Abstract
Flocculants type polyacrylamide (PAM) plays an important role in mineral processing circuits. It is common practice to reuse water from the thickening stages to other unit operations such as grinding and flotation. In the case of molybdenite flotation, an important depressing effect on mineral particles has been demonstrated by the residual flocculant dissolved in the recycled water. The aim of this work was to evaluate the effect of the degradation of a PAM type of flocculant on the flotation recovery of molybdenite. The study considered experiments of microflotation, adsorption isotherms, electrophoretic mobility, and intrinsic viscosity measurements. Molecular dynamic simulations were used to look for mechanisms of interactions between PAM and molybdenite. The results showed that molybdenite recovery was less affected by the flocculant when PAM molecules were subjected to more intense conditions of mechanical shearing. This behavior is related to the adsorption of flocculant on the mineral and the disposition and shape anisotropy of the flocculant chains.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
The efficiency of mineral separation processes depends on the quality of water. New water suitable for processing is limited, especially in northern Chile, where there are intense mineral exploitation activities [1, 2]. Consequently, wastewater from processing plants must have closed circuits in which the process water is recycled again. As a result of this operational practice, an important part of residual reagents dissolved in water is recirculated to flotation, causing flotation depression of some important minerals such as molybdenite [3].
Most of the flocculants used in mineral processing are high molecular weight polymers based on polyacrylamide. Polyacrylamide (PAM) is substantially a non-ionic uncharged polymer. However, industrial polyacrylamides are hydrolyzed to produce a series of acrylic units along the main chain. These acrylic units containing carboxylic groups make such copolymers weak to moderately anionic [4]. Figure 1 shows the chemical structure of anionic polyacrylamide.
The effectiveness of a flocculant is strongly influenced by the properties of the solution in which the polymer molecules are dissolved. The flocculant adsorption and the bridging process between the particles depend not only on the interactions with the mineral surface, but also on the molecular weight, the degree of anionicity and the conformation that the flocculant molecules take in a particular electrolyte solution [5, 6]. Applying excessive shear to high molecular weight flocculants can lead to chain breakage, substantially reducing bridging ability [7].
The main objective of this study is to investigate the effect of the presence of a polyacrylamide-type flocculant with a low degree of anionicity with three levels of degradation in the flotation of molybdenite in different conditions of concentration and pH.
Materials and Methods
Flocculant Preparation, Characterization, and Degradation
The flocculant used in this work was a polyacrylamide type, provided by SNF CHILE S.A. The sample was received as dry white granules and used without any further treatment or purification. According to the information provided by the manufacturer, this flocculant is anionic, high molecular weight, and low degree of anionicity. Flocculant preparation was done in a daily basis during the experimental program following the procedure described by Arinaitwe [4]. Three levels of mechanical shear degradation were considered: non sheared flocculant (NS-FLOC), moderately sheared flocculant (MS-FLOC)m and strongly sheared flocculant (SS-FLOC). Flocculant shearing was achieved using a 40 mm diameter bladed stirrer which was inserted into 100 mL of a 0.35 g/L flocculant fresh solution. The MS-FLOC was obtained by shearing for 30 min at 500 rpm, and the SS-FLOC was obtained by shearing for 3 h at 2000 rpm.
Fourier transform infrared (FTIR) spectra of the flocculant was obtained using a PerkinElmer 2000 FTIR spectrophotometer. The sodium and total organic carbon (TOC) concentration in the flocculant was measured using a PerkinElmer Optima 5300 DV ICP-OES and a Shimadzu TOC-L, respectively. The degree of anionicity (DA) of the flocculant under study was determined according to the electroneutrality criterion of polyelectrolyte solutions [4]. The DA of the flocculant can be obtained from the following expression:
Intrinsic viscosity ([η]) measurements were carried out in order to evaluate the degree of mechanical degradation of the flocculant. The method proposed by Fedors [8] and validated by Rao [9] and Ghimici and Popescu [8] was used to describe the viscosity of PAM. The intrinsic viscosity measurements were done following the procedure described by Klein and Conrad [9], i.e., 0.5 M NaCl and 25 ℃, and at natural pH. Solution viscosities (η) were measured using a Cannon-Fenske 200 viscometer at different flocculant concentrations.
