV-2 (Lokugamage et al. 2020). Clinical trials have been recently registered to evaluate a combination of lopinavir/ritonavir and IFN-α2b (ChiCTR2000029387) or a combination of lopinavir/ritonavir with ribavirin and IFN-β1b administered subcutaneously (NCT04276688) (Sallard et al. 2020). In an open clinical trial safety and efficacy trials of COVID-19 (NCT04315948), hospitalized adults were assessed in which subcutaneous IFN-β1a in combination with lopinavir/ritonavir is being compared to lopinavir/ritonavir alone, hydroxychloroquine, and remdesivir (Clinicaltrials.gov 2020).
12 Vaccination Strategies for COVID-19
Most of the vaccines under development against COVID-19 target the S-protein to elicit robust T and B cell responses, along with high viral neutralizing antibody production. Researchers have been in a race to develop ways to selectively target the most potent neutralizing epitopes likely to be critical for effective vaccines against SARS-CoV-2.
12.1 Viral Vector Vaccines
Adenovirus is an attractive vector candidate for the transfer of foreign genes because it is well characterized and comparatively easier to manipulate. Most adenoviruses are well tolerated and cause mild effects in immunocompetent human adults. Deletion of some crucial regions results in a replication-defective vector, which increases efficiency and reduces side-effects. For clinical use, they can be applied systemically as well as through the mucosal surface (Tatsis and Ertl 2004). Recombinant viruses can be used as vehicles for delivery of vaccines as the viral protein can act as potent adjuvants and can directly infect antigen-presenting cells (Rocha et al. 2004). The first report of using a chimpanzee adenovirus as a viral vectored vaccine demonstrated that chimpanzee adenovirus serotype 68 can express rabies glycoprotein and induce an immune response (Xiang et al. 2002). Viral vector vaccines induce cellular immune responses better than subunit vaccines (Draper and Heeney 2010).
12.1.1 Ad5-nCoV Vaccine
Ad5-nCoV vaccine is a genetically engineered vaccine which is delivered by a type-5 replication-defective adenovirus expressing the spike glycoprotein of SARS-CoV-2 (Sha et al. 2016). It contains the full length spike glycoprotein gene based on SARS-CoV-2 isolate Wuhan-Hu-1 with tissue plasminogen activator signal peptide into an E1 and E3 deleted Ad5 Vector. For phase 1 clinical trials, the vaccine contained 5 × 1010 viral particles per 0.5 mL/vial as a liquid formulation, injected intramuscularly into the arms of participants in three different dose groups (low/moderate/high). Systematic adverse reactions like fever, fatigue, headache and muscle pain or joint pain were observed which may be associated with viremia caused by Ad5 vector infection. RBD antibodies were observed from day 14 with a single dose eliciting a four-fold increase and showed higher antibody geometric titre based on infection assay using 1 × 1011 viral particles. Neutralizing antibodies against spike protein were found to be moderately at day 14. TNF-α was significantly lower in the low dose group and was higher in the high dose group. The vaccine was able to induce humoral and cellular response rapidly in most candidates; T-cell response peaked at day 14 and antibodies at day 28 after the vaccination. For phase 2 of the clinical trial, an intermediate dose was chosen and the trial is expected to be completed by 31 January 2021 (Zhu et al. 2020a, b).
12.1.2 ChAdOx1 nCoV-19
ChAdOx1 is a viral vector engineered as a replication-incompetent virus by Oxford University, UK. Previously, ChAdOx1 Chik has been tested for Chikungunya virus (CHIKV) which causes Chikungunya fever (CHIKF), an acute febrile illness leading to long-term arthralgia, especially in distal joints of the extremities (Kroon Campos et al. 2019). ChAdOx viral vector has previously been assessed for its safety and immunogenicity against a wide range of diseases such as influenza virus, plague, zika virus, tuberculosis, malaria, meningococcal group B bacteria (MenB) and MERS-CoV.
ChAdOx1 nCoV-19 vaccine is a chimpanzee derived adenovirus-vectored novel COVID-19 vaccine with replication deficient simian adenovirus expressing the full-length spike gene with a tissue plasminogen activator leader sequence inserted in to its genome. ChAdOx1 nCoV-19 vaccine was found to be immunogenic that elicited a robust anti-viral response in a murine model (van Doremalen et al. 2020). The vaccine has already completed phase 1 and 2 in a single-blinded, randomized controlled trial at six sites in the UK. Healthy adult participants aged 18–55 years with no exposure history of COVID-19 infection were chosen for the trial. No serious adverse effects related to ChAdOx1 nCoV-19 were observed and exhibited S-specific effector T-cell as early as day 7 which peaked on day 14. Anti-S IgG increased by day 28 capable of neutralizing the live SARS-CoV-2 virus. The booster dose resulted in the induction of both humoral as well as cellular immune responses. In rhesus macaques, this vaccine was capable of protecting against lower respiratory tract infection in primates. Clinical trial results thus far suggest ChAdOx1 nCoV-19 vaccine to be safe, tolerant and immunogenic (Folegatti 2020).During April 23 and Nov 4, 2020, 53 848 participants were enrolled for Phase 3 trial and 11636 participants (7548 in UK and 4088 in Brazil) were included in the interim primary efficacy study. The results show significant vaccine efficacy of 70.4% after two doses and protection of 64.1% after at least one dose against symptomatic disease. ChAdOx1 nCoV-19 showed acceptable safety profile and is efficacious against symptomatic COVID-19 patients. Vaccine is suitable for distribution as it can be stored and distributed in 2-8˚C (Voysey et al. 2021).
12.1.3 Sputnik V
Russia announced the launch of Sputnik V, heterologous COVID-19 vaccine consisting of two components, a recombinant adenovirus type 26 (rAd26) vector and a recombinant adenovirus type 5 (rAd5) vector, both carrying the gene for SARS-CoV-2 spike glycoprotein (rAd26-S and rAd5-S). The first interim analysis of Phase III trials of the Sputnik V vaccine revealed 92% efficacy in Covid-19 patients on 20 confirmed Covid-19 cases (Logunov et al. 2020). The interim data is based on the double-blind, randomised, placebo-controlled trials and is being conducted on 40,000 participants in Russia. Most adverse events were mild (pain at injection site, hyperthermia, headache, and muscle and joint pain) and no serious adverse events were detected. All participants produced antibodies to SARS-CoV-2 glycoprotein (Logunov et al. 2020). During September 2000, around 10,000 medics and other high-risk groups were administered Sputnik V under the civil use of the vaccine out of clinical trials. The III clinical trials of Sputnik V Phase are undergoing in Belarus, UAE, Venezuela and other countries, as well as Phase II-III in India (https://www.clinicaltrialsarena.com/news/russia-sputnik-v-efficacy). The preliminary results on the efficacy and safety of Gam-COVID-Vac (Sputnik V) of phase III trial shows that the vaccine is 91·6% (95% CI 85·6–95·2) efficacious against COVID-19 from the day of receiving second dose (day 21 after first dose). There were reports of serious adverse events in 45 (0·3%) of 16 427 participants, all of which were considered not due to the vaccine (Logunov et al. 2021).
12.2 Inactivated Vaccine Candidates
12.2.1 BBIBP-CorV
The strain 19nCoV-CDC-Tan-HB02 was considered for developing the inactivated SARS-CoV-2 vaccine, BBIBP-CorV. HB02 strain is homologous to other viral strains and the spike protein has 100% identity. BBIBP-CorV was capable of inducing high levels of neutralizing antibody in rats, mice, guinea pigs, rabbits, cynomolgus monkeys, and rhesus macaques, and found to be protective against SARS-CoV-2 infection. A lower two-dose immunization regime (2 mg/dose) provided highly efficient protection against SARS-CoV-2 in rhesus macaques with no immunopathological effects (Wang et al. 2020a, b, c, d, e, f, g).
