Abstract
Crop production in agriculture is affected by plant genetic background and environmental factors. The application of modern molecular biology tools to conventional plant breeding approaches has facilitated the plant genetic improvement attempts. After the extensive employment of recombinant DNA technology in diverse plant species and despite achievements, the use of transgenic crops has been encountered with public concerns due to the presence of transgenes. The advent of sequence-specific nuclease-based editing technologies especially clustered regularly interspaced short palindromic repeat-associated protein system (CRISPR/Cas) has opened a promising avenue in genetic engineering of plants. The importance of this approach is emphasized since it is a simple and robust tool, moreover, non-transgenic mutants can be selected in later generations. Following the successful use of the CRISPR/Cas9 editing tool in model plants, the applications of this system have been increasingly reported in different plant species. This chapter reviews the contribution of the CRISPR/Cas9 system in the development of genetically modified crops with improved yield, nutritional value, and response to biotic and abiotic stress factors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Abiotic stress factors
- Biotic stress factors
- Genome editing
- Improved yield
- Non-transgenic
- Nutritional quality
- Transgenic technology
16.1 Introduction
Food demand is the basic requirement of life and since the early ages of agriculture, farmers have attempted to enhance the quality and yield of plant species. However, agricultural products are threatened by biotic and abiotic stress factors. Global climate change and the emergence of new genotypes of plant pests and pathogens pose serious threats to the sustainable production of crops. Besides, the growing world population which is estimated to reach 9.7 billion by 2050 and the increase of 58–98% food demand during this time renders the conventional agricultural practices insufficient to secure the food supply (Valin et al. 2014). Therefore, the introduction of innovative technologies not only contributes to crop production by improving the tolerance of crop plants during stresses, but also improves the nutritional quality and yield of crops. The advent of recombinant DNA technology tackled many existing restrictions in plant breeding stages. Identification, characterization, and transformation of foreign genes into host plants, to confer desired features including resistance to biotic and abiotic stress factors, herbicide tolerance, improvement of nutritional qualities and yield, have been numerously reported (Pasquali et al. 2008; Tripathi 2012; Bakhsh et al. 2015, 2016; Khabbazi et al. 2016, 2018; Anayol et al. 2016). Modification of the plant genome using chemical and physical mutagenesis is another approach to achieve desired agricultural traits (Roychowdhury and Tah 2013). However, plant genetic modification through these mutagens has random effects on the host plant and consequently might lead to an unexpected outcome. To reduce the unwanted genome alterations, target-specific modifying tools are advantageous and could be exploited alternatively. Genome editing of plants has gained notable achievements since the advent of sequence-specific nucleases (SSN) (Shelake et al. 2019). These tools including zinc finger nucleases (ZNFs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9 system, have encouraged the creation of plant cultivars with desired traits. ZNFs- and TALENs-based genome editing has low-efficiency, is technically complex and cumbersome, therefore despite the reported researches (Lor et al. 2014; Sawai et al. 2014; Clasen et al. 2016) and due to the existing limitations, these methods are not employed any further (Kumar et al. 2018). CRISPR/Cas9 technology on the other hand is more accurate, simple and simultaneously can modify several targets in the genome. Moreover, improvement of a trait in crops using genome editing-based breeding methods requires an average of 4–6 years, while crossbreeding, mutation breeding, and transgene breeding approaches require 8–12 years (Chen et al. 2019). The discovery of CRISPR/Cas9 and its application in the determination of gene function and plant genetic modification studies have opened a promising avenue for the increased food supply in current global conditions (Yan et al. 2016; Minkenberg et al. 2017). The importance of this system is highlighted especially when it induces heritable targeted mutagenesis and contributes to the development of transgene-free plants (Wang et al. 2014; Pan et al. 2016). The introduction of non-transgenic edited plants has an unprecedented advantage to develop plants with desired traits, however maintenance of these genotypes is crucial as well. Hence, in vitro multiplication and generation of synthetic seeds could ensure the conservation and availability of the valuable genotypes (Ergül et al. 2018; Khabbazi et al. 2017, 2019). This chapter reviews the major CRISPR/Cas9-based genetic modifications in different plant species with improved yield, nutritional quality, and response to biotic and abiotic stress factors.
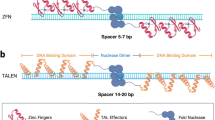
16.2 CRISPR/Cas System; Origin, Mechanism, and Its Use in Genome Editing
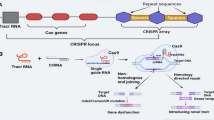
CRISPR/Cas is an adaptive immunity system naturally existing in bacteria and archaea and protects the host from invasive genetic elements like phages. Small nucleic acid fragments of invasive pathogens are incorporated into the host’s CRISPR loci (called spacer) and stored for future encounters (Amitai and Sorek 2016). Once the host cell is exposed to a new invasion, spacer sequences are transcribed, and as individual CRISPR RNAs (crRNAs) guide the Cas nuclease to the cognate nucleic acid sequences of the pathogen and cleave them (Barrangou et al. 2007). Based on the nature of interference and molecules involved, CRISPR/Cas systems have been divided into two classes and six types. CRISPR/Cas systems can target DNA (type I, II, V), RNA (type VI) or both DNA and RNA molecules (type III) (Koonin et al. 2017). Type II CRISPR/Cas9 isolated from Streptococcus pyogenes was the first demonstrated to show target-specific cleavage on the genome in eukaryotic cells and under in vitro conditions (Gasiunas et al. 2012; Jinek et al. 2012; Mali et al. 2013; Cong et al. 2013). This system is based on RNA-guided interference with DNA and has been employed in the development of plants with desired traits. CRISPR/Cas9 complex is comprised of Cas9 nuclease and a single guide RNA molecule (sgRNA). The sgRNA is an artificial fusion of a crRNA with a fixed transactivating crRNA (tracrRNA). A 20-nucleotide fragment at 5ʹ end of the sgRNA directs the complex to the target sites in genome adjoining to conserved protospacer-adjacent motifs (PAMs). The Cas9 enzyme induces double-strand breaks (DSBs) at specific genomic sites and exposes the broken DNA to either error-prone non-homologous end joining (NHEJ) repair or the precise homology-directed repair (HDR) mechanism. NHEJ may cause some unwanted indel mutations at the junction site of the repair whereas the HDR pathway stimulates accurate alteration of a gene sequence. HDR-mediated insertion or replacement of desired sequences in target sites of the genome provides an unprecedented tool for the genetic engineering of plants (Voytas and Gao 2014). Although, delivery of template DNA together with preparation of DSBs makes HDR mechanism more challenging than NHEJ, in vitro and in planta studies have reported the HDR-mediated genome editing in plants (Zhang et al. 2018a).