Samples and Reagents
The molybdenite sample used in this study was obtained after several collectorless flotation cleaning stages from an industrial molybdenite concentrate. The final sample is obtained after three washing steps with sodium hydrosulfide (NaSH) and acetone to remove residual flotation collectors and other organic reagents. The elimination of organic reagents was verified by analyzing the content of total organic carbon (TOC) of the liquid extracts obtained at different pH values, which did not show the presence of TOC, so it can be said that there was no presence of flotation reagents [10, 11]. Chemical analysis of the molybdenite sample shows a purity of 99.34%, with a chemical composition of 59.6% Mo and 39.8% S. The particle size of the molybdenite sample used in this study was in the range of −150 + 45 µm. The oxidation of the molybdenite samples was reduced following the procedure established by Ansari and Pawlik [12, 13] in which the ore samples were stored at 1 ℃ in sealed plastic bags previously blown with nitrogen. Methyl isobutyl carbinol (MIBC) obtained from Merck was used as frother. Sodium hydroxide (NaOH) obtained from Merck was used as pH modifier. A 0.01 M NaCl solution prepared using Milli-Q water of 18.2 MΩ cm at 25 ℃ and a chloride salt of sodium (Merck) was used as aqueous medium in the tests.
Microflotation Test
Molybdenite flotation was assessed by microflotation tests in a 150 mL Partridge and Smith glass cell with nitrogen gas at 80 mL/min. The experiments were done using 1 g of molybdenite which was initially conditioned for 2 min in 100 mL of solution containing the PAM reagent (nonsheared and sheared) at varying concentrations (2.5, 5.0, 7.5, and 10.0 ppm) and pH. Then, 25 ppm of MIBC were added along with 50 mL of solution to complete the volume of the microflotation cell and, lastly, mixed for additional 3 min. Then, the process of flotation was performed for 2 min, removing the froth every 10 s. The pulp level in the cell was controlled by adding a solution prepared at the same composition and pH of the original solution. Finally, concentrates and tailings were dried in an oven at 105 ℃ for 5 h, and molybdenite recovery was calculated as the ratio between the mass of molybdenite in the concentrate, and the mass in the concentrate plus the mass in the tailings. All tests were done in triplicate, and the results presented in the manuscript are average values with the error bars representing a standard deviation.
Adsorption Test
Adsorption of NS-FLOC, MS-FLOC, and SS-FLOC on molybdenite was measured through tests in which 1 g of molybdenite was mixed for 15 min with 150 mL of flocculant solutions of known concentrations. The suspensions were centrifuged, and the filtrated was analyzed for TOC, readings which were translated to flocculant concentrations through a calibration curve. The masses of adsorbed flocculant on molybdenite were calculated as the difference between the initial and final concentrations multiplied by the volume of solution (150 mL). Then, specific adsorption was calculated in mg of flocculant adsorbed per gram of molybdenite (mg/g) and the isotherm was drawn. Temperature was controlled all the time in these experiments by using a thermostatic bath, and the tests were done in duplicates with an average standard error of 4%.
Electrophoretic Mobility and Aggregation Measurements
Molybdenite and flocculant interactions were assessed through electrophoretic mobility measurements using a Zetacompact Z9000 from CAD instrument −20 microns molybdenite particles were tested, and the effects of pH and PAM changes were also studied.
Molecular Dynamics Simulation
PAM solution models were created using the package Materials Studio 2017 (Accelrys Inc., San Diego, CA), The PAM chain was composed of 20 repeating units of acrylamide monomers, of which 8% of the monomers were randomly replaced by sodium carboxylate monomers. The behavior of two PAM chains in aqueous solution was studied using molecular dynamics simulations with the package LAAMPS. (large-scale atomic/molecular massively parallel simulator) [14] at different concentrations of NaOH and at 298 K, employing periodic boundary conditions. For the PAM chain, the force field OPLS-AA was used (all-atom optimized potentials for liquid simulations). The SPC/E water model was used to describe the water. For the ions, 12–6 the Lennard–Jones parameters from Li and Mertz [15] were used. Then, the PAM molecules were embedded in a cube box of 6 × 6 × 5 nm, and then the ions were distributed in random positions, while maintaining a separation distance of 0.3 nm between the atoms. Finally, 5000 water molecules were added to the system. The system initially was simulated in an NVT run for 100 ps at 298 K, and the system was then annealed in an NpT run to accelerate its equilibration avoiding the formation of metastable states; during this stage, the temperature was kept at 298 K. The production step was then performed in an NVT simulation run of 40 ns at 300 K. The data was collected after 6 ns.
