Abstract
Magnetic resonance imaging (MRI) plays a central role in the diagnostic workup and clinical management of neuroimmunological diseases, including multiple sclerosis (MS). MRI is highly sensitive to visualize white matter signal abnormalities on T2-weighted images, and the characteristic appearance of demyelinating lesions has become well recognized. However, MRI is not pathologically specific, and differentiating MS from other (non-MS) conditions remains a challenge in the field. This chapter reviews the role of MRI in diagnosing and monitoring MS patients over time and discusses promising MR techniques that may increase the specificity of MRI for demyelination, including central vein imaging and cortical lesion detection, to help distinguish MS from other diagnoses. We also discuss possible applications of volumetric MRI as a way to monitor patients over time, and we highlight positron emission tomography (PET) methods that have been used in MS and may further our understanding of the biology of the disease, with particular focus on PET measures of microglial activation and remyelination.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Magnetic resonance imaging (MRI)
- Positron emission tomography (PET)
- Neuroimaging
- Multiple sclerosis (MS)
- Brain atrophy
-
1.
Because of its high sensitivity to detect white matter hyperintensities on T2-weighted images, magnetic resonance imaging (MRI) plays a central role in diagnosing and monitoring disease activity in multiple sclerosis (MS). MS plaques have a typical appearance on MRI (i.e., location, size, and morphology), which helps distinguish MS from other conditions. MRI can facilitate an earlier diagnosis of MS, often at first clinical presentation, by demonstrating dissemination in space and time within the central nervous system (CNS), the central tenet of MS Diagnostic Criteria.
-
2.
MS misdiagnosis is common when MRI criteria are not applied in the appropriate clinical context, particularly when the clinical presentation and/or MRI appearance is atypical or nonspecific. Detection of central veins within white matter lesions and/or demonstration of cortical lesions may increase the specificity of MRI for MS and are active areas of research currently.
-
3.
Neurodegeneration is a fundamental component of MS pathology. Whole brain volume decline on MRI can be measured reproducibly, is clinically relevant and modifiable with several available disease-modifying therapies, and has good face validity as a surrogate marker of neurodegeneration. There is also a high degree of interest in several regional volumes as MRI surrogate markers of neurodegeneration, including thalamus, other deep gray matter structures, and spinal cord.
-
4.
Positron emission tomography (PET) is a promising clinical research tool that can offer a high degree of pathologic specificity and can complement MRI to better understand MS pathology. PET can study aspects of the disease which are “MRI invisible,” such as microglial activation, or to which MRI is relatively insensitive, such as remyelination.
Introduction
Over the past few decades, magnetic resonance imaging (MRI) has evolved to play an essential role in the diagnosis and management of neuroimmunological diseases, including multiple sclerosis (MS), and has furthered our basic understanding of MS substantially. This chapter discusses MRI as a tool to diagnose and monitor MS, and to distinguish MS from other neurologic conditions. Volumetric MRI and positron emission tomography (PET) imaging are highlighted as specific areas of interest that are currently within the realm of MS clinical research, but may have applications for clinical care in the future.
The Role of MRI in the Diagnosis of MS
Despite having undergone multiple revisions, the central tenet of the diagnosis of MS has remained unchanged, that is, dissemination in space and time (DIS and DIT, respectively) within the central nervous system (CNS). Each iteration of the Diagnostic Criteria for MS, including the current 2010 Criteria [1] and the recently proposed 2017 revisions [2], has allowed the diagnosis of clinically definite MS to be made following two separate clinical events that demonstrate both DIS and DIT. MRI was first incorporated into the Diagnostic Criteria in 2001 [3] to facilitate earlier diagnosis at the time of the initial clinical presentation, that is, clinically isolated syndrome (CIS).
MRI is highly sensitive to visualize white matter (WM) signal abnormalities on T2-weighted images, and the characteristic appearance of demyelinating lesions has become well recognized. Typical demyelinating lesions are hyperintense on T2-weighted images, are round or ovoid in shape, are at least 3 mm in size (in-plane), and occur in characteristic locations within the CNS [4]. Because of the location of venules within the brain, classic periventricular demyelinating lesions are oriented perpendicularly to the long axis of the ventricles (so-called Dawson’s fingers; Fig. 3.1a, b). If demyelination involves the subcortical U-fibers, the lesions appear juxtacortical on MRI, which abut the cortex but respect the gray-white border (Fig. 3.1c). Demyelination often occurs in the infratentorium, commonly in the spinal cord, brainstem, middle cerebellar peduncle, or the deep WM of the cerebellum (Fig. 3.1d, f–h).
