Abstract
Nanoparticles have dimensions ranging from 1 to 100 nm and are fabricated using different processes. The broad range of physical and chemical properties of metal oxide nanoparticles (MONPs) allows them to exhibit variety of biological tasks. The MONPs are very adaptable and can be used in several biomedical applications. MONPs play a very significant role in biomedical research, as new biomaterials with enhanced performance and low toxicity, their demand is steadily increasing in recent years. Several investigations have been made in assessing the antibacterial properties of MONPs. The potential antibacterial outcomes revealed by MONPs emphasize the necessity to develop them into next generation antibacterial agents. There are different methods to synthesize MONPs and every procedure has important inference on the biological role of MONPs. Among all the synthesis methods, MONPs fabricated from plants via green nanotechnology are least toxic and these procedures are specifically appropriate for biomedical research. Underlying challenges in development of MONPs as antibacterial agents in clinics, their applications and future prospective are also discussed. Specifically, the current study emphasizes the use of green nanotechnology for fabrication of metal oxide nanoparticles from plant extracts and investigates their potential use as antibacterial agents.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Background
Nanoparticles (NPs) or nanomaterials are small size (1–100 nm) materials of different shapes and they exhibit many interesting physicochemical characteristics because of high surface area per unit volume and quantum confinement effect (Chen et al. 2018). Usually, NPs are made from pure elements such as carbon, metals (Au, Ag, Cu, Fe, Pd, Pt, and Zn), and from metal oxides like Ag2O, CuO, CeO2, FeO, NiO, TiO2, and ZnO. Many biological functions of the metal NPs and metal oxide NPs are directly related to their diverse chemistry and some of them include: antibacterial, anticancer, antifungal, anti-inflammatory antioxidant, antiparasitic, and antiviral activities (Teow et al. 2018; Cagno et al. 2018). As shown in Fig. 13.1, NPs can be synthesized using several physical, chemical and biological methods (Teow et al. 2018).
Examples include (a) physical methods (cathodic arc or physical vapor deposition, ion etching or suttering, thin film deposition, melt blending), (b) chemical methods (co-precipitation, photoinduced reduction, UV-initiated photoreduction, sol-gel, microemulsions, sonochemical) (c) biological methods (fabrication from plants, microbes, eukaryotes, yeasts, molds, enzymes, Metazoas, biological waste), and (d) hybrid methods (Teow et al. 2018; Ali et al. 2020; Saratale et al. 2018).
All these fabrication methods have both advantages and disadvantages; therefore, the selection of synthesis method is mainly dependent on the intended biomedical applications. The broad range of physical and chemical properties of NPs allows them to exhibit variety of biological tasks. The NPs are very adaptable and can be used in several biomedical applications. NPs play a very critical role in biomedical studies, as new biomaterials with improved performance and low toxicity, their necessity is steadily increasing in recent years. Because of low dimensions and better cell-permeating ability, nanoparticles are used in several biomedical engineering like bio-sensing, medical-imaging, disease recognition, delivery of pharmaceuticals, bio-coupling, heat stroke, and physiological therapeutics (Teow et al. 2018; Han et al. 2019). Recently, NPs have been extensively used in sensing and imaging applications, primarily because of their extraordinary localization ability in therapies (Han et al. 2019). Other benefits of nanoparticles are versatility in surface alteration, facile size regulation, and generation of considerably diminishable nanoparticles for in vivo therapeutics (Teow et al. 2018; Zhu et al. 2018). Furthermore, nanoparticles are used in bio-coupling and by conjugating with pharmaceuticals, they ease the targeted drug delivery process.
Detailed chemistry underlying bio-coupling of several pharmaceuticals and nanoparticles have been reported by Wadhawan et al. (2019). Also, the authors explained the application of NPs in drug delivery system for targeted therapies. The possible utilization of nanoparticles in heat stroke treatment is mostly to kill the tumor cells (Liu et al. 2020). With magnetic NPs, hyperthermia can be cured by targeting and killing the specific cancerous cells, while minimizing the damage to the healthy tissues (Jose et al. 2020; Shakil et al. 2019). Hence, NPs have a very crucial role in medical and biomedical research. Several investigations have been made in assessing the antibacterial properties of NPs. The potential antibacterial outcomes revealed by NPs emphasize the necessity to develop them into next generation antibacterial agents. In addition to cancer treatment, NPs are effective against diseases caused by Escherichia coli and Pseudomonas aeruginosa infections (Happy et al. 2019; Liao et al. 2019).
The exclusive properties of metal NPs (or MNPs) and metal oxide NPs (or MONPs) such as huge area, excellent elastic modulus, stability, high melting temperature makes them suitable for clinical applications like therapy, drug delivery, biosensors, anti-biofilms, biomedical imaging and infections (Teow et al. 2018; Khatami et al. 2018). Hydrophilic and hydrophobic properties of NPs make them appropriate for drug delivery applications (Fratoddi 2018).
MONPs can be synthesized by various physicochemical processes (Fig. 13.1). But, requirement of intensive power, complicated equipment, huge cost, less productivity, etc., are limitations of the physical methods (Jamkhande et al. 2019). The chemical methods for synthesis of MONPs are inexpensive, provides good product yield, and do not need complex experimental instrumentation (Akbari et al. 2018). Nevertheless, chemical methods make use of volatile and toxic chemical reagents (like aromatic amines, thiols, sodium borohydrate and hydrazine) that are very harmful, causes air pollution and also are non-ecofriendly in nature (Teow et al. 2018; Jamkhande et al. 2019; Wu and Tang 2018). Researchers are developing novel fabrication methods of MONPs that are sustainable and eco-friendly in nature (Singh et al. 2018). Therefore, the green nanotechnology protocols for fabrication of MONPs are gaining importance. The present study gives recent updates on antibacterial effects of MONPs fabricated from plants using green nanotechnology. Underlying challenges in developing MONPs as antibacterial agents in clinics, applications, and future prospective are also discussed.
