Abstract
Being sessile by nature, every plant is constantly exposed to myriad of both abiotic and biotic stresses in their immediate environment. All these different stresses negatively affect the plant’s growth, development, metabolism, reproduction, survival, productivity, and yield worldwide. It has been predicted by the Intergovernmental Panel on Climate Change reports that the whole scenario will be more frightening in the upcoming three decades due to the exponentially growing population, natural resources overexploitation, ozone layer depletion, and global warming. In this changing situation, heat stress has emerged as one of the major environmental stress worldwide. As a result, all the plant’s transcriptional, translational, molecular, cellular, organelle, metabolic, osmotic, and physiological profiles get altered. To cope up with the heat stress, plants regulate the complex interplay of gene expression, signal transduction cascades, and networks. One of the prominent signal transduction cascades that are affected upon the onset of heat stress is the mitogen-activated protein kinase (MAPK) cascade. Besides, this MAPK cascade also plays roles in plant’s vital processes and other stresses including osmotic, cold, and mechanical wounding. In the present book chapter, we have highlighted the involvement of the plant’s MAPK cascade under extreme environmental conditions, namely, heat stress. Additionally, we have also summarized the MAPK cascade’s role in heat-related secondary stresses such as osmotic, oxidative, and drought.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Stress signifies a set of conditions that deviate the organism from its “normal physiological conditions,” i.e., outside the optimum range in which the organism thrives. From seedling to the postharvest stage, plants encounter numerous stresses. The term “stress” can be categorized into two major groups, namely, biotic and abiotic stress (Gupta et al. 2020). The former one is a consequence of living disturbances, such as fungi, bacteria, viruses, algae, etc., and hampers the normal growth and development of the plants (Mehta et al. 2020). The latter one arises due to fluctuation in the plant’s physical environment (naturally occurring inanimate factors) like water, salinity, metal/metalloid toxicity, pollution, nutrient paucity, dwindling seasonal patterns, and temperature shifts (Husen 1997, 2010; Iqbal et al. 2015; Getnet et al. 2015; Embiale et al. 2016; Husen et al. 2018, 2019; Mehta et al. 2020; Pandey and Gautam 2020).
Compared to biotic stress, abiotic stresses are the first and foremost reason accounted for the loss of annual productivity rate (Sharma et al. 2019; Pandey and Gautam 2020). Interestingly, during the last five decades, one of the major global concerns besetting the crop biologists is the perpetually increasing temperature (Kaur et al. 2018; Rai 2020). The major reasons are global warming, increasing human population, overconsumption of fuel/resources, and anthropogenic activities. This is also reflected in the accumulation of greenhouse gases (GHGs) and increased heat entrapment in the immediate surroundings, famously known as global warming (Lesk et al. 2016; Yanni et al. 2020), which is supported by the data presented in Fig. 13.1. This GHGs-induced rise in ambient temperature ultimately inflicts a plunge in food production. In this chapter, we have highlighted the involvement of the plant’s MAPK cascade under extreme environmental conditions, namely, heat stress. Additionally, we have also summarized the MAPK cascade’s role in heat-related secondary stresses such as osmotic, oxidative, and drought.
(a) Graph showing the GISTEMP Seasonal Anomaly Cycle data based on MERRA2 reanalysis for the period 1880–2019. (b) Anomaly map depicting the globe surface temperature for the period 1880–2019. The data have been adapted from NASA-Goddard Institute for Space Studies (https://data.giss.nasa.gov/gistemp/). (Accessed on 24th January 2020)
2 Heat Stress
Biologically, a temperature condition which is hot enough for a persistent period, potentially jeopardizes the normal cellular functions, and results in a series of biochemical, morphological, physiological, and molecular changes that adversely affects the plant’s normal functioning is known as heat stress (Abdelrahman et al. 2020; Azhar et al. 2020). As temperature increases from the optimal threshold, plants adapt intricate mechanisms including cellular and molecular modifications to sustain cellular homeostasis. But prolonged exposure to abnormal temperature is competent enough to cause an irreversible menace to germination, plant growth, and development, reproduction, and finally yield loss, which is precisely addressed as heat stress (Liu et al. 2019; Ali et al. 2019). The optimum temperature varies from plant to plant, and any increase in temperature from the optimum for a prolonged period is categorized as heat stress. The effect of heat stress includes various physiological modifications at multiple levels throughout its ontogeny, i.e., protoplasm shrinkage, reduction in cell size, slough off leaves/flowers/fruits, reduced net assimilation rate, disturbed fertilization, general infertility, hormonal imbalance, increased respiration, early senescence, etc. (Abdelrahman et al. 2020; Azhar et al. 2020).
At the cellular level, it influences cell division and cell cycle by altering the phragmoplast microtubule elongation, formation of microtubule asters, and microtubule organization (Parrotta et al. 2016), whereas physiologically, changes like cell size reduction, rapid stomatal closure, enhanced number of xylem vessels in the root, and water loss have been diagnosed by the researchers (Lipiec et al. 2013; Haworth et al. 2018; Aliche et al. 2020). Depending on the extent and the temperature range, at the reproductive level, the plant experiences reduction in flower bud development, failure in the germination of the pollen tube and reduced viability of ovule, stigma anomalous positioning, and abnormal anther dehiscence (Raja et al. 2019; Aliche et al. 2020). Heat stress often comes along with drought (Loka et al. 2020). Therefore, protective measures including seed priming are preferred along with adaptive measures undertaken in drought conditions (Banerjee and Roychoudhury 2020). The overall effect of heat stress on plants and their responses are depicted in Fig. 13.2.
Additionally, the prolonged rise in temperature attenuates the photosynthetic efficiency due to many plausible reasons: (i) denaturation of the enzymes involved in the photosynthesis (e.g., RuBisCo) (Kumar et al. 2019), (ii) lipid peroxidation of chlorophyll and thylakoid membrane (Sharma et al. 2018), (iii) distortion of the grana stacking and PSII arrangement (Dongsansuk et al. 2017), (iv) disturbance of electron transport chain (Neves et al. 2019), and (v) loss of RuBP regeneration capacity (Chovancek et al. 2019). Due to all these abnormalities, Radiation Use Efficiency (RUE) of the plant reduces. On the other hand, an increase in respiration also leads to a higher rate of transpiration that causes permanent wilting and hence crop loss.
To control the water loss, plants shed their leaves, and in turn, photosynthesis is severely affected resulting in reduced grain weight (Bheemanahalli et al. 2019; Ali et al. 2019). Additionally, high temperature leads to a decrease in oil yield due to the reduction in linoleic acid content in Brassica, Helianthus, and seagrass (Beca-Carretero et al. 2018). All such injuries ultimately cause starvation, growth inhibition, reactive oxygen species (ROS) production, and ion flux reduction (Pucciariello et al. 2012; Baxter et al. 2014). In addition to all these effects, heat stress also causes both osmotic and oxidative stresses at the secondary level (Qi et al. 2011). As a result, it is one of the most challenging stresses that need to be tackled in order to achieve an optimum yield of the crop plants in the ideal case scenario where all other factors are taken care of.
3 Plant Response to Heat Stress
To maintain their cellular homeostasis in multiple natural adversities including high-temperature stress, plants have evolved various strategies during the course of time. Plants either tolerate or avoid the heat stress to some extent by some morphological and metabolic modifications (Matsui et al. 2019; Azhar et al. 2020). The mechanisms include induction of MAPK and Calcium-Dependent Protein Kinases (CDPK) cascades, scavenging the ROS, maintaining membrane stability, accumulation of compatible solutes, production of antioxidants, transcriptional activation and chaperone signaling, etc. (Fig. 13.2). All these mechanisms are regulated at the molecular level and help plants to fight against heat stress. Likewise, they also produce compatible solutes at the biochemical level, such as proline, polyols, tertiary and quaternary ammonium compounds, etc., that maintain the cell’s turgor pressure and help in the redox balance. In addition, there are multiple reports in the literature which elaborate the increase of late embryogenesis abundant (LEA) proteins, dehydrins, Pir proteins, ubiquitin, and heat shock proteins in the heat stress repercussions-surviving plants (Rurek 2010; Hand et al. 2011; Priya et al. 2019; Maher et al. 2019; Yadav et al. 2020). All the changes and modifications in the metabolism happen due to the changes in the upregulation of the genes that provide the plant with a range of osmoprotectants, transporters, regulatory proteins, and detoxifying enzymes. At the expression level, this results in heat tolerance that gets translated into acclimatization (in long-term “adaptation”) and depends directly on the signaling pathway. Para-heliotropism, altered membrane lipids, increased trichome density, etc., are the mechanisms undertaken by plants in heat avoidance; however, the signaling is involved here too (Thitz et al. 2017; Marcus 2019). Table 13.1 reviews the effect of high-temperature stress in different crop species.
4 Signal Transduction for Heat Stress
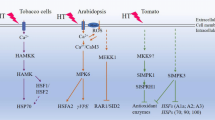
There is an array of signaling pathways involved in conferring heat stress resistance. While some of them control the expression and synthesis of heat shock proteins (HSPs), others are involved in the production and/or activation of different effector constituents (Yadav et al. 2020; Pereyra et al. 2020). To withstand the stressful conditions, plant upregulates various genes that get translated into a battery of proteins and enzymes that are the key players of stress signaling cascades to ultimately counter the stress (Kaur and Gupta 2005) (Fig. 13.3). The signaling cascades may operate independently or maybe in cross talk with various other pathways in the cell (Nakashima et al. 2014; Dunayevich et al. 2018; Muthuramalingam et al. 2020). Depending upon the signal transduction molecules, plant type, and subjected stress, there are broad groups of molecules along with transcription factors that activate the responsiveness of genes. These groups of molecules include Ca2+-dependent protein kinase (CDPKs), mitogen-activated protein kinase (MAPK/MPKs), NO, sugars, phytohormones (Azhar et al. 2020; Xalxo et al. 2020), etc. All these signaling increases the activity of antioxidants such as ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase, GST, CAT, glyoxalase I (Gly I), etc. (Fig. 13.2). This helps the plants to fight and survive in unfavorable conditions especially high-temperature and other-related stresses. Furthermore, various signaling pathways operate in a cell such as the AMPK signaling pathway, cAMP-dependent pathway, JAK-STAT signaling pathway, Nodal signaling pathway, Wnt signaling pathway, MAPK/ERK signaling pathway, and many more (Yu et al. 2019; Shumayla and Upadhyay 2019; Fu et al. 2020). Within all these highly conserved signaling cascades which play a central part in the heat stress conditions is the mitogen-activated protein kinase (MAPK) pathway (Yu et al. 2019; Ijaz et al. 2019). In simple words, mitogens are actually the agents that stimulate/promote cell division. Because of activation and deactivation of the enzyme by the kinases and phosphatases action, the signal passes down fast. Apart from heat stress, there are many studies in the literature which point out the importance of the MAPK pathway in growth, hormone signaling, and other stress too including cold, drought, ROS, salinity, wounding, ozone, and UV radiation (Qiu et al. 2019; Sözen et al. 2019; Mahmood et al. 2020). Table 13.1 tabulates the reported MAPKKK and associated components of MAPK signaling cascades in a few plant species.