Brain and spinal cord MRIs demonstrating typical demyelinating lesions from a patient with MS. (a) Ovoid, periventricular lesions oriented perpendicularly to the long axis of the lateral ventricles on axial FLAIR. (b) The same periventricular lesions from Panel A are seen in the sagittal plane, oriented perpendicularly to the ventricle and involving the corpus callosum, classically described as “Dawson’s fingers.” (c) Typical juxtacortical lesions (arrows) involving the subcortical U-fibers, abutting the cortex and respecting the gray-white border. (d) Demyelinating lesions in the pons and middle cerebellar peduncle, typical for infratentorial location. (e) An acute demyelinating lesion showing ring enhancement on post-contrast T1-weighted image. (f) Demyelinating lesion in the cervical spinal cord on sagittal T2-weighted image, which is located, (g) in a typical dorsolateral position in the spinal cord on axial T2. (h) After gadolinium administration, this lesion demonstrates enhancement on post-contrast T1-weighted sagittal image
Contrast-enhanced MRI can be useful in differentiating acute or “active” lesions from chronic or “inactive” lesions [5] (Fig. 3.1e, h). Acute inflammation leads to breakdown of the blood-brain barrier, allowing leakage of the paramagnetic contrast agent gadolinium into the brain parenchyma. Most acute MS lesions enhance for 2–4 weeks [6, 7], though this time window is dependent on the dose and timing of contrast administration, particularly the delay in acquisition of the post-contrast images following gadolinium injection. Most MS lesions enhance in either a nodular or ring pattern, though dynamic imaging has shown that both patterns can be seen in the same lesion depending on when the image is acquired [8]. Initially, contrast extravasates from the inflamed central vein, spreads centrifugally outward, and will be seen as nodular enhancement. However, in more established acute lesions, the leakage of contrast occurs at the inflamed margins of the lesions, resulting in ring enhancement, which spreads centripetally over minutes [9]. Periventricular or juxtacortical white matter lesions may enhance in an “open ring” fashion, with the open portion of the ring facing either the ventricle or the cortex [9]. Persistent enhancement beyond 6 weeks is uncommon in MS and should prompt consideration of other etiologies, such as malignancy or sarcoidosis.
In the 2010 Criteria [1], DIS can be demonstrated by >1 T2 lesion in ≥2 of the four typical locations for MS (periventricular, juxtacortical, infratentorial, and spinal cord; Fig. 3.1). DIT can be demonstrated by a new T2 lesion on any follow-up scan, irrespective of its timing, or by the simultaneous presence of gadolinium-enhancing and non-enhancing lesions on a single scan. From an MRI perspective, the proposed 2017 revisions to the Diagnostic Criteria [2] are largely similar, but with some important modifications. Whereas the 2010 Criteria did not allow the symptomatic lesion to be included in the lesion count to satisfy DIS, the 2017 revisions no longer distinguish between symptomatic and asymptomatic lesions. Perhaps the biggest proposed revision is that, for the first time, lesions in the optic nerve and/or cortical lesions, if present, can be used to demonstrate DIS. As with previous revisions, the 2017 Criteria aim to facilitate an earlier diagnosis of MS, which they may achieve with increased sensitivity, but reduced specificity for a second attack [10]. However, there are some important caveats in the real-world application of the proposed 2017 MRI Criteria, with particular caution advised regarding cortical lesions. Cortical lesions are not routinely seen using standard clinical MRI protocols, and early attempts to detect cortical lesions such as double inversion recovery (DIR) are highly artifact-prone and suffer from poor sensitivity, specificity, and interobserver agreement [11,12,13]. Efforts to visualize cortical lesions are ongoing in the field and will be of interest (see “Cortical Lesion Detection”).
Increasing the Specificity of MRI for CNS Demyelination
It is of fundamental importance to understand that each version of the Diagnostic Criteria for MS, including the MRI Criteria, has been devised to facilitate an early MS diagnosis by demonstrating DIS and DIT in patients who present with a clinical syndrome that is typical of CNS demyelination. Current and historic MRI criteria were not created to differentiate MS from other (non-MS) conditions, nor were they designed to be applied in clinical scenarios in which the symptoms and/or MRI lesions are atypical for CNS demyelinating disease. Although MRI is highly sensitive to detect white matter abnormalities, it is inherently nonspecific pathologically. T2-hyperintensity can result from any process that increases the water content of the tissue and therefore may reflect not only demyelination, but also inflammation, edema, gliosis, or any combination thereof. In recent years, an emerging literature focused on MS misdiagnosis has found that the misinterpretation of MRI findings is a common cause of misdiagnosis, particularly when the clinical presentation is nonspecific or atypical [14]. Entities commonly misdiagnosed as MS include migraine, nonspecific symptoms with abnormal MRI, fibromyalgia, and conversion or psychogenic disorders [15]. This underscores the need to develop and incorporate MRI techniques with improved specificity for demyelination into routine clinical care, which is an area of high interest in the field currently.
Radiologically Isolated Syndrome
Occasionally, typical demyelinating lesions may be demonstrated incidentally on MRI obtained for an unrelated indication, such as headache or trauma. When there are no clinical symptoms or signs of MS but MRI demonstrates demyelinating-appearing lesions that meet 2005 DIS Criteria without alternate explanation, radiologically isolated syndrome (RIS) may be diagnosed [16]. Observational studies suggest that over a period of 5 years, roughly 1/3 of RIS patients will develop clinical symptoms and therefore fulfill Criteria for CIS/RRMS or PPMS [17, 18] suggesting that RIS is a pre-symptomatic stage of MS for many patients. However, because of the lack of typical clinical symptoms that would ordinarily provide specificity for CNS demyelination (e.g., optic neuritis, partial myelitis, or brainstem syndrome), diagnosing RIS requires extreme caution. Nonspecific T2 WM changes are common, and RIS may be misdiagnosed if MRI criteria for DIS are inappropriately applied, which is especially concerning if treatment is initiated. RIS is a poignant example of the need to develop techniques that can increase the specificity of MRI for CNS demyelination.