16.3 Effectiveness and Versatility of CRISPR/Cas9 System in Genome Editing of Plants
The use of CRISPR/Cas9 in genome editing of plants was first indicated in model plants tobacco (Nicotiana benthamiana) and Arabidopsis (Li et al. 2013; Nekrasov et al. 2013). The versatility of CRISPR/Cas9 based genome editing has been studied by knocking out the phytoene desaturase gene (PDS) in different crop species such as potato, grape, sweet orange, and watermelon. Disruption of the PDS gene causes albino phenotype in mutants and acts as a visual marker for CRISPR/Cas9-based mutagenesis (Jia et al. 2014; Pan et al. 2016; Tian et al. 2017; Nakajima et al. 2017). The inheritance pattern of the pds mutants was investigated by monitoring the albino phenotype, sequencing, and genotyping (Pan et al. 2016). The proportion of PDS mutated cells is correlated with the cell age so that lower leaves of the plant display higher rates of the PDS mutation (Nakajima et al. 2017). The higher rates of mutagenesis in older leaves could be due to inefficient repair mechanism of old cells or the repeated induction of double-strand breaks (DSBs). The induced PDS mutagenesis in watermelon plant caused the albino phenotype either as a clear or mosaic pattern with no off-target effects (Tian et al. 2017). Transient expression of CRISPR/Cas9-gRNA-PDS in sweet orange leaves also disrupted the PDS gene without detecting any off-target effects. Pretreatment of the sweet orange leaves with a culture of Xanthomonas citri ssp. citri facilitated the agro-infiltration and enhanced the protein expression level in leaves (Jia and Wang 2014). To increase the efficiency of mutagenesis, studies have been carried out to optimize the Cas9 gene expression. Comparing zCas9 (Xing et al. 2014), AteCas9 (Schiml et al. 2014; Fauser et al. 2014) and Cas9p (Ma et al. 2015), AteCas9 was identified as the most efficient codon-optimized Cas9 enzyme in knocking out the flavanone-3-hydroxylase gene in carrot cells (Klimek-Chodaka et al. 2018). Numerous reports have supported the use of CRISPR/Cas9 for accurate mutagenesis in a variety of crop species with different aims of nutritional quality improvements or biotic and abiotic factor tolerance (reviewed in Ricroch et al. 2017; Santosh Kumar et al. 2020).
16.4 Genome Editing Technologies Contribute to Pathogen Resistance in Crops
Pathogens along with insect pests threaten sustainable crop production worldwide. Bacteria, viruses, and fungi are the most devastating pathogens causing serious economic losses (FAO 2017). Approximately 20–40% of global agricultural losses are caused by these pathogens (Savary et al. 2012). In certain crops such as rice, diseases caused by pathogens are the main reason for the yield losses (Heinrichs and Muniappan 2017). To meet the food demands of the growing global population, chemicals have traditionally been used to combat pathogenic diseases. Different aspects of chemical control from consumer health to imposed costs have brought the need for alternative approaches. Using classical plant breeding methods, resistant cultivars have been developed, however, it is a time-consuming and tough process. In the last two decades, the transformation of resistance genes has successfully enhanced the resistance of crops to pathogens. The gradual acquisition of pathogen resistance to chemicals and resistant varieties can render these approaches ineffective. Genome editing-based technologies have opened new avenues to control agricultural product losses and superseded the limitations of conventional breeding methods. Employment of engineered SSNs such as ZFNs, TALENs, and CRISPR/Cas9 has facilitated the genome modifications toward biotic stresses. Unlike ZFNs and TALENs which are low efficient and technically complex, CRISPR/Cas9 is highly effective and target-specific; however, the efficiency of the entire process remains species- and genotype-dependent. Moreover, off-target mutations, as well as unexpected damages, could be limiting in plant genetic manipulations. PEG-, gene gun- and Agrobacterium-mediated transformation methods and lately a nanodot based transformation method have been used to develop different strategies for stable and transient expression of Cas9/sgRNA constructs in crops (reviewed in Borrelli et al. 2018; Doyle et al. 2019). Employment of gene bombardment method ensures the availability of a sufficient amount of template DNA in host cells, however, the existence of excessive foreign genes and vector sequences might be contaminant for the recipient cell. A. tumefaciens-mediated transformations of plants results in the stable transformation of gene constructs and screening of the mutant plants containing foreign gene sequences in subsequent stages (Baltes et al. 2017). In the last few years, CRISPR/Cas9 has been widely used to improve the resistance of crops to different biotic stresses. These studies have mainly addressed viral, fungal, and bacterial diseases in plants (Table 16.1).
16.4.1 CRISPR/Cas-Based Engineering of Crops for Virus Resistance
Most of the biotic resistance studies in CRISPR-based edited plants are related to viruses (Borrelli et al. 2018). Based on their genome nature, plant viruses are classified into five groups: single-stranded DNA, single-stranded RNA, double-stranded RNA, positive-sense single-stranded RNA, negative-sense single-stranded RNA, and reverse-transcribing viruses. Virus-resistant plant species could be created through either targeting the viral genetic material or editing plant genome. Resistance obtained by disruption of viral genes requires the integration and permanent expression of Cas9/sgRNA constructs, therefore, such modifications subject the plants generated to biosafety regulations of genetically modified organisms (GMOs). In contrast, modifying plant susceptibility genes such as eukaryotic translation initiation factors necessary for the RNA virus life cycle, release non-transgenic virus-resistant plants (Sanfcon 2015).
CRISPR/Cas edited virus-resistant plants have been mostly developed for resistance to geminiviruses (Ji et al. 2015; Baltes et al. 2015; Ali et al. 2015, 2016). Geminiviruses cause substantial destructions in important families of plants including Fabaceae, Cucurbitaceae, Solanaceae, etc. (Zaidi et al. 2016). Most of these studies have been performed on model plants Arabidopsis thaliana and Nicotiana benthamiana (Table 16.1). Expression of Cas9/sgRNA constructs in host plants targeting the coding and non-coding regions of the virus reduced the virus accumulation. Targeting the virus coat protein (CP), replication protein (Rep) and intergenic regions (IR) effectively attenuates viral symptoms; however, mutations disrupting the virus in the non-coding IRs such as the stem-loop sequence within the origin of replication resulted in more reduction of virus replication and accumulation (Ali et al. 2015). Furthermore, targeting the virus in the IR inhibits the creation of new variants of the mutated virus which can potentially replicate and escape the CRISPR/Cas9 machinery (Ali et al. 2016). Targeting plant susceptibility genes such as eIF4E, eIF(iso)4E, and eIF4G has resulted in the development of non-transgenic plants resistant to RNA viruses of Potyviridae (Chandrasekaran et al. 2016; Pyott et al. 2016; Macovei et al. 2018).
In most of the studies, CRISPR-based edited plants have been developed using the SpCas9 enzyme isolated from Streptococcus pyogenes (reviewed in Ricroch et al. 2017). However, this enzyme only cuts double-stranded DNA molecules, therefore, remains inefficient for RNA viruses. Later studies isolated other CRISPR associated enzymes from Francisella novicida (FnCas9) and Leptotrichia wadei (LwaCas13a) which could target RNA molecules. FnCas9 and LwaCas13a have been used to develop plants resistant to cucumber mosaic virus, tobacco mosaic virus and turnip mosaic virus (Zhang et al. 2018b; Aman et al. 2018). Endonuclease activity of FnCas9 is not essential for enzymatic interference; therefore, FnCas9 could be utilized as a CRISPR interference tool (CRISPRi) (Zhang et al. 2018b).
