Abstract
Colorectal cancer (CRC) is a widespread cancer that starts in the digestive tract. It is the third most common cause of cancer deaths around the world. The World Health Organization (WHO) estimates an expected death toll of over 1 million cases annually. The limited therapeutic options as well as the drawbacks of the existing therapies necessitate the development of non-classic treatment approaches. Nanotechnology has led the evolution of valuable drug delivery systems thanks to their ability to control drug release and precisely target a wide variety of cancers. This has also been extended to the treatment of CRC. Herein, we shed light on the pertinent research that has been performed on the potential applications of nanoparticles in the treatment of CRC. The various types of nanoparticles in addition to their properties, applications, targeting approaches, merits, and demerits are discussed. Furthermore, innovative therapies for CRC, including gene therapies and immunotherapies, are also highlighted. Eventually, the research gaps, the clinical potential of such delivery systems, and a future outlook on their development are inspired.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC), which begins in the colon or rectum, is the third most common type of cancer worldwide. By 2035, it is expected that there will be 2.4 million people who will be diagnosed with colorectal cancer, which will result in 1.3 million deaths across the entire world [1, 2]. According to the American Joint Committee on Cancer, there are five distinct therapeutic stages for CRC [3]. When detected early, stage 0 colon cancer can be cured with surgical resection. Standard therapy for stages I and II is excision via surgery, with 5-year survival rates between 37 and 74%. In advanced stages of CRC, the survival rate falls to 6%, and chemotherapy is frequently prescribed after surgery [4, 5]. In the third and fourth stages, oxaliplatin, 5-fluorouracil, cisplatin, doxorubicin, and other medications are utilized. These medications have been associated with disagreeable side effects, such as vomiting, hair loss, and nausea, and have failed to show their effectiveness as promised [6]. Conventional treatments at this level involve adverse effects and restrictions due to poor chemical and physical characteristics, low absorption, and poor tissue selectivity of chemotherapeutic medicines. Patients may have several serious side effects during chemotherapy [7,8,9].
The limited therapeutic choices for CRC as well as the drawbacks of the existing therapies have prompted researchers to explore novel methods, such as improving conventional chemotherapeutics’ physicochemical and pharmacodynamic properties with a nanotechnology-based drug carrier system [10]. Lipid nanoparticles (LNPs) and polymeric nanoparticles are representative models of the application of nanodrug delivery systems (NanoDDS) for the treatment of CRC. Nanoparticles enable the delivery of chemotherapeutics, gene therapies, and vaccines to the desired site of action. Moreover, nanoparticles can improve the ex vivo and in vivo stability of the drugs in question, reduce their systemic toxicity, and overcome the evolution of chemoresistance [11,12,13].
Despite the increasing promise associated with nanomedicines, their clinical translation in the field of anticancer therapy is still limited by multiple challenges including sophisticated composition, poor scalability, variable in vitro–in vivo correlation, limited clinical data, and regulatory complications. Moreover, the heterogeneity of the various types of cancers makes it impractical to extrapolate the findings of certain studies to other types of cancers [14, 15]. Therefore, the precise tackling of these challenges requires a focused and comprehensive understanding of the properties and applications of various nanomaterials in each individual cancer type so as to customize the design of delivery systems and avoid clinical failure.
In the present article, we aimed to provide a focused overview of the various types of nanoparticles that are used for the delivery of drugs to CRC. The properties, applications, merits, and demerits of such delivery systems are discussed. Moreover, the most recent targeting approaches and mechanisms are compared, with an emphasis on the innovative and active targeting-based approaches in order to clarify the next steps in research as well as offer ideas for the development of nanoparticles for more effective treatment of CRC. Furthermore, the applications of nanoparticles in emerging therapeutic technologies such as gene therapy, immunotherapy, and vaccines against CRC are highlighted. Eventually, we discuss the clinical potential of the developed nanosystems and inspire a critical future outlook on the upcoming directions in this area of endeavor.
Pathophysiology of CRC and Its Molecular Features
The pathophysiology of CRC involves the uncontrolled growth of abnormal cells in the colon and rectum. These cells grow and divide uncontrollably, forming a tumor that can invade the surrounding tissues and organs. The exact cause of CRC is still unknown, but several risk factors have been identified. Some of these risk factors include age, family history, a diet with high levels of red and processed meat, obesity, smoking, and alcohol consumption [16]. The development of CRC occurs in several stages, starting from the formation of polyps in the colon and rectum. Polyps are small growths on the lining of the colon and rectum. They are usually benign, but some can develop into cancer over time. The majority of CRC develop from adenomatous polyps, which are a type of polyp that can become cancerous [17]. As the polyps grow, they can become larger and more numerous, eventually forming a tumor. The tumor can invade the wall of the colon or rectum and spread to the nearby lymph nodes. If left untreated, the cancer can spread to other parts of the body, such as the liver, lungs, and bones [18]. The pathophysiology of CRC also involves genetic mutations that contribute to the development and progression of the disease. Mutations in certain genes, such as the APC, KRAS, and TP53 genes, have been linked to the development of CRC. These genes play a critical role in regulating cell growth and division, and mutations in these genes can lead to the uncontrolled growth of abnormal cells [19].
Diagnosing CRC involves several tests and procedures, including a physical exam, blood tests, imaging-based examinations, and a colonoscopy. A colonoscopy is a procedure that uses a flexible tube with a camera on the end to examine the inside of the colon and rectum. If cancer is detected, further tests may be needed to determine the stage and extent of the disease [20]. Treatment options for CRC depend on the stage and extent of the disease. Surgery is the most common treatment option for early-stage CRC. In more advanced cases, chemotherapy and radiation therapy may be used in addition to surgery. Targeted therapy, which targets specific molecules involved in the growth and spread of cancer cells, may also be used in some cases [21]. Understanding the pathophysiology of CRC is crucial in developing effective treatments and preventing its occurrence. CRC is a complex disease that involves the uncontrolled growth of abnormal cells in the colon and rectum, genetic mutations, and several risk factors. Early detection and treatment are essential in improving outcomes for patients with CRC. Regular screening and a healthy lifestyle can help reduce the risk of developing CRC. In addition, understanding the unique molecular features of CRC is essential to facilitate the development of innovative therapies and precise targeting approaches, which are discussed in subsequent sections of this article.
Necessity for the Development of New Drug Delivery Systems
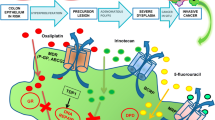
Most cases of CRC may be traced back to polyps, which are benign growths that are formed on the intestinal lining but can sometimes progress to malignancy. However, only around 10% of polyps have been shown to develop into a particularly dangerous cancer. This transformation takes a long time, typically more than 10–20 years, and is more likely to occur as polyps grow [22]. The need to improve the efficacy of standard chemotherapeutics and minimize off-target effects has prompted researchers to explore novel techniques, including the utilization of nanotechnology-based drug carrier systems. These systems aim to enhance chemotherapeutic agents’ physicochemical and pharmacodynamic properties and enable target-specific delivery of drugs to different types of tumor tissues, thereby optimizing treatment outcomes [10]. Nanoparticles are recognized as one of the most promising therapy methods for CRC and other malignancies because of their properties, which help to resolve the systemic drug delivery issues that diagnostics and anticancer drugs come across. More medications can be transported to be encapsulated within the core or adsorbed onto the surfaces of nanoparticles, which have massive surface area-to-volume ratios. Nanoparticles’ prolonged circulation and decreased renal clearance result from their small size, enhanced drug loading, and efficient encapsulation [23]. An additional benefit of using nanoparticles is that their selectivity can be increased towards the desired cells by functionalizing them with targeting ligands. At the same time, the likelihood of distributing the chemotherapeutics to the normal tissues is reduced. CRC chemotherapies can also employ various types of nanoparticles for such targeting (Fig. 1).
Nanodrug Delivery Systems (NanoDDS) Targeting CRC
Passively Targeted Nanoparticles
It is often thought that solid tumors show significant morphological and physiological differences from normal tissue. A solid tumor requires a substantial network of blood vessels. Meanwhile, the tumor has no functional lymphatic vessels. Since there is a significant amount of space between the tumor’s endothelial cells, it is possible for macromolecular drugs to extravasate and be retained. However, due to the slow lymphatic drainage, nanoparticles might enhance medicines’ concentrations in cancer cells for days or weeks after being injected into the body. Nanoparticle accumulation in tumor cells is caused by such an effect, which is referred to as the enhanced permeability and retention (EPR) effect (Fig. 2) [24,25,26]. There are evident disagreements over the effect of EPR on CRC. Scientists have realized over the past several years that there is a great deal of variety in how EPR might target tumors. This highlights the importance of enhancing the targeting capabilities of EPR-based nanoparticles by combining them with additional targeting techniques [27,28,29,30]. Whereas ionic cross-linking was utilized to encapsulate oxaliplatin into N,O-carboxymethyl chitosan nanoparticles (CMCS/OXE-NPs) for research, emulsification cross-linking was employed to encapsulate resveratrol (CMCS/Res-NPs). In comparison, the average particle size of CMCS/OXE-NPs was 190 nm, whereas the average particle size of CMCS/Res-NPs was 164.2 nm. CMCS/OXE-NPs had an encapsulation efficiency of 60% ± 1.67%, while CMCS/Res-NPs had an encapsulation efficiency of 65% ± 1.14%. The treatment that involved the use of both types of nanoparticles revealed much more potent anticancer action when compared to the use of either nanoparticle alone or free drugs. In in vivo investigations, the inhibition of cell proliferation by the combinational nanoparticle treatment was found to be more effective than using free medications or individual nanoparticles [26]. Emulsification cross-linking was employed to encapsulate curcumin into chitosan-gum Arabic nanoparticles. The results demonstrated that curcumin was effectively encapsulated in vehicles with a size of 136 nm and a high encapsulation efficacy of 95%. In vitro release studies revealed that curcumin nanoparticles would be stable against hydrolysis by gastric and intestinal enzymes, allowing for relatively large amounts of the active component to reach the colon. Additionally, due to the improved cellular uptake, curcumin nanoparticles demonstrated more efficacy against colon cancer than free curcumin [31]. Table I summarizes the information on some passively targeted nanomedicines that were applied for CRC therapy.