Central Vein Imaging
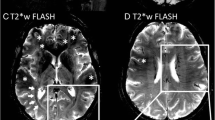
Central vein imaging is a promising technique to increase the specificity of MRI and was the topic of a recent Consensus Statement by the North American Imaging in MS (NAIMS) Cooperative [19]. That WM MS plaques form around venules was described pathologically over 100 years ago [20]. Susceptibility-based MR techniques (e.g., phase imaging, T2*, quantitative susceptibility mapping) are highly sensitive to iron and can be used to demonstrate the presence of a central vein within a T2-hyperintense lesion (Fig. 3.2a, b). One method with particular promise is FLAIR* [23], which combines 3D FLAIR and 3D T2*-weighted images in the post-processing setting (after they have been acquired). FLAIR* leverages the high sensitivity of FLAIR to demonstrate WM lesions, combined with the ability of T2* to detect blood vessels. Currently, FLAIR* is available on some commercial scanners, with increased availability across multiple MR manufacturers expected in the near future.
T2*-weighted image acquired at 7T demonstrating a hypointense central vein (arrow) on magnitude (a) and phase images (b). On phase images, a hypointense rim around the periphery of the lesion is also seen, which may suggest the presence of iron-laden microglia at the periphery of the demyelinating plaque. (c) High-resolution T2*-weighted image acquired at 7 T in axial plane showing an intracortical (Type 2) lesion (arrow). Source images in this figure were previously published and [21 (Panels A and B), 22 (Panel C)] and are used with permission
Further work is needed in order to implement central vein imaging into routine clinical care. The optimal MR technique to identify central veins is not known, and there is no standardized definition of the “central vein sign.” Relatively small studies using variable susceptibility-based MRI techniques and field strengths have demonstrated central veins in the majority (67–80%) of demyelinating lesions and the minority (20–30%) of WM lesions from other causes such as small vessel disease and vasculopathies [22,23,26]. Many of these studies have relied on manually counting the proportion of WM lesions that surround a central vessel, which is time-consuming and likely not feasible in clinical care. As such, simple, practical rules have been proposed to define and implement the central vein sign [19]. Once a standardized definition of the central vein sign is adopted, its operating characteristics (sensitivity, specificity, positive predictive value, and negative predictive value) will need to be determined prior to implementation into clinical care, not only in patients who present with typical demyelinating syndromes, but also in clinical scenarios with nonspecific/atypical symptoms and/or MRI findings, where it is perhaps needed most.
Cortical Lesion Detection
Cortical lesion detection may offer another approach to increase the specificity of MRI for CNS demyelination. Cortical lesions were described on histopathology several decades ago [27], but their clinical significance is not known due to difficulty visualizing them with current MR technology. Histopathologically, they are classified into Types 1, 2, and 3 (leukocortical, intracortical, and subpial, respectively) [28]. Subpial lesions are thought to be the most common, may span several gyri, and may be extensive, particularly in progressive MS [29]. Visualization of all three subtypes has been challenging, but particularly subpial lesions, which remain essentially undetected at conventional field strengths (1.5 and 3T). Multiple reasons for these challenges exist, including partial voluming from adjacent CSF, the small size of cortical lesions within an already thin cortex of 2–3 mm (which is often below the resolution of typical clinical images), and their relative lack of MR contrast due to their paucity of inflammation. Emerging MR techniques at higher resolutions and field strengths (7T) can generate better tissue contrast and may offer better visualization of these lesions (Fig. 3.2c). Recent work at 7T has described subpial lesions in the postmortem setting [30, 31]. However, translating this in vivo will require a clinically feasible scan time, which remains a challenge. Like central vein imaging, once cortical lesions can be reliably detected, further studies will be needed prior to implementation into routine clinical care to determine sensitivity and specificity prospectively in patients who present with typical demyelinating syndromes, and in clinical scenarios with nonspecific/atypical symptoms and/or MRI findings.
Role of MRI in Monitoring Patients with Established MS
New White Matter Lesion Formation
In addition to its utility in establishing the diagnosis of MS, MRI plays a central role in monitoring MS longitudinally. Formation of new lesions over time is one of the main hallmarks of MS, and detecting new lesions is one of the primary roles of MRI. The clinical relevance of new MRI lesion formation has been demonstrated conclusively as a predictor of clinical relapse in the short term [32] and of disability accrual in the longer term [33, 34]. Importantly, MRI lesions satisfy the stringent Prentice criteria as a statistically valid surrogate marker of clinical relapse both at the group level [35] and the individual level [36]. As such, in relapsing MS, it has become standard to use new MRI lesions as the primary endpoint in Phase 2 clinical trials to screen candidate drugs in the developmental pipeline. If a drug effectively prevents new MRI lesion formation in Phase 2, it has historically worked when tested in Phase 3, where clinical relapses are the primary endpoint.
Despite the clear implications of new MRI lesion formation, monitoring MS patients in clinical practice remains somewhat challenging. Although several national and international consortia have published recommendations for standardized MRI protocols by which to monitor MS patients in clinical practice [37, 38], these guidelines have not been adopted in the real world. Clinicians routinely face the challenge of manually comparing MRI scans that have been obtained on different MRI scanners with heterogeneous acquisition protocols, often with varying pulse sequences and tissue contrasts, and gaps between slices. Images are generally not realigned to facilitate lesion-by-lesion comparison. Manually determining new lesion formation can be particularly difficult in patients with a high lesion burden. Given these challenges, there has been interest in automated lesion detection algorithms, which may provide better power to detect new lesions [39, 40]. The output of these algorithms could be presented to clinicians to assist a manual reading and overall interpretation of the scan. Automated lesion detection remains an area of active interest.
