Abstract
As one of the promising upcoming alternatives to traditional drug therapy in central nervous system disorders and psychiatric disease, medical devices for neuromodulation have received a lot of attention. In addition to the invasive neural implant technologies used for deep-brain stimulation, a range of noninvasive brain stimulation (NIBS) techniques have recently been at the center of interest in research and therapy. Here, we provide an overview of the ever-growing family of NIBS methodologies, their clinical applications, and mechanisms of action involved. We suggest that NIBS technologies can be classified based on (1) the underlying technique (magnetic: transcranial magnetic stimulation, or electrical: transcranial electrical stimulation), (2) the targeted neurobiological process (ongoing processing, excitability/plasticity, oscillatory entrainment), or (3) the clinical domain of application (treatment, diagnosis, or prognosis). The current overview should prove valuable in understanding along which dimensions NIBS can be compared with traditional or alternative upcoming CNS modulation technologies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Noninvasive brain stimulation
- Transcranial magnetic stimulation
- Transcranial electrical stimulation
- Neuromodulation
- Plasticity
- Entrainment
- Clinical application
As one of the promising upcoming alternatives to traditional drug therapy in central nervous system disorders and psychiatric disease, medical devices for neuromodulation have received a lot of attention. In addition to the invasive neural implant technologies used for deep-brain stimulation, a range of non-invasive brain stimulation (NIBS) techniques have recently been at the center of interest in research and therapy. Here, we provide an overview of the ever-growing family of NIBS methodologies, their clinical applications, and mechanisms of action involved. We suggest that NIBS technologies can be classified based on (1) the underlying technique (magnetic: transcranial magnetic stimulation, or electrical: transcranial electrical stimulation), (2) the targeted neurobiological process (ongoing processing, excitability/plasticity, oscillatory entrainment), or (3) the clinical domain of application (treatment, diagnosis, or prognosis). The current overview should prove valuable in understanding along which dimensions NIBS can be compared with traditional or alternative upcoming CNS modulation technologies.
-
Gain an overview of the most commonly used NIBS techniques and protocols, including transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (TES).
-
Understand the physical and physiological mechanisms of action of TMS and TES.
-
Understand NIBS through three different classification schemes: (1) mechanisms of action, (2) targeted brain process, and (3) clinical application.
1 Introduction
We are now two decades from the “decade of the brain” (1990–2000), but progress in neuroscience has continued unabated. With no end in sight as of yet, the numbers of meaningful breakthroughs and neuroscience publications have only grown. In the wake of these developments, the last decade has seen an increase in more applied research as well as commercialization of brain-based treatment and products. For instance, treatment of depression with commercially available brain stimulation devices is now established, and the question is no longer whether such treatment is effective, but rather whether it should be considered as a first-line or even first-choice treatment. Such rapid progress was perhaps no surprise, as better understanding of the central nervous system (CNS) naturally led to developments of a range of techniques and tools that allow CNS interventions/modulation. Developments aiming both to facilitate ever more sophisticated research, and to measure (diagnosis, prognosis) and directly modulate (treatment) CNS activity in clinical applications.
Besides the invasive neural implant technologies used for deep-brain-stimulation, “non-invasive brain stimulation” (NIBS) has been referred to as one of the most promising families of devices/techniques. These tools have been around for a few decades now, but some of their recent incarnations and applications have exploded onto the clinical and research landscape only recently. Some of the newer applications are outlined in the companion chapter 8, where we also critically discuss limitations and unknowns. But roughly speaking, NIBS has in recent years demonstrated equivalent or even superior effects relative to alternative (e.g., drug) treatments of certain brain-based disorders, such as major depression disorder, with only minimal side effects. So, it seems useful to provide an overview of the NIBS toolkit in this chapter and then discuss the applications and limitations in the next chapter.
NIBS differs from other neuromodulation techniques in several key aspects. Firstly, it is non-invasive, in the sense that it does not penetrate (the skin remains intact) or introduce external substances into (e.g., drugs, neural implants, or electrodes) the body.
Definition
Non-invasive brain stimulation (NIBS): Altering neural activity through the (concurrent or preceding) application of a stimulus, such as electrical or magnetic, non-invasively through the intact skull.