Actively Targeted Nanoparticles
Active targeting, in conjunction with EPR-based passive targeting, is a strategy that can be utilized to increase the proportion of nanoparticles that are taken up selectively by the tumor tissue [25]. Employing a method involving the interaction of ligands and receptors, ligand-modified nanoparticles can actively accumulate in the tumor and deliver the medicine to the target cancer cells (Fig. 3) [32]. Cancer cells excessively express receptors and create paracrine or autocrine substances that promote cancer cell and tissue growth. Proteins, monoclonal antibodies, folic acid, aptamers, and tiny targeting ligands on nanocarriers are all examples of ligands that can bind specifically to the tumor cells exploiting the differentially expressed biomarkers that set them separate from the healthy cells [33, 34]. In recent years, the receptor-ligand binding strategy has formed the backbone of targeted delivery systems for CRC based on the idea of active targeting design. This strategy involves a wide variety of receptors that are highly expressed in CRC, including those for transferrin, folate, EGFR, CD44, epithelial cell adhesion molecule (EpCAM), mannose, and hyaluronic acid [35,36,37,38]. In conclusion, ligand-modified nanoparticles have the ability to concentrate in tumors through the process of passive targeting before penetrating tumor cells through the process of active targeting. This results in a therapeutic impact that is both more selective and more effective. In order to achieve accurate targeting, there is a need that the target is expressed in a sufficient amount. In an ideal scenario, its level should be linked to malignant actions, such as medicine resistance or cell growth, so that it can target potentially harmful tumors in a timely manner. Targeting can also make operations that help to distribute drugs more uncomplicated [39] Table II shows the different nanoparticles that can actively target CRC. The ligand linked to nanoparticles helped the chemotherapeutics agent to internalize the cells even though the nanoparticles were large in size and reached 300 nm. The nanoparticles used in that targeting were metallic nanoparticles such as gold nanoparticles, polymeric nanoparticles, and lipid nanoparticles. Most of the nanoformulations were converted from water-insoluble to free-soluble nanoparticles as an IV route of administration.
The efficiency of targeted nanocarriers is hinged on the specific ligand that is attached to them, as well as the overexpression of certain biomolecules that are present in the tumor. Active targeting, which is a more sophisticated approach, offers a higher degree of selectivity and versatility when compared to passive targeting. Passive targeting, on the other hand, takes advantage of the abnormal vasculature that is present in cancerous tissues. It is worth noting that there are notable differences between active and passive targeting approaches, and these differences are summarized in Table III.
Chitosan Polymeric Nanoparticles
Polymeric nanoparticles (PNPs) have a greater capacity with various characteristics for successful pharmaceutical delivery and therapeutic targeting. Polymeric nanoparticles are easy to synthesize, low-cost, biocompatible, biodegradable, environmentally friendly, non-immunogenic, and soluble in water [57]. PNPs may be nanospheres or nanocapsules [58]. To maximize the efficiency, producing PNPs involving the use of a natural polymer that blocks the P-glycoprotein efflux pump has been considered [59]. Chitosan is a linear polysaccharide that has a semi-crystalline structure. Chitosan is not found naturally in high concentrations but is easily produced by removing the acetyl group from the natural polymer chitin. Because of its unique biological and physicochemical qualities. The features of chitosan nanoparticles, such as their small size, enhanced stability, inexpensive price, simple production procedure, fewer side effects, and numerous administration modalities, made them ideal as pharmaceutical and gene carriers. These features have been incorporated into newer drug delivery systems that may be useful in the therapy of CRC [46, 60,61,62]. The cargo drugs are easily taken up into host cells owing to the cationic properties, electrostatic features, and biodegradability of chitosan nanoparticles [63]. In addition, some recent studies pointed to the intrinsic anticancer properties of chitosan or its capability of targeting certain tissues in vivo [64, 65].
In research, biocompatibility and biodegradability are two benefits of natural polymers like chitosan and chondroitin sulfate together with folic acid (FA) as conjugates. An example was bortezomib/chondroitin sulfate/chitosan-folic acid nanoparticles (Bor/Cs/Chs-FA). The average particle size of Bor/Cs/Chs-FA was 196.5 ± 1.2 nm, and the encapsulation efficiency was 21.4%. Meanwhile, the nanoparticles had a positive zeta potential of 28 mV. Bortezomib (Bor) was incorporated into self-assembled nanoparticles comprised of such two natural polymers and decorated to target the folate receptors, membrane-bound proteins, that are either undetected in normal cells or expressed at low levels, but are highly upregulated in many types of tumors, including CRC. The developed system was able to efficiently deliver Bor to cancerous cells, where it could decrease cell proliferation, induce cell death, and stimulate the immune cells [36]. In another study, researchers used oxaliplatin-loaded chitosan nanoparticles, referred to as chitosan-nanoparticles-oxaliplatin (CTNPOP), which are decorated with hyaluronic acid (HA) and encased into Eudragit S100 pellets. When comparing HA-coupled CTNPOP and uncoupled CTNPOP formulations, the average particle size was 152 ± 5.2 nm and 136 ± 6.0 nm, respectively. The drug entrapment efficiency of the former was 40% ± 3.9%, while that of the latter was 44% ± 4.2%. A significant difference was seen in the zeta potential, where CTNPOP showed a zeta potential of 40.3 ± 1.4 mV, whereas that of HA-CTNPOP was only 10.0 ± 0.5 mV [45, 66]. Folate-based targeting has been extensively coupled to the use of chitosan nanoparticles that target CRC [67]. For example, 5-fluorouracil (5-FU)-loaded chitosan (CS) nanoparticles coupled with folic acid (FA) were developed and tested for drug release and toxicity. Chitosan-5-fluorouracil nanoparticles (CS-5FU-NPs) measured 208 ± 15 nm in size, while FA-CS-5FU-NPs measured 235 ± 12 nm. The encapsulation efficiency of both formulations ranged between 55 and 59%. The result showed that the FA-modified nanoparticles had a significantly higher cytotoxicity than the unmodified chitosan nanoparticles [46]. In another study, the chemotherapeutic drug, doxorubicin (DOX), has been loaded into chitosan-modified nanoparticles. DOX induces programmed cell death, but it may cause severe adverse effects, such as mucous inflammation and irreversible cardiac arrhythmia, due to its lack of selectivity for cancer cells [68, 69]. Mesoporous silica nanoparticles (MSNs) were loaded with doxorubicin, and chitosan was used to coat the surface of MSNs. Chitosan’s role was to enhance the binding of aptamer and anti-microRNA-21 (anti-miR-21) to the nanoparticles’ surface. MSNs were found to have an average particle size of 87 ± 6 nm, with an encapsulation efficiency of 87.5%. The zeta potential of silica nanoparticles loaded with (DOX) was 16 mV and DOX-loaded silica nanoparticles included a loading concentration of 1% [50].
Compared to other nanoparticle systems, chitosan nanoparticles offer several advantages. Polymeric nanoparticles, such as poly(lactic-co-glycolic acid) (PLGA), have been extensively studied for drug delivery. However, they suffer from poor stability and rapid clearance from the body. Lipid-based nanoparticles, such as liposomes, have also been explored for CRC drug delivery. While they offer excellent biocompatibility, they are difficult to scale up and vulnerable to oxidation [70]. Polymeric and lipid-based delivery systems have shown promise in the field of CRC treatment. Polymeric delivery systems, such as chitosan nanoparticles, offer the advantage of being biocompatible and biodegradable, thus minimizing potential toxicity concerns [71, 72]. Lipid-based delivery systems, on the other hand, often provide enhanced drug solubility and stability. However, compared to lipid-based delivery systems, chitosan nanoparticles exhibit several unique properties that make them particularly well suited for CRC therapy [73, 74]. Chitosan nanoparticles have a high surface charge and mucoadhesive properties, allowing for efficient targeting and penetration of the colorectal mucosa. This enables the nanoparticles to directly deliver therapeutic agents to the site of the tumor, increasing drug accumulation, and reducing off-target effects. Furthermore, chitosan nanoparticles have the ability to encapsulate a wide range of therapeutic agents, including small molecules, proteins, and nucleic acids. This versatility makes them suitable for personalized medicine approaches in CRC treatment [75]. Chitosan nanoparticles offer superior stability and biocompatibility compared to PLGA nanoparticles. Their surface can be easily modified to enhance their cellular uptake and targeting efficiency. Furthermore, chitosan nanoparticles are highly versatile and can deliver a wide range of drugs, including hydrophobic and hydrophilic compounds [76]. Chitosan nanoparticles merge the advantages of several delivery systems. They are polymeric in nature, allowing for excellent stability and controlled drug release, but they are also biocompatible and show an enhanced cellular uptake, similar to that of lipid-based systems. Furthermore, chitosan nanoparticles are highly versatile and can be easily modified with targeting moieties, enhancing their ability to specifically target CRC cells [77]. In summary, our review of the literature views chitosan nanoparticles as a highly promising polymeric delivery system for CRC treatment that offers unique advantages over other nanodelivery systems such as biocompatibility, targeted delivery, and versatility in encapsulating various therapeutic agents.
