Abstract
Colorectal cancer (CRC) is a global health problem with limited therapeutic opportunities. It is the most common cancer death in the world, mainly due to its higher incidence and metastatic actions. The molecules expressed by the cancer tissues help to develop several novel approaches in cancer treatment. Nowadys, nanotechnology offers an advanced method for the identification and colon cancer therapy which are capable of overcoming biophysical and biochemical barriers in the body. The studies reveal a hope among the scientific community for developing innovative nanoparticles that possess high adaptability for both diagnosis and therapy. It is a most promising area in therapeutics with different design and formulations for the initiation of controlled drug release and uptake into colon cancer tissue. Different nanoparticles like liposomes, carbon nanotubes, nanoshells, polymeric nanoparticles, and dendrimers have been developed to transport a variety of antitumor agents including siRNA, chemo-modulators, and antiangiogenic mediators. Nanotechnology can significantly expand the precision of targeted drug delivery and helps to reduce the toxic side effects. It can also manipulate the interactions in the gut microbiome and the tumor environment which provide an innovative strategy for cancer treatment. This chapter focuses on the different roles of these nanoparticulate technologies and the potential use of nanoparticle formulations in colon cancer therapeutics. Even though several limitations are hindering in the development of nanotechnologies to function as nanotheranostic mediators, which is expected to pave the way for the fight against colon cancer malignancy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Colon cancer, also mentioned as colorectal cancer (CRC), is developed by the uncontrolled growth of tumor cells in the cecum, colon, or rectum. CRC is the one of the major causes of cancer mortality worldwide with approximately 1.4 million cases. It is recognized as the third most and second most cancer in men and women of around 10% and 9.2% of the entire worldwide cases, respectively (Ferlay et al. 2015). Compared to other malignancies, metastasis is the major cause of CRC . The primary site of CRC metastasis is mostly developed in the liver (Siegel et al. 2014). Therefore, the early stage of diagnosis helps in higher chance of survival rate. The CRC onset is mainly due to intrinsic features, such as gender, aging, and genetic factors. Chronic diseased conditions such as obesity, inflammatory bowel disease (IBD), and diabetes are also related with higher risk of colon cancer (Ma et al. 2013; Tsilidis et al. 2015). Gut microbial imbalance can also trigger inflammation, reactive oxygen species (ROS) production, and microbial metabolism products (toxins) that can promote genetic modifications leading to colon cancer. Lifestyle-associated factors, such as smoking, alcohol consumption, and high-fat and high processed meat intake, also have been connected to polyp growth and CRC development (Cross et al. 2010; Chen et al. 2015). Presently, various therapies are available for CRC, comprising chemotherapy, surgery, and radiation therapy. In late stages, chemotherapy is the main treatment line to reduce CRC progression.
Even though chemotherapy is the most common approach for cancer treatments, using individual chemotherapeutic agents results in a range of side effects that are still a major concern in chemotherapy. In conventional chemotherapy, 5-fluorouracil is a commom chemotherapeutic drug used in CRC along with some other drugs such as leucovorin, oxaliplatin and irinotecan. (Mazhar et al. 2006). Other than drugs, monoclonal antibodies like cetuximab are also recognized for CRC treatment (Van Cutsem et al. 2009). Chemotherapy has its own limitations, such as low circulation time, instability, lack of target specificity, and chance of overdosage; all these factors lead to toxicity and more side effects. Recently, there has been a higher interest in the field of nanotechnology that has shown a constant and target-specific delivery by using nanosized delivery systems which may pave a way for the novel ways toward effective and safe targeted colon therapies.
Nanotechnology as an emerging tool for colon targeted cancer therapy , which has received more interest and express significant therapeutic benefits. Nanotechnology can be a novel effective therapeutic strategy, which can overcome various challenges related with colon cancer therapy and drug development (Arshad et al. 2020). Various types of nanomaterials are developed and being investigated that could show improved cellular uptake, target specificity, and extended circulation for cancer treatment. In addition, their high surface area/volume ratio helps to incorporate several therapeutic drugs that are distributed to cancer tissues through EPR effect after entering into the leaky tumor vasculatures (Torchilin 2011; Prabhakar et al. 2013). Because of these characteristic features, nanomaterial-based therapeutics for cancer have shown similar or even better anticancer effect to marketable formulations, by exhibiting decreased side effects and providing new approaches to fight against colon cancer.
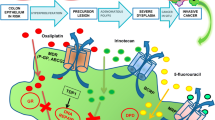
The advantage of nanosized carriers is that they can increase drug therapeutic index either encapsulated or conjugated to the nanocarrier’s surface. Size is a key factor in the delivery of nanotechnology-based cancer therapeutics (Fig. 8.1). Nanotherapeutic delivery primarily focuses on enhanced permeability and retention (EPR) effect by passive targeting on tumor tissue. This phenomenon is specific to tumor microenvironment, along with enlarged tumor vasculature permeability, which allows nanoparticles (<200 nm) to enter and accumulate in the cancer microenvironment. Besides, the site and time of drug release can also be controlled by stimulated actions, such as pH, ultrasound, material composition, or temperature. Although a few numbers of nanosized delivery carriers have been approved for human usage. Nanosized carriers have favorable surface characteristics, which is one of the benefits that enable them to be functionalized at the desired site of action (Torchilin 2011). These nanocarriers show higher efficiency and reduced side effects by lower accumulation rates in normal tissues, with higher accumulation and retention rates in cancer tissues (Prabhakar et al. 2013). It might also protect the drugs from inappropriate environmental conditions and enzymatic actions, as well as against early washout from the body. Moreover, due to their nanosize, it directly interacts with cell membrane and intracellular structures. The nanotechnology application has therefore provided a huge capability in overcoming intracellular mechanisms linked with drug resistance. Nanoparticles (NPs) have an important role in reversing multidrug resistance (MDR) by permitting sufficient drug to persist in the cytoplasm in both pump-dependent and pump-independent MDR. Nanocarriers induced enormous intracellular drug loading capacity, which overcomes many limitation progressions related to intake, metabolism, and efflux of drug, and noticeably increase the efficacy of chemotherapy.
