What You Will Learn in This Chapter
One of the fast growing 3D mammalian cell culture platforms are hydrogels—three-dimensional, crosslinked networks of polymers. In this chapter we describe the fundamentals of hydrogels and provide an overview of sources from which hydrogels can be derived, such as animal, non-animal, synthetic, or combinations. The physical/mechanical requirements of hydrogels are discussed in order that they produce a physiologically relevant environment for 3D cell cultivation. We review the characterization methods used for hydrogels and how this impacts application in 3D cell culture and regenerative medicine. Modification of hydrogels by crosslinking affords them the property of tunability and degradation to recover cells for downstream analysis provides an opportunity to gather additional supportive data, these areas are examined along with the physical properties needed for optimum use in cell monitoring and analysis techniques. Creation of gradient hydrogels and incorporation into microfluidic and organ-on-a-chip models opens the possibility to recapitulate more closely the in vivo environment. We cover the areas in which hydrogels are being applied to support 3D cell culture such as bioprinting and bioprocessing and discuss the future potential of these versatile materials in taking cell research from the bench to the clinic.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara What You Will Learn in This ChapterOne of the fast growing 3D mammalian cell culture platforms are hydrogels—three-dimensional, crosslinked networks of polymers. In this chapter we describe the fundamentals of hydrogels and provide an overview of sources from which hydrogels can be derived, such as animal, non-animal, synthetic, or combinations. The physical/mechanical requirements of hydrogels are discussed in order that they produce a physiologically relevant environment for 3D cell cultivation. We review the characterization methods used for hydrogels and how this impacts application in 3D cell culture and regenerative medicine. Modification of hydrogels by crosslinking affords them the property of tunability and degradation to recover cells for downstream analysis provides an opportunity to gather additional supportive data, these areas are examined along with the physical properties needed for optimum use in cell monitoring and analysis techniques. Creation of gradient hydrogels and incorporation into microfluidic and organ-on-a-chip models opens the possibility to recapitulate more closely the in vivo environment. We cover the areas in which hydrogels are being applied to support 3D cell culture such as bioprinting and bioprocessing and discuss the future potential of these versatile materials in taking cell research from the bench to the clinic.
5.1 Introduction: What Is a Hydrogel?
Hydrogels are three-dimensional network structures permeable to oxygen and nutrients and capable of taking on large amounts of water, which make them attractive for use in biological applications. Presence of chemical or physical crosslinks and/or chain entanglements typically prevents the hydrogel from dissolving, therefore retaining structure and stiffness. They can be manufactured synthetically or extracted from natural sources, e.g. collagen, gelatin, alginate, and nanofibrillar cellulose. When used for 3D cell culture the hydrogel properties can be adapted to match the specific use, important as different body tissues have different physical and biochemical requirements. The inherent and versatile properties of hydrogels have seen them used in many applications including controlled drug delivery systems, biosensors, tissue engineering scaffolds, artificial organs, wound healing bandages, physiological membranes, contact lenses, and microfluidic valves.
In cell culture hydrogels were initially used to coat tissue culture vessels providing a 2.5D environment for adherent cell growth. With a drive to bridge the gap between in vitro and in vivo conditions there has been a shift away from the 2D model toward 3D to create more human relevant data. Hydrogels have been the natural choice for development of these new 3D cell culture systems.
5.2 Hydrogel Classification
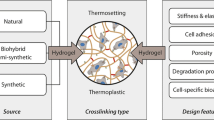
There are different categories into which hydrogels can be classified: (1) structural composition (e.g., homopolymers, copolymers, or interpenetrating networks), (2) origin/source of polymers (e.g., natural, semi-synthetic, or synthetic hydrogels), (3) crosslinking (e.g., photo, physically or chemically crosslinked hydrogels), (4) responsiveness to stimuli (e.g., temperature-responsiveness and pH-responsiveness), (5) molecular charge (cationic, anionic, neutral, ampholytic), and (6) crosslinking reversibility (reversibly or irreversibly crosslinked hydrogels) [1,2,3] (Fig. 5.1). Additionally, hydrogels can be classified according to their design features (physical, biological, or mass-transport design features) [3].
5.2.1 Structural Composition
Classification by structural composition divides hydrogels into homopolymeric (derived from the single monomer species), copolymeric (derived from two or more monomer species), and multipolymeric (also called interpenetrating networks), derived from two independent crosslinked polymers, where at least one of them being synthesized and/or crosslinked within the immediate presence of the other and without any covalent bonds between them [4].
The most widely used homopolymeric hydrogels in 3D mammalian cell culture are collagen [5], fibrin [6], and nanofibrillar cellulose [7]. Gelatin methacryloyl (GelMA) [8, 9] and PEG-fibrinogen [10, 11] hydrogels are macromonomeric homopolymer hydrogels. Copolymeric hydrogels suitable for 3D cell culture are represented by alginates [12], hyaluronic acid and poly(N-isopropolyacrylamide) hydrogels [13], PEG-based copolymers (PEGMEMA–MEO2MA–PEGDA) [14], and synthetic saccharide−peptide hydrogels [15]. Some examples of multipolymeric hydrogels are networks of dextran and gelatin [16], gelatin and silk fibroin [17], or alginate and reconstituted basement membrane matrix hydrogels [18].