12.2.2 PiCoVacc
PiCoVacc is a purified inactivated SARS-CoV-2 vaccine candidate developed from CN2 strains in conjunction with CN1, CN3-CN5, and OS1-OS6, which were used as pre-clinical challenge strains. PiCoVacc was able to induce SARS-CoV-2 specific neutralizing antibodies in mice, rats, and non-human primates. To assess the immunogenicity of PiCoVacc, BALB/c mice were injected with various doses of PiCoVacc mixed with an alum adjuvant. No inflammation or other adverse effects were observed. SARS-CoV-2 S-specific and RBD-specific IgG antibodies were generated and the titer peaked at week 6 accounting for half of the S-induced antibody responses. Two immunization doses, 3 μg and 6 μg, provided partial or complete protection in macaques against a SARS-CoV-2 challenge without demonstrating any observable antibody dependent enhancement of the infection (Gao et al. 2020a, b, c, d).
12.3 Nucleic Acid Vaccine Candidates
12.3.1 mRNA 1273 (RNA Vaccine Candidate)
This is an mRNA vaccine that encodes for S-2P antigen which is a SARS-CoV-2 spike glycoprotein trimer with a transmembrane anchor and an intact S1-S2 cleavage site. S-2P is stabilized by two consecutive proline substitutions at amino acid positions 986 and 987, at the top of heptad repeat 1, which prevent structural rearrangements of the fusion S2 subunit and retain its prefusion conformation. The nucleoside-modified messenger RNA (mRNA) is encapsulated in a lipid nanoparticle capsule, formulated in a fixed ratio of mRNA and lipid. The mRNA is suspended in a sterile liquid for injection at a concentration of 0.5 mg per mL. This vaccine is developed by Moderna in collaboration with the National Institute of Allergy and Infectious Disease Vaccine Research Centre. The vaccine underwent an open-label phase 1 clinical trial which started for 6 weeks in three dose cohorts (25 μg, 100 μg and 250 μg) via intramuscular injection in the upper arm. A Phase II trial with 600 healthy participants in two cohorts treated with a placebo, a 50 μg or a 250 μg dose also started recently. The vaccine was found to be immunogenic in murine models, capable of inducing IgG2a and IgG1 antibodies. It could also stimulate higher secretions of IFN-γ than IL-4, IL-5 or IL-3 upon re-stimulation with peptide pools and induce robust CD8+T cell response to the S1 peptide pool with balanced Th1/Th2 antibody isotype (Corbett et al. 2020).On 26 January, 2021 WHO announcedModerna mRNA 1273 vaccine to have an efficacy of approximately 92 % in protecting against COVID-19, 14 days after the first dose. The vaccine has been found to be safe and effective in people with medical conditions associated with increased risk of hypertension, diabetes, asthma, pulmonary, liver or kidney disease, as well as chronic infections that are stable and controlled. The new variants of SARS-CoV-2, including the B.1.1.7 and the 501Y.V2, do not alter the effectiveness of the Moderna mRNA vaccine (WHO Report 2021c).
12.3.2 BNT162 (RNA Vaccine Candidate)
BNT162 is a Pfizer licensed BioN TechmRNA vaccine candidate: there are four vaccine candidates under this program, two for coding the SARS-CoV-2 S-protein and two for the RBD of the S-protein made up of three different mRNA formats. During the preclinical studies among the four BNT162 mRNA vaccine candidates, BNT162b1 and BNT162b2 emerged as strong candidates on the basis of their immune response and safety. In clinical phase 1 and 2 trials, results conducted on up to 120 patients exhibited that BNT162b2 had a favorable tolerability profile over BNT162b1 and also showed high CD4+ and CD8+ T cell responses. BNT162b2 has been chosen for phase 2 and 3 trials where participants were chosen between the age of 18 to 85 years (BioNTech n.d.).BNT162b2 is a lipid nanoparticle formulated nucleoside modified RNA vaccine. In phase 2/3, two-dose regimen of BNT162b2 given at an interval of 21 days to 43,548 participants, of whom 43,448 received injections (21,720 with BNT162b2 and 21,728 with placebo). A two-dose regimen of BNT162b2 conferred 95% protection against Covid-19 in persons 16 years of age or older. Systematic reactogenicity was more common and severe after the second dose. Severe fatigue was also observed in 4% of BNT162b2 recipient. These reactogenicity events (short-term, mild-to-moderate pain at the injection site, fatigue, and headache) were transient and resolved within a couple of days. (Polack F. 2020). Children, pregnant women and immunocompromised persons were not included in this 2/3 phase trial.
12.3.3 DNA Vaccine
A prototype DNA vaccine expressing six variants of the SARS-CoV-2 spike protein: (a) full-length, (b) cytoplasmic tail deleted, (c) transmembrane domain deleted and cytoplasmic tail reflecting the soluble ectodomain, (d) S1 domain with a foldon trimerization tag, (e) RBD with a fold-on trimerization tag, and (f) a prefusion stabilized soluble ectodomain with furin cleavage site deleted, was constructed. The vaccine was tested on rhesus macaques which developed humoral and cellular immune responses, including neutralizing antibody titers that were comparable to macaques infected with SARS-CoV-2. The vaccine elicited neutralizing antibody inducing protection (Yu et al. 2020).
12.3.4 INO-4800
INO-4800, is an optimized S- protein of SARS-CoV-2 viral DNA plasmids developed by Inovio (Pharmaceuticals 2020). Phase I trial started on April 3, 2020 to evaluate the safety, tolerability and immunogenicity of the vaccine. INO-4800 with a regime of three different doses are being administered intradermally followed by electroporation in healthy volunteers (120 participants).
12.4 Protein-based vaccines
SCB-2019 is a protein subunit vaccine candidate containing a stabilised trimeric form of the spike (S)-protein (S-Trimer) combined with two different adjuvants. The difference of SCB-2019 with other vaccine is that it uses a stabilised protein trimer as the antigen. The Trimer-Tag is a protein, derived from the C-terminus of human type I procollagen which preserves the trimeric conformation of the SARS-CoV-2 spike protein (Blakney and McKay 2021). The efficacy and safety of SCB-2019 was assessed as the S-Trimer protein alone (non-adjuvanted), or as one of two adjuvanted formulations with either AS03 or CpG/Alum. The Phase I trial suggested non-adjuvanted SCB-2019 to be poorly immunogenic, but in combination with the adjuvant system (AS03 or CpG/Alum) robust increase in functional immune responses were observed with SARS-CoV-2 neutralising activity that correlated well with IgG antibodies against SCB-2019 or ACE2-competitive blocking antibodies (Richmond et al. 2021).
13 Perspectives
Last 18 months have seen an explosion of information about SARS-CoV-2 genome, virulence factors, mode of entry into the target cells, receptors and co-receptors/proteases, and host immune response. The fact that a large proportion of individuals can be infected and remain asymptomatic bodes well for the role of the innate immunity in engineering a host-pathogen stand-off. Whether this reflects on the threshold of virus latency remains to be understood. Whether the complement system has a protective role is yet to be established: there are viral proteins other that Spike protein that can activate complement, specifically the lectin pathway. What is remarkably clear is that the complement activation contributes considerably in microangiopathy and coagulopathy in severe COVID-19 patients. Complement activation also seems to contribute to coagulopathy seen in very few individuals who were administered AstraGeneca (Oxford) vaccines. The roles that T cells, NK cells and DCs play in mounting a protective response against SARS-CoV-2 are being recognized as well. The presence of specific antibodies, some of them being neutralizing, is also well-documented. Vaccine trials have relied mostly on the use of Spike protein: results have been promising and several countries have double vaccinated a vast majority of their adult population. However, levels of neutralizing antibodies and persistence of B cell response remain a concern. These limitations apply to infection as well as vaccination trials. To continue to assess likely alterations in the viral genome (with or without a fitness cost), uncertaintly of neutralizing antibody titres, and human-to-human transmission rates, are going to be major issues in short- and medium-terms However, like other vaccines, one would expect that SARS-CoV-2 vaccines will also work following the principle of immunology, i.e. after two doses of vaccinations, subsequent virus exposure should recall memory cells and hence swift protective immune response. There are discussions about giving third dose of the vaccine in winter that may coincidewith rise in flu.
