Abstract
About 40% of all patients suffer at least one perioperative complication after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC), with a small number needing ICU intervention for organ support. Despite chemotherapy-induced nephropathy and extensive perioperative volume/fluid shifts, the incidence of acute kidney injury with the need for renal replacement therapy is low (1.3–5.7%) [4], although many patients have a temporary increase in serum creatinine levels. Perioperative kidney injury is an important independent risk factor increasing mortality as much as 6.5 times. As a result a detailed risk assessment with focus on renal function should be performed preoperatively. Risk factors include chronic kidney disease, high BMI, hyperglycemia, preoperative hypoalbuminemia, scheduled OR time over 600 minutes, transfusion of blood products, and an expected blood loss of over 60 ml/kg [4].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Perioperative Complications
About 40% of all patients suffer at least one perioperative complication after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC), with a small number needing ICU intervention for organ support. Despite chemotherapy-induced nephropathy and extensive perioperative volume/fluid shifts, the incidence of acute kidney injury with the need for renal replacement therapy is low (1.3–5.7%) [4], although many patients have a temporary increase in serum creatinine levels. Perioperative kidney injury is an important independent risk factor increasing mortality as much as 6.5 times. As a result a detailed risk assessment with focus on renal function should be performed preoperatively. Risk factors include chronic kidney disease, high BMI, hyperglycemia, preoperative hypoalbuminemia, scheduled OR time over 600 minutes, transfusion of blood products, and an expected blood loss of over 60 ml/kg [4].
In addition, perioperative optimization of fluid balance, cardiac output, and oxygen supply should be achieved through the implementation of goal-directed therapy and the use of hemodynamic monitoring (see Chap. 35). Furthermore, nephrotoxic drugs should be avoided whenever possible, and an adequate renal perfusion should be sought. Perioperative pulmonary complications are also a major cause of morbidity after CRS and HIPEC procedures. Non-invasive ventilation or nasal high-flow systems should be used prophylactically after extubation to avoid atelectasis and reduce recruitment/de-recruitment damage, and thoracic epidural anesthesia should be implemented routinely for preventative therapy [8]. Septic shock is the leading cause of death after CRS and HIPEC. Optimal perioperative thermoregulation, improved fluid management, and multimodal pain therapy should be a focus for every anesthesiologist.
Other complications that should be considered perioperatively are the side effects of chemotherapy such as anaphylactic reactions, hypomagnesaemia following cisplatin application with the risk of amiodarone-refractory ventricular tachycardia, long QT syndrome following cisplatin infusion, arrhythmias or cardiomyopathies after doxorubicin or mitomycin C treatment, hyponatremia, lactate acidosis, or hyperglycemia after HIPEC with oxaliplatin in a dextrose-based carrier solution.
2 Thermoregulation
Maintaining normothermia throughout the procedure is an important goal during perioperative management.
During CRS, hypothermia should be minimized given the large abdominal wound surface. This can be achieved through convective heat or heated infusions. Perioperative hypothermia is a common complication and occurs in up to 70% of all cases. Consequences of hypothermia may include increased blood loss through the attenuated platelet and clotting-factor function, increased incidence of wound infections, weakened immune system, and tachycardia with increased oxygen consumption as well as potential myocardial ischemia and cardiac arrhythmias [5].
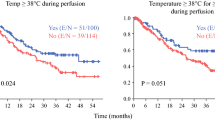
Systemic hyperthermia is also a possible risk due to the intra-abdominal temperatures of up to 42 °C during HIPEC. This can lead to an increased metabolic rate and oxygen imbalance with consequent tachycardia, increased end-tidal CO2 levels, and metabolic acidosis. In addition, the development of myocardial ischemia is a potential complication, especially in patients with pre-existing coronary heart disease [5]. Pulmonary edema, ARDS, and neurocognitive dysfunction are possible. Furthermore, it has been shown that temperatures above 42 °C can lead to neurological and electrophysical changes in peripheral nerves such as the phrenic nerve, the vagus, and the recurrent laryngeal nerve, resulting in dysfunction and dysesthesia [5].
3 Coagulation Management
CRS and HIPEC is often associated with significant blood loss [8].
This is due not only to the extent of the surgical procedure but also to an increased bleeding tendency, which is due to coagulation disorders in the context of hypo-/hyperthermia and to chemotherapeutic agents, cancer entity, or hemodilution and fluid shifts.