16.4.2 CRISPR/Cas-Based Genetic Modification of Plants Against Fungal Disease
Studies over the plant-pathogen interactions have elucidated the molecular mechanisms underlying pathogen infection and plant immune system. The presence of plant genes serving pathogens can lead to the emergence of diseases. Targeting the susceptibility genes corresponding powdery mildew (MLO-A1, MLO-1, and MLO-7), transcription factors involved in stress responses (WRKY52 and ERF922), regulators associated with host immune system (NPR3) and subunits of the exocyst protein complex (SEC3A), has conferred resistance to fungal diseases in a variety of annual and perennial plants (reviewed in Borrelli et al. 2018).
Till date, resistance against powdery mildew (Wang et al. 2014; Malnoy et al. 2016; Nekrasov et al. 2017), gray mold (Wang et al. 2018), black pod disease (Fister et al. 2018), and blast disease of rice (Wang et al. 2016; Ma et al. 2018) has been improved in several crops such as wheat, rice, tomato, grapevine, and cacao tree. Self-pollination and subsequent selection of individuals with on-target mutations but lacking Cas9/sgRNA constructs introduce edited non-transgenic plants (Nekrasov et al. 2017). Generally, CRISPR/Cas constructs are transferred to host plants through vector plasmids however this method is intercepted by the biosafety regulations of transgenic crops. Implementing genome editing of plants without using DNA plasmid attempts to reduce the social distrust related to the transformations of foreign genes. Transient expression of plasmid-free genome editing constructs is critical particularly in perennial fruit crops due to the longer time required for segregation and backcrossing. In this regard, Malnoy et al (2016) delivered the purified CRISPR/Cas9 ribonucleoproteins (RNPs) to the protoplast of apple and grape cultivars. This method could increase the public acceptance of the genetically modified (GM) crops and thereby could be exempted from the existing GMO regulations (Waltz 2012; Jones 2015).
16.4.3 Bacterial Resistance Achieved Through CRISPR/Cas9
Unlike other pests and pathogens, bacterial diseases of plants cannot be controlled by chemicals, and the only way to cope is to prevent the disease by using different approaches such as efficient agricultural practices, cultivating healthy plants, and developing resistant varieties (Janse 2001). CRISPR/Cas9-based targeted mutagenesis on plant susceptibility genes has conferred resistance to bacterial infection in host plants. Canker is one of the serious diseases of citrus cultivars around the world. The disease is caused by Xanthomonas citri subsp. citri (Xcc) and CsLOB1 is the susceptibility gene in the host induced by the pathogenicity factor PthA4 (Hu et al. 2014). Modifying the PthA4 effector binding elements (EBEs) in the promoter region of CsLOB1 gene in Duncan grapefruit variety interfered with the binding of PthA4, therefore, decreased the typical symptoms of bacterial canker (Jia et al. 2016). Later CRISPR/Cas9-mediated disruption of EBEPthA4 confirmed the presence of the link between CsLOB1 promoter activity and the susceptibility against Xcc in Wanjincheng orange (Citrus sinensis Osbeck) (Peng et al. 2017).
Expression of OsSWEET13, a member of sucrose transporters family proteins, is required for bacteria-host interactions. OsSWEET13 activity is induced by Xanthomonas oryzae pv. Oryzae PthXo2 effector protein and CRISPR/Cas9-mediated knockout of OsSWEET13 susceptibility gene better explored the role of PthXo2 in the emergence of the bacterial blight of rice (Zhou et al. 2015). Recently, CRISPR-based genome-edited SWEET gene promoters (SWEET11/SWEET13/SWEET14) introduced rice lines with broad-spectrum resistance to bacterial blight disease (Oliva et al. 2019).
16.5 Tolerance to Herbicides and Abiotic Stress Factors via CRISPR/Cas9
Weeds are the biotic restraint that causes significant losses in agricultural production and if not controlled timely and effectively reduces the crop yield by up to 50% (Oerke 2006). Transgenic soybean, canola, cotton, and corn were the first glyphosate and glufosinate tolerant crops released to the market in 1996–1997. According to a report, more than 100 million hectares worldwide are cultivated with genetically modified (GM) crops with at least one herbicide tolerance gene (ISAAA 2012). Adoption of herbicide-tolerant (HT) transgenic crops reduces the use of chemicals and greenhouse gas emissions resulting from agricultural practices; moreover, it increases the product yield, farmer income (Cerdeira and Duke 2006; Martino-Catt and Sachs 2008). However, the expression pattern of the transgene is influenced by genetic elements such as the gene promoter and intron fragments (reviewed by Huang et al. 2015). The repeated cultivation of a single herbicide-tolerant crop causes the evolution of weeds resistant to the frequently used chemicals (James 2014). To address this issue, gene stacking has contributed successfully.
Herbicides bind to vital proteins in the host and affect the normal functioning of the cell, inhibit plant growth and consequently make weeds unable to survive (Schonbrunn et al. 2001). Modifying the structure of the target proteins such that their functions are not affected but at the same time it disables the binding of herbicides, is an efficient method to confer tolerance to herbicides. Precise mutagenesis in phosphoenol pyruvate binding site within 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) is an example in this regard which inhibits the binding of glyphosate to phosphoenol pyruvate. Employment of CRISPR/Cas9 tool enables the accurate modification of target sites in the plant genome (Table 16.2). An example in this regard is the substitutions in T178I and P182A within phosphoenol pyruvate binding (PEP) binding site in Linum usitatissimum (Sauer et al. 2016). Comparing the edited plants with wild type flax indicated at the higher levels of tolerance to glyphosate. Likewise, induction of such mutations in EPSPS gene of rice plant confirmed the efficiency of the CRISPR/Cas9, however, based on results, only those individuals containing heterozygous gene were viable and showed tolerance to glyphosate (Li et al. 2016a). In addition to EPSPS, acetolactate synthase (ALS) gene has been targeted by CRISPR/Cas system to apply mutagenesis with the aim of increasing tolerance to herbicides. Acetolactate synthase is a conserved protein required for biosynthesis of branched-chain amino acids such as valine, leucine, and isoleucine. This protein is a primary target for some classes of herbicides including pyrimidinyl thiobenzoates, imidazolinones, triazolopyrimidines, sulfonylureas, and sulfonyl-aminocarbonyl triazolinones (Baltes et al. 2017). CRISPR-based ALS mutations conferred tolerance to such herbicides in Solanum tuberosum, Oryza sativa, Zea mays, and Glycine max species (Svitashev et al. 2015; Li et al. 2015; Butler et al. 2016; Sun et al. 2016; Endo et al. 2016; Butt et al. 2017) (Table 16.2).
In addition to the crop losses imposed by weeds, factors such as unfavorable drought, salinity, and temperature induce metabolic stresses that affect the growth of plants. CRISPR/Cas-mediated precise disruption of target sites in the genome has been employed to confer tolerance to stress factors (Table 16.2). Ethylene plays an important role in regulating plant response to water deficits and high temperatures (Hays et al. 2007; Kawakami et al. 2010, 2013). Reducing plants’ ethylene biosynthesis or decreasing the sensitivity of plants to ethylene can improve grain yield under drought conditions (Habben et al. 2014; Shi et al. 2015). ARGOS8 gene is a negative regulator of ethylene responses. In maize, the expression of ARGOS8 gene is relatively low and therefore a constitutive overexpression enhanced the expression level of ARGOS8 and consequently led to the increase in grain yield under drought stress (Shi et al. 2015). Utilization of CRISPR/Cas system via the HDR pathway contributed to precise allelic variation in ARGOS8 thereby enhancing the grain yield under drought conditions (Shi et al. 2017). HDR-dependent mutation of mir169a improved the drought tolerance in model plant A. thaliana such that more than 50% of mutant plants exhibited tolerance to drought stress whereas no wild individual survived (Zhao et al. 2016).