Results
Flocculant Characterization
Figure 2 shows the FTIR spectrum of the flocculant under study. This spectrum was obtained using a solid polyacrylamide sample supported on a KBr disk, and it shows the different transmission bands corresponding to the different vibrational modes associated with the functional groups of polyacrylamide (PAM) reported by Sundaraganesan [16]. The analysis will focus on the amino groups (NH2), carbonyl (C = O), acrylate (COO−), and the C-N bond of the amide group (CONH2). The IR band at 3424 cm−1 arises from the asymmetric stretching vibration of the NH2 group, the band at 3189 cm−1 corresponds to the symmetric stretching vibration of the NH2 group, the band at 1653 cm−1 is from the stretching vibration of the bond C = O in -CONH2, the band at 1406 cm−1 indicates the stretching vibration of the CN bond, and the band present at 1551 cm−1 is attributed to acrylate (COO−).
The TOC analysis of the flocculant sample indicates that the amount of carbon is close to 720 mg/L, and the ICP-OES analysis reveals that the sodium concentration in the flocculant is 37.5 mg/L; the sodium content in the flocculant sample is high indicating that this flocculant is sodium; therefore, using Eq. 1, the degree of anionicity of the flocculant is equivalent to 8%, value similar to that reported by Deng et al. [17]. Intrinsic viscosities of the NS-FLOC, MS-FLOC, and SS-FLOC were obtained from method proposed by Fedors [8] as shown in Fig. 3. The insert shows the obtained intrinsic viscosities. As expected, shearing has a significant effect on the viscosity of the flocculant.
Microflotation
Figure 4 microflotation shows the recovery of molybdenite as a function of the concentration of flocculant NS-FLOC, MS-FLOC, and SS-FLOC at pH 7, 9, and 11. The results of molybdenite flotation in the presence of the PAM flocculant subjected to different degrees of mechanical shearing show that the NS-FLOC had the strongest depressing effect on molybdenite flotation followed by the MS-FLOC. The depressing effect of the SS-FLOC is minor.
Adsorption
Figure 5 shows the results of specific adsorption of NS-FLOC, MS-FLOC and SS-FLOC on molybdenite at different values of pH. The results show that the strongest the mechanical shearing of the PAM the more adsorption of the resulting molecules on the molybdenite surfaces. The adsorptions of flocculants on molybdenite tend to increase with pH.
Electrophoretic Mobility
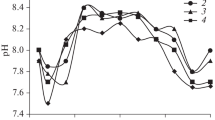
Figure 6 shows the results of electrophoretic mobility distributions of molybdenite in the absence and presence of flocculant, at pH 7, 9, and 11. The results show that the electrophoretic mobility distributions of molybdenite with no flocculant added move toward more negative values as the pH increases which agrees with previous studies [18]. In the presence of flocculant, the electrophoretic mobility distributions move toward less negative values. These experimental results indicate that that PAM flocculants obtained under different shearing conditions slightly reduce the negative surface charge of molybdenite, and that the more sheared the PAM the strongest the neutralizing effect.
Molecular Dynamics Simulation
Figure 7 shows the results of the radius of gyration \({R}_{g}={\left(\left({\sum }_{i}{\Vert {r}_{i}\Vert }^{2}{m}_{i}/{\sum }_{i}{m}_{i}\right)\right)}^{1/2}\) of PAM calculated by molecular dynamic simulation at different PH values. The results show that when the pH is increased with NaOH, the radius of gyration of the PAM molecules decreases which agrees with previous studies [19].