About one-third to one-half of patients require perioperative red blood cell concentrates. It should be kept in mind that allogeneic blood transfusions are an independent prognostic risk factor for the long-term survival of cancer patients. Blood transfusions are associated with increased morbidity and mortality in oncological surgery. For this reason, in addition to minimally invasive surgical work, the use of a cellsaver and consecutive radiotherapy with 50 Gy to eliminate tumor cells and retransfusion are an option to reduce allogeneic blood transfusions. Irradiated cellsaver blood has a markedly higher rate of morphologically intact red blood cells with higher levels of 2,3-diphosphoglycerate than allogenic RBCs [2, 3, 8]. Further prospective studies are needed to establish the long-term effects of irradiated cellsaver blood in cancer patients. In any case, the transfusion regime should be restrictive. Of course, the transfusion trigger depends on many perioperative factors, including patient morbidity, but the “cross-section guidelines of the German Medical Association on blood component therapy” with a transfusion trigger of 6–8 g/dl (3.7–5.0 mmol/l) should serve as a manifest basis [2].
About one-third of all HIPEC patients develop a clinically relevant bleeding tendency intraoperatively, resulting in a high transfusion rate of fresh frozen plasma (FFP). An international survey showed that >60% of all HIPEC centers perform pre-emptive therapy with FFP before any coagulopathy is evident clinically [1]. Routine application of FFP is currently performed in almost half of all HIPEC centers, whereas only 14% reported regular administration of tranexamic acid. A fall in fibrinogen levels may trigger bleeding during CRS and HIPEC. A regime of tranexamic acid and cryoprecipitate has shown promise in reducing the need for FFP or red blood cell concentrates [10]. In this context, it should be pointed out again that, following European and German transfusion guidelines (“cross-sectional guidelines” [2]), the transfusion of FFP is recommended only in patients with clinically evident bleeding after substitution of single factors such as fibrinogen or PPSB and exclusion of hyperfibrinolysis, since the application of FFP is associated with an increased risk of developing multi-organ failure and ARDS. Some HIPEC centers confirm this approach and report a significantly reduced transfusion rate of FFP of only 5% [4].
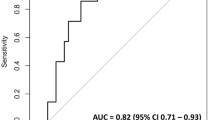
In more than 90% of all HIPEC centers, coagulation management so far has been controlled by standard coagulation laboratory parameters. Only about a fifth of all hospitals surveyed control coagulation by point-of-care (POC) systems such as thromboelastometry and/or platelet function analyzers [1]. However, it has been shown that, with the aid of these POC systems, the complex pathophysiological changes in coagulation are analyzed much better perioperatively and could be targeted better. Therefore, these devices may have an important role to play in coagulation management of patients undergoing CRS and HIPEC [8]. Last but not least, pre-emptive administration of 1 g tranexamic acid should be considered every 8 hours to prevent hyperfibrinolysis in patients with CRS and HIPEC since this can reduce blood loss and the use of red cell transfusions. A recent study has shown human fibrinogen concentrate may also be useful in managing bleeding during CRS and HIPEC when used with thromboelastometry [9].
4 Pain Therapy
It is generally accepted and evident that thoracic epidural anesthesia supplementing general anesthesia in the control of perioperative pain is clearly superior to general anesthesia alone.
Epidural anesthesia (EA) allows early postoperative extubation and mobilization, reduces postoperative pulmonary complications, reduces morbidity after CRS and HIPEC, and significantly improves patient satisfaction [8]. Postoperative ventilation was significantly shortened in patients after HIPEC, and the application of intravenous opioids, which in turn can lead to complications such as gastric atony, was reduced [5, 8]. It is not surprising that some studies have observed a significant reduction in postoperative ileus with epidural anesthesia. Many patients who undergo CRS and HIPEC are dependant on opioids pre-operatively with chronic pain and reduced quality of life, so adequate postoperative pain management can be challenging but is vital.
Supportive EA is now regarded as the gold standard in patients undergoing CRS and HIPEC. With EA it was possible to significantly reduce opioid and non-opioid analgesia as well as numeric rating scale (NRS) levels within the first 60 h after surgery. Residual functional capacity and vital capacity as well as the FEV1 of the lungs were improved and the balance between oxygen consumption and uptake reduced the risk of myocardial ischemia [5]. The fear that EA-induced sympathetic blockade together with the systemic effects of HIPEC leads to hemodynamic instability is unjustified, since these thermodynamic alterations can be avoided with targeted optimization of fluid administration [8].
As thrombocytopenia and coagulation disorders are often seen following CRS and HIPEC, an increased risk of an epidural hematoma has been suggested. Recent studies have shown that, for patients undergoing CRS and HIPEC, epidural anesthesia is a safe treatment option, and the risk of epidural hematoma is not higher than other surgical groups (1:6628) [6]. Considering that the main reasons for an epidural hematoma are the insertion of the catheter and a difficult and traumatic puncture, preoperative detailed coagulation tests as well as an atraumatic puncture and catheter insertion by an experienced anesthetist appear to reduce the risk of bleeding complications.
