Abstract
The current study investigated the effect of 1-methylcyclopropene (1-MCP), an ethylene inhibiting compound, in alleviating the detrimental effect of drought on cotton plants. The experiment was conducted in a growth chamber in 2006 and 2007. Treatments consisted of (T1) an untreated control well-watered, (T2) 1-MCP at 10 g ai/ha well-watered, (T3) an untreated control water-stressed, and (T4) 1-MCP at 10 g ai/ha water-stressed. Water-stress treatment consisted of withholding water from the pots until stomatal closure. The water-stress regime and the 1-MCP treatments were imposed at the pinhead-square stage, approximately 4 weeks after planting. Water-stressed plants treated with 1-MCP had a higher stomatal resistance, less negative water potential, higher activity of antioxidant enzymes, and better maintenance of membrane integrity. The greatest effects on stomatal resistance were observed at 5 days after treatment initiation, in which water-stressed 1-MCP-treated plants exhibited stomatal resistance of 0.079 m2 s mmol−1, whereas water-stressed untreated plants exhibited only 0.047 m2 s mmol−1. There was no significant effect of 1-MCP on water-use efficiency, transpiration, and dry matter production. These results indicated that application of 1-MCP to water-stressed cotton may have the potential to lower levels of stress in treated plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among all abiotic stresses, drought is the major environmental constraint to crop productivity worldwide (Sharp and others 2004). According to Bot and others (2000), 45% of the world’s agricultural lands are subject to continuous or frequent drought conditions. Even though cotton originates from dry regions, high yields are positively correlated with the amount of rainfall or irrigation supplied (McWilliams 2003). In many regions of the U.S. Cotton Belt, higher cotton yields are limited by inadequate amounts, or inadequate distribution, of rainfall (Basal and others 2005).
Ethylene is a stress hormone often associated with plant senescence (Abeles and others 1992) and has been implicated in plant response to water deficit (Apelbaum and Yang 1981; Morgan and Drew 1997). Morgan and Drew (1997) concluded that rapid desiccation of detached leaves promoted synthesis of ethylene; however, when soil dried slowly, plants exhibited a decrease in ethylene production. In cotton, the synthesis of ethylene decreased after cessation of irrigation (Morgan 1990). In soybean (Glycine max L. Merr.), slow soil drying promoted decreased ethylene levels, whereas rapid soil drying caused an initial increase followed by a decline in ethylene production (Chang-Cheng and Qi 1993). Furthermore, increased ethylene synthesis induced by drought stress has been reported in tomato (Lycopersicon esculentum, Sobeih and others 2004), cotton (Gossypium hirsutum, McMichael and others 1972), and Cleopatra mandarin (Citrus reshni, Gomez-Cadenas and others 1996). The explanation for these conflicting reports might be related to the trigger for synthesis of abscisic acid (ABA) by plants under water stress, which can inhibit ethylene production (Spollen and others 2000; Sharp 2002). Sharp (2002), studying the interaction between ABA and ethylene, concluded that inhibition of ethylene synthesis by ABA could be a protective mechanism of some plant species to maintain their growth under water-stress conditions. An antagonistic relationship between ethylene and ABA on stomatal closure of water-stressed plants has also been reported (Tanaka and others 2005; Wilkison and Davies 2009).
It is likely that water-stressed plants exhibit a higher sensitivity to ethylene (Morgan 1990). Ethylene receptor complexes work by inactivating the receptor sites to transfer the ethylene signal (Binder and Bleecker 2003). Therefore, ethylene effectiveness is negatively regulated by the number of receptor sites available such that stressed plants with abundant receptors have the advantage of repressing the ethylene response (Hua and Meyerowitz 1998). Thus, it is possible that water-stressed plants exhibit fewer receptor sites; this would increase their sensitivity to ethylene and result in the initiation of senescence and abscission, even in plants that produce low amounts of ethylene. It is clear that ethylene plays a major role in the stress response of water-stressed plants. Apparently, ethylene acts as a negative regulator of plant resistance to drought, and plants have developed a mechanism to overcome ethylene production to increase their resistance to water shortage (Young and others 2004; Pierik and others 2007).