Diagrammatic representation to show the core of MAPK pathway which contains three components MAPKKK-MAPKK-MAPK and along with that the downstream targets of the pathway. The downstream targets generally are transcription factor (WRKY), ribosomal protein (RPS), protein kinases/lipase/phosphatases, microtubule binding protein, and cytoskeleton-associated proteins
5 MAPK Pathway in Plants
A cell has multiple MAP kinase pathways that control distinct cellular responses. The pathway is composed of three protein kinases: a terminal MAP kinase and two upstream kinases, namely, MAPK kinase (MKKs) and MAPK kinase kinases (MKKKs) (Sözen et al. 2019). These kinases sequentially channelize, integrate, and amplify the cellular external environment response. MAPKs were first discovered because of their ability to phosphorylate the microtubule-associated protein-2 (MAP2), and that is why it was initially named as MAP2 kinases (Ray and Sturgill 1987). Later, it was discovered that these proteins show homology with certain mitogen-stimulated proteins (such as p42 protein), and they were renamed to mitogen-activated protein kinase (Cooper et al. 1982). With the continued research in the same field, it is now known that there are certain MAP kinases (a member of MAPKs family) collectively known as extracellular signal-regulated kinases (ERK). The first ERK (an insulin-activated protein kinase) was discovered from the Chinese hamster ovary. MAPKs need activation in the form of phosphorylation (Ijaz et al. 2019), and the activators are known as MEK (for MAPK/ERK Kinase). MEKs are in turn phosphorylated by MEKs activator, and they are known as MEKK (MEK Kinase) (Wang et al. 2015). The first MAPK pathway was characterized in Arabidopsis, and AtMEKK1, AtMKK2/MEK1, and ATMPK4/AtMPK6 constitute the whole pathway. It is vital for the plant’s innate immunity and also regulates the response in conditions like salt, cold, and drought stress (Blanco et al. 2006; Xing et al. 2008). In interaction studies, it was established that AtMEKK1interacts with AtMKK2 and MEK1. AtMPK4 interacts with both AtMKK2 and MEK1, sometimes directly to AtMEKK1. MEK1 primarily phosphorylates the threonine residue of AtMPK4, but tyrosine phosphatase can deactivate AtMPK4. This suggests that in plants either the MEK1 doesn’t have dual specificity and thus tyrosine phosphorylation is simultaneously done by second MEK (Ichimura et al. 1998) or tyrosine gets autophosphorylated and thus MAPK is activated. MAPK cascade has a very important role to play in signal transduction for the multitude of stress responses (Fig. 13.3).
5.1 MAPKs
MAPKs are serine/threonine kinases activated by MEKs which either move to the nucleus to phosphorylate other specific transcription factors or stay in the cytoplasm to pass the signal to cytoskeleton binding proteins or some enzymes (protein kinases, phosphatases, phospholipases, etc.) for further signal transmission. Upon activation, MAPK disassociates from MEK and get arranged in a homodimer form to expose a domain called as MAP kinase insertion domain for facilitating active nuclear import (Pitzschke 2015; Wu et al. 2015). In MAPKs, the substrate phosphorylation occurs specifically at serine/threonine residues that are followed by a proline residue, i.e., PXS/T where X can be basic or neutral, but −2 position proline is not required (Clark-Lewis et al. 1991; Gonzalez et al. 1991). This mechanism provides specificity in substrate recognition, and the P + 1 loop present in the substrate-binding pocket regulates the substrate binding. Binding to the proline of the substrate is only possible when kinase is in active form (Canagarajah et al. 1997). A secondary structure called activation loop is present which forms the mouth of the active site (Zhang et al. 1995). The dual phosphorylation motif (TXY: threonine–X–tyrosine) is present on the activation loop in kinase sub-domain VIII (Payne et al. 1991; Gartner et al. 1992), and phosphorylation on both tyrosine and threonine residues is required for the full activation of the MAPKs; as without phosphorylation, the binding site will be blocked. Distinct MAPKs have distinct dual phosphorylation motif, and the length of the loop also varies (length of the loop controls the autophosphorylation of the protein) (Jiang et al. 1997). Mammalian MAP kinases can be divided into three families, and each family has multiple members and multiple activators (MEK and MEKK) that are present upstream to MAPK. The first family is ERK/MAP kinases, and the members are many times activated when receptor tyrosine kinase is activated by EGF (epidermal growth factor), and the activation sequence for this family is threonine–glutamic acid–tyrosine (TEY). The other family is JNK/SAPK (Jun N-terminal kinase/stress-activated protein kinase), and they are activated by stress or inflammatory cytokines with the activation sequence: threonine–aspartic acid–tyrosine (TDY). The last family is p38/Hog which is activated by cytokines, endotoxins, and osmotic stress with activation sequence: threonine–glycine–tyrosine (TGY). As EGF, which activates both ERK and JNK/SAPK pathways, extracellular stimuli may activate more than one pathway (Cano and Mahadevan 1995). Plant MAPKs shares high homology with the ERK subfamily. Plant MAPKs mainly cluster in one group which is known as PERKα, and very few (three) clusters in a group denoted as PERKβ. Based on sequence similarity, the PERKα family can be divided into five different subfamilies named as PERKα1–5. The activation loop length varies among the PERK groups which are 25, 22, and 21 amino acids long for PERKβ, PERKα5, and PERKα1–4, respectively (Ligterink and Hirt 2001).
Generally, all plant MAPKs have a TEY motif at the site of dual phosphorylation, except in Arabidopsis and alfalfa MAPK because they have TDY as the motif. Additional to this, the MAPKs having the TDY motif have an additional C-terminal extension (Mizoguchi et al. 1997; Schoenbeck et al. 1997) compared to the MAPKs that have a TEY motif. Generally, C-terminal (not extended) has a CD (common docking) domain which acts as the docking site for MPKKs, phosphatase, and substrate proteins (Ichimura et al. 2002). The amino acid sequence [LH][LHY]Dxx[DE]xx[DE]EPxC (where x is any amino acid residue) clearly denotes the importance of acidic residues D (aspartate) and E (glutamate) in interacting with the basic (K-lysine and R-arginine) counterparts that are present as a cluster on the MPKKs. Based on the sequence of the activation loop, the MAPKs can be divided into four groups (A, B, C, and D). Groups A, B, and C have a TEY sequence motif, whereas group D has a TDY sequence motif. Group A members are involved in environmental and hormonal responses, e.g., MPK6 (Arabidopsis), and its orthologs in other plants are activated by various environmental cues. Group B members are involved in environmental stress responses and cell division, e.g., MPK4 (Arabidopsis) is induced by both biotic and abiotic stress induction, and subgroup B2 members MPK13 (Arabidopsis), Ntf6 (Nicotiana), and MMK3 (Alfalfa) have cell cycle-dependent activation. Group C member MPK7 (Arabidopsis) shows expression that is regulated by circadian rhythm. Some group D members (BMWK1 from rice and TDY1 from alfalfa) are induced by pathogen attack and wounds. Group D members lack the CD domain, but some like MPK8, MPK9, and MPK15 (Arabidopsis) have a small (60–80 amino acids) N-terminal extension (Ichimura et al. 2002). Table 13.2 summarizes about the reported MAPKs and their associated activators in plant species using Google scholar (https://scholar.google.co.in/).
5.2 MKKs
MKKs are dual-specificity protein kinase which activates MAPKs by phosphorylating both tyrosine and threonine residues on the TXY motif of the activation loop. As protein kinases are generally specific for either serine/threonine or tyrosine phosphorylation, it was hypothesized that MAPK might require two protein kinases for its activation. Surprisingly, a single dual-specificity kinase (MEK) activates the MAPK by phosphorylating both tyrosine and threonine residues on the TXY motif (Matsuda et al. 1992; Pitzschke 2015). In the signaling cascade, MKKs can in turn get activated by phosphorylation on the two conserved serine or threonine residues which are present in between the domain VII and domain VIII (Alessi et al. 1995; Zheng and Guan 1994), and these conserved amino acids form a motif S/TXXX S/T in most MKKs. In most yeast and animal MKKs, the motif is SXXXS/T, while in plants, S/TXXXXXS/T is the motif (Ligterink and Hirt 2001). The activity of MKKs can be regulated at the posttranslational level by phosphorylation of the residues other than the conserved serine or threonine residues that negatively regulates MKKs (Brunet et al. 1994; Rossomando et al. 1994). Along with the posttranslational regulation, MKKs can be regulated at the posttranscriptional level by differential splicing (English et al. 1995). The substrate specificity for the MKKs is restricted, and the specificity is defined by multiple domains of MAPKs, and MKKs in turn bind to the tertiary structure of the MAPKs, thus restricting the substrate specificity (Seger et al. 1992). That is why MKKs are considered to be the convergence points of the pathway as it can receive many signals that it can feed into the MAPKs pathway. A conserved N-terminal putative MAPK docking site [K/R][K/R][K/R]x(1–5)[L/I]x[L/I] (basic –K and R at the extreme N-terminal to the hydrophobic L and I at inwards N-terminal) is the docking site for the MAPKs (Bardwell and Thorner 1996), and MKKs are known to regulate the kinetics of the cascade. Specificity is enhanced when both MAPK and MEK interact with the scaffold protein (e.g., MP1; Schaeffer et al. 1998) that also linearly guides the interaction between the components of the cascade (Fig. 13.3). The complex formed by pathway components with the scaffold protein is known as signalosome, and such interactions restrict any cross talk that can happen with the multitude of different pathways and within the pathway (Chang and Karin 2001; Whitmarsh and Davis 1998). Normally, MKKKs (to perceive myriad of stimuli) are present more than MKKs, and thus, it can be hypothesized that they also function in signal integration (Ferrell 1996), and an MKK can activate multiple MAPKs (they are also in excess) in the cascade, and this is the step of signal amplification (Ichimura et al. 2002). The first-ever MKK reported in plants was from Nicotiana, and thus, it was named NPK2 (nucleus- and phragmoplast-localized protein kinase, renamed from Nicotiana protein kinase 2). Plant MKKs are divided into three subfamilies, namely, PMKK1, PMKK2, and PMKK3, from which the members of PMKK3 have additional long non-catalytic C-terminal which is not present in other PMKKs subfamilies (Ligterink and Hirt 2001). Based on new advances, the plant MKKs can be divided into four groups (A, B, C, and D). Group A members are involved in multiple abiotic stress responses, e.g., MKK1 and MKK2 (upstream of MPK4 in Arabidopsis), whereas PRKK (pathogen-responsive MPKK in alfalfa) involved in the transduction of elicitor signals, and MKK6 (Arabidopsis) along with NtMEK1 (Nicotiana) is involved in cell division. Group B members MKK3 (Arabidopsis) and NPK2 (Nicotiana) have an extended C-terminal, and the extended C-terminal consists of a nuclear transport factor (NTF2) for nuclear localization. Group C members are stress-responsive, and downstream, they signal group A members of MAPKs, e.g., SIMKK has both salt and elicitor-induction specificity and NtMEK2 (Nicotiana) can induce SIMK (salt) and WIPK (wound).