Lipid Nanoparticles (LNPs)
In the field of nanomedicine, liposomes were the pioneering nanoparticle platform. Research interest in phospholipid membrane systems has a long history that precedes nanotechnology because of the lipid bilayer’s key role in the cell membrane structure [78]. As drug delivery vehicles, liposomes offer various advantageous qualities. First, both hydrophilic and hydrophobic substances can be carried by them. Liposomes can encapsulate hydrophilic medicines owing to their hydrophilic core [79]. On the other hand, hydrophobic substances can be loaded into the lipid shells. When a therapeutic cargo is administered in a liposomal form, the distribution in the body and pharmacokinetics of the cargo is modified. Such actions can boost the therapeutic efficacy and minimize toxicity. In addition, treatments that need to avoid the lysosomal pathway, such as nucleic acids, are ideally loaded into liposomes [80]. Thirty treatments utilizing liposomes or lipid nanoparticles are now undergoing clinical trials. The study of ligand-targeted liposomes has grown rapidly, and numerous investigations have given insight into some of the aspects responsible for the success and failure of ligand-targeted versus passively targeted liposomes. The reader is directed to a variety of articles that provide extensive coverage of this topic for further reading [81,82,83,84,85].
Recent research showed that urotensin II (UT II) is more effective at constricting blood vessels, outperforming endothelin-1, noradrenalin, and serotonin [86, 87]. Numerous research focused on UT II and urotensin II receptor (UTR) and how they play a part in human cancers [88]. Specifically, UTR was overexpressed in the colon cancer cells. Increasing the specificity and effectiveness of drug delivery systems is possible by using ligands conjugated to the liposome surface, which can bind to such overexpressed receptors on CRC cells [89]. A representative study in this direction compared doxorubicin-loaded LNPs (Lipo-dox) and UT-targeted LNPs (LipoUTdox), which had an average particle size of 148.9 ± 8.27 nm and 190.9 ± 17.42 nm, while their encapsulation efficiencies were 90% and 96%, respectively [41]. In another study, hyaluronic acid (HA) that targets the overexpressed CD44 receptors in CRC was used as a targeting moiety. In that study, liposomes coated with HA were recruited for the targeted delivery of 5-FU to CRC [55]. While both the unmodified and HA-modified formulations showed comparable particle sizes, the HA-modified formulation showed a higher apoptosis induction in CRC cells [55].
Gold Nanoparticles
Gold nanoparticles (AuNPs) have been used in several areas of biotechnology drug delivery such as diagnostics, biosensing, bio-imaging, and therapeutic administration because of their desirable features. Medications coupled with AuNPs have shown significant increases in therapeutic efficacy and delivery efficacy in various cancer types, including CRC, breast cancer, and prostate cancer [90,91,92,93,94]. Recent studies demonstrated that AuNPs can increase the tumor vessel perfusion and oxygen supply to achieve optimal clinical outcomes. According to the research, approximately 60–80% of CRC tumors have a poor prognosis when they are overexpressing the epidermal growth factor receptor (EGFR) [42, 95]. Cetuximab and panitumumab as anti-EGFR monoclonal antibodies have an important role in treating advanced CRC. Researchers believe that attaching cetuximab to AuNPs can increase the cytotoxicity towards cancer cells and cause a shift in the expression of the associated markers on the surface of cancer cells. Enhanced EGFR endocytosis and decreased downstream signaling pathway in cetuximab-AuNPs led to a reduction in cell proliferation in comparison to either AuNPs or cetuximab alone [49, 96,97,98].
Cyclodextrin-Based Nanoparticles
Cyclodextrins have served as excellent bases for nanostructured materials, leading to new cancer therapy alternatives [99]. Recent studies have shown that vascular endothelial growth factor (VEGF)–targeting biological medications like bevacizumab, aflibercept, and regorafenib (RG) improve CRC patients’ responses to therapy [100, 101]. RG is a multikinase inhibitor that blocks many kinases involved in angiogenesis, which has major benefits for slowing the development of CRC [102, 103]. RG has poor drug characteristics, which diminish the benefits of therapy and worsen adverse consequences [104, 105]. The mannose receptor, also known as MR, is a type of endocytic receptor that is very efficient and has the function of promoting the process by which cells take in and transport mannose-terminated particles or nanoparticles. Due to the fact that both cells with CRC and colonic macrophages overexpress MR on their surfaces, mannose alteration can be included in the development of CRC-specific nanotechnology as an effective dual-targeting approach [106, 107]. This could be accomplished by adding mannose to cyclodextrins, which resulted in the formation of a modified host known as mannose cyclodextrins (M-CD). To create the interlocked molecule RG-M-CD, the M-CD molecule encapsulates RG within its cavity so that the molecular recognition motifs can recognize it. The average size of RG-M-CD was 100 nm. As a tailored nanomedicine, RG-M-CD NPs achieved the most effective inhibition of malignancy both in vitro and in vivo. In contrast, RG-CD NPs that lacked mannose alteration exhibited a relatively weaker inhibition than RG-M-CD NPs [37].
Mesoporous Silica Nanoparticles (MSNs)
Substantial new drug delivery approaches have focused on mesoporous silica nanoparticles (MSNs), which have attractive qualities such as large surface areas, varied sizes, flexible alteration of surface chemistry, and facile production. MSNs have been the topic of substantial research in the discipline of treatment for cancer [108,109,110,111]. MSNs also possess other useful characteristics, such as the ability to alter the size of their cavities between the ranges of 50–300 and 2–6 nm, respectively [112]. In the research, as an overexpressed receptor on CRC cells, EGFR is strongly correlated with a poor prognosis [86]. Cetuximab (CTX), a monoclonal antibody (mAb) that preferentially binds to EGFR, has suppressed EGFR-associated tumor development and progression. In addition, when CTX is administered alongside chemotherapy medications such as 5-FU, established tumors can be eliminated while also preventing the growth of new cancer cells due to a synergistic antitumor effect [113,114,115]. The water-soluble chemotherapeutic drug 5-FU was incorporated into nanoparticles to create MSN/ 5-FU. SLB-MSN/5-FU was successfully formed by encapsulating 5-FU-loaded MSN within PEGylated supported lipid bilayers (SLB). Ultimately, CTX was coupled to nanoparticles to produce CTX-SLB-MSN/5-FU. the encapsulation efficiency was 37.67%. The active targeting effect for tumor-specific recognition of targeted nanoplatforms was provided by the specific interaction between EGFR and CTX, according to in vitro and in vivo research [56].
Methoxy Poly(ethylene glycol)-poly(ɛ-caprolactone) (MPEG-PCL) Nanoparticles
Methoxy poly(ethylene glycol)-poly(ɛ-caprolactone) (MPEG-PCL) is a block copolymer that is widely used in the preparation of polymeric nanoparticles. The hydrophobic PCL segments of the copolymer can naturally combine into a core that surrounds an insoluble water drug when the polymers are in aqueous solutions [116, 117]. MPEG-PCL copolymers loaded with hydrophobic medicines can maintain drug release and enhance medication bioavailability [118, 119]. In addition, the biodegradability and nanoscale characteristics of amphiphilic MPEG-PCL micelles make them suitable drug carriers for systemic administration. In previous research, FA/NanoCur micelles were produced by trapping curcumin (Cur) in the hydrophobic core of a self-assembled FA/MPEG-PCL micelle. This was done to investigate the effects of curcumin on FA/NanoCur micelles. It was expected that FA/NanoCur would be capable of targeting FA receptors that are present in tumor cells, hence increasing the tumor-specific administration of medicine. FA/NanoCur micelle particle size was determined to be 30.47 nm. Moreover, the drug encapsulation efficiency was determined to be 98%. Both in vitro and in vivo experiments showed that FA/NanoCur and NanoCur triggered significantly more cell death at a given dose than Free Cur. Moreover. The FA alteration significantly raised the cytotoxicity of NanoCur, as evidenced by a greater number of apoptotic cells in the FA/NanoCur treatment group relative to the NanoCur treated group [32].
Beyond Classic Therapeutics: Nanomedicines Delivering Biotherapeutics to CRC
In the previous sections, we discussed representative examples of nanomedicines that are used to deliver conventional drugs to CRC. In the present section, we attempted to shed light on the applications of nanomedicines in delivering biotherapeutics to CRC as emerging technologies for cancer treatment. Two representative technologies are highlighted; namely gene therapy and immunotherapy.