8.2 Nanomedicines/Nanodevices for Colorectal Cancer Treatment
Nanomedicine can be delivered by oral, inhalation, rectal, or systemic route. Administration through oral route appears to be mostly improved and acceptable in the gastrointestinal disease treatment for patient’s comfort (Homayun et al. 2019). The major challenge in oral intake for colon-specific drug delivery system (CDDS) is the protection of drug against its release and absorption from gastrointestinal tract (GIT) to the colon. Also, it should not be degraded in GIT sites and only released and absorbed at the colon-specific site (Banerjee and Mitragotri 2017). Nanotechnological studies mainly concentrate on inorganic nanomaterials, such as carbon nanotubes (CNTs), silica nanoparticles (NPs), fullerenes, and nanoshells, and organic nanomaterials, such as liposomes, polymeric nanoparticles, dendrimers, polymeric micelles, etc. (Table 8.1). Nanoparticles also offer various CRC treatment strategies combined with chemotherapeutic drugs, including 5-fluorouracil (5-FU) and irinotecan, as well as targeted therapies using antibodies (cetuximab, rapamycin). The therapeutic approaches of nanoparticles through photosensitizing or thermal destruction of malignant cells via intratumoral release can also be an innovative approach for tumor cell destruction.
8.2.1 Organic Nanomaterials for Colon Cancer Therapies
Organic polymers possess very good properties such as biocompatibility and degradability. Organic-based nanomaterials from natural or synthetic polymer have been widely used in the field of CRC therapies nowadays. The organic nanostructures can be divided into polymeric nanoparticles (NPs), liposomes, dendrimers, polymeric micelles, hydrogel, and nanoemulsion.
8.2.1.1 Polymeric Nanoparticles
In the field of nanomedicine, polymeric NPs possess greater pharmacokinetics, drug loading capability, and stability than polymeric micelles. Polymeric matrix comprises media for the drug absorption, entrapment, and encapsulation (Masood 2016; Devulapally and Paulmurugan 2014). Polymeric nanoparticle structures range in size between 10 and 100 nm. The outer surface of polymeric NPs comprises of nonionic surfactants, which helps to minimize immunological interactions (Torchilin 2008; Bilensoy et al. 2009). The two main polymeric NPs that have been permitted by US FDA are poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL) (Zhang et al. 2007).
5-Fluorouracil , which is employed in the initial phase for CRC therapy, also affects the healthy cells, and the bioavailability is also not promising in the colon region. Several studies stated that the 5-FU delivery can cause harmful general side effects, such as diarrhea, mucositis, changes in gut microflora, and bacterial translocations. Jain et al. have developed hyaluronic acid (HA)-coupled chitosan nanoparticles (HACTNP) incorporated with 5-fluorouracil (5-FU) and oxaliplatin for effective colon cancer delivery system (Jain and Jain 2008; Jain 2010). It shows a significant uptake by HT-29, colonic cancer cells, and higher cellular toxicity as compared to the 5-FU solution. In another study, 5-FU-incorporated nanoparticles with citrus pectin and pH-responsive enteric polymer (Eudragit S100) were used as targeted drug delivery systems for colon cancer (Subudhi et al. 2015). They concluded that polymer pectin was a promising carrier for the development of colon-specific delivery system. The toxicity and efficacy of Eudragit S100-coated CPN-loaded 5-FU in CRC were also studied in both cellular and animal models (Subudhi et al. 2015; Tummala et al. 2015). Eudragit S100 is a pH-responsive enteric polymer that does not degrade below pH 7 and is mainly used for coating nanoparticles. The main advantage of Eudragit S100 coating was to avoid drug release in gastrointestinal tract and only release at targeted colon site (Obeidat and Price 2006; Asghar and Chandran 2008). 5-FU may also exhibit toxicity on the gut bacteria that are involved in dietary fiber metabolism including polysaccharides such as chitosan, xanthan gum (XG), guar gum (GG), and amylose. Site-targeted delivery of 5-FU carrier was coated with natural gums such as GG and XG, which permit drug release in the colon and also provide nutrients for probiotics, thereby enabling gut homeostasis. On the other hand, the coadministration of probiotics can overcome the 5-FU nanoparticle-linked side effects on the colon bacteria (Singh et al. 2015).
8.2.1.2 Liposomes
Liposomes are first nanocarriers introduced by Bangham et al. in 1961 (Bangham et al. 1965). Liposomes are the first drug delivery system approved by FDA for clinical purposes (Pattni et al. 2015). Nanoliposomes are tiny, spherical nontoxic carriers enclosing an aqueous core with phospholipid bilayer and cholesterol. Liposomes are promising drug delivery systems for cells, due to their size, ability to incorporate numerous substances, slow-releasing capability, targeted delivery, and reduced side effects (Suntres 2011; Patil and Jadhav 2014). Nanoliposomes are most commonly used nanoparticles for delivering proteins, small peptides, and nucleic acids. In cancer treatment, to enhance the site-specific delivery of drugs, cell-specific receptors and ligands are inserted within a lipid bilayer. Because of their amphiphilic nature, nanoliposomes are also used for site-specific delivery of hydrophobic anticancer drugs (Andresen et al. 2005; Huynh et al. 2009; Abreu et al. 2011).
Nanoliposomes are mainly divided into the following:
-
I.
Stealth liposomes or long-circulating liposomes: Here, the liposome bilayer is reformed by adding polyethylene glycol (PEG) or gangliosides, which aid to avoid the binding of plasma opsonins to the surface of liposome and allow it to remain stable in the circulation (Nag and Awasthi 2013; Noble et al. 2014).
-
II.
Active nanoliposomes: Liposome nanoparticle targets antibodies, peptides, glycoside residues, receptors, and hormones.
-
III.
Sensitive nanoliposomes: Liposomes with specific properties such as pH-sensitive, thermosensitive, and magnetic (Akbarzadeh et al. 2013).
