Abstract
Many arthropod species have adopted the blood of vertebrates as their main food. Blood is rich in nutrients and, except for the presence of parasites, otherwise sterile. This food, however, is not freely available nor is its obtention devoid of risk; it circulates inside vessels hidden underneath the skin of mobile hosts, which are able to defend themselves and even predate the insects attempting to feed on them. Thus, the haematophagous lifestyle is associated with major morphological, physiological and behavioural adaptations that have accumulated throughout the evolutionary history of the various lineages of blood-sucking arthropods, including triatomines. These adaptations have, on the other hand, significant consequences for the evolution of parasites, as well as for the epidemiology of vector-transmitted diseases. This review article analyses various aspects of the behaviour of triatomine bugs to illustrate how behavioural traits represent particular adaptations to their close association with hosts, which may easily turn into predators. The aim is to offer to the reader an up-to-date integrative view of the behaviour of Chagas disease vectors from a personal perspective, with the hope of encouraging young and experienced colleagues to explore this fascinating aspect of triatomine biology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Kissing bugs, in particular Rhodnius prolixus , exhibit the double interest of being considered as a classical model in insect physiology and also a vector of a major health problem in the New World, that is, Chagas disease. Understanding the behaviour of kissing bugs, as well as its underlying physiological mechanisms, is crucial for better understanding their role as vectors of Trypanosoma cruzi and may be adapting strategies for better controlling the transmission of Chagas disease.
Not far in the past, it was sometimes laborious persuading certain scientific and non-scientific fora about the necessity of a deep biological knowledge for designing and adapting efficient control campaigns. The reason was a fair point: the urgency for stopping disease transmission masked the necessity of scrutinising fundamental aspects. Control campaigns have been relatively successful and important progress has been achieved in eradicating bugs from vast regions. Yet, some problems, such as the development of resistance to control actions and resilient house reinfestation, still require digging deeper in biological aspects; not in the search for a ‘silver bullet’, but for making control as effective as possible.
In recent years, much effort has been devoted to the study of triatomine behaviour. This interest is not only inspired by the need to control bugs but also because these insects constitute an excellent model for the study of fundamental aspects of haematophagy and general questions on insect physiology, which have been pondered since the time of V.B. Wigglesworth. The present document presents an analytical review of our knowledge about triatomine behaviour and suggests possible future research topics regarding particular aspects for which a better understanding would be beneficial. Besides, the author is convinced that triatomines, and in particular Rhodnius prolixus , still constitute excellent model systems for unravelling fundamental biological mechanisms. Recently, the genome of Rhodnius prolixus has been sequenced (Mesquita et al. 2015), representing a major breakthrough in our comprehension of the genetic bases of the adaptations of insects to haematophagy and the interaction of blood-sucking insects with parasites and hosts. This achievement opened multiple research lines on lesser understood biological aspects and gave place to novel hypotheses, many of which wait for a functional validation. The timing for this review seems appropriate to open novel research possibilities in functional genetics of insect behaviour.
Most of our current knowledge on the behaviour of kissing bugs comes from the exhaustive study of a group of species considered as major vectors of Chagas disease , in particular Rhodnius prolixus and Triatoma infestans, but also Panstrongylus megistus, Triatoma brasiliensis, Triatoma dimidiata and few others. Yet, given the diversity of species, habitats and hosts associations present in the group, we can expect differences in the behavioural adaptations across triatomine species, in particular those inhabiting isolated ecotopes or challenging to rear in the laboratory. As a consequence, the information presented here should not be taken as valid for every species of the subfamily, but as representative of well-established strategies and mechanisms, serving as a rational basis for the analysis of behavioural traits and adaptations of other species to their specific way of life. An extensive revision of the behaviour of triatomines has been published some years ago (Lazzari et al. 2013); this chapter presents a more synthetic and updated view.
2 Host Search and Feeding Behaviour
Triatomines are documented as nocturnal insects that during daylight hours remain hidden and virtually immobile (i.e. akinesis) in dark places. During the first hours after dusk, bugs awake and begin exploring the surroundings, looking for distinctive cues revealing the presence of warm-blooded vertebrates. Host detection is achieved via air currents carrying distinctive odours, water vapour and heat. For this, triatomines exhibit not only a high level of sensitivity, but also the capacity of integrating multimodal information. Conversely, inhibitory mechanisms keep the insects away from hosts when obtaining a blood meal that is not absolutely necessary (Guerenstein and Lazzari 2009).
2.1 Orienting Cues
When insects are motivated to search for a resource, as a hungry bug leaving its refuge to search for food, the sensory cues associated with that resource are not necessarily apparent. Most insects do not sit and stay until something appears, nor move randomly looking for signs . Instead, they orient themselves relative to air currents potentially transporting odours of interest. The way insects use air currents varies according to the stability of their direction (Sabelis and Schippers 1984; Bau and Cardé 2015). Even though inside a nest or house one cannot expect intense winds, air always moves (e.g. convective currents). Upon detection of host-associated cues, appetitive search and long-range orientation are triggered (Barrozo et al. 2017). It should be noted that, when detected at a distance, olfactory cues do not provide directional information by themselves, but they can trigger instead positive anemotaxis, that is, upwind displacement (Guerenstein and Guerin 2001; Guerenstein and Lazzari 2009; Núñez 1982; Taneja and Guerin 1995). When detected at a close range, chemical cues can stimulate the insects to follow the concentration gradient and attract them. As in virtually every blood-sucking insect, carbon dioxide modifies the behaviour of triatomines (Barrozo and Lazzari 2004a; Guerenstein and Hildebrand 2008). Triatomine responses to carbon dioxide are modulated along the day by an endogenous circadian rhythm, making insects responsive when feeding motivation is also higher during the first hours of the scotophase (Barrozo et al. 2004a; Bodin et al. 2008; Lorenzo and Lazzari 1998). The responsiveness of triatomines to carbon dioxide is also dependent upon their physiological condition. Long starvation periods do not seem to strengthen the response (Barrozo and Lazzari 2004a), but recent feeding switches the effect of carbon dioxide from attraction to repellence (Bodin et al. 2009b). Moulting and reproduction (Bodin et al. 2009a, b) also affect the responses of bugs to carbon dioxide. In contrast to other haematophagous insects, carbon dioxide is not an essential component for the attraction of triatomines, although air currents loaded with relatively low quantities of carbon dioxide are able to evoke attraction by themselves (Barrozo and Lazzari 2004a). Some host odorants , such as nonanal, increase bug activity (Guerenstein and Guerin 2001) and others that are found in human sweat (Cork and Park 1996) such as isobutyric acid and 1-octen-3-ol induce their attraction (Barrozo and Lazzari 2004b; Guerenstein and Guerin 2001). Finally, ammonia , which is present in the sweat and urine of vertebrates, is able to evoke both activation and attraction in Triatoma infestans (Taneja and Guerin 1997), antennal responses to this compound being higher with increasing starvation (Reisenman 2014).
In nature, kissing bugs are confronted with mixtures of odours rather than with isolated volatiles. The components of such mixtures often interact in a synergistic form. In the case of triatomines, the response threshold for pure carbon dioxide is beyond 300 ppm above atmospheric concentration (Barrozo and Lazzari 2004a). Interestingly, pure L-lactic acid and short-chain fatty acids that are present in human sweat and on the skin (Bernier et al. 2000; Cork and Park 1996) are completely ineffective for triggering anemotaxis over a wide range of concentrations (Barrozo and Lazzari 2004a, b). Nevertheless, when sub-threshold amounts of carbon dioxide are combined with L-lactic acid and fatty acids in appropriate proportions, the attractiveness of the mixture becomes similar to that of a living host (Barrozo and Lazzari 2004b). Recently obtained experimental evidence supports the idea that microorganisms associated with human skin produce volatiles that are attractive to Rhodnius prolixus (Ortiz and Molina 2010; Tabares et al. 2018). These findings add a novel dimension to the analysis of how Chagas disease vectors recognize and localize their hosts.
Even though both vision and vibro-reception are well-developed triatomine senses, so far there is no evidence suggesting a role in host orientation. Mechanoreception is involved in long-range orientation, but not in relation with hosts, but with the detection of air currents guiding upwind displacement. The most important host-associated physical cue perceived by triatomine bugs is heat . Even if the heat emitted by host bodies is widely used as an orienting cue for many blood-sucking insects, its role remains relatively unknown for the vast majority (Lazzari 2009, 2019). In contrast, the thermal sense of triatomines has been the object of detailed studies. The sensitivity of triatomines to heat is extremely high, such that the insect can detect differences of thermal energy in the order of a few μWatt/cm2 (Lazzari and Núñez 1989b; Lorenzo et al. 1999a, b; Lazzari 2009). It has been calculated that Triatoma infestans is capable of detecting the heat emitted by a human face from a distance of approximately 2 m and by a mammal the size of a dog from several meters (Lorenzo et al. 1999a, b).