This sets it apart from otherwise conceptually overlapping techniques such as deep brain stimulation (Aum and Tierney 2018), electroconvulsive therapy (ECT) (Lisanby 2007), optogenetics (Kim et al. 2017; Henderson et al. 2009), or chemical neuromodulation (Robbins 2000). Secondly, NIBS is considered a local (brain) neuromodulation approach, for instance able to target a cortical site of around a centimeter squared (Deng et al. 2013), as opposed to neuromodulation with chemicals that flood the system or system-level approaches such as neurofeedback (Sitaram et al. 2017). Thirdly, NIBS involves direct neuromodulation (stimulation), in the sense that it actively induces action potentials or modulates membrane potentials (Romero et al. 2019; Jackson et al. 2016). As such, NIBS directly affects activity in the building blocks of the CNS: neuronal firing.
Yet, even if NIBS is a more restrictive term than “neuromodulation,” it still encompasses a wide, and rapidly growing, family of techniques and approaches. In fact, the range of NIBS techniques and applications has grown to the point where it is not trivial to decide what sort of taxonomy (classification scheme) makes most sense. At the same time, it is crucial to maintain a meaningful overview and to allow evaluations and comparisons of NIBS techniques relative to other options for both research and clinical application within the dynamic landscape of CNS medical devices and approaches. Depending on one’s question, it might be useful to classify NIBS techniques according to the underlying physical mechanisms. This is a good starting point and perhaps the classical approach to categorizing the various techniques. Alternatively, it might actually be more valuable to classify NIBS techniques according to the biological mechanism they target/modulate. Indeed, it turns out that very different NIBS approaches might be interchangeable for certain interventions, at least conceptually (Dunlop et al. 2017; Blumberger et al. 2013). Lastly, it makes sense to evaluate the contributions of NIBS in different clinical settings, discussing NIBS approaches in the context of diagnosis, prognosis, or treatment. All three of these taxonomy schemes have merit, they are largely orthogonal, and together they offer a complete picture of the physical mechanisms underlying the modulation, the biological mechanisms modulated, and the range of resulting applications of NIBS in the lab and the clinic.
1.1 NIBS Techniques (◘ Table 7.1)
From the perspective of mechanisms of action, NIBS can be divided into magnetic and electrical techniques. Transcranial magnetic stimulation (TMS) allows magnetic stimulation (Barker et al. 1985).
Definition
TMS: transcranial magnetic stimulation. TMS involves the non-invasive delivery of a magnetic pulse to a brain region. This pulse can induce electric field/current that, if sufficiently strong, can depolarize neurons to induce action potentials.
TMS hardware includes a generally non-portable stimulation device positioned on a table or trolley, with a port to which different TMS coils can be connected. Inside the device, a large capacitor can charge up to high voltage, leading to a strong electrical current if a TMS coil is connected and an internal switch is flipped to close the circuit. The current flows through the TMS coil, which consists of one (circular coil) or two neighboring (butterfly or figure-8 coil) windings, housed in a synthetic protective casing which is placed on the skull of the patient (or over peripheral nervous system). Due to well-established physical principles of electromagnetic induction, the following sequence of events occurs: (1) the electric current through the TMS coil gives rise to a perpendicular magnetic field. Since the electric current is rapidly changing, the magnetic field is rapidly changing in proportion, becoming a magnetic “pulse.” (2) The magnetic pulse crosses the scalp and skull unhindered, noninvasively. (3) In the conductive neuronal tissue reached by the magnetic field, electrical activity is again induced (Polson et al. 1982; Walsh and Rushworth 1999; Jalinous 1991; Hallett 2007; Rossini et al. 2015; Kammer et al. 2001). The strength of the induced electric field and electric currents is proportional to the rate of change of the magnetic field, rather than the strength of the magnetic field directly (which, incidentally, is why an MRI scan does not stimulate the neurons) (Jalinous 1991; Barker 1991). This is also why different “waveforms” of the electric currents through the TMS coil can have different effects on the affected neurons (Kammer et al. 2001; Groppa et al. 2012). Irrespectively, the mechanism of TMS is that the magnetic pulse and associated induced electric field are sufficient to depolarize (most likely the axons of cortico-cortical inter-) neurons to achieve action potentials with each TMS pulse (Romero et al. 2019; Pashut et al. 2011).