Doxorubicin (Doxil) and (DaunoXome), anticancer drugs loaded with liposome, have been approved by FDA for CRC (Barenholz 2012). Another nanoliposomal drug is ThermoDox (thermosensitive liposome doxorubicin) that targets colon liver metastasis in combination with radiofrequency. This nanoliposomal formulation releases the drug upon a slight hyperthermic trigger. ThermoDox has shown 25-fold more site-specific delivery of doxorubicin than IV doxorubicin (Stang et al. 2012). In addition to this liposome, nanocarriers, viz., CPX1, LE-SN38, and CPX-1 (irinotecan HCl: floxuridine), have cleared phase II trials for the treatment of CRC (Patel 2008; Loira-Pastoriza et al. 2014; Jefremow and Neurath 2020). Magnetoliposomes show many interesting properties, like magnetically directed drug delivery, hyperthermia-induced drug delivery, and high drug loading efficiency. To improve the antitumor activity against CRC in rat model, a monoclonal antibody-coupled liposomal 5-fluoro-2′-deoxyuridine nanocarrier is developed (Koning et al. 2002). A cationic nanoliposome is used to investigate the therapeutic effect of double gene therapy combined with immunotherapy for tumor cells. It has significant result in improving the survival rate and quality of life of CRC in mouse model (Sun et al. 2012). Handali et al. developed a novel folic acid-conjugated liposome for the targeted delivery of 5-fluorouracil to colorectal cancer cells (Handali et al. 2018). It might increase the therapeutic efficacy of drug while reducing the toxic side effects. One of the main challenges in the treatment of CRC is to overcome the multidrug resistance (MDR) protein-induced tumor resistance. A liposomal formulation developed with hyaluronic acid coating is found to be very efficient for the delivery of drug imatinib mesylate against MDR colorectal cancer (Negi et al. 2015). Several liposome-based nanosystems are under preclinical development, liposomal curcumin , liposomes (PEG-liposomal L-OHP), etc. (Patel 2008; Cay et al. 1997).
8.2.1.3 Dendrimers
Dendrimers are monodisperse molecules characterized with an extremely branched 3D structure (Bharali et al. 2009; Somani and Dufès 2014). They are highly water soluble due to the presence of hydrophilic functional groups (Nanjwade et al. 2009). Dendrimers can load drugs as well as gene through covalent conjugations and electrostatic interactions. They consist of vacant inner cavities and a bulk of surface functional groups, which possess a promising carrier for anticancer therapy. Due to comparatively small size (1–15 nm), dendritic carriers can be easily cleared off from the body through kidneys, thereby reducing in vivo toxicity (Padilla De Jesús et al. 2002). Among all the dendrimers, the most broadly studied dendrimer is polyamidoamine (PAMAM), and its surface is covered with a huge number of amine groups, which helps to conjugate with other functional moieties. It is one of the most sophisticated nanotechnology devices for targeted drug delivery (Semwal et al. 2010). Tomalia et al. described first PAMAM dendrimers in 1985 (Tomalia et al. 1985). Due to their extremely branched structure, dendrimers have open spaces between branches, so they can easily encapsulate drugs (Bhadra et al. 2003). Mignani et al. have demonstrated DOX dendrimer was 10 times less toxic than DOX alone solution after exposure to colon carcinoma cells for 72 h. Administration of DOX dendrimer to BALB/c mice bearing colon carcinoma tumors also resulted in higher uptake than plain DOX at 48 h (Mignani and Majoral 2013).
8.2.1.4 Polymeric Micelles (PMs)
Polymeric micelles (PMs) are nanosized particles with both hydrophilic shell and hydrophobic core (Torchilin 2007; Wu et al. 2013a). The hydrophilic shell of micelle is commonly constructed with PEG, which helps to stabilize and protect the carriers from degradation in vivo (Kataoka et al. 2012; Zhou et al. 2017). For hydrophobic core construction, several natural as well as synthetic polymers have been commonly used, including polysaccharides, PCL, PLA, and PLGA. The core provides a better medium to encapsulate hydrophobic drugs, thereby solving their poor water solubility. Oxaliplatin NPs were made up of chitosan oligosaccharide and stearic acid polymer, which helps in polymeric micelle development. These micelles exhibited good internalization capacity and higher oxaliplatin accumulation in tissues. Moreover, it also showed higher cytotoxicity against most of the tumor cells when compared to oxaliplatin alone (Wang et al. 2011). Another targeted therapy of CRC was developed using a TORC1 signaling complex inhibitor called rapamycin in pegylated octadecyl lithocholate micelles linked with LTTHYKL peptide against CRC. These micelles showed a higher beneficial efficacy than free rapamycin drug in mice model, with significantly lower toxicity (Khondee et al. 2015). Another predictive factor PLK1 is considered to classify patients expected to respond to randomized controlled trial (RCT) (Rödel et al. 2010; Fernandez-Acenero et al. 2016). Nowadays, several inhibitors are targeting PLK for the delivery system development mixture mode .
8.2.2 Inorganic Nanomaterials for Colon Cancer Therapies
In addition to organic NPs , several other inorganic materials with exciting confirmation as well as exclusive chemico-physical properties have also been identified as NPs for colon cancer therapies. Some of these are discussed below.