The effect of ethylene on antioxidant enzymes is not well documented in the literature. However, because ethylene is the main trigger for senescence in plants (Abeles and others 1992), it is possible that an indirect relationship exists between ethylene and antioxidant enzymes. The production of reactive oxygen species (ROS) such as superoxide radical (O2 −), hydroxyl radicals (OH), and hydrogen peroxide (H2O2) is known to be a physiological response of plants to water and salt stress (Miller and others 2009). Reactive oxygen species are highly reactive and can cause damage to cell structure and function (Elstner 1991; Asada 1999). Decreases in plant growth under stress conditions are associated mainly with increases in ROS synthesis (Grene 2002). Plants respond to ROS by increasing the synthesis of antioxidant enzymes, as a protective mechanism against cell damage (Scandalios 1997). Antioxidant enzymes in plant cells play a major role in the preservation of membrane integrity, protection of DNA, and protein degradation (Scandalios 1997). Measurements of antioxidant enzyme concentrations and membrane-integrity parameters have been used as an indication of the level of stress in plants.
1-Methylcyclopropene (1-MCP) is an antiethylene compound that hinders the action of ethylene by occupying its receptor sites (Sisler and Serek 1997). The product works by decreasing or delaying the effect of ethylene by occupying ethylene receptors such that ethylene cannot bind and elicit action (Blankenship and Dole 2003). Because 1-MCP acts as an inhibitor of the hormone ethylene, the use of 1-MCP could provide a possible short-term solution to situations of water deficit. The plant growth regulator 1-MCP has been approved by the EPA for use in fruit and vegetables. In cotton, the use of 1-MCP has been reported to result in increased boll retention and increased fiber yield (Mark L. Dahmer, personal communication). These data appear to be a result of a decrease in cotton boll abortion by the effect of 1-MCP inhibiting ethylene action induced by biotic or abiotic stresses.
We hypothesized that 1-MCP sprayed on cotton plants under drought conditions would inhibit the action of ethylene and alleviate plant stress during dry periods and thereby prevent yield loss. Therefore, the objective this study was to investigate the effect of the plant growth regulator 1-MCP on the physiology and growth of cotton plants under water-deficit stress and well-watered conditions.
Materials and Methods
The studies were conducted in the Altheimer Laboratory, Arkansas Agricultural Research and Extension Center, in October 2006 and repeated in June 2007. The cotton (Gossypium hirsutum L.) cultivar DP444 BG/RR was planted in 1-L pots filled with Sunshine potting mix (Sun Gro Horticultural Distribution Inc., Bellevue, WA). Pots were arranged in a large growth chamber (Model PGW36, Conviron, Winnipeg, Canada) with a day/night temperature regime of 30/20°C, 12-h photoperiods (700 μmol m−2 s−1 photosynthetically active radiation), and a relative humidity of 60%. Plants were watered daily with a half-strength complete balanced (10-10-10) Peter’s nutrient solution (Spectrum Group, St. Louis, MO). The pots were wrapped with plastic bags sealed around the plant base to prevent water evaporation from the soil and to confine water loss to transpiration only.
The experiment was arranged in a completely randomized design with two factors that consisted of 1-MCP treatment and water regime. Each plant was considered an experimental unit and five replications were used to conduct the experiments. At 28 days after planting (DAP), 1-MCP was sprayed according to the following treatments: (T1) an untreated control well-watered, (T2) 1-MCP at 10 g ai/ha well-watered, (T3) an untreated control water-stressed, and (T4) 1-MCP at 10 g ai/ha water-stressed. The well-watered condition was maintained by watering the pots back to 80% of field capacity daily by weighing the pots and monitoring stomatal resistance. Soil field capacity was determined by the classical method of weighing the pots before and watering to saturation and allowing for excess drainage. A water-stress cycle consisted of withholding water from the pots until stomatal closure, after which the stressed plants were rewatered to 80% of field capacity. This process was repeated three times for a total of three water-stress cycles. After treatment initiation, all plants were watered with distilled water only. The 1-MCP was applied with a CO2 backpack sprayer calibrated to deliver 187 l ha−1. All 1-MCP treatments were applied with the adjuvant AF-400 (Rohm Hass, Philadelphia, PA) at 0.375% v/v.
Stomatal resistance was recorded daily using a LICOR 1600 porometer (LICOR Inc., Lincoln, NE) and results were expressed in m2 s mmol−1. Leaf water potential was measured at the end of each stress cycle using leaf end-window thermocouple psychrometers (Model 84-1 V, J.R.D Merrill Specialty Equipment, Logan, UT), a microvoltimeter (Model Zero Centurion Elite, J.R.D Merrill Specialty Equipment), and a high-precision water bath (Model 7011, Hart Scientific, American Fork, UT). A 1-cm-diameter leaf disk from the upper fully-expanded main-stem leaf four nodes below the terminal of the plant was placed in the thermocouple psychrometer chambers, and the measurements were made using the procedure described by Oosterhuis (2003). Thermocouple psychrometer chambers with leaf samples were equilibrated for 4 h in a water bath at 25°C, after which measurements were taken using a microvoltimeter. The stomatal resistance and leaf water potential measurements were recorded between 12:00 p.m. and 2:00 p.m. Membrane leakage measurements were made according to Gonias and others (2008). A 1-cm-diameter leaf disk was sampled from the upper fully-expanded main-stem leaf four nodes below the terminal and incubated for 48 h in a cell with 2 ml of deionized water. Measurements were recorded with an automatic seed analyzer (Applied Intelligent Systems Inc., Ann Arbor, MI) at the end of the third stress cycle.