Single suprathreshold TMS pulse and associated electric field are sufficient to depolarize neurons.
As outlined below, even such single TMS pulses can be used both in research and clinically. However, much of the excitement surrounding TMS as a research and treatment tool is based on repetitive application of single pulses. Repetitive TMS (rTMS) involves multiple pulses applied rhythmically in patterns of single or even multiple frequencies. Classical rTMS protocols such as 1 Hz rTMS and 10 Hz rTMS were soon found to affect targeted brain regions even beyond the period of stimulation (Pascual-Leone et al. 1994; Muellbacher et al. 2000). Recent patterned protocols, such as the 40-second continuous theta burst stimulation (cTBS) and the 3-minute intermittent theta burst stimulation (iTBS) protocols, were shown to have much longer after-effects on cortical excitability than the protocol duration; for cTBS up to an hour after the end of the stimulation (Huang et al. 2005). The cTBS protocol involves triplets of single pulses at 50 Hz, which themselves are presented in a 5 Hz rhythm, until 600 pulses are delivered. The iTBS protocol adds another pattern, that is, 2 seconds of such theta burst stimulation followed by 8 seconds of rest, until again 600 pulses are administered in total. Importantly, effects on excitability are (on average) inhibitory (1 Hz rTMS, cTBS) or excitatory (10 Hz rTMS, iTBS), depending on the precise parameters of the rTMS protocols. It has been suggested that both classical and patterned rTMS protocols engage synaptic plasticity mechanisms, such as long-term potentiation (LTP) and long-term depression (LTD), to achieve these impressive modulations of neuronal activity (Huang et al. 2007; Teo et al. 2007; Cirillo et al. 2017). However, the precise mechanisms involved remain unclear and may differ between rTMS protocols.
rTMS protocols are capable of affecting targeted brain regions beyond the period of stimulation. Depending on stimulation parameters, effects on excitability can be (on average) inhibitory (1 Hz rTMS, cTBS) or excitatory (10 Hz rTMS, iTBS).
The second family of NIBS applications involves transcranial electrical stimulation (TES).
Definition
TES: Transcranial electrical stimulation. Also referred to as low-intensity transcranial current stimulation, among other labels. Low-intensity current is administered to a brain region, not sufficiently strong to cause pain or to induce action potentials, yet sufficiently strong to modulate membrane potentials to change excitability. It can also have after-effects on excitability.
TES requires a, usually portable, stimulation device that primarily includes a battery and contact points for two connected electrodes. The latter come in different shapes and sizes, which directly affect the spatial configuration and intensity of stimulation in the brain (Woods et al. 2016). Low-intensity electric current flows between both electrodes, from the “anodal” electrode to the “cathodal” electrode (Paulus 2011). If continuous, these are referred to as anodal or cathodal transcranial direct current stimulation (tDCS) (Nitsche et al. 2008). Electric current can also be directed back and forth between both electrodes, which rhythmically switch polarity at a user-defined frequency, in transcranial alternating current stimulation (tACS) (Paulus 2011). A different setting on these machines causes current to switch direction much more often and at differing frequencies, which is called “transcranial random noise stimulation” (tRNS) (Terney et al. 2008). No matter which of these protocols are applied, in contrast to TMS (and also to electroconvulsive therapy (ECT), for example), TES primarily does not actually excite neurons to the point of action potentials. Instead, it achieves neuromodulation by changing the resting membrane potentials of affected neurons (Jackson et al. 2016; Radman et al. 2009). For instance, anodal tDCS depolarizes neurons slightly, bringing them “closer to threshold,” which means fewer excitatory inputs will be required to induce action potentials (Liebetanz et al. 2002). Cathodal tDCS instead hyperpolarizes, achieving the opposite effect on excitability. tRNS may increase excitability, but by less straightforward mechanisms (Antal and Herrmann 2016). tACS sinusoidally changes membrane potentials, mimicking naturally occurring neuronal oscillations (Krause et al. 2019). Interestingly, all of these modulations of cortical excitability seem to last beyond the period of stimulation, just as in rTMS (Bindman et al. 1964).
TES primarily does not actually excite neurons to the point of action potentials. Instead, it achieves neuromodulation by changing the resting membrane potentials of affected neurons.