8.2.2.1 Carbon Nanotubes
Carbon nanotubes (CNTs) are allotropes of carbon with a cylindrical-shaped nanostructure (Wang et al. 2009). CNTs have unique properties that include its improved geometrical, mechanical, and electrical properties, stiffness, thermal conductivity, strength, etc. These unique properties are exploited in the field of nanotechnology, electronics, optics, material science, and technology (Gullapalli and Wong 2011; Lim et al. 2014). Based on their structure, CNTs are classified into single-walled nanotubes (SWNTs), consisting of a single graphite sheet covered into a cylindrical tube, and multi-walled nanotubes (MWNTs) comprising group of nanotubes concentrically nested together as the rings of a tree trunk. Functionalized CNTs are used as novel nanocarriers for proteins, drugs, and genes (Ghorbani and Karimi 2015). The anticancer activity of paclitaxel (PAX)-loaded nanotubes and its cellular interactions were studied using poly(2-(dimethylamino)ethyl methacrylate-co-methacrylic acid) on MC38 murine colon cell line . It has shown effective anticancer action against CRC (Lee and Geckeler 2012). MWCNT (Multi-walled carbon nanotubes) is combined with embryonic stem cells (ESC) as cellular agents exhibiting anticancer activity against MC38 cancer cells (Mocan and Iancu 2011). MWCNT conjugated with folic acid is used as nanosystem for the delivery of photothermal drug against CRC and the drug-targeting efficiency improved by folic acid conjugation (Wen et al. 2013). MWCNT conjugated with hyaluronic acid and PEG shows sustained gemcitabine (GEM) release against colon adenocarcinoma cell lines (HT-29), and the side effects of GEM can be reduced as a result of hydrolysis within cancer cells (Prajapati et al. 2019). MWCNTs loaded with oxaliplatin also confirmed effective drug release against HT-29 cells (Wu et al. 2013b). In addition to MWCNTs, SWCNT modified with antibody C225 can be an active nanosystem for specific delivery of antitumor drug 7-ethyl-10-hydroxy-camptothecin (SN38) into EGFR-expressing colorectal cancer cells (Lee et al. 2013). CNTs are one of the promising nanocarriers used in the treatment and diagnosis of various types of cancer. Many studies have been carried out on CNTs as nanosystems; not much work is reported on colorectal cancer. Moreover, further research is required to show any disadvantages or side effects caused by CNTs .
8.2.2.2 Nanoshells
Nanoshells are spherical nanoparticles containing a dielectric core which is surrounded by a shell (Fuchigami et al. 2012). These nanoparticles can be designed to have unique chemical and optical properties by varying the geometry, such that they can be useful for biological applications. Nanoshell materials can be made from metals, semiconductors, or insulators. Gold nanoshells are metal nanoshells, which are mainly used for in vitro cancer detection, imaging, and treatment (Mody et al. 2010). Nanoshells are used for site-specific delivery of certain anticancer drugs like paclitaxel, doxorubicin, and siRNA. These are coated with PEG or other functional groups, which enhance the efficacy of delivery and bioavailability (Mudshinge et al. 2011). For the treatment of colon cancer cells, plasmonic photothermal therapy using gold nanoshell exhibited promising results (Koohi et al. 2017). Platinum drug-loaded gold nanoshells are used to study the combined effect of chemo-photothermal therapy in CRC. Poly[2-(N,N-dimethylamino)ethyl methacrylate]-poly(ε-caprolactone) micellar template-based gold nanoshell has been used as a nanosystem for the delivery of platinum-based drug, dichloro(1,2-diaminocyclohexane)platinum(II) (DACHPt) (Lee and Shieh 2020). This DACHPt-loaded nanoshell showed combined chemo-photothermal therapy which results in tumor growth inhibition with less side effects (Lee and Shieh 2020). Guanylyl cyclase C is targeted by bacterial heat-stable enterotoxin conjugated gold nanoshell, which helps to induce thermal abalation in metastatic colorectal cancer by heat release during excitation by using near-infrared (NIR) light exposure (Waldman et al. 2006).
8.2.2.3 Fullerene Derivatives
Fullerenes are carbon-based compounds made in the form of hollow sphere, tube, or ellipsoid. The most stable, common, and high symmetry fullerene is C60. C70, C20, carbon nano-onions, nanobuds, and CNs (elongated, tube-structured fullerene) are other fullerene variants (Nasibulin et al. 2007). Their size, three-dimensionality, hydrophobicity, electronic configurations, and photoexcitation make them an interesting topic in various medical fields. C60 is insoluble and aggregates easily in aqueous media. Generally, carriers like calixarenes, cyclodextrins, polyvinylpyrrolidone, liposomes, and micelles are used for fullerenes encapsulation. Chemical functionalization can increase the hydrophilicity mainly done with carboxylic acid, amino acid, amphiphilic polymers, and polyhydroxyl group. Fullerene can be used as antioxidant and radical scavenger (Caruso et al. 2014). Moreover, for drug and gene delivery, fullerenes have been used as a nanocarrier. In vitro studies with C60 fullerene derivatives decrease the migration and invasion of HT-29 CRC cells (Lucafo et al. 2014). Fullerenes are used as a photosensitizer to mediate intraperitoneal photodynamic therapy for abdominal dissemination of colon adenocarcinoma in mouse model. Fullerenol shows protective effects on cardiotoxicity and hepatotoxicity caused by doxorubicin in rats with CRC (Injac et al. 2009). The C60 fullerenol nanoparticles (FNP) efficiently inhibited the development of dysplastic aberrant crypt foci in dimethylhydrazine-induced rat model of CRC (Perše et al. 2011). The antineoplastic activity of 5-fluorouracil (5-FU) and pyrrole derivative 1-(4-Cl-benzyl)-3-Cl-4-(CF3-fenylamino)-1H-pyrrol-2.5-dione (MI-1) are compared with pristine C60 fullerene (C60FAS) in rat model which induced CRC by 1,2-dimethylhydrazine (DHM). It is found that when rats are treated with C60FAS, 5-FU, and MI-1, the number of tumors and lesion area is reduced and it also decreases the side effects of antitumor therapy (Lynchak et al. 2017).
8.2.2.4 Silica Nanoparticles
Silica nanoparticles exhibit a porous framework with several benefits including higher biocompatibility and functionalization (Amato 2010; Wei et al. 2010). These nanoparticles are like a porous construction like a beehive, which is able to entrap large quantities of various bioactive components. Important features of mesoporous silica nanoparticles include adjustable size range of 50–300 and cavities of 2–6 nm (Stang et al. 2012). And it also shows lower toxicity, easy endocytosis, and resistance against external factors like temperature and pH (Bharti et al. 2015). Radhakrishnan et al. developed mesoporous silica nanoparticle (MSN) with protamine hybrid system to control drug release in cancer cells where definite enzymes can activate the drug activity (Radhakrishnan et al. 2014). This study also significantly enhanced the cell death in comparison to the free drug with MSN-PRM system. Cellular uptake was improved in DOX-loaded HA-MSNs, and it is also shown that DOX-HA-MSNs exhibit more cytotoxicity to HCT-116 cell lines than free DOX drug (Gidding et al. 1999).
