Abstract
Abdominal compartment syndrome (ACS) and intra-abdominal hypertension present as the pathological sequelae of ongoing pressure changes and/or interstitial swelling which affect tissue viability and organ function. Surgical decompression with midline laparotomy is the standard treatment once ACS with organ dysfunction has occurred. While the operating room remains the ideal location for general surgery-bedside decompressive laparotomy in the Intensive Care Unit can help avoid delays in care, mitigate cost while rapidly improved hemodynamics, respiratory and renal parameters.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Abdominal compartment syndrome
- Intra-abdominal hypertension
- Bedside laparotomy
- Decompressive
- Intensive Care Units
Introduction
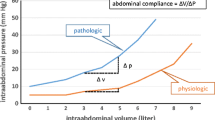
Abdominal compartment syndrome (ACS) and intra-abdominal hypertension (IAH) are terms that present the pathological consequences of “a spectrum of pressures that can affect intra-abdominal tissue viability and organ function” [1]. Interstitial swelling or pressure changes caused by ongoing hemorrhage, hematoma, or ascites can result in a pathological progression of increasing intra-abdominal pressure or IAH. The resulting pressure changes impede kidney and abdominal visceral perfusion and may also impact ventilation and cardiac output [1]. Normal intra-abdominal pressures are described as ranging from 5 to 7 mm Hg in the non-obese individual. Pressures greater than or equal to 12 mm Hg represent IAH, and pressures >20 mm Hg with concomitant new organ dysfunction are defined as ACS. Surgical decompression with midline laparotomy is the standard treatment once ACS with organ dysfunction has occurred [1].
The Operating Room (OR) remains the ideal location for general surgery. In this setting, hygiene, lighting, equipment, staffing, and infrastructure are optimized [2]. However, in patients with an acute intra-abdominal conditions and indications for emergent laparotomy but with concomitant severe cardiopulmonary instability which precludes transport, a bedside resuscitative laparotomy in the Intensive Care Unit may be considered a potential treatment option [3]. In addition, as more complex patients with higher acuities are being transferred to larger academic medical centers there can be difficulty obtaining OR time for non-elective procedures [4]. Bedside laparotomies may allow some simple abdominal procedures to be performed in ICU and avoid the delays and costs of OR time.
Indications
Clinical indications for bedside decompressive laparotomy (DL) include suspected intra-abdominal hypertension/abdominal compartment syndrome. Overall, bedside DL can be considered for those patients who require urgent/emergent intervention or for those with diagnosed or impending ACS. IAH/ACS is seen in a variety of medical and surgical conditions including trauma, sepsis, pancreatitis, massive ascites, retroperitoneal pathologies (ruptured abdominal aortic aneurysm, pelvic fractures with ongoing hemorrhage, etc.), ischemia, burns, and peritonitis [5]. IAH can be further subdivided into grades according to the severity, etiology, and rapidity of onset [6]. Abdominal compartment syndrome (ACS) is defined by a sustained IAP > 20 mg Hg pressure which adversely affects circulation and tissue perfusion – ultimately causing organ dysfunction [1]. Increased pressure compromises venous return, cardiac output, and systemic oxygen delivery with confounding effects. As pressure worsens, visceral edema impedes diaphragm movement and ultimately limits alveolar recruitment while elevating pleural and intra peritoneal pressures which further limits venous return and cardiac function [6]. The formation of oxygen free radicals, release of cytokines, and decreased production of adenosine triphosphate may lead to translocation of bacteria and worsening visceral edema further confounding the cycle and predisposing the patient to multiorgan dysfunction [7] (Table 32.1).
Contraindications
Patients with grave underlying conditions such as terminal cancer or severe TBI with suspected herniation, who will not gain any benefit from the risk of decompressive laparotomy, remain an absolute contraindication to the procedure. Another absolute contraindication to bedside decompression would be suspected ruptured abdominal aneurysm, where Operating Room exposure to control active bleeding is mandatory. Relative contraindications to bedside decompression would be significant coagulopathy or suspected severe uncontrolled abdominal hemorrhage, due to the likely need for more exposure and operating room resources for the control of bleeding. Lastly, patients with significant past abdominal surgeries would represent a relative contraindication to bedside laparotomy for decompression given the higher risk of visceral injury and the need for better exposure in OR for any lysis of adhesions needed to adequately decompress.