5.2.2 Origin of Polymers
Natural hydrogels are made of polysaccharides (alginate, agarose, glucan, hyaluronic acid, nanocellulose, and chitosan) or proteins (collagen, albumin, fibrin, and silk proteins), derived by extractions from biological sources. Collagen, fibrin, and hyaluronic acid are natural constituents of the ECM , while alginate and agarose are derived from marine algae. Another new natural source of hydrogels is nanofibrillar cellulose, which is extracted from wood [19]. The major advantage of natural hydrogels is their biocompatibility and closest proximity to the in vivo cell microenvironment . Animal ECM-derived hydrogels perfectly support cell adhesion, while hydrogels of non-animal (non-human) origin are readily available and avoid possible viral contamination. There are a few notable disadvantages associated with animal derived hydrogels, such as batch-to-batch variation and lower tunability [20].
Synthetic hydrogels include, for example, poly(ethylene glycol) (PEG), poly(acrylic acid) (PAA), poly(ethylene oxide) (PEO), poly(vinyl alcohol) (PVA), poly(hydroxyethyl methacrylate) (PHEMA), and poly(methacrylic acid) (PMMA) [20, 21]. Synthetic structures of such hydrogels offer no biological information to cells, but can be easily tuned according to the mechanical (viscoelastic) requirements and have high uniform quality as well as defined structure [3, 22]. The choice of the hydrogel is dependent on the experimental setup (i.e., required stiffness, optical properties, conductive properties), material availability, and cost. There is no shortage of materials to evaluate for application development, given the great variety of natural and synthetic hydrogels available for 3D cell culture [23].
Semi-synthetic biohybrid hydrogels combine the best properties of both the abovementioned hydrogels. The resulting hydrogels have highly defined technical (and mechanical) properties and at the same time offer biological information to cells. Semi-synthetic hydrogels are often produced in co-polymerization reactions between the polymer precursor and the biological conjugate. Some examples of such combinations are hydrogels which consist of PEG (which is inert to cells) modified with peptide sequences (e.g., RGD) for cell adhesion [24] or PEG modified with fibrinogen [10]. Another example of biohybrid hydrogels is GelMA—here, cell promoting gelatin is derivatized with methacrylamide and methacrylate groups, which provide the hydrogel with shape fidelity and stability at physiological temperature [8].
5.2.3 Crosslinking
The crosslinks between individual polymer molecules maintain the entire 3D structure of the hydrogel after swelling in water. For use in 3D cell culture (especially in the case of cell encapsulation prior polymerization) not only the polymer material, but also crosslinking reaction must be cell-friendly, which means that reaction conditions, substrates, and products should not affect cell viability. One of the widely used strategies is crosslinking with visible or UV light through photopolymerization of acrylate or methacrylate-modified hydrogel polymers. Here, photo-initiator is used to create free radicals which attack the vinyl groups of precursor molecules, resulting in covalent crosslinking of the hydrogel within seconds or minutes upon irradiation [8, 25]. Some hydrogels can be crosslinked by physical methods, such as ionic crosslinking of alginate [26], thermally induced gelation [27], or self-assembling amphiphiles [28]. Hydrogels can be covalently crosslinked in polymerization reactions (see also Chap. 5 “Biological, natural and synthetic 3D matrices”), which involve gentle chemistries under physiological conditions (bio-orthogonal chemistry). Hydrogels can be also crosslinked enzymatically. Here, transglutaminase is widely used to crosslink peptide-functionalized hydrogel materials [29, 30].
Choosing the polymerization strategy of the hydrogel for use in 3D cell culture, the researcher must also take into account the time of polymerization (gelation kinetics)—some polymerization reactions are too fast to ensure an even cell distribution and some are too slow, so that cells sediment to the bottom of the construct before complete polymerization.
5.2.4 Stimuli-Responsive Hydrogels
Although 3D structure of hydrogels brings in vitro cell culture closer to the physiological in vivo conditions, static materials cannot fully mimic the dynamicity of native microenvironment [31]. Stimuli-responsive hydrogels can change their physical and chemical properties depending on the external stimuli, which makes them an important tool for basic research and biomedical applications. Depending on the ability to react to these stimuli, hydrogels can be divided into pH-responsive [32], temperature-responsive [33], light/photo-responsive [34], and electric field-responsive hydrogels [35]. These hydrogels can provide cells with irreversible or reversible spatiotemporal modulation of cues, directing cell behavior [31].
5.2.5 Molecular Charge and Reversibility of Crosslinking
Finally, hydrogels can be sorted by molecular charge and reversibility of crosslinking. Bilayer phospholipid membranes of cells are negatively charged and positively charged cationic hydrogels can facilitate cell attachment [36]. Anionic hydrogels have been shown to induce formation of the bone mineral hydroxyapatite by the cells [37] and can be used as a bone regeneration matrix [38].
Reversibility of crosslinking plays an important role in cell recovery and analysis. If hydrogels are crosslinked chemically the junction points are usually permanent covalent bonds [39]. If such hydrogels cannot be degraded by the cells, it limits cell spreading and migration. Hydrogels crosslinked physically are usually reversible. So, alginates, for example, can be easily dissociated by calcium chelators (e.g., EDTA and sodium citrate).