Triatomines remain the only group of blood-sucking insects whose ability to perceive host-emitted infrared radiation has been demonstrated (Lazzari and Núñez 1989b; Schmitz et al. 2000). This ability has important implications for successful host finding because infrared radiation propagation is not disrupted by air currents (which do disrupt conductive and convective heat transfer) or by the relative position of the insect with respect to the thermal source (which does influence convective heat transfer) (Lazzari 2009, 2019).
A remarkable characteristic of Triatoma infestans is the ability to detect the temperature and distance of heat sources, independently of their sizes, and based on thermal information alone. This seems to be possible due to their capacity to perceive radiant heat (Lazzari 2009; Lazzari and Núñez 1989b). A hypothetical model on how these bugs distinguish the temperature, size, and distance of objects based on thermo-receptive inputs and active antennal movements has been proposed by Lazzari (2009). It provides an explanation for the ability of triatomines to discriminate distant (or small) burning objects from closer (or large) tepid ones, even if the amount of thermal energy reaching their antennae is of the same magnitude.
The extreme thermal sensitivity of triatomines, together with their ability to discriminate the temperature of distant objects, raises the question about whether the absolute temperature of a heat source and the difference between the temperature of objects and their background are in fact used by these insects to recognize their hosts. This question is related to another, more practical one: are triatomines able to perceive objects at the temperature of a host when the ambient temperature is higher? Experiments were performed for testing the response of bugs to an object presented at different temperatures, in a chamber providing a background temperature that could also be modified (Fresquet and Lazzari 2011). Bugs responded by trying to bite objects showing temperatures between 30 °C and 40 °C, but only if the surrounding environment was colder than the objects themselves. Therefore, their ability to measure the temperature of objects is only effective when a positive difference exists between object and air temperature.
It should be emphasized that, in a natural context, triatomines are not exposed to single cues such as specific odours or heat , but to multiple cue combinations detected by different sensory modalities (mechanical, chemical, thermal and hydric). In the context of food search , multimodal convergence may increase the ability of bugs to find a host. For instance, it has been shown that water vapour , which constitutes a close-range orientation cue by itself, also increases bug responsiveness to heat (Barrozo et al. 2003). This can be due to the convergence of different sensory inputs into the insect brain or to a physical phenomenon (i.e. moist air transports more heat than dry air) increasing sensory stimulation.
2.2 Orientation Mechanisms
Sensory information can be used in different ways for locating a resource. In some cases, the source of stimuli is the resource itself, as the colour of a flower, but in others, external cues become relevant. For instance, the sun, the moon and the polarization pattern of the sky guide the displacements of ants, bees, beetles and other insects. In a similar way, the wind plays a major role in the orientation of insects that follow odour tracks. This is necessary because not all stimuli provide spatial information in the same way, nor the sensory system is able to extract precise spatial information from them. It is possible to locate a light spot in the night without difficulty, even with just one open eye, because light produces a discrete spatial pattern, propagates radially, it is not altered by air currents and our eyes are capable of producing a detailed image, even monocularly. This is not the case, however, for all potential stimuli, nor for all sensory systems (Lazzari 2009).
If we consider two main cues emitted by hosts, that is, odours (kairomones) and heat , each modality presents its own particularities and requires specific mechanisms in order for them to serve as orienting cues.
Odours disperse in the air and are transported by the wind and, as a consequence, only provide spatial information in the close proximity of the source. Insects, in general, and kissing bugs, in particular, use different mechanisms for olfactory orientation , according to the situation. Those chemical stimuli relevant for locating and recognizing elements in close proximity or physical contact usually act in high concentration and form stable concentration gradients, which can be followed by the insect. For instance, this is the way chemical tastants on the host skin are recognized before deciding to bite or not (Barrozo 2019). On the other hand, the detection of volatiles emitted by a distant host does not provide enough spatial information. In that case, upon detection of host-associated chemicals, bugs turn upwind, following the direction of the air current transporting the odour.
Heat disperses radially as light, but there is no ‘thermal retina’ for its detection, but specialized receptors located in the antennae (Zopf et al. 2014a, b). Then, from the point of view of a kissing bug, the spatial information delivered by a warm object is not as precise as that of a light source, but nor as imprecise as that of a source of odours. As indicated above, triatomines use thermal cues for locating a host and, once in contact with its skin, to locate the most adequate spot to be bitten (see below). Interestingly, each task involves a different use of sensory information or orientation mechanism (Wigglesworth and Gillett 1934, Flores and Lazzari 1996). The approach to a warm object can be performed using just one antenna and the bugs can determine the direction and follow thermal gradients for approaching, using a mechanism called ‘telotaxis ’ (i.e. enough information is provided by only one bilateral organ). Yet, when they extend their proboscis, bilateral inputs from both antennae need to be integrated in order to reach the target precisely, that is, ‘tropotaxis ’ (i.e. the contribution of both bilateral organs is necessary, Flores and Lazzari 1996; Lazzari 2009,).
The consideration of these possible alternatives (i.e. orientation mechanisms) in the use of sensory information is crucial in the design of experiments. For instance, the use of olfactometers using air currents for transporting odours is a very usual way for testing the response of insects to odours, kissing bugs included. Yet, this type of device is conceived to evoke odour-triggered anemotaxis, but this is not the only way a chemical compound may attract an individual, and a negative result does not necessarily mean that the insect does not respond or does not detect the odour. Triatomines do not respond to their arresting pheromone left on walked substrates (Lorenzo Figueiras and Lazzari 1998b).
2.3 Biting and Feeding
In addition to its role as an orienting signal during host search , heat is also used to locate blood vessels hidden under the host skin (Ferreira et al. 2007). By analysing the bugs feeding behaviour on live hosts, it has been shown that the insects do not bite randomly; instead, they extend their proboscises mostly towards vessels. When the host skin was replaced by a vessel-shaped heat source placed on a heated metal plate, both with independently controlled temperature, a similar precisely directed choice was observed for the warmest linear area. This suggests that heat discontinuities over the skin surface area are used to guide the bite. Biting the warmest patch of skin surface requires a bilateral integration of the thermal inputs provided by both antennae. If this integration is experimentally altered by a unilateral or bilateral antennectomy, bugs either miss the target (unilateral) or do not exhibit proboscis extension responses at all (bilateral antennectomy). This suggests that, if present, rostral thermoreceptors do not provide information for triggering or guiding biting (Ferreira et al. 2007). Interestingly, heat is a key factor for host finding and biting, but once a blood is contacted, heat becomes no longer relevant (Lazzari and Núñez 1989a, b).
The decision to bite the host skin and ingest its blood is a complex process for triatomines. Classical work revealed that food recognition in triatomines is based on the analysis of specific food properties, such as osmolarity (Guerenstein and Núñez 1994) and the presence of phagostimulant compounds (Friend and Smith 1977). For a long time, this remained the only information available. Yet, recent work has shed supplementary light on the process, revealing an elaborated feeding decision-making system, depending on specific sensory pathways and cognitive processes (Barrozo 2019).
Not all triatomines are obligate haematophagous insects. Entomophagy has been frequently reported in different species of kissing bugs. This habit, which can also be expressed towards conspecifics, involves either haemolymphagy or cleptoheamatophagy (i.e. stealing part of the vertebrate blood ingested by a conspecific). For instance, these phenomena have been reported to occur in Triatoma infestans and in Rhodnius prolixus (Schaub et al. 1989; Alves et al. 2011; Lazzari et al. 2018), eventually resulting in direct Trypanosoma cruzi transmission amongst Chagas disease vectors (Schaub 1988). Interspecific entomophagy is also frequent (Garrouste 2009; Pontes et al. 2011; Duran et al. 2016). Even though haemolymphagy and cleptohaematophagy can be considered two forms of cannibalism; there are different processes. Cleptohaematophagy is usually intraspecific and the gathered food is similar to that obtained from a vertebrate host, that is, blood. Haemolymphagy can occur against conspecifics or heterospecifics and the food is not vertebrate blood. It can be concluded then that the triatomine saliva is not only adapted to gathering blood from vertebrates, but also insect haemolymph (Alves et al. 2011).
Intriguingly enough, recent reports describe Rhodnius prolixus consuming a sugar solution (Diaz-Albiter et al. 2016) and water from a drop (Páez-Rondón et al. 2018) in the laboratory. These observations deserve to be further investigated, in order to evaluate their biological implications for triatomines.
3 Sexual Behaviour
Mating is a major biological necessity and insects have developed complex mechanisms for locating, attracting and choosing potential partners. Triatomines are not an exception and, even though no elaborated courtships can be observed as in other insects, mating relies on specific interactions between male and female individuals. One of them is polyandry , which has been observed in females of different bug species (Baldwin et al. 1971; Manrique and Lazzari 1994, 1995; Vitta and Lorenzo 2009; De Simone et al. 2018).