5.3 MKKKs
MKKKs are serine/threonine kinase that regulates the MKKs activation. Structurally, MKKKs are different from MAPKs and MKKs, and different MKKKs have different regulatory motifs such as Pleckstrin homology (PH) domains, proline-rich sequences involved in SH3 binding, zinc finger motifs, leucine zippers, and binding sites for G-proteins (Garrington and Johnson 1999; Pitzschke 2015). MKKKs have multiple tyrosine and serine/threonine phosphorylation sites, and thus, it can be activated by a different mechanism like it can be phosphorylated by MKKKs and PKCs, by interaction with G-protein, or by the cellular two-component system (Whitmarsh and Davis 1998; Fanger et al. 1997). This much diversity in the structure and the mode of activation provide flexibility to respond to different stimuli. MKKKs are the branching point and mediate cross talk between signaling pathways. Many MKKKs have been identified by various scientists; however, Raf is well studied and documented (serine/threonine-protein kinase). Mostly, plant MKKKs show homology with yeast MEKK/STE11 and mammalian Raf (related to retroviral oncogenes), and hence, they are known as PMEKKs (e.g., AtANP1 and AtMEKK1 with a conserved sequence G (T/S) Px (W/Y/F) MAPEV) and PRaf (e.g., AtCTR1 and AtEDR1 with a conserved sequence GTxx (W/Y) MAPE) (Rao et al. 2010). The PMEKKs can be subdivided into three groups, i.e., PMEKK1, PMEKK2, and PMEKK3, whereas PRaf can be subdivided into two groups, i.e., PRaf1 and PRaf2 (Ligterink and Hirt 2001). Some sequences in the plant also share homology with the mixed lineage kinase (MLK), thus widening the range of diversity of MKKKs in plants (Ligterink and Hirt 2001). Ichimura et al. (2002) has also provided a way of dividing MKKKs in a plant in which group A members are MEKKK1 type and group B and C members are RAF kinase type. Group A further can be divided into five subgroups and subgroup A1 (AtMEKKK1, AtMEKKK2, AtMEKKK3, and AtMEKKK4) members active in drought, high salinity, and touch. AtMEKKK1 is present upstream of MPK4, MKK1, and MKK2. AtMEKKK4 has an extended N-terminal which is a unique feature as it has several domains such as a glycine-rich region, WRKY domain, paired-amphipathic-helix repeat, TIR domain, leucine-rich repeat (LRR), NB-ARC domain, and a protein kinase domain. WRKY proteins are Zn-finger transcription factors that are specific to plants as they regulate plant defense response and capacity to deal with drought condition, and WRKY domain provide the direct DNA binding capacity to the protein. Along with that, the presence of the TIR-NB-LRR domain further confirms the role of AtMEKKK1 in plant defense. Subgroup A3 members like ANP1, ANP2, and ANP3 have a C-terminal regulatory region, and NPK1 is a positive regulator of cytokinesis (Nishihama et al. 2001) and a negative regulator of the stress response (Krysan et al. 2002). But all of these MPKKKs work in oxidative stress response as a negative regulator of the auxin-response pathway (Kovtun et al. 2000). Subgroup A4 members AtMAP3Kε1 and AtMAP3Kε2 are involved in cell division (Jouannic et al. 2001), and the function of subgroup A2 members is not determined. Group B members are RAF kinase such as CTR1 which is involved in ethylene signaling, and EDR1 is involved in disease resistance signaling. They have an extended N-terminal (regulatory) and a C-terminal kinase domain, whereas specifically, subgroup B2 N-terminal has PAS (Per, Arnt, and Sim) domains and PAC (PAS-associated C-terminal) domains. Group C members are also RAF kinase, e.g., ATN1 and AtMRK1. Broadly, functions of the group members are unknown. There is some information about the domains that are present like the N-terminal of subgroup C1 members having an ankyrin like a domain that is known for protein-protein interaction. Subgroup C2 has an aspartokinase, chorismate mutase, and Try A (ACT) domain which is known for sensing amino acid concentration and then regulating the activity of many metabolic enzymes (Aravind and Koonin 1999). There is another group present in cucumber MEKKs family known as ZIK like kinases, and they have an N-terminal kinase domain having a signature sequence GTPEFMAPE (L/V) Y. ZIK like kinases are also known as WNK (with no lysine (K)) which are involved in controlling circadian/internal rhythms (Murakami-Kojima et al. 2002) and in responding to abiotic stress (Kumar et al. 2011) without showing any evident phosphorylation of the MKKs in plants (Kong et al. 2013b) such as At3g04910 in (Arabidopsis). There are chances that with further studies there will be additional groups that will add up to already mentioned groups of MKKKs in plants. Members of MEKKs have structural diversity and don’t share any general structure, whereas Raf protein kinase has similar structural organization both in plants and animals, i.e., catalytic domain present at C-terminal and a long non-catalytic extension at the N-terminal (Ligterink and Hirt 2001). In addition, the non-catalytic domains both in plants and in animals are rich in serine and cysteine (Ligterink and Hirt 2001). The sequence and structural diversity of MKKKs in plants suggest that even in plants, MKKKs have a wide variety of substrates and diversity in mode of regulation (Ligterink and Hirt 2001). NPK1 forms the largest group of MKKKs plant and regulation of homologs of NPK1 in Arabidopsis (ANP1) occurs by differential splicing resulting in ANP1L (large) and ANP1S (small) forms and ANP1S shows higher activity than the ANP1L spliced form which highlights the role of splicing in the regulation of MKKKs in plants (Nishihama et al. 1997). The cascade does not always work in a linear direction as MAPKs and MKKs can phosphorylate the MKKKs or the upstream regulating components, and this serves as the negative feedback loop (Ueki et al. 1994). At the same time, MAPKs can interact with MKKKs (thus activating) in the positive feedback mechanism (Zimmermann et al. 1997; Pitzschke 2015).
5.4 Upstream of MAPK Cascade
In upstream of MKKK, different effectors are known to function and activate the MAPK pathway. Several kinases act upstream of MKKKs in yeast and mammalian systems such as receptor tyrosine kinases (RTK) and G-protein-coupled receptors. When RTK is active, it can stimulate the exchange of guanosine triphosphate (GTP) for the guanosine diphosphate (GDP) on G-protein Ras. Activated Ras can then interact with potential partners including Raf (Morris 2001; Pitzschke 2015). Yeast on contrast doesn’t possess RTKs and instead has two components, i.e., histidine-protein kinase and G-coupled protein receptors. Yeast and mammalian MKKKs can be divided into two subfamilies. The first one is the STE20/PAK subfamily, and it is characterized by the catalytic domain at C-terminal and putative G-protein binding motif at the N-terminal. The other subfamily is GCK/SPS1, and this subfamily has a characteristic catalytic domain at the N-terminal and a long kinase-unrelated region which is mostly activated by stress at the C-terminal (Fanger et al. 1997). In plants, RTKs are not present, but instead, receptor-like kinases (RLKs) are present, and RLKs are transmembrane serine/threonine protein kinases (Stone and Walker 1995). In the plant system, there are two putative MKKKs, i.e., BnMAP4Kα1 and − 2 from rapeseed (Leprince et al. 1999), and one MKKK, i.e., SIK1 (for stress-induced kinase1) from Arabidopsis which can be grouped under GCK/SPS1 subfamily. Many genomic sequences that are candidates for the plant MKKKs can be grouped under STE20/PAK. In yeast and mammals, the presence of PKC and small G-proteins to activate MKKKs somewhere indicates that similar regulation of activation exists in plants upstream of the MAPK cascade (Ligterink and Hirt 2001).