5.3 Physical Requirements for Cell Culture
The extracellular matrix (ECM) surrounds most cells in tissues of complex organisms, protecting them from stress and regulating cellular functions such as spreading, migration, proliferation, and stem cell differentiation. Stiffness of the ECM is considered to have implications for development, differentiation, disease, and regeneration [40].
In Fig. 5.2 the relationship between ECM stiffness and cell type is depicted. There is a large variation in the in vivo ECM environment with neural cells at the softer end and cartilage and bone cells at the stiffer end of the range. Studies have shown that by adjusting the stiffness of the matrix rather than making changes just to the biochemical environment (i.e., use of growth factors or defined media) then directed differentiation can be achieved. Matching the stiffness of the hydrogel to the tissue is of interest particularly when targeting MSC fates, since MSCs (and numerous other cell types) can convert external mechanical clues to intracellular biochemical signals. This ability to sense mechanical microenvironment called mechanosensing is described in several studies and reviews [41, 42]. Those MSCs cultured in lower stiffness hydrogel (2 kPa) show a tendency to differentiate toward cells expressing neural markers; those cultured in hydrogel with a kPa of 10 formed myocytes and those cultured on rigid substrates (40 kPa) become osteoblasts [43].
There are many examples of studies where matrix stiffness has been shown to play a role in cell development, migration or differentiation, for example, neural cells, MSC differentiation, muscle cells, breast cancer cells, and bone [43,44,45,46,47].
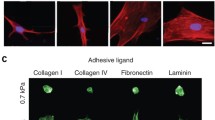
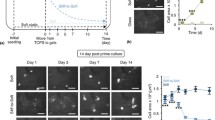
Anchorage-dependent cells are highly responsive to hydrogel properties (stiffness and pore structure) and encapsulated cells demonstrate higher spreading in low stiffness hydrogels and no spreading by high stiffness [8, 48, 49]. So, MSCs show good spreading already starting on day 1 after encapsulation in Gelatin-Methacryloyl (GelMA) hydrogel with a low degree of functionalization (final stiffness 24 Pa) and no spreading in the same hydrogel with stiffness of 1537 Pa (Fig. 5.3 and supplementary Video 1).
Microscopic analysis of hAD-MSCs and HUVECs co-culture after 3 days of cultivation in GelMA hydrogels with stiffness of 24 Pa and 1537 Pa: (a) Confocal microscopy pictures (green—hAD-MSCs, red—HUVECs), (b) microphotographs, (c) screenshot of time-lapse, and (d) QR code for time-lapse video of hAD-MSCs spreading
It should be noted that if the pore size within the hydrogel is too small or the hydrogel cannot be proteolytically degraded by the cells, anchorage-dependent cells will not survive long. In addition, stability of the hydrogel is of importance as the matrix needs to be able to withstand standard cell culture operations, such as transfer to and from the microscope and media change without loss or degradation for the duration of the experiment. Ideally the best case would be a slowly biodegradable hydrogel which can be replaced by de novo formed ECM .
5.4 Material Characterization
Precise control of hydrogel properties belongs to the essential routines of hydrogel-based 3D cell culture. As already mentioned, most cells are sensitive to the mechanical microenvironment and knowledge as well as control of the mechanical properties of a hydrogel , like stiffness or viscoelasticity, plays a crucial role in the establishment of desired cultivation conditions. Hydrogel mechanical properties and polymerization dynamics (gelation) can be characterized using rheology [3]. Using only relatively small sample volumes (100–1000 μL), modern rheometers can quickly and sensitively measure the mechanical properties of hydrogels. The hydrogel is placed between parallel plates (alternatively cone-plate or concentric cylinders) and torsional oscillation generates shear flow in the sample (Fig. 5.4). Protocols for the rheological characterization of hydrogels and different sweep experiments are well-established and described [50]. Time sweep experiments determine the gelation time of hydrogels, strain sweep experiments measure the linear viscoelastic region of the hydrogel in dependency to the applied strain. Frequency sweep experiments determine the linear modulus plateau of the hydrogel . Rheometers are available from various manufacturers (Anton Paar, TA Instruments, Malvern or Thermo Fisher). Typical equipment for hydrogel characterization is a rotational- and oscillatory rheometer with parallel plate geometry, Peltier-element (for precise temperature settings), and a UV-curing system (for UV photo-crosslinkable hydrogels). Precise temperature settings are crucial for characterization of hydrogels with crosslinking via temperature transition or enzymatic crosslinking [51]. Temperature transition, enzymatic or photo-crosslinking reactions trigger the hydrogel development from its original liquid state to its fully polymerized state (sol-gel transition). Major viscoelastic properties of hydrogels are the storage modulus (G’), which measures the stiffness, and the loss modulus (G”), reflecting the hydrogel viscosity [52]. Taken together, G’ and G” represent the shear modulus G of a hydrogel , according to Eq. (5.1):
Another important characteristic of hydrogels is their swelling behavior. Hydrogel swelling characteristics influence the materials’ mechanical properties, shape fidelity and diffusion of nutrients, and depend on crosslinking density, hydrophilicity of the polymer, and interactions with medium or other solvent [53]. For determination of swelling, the polymerized sample is placed into solvent/medium for 24 h until equilibrium, weighed, freeze-dried, and re-weighed again. The mass-swelling ratio is calculated as the ratio of swollen hydrogel mass to the mass of dry material [54]. The swelling degree of hydrogels is usually inversely proportional to the hydrogel concentration and degree of crosslinking—the higher the crosslinking, the lower the swelling.