In addition to new/enlarging T2 lesions, the detection of contrast-enhancing lesions is standard in clinical practice to monitor disease activity. Although there are certain clinical scenarios in which the detection of gadolinium enhancement is very useful (e.g., upon diagnosis, to exclude alternate diagnoses, or during a clinical relapse), there are limitations of relying on gadolinium enhancement to detect new MS disease activity on routine follow-up MRIs, which are often obtained annually. Because new MS lesions enhance with gadolinium for an average of 4 weeks [6, 7], detecting gadolinium enhancement on an annual MRI scan is essentially random, and new lesion formation can typically be identified on T2-weighted images without contrast. Moreover, recent descriptions of gadolinium accumulation in the brain [41] have raised concerns about administering repeated dosages of gadolinium over time. Whether gadolinium accumulation has long-term clinical effects is unknown; nonetheless, the North American Consortium of Multiple Sclerosis Centers (CMSC) MRI working group revised their guidelines to address this concern [38]. The new guidelines recommend judicious use of gadolinium, recognizing that gadolinium is essential when monitoring a patient with highly active disease, especially in the first few years, when there is an unexpected decline in the patient’s clinical status, upon first clinical presentation (i.e., at CIS), and when there is question of an alternative diagnosis.
Recommended MRI Protocol and Clinical Guidelines in Diagnosing and Monitoring MS
The reader is referred to published CMSC recommendations for standardized MRI protocols and clinical guidelines, most recently revised in 2018 [38]. Scans should be of good quality, with adequate signal-noise ratio (SNR) and spatial resolution (in slice pixel resolution of ≤1 mm × 1 mm), cover the whole brain, and have ≤3 mm slice thickness without gaps for 2D acquisition or 3D reconstruction. 3D acquisitions (≤1 mm × 1 mm × 1 mm isotropic voxel size) are generally recommended, but options for 2D acquisitions are also provided. Recommended core sequences include 2D/3D sagittal and axial FLAIR, 2D/3D axial T2, axial 2D DWI, 3D gradient echo T1, and post gadolinium 2D/3D axial T1 as required. The CMSC recommends a baseline scan and at 6 months after initiation of a DMT. Thereafter, a periodic brain MRI, typically annually, should be performed to assess subclinical disease activity. Interim imaging is indicated when there is unexpected decline or suspicion for a relapse or PML. Cervical spinal cord imaging is recommended at the time of diagnosis and if new symptoms develop that are referable to the spinal cord. Spinal cord MRI may also be useful to increase specificity while establishing the diagnosis in atypical presentations.
Volumetric MRI
Neurodegeneration is a fundamental component of MS pathology and probably results from multiple mechanisms, including axonal transection within WM lesions [42] causing downstream degeneration, as well as glutamate excitotoxicity, iron accumulation, mitochondrial dysfunction, and microglial activation. These mechanisms may lead to a final common pathway of oxidative stress that eventually overwhelms cellular compensatory mechanisms and results in neuronal cell death [29]. Whole brain volume decline on MRI likely represents the net accumulation of tissue damage in MS, including neuroaxonal, myelin, and glial cell loss, and reduced synaptic density. Neuroaxonal loss is thought to be the major pathologic substrate of irreversible clinical disability in MS [43]; as such, understanding and preventing neuroaxonal loss has become a major focus in the field.
Whole brain volume has been studied extensively in MS [44, 45]. Most studies suggest that the rate of whole brain atrophy in MS averages −0.7% per year, which is about 3x the rate of healthy controls [46]. In MS, whole brain volume loss correlates with several clinical endpoints, including ambulation [47], cognition [48], and quality of life [49]. Several available DMTs can slow the rate of whole brain atrophy significantly [44], and it has now become standard to include whole brain volume decline as a secondary or tertiary outcome in clinical trials testing primarily anti-inflammatory agents, and/or to use whole brain volume as the primary endpoint in phase 2 trials testing primarily neuroprotective agents [50].
Despite its many practical advantages, there are limitations of whole brain volume measurements. Whole brain volume may lack sensitivity in early phases of MS, such as CIS and RIS, though data in these phases are mixed [45]. Whole brain volume measurements, particularly at a single time point, can be confounded by several other factors that reduce the specificity for neurodegeneration, such as diurnal fluctuations [51], patient hydration status [52], or corticosteroid administration and/or newly initiated DMT (so-called pseudoatrophy [53]). Because of these limitations, interest has emerged in regional brain atrophy metrics, which may be more sensitive in earlier phases of MS and should be less confounded by tissue fluid dynamics. Thalamic volume loss, for example, is an early occurrence in MS and has been documented in RIS, CIS, and pediatric MS [52,53,54,57]. Thalamic volume declines persistently throughout the MS disease duration [58], correlates with clinical endpoints including cognition [56,57,60], and appears to provide feasible sample sizes as a primary MRI endpoint [58]. For these reasons, regional gray matter metrics, such as thalamic and other deep gray matter volumes, cortical thickness, and spinal cord volumes, are an active area of ongoing research in the field.
Despite a high degree of interest in volumetrics and their face validity as measures of neurodegeneration, several barriers exist in incorporating brain volume measurements into MS clinical practice, a topic which has been recently reviewed [61, 62]. The heterogeneous images that are collected in clinical practice present major challenges to current image processing software. No “gold standard” software to measure brain volumes has been identified. Most published work has assessed brain volume loss at the group level; the optimal statistical methods to translate brain volumes into a clinically meaningful metric at the individual level remain to be defined. Efforts have been made to define pathologic “cutoff” values for whole brain volume decline [63], but further work is needed to refine these values, including an adjustment for age. Because of the aforementioned fluctuations, brain volume decline over multiple time points may be more useful than single time point measurements, but this is difficult to implement in a clinical setting. Robust data from a large reference population that include both MS patients and healthy controls will be needed to develop an individual level metric. Statistical methods to adjust for variation from scan parameters, tissue fluid status, etc., could be developed, but these will need validation before implementing in the clinic [62].