6 Role of MAPK Pathway in Heat and Other Related Stresses in Plants
6.1 High-Temperature Stress
The first report of induction of MAPKs under heat stress is from the group of Sangwan who highlighted the heat-activated mitogen kinase (HAMK) getting induced in alfalfa (Sangwan et al. 2002). Following this, there were many reports in the literature that have shown that high temperatures induce the expression of many MAPK components in varied plants. OsMSRMK2 is a rice multi-stress-responsive gene of the MAP kinase family (Agrawal et al. 2002). In one of the preliminary study, it was found out that OsMSRMK2 can sense the change in temperature as the transcripts shows enhancement (37 °C) and no induction (25 °C and 12 °C) with in a period of 15 min. Whereas it showed a transient nature because at 37 °C, the transcript level decreases at the time point of 30 min and beyond and at 25 ºC the transcripts started increasing at 30 min and then drastrically decreased after that, interestingly, at low temperature (12 ºC) the transcripts started accumulating at 60 min and reached to a height at 90 min and then shown a decrease at 120 min. But a rapid accumulation at high temperature suggests that it helps the plant in sensing the adverse temperature condition and thus prepares the plant to thrive in it (Agrawal et al. 2002). Another report by Link et al. (2002) showed that the heat activation of the MAPK pathway in tomato is calcium as well as heat stress factor 1 (Hsf1) dependent. The phosphorylation of Hsf1 at the tyrosine by the involved MAPK activates Hsf1, and the activated Hsf1 further activates the Hsps which induces thermo-tolerance in the plant (Link et al. 2002). On the other hand, HAMK, a 46 KDa protein, becomes active in tobacco (Nicotiana tabacum) and SlMAPK1 in tomato (Ding et al. 2018) at the advent of heat stress, and in Arabidopsis, the expression of AtMPK6 increases during the heat stress (Li et al. 2014a; Li et al. 2014b). AtMPK6 mediates the activation of γVPE which is a vacuolar-localized cysteine protease with a Caspase1 like activity, and it basically activates the downstream hydrolytic enzymes in the vacuole which are responsible for the induction of hypersensitive reaction. This leads to cell death and tissue senescence (Albertini et al. 2014). Thus, it was postulated by that γVPE plays a role in heat-induced programmed cell death (PCD). γVPE is expressed in guard cells of Arabidopsis, and Albertini et al. 2014 deciphered its involvement in water stress which is one of the secondary stress of high-temperature stress. In another report by Evrard et al. 2013, it was reported that AtMPK6 negatively regulates heat stress by phosphorylating HSF2, which is a known heat shock factor and plays a role in heat stress response. In similar reports, it was reported that the mpk6 Arabidopsis mutant shows higher tolerance than the wild type. A study undertaken by Ding et al. 2008 on tomato has helped in deciphering the mechanism of action of the MAPK signaling pathway in alleviating the high-temperature stress. In the case of tomato, there is 16 putative family of SlMPK which have been grouped into four major groups (A–D) (Kong et al. 2013a; Kong et al. 2013b). Silencing of MPK1/2 (SlMPK) results in compromised tolerance toward heat, cold, and oxidative stress (Nie et al. 2013; Zhou et al. 2014; Lv et al. 2017). But another report from Ding et al. (2018) showed that silencing SlMPK 2 can increase the tolerance of plants toward high-temperature stress. SlMPK1 (ortholog of AtMPK6, NtSIPK, and OsMPK6) is a negative regulator of heat stress responses (Ding et al. 2018). The tomato plants with silenced SlMPK1 gene show the expression of many proteins that are involved in various functions such as protein folding, lipid metabolism, translation, amino acid biosynthesis, and oxide reduction. The silenced lines show no chlorophyll degeneration as compared to the wild type. Thus, Ding et al. (2018) postulated that it is due to the activation of CPN-60 (role in chloroplast biogenesis and plastid division) (Ahsan et al. 2010), and CPN-60B is known to play a vital role in acclimatizing photosynthesis to high temperature (by protecting the thermal denaturation of RuBisCo activase). The lines that are having suppressed expression of SIMPK1 have accumulated redox buffers. The main mechanism behind the attained tolerance was the reduction of the high temperature-induced oxidative damage to maintain cellular redox homeostasis (Ding et al. 2018). SIMKK9 (homolog of AtMKK9) interacts with SlMPK1, and it is an upstream component of the pathway, and the downstream partner of SlMPK1 is SISPRH1 (homolog of At1g04330), but it is a protein of unknown function and has a putative phosphorylation site at Ser-44 (Ding et al. 2018). Later, it was deciphered that this phosphorylation site is very important for the enzyme activity as a mutation at this residue can block the SlMPK1-mediated inhibition under high temperature (Ding et al. 2018). High temperature induces inactivation of a 50KDa kinase, and the ability of this kinase to phosphorylate myelin basic protein (MBP) suggests that it is a member of the MAPK family (Heider et al. 1998). While the induction of the heat shock genes by the transcription factor Hsf1 is a general response in heat stress, this is repressed by the phosphorylation of Hsf1 by the ERK1 resulting in the silencing of heat-inducible genes in unstressed conditions (Chu et al. 1996). Most of the studies conducted by researchers confirmed MAPKs except CsMPK3 and CsMPK7 are overexpressed under heat and drought stress (Wang et al. 2015). It has also been studied that SlMPK3 plays a major role during various biotic and abiotic stresses. Previously, it was known that knockout of SlMPK3 results in reduced drought tolerance and disease resistance to Botrytis cinerea. But SlMPK3 also gets influenced by heat stress as its relative expression gets downregulated, and its knockout provides tolerance to heat stress (Yu et al. 2019). Both ion leakage and MDA content were significantly lower in the knockout mutants. This suggested that SlMPK3 acts as a negative regulator of heat stress whose knockout maintains the relative integrity of the cell membrane and reduces cell membrane damage. Elevated levels of SlHSP70, SlHSP90, SlHSP100, and SlHSFA1a, SlHSFA2, and SlHSFA3 were observed in SlMPK3 mutants, indicating the increase in HSPs and HSFs genes’ relative expression might be associated with SlMAPK3-mediated heat stress response in tomato plants (Yu et al. 2019).
6.2 Oxidative Stress
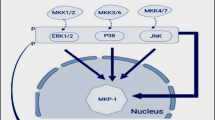
Oxidative stress is the most common secondary stress in biotic and abiotic stress conditions as in most of the stressed conditions, the disruption of metabolic imbalance of cell takes place, and that hampers the cellular redox homeostasis. But this is also true that ROS plays a vital role in signaling in the lower concentrations. In support of that, investigation done by Kovtun et al. (2000) helped in understanding the effect of H2O2 on MAPK activation under stress condition that will aid the plant to somehow deal with the adverse condition (in this case under pathogen attack). In Arabidopsis protoplast study, they found out two H2O2-activated (independent of other activators like ethylene, SA, and JA) MBP kinases (44 and 42 KDa mass). They also elucidated that H2O2 activated promoters of oxidative stress-responsive gene GST6 and an HSP named HSP 18.2. The fact that H2O2 is involved in cell cycle under nonstress conditions and also the involvement of MAPK in cell cycle regulation instigated the idea that the tobacco cell cycle regulating MAPK-NPK1 (A class of MEK kinases) might be mediating oxidative stress responses in plant cells (Nakashima et al. 2014). The homologs of NPK1 in Arabidopsis MEK kinases are ANP1, ANP2, and ANP3 which can activate two MBP kinases (same molecular masses) as activated by H2O2. Later, it was established that ATMPK3 and ATMPK6 are the substrates of ANP1, ANP2, and ANP3. Activation of MAPK suppresses the oxidative stress. But sometimes, there are activators of ROS that don’t involve in the activation of MAPK, and this suggests that MAP kinase activation is either independent or upstream of oxidative burst. In a study conducted by Xing et al. (2007), it was found out that MAP2K inhibits the ABA-dependent activation of the CAT1 enzyme. Nakagami et al. (2006) showed that MEKK1-MPK4 cascade has a role to play in ROS metabolism. Not only the MAPK pathway mediates the oxidative stress responses but also it regulates the concentration of ROS in the system by hampering the expression of the CAT enzyme (Kong et al. 2013b). An alfalfa MAPKKK, namely, OMTK1 (oxidative stress-activated MAP triple-kinase 1), is a key response regulator, and it further regulates the downstream components that are MAPK and MMK3 (Nakagami et al. 2004). The role of MAPKs in oxidative stress was also similarly deduced by the many other researchers.
6.3 Osmotic Stress
One of the secondary stresses that come in the picture due to the high temperature with which the plant has to deal is the osmotic stress. Plants have protein kinases that deal with the changes in the osmolarity. In green algae, Dunaliella tertiolecta hypoosmotic stress induces a 40 KDa kinase, and this kinase can phosphorylate MBP and histone, while the hyperosmotic stress induces a 40 KDa (can phosphorylate MBP, histone, and casein) kinase and a 45 KDa (can phosphorylate MBP only) kinase (Yuasa and Muto 1996). The activity of these kinases is independent of the presence of calcium, and thus, they are not CDPKs and thus possibly can be MAPKS. But MAPKs don’t use casein as a substrate, while histone can be used as a substrate by MAPK in plants (Wilson et al. 1995; Zhang and Klessig 1997). This suggests that the 40 KDa protein kinase induced in hyperosmotic stress cannot be a member of the MAPK family, while others can be MAPKs. Another response that is evident under osmotic stress in D. tertiolecta cells is that the cell volume changes in response to the extracellular osmolarity, and to gain the original volume, the cell induces MAPK cascade to nullify the effect of the osmolarity changes. In several experiments where the application of protein kinase inhibitor can block the recovery process, the involvement of protein kinases in the cell osmolarity balance kinetics was established. Thus, all these instances indicate the involvement of stress-induced protein kinases in osmotic tolerance in D. tertiolecta (Yuasa and Muto 1996), and these protein kinases can be MAPKs. Extending the studies to the higher plants indicates that MAPKs are involved in osmotic stress tolerance in plants too. In an in vitro study where tobacco-suspension-cultured cells were given hyperosmotic stress, it resulted in the activation of MAPK like kinases. The hypoosmotic stress resulted in the activation of MBP kinases of 50, 70, and 80 KDa molecular weight (Takahashi et al. 1997). 50 KDa protein kinase exhibits all the characteristic properties of an MAPK as the activation and inactivation through phosphorylation and dephosphorylation events (Takahashi et al. 1997). Even yeast has osmosensors to sense hyperosmotic stress, and one of which is a part of a two-component regulatory system. Yeast osmosensor SLN1 gets autophosphorylated at histidine which is present at the N-terminal sensor domain. The phosphate is then transferred to YPD and then to the aspartic residue of the C-terminal sensory domain of the SSK1 which is a response regulator. Then SSK1 feeds the signal into the HOG1 MAPK pathway (Posas et al. 1996). A similar pathway or mechanism of response exists in plants as Arabidopsis SLN1 homolog AtHK1 act as osmosensor in complementation studies with SLN1-deficient yeast cells (Shinozaki and Yamaguchi-Shinozaki 1997). Similarly, a pea MAPK, i.e., PsD5, can complement HOG1-deficient yeast mutant (Pöpping et al. 1996), and an alfalfa MAPK, i.e., MMK2, can complement yeast MPK1 kinase (Jonak et al. 1995). Some stress-activated MAPKs like AtMPK3 and stress-activated MAPK (previously called as MMK4) also get upregulated in drought, cold, heat, touch, and salt stress, and all these stresses result in a condition of dehydration. So this can be concluded that AtMEKK1/AtMPK3 and SAMK can deal with general dehydration conditions and thus can provide tolerance to the plant against the osmotic changes (Ligterink and Hirt 2001).