At the end of the experiments the upper fully-expanded main-stem leaf four nodes below the terminal of the plant was collected and stored at -80°C. The leaf extraction procedure for enzymes described by Gomez and others (2004) was followed. A 1-g leaf sample was ground using a mortar and pestle with liquid nitrogen. The ground sample was placed in a 50-ml tube containing 0.5 g of polyvinylpyrroline, one drop of antifoam A, and 4 ml of extraction buffer solution. Homogenization was carried out for 3 min using a Polytron homogenizer (Brinkmann Instruments Inc., Palo Alto, CA). The samples were then centrifuged for 20 min at 13,000 rpm (21,000 g) and 4°C in a Hermle centrifuge (Labnet International, Inc., Edison, NJ). The supernatant was collected and desalted by passing it through a PD-10 column (GE Healthcare Bio-Sciences Corp., Piscataway, NJ).
The glutathione reductase (GR) measurements were done following the method of Schaedle and Bassham (1977). The assay was initiated by placing 950 μl of a reaction solution and 50 μl of plant extracted sample in a 1-ml quartz cuvette. The reaction solution was composed of 50 mM Tris, 0.15 mM NADPH+H, 0.5 mM oxidized glutathione, and 3 mM MgCl2 (Sigma, St. Louis, MO). The GR activity was measured according to the oxidation of NADPH+H with a Biospec 1601 UV/VIS spectrophotometer (Shimadzu, Columbia, MD). The instrument was regulated to display a wavelength of 340 nm, and measurements were made during a period of 1 min. GR activity was expressed as mmol g−1 FW min−1.
The superoxide dismutase (SOD) measurements were recorded following the method of Lu and Foo (2000, 2001). A 0.1-ml aliquot of aqueous SOD standard solution (Sigma), (0, 1, 2.5, 5, 10, and 25 units/ml) and 0.1 ml of leaf extraction solution (described above) were added to separate test tubes containing 1.0 ml of solution composed of 0.4 mM xanthine and 0.24 mM nitroblue tetrazolium chloride (NBT) in 0.1 M phosphate buffer (pH 8.0). A 1.0-ml solution of xanthine oxidase (0.049 unit/ml), diluted in 0.1 M phosphate buffer (pH 8.0), was added to each tube and the resulting mixture was incubated in a water bath at 37°C for 20 min. The reaction was terminated by adding 2.0 ml of an aqueous solution of 69 mM sodium dodecylsulfate (SDS), and the absorbance of NBT was measured at 560 nm. SOD activity was determined from the standard curve and was expressed as SOD units g−1 FW.
Plant transpiration was measured by weighing the pots daily and by recording the amount of water that each pot received throughout the experiment. Water-use efficiency (WUE) was calculated by dividing the total plant aerial dry matter produced by the total amount of water used. At the end of the third stress cycle, the numbers of squares and main-stem nodes were recorded and plants were harvested. For dry matter (DM) determination, plants were cut at soil level, and leaves, squares, and stems were separated and dried in an oven (Fisher Scientific, Atlanta, GA) at 55°C for 5 days before weighing. Leaf area was measured before drying using a LICOR 3100 electronic leaf area meter.
A two-factor factorial statistical analysis with six replications was utilized to evaluate the results using the software JMP 7 (SAS Institute, Cary, NC). The factors were experiment, 1-MCP treatment, and water regime. Analysis of variance (ANOVA) and conventional LSD (α = 0.05) using Student’s t test were used to analyze statistical significance between means. The variable days was not considered a factor; a single ANOVA was done for each day to compare differences among treatment combinations. A probability of less than 0.05 was considered significant.
Results
Stomatal Resistance
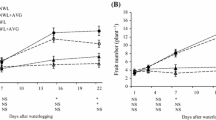
The stomatal resistance data analysis showed a violation of the equal-variance assumption in the ANOVA and therefore values were transformed using the natural logarithm. The results of the transformed stomatal resistance data (Fig. 1) indicated a significant interaction effect between 1-MCP treatment and water regime at day 5 (P = 0.005) and day 9 (P = 0.023) after 1-MCP application. A significant water regime effect was detected at days 3 (P ≤ 0.001), 4 (P ≤ 0.001), 6 (P = 0.002), 7 (P ≤ 0.001), 8 (P ≤ 0.001), 10 (P ≤ 0.001), 13 (P ≤ 0.001), and 14 (P ≤ 0.001), with the water-stressed plants as expected exhibiting higher stomatal resistance than the well-watered plants (data not shown).