8.3 Conclusions
In this chapter, different types of nanodevices were discussed including organic (polymeric NPs, liposomes, dendrimers, polymeric micelles) and inorganic (carbon nanotubes, nanorods, fullerenes) nanoparticles. These devices are developed by improving their structural and cellular targeting abilities, which promoted more effective therapeutic delivery. Of all the nanomaterials presented in this chapter, liposomes are highly advanced and are clinically permitted for clinical trials with some formulations already available in the market, while other inorganic-based formulations have not received such approval. Though some nanomaterial formations are already approved for various cancer therapies, e.g., Abraxane, Doxil, and Embosphere., successful clinical studies are limited, possibly due to the inconsistency of patients and different tumor pathological characteristics, opsonization, pharmacokinetics, tumor accessibility, as well as biodistribution. Certainly, almost all therapeutic methods are depending on EPR effect which intensely differs from one patient to other and from tumor to tumor.
Regardless of outstanding health benefits, the effective conversion of nanomaterial therapeutics into medical practice still has many concerns and challenges including pharmacokinetics and targeting efficacy. In the near future, scientifically validated nanomaterials for medical usage will be developed. The important restriction which delays the transformation of nanodevices to bedside is the difficulties to meet drug regulatory approval for the design and scaling up of nanodevices. The development of new nanodevices for colon cancer treatment is probably another way to have more innovative clinical trials in the future.
Abbreviations
- 5-FU:
-
5-Fluorouracil
- AuNPs:
-
Gold nanoparticles
- CDDS:
-
Colon-specific drug delivery system
- CNTs:
-
Carbon nanotubes
- CRC:
-
Colorectal cancer
- EPR:
-
Enhanced permeability and retention
- ESC:
-
Embryonic stem cells
- FDA:
-
Food and Drug Administration
- GG:
-
Guar gum
- GIT:
-
Gastrointestinal tract
- HA:
-
Hyaluronic acid
- IBD:
-
Inflammatory bowel disease
- LP:
-
Lycopene
- MDR:
-
Multidrug resistance
- MSN:
-
Mesoporous silica nanoparticle
- MWCNT:
-
Multi-walled carbon nanotubes
- MWNTs:
-
Multi-walled nanotubes
- NPs:
-
Nanoparticles
- PAMAM:
-
Polyamidoamine
- PAX:
-
Paclitaxel
- PCL:
-
Polycaprolactone
- PEG:
-
Polyethylene glycol
- PLA:
-
Polylactide
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PLK-1:
-
Polo-like kinase 1
- RCT:
-
Randomized controlled trial
- ROS:
-
Reactive oxygen species
- SWNTs:
-
Single-walled nanotubes
- US FDA:
-
United States Food and Drug Administration
- XG:
-
Xanthan gum
References
Abreu, A. S., Castanheira, E. M., Queiroz, M. J., Ferreira, P. M., Vale-Silva, L. A., & Pinto, E. (2011). Nanoliposomes for encapsulation and delivery of the potential antitumoral methyl 6-methoxy-3-(4-methoxyphenyl)-1H-indole-2-carboxylate. Nanoscale Research Letters, 6(1), 482.
Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., Joo, S. W., Zarghami, N., Hanifehpour, Y., Samiei, M., Kouhi, M., & Nejati-Koshki, K. (2013). Liposome: Classification, preparation, and applications. Nanoscale Research Letters, 8(1), 102.
Amato, G. I. (2010). Silica-encapsulated efficient and stable Si quantum dots with high biocompatibility. Nanoscale Research Letters, 5(7), 1156–1160.
Andresen, T. L., Jensen, S. S., & Jørgensen, K. (2005). Advanced strategies in liposomal cancer therapy: Problems and prospects of active and tumor specific drug release. Progress in Lipid Research, 44(1), 68–97.
Arshad, U., Sutton, P. A., Ashford, M. B., Treacher, K. E., Liptrott, N. J., Rannard, S. P., Goldring, C. E., & Owen, A. (2020). Critical considerations for targeting colorectal liver metastases with nanotechnology. Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology, 12(2), e1588. https://doi.org/10.1002/wnan.1588.
Asghar, L. F., & Chandran, S. (2008). Design and evaluation of matrices of Eudragit with polycarbophil and carbopol for colon-specific delivery. Journal of Drug Targeting, 116(10), 741–757.
Banerjee, A., & Mitragotri, S. (2017). Intestinal patch systems for oral drug delivery. Current Opinion in Pharmacology., 36, 58–65.
Bangham, A. D., Standish, M. M., & Watkins, J. C. (1965). Diffusion of univalent ions across the lamellae of swollen phospholipids. Journal of Molecular Biology, 13(1), 238–IN27.
Barenholz, Y. C. (2012). Doxil®—The first FDA-approved nano-drug: Lessons learned. Journal of Controlled Release, 160(2), 117–134.
Bhadra, D., Bhadra, S., Jain, S., & Jain, N. K. (2003). A PEGylated dendritic nanoparticulate carrier of fluorouracil. International Journal of Pharmaceutics, 257(1–2), 111–124.
Bharali, D. J., Khalil, M., Gurbuz, M., Simone, T. M., & Mousa, S. A. (2009). Nanoparticles and cancer therapy: A concise review with emphasis on dendrimers. International Journal of Nanomedicine, 4, 1.
Bharti, C., Nagaich, U., Pal, A. K., & Gulati, N. (2015). Mesoporous silica nanoparticles in target drug delivery system: A review. International Journal of Pharmaceutical Investigation, 5(3), 124.
Bilensoy, E., Sarisozen, C., Esendağlı, G., Doğan, A. L., Aktaş, Y., Şen, M., & Mungan, N. A. (2009). Intravesical cationic nanoparticles of chitosan and polycaprolactone for the delivery of Mitomycin C to bladder tumors. International Journal of Pharmaceutics, 371(1–2), 170–176.