6.4 Drought Stress
The high temperature in absence of irrigation/rainfall generally results in a condition called drought. In situations of dehydration, AtMEKK1 and AtMPK3 are transcriptionally activated (Mizoguchi et al. 1996). A ribosomal S6 kinase is activated by MAPK after phosphorylation in the mammalian system (Sturgill et al. 1988; Gregory et al. 1989), and a homolog of the same exists in Arabidopsis, i.e., AtPK19 which shows accumulation in the drought stress (Mizoguchi et al. 1996). This somewhere indicates that AtPK19 is activated by AtMPK3 following a similar pathway as in the mammalian system. Transcriptional and translational activation of SAMK is also reported, but no significant changes in the protein level were found (Jonak et al. 1996). The drought-stressed leaves of alfalfa show the activation of p44MKK4 kinase within 5 min (Jonak et al. 1996). Although the activation was transient and ABA independent (Jonak et al. 1996), after full activation, it shows a decrease in activity (after 20–30 min). But the activation of p44MKK4 under high temperature (37 °C) was not seen in lab experiments. The drought-like condition induces the activation of OsMSRMK2 and OsMAPK5 in rice plants. DSM1, a putative MPKKK of rice, when overexpressed can increase the tolerance of the plant toward dehydration (Ning et al. 2010).
7 Concluding Remarks and Future Prospects
Due to the fact that the MAPK cascade is the major multitier player network for stress signaling transductions involved in various environmental biotic and abiotic stresses, MAPKs and other components involved in the perception of various signals are chosen as targets by multiple biologists, breeders, and bioinformaticians worldwide. Various omics approaches such as transcriptomics, proteomics, miRNAomics, metabolomics, and bioinformatics along with high-throughput DNA sequencing have allowed precise analysis of MAPK pathway cross-networking under various abiotic stresses. Due to the increase in ambient temperature around the plants, it has posed a serious threat on the yield and productivity. As a result, a huge plethora of studies has been conducted to solve the problem of making the plants more tolerant to high-temperature stress by different means of genetic modifications in order to sustain the crop yield for every second increasing population. By implying this knowledge to wheat, ryegrass, Chinese clematis, black raspberry, and other 580+ sequenced plants, the orthologous genes can be discovered and can be used to improve every important crop plant against heat and other abiotic stresses. Consequently, as already discussed above, overexpressing the positive regulators of MAPK pathway components and knocking out or editing the negatively regulating components are the ways toward climate-resilient agriculture. In a long run to the future, the rewiring of circuits will ultimately enable the smooth cultivation of crop plants such as rice, wheat, tomato, potato, lemongrass, jute, cotton, and many more even in harsh conditions.
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- APX:
-
Ascorbate peroxidase
- cAMP:
-
Cyclic adenosine monophosphate
- CAT:
-
Catalase
- CDPK:
-
Calcium-dependent protein kinase
- DHAR:
-
Dehydroascorbate reductase
- ERK:
-
Extracellular signal-regulated kinase
- GHGs:
-
Greenhouse gases
- Gly I:
-
Glyoxalase I
- GR:
-
Glutathione reductase
- GST:
-
Glutathione-S-transferase
- H2O2:
-
Hydrogen peroxide
- Hsf1:
-
Heat shock factor 1
- HSPs:
-
Heat shock proteins
- JAK-STAT:
-
Janus kinase/signal transducers and activators of transcription
- KDa:
-
Kilo Daltons
- LEA:
-
Late embryogenesis abundant
- MAPK:
-
Mitogen-activated protein (MAP) kinase
- MAPKK:
-
Mitogen-activated protein (MAP) kinase kinase
- MAPKKK:
-
Mitogen-activated protein (MAP) kinase kinase kinase
- MDHAR:
-
Monodehydroascorbate reductase
- NPK2:
-
Nucleus- and phragmoplast-localized protein kinase
- NTF2:
-
Nuclear transport factor 2
- OMTK1:
-
Oxidative stress-activated MAP triple-kinase 1
- PSII:
-
Photosystem II
- ROS:
-
Reactive oxygen species
- RuBisCo:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- RUE:
-
Radiation use efficiency
- SIMK:
-
Salt-induced mitogen kinase
- UV:
-
Ultraviolet
- WIPK:
-
Wound-induced mitogen kinase
References
Abdelrahman M, Burritt DJ, Gupta A, Tsujimoto H, Tran LSP (2020) Heat stress effects on source–sink relationships and metabolome dynamics in wheat. J Exp Bot 71:543–554
Agrawal GK, Rakwal R, Iwahashi H (2002) Isolation of novel rice (Oryza sativa L.) multiple stress responsive MAP kinase gene, OsMSRMK2, whose mRNA accumulates rapidly in response to environmental cues. Biochem Biophys Res Commun 294:1009–1016
Ahsan N, Donnart T, Nouri MZ, Komatsu S (2010) Tissue-specific defense and thermo-adaptive mechanisms of soybean seedlings under heat stress revealed by proteomic approach. J Proteome Res 9:4189–4204
Albertini A, Simeoni F, Galbiati M, Bauer H, Tonelli C, Cominelli E (2014) Involvement of the vacuolar processing enzyme γVPE in response of Arabidopsis thaliana to water stress. Biol Plant 58:531–538
Alessi DR, Gomez N, Moorhead G, Lewis T, Keyse SM, Cohen P (1995) Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol 5:283–295
Ali F, Waters DL, Ovenden B, Bundock P, Raymond CA, Rose TJ (2019) Australian rice varieties vary in grain yield response to heat stress during reproductive and grain filling stages. J Agron Crop Sci 205:179–187
Aliche EB, Prusova-Bourke A, Ruiz-Sanchez M, Oortwijn M, Gerkema E, Van As H, Visser RG, van der Linden CG (2020) Morphological and physiological responses of the potato stem transport tissues to dehydration stress. Planta 251:1–15
Aravind L, Koonin EV (1999) Gleaning non-trivial structural, functional and evolutionary information about proteins by iterative database searches. J Mol Biol 287:1023–1040
Asif MH, Lakhwani D, Pathak S, Bhambhani S, Bag SK, Trivedi PK (2014) Genome-wide identification and expression analysis of the mitogen-activated protein kinase gene family from banana suggest involvement of specific members in different stages of fruit ripening. Funct Integr Genomics 14:161–175
Awasthi R, Gaur P, Turner NC, Vadez V, Siddique KH, Nayyar H (2017) Effects of individual and combined heat and drought stress during seed filling on the oxidative metabolism and yield of chickpea (Cicer arietinum) genotypes differing in heat and drought tolerance. Crop Pasture Sci 68:823–841
Azhar MT, Wani SH, Chaudhary MT, Jameel T, Kaur P, Du X (2020) Heat tolerance in cotton: morphological, physiological, and genetic perspectives. Heat stress tolerance in plants: physiological, molecular and genetic perspectives. Wiley, USA, pp 1–22
Banerjee A, Roychoudhury A (2020) Seed priming as a method to generate heat-stress tolerance in plants: a Minireview. Heat stress tolerance in plants: physiological, molecular and genetic perspectives. Wiley, USA, pp 23–32
Bardwell L, Thorner J (1996) A conserved motif at the amino termini of MEKs might mediate high-affinity interaction with the cognate MAPKs. Trends Biochem Sci 21:373–374
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65:1229–1240
Beca-Carretero P, Guihéneuf F, Marín-Guirao L, Bernardeau-Esteller J, García-Muñoz R, Stengel DB, Ruiz JM (2018) Effects of an experimental heat wave on fatty acid composition in two Mediterranean seagrass species. Mar Pollut Bull 134:27–37
Bheemanahalli R, Sunoj VS, Saripalli G, Prasad PV, Balyan HS, Gupta PK, Grant N, Gill KS, Jagadish SV (2019) Quantifying the impact of heat stress on pollen germination, seed set, and grain filling in spring wheat. Crop Sci 59:684–696
Blanco FA, Zanetti ME, Casalongué CA, Daleo GR (2006) Molecular characterization of a potato MAP kinase transcriptionally regulated by multiple environmental stresses. Plant Physiol Biochem 44:315–322
Brunet A, Pages G, Pouysse J (1994) Growth factor-stimulated MAP kinase induces rapid retrophosphorylation and inhibition of MAP kinase kinase (MEK1). FEBS Lett 346:299–303
Cakir B, Kılıçkaya O (2015) Mitogen-activated protein kinase cascades in Vitis vinifera. Front Plant Sci 6:556
Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ (1997) Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell 90:859–869
Cano E, Mahadevan LC (1995) Parallel signal processing among mammalian MAPKs. Trends Biochem Sci 20:117–122
Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410:37–40
Chen L, Hu W, Tan S, Wang M, Ma Z, Zhou S, Deng X, Zhang Y, Huang C, Yang G (2012) Genome-wide identification and analysis of MAPK and MAPKK gene families in Brachypodium distachyon. PLoS One 7:e46744
Chovancek E, Zivcak M, Botyanszka L, Hauptvogel P, Yang X, Misheva S, Hussain S, Brestic M (2019) Transient heat waves may affect the photosynthetic capacity of susceptible wheat genotypes due to insufficient photosystem I photoprotection. Plan Theory 8:282
Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK (1996) Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem 271:30847–30857
Clark-Lewis I, Sanghera JS, Pelech SL (1991) Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem 266:15180–15184
Cooper JA, Bowen-Pope DF, Raines E, Ross R, Hunter T (1982) Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell 31:263–273
Cui L, Yang G, Yan J, Pan Y, Nie X (2019) Genome-wide identification, expression profiles and regulatory network of MAPK cascade gene family in barley. BMC Genomics 20:750
Delahunty A, Nuttall J, Nicolas M, Brand J (2015) Genotypic heat tolerance in lentil. In Proceedings of the 17th ASA Conf, pp 20–24