Discussion
The effect of different polymers on the flotation process is a subject which is under continuous research. Polymers of different nature and molecular weights have been used as dispersants/depressants, and polymers of high molecular weights have been usually used as flocculants in mineral processing plants. All these polymers are water soluble; thus, they are highly hydrophilic macromolecules which may affect the surface properties of different minerals.
Molybdenite particles are formed by non-polar and highly hydrophobic surfaces called faces and polar and highly hydrophilic surfaces called edges. Therefore, molybdenite can be described as an anisotropic mineral with varying surface properties on the edges and faces. Therefore, the negative surface charge of the molybdenite edges and faces increases with pH which agrees with the experimental data presented in Fig. 6 that show that the electrophoretic mobility distributions move to more negative values as pH is increased from 7 to 11. The microflotation results presented in Fig. 4 indicate that the depressing effect of the NS-FLOC and MS-FLOC on molybdenite flotation slightly increases with pH, which is in agreement with the theory raised regarding the possible interactions between polymers and the hydroxylated metallic sites existing on the mineral surfaces.
Figure 3 shows that shearing induces strong changes on the molecular weights of the flocculant as measured by their intrinsic viscosities. The flotation results presented in Fig. 4 indicate that molybdenite recovery is strongly depressed by the NS-FLOC, and to a lesser extent by MS-FLOC; however, molybdenite flotation is only slightly affected by SS-FLOC. Castro and Laskowski [3] reported that molybdenite depression by a polyacrylamide flocculant of a DA of 18.1% occurs as other applications in which hydrophobic minerals are depressed by hydrophilic polymer. These authors also indicated that that when PAM molecules are mechanically sheared their depressing effect on molybdenite flotation is maintained and the ability to flocculate quartz particles is lost. The results obtained in this work show that the depressing effect of PAM on the flotation of molybdenite takes place only if this flocculant is sheared at moderate shearing conditions. At strongly shearing conditions, the PAM flocculant has almost no effect on molybdenite flotation.
The adsorption results presented in Fig. 5 indicate that the strongest the shearing the more the resulting molecules adsorb on molybdenite particles. Although the depressing effect of the SS-FLOC on molybdenite flotation is minor, the adsorption of these molecules on the mineral particles is high. In contrast, the adsorption of the NS-FLOC on molybdenite is lower than the adsorption of the MS-FLOC and SS-FLOC, but the observed depressing effect on molybdenite flotation is by far the strongest. The adsorption results correlate with the changes in the electrophoretic mobility distributions presented in Fig. 6 that show that the largest shifts of the distributions are obtained with the MS-FLOC and SS-FLOC.
Figure 7 shows that increasing the pH with NaOH the radius of gyration of the polyacrylamide molecules decreases. These results are a consequence of the neutralization of charges by the sodium ion in the polymer chains; this results in chains less extended and less negative. The simulation of radius of gyration results correlate with the changes in the electrophoretic mobility distributions presented in Fig. 6 that show less negative values with increasing pH.
Conclusions
Based our experimental observations, the following conclusions were drawn:
-
Molybdenite flotation is strongly depressed by the NS-FLOC, and to a lesser extent by MS-FLOC; however, molybdenite flotation is only slightly affected by SS-FLOC.
-
The results obtained in this work show that the depressing effect of PAM on the flotation of molybdenite takes place only if this flocculant is sheared at moderate shearing conditions. At strongly shearing conditions, the PAM flocculant has almost no effect on molybdenite flotation.
-
The strongest the shearing of flocculant, the more the resulting molecules adsorb on molybdenite particles.
-
The adsorption results correlate with the changes in the electrophoretic mobility distributions. when the pH is increased, the electrophoretic mobility of molybdenite tends to less negative values in the presence of flocculant.
-
Molecular dynamics simulation shows that increasing the pH with NaOH the radius of gyration of the polyacrylamide molecules decreases.