13.2 Phyto-fabrication of MONPs
13.2.1 Green Nanotechnology
There are different methods to synthesize MONPs and every procedure has important inference on the biological role of MONPs (Fig. 13.1). Among all the available nanoparticle synthesis methods, MONPs produced from green nanotechnology methods are less toxic, inexpensive, and efficient. These procedures are specifically appropriate for biomedical research. In general, fabrication of MONPs using biological methods has additional advantages, while compared to their synthesis using physicochemical processes. The biological methods for the synthesis of NPs are facile, efficient, simple to step-up (manufacture), and they require less power consumption (Teow et al. 2018).
The “greener” nanotechnology methods are sustainable and they employ nonhazardous reagents and produces environmentally harmless substances (Singh et al. 2018; El Shafey 2020). The developed green nanotechnology methods are appropriate and can be useful for the production of a wider range of biomedical products like pharmaceuticals, food, and cosmetics (Teow et al. 2018; El Shafey 2020; Soni et al. 2018). Relatively, chemical and physical methods are expensive and frequently employ hazardous and toxic chemicals that are more harmful to human cells. Moreover, the green nanotechnology methods do not rigorously need more energy, high pressure, and temperature conditions for the preparation of MONPs (Teow et al. 2018). Nevertheless, parameters like chemical concentration, pH, reaction time, and temperature are very crucial for constant production of biologically important MONPs (El Shafey 2020; Hasan et al. 2018). Moreover, fabrication of MONPs from the plants does not require other maintenance costs like for growth of microbial culture and thus, reduces the unnecessary costs for isolation of microorganisms and preparation of microbial culture media (El Shafey 2020). As shown in Table 13.1, majority of MONPs obtained from plants have sizes <100 nm. Some larger size particles (100–500 nm) are also observed (Teow et al. 2018; Thatoi et al. 2016; Tippayawat et al. 2016). The phyto-fabricated nanoparticles are nontoxic to many cells like HEK293 (human embryonic kidney), NIH3T3 (mouse embryonic fibroblast), VSMCs (rat aortic vascular smooth muscle cells), and PBMCs (peripheral blood mononuclear cells).
13.2.2 MONPs Synthesis from Plants
MONPs fabrication from plants is effective in achieving high productivity, while compared to their production from other biological sources like fungi and microbes. Plants have many biochemicals (like polyphenols) and metabolites, which can function as reducing and stabilizing agents during the production of biogenic MONPs. As shown in Fig. 13.2, phyto-fabrication of MONPs is simple, economical, and eco-friendly, thus prohibiting the use of harmful chemicals (Singh et al. 2020). MONPs obtained from the plant extracts were found to be relatively stable, while compared to those obtained from fungal and microbial extracts. Phyto-fabrication of MONPs is broadly categorized into (a) within cell synthesis, (b) extracellular generation, and (c) fabrication using plant chemicals. Intracellular synthesis of MONPs occurs within the cells of plant tissues via specific enzymes present in the cells.
After the synthesis, the MONPs are recovered upon rupture of plant cell membranes. Other methods that use plant-based extracts as raw materials are preferred over the intracellular synthesis methods for production of MONPs. Fabrication of MONPs from the plant extracts is moderately cheaper process and it often gives higher yields because of the availability of significant quantities of plant chemicals in the extracts, which reduces or stabilizes the oxidation state of metal in MNPs (Mohammadinejad et al. 2019). Plant chemicals dependent fabrication of metal-based nanoparticles is difficult because of involvement of specific phytochemicals required for producing stable nanoparticles (Dauthal and Mukhopadhyay 2016). Spherical silver nanoparticles were fabricated using the leaf extracts from Azadirachta indica by Ahmed et al. (2016a, b). These investigations revealed that the extracts containing phytochemicals and bioflavonoids function like stabilizing and reducing agents during the production of silver nanoparticles. They showed high potential to be used as antimicrobial agents. Gold nanoparticles were obtained from the extracts of Morinda citrifolia roots that also exhibited antipathogenic activity (Suman et al. 2014).
13.2.3 Purification of MONPs
After phyto-fabrication, appropriate purification of biocompatible MONPs is required prior to their utilizing in biomedical engineering research. For many years, centrifugation techniques have been used to isolate MONPs because of easy and fast operation steps. Hence, multiple washings and high-speed centrifugations are employed to separate and purify biologically synthesized metal-based NPs and to remove any unreacted bioactive molecules from the products (Dauthal and Mukhopadhyay 2016). But, the centrifugation method have some limitations like the centrifugation step can often result in agglomeration of MONPs and destabilization of MONPs because of detachment of inert agents from the MONPs surface, thus changing the MONPs inherent properties. Alternatively, dialysis technique uses suitable cutoff membrane for separation of MONPs. Dialysis membrane separates mixture of molecules present in the plant extracts by selectively retaining larger biomolecules inside the membrane and expelling the smaller unwanted biomolecules out of the membrane. The larger biomolecules that act as inert agents on the surface of MONPs are coupled tightly with them. Later, MONPs are collected from the dialysis bag during the washing step. Dialysis purification process usually requires a day (time consuming) to isolate the MONPs from the plant extracts. But, the diafiltration technique cannot be employed for MONPs because of their aqueous insolubility. MONPs such as Fe2O3 and Fe3O4 can be detached simply by using the field of magnet (physical method). But, eliminating chemically bound molecules from the MONPs surface is another key task that needs significant knowledge of chemistry.
