Abstract
Marine and freshwater algae are similar to other plants; they produce different bioactive compounds collectivity referred to as natural products which are synthesized by these microorganisms at the end of their growth phase due to the metabolic changes induced by various environmental stress factors/conditions. From these active ingredients are plant hormones and other growth regulators (such as gibberellin, auxin, ethylene, cytokinin, etc.). The hormonal substances are produced not only by the highly evolved terrestrial plants but also by the lower primitive thallophytic algae, and they function similarly. The present chapter focused mainly on plant hormone production from marine macroalgae and the effects of biotic and abiotic stresses on productions of each kind of phytohormones from these organisms which were rarely cultured and studied in the laboratory. So the stress factors on algae were considered in these cases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Plant growth regulators (such as auxins, cytokinins, gibberellin, abscisic acid, and ethylene) play an important role in mediating growth of different plant species at very low concentrations as well as signaling environmental alterations or changes, initiating stress responses (biotic and abiotic) and indicator molecules in the regulation of almost all phases of plant growth and development (maturation) from embryogenesis to senescence (Li et al. 2010).
Seaweed and microalgae extracts are used as growth stimulants or growth regulators in cultivation of agricultural species due to its content from the plant growth regulator (hormones) concentrations (Stirk and Van Staden 2006).
In various algal species, phytohormones were recorded in significant amount when compared with their amount in plants, and the various biological activities of algal hormones corresponded to the functions of hormones in higher plant (Tarakhovskaya et al. 2007).
El Shoubaky and Salem (2009) investigated green macroalgae (Ulva lactuca and Enteromorpha clathrate) as biofertilizers due to their high concentrations of inorganic nutrients in addition to organic compounds and plant growth hormones. Phytohormones of microalgae and macroalgae are exogenous growth regulators, affecting the tolerance ability to different factors of various (abiotic and biotic) stress conditions (Romanenko et al. 2015).
Hormonal level may undergo changes when the alga is exposed to an alteration in natural environmental or laboratory conditions such as light (quantity, quality, and duration), temperature, salinity, etc.
Moreover, Nimura and Mizuta (2002) reported that the endogenous abscisic acid (ABA) content of laminaria sporophyte increased as a result of its transition from the vegetative growth stage to the reproductive state. Also, in some microalgae, ABA was increased under salt stress or lowered moisture content. Polyamines and betaines are important active signal factors required for different processes in plant and algal development and participate in biotic and abiotic stress responses as illustrated by Kusano et al. (2008). These factors were recorded in different stressed microalgal and seaweed species (Mackinnon et al. 2010; Gebser and Pohnert 2013).
In the following sections, we will summarize the major plant hormones and its analogues present in algal species and its physiological functions and methods of extractions and determinations and if any abiotic stress factors can affect the accumulation of these regulators inside the algal species.

The chemical structure of major phytohormones and other regulators produced by algae
2 Algal Hormones
2.1 Auxins
It is interesting to know that not only the hormonal substances produced by the highly evolved terrestrial plants are already produced by the lower primitive thallophytic algae, but they also function similarly. Regarding auxins, there have been numerous investigations that dealt with auxin production (especially IAA) in many algal species (microalgae, macroalgae, and cyanobacteria).
Starting with Du Buy and Olson (1937) who reported the presence of auxin in the tissue of Fucus vesiculosus , occasionally, other scientists recorded the presence of auxin in Bryopsis muscosa. Also, indole acetic acid (IAA) were found in the brown seaweeds Fucus, Macrocystis, and Desmarestia sp. Few years later (Skibola 2004; Tarakhovskaya et al. 2007; Li et al. 2007), a growth substance similar to IAA was found in Laminaria agardhii. These previous investigations proved their findings by the use of bioassay of Avena coleoptile curvature.
This was followed by a lot of investigations which dealt with the presence of auxinic substance in many algal species as well as the isolation and identification of indolic substance using different analytical methods. The previously recorded studies confirmed the production of auxins in various algal species belonging to different divisions, but they also proved that the hormonal function and its catabolism followed the same pathway as that in angiosperms (Sitnik et al. 2003; Stirk et al. 2009). Researches continued in this field till now, recording the presence of one or more plant hormones in different algal species, identifying its (or their) chemical structure by chromatographic analysis, and confirming its hormonal properties by specific plant bioassays (Table 14.1 and Figs. 14.1, 14.2, and 14.3). Auxins in algal thalli varied from season to season, and developmental stage and highest concentration were recorded especially in summer season and in vegetative tissues (EL Shoubaky and Salem 2016; Mori et al. 2017).
The effect of different culture conditions especially a biotic stress (such as concentration of l-tryptophan, acidity degree, and light conditions) on the synthesis of indole by Spirulina sp. was reported by Ahmed et al. (2010). It was found that the formation of IAA in Spirulina sp. was organized by 1.5 μg/mL l-tryptophan concentration. Moreover, the height amount of IAA was found at pH 6 in light-dark cycle 8:16 h. However, in the dark, auxin synthesis was not observed.