There are ongoing developments in both these families of NIBS methodologies. For instance, TES is becoming more sophisticated by the use of more focal electrode montages (e.g., a small center electrode surrounded by a ring electrode or several small surrounding electrodes) (Sehm et al. 2013) and research in computational modeling to better understand the achieved distributions of induced electric fields for different electrode montages (Saturnino et al. 2018; Thielscher et al. 2015). In TMS, research to better understand the precise biological mechanisms affected by different protocols; how to tailor rTMS protocols to individual physiology; and how best to design coils and waveforms to achieve reliable or deeper (subcortical) modulation is underway (Deng et al. 2013; Romero et al. 2019; Cirillo et al. 2017; Cuypers et al. 2014; Banerjee et al. 2017; Medaglia et al. 2019). Fundamentally different tools that do not involve TMS or TES are arising as well. Still under investigation is “static magnetic stimulation” which involves a strong local static magnet (Oliviero et al. 2011), and recently “focused ultrasound” stimulation (Legon et al. 2014) has been receiving attention as a new alternative to TMS/TES with similar potential applications and a new set of pros and cons. Here, ultrasound at particular frequencies is directed toward particular brain regions, possibly mechanically causing neurites to “vibrate” and depolarize through entirely different mechanisms as compared to TMS (Krasovitski et al. 2011). While not yet as mainstream as TMS or TES, this ultrasound approach is an interesting avenue to follow going forward. We will restrict our further discussion to TMS and TES technologies.
1.2 The Biological Mechanisms in the CNS Targeted by NIBS
We provided an overview of NIBS approaches, mainly TMS and TES with various specifications, based on how these techniques work: the underlying physics. A very different perspective, and indeed different classification scheme, arises when we focus on their effects in the brain. In other words, we can also classify NIBS technologies according to the biological mechanism affected. We find it useful to delineate three targeted biological mechanisms, or three neuromodulation targets, to capture most NIBS applications.
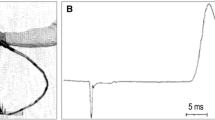
Non-repetitive TMS is often referred to as “single-pulse TMS” or “event-related TMS,” but this seems a bit restrictive. The point is that NIBS is used to excite neurons briefly. Every such administration of NIBS is momentary and delivers a datapoint. In research, event-related TMS can be used for “chronometric studies” for example, where a particular event (e.g., the presentation of a visual image on screen) is time-locked to TMS pulses (e.g., a single pulse 100 milliseconds after image presentation) to evaluate the causal role of the targeted cortical region (e.g., occipital cortex) for a particular function (e.g., image discrimination: an occipital pulse around 100 ms will impair discrimination or even make the image invisible) (de Graaf et al. 2011). Clinically, single TMS pulses can be applied to motor cortex to elicit motor-evoked potentials (MEPs) that can be measured with electromyography (EMG) (Rothwell et al. 1999). The amplitude of the MEP, which is simply a quantification of a TMS-induced motor twitch, has clinical implications. As we discuss below, this can be in diagnosis and prognosis, in isolation or coupled with neuroimaging techniques. Also under this category, one might consider paired-pulse TMS, which involves two TMS pulses administered in quick succession (Valls-Sole et al. 1992). When applied to the motor cortex, a subthreshold TMS pulse preceding a suprathreshold TMS pulse by around 2–7 milliseconds will reduce the MEP elicited by the latter pulse (Valls-Sole et al. 1992; Kujirai et al. 1993). This reduction is called short-interval cortical inhibition (SICI) and has clinical implications, as do long-interval cortical inhibition (LICI) and intracortical facilitation (ICF), all of which involve paired TMS pulses applied to motor cortex in different configurations of TMS intensity and inter-pulse intervals (Berardelli et al. 2008; McClintock et al. 2011; Rossini et al. 1994). By using two TMS coils, one over motor cortex and the other over anatomically/functionally connected regions, it is possible to quantify similar modulations of MEP by prior excitation of other motor network nodes (Hampson and Hoffman 2010). Interesting work has taken similar approaches beyond the motor system to understand cortical information flows (Pascual-Leone and Walsh 2001). Ultimately, these are all instances of momentary NIBS excitation of neurons to achieve different goals.