Structural characteristics of hydrogels can be evaluated by several techniques: scanning electron microscopy (SEM), cryosectioning, or confocal microscopy of fluorescently stained hydrogels. SEM micrographs of the three-dimensional polymer network and the pores of hydrogels provide information about morphological structure and pore architecture—here the effect of modifications can be estimated on the hydrogel pores [55]. For SEM, hydrogels are usually first swollen, then frozen in liquid nitrogen, freeze-dried, and sputtered with gold prior to the observation. For cryosectioning, hydrogels are frozen in the optimal cutting temperature compound (Tissue-Tek®) and sections are prepared and collected on slides using cryostat [56]. Additionally, atomic force microscopy (AFM) is used for high-resolution characterization of hydrogel topography, as well as for probing the elastic modulus (elastic moduli map), disclosing the surface roughness and stiffness of the hydrogel constructs [57].
5.5 Gradient Hydrogels
Oxford dictionary defines gradients as “an increase or decrease in the magnitude of a property (e.g., temperature, pressure, or concentration) observed in passing from one point or moment to another” (https://www.lexico.com/definition/gradient). In vivo, gradients are the essential part of all living organisms, beginning already in the early embryogenesis as the gradient distribution of the transcriptional factors. In all multicellular organisms (or colonies of unicellular organisms) gradients of different nature and temporal resolution can be found. Gradients can be stable or transitional, physiological or pathological. By the nature, gradients can be classified as physical, chemical, and biological (Fig. 5.5). Stable physiological mechanical gradients can be found in different tissues like cartilage, ligaments, tendon, bone, and tooth [58,59,60]. Stable chemical physiological gradients (oxygen concentrations, pH gradient as a result of catabolite distribution) go along the increasing distances from blood vessels [61]. Stable pathological gradients (chemical, physical, and biological) can be found in tumors, where fast growing cellular mass breaks tissue homeostasis [62, 63]. Transitional physiological gradients direct embryonic development, growth of blood vessels, and tissues [64,65,66]. Transitional pathological gradients are present in wounds, scars, during mineralization of the artery walls or fibrogenesis in kidney [59, 67, 68].
Cellular fate is strongly influenced by the composition of the tissue microenvironment and most cell types sense physical, chemical, and biological characteristics of their external microenvironment and convert them to intracellular biochemical signals. The influence of different microenvironmental signals, alone or in combination with each other, can be studied in hydrogel-based 3D cultivation systems. The above discussed tunable properties of hydrogels allow not only creation of desired in situ mechanical, biological, and architectural microenvironments, but also give the opportunities to create gradients inside of the bulk hydrogel constructs. Fabrication of gradients in hydrogels (1) enables the recapitulation of in vivo gradients and (2) can help to find an optimal niche for different cell types and co-cultures [69]. Similarly to the in vivo gradients, gradient hydrogels can be divided into three major groups: physical (mechanical properties of material), biological (bioactive molecules incorporation), and chemical (material composition) gradient hydrogels (Fig. 5.6) [69, 70]. Gradients in hydrogels can be continuous or stepped. By profiles gradients are divided into linear, radial, exponential, or sigmoidal [70].
There are many different methods to create gradient hydrogels, some of them are presented in Fig. 5.7. Mechanical (hydrogel stiffness and pore architecture) gradients can be created by two main strategies: (1) variation of crosslinker concentration in the pre-polymer solution and (2) variation of polymerization intensity. Variation of crosslinker concentration can be made by dynamic mixing with the help of two-syringe pump system [49, 71, 72], microfluidic techniques [73,74,75,76], or limited mixing in the Hele-Shaw cell device [77] (Fig. 5.7). Variation of polymerization can be created (in the case of photopolymerized hydrogels) by the use of sliding mask [78] or photolithographic patterning [79].
Most of the abovementioned techniques can be also used to create biological and chemical gradients [69]. So, e.g., differentiation factors such as bone morphogenic protein 2 (BMP-2) and transforming growth factor ß1 (TGF-ß1), incorporated in heparin-alginate hydrogels in opposite directions (Fig. 5.3a) led to higher osteogenic differentiation of mesenchymal stem cells along increasing BPM-2 and higher chondrogenic differentiation in the direction of the TGF-ß1 concentration growth [72]. Such biological gradient hydrogels were also engineered for in vitro disease model application, like gradients of epidermal growth factor to study of tumor cell intravasation [80]. Moreover, new methods to fabricate hydrogels with combined multiple gradients of different natures were reported [81]. Using these combinatory gradients, complex disease models can be created and better functioning tissue engineered constructs can be produced.
5.6 Cell Analysis, Sample Recovery, and Downstream Analysis
Different analytical techniques may be performed directly on the in vitro 3D disease model or TE construct such as cell morphology, cell viability, differentiation or expression biomarkers using methods such as phase contrast, fluorescence, or confocal microscopy [82]. However, this non-invasive monitoring often does not provide the complete picture and supplemental data obtained from RNA isolation, protein extraction, single cell isolation and following analysis by, e.g., Western blot, flow cytometry, and qPCR among other downstream applications may be required to support experimental findings and hypothesis. Moreover, recovery of spheroids, organoids, or tissue explants followed by staining and sectioning can reveal detailed information on cell structure, morphology, and organization, which can be invaluable when attempting to replicate in vivo tumor structure for the development of disease models, new drugs and/or treatment regimes. Downstream techniques require cells, spheroid, organoid, or tissue explants to be recovered quickly, easily, and intact with no residual matrix present that may interfere with the process and data ultimately generated.