Under water-deficit stress, 1-MCP-treated plants exhibited higher stomatal resistance than the untreated water-stressed control. The highest stomatal resistance value was recorded at day 5 when that of 1-MCP-treated plants under water stress reached 0.079 m2 s mmol−1. Significant differences were observed at days 5 and 9, although the magnitude of the dissimilarity between 1-MCP treatments was much greater at day 5 than at day 9. At day 5 the difference was about 0.032 m2 s mmol−1, whereas at day 9 the difference was close to 0.009 m2 s mmol−1 (Fig. 1).
Leaf Water Potential
Similar to stomatal resistance, leaf water potential showed a significant interaction effect between 1-MCP treatment and water regime at day 5 (P = 0.0189) (Fig. 2). At day 5 the water-stress 1-MCP treatment was statistically different compared to the untreated control under water stress, with the 1-MCP treatment exhibiting an increase of 0.71 MPa compared to the untreated control. Less negative values were observed in the 1-MCP treatment, indicating lower levels of stress caused by the drought regime. By the end of the second stress cycle (day 10), only a water-stress effect was observed (P ≤ 0.001), and at the end of the experiment (day 14), no significant effect of any treatment was recorded.
Membrane Leakage
An interaction effect between treatment and water regime was not significant in the membrane integrity measurements, and the water regime and 1-MPC treatments were analyzed by averaging regime treatments across 1-MCP treatments and 1-MCP treatments across water regime treatments (Fig. 3). The 1-MCP treatment lowered the electrical conductivity (that is, less membrane leakage) of the leaf incubation solution (P = 0.012), indicating better maintenance of cell membrane integrity. As expected, water-stressed plants exhibited higher values of electrical conductivity than well-watered plants (P = 0.046).
Effect of 1-MCP and water regime treatment on leaf membrane leakage. Groups of columns within each treatment with the same letters are not significantly different (P = 0.05). Error bars represent ±1 standard error. Measurements were made at the end of the experiment. Data were averaged across regime treatment for 1-MCP effect, and data were averaged across 1-MCP treatment for regime effect
Antioxidant Enzymes (GR and SOD)
Both GR activity (Fig. 4) and SOD units (Fig. 5) showed an interaction effect between 1-MCP treatment and water regime. The P values were 0.0408 for GR and 0.0404 for SOD.
The GR activity indicated that there was no effect of 1-MCP under well-watered conditions (Fig. 4). However, in the water-stressed regime, 1-MCP application significantly increased GR activity compared to the untreated control. The overall analyses comparing the four treatments showed no significant differences among untreated well-watered, 1-MCP-treated well-watered, and untreated water-stressed plants. Only the water-stressed plants that received the 1-MCP application exhibited a significant increase in GR activity. This increase in antioxidant activity in plants accompanying the application of 1-MCP could be an important key to protecting cotton plants from reactive oxygen species under drought stress, as indicated by Jiang and Zhang (2002b).
The SOD measurements also showed a significant effect of 1-MCP under water-deficit conditions, that is, the 1-MCP-treated plants exhibited significantly higher SOD units compared with untreated plants (Fig. 5). Plants treated with 1-MCP sustained their levels of SOD equally to nonstressed plants, whereas water-stressed plants without 1-MCP showed a significant decrease in SOD content. Similar to GR activity, this maintenance of SOD production could be helpful for plants to overcome cellular damage from drought stress in field conditions.
Dry Matter, Leaf Area, Number of Nodes, Number of Squares, and Water-Use Efficiency
Measurements of total plant dry matter, which included leaf, stem, and squares, indicated a significant effect of water regime but no significant effect of 1-MCP (Table 1). Only a slight consistent numerical increase in dry matter production was observed in 1-MCP-treated compared to untreated plants. As expected, water stress significantly decreased all dry matter measurements analyzed.
Similar results were observed for leaf area, number of nodes, number of squares, and water-use efficiency parameters (Table 2). Water-stressed plants exhibited significantly lower leaf area and numbers of nodes and squares. In contrast, water-stressed plants had higher water-use efficiency than well-watered plants. However, 1-MCP application had no effect on water-use efficiency.
