Abstract
Autoimmune neurologic disorders with overlapping clinical presentation are associated with a growing number of autoantibodies. Detection of these neural autoantibodies can play an important role in the diagnosis, prognosis, and management of patients with autoimmune neurologic disorders. Neural autoantibodies are commonly divided into categories based on the subcellular location of the antigens targeted. Autoantibodies against intracellular antigens are associated with paraneoplastic syndromes, whereas those against extracellular antigens are associated with a variety of disorders including autoimmune encephalitis and autoimmune neuromuscular junction diseases. Different methods are used in order to optimize the detection of these groups of autoantibodies with high sensitivity and specificity. In this chapter, we describe methods used to detect neural autoantibodies including their advantages and disadvantages, discuss the sensitivity and specificity of the assays and which biologic fluid(s) to test, and provide an introduction to the role of neural autoantibodies in the pathogenesis of autoimmune neurologic disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Autoantibody
- Tissue-based assay
- Cell-based assay
- Immunoblot
- Immunoprecipitation
- ELISA
- Paraneoplastic
- Autoimmune encephalitis
-
1.
Neural autoantibodies are markers of autoimmune neurologic disorders, with only a few shown to be pathogenic.
-
2.
Detection of neural autoantibodies can play an important role in the diagnosis, prognosis, and management of patients with autoimmune neurologic disorders.
-
3.
Failure to detect a neural autoantibody does not rule out an autoimmune neurologic disorder.
-
4.
Tests for detecting neural autoantibodies have complexities that must be considered, including the performance characteristics of the method used and the specimen type evaluated.
-
5.
Results of neural antibody testing must be interpreted within the clinical context; taking them as conclusive evidence of autoimmune neurologic disorder could be a mistake.
-
6.
The number of neural autoantibodies continues to grow, as does the number of specimens tested. This presents a challenge for both clinicians and laboratories in determining which autoantibodies to test, by which methods, and whether testing should be performed independently or in which combinations.
Introduction
Autoimmune neurology is a rapidly evolving field largely driven by the discovery of new autoantibodies (Table 2.1). Autoimmune neurologic disorders (ANDs) are a heterogeneous group of diseases thought to occur as a result of an aberrant immune response targeting the nervous system. Patients with these disorders are frequently identified by the detection of an autoantibody in their serum or cerebrospinal fluid (CSF), and thus the response is considered antigen specific. ANDs typically present with a subacute onset with rapid progression of symptoms that may affect any and often multiple parts of the nervous system. Thus, they can present with a wide array of symptoms ranging from nonspecific flu-like symptoms such as fever, headache, and pain to more specific neurologic symptoms including seizures, cognitive issues, movement disorders, dysautonomia, and psychiatric symptoms and can even result in loss of consciousness or death. Due to this wide array of symptoms, there are a number of other potential causes including infectious, metabolic, genetic, and toxic etiologies that need to be ruled out in order to diagnose a patient with an AND [1].
The workup for a suspected AND includes brain magnetic resonance imaging (MRI) and/or positron-emission tomography (PET) to screen for hyperintensities or metabolic abnormalities, respectively; electroencephalography (EEG) to confirm or exclude seizures; CSF studies to evaluate for the presence of elevated levels of white blood cells, protein and/or immunoglobulin type G (IgG) and oligoclonal bands, as well as molecular methods or culture to explore infectious causes; and serum studies to evaluate for other potential autoimmune causes or indications of an autoimmune tendency and the presence of neural autoantibodies [1]. Depending on the results of these studies, additional testing may be performed to evaluate for malignancy. The diagnostic workup for various ANDs is discussed in detail in Part III of this book.
Neural autoantibodies are commonly divided into two categories based on the subcellular location of the antigens targeted [2]. One group of autoantibodies recognizes intracellular targets including RNA-binding proteins, transcription factors, and other nuclear and cytoplasmic proteins. Paraneoplastic syndromes (PNS), ANDs classically associated with malignancy, are most frequently associated with autoantibodies against intracellular targets (discussed in Chap. 16). The second group of autoantibodies recognizes cell-surface proteins including ion channels, water channels, and neurotransmitter receptors. Autoantibodies against cell-surface proteins have been associated with a variety of disorders, with two of the most common being autoimmune encephalitis (Chap. 12) and autoimmune neuromuscular junction disease (Chap. 19). Detection of any of these neural autoantibodies can play a significant role in the diagnosis, prognosis, and management of patients with ANDs.