Nanomedicines Enabling the Gene Therapy of CRC
It has been well recognized that carcinogenesis is a complex process that involves the recruitment of multiple cellular signaling pathways. The recent progress in molecular studies has enabled the detection of several differentially expressed genes, either upregulated or downregulated, in association with oncogenesis [120]. Moreover, several mutations have been detected in a wide diversity of genes in the cancerous cells compared to the normal adjacent tissues. Subsequently, the concept of gene therapy has been coined to correct such mutations, knock down the overexpressed oncogenes, or replace the missing downregulated tumor suppressor genes [121, 122]. Gene therapeutic modalities include gene introduction tools (plasmid DNA, pDNA, or messenger RNA, mRNA), gene silencing tools (e.g., antisense oligonucleotides, ASO, small interfering RNA, siRNA, small hairpin RNA, shRNA, and micro RNA, miRNA), or genome-editing tools (e.g., clustered regularly interspaced short palindromic repeats, CRISPR, and their associated protein, Cas9). The aforementioned modalities and general strategies have been reviewed in previous works [11, 123, 124]. In the present subsection, we focus on those applicable to CRC treatment.
Tumor necrosis factor–related apoptosis–inducing ligand (TRAIL) is a natural tumor suppressor, produced by the natural killer (NK) cells, which induces apoptosis in faulty or precancerous cells via binding to death receptors 4 (DR4) or death receptors 5 (DR5) in the target cells to recruit caspase-3 and caspase-8 [125]. Previous reports pointed to the downregulation of the TRAIL gene in a wide range of cancers, suggesting that its replacement would provide a therapeutic benefit to patients [126]. Nevertheless, the administration of TRAIL as a recombinant protein failed to show a significant outcome in phase II clinical trials and resulted in undesired systemic toxicities [127]. Pishava and coworkers investigated the delivery of TRAIL in the form of pDNA to CRC both in vitro and in vivo utilizing polymeric nanoparticles based on the branched polymer, polyamidoamine dendrimer (PAMAM), that was modified with cholesteryl chloroformate and alkyl-PEG chains. The developed nanoparticles enabled the codelivery of TRAIL pDNA and doxorubicin efficiently to CRC cells and significantly inhibited the growth of the C26 colon carcinoma tumor model in mice [128].
Resistance to chemotherapy, known as chemoresistance, is one of the major obstacles that encounter efficient cancer treatment. The discovery of some genes that are linked to chemoresistance has opened a window for gene therapy that can overcome chemoresistance and promote anticancer chemotherapy. Ju et al. [129] delivered shRNA against the overexpressed glucose-6-phosphate dehydrogenase (G6PD) gene in CRC using poly (amino-co-ester) polyplexes. G6PD knockdown disrupted the redox homeostasis in CRC cells and increased the sensitivity to the cytotoxic drug, oxaliplatin, in both cell culture–derived and patient-derived xenografts [129].
LNPs have shown a great potential for mRNA delivery in vivo [130]. The combination of LNPs and other nanotechnologies can maximize their gene delivery capacity. In a recent study, Gao et al. [131] designed a hybrid multifunctional nanosystem called DMP-039, for the delivery of the suicide mRNA, Bcl-2-like 11 (BIM), to CRC. BIM has the ability to induce mitochondrial apoptosis (i.e., suicide) in cancer cells through the inhibition of Bcl-2 activity and activation of BAX-BAK1 proteins. DMP-039 was fabricated by self-assembling the cationic lipid, 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), and the biodegradable copolymer, methoxypoly(ethylene glycol)poly(ε-caprolactone) (mPEG-PCL). The hybrid nanosystem was functionalized with the cell-penetrating peptide, cRGD-R9, to promote its cellular uptake and mRNA delivery capacity. mRNA-loaded DMP-039 inhibited the growth of either the local C26 tumor model or its associated pulmonary metastasis in mice [131].
Nanomedicines as Anti-CRC Vaccines and Immunotherapies
Recently, there has been a growing interest in boosting the immune system to fight cancer as an alternative approach to the classic therapeutic approaches that rely on external interventions. This can minimize the hazards associated with conventional chemotherapy or radiotherapy. Success stories in this area can be seen in the efforts of Ugur Sahin, a cofounder of BioNTech®, which involved either developing anticancer vaccines on the prophylactic level or immunotherapies to cope with the existing tumors [132,133,134,135]. These achievements have also established the infrastructure that led to the evolution of COVID-19 mRNA vaccines during the last COVID-19 pandemic [13]. Furthermore, the discovery of immune check point pathways, programmed death molecule-1 and its ligand (PD1/PDL1), and cytotoxic T-lymphocyte antigen-4 (CTLA-4), by which cancer cells evade immune surveillance, has enabled the development of immune check point inhibitors, the achievement that was crowned by the Nobel prize in physiology or medicine for Tasuku Honjo and James Allison in 2018 [136]. Further details on anticancer immunotherapy have been reviewed elsewhere [137, 138]. Herein, representative examples of immunotherapies for CRC which rely on nanoparticles as a delivery platform are highlighted.
Ni and colleagues [139] developed a bi-adjuvant neoantigen nanovaccine (banNV) for the immunotherapy of CRC. A self-assembled delivery system based on maleimide-functionalized poly (ethylene oxide)-block-poly (d,l-lactic acid) (MAL-PEG-b-PLA) micelles was prepared and loaded with a peptide neoantigen, Adpgk, and two adjuvants, R848 and CpG, that target Toll-like receptors (TLR) 7/8 and TLR9, respectively. The developed nanovaccine demonstrated a high immunostimulation and sensitized PD1 receptors on T cells for synergistic immunotherapy together with an immune checkpoint inhibiting therapy, anti-PD1, in mice [139].
Cheng et al. [140] engineered bacteria-derived outer membrane vesicles (OMVs) to display multiple neoantigens through protein catchers on their surface. The developed nanovesicles demonstrated excellent biocompatibility and synergistic anticancer immune responses against CRC in vivo.
Moreover, tumor-triggered inorganic nanoparticles can be invested to induce anticancer immune response. Chang et al. [141] designed a core-shell Cu2O@CaCO3 nanoparticles that can be triggered by the pH microenvironment in CRC to decompose the CaCO3 shell and release Cu2O, which subsequently reacts with the endogenous H2S in the tumor microenvironment to generate Cu31S16 nanocrystals with a strong optical properties. Thanks to its strong absorption of near-infrared (NIR) radiation and intratumoral release of calcium, the developed system can be applied for multimodal therapy of CRC via photothermal/photodynamic/chemodynamic/calcium overload. In addition, the excessive generation of hyperthermia and oxidative stress reprogrammed the tumor-associated macrophages from the protumorous M2 phenotype into the antitumorus M1 phenotype, which subsequently elicits anticancer immune response [141].
Discussion
The research and development of anticancer nanotechnology over the past two decades have resulted in hundreds of articles and various therapeutic formulations that have shown potential for the treatment of solid tumors and hematological cancers. The enhanced permeability and retention (EPR) effect has emerged as a critical factor in the development of anticancer nanomedicines. This impact is most prominent when applied to solid tumors. Researchers have shown that nanoparticles between 10 and 100 nm in size have a greater chance of being taken up by tumors during their prolonged circulation time. The kidneys filter out particles as small as 10 nm and the liver takes up those over 100 nm. Nevertheless, a significant amount of work still needs to be conducted to achieve a full understanding of nanoparticle size selection and determine the optimal size of nanoparticles. Researchers delivered nanoparticles that target CRC by active targeting in the same process to develop nanotechnology that targets CRC. This is accomplished by first concentrating on tumors via passive targeting before entering tumor cells through active targeting.
Active targeting with the assistance of cancer cells that excessively express certain types of receptors and produce chemicals that can encourage the development of cancer cells in the body. In the past few years, the ligand-receptor approach has formed the basis of the active targeting for nanotechnology-targeted drug delivery systems for CRC. This is due to the fact that this strategy allows receptors to bind to their ligands. This approach uses a wide number of receptors, many of which are highly expressed in CRC, including the transferrin, folate, EGFR, CD44, EpCAM, mannose, and hyaluronic acid receptors. Nanoparticles aimed at folate receptors and EGFR were the subject of additional research, and attention was directed to them. Recent scientific literature suggests that active targeting results in fewer harmful effects on healthy tissues due to the differential expression of the target receptors on cancerous cells. Chitosan-folate-hesperetin nanoparticles (CFH) were produced by Mary Lazer and her colleagues [52] by covalently conjugating folic acid molecules with chitosan molecules. These nanoparticles had an average particle size of 457 nm and utilized the EPR effect due to the gaps that are present in tumor blood vessels. In addition, they used ligand-based targeting to enhance the selectivity and efficacy of nanoparticle accumulation in the tumor cells. The authors found that the CFH efficacy in inhibiting CRC growth is better with ligand modification compared to the unmodified nanocarriers.