13.2.4 MONPs Processing Parameters
Considerable number of research works is being carried out globally to investigate the effect of temperature on phyto-fabrication of MONPs. Temperature is the single most controlling factor that influences the size, morphology and shape of the MONPs and the rate at which they are synthesized. The different shapes (octahedral platelets, triangular, rod-like, and spherical structures), change in the size and reaction time for MONPs preparation are related to the temperature. With the increase in temperature, a simultaneous rise in rate of the reaction is observed and formation of nucleation centers of NPs is observed (Sneha et al. 2010). Acidity or basicity of the reactant mixture is critical in phyto-fabrication of MONPs. It influences the creation of seeding centers for growth of MONPs. With the increase in pH, an instantaneous growth in centers for nucleation process was noticed, thus improving the metal-based NPs formation. The pH takes a vital role in regulating the morphology and size of the MONPs. In addition to temperature and pH, reaction time is another major factor that affects the morphology of MONPs. The role of the time on dimensions of pure zinc oxide and cerium-doped zinc oxide particles was investigated by Flor et al. (2004). The authors reported that the particle size rises linearly with the reaction time. Also, the authors observed that the size of cerium-doped ZnO particles is relatively larger than the size of ZnO particles for a given constant reaction time.
13.3 Antibacterial Behavior of MONPs
13.3.1 MONPs Antibacterial Activities
MONPs fabricated from plants illustrated bactericidal properties and have greater prospective for becoming next generation antibacterial agents, primarily because of their low toxicity (Gupta et al. 2017). Earlier reports indicates that NPs inhibit gram-positive bacteria like Bacillus spp., Streptococcus spp. and Staphylococcus spp. and gram-negative bacteria like Escherichia spp., Proteus spp., Salmonella spp., Vibrio spp., Shigella spp., and Pseudomonas spp. (Teow et al. 2018; Singh et al. 2020; Kanjikar et al. 2018; Manikandan et al. 2017; Vijayakumar et al. 2016; Banumathi et al. 2017). In addition, NPs inhibit antibiotic-resistant bacteria like anti-drug MRSA and E. coli (Teow et al. 2018; Jadhav et al. 2016; Azizi et al. 2017). Table 13.1 summarizes the bactericidal nature of MONPs, data obtained from literature (PubMed journal directory) published during 2016–2017 (Teow et al. 2018). About 107 articles was found using the online literature search engine (NCBI database) with the input words like green synthesis, nanoparticles, plants, and antibacterial (NCBI 2018; Teow et al. 2018). Among the 107 searched articles, 17 papers were eliminated because of their irrelevant data for the current book chapter, which comprised of reports, retracted articles and non-phyto related nanoparticles information. About 90 articles are considered for the present study, with the relevant data of classified MONPs is listed in Table 13.1 (Teow et al. 2018). Because of many fabrication techniques for producing MONPs (Fig. 13.1), the phytosynthesis source of the MONPs (Table 13.1) differs with the type of metal oxide, although they are obtained from same common precursor i.e., plant extracts. In general, extensively studied nanoparticles include silver, gold, transition metals based MNPs or MONPs and their composites. Transition metals are anticipated to be the ideal candidates for the synthesis of metal-based nanoparticles because they possess partially filled d-orbitals that makes them redox-active (cations are reduced to zero valence states), a quality that assists aggregation of MONPs (Sánchez-López et al. 2020).
From Table 13.1, MONPs have the size of less than 100 nm except for a couple of them (Teow et al. 2018; Thatoi et al. 2016; Rezaie et al. 2017). Irrelevant of the MONPs type, majority of them were exclusively obtained from the leaf extracts, except in few cases from other plant sources like fruits, seeds, crude, etc. Table 13.2 presents bacteria (gram +ve and gram −ve) that are targeted using different MONPs along with the number of bacteria-related research articles (Teow et al. 2018). Gram −ve bacteria considered for current study include Escherichia coli and Pseudomonas aeruginosa, while gram +ve bacteria investigated include B. subtilis, B. cereus and S. aureus. Relatively, gram −ve bacteria are affected by metal-based NPs in comparison to gram +ve bacteria. Metal-based nanoparticles act as antibacterial agents because they can efficiently penetrate gram −ve bacteria cell membrane (well-known for impermeable multilayered cell surface) and kill them (Zgurskaya et al. 2015). Recently, the potential of AgNPs in killing K. pneumonia was demonstrated by Acharya et al. (2018).
Scanning electron microscopy (SEM) images of K. pneumonia after treating with Ag NPs illustrate the disruption caused by the Ag NPs to the cell surfaces and resulted in cell death. Figure 13.3a shows the FESEM image of untreated and intact K. pneumonia cells. After treatment with Ag NPs, the FESEM image of Fig. 13.3b clearly shows the disruption (black arrows) caused by Ag NPs to the K. pneumonia cell surfaces. Also, in another study, the SEM images showed disruption/lysis of cell surface by AgNPs and outflow of the cellular fluids of Escherichia coli and their random distribution into different regions (Das et al. 2017). Previous reports reveal that metal-based NPs can be improved as antibacterial agents against skin infection–linked bacteria (S. aureus) and gut-associated bacteria (P. aeruginosa, E. coli, K. pneumoniae, S. typhi, S. flexneri, and B. cereus). Silver nanoparticles are most effective against the skin bacterial infection (S. epidermidis and S. aureus) and the gut-associated bacteria. Au NPs are efficient in killing S. aureus and E. coli. Some metal-based NPs are efficient against specific type of bacteria, like ZnO NPs are efficient against S. aureus and P. aeruginosa.
FESEM images of (a) untreated and (b) Ag NPs treated K. pneumoniae cells. (Adapted from Acharya et al. 2018)
13.3.2 MONPs-Based Antibiotics
In addition, phyto-fabricated metal-based NPs can deliver drugs or antibacterial compounds in cells as nanoemulsions and by via bioconjugation, thus symbiotically improving bactericidal results (Pagar and Darekar 2019; Wadhawan et al. 2019). Ag NPs fabricated using plant extracts resulted in killings of food borne pathogenic bacteria, while they were used in association with other antibiotics (Thatoi et al. 2016). Au NPs improve the Amoxicillin killings of gram +ve bacteria (Bacillus and Staphylococcus) and gram +ve bacteria (Escherichia coli) (Kalita et al. 2016). The metal-based NPs reversed the progress of resistance induced from antibiotics via death of MRSA bacteria using mice MRSA disease prototypes (Kalita et al. 2016). Figure 13.4 shows a schematic of the timeline for the development of antibiotics in treatment of different bacterial infections and subsequent timeline for the development of resistance by the bacteria to the antibiotics (Sánchez-López et al. 2020).