The sample recovery technique employed will depend on the type of hydrogel used for the culture and can range from depolymerization, enzymatic digestion (e.g., collagenase, trypsin, dispase, or cellulase), and mechanical processes, or a combination used in parallel which is often the case.
Recovery of cells grown on animal derived matrices such as collagen, gelatin, and basement matrix is most often achieved by the use of enzymatic digestion and mechanical agitation. For example, recovery from Matrigel® can be performed by use of proteases that depolymerize the matrix within a few hours on ice using gentle agitation via a flatbed shaker. The sample is then washed several times with PBS and cells pelleted. Alternatively, dispase, a metalloenzyme which gently releases cells, can be used in combination with mechanical agitation. Protocols for recovery of cells from collagen recommend use of a collagenase/dispase solution where the sample is pipetted up and down to break up the gel completely, followed by addition of an EDTA/EGTA-containing solution to quench the reaction. Care should be taken to ensure the correct type of collagenase is used as this may impact cell viability should further culture of recovered cells be required.
Hyaluronic acid (HA) hydrogel from thiol-modified HA can be returned to solution phase by addition of dithiothreitol as demonstrated with L-929 murine fibroblasts [83].
Protocols for PEG-based hydrogels employ an enzyme α-chymotrypsin to release spheroids from the matrix in combination with mechanical shaking [84]. Block copolymers based on disulfide-containing polyethylene glycol diacrylate crosslinkers have been shown to be dissociated using the thiol–disulfide exchange reaction in the presence of N-acetyl-cysteine or glutathione, this dissolves the hydrogel network and cells recovered by centrifugation [85]. Examples of cells recovered in this manner include murine NIH 3T3 fibroblasts, human HepG2 C3A hepatocytes, human bone marrow-derived mesenchymal stem cells (MSCs), and human umbilical vein endothelial cells (HUVECs).
When recovering cells from a natural non-animal product such as nanocellulose, cellulase enzymes may be used to digest the cellulose fibers [86]. These enzymes break the cellulose fibers into glucose molecules removing the hydrogel structure to form a solution. The digestion can be done in situ without mechanical agitation, which is an advantage when trying to preserve cell structures for sectioning. Ionic alginate hydrogels require the addition of chelating agents (e.g., EDTA and sodium citrate) to reverse the crosslinking and release the encapsulated cells [87].
5.7 Technologies into Which Hydrogels Can be Incorporated
All of the hydrogel properties described above make them useful tools in a wide variety of applications. In 3D cell culture and regenerative medicine hydrogels are widely used as bioinks in bioprinting (Chap. 11). Here, cells resuspended in unpolymerized hydrogels are printed in 3D structures, which allow precise control of the 3D construct geometry and spatial cell distribution. Hydrogel polymerization, if required for stabilization of the construct, takes place during or directly after printing. Another frequently used application of hydrogels is their implementation into microfluidic systems and organ-on-chips (Chap. 10). The use of hydrogels in microfluidic chips helps to better recapitulate the in vivo microenvironment providing cells with ECM-like surrounding. On the other hand, microfluidic allows creation of spatiotemporal gradients of bioactive molecules, nutrients, and oxygen in hydrogels in a very small scale [88]. Moreover, microfluidic systems can be created directly from hydrogels [89]. Hydrogels can also be used for expansion of various cell types in stirred tank reactors—here cells can be encapsulated in hydrogel beads or can grow on the surface of hydrogel microcarriers [90, 91]. Recently, hydrogels were used to enable 3D isolation of MSCs, resulting in cell material never exposed to plastic adherence in a 2D environment [92]. In the clinic, besides tissue and organ reconstruction by tissue engineering, injectable hydrogels are used to protect and support cells for delivery to treatment sites. Injectable hydrogels can be, for example, used for restoring the lost functions of nervous system as drug, liposome and cell delivery systems [93] or wound dressing for skin wounds [94].
5.8 Future Perspectives
The need for more biologically relevant disease models to bridge the gap between in vitro and in vivo conditions has resulted in significant advances being made in the area of 3D cell culture. Hydrogels have been shown to offer great potential in the development of 3D models due to their properties such as high water retention, oxygen and nutrient diffusion and tunability.
The first use of hydrogels for cell culture was reported by Ehrmann and Gey, who in 1956 reconstituted rat tail collagen and used it as substrate for cell growth [95]. Nowadays, hydrogels are used by the scientific community for modeling physiological and pathological tissues, for advanced drug screening and in tissue engineering. In 3D cell culture, the rapidly developing field of 3D bioprinting requires further development of cell promoting, but mechanically stable hydrogels with optimal gelation dynamics, high biocompatibility, and possible biodegradability.
Traditionally animal derived hydrogels have been used but more recently new synthetic, semi-synthetic, and bio-based hydrogels, such as those manufactured from peptides and wood, offer realistic alternatives. When following the journey from cell research in the laboratory to cell therapy at the clinic, it is not possible to utilize a material with animal components. Bio-based, semi-synthetic, and synthetic products offer a clear advantage here, in addition to providing more reproducible manufacturing. Hydrogels produced from recombinant animal proteins also have more potential for clinical applications than ones of animal origin or ones derived from human blood material.