One aspect deserving major interest is the chemical attraction between sexes. The study of sexual pheromones has been particularly challenging in triatomines, with respect to their origin, biological significance and composition. Despite a considerable amount of experimental work and published observations on the chemical ecology of triatomines (Cruz-Lopez et al. 2001), it took time to understand how sexual pheromones work in this group. The first evidence of their occurrence in triatomines was obtained in Rhodnius prolixus (Baldwin et al. 1971) and much later in Triatoma infestans (Manrique and Lazzari 1995). In these species, mating couples release volatiles that attract and gather males, who eventually mate with a single female.
The origin of sexual volatiles has been a matter of controversy due to early reports describing the detection of isobutyric acid, produced by Brindleys glands, in the ‘headspace’ (i.e. air surrounding) of mating pairs (e.g. Fontan et al. 2002). It is worth highlighting that this compound is usually released when adult triatomine bugs are disturbed (Barrett et al. 1979). However, experiments occluding different exocrine glands of males and females alternatively revealed that female metasternal glands are the source of sexual signals, but not Brindleys glands (Crespo and Manrique 2007; Pontes et al. 2008; Vitta et al. 2009). Chemical analysis subsequently confirmed that isobutyric acid is not present in metasternal glands (Manrique et al. 2006) and does not make part of the sexual signal. Further evidence supports that female metasternal glands odours are emitted preferentially during the scotophase (Pontes et al. 2008), and that the pheromone is capable of inducing males to leave their shelters (Pontes 2010), take flight (Zacharias et al. 2010), aggregate (Pontes and Lorenzo 2012) and display odour-modulated anemotaxis in airstreams associated with female odours (Vitta et al. 2009; Pontes et al. 2014; May-Concha et al. 2013). The secretion of metasternal glands can be a complex mixture eventually including ketones, alcohols, dioxolanes and aldehydes (Manrique et al. 2006; Pontes et al. 2008; Vitta et al. 2009; May-Concha et al. 2013). Chemical and behavioural analyses allowed the recent development of a synthetic female-pheromone blend, capable of attracting males of Rhodnius prolixus in the laboratory (Bohman et al. 2018).
Additionally, female cuticular hydrocarbons seem to play a role in mate recognition (Cocchiararo-Bastias et al. 2011).
Non-receptive females reject male copulatory attempts by displaying various evasive behaviours including stridulation (Manrique and Lazzari 1994). Although differences have been reported for the mating behaviour of various triatomine species, stridulation by unreceptive females during male mating attempts seems to be a frequent feature (Lazzari et al. 2006).
4 Aggregation and Alarm
Triatomine bugs are gregarious insects sharing shelters. Depending on the species, this behaviour can be mediated by two chemical signals: a volatile aggregation pheromone emitted by bug faeces and a cuticular contact factor deposited on substrates. These signals act parallelly to the known tendency to maintain physical contact with the substrate and conspecifics (thigmotaxis) and by visual cues (i.e. darkness).
The faeces of triatomines are a source of aggregation pheromones that attract and gather bugs in their proximity (Lorenzo Figueiras et al. 1994; Schofield and Patterson 1977). It was originally suggested that the functionality of this signal would be to indicate a suitable food source (Schofield and Patterson 1977). Further studies revealed that faeces accumulate in the access path to refuges and play a major role as chemical landmarks for finding shelters (Lorenzo and Lazzari 1996). The pheromones emitted by bug excrement do not seem to have species-specific effects as they are capable of assembling bugs of other species (Lorenzo Figueiras and Lazzari 1998a; Pires et al. 2002a, b, c). They can be extracted using polar solvents, and chemical analysis has revealed multiple compounds whose biological role is not yet clear (Alzogaray et al. 2005; Cruz-Lopez et al. 2001; Mota et al. 2014).
As said above, bugs of some species deposit on substrates a non-volatile footprints signal promoting aggregation . This signal can be extracted with non-polar solvents and seems to be composed by a mixture of hydrocarbons of cuticular origin (Lorenzo Figueiras et al. 2009) and insects require physical contact for its detection (Lorenzo Figueiras and Lazzari 1998b). This pheromone, therefore, can be considered to be an arresting factor rather than an attractive one. It is worth noting that R. prolixus and T. brasiliensis do not seem to use footprints to promote arrestment (Lorenzo Figueiras and Lazzari 2002; Vitta et al. 2007), suggesting that mechanisms promoting bug aggregation differ across species.
Bugs exhibit a tendency to avoid illuminated environments, that is, negative phototaxis, which is mediated by the compound eyes and, in adult bugs, also by the ocelli (Lazzari et al. 1998; Reisenman et al. 1998). This photophobic behaviour also mediates shelter choice (Reisenman et al. 2000).
It is a frequent observation that mechanically disturbed adult triatomines release a pungent odour, which origin is the secretion of their Brindleys glands. As indicated above, the secretion is composed of isobutyric acid, together with a complex mixture of other volatiles (Cruz-Lopez et al. 2001; Guerenstein and Guerin 2004; Manrique et al. 2006; Palottini and Manrique 2016) produced by these glands. Triatomines respond by walking away from conspecifics releasing the Brindleys glands secretion (Manrique et al. 2006); yet, some reports also indicate that bugs approach to sources releasing low quantities of isobutyric acid (Ward 1981; Guerenstein and Guerin 2001). This dual response has not facilitated the distinction of the biological significance of this compound in triatomine biology. Its release by physically disturbed bugs and its repulsive role on other individuals have allowed considering the secretion of Brindleys glands as an alarm pheromone . It should be noted that Brindleys and metasternal glands are only present in adults. Given that the latter are involved in sexual communication , its exclusively imaginal character can be easily understood. Nevertheless, the fact that nymphs could respond to, but not produce alarm pheromones, remains to be interpreted in adaptive terms. As in other insect species, the response of bugs to their own alarm pheromone is modulated by the individual experience (Minoli et al. 2013).
5 Learning and Memory
In the past, insects were considered to be ‘reflex machines ’ whose behaviour were mostly ruled by stereotyped, innate responses to external stimuli (Giurfa 2004). At present, their ability to adapt their behavioural responses as a function of their previous experience is widely recognized. Despite possessing tiny brains and much less neurons than vertebrates, they have largely proven to be capable of complex forms of learning, including the acquisition of rules and concepts (Giurfa 2003; Giurfa et al. 2001). As a consequence, insects became major models in the study of learning and memory. Most studies in this area have focused only on a handful of species, and particularly, on vinegar flies and honeybees . Provided that none of them presents a haematophagous habit, the selective pressures that have modelled their cognitive abilities are much different to those acting on blood-sucking arthropods. Yet, the potential learning abilities of disease vector insects may have significant epidemiological consequences, because they can induce heterogeneous biting patterns within host populations (Kelly 2001; Kelly and Thompson 2000). Due to the strong selection pressures to which blood-sucking insects are exposed, it is reasonable to ask to what extent their cognitive abilities are developed and how could they modulate vector behaviour. For instance, learning to recognize the most vulnerable hosts through individual experience could influence host selection and, as a result, affect parasite transmission patterns (McCall and Kelly 2002; Vinauger et al. 2016).
Many field and semi-field studies have evinced behavioural responses that might indicate learning capabilities in disease vectors . Nevertheless, only a few controlled studies have succeeded in demonstrating conclusive experimental evidence (Alonso and Schuck-Paim 2006; Alonso et al. 2003).
Amongst Chagas disease vectors , early experiments failed to demonstrate learning capabilities (Abramson et al. 2005; Aldana et al. 2005, 2008). In most cases, the absence of positive results can be associated with issues that often plague this kind of studies. Learning experiments need a rigorous control over experimental variables (insect motivational state, time of the day etc.), adequate neutral stimuli (i.e. unquestionably perceived by the insect, but ineffective in evoking the evaluated response) and finally, a deep knowledge of the experimental model. It should also be noted that experimental psychology imposes a strict theoretical framework and numerous control experiments in order to properly assess the learning capabilities of an animal. In contrast to the study of honeybees and Drosophila, this has not always been respected in studies dealing with disease vectors (Alonso and Schuck-Paim 2006). In many cases ‘learning-compatible’ results are considered as evidence of learning to occur, without adequately excluding other, more parsimonious, explanations.
Triatomines are adequate models for the experimental study of the cognitive capabilities of blood-sucking insects because: (1) they can be reared in the laboratory under controlled conditions and fed using artificial feeders; (2) they are haematophagous during their whole life, thus facilitating experimentation with juveniles and adults; (3) their size facilitates testing them in constrained conditions (i.e. fixation to a holding support or to a locomotion compensator); (4) they express a widely exploited response in insect learning studies, the proboscis extension reflex or PER . Last but not least, our knowledge about the behavioural biology and the sensory ecology of triatomines is one of the deepest amongst disease vectors. This knowledge allows investigating cognitive abilities in meaningful biological contexts, such as blood feeding, aggregation and escape.