CRISPR/Cas9 tool has also been used to characterize the transcriptional response of rice RAV genes (OsRAVs) to salt stress. In this study, the expression patterns of the five members of OsRAVs were examined under salt stress, and it was observed that only OsRAV2 was stably induced by salt treatment. Further analysis on the OsRAV2 promoter region elucidated that pOsRAV2 is induced by salt. The GT-1 element located at pOsRAV2 is necessary for salt induction of the promoter (Duan et al. 2016).
16.6 Improvement of Crop Yield, Nutritional Quality and Storage Using CRISPR/Cas9
The effectiveness of the CRISPR/Cas9 system in the disruption of negative regulators of undesirable traits in plants has led to the improvement of yield, nutritional value, and shelf-life of crops, such that these researches share the highest proportion among the CRISPR-based studies (Table 16.3 and Fig. 16.1). Improving the yield is a crucial step to ensure food supply for the growing population. Crop yield is assessed considering various factors such as grain number, size, and weight as well as tiller number and panicle size. As a quantitative trait, yield depends upon many regulating factors. Disruption of genes responsible for the negative regulation of the aforementioned yield factors has contributed to the development of crops with improved yield quality (reviewed by Chen et al. 2019). Knocking out the OsGn1a, OsGS3, TaGW2, OsGW5, OsGLW2, TaGASR7, OsDEP1, TaDEP1, and OsAAP3 genes has indicated the effectiveness of CRISPR/Cas system in improving the crop yield by the creation of targeted loss-of-function mutations in plants (Li et al. 2016d; Liu et al. 2017; Lu et al. 2018; Zhang et al. 2016, 2018c). Simultaneous knock out of three genes including GW2, GW5, and TGW6 increased the grain weight in rice which demonstrated the effectiveness of CRISPR/Cas use in trait pyramiding in plants (Xu et al. 2016).
Crop quality traits such as starch, oil, and secondary metabolite content have also been improved by the CRISPR/Cas-based gene editing. Starch produced from crops like potato has many applications in the food and industrial sectors. Starch has two components including amylose and amylopectin and modification of amylose or amylopectin alters the properties of starch (Zeeman et al. 2010). Granule-bound starch synthase (GBSS) is the enzyme responsible for amylose synthesis in many plant species. In potato plant (Solanum tuberosum), the GBSS enzyme is encoded by a single locus (GBSS1) with four alleles. High amylopectin potato genotypes (Waxy potato) have been developed by gene silencing technologies and traditional mutational breeding methods (Andersson et al. 2003; Muth et al. 2008). Recently, CRISPR/Cas-based multiallelic indel mutations of potato GBSS gene reduced the amylose, and increased the amylopectin content of the starch (Andersson et al. 2017, 2018). In Zea mays, knock out of the endogenous waxy gene led to the production of grains with high amylopectin content (Waltz 2016). Consumption of cereals with enriched amylose or resistant starch benefits human health and reduces the risks of non-infectious serious diseases such as diabetes (Regina et al. 2006). Using the CRISPR/Cas tool, mutagenesis was implemented in the starch branching enzyme gene (SBEIIb), leading to an increased proportion of amylose and resistant starch in rice grains (Sun et al. 2017).
Modifications of the fatty acid composition of plant oils have also been carried out by the CRISPR genome editing tool. Targeting the Fad2 (Morineau et al. 2016; Jiang et al. 2017) and CsDGAT1 and CsPDAT1 (Aznar-Moreno and Durrett 2017) genes in Camelina sativa altered the fatty acid composition of the seed oil. CRISPR/Cas9 tool was used to interfere with the ripening process of tomato and enhanced the fruit shelf-life by targeting RIN, SLALC, and lncRNA1459 genes (Ito et al. 2015; Yu et al. 2017; Li et al. 2018). Precise mutations of RIN, SLALC and lncRNA1459 genes of tomato interfered with the ripening process and enhanced the fruit shelf-life (Ito et al. 2015; Yu et al. 2017; Li et al. 2018). Other studies carried out with the aim of improving quality, yield, and breeding-related attempts has been summarized in Table 16.3.
16.7 Conclusion
CRISPR/Cas is a simple, versatile, and robust tool to induce target-specific mutations in different plant species. The employment of the CRISPR/Cas system has indicated an unprecedented contribution to the genome editing of crops. This tool has paved the way for conferring desired characteristics to various plant species and eliminating foreign genes in subsequent generations. Using this technology along with the high throughput system, quantitative traits with polygenic inheritance can be improved by simultaneously targeting the gene loci in crops. CRISPR/Cas system is also useful in the identification of the roles of genetic elements involved in different metabolic pathways. Despite the advances achieved to date, the existence of impediments such as off-target effects, delivery of the CRISPR reagents to host cells, and plant regeneration are still a limiting factor for some species. Unlike the commonly reported knockout-based mutation studies, knock-in researches are less reported and have been carried out on a limited scale. The recent use of the prime editing method in human genome editing has raised the hope for more efficient and precise base editing attempts without relying upon double-strand breaks or donor DNA (Anzalone et al. 2019). The prime editing technology is also expected to be used for plant cells, which will lead to new directions in the precise engineering of the plant genome (Xu et al. 2020).