Caruso, G., Merlo, L., & Caffo, M. (2014). Nanoparticles potential: Types, mechanisms of action, actual in vitro and animal studies, recent patents. Innovative Brain Tumor Therapy. Oxford., 53–150.
Cay, O., Kruskal, J. B., Nasser, I., Thomas, P., & Clouse, M. E. (1997). Liver metastases from colorectal cancer: Drug delivery with liposome-encapsulated doxorubicin. Radiology, 205(1), 95–101.
Chen, W. K., Ren, L., Wei, Y., Zhu, D. X., Miao, C. H., & Xu, J. M. (2015). General anesthesia combined with epidural anesthesia ameliorates the effect of fast-track surgery by mitigating immunosuppression and facilitating intestinal functional recovery in colon cancer patients. International Journal of Colorectal Disease, 30(4), 475–481.
Cross, A. J., Ferrucci, L. M., Risch, A., Graubard, B. I., Ward, M. H., Park, Y., Hollenbeck, A. R., Schatzkin, A., & Sinha, R. (2010). A large prospective study of meat consumption and colorectal cancer risk: An investigation of potential mechanisms underlying this association. Cancer Research, 70(6), 2406–2414.
Devulapally, R., & Paulmurugan, R. (2014). Polymer nanoparticles for drug and small silencing RNA delivery to treat cancers of different phenotypes. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 6(1), 40–60.
Ferlay, J., Soerjomataram, I., Ervik, M., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D. M., Forman, D., & Bray, F. (2015). GLOBOCAN 2012 v1. 1, Cancer incidence and mortality worldwide: IARC CancerBase No. 11. Lyon: International Agency for Research on Cancer.
Fernandez-Acenero, M. J., Cortes, D., Del Pulgar, T. G., Cebrian, A., Estrada, L., Martinez-Useros, J., Celdrán, A., García-Foncillas, J., & Pastor, C. (2016). Plk-1 expression is associated with histopathological response to neoadjuvant therapy of hepatic metastasis of colorectal carcinoma. Pathology & Oncology Research, 22(2), 377–383.
Fuchigami, T., Kitamoto, Y., & Namiki, Y. (2012). Size-tunable drug-delivery capsules composed of a magnetic nanoshell. Biomatter, 2(4), 313–320.
Ghorbani, M., & Karimi, H. (2015). Role of biotechnology in cancer control. International Journal of Scientific Research in Science and Technology (IJSRST)., 1(5), 180–185.
Gidding, C. M., Kellie, S. J., Kamps, W. A., & de Graaf, S. N. (1999). Vincristine revisited. Critical Reviews in Oncology/Hematology, 29(3), 267–287.
Gullapalli, S., & Wong, M. S. (2011). Nanotechnology: A guide to nano-objects. Chemical Engineering Progress, 107(5), 28–32.
Handali, S., Moghimipour, E., Rezaei, M., Ramezani, Z., Kouchak, M., Amini, M., Angali, K. A., Saremy, S., & Dorkoosh, F. A. (2018). A novel 5-Fluorouracil targeted delivery to colon cancer using folic acid conjugated liposomes. Biomedicine & Pharmacotherapy, 108, 1259–1273.
Homayun, B., Lin, X., & Choi, H. J. (2019). Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics, 11(3), 129.
Huynh, N. T., Passirani, C., Saulnier, P., & Benoît, J. P. (2009). Lipid nanocapsules: A new platform for nanomedicine. International Journal of Pharmaceutics, 379(2), 201–209.
Injac, R., Perse, M., Cerne, M., Potocnik, N., Radic, N., Govedarica, B., Djordjevic, A., Cerar, A., & Strukelj, B. (2009). Protective effects of fullerenol C60 (OH) 24 against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats with colorectal cancer. Biomaterials, 30(6), 1184–1196.
Jain, K. K. (2010). Advances in the field of nanooncology. BMC Medicine, 8(1), 83.
Jain, A., & Jain, S. K. (2008). In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. European Journal of Pharmaceutical Sciences, 35(5), 404–416.
Jefremow, A., & Neurath, M. F. (2020). Nanoparticles in gastrooncology. Visceral Medicine, 36(2), 88–94.
Kataoka, K., Harada, A., & Nagasaki, Y. (2012). Block copolymer micelles for drug delivery: Design, characterization and biological significance. Advanced Drug Delivery Reviews, 64, 37–48.
Khondee, S., Rabinsky, E. F., Owens, S. R., Joshi, B. P., Qiu, Z., Duan, X., Zhao, L., & Wang, T. D. (2015). Targeted therapy of colorectal neoplasia with rapamycin in peptide-labeled pegylated octadecyl lithocholate micelles. Journal of Controlled Release, 199, 114–121.
Koning, G. A., Kamps, J. A., & Scherphof, G. L. (2002). Efficient intracellular delivery of 5-fluorodeoxyuridine into colon cancer cells by targeted immunoliposomes. Cancer Detection and Prevention, 26(4), 299–307.
Koohi, S. R., Derakhshan, M. A., Faridani, F., Nejad, S. M., Amanpour, S., Tajerian, R., Yarmahmoodi, M., & Faridi-Majidi, R. (2017). Plasmonic photothermal therapy of colon cancer cells utilising gold nanoshells: An in vitro study. IET Nanobiotechnology, 12(2), 196–200.
Lee, Y., & Geckeler, K. E. (2012). Cellular interactions of a water-soluble supramolecular polymer complex of carbon nanotubes with human epithelial colorectal adenocarcinoma cells. Macromolecular Bioscience, 12(8), 1060–1067.
Lee, S. Y., & Shieh, M. J. (2020). Platinum (II) drug-loaded gold nanoshells for chemo-photothermal therapy in colorectal cancer. ACS Applied Materials & Interfaces, 12(4), 4254–4264.
Lee, P. C., Chiou, Y. C., Wong, J. M., Peng, C. L., & Shieh, M. J. (2013). Targeting colorectal cancer cells with single-walled carbon nanotubes conjugated to anticancer agent SN-38 and EGFR antibody. Biomaterials, 34(34), 8756–8765.