Hydrogels are not limited to plate-based 3D cell culture but have application in organ-on-a-chip models, microfluidic devices, in drug delivery and 3D bioprinting for tissue engineering and regenerative medicine. In all of these applications the ability to support cell health and viability whilst retaining cell morphology and function is paramount. Hydrogels are capable of fulfilling all of these requirements and indeed offer an exciting way forward to bridge the in vitro/in vivo gap and take cell research from the bench to therapy in the clinic.
Take-Home Messages
-
Hydrogels are 3D network structures able to imbibe large amounts of water.
-
Hydrogels can be isolated from natural animal and non-animal sources, synthesized or a combination of natural and synthetized molecules.
-
Unpolymerized hydrogels are liquid and by polymerization become solid (sol-gel transition).
-
Hydrogel classification is based on structural composition, origin/source of polymers, responsiveness to stimuli, molecular charge, and crosslinking reversibility.
-
Stiffness of the hydrogel plays a role in determining cell behavior and fate and can be adjusted via crosslinking or changing concentration.
-
Tunable properties of hydrogels allow creation of various physical/mechanical in vitro microenvironments.
-
Hydrogel properties can be characterized by rheology, SEM, confocal microscopy, and AFM.
-
Hydrogels can be used to create in vitro gradients.
-
Easy removal of the hydrogel is required for cell recovery and downstream analysis but should not damage or affect cell viability.
-
Hydrogels are used in standard 3D cell culture, bioprinting, microfluidic devices, organ-on-a-chip models and as carrier material in bioreactors for cell expansion.
-
They offer a way to bridge the gap between research and the clinic having use in cell therapy and tissue engineering .
References
Patel A, Mequanint K. Hydrogel biomaterials. In: Fazel-Rezai R, editor. Biomedical engineering - frontiers and challenges. Rijeka: InTech; 2011.
Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–51.
Ruedinger F, et al. Hydrogels for 3D mammalian cell culture: a starting guide for laboratory practice. Appl Microbiol Biotechnol. 2015;99(2):623–36.
Dragan ES. Design and applications of interpenetrating polymer network hydrogels. A review. Chem Eng J. 2014;243:572–90.
Dinescu S, et al. Collagen-based hydrogels and their applications for tissue engineering and regenerative medicine. In: Mondal M, editor. Cellulose-based superabsorbent hydrogels. Polymers and polymeric composites: a reference series. Cham: Springer; 2018.
Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14(2):199–215.
Curvello R, Raghuwanshi VS, Garnier G. Engineering nanocellulose hydrogels for biomedical applications. Adv Colloid Interf Sci. 2019;267:47–61.
Pepelanova I, et al. Gelatin-methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering (Basel). 2018;5(3):55.
Chen YC, et al. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater. 2012;22(10):2027–39.
Dikovsky D, Bianco-Peled H, Seliktar D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials. 2006;27(8):1496–506.
Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336(6085):1124–8.
Andersen T, Auk-Emblem P, Dornish M. 3D cell culture in alginate hydrogels. Microarrays. 2015;4(2):133–61.
Ekerdt BL, et al. Thermoreversible hyaluronic acid-PNIPAAm hydrogel systems for 3D stem cell culture. Adv Healthc Mater. 2018;7(12):1800225.
Hassan W, Dong Y, Wang W. Encapsulation and 3D culture of human adipose-derived stem cells in an in-situ crosslinked hybrid hydrogel composed of PEG-based hyperbranched copolymer and hyaluronic acid. Stem Cell Res Ther. 2013;4(2):32.
Chawla K, et al. Biodegradable and biocompatible synthetic saccharide− peptide hydrogels for three-dimensional stem cell culture. Biomacromolecules. 2011;12(3):560–7.
Liu Y, Chan-Park MB. Hydrogel based on interpenetrating polymer networks of dextran and gelatin for vascular tissue engineering. Biomaterials. 2009;30(2):196–207.
Xiao W, et al. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011;7(6):2384–93.
Wisdom K, Chaudhuri O. 3D cell culture in interpenetrating networks of alginate and rBM matrix. In: 3D cell culture. Totowa, NJ: Springer; 2017. p. 29–37.
Bhattacharya M, et al. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J Control Release. 2012;164(3):291–8.
Peppas NA, et al. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345–60.
Thiele J, et al. 25th anniversary article: designer hydrogels for cell cultures: a materials selection guide. Adv Mater. 2014;26(1):125–47.
Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103(4):655–63.
Thiele J, et al. DNA-functionalized hydrogels for confined membrane-free in vitro transcription/translation. Lab Chip. 2014;14(15):2651–6.
Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31(17):4639–56.
Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26(15):2467–77.
Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci (Oxf). 2012;37(1):106–26.
Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68(1):34–45.
Ryan DM, Nilsson BL. Self-assembled amino acids and dipeptides as noncovalent hydrogels for tissue engineering. Polym Chem. 2012;3(1):18–33.
Heck T, et al. Enzyme-catalyzed protein crosslinking. Appl Microbiol Biotechnol. 2013;97(2):461–75.