Although 1-MCP treatments were shown to have an effect on stomatal resistance of plants under water stress, data on daily plant transpiration (Fig. 6) did not show the same results. Transpiration measurements were not significantly affected by 1-MCP treatments, and only a significant water regime effect was observed, with well-watered plants exhibiting higher transpiration compared to water-stressed plants from day 2 through day 14, with P < 0.0001 (Fig. 6).
Discussion
Ethylene has been associated with both stomatal closure and stomatal opening (Acharya and Assmann 2009). Our results indicated that in water-deficit conditions 1-MCP increased stomatal resistance; thus, in the case of drought-stressed cotton, ethylene might be closely related to stomatal opening. The explanation of these results could be related to the findings of Tanaka and others (2005) in which they observed that ABA-induced stomatal closure in Arabidopsis was inhibited by application of ethylene or 1-aminocyclopropane-1-carboxylic acid (ethylene precursor). The ethylene inhibition of ABA in stomatal closure was not recorded with application of 1-MCP or in ethylene-insensitive mutants. The authors suggest that this physiological ABA inhibition by ethylene in water-stressed plants might be a mechanism to maintain a minimum supply of carbon dioxide for the photosynthesis process or an effect of ethylene on promoting drought-induced leaf senescence. Desikan and others (2006) showed that application of ethylene alone causes stomatal closure in Arabidopsis by ethylene-induced H2O2 synthesis. However, they observed that this process is evident only in the absence of ABA, whereas in the presence of ABA, ethylene was reported to inhibit ABA-induced stomatal closure. Wilkison and Davies (2009), studying the effect of ozone, ABA, and ethylene on stomatal closure of water-stressed Leontodon hispidus, proposed that the effect of ethylene on inhibiting ABA-induced stomatal closure might be significant only under severe stress conditions. Our results agree with Wilkison and Davies (2009) because the effect of 1-MCP on stomatal resistance was observed only under water-stress conditions. Thus, according to our data, the possible physiological effect of ethylene inhibiting ABA-induced stomatal closure is plausible for water-stressed cotton plants. Furthermore, we observed a highly significant effect of 1-MCP on stomatal resistance of water-stressed plants only 5 days after application, indicating that 1-MCP spray application has a short life effectiveness of only a few days, as has been reported by Bugby (personal communication).
The effects of 1-MCP on leaf water potential can be explained by the stomatal resistance data in which higher stomatal resistance would result in decreased water losses, thus increasing leaf water potential values. At day 5 a higher stomatal resistance in the 1-MCP-treated plants under water deficit resulted in higher water potential values, whereas at day 10 the 1-MCP treatment effect was small, with only a slight positive effect on stomatal resistance which resulted in only a numerical increase in the leaf water potential. At day 14, no 1-MCP effect was detected in either measurement. During the three measurements, the leaf water potential in the water-stressed treatments became less negative, which could be due to plant acclimation to water deficit.
Studying the effect of water stress in mulberry (Morus alba L.) plants, Reddy and others (2004) also observed an increase in cell electrolyte leakage that they attributed to high membrane fluidity caused by membrane lipid peroxidation. Our results showed that the maintenance of membrane integrity in cotton plants could be improved by application of 1-MCP. Ethylene was therefore responsible for some of the water-stress effect on increasing leaf cell membrane leakage. Membrane lipids are extremely important for maintenance of vital cell physiological processes, and damaged cell membranes due to water stress could impair physiological functions.
Because ethylene is related to the onset of senescence in plant cells, it is likely that it has an effect on increasing membrane leakage of plants. Applications of ethylene to petals of hybrid Tradescantia (Tradescantia occidentalis × Tradescantia ohiensis) hastened the increase of membrane permeability during senescence (Suttle and Kende 1980). Similar results were observed in cut carnation flowers (Dianthus caryophyllus L.), where ethylene caused rapid flower senescence by increasing membrane permeability, a process prevented by the application of silver thiosulfate, an antiethylene agent (Thompson and others 1982). Serek and others (1995) tested the effect of 1-MCP on petunia flowers (Petunia hybrida) treated with ethylene and found that ethylene increased membrane electrolyte leakage, and that 1-MCP protected the plant against ethylene-induced membrane damage, similar to our findings.