One of the most generalized forms of learning is establishing novel associations between pairs of stimuli by classical or Pavlovian conditioning. In its most basic form of classical conditioning, a ‘neutral’ stimulus , known to be perceived by the animal, but unable to trigger a given response (e.g. orientation) by itself, is paired with another stimulus, the ‘unconditioned stimulus ’, which innately evokes the desired response. After presenting them together several times, the originally neutral stimulus may acquire the capacity of evoking the same response, turning in what is called a ‘conditioned stimulus ’.
Classical conditioning protocols have been adapted for the appetitive and aversive training of Rhodnius prolixus . Vinauger et al. (2011a, b) successfully conditioned bugs to the same neutral odour, either making it act as an appetitive (i.e. inducing attraction) or aversive (i.e. inducing avoidance) conditioned stimulus. In that study bugs learnt to associate lactic acid , which is perceived but does not evoke any oriented behavioural response by itself in bugs, with either a blood meal or with mechanical disturbance. After being trained to reinforce a specific association, bugs were confronted with an air current loaded with lactic acid to test whether their innate behaviour changed. Not surprisingly, they manifested either significant attraction or significant repulsion to lactic acid-associated air currents , depending on their previous appetitive or aversive individual experience. This demonstrates not only the ability of bugs to learn and to remember information, but also their ability to use that knowledge in different contexts, for example, use in orientation tests in an olfactometer, what they have learnt when confronted to an artificial feeder (Vinauger et al. 2011a, b).
The ability of triatomines to modify their responses to chemical stimuli as a function of their individual experience has been proven to affect their host choices (Vinauger et al. 2012).
The conditioning of the PER is another classical insect learning paradigm in insects, which has been successfully applied with triatomines (Vinauger et al. 2013). This well-characterized response used with bees, flies, butterflies and bumblebees constitutes a simple bioassay for investigating different facets of insect learning and memory. In the case of triatomines, the PER could be aversively conditioned in Rhodnius prolixus and revealed that these bugs can remember novel associations for at least 72 h (Vinauger et al. 2013). Furthermore, those experiments showed that they are only able to learn during the night hours, that is, in the temporal context when they display their daily activity (Vinauger and Lazzari 2015).
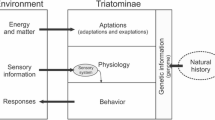
The successful application of different learning protocols in experiments with Triatoma infestans and Rhodnius prolixus also provided relevant information about how can the previous experience modify their responses to alarm and aggregation pheromones (Minoli et al. 2013; Mengoni et al. 2016), which are genetically determined, but modifiable by experience. Learning protocols also allowed demonstrating that kissing bugs can generalize and discriminate between different bitter compounds (Asparch et al. 2016), proving the utility of this approach for investigating basic sensory principles (Fig. 1).
Schematic representation of factors modulating host search in triatomines and other blood-sucking insects . The search for a host depends on external stimuli, helping the insect to find a potential blood source, and internal factors modulating its motivational state. The balance between inducing and inhibiting drivers will determine whether or not the insect will respond to the presence of a host, as well as the timing of these responses and the selection of the most appropriate host to feed on
6 Triatomine Chronobiology
A salient characteristic of the biology of triatomines is the marked temporal organization of their behaviour. It is known that selective pressures have acted to adjust the biting activity of blood-sucking insects to the time of the day when hosts are less active (reviewed by Barrozo et al. 2004b). However, the degree of synchronization of the various behavioural and physiological processes that have been proven to occur in triatomines is quite unusual. This temporal arrangement begins at a very early stage in the life of these bugs. Even ‘once-in-a-lifetime’ events in their biology are controlled by circadian clocks and not by the direct effect of the environment. First-instar larvae hatch from eggs in the early morning when the environmental relative humidity reaches a daily maximum. Nevertheless, this is not triggered by environmental changes but by an endogenous circadian clock (Lazzari 1991a; Schilman et al. 2009). The same temporal window is used for ecdysis across the various instars. Again, a circadian clock has been proven to control this process (Ampleford and Steel 1982). The temporal synchronization of egg hatching and ecdysis has an evident adaptive value that is probably related to the deleterious effect of low relative humidity on these processes. As indicated above, some triatomine species exhibit a marked xeropreference and this preference is not modified at egg laying activity nor for moulting (Guarneri et al. 2002; Roca and Lazzari 1994). Thus, we can conclude that triatomines exhibit a temporal hygropreference rather than a spatial one to adapt their needs at critical moments of their life. Instead of moving to humid places to perform humidity-sensitive activities, they perform them at a specific phase of the day that usually presents high humidity.
Even though triatomines are frequently described as nocturnal insects, their general, spontaneous activity can be categorized as bimodal, provided that it splits into two different temporal windows: one that encompasses the first hours after dusk and a second during the first hour after dawn, each controlled by a different internal oscillator (Lazzari 1992). These two peaks of activity comprise all the various activities displayed by these bugs, including the modulation of related sensory sensitivities. As a result, part of these processes take place during the first activity period, for example, host search, feeding, departure from refuges, egg laying and dispersion by flight, while others occur during the second activity peak as, for example, ecdysis, egg hatching, refuge search and bug aggregation (Barrozo et al. 2004a, b; Constantinou 1984) (Fig. 2).
Host and refuge search are two linked, but mutually excluding behaviours in triatomines. When the animal has consumed its nutritional reserves, it should go out from its refuge to look for a potential host. Once gorged, the bug looks for shelter. The figure synthesises the different steps and stimuli associated with these two activities, in the form of a loop that makes bugs alternating between food and shelter search
The behavioural response to external stimuli is also rhythmically modulated as evidenced by the fact that the sensitivity of the insects is higher at the time in which a given cue or signal becomes biologically relevant. As an example, bug eyes become more sensitive to light during the night thus enhancing the negative phototactic response of bugs (Reisenman et al. 2002; Reisenman et al. 1998). The responsiveness to odours also varies in a very specific manner, as it is maximal to host odours during the nocturnal activity peak, but during the early morning peak in the case of aggregation pheromones. These modulatory changes match the time of the day in which each olfactory stimulus has biological relevance, maximizing response efficiency (Barrozo et al. 2004a, b; Bodin et al. 2008).
A rhythmic change in the thermopreference of bugs has been described in several triatomines species. It causes bugs to expose themselves to relatively higher temperatures at the beginning of the night, before displaying the first activity peak. In the early photophase at the end of the second peak, the bugs return to cooler places. This rhythm is also controlled by an internal circadian clock (Lazzari 1991b; Minoli and Lazzari 2003). It is suggested that this thermopreference rhythm is intended to increase the body temperature of bugs and, in turn, their metabolic rate , just before exhibiting their regular daily activity. Conversely, both body temperature and metabolism would be reduced during resting periods.
In summary, many triatomine activities and processes are modulated in the form of daily rhythms. Most of them express at the individual level (activity, oviposition etc.), while others take the form of population rhythms (egg hatching and ecdysis). Interestingly, all daily rhythms have an endogenous basis and are controlled by circadian clocks with the exception of the modulation of aggregation pheromone sensitivity (Bodin et al. 2008) and the adaptation of ocelli to light (Lazzari et al. 2011). The precise temporal allocation of every sensitivity and action would be significant enough to cause the development of dedicated, specific clocks by natural selection. The fact that each activity has a particular temporal window is not just the result of its association with one of the two activity periods but it is a result of the temporal adjustment of that activity to a particularly adequate temporal context, for example, host resting period. The study of the chronobiology of triatomines also contributes to the understanding of the insect clock structure and the manner in which it coordinates different physiological processes (Steel and Vafopoulou 2006; Vafopoulou et al. 2007; Vafopoulou et al. 2010), revealing once more the value of these bugs as model systems to analyse fundamental biological questions.
One important consequence for researchers dealing with this strong temporal organization of triatomine physiology and behaviour is the conclusion that any process of interest must be studied in its appropriate temporal context . If the proper timing is not respected in studies of this nature, the results obtained may be biased, therefore masking the significant patterns (Lazzari et al. 2004) (Fig. 3).
The daily organisation of a kissing bug’s life. Typically nocturnal, these insects distribute their different activities in two temporal windows, one at dusk and another at dawn. Bug sensitivity for stimuli such as odours and light is also modulated accordingly, in order to render them more sensitive and more prone to learn about their environment during the night hours
7 Behavioural Manipulation
Controlling disease vectors by exploiting their behaviour requires a deep knowledge of their biology. Information such as the identity of relevant attractive cues, the profile of preferred shelters and environments, as well as the estimation of their ability to escape from traps is potentially useful for the planning of sampling, detection and killing activities designed for controlling these insects. It is also critical that the stimuli used to attract the insects, as well as their presentation context (i.e. environment, timing), were biologically relevant and coherent to the insect, and not just a combination of all potentially attractive stimuli (pheromones, host odours, light); any association of stimuli must be significant for the insect. Here, ‘biological context’ means a clear message (i.e. host, refuge, partner), an adequate spatial setting (indoors, outdoors, ground, wall, roof) and timing that matches the proposed information to the insect needs (e.g. time for host search or for refuge localization).
