Abstract
The introduction of direct-acting antivirals (DAAs) has transformed the landscape of chronic hepatitis C virus (HCV) treatment; making the ambitious target of HCV elimination is possibly feasible. The global burden of chronic hepatitis C virus (HCV) infection is currently estimated by 71 million infected patients worldwide. Most infected patients reside in Eastern Mediterranean and European Regions with a reported prevalence of HCV infection of 2.3% and 1.5%, respectively. Egypt is one of the highest prevalence countries for HCV infection who took excellent steps towards HCV elimination since the establishment of the National Committee for Control of Viral Hepatitis (NCCVH) in 2006 with an integrated teamwork of government, researchers, healthcare providers, policymakers, and the pharmaceutical companies. Herein, we will present the Egyptian journey towards the successful elimination of HCV as a model of care for a low-income country.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Hepatitis C virus (HCV) infection is a major public challenge that affects more than 71 million people all over the world [1]. Egypt is one of the world’s highest prevalence rates of HCV infection. According to Egyptian Demographic Health Survey (EDHS) conducted in 2008, a substantial proportion of Egyptian population was affected by the virus, with an overall estimate of HCV antibody and HCV RNA positivity among the 15–59-year age group of 14.7% and 9.9%, respectively [2]. In 2015, the Egyptian Health Issues Survey (EHIS) was done to re-estimate the prevalence of HCV infection in Egypt, HCV population burden was estimated by around six million patients, and the overall prevalence of HCV antibody and HCV RNA positivity among 15–59-years-age group went down to 10.0% and 7.0%, respectively [3], with 29% reduction in HCV RNA seroprevalence since 2008. The decline in HCV prevalence, although looks encouraging, is not pure decline; stock of patients who were infected due to bilharzial treatment are age shifted so that majority of them are now older than 60 years so are not included in 2015 survey while were included in 2008 survey. Also, no obvious impact of direct-acting antivirals (DAAs) was expected although it is believed that a related true decline will be proved in future surveys.
The burden of HCV infection in Egypt is largely attributed to the community-wide campaigns that were conducted by the Egyptian Ministry of Health (EOH) during the period from 1950 to 1980, aiming at the eradication of schistosomiasis which was the major public health problem and a major cause of liver disease at that time [4,5,6]. This great effort gave rise to a huge reservoir of HCV genotype four in the country due to exposure of the patients to unsafe injection practices [6]. In addition, health-care related transmission affects about 150,000 Egyptian patients per year [7].
HCV may complicate the course of schistosomiasis and vice versa (possibly through synergistic effect). Liver cirrhosis occurs at a much higher rate in those who have co-infection of HCV with schistosomiasis (48%), compared with those who are mono-infected with either HCV or schistosomiasis (15% and 0%, respectively) [8].
HCV is a small (55–65 nm in size), enveloped, strictly blood-borne RNA virus in Flaviviridae family [9]. It can survive infectious in dried blood for weeks [10]. The major routes for its spread include injection drug use with a shared unsterilized needle, medical and dental procedures in settings with inadequate infection control, body piercing with reused needles, and transfusion of unscreened blood or blood products. Less common modes of HCV spread include sexual transmission and viral transmission from an infected mother to her baby [11]. In Egypt, approximately 150,000 individuals acquire HCV infection annually primarily through a healthcare-related transmission which is considered the primary mode of HCV transmission in Egypt [7].
Nearly 75%–85% of HCV-infected patients do not clear infection within 6 months, and subsequently, they develop chronic hepatitis [8, 12]. Many factors affect the rate of HCV chronicity, including patients’ age at the time of infection, gender, ethnicity, and the development of jaundice during the acute phase of infection [13].
Vulnerable groups for acquiring HCV infection, to which screening programs are directed, include healthcare, emergency medical, or public safety workers who are in contact with HCV-positive blood, injection drug users, patients who received a blood transfusion or organ transplant before July 1992 or clotting factor concentrates produced before 1987, long-term hemodialysis patients, and babies born to HCV-positive mothers.