The effect of drought on antioxidant enzymes in cotton seems to be variable and cultivar dependent. Mahan and Wanjura (2005), studying the activity of antioxidant enzymes in water-stressed cotton, observed an increase in ascorbate and ascorbate peroxidase but no increase of GR. On the other hand, Burke and others (1985) reported a significant increase of GR in leaves of water-stressed cotton plants. High antioxidant enzyme levels have been shown to be a good indicator for screening for salt-tolerant cotton cultivars (Gosset and others 1994). Salt-tolerant cotton cultivars grown in nutrient solution showed an increase in SOD and GR in response to increased salt concentration, whereas sensitive cultivars exhibited no change in either enzyme (Meloni and others 2003). Similar results were also observed in wheat (Triticum aestivum), where drought-tolerant genotypes under water stress exhibited high antioxidant enzyme activity leading to lower accumulations of H2O2 and lower lipid membrane peroxidation (Sairam and others 1997, 1998). According to these studies, the increase of GR activity and SOD observed in our experiments with 1-MCP application is a positive effect that could improve the tolerance of cotton plants to drought. This increase in the activity of GR and SOD enzymes of treated plants could also partially explain the results of 1-MCP lowering cell membrane leakage. A better protection mechanism against reactive oxygen species would help maintain cell membrane integrity during stress situations. Furthermore, a potential explanation for the increase in GR with application of 1-MCP under water-stress conditions could also be related to ABA. Many researchers have concluded that ABA can induce antioxidant activity in plants (Prasad and others 1994; Bueno and others 1998; Jiang and Zhang 2001, 2002a). In cotton, drought stress and ABA have also been reported to induce antioxidant enzyme activity (Bellaire and others 2000). Thus, in our study it is possible that similar to stomatal closure, ethylene could also be inhibiting the effect of ABA-inducing antioxidant enzymes in water-stressed cotton plants. However, this effect of ethylene inhibiting ABA-induced antioxidants in water-stressed plants requires further research.
The significantly lower levels of SOD in water-stressed plants observed in our research could be explained by the findings of Wang and others (2008) who found that the levels of SOD in leaves of Puccinellia tenuiflora under salt stress first increased and then decreased as the salt stress continued. Therefore, it is possible that in water-stressed plants the levels of SOD were higher at a certain point during the first or second water-deficit cycle than in well-watered plants. Our studies have shown that water-stressed cotton plants exhibited only a decreasing trend in GR activity. Meloni and others (2003) found similar decreases in salt-sensitive cotton plants, and Garrat and others (2002) showed significantly lower GR activity in salt-sensitive cotton plants grown under salt stress.
Higher growth exhibited by well-watered compared to water-stressed plants was also observed by Pace and others (1999). They studied the effect of drought in field-grown cotton and concluded that plants under water deficit exhibited a lower number of nodes, dry matter, and leaf area than the well-watered control. There are many reports that show that plants under mild water deficits induce partial stomatal closure which can increase water-use efficiency (Begg and Turner 1976; Davies and others 1978; Turner 1997). These reports support our results that indicated higher water-use efficiency of plants in water deficit compared to well-watered plants.
Water-stressed 1-MCP-treated plants showed that there was a significant effect on stomatal resistance (Fig. 1); however, no effect was observed on plant transpiration. Bugby (personal communication), studying the effect of 1-MCP in cotton, observed that 1-MCP-treated plants exhibited a decrease in stomatal resistance. Our results of stomatal resistance were based on a daily single measurement at midday; thus, it is possible that at a different time of day our water-stressed 1-MCP-treated plants exhibited a significant decrease in stomatal resistance compared to untreated plants. This could explain the absence of an effect of 1-MCP on transpiration of water-stressed plants. An experiment is planned to test this hypothesis by analyzing the 1-MCP effect on stomatal resistance of water-stressed cotton plants throughout the day. We found no reports in the literature on the effect of 1-MCP on water-use efficiency, and in our study 1-MCP application did not show a significant effect on plant water-use efficiency. This result can be supported by the absence of a 1-MCP effect on plant transpiration and dry matter production.
We conclude that the application of 1-MCP had positive effects on the physiological parameters measured under water deficit. Application of 1-MCP resulted in higher stomatal resistance, higher water potential, higher antioxidant enzyme activity, and lower membrane leakage. In this experiment the effect of 1-MCP on inhibiting ethylene action lasted for only about 5 days. The 1-MCP treatments did not have an effect on transpiration, which explains the absence of an effect of 1-MCP on plant water-use efficiency. According to our previous studies (unpublished data), the cotton cultivar DP444 BG/RR is apparently susceptible to drought, and our results have shown that 1-MCP application could improve its tolerance to water-stress situations. However, 1-MCP application did not have an effect on the productivity parameters analyzed. It is possible that under water deficit, multiple applications of 1-MCP applied at 5-day intervals may be needed to maintain these positive benefits for improved productivity.