Ding H, He J, Wu Y, Wu X, Ge C, Wang Y, Zhong S, Peiter E, Liang J, Xu W (2018) The tomato mitogen-activated protein kinase slmpk1 is as a negative regulator of the high-temperature stress response. Plant Physiol 177:633–651
Ding X, Richter T, Chen M, Fujii H, Seo YS, Xie M, Zheng X, Kanrar S, Stevenson RA, Dardick C, Li Y (2008) A rice kinase-protein interaction map. Plant Physiol 149:1478–1492
Djanaguiraman M, Prasad PV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48:999–1007
Djanaguiraman M, Prasad PVV, Al-Khatib K (2011) Ethylene perception inhibitor 1-MCP decreases oxidative damage of leaves through enhanced antioxidant defense mechanisms in soybean plants grown under high temperature stress. Environ Exp Bot 71:215–223
Dongsansuk A, Theerakulpisut P, Pongdontri P (2017) Short-term heat exposure effect on PSII efficiency and growth of Rice (Oryza sativa L.). Pertanika J Trop Agric Sci 40:621–628
Dunayevich P, Baltanás R, Clemente JA, Couto A, Sapochnik D, Vasen G, Colman-Lerner A (2018) Heat-stress triggers MAPK crosstalk to turn on the hyperosmotic response pathway. Sci Rep 8:1–15
Embiale A, Hussein M, Husen A, Sahile S, Mohammed K (2016) Differential sensitivity of Pisum sativum L. cultivars to water-deficit stress: changes in growth, water status, chlorophyll fluorescence and gas exchange attributes. J Agron 15:45–57
English JM, Vanderbilt CA, Xu S, Marcus S, Cobb MH (1995) Isolation of MEK5 and differential expression of alternatively spliced forms. J Biol Chem 270:28897–28902
Evrard A, Kumar M, Lecourieux D, Lucks J, von Koskull-Döring P, Hirt H (2013) Regulation of the heat stress response in Arabidopsis by MPK6-targeted phosphorylation of the heat stress factor HsfA2. PeerJ 1:e59
Fahad S, Hussain S, Saud S, Hassan S, Ihsan Z, Shah AN, Wu C, Yousaf M, Nasim W, Alharby H, Alghabari F (2016) Exogenously applied plant growth regulators enhance the morpho-physiological growth and yield of rice under high temperature. Front Plant Sci 7:1250
Fanger GR, Gerwins P, Widmann C, Jarpe MB, Johnson GL (1997) MEKKs, GCKs, MLKs, PAKs, TAKs, and tpls: upstream regulators of the c-Jun amino-terminal kinases? Curr Opin Genet Dev 7:67–74
Ferrell JE (1996) Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem Sci 21:460–466
Fu L, Wang P, Xiong Y (2020) Target of rapamycin signaling in plant stress responses. Plant Physiol 182:1613–1623
Garrington TP, Johnson GL (1999) Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol 11:211–218
Gartner A, Nasmyth K, Ammerer G (1992) Signal transduction in Saccharomyces cerevisiae requires tyrosine and threonine phosphorylation of FUS3 and KSS1. Genes Dev 6:1280–1292
Getnet Z, Husen A, Fetene M, Yemata G (2015) Growth, water status, physiological, biochemical and yield response of stay green sorghum {Sorghum bicolor (L.) Moench} varieties-a field trial under drought-prone area in Amhara regional state, Ethiopia. J Agron 14:188–202
Gonzalez FA, Raden DL, Davis RJ (1991) Identification of substrate recognition determinants for human ERK1 and ERK2 protein kinases. J Biol Chem 266:22159–22163
Gregory JS, Boulton TG, Sang BC, Cobb MH (1989) An insulin-stimulated ribosomal protein S6 kinase from rabbit liver. J Biol Chem 264:18397–18401
Gunawardhana MDM, De Silva CS (2011) Impact of temperature and water stress on growth yield and related biochemical parameters of okra. Trop Agric Res 23:77–83
Gupta KJ, Mur LA, Wany A, Kumari A, Fernie AR, Ratcliffe RG (2020) The role of nitrite and nitric oxide under low oxygen conditions in plants. New Phytol 225:1143–1151
Hand SC, Menze MA, Toner M, Boswell L, Moore D (2011) LEA proteins during water stress: not just for plants anymore. Annu Rev Physiol 73:115–134
Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684
Haworth M, Marino G, Brunetti C, Killi D, De Carlo A, Centritto M (2018) The impact of heat stress and water deficit on the photosynthetic and stomatal physiology of olive (Olea europaea L.)—a case study of the 2017 heat wave. Plants 7:76
Heider H, Boscheinen O, Scharf KD (1998) A heat-stress pulse inactivates a 50 kDa myelin basic protein kinase in tomato. Bot Acta 111:398–401
Hinojosa L, Matanguihan JB, Murphy KM (2019) Effect of high temperature on pollen morphology, plant growth and seed yield in quinoa (Chenopodium quinoa Willd.). J Agron Crop Sci 205:33–45
Hurkman WJ, Vensel WH, Tanaka CK, Whitehand L, Altenbach SB (2009) Effect of high temperature on albumin and globulin accumulation in the endosperm proteome of the developing wheat grain. J Cereal Sci 49:12–23
Husen A (1997) Impact of air pollution on the growth and development of Datura innoxia Mill. M.Sc. Dissertation, Jamia Hamdard, Hamdard Nagar, New Delhi, India
Husen A (2010) Growth characteristics, physiological and metabolic responses of teak (Tectona grandis Linn. f.) clones differing in rejuvenation capacity subjected to drought stress. Silva Genet 59:124–136
Husen A, Iqbal M, Khanum N, Aref IM, Sohrab SS, Meshresa G (2019) Modulation of salt-stress tolerance of Niger (Guizotia abyssinica), an oilseed plant, by application of salicylic acid. J Environ Biol 40:94–104
Husen A, Iqbal M, Sohrab SS, Ansari MKA (2018) Salicylic acid alleviates salinity-caused damage to foliar functions, plant growth and antioxidant system in Ethiopian mustard (Brassica carinata A. Br.). Agric Food Sec 7:44
Ichimura K, Mizoguchi T, Irie K, Morris P, Giraudat J, Matsumoto K, Shinozaki K (1998) Isolation of ATMEKK1 (a MAP Kinase Kinase Kinase)-interacting proteins and analysis of a MAP kinase cascade in Arabidopsis. Biochem Biophys Res Commun 253:532–543
Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, Champion A, Kreis M, Zhang S, Hirt H, Wilson C (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7:301–308
Ijaz M, Qamar S, Bukhari SA, Malik K (2019) Abiotic stress signaling in rice crop. In Advances in rice research for abiotic stress tolerance. Woodhead Publishing, United Kingdom, pp 551–569
Iqbal M, Ahmad A, Ansari MKA, Qureshi MI, Aref IM, Khan PR, Hegazy SS, El-Atta H, Husen A, Hakeem KR (2015) Improving the phytoextraction capacity of plants to scavenge metal(loid)-contaminated sites. Environ Rev 23:44–65
Jiang M, Chu Z (2018) Comparative analysis of plant MKK gene family reveals novel expansion mechanism of the members and sheds new light on functional conservation. BMC Genomics 19:407
Jiang Y, Li Z, Schwarz EM, Lin A, Guan K, Ulevitch RJ, Han J (1997) Structure-function studies of p38 mitogen-activated protein kinase LOOP 12 influences substrate specificity and autophosphorylation, but not upstream kinase selection. J Biol Chem 272:11096–11102
Jonak C, Kiegerl S, Hirt H, Lloyd C, Chan J (1995) MMK2, a novel alfalfa MAP kinase, specifically complements the yeast MPK1 function. Mol Gen Genet 248:686–694
Jonak C, Kiegerl S, Ligterink W, Barker PJ, Huskisson NS, Hirt H (1996) Stress signaling in plants: a mitogen-activated protein kinase pathway is activated by cold and drought. Proc Natl Acad Sci U S A 93:11274–11279
Jouannic S, Champion A, Segui-Simarro JM, Salimova E, Picaud A, Tregear J, Testillano P, Risueño MC, Simanis V, Kreis M, Henry Y (2001) The protein kinases AtMAP3Kε1 and BnMAP3Kε1 are functional homologues of S. pombe cdc7p and may be involved in cell division. Plant J 26:637–649
Jumrani K, Bhatia VS (2018) Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol Mol Biol Plants 24:37–50
Kaur G, Asthir B, Bains NS (2018) Biochemical and molecular mechanisms of high-temperature stress in crop plants. In: Metabolic adaptations in plants during abiotic stress. CRC Press, USA, pp 65–72
Kaur N, Gupta AK (2005) Signal transduction pathways under abiotic stresses in plants. Curr Sci 88(11):1771–1780
Kong X, Lv W, Zhang D, Jiang S, Zhang S, Li D (2013a) Genome-wide identification and analysis of expression profiles of maize mitogen-activated protein kinase kinase kinase. PLoS One 8:e57714
Kong X, Lv W, Zhang D, Jiang S, Zhang S, Li D (2013b) Genome-wide identification and analysis of expression profiles of maize mitogen-activated protein kinase kinase kinase. PLoS One 8:57714
Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci U S A 97:2940–2945
Krysan PJ, Jester PJ, Gottwald JR, Sussman MR (2002) An Arabidopsis mitogen-activated protein kinase kinase kinase gene family encodes essential positive regulators of cytokinesis. Plant Cell 14:1109–1120
Kumar K, Rao KP, Biswas DK, Sinha AK (2011) Rice WNK1 is regulated by abiotic stress and involved in internal circadian rhythm. Plant Signal Behav 6:316–320
Kumar RR, Goswami S, Dubey K, Singh K, Singh JP, Kumar A, Rai GK, Singh SD, Bakshi S, Singh B, Pathak H (2019) RuBisCo activase—a catalytic chaperone involved in modulating the RuBisCo activity and heat stress-tolerance in wheat. J Plant Biochem Biotechnol 28:63–75
Leprince AS, Jouannic S, Hamal A, Kreis M, Henry Y (1999) Molecular characterisation of plant cDNAs BnMAP4Kα1 and BnMAP4Kα2 belonging to the GCK/SPS1 subfamily of MAP kinase kinase kinase kinase. Biochim Biophys Acta 1444:1–13
Lesk C, Rowhani P, Ramankutty N (2016) Influence of extreme weather disasters on global crop production. Nature 529:84–87
Li C, Chang PP, Ghebremariam KM, Qin L, Liang Y (2014a) Overexpression of tomato SpMPK3 gene in Arabidopsis enhances the osmotic tolerance. Biochem Biophys Res Commun 443:357–362
Li X, Yang Y, Sun X, Lin H, Chen J, Ren J, Hu X, Yang Y (2014b) Comparative physiological and proteomic analyses of poplar (Populus yunnanensis) plantlets exposed to high temperature and drought. PLoS One 9:e107605