In addition to light-traps employed for trapping nocturnal insects , whose attractivity principle is not fully understood, trapping triatomines and other blood-sucking insects is mostly based on the use of baits delivering host-associated cues. The most efficient is, as expected, the use of live hosts which usually consist of a mouse or baby chicken (Abad-Franch et al. 2000; Noireau et al. 1999, 2002). The use of live hosts as bait can sometimes be impractical due to the difficulty of providing them adequate care during extended field campaigns. Some alternative baits have been proposed and have already passed both laboratory and field testing. The simplest is using Baker’s yeast cultures (a simple mixture of yeast, sugar and water) producing carbon dioxide and other volatiles that are attractive for kissing bugs (Guerenstein et al. 1995; Lorenzo et al. 1998, 1999a, b; Pedrini et al. 2009; Pires et al. 2000). In addition, a relatively simple combination of multimodal cues including heat , water vapour, carbon dioxide and short-chain fatty acids has been reported (Ryelandt et al. 2011). Both types of baits described above deliver compounds recognized to be attractive to many haematophagous arthropods and are useful for capturing not only blood-sucking bugs, but also mosquitoes and ticks (Ryelandt et al. 2011; Smallegange et al. 2005).
Artificial refuges operate in a different manner than baited traps. Rather than attracting the bugs, they provide convenient resting places. Due to the physical structure that induces bugs to enter and remain inside, they can even be used without chemical baits. Eventually, the bugs will produce ‘their own bait’ when their excrement, containing aggregation pheromones , starts to accumulate, attracting other bugs searching for shelters. Cardboard boxes simulating refuges have been used as part of the intra-domiciliary surveillance of Chagas disease vectors in endemic areas of Latin America (Gómez-Núñez 1965; Wisnivesky-Colli et al. 1987). Other refuge-like devices , composed of simple, resistant materials, have also proven to be effective for outdoor bug detection (Vazquez-Prokopec et al. 2002). An attractive perspective for the use of shelter-like devices is the incorporation of chemical lures into artificial refuges, as tested by Mota et al. (2014) and Forlani et al. (2015). Some devices combine attractive baits with killing or trapping tools (Pedrini et al. 2009; Rojas de Arias et al. 2012).
8 Perspectives and Research Needs
Our present knowledge allows identifying relevant aspects of the triatomine biology needing additional research efforts. Moreover, further investigation could provide important insight into the fundamental aspects of haematophagy and help in the development of novel control tools. The availability of the genome sequence of Rhodnius prolixus is an extraordinary opportunity to dig deeper into the genetic and molecular bases of haematophagous behaviour, confirming once more this species as one of the most useful and fascinating models available in insect science. I will mention here only three aspects that, in the opinion of the author, need to be prioritized.
Dispersion
Dispersion is a key element for the spread of Chagas disease and for the reinfestation of treated houses (Abrahan et al. 2011). Several relevant questions remain to be answered, in particular, concerning the sensory ecology of long-distance displacements (e.g. the use of terrestrial and celestial navigation cues, orientation mechanisms).
Behavioural Impacts of Infection
It is well known that many parasites modify the physiology and behaviour of vertebrate hosts and insect vectors to their own advantage (Lehane 2005; Schaub 2006). The behaviour of parasitized bugs is a major issue for which very restricted data are available. Trypanosoma cruzi infections seem to have physiological consequences for the insects (Vallejo et al. 2009; Fellet et al. 2014; Elliot et al. 2015), and it is highly probable that their behaviour is also affected. It has been shown that some key behaviours, like feeding and activity, differ between infected and non-infected bugs (Botto-Mahan et al. 2006; Marliere et al. 2015), yet the impact of infection on other behavioural traits needs to be further investigated, along with the underlying mechanisms (Takano-Lee and Edman 2002).
Behavioural Manipulation
Synthetic pheromones could be used to interfere with bug communication in the sexual, alarm and aggregation contexts. Even though these alternatives appear as promissory options and despite the steadily advancing knowledge on this field, the use of synthetic pheromones for triatomine behavioural manipulation still requires further research efforts to be useful at an operational level.
References
Abad-Franch F, Noireau F, Paucar A, Aguilar HM, Carpio C, Racines J (2000) The use of live-bait traps for the study of sylvatic Rhodnius populations (Hemiptera: Reduviidae) in palm trees. Trans R Soc Trop Med Hyg 94:629–630
Abrahan LB, Gorla DE, Catala SS (2011) Dispersal of Triatoma infestans and other Triatominae species in the arid Chaco of Argentina – flying, walking or passive carriage? The importance of walking females. Mem Inst Oswaldo Cruz 106:232–239
Abramson CI, Romero ES, Frasca J, Fehr R, Lizano E, Aldana E (2005) Psychology of learning: a new approach to study behavior of Rhodnius prolixus Stål under laboratory conditions. Psychol Rep 97:721–731
Aldana E, Otalora F, Abramson CI (2005) A new apparatus to study behavior of triatomines under laboratory conditions. Psychol Rep 96:825–832
Aldana E, Abramson CI, Lizano E, Vegas R, Sulbaran-Romero E (2008) Learning and orientation to odor in the bug Rhodnius prolixus Stal 1859 under laboratory conditions. Parasitol Res 103:587–594
Alonso WJ, Schuck-Paim C (2006) The ‘ghosts’ that pester studies on learning in mosquitoes: guidelines to chase them off. Med Vet Entomol 20:157–165
Alonso WJ, Wyatt TD, Kelly DW (2003) Are vectors able to learn about their hosts? A case study with Aedes aegypti mosquitoes. Mem Inst Oswaldo Cruz 98:665–672
Alves CL, Araujo RN, Gontijo NF, Pereira MH (2011) Importance and physiological effects of hemolymphagy in triatomines (Hemiptera: Reduviidae). J Med Entomol 48:372–381
Alzogaray RA, Fontan A, Camps F, Masuh H, Santo OP, Fernandez D, Cork A, Zerba E (2005) Behavioural response of Triatoma infestans (Klug) (Hemiptera: Reduviidae) to quinazolines. Molecules 10:1190–1196
Ampleford EJ, Steel CGH (1982) Circadian control of ecdysis in Rhodnius prolixus (Hemiptera). J Comp Physiol A 147:281–286
Asparch Y, Pontes G, Masagué S, Minoli S, Barrozo RB (2016) Kissing bugs can generalize and discriminate between different bitter compounds. J Physiol Paris 110(3):99–106
Baldwin WF, Knight AG, Lyn KR (1971) A sex pheromone in the insect Rhodnius prolixus (Hemiptera: Reduviidae). Can Entomol 103:18–22
Barrett FM, Millen BF, Laifook J (1979) Brindley’s glands of Rhodnius prolixus. 1. Structure of the mature gland. Can J Zool 57:1109–1119
Barrozo RB (2019) Food recognition in hematophagous insects. Curr Opin Insect Sci 34:55–60
Barrozo RB, Lazzari CR (2004a) The response of the blood-sucking bug Triatoma infestans to carbon dioxide and other host odours. Chem Senses 29:319–329
Barrozo RB, Lazzari CR (2004b) Orientation behaviour of the blood-sucking bug Triatoma infestans to short-chain fatty acids: synergistic effect of L-lactic acid and carbon dioxide. Chem Senses 29:833–841
Barrozo RB, Manrique G, Lazzari CR (2003) The role of water vapour in the orientation behaviour of the blood-sucking bug Triatoma infestans (Hemiptera, Reduviidae). J Insect Physiol 49:315–321
Barrozo RB, Minoli SA, Lazzari CR (2004a) Circadian rhythm of behavioural responsiveness to carbon dioxide in the blood-sucking bug Triatoma infestans (Heteroptera: Reduviidae). J Insect Physiol 50:249–254