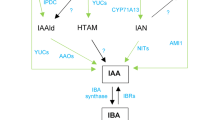
IAA is the naturally occurring growth regulator in the kingdom of plant. It is present in very low concentration (0.5–15 μg/kg) and is in equilibrium with bound forms (such as glucose, ester, aldehyde, and more complex forms as glucobrassicin).
IAA is synthesized in different plants from the tryptophan (amino acid) by different biosynthetic routes.
2.1.1 Physiological Properties of Auxins
-
1.
Initiation of root formation.
-
2.
Apical dominance.
-
3.
Tropisms.
-
4.
Differentiation of phloem elements.
-
5.
Induction of elongation.
2.1.2 Separation and Detection
Auxins can be separated by paper chromatography (PC) using the mobile phase/isopropanol/ammonium hydroxide/water (8:1:1).
It can be separated by thin layer chromatography (TLC F254) using the mobile phase/chloroform/ethyl acetate/formic acid (5:4:1); standard indoles are used for comparison.
To detect the separated spots, they can be sprayed with the coloring reagent DMAC (0.1 g of p-dimethylaminocinnamaldehyde in 10 mL concentrated HCl, then diluting to 200 mL with acetone). In case of using PC for separation, the PC paper will be dipped in the reagent, dried, and then heated at 65 °C for 2.5 min. Another coloring reagent can be used in case of PC which is called Salkowski reagent (0.001 M ferric chloride in 5% perchloric acid), giving pink spots with FeCl3/perchloric acid/ethanol reagent. Using TLC for separation of the indolic extract, the spray reagent is used as 0.25% of DMCA in ethanol/conc. HCl (1:1) and the color will develop over night at room temperature (purple spot).
2.1.3 Identification and Determination
For identification of indoles, spectral measurements in methanol must be at wavelength 220–320 nm. Indole acetic acid has fluorescence peak of 365 nm and activity peak of 285 nm. Identification of auxins are performed by LC/MS, GLC/MS, HPLC/MS, and GC/MS, which identify the indolic compound(s) (compared with either standard or not) at its specific retention time, and recording its chemical structure and formula.
2.1.4 Measurement of Its Characteristic Biological Activity
-
1.
Using suitable plant bioassays (for each hormone compared to the control of synthetic standard).
-
2.
Avena coleoptile curvature test.
-
3.
Elongation of wheat (or barley) coleoptile sections.
-
4.
Induction of rooting in cutting stem of mung bean.
2.2 Cytokinins
They are group of plant growth regulators consist of purines substituted in the six position. The first naturally occurring cytokinin was zeatin (from Zea mays) which was commonly found as riboside forms. Cytokinin was found to initiate all division in plant tissue culture during growth. It interferes with auxins in many developmental stages controlled by the balance on the ratios of cytokinin to indoles which may influence shoot and root differentiation and growth of lateral buds or remain at the undifferentiated callus stage. Cytokinins are synthesizing from adenosine-5-monophosphate producing iso-pentenyladenine (ip) which was believed to be the precursor of all other naturally occurring cytokinins. Different cytokinin-like substances were reported in different algal species belonging to various algal groups, as zeatin, dihydrozeatin, iso-pentenyladenine (ip), N6-methylaminopurine, and N6,8,8-dimethyl-allylaminopurine.
In Phaeophyta, Stirk et al. (2003) recorded cytokinin-like activity in Fucus serratus, Ascophyllum nodosum, Ecklonia maxima, Laminaria saccharina, Fucus vesiculosus, Dictyota sp., and Sargassum heterophyllum (coincide with the release of gametes), while Stirk and Van staden (1997) reported the cytokinin activity in the green seaweeds: Ulva sp., Cladophora contexta, Codium capitatum, C. extricatum, Halimeda cuneata, Caulerpa racemosa, and Valonia macrophysa.
Detection of cytokinin-like activity in the red seaweeds (Rhodophyta ) was achieved by Yokoya et al. (2010) and Mori et al. (2017), who reported that this activity was demonstrated in Galaxaura diesingiana, Gelidium amansii, Amphiroa bowbankii, A. ephedraea, Arthrocardia sp., Cheilosiphorum sagiltatum, Jania crassa, Plocamium corallorhiza, Hypnea rosea, H. spicifera, Spyridia hypnoides, Pyropia yezoensis, and Bangia fuscopurpurea. In various algal groups (Stirk et al. 2009; Mori et al. 2017), aromatic cytokinins (topolins) were recorded as shown in Table 14.2 and Figs. 14.4, 14.5, and 14.6.