Positron Emission Tomography (PET)
Although MRI is an invaluable tool in MS clinical practice and clinical research, it has limitations. MRI may not detect “other” types of inflammation besides WM lesions that are present in MS, such as microglial activation, and MRI does not reliably detect diffuse pathology in the NAWM and/or cortical GM. In addition, because MRI is pathologically nonspecific, its ability to measure remyelination is limited. Positron emission tomography (PET) can investigate aspects of MS not visualized by MRI and can provide a higher degree of specificity depending on the ligand used. PET measures radiation (positrons) emitted by specific radioisotopes tagged to a specific ligand, which binds to a target of interest. The signal measured by PET can provide in vivo quantitative information about the concentration of these target molecules. The use of PET in MS has been reviewed elsewhere [64, 65]. Herein, we focus on current PET approaches to measure microglial activation and remyelination.
Activated Microglia
Microglia are the key component of the innate CNS immune system and are activated in the setting of chronic inflammation [66]. Although activated microglia are an area of high interest in MS, their role in disease pathophysiology is not entirely clear. Histopathologically, activated microglia are seen at the edge of chronically active and expanding (aka “smoldering”) MS lesions, but not at chronic inactive lesions [67]. Both blood-derived monocytes and resident CNS microglia may contribute to neuronal damage by releasing pro-inflammatory cytokines and reactive oxygen species, leading to oxidative stress [68], which is thought to be an important mechanism in the pathophysiology of progressive MS [29]. Chronic, “smoldering” inflammation occurs behind an intact blood-brain barrier and is MRI-invisible, but it can be detected by PET strategies targeting activated microglia. This could enhance our fundamental understanding of MS and ultimately lead to the development of new therapeutic targets for progressive MS, which are urgently needed in the field.
Translocator protein (TSPO) is an 18 KDa protein that is expressed on the outer mitochondrial membrane of activated microglia and is the most commonly studied radiotracer target to visualize microglial activation in MS. Formerly known as the peripheral benzodiazepine receptor, TSPO is expressed predominantly on activated CNS resident microglia, but it is also found on blood-derived macrophages, reactive astrocytes, and vascular endothelial and smooth muscle cells [66]. TSPO is also expressed in the normal human brain, mainly neurons [69]. The first-generation radioligand, [11C]PK11195, has high specificity for TSPO, but binds to multiple cell types that express TSPO including reactive astrocytes, endothelial cells, and plasma proteins, in addition to activated microglia. [11C]PK11195 has a short half-life of about 20 minutes and a low signal-to-noise ratio, and signal quantification can be difficult. Typically, [11C]PK11195 binding quantification uses a normal reference region, but because such a region does not exist in MS, relatively complex mathematical modeling is required. The second-generation TSPO radioligands, such as [11C]PBR28 and [18F]PBR111, have higher binding affinity and better signal-to-noise ratio, but their binding affinity is affected by TSPO gene polymorphisms, making genetic testing mandatory for proper interpretation of the PET signal [70]. Like [11C]PK11195, the second-generation TSPO ligands also bind to activated astrocytes and endothelial cells [71, 72].
TSPO uptake is increased in MS plaques during relapse [73] (Fig. 3.3) and in some but not all chronic lesions [70, 75], potentially consistent with the “smoldering” inflammation described pathologically in chronic active lesions. Diffuse TSPO binding in NAWM has been shown in RRMS patients compared to healthy controls [76], and may be more pronounced in SPMS compared to RRMS [77]. TSPO uptake in the cortex, cortical lesions, deep GM, and NAWM in MS patients is also associated with worse clinical disability, cognitive function, and more cortical thinning on MRI [77].
MRI and 11C-PBR28 PET images from a patient with relapsing multiple sclerosis. Panels a–c show post-contrast T1-weighted MR images demonstrating an acute lesion that enhances following gadolinium administration (arrows) in sagittal (a), axial (b), and coronal (c) plane. Panels d–f show the corresponding VT (volume of distribution) parametric map demonstrating a focal increase (arrows) in uptake of 11C-PBR28, a second-generation TSPO ligand, perhaps suggesting the presence of activated microglia within these acute lesions. Source images in this figure were previously published and are used with permission [74]
It has been thought that TSPO expression on the surface of microglia is upregulated upon exposure to pro-inflammatory stimuli. However, TSPO was recently shown to be downregulated on macrophages exposed to pro-inflammatory stimuli and unchanged upon exposure to anti-inflammatory stimuli [78]. In another study, TSPO was upregulated upon exposure to pro-inflammatory stimuli in rodents, but did not change in human microglia exposed to the same stimulus [79]. The implications of these findings are not clear in humans with MS, but further study is needed to understand TSPO localization in MS brain tissue in situ [66] and the correct interpretation of TSPO binding in vivo in MS. It is possible that TSPO expression measured by PET may reflect microglial/macrophage density rather than activation status [79].
Myelination
Imaging myelin content is perhaps one of the most promising applications of PET imaging in MS. Developing remyelinating agents, which are currently lacking in the field, has become a major focus in MS clinical research. Remyelination should not only improve functional recovery after demyelinating injury in the short term, but should also protect axons in the long term, as chronically denuded axons are more susceptible to inflammatory insults that eventually lead to neurodegeneration [29]. One major barrier to developing remyelinating agents has been evaluating their efficacy in vivo. Compared to MR-based methods that have been used for this purpose (e.g., magnetization transfer ratio, diffusion tensor imaging, myelin water imaging), PET offers a more direct and specific measure of myelin and is thus a promising approach.