Barrozo RB, Schilman PE, Minoli SA, Lazzari CR (2004b) Daily rhythms in disease-vector insects. Biol Rhythm Res 35:79–92
Barrozo RB, Reisenman CE, Guerenstein PG, Lazzari CR, Lorenzo MG (2017) An inside look at the sensory biology of triatomines. J Insect Physiol 97:3–19
Bau J, Cardé RT (2015) Modelling optimal strategies for finding a resource-linked, windborne odor plume: theories, robotics, and biomimetic lessons from flying insects. Integr Comp Biol 55:461–477
Bernier UR, Kline DL, Barnard DR, Schreck CE, Yost RA (2000) Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal Chem 72:747–756
Bodin A, Barrozo RB, Couton L, Lazzari CR (2008) Temporal modulation and adaptive control of the behavioural response to odours in Rhodnius prolixus. J Insect Physiol 54:1343–1348
Bodin A, Vinauger C, Lazzari CR (2009a) Behavioural and physiological state dependency of host-seeking in the bloodsucking insect Rhodnius prolixus. J Exp Biol 212:2386–2393
Bodin A, Vinauger C, Lazzari CR (2009b) State-dependency of host-seeking in Rhodnius prolixus: the post-ecdysis time. J Insect Physiol 55:574–579
Bohman B, Weinstein AM, Unelius CK, Lorenzo MG (2018) Attraction of Rhodnius prolixus males to a synthetic female-pheromone blend. Parasite Vector 11:418
Botto-Mahan C, Cattan PE, Medel R (2006) Chagas disease parasite induces behavioural changes in the kissing bug Mepraia spinolai. Acta Trop 98:219–223
Cocchiararo-Bastias LM, Mijailovsky SJ, Calderon-Fernández GM, Lorenzo Figueiras AN, Juarez P (2011) Epicuticle lipids mediate mate recognition in Triatoma infestans. J Chem Ecol 37:246–252
Constantinou C (1984) Circadian rhythm of oviposition in the blood-sucking bugs, Triatoma phyllosoma, Triatoma infestans and Panstrongylus megistus (Hemiptera, Reduviidae). J Interdiscip Cycle Res 15:203–211
Cork A, Park KC (1996) Identification of electrophysiological-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med Vet Entomol 10:269–276
Crespo JG, Manrique G (2007) Mating behavior of the hematophagous bug Triatoma infestans: role of Brindley’s and metasternal glands. J Insect Physiol 53:708–714
Cruz-Lopez L, Malo EA, Rojas JC, Morgan ED (2001) Chemical ecology of triatomine bugs: vectors of Chagas disease. Med Vet Entomol 15:351–357
De Simone GA, Manrique G, Pompilio L (2018) Females’ sequential mating decisions depend on both the quality of the courting male and the quality of the potential mates in a blood-sucking bug. Behav Ecol Sociobiol 72:145
Diaz-Albiter HM, Ferreira TN, Costa SG, Rivas GB, Gumiel M, Cavalcante DR, Pavan MG, Gonzalez MS, de Mello CB, Dillon VM, Bruno RV, Garcia ED, Lima MM, de Castro DP, Dillon RJ, de Azambuja P, Genta FA (2016) Everybody loves sugar: first report of plant feeding in triatomines. Parasit Vectors 9
Duran P, Siñani E, Depickère S (2016) On triatomines, cockroaches and haemolymphagy under laboratory conditions: New discoveries. Mem Inst Oswaldo Cruz 111(10):605–613
Elliot SL, Rodrigues JO, Lorenzo MG, Martins-Filho OA, Guarneri AA (2015) Trypanosoma cruzi, etiological agent of Chagas disease, is virulent to its triatomine vector Rhodnius prolixus in a temperature-dependent manner. PLoS Negl Trop Dis 9:3
Fellet MR, Lorenzo MG, Elliot SL, Carrasco D, Guarneri AA (2014) Effects of infection by Trypanosoma cruzi and Trypanosoma rangeli on the reproductive performance of the vector Rhodnius prolixus. PLoS One 9(8)
Ferreira RA, Lazzari CR, Lorenzo MG, Pereira MH (2007) Do haematophagous bugs assess skin surface temperature to detect blood vessels? PLoS One 2:e932
Flores GB, Lazzari CR (1996) The role of the antennae in Triatoma infestans: orientation towards thermal sources. J Insect Physiol 42:433–440
Fontan A, Audino PG, Martinez A, Alzogaray RA, Zerb EN, Camps F, Cork A (2002) Attractant volatiles released by female and male Triatoma infestans (Hemiptera: Reduviidae), a vector of Chagas disease: chemical analysis and behavioral bioassay. J Med Entomol 39:191–197
Forlani L, Pedrini N, Girotti JR, Mijailovsky SJ, Cardozo RM, Gentile AG, Hernandez-Suarez CM, Rabinovich JE, Juarez MP (2015) Biological control of the Chagas disease vector Triatoma infestans with the entomopathogenic fungus Beauveria bassiana combined with an aggregation cue: field, laboratory and mathematical modelling assessment. PLoS Negl Trop Dis 9
Fresquet N, Lazzari CR (2011) Response to heat in Rhodnius prolixus: the role of the thermal background. J Insect Physiol 57:1446–1449
Friend WG, Smith JJ (1977) Factors affecting feeding by bloodsucking insects. Annu Rev Entomol 22:309–331
Garrouste R (2009) La première observation in natura de l’entomophagie de Panstrongylus geniculatus (Latreille 1811) hématophage vecteur de la maladie de Chagas (Hemiptera: Reduviidae). Ann Soc Entomol France 45:302–304
Giurfa M (2003) Cognitive neuroethology: dissecting non-elemental learning in a honeybee brain. Curr Opin Neurobiol 13:726–735
Giurfa M (2004) Comportement et cognition: ce que nous apprend un mini-cerveau. In: Vauclair J, Kreutzer M (eds) L’Ethologie cognitive, pp 83–99
Giurfa M, Zhang S, Jenett A, Menzel R, Srinivasan MV (2001) The concepts of ‘sameness’ and ‘difference’ in an insect. Nature 410:930–933
Gómez-Núñez JC (1965) Desarrollo de un nuevo método para evaluar la infestación intradomiciliaria por Rhodnius prolixus. Acta Cient Venez 16:26–31
Guarneri AA, Lazzari C, Diotaiuti L, Lorenzo MG (2002) The effect of relative humidity on the behaviour and development of Triatoma brasiliensis. Physiol Entomol 27:142–147
Guerenstein PG, Guerin PM (2001) Olfactory and behavioural responses of the blood-sucking bug Triatoma infestans to odours of vertebrate hosts. J Exp Biol 204:585–597
Guerenstein PG, Guerin PM (2004) A comparison of volatiles emitted by adults of three triatomine species. Entomol Exp Appl 111:151–155
Guerenstein PG, Hildebrand JG (2008) Roles and effects of environmental carbon dioxide in insect life. Annu Rev Entomol 53:161–178
Guerenstein PG, Lazzari CR (2009) Host-seeking: how triatomines acquire and make use of information to find blood. Acta Trop 110:148–158
Guerenstein PG, Núñez JA (1994) Feeding response of the hematophagous bugs Rhodnius prolixus and Triatoma infestans to saline solutions. A comparative-study. J Insect Physiol 40:747–752
Guerenstein PG, Lorenzo MG, Núñez JA, Lazzari CR (1995) Baker’s yeast, an attractant for baiting traps for Chagas’ disease vectors. Experientia 51:834–837
Kelly DW (2001) Why are some people bitten more than others? Trends Parasitol 17:578–581
Kelly DW, Thompson CE (2000) Epidemiology and optimal foraging: modelling the ideal free distribution of insect vectors. Parasitology 120:319–327
Lazzari CR (1991a) Circadian rhythm of egg hatching in Triatoma infestans (Hemiptera, Reduviidae). J Med Entomol 28:740–741
Lazzari CR (1991b) Temperature preference in Triatoma infestans (Hemiptera, Reduviidae). Bull Entomol Res 81:273–276
Lazzari CR (1992) Circadian organization of locomotion activity in the hematophagous bug Triatoma infestans. J Insect Physiol 38:895–903
Lazzari CR (2009) Orientation towards hosts in haematophagous insects: an integrative perspective. Adv Insect Physiol 37:1–58
Lazzari CR (2019) The thermal sense of blood-sucking insects: why physics matters. Curr Opin Insect Sci 34:112–116
Lazzari CR, Núñez JA (1989a) Blood temperature and feeding behavior in Triatoma infestans (Heteroptera, Reduviidae). Entomol Gener 14:183–188
Lazzari CR, Núñez JA (1989b) The response to radiant heat and the estimation of the temperature of distant sources in Triatoma infestans. J Insect Physiol 35:525–529
Lazzari CR, Reiseman CE, Insausti TC (1998) The role of the ocelli in the phototactic behaviour of the haematophagous bug Triatoma infestans. J Insect Physiol 44:1159–1162
Lazzari CR, Minoli SA, Barrozo RB (2004) Chemical ecology of insect vectors: the neglected temporal dimension. Trends Parasitol 20:506–507