Liang W, Yang B, Yu B-J, Zhou Z, Li C, Jia M, Sun Y, Zhang Y, Wu F, Zhang H (2013) Identification and analysis of MKK and MPK gene families in canola (Brassica napus L.). BMC Genomics 14:1–24
Ligterink W, Hirt H (2001) Mitogen-activated protein (MAP) kinase pathways in plants: versatile signaling tools. In International review of cytology, pp 209–258
Link V, Sinha AK, Vashista P, Hofmann MG, Proels RK, Ehness R, Roitsch T (2002) A heat-activated MAP kinase in tomato: a possible regulator of the heat stress response. FEBS Lett 531:179–183
Lipiec J, Doussan C, Nosalewicz A, Kondracka K (2013) Effect of drought and heat stresses on plant growth and yield: a review. Int Agrophys 27:463–477
Liu Y, Li J, Zhu Y, Jones A, Rose RJ, Song Y (2019) Heat stress in legume seed setting: effects, causes, and future prospects. Front Plant Sci 10:938
Liu Z, Zhang L, Xue C, Fang H, Zhao J, Liu M (2017) Genome-wide identification and analysis of MAPK and MAPKK gene family in Chinese jujube (Ziziphus jujuba Mill.). BMC Genomics 18:1–13
Loka DA, Oosterhuis DM, Baxevanos D, Noulas C, Hu W (2020) Single and combined effects of heat and water stress and recovery on cotton (Gossypium hirsutum L.) leaf physiology and sucrose metabolism. Plant Physiol Biochem 148:166–179
Lv X, Ge S, Jalal Ahammed G, Xiang X, Guo Z, Yu J, Zhou Y (2017) Crosstalk between nitric oxide and MPK1/2 mediates cold acclimation-induced chilling tolerance in tomato. Plant Cell Physiol 58:1963–1975
Maher T, Mirzaei M, Pascovici D, Wright IJ, Haynes PA, Gallagher RV (2019) Evidence from the proteome for local adaptation to extreme heat in a widespread tree species. Funct Ecol 33:436–446
Mahmood T, Khalid S, Abdullah M, Ahmed Z, Shah MKN, Ghafoor A, Du X (2020) Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cell 9:105
Marcus Y (2019) Heliotropism: plants follow the Sun. In: Handbook of plant and crop stress, 4th edn. CRC Press, USA, pp 385–387
Matsuda S, Kosako H, Takenaka K, Moriyama K, Sakai H, Akiyama T, Gotoh Y, Nishida E (1992) Xenopus MAP kinase activator: identification and function as a key intermediate in the phosphorylation cascade. EMBO J 11:973–982
Matsui A, Nakaminami K, Seki M (2019) Biological function of changes in RNA metabolism in plant adaptation to abiotic stress. Plant Cell Physiol 60:1897–1905
Mehta S, Lal SK, Sahu KP, Venkatapuram AK, Kumar M, Sheri V, Varakumar P, Vishwakarma C, Yadav R, Jameel MR, Ali M, Achary VMM, Reddy MK (2020) CRISPR/Cas9-edited rice: a new frontier for sustainable agriculture. In: New frontiers in stress management for durable agriculture. Springer Nature, Singapore, pp 427–458
Mizoguchi T, Ichimura K, Shinozaki K (1997) Environmental stress response in plants: the role of mitogen-activated protein kinases. Trends Biotechnol 15:15–19
Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, Shinozaki K (1996) A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc Natl Acad Sci U S A 93:765–769
Mohammed AR, Tarpley L (2010) Effects of high night temperature and spikelet position on yield-related parameters of rice (Oryza sativa L.) plants. Eur J Agron 33:117–123
Mohanta TK, Arora PK, Mohanta N, Parida P, Bae H (2015) Identification of new members of the MAPK gene family in plants shows diverse conserved domains and novel activation loop variants. BMC Genomics 16:58
Morris PC (2001) MAP kinase signal transduction pathways in plants. New Phytol 151:67–89
Murakami-Kojima M, Nakamichi N, Yamashino T, Mizuno T (2002) The APRR3 component of the clock-associated APRR1/TOC1 quintet is phosphorylated by a novel protein kinase belonging to the WNK family, the gene for which is also transcribed rhythmically in Arabidopsis thaliana. Plant Cell Physiol 43:675–683
Muthuramalingam P, Jeyasri R, Bharathi RKAS, Suba V, Pandian STK, Ramesh M (2020) Global integrated omics expression analyses of abiotic stress signaling HSF transcription factor genes in Oryza sativa L.: an in silico approach. Genomics 112:908–918
Nakagami H, Kiegerl S, Hirt H (2004) OMTK1, a novel MAPKKK, channels oxidative stress signaling through direct MAPK interaction. J Biol Chem 279:26959–26966
Nakagami H, Soukupová H, Schikora A, Zárský V, Hirt H (2006) A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem 281:38697–38704
Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K (2014) The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front Plant Sci 5:170
Neupane A, Nepal MP, Benson BV, MacArthur KJ, Piya S, behavior (2013a) Evolutionary history of mitogen-activated protein kinase (MAPK) genes in Lotus, Medicago, and Phaseolus. Plant Signal Behav 8:e27189
Neupane A, Nepal MP, Piya S, Subramanian S, Rohila JS, Reese RN, Benson BV (2013b) Identification, nomenclature, and evolutionary relationships of mitogen-activated protein kinase (MAPK) genes in soybean. Evol Bioinform 9:EBO. S12526
Neupane S, Schweitzer SE, Neupane A, Andersen EJ, Fennell A, Zhou R, Nepal MP (2019) Identification and characterization of mitogen-activated protein kinase (MAPK) genes in sunflower (Helianthus annus L.). Plants 8:28
Neves LH, Santos RIN, dos Santos Teixeira GI, de Araujo DG, Silvestre WVD, Pinheiro HA (2019) Leaf gas exchange, photochemical responses and oxidative damages in assai (Euterpe oleracea Mart.) seedlings subjected to high temperature stress. Sci Hortic-Amsterdam 257:108733
Nie WF, Wang MM, Xia XJ, Zhou YH, Shi K, Chen Z, Yu JQ (2013) Silencing of tomato RBOH1, MPK2 abolishes brassinosteroid-induced H2O2 generation and stress tolerance. Plant Cell Environ 36:789–803
Ning J, Li X, Hicks LM, Xiong L (2010) A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol 152:876–890
Nishihama R, Banno H, Kawahara E, Irie K, Machida Y (1997) Possible involvement of differential splicing in regulation of the activity of Arabidopsis ANP1 that is related to mitogen-activated protein kinase kinase kinases (MAPKKKs). Plant J 12:39–48
Nishihama R, Ishikawa M, Araki S, Soyano T, Asada T, Machida Y (2001) The NPK1 mitogen-activated protein kinase kinase kinase is a regulator of cell-plate formation in plant cytokinesis. Genes Dev 15:352–363
Pandey AK, Gautam A (2020) Stress responsive gene regulation in relation to hydrogen sulfide in plants under abiotic stress. Physiol Plant 168:511–525
Parrotta L, Faleri C, Cresti M, Cai G (2016) Heat stress affects the cytoskeleton and the delivery of sucrose synthase in tobacco pollen tubes. Planta 243:43–63
Payne DM, Rossomando AJ, Martino P, Erickson AK, Her JH, Shabanowitz J, Hunt DF, Weber MJ, Sturgill TW (1991) Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J 10:885–892
Pereyra CM, Aznar NR, Rodriguez MS, Salerno GL, Martínez-Noël GM (2020) Target of rapamycin signaling is tightly and differently regulated in the plant response under distinct abiotic stresses. Planta 251:21
Pitzschke A (2015) Modes of MAPK substrate recognition and control. Trends Plant Sci 20:49–55
Pöpping B, Gibbons T, Watson MD (1996) The Pisum sativum MAP kinase homologue (PsMAPK) rescues the Saccharomyces cerevisiae hog1 deletion mutant under conditions of high osmotic stress. Plant Mol Biol 31:355–363
Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H (1996) Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1–YPD1–SSK1 “two-component” osmosensor. Cell 86:865–875
Priya M, Dhanker OP, Siddique KH, Hanumantha Rao B, Nair RM, Pandey S, Singh S, Varshney RK, Prasad PV, Nayyar H (2019) Drought and heat stress-related proteins: an update about their functional relevance in imparting stress tolerance in agricultural crops. Theor Appl Genet 132(6):1607–1638
Pucciariello C, Banti V, Perata P (2012) ROS signalling as common element in low oxygen and heat stresses. Plant Physiol Biochem 59:3–10
Purayannur S, Kumar K, Kaladhar VC, Verma PK (2017) Phylogenomic analysis of MKKs and MAPKs from 16 legumes and detection of interacting pairs in chickpea divulge MAPK signalling modules. Sci Rep 7:1–11
Qi Y, Wang H, Zou Y, Liu C, Liu Y, Wang Y, Zhang W (2011) Over-expression of mitochondrial heat shock protein 70 suppresses programmed cell death in rice. FEBS Lett 585:231–239
Qiu YW, Zhe FENG, Fu MM, Yuan XH, Luo CC, Yu YB, Feng YZ, Qi WEI, Li FL (2019) GsMAPK4, a positive regulator of soybean tolerance to salinity stress. J Integr Agric 18:372–380
Rahman MA, Chikushi J, Yoshida S, Karim AJMS (2009) Growth and yield components of wheat genotypes exposed to high temperature stress under control environment. Bangladesh J Agric Res 34:360–372
Rai R (2020) Heat stress in crops: driver of climate change impacting global food supply. In: Contemporary environmental issues and challenges in era of climate change. Springer, Singapore, pp 99–117
Raja MM, Vijayalakshmi G, Naik ML, Basha PO, Sergeant K, Hausman JF, Khan PSSV (2019) Pollen development and function under heat stress: from effects to responses. Acta Physiol Plant 41:47
Rao KP, Tambi R, Kundan K, Badmi R, Sinha AK (2010) In silico analysis reveals 75 members of mitogen-activated protein kinase kinase kinase gene family in rice. DNA Res 17:139–153
Rashid M, Hampton JG, Rolston MP, Trethewey JA, Saville DJ (2018) Forage rape (Brassica napus L) seed quality: impact of heat stress in the field during seed development. Field Crop Res 217:172–179
Ray LB, Sturgill TW (1987) Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc Natl Acad Sci U S A 84:1502–1506
Rossomando AJ, Dent P, Sturgill TW, Marshak DR (1994) Mitogen-activated protein kinase kinase 1 (MKK1) is negatively regulated by threonine phosphorylation. Mol Cell Biol 14:1594–1602
Rurek M (2010) Diverse accumulation of several dehydrin-like proteins in cauliflower (Brassica oleracea var. botrytis), Arabidopsis thaliana and yellow lupin (Lupinus luteus) mitochondria under cold and heat stress. BMC Plant Biol 10:181