Regarding the response of algal cells to abiotic stress condition and its relation to phytohormone accumulation, Maršálek et al. (1992) found that during cultivation of microalgae species, the concentration of ABA in mother algal cultures was three times folded when compared with new culture. An increase in ABA level was observed during the first 24 h of microalgae cultivation in the absence of light condition and decreased in the following 24 h; at the same time, the amount of ABA was decreased gradually in light and dark environment conditions (14:10) (Stirk et al. 2014). Also, ABA softened the effect of various oxidative stress conditions, with positive relation to the activity of AO enzymes such as glutathione S-transferase, peroxidase, and catalase (Yoshida et al. 2003).
2.2.1 Separation and Detection
Separate the ammoniacal fraction by descending paper chromatography using propanol/ammonia/water (10:1:1) as described by Stirk and Van staden (1997) and using standard cytokinin (as kinetin) for comparison. t-Butanol/conc. NH4OH/H2O (3:1:1) or n-butanol/acetic acid/water (4:1:1) can be used as mobile systems. The separated cytokinin appears as dark spots in short UV light. Using TLC of alumina G, separation of cytokinin (as zeatin) can be performed using butanone saturated with water or EA saturated with H2O or by chloroform/EthOH (9:1). Detection done using Dische reagent (spraying with 0.5 g cysteine hydrochloride in 3MH2SO4 giving a pink color after 20 min).
2.2.2 Identification and Determination
Occur by using HPLC.
2.2.3 Bioassay/Biological Activity
The ability of cytokinin to promote growth of secondary phloem of carrot. The effect of cytokinin on barley germination.
The cytokinin-like activity promoting cell division can be assayed by soybean callus culture.
2.2.4 Physiological Properties of cytokinins
-
1.
Shoot and Root differentiation in tissue culture.
-
2.
Growth of lateral buds and leaf expansion.
-
3.
Chloroplast development.
-
4.
Leaf senescence.
-
5.
Morphogenesis in cultured tissues.
2.3 Gibberellins (GAs)
It is clear from literatures that gibberellin-like substances are synthesized by different types of macroalgae. Bently (1960) suggested the presence of GA3 like substances in microalgae and macroalgae. She revealed that there are two unknown components in acidic extracts of phytoplankton which have some growth stimulatory characteristics. Meanwhile, Stirk et al. (2013a, b) extracted gibberellin-like substance from Fucus vesiculosus, purified it by PC and identified one or an analogue of GA1, GA3, and GA6 (~10 μg/kg F.wt) (Rf = 0.3–0.4). Furthermore, other data found gibberellin-like activity in Fucus spiralis (GA1 or GA3).
Detection of gibberellins in other brown seaweeds was recorded by many investigations (EL shoubaky and Salem 2016). Moreover, these scientists recorded gibberellin in the red seaweeds Hypnea musciformis, Gracilaria corticata, and Porphyra leucostricta. Table 14.3 and Figs. 14.7, 14.8, and 14.9 recorded some algal species producing gibberellins. Researches continued till now searching for plant hormones in seaweed species and applying recent techniques for their extraction, separation, identification, and determination (Stirk et al. 2013a, b). Gibberellins are group of hormones (belongs to diterpenoids) which stimulate plant growth and are widespread in plants and algae.
In fact, more than 40 compounds of gibberellin structure have been recognized till now. The most familiar is gibberellic acid (GA3).
2.3.1 Physiological Properties of Gibberellins
-
1.
It promotes seed germination and organ differentiation.
-
2.
It stimulates stem elongation and shoot growth.
-
3.
It interferes with leaf expansion, development, and fruit maturity (Yamaguchi 2008; Sun 2010). It has positive effect with IAA to differentiation of cell and elongation of root but has negative effect with abscisic acid on growth and germination. Negative and positive effects depend on environmental conditions and factors with stress-related ethylene and negative effects with cytokinin concentration (Weiss and Ori 2007; Yamaguchi 2008). Gibberellins were synthesized from glyceraldehyde 3-phosphate in young shoot tissues and developing seeds.
2.3.2 Separation and Detection
Gibberellins contain more than 40 chemically closely related compounds which are difficult to separate and distinguish. Separation of gibberellins can be performed on column of 5% OV-22, on DMCS-treated chromosorb W.
Gibberellins are separated on silica gel plates with the solvent system benzene/butanol/acetic acid (70:25:5) and benzene/acetic acid/water (50:19:31). Detection was carried out by H2SO4/water (7:3) spraying the plate and then exposure at 120 °C; GA3 appear as spots with yellow-green.
2.3.3 Identification and Determination
The most satisfactory method for gibberellin determination is by GC/MS; using GLC, gibberellins converted first to their methyl ester (by Methylation) or TMS esters (silylation).
2.3.4 Plant Bioassays (Measurement of Gibberellin Biological Activity)
-
1.
Lettuce hypocotyl.
-
2.
α-amylase,
-
3.