References
Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in plant biology, 2nd edn. Academic Press, San Diego, CA
Acharya BR, Assmann SM (2009) Hormone interactions in stomatal function. Plant Mol Biol 69:451–462
Apelbaum A, Yang SF (1981) Biosynthesis of stress ethylene induced by water deficit. Plant Physiol 68:594–596
Asada K (1999) The water-water cycle in chloroplast: scavenging of active oxygen and dissipation of excess photons. Ann Rev Plant Physiol Biol 50:601–639
Basal H, Smith CW, Thaxton PS, Hemphill JK (2005) Seedling drought tolerance in upland cotton. Crop Sci 45:766–771
Begg JE, Turner NC (1976) Crop water deficits. Adv Agron 28:161–217
Bellaire BA, Carmody J, Braud J, Gossett DR, Banks SW, Lucas MC, Fowler TE (2000) Involvement of abscisic acid-dependent and independent pathways in the upregulation of antioxidant enzyme activity during NaCl stress in cotton callus tissue. Free Radic Res 33:531–545
Binder BM, Bleecker AB (2003) A model for ethylene receptor function and 1-methylcyclopropene action. Acta Hortic 628:177–187
Blankenship SM, Dole JM (2003) 1-Methylcyclopropene: a review. Postharvest Biol Technol 28:1–25
Bot AJ, Nachtergaele FO, Young A (2000) Land resource potential and constraints at regional and country levels. World Soil Resources Reports 90. Land and Water Development Division, FAO, Rome
Bueno P, Piqueras A, Kurepa J, Savoure A, Verbruggen N, Van Montagu M, Inze D (1998) Expression of antioxidant enzymes in response to abscisic acid and high osmoticum in tobacco BY-2 cell cultures. Plant Sci 138:27–34
Burke JJ, Gamble PE, Hatfield JL, Quisenberry JE (1985) Plant morphological and biochemical responses to field water deficits. Plant Physiol 79:415–419
Chang-Cheng X, Qi Z (1993) Effect of drought on lipoxygenase activity, ethylene and ethane production in leaves of soybean plants. Acta Bot Sin 35:31–37
Davies WJ, Mansfield TA, Ortron PJ (1978) Strategies employed by plants to conserve water: can we improve them? Proc Brit Crop Protect Council 8:45–54
Desikan R, Last K, Harrett-Williams R, Tagliavia C, Harter K, Hooley R, Hancock JT, Neill SJ (2006) Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J 47:907–916
Elstner EF (1991) Mechanisms of oxygen activation in different compartments of plant cells. In: Pell EJ, Steffen KL (eds) Active oxygen species, oxidative stress, and plant metabolism. American Society of Plant Physiologists, Rockville, MD, pp 13–25
Garrat LC, Janagoudar BS, Lowe KC, Anthony P, Power JB (2002) Salinity tolerance and antioxidant status in cotton cultures. Free Radic Biol Med 33:502–511
Gomez SK, Oosterhuis DM, Rajguru SN, Johnson DR (2004) Foliar antioxidant enzyme responses in cotton after aphid herbivory. J Cotton Sci 8:99–104
Gomez-Cadenas A, Tadeo FR, Talon M, Primo-Millo E (1996) Leaf abscission induced by ethylene in water-stressed intact seedlings of Cleopatra mandarin requires previous abscisic acid accumulation in roots. Plant Physiol 112:401–408
Gonias ED, Oosterhuis DM, Bibi AC (2008) Physiologic response of cotton to the insecticide imidacloprid under high-temperature stress. J Plant Growth Regul 27:77–82
Gosset DR, Millhollon EP, Lucas MC (1994) Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci 34:706–714
Grene R (2002) Oxidative stress and acclimation mechanisms in plants. In: Somerville CR, Myerowitz EM (eds) The Arabidopsis book. American Society of Plant Biologists, Rockville, MD. Available at http://www.aspb.org/publications/Arabidopsis/
Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94:261–271
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen leaves of maize seedlings. Plant Cell Physiol 42:1265–1273
Jiang M, Zhang J (2002a) Involvement of plasma-membrane NADPH oxidase in abscisic acid and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215:1022–1030
Jiang M, Zhang J (2002b) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant in maize leaves. J Exp Bot 53:2401–2410
Lu Y, Foo LY (2000) Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem 68:81–85
Lu Y, Foo LY (2001) Antioxidant activities of polyphenols from sage (Salvia officinalis). Food Chem 75:197–202
Mahan JR, Wanjura DF (2005) Seasonal patterns of glutathione and ascorbate metabolism in field-grown cotton under water stress. Crop Sci 45:193–201
McMichael BL, Jordan WR, Powekk RD (1972) Effect of water stress on ethylene production by intact cotton petioles. Plant Physiol 49:658–662
McWilliams D (2003) Drought strategies for cotton. CES Circular 583, New Mexico State University