13.3.3 MONPs Antibacterial Mechanism
The exact controlling mechanisms of metal-based NPs synergism with antibiotics still remain unknown. Some possibilities include: (a) production of bactericidal hydroxyl radicals by the NPs, (b) generation of more bactericidal metal ions by the NPs, and (c) efficient blocking of the flow for killings of drug-resistant bacteria (Gupta et al. 2017; Deng et al. 2016; Hwang et al. 2012). Other possible antibacterial effects of metal-based NPs include: (a) facilitation of columbic attraction (+ve nanoparticles and −ve bacteria), (b) many nanoparticles binding to the surface of bacteria that stops respiration and permeability-related processes, (c) damage or breakage of bacterial genes after effective penetration of the NPs into the cells, and (d) degradation and/or inactivation of vital proteins present in the bacteria (Manikandan et al. 2017; Reidy et al. 2013; Guzman et al. 2012). Ag NPs enter bacterial cells and results in considerable damage to DNA via interacting with phosphorus- and sulfur-containing molecules (Swamy et al. 2015; Ramesh et al. 2015). Also, Ag NPs release Ag+ ions and radicals in bacteria cells. They are very reactive enough to cause antibacterial effects (Ovington 2004). The Ag+ ions in association with sulfur-rich proteins of bacteria surface result many damages to cells (Reidy et al. 2013). Ag nanoparticles deactivate enzymes present in bacteria and produce harmful substance (H2O2) that causes killings of the bacteria (Raffi et al. 2008).
13.3.4 MONPs-ROS Induced Cell Damage
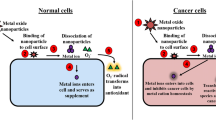
The precise mechanism of metal-based nanoparticles against bacteria is still not fully understood and several investigations are being carried out across the globe. Scientists have revealed that metal-based nanoparticles can induce harmful effects by disrupting the outside surface of bacteria cells (Fig. 13.3). Silver nanoparticles create dents and cracks on the outer cell layers of bacteria and eject charged species that promote interaction of sulfhydryl/disulfide moieties of proteins and charged species, thus resulting in disturbance of metabolic pathway and ultimately leading to the bacterial cell death (Kailasa et al. 2019). As shown in Fig. 13.2, metal oxide nanoparticles generate reactive oxygen species (ROS), which stimulates oxidative stress inside bacteria cells and causes the cell death in bacteria (Dizaj et al. 2014). Ag NPs synthesized using green nanotechnology kills drug-resistant bacteria through ROS mediated damage of bacterial cell membrane (Das et al. 2017). ZnO NPs enhances the ROS production on the surface of bacterial cell membrane, thus resulting in the malfunction of the membrane and finally leading to the death of bacterial cells (Santhoshkumar et al. 2017). Similarly, examination of the mechanistic aspects of stress induced from reactive oxygen species production due to treatment of bacteria with titania nanoparticles was done by Allahverdiyev et al. (2011). The ROS production induced by TiO2 NPs can result in lipid peroxidation, which further influences the integrity and fluidity of bacterial cell membrane. The toxic and inhibitory effects of MONPs against different bacteria depend on the concentration of ejected charged species inside the cells by MONPs. The concentration of charged species increases with the amount of ROS that is generated within the cells.
The precise cell signaling mechanism mediated via MONPs for their bactericidal role is unknown. But, metal-based NPs stimulate two different pathways in exhibiting the bactericidal influence; producing charged species (by MNPs) and ROS (by MONPs), as shown in Fig. 13.2. The freed ROS/charged species via oxidation cause harm to cell surface, lipids, or nucleic acids (Sintubin et al. 2011). During MONPs fabrication, the reactive compounds present in the microbial and plant extracts can also reduce them (Gautam et al. 2019). These molecules give high stability to the phyto-fabricated MONPs and also attach diverse functional moieties on to the MONPs surface. The moieties facilitate chemical bonding and provide specific regions that enhance MNPs-cell physical interaction , which is required for bactericidal activity (Baker et al. 2017). Growth of resistance to antibiotics by bacteria is the main problem of research for the scientists (Sánchez-López et al. 2020). In another work, zinc oxide nanoparticles based antibiotics was found to efficiently kill MRSA therapeutical segregates (Ali et al. 2016).
13.4 Applications of Antibacterial MONPs
To date, EMA and FDA have approved many metal-based NPs for different clinical studies that include cure of anemia (Vifor-based Fe-substitute treatment), imaging (Resovist), delivery of anticancer drugs (Mepact and Onivyde), fungal infection (AmBisome), vaccines for viral diseases like hepatitis A (Epaval), influenza (Inflexal V) and many others are in pipeline (Carnevale and Ko 2016; Bovier 2008; Herzog et al. 2009; Rivnay et al. 2019). But, none of these approved metal-based NPs are utilized for the treatment of bacterial infections. At present, only one liposomal NP formulation (CAL02) is going through clinical trials for the treatment of bacterial pneumonia (Anselmo and Mitragotri 2019).
13.4.1 Challenges for Antibacterial MONPs
Several challenges exist during the development of MONPs into antibacterial agents that are clinically tested and approved by EMA and/or FDA. Table 13.3 summarizes several limitations and challenges of using phyto-fabricated MONPs as antibacterial agents. Certainly, the nanoscale dimensions of metal-based NPs assist them in penetrating through the cell membrane and even in crossing of the blood–brain barrier (BBB) (Nowak et al. 2020; Tosi et al. 2020). This improves the biological activity and target specificity of the MONPs. But, the small size of the MONPs can often be a challenge during the clinical studies.