Dwarf rice leaf.
2.4 Abscisic Acid (ABA) and Lunularic Acid
It is a sesquiterpenoid growth inhibitor (inhibitor of elongation), but it may significantly stimulate maize root elongation at some concentrations. ABA is present in several plant species in root tips and root caps. White light and stresses (mineral starvation and leaf dehydration) induce high content of ABA in leaves. In the brown algae of genus Ascophyllum (A. nodosum) and some species of Laminaria (Nimura et al. 2002), a hormone was detected which suppressed plant growth in bioassay.
In various algal groups, the growth-inhibiting complex includes lunularic acid and abscisic acid, and other undifferentiated biologically active compounds were recorded.
Lunularic acid is a kind of growth inhibitor which was detected in liverworts. Its structure, activity, and metabolism resemble those of ABA. It suppressed the growth of cut discs from cultured Laminaria japonica and induced reproductive tissue formation at the same concentration used in higher plants (10−6 to 10−4 M). Also, ABA induced the morphogenesis of Hypnea pluvialis cells to form cysts. In some microalgae, the endogenous ABA content increased under stress conditions (salinity, light intensity, drought, etc.).
El shoubaky and Salem (2016) recorded ABA in the green seaweeds Ulva rigida and Ulva lactuca as well as in the red Sarconema filiforme, and higher concentration of ABA was recorded in U. lactuca where the ABA profile (by GC/MS) contained cis-, trans-ABA-l-alanine methyl ester, cis-, trans-ABA-l-valine, and cis-, trans-ABA-l-alanine. Stirk et al. (2009) detected endogenous ABA in the green Ulva fasciata and the brown Dictyota humifusa. It was also detected in different seaweed commercial extracts as Kelpak R from Ecklonia maxima. Also, red seaweeds were found to produce ABA as in the case of Bangia fuscopurpurea and Pyropia yezoensis by Mori et al. (2017), as well as red algae of Brazil (Yokoya et al. 2010), as illustrated in Table 14.4 and Figs. 14.10 and 14.11.
2.4.1 Separation, Detection, and Determination
Using paper chromatography for separation of ABA in the algal extract and standard ABA (at conc. 10−6 M) for comparison. Detect the isolated spots by UV light of 254 nm as dark absorbed spots. The most widely used techniques for quantification are GC/MS, GLC/MS, and HPLC/MS.
2.4.2 The Biological Activity of ABA Using Specific Bioassays
-
1.
Inhibition of elongation of wheat coleoptile sections (ABA of more than 10−8 M).
-
2.
Induction of stomatal closure.
2.5 Ethylene
Plants and macroalgae produce a range of volatile compounds, such as alcohols, alkane, alkenes, esters, etc.
These volatile compounds are produced in response to biotic or abiotic stimuli. These compounds have several biological roles in higher plants such as promotion of seed germination, inhibition of the stem, root elongation, ripening of fruits, senescence of leaves and flowers, and sex determination as reported by Bleecker and Kenode (2000), Klee (2004), Grennan (2008), Holopainen and Gershenzon (2010), and Loreto and Schnitzler (2010). Most of the biosynthetic pathways of volatile compound production depend on S-adenosylmethionine compound which may act as a substrate for the enzyme reactions or as a source of methyl group (as in the synthesis of jasmonates, salicylates, and brassinosteroids). Ethylene and dimethyl sulfide are examples of etherial compounds which are produced from the red alga Gelidium sp.
The concentrations and types of these compounds were affected by various abiotic stress factors as salinity, light quality, and exogenous ethylene. The period of light and darkness causes the production of amines and methyl alkyl compounds.
Reaction oxygen species (ROS) act as a secondary messenger initiating a signal cascade which stimulate ethylene synthesis (Mackerness 2000). Accumulation of volatile compounds was recorded after the exposure to red light and application of exogenous ethylene.
The level of dimethyl sulfide (DMS) which emitted in all conditions didn’t increase after incubation with ethylene (they appear to be not coordinated as reported in the red alga G. arbuscula. In Acetabularia mediterranea, the rate of algal development decreases with increase of ethylene production.
In Enteromorpha intestinalis, reduction of chlorophyll content below that of control occurred on the addition of ethephon which decompose to generate ethylene as reported by Garcia-Jimenez et al. (2013). Moreover, ethylene was involved in growth of the red alga Pterocladiella capillacea (Garcia-Jimenez and Robaina 2012).
When the acclimatized Ulva intestinalis to low light intensity was transferred to high light condition, ethane level was increased causing an inhibition of chlorophyll content (by 30%). Table 14.5 and Fig. 14.12a, b recorded some algal species producing ethylene.
2.5.1 Gas Chromatography/MS Analysis of the Released Volatiles (GC/ MS)
Volatile compounds are analyzed using Varian 431GC/210MS with capillary column and He as a carrier gas.