Plasticity targeted NIBS refers to the collection of NIBS tools and protocols that likely engage either LTD or LTP to transiently decrease or increase cortical excitability, respectively. To decrease cortical excitability, there are cathodal tDCS, classical inhibitory rTMS (1 Hz), and cTBS. To increase excitability, there are anodal tDCS, tRNS, classical excitatory rTMS (10 Hz), and iTBS (Rossini et al. 2015). It is not unequivocally established that all these protocols indeed similarly engage LTD and LTP, or that they rely on the same mechanisms of action in the brain, but that is not what is relevant here. What is relevant is that they all can serve the same functional purpose. If one wishes to increase excitability beyond the period of stimulation, or decrease it, there are these various – in many ways very different – options. One can weigh the pros and cons according to the use case, or the patient. In research, temporarily changing cortical excitability in a local brain region allows assessment of the causal contribution that region makes to various tasks (e.g., decreasing excitability might induce a task impairment). In clinical applications, treatment with NIBS builds on this foundation, though an additional mechanism of plasticity is somehow involved which remains imperfectly understood. After all, the effects of these NIBS protocols are in the range of minutes to hours, not weeks to years (Cirillo et al. 2017). Yet, the clinical efficacy of repeating such protocols over weeks has indeed been reported to last for such extended periods of time (Dunlop et al. 2017; Sonmez et al. 2019; Blumberger et al. 2018).
Entrainment is the final NIBS application to mention here. The human brain operates in large part by means of naturally occurring oscillations. The power and phase of these oscillations, in different frequency bands, have been related to various sorts of motor, cognitive, and perceptual functions (Ward 2003). In turn, neuromodulation of these oscillations is possible. Especially for research, short bursts of TMS pulses can briefly increase the power of oscillations in a particular frequency band in a particular region to evaluate the causal contribution of these oscillations to a task of interest (Thut et al. 2011). tACS can achieve the same for a longer period of time (Polania et al. 2012; Pogosyan et al. 2009). In fact, alpha power was increased even beyond the period of alpha-frequency tACS (Helfrich et al. 2014). Both methods allow the investigation and modulation of both oscillatory power and phase. Since many psychiatric disorders have been related to oscillatory/connectivity dysfunction, the direct NIBS-targeting of oscillations has the potential to make unique neuromodulatory contributions not only to the lab but also to the clinic (Hong et al. 2010; Michelini et al. 2018; Schnitzler and Gross 2005).
1.3 Clinical Applications of NIBS
In our companion Chapter 8, we place NIBS in the context of traditional clinical tools. Here, we would like to delineate the three core clinical applications of NIBS, providing a classification scheme along a third, more applied, dimension. These applications are diagnosis, prognosis, and treatment.
Diagnosis and prognosis currently primarily involve non-repetitive TMS, that is, either single-pulse TMS or paired-pulse paradigms as outlined above. In all cases, the principal idea is to assess responses to TMS pulses to obtain information about the current brain state (diagnosis) which may also have predictive value regarding the further development of a disorder/disease (prognosis). To illustrate, single-pulse TMS applied over primary motor cortex can typically elicit muscle twitches (motor-evoked potentials; MEP). However, damage to the corticospinal tract can cause subtle changes of MEP amplitudes and latencies, or even a complete absence of motor responses (Kobayashi and Pascual-Leone 2003). TMS thus allows probing the integrity of the motor system, which is a critical diagnostic step in the acute phase after stroke, a useful tool for monitoring changes during stroke rehabilitation, and even of prognostic value as the presence/absence of MEPs is indicative of the potential for long-term functional recovery (Di Pino et al. 2014).
Similarly, the minimum intensity required to observe a TMS-induced motor response (motor threshold) is an established measure of cortical excitability. While inter-individual variability of cortical excitability currently poses some limits in terms of specificity, there are promising applications of TMS as a diagnostic marker in epilepsy (Kimiskidis et al. 2014), and monitoring excitability changes over time can help in determining which particular antiepileptic drug effectively decreases cortical excitability without relying on the occurrence of seizures as a marker of treatment success (Badawy et al. 2012).