References
Alagoz Y, Gurkok T, Zhang B, Unver T (2016) Manipulating the biosynthesis of bioactive compound alkaloids for next-generation metabolic engineering in opium poppy using CRISPR-Cas 9 genome editing technology. Sci Rep 6:30910. https://doi.org/10.1038/srep30910
Ali Z, Abulfaraj A, Idris A, Ali S, Tashkandi M, Mahfouz MM (2015) CRISPR/Cas9-mediated viral interference in plants. Genome Biol 16:238. https://doi.org/10.1186/s13059-015-0799-6
Ali Z, Ali S, Tashkandi M, Shan S, Zaidi A, Mahfouz MM (2016) CRISPR/Cas9-mediated immunity to geminiviruses: differential interference and evasion. Sci Rep 6:26912. https://doi.org/10.1038/srep26912
Aman R, Ali Z, Butt H, Mahas A, Aljedaani F, Khan MZ et al (2018) RNA virus interference via CRISPR/Cas13a system in plants. Genome Biol 19:1. https://doi.org/10.1186/s13059-017-1381-1
Amitai G, Sorek R (2016) CRISPR-Cas adaptation: insights into the mechanism of action. Nat Rev Microbiol 14:67–76
Anayol E, Bakhsh A, Karakoç ÖC, Onarıcı S, Köm D, Aasim M, Özcan SF, Barpete S, Khabbazi SD, Önol B et al (2016) Towards better insect management strategy: restriction of insecticidal gene expression to biting sites in transgenic cotton. Plant Biotechnol Rep 10:83–94
Andersson M, Trifonova A, Andersson AB, Johansson M, Bulow L, Hofvander P (2003) A novel selection system for potato transformation using a mutated AHAS gene. Plant Cell Rep 22:261–267
Andersson M, Turesson H, Nicolia A, Fält AS, Samuelsson M, Hofvander P (2017) Efficient targeted multiallelic mutagenesis in tetraploid potato (Solanum tuberosum) by transient CRISPR-Cas9 expression in protoplasts. Plant Cell Rep 36:117–128. https://doi.org/10.1007/s00299-016-2062-3
Andersson M, Turesson H, Olsson N, Falt AS, Ohlsson P, Gonzalez MN, Samuelsson M, Hofvander P (2018) Genome editing in potato via CRISPR/Cas9 ribonucleoprotein delivery. Physiol Planta 164:119–240
Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW et al (2019) Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576(7785):149–157
Aznar-Moreno JA, Durrett TP (2017) Simultaneous targeting of multiple gene homeologs to alter seed oil production in Camelina sativa. Plant Cell Physiol 58:1260–1267. https://doi.org/10.1093/pcp/pcx058
Bakhsh A, Khabbazi SD, Baloch FS, Demirel U, Çalişkan ME, Hatipoğlu R, Özcan S, Özkan H (2015) Insect resistant transgenic crops: retrospects and challenges. Turk J Agric For 39:531–548
Bakhsh A, Anayol E, Khabbazi SD, Karakoç ÖC, Sancak C, Özcan S (2016) Development of insect-resistant cotton lines with targeted expression of insecticidal gene. Arch Biol Sci 68(4):773–780
Baltes NJ, Hummel AW, Konecna E, Cegan R, Bruns AN, Bisaro DM et al (2015) Conferring resistance to geminiviruses with the CRISPR–Cas prokaryotic immune system. Nat Plants 1:15145. https://doi.org/10.1038/nplants.145
Baltes NJ, Gil-Humanes J, Voytas DF (2017) Genome engineering and agriculture: opportunities and challenges. Calyxt, Inc., New Brighton, MN, United States. Progress in molecular biology and translational science, vol 149. Elsevier Inc. http://dx.doi.org/10.1016/bs.pmbts.2017.03.011
Barrangou R, Fremaux C, Deveau H, Richards M et al (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315(5819):1709–1712
Borrelli VMG, Brambilla V, Rogowsky P, Marocco A, Lanubile A (2018) The enhancement of plant disease resistance using CRISPR/Cas9 technology. Front Plant Sci 9:1245. https://doi.org/10.3389/fpls.01245
Butler NM, Baltes NJ, Voytas DF, Douches DS (2016) Geminivirus-mediated genome editing in potato (Solanum tuberosum L.) using sequence-specific nucleases. Front Plant Sci 7:1045
Butt H, Eid A, Ali Z, Atia MAM, Mokhtar MM, Hassan N et al (2017) Efficient CRISPR/Cas9-mediated genome editing using a chimeric single-guide RNA molecule. Front Plant Sci 8:1441
Cerdeira AL, Duke SO (2006) The current status and environmental impacts of glyphosate-resistant crops: a review. J Environ Qual 35(5):1633–1658
Chandrasekaran J, Brumin M, Wolf D, Leibman D, Klap C, Pearlsman M et al (2016) Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol Plant Pathol 17:1140–1153
Chen K, Wang Y, Zhang R, Zhang H, Gao C (2019) CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu Rev Plant Biol 70:667–697
Clasen BM, Stoddard TJ, Luo S, Demorest ZL, Li J, Cedrone F, Tibebu R, Davison S, Ray EE, Daulhac A, Coffman A, Yabandith A, Retterath A, Haun W, Baltes NJ, Mathis L, Voytas DF, Zhang F (2016) Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol J 14:169–176
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823
Doyle C, Higginbottom K, Swift TA, Winfield M, Bellas C, Benito-Alifonso D, Fletcher T, Galan CM, Edwards K, Whitney HM (2019) A simple method for spray-on gene editing in planta. bioRxiv 1:805036
Duan YB, Li J, Qin RY, Xu RF, Li H, Yang YC et al (2016) Identification of a regulatory element responsible for salt induction of rice OsRAV2 through ex situ and in situ promoter analysis. Plant Mol Biol 90:49–62. https://doi.org/10.1007/s11103-015-0393-z
Endo M, Mikami M, Toki S (2016) Biallelic gene targeting in rice. Plant Physiol 170:667–677
Ergül A, Khabbazi SD, Oğuz MÇ, Özmen CY, Gürel S, Gürel E (2018) In vitro multiplication of wild relatives in genus Beta conserves the invaluable threatened germplasms. Plant Cell, Tissue and Organ Culture (PCTOC):1–7
FAO (2017) The future of food and agriculture—trends and challenges. FAO, Rome
Fauser F, Schiml S, Puchta H (2014) Both CRISPR/Cas-based nucleases and nickases can be used efficiently for genome engineering in Arabidopsis thaliana. Plant J 79:348–359
Fister AS, Landherr L, Maximova SN, Guiltinan MJ (2018) Transient expression of CRISPR/Cas9 machinery targeting TcNPR3 enhances defense response in Theobroma cacao. Front Plant Sci 9:268. https://doi.org/10.3389/fpls.00268
Gasiunas G, Barrangou R, Horvath P, Siksnys V (2012) Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. PNAS 109:E2579–E2586
Habben JE, Bao X, Bate NJ, DeBruin JL, Dolan D, Hasegawa D, Helentjaris TG et al (2014) Transgenic alteration of ethylene biosynthesis increases grain yield in maize under field drought-stress conditions. Plant Biotechnol J 12:685–693
Hays DB, Do JH, Mason RE, Morgan G, Finlayson SA (2007) Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Sci 172:1113–1123
Heinrichs EA, Muniappan R (2017) IPM for tropical crops: rice. CAB Reviews 12:1–31
Hu Y, Zhang J, Jia H, Sosso D, Li T, Frommer WB, Yang B et al (2014) Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc Natl Acad Sci USA 111:E521–E529
Huang J, Ellis C, Hauge B, Qi Y, Varagona MJ (2015) Herbicide tolerance. Recent advancements in gene expression and enabling technologies in crop plants. Springer, New York, NY, pp 213–237