Metal-based NPs have shorter life time, low stability, and poor bioavailability under biological conditions of host cells (Schubert and Chanana 2019). Several enzymes and proteins present in the human blood attacks the MNPs and degrade them before they reach to the intended target cells (Bondarenko et al. 2020; Jain et al. 2018). To counter this drawback, various strategies to modify the MNPs have been implemented like alteration of MNPs surface chemistry, production of stable nanoemulsions of MNPs, conjugation of the MNPs with protein stabilizer (serum albumin), functionalization and improvements of the combined nanoparticles (Pagar and Darekar 2019; Guo et al. 2020; Ling et al. 2019). Moreover, the morphology and shape of the MNPs play a crucial role in their toxicity and biological actions against the bacteria (Teow et al. 2018; Ling et al. 2019). Certain nanoparticles combine together and form aggregates due to their surface chemistry. As a result, unnecessary harmfulness to the healthy cells and also considerable reduction in entrance of metal-based nanoparticles into the intended cells can be observed (Enea et al. 2019). Lethality accounts for many characteristics of metal-based nanoparticles (chemistry, stability, retention rate, specificity, and biodistribution), target cells and method of their administration (Enea et al. 2019).
13.4.2 Challenges in MONPs Production
As listed in Table 13.3, numerous setbacks exist during the production of MONPs for biomedical application (Teow et al. 2018). Because the biological roles of MONPs vastly rely on their size, shape, cell-permeability, physical and chemical properties, fabrication of MONPs at industrial scale must firmly stick to the tightly-regulated, reliable, and repeatable SOPs and GMPs. An additional challenge to MONPs research is the heterogeneous nature of several bacterial infections in humans. MONPs therapeutic studies on diseased patients are complex, since diverse patients have different immunological response although they have been infected from the same common bacterial source. Considering the huge list of potential MONPs, the demand for clinical laboratory testing facility that involves immense performance operation for physiological sorting out of the candidate MONPs also raises proportionately. This is directly related to the automation of the processes that permits cost-efficient production of MONPs, modeling and computing technologies that would assess the toxicities or efficacies of MONPs on the intended cells. Developments of immense performance computation as well as screening strategies certainly further metal oxide nanoparticles usage in biomedical research.
13.5 Conclusions and Future Outlook
The current book chapter presents the new developments of phyto-fabricated nanoparticles that act as potential antibacterial agents. The MONPs obtained using green nanotechnology methods are promising as next generation antibacterial agents and they are cable of synergistically improving the efficiency of the antibiotics. Although the precise controlling mechanism(s) remain still unidentified, a considerable research is in progress to introduce potential antibacterial MONPs in therapeutic trials. Knowledge on antibacterial mechanisms of metal-based NPs is very crucial in controlling and overcoming the growing concern of bacterial resistance to the MONPs. As outlined, several drawbacks and impending challenges have to be surmounted to maximize the usage of MONPs in clinical research. Biological properties such as homogeneity, specificity, stability, and toxicity of MONPs are the primary controlling factors that decide the MONPs scope in therapeutic trials. Considering the manufacturing aspects, growth of green nanotechnology techniques and equipments are critical for manufacturing MONPs at industrial scale amounts for the clinical purposes. New advancements in nanotechnology and biotechnology are expected to further the usage of phyto-fabricated metal oxide-based nanoparticles as antibacterial agents in clinical and biomedical industries. Very few reports exist on green nanotechnology that uses plants as a chief source for the production of MONPs. In this study, brief background on green nanotechnology methods for the phyto-fabrication of MONPs, the controlling antibacterial mechanisms of MONPs, challenges in the development and future prospective of MONPs as antibacterial agents were discussed.
References
Acharya, D., Singha, K. M., Pandey, P., Mohanta, B., Rajkumari, J., & Singha, L. P. (2018). Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria. Scientific Reports, 8, 201.
Ahmed, S., Ahmad, M., Swami, B. L., & Ikram, S. (2016a). A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. Journal of Advanced Research, 7, 17–28.
Ahmed, S., Saifullah, Ahmad, M., Swami, B. L., & Ikram, S. (2016b). Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. Journal of Radiation Research and Applied Science, 9, 1–7.
Akbari, A., Amini, M., Tarassoli, A., Eftekhari-Sis, B., Ghasemian, N., & Jabbari, E. (2018). Transition metal oxide nanoparticles as efficient catalysts in oxidation reactions. Nano-Struct Nano-Object, 14, 19–48.
Ali, K., Dwivedi, S., Azam, A., Saquib, Q., Al-Said, M. S., Alkhedhairy, A. A., & Musarrat, J. (2016). Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolate. Journal of Colloid and Interface Science, 472, 145–156.
Ali, M., Ahmed, T., Wu, W., Hossain, A., Hafeez, R., Islam Masum, M., Wang, Y., An, Q., Sun, G., & Li, B. (2020). Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials, 10(6), 1146.
Allahverdiyev, A. M., Abamor, E. S., Bagirova, M., & Rafailovich, M. (2011). Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiology, 6, 933–940.
Anselmo, A. C., & Mitragotri, S. (2019). Nanoparticles in the clinic: An update. Bioengineering & Translational Medicine, 4(3), e10143.
Azizi, S., Mohamad, R., & Mahdavi Shahri, M. (2017). Green microwave-assisted combustion synthesis of zinc oxide nanoparticles with Citrullus colocynthis L. schrad: Characterization and biomedical applications. Molecules, 22, E301.
Baker, S., Pasha, A., & Satish, S. (2017). Biogenic nanoparticles bearing antibacterial activity and their synergistic effect with broad spectrum antibiotics: Emerging strategy to combat drug resistant pathogens. Saudi Pharmaceutical Journal, 25, 44–51.
Banumathi, B., Vaseeharan, B., Ishwarya, R., Govindarajan, M., Alharbi, N. S., Kadaikunnan, S., Khaled, J. M., & Benelli, G. (2017). Toxicity of herbal extracts used in ethno-veterinary medicine and green-encapsulated ZnO nanoparticles against Aedes aegypti and microbial pathogens. Parasitology Research, 116, 1637–1651.