Lastly, the vast majority of studies have focused on diagnostic and prognostic applications of TMS in the motor system because of the simplicity of MEP recordings. However, the potential of TMS dramatically increases when combined with neuroimaging. In recent years, EEG has become very popular to assess brain responses to TMS pulses outside the motor system. In a pioneering research line, the simultaneous combination of TMS and EEG has been used to reveal how TMS-induced activity spreads throughout the brain in various disorders of consciousness. Strikingly, the complexity of the brain network response was sufficient to allow researchers to accurately classify individual patients as being in unresponsive wakefulness syndrome, a minimally conscious state, or locked-in syndrome (Sarasso et al. 2014). Admittedly, the technical complexities of such multimodal approaches currently constrain their application in clinical practice, but fully integrated systems to record TMS-induced changes in EEG activity are already emerging on the market.
Not only can NIBS be used to help establish a diagnosis, or inform a prognosis, it is perhaps most well-known for its application as actual brain-based treatment, in neurorehabilitation but especially also for psychiatric disorders. This application relies on the lasting effects on plasticity described above. In its currently most widespread clinical application, NIBS is applied to either increase cortical excitability in left frontal cortex or decrease cortical excitability in right frontal cortex to treat depression (Santre et al. 1995; O’Reardon et al. 2007; Hoppner et al. 2003). The evidence for efficacy is strongest for high-frequency left frontal rTMS (O’Reardon et al. 2007). As discussed above, anodal tDCS may have similar effects, since it should achieve the same thing: an increase in excitability (Brennan et al. 2017). This particular example at the same time exemplifies that things are never as straightforward as they seem: left frontal rTMS works well for treatment-resistant depression patients, while left frontal anodal tDCS actually receives more evidence for efficacy in non-treatment-resistant patients (Blumberger et al. 2013). This, as well as NIBS treatment efficacy in a range of other disorders, is under intense investigation. It seems NIBS might be helpful in the treatment of not only mood disorders but also neuropathic pain, motor disorders, anxiety disorders, and a range of other brain-based malfunctions (Chen et al. 2017; Chalah and Ayache 2019). Excellent, and at the same time exhaustive, reviews on the precise level of evidence for both rTMS and tDCS can be found in overview articles by Lefaucheur et al. (Lefaucheur et al. 2017; Lefaucheur et al. 2014) for rTMS and by O’Reardon et al. (2007) for tDCS. In fact, as elaborated in the companion chapter, an updated overview was recently published by an overlapping group of experts (Lefaucheur et al. 2020).
Conclusion
Non-invasive brain stimulation (NIBS) is an umbrella term for a wide and growing range of techniques and applications. There are so many techniques, and in fact we have presented three different classification schemes to create an overview of NIBS. NIBS applications were classified according to their physical principles, the biological mechanisms they targeted, and clinical applications. The value of NIBS in research is established. Its value in clinical applications is becoming increasingly clear and has received sufficient empirical support that implementation is widespread. But such rapid growth and acknowledgment come with a risk. NIBS to “improve” the healthy human brain (neuroenhancement) and as a sort of mental panacea (to cure all brain problems) has captured the public imagination, while at least some clinicians remain more wary. NIBS is a technique with such wide applications, at low cost, with minimal side effects, with minimal risks involved – does it not sound too good to be true? We address this question in the companion Chapter 8.