Finally, retrospective clinical investigations have shown an improvement in the long-term outcome and a reduction in metastatic growth after cancer surgery with supplemental EA [8]. Currently, three quarters of all HIPEC centers perform supportive EA [1, 7, 11].
5 Monitoring
Not all patients need to be monitored in the ICU after CRS and HIPEC procedures. Depending on the Eastern Cooperative Oncology Group (ECOG) performance status, the patient’s nutritional status, age, suspected blood loss, and the extent of cytoreduction, the indication for intensive care monitoring should be rigorous and take into consideration the potential complications such as the risk of infections and the costs of ICU stay.
In practice, most of the patients (67–100%) are admitted postoperatively to the ICU, as the fluid loss of up to 10 liters per day within the first 72 h after surgery is very high. It is similar with perioperative coagulation disorders and the ensuing bleeding, which usually occur within the first 24 h after surgery before returning to normal.
6 Conclusion
In order to avoid perioperative complications, the anesthesiologist and intensive care physician should focus especially on fluid management, thermoregulation, coagulation therapy, and adequate pain management.
References
Bell JC, Rylah BG, Chambers RW, Peet H, Mohamed F, Moran BJ. Perioperative management of patients undergoing cytoreductive surgery combined with heated intraperitoneal chemotherapy for peritoneal surface malignancy: a multi-institutional experience. Ann Surg Oncol. 2012;19:4244–51.
Bundesärztekammer. Querschnitts-Leitlinien (BÄK) zur Therapie mit Blutkomponenten und Plasmaderivaten. 2014. www.bundesaerztekammer.de/fileadmin/user_upload/downloads/QLL_Haemotherapie_2014.pdf. Last accessed 31.10.2019.
Hansen E, Bechmann V, Altmeppen J. Intraoperative blood salvage with irradiation of blood in cancer surgery: answers to current queries. Anasthesiol Intensivmed Notfallmed Schmerzther. 2002;37:740–4.
Kajdi ME, Beck-Schimmer B, Held U, Kofmehl R, Lehmann K, Ganter MT. Anaesthesia in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: retrospective analysis of a single centre three-year experience. World J Surg Oncol. 2014;12:136.
Kerscher C, Ried M, Hofmann HS, Graf BM, Zausig YA. Anaesthetic management of cytoreductive surgery followed by hyperthermic intrathoracic chemotherapy perfusion. J Cardiothorac Surg. 2014;9:125.
Korakianitis O, Daskalou T, Alevizos L, Stamou K, Mavroudis C, Iatrou C, et al. Lack of significant intraoperative coagulopathy in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) indicates that epidural anaesthesia is a safe option. Int J Hyperth. 2015;31(8):857–62.
Osseis M, Weyrech J, Gayat E, Dagois S, Lo Dico R, Pocard M, et al. Epidural analgesia combined with a comprehensive physiotherapy program after cytoreductive surgery and HIPEC is associated with enhanced post-operative recovery and reduces intensive care unit stay: a retrospective study of 124 patients. Eur J Surg Oncol. 2016;42(12):1938–43.
Raspé C, Flöther L, Schneider R, Bucher M, Piso P. Best practice for perioperative management of patients with cytoreductive surgery and HIPEC. Eur J Surg Oncol. 2017;43:1013–27.
Roy A, Stanford S, Nunn S, Alves S, Sargant N, Rangarajan S, et al. Efficacy of fibrinogen concentrate in major abdominal surgery – a prospective, randomized, controlled study in cytoreductive surgery for pseudomyxoma peritonei. J Thromb Haemost. 2020;18(2):352–63.
Sargant N, Roy A, Simpson S, Chandrakumaran K, Alves S, Coakes J, et al. A protocol for management of blood loss in surgical treatment of peritoneal malignancy by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Transfus Med. 2016;26(2):118–22.
Teoh DA, Hutton MJH, Else S, Walker A, Lee A, Mack LA. Epidural analgesia? A prospective analysis of perioperative coagulation in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Am J Surg. 2019;217(5):887–92.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Raspé, C., Rückert, F.F. (2021). Preventing Complications of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. In: Rau, B., Königsrainer, A., Mohamed, F., Sugarbaker, P.H. (eds) Peritoneal Tumors and Metastases. Springer, Cham. https://doi.org/10.1007/978-3-030-62640-2_33
Download citation
DOI: https://doi.org/10.1007/978-3-030-62640-2_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62639-6
Online ISBN: 978-3-030-62640-2
eBook Packages: MedicineMedicine (R0)