Risks/Benefits
In critically ill patients with severe or profound metabolic acidosis who require bedside decompressive laparotomy, there is an associated high mortality rate. Additionally, “even when controlling for other known predictors of mortality, IAH is an independent predictor of mortality” [8]. For those patients with intra-abdominal hypertension or abdominal compartment syndrome secondary to resuscitation (e.g., burns, sepsis), and not a primary abdominal process (intraabdominal or retroperitoneal bleeding), consideration may be given to perform an ultrasound of the abdomen in order to determine if a paracentesis or abdominal drain to decompress the ascites would be adequate to avoid the high risk of decompressive laparotomy. Patients who require decompressive laparotomy are severely ill. However, for those patients with IAH, a decompressive laparotomy can result in significantly lower intra-abdominal pressures and improve hemodynamic and respiratory and renal parameters [9]. Other risk factors for the bedside DL include recurrent hemorrhage, unanticipated need for other specific equipment/instrumentation, crisis management issues (location, spacing, movement of staff in and out of area), device failure, or unanticipated cardiac arrest [4].
As patient’s acuity and complexity arise, transportation out of the ICU to the operating room has its own inherent risks including device failure, inadvertent, or unplanned device removal, attendant support or issues, or lack of medications or equipment needed for unplanned events [4]. In such cases, operating in the ICU is a viable and reasonable option, with proper preparation.
Equipment for IAP Monitoring (Fig. 32.1)
-
Three way set up
-
Pressure cable
-
Transducer set
-
Urinary catheter
-
Urinary collection bag
-
Luer lock syringe
-
500 cc sterile 0.9% sodium chloride
-
Clamp
-
Gloves
-
Chlorhexidine or alcohol prep
Additional Equipment
Bedside monitor.
Measuring IAP
-
Collect supplies
-
Place patient in supine position.
-
Connect a standard sterile IV saline infusion set to a three-way stop cock with an installation syringe. Attach a disposable pressure transducer.
-
Attach pressure tubing to the urine sample port (aka “Luer lock”) between a standard Foley catheter and urinary drainage tubing (Fig. 32.2).
-
Flush tubing to the Foley catheter with sterile saline and “zero” the transducer to atmospheric pressure at the iliac crest at the mid axillary line (Figs. 32.3 and 32.4).
-
Insert ~25 ml or less of sterile saline into the urinary catheter via the closed system and immediately clamp the tubing.
-
Wait for ~30–60 seconds after installing to allow for detrusor muscle relaxation and obtain bladder pressure at end expiration.
-
Reporting of 1 mm Hg = 1.36 cm H20 is recommended for standardization.
-
Remove clamp from urine drainage tubing so that bladder can drain.
-
Obtain measurements every 4–6 hours as clinically indicated [1].
Preparation for Bedside Laparotomy
-
If not emergent procedure, obtain informed consent.
-
Key personnel include surgeon, surgical assistant (advance practice provider, resident physician, medical student, registered nurse first assist, etc.), registered nurse, respiratory therapy, and extra runner (for any needed supplies as the bedside RN will not be able to leave).
-
Review medical record: current medications, allergies, recent or pertinent laboratory and radiographic studies.
-
Optimize ventilator settings – place on mandatory rate with 100% fraction of inspired oxygen (FIO2).
-
Ensure ongoing monitoring of vital signs, cardiac, pulse oximetry, end-tidal carbon dioxide (ETCO2), or other invasive monitoring as indicated.
-
Ensure sterile perimeter in room – all individuals in room who are not operating need to be wearing surgical cap, mask, eye protection, gloves, and appropriate personal protective equipment.
-
Chlorhexidine gluconate agent of choice for skin preparation.
-
Anesthesia: sedation, analgesia, and paralytics if indicated.
-
Cross-check equipment function, e.g., suction, electrocautery, etc.
-
Prior to starting: Surgeon to and any necessary equipment (e.g. wound vac or improvised vacuum dressing setup) anticipated duration, and contingency plan and perform “time-out.”
-
Post procedure – collect sharps and instruments.