Stilbene derivative radiotracers bind to intact myelin sheath proteins such as PLP, MBP, or the sites of interactions between myelin lipids and these proteins [80]. Amyloid tracers such as Pittsburgh Compound B (PiB) also bind to myelin and may be useful. PiB has been shown to be highly sensitive to detect myelin loss in EAE [81] and has been used in humans longitudinally to create a global index of myelin content change in a voxel-wise, individual-level analysis [82]. However, PiB must be mixed in an on-site cyclotron prior to administration to the patient, which many centers do not have; newer fluorinated amyloid tracers such as florbetapir, flutemetamol, and florbetaben may improve availability because they are more stable and do not require a cyclotron.
In addition to its low availability, several limitations of PET exist. PET has a low spatial resolution (typically 2–3 mm at best), which can make changes in small lesions difficult to detect due to partial voluming. PET is an expensive technique that requires a high degree of on-site expertise, and data analysis/signal quantification can be challenging. Finally, radiation exposure is a concern with PET, particularly in longitudinal studies.
Conclusion
MRI plays a central role in the diagnosis and clinical management of MS due to its high sensitivity, but approaches such as central vein imaging and cortical lesion detection are needed to increase specificity for CNS demyelination. MRI is a useful tool to monitor disease activity and assess efficacy of DMTs both in clinical trials and in clinical practice. Brain volume decline is clinically relevant, but more work is needed before incorporation of volumetrics into routine clinical care. Finally, PET may further our understanding of the disease biology by its ability to study specific aspects of the disease not visible with MRI.
References
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302.
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–73.
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–7.
Fazekas F, Barkhof F, Filippi M, Grossman RI, Li DKB, McDonald WI, et al. The contribution of magnetic resonance imaging to the diagnosis of multiple sclerosis. Neurology. 1999;53(3):448–56.
Filippi M, Rocca MA, Barkhof F, Brück W, Chen JT, Comi G, et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012;11(4):349–60.
Filippi M. Enhanced magnetic resonance imaging in multiple sclerosis. Mult Scler. 2000;6(5):320–6.
Cotton F, Weiner HL, Jolesz FA, Guttmann CRG. MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology. 2003;60(4):640–6.
Lassmann H. A dynamic view of the blood-brain barrier in active multiple sclerosis lesions. Ann Neurol. 2011;70(1):1–2.
Gaitán MI, Shea CD, Evangelou IE, Stone RD, Fenton KM, Bielekova B, et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann Neurol. 2011;70(1):22–9.
Van Der Vuurst De Vries RM, Mescheriakova JY, YYM W, Runia TF, Jafari N, Samijn JP, et al. Application of the 2017 revised McDonald criteria for multiple sclerosis to patients with a typical clinically isolated syndrome. JAMA Neurol. 2018;75(11):1392–8.
Geurts JJG, Roosendaal SD, Calabrese M, Ciccarelli O, Agosta F, Chard DT, et al. Consensus recommendations for MS cortical lesion scoring using double inversion recovery MRI. Neurology. 2011;76(5):418–24.
Seewann A, Kooi EJ, Roosendaal SD, Pouwels PJW, Wattjes MP, Van Der Valk P, et al. Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology. 2012;78(5):302–8.
Sethi V, Muhlert N, Ron M, Golay X, Wheeler-Kingshott CA, Miller DH, et al. MS cortical lesions on DIR: not quite what they seem? PLoS One. 2013;8(11):8–12.
Solomon AJ, Bourdette DN, Cross AH, Applebee A, Skidd PM, Howard DB, et al. The contemporary spectrum of multiple sclerosis misdiagnosis. Neurology. 2016;87(13):1393–9.
Solomon AJ, Corboy JR. The tension between early diagnosis and misdiagnosis of multiple sclerosis. Nat Rev Neurol. 2017;13(9):567–72.
Okuda DT, Mowry EM, Beheshtian A, Waubant E, Baranzini SE, Goodin DS, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology. 2009;72(9):800–5.
Okuda DT, Siva A, Kantarci O, Inglese M, Katz I, Tutuncu M, et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One. 2014;9(3):e90509.
Kantarci OH, Lebrun C, Siva A, Keegan MB, Azevedo CJ, Inglese M, et al. Primary progressive multiple sclerosis evolving from radiologically isolated syndrome. Ann Neurol. 2016;79(2):288–94.
Sati P, Oh J, Todd Constable R, Evangelou N, Guttmann CRG, Henry RG, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the north American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12(12):714–22.
Dawson JW. The histology of disseminated sclerosis. Trans R Soc Edinburgh. 1916;50(03):517–740.
Hammond KE, Metcalf M, Carvajal L, Okuda DT, Srinivasan R, Vigneron D, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol 2008;64:707–13.
Metcalf M, Xu D, Okuda DT, Carvajal L, Srinivasan R, Kelley DAC, et al. High-resolution phased-array MRI of the human brain at 7 Tesla: Initial experience in multiple sclerosis patients. J Neuroimaging 2010;20:141–47.
Sati P, George IC, Shea CD, Gaitán MI, Reich DS. FLAIR*: a combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology. 2012;265(3):926–32.
Tallantyre EC, Brookes MJ, Dixon JE, Morgan PS, Evangelou N, Morris PG. Demonstrating the perivascular distribution of MS lesions in vivo with 7-tesla MRI. Neurology. 2008;70(22):2076–9.