The journey of Egyptian battle against hepatitis C started in 2001 by the great efforts of Egyptian Ministry of Health (MOH) in the execution of a multitask strategic program aiming at reduction of HCV transmission in Egypt. The program involved the foundation of the first HCV surveillance unit at the MOH as well as the release of the first national guidelines for HCV infection control. Both actions contributed to reduction in the annual incidence of de novo HCV infection among dialysis patients from 28% to 6% in 2008 [14].
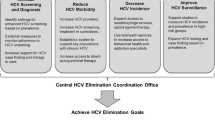
The second major achievement for the Egyptian Ministry of Health (MOH) in regard to HCV infection control was the establishment of the National Committee for Control of Viral Hepatitis (NCCVH) in 2006 in collaboration with ad hoc experts from the University of California in San Francisco (United States) and Pasteur Institute in Paris (France) [15, 16]. The NCCVH set its mission to eradicate HCV in Egypt by 2030 through setting up, implementing, and maintaining a nationwide HCV control strategy aiming at the reduction of the infection rate from currently 8% to 2% (international prevalence disease). NCCVH also offers antiviral medications for HCV-infected patients at minimum cost or even totally free of charge. Nowadays, patients with viral hepatitis are served through more than 60 treatment centers all over the country [17].
12.1 Strategic and Action Plan Evolution During Egyptian Journey Towards HCV Elimination
“Elimination of HCV in Egypt is critically dependent on the prevention of new infections, through the adoption of universal infection control strategies at all levels of the health system hand in hand with the implementation of universal screening program and treatment of all diagnosed cases.”
12.1.1 Addressing HCV Problem and Raising the Awareness Against HCV Transmission
In 2011, the national services for awareness against HCV were limited to few media-based public awareness campaigns and educational campaigns targeting university students (nearly 100,000 university undergraduates in different areas). During the period between 2014 and 2018, the NCCVH adopted a novel “Action Plan” addressing all aspects of control of viral hepatitis with support from the World Health Organization (WHO), the US Centers for Disease Control and Prevention (US-CDC), and the Pasteur Institute, Paris. The “Action Plan” strategies were to improve current HCV surveillance practices, ensure blood and blood products safety, promote infection control measures, increase public awareness about viral hepatitis prevention, and improve care and treatment of viral hepatitis-related liver disease and cancer [16].
12.1.2 Implementing Universal Screening
Eradication of HCV is a possible target since the disease meets most of the criteria for disease eradicability [18, 19]. However, such target would not be achievable without improvement of screening effectiveness [20]. The current risk-based screening approach is problematic since a significant number of patients are unaware of their infection status [21]. This underdiagnosis comprises a missed opportunity for infected patients to benefit from early access to treatment and allows the spread of infection from the untreated patient (untreated patient could transmit the infection to an average of three to four patients during his lifetime). Therefore, an extension of risk-based screening strategy to universal screening could possibly increase the detection rate of undiagnosed HCV cases and subsequently significantly reduces the disease burden.
Great efforts have been made in that context; Cairo University launched regular screening and awareness campaigns for its workers and students. The innovative initiatives from professor Gamal Shiha (towards a free village from viral hepatitis 2014–2019) establishing exemplary village free from viral hepatitis through implementing community-based outreach interventions and provision of accessible and comprehensive treatment and care services that address HCV and the steps taken by the NCCVH to unmask the hidden part of unrevealed HCV patients by screening different populations and relatives of diagnosed HCV patients are good examples of such efforts.
Media awareness campaigns were launched on radio and TV to increase awareness about this hidden epidemic and encourage people to get tested for hepatitis C in collaboration with community leaders and social stars.
12.1.3 Treating HCV Patients and Ending Hepatitis C Transmission with Antiviral Medication
Biologically, HCV is an amenable target for eradication as there is no known non-human reservoir as well as no latent cellular reservoir [22]; thus, treatment-based elimination of HCV could be an option [23]. The most prevalent HCV RNA genotype in Egypt is genotype 4, accounting for >90% of all HCV cases [24].