There is a terrible beauty about how viruses such as SARS-CoV-2 have evolved to be so efficient at causing infection. The intricate molecular interactions between the viral and host cellular molecules and the tango between the immune response and the viral mechanisms that seek to subvert it are complex and fascinating. It is also truly amazing that in such a short period of time, we have learnt so much about the both SARS-CoV-2 and COVID-19. An understanding of COVID-19 requires us to look at the molecular level (for example during replication of the virus), the cellular (internalization of the virus), at the level of the tissue or organ (local inflammatory response) and the organism (adaptive immune responses). We also need to understand interactions between people, and between people and their environment, and social issues such as a flow of people across the world and the impact of socioeconomic factors on viral propagation. Interventions on all these levels are needed—there will not be a single ‘magic bullet’.
Some will argue that this is pessimistic. Vaccination will allow us to deal with SARS-CoV-2. However, while development of an effective vaccination is an incredibly useful tool in helping us cope with the virus, we should not rely on it. In part, because it may be more difficult than we hope to produce an effective vaccine that results in long term immunity (the failure to produce effective vaccines to other forms of CoV should give us cause for concern). However, even with an effective vaccine, it took many years for us to eradicate smallpox. Salk developed the first effective polio vaccine in 1952, and despite a worldwide effort, there are still some pockets of polio in Asia. These are diseases with no animal reservoirs of infection.
This is not a message of gloom. We will be able to control the virus, but it will need a multi-pronged approach to do so. We also need to develop our science base and understanding so that when the next pandemic strikes (which it will do), we can rapidly mobilise to contain and control it. We need to develop public understanding of the science (for example, to counter the ant-vaccination movement) so we learn from SARS-CoV-2 to be more resilient to future threats.
References
Aftab SO, Ghouri MZ, Masood MU et al (2020) Analysis of SARS-CoV-2 RNA-dependent RNA polymerase as a potential therapeutic drug target using a computational approach. J Transl Med 18:275. https://doi.org/10.1186/s12967-020-02439-0
Alsaadi E, Jones I (2019) Membrane binding proteins of corona viruses. Futur Virol 14(4):275–286
Andrei G, De Clercq E (1993) Molecular approaches for the treatment of hemorrhagic fever virus infections. Antiviral Res 22:45–75. https://www.roche.com/media/releases/med-cor-2020-03-19.htm~
Backer JA, Klinkenberg D, Wallinga J (2020) Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Eurosurveillance. 25:2000062
Balakrishnan VS (2020) The arrival of Sputnik V-Controversy continues to brew around Russia’s newly minted COVID-19 vaccine, Sputnik V. Lancet .20; 1128. published online Sept 4. https://doi.org/10.1016/S0140-6736(20)31866-3
Barbulescu A, Delcoigne B, Askling J, et al (2020)Gastrointestinal perforations in patients with rheumatoid arthritis treated with biological disease-modifying antirheumatic drugs in Sweden: a nationwide cohort study. RMD Open6:e001201. https://doi.org/10.1136/rmdopen-2020-001201
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC et al (2020) Remdesivir for the treatment of covid-19—preliminary report. N Engl J Med 383(19):1813–1826. https://doi.org/10.1056/nejmoa2007764
Bertram S, Glowacka I, Muller MA, Lavender H, Gnirss K, Nehlmeier I, Niemeyer D, He Y, Simmons G, Drosten C et al (2011) Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J Virol 85:13363–13372. https://doi.org/10.1128/jvi.05300-11
Bianchi M, Benvenuto D, Giovanetti M, Angeletti S, Cicozzi M, Pascarella S (2020) Sars-CoV-2 envelope and membrane proteins: structural differences linked to virus characteristics? Biomed Res Int 2020:1–6. https://doi.org/10.1155/2020/4389089
BioNTech (n.d.) mRNA therapeutics|BioNTech. https://biontech.de/how-we-translate/mrna-therapeutics. Accessed 22 Aug 2020
Blakney AK, McKay PF (2021) Next-generation COVID-19 vaccines: here come the proteins. Lancet. Vol 397 February 20, 2021. https://doi.org/10.1016/S0140-6736(21)00258-0
Bleyzac N, Goutelle S, Bourguignon L, Tod M (2020) Azithromycin for COVID-19: more than just an antimicrobial? Clin Drug Investig 40(8):683–686. https://doi.org/10.1007/s40261-020-00933-3
Bonomi L, Ghilardi L, Arnoldi E, Tondini CA, Bettini AC (2020) A rapid fatal evolution of coronavirus disease-19 (COVID-19) in an advanced lung cancer patient with a long time response to nivolumab. J Thorac Oncol 15(6):e83–e85. https://doi.org/10.1016/j.jtho.2020.03.021
Boriskin Y, Leneva I, Pecheur E-I, Polyak S (2008) Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem 15:997–1005
Cai X (2020) An insight of comparison between COVID-19 (2019-nCoV disease) and SARS in pathology and pathogenesis. Open Science Framework. https://doi.org/10.31219/osf.io/hw34x
Calisher C, Childs J, Field H, Holmes K, Schountz T (2006) Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev 19(3):531–545. https://doi.org/10.1128/CMR.00017-06
Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM (2020) The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res 178:104787. https://doi.org/10.1016/j.antiviral.2020.104787
Candido DS, Claro IM, de Jesus JG, et al. (2020). Evolution and epidemic spread of SARSCoV-2 in Brazil. Science. 369(6508):1255–60. https://doi.org/10.1126/science.abd2161
Cao B, Wang Y, Wen D et al (2020) A trial of Lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382(19):1787–1799. https://doi.org/10.1056/NEJMoa2001282
Cavanagh D (1995) In: Siddell SG (ed) The Coronaviridae. Plenum, New York, pp 73–113
CDC. COVID-19: SARS-CoV-2 variant classifications and definitions. Atlanta, GA: US Department of Health and Human Services, CDC; 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html
Chan JFW, Yao Y, Yeung ML, Deng W, Bao L, Jia L, Li F, Xiao C, Gao H, Yu P, Cai JP, Chu H, Zhou J, Chen H, Qin C, Yuen KY (2015) Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERSCoV infection in a nonhuman primate model of common marmoset. J Infect Dis 212:1904–1913. https://doi.org/10.1093/infdis/jiv392
Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J et al (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395:514–523
Chandrashekar A et al (2020) SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 369(6505):812–817. https://doi.org/10.1126/science.abc4776
Channappanavar R, Perlman S (2017) Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 39:529–539. https://doi.org/10.1007/s00281-017-0629-x
Charlton CL, Babady E, Ginocchio CC et al (2018) Practical guidance for clinical microbiology laboratories: viruses causing acute respiratory tract infections. Clin Microbiol Rev 32(1):e00042–e00018. https://doi.org/10.1128/CMR.00042-18
Chen F, Chan KH, Jiang Y, Kao RYT, Lu HT, Fan KW, Cheng VCC, Tsui WHW, Hung IFN, Lee TSW, Guan Y, Peiris JSM, Yuen KY (2004) In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol 31:69–75. https://doi.org/10.1016/j.jcv.2004.03.003
Chen PL, Zhang TX, Hu YH, Zhou CF, Wang SD (2005) A multicenter randomized controlled clinical study of recombinant human interferon-typeα1b in the treatment of viral pneumonia in children. Lin Chuang ErKe Za Zhi 23:244–245. (in Chinese)
Chen H, Wang F, Zhang P, Zhang Y, Chen Y, Fan X et al (2019) Management of cytokine release syndrome related to CAR-T cell therapy. Front Med 13:610–617. https://doi.org/10.1007/s11684-019-0714-8
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H et al (2020a) Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest 130:2620–2629. https://doi.org/10.1101/2020.02.16.20023903
Chen L, Liu H, Liu W, Liu J, Liu K, Shang J et al (2020b) Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. ZhonghuaJie He He Hu Xi Za Zhi 43:203–208. https://doi.org/10.3760/cma.j.issn.1001-0939.2020.0005
Chen N, Zhou M, Dong X et al (2020c) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513. https://doi.org/10.1016/S0140-6736(20)30211-7