Lazzari CR, Manrique G, Schilman PE (2006) Vibrational communication in Triatominae (Heteroptera: Reduviidae). In: Drumond JA, Claridge M (eds) Insect sounds and communication. Physiology, behaviour, ecology and evolution. CRC Press, Boca Raton, pp 297–304
Lazzari CR, Fischbein D, Insausti TC (2011) Differential control of light-dark adaptation in the ocelli and compound eyes of Triatoma infestans. J Insect Physiol 57:1545–1155
Lazzari CR, Pereira MH, Lorenzo MG (2013) Behavioural biology of Chagas disease vectors. Mem Inst Oswaldo Cruz 108(Suppl. I):34–47
Lazzari CR, Fauquet A, Lahondère C (2018) Keeping cool: kissing bugs avoid cannibalism by thermoregulating. J Insect Physiol 107:29–33
Lehane MJ (2005) The biology of blood-sucking in insects. Cambridge University Press, New York, pp xiii–321
Lorenzo Figueiras AN, Lazzari CR (1998a) Aggregation behaviour and interspecific responses in three species of Triatominae. Mem Inst Oswaldo Cruz 93:133–137
Lorenzo Figueiras AN, Lazzari CR (1998b) Aggregation in the haematophagous bug Triatoma infestans: a novel assembling factor. Physiol Entomol 23:33–37
Lorenzo Figueiras AN, Lazzari CR (2002) Aggregation behaviour and interspecific responses in Rhodnius prolixus Stål. Mem Inst Oswaldo Cruz 97:569–571
Lorenzo Figueiras AN, Kenigsten A, Lazzari CR (1994) Aggregation in the hematophagous bug Triatoma infestans – chemical signals and temporal pattern. J Insect Physiol 40:311–316
Lorenzo Figueiras AN, Girotti JR, Mijailovsky SJ, Juarez MP (2009) Epicuticular lipids induce aggregation in Chagas disease vectors. Parasit Vectors 2:8
Lorenzo MG, Lazzari CR (1996) The spatial pattern of defaecation in Triatoma infestans and the role of faeces as a chemical mark of the refuge. J Insect Physiol 42:903–907
Lorenzo MG, Lazzari CR (1998) Activity pattern in relation to refuge exploitation and feeding in Triatoma infestans (Hemiptera: Reduviidae). Acta Trop 70:163–170
Lorenzo MG, Reisenman CE, Lazzari CR (1998) Triatoma infestans can be captured under natural climatic conditions using yeast-baited traps. Acta Trop 70:277–284
Lorenzo MG, Flores GB, Lazzari CR, Reisenman CE (1999a) Sensory ecology. A: orientation. In: Carcavallo RU, Galindez-Giron I, Jurberg J, Lent H, Vol III (eds) Atlas of Chagas’ disease vectors in America. Fiocruz, Rio de Janeiro, pp 1071–1087
Lorenzo MG, Manrique G, Pires HH, de Brito Sanchez MG, Diotaiuti L, Lazzari CR (1999b) Yeast culture volatiles as attractants for Rhodnius prolixus: electroantennogram responses and captures in yeast-baited traps. Acta Trop 72:119–124
Manrique G, Lazzari CR (1994) Sexual behaviour and stridulation during mating in Triatoma infestans (Hemiptera: Reduviidae). Mem Inst Oswaldo Cruz 89:629–633
Manrique G, Lazzari CR (1995) Existence of a sex pheromone in Triatoma infestans (Hemiptera: Reduviidae): I. Behavioural evidence. Mem Inst Oswaldo Cruz 90:645–648
Manrique G, Vitta AC, Ferreira RA, Zani CL, Unelius CR, Lazzari CR, Diotaiuti L, Lorenzo MG (2006) Chemical communication in Chagas disease vectors. Source, identity, and potential function of volatiles released by the metasternal and Brindley’s glands of Triatoma infestans adults. J Chem Ecol 32:2035–2052
Marliere NP, Latorre-Estivalis JM, Lorenzo MG, Carrasco D, Alves-Silva J, Rodrigues JD, Ferreira LD, Lara LD, Lowenberger C, Guarneri AA (2015) Trypanosomes modify the behavior of their insect hosts: effects on locomotion and on the expression of a related gene. PLoS Negl Trop Dis 9
May-Concha I, Rojas JC, Cruz-Lopez L, Millar JG, Ramsey JM (2013) Volatile compounds emitted by Triatoma dimidiata, a vector of Chagas disease: chemical analysis and behavioural evaluation. Med Vet Entomol 27:165–174
McCall PJ, Kelly DW (2002) Learning and memory in disease vectors. Trends Parasitol 18:429–433
Mengoni SL, Lorenzo Figueiras AN, Minoli SA (2017) Experience-dependent modulation of the attraction to faeces in the kissing bug Triatoma infestans. J Insect Physiol 98:23–28
Mesquita RD, Vionette-Amaral RJ, Lowenberger C et al (2015) Genome of Rhodnius prolixus, an insect vector of Chagas disease, reveals unique adaptations to hematophagy and parasite infection. Proc Natl Acad Sci U S A 112:14936–14941
Minoli SA, Lazzari CR (2003) Chronobiological basis of thermopreference in the haematophagous bug Triatoma infestans. J Insect Physiol 49:927–932
Minoli SA, Palotini F, Manrique G (2013) The main component of an alarm pheromone of kissing bugs plays multiple roles in the cognitive modulation of the escape response. Front Behav Neurosci 7:77
Mota T, Vitta ACR, Lorenzo-Figueiras AN, Barezani CP, Zani CL, Lazzari CR, Diotaiuti L, Jeffares L, Bohman B, Lorenzo MG (2014) A multi-species bait for Chagas disease vectors. PLoS Negl Trop Dis 8
Noireau F, Flores R, Vargas F (1999) Trapping sylvatic Triatominae (Reduviidae) in hollow trees. Trans R Soc Trop Med Hyg 93:13–14
Noireau F, Abad-Franch F, Valente SA, Dias-Lima A, Lopes CM, Cunha V, Valente VC, Palomeque FS, de Carvalho-Pinto CJ, Sherlock I, Aguilar M, Steindel M, Grisard EC, Jurberg J (2002) Trapping Triatominae in sylvatic habitats. Mem Inst Oswaldo Cruz 97:61–63
Núñez JA (1982) Food source orientation and activity in Rhodnius prolixus Stal (Hemiptera, Reduviidae). Bull Entomol Res 72:253–262
Ortiz MI, Molina J (2010) Preliminary evidence of Rhodnius prolixus (Hemiptera: Triatominae) attraction to human skin odour extracts. Acta Trop 113:174–179
Páez-Rondón O, Aldana EJ, Dickens J, Otálora-Luna F (2018) Ethological description of a fixed action pattern in a kissing bug (Triatominae): vision, gustation, proboscis extension and drinking of water and guava. J Ethol 36:107
Palottini F, Manrique G (2016) Compounds released by disturbed adults of the haematophagous bug Triatoma infestans (Hemiptera: Reduviidae): behavioural effects of single compounds and binary mixtures. Physiol Entomol 41:234–240
Pedrini N, Mijailovsky SJ, Girotti JR, Stariolo R, Cardozo RM, Gentile A, Juarez MP (2009) Control of pyrethroid-resistant Chagas disease vectors with entomopathogenic fungi. PLoS Negl Trop Dis 3(5):e434
Pires HH, Lazzari CR, Diotaiuti L, Lorenzo MG (2000) Performance of yeast-baited traps with Triatoma sordida, Triatoma brasiliensis, Triatoma pseudomaculata, and Panstrongylus megistus in laboratory assays. Rev Panam Salud Publica 7:384–388
Pires HHR, Abrao DO, Machado EMD, Schofield CJ, Diotaiuti L (2002a) Eye colour as a genetic marker for fertility and fecundity of Triatoma infestans (Klug, 1834) Hemiptera, Reduviidae, Triatominae. Mem Inst Oswaldo Cruz 97:675–678
Pires HH, Lazzari CR, Schilman PE, Diotaiuti L, Lorenzo MG (2002b) Dynamics of thermopreference in the Chagas disease vector Panstrongylus megistus (Hemiptera: Reduviidae). J Med Entomol 39:716–719
Pires HH, Lorenzo MG, Diotaiuti L, Lazzari CR, Lorenzo Figueiras AN (2002c) Aggregation behaviour in Panstrongylus megistus and Triatoma infestans: inter and intraspecific responses. Acta Trop 81:47–52
Pontes GB (2010) Comportamento sexual de Rhodnius prolixus. Doctoral dissertation. Fundação Oswaldo Cruz, Belo Horizonte, 160 pp
Pontes GB, Lorenzo MG (2012) Female metasternal gland odours mediate male aggregation in Rhodnius prolixus, a triatomid bug. Med Vet Entomol 26:33–36
Pontes GB, Bohman B, Unelius CR, Lorenzo MG (2008) Metasternal gland volatiles and sexual communication in the triatomine bug, Rhodnius prolixus. J Chem Ecol 34:450–457
Pontes GB, Noireau F, Lorenzo MG (2011) Behavioral evidence of an ectoparasitic interaction between Triatoma pseudomaculata Corrêa e Espínola (Heteroptera: Reduviidae) and Periplaneta americana (L.) (Blattodea: Blattidae). Neotrop Entomol 240(6):708–710
Pontes G, Zacharias CA, Manrique G, Lorenzo MG (2014) Female odours promote the activation of sheltered kissing bug Rhodnius prolixus males and modulate their orientation. Med Vet Entomol 28:257–263