Bondarenko, L. S., Kovel, E. S., Kydralieva, K. A., Dzhardimalieva, G. I., Illés, E., Tombácz, E., Kicheeva, A. G., & Kudryasheva, N. S. (2020). Effects of modified magnetite nanoparticles on bacterial cells and enzyme reactions. Nanomaterials, 10(8), 1499.
Bovier, P. A. (2008). Epaxal®: A virosomal vaccine to prevent hepatitis A infection. Expert Review of Vaccines, 7, 1141–1150.
Cagno, V., Andreozzi, P., D’Alicarnasso, M., Silva, P. J., Mueller, M., Galloux, M., Le Goffic, R., Jones, S. T., Vallino, M., Hodek, J., & Weber, J. (2018). Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nature Materials, 17(2), 95–203.
Carnevale, J., & Ko, A. H. (2016). MM-398 (nanoliposomal irinotecan): Emergence of a novel therapy for the treatment of advanced pancreatic cancer. Future Oncology, 12, 453–464.
Chen, Q., Tong, R., Chen, X., Xue, Y., Xie, Z., Kuang, Q., & Zheng, L. (2018). Ultrafine ZnO quantum dot-modified TiO2 composite photocatalysts: The role of the quantum size effect in heterojunction-enhanced photocatalytic hydrogen evolution. Catalysis Science & Technology, 8(5), 1296–1303.
Das, B., Dash, S. K., Mandal, D., Ghosh, T., Chattopadhyay, S., Tripathy, S., Das, S., Dey, S. K., Das, D., & Roy, S. (2017). Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arabian Journal of Chemistry, 10, 862–876.
Dauthal, P., & Mukhopadhyay, M. (2016). Noble metal nanoparticles: Plant-mediated synthesis, mechanistic aspects of synthesis, and applications. Industrial and Engineering Chemistry Research, 55, 9557–9577.
Deng, H., McShan, D., Zhang, Y., Sinha, S. S., Arslan, Z., Ray, P. C., & Yu, H. (2016). Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environmental Science & Technology, 50, 8840–8848.
Dizaj, S. M., Lotfipour, F., Barzegar-Jalali, M., Zarrintan, M. H., & Adibkia, K. (2014). Antimicrobial activity of the metals and metal oxide nanoparticles. Materials Science and Engineering: C, 44, 278–284.
El Shafey, A. M. (2020). Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Processing and Synthesis, 9(1), 304–339.
Elemike, E. E., Onwudiwe, D. C., Ekennia, A. C., Sonde, C. U., & Ehiri, R. C. (2017). Green synthesis of Ag/Ag2O nanoparticles using aqueous leaf extract of Eupatorium odoratum and its antimicrobial and mosquito larvicidal activities. Molecules, 22, E674.
Enea, M., Peixoto de Almeida, M., Eaton, P., Dias da Silva, D., Pereira, E., Soares, M. E., Bastos, M. D., & Carmo, H. (2019). A multiparametric study of gold nanoparticles cytotoxicity, internalization and permeability using an in vitro model of blood–brain barrier. Influence of size, shape and capping agent. Nanotoxicology, 13(7), 990–1004.
Ezhilarasi, A. A., Vijaya, J. J., Kaviyarasu, K., Maaza, M., Ayeshamariam, A., & Kennedy, L. J. (2016). Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: Cytotoxicity effect of nanoparticles against HT-29 cancer cells. Journal of Photochemistry and Photobiology B: Biology, 164, 352–360.
Flor, J., de Lima, S. M., & Davolos, M. R. (2004). Effect of reaction time on the particle size of ZnO and ZnO:Ce obtained by a Sol–gel method. In Surface and colloid science (pp. 239–243). Berlin: Springer.
Fratoddi, I. (2018). Hydrophobic and hydrophilic Au and Ag nanoparticles. Breakthroughs and perspectives. Nanomaterials, 8(1), 11.
Gautam, P. K., Singh, A., Misra, K., Sahoo, A. K., & Samanta, S. K. (2019). Synthesis and applications of biogenic nanomaterials in drinking and wastewater treatment. Journal of Environmental Management, 231, 734–748.
Guo, X., Wei, X., Chen, Z., Zhang, X., Yang, G., & Zhou, S. (2020). Multifunctional nanoplatforms for subcellular delivery of drugs in cancer therapy. Progress in Materials Science, 107, 100599.
Gupta, A., Saleh, N. M., Das, R., Landis, R. F., Bigdeli, A., Motamedchaboki, K., Campos, A. R., Pomeroy, K., Mahmoudi, M., & Rotello, V. M. (2017). Synergistic antimicrobial therapy using nanoparticles and antibiotics for the treatment of multidrug-resistant bacterial infection. Nano Futures, 1, 015004.
Guzman, M., Dille, J., & Godet, S. (2012). Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomedicine: Nanotechnology, Biology and Medicine, 8, 37–45.
Han, X., Xu, K., Taratula, O., & Farsad, K. (2019). Applications of nanoparticles in biomedical imaging. Nanoscale, 11(3), 799–819.
Happy, A., Soumya, M., Kumar, S. V., Rajeshkumar, S., Sheba, R. D., Lakshmi, T., & Nallaswamy, V. D. (2019). Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochemistry and Biophysics Reports, 17, 208–211.
Hasan, M., Ullah, I., Zulfiqar, H., Naeem, K., Iqbal, A., Gul, H., Ashfaq, M., & Mahmood, N. (2018). Biological entities as chemical reactors for synthesis of nanomaterials: Progress, challenges and future perspective. Mater Today Chemistry, 8, 13–28.
Herzog, C., Hartmann, K., Künzi, V., Kürsteiner, O., Mischler, R., Lazar, H., & Glück, R. (2009). Eleven years of inflexal® V—A virosomal adjuvanted influenza vaccine. Vaccine, 27, 4381–4387.
Hwang, I. S., Hwang, J. H., Choi, H., Kim, K. J., & Lee, D. G. (2012). Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. Journal of Medical Microbiology, 61, 1719–1726.