2.5.2 Physiological Properties of Ethylene
Ethylene production increased during leaf abscission, flower senescence, and fruit ripening.
Physiological stresses and wounding induce ethylene biosynthesis.
During storage of fruits, vegetables, and flowers, an effective ethylene absorbent is used (KMnO4, pot. permanganate) to reduce ethylene concentration in the storage area (extending the storage life of the fruits).
Inhibition of Ethylene Action.
Ethylene effects can be antagonized by silver ion Ag+ [in the form of AgNO3 or silver thiosulfate Ag(S2O3)2]. Also, CO2 at high concentrations (5–10%) inhibit many ethylene effects.
3 Growth Substances (Growth Regulators)
There are five classical phytohormones detected in angiosperms and in the lower plants as algae. They are auxins, cytokinins, gibberellins, abscisic acid, and ethylene which control different physiological and developmental processes. Their extraction, separation, detection, and determination by various methods are well known. Also, their synthetic pathway(s) and biological functions and bioassays have long been documented. Different chemical compounds were reported to control growth and ameliorate plant (or algal) defensive system against biotic or abiotic stresses. These compounds are termed growth substances or growth regulators; they include brassinosteroids, jasmonic acid, salicylic acid, polyamines, and betaines. Higher plants and lower plants (as algae) were reported to produce different growth regulators (Mikami et al. 2016; Mori et al. 2017).
3.1 Brassinosteroids (BRs)
They are group of polyhydroxylated steroid growth regulators which have a remarkable role in various biochemical and development processes in different organisms such as plants and algae, including elongation, reproduction, and cell division, in stems and roots, stress responses, leaf senescence, and photomorphogenesis. The most active components of brassinosteroids are termed brassinolide and castasterone which are widely reported in various tissues of seeds, flowers, leaves, pollens, stems, and roots (Bajguz and Hayat 2009). The precursor of brassinolide is the campesterol (C28-sterol) by oxidation at C6 and addition of OH groups into the β-ring. Brassinazole is an inhibitor of brassinosteroid biosynthesis.
Brassinosteroids have been recorded in Hydrodictyon reticulatum by Bajguz and Hayat (2009) as well as in angiosperms, gymnosperms, the pteridophyte Equisetum arvense, and the bryophyte Marchantia polymorpha (Bajguz and Tretyn 2003). Table 14.6 and Fig. 14.13 recorded some seaweed species producing growth substances.
Secondary internode bioassay of beans is used, where brassins cause both cell elongation and cell division as well as bending, swelling, and splitting of the second internode. Brassinosteroids act locally near their site of synthesis and transported in the xylem.
Brassinosteroids have usually effect on the transport of auxin by indirect way (Symons et al. 2008), increase the percentage of ethylene accumulation, and have an additive effect with GA3, in addition to its synergistic effect with auxins (IAA). Moreover, Brassinosteroids have effects on increase cytokinin and jasmonic acid production and decrease ABA responses. In the seaweed extract of Ecklonia maxima (Phaeophyta) called Kelpak (Stirk et al. 2013a, b), auxins, cytokinin, GAs, ABA, and brassinosteroids were detected.
3.2 Jasmonic Acid (JA)
Jasmonic acid (is a fatty acid) and its volatile methyl ester (jasmonate) were detected in some seaweed’s species. Also, a hydroxylated compound called tuberonic acid, its ME, and its glucosides control potato tuberization development.
Jasmonic acid is produced from the fatty acid linolenic acid (18:3) and plays an important role in plant defense by inducing the synthesis of proteinase inhibitors.
Jasmonates inhibit seed and growth germination and promote abscission, fruit pigmentation , and ripening formation. Jasmonic acid and methyl jasmonate were detected in many species of microalgae and cyanobacteria. Moreover, it was observed in the red seaweed Gelidium latifolium and in brown seaweeds (oxilipins and lipoxygenases) as reported by Arnold et al. (2001) shown in Table 14.6 and Fig. 14.13.
3.3 Polyamines (Aliphatic Amines)
Different seaweed species produced polyamines in the red macroalgae Gelidium canariensis, Grateloupia doryphora, and Cyanidium caldarium, in the brown Dictyota dichotoma, as well as in the green Ulva rigida (Table 14.6 and Fig. 14.13). The content as well as the biosynthesis of polyamines in algae doesn’t differ from that in higher plants (50–150 μg/g F.wt).
Polyamine content in macroalgae changes with seasons and developmental stage (Marián et al. 2000; Sacramenta et al. 2004; Alcazar et al. 2010). Polyamines belong to the putrescine group (putrescine, spermine, and spermidine). It derived from the carboxylation of the amino acids, arginine and ornithine, putrescine (diamine) → spermidine (triamine) → spermine (quadramine).