References
Romero H, Méndez M, Smith P (2012) Mining development and environmental injustice in the atacama desert of Northern Chile. Environ Justice 5(2):70–76. https://doi.org/10.1089/env.2011.0017
Oyarzún J, Oyarzún R (2011) Sustainable development threats, inter-sector conflicts and environmental policy requirements in the arid, mining rich, Northern Chile Territory. Sustain Develop 19(4):263–274. https://doi.org/10.1002/sd.441
Castro S, Laskowski JS (2015) Depressing effect of flocculants on molybdenite flotation. Miner Eng 74:13–19. https://doi.org/10.1016/j.mineng.2014.12.027
Arinaitwe E, Pawlik M (2009) A method for measuring the degree of anionicity of polyacrylamide-based flocculants. Int J Miner Process 91(1–2):50–54. https://doi.org/10.1016/j.minpro.2008.12.002
Owen AT, Fawell PD, Swift JD, Farrow JB (2002) The impact of polyacrylamide flocculant solution age on flocculation performance. Int. J Miner Process 67(1–4):123–144. https://doi.org/10.1016/S0301-7516(02)00035-2
Hogg R (2000) Flocculation and dewatering. Int J Miner Process 58(1–4):223–236. https://doi.org/10.1016/S0301-7516(99)00023-X
Scott JP, Fawell PD, Ralph DE, Farrow JB (1996) The shear degradation of high-molecular-weight flocculant solutions. J Appl Polym 62:2097–2106
Ghimici L, Popescu F (1998) Determination of intrinsic viscosity for some cationic polyelectrolytes by Fedors method. Eur Polym J 34(1):13–16. https://doi.org/10.1016/S0014-3057(97)00072-4
Klein J, Conrad KD (1978) Molecular weight determination of poly(acrylamide) and poly(acrylamide-co-sodium acrylate). Die Makromol Chemie 179(6):1635–1638. https://doi.org/10.1002/macp.1978.021790624
Ramirez A, Gutierrez L, Vega-Garcia LD, Reyes-Bozo L (2020) The depressing effect of Kaolinite on Molybdenite flotation in seawater. Minerals 10(6):578. https://doi.org/10.3390/min10060578
Ramirez A, Gutierrez L, Laskowski JS (2020) Use of ‘oily bubbles’ and dispersants in flotation of molybdenite in fresh and seawater. Miner Eng 148:106197. https://doi.org/10.1016/j.mineng.2020.106197
Ansari A, Pawlik M (2006) Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part I. Adsorption Studies Miner Eng 20(6):600–608. https://doi.org/10.1016/j.mineng.2006.12.007
Ansari A Pawlik M (2007) Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part II. Hallimond tube flotation. Miner Eng 20(6):609–616. https://doi.org/10.1016/j.mineng.2006.12.008
Plimpton S (1997) Short-range molecular dynamics. J Comput Phys 117(6):1–42. https://doi.org/10.1006/jcph.1995.1039
Li P, Merz KM (2014) Taking into account the ion-induced dipole interaction in the nonbonded model of ions. J Chem Theory Comput 10(1):289–297. https://doi.org/10.1021/ct400751u
Sundaraganesan N, Puviarasan N, Mohan S (2001) Vibrational spectra, assignments and normal coordinate calculation of acrylamide. Talanta 54(2):233–241. https://doi.org/10.1016/S0039-9140(00)00585-3
Deng Y, Dixon JB, White GN (2006) Adsorption of Polyacrylamide on Smectite, Illite, and Kaolinite. Soil Sci Soc Am J 70(1):297–304. https://doi.org/10.2136/sssaj2005.0200
Castillo I, Gutierrez L, Hernandez V, Diaz E, Ramirez A (2020) Hemicelluloses monosaccharides and their effect on molybdenite flotation. Powder Technol 373:758–764. https://doi.org/10.1016/j.powtec.2020.07.032
Hirst LS (2012) Fundamentals of soft matter science. CRC Press, Boca Raton
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Echeverry, L., Estrada, D., Gutierrez, L. (2021). Flotation of Molybdenite in the Presence of Sheared Polyacrylamide Flocculants. In: Lee, J., Wagstaff, S., Anderson, A., Tesfaye, F., Lambotte, G., Allanore, A. (eds) Materials Processing Fundamentals 2021. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-65253-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-65253-1_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65252-4
Online ISBN: 978-3-030-65253-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)