Yang G, et al. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue-derived stromal cells. PeerJ. 2016;4:e2497.
Mohamed MA, et al. Stimuli-responsive hydrogels for manipulation of cell microenvironment: from chemistry to biofabrication technology. Prog Polym Sci. 2019;98:101147.
Qu J, et al. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017;58:168–80.
Nagahama K, Ouchi T, Ohya Y. Temperature-induced hydrogels through self-assembly of cholesterol-substituted star PEG-b-PLLA copolymers: an injectable scaffold for tissue engineering. Adv Funct Mater. 2008;18(8):1220–31.
Kloxin AM, Tibbitt MW, Anseth KS. Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nat Protoc. 2010;5(12):1867.
Lim HL, et al. Dynamic electromechanical hydrogel matrices for stem cell culture. Adv Funct Mater. 2011;21(1):55–63.
Shen Z, et al. A thermally responsive cationic nanogel-based platform for three-dimensional cell culture and recovery. RSC Adv. 2014;4(55):29146–56.
Amosi N, et al. Acidic peptide hydrogel scaffolds enhance calcium phosphate mineral turnover into bone tissue. Acta Biomater. 2012;8(7):2466–75.
Green H, et al. RGD-presenting peptides in amphiphilic and anionic β-sheet hydrogels for improved interactions with cells. RSC Adv. 2018;8(18):10072–80.
Wang H, Heilshorn SC. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv Mater. 2015;27(25):3717–36.
Discher DE, Janmey P, Wang Y-l. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–43.
Rosales AM, et al. Hydrogels with reversible mechanics to probe dynamic cell microenvironments. Angew Chem Int Ed. 2017;56(40):12132–6.
Chen Y, et al. Receptor-mediated cell mechanosensing. Mol Biol Cell. 2017;28(23):3134–55.
Alakpa EV, et al. Tunable supramolecular hydrogels for selection of lineage-guiding metabolites in stem cell cultures. Chem. 2016;1(2):298–319.
Cha C, et al. Decoupled control of stiffness and permeability with a cell-encapsulating poly (ethylene glycol) dimethacrylate hydrogel. Biomaterials. 2010;31(18):4864–71.
Ledo AM, et al. Extracellular matrix mechanics regulate transfection and SOX9-directed differentiation of mesenchymal stem cells. Acta Biomater. 2020;110:153–63.
Cosgrove BD, et al. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat Mater. 2016;15(12):1297–306.
Cavo M, et al. A new cell-laden 3D Alginate-Matrigel hydrogel resembles human breast cancer cell malignant morphology, spread and invasion capability observed “in vivo”. Sci Rep. 2018;8(1):1–12.
Yosef A, et al. Fibrinogen-based hydrogel modulus and ligand density effects on cell morphogenesis in two-dimensional and three-dimensional cell cultures. Adv Healthc Mater. 2019;8(13):1801436.
Lavrentieva A, et al. Fabrication of stiffness gradients of GelMA hydrogels using a 3D printed micromixer. Macromol Biosci. 2020;20(7):e2000107.
Zuidema JM, et al. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J Biomed Mater Res B Appl Biomater. 2014;102(5):1063–73.
Shachaf Y, Gonen-Wadmany M, Seliktar D. The biocompatibility of PluronicF127 fibrinogen-based hydrogels. Biomaterials. 2010;31(10):2836–47.
Plotkin M, et al. The effect of matrix stiffness of injectable hydrogels on the preservation of cardiac function after a heart attack. Biomaterials. 2014;35(5):1429–38.
Kim SW, Bae YH, Okano T. Hydrogels: swelling, drug loading, and release. Pharm Res. 1992;9(3):283–90.
Kirsch M, et al. Gelatin-methacryloyl (GelMA) formulated with human platelet lysate supports mesenchymal stem cell proliferation and differentiation and enhances the hydrogel’s mechanical properties. Bioengineering. 2019;6(3):76.
Rahman MS, et al. Morphological characterization of hydrogels. In: Mondal MIH, editor. Cellulose-based superabsorbent hydrogels. Cham: Springer International Publishing; 2019. p. 819–63.
Shi J, et al. Cell-compatible hydrogels based on a multifunctional crosslinker with tunable stiffness for tissue engineering. J Mater Chem. 2012;22(45):23952–62.
Iturri J, Toca-Herrera JL. Characterization of cell scaffolds by atomic force microscopy. Polymers. 2017;9(8):383.
Barani A, Bush MB, Lawn BR. Effect of property gradients on enamel fracture in human molar teeth. J Mech Behav Biomed Mater. 2012;15:121–30.
Xia T, Liu W, Yang L. A review of gradient stiffness hydrogels used in tissue engineering and regenerative medicine. J Biomed Mater Res A. 2017;105(6):1799–812.
Laasanen MS, et al. Biomechanical properties of knee articular cartilage. Biorheology. 2003;40(1–3):133–40.
Tsai AG, Johnson PC, Intaglietta M. Oxygen gradients in the microcirculation. Physiol Rev. 2003;83(3):933–63.
Oudin MJ, Weaver VM. Physical and chemical gradients in the tumor microenvironment regulate tumor cell invasion, migration, and metastasis. Cold Spring Harb Symp Quant Biol. 2016;81:189–205.