Hummel AW, Chauhan RD, Cermak T, Mutka AM, Vijayaraghavan A, Boyher A et al (2018) Allele exchange at the EPSPS locus confers glyphosate tolerance in cassava. Plant Biotechnol J 16:1275–1282
Iaffaldano B, Zhang Y, Cornish K (2016) CRISPR/cas9 genome editing of rubber producing dandelion Taraxacum kok-saghyz using Agrobacterium rhizogenes without selection. Ind Crops Prod 89:356–362. https://doi.org/10.1016/j.indcrop.2016.05.029
Ito Y, Nishizawa-Yokoi A, Endo M, Mikami M, Toki S (2015) CRISPR/Cas9-mediated mutagenesis of the RIN locus that regulates tomato fruit ripening. Biochem Biophys Res Commun 467:76–82. https://doi.org/10.1016/j.bbrc.2015.09.117
James C (2014/2012) Global status of commercialized biotech/GM crops. ISAAA Briefs (49). ISAAA (International Service for the Acquisition of Agri-biotech Applications). Ithaca, NY
Janse JD (2001) Control of bacterial diseases through pathogen freedom of planting material. In: De Boer SH (ed) Plant Pathogenic Bacteria. Springer, Dordrecht
Ji X, Zhang H, Zhang Y, Wang Y, Gao C (2015) Establishing a CRISPR–Cas-like immune system conferring DNA virus resistance in plants. Nat Plants 1:15144. https://doi.org/10.1038/nplants.2015.144
Jia H, Orbovic V, Jones JB, Wang N (2016) Modification of the PthA4 effector binding elements in type I CsLOB1 promoter using Cas9/sgRNA to produce transgenic Duncan grapefruit alleviating XccΔpthA4:dCsLOB1.3 infection. Plant Biotechnol J 14:1291–1301
Jia H, Wang N (2014) Xcc-facilitated agroinfiltration of citrus leaves: a tool for rapid functional analysis of transgenes in citrus leaves. Plant Cell Rep 33:1993–2001
Jia W, Yang B, Weeks DP (2014) Efficient CRISPR/Cas9-mediated gene editing in Arabidopsis thaliana and inheritance of modified genes in the T2 and T3 generations. PLoS One 9:e99225
Jia H, Zhang Y, Orbovic V, Xu J, White FF, Jones JB, Wang N (2017) Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol J 15:817–823
Jiang WZ, Henry IM, Lynagh PG, Comai L, Cahoon EB, Weeks DP (2017) Significant enhancement of fatty acid composition in seeds of the allohexaploid, Camelina sativa, using CRISPR/Cas9 gene editing. Plant Biotechnol J 15:648–657. https://doi.org/10.1111/pbi.12663
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
Jones HD (2015) Regulatory uncertainty over genome editing. Nat Plants 1:14011. https://doi.org/10.1038/nplants.2014.11
Kapusi E, Corcuera-Gómez M, Melnik S, Stoger E (2017) Heritable genomic fragment deletions and small indels in the putative ENGase gene induced by CRISPR/Cas9 in barley. Front Plant Sci 8:540. https://doi.org/10.3389/fpls.2017.00540
Kawakami EM, Oosterhuis DM, Snider JL (2010) Physiological effects of 1-methylcyclopropene on well-watered and water-stressed cotton plants. J Plant Growth Regul 29:280–288
Kawakami EM, Oosterhuis DM, Snider J (2013) High temperature and the ethylene antagonist 1-methylcyclopropene alter ethylene evolution patterns, antioxidant responses, and boll growth in Gossypium hirsutum. Am J Plant Sci 4:1400–1408
Khabbazi SD, Bakhsh A, Sancak C, Özcan S (2016) Molecular Characterization of Snowdrop Lectin (GNA) and its comparison with reported lectin sequences of Amaryllidaceae. Czech J Genet Plant 52(3):94–100
Khabbazi SD, Barpete S, Sancak C, Ozcan S (2017) Preconditioning effect of BAP on in vitro multiplication of mature embryo of lentil (Lens culinaris Medik). Fresen Environ Bull 26:919–925
Khabbazi SD, Khabbazi AD, Özcan SF et al (2018) Expression of GNA and biting site-restricted cry1Ac in cotton; an efficient attribution to insect pest management strategies. Plant Biotechnol Rep 12:273. https://doi.org/10.1007/s11816-018-0493-8
Khabbazi SD, Yüksel Özmen C, Ergül A (2019) Synthetic seeds of wild beet: basic concepts and related methodologies. In: Faisal M, Alatar A (eds) Synthetic Seeds. Springer, Cham
Klimek-Chodaka M, Oleszkiewicz T, Lowder LG, Baranski QiR (2018) Efficient CRISPR/Cas9-based genome editing in carrot cells. Plant Cell Rep 37:575–586
Koonin EV, Makarova KS, Zhang F (2017) Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol 37:67–78
Kui L, Chen H, Zhang W, He S, Xiong Z, Zhang Y et al (2017) Building a genetic manipulation tool box for orchid biology: identification of constitutive promoters and application of CRISPR/Cas9 in the orchid, Dendrobium officinale. Front Plant Sci 7:2036. https://doi.org/10.3389/fpls.2016.02036
Kumar N, Stanford W, deSolis C, Aradhana Abraham ND, Dao TJ, Thaseen S, Sairavi A, Gonzalez CU, Ploski JE (2018) The development of an AAV-based CRISPR SaCas9 genome editing system that can be delivered to neurons in vivo and regulated via doxycycline and Cre-recombinase. Front Mol Neurosci 11:413
Lawrenson T, Shorinola O, Stacey N, Li C, Østergaard L, Patron N et al (2015) Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol 16:258. https://doi.org/10.1186/s13059-015-0826-7
Lee SK, Eom JS, Hwang SK, Shin D, An G, Okita TW et al (2016) Plastidic phosphoglucomutase and ADP-glucose pyrophosphorylase mutants impair starch synthesis in rice pollen grains and cause male sterility. J Exp Bot 67:5557–5569. https://doi.org/10.1093/jxb/erw324
Li JF, Norville JE, Aach J, McCormack M, Zhang D, Bush J, Church GM, Sheen J (2013) Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol 31:688–691
Li Z, Liu ZB, Xing A, Moon BP, Koellhoffer JP, Huang L et al (2015) Cas9-guide RNA directed genome editing in soybean. Plant Physiol 169:960–970
Li J, Meng X, Zong Y, Chen K, Zhang H, Liu J et al (2016a) Gene replacements and insertions in rice by intron targeting using CRISPR-Cas9. Nat Plants 2:16139
Li Q, Zhang D, Chen M, Liang W, Wei J, Qi Y et al (2016b) Development of japonica photo-sensitive genic male sterile rice lines by editing carbon starved anther using CRISPR/Cas9. J Genet Genomics 43:415–419. https://doi.org/10.1016/j.jgg.2016.04.011
Li M, Li X, Zhou Z, Wu P, Fang M et al (2016c) Reassessment of the four yield-related genes gn1a, dep1, gs3, and ipa1 in rice using a CRISPR/Cas9 system. Front Plant Sci 7:377
Li S, Gao F, Xie K, Zeng X, Cao Y et al (2016d) The OsmiR396c-OsGRF4-OsGIF1 regulatory module determines grain size and yield in rice. Plant Biotechnol J 14:2134–2146
Li B, Cui G, Shen G, Zhan Z, Huang L, Chen J et al (2017a) Targeted mutagenesis in the medicinal plant Salvia miltiorrhiza. Sci Rep 7:43320. https://doi.org/10.1038/srep43320
Li C, Liu C, Qi X, Wu Y, Fei X, Mao L et al (2017b) RNA-guided Cas9 as an in vivo desired-target mutator in maize. Plant Biotechnol J. https://doi.org/10.1111/pbi.12739
Li X, Zhou W, Ren Y, Tian X, Lv T, Wang Z et al (2017c) High-efficiency breeding of early-maturing rice cultivars via CRISPR/Cas9-mediated genome editing. J Genet Genomics 44:175–178. https://doi.org/10.1016/j.jgg.2017.02.001