References
Antal A, Herrmann CS (2016) Transcranial alternating current and random noise stimulation: possible mechanisms. Neural Plast 2016:3616807
Aum DJ, Tierney TS (2018) Deep brain stimulation: foundations and future trends. Front Biosci (Landmark Ed) 23(1093–4715 (Electronic)):162–182
Badawy RAB et al (2012) Cortical excitability and refractory epilepsy: a three-year longitudinal transcranial magnetic stimulation study. Int J Neural Syst 23(01):1250030
Banerjee J et al (2017) Immediate effects of repetitive magnetic stimulation on single cortical pyramidal neurons. PLoS One 12(1):e0170528
Barker AT (1991) An introduction to the basic principles of magnetic nerve stimulation. J Clin Neurophysiol 8(1):26–37
Barker AT, Jalinous R, Freeston IL (1985) Non-invasive magnetic stimulation of human motor cortex. Lancet 325(0140–6736 (Print)):1106–1107
Berardelli A et al (2008) Consensus paper on short-interval intracortical inhibition and other transcranial magnetic stimulation intracortical paradigms in movement disorders. Brain Stimul 1(3):183–191
Bindman LJ, Lippold OC, Redfearn JW (1964) The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol 172:369–382
Blumberger DM, Mulsant BH, Daskalakis ZJ (2013) What is the role of brain stimulation therapies in the treatment of depression? Curr Psychiatry Rep 15(7):368
Blumberger DM et al (2018) Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 391(10131):1683–1692
Brennan S et al (2017) Anodal transcranial direct current stimulation of the left dorsolateral prefrontal cortex enhances emotion recognition in depressed patients and controls. J Clin Exp Neuropsychol 39(4):384–395
Chalah MA, Ayache SS (2019) Noninvasive brain stimulation and psychotherapy in anxiety and depressive disorders: a viewpoint. Brain Sci 9(4):82
Chen ML et al (2017) Non-invasive brain stimulation interventions for management of chronic central neuropathic pain: a scoping review protocol. BMJ Open 7(10):e016002
Cirillo G et al (2017) Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul 10(1):1–18
Cuypers K, Thijs H, Meesen RL (2014) Optimization of the transcranial magnetic stimulation protocol by defining a reliable estimate for corticospinal excitability. PLoS One 9(1):e86380
Deng Z-D, Lisanby SH, Peterchev AV (2013) Electric field depth–focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul 6(1):1–13
Di Pino G et al (2014) Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 10(10):597–608
Dunlop K, Hanlon CA, Downar J (2017) Noninvasive brain stimulation treatments for addiction and major depression. Ann N Y Acad Sci 1394(1):31–54
de Graaf TA, Herring J, Sack AT (2011) A chronometric exploration of high-resolution ‘sensitive TMS masking’ effects on subjective and objective measures of vision. Exp Brain Res 209(1):19–27
Groppa S et al (2012) A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 123(5):858–882
Hallett M (2007) Transcranial magnetic stimulation: a primer. Neuron 55(2):187–199
Hampson M, Hoffman RE (2010) Transcranial magnetic stimulation and connectivity mapping: tools for studying the neural bases of brain disorders. Front Syst Neurosci 4:40
Helfrich RF et al (2014) Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol 24(3):333–339
Henderson JM, Federici T, Boulis N (2009) Optogenetic neuromodulation. Neurosurgery 64(5):796–804
Hong LE et al (2010) Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology 35(3):632–640
Hoppner J et al (2003) Antidepressant efficacy of two different rTMS procedures. High frequency over left versus low frequency over right prefrontal cortex compared with sham stimulation. Eur Arch Psychiatry Clin Neurosci 253(2):103–109
Huang YZ et al (2005) Theta burst stimulation of the human motor cortex. Neuron 45(2):201–206
Huang Y-Z et al (2007) The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol 118(5):1028–1032
Jackson MP et al (2016) Animal models of transcranial direct current stimulation: methods and mechanisms. Clin Neurophysiol 127(11):3425–3454
Jalinous R (1991) Technical and practical aspects of magnetic nerve stimulation. J Clin Neurophysiol 8(1):10–25
Kammer T et al (2001) Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin Neurophysiol 112(2):250–258
Kim CK, Adhikari A, Deisseroth K (2017) Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci 18(4):222–235
Kimiskidis VK, Valentin A, Kalviainen R (2014) Transcranial magnetic stimulation for the diagnosis and treatment of epilepsy. Curr Opin Neurol 27(2):236–241
Kobayashi M, Pascual-Leone A (2003) Transcranial magnetic stimulation in neurology. Lancet Neurol 2(3):145–156
Krasovitski B et al (2011) Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc Natl Acad Sci U S A 108(8):3258–3263
Krause MR et al (2019) Transcranial alternating current stimulation entrains single-neuron activity in the primate brain. Proc Natl Acad Sci U S A 116(12):5747–5755
Kujirai T et al (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Lefaucheur JP et al (2014) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 125(11):2150–2206