Mistry N, Dixon J, Tallantyre E, Tench C, Abdel-Fahim R, Jaspan T, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70(5):623–8.
Sparacia G, Agnello F, Gambino A, Sciortino M, Midiri M. Multiple sclerosis: high prevalence of the ‘central vein’ sign in white matter lesions on susceptibility-weighted images. Neuroradiol J. 2018;31(4):356–61.
Lumsden C. The neuropathology of multiple sclerosis. In: Vinken PJ, Bruyn G, editors. Handbook of clinical neurology. Amsterdam: Elsevier; 1970. p. 217–309.
Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68(22 Supp):S22–31.
Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183–93.
Pitt D, Boster A, Pei W, Wohleb E, Jasne A, Zachariah CR, et al. Imaging cortical lesions in multiple sclerosis with ultra–high-field magnetic resonance imaging. Arch Neurol. 2010;67(7):812–8.
Kilsdonk ID, Jonkman LE, Klaver R, Van Veluw SJ, Zwanenburg JJM, Kuijer JPA, et al. Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: a post-mortem verification study. Brain. 2016;139(5):1472–81.
Sormani MP, Bruzzi P. MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol. 2013;12(7):669–76.
Fisniku LK, Brex PA, Altmann DR, Miszkiel KA, Benton CE, Lanyon R, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008;131(3):808–17.
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60(2):236–42.
Sormani MP, Bonzano L, Roccatagliata L, Cutter GR, Mancardi GL, Bruzzi P. Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol. 2009;65(3):268–75.
Sormani MP, Stubinski B, Cornelisse P, Rocak S, Li D, De Stefano N. Magnetic resonance active lesions as individual-level surrogate for relapses in multiple sclerosis. Mult Scler J 2011;17(5):541–549.
Traboulsee A, Létourneau-Guillon L, Freedman MS, O’Connor PW, Bharatha A, Chakraborty S, et al. Canadian expert panel recommendations for MRI use in MS diagnosis and monitoring. Can J Neurol Sci. 2015;42(3):159–67.
Consortium of MS centers MRI protocol and clinical guidelines for the diagnosis and follow-up of MS: 2018 Revised Guidelines [Internet]. Available from: www.mscare.org/mri
Moraal B, Wattjes MP, Geurts JJG, Knol DL, van Schijndel RA, Pouwels PJW, et al. Improved detection of active multiple sclerosis lesions: 3D subtraction imaging. Radiology. 2010 Apr;255(1):154–63.
Moraal B, Van Den Elskamp IJ, Knol DL, Uitdehaag BMJ, Geurts JJG, Vrenken H, et al. Long-interval T2-weighted subtraction magnetic resonance imaging: a powerful new outcome measure in multiple sclerosis trials. Ann Neurol. 2010;67(5):667–75.
McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275:150025.
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278–85.
Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206(2):165–71.
De Stefano N, Airas L, Grigoriadis N, Mattle HP, O’Riordan J, Oreja-Guevara C, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs. 2014;28(2):147–56.
Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol. 2006;5(2):158–70.
Vollmer T, Signorovitch J, Huynh L, Galebach P, Kelley C, DiBernardo A, et al. The natural history of brain volume loss among patients with multiple sclerosis: a systematic literature review and meta-analysis. J Neurol Sci. 2015;357(1–2):8–18.
Fisher E, Rudick RA, Simon JH, Cutter G, Baier M, Lee JC, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology. 2002;59(9):1412–20.
Rocca MA, Amato MP, De Stefano N, Enzinger C, Geurts JJ, Penner IK, et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302–417.
Mowry EM, Beheshtian A, Waubant E, Goodin DS, Cree BA, Qualley P, et al. Quality of life in multiple sclerosis is associated with lesion burden and brain volume measures. Neurology. 2009;72(20):1760–5.
Fox RJ, Coffey CS, Conwit R, Cudkowicz ME, Gleason T, Goodman A, et al. Phase 2 trial of Ibudilast in progressive multiple sclerosis. N Engl J Med. 2018;379(9):846–55.
Nakamura K, Brown RA, Narayanan S, Collins DL, Arnold DL. Diurnal fluctuations in brain volume: statistical analyses of MRI from large populations. NeuroImage. 2015;118:126–32.
Nakamura K, Brown RA, Araujo D, Narayanan S, Arnold DL. Correlation between brain volume change and T2 relaxation time induced by dehydration and rehydration: implications for monitoring atrophy in clinical studies. NeuroImage Clin. 2014;6:166–70.
Miller DH, Soon D, Fernando KT, MacManus DG, Barker GJ, Yousry TA, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68(17):1390–401.
Azevedo CJ, Overton E, Khadka S, Buckley J, Liu S, Sampat M, et al. Early CNS neurodegeneration in radiologically isolated syndrome. Neurol Neuroimmunol NeuroInflamm. 2015;2(3):e102.
Henry RG, Shieh M, Okuda DT, Evangelista A, Gorno-Tempini ML, Pelletier D. Regional grey matter atrophy in clinically isolated syndromes at presentation. J Neurol Neurosurg Psychiatry. 2008;79(11):1236–44.
Aubert-Broche B, Fonov V, Ghassemi R, Narayanan S, Arnold DL, Banwell B, et al. Regional brain atrophy in children with multiple sclerosis. NeuroImage. 2011;58(2):409–15.
Henry RG, Shieh M, Amirbekian B, Chung SW, Okuda DT, Pelletier D. Connecting white matter injury and thalamic atrophy in clinically isolated syndromes. J Neurol Sci. 2009;282(1–2):61–6.