NCCVH issues the national treatment protocols and generalizes it to all its affiliated specialized treatment centers. These treatment protocols are regularly updated by expert hepatologist panel regarding patients’ treatment eligibility criteria, pretreatment assessments, drug regimens, and treatment of special populations.
12.2 Revulsions of Patients’ Assessments in NCCVH Affiliated Centers
-
Initial pretreatment evaluation of hepatic fibrosis stage based on FibroScan; now NCCVH affiliated centers depend on FIB4 score to assess patients’ fibrosis stage.
-
The target population for HCV treatment was initially patient with fibrosis stages 3 and 4 being the most vulnerable group for HCV-related complications and hepatic decompensation. Currently, treatment of HCV is offered to all HCV-infected patients regardless the fibrosis stage aiming at the reduction of infection transmission.
-
Before the era of direct acting antivirals (DAA), between 2007 and 2014, the available choices for treatment of HCV were very limited. The NCCVH offered HCV- infected patients with a combination of pegylated interferon (Peg IFN) and ribavirin (RBV) which was the standard of care (SOC) at that time, leading to a sustained virologic response (SVR) that didn’t reach 65%. The treatment options for HCV have been revolutionized since the introduction of DAAs on 2014 to a highly efficacious and well-tolerated therapy for nearly all HCV-infected patients with a significant increment in SVR rates from 40 to 50% with Peg IFN/RBV [25] to higher than 90% response rates. In real-life experience of Egyptian NCCVH with DAAs, a mass treatment plan [14] that basically depended on sofosbuvir-based regimen using its combination with ribavirin (dual therapy) or Peg IFN/RBV (triple therapy) was implemented in September 2014 with SVR-12 rates for those who received dual (n = 5667) and triple therapy (n = 8742) as 79% and 94%, respectively. Between May 2015 and November 2015, 6211 patients received combined sofosbuvir and simeprevir therapy and achieved 94% SVR-12 [26]. Since November 2015, the protocol of NCCVH is directed to treat HCV patients with two DAAs ± RBV. Since that time, sofosbuvir/daclatasvir regimen with or without ribavirin took the lead of management (n = 18,378) with an overall SVR-12 of 95.08%. Sofosbuvir/ledipasvir with or without ribavirin and paritaprevir/ritonavir/ombitasvir + ribavirin regimens are also available.
-
The concern about HCC recurrence in patients treated with DAAs for hepatitis C following curative tumor therapy reflected on NCCVH treatment protocol for this subset of patients. Patients with HCV-related hepatocellular carcinoma are currently eligible to receive treatment for their HCV with DAA after completing 6 months after successful curative interventions that aimed complete HCC ablation and so concurrent no evidence of activity by dynamic imaging (CT, MRI) before starting HCV treatment.
12.3 Addressing DAA Availability
DAAs have considered the ideal choice for treatment of chronic HCV infection being effective medications (SVR > 90%) with minimal adverse effects. The main barrier to universal use of DAA treatment has stemmed from their exorbitant prices; in the United States, a 12-week course of treatment with sofosbuvir and simeprevir costs around USD84,000 and USD66,000, respectively [27]. Egypt took many steps to obtain the widest possible treatment coverage for Egyptian HCV-infected patients. The negotiation between the government in the form of “NCCVH” and Gilead sciences resulted in the importation of Gilead’s sofosbuvir (Sovaldi) in 1% of its original price and sofosbuvir/ledipasvir (Harvoni). Gilead also allowed 11 Indian and 2 Egyptian pharmaceutical companies to make sofosbuvir under license and to price it as they like. In real-life experience, many Egyptian studies concluded that the efficacy of generic drugs in Egyptian population was proven to be comparable to brand one [28].
The NCCVH tests the effectiveness of each individual new DAA before its rollout in the Egyptian market through conduction of multicenter, randomized, active-controlled studies in its well-equipped hepatology centers. The results of those trials are analyzed and published in order to give a pure view on the effectiveness of the candidate medication in treatment of HCV genotype 4 in Egypt.