Equipment List for Bedside Laparotomy
-
Electrocautery
-
Wall suction
-
In-line suction
-
Warm balanced crystalloid solution
-
Standard bedside laparotomy tray with suture
-
Safety towels
-
10/19 French JP drains
-
Negative pressure dressings (improvised vacuum dressing if needed, KCI Abthera, etc.)
Procedure for Bedside Laparotomy
The abdomen is prepped widely with chlorhexidine gluconate or betadine and draped with towels. A large drape is applied. The required equipment is assembled and organized on the drape for ease of access. Once the surgeon, RT, bedside nurse, and assistant are ready to proceed, a vertical midline incision is made into the skin sharply and carried down to the fascia with electrocautery. The fascia is opened with extreme caution using cautery or knife, and the abdomen is decompressed. This opening of the fascia is done with great care as the abdominal viscera are under pressure and are at risk for injury during decompression. Usually, for true ACS, this will eviscerate a large volume of fluid, blood, or swollen bowel under pressure (depending on cause for ACS). The fluid is evacuated and the remainder of the fascia is opened from xiphoid to pubis, enabling maximal decompression of the abdomen. Alternatively, if the abdomen was recently closed after a laparotomy and requires reopening, skin staples or wound vac are removed. The fascial sutures are located, cut with a heavy scissors, and removed, opening the entire fascia and allowing evacuation of abdominal contents. This rapid decompression usually results in immediate improvement in venous return and thus improved blood pressure and heart rate. There is the risk of cardiac arrest on opening the abdomen due to profound hemodynamic change. This should be prepared for with code medications and volume loading rapidly available. The abdomen is then inspected for etiology of ACS. Sources such as ascites and hemoperitoneum should have been readily identified on opening. Alternatively, bowel edema, retroperitoneal hemorrhage, and retroperitoneal edema are also possible causes of ACS. The liver is inspected, and the bowel is quickly run from stomach to rectum for obvious ischemia or necrosis. The lesser sac is opened and examined to evaluate for saponification from pancreatitis. Any obvious edema or retroperitoneal hematoma is noted. Any obvious bleeding is controlled and any frankly necrotic bowel is removed. This is beyond the scope of this chapter and may require an operating room, depending on location of ischemia. The abdomen is irrigated copiously and a negative pressure dressing applied. This becomes an open abdomen. Please see chapter on open abdomen and temporary closures.
Complications
Given the physiologic status of the patient, which is frequently nearly moribund, the risk of this procedure is high. The immediate complications include uncontrolled hemorrhage and cardiac arrest from profound hypovolemic shock. If large volume blood loss is anticipated, more consideration for transporting to OR for adequate exposure and lighting is needed. If this is not possible, adequate preparation with blood products, hemostatic agents, suture, and better lighting should strongly be considered. Preparation with crystalloid, blood products, and ACLS drugs should be standard for opening an abdomen in the ICU.
Delayed complications may occur from days to months later. These include possible infectious complications, including intra-abdominal abscess, unrecognized enterotomy, and enterocutaneous fistula. These may be secondary to the underlying insult (i.e., intra-abdominal sepsis) or secondary to iatrogenic injury from the operative technique or the ensuing open abdomen management. Additional iatrogenic injuries to other abdominal viscera are possible but less common. Standard sterile procedure and meticulous technique should minimize these complications as much as possible. Lastly, a common late complication of open abdominal management after decompressive laparotomy for ACS remains a ventral hernia. After decompression, the fascia is sequentially pulled apart from massive edema, and sometimes it is unable to be closed primarily. At this point, the skin may be closed over the open fascia or an absorbable mesh is used to create a temporary barrier between the viscera and the outside environment (and frequently the negative pressure dressing). The mesh is allowed to granulate and eventually a split thickness skin graft is performed for closure of the skin. The patient will have a planned ventral hernia, which may be repaired at a later date after his or her full recovery from his acute illness.
Keys to Success, Perils and Pitfalls
Prevention, prevention, prevention! Luckily, abdominal compartment syndrome has fallen in frequency over recent years. This is largely due to our understanding of the harm of high-volume crystalloid resuscitations. High-volume resuscitations should be avoided in favor of goal-directed therapy and patients receiving high-volume resuscitations should be monitored closely with frequent intra-abdominal bladder pressures (IABP) to identify and follow intra-abdominal hypertension.
Preparation is key! The more prepared you are for this procedure, the easier things will go.