Lewis DM, et al. Intratumoral oxygen gradients mediate sarcoma cell invasion. Proc Natl Acad Sci. 2016;113(33):9292–7.
Sansom SN, Livesey FJ. Gradients in the brain: the control of the development of form and function in the cerebral cortex. Cold Spring Harb Perspect Biol. 2009;1(2):a002519.
Wartlick O, Kicheva A, Gonzalez-Gaitan M. Morphogen gradient formation. Cold Spring Harb Perspect Biol. 2009;1(3):a001255.
Akeson A, et al. Endothelial cell activation in a VEGF-A gradient: relevance to cell fate decisions. Microvasc Res. 2010;80(1):65–74.
Pedron S, Becka E, Harley BA. Spatially gradated hydrogel platform as a 3D engineered tumor microenvironment. Adv Mater. 2015;27(9):1567–72.
Moeendarbary E, et al. The soft mechanical signature of glial scars in the central nervous system. Nat Commun. 2017;8:14787.
Wang L, et al. Hydrogel-based methods for engineering cellular microenvironment with spatiotemporal gradients. Crit Rev Biotechnol. 2016;36(3):553–65.
Smith Callahan LA. Gradient material strategies for hydrogel optimization in tissue engineering applications. High-throughput. 2018;7(1):1.
Diederich VE, et al. Bioactive polyacrylamide hydrogels with gradients in mechanical stiffness. Biotechnol Bioeng. 2013;110(5):1508–19.
Jeon O, et al. Biochemical and physical signal gradients in hydrogels to control stem cell behavior. Adv Mater. 2013;25(44):6366–72.
Burdick JA, Khademhosseini A, Langer R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir. 2004;20(13):5153–6.
Zaari N, et al. Photopolymerization in microfluidic gradient generators: microscale control of substrate compliance to manipulate cell response. Adv Mater. 2004;16(23–24):2133–7.
Sundararaghavan HG, et al. Neurite growth in 3D collagen gels with gradients of mechanical properties. Biotechnol Bioeng. 2009;102(2):632–43.
Orsi G, et al. A new 3D concentration gradient maker and its application in building hydrogels with a 3D stiffness gradient. J Tissue Eng Regen Med. 2017;11(1):256–64.
Lee D, et al. Fabrication of hydrogels with a stiffness gradient using limited mixing in the Hele-Shaw geometry. Exp Mech. 2019;59(9):1249–59.
Sunyer R, et al. Fabrication of hydrogels with steep stiffness gradients for studying cell mechanical response. PLoS One. 2012;7(10):e46107.
Nemir S, Hayenga HN, West JL. PEGDA hydrogels with patterned elasticity: novel tools for the study of cell response to substrate rigidity. Biotechnol Bioeng. 2010;105(3):636–44.
Zervantonakis IK, et al. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci U S A. 2012;109(34):13515–20.
Vega SL, et al. Combinatorial hydrogels with biochemical gradients for screening 3D cellular microenvironments. Nat Commun. 2018;9(1):614.
Peerani E, Candido JB, Loessner D. Cell recovery of hydrogel-encapsulated cells for molecular analysis. In: Theranostics. New York: Springer; 2019. p. 3–21.
Shu XZ, et al. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3(6):1304–11.
Raza A, Lin C-C. Generation and recovery of β-cell spheroids from step-growth PEG-peptide hydrogels. J Vis Exp. 2012;70:e50081.
Zhang J, Skardal A, Prestwich GD. Engineered extracellular matrices with cleavable crosslinkers for cell expansion and easy cell recovery. Biomaterials. 2008;29(34):4521–31.
Lou Y-R, et al. The use of nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells. Stem Cells Dev. 2014;23(4):380–92.
Bidarra SJ, Barrias CC. 3D culture of mesenchymal stem cells in alginate hydrogels. In: Stem cell niche. New York: Springer; 2018. p. 165–80.
Lee SH, et al. Hydrogel-based three-dimensional cell culture for organ-on-a-chip applications. Biotechnol Prog. 2017;33(3):580–9.
Paguirigan A, Beebe D. Gelatin based microfluidic devices for cell culture. Lab Chip. 2006;6(3):407–13.
Huang L, et al. Biopolymer-based microcarriers for three-dimensional cell culture and engineered tissue formation. Int J Mol Sci. 2020;21(5):1895.
Kumar A, Starly B. Large scale industrialized cell expansion: producing the critical raw material for biofabrication processes. Biofabrication. 2015;7(4):044103.
Egger D, et al. From 3D to 3D: isolation of mesenchymal stem/stromal cells into a three-dimensional human platelet lysate matrix. Stem Cell Res Ther. 2019;10(1):248.
Pakulska MM, Ballios BG, Shoichet MS. Injectable hydrogels for central nervous system therapy. Biomed Mater. 2012;7(2):024101.
Qu J, et al. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–99.
Ehrmann RL, Gey GO. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst. 1956;16(6):1375–403.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lavrentieva, A., Spencer-Fry, J. (2021). Hydrogels for 3D Cell Culture. In: Kasper, C., Egger, D., Lavrentieva, A. (eds) Basic Concepts on 3D Cell Culture . Learning Materials in Biosciences. Springer, Cham. https://doi.org/10.1007/978-3-030-66749-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-66749-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66748-1
Online ISBN: 978-3-030-66749-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)