12.4 It Could Be Very Soon
In 2014, the government set ambitious goals to increase the number of newly diagnosed cases from 150,000/year in 2015 to 340,000/year in 2018 and to target treatment of 325,000 patients annually from 2018. By that, Egypt might be able to achieve hepatitis C elimination by 2030 [29]. Eventually, with a marked actual jump in the numbers of really treated patients, eradication of HCV could be achieved earlier in time.
References
World Health Organization. Hepatitis C [online]. 2018. http://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c. Accessed 1 Jun 2018.
El-Zanaty F, Way A. Egypt demographic and health survey 2008. Cairo, Egypt: Ministry of Health and Population; 2009.
Ministry of Health, Egypt, El-Zanaty and Associates, Egypt and ICF International. Egypt Health Issues Survey 2015. Cairo, Egypt/Rockville, MD: Ministry of Health and ICF International; 2015.
Strickland G. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology. 2006;43(5):915–22.
Rao M, Naficy A, Darwish M, Darwish N, Schisterman E, Clemens J, Edelman R. Further evidence for association of hepatitis C infection with parenteral schistosomiasis treatment in Egypt. BMC Infect Dis. 2002;2(1):29.
Frank C, Mohamed M, Strickland G, Lavanchy D, Arthur R, Magder L, Khoby T, Abdel-Wahab Y, Ohn E, Anwar W, Sallam I. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355(9207):887–91.
Hajarizadeh B, Grebely J, Dore G. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10(9):553–62.
Struthers A. From schistosomiasis to hepatitis C: the spread of HCV in Egypt. Med J Therapeut Africa. 2007;3:213–21. http://mjota.org/images/SpreadofHCVEgypt.pdf. Accessed 16 Feb 2016.
Miller F, Abu-Raddad L. Evidence of intense ongoing endemic transmission of hepatitis C virus in Egypt. Proc Natl Acad Sci. 2010;107(33):14757–62.
Paintsil E, Binka M, Patel A, Lindenbach B, Heimer R. Hepatitis C virus maintains infectivity for weeks after drying on inanimate surfaces at room temperature: implications for risks of transmission. J Infect Dis. 2013;209(8):1205–11.
Yeung C. Vertical transmission of hepatitis C virus: current knowledge and perspectives. World J Hepatol. 2014;6(9):643.
Hoofnagle J. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997;26(S3):15S–20S.
Chen S, Morgan T. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47–52.
CDC C. Progress toward prevention and control of hepatitis C virus infection—Egypt, 2001–2012.—PubMed-NCBI. [online] 2018. Ncbi.nlm.nih.gov. https://www.ncbi.nlm.nih.gov/pubmed/22832935. Accessed 1 Jun 2018.
El-Akel W, El-Sayed M, El Kassas M, El-Serafy M, Khairy M, Elsaeed K, Kabil K, Hassany M, Shawky A, Yosry A, Shaker M, ElShazly Y, Waked I, Esmat G, Doss W. National treatment programme of hepatitis C in Egypt: hepatitis C virus model of care. J Viral Hepat. 2017;24(4):262–7.
Gomaa A, Allam N, Elsharkawy A, El Kassas M, Waked I. Hepatitis C infection in Egypt: prevalence, impact and management strategies [Corrigendum]. Hepat Med Evid Res. 2017;9:35–6.
El Kassas M, Elbaz T, Elsharkawy A, Omar H, Esmat G. HCV in Egypt, prevention, treatment and key barriers to elimination. Expert Rev Anti-Infect Ther. 2018;16(4):345–50.
Hellard M, Doyle J, Sacks-Davis R, Thompson A, McBryde E. Eradication of hepatitis C infection: the importance of targeting people who inject drugs. Hepatology. 2013;59(2):366–9.
Hopkins D. Disease Eradication. N Engl J Med. 2013;368(1):54–63.