Chen T, Wu D, Chen H et al (2020d) Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368:m1091
Chhaiya SB, Mehta DS, Kataria BC (2012) Ivermecin: pharmacology and therapeutic applications. Int J Basic Clin Pharmacol 1:132–139
Chung MP, Park MS et al (2020) Safety and efficacy of pirfenidone in advanced idiopathic pulmonary fibrosis: a nationwide post-marketing surveillance study in Korean patients. Adv Ther 37(5):2303–2316. https://www.clinicaltrials.gov/ct2/show/NCT04412486 (as appeared on 03.09.2020)
COG-UK (2020) https://www.cogconsortium.uk/wp-content/uploads/2020/07/25th-June-2020-Report-COVID-19-Genomics-UK-COG-UK-Consortium.pdf (accessed on 12th April, 2021)
Corbett KS et al (2020) SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586(7830):567–571. https://doi.org/10.1038/s41586-020-2622-0
Costanzo L, Palumbo FP, Ardita G, Antignani PL, Arosio E, Failla G (2020) Coagulopathy, thromboembolic complications, and the use of heparin in COVID-19 pneumonia. J Vasc Surg Venous Lymphat Disord 8(5):711–716
Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E (2020) The spike glycoprotein of the new coronavirus 2019- nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res 176:104742
D’Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L (2020) Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clin Gastroenterol Hepatol 18(8):1663–1672. https://doi.org/10.1016/j.cgh.2020.04.001
Daruich A et al (2020) Unilateral conjunctivitis as first presentation of coronavirus disease 2019 (COVID-19): a telemedicine diagnosis. J Fr Ophtalmol 43(5):e167–e168. https://doi.org/10.1016/j.jfo.2020.04.001
Davies NG, Barnard RC, Jarvis CI et al. (2020) Estimated Transmissibility and Severity of Novel SARS-CoV-2 Variant of Concern 202012/01 in England. medRxiv 2020
Deng G, Yin M, Chen X, Zeng F (2020) Clinical determinants for fatality of 44,672 patients with COVID-19. Crit Care 24:179
Dong L, Hu S, Gao J (2020) Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther 14:58–60. https://doi.org/10.5582/ddt.2020.01012
Draper SJ, Heeney JL (2010) Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol 8:62–73
Drosten C, Günther S, Preiser W, Van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RAM et al (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348:1967–1976. https://doi.org/10.1056/NEJMoa030747
Duan K et al (2020) Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A 117(17):9490–9496. https://doi.org/10.1073/pnas.2004168117
Ekdahl KN, Nilsson B (1995) Phosphorylation of complement component C3 and C3 fragments by a human platelet protein kinase. Inhibition of factor I-mediated cleavage of C3b. J Immunol 154:6502–6510
Eriksson B, Helgstrand E, Johansson NG et al (1977) Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob Agents Chemother 11:946–951
Fears AC, Klimstra WB, Duprex P, Weaver SC, Plante JA, Aguilar PV et al (2020) Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis 26(9):2168–2171. https://doi.org/10.3201/eid2609.201806
Ferluga J, Kishore U, Sim RB (2014) A potential anti-coagulant role of complement factor H. Mol Immunol 59(2):188–193. https://doi.org/10.1016/j.molimm.2014.02.012
Ferrara F, Granata G, Pelliccia C et al (2020) The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2. Eur J Clin Pharmacol 76(11):1615–1618
Flanagan KL, Fink AL, Plebanski M, Klein SL (2017) Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol 33:577–599
Folegatti PM (2020) Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396(10249):467–478. https://doi.org/10.1016/S0140-6736(20)31604-4
Forgie S, Marrie TJ (2009) Healthcare-associated atypical pneumonia. Semin Respir Crit Care Med 30(1):67–85. https://doi.org/10.1055/s-0028-1119811
Funk CJ. Wang J. Ito Y. Travanty EA et al (2012) Infection of human alveolar macrophages by human coronavirus strain 229E. J Gen Virol. 93(Pt 3): 494–503.
Furuta Y, Gowen BB, Takahashi K et al (2013) Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 100:446–454
Galloway SE. Paul P. MacCannell DR, et al (2021) Emergence of SARS-CoV-2 B.1.1.7 Lineage-United States, December 29,2020. MMWR Morb Mortal Wkly Rep. 70:95-99. https://doi.org/10.15585/mmwr.mm7003e2
Gao H, Zhang LL, Qin C, Duan ZJ, Tu XM, Yu ZA et al (2005) Experimental study on the prevention and treatment of SARS-CoV infection in rhesus monkeys with recombinant human interferon 2b nasal spray. Chin J Exp Clin Virol 19:207–211. (in Chinese)
Gao Q et al (2020a) Development of an inactivated vaccine candidate for SARS-CoV-2. Science 369:77–81
Gao T, Hu M, Zhang X, Li H, Zhu L, Liu H, Dong Q, Zhang Z, Wang Z, Hu Y, et al (2020b) Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv. 2020.03.29.20041962. https://doi.org/10.1101/2020.03.29.20041962
Gao Y, Li T, Han M, Li X, Wu D, Xu Y et al (2020c) Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 92:791–796. https://doi.org/10.1002/jmv.25770
Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, et al (2020d) Rapid development of an inactivated vaccine candidate for SARS-CoV-2. Science https://doi.org/10.1126/science.abc1932
George PM, Wells AU, Jenkins RG (2020) Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med 8(8):807–815. https://doi.org/10.1016/s2213-2600(20)30225-3
Ghebrehiwet B, Silverberg M, Kaplan AP (1981) Activation of the classical pathway of complement by Hageman factor fragment. J Exp Med 153:665–676. [PubMed: 7252410]
Ghebrehiwet B, Randazzo BP, Dunn JT, Silverberg M, Kaplan AP (1983) Mechanisms of activation of the classical pathway of complement by Hageman factor fragment. J Clin Invest 71:1450–1456. [PubMed: 6304147]
Ghinai I et al (2020) First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet 395:1137–1144. https://doi.org/10.1016/S0140-6736(20)30607-3
Gómez CE, Perdiguero B, Esteban M (2021) Emerging SARS-CoV-2 Variants and Impact in Global Vaccination Programs against SARS-CoV-2/COVID-19. Vaccines, 9, 243. https://doi.org/10.3390/vaccines9030243
Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, Gotte M (2020) Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem 295(20):6785–6797. https://doi.org/10.1074/jbc.RA120.013679
Graci JD, Cameron CE (2006) Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol 16(1):37–48
Gralinski LE, Menachery VD (2020) Return of the coronavirus: 2019-nCoV. Viruses 12:135. (in English). https://doi.org/10.3390/v12020135
Grein J, Ohmagari N, Shin D, Diaz G (2020) Compassionate use of Remdesivir for patients with severe Covid-19. N Engl J Med 382(25):e101. https://doi.org/10.1056/NEJMoa2007016
Grifoni A et al (2020) Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181:1489–1501
Guan W, Ni Z, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720. https://doi.org/10.1056/NEJMoa2002032
Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, Franceschini E, Cuomo G, Orlando G, Borghi V et al (2020) Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2(8):e474–e484. https://doi.org/10.1016/S2665-9913(20)30173-9
Gulla KC, Gupta K, Krarup A, Gal P, Schwaeble WJ, Sim RB, O’Connor CD, Hajela K (2010) Activation of mannan-binding lectin-associated serine proteases leads to generation of a fibrin clot. Immunology 129:482–495
Hachim A, Kavian N, Cohen CA et al (2020) ORF8 and ORF3b antibodies are accurate serological markers of early and late SARS-CoV-2 infection. Nat Immunol 21(10):1293–1301. https://doi.org/10.1038/s41590-020-0773-7
Hack CE, Nuijens JH, Felt-Bersma RJ, Schreuder WO, Eerenberg-Belmer AJ, Paardekooper J, Bronsveld W, Thijs LG (1989) Elevated plasma levels of the anaphylatoxins C3a and C4a are associated with a fatal outcome in sepsis. Am J Med 86:20–26
Hajishengallis G, Lambris JD (2010) Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol 31:154–163
Hamad OA, Nilsson PH, Lasaosa M, Ricklin D, Lambris JD, Nilsson B, Ekdahl KN (2010) Contribution of chondroitin sulfate A to the binding of complement proteins to activated platelets. PLoS One 5:e12889. [PubMed: 20886107]
Hao X et al (2020) Reconstruction of the full transmission dynamics of COVID-19 in Wuhan. Nature 584(7821):420–424. https://doi.org/10.1038/s41586-020-2554-8
Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J (2005) C5a negatively regulates toll like receptor 4-induced immune responses. Immunity 22:415–426
Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S (2004) Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526–1529
Ho JC, Chan KN, Hu WH et al (2001) The effect of aging on nasal mucocilliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med 163:983–988
Hoffmann M, Kleine-Weber H, Schroeder S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:1–10. https://doi.org/10.1016/j.cell.2020.02.052
Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, et al 2020 Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. https://doi.org/10.1101/2020.06.22.20137273, 06.22.20137273
Hsieh MH, Beirag N, Murugaiah V, Chou Y-C, Kuo W-S, Kao H-F, Madan T, Kishore U, Wang J-Y. 2021. A recombinant fragment of Human Surfactant Protein D binds SARS-CoV-2 Spike protein and acts as an entry inhibitor of Pseudotyped viral particles. Frontiers in Immunology, 12:641360. https://doi.org/10.3389/fimmu.2021.641360
https://clinicaltrials.gov/ct2/show/NCT04315948. 22.03.2020
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Hung IF, Lung K, Tso EY, Liu R, Chung TW, Chu MY et al (2020) Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395(10238):1695–1704. https://doi.org/10.1016/s0140-6736(20)31042-4
Jin J-M, Bai P, He W, Wu F, Liu X-F, Han D-M, Liu S, Yang J-K (2020) Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health 8:152. https://doi.org/10.3389/fpubh.2020.00152
Juno JA, Tan H-X, Lee WS, Reynaldi A, Kelly HG, Wragg K et al (2020) Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat Med 26(9):1428–1434. https://doi.org/10.1038/s41591-020-0995-0
Kalaiyarasu S, Kumar M, Senthil Kumar D, Bhatia S, Dash SK, Bhat S et al (2016) Highly pathogenic avian influenza H5N1 virus induces cytokine dysregulation with suppressed maturation of chicken monocyte-derived dendritic cells. Microbiol Immunol 60:687–693. https://doi.org/10.1111/1348-0421.12443
Kam Y, Kien F, Roberts A, Cheung Y, Lamirande E, Vogel L, Chu S, Tse J, Guarner J, Zaki S, Subbarao K, Peiris M, Nal B, Altmeyer R (2007) Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcγRII-dependent entry into B cells in vitro. Vaccine 25(4):729–740
Kam Y, Okumura Y, Kido H, Ng L, Bruzzone R, Altmeyer R (2009) Cleavage of the SARS coronavirus spike glycoprotein by airway proteases enhances virus entry into human bronchial epithelial cells in vitro. PLoS One 4(11):e7870
Kemp SA, Harvey WT, Datir RP, et al. (2021). Recurrent emergence and transmission of SARS-CoV-2 spike deletion ΔH69/V70. bioRxiv [Preprint posted online January 14, 2021]. https://www.biorxiv.org/content/10.1101/2020.12.14.422555v4
Kerslake R, Hall M, Randeva H, Spandidos D, Chatha K, Kyrou I, Karteris E (2020) Co-expression of peripheral olfactory receptors with SARS-CoV-2 infection mediators: potential implications beyond loss of smell as a COVID-19 symptom. Int J Mol Med 46(3):949–956. https://doi.org/10.3892/ijmm.2020.4646
Killerby ME, Biggs HM, Midgley CM, Gerber SI, Watson JT (2020) Middle East respiratory syndrome coronavirus transmission. Emerg Infect Dis 26:191–198. https://doi.org/10.3201/eid2602.190697
Kim D, Lee J, Yang J, Kim J, Kim V, Chang H (2020) The Architecture of SARS-CoV-2 Transcriptome. Cell, 181(4), pp.914-921.e10.
Kishore U, Sim RB (2012) Factor has a regulator ofthe classical pathway activation. Immunobiology 217:162–168
Klein SL (2012) Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays 34:1050–1059
Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16:626–638
Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM et al (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191:145–147. https://doi.org/10.1016/j.thromres.2020.04.013
Krarup A, Wallis R, Presanis JS, Gal P, Sim RB (2007) Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One 2:e623
Krilov LR (2001) Respiratory syncytial Vrus: update on infection, treatment, and prevention. Curr Infect Dis Rep 3:242–246
Kristina K, Waldenstrom J, Tang K, Lagging M (2020) Ribavirin: pharmacology, multiple modes of action and possible future perspectives. Future Virol 14(3):153–160. https://doi.org/10.2217/fvl-2018-0166
Kritas SK et al (2020) Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J Biol Regul Homeost Agents 34(1):9–14. https://doi.org/10.23812/20-Editorial-Kritas
Kroon Campos R, Preciado-Llanes L, Azar S, Lopez-Camacho C, Reyes-Sandoval A, Rossi S (2019) A single and un-adjuvanted dose of a chimpanzee adenovirus-vectored vaccine against Chikungunya virus fully protects mice from lethal disease. Pathogens 8(4):231
Lan J, Ge J, Yu J et al (2020) Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581:215–220. https://doi.org/10.1038/s41586-020-2180-5
Lau SK, Woo PC, Li KS et al (2005) Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A 102:14040–14045. https://doi.org/10.1073/pnas.0506735102
Le Berre A et al (2020) Concomitant acute aortic thrombosis and pulmonary embolism complicating COVID-19 pneumonia. Diagn Interv Imaging 101(5):321–322. https://doi.org/10.1016/j.diii.2020.04.003
Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, Chng MHY, Lin M, Tan N, Linster M et al (2020) SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584(7821):457–462
Lei J, Li J, Li X, Qi X (2020) CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295(1):18. https://doi.org/10.1148/radiol.2020200236
Leth-Larsen R. Zhong F. Chow VTK. Holmskov U. Lu J (2007). The SARS coronavirus spike glycoprotein is selectively recognized by lung surfactant protein D and activates macrophages.Immunobiology. 212(3): 201–211
Li F, Li W, Farzan M, Harrison SC (2005) Structural biology: structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 309:1864–1868. https://doi.org/10.1126/science.1116480
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y et al (2020a) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207. https://doi.org/10.1056/NEJMoa2001316
Li L et al (2020b) Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19. JAMA 324:1–11
Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y et al (2020c) Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 146(1):110–118. https://doi.org/10.1016/j.jaci.2020.04.006
Liang W et al (2020) Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 21:335–337
Ling Z, Xu X, Gan Q, Zhang L, Luo L, Tang X, Liu J (2020) Asymptomatic SARS-CoV-2 infected patients with persistent negative CT findings. Eur J Radiol 126:108956. https://doi.org/10.1016/j.ejrad.2020.108956
Liu P, Shi L, Zhang W et al (2017) Prevalence and genetic diversity analysis of human coronaviruses among cross-border children. Virol J 14(1):230. https://doi.org/10.1186/s12985-017-0896-0
Liu BM, Yang QQ, Zhao LY, Xie W, Si XY (2020a) Epidemiological characteristics of COVID-19 patients in convalescence period. Epidemiol Infect 148:e108. https://doi.org/10.1017/s0950268820001181
Liu J, Liu Y, Xiang P et al (2020b) Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med 18:206. https://doi.org/10.1186/s12967-020-02374-0
Logunov DY, Dolzhikova IV, Zubkova OV, etal (2020) Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet; 396: 887–97 Published Online September 4, 2020 https://doi.org/10.1016/S0140-6736(20)31866-3
Logunov DY, Dolzhikova IV, Shcheblyakov AV. et al. 2021. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397:10275 P671-681. https://doi.org/10.1016/S0140-6736(21)00234-8
Lokugamage KG, Schindewolf C, Menachery VD, 2020. SARS-CoV-2 sensitive to type I interferon pretreatment. BioRxiv. https://doi.org/10.1101/2020.03.07.982264
Long Q, Liu B, Deng H et al (2020a) Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26(6):845–848. https://doi.org/10.1038/s41591-020-0897-1
Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, Hu JL, Xu W, Zhang Y, Lv FJ et al (2020b) Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 26:1200–1204
Loutfy MR, Blatt LM, Siminovitch KA, Ward S, Wolff B, Lho H, Pham DH, Deif H, LaMere EA, Chang M, Kain KC, Farcas GA, Ferguson P, Latchford M, Levy G, Dennis JW, Lai EKY, Fish EN (2003) Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. J Am Med Assoc 290:3222–3228. https://doi.org/10.1001/jama.290.24.3222