Lim, D. J., Sim, M., Oh, L., Lim, K., & Park, H. (2014). Carbon-based drug delivery carriers for cancer therapy. Archives of Pharmacal Research, 37(1), 43–52.
Loira-Pastoriza, C., Todoroff, J., & Vanbever, R. (2014). Delivery strategies for sustained drug release in the lungs. Advanced Drug Delivery Reviews, 75, 81–91.
Lucafo, M., Pelillo, C., Carini, M., Da Ros, T., Prato, M., & Sava, G. (2014). A cationic [60] fullerene derivative reduces invasion and migration of HT-29 CRC cells in vitro at dose free of significant effects on cell survival. Nano-Micro Letters., 6(2), 163–168.
Lynchak, O. V., Prylutskyy, Y. I., Rybalchenko, V. K., Kyzyma, O. A., Soloviov, D., Kostjukov, V. V., Evstigneev, M. P., Ritter, U., & Scharff, P. (2017). Comparative analysis of the antineoplastic activity of C 60 fullerene with 5-fluorouracil and pyrrole derivative in vivo. Nanoscale Research Letters, 12(1), 1–6.
Ma, Y., Yang, Y., Wang, F., Zhang, P., Shi, C., Zou, Y., & Qin, H. (2013). Obesity and risk of colorectal cancer: A systematic review of prospective studies. PLoS One, 8(1), e53916.
Masood, F. (2016). Polymeric nanoparticles for targeted drug delivery system for cancer therapies. Materials Science and Engineering: C, 60, 569–578.
Mazhar, D., Stebbing, J., & Heller, W. (2006). Recent advances in the systemic management of colorectal cancer. Future Oncology, 2, 643–650.
Mignani, S., & Majoral, J. P. (2013). Dendrimers as macromolecular tools to tackle from colon to brain tumor types: A concise overview. New Journal of Chemistry, 37(11), 3337–3357.
Mocan, T., & Iancu, C. (2011). Effective colon cancer prophylaxis in mice using embryonic stem cells and carbon nanotubes. International Journal of Nanomedicine, 6, 1945.
Mody, V. V., Siwale, R., Singh, A., & Mody, H. R. (2010). Introduction to metallic nanoparticles. Journal of Pharmacy and Bioallied Sciences., 2(4), 282.
Mudshinge, S. R., Deore, A. B., Patil, S., & Bhalgat, C. M. (2011). Nanoparticles: Emerging carriers for drug delivery. Saudi Pharmaceutical Journal, 19(3), 129–141.
Nag, O. K., & Awasthi, V. (2013). Surface engineering of liposomes for stealth behavior. Pharmaceutics., 5(4), 542–569.
Nanjwade, B. K., Bechra, H. M., Derkar, G. K., Manvi, F. V., & Nanjwade, V. K. (2009). Dendrimers: Emerging polymers for drug-delivery systems. European Journal of Pharmaceutical Sciences, 38(3), 185–196.
Nasibulin, A. G., Pikhitsa, P. V., Jiang, H., Brown, D. P., Krasheninnikov, A. V., Anisimov, A. S., Queipo, P., Moisala, A., Gonzalez, D., Lientschnig, G., & Hassanien, A. (2007). A novel hybrid carbon material. Nature Nanotechnology, 2(3), 156.
Negi, L. M., Jaggi, M., Joshi, V., Ronodip, K., & Talegaonkar, S. (2015). Hyaluronan coated liposomes as the intravenous platform for delivery of imatinib mesylate in MDR colon cancer. International Journal of Biological Macromolecules, 73, 222–235.
Noble, G. T., Stefanick, J. F., Ashley, J. D., Kiziltepe, T., & Bilgicer, B. (2014). Ligand-targeted liposome design: Challenges and fundamental considerations. Trends in Biotechnology, 32(1), 32–45.
Obeidat, W., & Price, J. C. (2006). Preparation and evaluation of Eudragit S 100 microspheres as pH-sensitive release preparations for piroxicam and theophylline using the emulsion-solvent evaporation method. Journal of Microencapsulation, 23(2), 195–202.
Padilla De Jesús, O. L., Ihre, H. R., Gagne, L., Fréchet, J. M., & Szoka, F. C. (2002). Polyester dendritic systems for drug delivery applications: In vitro and in vivo evaluation. Bioconjugate Chemistry, 13(3), 453–461.
Patel, D. K. (2008). Clinical use of anti-epidermal growth factor receptor monoclonal antibodies in metastatic colorectal cancer. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 28(11P2), 31S–41S.
Patil, Y. P., & Jadhav, S. (2014). Novel methods for liposome preparation. Chemistry and Physics of Lipids, 177, 8–18.
Pattni, B. S., Chupin, V. V., & Torchilin, V. P. (2015). New developments in liposomal drug delivery. Chemical Reviews, 115(19), 10938–10966.
Perše, M., Injac, R., Djordjevic, A., Štrukelj, B., & Cerar, A. (2011). Protective effect of fullerenol nano particles on colon cancer development in dimethylhydrazine rat model. Digest Journal of Nanomaterials & Biostructures (DJNB), 6(4).
Prabhakar, U., Maeda, H., Jain, R. K., Sevick-Muraca, E. M., Zamboni, W., Farokhzad, O. C., Barry, S. T., Gabizon, A., Grodzinski, P., & Blakey, D. C. (2013). Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Research, 73(8), 2412–2417.
Prajapati, S. K., Jain, A., Shrivastava, C., & Jain, A. K. (2019). Hyaluronic acid conjugated multi-walled carbon nanotubes for colon cancer targeting. International Journal of Biological Macromolecules, 123, 691–703.
Radhakrishnan, K., Gupta, S., Gnanadhas, D. P., Ramamurthy, P. C., Chakravortty, D., & Raichur, A. M. (2014). Protamine-capped mesoporous silica nanoparticles for biologically triggered drug release. Particle & Particle Systems Characterization, 31(4), 449–458.
Rödel, F., Keppner, S., Capalbo, G., Bashary, R., Kaufmann, M., Rödel, C., Strebhardt, K., & Spänkuch, B. (2010). Polo-like kinase 1 as predictive marker and therapeutic target for radiotherapy in rectal cancer. The American Journal of Pathology, 177(2), 918–929.