Irshad, R., Tahir, K., Li, B., Ahmad, A., Siddiqui, A. R., & Nazir, S. (2017). Antibacterial activity of biochemically capped iron oxide nanoparticles: A view towards green chemistry. Journal of Photochemistry and Photobiology B: Biology, 170, 241–246.
Jadhav, K., Dhamecha, D., Bhattacharya, D., & Patil, M. (2016). Green and ecofriendly synthesis of silver nanoparticles: Characterization, biocompatibility studies and gel formulation for treatment of infections in burns. Journal of Photochemistry and Photobiology B: Biology, 155, 109–115.
Jafarirad, S., Mehrabi, M., Divband, B., & Kosari-Nasab, M. (2016). Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: A mechanistic approach. Materials Science & Engineering. C, Materials for Biological Applications, 59, 296–302.
Jain, A., Singh, S. K., Arya, S. K., Kundu, S. C., & Kapoor, S. (2018). Protein nanoparticles: Promising platforms for drug delivery applications. ACS Biomaterials Science & Engineering, 4(12), 3939–3961.
Jamkhande, P. G., Ghule, N. W., Bamer, A. H., & Kalaskar, M. G. (2019). Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. Journal of Drug Delivery Science and Technology, 53, 101174.
Jose, J., Kumar, R., Harilal, S., Mathew, G. E., Prabhu, A., Uddin, M. S., Aleya, L., Kim, H., & Mathew, B. (2020). Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environmental Science and Pollution Research, 27(16), 19214–19225.
Kailasa, S. K., Park, T. J., Rohit, J. V., & Koduru, J. R. (2019). Antimicrobial activity of silver nanoparticles. Nanoparticles in Pharmacotherapy, 1, 461–484.
Kalita, S., Kandimalla, R., Sharma, K. K., Kataki, A. C., Deka, M., & Kotoky, J. (2016). Amoxicilin functionalized gold nanoparticles reverts MRSA resistance. Materials Science & Engineering. C, Materials for Biological Applications, 61, 720–727.
Kanjikar, A. P., Hugar, A. L., & Londonkar, R. L. (2018). Characterization of phyto-nanoparticles from Ficus krishnae for their antibacterial and anticancer activities. Drug Development and Industrial Pharmacy, 44, 377–384.
Khatami, M., Alijani, H. Q., & Sharifi, I. (2018). Biosynthesis of bimetallic and core–shell nanoparticles: Their biomedical applications—A review. IET Nanobiotechnology, 12(7), 879–887.
Liao, S., Zhang, Y., Pan, X., Zhu, F., Jiang, C., Liu, Q., Cheng, Z., Dai, G., Wu, G., Wang, L., & Chen, L. (2019). Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. International Journal of Nanomedicine, 14, 1469–1487.
Ling, W., Wang, M., Xiong, C., Xie, D., Chen, Q., Chu, X., Qiu, X., Li, Y., & Xiong, X. (2019). Synthesis, surface modification, and applications of magnetic iron oxide nanoparticles. Journal of Materials Research and Technology, 34(11), 1828–1844.
Liu, D., Hong, Y., Li, Y., Hu, C., Yip, T. C., Yu, W. K., Zhu, Y., Fong, C. C., Wang, W., Au, S. K., & Wang, S. (2020). Targeted destruction of cancer stem cells using multifunctional magnetic nanoparticles that enable combined hyperthermia and chemotherapy. Theranostics, 10(3), 1181.
Manikandan, V., Velmurugan, P., Park, J. H., Chang, W. S., Park, Y. J., Jayanthi, P., Cho, M., & Oh, B. T. (2017). Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3 Biotech, 7(1), 72.
Maqbool, Q., Nazar, M., Naz, S., Hussain, T., Jabeen, N., Kausar, R., Anwaar, S., Abbas, F., & Jan, T. (2016). Antimicrobial potential of green synthesized CeO2 nanoparticles from Olea europaea leaf extract. International Journal of Nanomedicine, 11, 5015–5025.
Mohammadinejad, R., Shavandi, A., Raie, D. S., Sangeetha, J., Soleimani, M., Hajibehzad, S. S., Thangadurai, D., Hospet, R., Popoola, J. O., Arzani, A., & Gómez-Lim, M. A. (2019). Plant molecular farming: Production of metallic nanoparticles and therapeutic proteins using green factories. Green Chemistry, 21, 1845–1865.
Nadaroglu, H., Onem, H., & Gungor, A. A. (2017). Green synthesis of Ce2O3 NPs and determination of its antioxidant activity. IET Nanobiotechnology, 11, 411–419.
NCBI (National Center for Biotechnology Information). (2018). Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. Retrieved from https://www.ncbi.nlm.nih.gov/.
Nowak, M., Brown, T. D., Graham, A., Helgeson, M. E., & Mitragotri, S. (2020). Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow. Bioengineering & Translational Medicine, 5(2), e10153.
Ovington, L. G. (2004). The truth about silver. Ostomy/Wound Management, 50, 1S–10S.
Pagar, K. R., & Darekar, A. B. (2019). Nanoemulsion: A new concept of delivery system. Asian Journal of Research in Pharmaceutical Sciences, 9(1), 39–46.
Raffi, M., Hussain, F., Bhatti, T. M., Akhter, J. I., Hameed, A., & Hasan, M. M. (2008). Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. Journal of Materials Science and Technology, 24, 192–196.
Ramesh, P. S., Kokila, T., & Geetha, D. (2015). Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 142, 339–343.
Reidy, B., Haase, A., Luch, A., Dawson, K. A., & Lynch, I. (2013). Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials, 6, 2295–2350.
Rezaie, A. B., Montazer, M., & Rad, M. M. (2017). Photo and biocatalytic activities along with UV protection properties on polyester fabric through green in-situ synthesis of cauliflower-like CuO nanoparticles. Journal of Photochemistry and Photobiology B: Biology, 176, 100–111.