Lu H (2020) Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends 14(1):69–71. https://doi.org/10.5582/bst.2020.01020
Madan T, Biswas B, Varghese P, Subedi R, Pandit H, Idicula-Thomas S, et al (2021) A recombinant fragment of Human surfactant protein D binds Spike protein and inhibits infectivity and replication of SARS-CoV-2 in clinical samples.Am J Respir Cell Mol Biol. 65 (1): 41–53. https://doi.org/10.1165/rcmb.2021-0005OC
Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J et al (2020) Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 220:1–13. https://doi.org/10.1016/j.trsl.2020.04.007
Mallapaty S (2020) Will antibody tests for the coronavirus really change everything? Nature 580:571–572
Martines RB et al (2020) Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis 26(9):2005–2015
Mason RJ (2020) Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J 55:2000607. https://doi.org/10.1183/13993003.00607-2020
Mastaglio S, Ruggeri A, Risitano AM, Angelillo P, Yancopoulou D, Mastellos DC et al (2020) The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol 215:108450. https://doi.org/10.1016/j.clim.2020.108450
Matsuyama S, Nagata N, Shirato K, Kawase M, Takeda M, Taguchi F (2010) Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol 84:12658–12664. https://doi.org/10.1128/JVI.01542-10
Mazumder A, Arora M, Bharadiya V et al (2020) SARS-CoV-2 epidemic in India: epidemiological features and in silico analysis of the effect of interventions [version 1; peer review: awaiting peer review]. F1000 Res 9:315. https://doi.org/10.12688/f1000research.23496.1
McCarthy KR, Rennick LJ, Namnulli S, et al. (2020) Natural deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. bioRxiv [Preprint posted online November 19, 2020]. https://www.biorxiv.org/content/10.1101/2020.11.19.389916v1
Mehta V et al (2020) Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov 10(7):935–941. https://doi.org/10.1158/2159-8290.CD20-0516
Mikkonen L, Pihlajamaa P, Sahu B, Zhang FP, Janne OA (2010) Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol 317:14–24
Miller JP, Kigwana LJ, Streeter DG et al (1997) The relationship between the metabolism of ribavirin and its proposed mechanism of action. Ann N Y Acad Sci 284:211–229
Mizumoto K, Kagaya K, Zarebski A, Chowell G (2020) Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the diamond princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 25(10):2000180. https://doi.org/10.2807/1560-7917.ES.2020.25.10.2000180
Monto AS, DeJonge P, Callear AP et al (2020) Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis 222(1):9–16. https://doi.org/10.1093/infdis/jiaa161
Montopoli M et al (2020) Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (n = 4532). Ann Oncol 31(8):1040–1045. https://doi.org/10.1016/j.annonc.2020.04.479
Moreno-Eutimio MA, Lopez-Macias C, Pastelin-Palacios R (2020) Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microbes Infect 22(4-5):226–229. https://doi.org/10.1016/j.micinf.2020.04.009
Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl J (2005) Ribavirin and interferon-β synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun 326:905–908. https://doi.org/10.1016/j.bbrc.2004.11.128
Muller F, Mutch NJ, Schenk WA, Smith SA, Esteri L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T (2009) Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 139:1143–1156
National Health Commission of People’s Republic of China (2020) Diagnosis and treatment of pneumonia caused by novel coronavirus (trial version 4)
National Institute for Health Research (2020). First drug to reduce mortality in hospitalised patients with respiratory complications of COVID-19 found. https://www.nihr.ac.uk/news/first-drug-to-reduce-mortality-in-hospitalised-patients-withrespiratory-complications-of-covid-19-found/25061. Accessed 23 June 2020
Navarro-Millan I, Sattui S, Lakhanpal A, Zisa D, Siegel C, Crow M (2020) Use of Anakinra to prevent mechanical ventilation in severe COVID-19: a case series. Arthritis Rheumatol 72(12):1990–1997. https://doi.org/10.1002/art.41422
Neuman BW, Kiss G, Kunding AH et al (2011) A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol 174(1):11–22. https://doi.org/10.1016/j.jsb.2010.11.021
Ni L, Ye F, Cheng M-L, Feng Y, Deng Y-Q, Zhao H et al (2020) Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity 52(6):971–977.e3. https://doi.org/10.1016/j.immuni.2020.04.023
Nishiura H et al (2020) Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int J Infect Dis 94:154–155. https://doi.org/10.1016/j.ijid.2020.03.020
Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S (2014) Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir Res 105:17–21. https://doi.org/10.1016/j.antiviral.2014.02.014
Oikonomopoulou K (2012) Interactions between coagulation and complement—their role in inflammation. Katerina SeminImmunopathol 34(1):151–165. https://doi.org/10.1007/s00281-011-0280-x
Oliver CM, Campbell M, Dulan O et al (2020) Appearance and management of COVID-19 laryngo-tracheitis: two case reports [version 1; peer review: awaiting peer review]. F1000 Res 9:310. https://doi.org/10.12688/f1000research.23204.1
Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY, Almakhlafi GA, Albarrak MM, Memish ZA, Albarrak AM (2014) Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis 14:1090–1095. https://doi.org/10.1016/S1473-3099(14)70920-X
Onder G, Rezza G, Brusaferro S (2020) Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 323(18):1775–1776. https://doi.org/10.1001/jama.2020.4683
Pan Y, Zhang D, Yang P, Poon LLM, Wang Q (2020a) Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 20(4):411–412. https://doi.org/10.1016/s1473-3099(20)30113-4
Pan X, Chen D, Xia Y, Wu X, Li T, Ou X et al (2020b) Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis 20(4):410–411. https://doi.org/10.1016/s1473-3099(20)30114-6
Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V (2020) Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 18:1738–1742
Pastorino B, Touret F, Gilles M, de Lamballerie X, Charrel RN (2020) Prolonged infectivity of SARS-CoV-2 in fomites. Emerg Infect Dis 26(9):2256–2257. https://doi.org/10.3201/eid2609.201788
Pharmaceuticals I (2020) Innovations C for EP. Safety, tolerability and immunogenicity of INO-4800 for COVID-19 in healthy volunteers. https://clinicaltrials.gov/ct2/show/record/NCT04336410. Accessed 18 May 2020
Polack F, Thomas S, Kitchin N, Absalon J, Gurtman A, Lockhart S et al (2020) Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal Of Medicine, 383(27), 2603-2615. https://doi.org/10.1056/nejmoa2034577
Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England—technical briefing 17. London, United Kingdom: Public Health England; 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/997418/Variants_of_Concern_VOC_Technical_Briefing_17.pdf
Qi F, Qian S, Zhang S, Zhang Z (2020) Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun 526(1):135–140. https://doi.org/10.1016/j.bbrc.2020.03.044
Rambaldi A, Gritti G, Micò MC, Frigeni M, Borleri G et al (2020) Endothelial injury and thrombotic microangiopathy in COVID-19: treatment with the lectin-pathway inhibitor Narsoplimab. Immunobiology 225(6):152001. https://doi.org/10.1016/j.imbio.2020.152001
Ramos-Casals M, Brito-Zeron P, Lopez-Guillermo A, Khamashta MA, Bosch X (2014) Adult haemophagocytic syndrome. Lancet 383:1503–1516
Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M et al (2020) The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 18(7):1747–1751. https://doi.org/10.1111/jth.14854
Ren LL, Wang YM, Wu ZQ et al (2020) Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J 133(9):1015–1024. https://doi.org/10.1097/CM9.0000000000000722
Renne T, Schmaier AH, Nickel KF, Blomback M, Maas C (2012) In vivo roles of factor XII. Blood 120:4296–4303
Resende PC, Bezerra JF, de Vasconcelos RHT et al. (2021) Spike E484K mutation in the first SARS-CoV-2 reinfection case confirmed in Brazil. 10 January. https://virological.org/t/spike-e484k-mutation-in-the-firstsars-cov-2-reinfection-case-confirmed-in-brazil-2020/584
Richmond P, Hatchuel L, Dong M, et al (2021) Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 397: 682–94 https://doi.org/10.1016/S0140-6736(21)00241-5