Li X, Wang Y, Chen S, Tian H, Fu D, Zhu B, Luo Y, Zhu H (2018) Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00559
Liu L, Zheng C, Kuang B, Wei L, Yan L, Wang T (2016) Receptor-like kinase RUPO interacts with potassium transporters to regulate pollen tube growth and integrity in rice. PLoS Genet 12:e1006085. https://doi.org/10.1371/journal.pgen.1006085
Liu J, Chen J, Zheng X, Wu F, Lin Q et al (2017) GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat Plants 3:17043
Lor VS, Starker CG, Voytas DF, Weiss D, Olszewski NE (2014) Targeted mutagenesis of the tomato PROCERA gene using transcription activator-like effector nucleases. Plant Physiol 166:1288–1291
Lu K, Wu B, Wang J, Zhu W, Nie H et al (2018) Blocking amino acid transporter OsAAP3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol J 16:1710–1722
Ludman M, Burgyán J, Fátyol K (2017) CRISPR/Cas9 mediated inactivation of argonaute 2 reveals its differential involvement in antiviral responses. Sci Rep 7:1010. https://doi.org/10.1038/s41598-017-01050-6
Ma L, Zhang D, Miao Q, Yang J, Xuan Y, Hu Y (2017) Essential role of sugar transporter OsSWEET11 during the early stage of rice grain filling. Plant Cell Physiol 58:863–873. https://doi.org/10.1093/pcp/pcx040
Ma J, Chen J, Wang M, Ren Y, Wang S, Lei C et al (2018) Disruption of OsSEC3A increases the content of salicylic acid and induces plant defense responses in rice. J Exp Bot 69:1051–1064. https://doi.org/10.1093/jxb/erx458
Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, Qiu R, Wang B, Yang Z, Li H, Lin Y, Xie Y, Shen R, Chen S, Wang Z, Chen Y, Guo J, Chen L, Zhao X, Dong Z, Liu YG (2015) A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicotplants. Mol Plant 8:1274–1284
Macovei A, Sevilla NR, Cantos C, Jonson GB, Slamet-Loedin I, Cermak T et al (2018) Novel alleles of rice eIF4G generated by CRISPR/Cas9-targeted mutagenesis confer resistance to Rice tungro spherical virus. Plant Biotechnol J. https://doi.org/10.1111/pbi.12927 [Epub ahead of print]
Mali P, Yang L, Esvelt KM, Aach J, Guell M et al (2013) RNA-guided human genome engineering via Cas9. Science 339:823–826
Malnoy M, Viola R, Jung MH, Koo JO, Kim S, Kim JS et al (2016) DNAfree genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front Plant Sci 7:1904. https://doi.org/10.3389/fpls.01904
Martino-Catt SJ, Sachs ES (2008) Editor’s choice series: the next generation of biothech crops. Plant Physiol 147(1):3–5
Miao J, Guo D, Zhang J, Huang Q, Qin G, Zhang X et al (2013) Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res 23:1233–1236
Minkenberg B, Xie K, Yang Y (2017) Discovery of rice essential genes by characterizing a CRISPR-edited mutation of closely related rice MAP kinase genes. Plant J 89:636–648. https://doi.org/10.1111/tpj.13399
Morineau C, Bellec Y, Tellier F, Gissot L, Kelemen Z, Nogué F et al (2016) Selective gene dosage by CRISPR-Cas9 genome editing in hexaploid Camelina sativa. Plant Biotechnol 15:729–739. https://doi.org/10.1111/pbi.12671
Muth J, Hartje S, Twyman RM, Hofferbert H-R, Tacke E, Pruefer D (2008) Precision breeding for novel starch variants in potato. Plant Biotechnol J 6:576–584
Nakajima I, Ban Y, Azuma A, Onoue N, Moriguchi T, Yamamoto T, Toki S, Endo M (2017) CRISPR/Cas9-mediated targeted mutagenesis in grape. PLoS One 12:e0177966. https://doi.org/10.1371/journal.pone.0177966
Nekrasov V, Staskawicz B, Weigel D, Jones JD, Kamoun S (2013) Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat Biotechnol 31:691–693
Nekrasov V, Wang C, Win J, Lanz C, Weigel D, Kamoun S (2017) Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci Rep 7:482
Oerke E (2006) Crop losses to pests. J Agri Cult Sci 144(1):31–43
Oliva R, Ji C, Atienza-Grande G, Huguet-Tapia JC, Perez-Quintero A et al (2019) Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat Biotechnol 37(11):1344–1350
Ortigosa A, Gimenez-Ibanez S, Leonhardt N, Solano R (2018) Design of a bacterial speck resistant tomato by CRISPR/Cas9-mediated editing of SlJAZ2. Plant Biotechnol J. https://doi.org/10.1111/pbi.13006
Pan C, Ye L, Qin L, Liu X, He Y, Wang J et al (2016) CRISPR/Cas9-mediated efficient and heritable targeted mutagenesis in tomato plants in the first and later generations. Sci Rep 6:24765. https://doi.org/10.1038/srep24765
Pasquali G, Biricolti S, Locatelli F, Baldoni E, Mattana M (2008) Osmyb4 expression improves adaptive responses to drought and cold stress in transgenic apples. Plant Cell Rep 27:1677–1686
Peng A, Chen S, Lei T, Xu L, He Y, Wu L et al (2017) Engineering canker resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol J 10:1011–1013
Pyott DE, Sheehan E, Molnar A (2016) Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol Plant Pathol 17:1276–1288. https://doi.org/10.1111/mpp.12417
Regina A, Bird A, Topping D, Bowden S, Freeman J, Barsby T et al (2006) High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc Natl Acad Sci USA 103:3546–3551. https://doi.org/10.1073/pnas.0510737103
Ricroch A, Clairand P, Harwood W (2017) Use of CRISPR systems in plant genome editing: toward new opportunities in agriculture. Emerg Top Life Sci 1:169–182
Roychowdhury R, Tah J (2013) Mutagenesis—a potential approach for crop improvement. In: Hakeem K, Ahmad P, Ozturk M (eds) Crop improvement. Springer, Boston, MA
Sanfcon H (2015) Plant translation factors and virus resistance. Viruses 7:3392–3419
Santosh Kumar VV, Verma RK, Yadav SK et al (2020) CRISPR-Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in indica mega rice cultivar MTU1010. Physiol Mol Biol Plants 26:1099–1110. https://doi.org/10.1007/s12298-020-00819-w
Sauer NJ, Narváez-Vásquez J, Mozoruk J, Miller RB, Warburg ZJ, Woodward MJ et al (2016) Oligonucleotide-mediated genome editing provides precision and function to engineered nucleases and antibiotics in plants. Plant Physiol 170:1917–1928
Savary S, Ficke A, Aubertot J, Hollier C (2012) Crop losses due to diseases and their implications for global food production losses and food security. Food Secur 4:519–537
Sawai S, Ohyama K, Yasumoto S, Seki H, Sakuma T, Yamamoto T, Takebayashi Y, Kojima M, Sakakibara H, Aoki T, Muranaka T, Saito K, Umemoto N (2014) Sterol side chain reductase 2 is a key enzyme in the biosynthesis of cholesterol, the common precursor of toxic steroidal glycoalkaloids in potato. Plant Cell 26:3763–3774
Schiml S, Fauser F, Puchta H (2014) The CRISPR/Cas system can be used as nuclease for in planta gene targeting and as paired nickases for directed mutagenesis in Arabidopsis resulting in heritable progeny. Plant J 80:1139–1150