As alluded to above, the biopolymer with the second highest level of acceptability, after cellulose, is chitosan. Chitosan is derived from shellfish. It is highly abundant and its yearly output is greater than one hundred million tons. Among the polymeric nanoparticles, chitosan stands out as a highly effective, economically viable, and environment-friendly nanocarrier source. The development of chitosan nanoparticles has attracted a lot of attention thanks to their characteristic profile. Combining the beneficial properties of polymers and nanotechnology is seen as a promising technique for improving the stability and bioavailability of numerous active substances, and chitosan nanoparticles are at the forefront of this movement. Together with a member of his team in a study [62], Herdiana was able to discover and explain how the encapsulated medicine with chitosan enhances the inhibition of tumor growth in colon cancer. According to our current understanding, chitosan nanoparticles induce the programmed death of cancerous cells, the process that is called apoptosis. They accomplish this effect by altering the cellular metabolism and inhibiting tumor-derived cellular growth. Previous research on chitosan nanoparticles has demonstrated that they are likely to be removed from the systemic circulation through macrophages. The highly positive surface charge of chitosan nanoparticles adsorbs plasma proteins to them, which subsequently allows macrophages to recognize and ingest them. Additionally, chitosan nanoparticles have shown promise in the oral route of drug delivery. Furthermore, research has been conducted on ligand-modified chitosan nanoparticles for an active approach. The interaction between chitosan and colon mucosa is crucial for the successful accumulation of drugs into colon cancer cells. Chitosan’s positive surface charge may accelerate the drug accumulation process. Although the interaction is complex and challenging to predict, there is still hope for a better understanding in the future. Nanoparticles based on chitosan and its derivatives have been proven to be effective in improving the stability of drugs and are aimed at treating CRC when administered orally, thanks to their physicochemical properties. Excitingly, in the upcoming few years, more research will be aimed at improving chitosan characteristics through physical and chemical modifications. This will ultimately lead to an increased specific drug accumulation within CRC cells. Looking at the bright side, we can ensure their long-term safety and effectiveness in anticancer treatment by studying the toxicity and immunogenicity of chitosan nanocarriers toward normal cell lines. Even though polymers are often biodegradable and promise the simple removal of their oligomers via widespread metabolic pathways, the clinical use of polymeric nanoparticles still needs to be strictly regulated because of the potential toxicity of their constituents. The stability of nanoparticles is substantially dependent on their charge. The zeta potential is a crucial property that significantly impacts the efficacy of nanomedicine. Maintaining good physicochemical stability of nanoparticles is contingent upon the zeta potential (ZP) being greater than 10 mV. This is due to the fact that large repulsive forces serve as an obstacle to aggregation. In 2022, Shafi Ullah and colleagues successfully encapsulated 5-FU within chitosan nanoparticles which were conjugated to folic acid, producing a folic acid–chitosan conjugate (FA-CS-5FU). The size, zeta potential, encapsulation efficiency, and drug-loading efficiency of the prepared nanoparticles fall within acceptable ranges. The developed nanoparticles showed high efficiency owing to merging the characteristics of folic acid–based targeting and chitosan nanoparticle technologies. The results also revealed the high stability of such a delivery system [46]. Collectively, chitosan nanoparticles possess multiple attractive features that make them promising for wide applications in targeted drug delivery to CRC.
Despite the promising results obtained with nanoparticles, there are still some research gaps that need to be addressed. First, there is a need for standardized protocols and guidelines for characterizing nanoparticles used in CRC treatment. This will ensure consistency and reliability in nanoparticle formulation and characterization, facilitating the comparison of different studies and improving reproducibility [15]. Second, while targeted nanoparticles have shown the ability to accumulate in solid tumors, further research is needed to identify the most effective targeting strategies for CRC. This includes determining the optimal ligands or antibodies that can specifically recognize and bind to CRC cells, as well as understanding the factors that influence nanoparticle uptake and internalization by tumor cells [142]. Furthermore, to improve the targeting capabilities of nanoparticles, it is crucial to identify which specific biomarkers are overexpressed in CRC cells [143]. This can help in the development of targeted nanoparticles that can selectively bind to these biomarkers and deliver therapeutic agents specifically to the cancer cells. Third, although nanoparticles can efficiently deliver drugs to tumor cells, there is a need for further research to optimize the release kinetics of the drugs from the nanoparticles. This includes studying the factors that influence drug release, such as the composition and structure of the nanoparticles, as well as the physiological conditions at the tumor site [144]. Fourth, while targeted nanoparticles have the potential to reduce systemic side effects by delivering drugs specifically to tumor cells, more research is needed to evaluate the potential systemic side effects of targeted nanoparticles in CRC treatment [145]. It is not yet clear how targeted nanoparticles interact with the immune system and whether they can trigger an immune response. It is also not clear how the size, shape, and surface properties of targeted nanoparticles affect their bioreactivity in vivo [146]. In addition, targeted nanoparticles have the potential to be used in combination with other treatment modalities, such as chemotherapy, radiation therapy, and immunotherapy. Further research is needed to investigate the potential synergistic effects of combining targeted nanoparticles with other therapies for CRC. Additional research is required to understand the long-term effects of targeted nanoparticles on normal cells and tissues, as well as potential immune responses and toxicity profiles. While preclinical research has shown promising results in preclinical studies, it is essential to evaluate the efficacy and clinical outcomes of targeted nanoparticle-based treatments in human clinical trials. This will help in determining the effectiveness of these treatments in real scenarios and providing valuable information on patient responses, survival rates, and potential adverse effects. Eventually, CRC is a complex disease with significant inter-tumoral and intra-tumoral heterogeneity. Further research is needed for a better understanding of the heterogeneity of CRC and how it may impact the effectiveness of targeted nanoparticle-based treatments [147]. Personalized medicine aims to tailor treatment strategies based on the individual’s unique characteristics, including genetic profile and tumor molecular markers. Further research is needed to determine how personalized approaches can be integrated with targeted nanoparticle-based treatments for CRC.
Concluding Remarks and Future Outlook
Therapeutic substances can be transported to a target area of the body via nanocarrier-based delivery systems, which have proven to be more effective than free drugs. The use of polymeric nanocarriers encapsulating therapeutic drugs with or without targeting ligands offers considerable potential for the creation of colon-targeted drug delivery systems that are safe and require minimal monitoring of patients. CRC-specific microenvironment has significant implications on the development of nanoparticles with a high capacity for targeting; thus, active targeting strategies have a better chance for tumor-specific delivery of therapeutic cargos. Although actively targeted nanopreparations have shown promise in basic research, their translation into clinics has been slow due to factors such as preparation instability and discrepancies between animal and human CRC. The sophisticated nature of several reported nanomedicines complicates the process of scale-up in industry. In addition, some laboratory-based preparation methods of nanoparticles are multi-step, tedious, and time-consuming, which collectively increase the production cost and implicate the uniformity of the end product upon preparation at a large scale. To boost the clinical translatability of anticancer nanomedicines, the composition of the developed systems should be simplified, and scalable one-step preparation methods such as microfluidic devices should be adopted instead of the traditional preparation methods. Moreover, more representative experimental and patient-derived animal models should be recruited for improved clinical relevance [148]. Furthermore, the significant benefits of some biopolymers such as chitosan, including its mucoadhesive properties, non-toxic nature, facile modifiability, ability to form complexes with proteins or DNA, biodegradability, and cost-effectiveness, have led to the ongoing development of these advantageous carriers for efficient drug delivery to colon cancer. In conclusion, actively targeted nanomedicines can offer promising non-classic therapies for CRC. However, additional research is required to address the challenges that limit their clinical translation and expand their applicability to other types of tumors.
References
Douaiher J, Ravipati A, Grams B, Chowdhury S, Alatise O, Are C. Colorectal cancer—global burden, trends, and geographical variations. J Surg Oncol. 2017;115:619–30.
Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Transl Med. 2019;7:609–609.
Yang C, Merlin D. Lipid-based drug delivery nanoplatforms for colorectal cancer therapy. Nanomaterials. MDPI AG; 2020. p. 1–32.
Tian Q, Liu Y, Zhang Y, Song Z, Yang J, Zhang J, et al. THBS2 is a biomarker for AJCC stages and a strong prognostic indicator in colorectal cancer. JBUON. 2018;23:1331–6.
Bennedsgaard K, Ventzel L, Themistocleous AC, Bennett DL, Jensen AB, Jensen AR, et al. Long-term symptoms of polyneuropathy in breast and colorectal cancer patients treated with and without adjuvant chemotherapy. Cancer Med. 2020;9:5114–23.
Duran G, Cruz R, Simoes AR, Barros F, Giráldez JM, Bernárdez B, et al. Efficacy and toxicity of adjuvant chemotherapy on colorectal cancer patients: how much influence from the genetics? J Chemother. 2020;32:310–22.
Alomrani A, Badran M, Harisa GI, ALshehry M, Alhariri M, Alshamsan A, et al. The use of chitosan-coated flexible liposomes as a remarkable carrier to enhance the antitumor efficacy of 5-fluorouracil against colorectal cancer. Saudi Pharm J. 2019;27:603–11.
Son HS, Lee WY, Lee WS, Yun SH, Chun HK. Compliance and effective management of the hand-foot syndrome in colon cancer patients receiving capecitabine as adjuvant chemotherapy. Yonsei Med J. 2009;50:796–802.
Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–91.
Arumov A, Trabolsi A, Schatz JH. Potency meets precision in nano-optimized chemotherapeutics. Trends Biotechnol. 2021;39:974–7.