Rittirsch D, Flierl MA, Ward PA (2008) Harmful molecular mechanisms in sepsis. Nat Rev Immunol 8:776–787
Roboz, J., (1977) The relationship between the metabolism of ribavirin and its proposed mechanism of action. Ann N Y Acad Sci 284:211–229
Rocha CD, Caetano BC, Machado AV, Bruna-Romero O (2004) Recombinant viruses as tools to induce protective cellular immunity against infectious diseases. Int Microbiol 7:83–94
Rogers TF et al (2020) Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 369(6506):956–963. https://doi.org/10.1126/science.abc7520
Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C et al (2020) Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 382(10):970–971. https://www.nejm.org/doi/pdf/10.1056/NEJMc2001468?articleTools=true
Rottier PJM (1995) In: Siddell SG (ed) The Coronaviridae. Plenum, New York, pp 115–139
Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46:846–848. https://doi.org/10.1007/s00134-020-06028-z
Sallard E, Lescure F-X, Yazdanpanah Y, Mentre F et al (2020) Type 1 interferons as a potential treatment against COVID-19. Antivir Res 178:104791. https://doi.org/10.1016/j.antiviral.2020.104791
Samuel C (2001) Antiviral actions of interferons. Clin Microbiol Rev 14(4):778–809
Seguin A, Galicier L, Boutboul D, Lemiale V, Azoulay E (2016) Pulmonary involvement in patients with hemophagocytic lymphohistiocytosis. Chest 149:1294–1301
Sekine T, Perez-Potti A, Rivera-Ballesteros O (2020) Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 183(1):158–168.e14. https://doi.org/10.1016/j.cell.2020.08.017
Sha J, Kirtley ML, Klages C, Erova TE, Telepnev M et al (2016) A replication-defective human type 5 adenovirus-based trivalent vaccine confers complete protection against plague in mice and nonhuman primates. Clin Vaccine Immunol 23:586–600. https://doi.org/10.1128/CVI.00150-16
Shang YX, Huang Y, Liu EM, Chen Q, Cao L, Lu M et al (2014) A multicenter study on the treatment of acute bronchiolitis by nebulized human recombinant human interferon-alpha 1b. Zhongguo Shi Yong ErKe Za Zhi 29:840–844. (in Chinese)
Shang J, Ye G, Shi K et al (2020a) Structural basis of receptor recognition by SARS-CoV-2. Nature 581:221–224. https://doi.org/10.1038/s41586-020-2179-y
Shang J, Wana Y, Luoa C, Yea G, Genga Q, Auerbacha A, Lia F (2020b) Cell entry mechanisms of SARS-CoV-2. PNAS 117(21):11727–11734. https://www.pnas.org/cgi/doi/10.1073/pnas.2003138117
Sheahan TP, Sims AC, Leist SR et al (2020) Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 11:222
Shen KL, Yang YH (2020) Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue. World J Pediatr 16(3):219–221. https://doi.org/10.1007/s12519-020-00344-6
Shen KL, Shang YX, Zhang GC, Xu BP, Fu Z, Cao L et al (2018) Rational use of interferon alpha in pediatrical clinical practice: experts’ consensus statement. Zhonghua Shi Yong ErKe Za Zhi 33:1301–1308. (in Chinese)
Shen Q, Xiao X, Aierken A, Liao M, Hua J (2020) The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J Cell Mol Med 24(16):9472–9477. https://doi.org/10.31219/osf.io/fs5hd
Shiraki K, Daikoku T (2020) Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. PharmacolTher 209:107512
Silva JC, Mariz HA, Rocha LF Jr, Oliveira PS, Dantas AT, Duarte AL et al (2013) Hydroxychloroquine decreases Th-17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics (Sao Paulo) 68:766–771
Skendros P et al (2020) Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest 130(11):6151–6157. https://doi.org/10.1172/JCI141374
Song HD, Tu CC, Zhang GW, Wang SY et al (2005) Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc Natl Acad Sci U S A 102:2430–2435. https://doi.org/10.1073/pnas.0409608102
Sun D (2020) Remdesivir for treatment of COVID-19: combination of pulmonary and IV administration may offer additional benefit. AAPS J 22:77. https://doi.org/10.1208/s12248-020-00459-8
Sun B, Feng Y, Mo X, Zheng P, Wang Q, Li P, Peng P, Liu X, Chen Z, Huang H, Zhang F, Luo W, Niu X, Hu P, Wang L, Peng H, Huang Z, Feng L, Li F, Zhang F, Li F, Zhong N, Chen L (2020a) Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbe Infect 9(1):940–948
Sun J, Zhu A, Li H, Zheng K, Zhuang Z, Chen Z et al (2020b) Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg Microbes Infect 9:991–993
Sungnak, W., Huang, N., Bécavin, C., Berg, M., Queen et al. (2020). SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med;26(5):681-687 doi:https://doi.org/10.1038/s41591-020-0868-6
Sutton D, Fuchs K, D'Alton M, Goffman D (2020) Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 382:2163–2164. https://doi.org/10.1056/NEJMc2009316
Takahashi T, Ellingson MK, Wong P et al (2020) Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 588(7837):315–320. https://doi.org/10.1038/s41586-020-2700-3
Tan LA, Yu B, Sim FCJ, Kishore U, Sim RB (2010) Complement activation by phospholipids: the interplay of factor H and C1q. Protein Cell 1:1033–1049
Taneja V (2018) Sex hormones determine immune response. Front Immunol 9:1931. https://doi.org/10.3389/fimmu.2018.01931
Tang N, Li D, Wang X, Sun Z (2020a) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18:844–847
Tang T, Bidon M, Jaimes JA, Whittaker GR, Daniel S (2020b) Coronavirus membrane fusion mechanism offers as a potential target for antiviral development. Antiviral Res 178:104792. https://doi.org/10.1016/j.antiviral.2020.104792
Tatsis N, Ertl HC (2004) Adenoviruses as vaccine vectors. Mol Ther 10(4):616–629. https://doi.org/10.1016/j.ymthe.2004.07.013
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP (2020) The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20(6):363–374. https://doi.org/10.1038/s41577-020-0311-8
To KK et al (2020) Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 20(5):565–574. https://doi.org/10.1016/S1473-3099(20)30196-1
Tong ZD et al (2020) Potential presymptomatic transmission of SARS-CoV-2, Zhejiang Province, China. Emerg Infect Dis 26:1052–1054. https://doi.org/10.3201/eid2605.200198
Tooze J, Tooze S, Warren G (1984) Replication of coronavirus MHV-A59 in sac-cells: determination of the first site of budding of progeny virions. Eur J Cell Biol 33:281–293. PMID: 6325194
Vaira LA, Salzano G, Deiana G, De Riu G (2020) Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope 130:1787. https://doi.org/10.1002/lary.28692
van der Hoek L, Sure K, Ihorst G et al (2005) Croup is associated with the novel coronavirus NL63. PLoS Med 2:e240. https://doi.org/10.1371/journal.pmed.0020240
van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI et al (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382(16):1564–1567
Van Voris LP, Newell PM (1993) Antivirals for the chemoprophylaxis and treatment of influenza. Semin Respir Infect 7:61–70
Varghese PM et al (2020) Host-pathogen interaction in COVID-19: potential therapeutics and vaccination strategies. Immunobiology 225(6):152008
Vasques Nonaka CK, Miranda Franco M, Gräf T, et al. (2021) Genomic Evidence of a Sars-CoV-2 Reinfection Case With E484K Spike Mutation in Brazil. https://www.preprints.org/manuscript/202101.0132/v1
Verdia-Baguena C, Nieto-Torres JL, Alcaraz A, DeDiego ML, Torress J, Anguilella VM, Enjuanes L (2012) Coronavirus E protein forms ion channes with functionally and structurally-involved membrane lipids. Virology 432:485–494
Villalaín J (2010) Membranotropic effects of Arbidol, a broad anti-viral molecule, on phospholipid model membranes. J Phys Chem B 114(25):8544–8554
Vincent MJ, Bergeron E, Benjannet S et al (2005) Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2:69. https://doi.org/10.1186/1743-422X-2-69S
Volz E, Hill V, McCrone JT, et al (2021) COG-UK Consortium. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell 184:64–75.e11
Voysey M, Clemens SAC, Madhi SA, et al (2021) Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet; 397: 99–111
Walls AC et al (2020a) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181:281–292
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D (2020b) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181:281–292.e6
Wang H, Zhang L (2020) Risk of COVID-19 for patients with cancer. Lancet Oncol 21:e181