Azevedo CJ, Cen SY, Khadka S, Liu S, Kornak J, Shi Y, et al. Thalamic atrophy in multiple sclerosis: a magnetic resonance imaging marker of neurodegeneration throughout disease. Ann Neurol. 2018;83(2):223–34.
Zivadinov R, Bergsland N, Dolezal O, Hussein S, Seidl Z, Dwyer MG, et al. Evolution of cortical and thalamus atrophy and disability progression in early relapsing-remitting MS during 5 years. Am J Neuroradiol. 2013;34(10):1931–9.
Houtchens MK, Benedict RHB, Killiany R, Sharma J, Jaisani Z, Singh B, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69(12):1213–23.
Rocca MA, Battaglini M, Benedict RHB, De Stefano N, Geurts JJG, Henry RG, et al. Brain MRI atrophy quantification in MS. Neurology. 2017;88(4):403–13.
Azevedo CJ, Pelletier D. Whole-brain atrophy: ready for implementation into clinical decision-making in multiple sclerosis? Curr Opin Neurol. 2016;29(3):237–42.
De Stefano N, Stromillo ML, Giorgio A, Bartolozzi ML, Battaglini M, Baldini M, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(1):93–9.
Moccia M, Ciccarelli O. Molecular and metabolic imaging in multiple sclerosis. Neuroimaging Clin N Am. 2017;27(2):343–56.
De Paula Faria D, Copray S, Buchpiguel C, Dierckx R, De Vries E. PET imaging in multiple sclerosis. J Neuroimmune Pharmacol. 2014;9(4):468–82.
Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35(3):306–28.
Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(5):1175–89.
Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, et al. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain. 2012;135(3):886–99.
Doble A, Malgouris C, Daniel M, Daniel N, Imbault F, Basbaum A, et al. Labelling of peripheral-type benzodiazepine binding sites in human brain with [3H]PK 11195: anatomical and subcellular distribution. Brain Res Bull. 1987;18(1):49–61.
Airas L, Nylund M, Rissanen E. Evaluation of microglial activation in multiple sclerosis patients using positron emission tomography. Front Neurol. 2018;9:181.
Dickens AM, Vainio S, Marjamaki P, Johansson J, Lehtiniemi P, Rokka J, et al. Detection of microglial activation in an acute model of neuroinflammation using PET and radiotracers 11C-(R)-PK11195 and 18F-GE-180. J Nucl Med. 2014;55(3):466–72.
Rizzo G, Veronese M, Tonietto M, Zanotti-Fregonara P, Turkheimer FE, Bertoldo A. Kinetic modeling without accounting for the vascular component impairs the quantification of [11C]PBR28 brain PET data. J Cereb Blood Flow Metab. 2014;34(6):1060–9.
Debruyne JC, Versijpt J, Van Laere KJ, De Vos F, Keppens J, Strijckmans K, et al. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur J Neurol. 2003;10(3):257–64.
Park E, Gallezot JD, Delgadillo A, Liu S, Planeta B, Lin SF, et al. 11C–PBR28 imaging in multiple sclerosis patients and healthy conrols: test-retest reproducibility and focual visualization of active white matter areas. Eur J Nucl Med Mol Imaging 2015;42:1081–92.
Rissanen E, Tuisku J, Rokka J, Paavilainen T, Parkkola R, Rinne JO, et al. In vivo detection of diffuse inflammation in secondary progressive multiple sclerosis using PET imaging and the radioligand 11C-PK11195. J Nucl Med. 2014;55(6):939–44.
Sucksdorff M, Rissanen E, Tuisku J, Nuutinen S, Paavilainen T, Rokka J, et al. Evaluation of the effect of fingolimod treatment on microglial activation using serial PET imaging in multiple sclerosis. J Nucl Med. 2017;58(10):1646–51.
Herranz E, Giannì C, Louapre C, Treaba CA, Govindarajan ST, Ouellette R, et al. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann Neurol. 2016;80(5):776–90.
Narayan N, Mandhair H, Smyth E, Dakin SG, Kiriakidis S, Wells L, et al. The macrophage marker translocator protein (TSPO) is down-regulated on pro-inflammatory ‘M1’ human macrophages. PLoS One. 2017;12(10):1–19.
Owen DR, Narayan N, Wells L, Healy L, Smyth E, Rabiner EA, et al. Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. J Cereb Blood Flow Metab. 2017;37(8):2679–90.
Wu C, Tian D, Feng Y, Polak P, Wei J, Sharp A, et al. A novel fluorescent probe that is brain permeable and selectively binds to myelin. J Histochem Cytochem. 2011;54(9):997–1004.
De Paula Faria D, Copray S, Sijbesma JW, Willemsen AT, Buchpiguel CA, Dierckx RA, et al. PET imaging of focal demyelination and remyelination in a rat model of multiple sclerosis: comparison of [11C]MeDAS, [11C]CIC and [11C]PIB. Eur J Nucl Med Mol Imaging. 2014;41(5):995–1003.
Bodini B, Veronese M, García-Lorenzo D, Battaglini M, Poirion E, Chardain A, et al. Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann Neurol. 2016;79(5):726–38.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rukmangadachar, L.A., Azevedo, C.J. (2021). Neuroimaging Modalities in Neuroimmunology. In: Piquet, A.L., Alvarez, E. (eds) Neuroimmunology. Springer, Cham. https://doi.org/10.1007/978-3-030-61883-4_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-61883-4_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-61882-7
Online ISBN: 978-3-030-61883-4
eBook Packages: MedicineMedicine (R0)