Rivnay, B., Wakim, J., Avery, K., Petrochenko, P., Myung, J. H., Kozak, D., Yoon, S., Landrau, N., & Nivorozhkin, A. (2019). Critical process parameters in manufacturing of liposomal formulations of amphotericin B. International Journal of Pharmaceutics, 565, 447–457.
Saleem, S., Ahmed, B., Khan, M. S., Al-Shaeri, M., & Musarrat, J. (2017). Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microbial Pathogenesis, 111, 375–387.
Sánchez-López, E., Gomes, D., Esteruelas, G., Bonilla, L., Lopez-Machado, A. L., Galindo, R., Cano, A., Espina, M., Ettcheto, M., Camins, A., & Silva, A. M. (2020). Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials, 10, 292.
Santhoshkumar, J., Kumar, S. V., & Rajeshkumar, S. (2017). Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resource-Efficient Technologies, 3, 459–465.
Saratale, R. G., Saratale, G. D., Shin, H. S., Jacob, J. M., Pugazhendhi, A., Bhaisare, M., & Kumar, G. (2018). New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environmental Science and Pollution Research, 25(11), 10164–10183.
Schubert, J., & Chanana, M. (2019). Coating matters: Review on colloidal stability of nanoparticles with biocompatible coatings in biological media, living cells and organisms. Current Medicinal Chemistry, 25(35), 4556.
Shakil, M. S., Hasan, M., & Sarker, S. R. (2019). Iron oxide nanoparticles for breast cancer theranostics. Current Drug Metabolism, 20(6), 446–456.
Singh, J., Dutta, T., Kim, K. H., Rawat, M., Samddar, P., & Kumar, P. (2018). ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. Journal of Nanbiotechnology, 16(1), 84.
Singh, A., Gautam, P. K., Verma, A., Singh, V., Shivapriya, P. M., Shivalkar, S., Sahoo, A. K., & Samanta, S. K. (2020). Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnology Reports, 25, e00427.
Sintubin, L., De Gusseme, B., Van der Meeren, P., Pycke, B. F., Verstraete, W., & Boon, N. (2011). The antibacterial activity of biogenic silver and its mode of action. Applied Microbiology and Biotechnology, 91, 153–162.
Sneha, K., Sathishkumar, M., Kim, S., & Yun, Y. S. (2010). Counter ions and temperature incorporated tailoring of biogenic gold nanoparticles. Process Biochemistry, 45, 1450–1458.
Soni, M., Mehta, P., Soni, A., & Goswami, G. K. (2018). Green nanoparticles: Synthesis and applications. IOSR Journal of Biotechnology and Biochemistry, 4, 78–83.
Suman, T. Y., Rajasree, S. R., Ramkumar, R., Rajthilak, C., & Perumal, P. (2014). The green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 118, 11–16.
Surendra, T. V., & Roopan, S. M. (2016). Photocatalytic and antibacterial properties of photosynthesized CeO2 NPs using Moringa oleifera peel extract. Journal of Photochemistry and Photobiology B: Biology, 161, 122–128.
Swamy, M. K., Sudipta, K. M., Jayanta, K., & Balasubramanya, S. (2015). The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulate leaf extract. Applied Nanoscience, 5, 73–81.
Teow, S. Y., Wong, M. M., Yap, H. Y., Peh, S. C., & Shameli, K. (2018). Bactericidal properties of plants-derived metal and metal oxide nanoparticles (NPs). Molecules, 23(6), 1366.
Thatoi, P., Kerry, R. G., Gouda, S., Das, G., Pramanik, K., Thatoi, H., & Patra, J. K. (2016). Photo-mediated green synthesis of silver and zinc oxide nanoparticles using aqueous extracts of two mangrove plant species, Heritiera fomes and Sonneratia apetala and investigation of their biomedical applications. Journal of Photochemistry and Photobiology B: Biology, 163, 311–318.
Tippayawat, P., Phromviyo, N., Boueroy, P., & Chompoosor, A. (2016). Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ, 4, e2589.
Tosi, G., Duskey, J. T., & Kreuter, J. (2020). Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opinion on Drug Discovery, 17(1), 23–32.
Velmurugan, P., Park, J. H., Lee, S. M., Yi, Y. J., Cho, M., Jang, J. S., Myung, H., Bang, K. S., & Oh, B. T. (2016). Eco-friendly approach towards green synthesis of zinc oxide nanocrystals and its potential applications. Artificial Cells, Nanomedicine, and Biotechnology, 44, 1537–1543.
Vijayakumar, S., Vaseeharan, B., Malaikozhundan, B., & Shobiya, M. (2016). Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomedicine & Pharmacotherapy, 84, 1213–1222.
Wadhawan, A., Chatterjee, M., & Singh, G. (2019). Present scenario of bioconjugates in cancer therapy: A review. International Journal of Molecular Sciences, 20(21), 5243.
Wu, T., & Tang, M. (2018). Review of the effects of manufactured nanoparticles on mammalian target organs. Journal of Applied Toxicology, 38(1), 25–40.
Zgurskaya, H. I., Lopez, C. A., & Gnanakaran, S. (2015). Permeability barrier of gram-negative cell envelopes and approaches to bypass it. ACS Infectious Diseases, 1, 512–522.
Zhu, N., Ji, H., Yu, P., Niu, J., Farooq, M. U., Akram, M. W., Udego, I. O., Li, H., & Niu, X. (2018). Surface modification of magnetic iron oxide nanoparticles. Nanomaterials, 8(10), 810.
Acknowledgments
The author would like to thank Department of Materials Science at Central University of Tamil Nadu for providing the necessary facilities to complete the work. No external financial support was received for carrying out the current project.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The work is original investigation by the author and there are no ethical issues.
Conflict of Interest
The author declares that there is no conflict of interest.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bogala, M.R. (2021). Phyto-fabricated Metal Oxide Nanoparticles as Promising Antibacterial Agents. In: Pal, K. (eds) Bio-manufactured Nanomaterials. Springer, Cham. https://doi.org/10.1007/978-3-030-67223-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-67223-2_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67222-5
Online ISBN: 978-3-030-67223-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)