Younis MA, Khalil IA, Harashima H. Gene therapy for hepatocellular carcinoma: highlighting the journey from theory to clinical applications. Adv Ther. 2020;3:2000087.
Younis MA, Khalil IA, Elewa YHA, Kon Y, Harashima H. Ultra-small lipid nanoparticles encapsulating sorafenib and midkine-siRNA selectively-eradicate sorafenib-resistant hepatocellular carcinoma in vivo. J Control Release. 2021;331:335–49.
Abdellatif AAH, Younis MA, Alsowinea AF, Abdallah EM, Abdel-Bakky MS, Al-Subaiyel A, et al. Lipid nanoparticles technology in vaccines: shaping the future of prophylactic medicine. Colloids Surf B Biointerfaces. 2023;222: 113111.
Abdellatif AAH, Scagnetti G, Younis MA, Bouazzaoui A, Tawfeek HM, Aldosari BN, et al. Non-coding RNA-directed therapeutics in lung cancer: delivery technologies and clinical applications. Colloids Surf B Biointerfaces. 2023;229: 113466.
Younis MA, Tawfeek HM, Abdellatif AAH, Abdel-Aleem JA, Harashima H. Clinical translation of nanomedicines: challenges, opportunities, and keys. Adv Drug Deliv Rev. 2022;181: 114083.
Alzahrani S, Al Doghaither H, Al-Ghafari A. General insight into cancer: an overview of colorectal cancer (Review). Mol Clin Oncol. 2021;15:271.
Balchen V, Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967–76.
Tanaka T. Colorectal carcinogenesis: review of human and experimental animal studies. J Carcinog. 2009;8:5.
Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19–27.
Colorectal Cancer Early Detection, Diagnosis, and Staging [Internet]. Available from: https://seer.cancer.gov/csr/1975_2016/. Accessed 15 Nov 2023.
Krasteva N, Georgieva M. Promising therapeutic strategies for colorectal cancer treatment based on nanomaterials. Pharmaceutics. 2022;14:1213.
Pickhardt PJ, Pooler BD, Kim DH, Hassan C, Matkowskyj KA, Halberg RB. The natural history of colorectal polyps: overview of predictive static and dynamic features. Gastroenterol Clin North Am. W.B. Saunders; 2018. p. 515–36.
Kundu M, Chatterjee S, Ghosh N, Manna P, Das J, Sil PC. Tumor targeted delivery of umbelliferone via a smart mesoporous silica nanoparticles controlled-release drug delivery system for increased anticancer efficiency. Mater Sci Eng C. 2020;116:111239.
Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical q overview of the prototype polymeric drug SMANCS. J Control Release. 2001;74:47–61.
Shi Y, Shan S, Li C, Song X, Zhang C, Chen J, et al. Application of the tumor site recognizable and dual-responsive nanoparticles for combinational treatment of the drug-resistant colorectal cancer. Pharm Res. 2020;37:72.
Wang Y, Ma J, Qiu T, Tang M, Zhang X, Dong W. In vitro and in vivo combinatorial anticancer effects of oxaliplatin- and resveratrol-loaded N, O-carboxymethyl chitosan nanoparticles against colorectal cancer. Eur J Pharm Sci. 2021;163:105864.
Nichols JW, Bae YH. EPR: evidence and fallacy. J Control Release. 2014;190:451–64.
Golombek SK, May JN, Theek B, Appold L, Drude N, Kiessling F, et al. Tumor targeting via EPR: strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17–38.
Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63:170–83.
Anitha A, Maya S, Sivaram AJ, Mony U, Jayakumar R. Combinatorial nanomedicines for colon cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:151–9.
Udompornmongkol P, Chiang BH. Curcumin-loaded polymeric nanoparticles for enhanced anti-colorectal cancer applications. J Biomater Appl. 2015;30:537–46.
Hu Y, He Y, Ji J, Zheng S, Cheng Y. Tumor targeted curcumin delivery by folate-modified MPEG-PCL self-assembly micelles for colorectal cancer therapy. Int J Nanomedicine. 2020;15:1239–52.
Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12:137.
Yusefi M, Chan HY, Teow SY, Kia P, Lee-Kiun Soon M, Sidik NABC, et al. 5-fluorouracil encapsulated chitosan-cellulose fiber bionanocomposites: synthesis, characterization and in vitro analysis towards colorectal cancer cells. Nanomaterials. 2021;11:1691.
Ge P, Niu B, Wu Y, Xu W, Li M, Sun H, et al. Enhanced cancer therapy of celastrol in vitro and in vivo by smart dendrimers delivery with specificity and biosafety. Chem Eng J. 2020;383:123228.
Soe ZC, Poudel BK, Nguyen HT, Thapa RK, Ou W, Gautam M, et al. Folate-targeted nanostructured chitosan/chondroitin sulfate complex carriers for enhanced delivery of bortezomib to colorectal cancer cells. Asian J Pharm Sci. 2019;14:40–51.
Bai H, Wang J, Phan CU, Chen Q, Hu X, Shao G, et al. Cyclodextrin-based host-guest complexes loaded with regorafenib for colorectal cancer treatment. Nat Commun. 2021;12:759.
Pan DC, Krishnan V, Salinas AK, Kim J, Sun T, Ravid S, et al. Hyaluronic acid–doxorubicin nanoparticles for targeted treatment of colorectal cancer. Bioeng Transl Med. 2021;6:e10166.
Afzal M, Ameeduzzafar, Alharbi KS, Alruwaili NK, Al-Abassi FA, Al-Malki AAL, et al. Nanomedicine in treatment of breast cancer – a challenge to conventional therapy. Semin Cancer Biol. 2021;69:279-92.
Wang K, Shen R, Meng T, Hu F, Yuan H. Nano-drug delivery systems based on different targeting mechanisms in the targeted therapy of colorectal cancer. Molecules. 2022;27:2981.
Zappavigna S, Abate M, Cossu AM, Lusa S, Campani V, Scotti L, et al. Urotensin-II-targeted liposomes as a new drug delivery system towards prostate and colon cancer cells. J Oncol. 2019;2019:9293560.
El Hallal R, Lyu N, Wang Y. Effect of cetuximab-conjugated gold nanoparticles on the cytotoxicity and phenotypic evolution of colorectal cancer cells. Molecules. 2021;26:567.
Bhattacharya S. Anti-EGFR-mAb and 5-fluorouracil conjugated polymeric nanoparticles for colorectal cancer. Recent Pat Anticancer Drug Discov. 2020;16:84–100.
Wei Y, Gu X, Sun Y, Meng F, Storm G, Zhong Z. Transferrin-binding peptide functionalized polymersomes mediate targeted doxorubicin delivery to colorectal cancer in vivo. J Control Release. 2020;319:407–15.
Jain A, Jain SK, Ganesh N, Barve J, Beg AM. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomedicine. 2010;6:179–90.
Ullah S, Azad AK, Nawaz A, Shah KU, Iqbal M, Albadrani GM, et al. 5-Fluorouracil-loaded folic-acid-fabricated chitosan nanoparticles for site-targeted drug delivery cargo. Polymers (Basel). 2022;14:2010.
Lee KJ, Ko EJ, Park YY, Park SS, Ju EJ, Park J, et al. A novel nanoparticle-based theranostic agent targeting LRP-1 enhances the efficacy of neoadjuvant radiotherapy in colorectal cancer. Biomaterials. 2020;255:120151.
Ben Djemaa S, David S, Hervé-Aubert K, Falanga A, Galdiero S, Allard-Vannier E, et al. Formulation and in vitro evaluation of a siRNA delivery nanosystem decorated with gH625 peptide for triple negative breast cancer theranosis. Eur J Pharm Biopharm. 2018;131:99–108.
Leve F, Bonfim DP, Fontes G, Morgado-Díaz JA. Gold nanoparticles regulate tight junctions and improve cetuximab effect in colon cancer cells. Nanomedicine. 2019;14:1665–78.
Khatami F, Matin MM, Danesh NM, Bahrami AR, Abnous K, Taghdisi SM. Targeted delivery system using silica nanoparticles coated with chitosan and AS1411 for combination therapy of doxorubicin and antimiR-21. Carbohydr Polym. 2021;266:118111.
DuRoss AN, Landry MR, Thomas CR, Neufeld MJ, Sun C. Fucoidan-coated nanoparticles target radiation-induced P-selectin to enhance chemoradiotherapy in murine colorectal cancer. Cancer Lett. 2021;500:208–19.
Mary Lazer L, Sadhasivam B, Palaniyandi K, Muthuswamy T, Ramachandran I, Balakrishnan A, et al. Chitosan-based nano-formulation enhances the anticancer efficacy of hesperetin. Int J Biol Macromol. 2018;107:1988–98.
Bagheri E, Abnous K, Farzad SA, Taghdisi SM, Ramezani M, Alibolandi M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020;261:118369.
Xu M, Wen Y, Liu Y, Tan X, Chen X, Zhu X, et al. Hollow mesoporous ruthenium nanoparticles conjugated bispecific antibody for targeted anti-colorectal cancer response of combination therapy. Nanoscale. 2019;11:9661–78.