Schonbrunn E, Eschenburg S, Shuttleworth WA et al (2001) Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. Proc Natl Acad Sci USA 98(4):1376–1380
Shelake RM, Pramanik D, Kim JY (2019) Evolution of plant mutagenesis tools: a shifting paradigm from random to targeted genome editing. Plant Biotechnol Rep 13:423–445
Shi J, Habben JE, Archibald RL, Drummond BJ, Chamberlin MA, Williams RW, Lafitte HR et al (2015) Overexpression of ARGOS genes modifies plant sensitivity to ethylene, leading to improved drought tolerance in both Arabidopsis and maize. Plant Physiol 169:266–282
Shi J, Gao H, Wang H, Lafitte HR, Archibald RL, Yang M et al (2017) ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J 15:207–216
Shimatani Z, Kashojiya S, Takayama M, Terada R, Arazoe T, Ishii H et al (2017) Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat Biotechnol 35:441–443. https://doi.org/10.1038/nbt.3833
Sun Y, Zhang X, Wu C, He Y, Ma Y, Hou H et al (2016) Engineering herbicideresistant rice plants through CRISPR/Cas9-mediated homologous recombination of acetolactate synthase. Mol Plant 9:628–631
Sun Y, Jiao G, Liu Z, Zhang X, Li J, Guo X et al (2017) Generation of high-amylose rice through CRISPR/Cas9-mediated targeted mutagenesis of starch branching enzymes. Front Plant Sci 8:298. https://doi.org/10.3389/fpls.2017.00298
Svitashev S, Young JK, Schwartz C, Gao H, Falco SC, Cigan AM (2015) Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol 169:931–945
Tian S, Jiang L, Gao Q, Zhang J, Zong M, Zhang H et al (2017) Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Rep 36:399–406. https://doi.org/10.1007/s00299-016-2089-5
Tripathi L (2012) Transgenics in crop improvement research at IITA. IITA Res Dev (R4D) Rev 8:58–60
Ueta R, Abe C, Watanabe T, Sugano SS, Ishihara R, Ezura H et al (2017) Rapid breeding of parthenocarpic tomato plants using CRISPR/Cas9. Sci Rep 7:507. https://doi.org/10.1038/s41598-017-00501-4
Valin H, Sands RD, van der Mensbrugghe D et al (2014) The future of food demand: understanding differences in global economic models. Agric Econ 45(1):51–67
Voytas DF, Gao C (2014) Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol 12:e1001877
Waltz E (2012) Tiptoeing around transgenics. Nat Biotechnol 30:215–217. https://doi.org/10.1038/nbt.2143
Waltz E (2016) CRISPR-edited crops free to enter market, skip regulation. Nat Biotechnol 34:582
Wang Y, Cheng X, Shan Q, Zhang Y, Liu J, Gao C et al (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol 32:947–951. https://doi.org/10.1038/nbt.2969
Wang F, Wang C, Liu P, Lei C, Hao W, Gao Y et al (2016) Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE 11:e0154027
Wang W, Pan Q, He F, Akhunova A, Chao S, Trick H et al (2018) Transgenerational CRISPR-Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. CRISPR J. 1:65–74. https://doi.org/10.1089/crispr.2017.0010
Xing HL, Dong L, Wang ZP, Zhang HY, Han CY, Liu B, Wang XC, Chen QJ (2014) A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol 14:327
Xu R, Yang Y, Qin R, Li H, Qiu C et al (2016) Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J Genet Genom 43:529–532
Xu R, Li J, Liu X, Shan T, Qin R, Wei P (2020) Development of plant prime-editing systems for precise genome editing. Plant Commun 1(3). https://doi.org/10.1016/j.xplc.2020.100043
Yan W, Chen D, Kaufmann K (2016) Efficient multiplex mutagenesis by RNA-guided Cas9 and its use in the characterization of regulatory elements in the AGAMOUS gene. Plant Methods 12:23. https://doi.org/10.1186/s13007-016-0125-7
Yasui Y, Tanaka W, Sakamoto T, Kurata T, Hirano HY (2017) Genetic enhancer analysis reveals that FLORAL ORGAN NUMBER2 and OsMADS3 co-operatively regulate maintenance and determinacy of the flower meristem in rice. Plant Cell Physiol 58:893–903. https://doi.org/10.1093/pcp/pcx038
Yin X, Biswal AK, Dionora J, Perdigon KM, Balahadia CP, Mazumdar S et al (2017) CRISPR-Cas9 and CRISPR-Cpf1 mediated targeting of a stomatal developmental gene EPFL9 in rice. Plant Cell Rep 36:745–757. https://doi.org/10.1007/s00299-017-2118-z
Yu Q, Wang BLiN, Tang Y, Yang S, Yang T, Xu J (2017) CRISPR/Cas9-induced targeted mutagenesis and gene replacement to generate long-shelf life tomato lines. Sci Rep 7:11874
Yuan J, Chen S, Jiao W, Wang L, Wang L, Ye W et al (2017) Both maternally and paternally imprinted genes regulate seed development in rice. New Phytol 216:373–387. https://doi.org/10.1111/nph.14510
Zaidi SSeA, Tashkandi M, Mansoor T, Mahfouz MM (2016) Engineering plant immunity using CRISPR/Cas9 to generate virus resistancs. Front Plant Sci 7:1673
Zeeman SC, Kossmann J, Smith AM (2010) Starch: its metabolism, evolution, and biotechnological modification in plants. Annu Rev Plant Biol 61:209–234
Zhang Y, Liang Z, Zong Y, Wang Y, Liu J et al (2016) Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat Commun 7:12617
Zhang Y, Bai Y, Wu G, Zou S, Chen Y, Gao C, Tang D (2017) Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J 91:714
Zhang Y, Massel K, Godwin ID, Gao C (2018a) Applications and potential of genome editing in crop improvement. Genome Biol 19:210
Zhang Y, Zheng Q, Yi X, An H, Zhao Y, Ma S, Zhou G (2018b) Establishing RNA virus resistance in plants by harnessing CRISPR immune system. Plant Biotechnol J 16(8):1415–1423
Zhang Y, Li D, Zhang D, Zhao X, Cao X, Dong L, Liu J, Chen K, Zhang H, Gao C, Wang D (2018c) Analysis of the functions of homoeologs in wheat grain weight and protein content traits. Plant J 94(5):857–866
Zhao Y, Zhang C, Liu W, Gao W, Liu C, Song G et al (2016) An alternative strategy for targeted gene replacement in plants using a dual-Sgrna/Cas9 design. Sci Rep 6:23890. https://doi.org/10.1038/srep23890
Zhou J, Peng Z, Long J, Sosso D, Liu B, Eom JS et al (2015) Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J 82:632–643
Acknowledgments
The corresponding author (Dr. Saber D. Khabbazi) is grateful to The Scientific and Technological Research Council of Turkey (TUBITAK-BIDEB) for providing postdoctoral fellowship (Code 2216) to carry out research activities in plant biotechnology at Ankara University.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Khabbazi, S.D., Khabbazi, A.D., Cevik, V., Ergül, A. (2021). CRISPR/Cas9 System, an Efficient Approach to Genome Editing of Plants for Crop Improvement. In: Tang, G., Teotia, S., Tang, X., Singh, D. (eds) RNA-Based Technologies for Functional Genomics in Plants. Concepts and Strategies in Plant Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-64994-4_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-64994-4_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64993-7
Online ISBN: 978-3-030-64994-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)