It exerts regulatory control on the development and growth at very low concentration (especially cell division and morphology). In carrot tissue culture, when polyamine content is low, callus growth only occurs, but at higher concentration, the embryo is formed (polyamines are released to the outer growth media). They aren’t recorded in the commercial seaweed products till now.
3.4 Salicylic Acid (SA)
It is recognized recently as potential regulatory compound. It is produced from phenylalanine (AA).
Salicylic acid plays a role in the pathogen’s resistance. It was reported that SA enhance flower longevity, inhibit biosynthesis of ethylene and seed germination, and reverse the effect of ABA.
Salicylic acid was detected in the red seaweeds Pyropia yezoensis and Bangia fuscopurpurea (Mori et al. 2017). The precursors of SA are cinnamic acid and benzoic acid. To quantify the amount of SA in an extract, methyl salicylate (ortho-anisic acid) can be used as an internal standard (HPLC analysis) (Forcat et al. 2008).
3.5 Signal Peptides
Small molecular weight peptides (as systemin) were found to have regulatory properties in plants which travel in phloem from attacked leaves by herbivore insects to the distant leaves to protect them from insect attack. The traveled signal peptides induce an increase in the production of jasmonic acid and proteinase in the distant leaves for protection.
Signal peptides produced by plants many have a role in:
-
1.
Activation of defensive responses.
-
2.
Cell proliferation promotion.
-
3.
Nodule formation (in legumes).
No known recorded studies that extracted, separated, and identified such signal peptides in seaweeds till now.
3.6 Small RNA Molecules
Recently, many small RNA molecules of single-stranded RNA that consist of 21–22 nucleotides have been identified in plant phloem which may act as transportable signals that regulate gene expression involved in plant defense against viruses. Many of these micro-RNAs (miRNAs) have been identified which means that they may represent a more general means of regulating gene expression.
No such RNA molecules have been identified in seaweeds.
3.7 Rhodomorphin
This regulator was detected in Griffithsia pacifica (red alga) following morphogenetic effects in this alga (Table 14.6 and Fig. 14.13). When an intercalary cell in the filament is removed, the basal cell of the filament starts to secrete the regulator rhodomorphin which increases the formation of reparatory cell. Further studies showed that rhodomorphin is a glycoprotein with molecular weight of 14 kDa. Similar glycoproteins were recorded in the green alga Volvox sp. where it acts as a pheromone facilitating the gamete adhesion and fusion. So, the function of these glycoproteins in algae was to provide adhesion and fusion of gametes during sexual reproduction.
3.8 Commercial Seaweed Concentrates (or Extracts)
Many reports were published in literature concerning the presence of plant growth hormones in brown, green, and red seaweeds (Crouch and Van Staden 1993; Stirk et al. 2013a, b; Tuhy et al. 2013). Their presence was determined and confirmed by plant bioassays and chromatographic analysis (TLC/LC/MS, HPLC/MS, and GC/MS).
A commercial seaweed concentrate was firstly prepared from the water zone occupied by Fucus and Ascophyllum sp. which was tested to have cytokinin-like activity due to the presence of isopentenyladenine (ipA).
During the past two decades, utilization of commercial seaweed products increased as natural sources of fertilizers, biostimulants, and soil ameliorants. It improves plant growth is relatively cheap and easy to apply either as soil manure or as foliar spray. It was suggested that bioactive organic compounds in the seaweed concentrate are responsible for the increase in crop yield (Crouch et al. 1992). Recent researches showed that macroalgae have recorded the ability as a source for products that contain growth regulators and plant hormones and many of the observed effects to treated crops are now attributed to these constituents. The chemical composition of seaweeds revealed that all the major plant nutrients and trace elements are present in marine seaweeds.
Many investigators recently reviewed the presence of antibiotic, antiviral, antibacterial, and antioxidant activities due to the active substances obtained from marine algae. These substances may be responsible for the reduced harmful effects of some plant pathogens (Hamed et al. 2018).
Some of commercial seaweed products (have commercial names):
-
1.
Maxi-crop/Seasol.
-
2.
Algifert/Algimex/Algistim.
-
3.
SM3/Seamac/Marinure.
-
4.
SWC (Kelpak66).
They contain gibberellin-like activity, betaines, ABA, ethylene, and cytokinins. The commercial seaweed extracts use the seaweeds Ascophyllum nodosum, Ecklonia maxima, Enteromorpha compressa, Durvillaea potatorum, Fucus serratus, Porphyra perforata, Sargassum muticum, Laminaria japonica, Macrocystis pyrifera, Ectocarpus confervoides, and Pylaiella littoralis.