Mansoori B, Mohammadi A, Abedi-Gaballu F, Abbaspour S, Ghasabi M, Yekta R, et al. Hyaluronic acid-decorated liposomal nanoparticles for targeted delivery of 5-fluorouracil into HT-29 colorectal cancer cells. J Cell Physiol. 2020;235:6817–30.
Chen R, Huang Y, Wang L, Zhou J, Tan Y, Peng C, et al. Cetuximab functionalization strategy for combining active targeting and antimigration capacities of a hybrid composite nanoplatform applied to deliver 5-fluorouracil: toward colorectal cancer treatment. Biomater Sci. 2021;9:2279–94.
Jasmine MDC, Prabhu VV. Polymeric nanoparticles-the new face in Drug Delivery and Cancer Therapy. Malaya J Biosci. 2014;1:1–7.
Zielinska A, Carreiró F, Oliveira AM, Neves A, Pires B, Nagasamy Venkatesh D, et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25:3731.
Hoosain FG, Choonara YE, Tomar LK, Kumar P, Tyagi C, Du Toit LC, et al. Bypassing P-glycoprotein drug efflux mechanisms: possible applications in pharmacoresistant schizophrenia therapy. Biomed Res Int. 2015;2015:484963.
Zhang M, Kim YK, Cui P, Zhang J, Qiao J, He Y, et al. Folate-conjugated polyspermine for lung cancer–targeted gene therapy. Acta Pharm Sin B. 2016;6:336–43.
Ravishankar K, Dhamodharan R. Advances in chitosan-based hydrogels: evolution from covalently crosslinked systems to ionotropically crosslinked superabsorbents. React Funct Polym. 2020;149:104517.
Herdiana Y, Wathoni N, Shamsuddin S, Joni IM, Muchtaridi M. Chitosan-based nanoparticles of targeted drug delivery system in breast cancer treatment. Polymers (Basel). 2021;13:1717.
Shanmuganathan R, Edison TNJI, LewisOscar F, Kumar P, Shanmugam S, Pugazhendhi A. Chitosan nanopolymers: an overview of drug delivery against cancer. Int J Biol Macromol. 2019;130:727–36.
Tawfeek HM, Younis MA, Aldosari BN, Almurshedi AS, Abdelfattah A, Abdel-Aleem JA. Impact of the functional coating of silver nanoparticles on their in vivo performance and biosafety. Drug Dev Ind Pharm. 2023;49:349–56.
Abdellatif AAH, Abdelfattah A, Younis MA, Aldalaan SM, Tawfeek HM. Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells. Nanotechnol Rev. 2023;12:20220546.
Culy CR, Clemett D, Wiseman LR. Oxaliplatin A review of its pharmacological properties and clinical efficacy in metastatic colorectal cancer and its potential in other malignancies. Drugs. 2000;60:895–924.
Gaspar VM, Costa EC, Queiroz JA, Pichon C, Sousa F, Correia IJ. Folate-targeted multifunctional amino acid-chitosan nanoparticles for improved cancer therapy. Pharm Res. 2015;32:562–77.
Chen K, Cai H, Zhang H, Zhu H, Gu Z, Gong Q, et al. Stimuli-responsive polymer-doxorubicin conjugate: antitumor mechanism and potential as nano-prodrug. Acta Biomater. 2019;84:339–55.
Xia P, Chen J, Liu Y, Fletcher M, Jensen BC, Cheng Z. Doxorubicin induces cardiomyocyte apoptosis and atrophy through cyclin-dependent kinase 2-mediated activation of forkhead box O1. J Biol Chem. 2020;295:4265–76.
Yang F, Cabe M, Nowak HA, Langert KA. Chitosan/poly(lactic-co-glycolic)acid nanoparticle formulations with finely-tuned size distributions for enhanced mucoadhesion. Pharmaceutics. 2022;14:95.
Upadhyay J, Shah K. Implementation of factorial experimental design in chitosan - tripolyphosphate nanoparticles development by ionotropic gelation. Int J Health Sci (Qassim). 2022;6:8529–43.
Chaichanasak N, Rojanapanthu P, Yoon Y, Gritsanapan W, Chirachanchai S, Sathirakul K, et al. Chitosan-based nanoparticles with damnacanthal suppress CRM1 expression. Oncol Lett. 2018;16:7029–34.
Tang X, Zeng B, Gao J-K, Liu H-Q. Molecular mechanism of enhanced anticancer effect of nanoparticle formulated LY2835219 via p16-CDK4/6-pRb pathway in colorectal carcinoma cell line. J Nanomater. 2016;2016:2095878.
Orkhan F, Melike U, Cihan G, Faruk DO, Samet B, Ilknur U, Alemdar J. RBD and ACE2 embedded chitosan nanoparticles as a prevention approach for SARS-COV 2. Biomed J Sci Tech Res. 2021;37:29193–7.
Zhou T, Liu Y, Lei K, Liu J, Hu M, Guo L, et al. A “Trojan Horse” strategy: the preparation of bile acid-modifying irinotecan hydrochloride nanoliposomes for liver-targeted anticancer drug delivery system study. Molecules. 2023;28:1577.
Mikušová V, Mikuš P. Advances in chitosan-based nanoparticles for drug delivery. Int J Mol Sci. 2021;22:9652.
Aibani N, Rai R, Patel P, Cuddihy G, Wasan EK. Chitosan nanoparticles at the biological interface: implications for drug delivery. Pharmaceutics. 2021;13:1686.
Min Y, Caster JM, Eblan MJ, Wang AZ. Clinical translation of nanomedicine. Chem Rev. 2015;115:11147–90.
Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48.
Younis MA, Sato Y, Elewa YHA, Harashima H. Reprogramming activated hepatic stellate cells by siRNA-loaded nanocarriers reverses liver fibrosis in mice. J Control Release. 2023;361:592–603.
Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750–63.
Sawant RR, Torchilin VP. Challenges in development of targeted liposomal therapeutics. AAPS Journal. 2012;14:303–15.
Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161:175–87.
JØlck RI, Feldborg LN, Andersen S, Moghimi SM, Andresen TL. Engineering liposomes and nanoparticles for biological targeting. Adv Biochem Eng Biotechnol. 2011;125:251–80.
Sapra P, Tyagi P, Allen TM. Ligand-targeted liposomes for cancer treatment. Curr Drug Deliv. 2005;2:369–81.
Grieco P, Rovero P, Novellino E. Recent structure-activity studies of the peptide hormone urotensin-II, a potent vasoconstrictor. Curr Med Chem. 2004;11:969–79.
Maguire JJ, Davenport AP. Is urotensin-II the new endothelin? Br J Pharmacol. 2002;137:579–88.
Takahashi K, Totsune K, Murakami O, Shibahara S. Expression of urotensin II and urotensin II receptor mRNAs in various human tumor cell lines and secretion of urotensin II-like immunoreactivity by SW-13 adrenocortical carcinoma cells. Peptides. 2001;22:1175–9.
Federico A, Zappavigna S, Romano M, Grieco P, Luce A, Marra M, et al. Urotensin-II receptor is over-expressed in colon cancer cell lines and in colon carcinoma in humans. Eur J Clin Invest. 2014;44:285–94.
Banu H, Sethi DK, Edgar A, Sheriff A, Rayees N, Renuka N, et al. Doxorubicin loaded polymeric gold nanoparticles targeted to human folate receptor upon laser photothermal therapy potentiates chemotherapy in breast cancer cell lines. J Photochem Photobiol B. 2015;149:116–28.
Mackey MA, El-Sayed MA. Chemosensitization of cancer cells via gold nanoparticle-induced cell cycle regulation. Photochem Photobiol. 2014;90:306–12.
Cui L, Her S, Dunne M, Borst GR, De Souza R, Bristow RG, et al. Significant radiation enhancement effects by gold nanoparticles in combination with cisplatin in triple negative breast cancer cells and tumor xenografts. Radiat Res. 2017;187:147–60.
Zhao X, Pan J, Li W, Yang W, Qin L, Pan Y. Gold nanoparticles enhance cisplatin delivery and potentiate chemotherapy by decompressing colorectal cancer vessels. Int J Nanomedicine. 2018;13:6207–21.
Agabeigi R, Rasta SH, Rahmati-Yamchi M, Salehi R, Alizadeh E. Novel chemo-photothermal therapy in breast cancer using methotrexate-loaded folic acid conjugated Au@SiO2 nanoparticles. Nanoscale Res Lett. 2020;15:62.
Liu D, Sun J, Zhu J, Zhou H, Zhang X, Zhang Y. Expression and clinical significance of colorectal cancer stem cell marker EpCAMhigh/CD44+ in colorectal cancer. Oncol Lett. 2014;7:1544–8.
Qian Y, Qiu M, Wu Q, Tian Y, Zhang Y, Gu N, et al. Enhanced cytotoxic activity of cetuximab in EGFR-positive lung cancer by conjugating with gold nanoparticles. Sci Rep. 2014;4:7490.
Kao HW, Lin YY, Chen CC, Chi KH, Tien DC, Hsia CC, et al. Biological characterization of cetuximab-conjugated goldnanoparticles in a tumor animal model. Nanotechnology. 2014;25:295102.