Sangwan V, Örvar BL, Beyerly J, Hirt H, Dhindsa RS (2002) Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J 31:629–638
Schaeffer HJ, Catling AD, Eblen ST, Collier LS, Krauss A, Weber MJ (1998) MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 281:1668–1671
Schoenbeck MA, Trepp GB, Gregerson RG, Samac DA, Gantt JS, Vance CP (1997) The alfalfa TDY1 gene encodes a MAP kinase homologue. Plant Physiol 114:270
Seger R, Ahn NG, Posada J, Munar ES, Jensen AM, Cooper JA, Cobb MH, Krebs EG (1992) Purification and characterization of mitogen-activated protein kinase activator(s) from epidermal growth factor-stimulated A431 cells. J Biol Chem 267:14373–14381
Sharma D, Rachhoya HK, Sharma M, Agarwal R (2018) Effect of rising temperature stress on growth and physiology of domestic crops of Rajasthan, India. Int J Curr Microbiol App Sci 7:1426–1440
Sharma P, Jha AB, Dubey RS (2019) Oxidative stress and antioxidative defense system in plants growing under abiotic stresses. In: Handbook of plant and crop stress, 4th edn. CRC Press, USA, pp 93–136
Shinozaki K, Yamaguchi-Shinozaki K (1997) Gene expression and signal transduction in water-stress response. Plant Physiol 115:327
Shumayla ST, Upadhyay SK (2019) Receptor-like kinases and environmental stress in plants. In: Molecular approaches in plant biology and environmental challenges. Springer, Singapore, pp 79–102
Sözen C, Schenk ST, Boudsocq M, Chardin C, Almeida-Trapp M, Krapp A, Hirt H, Mithöfer A, Colcombet J (2019) Wounding and insect feeding trigger two independent MAPK pathways with distinct regulation and kinetics. Plant Cell 32:1988–2003
Stone JM, Walker JC (1995) Plant protein kinase families and signal transduction. Plant Physiol 108:451–457
Sturgill TW, Ray LB, Erikson E, Maller JL (1988) Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature 334:715–718
Sun M, Xu Y, Huang J, Jiang Z, Shu H, Wang H, Zhang S (2017) Global identification, classification, and expression analysis of MAPKKK genes: functional characterization of MdRaf5 reveals evolution and drought-responsive profile in apple. Sci Rep 7:1–14
Sun Y, Wang C, Yang B, Wu F, Hao X, Liang W, Niu F, Yan J, Zhang H, Wang B (2014) Identification and functional analysis of mitogen-activated protein kinase kinase kinase (MAPKKK) genes in canola (Brassica napus L.). J Exp Bot 65:2171–2188
Suwa R, Hakata H, Hara H, El-Shemy HA, Adu-Gyamfi JJ, Nguyen NT, Kanai S, Lightfoot DA, Mohapatra PK, Fujita K (2010) High temperature effects on photosynthate partitioning and sugar metabolism during ear expansion in maize (Zea mays L.) genotypes. Plant Physiol Biochem 48:124–130
Takahashi K, Isobe M, Muto S (1997) An increase in cytosolic calcium ion concentration precedes hypoosmotic shock-induced activation of protein kinases in tobacco suspension culture cells. FEBS Lett 401:202–206
Tan W, wei Meng Q, Brestic M, Olsovska K, Yang X (2011) Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J Plant Physiol 168:2063–2071
Thitz P, Possen BJHM, Oksanen E, Mehtätalo L, Virjamo V, Vapaavuori E (2017) Production of glandular trichomes responds to water stress and temperature in silver birch (Betula pendula) leaves. Can J For Res 47:1075–1081
Ueki K, Matsuda S, Tobe K, Gotoh Y, Tamemoto H, Yachi M, Akanuma Y, Yazaki Y, Nishida E, Kadowaki T (1994) Feedback regulation of mitogen-activated protein kinase kinase kinase activity of c-Raf-1 by insulin and phorbol ester stimulation. J Biol Chem 269:15756–15761
Wang G, Wang T, Jia Z-H, Xuan J-P, Pan D-L, Guo Z-R, Zhang J-YJ (2018a) Genome-wide bioinformatics analysis of MAPK gene family in kiwifruit (Actinidia chinensis). Int J Mol Sci 19:2510
Wang H, Gong M, Guo J, Xin H, Gao Y, Liu C, Dai D, Tang L (2018b) Genome-wide identification of Jatropha curcas MAPK, MAPKK, and MAPKKK gene families and their expression profile under cold stress. Sci Rep 8:1–17
Wang J, Pan C, Wang Y, Ye L, Wu J, Chen L, Zou T, Lu G (2015) Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber. BMC Genomics 16:386
Wang L, Hu W, Tie W, Ding Z, Ding X, Liu Y, Yan Y, Wu C, Peng M, Xu B (2017) The MAPKKK and MAPKK gene families in banana: identification, phylogeny and expression during development, ripening and abiotic stress. Sci Rep 7:1–15
Wang M, Yue H, Feng K, Deng P, Song W, Nie X (2016) Genome-wide identification, phylogeny and expressional profiles of mitogen activated protein kinase kinase kinase (MAPKKK) gene family in bread wheat (Triticum aestivum L.). BMC Genomics 17:668
Whitmarsh AJ, Davis RJ (1998) Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem Sci 23:481–485
Wilson C, Anglmayer R, Vicente O, Heberle-Bors E (1995) Molecular cloning, functional expression in Escherichia coli, and characterization of multiple mitogen-activated-protein kinases from tobacco. Eur J Biochem 233:249–257
Wu C, Cui K, Wang W, Li Q, Fahad S, Hu Q, Huang J, Nie L, Peng S (2016) Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice. Sci Rep 6:34978
Wu J, Wang J, Pan C, Guan X, Wang Y, Liu S, He Y, Chen J, Chen L, Lu G (2014) Genome-wide identification of MAPKK and MAPKKK gene families in tomato and transcriptional profiling analysis during development and stress response. PLoS One 9:e103032
Wu L, Zu X, Zhang H, Wu L, Xi Z, Chen Y (2015) Overexpression of ZmMAPK1 enhances drought and heat stress in transgenic Arabidopsis thaliana. Plant Mol Biol 88:429–443
Wu P, Wang W, Li Y, Hou X (2017) Divergent evolutionary patterns of the MAPK cascade genes in Brassica rapa and plant phylogenetics. Hortic Res 4:1–12
Xalxo R, Yadu B, Chandra J, Chandrakar V, Keshavkant S (2020) Alteration in carbohydrate metabolism modulates thermotolerance of plant under heat stress. Heat stress tolerance in plants: physiological, molecular and genetic perspectives. Wiley, USA, pp 77–115
Xing Y, Jia W, Zhang J (2007) AtMEK1 mediates stress-induced gene expression of CAT1 catalase by triggering H2O2 production in Arabidopsis. J Exp Bot 58:2969–2981
Xing Y, Jia W, Zhang J (2008) AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J 54:440–451
Yadav A, Singh J, Ranjan K, Kumar P, Khanna S, Gupta M, Kumar V, Wani SH, Sirohi A (2020) Heat shock proteins: master players for heat-stress tolerance in plants during climate change. Heat stress tolerance in plants: physiological, molecular and genetic perspectives. Wiley, USA, pp. 189–211
Yan Y, Wang L, Ding Z, Tie W, Ding X, Zeng C, Wei Y, Zhao H, Peng M, Hu W (2016) Genome-wide identification and expression analysis of the mitogen-activated protein kinase gene family in cassava. Front Plant Sci 7:1294
Yanni SF, Helgason BL, Janzen HH, Ellert BH, Gregorich EG (2020) Warming effects on carbon dynamics and microbial communities in soils of diverse texture. Soil Biol Biochem 140:107631
Ye J, Yang H, Shi H, Wei Y, Tie W, Ding Z, Yan Y, Luo Y, Xia Z, Wang W (2017) The MAPKKK gene family in cassava: genome-wide identification and expression analysis against drought stress. Sci Rep 7:1–12
Yin Y, Li S, Liao W, Lu Q, Wen X, Lu C (2010) Photosystem II photochemistry, photoinhibition, and the xanthophyll cycle in heat-stressed rice leaves. J Plant Physiol 167:959–966
Yu W, Wang L, Zhao R, Sheng J, Zhang S, Li R, Shen L (2019) Knockout of SlMAPK3 enhances tolerance to heat stress involving ROS homeostasis in tomato plants. BMC Plant Biol 19:1–13
Yuasa T, Muto S (1996) Activation of 40-kDa protein kinases in response to hypo-and hyperosmotic shock in the halotolerant green alga Dunaliella tertiolecta. Plant Cell Physiol 37:35–42
Zhan H, Yue H, Zhao X, Wang M, Song W, Nie X (2017) Genome-wide identification and analysis of MAPK and MAPKK gene families in bread wheat (Triticum aestivum L.). Genes 8:284
Zhang J, Zhang F, Ebert D, Cobb MH, Goldsmith EJ (1995) Activity of the MAP kinase ERK2 is controlled by a flexible surface loop. Structure 3:299–307
Zhang S, Klessig DF (1997) Salicylic acid activates a 48-kD MAP kinase in tobacco. Plant Cell 9:809–824
Zhang S, Xu R, Luo X, Jiang Z, Shu H (2013) Genome-wide identification and expression analysis of MAPK and MAPKK gene family in Malus domestica. Gene 531:377–387
Zhang X, Xu X, Yu Y, Chen C, Wang J, Cai C, Guo W (2016) Integration analysis of MKK and MAPK family members highlights potential MAPK signaling modules in cotton. Sci Rep 6:1–13
Zheng CF, Guan KL (1994) Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J 13:1123–1131
Zhou H, Ren S, Han Y, Zhang Q, Qin L, Xing YJ (2017a) Identification and analysis of mitogen-activated protein kinase (MAPK) cascades in Fragaria vesca. Int J Mol Sci 18:1766
Zhou H, Suyue R, Yuanfang H, Qing Z, Ling Q, Yu X (2017b) Identification and analysis of mitogen-activated protein kinase (MAPK) cascades in Fragaria vesca. Int J Mol Sci 18:1766
Zhou J, Xia XJ, Zhou YH, Shi K, Chen Z, Yu JQ (2014) RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. J Exp Bot 65:595–607
Zimmermann S, Rommel C, Ziogas A, Lovric J, Moelling K, Radziwill G (1997) MEK1 mediates a positive feedback on Raf-1 activity independently of Ras and Src. Oncogene 15:1503–1511
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bharti, J. et al. (2021). Mitogen-Activated Protein Kinase, Plants, and Heat Stress. In: Husen, A. (eds) Harsh Environment and Plant Resilience. Springer, Cham. https://doi.org/10.1007/978-3-030-65912-7_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-65912-7_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65911-0
Online ISBN: 978-3-030-65912-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)