Lefaucheur JP et al (2017) Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 128(1):56–92
Lefaucheur J-P et al (2020) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol 131(2):474–528
Legon W et al (2014) Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci 17(2):322–329
Liebetanz D et al (2002) Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125(Pt 10):2238–2247
Lisanby SH (2007) Electroconvulsive therapy for depression. N Engl J Med 357(19):1939–1945
McClintock SM et al (2011) Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry 70(1):19–27
Medaglia JD et al (2019) Personalizing neuromodulation. Int J Psychophysiol 154:101–110
Michelini G et al (2018) Shared and disorder-specific event-related brain oscillatory markers of attentional dysfunction in ADHD and bipolar disorder. Brain Topogr 31(4):672–689
Muellbacher W et al (2000) Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol 111(1388–2457 (Print)):1002–1007
Nitsche MA et al (2008) Transcranial direct current stimulation: state of the art 2008. Brain Stimul 1(3):206–223
O’Reardon JP et al (2007) Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 62(11):1208–1216
Oliviero A et al (2011) Transcranial static magnetic field stimulation of the human motor cortex. J Physiol 589(Pt 20):4949–4958
Pascual-Leone A, Walsh V (2001) Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science 292(5516):510–512
Pascual-Leone A et al (1994) Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117(Pt 4):847–858
Pashut T et al (2011) Mechanisms of magnetic stimulation of central nervous system neurons. PLoS Comput Biol 7(3):e1002022
Paulus W (2011) Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol Rehabil 21(5):602–617
Pogosyan A et al (2009) Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr Biol 19(19):1637–1641
Polania R et al (2012) The importance of timing in segregated theta phase-coupling for cognitive performance. Curr Biol 22(14):1314–1318
Polson MJ, Barker AT, Freeston IL (1982) Stimulation of nerve trunks with time-varying magnetic fields. Med Biol Eng Comput 20(2):243–244
Radman T et al (2009) Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul 2(4):215–228, 228 e1-3
Robbins TW (2000) Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res 133(1):130–138
Romero MC et al (2019) Neural effects of transcranial magnetic stimulation at the single-cell level. Nat Commun 10(1):2642
Rossini PM et al (1994) Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91:79–92
Rossini PM et al (2015) Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. committee. Clin Neurophysiol 126(6):1071–1107
Rothwell JC et al (1999) Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 52:97–103
Santre C et al (1995) Amikacin levels in bronchial secretions of 10 pneumonia patients with respiratory support treated once daily versus twice daily. Antimicrob Agents Chemother 39(1):264–267
Sarasso S et al (2014) Quantifying cortical EEG responses to TMS in (un)consciousness. Clin EEG Neurosci 45(1):40–49
Saturnino GB et al (2018) SimNIBS 2.1: a comprehensive pipeline for individualized electric field modelling for transcranial brain stimulation. bioRxiv:500314
Schnitzler A, Gross J (2005) Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 6(4):285–296
Sehm B et al (2013) A novel ring electrode setup for the recording of somatosensory evoked potentials during transcranial direct current stimulation (tDCS). J Neurosci Methods 212(2):234–236
Sitaram R et al (2017) Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci 18(2):86–100
Sonmez AI et al (2019) Accelerated TMS for depression: a systematic review and meta-analysis. Psychiatry Res 273:770–781
Teo JT, Swayne OB, Rothwell JC (2007) Further evidence for NMDA-dependence of the after-effects of human theta burst stimulation. Clin Neurophysiol 118(7):1649–1651
Terney D et al (2008) Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci 28(52):14147–14155
Thielscher A, Antunes A, Saturnino GB (2015) Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS? In: 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC)
Thut G et al (2011) Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol 21(14):1176–1185
Valls-Sole J et al (1992) Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85(6):355–364
Walsh V, Rushworth M (1999) A primer of magnetic stimulation as a tool for neuropsychology. Neuropsychologia 37(2):125–135
Ward LM (2003) Synchronous neural oscillations and cognitive processes. Trends Cogn Sci 7(12):553–559
Woods AJ et al (2016) A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 127(2):1031–1048
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
de Graaf, T.A., Thomson, A., Duecker, F., Sack, A.T. (2021). The Various Forms of Non-invasive Brain Stimulation and Their Clinical Relevance. In: Schreiber, R. (eds) Modern CNS Drug Discovery . Springer, Cham. https://doi.org/10.1007/978-3-030-62351-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-62351-7_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62350-0
Online ISBN: 978-3-030-62351-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)