An extra set of hands is crucial for this procedure. While an experienced set of hands is ideal, an inexperienced provider can provide adequate retraction for the surgeon, in order to explore the abdomen, control hemorrhage, and apply a negative pressure dressing.
The surgical supplies of drapes, suction, electrocautery, and a basic laparotomy pan are mandatory. A linear stapler should also be considered if there is concern for bowel ischemia which may require a resection. A skin stapler is very useful for securing all drapes and adjuncts safely on the table. If there is large volume ascites, there may be liters of fluid for evacuation. Therefore, multiple suction canisters are needed on opening the abdomen for ACS.
Resuscitation is ongoing during this procedure, and additional IV crystalloid should be readily available as well as blood products if needed. Code drugs, including epinephrine, calcium, atropine, and bicarbonate are needed to be readily accessible.
CPT Coding
XLAP
XLAP 35840 – Exploration for postoperative hemorrhage, thrombosis or infection; abdomen
XLAP 49002 – Reopening of recent laparotomy
M79.A3. Non traumatic abdominal compartment syndrome
T79.A3XA. Traumatic abdominal compartment syndrome
Summary
Failure to recognize IAH prior to progression of ACS results in hypoperfusion and may ultimately lead to multisystem organ failure and death. ACS can affect the function of nearly every organ system. Bladder pressure monitoring is the standard method to screen for IAH and ACS. Surgical decompression should not be delayed in patients with ACS and may lead to increased mortality [5].
References
Rogers WK, Garcia L. Intraabdominal hypertension, abdominal compartment syndrome, and the open abdomen. Chest. 2018;153(1):238–50. https://doi.org/10.1016/j.chest.2017.07.023.
Van Beuzekom M, Boer F, Akerboom S, Hudson P. Patient safety in the operating room: an intervention study on latent risk factors. BMC Surg. 2012;12:10. https://doi.org/10.1186/1471-2482-12-10. Available from https://www.ncbi.nlm.nih.gov/pubmed/22726757
Schreiber J, Nierhaus A, Vettorazzin E, Braune SA, Frings DP, Vashist Y, Izbicki JR, Klunge S. Rescue bedside laparotomy in the intensive care unit in patients too unstable for transport to the operating room. Crit Care. 2014;18(3):R123. https://doi.org/10.1186/cc13925. Available from https://www-ncbi-nlm-nih-gov.ahecproxy.ncahec.net/pmc/articles/PMC4231096/.
Piper GL, Maerz LL, Schuster KM, Maung AA, Luckianow GM, Davis KA, Kaplan LJ. When the ICU is the operating room. J Trauma Acute Care Surg. 2013;74(3):871–5. https://doi.org/10.1097/TA.0b013e31827e9c52.
Gestring M. Abdominal compartment syndrome. UpToDate. Waltham: UpToDate Inc. This topic last updated Mar 5, 2020. https://www.uptodate.com/contents/abdominal-compartment-syndrome-in-adult.
Maerz L, Kaplan LJ. Abdominal compartment syndrome. Crit Care Med. 2008;36(4):S212–5. https://doi.org/10.1097/CCM.0b013e318168e333.
Walker J, Criddle LM. Pathophysiology and management of abdominal compartment syndrome. Am J Crit Care. 2003;12(4):367–71, quiz 372–3
Murphy PB, Parry NG, Sela N, Leslie L, Vogt K, et al. Intra-Abdominal Hypertension is more common than previously thought: a prospective study in a mixed medical surgical ICU. Crit Care Med. 2018;46(6):958–64.
Van Damme L, & De Waele JJ. Effect of decompressive laparotomy on organ function in patients with abdominal compartment syndrome: a systematic review and meta-analysis. Crit Care. 2018;22(1):179. https://doi.org/10.1186/s13054-018-2103-0 . Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6060511/
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gulledge, M., Lauer, C.W. (2021). Decompressive Laparotomy. In: Taylor, D.A., Sherry, S.P., Sing, R.F. (eds) Interventional Critical Care. Springer, Cham. https://doi.org/10.1007/978-3-030-64661-5_32
Download citation
DOI: https://doi.org/10.1007/978-3-030-64661-5_32
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64660-8
Online ISBN: 978-3-030-64661-5
eBook Packages: MedicineMedicine (R0)