Reisenman CE (2014) Hunger is the best spice: effects of starvation in the antennal responses of the blood-sucking bug Rhodnius prolixus. J Insect Physiol 71:8–13
Reisenman CE, Lazzari CR, Giurfa M (1998) Circadian control of photonegative sensitivity in the haematophagous bug Triatoma infestans. J Comp Physiol A 183:533–541
Reisenman CE, Figueiras ANL, Giurfa M, Lazzari CR (2000) Interaction of visual and olfactory cues in the aggregation behaviour of the haematophagous bug Triatoma infestans. J Comp Physiol A 186:961–968
Reisenman CE, Insausti TC, Lazzari CR (2002) Light-induced and circadian changes in the compound eye of the haematophagous bug Triatoma infestans (Hemiptera: Reduviidae). J Exp Biol 205:201–210
Roca MJ, Lazzari CR (1994) Effects of relative humidity on the hematophagous bug Triatoma infestans: Hygropreference and eclosion success. J Insect Physiol 40:901–907
Rojas de Arias A, Abad-Franch F, Acosta N, López E, González N, Zerba E, Tarelli G, Masuh H (2012) Post-control surveillance of Triatoma infestans and Triatoma sordida with chemically-baited sticky traps. PLoS Negl Trop Dis 6(9):e1822
Ryelandt J, Noireau F, Lazzari CR (2011) A multimodal bait for trapping blood-sucking arthropods. Acta Trop 117:131–136
Sabelis MW, Schippers P (1984) Variable wind directions and anemotactic strategies of searching for an odour plume. Oecologia 63:225–228
Schaub GA (1988) Direct transmission of Trypanosoma cruzi between vectors of Chagas’ disease. Acta Trop 45:11–19
Schaub GA (2006) Parasitogenic alterations of vector behaviour. Int J Med Microbiol 296(Suppl 40):37–40
Schaub G, Böker CA, Jensen C, Reduth JD (1989) Cannibalism and coprophagy are modes of transmission of Blastocrithidia triatomae (Trypanosomatidae) between triatomines. J Protozool 36:171–175
Schilman PE, Minoli SA, Lazzari CR (2009) The adaptive value of hatching towards the end of the night: lessons from eggs of the haematophagous bug Rhodnius prolixus. Physiol Entomol 34:231–237
Schmitz H, Trenner S, Hofmann MH, Bleckmann H (2000) The ability of Rhodnius prolixus (Hemiptera; Reduviidae) to approach a thermal source solely by its infrared radiation. J Insect Physiol 46:745–751
Schofield CJ, Patterson JW (1977) Assembly pheromone of Triatoma infestans and Rhodnius prolixus nymphs (Hemiptera: Reduviidae). J Med Entomol 13:727–734
Smallegange RC, Qiu YT, van Loon JJ, Takken W (2005) Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae). Chem Senses 30:145–152
Steel CG, Vafopoulou X (2006) Circadian orchestration of developmental hormones in the insect, Rhodnius prolixus. Comp Biochem Physiol A Mol Integr Physiol 144:351–364
Tabares M, Ortiz M, González Montoya MC, Carazzone C, Vives M, Molina J (2018) Behavioral responses of Rhodnius prolixus to volatile organic compounds released in vitro by bacteria isolated from human facial skin. PLoS Negl Trop Dis 12(4):e0006423
Takano-Lee M, Edman JD (2002) Lack of manipulation of Rhodnius prolixus (Hemiptera: Reduviidae) vector competence by Trypanosoma cruzi. J Med Entomol 39:44–51
Taneja J, Guerin PM (1995) Oriented responses of the triatomine bugs Rhodnius prolixus and Triatoma infestans to vertebrate odors on a servosphere. J Comp Physiol A 176:455–464
Taneja J, Guerin PM (1997) Ammonia attracts the haematophagous bug Triatoma infestans: behavioural and neurophysiological data on nymphs. J Comp Physiol A 181:21–34
Vafopoulou X, Steel CG, Terry KL (2007) Neuroanatomical relations of prothoracicotropic hormone neurons with the circadian timekeeping system in the brain of larval and adult Rhodnius prolixus (Hemiptera). J Comp Neurol 503:511–524
Vafopoulou X, Terry KL, Steel CGH (2010) The circadian timing system in the brain of the fifth larval instar of Rhodnius prolixus (Hemiptera). J Comp Neurol 518:1264–1282
Vallejo GA, Guhl F, Schaub GA (2009) Triatominae-Trypanosoma cruzi/T. rangeli: vector-parasite interactions. Acta Trop 110:137–147
Vazquez-Prokopec GM, Ceballos LA, Salomon OD, Gurtler RE (2002) Field trials of an improved cost-effective device for detecting peridomestic populations of Triatoma infestans (Hemiptera: Reduviidae) in rural Argentina. Mem Inst Oswaldo Cruz 97:971–977
Vinauger C, Lazzari CR (2015) Circadian modulation of learning abilities in a disease vector insect, Rhodnius prolixus. J Exp Biol 218:3110–3117
Vinauger C, Buratti L, Lazzari CR (2011a) Learning the way to blood: first evidence of dual olfactory conditioning in a blood-sucking insect, Rhodnius prolixus. Part I: Appetitive learning. J Exp Biol 214:3032–3038
Vinauger C, Buratti L, Lazzari CR (2011b) Learning the way to blood: first evidence of dual olfactory conditioning in a blood-sucking insect, Rhodnius prolixus. Part II: Aversive learning. J Exp Biol 214:3039–3045
Vinauger C, Pereira MH, Lazzari CR (2012) Learned host preference in a Chagas disease vector, Rhodnius prolixus. Acta Trop 122:24–28
Vinauger C, Lallement H, Lazzari CR (2013) Learning and memory in Rhodnius prolixus: habituation and aversive operant conditioning of the proboscis extension response (PER). J Exp Biol 216:892–900
Vinauger C, Lahondère C, Cohuet A, Lazzari CR, Riffell J (2016) Learning and memory in disease vector insects. Trends Parasitol 32:761–771
Vitta ACR, Lorenzo MG (2009) Copulation and mate guarding behavior in Triatoma brasiliensis (Hemiptera: Reduviidae). J Med Entomol 46:789–795
Vitta ACR, Mota TRP, Diotaiuti L, Lorenzo MG (2007) The use of aggregation signals by Triatoma brasiliensis (Heteroptera: Reduviidae). Acta Trop 101:147–152
Vitta ACR, Serrao JE, Lima ER, Vilela EF (2009) The Metasternal and Brindley’s glands of Triatoma brasiliensis Neiva (Hemiptera: Reduviidae). Neotrop Entomol 38:231–236
Ward JP (1981) A comparison of the behavioral responses of the hematophagous bug, Triatoma infestans, to synthetic homologs of 2 naturally-occurring chemicals (N-Butyric and Iso-Butyric Acid). Physiol Entomol 6:325–329
Wigglesworth VB, Gillett JD (1934) The function of the antennae in Rhodnius prolixus (Hemiptera) and the mechanism of orientation to the host. J Exp Biol 11:120–138
Wisnivesky-Colli C, Paulone I, Perez A, Chuit R, Gualtieri J, Solarz N, Smith A, Segura EL (1987) A new tool for continuous detection of the presence of triatomine bugs, vectors of Chagas disease, in rural households. Medicina (B Aires) 47:45–50
Zacharias CA, Pontes GB, Lorenzo MG, Manrique G (2010) Flight initiation by male Rhodnius prolixus is promoted by female odors. J Chem Ecol 36:449–451
Zopf L, Lazzari CR, Tichy H (2014a) Infrared detection without specialized infrared receptors in the bloodsucking bug Rhodnius prolixus. J Neurophysiol 112:1606–1615
Zopf L, Lazzari CR, Tichy H (2014b) Differential effects of ambient temperature on warm cell responses to infrared radiation in the bloodsucking bug Rhodnius prolixus. J Neurophysiol 111:1341–1349
Acknowledgements
The author admits that the work of many colleagues could not be included in this review because of space restrictions and he wants to apologize for this. Much of the work and ideas presented here have been possible thanks to innumerable discussions with a lot of colleagues along many years and to the support of international (WHO) and national agencies of different countries: ANR, CNRS, Le Studium and the University of Tours (France), CNPq, FIOCRUZ and FAPEMIG (Brazil), University of Buenos Aires and CONICET (Argentina).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lazzari, C.R. (2021). The Behaviour of Kissing Bugs. In: Guarneri, A., Lorenzo, M. (eds) Triatominae - The Biology of Chagas Disease Vectors . Entomology in Focus, vol 5. Springer, Cham. https://doi.org/10.1007/978-3-030-64548-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-64548-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64547-2
Online ISBN: 978-3-030-64548-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)