Wang C, Horby PW, Hayden FG, Gao GF (2020a) A novel coronavirus outbreak of global health concern. Lancet 395(10223):470–473. https://doi.org/10.1016/S0140-6736(20)30185-9
Wang D, Hu B, Hu C et al (2020b) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323(11):1061–1069. https://doi.org/10.1001/jama.2020.1585
Wang H et al (2020c) Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell 182(3):713–721.e9. https://doi.org/10.1016/j.cell.2020.06.008
Wang L, Gao Y-H, Lou L-L et al (2020d) The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur Respir J 55(4):2000398. https://doi.org/10.1183/13993003.00398-2020
Wang M, Cao R, Zhang L, Yang X et al (2020e) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitri. Cell Res 30(3):269–271. https://doi.org/10.1038/s41422-020-0282-0
Wang Y, Liu Y, Liu L, Wang X, Luo N, Ling L (2020f) Clinical outcome of 55 asymptomatic cases at the time of hospital admission infected with SARS-Coronavirus-2 in Shenzhen, China. J Infect Dis 221(11):1770–1774
Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, Xu W, Zhao Y, Li N, Zhang J, Liang H, Bao L, Xu Y, Ding L, Zhou W, Gao H, Liu J, Niu P, Zhao L, Zhen W, Fu H, Yu S, Zhang Z, Xu G, Li C, Lou Z, Xu M, Qin C, Wu G, Gao G, Tan W, Yang X (2020g) Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell 182(3):713–721.e9
Warren T, Jordan R, Lo M et al (2016) Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 531:381–385. https://doi.org/10.1038/nature17180
Wei X et al (2020) Sex differences in severity and mortality among patients with COVID-19: evidence from pooled literature analysis and insights from integrated bioinformatic analysis. arxiv
Report of the WHO-China joint Mission on coronavirus disease 2019 (COVID-19) 16–24 February 2020. Geneva: World Health Organization; 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
WHO Coronavirus disease (COVID-19) Dashboard https://covid19.who.int/?gclid=Cj0KCQjwhb36BRCfARIsAKcXh6HCzQZUKFtQ-S7SfYgwOXj3Z4KeQ-L1rJnTjNMSb3EaQiUylzMSf_saAnrbEALw_wcB. 03.09.2020
WHO Report (2021a) Coronavirus disease (COVID-19) advice for the public: Mythbusters. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters#dexamethasone (as accessed on 14th April, 2021)
WHO Report (2021b) Coronavirus disease (COVID-19) advice for the public: Mythbusters.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice for public/mythbusters?gclid=EAIaIQobChMIpvm9j__97wIVkH4rCh3TlwU6EAAYASAAEgKlBvD_BwE#chloroquine (as accessed on 14th April, 2021)
WHO Report (2021c) The Moderna COVID-19 (mRNA-1273) vaccine: what you need to know.HYPERLINK “https://www.who.int/news-room/feature-stories/detail/the-moderna-covid-19-mrna-1273-vaccine-what-you-need-toknow?gclid=EAIaIQobChMI9rHG7LH%207wIVBqyWCh (accessed on 15th April, 2021)
Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C et al (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469
Woloshin S, Patel N, Kesselheim AS (2020) False negative tests for SARSCoV-2 infection—challenges and implications. N Engl J Med 383(6):e38
World Health Organization (2020) WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020 [Internet]. Geneva (Switzerland), World Health Organization. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367:1260–1263
Wu Z, McGoogan JM (2020) Characteristics of and important lesions from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Centre for Disease Control and Prevention. JAMA 323(13):1239–1242. PMID: 32091533. https://doi.org/10.1001/jama.2020.2648
Wu D, Tu C, Xin C, Xuan H et al (2005) Civets are equally susceptible to experimental infection by two different severe acute respiratory syndrome coronavirus isolates. J Virol 79:2620–2625. https://doi.org/10.1128/JVI.79.4.2620-2625.2005
Wu YP. Liu ZH, Wei R, Pan SD et al (2009) Elevated Plasma Surfactant Protein D (SP-D) levels and a direct correlation with Anti-Severe Acute Respiratory Syndrome Coronavirus-specific IgG antibody in SARS Patients. Scand J Immunol. 69(6):508-15. https://doi.org/10.1111/j.1365-3083.2009.02245.x
Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G et al (2020a) A new coronavirus associated with human respiratory disease in China. Nature 579:265–269
Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X et al (2020b) Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 5(5):434–435
Xia Y, Jin R, Zhao J, Li W, Shen H (2020) Risk of COVID-19 for patients with cancer. Lancet Oncol 21:e180
Xiang Z-Q, Gao GG, Reyes-Sandoval A, Cohen CJ, Li Y, Bergelson JM, Wilson JM, Ertl HCJ (2002) Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol 76:2667–2675
Xiang P, Xu XM, Gao LL, Wang HZ, Xiong HF, Li RH, et al (2020) First case of 2019 novel coronavirus disease with encephalitis. ChinaXiv T202003.00015
Xu X, Han M, Li T, Sun W, Wang D et al (2020a) Effective treatment of severe COVID-19 patients with Tocilizumab. Proc Natl Acad Sci U S A 117:11970–11975. https://doi.org/10.1073/pnas.2005615117
Xu Z et al (2020b) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8:420–422. https://doi.org/10.1016/S2213-2600(20)30076-X
Xu K et al (2020c) Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis 71(15):799–806. https://doi.org/10.1093/cid/ciaa351
Yang M (2020) Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection. SSRN. https://doi.org/10.2139/ssrn.3527420
Yasmin H, Kishore U (2021) Biological Activities of SP-A and SP-D Against Extracellular and Intracellular Pathogens. The Collectin Protein Family and Its Multiple Biological Activities, 103–133. https://doi.org/10.1007/978-3-030-67048-1_5
Ye M, Ren Y, Lv T (2020) Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun 88:945–946
Younger JG, Bracho DO, Chung-Esaki HM, Lee M, Rana GK, Sen A, Jones AE (2010) Complement activation in emergency department patients with severe sepsis. Acad Emerg Med 17:353–359
Yu J, Tostanoski LH, Peter L, Mercado NB, McMahan K, Mahrokhian SH, Nkolola JP, Liu J, Li Z, Chandrashekar A et al (2020) DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 369(6505):806–811. https://doi.org/10.1126/science.abc6284
Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367:1814–1820. https://doi.org/10.1056/NEJMoa1211721
Zhang L, Zhu F, Xie L, Wang C et al (2020a) Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol 31(7):894–901. https://doi.org/10.1016/j.annonc.2020.03.296
Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W et al (2020b) Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 382(17):e38. https://doi.org/10.1056/nejmc2007575
Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y et al (2020) Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis 71(16):2027–2034
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273
Zhu N, Zhang D, Wang W et al (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382(8):727–733. https://doi.org/10.1056/NEJMoa2001017
Zhu F, Guan X, Hou L et al (2020a) Immunogenicity and safety of recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 396(10249):479–488. https://doi.org/10.1016/S0140-6736(20)31605-6
Zhu F, Li Y, Guan X, Hou L, Wang W, Li, et al. (2020b) Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 395(10240):1845–1854. https://doi.org/10.1016/s0140-6736(20)31208-3
Zmora P, Hoffmann M, Kollmus H, Moldenhauer A, Danov O, Braun A, Winkler M, Schughart K, Pöhlmann S (2018) TMPRSS11A activates the influenza A virus hemagglutinin and the MERS coronavirus spike protein and is insensitive against blockade by HAI-1. J Biol Chem 293(36):13863–13873
Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M et al (2020) Neutrophil extracellular traps in COVID-19. JCI Insight 5(11):e138999. https://doi.org/10.1172/jci.insight.138999
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yasmin, H., Saha, S., Butt, M.T., Modi, R.K., George, A.J.T., Kishore, U. (2021). SARS-CoV-2: Pathogenic Mechanisms and Host Immune Response. In: Kishore, U. (eds) Microbial Pathogenesis. Advances in Experimental Medicine and Biology, vol 1313. Springer, Cham. https://doi.org/10.1007/978-3-030-67452-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-67452-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67451-9
Online ISBN: 978-3-030-67452-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)