Andrade LM, Martins EMN, Versiani AF, Reis DS, da Fonseca FG, Souza IP de, et al. The physicochemical and biological characterization of a 24-month-stored nanocomplex based on gold nanoparticles conjugated with cetuximab demonstrated long-term stability, EGFR affinity and cancer cell death due to apoptosis. Mater Sci Eng C. 2020;107:110203.
Uekama K, Hirayama F, Irie T. Cyclodextrin drug carrier systems. Chem Rev. 1998;98:2045–76.
Weng W, Feng J, Qin H, Ma Y. Molecular therapy of colorectal cancer: progress and future directions. Int J Cancer. 2015;136:493–502.
Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519–27.
Waddell T, Cunningham D. Evaluation of regorafenib in colorectal cancer and GIST. Lancet. 2013;381:273–5.
Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Effi cacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302.
Mir O, Brodowicz T, Italiano A, Wallet J, Blay JY, Bertucci F, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17:1732–42.
Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17:79–92.
Xiong M, Lei Q, You X, Gao T, Song X, Xia Y, et al. Mannosylated liposomes improve therapeutic effects of paclitaxel in colon cancer models. J Microencapsul. 2017;34:513–21.
Fan NJ, Chen HM, Song W, Zhang ZY, Zhang MD, Feng LY, et al. Macrophage mannose receptor 1 and S100A9 were identified as serum diagnostic biomarkers for colorectal cancer through a label-free quantitative proteomic analysis. Cancer Biomark. 2016;16:235–43.
García-Fernández A, Aznar E, Martínez-Máñez R, Sancenón F. New advances in in vivo applications of gated mesoporous silica as drug delivery nanocarriers. Small. 2020;16:e1902242.
Kankala RK, Han YH, Na J, Lee CH, Sun Z, Wang S Bin, et al. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv Mater. 2020;32:e1907035.
Wang Y, Huang HY, Yang L, Zhang Z, Ji H. Cetuximab-modified mesoporous silica nano-medicine specifically targets EGFR-mutant lung cancer and overcomes drug resistance. Sci Rep. 2016;6:25468.
Brar B, Ranjan K, Palria A, Kumar R, Ghosh M, Sihag S, et al. Nanotechnology in colorectal cancer for precision diagnosis and therapy. Front Nanotechnol. 2021;3:699266.
Stang J, Haynes M, Carson P, Moghaddam M. A preclinical system prototype for focused microwave thermal therapy of the breast. IEEE Trans Biomed Eng. 2012;59:2431–8.
Blick SKA, Scott LJ, Ciardiello F, Magrassi F, Lanzara A, Galizia G. Cetuximab: A review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer. Drugs. 2007;67:2585–607.
Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–57.
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45.
Danafar H, Sharafi A, Kheiri Manjili H, Andalib S. Sulforaphane delivery using mPEG–PCL co-polymer nanoparticles to breast cancer cells. Pharm Dev Technol. 2017;22:642–51.
Zamani M, Shirinzadeh A, Aghajanzadeh M, Andalib S, Danafar H. In vivo study of mPEG–PCL as a nanocarriers for anti-inflammatory drug delivery of simvastatin. Pharm Dev Technol. 2019;24:663–70.
Gou M, Men K, Shi H, Xiang M, Zhang J, Song J, et al. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale. 2011;3:1558–67.
Gou M, Wei X, Men K, Wang B, Luo F, Zhao X, et al. PCL/PEG copolymeric nanoparticles: potential nanoplatforms for anticancer agent delivery. Curr Drug Targets. 2011;12:1131–50.
Xue J, Liu Y, Wan L, Zhu Y. Comprehensive analysis of differential gene expression to identify common gene signatures in multiple cancers. Med Sci Monit. 2020;26:e919953-1–13.
Younis MA, Khalil IA, Abd Elwakil MM, Harashima H. A multifunctional lipid-based nanodevice for the highly specific codelivery of sorafenib and midkine siRNA to hepatic cancer cells. Mol Pharm. 2019;16:4031–44.
Belete TM. The current status of gene therapy for the treatment of cancer. Biologics. 2021;15:67–77.
Nakamura T, Sato Y, Yamada Y, Abd Elwakil MM, Kimura S, Younis MA, et al. Extrahepatic targeting of lipid nanoparticles in vivo with intracellular targeting for future nanomedicines. Adv Drug Deliv Rev. 2022;188: 114417.
Khalil IA, Younis MA, Kimura S, Harashima H. Lipid nanoparticles for cell-specific in Vivo Targeted Delivery of Nucleic Acids. Biol Pharm Bull. 2020;43:584–95.
Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural Killer (NK) Cell–mediated cytotoxicity: differential use of TRAIL and fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188:2375–80.
Deng D, Shah K. TRAIL of hope meeting resistance in cancer. Trends Cancer. 2020;6:989–1001.
Lemke J, von Karstedt S, Zinngrebe J, Walczak H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014;21:1350–64.
Pishavar E, Ramezani M, Hashemi M. Co-delivery of doxorubicin and TRAIL plasmid by modified PAMAM dendrimer in colon cancer cells, in vitro and in vivo evaluation. Drug Dev Ind Pharm. 2019;45:1931–9.
Ju H-Q, Lu Y-X, Wu Q-N, Liu J, Zeng Z-L, Mo H-Y, et al. Disrupting G6PD-mediated Redox homeostasis enhances chemosensitivity in colorectal cancer. Oncogene. 2017;36:6282–92.
Younis MA, Sato Y, Elewa YHA, Kon Y, Harashima H. Self-homing nanocarriers for mRNA delivery to the activated hepatic stellate cells in liver fibrosis. J Control Release. 2023;353:685–98.
Gao Y, Men K, Pan C, Li J, Wu J, Chen X, et al. Functionalized DMP-039 hybrid nanoparticle as a novel mRNA vector for efficient cancer suicide gene therapy. Int J Nanomedicine. 2021;16:5211–32.
Vascotto F, Petschenka J, Walzer KC, Vormehr M, Brkic M, Strobl S, et al. Intravenous delivery of the toll-like receptor 7 agonist SC1 confers tumor control by inducing a CD8+ T cell response. Oncoimmunology. 2019;8: e1601480.
Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics — developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–80.
Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512:324–7.
Reinhard K, Rengstl B, Oehm P, Michel K, Billmeier A, Hayduk N, et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. 1979;2020(367):446–53.
Huang P-W, Chang JW-C. Immune checkpoint inhibitors win the 2018 Nobel Prize. Biomed J. 2019;42:299–306.
Esfahani K, Roudaia L, Buhlaiga N, Del Rincon SV, Papneja N, Miller WH. A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol. 2020;27:87–97.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–68.
Ni Q, Zhang F, Liu Y, Wang Z, Yu G, Liang B, et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci Adv. 2020;6:eaaw6071.
Cheng K, Zhao R, Li Y, Qi Y, Wang Y, Zhang Y, et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat Commun. 2021;12:2041.
Chang M, Hou Z, Jin D, Zhou J, Wang M, Wang M, et al. Colorectal tumor microenvironment‐activated bio‐decomposable and metabolizable Cu2O@CaCO3 nanocomposites for synergistic oncotherapy. Adv Mater. 2020;32:e2004647.
Ginghină O, Hudiță A, Zaharia C, Tsatsakis A, Mezhuev Y, Costache M, et al. Current landscape in organic nanosized materials advances for improved management of colorectal cancer patients. Materials. 2021;14:2440.
Sun J, Zhao J, Jiang F, Wang L, Xiao Q, Han F, et al. Identification of novel protein biomarkers and drug targets for colorectal cancer by integrating human plasma proteome with genome. Genome Med. 2023;15:75.
Modi S, Anderson BD. Determination of drug release kinetics from nanoparticles: overcoming pitfalls of the dynamic dialysis method. Mol Pharm. 2013;10:3076–89.
Yalikong A, Li X-Q, Zhou P-H, Qi Z-P, Li B, Cai S-L, et al. A triptolide loaded HER2-targeted nano-drug delivery system significantly suppressed the proliferation of HER2-positive and BRAF mutant colon cancer. Int J Nanomedicine. 2021;16:2323–35.
Zolnik BS, González-Fernández A, Sadrieh N, Dobrovolskaia MA. Minireview: nanoparticles and the immune system. Endocrinology. 2010;151:458–65.
Molinari C, Marisi G, Passardi A, Matteucci L, De Maio G, Ulivi P. Heterogeneity in colorectal cancer: a challenge for personalized medicine? Int J Mol Sci. 2018;19:3733.
Abdellatif AA, Younis MA, Alsharidah M, Al Rugaie O, Tawfeek HM. Biomedical applications of quantum dots: overview, challenges, and clinical potential. Int J Nanomedicine. 2022;17:1951–70.
Author information
Authors and Affiliations
Contributions
• Ahmed A. H. Abdellatif: conceptualization; design; validation; supervision; writing the original draft of the manuscript.
• Abdulmajeed S. Alshubrumi: data collection; presentation; visualization; writing the original draft of the manuscript.
• Mahmoud A. Younis: conceptualization; design; visualization, writing review and editing.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdellatif, A.A.H., Alshubrumi, A.S. & Younis, M.A. Targeted Nanoparticles: the Smart Way for the Treatment of Colorectal Cancer. AAPS PharmSciTech 25, 23 (2024). https://doi.org/10.1208/s12249-024-02734-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02734-9