Meloni DA, Oliva MA, Maritnez CA, Cambraia J (2003) Photosynthesis and activity of superoxidase dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2009) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ (in press)
Morgan PW (1990) Effects of abiotic stress on plant hormone systems. In: Alscher R, Cummings J (eds) Stress responses in plants: adaptation mechanisms. Wiley-Liss, New York, pp 313–314
Morgan PW, Drew M (1997) Ethylene and plant responses to stress. Physiol Plant 100:620–630
Oosterhuis DM (2003) Psychrometry for Measuring Plant and Soil Water Status: Theory, Types and Uses. In: Stewart BA, Howell T (eds) Encyclopedia of water science. Marcel Dekker, New York, pp 751–755
Pace PF, Cralle HT, El-Halawany SH, Cothren JT, Senseman SA (1999) Drought-induced changes in shoot and root growth of young cotton plants. J Cotton Sci 3:183–187
Pierik R, Sasidharan R, Voesenek LAC (2007) Growth control by ethylene: adjusting phenotypes to the environment. J Plant Growth Regul 26:188–200
Prasad TK, Anderson MD, Stewart CR (1994) Acclimation, hydrogen peroxide, and abscisic acid protect mitochondria against irreversible chilling injury in maize seedlings. Plant Phyiol 105:619–627
Reddy AR, Chaitanya KV, Jutur PP, Sumithra K (2004) Differential antioxidative responses to water stress among five mulberry (Morus alba L.) cultivars. Environ Exp Bot 52:33–42
Sairam RK, Siiukla DS, Saxena DC (1997) Stress induced injury and antioxidant enzymes in relation to drought tolerance in wheat genotypes. Biol Plant 40:357–364
Sairam RK, Deshmukh PS, Saxena DC (1998) Role of antioxidant systems in wheat genotypes’ tolerance to water stress. Biol Plant 41:387–394
Scandalios JG (1997) Molecular genetics of superoxide dismutase in plants. In: Scandalios JG (ed) Oxidative Stress and Molecular Biology of Antioxidant Defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 527–568
Schaedle M, Bassham JA (1977) Chloroplast glutathione reductase (in spinach). J Plant Physiol 59:1011–1012
Serek M, Tamari G, Sisler EC, Borochov A (1995) Inhibition of ethylene-induced cellular senescence symptoms by 1-methylcyclopropene, a new inhibitor of ethylene action. Physiol Plant 94:229–232
Sharp RE (2002) Interaction with ethylene: changing views on the role of abscisic acid in roots and shoot growth responses to water stress. Plant Cell Environ 25:211–222
Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT (2004) Root growth maintenance during water deficits: physiology to functional genomics. J Exp Bot 55:2343–2351
Sisler EC, Serek M (1997) Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiol Plant 100:577–582
Spollen WG, LeNoble ME, Samuels TD, Bernstein N, Sharp RE (2000) Abscisic acid accumulation maintains maize primary root elongation and low water potential by restricting ethylene production. J Plant Physiol 122:967–976
Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ (2004) Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. J Exp Bot 55:2353–2363
Suttle JC, Kende H (1980) Ethylene action and loss of membrane integrity during petal senescence in Tradescantia. J Plant Physiol 65:1067–1072
Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S (2005) Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol 138:2337–2343
Thompson JE, Mayak S, Shinitzky M, Halevy A (1982) Acceleration of membrane senescence in cut carnation flowers by treatment with ethylene. Plant Physiol 69:859–863
Turner NC (1997) Further progress in crop water relations. Adv Agron 58:293–338
Wang Y, Sun G, Suo B, Chen G, Wang J, Yan Y (2008) Effects of Na2CO3 and NaCl stresses on the antioxidant enzymes of chloroplasts and chlorophyll fluorescence parameters of leaves Puccinellia tenuiflora (Turcz.) scribn. et Merr. Acta Physiol Plant 30:143–150
Wilkison S, Davies WJ (2009) Ozone suppresses soil drying and abscisic acid (ABA)-induced stomatal closure via an ethylene-dependent mechanism. Plant Cell Environ 32:949–959
Young TE, Meeley RB, Gallie DR (2004) ACC synthase expression regulates leaf performance and drought tolerance in maize. Plant J 40:813–825
Acknowledgment
We thank the University of Arkansas Agricultural Research and Extension Center for supporting this research and Agrofresh Inc. for partial funding of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawakami, E.M., Oosterhuis, D.M. & Snider, J.L. Physiological Effects of 1-Methylcyclopropene on Well-Watered and Water-Stressed Cotton Plants. J Plant Growth Regul 29, 280–288 (2010). https://doi.org/10.1007/s00344-009-9134-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-009-9134-3