Wedemeyer H, Duberg A, Buti M, Rosenberg W, Frankova S, Esmat G, Örmeci N, Van Vlierberghe H, Gschwantler M, Akarca U, Aleman S, Balık İ, Berg T, Bihl F, Bilodeau M, Blasco A, Brandão Mello C, Bruggmann P, Calinas F, Calleja J, Cheinquer H, Christensen P, Clausen M, Coelho H, Cornberg M, Cramp M, Dore G, Doss W, El-Sayed M, Ergör G, Estes C, Falconer K, Félix J, Ferraz M, Ferreira P, García-Samaniego J, Gerstoft J, Giria J, Gonçales F, Guimarães Pessôa M, Hézode C, Hindman S, Hofer H, Husa P, Idilman R, Kåberg M, Kaita K, Kautz A, Kaymakoglu S, Krajden M, Krarup H, Laleman W, Lavanchy D, Lázaro P, Marinho R, Marotta P, Mauss S, Mendes Correa M, Moreno C, Müllhaupt B, Myers R, Nemecek V, Øvrehus A, Parkes J, Peltekian K, Ramji A, Razavi H, Reis N, Roberts S, Roudot-Thoraval F, Ryder S, Sarmento-Castro R, Sarrazin C, Semela D, Sherman M, Shiha G, Sperl J, Stärkel P, Stauber R, Thompson A, Urbanek P, Van Damme P, van Thiel I, Vandijck D, Vogel W, Waked I, Weis N, Wiegand J, Yosry A, Zekry A, Negro F, Sievert W, Gower E. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21:60–89.
Denniston M, Klevens R, McQuillan G, Jiles R. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55(6):1652–61.
Shop.lww.com. Fields Virology. [online]. 2018. https://shop.lww.com/Fields-Virology/p/9781451105636. Accessed 1 Jun 2018.
Pawlotsky J. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146(5):1176–92.
Ray S, Arthur R, Carella A, Bukh J, Thomas D. Genetic epidemiology of hepatitis C virus throughout Egypt. J Infect Dis. 2000;182(3):698–707.
Asselah T, De Muynck S, Broët P, Masliah-Planchon J, Blanluet M, Bièche I, Lapalus M, Martinot-Peignoux M, Lada O, Estrabaud E, Zhang Q, El Ray A, Vidaud D, Ripault M, Boyer N, Bedossa P, Valla D, Vidaud M, Marcellin P. IL28B polymorphism is associated with treatment response in patients with genotype 4 chronic hepatitis C. J Hepatol. 2012;56(3):527–32.
Eletreby R, Elakel W, Said M, El Kassas M, Seif S, Elbaz T, El Raziky M, Abdel Rehim S, Zaky S, Fouad R, Gamal Eldeen H, Abdo M, Korany M, Yosry A, El Serafy M, El-Sayed M, ElShazly Y, Waked I, Doss W, Esmat G. Real life Egyptian experience of efficacy and safety of Simeprevir/Sofosbuvir therapy in 6211 chronic HCV genotype IV infected patients. Liver Int. 2016;37(4):534–41.
Project Inform. Fair pricing coalition condemns Gilead for exorbitant price of new hep C drug—Project Inform. [online]. 2018. https://www.projectinform.org/hepc/fair-pricing-coalition-condemns-gilead-for-exorbitant-price-of-new-hep-c-drug/. Accessed 1 Jun 2018.
P150: Effect of Sofosbuvir. Brand drug (Sofaldi) versus generic (Viropack) in treating chronic HCV infection among Egyptian patients. J Viral Hepat. 2015;22:94.
Waked I, Doss W, El-Sayed M, Estes C, Razavi H, Shiha G, Yosry A, Esmat G. The current and future disease burden of chronic hepatitis C virus infection in Egypt. Arab J Gastroenterol. 2014;15(2):45–52.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Omar, H., Elbaz, T., Esmat, G. (2021). Egypt: Towards Successful Elimination of HCV in Low-Income Countries. In: Hatzakis, A. (eds) Hepatitis C: Epidemiology, Prevention and Elimination . Springer, Cham. https://doi.org/10.1007/978-3-030-64649-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-64649-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64648-6
Online ISBN: 978-3-030-64649-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)