4 Conclusion
From the obtained data, we can conclude that algae (micro and macro) extracts are rich with plant hormones and other growth regulator substances. So we can use these species commercially as growth stimulants in different agricultural sectors. Some of these algal species can be used as organic fertilizer and biofertilizers due to its ability for nitrogen fixation (in case of species with heterocysts) and its content from inorganic chemical substances (such as phosphorus and potassium), in addition to organic substances and hormones. The wider distribution, high adaptability to different cultural factor conditions (biotic and abiotic stresses), and high growth rates led algae to be considered as an attractive feedstock for developing fertilizer and biorefinery products, in addition to the ability of these algal species to increase the accumulation of phytohormones when exposed to different abiotic stress conditions such as salinity, drought, light intensity, chemical substances, etc.
References
Ahmed M, Stal LJ, Hasnain S (2010) Production of indole-3-acetic acid by the cyanobacterium Arthrospira platensis strain MMG-9. J Microbiol Biotechnol 20(9):1259–1265
Arnold TM, Targett NM, Tanner CE, Halch WI, Ferrari KE (2001) Evidence for methyl Jasmonate induced phlorotannin production in Fucus vesiculosus (Phaeophyceae). J Phycol 37:1026–1029
Alcazar R, Altabbella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Bajguz A, Hayat S (2009) The effect of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Bajguz A, Tretyn A (2003) The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 62:1027–1046
Bently JA (1960) Role of plant hormones in algal metabolism and ecology. Nature 181:1499–1502
Bleecker AB, Kenode H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16:1–18
Crouch IJ, Van Staden J (1993) Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul 13:21–29
Crouch IJ, Smith MT, Van Staden J, Lewis MJ, Hoad GV (1992) Identification of auxins in a commercial seaweed concentrate. J Plant Physiol 138:590–594
Du Buy HG, Olson RA (1937) The presence of growth regulators during the early development of Fucus. Am J Bot 24:609–611
El Shoubaky GA, Salem EA (2009) Biodiversity in Timsah Lake as a biofertilizer source to the economic plants. Egypt J Bot 49:53–69
El Shoubaky GA, Salem EA (2016) Effect of abiotic stress on endogenous phytohormones profile in some seaweeds. Int J Pharm Phytochem Res 8(1):124–134
Forcat S, Bennett MH, Mansfield JW, Grant MR (2008) A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress. Plant Methods 4:16–23
Garcia-Jimenez P, Robaina RR (2012) Effects of ethylene on tetrasporogenesis in Pterocladiella capillacea (Rhodophyta). J Phycol 48:710–715
Garcia-Jimenez P, Brito-Romano O, Robaina RR (2013) Production of volatiles by the red seaweed Gelidium arbuscula (Rhodophyta): emission of ethylene and dimethyl sulfide. J Phycol 49:661–669
Gebser B, Pohnert G (2013) Synchronized regulation of different zwitterionic metabolites in the osmoadaption of phytoplankton. Mar Drugs 11:2168–2182
Grennan AK (2008) Ethylene response factors in Jasmonate signaling and defense response. Plant Physiol 146:1457–1458
Hamed SM, Abd EL-Rhman AA, Abdel-Raouf N, Ibraheem IBM (2018) Role of marine macro algae in plant protection and improvement for sustainable agriculture technology. Beni-Suef Univ J Basic Appl Sci 7:104–110
Holopainen IK, Gershenzon J (2010) Multiple stress factors and emission of plant VOCs. Trends Plant Sci 15:176–184
Klee H (2004) Ethylene signal transduction. Moving beyond Arabidopsis. Plant Physiol 135:660–667
Kusano T, Berberich T, Tateda C, Takahashi Y (2008) Polyamines: essential factors for growth and survival. Planta 228:367–381
Li T, Wang CG, Miao J (2007) Identification and quantification of indole-3- acetic acid in the kelp Laminaria japonica Areschoug and its effect on growth of marine micro algae. J Appl Phycol 19:479–484
Li XG, Su YH, Zhao XY, Li W, Gao XQ, Zhang XS (2010) Cytokinin over production caused alteration of flower development is partially mediated by CUC2 and CUC3 in Arabidopsis. Gene 450:109–120
Loreto F, Schnitzler JP (2010) Abiotic stress and induced BVOCs. Trends Plant Sci 15:154–166
Mackerness SAH (2000) Plant responses to ultraviolet-B (UV-B:280-320nm) stress. What are the key regulators? Invited review. Plant Growth Regul 32:27–39
Mackinnon SA, Craft CA, Hilty D, Ugate R (2010) Improved method of analysis for betaines in Ascophyllum nodosum and its commercial seaweed extracts. J Appl Phycol 22:489–494