Semwal, R., Semwal, D. K., Madan, A. K., Paul, P., Mujaffer, F., & Badoni, R. (2010). Dendrimers: A novel approach for drug targeting. Journal of Pharmacy Research, 3(9), 2238–2247.
Siegel, R., DeSantis, C., & Jemal, A. (2014). Colorectal cancer statistics, 2014. CA: A Cancer Journal for Clinicians, 64(2), 104–117.
Singh, S., Kotla, N. G., Tomar, S., Maddiboyina, B., Webster, T. J., Sharma, D., & Sunnapu, O. (2015). A nanomedicine-promising approach to provide an appropriate colon-targeted drug delivery system for 5-fluorouracil. International Journal of Nanomedicine, 10, 7175.
Somani, S., & Dufès, C. (2014). Applications of dendrimers for brain delivery and cancer therapy. Nanomedicine, 9(15), 2403–2414.
Stang, J., Haynes, M., Carson, P., & Moghaddam, M. (2012). A preclinical system prototype for focused microwave thermal therapy of the breast. IEEE Transactions on Biomedical Engineering, 59(9), 2431–2438.
Subudhi, M. B., Jain, A., Jain, A., Hurkat, P., Shilpi, S., Gulbake, A., & Jain, S. K. (2015). Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-fluorouracil. Materials, 8(3), 832–849.
Sun, N. F., Meng, Q. Y., Tian, A. L., Hu, S. Y., Wang, R. H., Liu, Z. X., & Xu, L. (2012). Nanoliposome-mediated FL/TRAIL double-gene therapy for colon cancer: In vitro and in vivo evaluation. Cancer Letters, 315(1), 69–77.
Suntres, Z. E. (2011). Liposomal antioxidants for protection against oxidant-induced damage. Journal of Toxicology, 2011, 152474.
Tomalia, D. A., Baker, H., Dewald, J., Hall, M., Kallos, G., Martin, S., Roeck, J., Ryder, J., & Smith, P. (1985). A new class of polymers: Starburst-dendritic macromolecules. Polymer Journal, 17(1), 117–132.
Torchilin, V. P. (2007). Micellar nanocarriers: Pharmaceutical perspectives. Pharmaceutical Research, 24(1), 1.
Torchilin, V. (2008). Multifunctional pharmaceutical nanocarriers: Development of the concept. In Multifunctional pharmaceutical nanocarriers (pp. 1–32). New York: Springer.
Torchilin, V. (2011). Tumor delivery of macromolecular drugs based on the EPR effect. Advanced Drug Delivery Reviews, 63(3), 131–135.
Tsilidis, K. K., Kasimis, J. C., Lopez, D. S., Ntzani, E. E., & Ioannidis, J. P. (2015). Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ, 350, g7607.
Tummala, S., Kumar, M. S., & Prakash, A. (2015). Formulation and characterization of 5-Fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharmaceutical Journal., 23(3), 308–314.
Van Cutsem, E., Köhne, C. H., Hitre, E., Zaluski, J., Chang Chien, C. R., Makhson, A., D’Haens, G., Pintér, T., Lim, R., Bodoky, G., & Roh, J. K. (2009). Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. New England Journal of Medicine, 360(14), 1408–1417.
Waldman, S. A., Fortina, P., Surrey, S., Hyslop, T., Kricka, L. J., & Graves, D. J. (2006). Opportunities for near-infrared thermal ablation of colorectal metastases by guanylyl cyclase C-targeted gold nanoshells. Future Oncology, 705–716.
Wang, X., Li, Q., Xie, J., Jin, Z., Wang, J., Li, Y., Jiang, K., & Fan, S. (2009). Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Letters, 9(9), 3137–3141.
Wang, K., Liu, L., Zhang, T., Zhu, Y. L., Qiu, F., Wu, X. G., Wang, X. L., Hu, F. Q., & Huang, J. (2011). Oxaliplatin-incorporated micelles eliminate both cancer stem-like and bulk cell populations in colorectal cancer. International Journal of Nanomedicine, 6, 3207.
Wei, L., Hu, N., & Zhang, Y. (2010). Synthesis of polymer—Mesoporous silica nanocomposites. Materials, 3(7), 4066–4079.
Wen, S., Liu, H., Cai, H., Shen, M., & Shi, X. (2013). Targeted and pH-responsive delivery of doxorubicin to cancer cells using multifunctional dendrimer-modified multi-walled carbon nanotubes. Advanced Healthcare Materials, 2(9), 1267–1276.
Wu, H., Zhu, L., & Torchilin, V. P. (2013a). pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials, 34(4), 1213–1222.
Wu, L., Man, C., Wang, H., Lu, X., Ma, Q., Cai, Y., & Ma, W. (2013b). PEGylated multi-walled carbon nanotubes for encapsulation and sustained release of oxaliplatin. Pharmaceutical Research, 30(2), 412–423.
Zhang, L., Radovic-Moreno, A. F., Alexis, F., Gu, F. X., Basto, P. A., Bagalkot, V., Jon, S., Langer, R. S., & Farokhzad, O. C. (2007). Co-delivery of hydrophobic and hydrophilic drugs from nanoparticle–aptamer bioconjugates. ChemMedChem, 2(9), 1268–1271.
Zhou, Q., Hou, Y., Zhang, L., Wang, J., Qiao, Y., Guo, S., Fan, L., Yang, T., Zhu, L., & Wu, H. (2017). Dual-pH sensitive charge-reversal nanocomplex for tumor-targeted drug delivery with enhanced anticancer activity. Theranostics, 7(7), 1806.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Reshmitha, T.R., Shini, V.S., Nisha, P. (2021). Nanotechnology Approaches for Colorectal Cancer Diagnosis and Therapy. In: Vishvakarma, N.K., Nagaraju, G.P., Shukla, D. (eds) Colon Cancer Diagnosis and Therapy. Springer, Cham. https://doi.org/10.1007/978-3-030-64668-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-64668-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64667-7
Online ISBN: 978-3-030-64668-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)