Marián FD, Garcia-Jiménez P, Robaina RR (2000) Polyamines in marine macro algae: levels of putrescine, spermidine and spermine in the thalli and changes in their concentration during glycerol-induced cell growth in vitro. Physiol Plant 110:530–534
Maršálek B, Zahradníčková H, Hronková M (1992) Extracellular abscisic acid produced by cyanobacteria under salt stress. J Plant Physiol 139(4):506–508
Mikami K, Mori IC, Matsuura T, Ikeda Y, Kojima M, Sakakibara H, Hirayama T (2016) Comprehensive quantification and genome survey reveal the presence of novel phytohormone action modes in red seaweeds. J Appl Phycol 28:2539–2548
Mori IC, Ikeda Y, Matsuura T, Hirayama T, Mikami K (2017) Phytohormones in red seaweeds : a technical review of methods for analysis and a consideration of genomic data. Bot Mar 60(2):153–170
Nimura K, Mizuta H (2002) Inducible effects of abscisic acid on sporophyte discs from Laminaria japonica Areschoug (Laminariales, Phaeophyceae). J Appl Phycol 14:159–163
Romanenko EA, Kosakovskaya IV, Romanenko PA (2015) Phytohormones of microalgae: biological role and involvement in the regulation of physiological processes. Pt I. auxins, abscisic acid, ethylene. Int J Algae 17(3):275–289
Sacramenta AT, Garcia-Jimenez P, Alcazar R, Tiburcio AF, Robaina RR (2004) Influence of polyamines on the sporulation of Grateloupia (Halymeniaceae, Rhodophyta). J Phycol 40:887–894
Sitnik KM, Musatenko LI, Vosyuk VA, Vedenicheva NP, Generalova VM, Martin GG, Nesterova AN (2003) Gormonal’nii Kompleks roslin i gribiv (Hormonal complex in plants and fungi). Akademperiodika, Kiev
Skibola CF (2004) The effect of Fucus vesiculosus, an edible brown seaweed, upon menstrual cycle length and hormonal status in three pre-menopausal women: a case report. BMC Complement Altern Med 4:10–18
Stirk WA, Van Staden J (1997) Comparison of cytokinin and auxin-like activity in some commercially used seaweed extracts. J Appl Phycol 8:503–508
Stirk WA, Van Staden J (2006) Seaweed products as biostimulants in agriculture. In: Critchley AT, Ohno M, Largo DB (eds) World seaweed resources. ETI Information Services Ltd., Wokingham, pp 1–32, (DVD ROM)
Stirk WA, Novák O, Strnad M, Van Staden J (2003) Cytokinins in macroalgae. Plant Growth Regul 41:13–24
Stirk WA, Novák O, Hradecká V, Pӗnčik A, Rolčik J, Strnad M, Van Standen J (2009) Endogenous cytokinins, auxins and abscisic acid in Ulva fasciata (Chlorophyta) and Dictyota humifusa (Phaeophyta): towards understanding their biosynthesis and homoeostasis. Eur J Phycol 44(2):231–240
Stirk WA, Balint P, Tarkowská D, Novák O, Strnad M, Ordog V, Van Staden J (2013a) Hormone profiles in microalgae: gibberellins and brassinosteroids. Plant Physiol Biochem 70:348–353
Stirk WA, Tarkowská D, Turecová V, Strnad M, Van Staden J (2013b) Abscisic acid, gibberellins and brassinosteroids in Kelpak R, a commercial seaweed extract made from Ecklonia maxima. J Appl Phycol 26(1):561–567
Stirk WA, Tarkowska D, Turecová V, Strnad M, Van Staden I (2014) Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. J Appl Phycol 26:561–567
Sun TP (2010) Gibberellin-GIDI-DELLA: a pivotal regulatory module for plant growth and development. Plant Physiol 154:567–570
Symons GM, Ross JJ, Jager CE, Reid JB (2008) Brassinosteroid transport. J Exp Bot 59:17–24
Tarakhovskaya ER, Maslov YI, Shishova MF (2007) Phytohormones in algae. J Plant Physiol 54:163–170
Tuhy L, Chowanska J, Chojnacka K (2013) Seaweed extracts as bio-stimulants of plant growth: review. Chemik 67(7):636–641
Weiss D, Ori N (2007) Mechanisms of cross talk between gibberellins and other hormones. Plant Physiol 144:1240–1246
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251
Yokoya NS, Stirk WA, Van staden J, Novák O, Tureckova V, Pencik A, Strnad M (2010) Endogenous cytokinins, auxins and abscisic acid in red algae from Brazil. J Phycol 46:1198–1205
Yoshida K, Igarashi E, Mukai M, Hirata K, Miyamoto K (2003) Induction of tolerance to oxidative stress in the green alga Chlamydomonas reinhardtii by abscisic acid. Plant Cell Environ 26:451–457
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shanab, S.M., Shalaby, E.A. (2021). Production of Plant Hormones from Algae and Its Relation to Plant Growth. In: Mohamed, H.I., El-Beltagi, H.ED.S., Abd-Elsalam, K.A. (eds) Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management. Springer, Cham. https://doi.org/10.1007/978-3-030-66587-6_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-66587-6_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66586-9
Online ISBN: 978-3-030-66587-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)