Methods for the Detection of Neural Autoantibodies
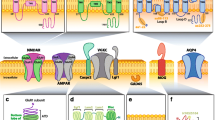
A variety of techniques are used to detect the presence of neural autoantibodies. These include the following: (1) tissue-based assays, (2) Western blot or line immunoblot assays, (3) immunoprecipitation assays, (4) cell-based assays, (5) enzyme-linked immunosorbent assays (ELISAs), and (6) primary culture-based immunofluorescence assays, with this last methodology primarily performed on a research basis (Table 2.1, Fig. 2.1) [3, 4].
Overview of methods for the detection of neural autoantibodies. (a) Tissue-based assays are performed using sections of primate or rodent neural tissue(s), patient serum or CSF is added; bound autoantibodies are detected with a fluorescent- or enzyme-conjugated anti-human IgG secondary antibody; substrate is added to induce a color change when an enzyme-conjugated antibody is used; and the presence and pattern of bound autoantibodies is determined by microscopy. (b) Western blot or line immunoblot assays are performed using strips of membrane containing neural proteins, patient serum or CSF is added, bound autoantibodies are detected using an enzyme-conjugated antibody against human IgG, which after addition of the substrate are visualized as a change in color at a specific position on the strip. (c) Enzyme-linked immunosorbent assays are performed using plastic wells coated with neural proteins, patient serum or CSF is added, bound autoantibodies are detected by addition of biotin-conjugated protein of interest, which after addition of enzyme-conjugated streptavidin and substrate are visualized as a change in color measured by spectrophotometry. (d) Cell-based assays are performed using cells expressing the neural antigen and/or receptor of interest, patient serum or CSF is added, bound autoantibodies are detected using a fluorochrome-conjugated antibody against human IgG, which are visualized by either microscopy or flow cytometry. (e) Radioimmununoprecipitation assays are performed using radioactively labeled proteins, patient serum or CSF is added, bound autoantibodies are precipitated with an anti-human IgG secondary antibody, radioactivity in pelleted immune complexes is measured with a gamma counter
The first autoantibodies associated with PNS were identified by incubating patient serum or CSF with brain tissue sections and observing autoantibodies binding to intracellular neural proteins [4]. The majority of neural autoantibodies can be screened for using this tissue-based assay (TBA) method on sections of the cerebellum and the hippocampus, with the exception of autoantibodies against neuromuscular junction antigens, since they are not present in these tissues. However, detection by TBA must be followed by testing using a different methodology in order to identify the specific antigen recognized by the autoantibody. Autoantibodies against intracellular neural antigens primarily recognize linear epitopes. Western blot or line immunoblot assays are frequently used to identify these autoantibodies. In contrast, autoantibodies against cell-surface or synaptic neural antigens primarily recognize conformational epitopes. Thus, different methodologies are preferred for the detection of these autoantibodies. Cell-based assays (CBAs) are the method of choice for autoantibodies against cell-surface receptors, and radioimmunoprecipitation assays (RIAs) are preferred for the detection of autoantibodies against many of the synaptic receptors.
Tissue-Based Assays
TBAs for the detection of neural autoantibodies using indirect immunofluorescence assay (IFA) or immunohistochemistry (IHC) are performed by incubating patient serum or CSF on sections of primate or rodent neural tissue(s), bound autoantibodies are detected with a fluorescent- or enzyme-conjugated anti-human IgG secondary antibody, and the presence and pattern of bound autoantibodies are determined by microscopy (Fig. 2.1a). An important consideration for the optimal detection of neural autoantibodies is the region of the brain used and preparation of the tissue sections with regard to pretreatment and fixation, which differs between intracellular and cell-surface antigens [4, 5]. Primate cerebellum snap-frozen, sectioned using a cryostat and fixed with paraformaldehyde or acetone is the preferred substrate for the detection of autoantibodies against intracellular neural antigens. Whereas, rat hippocampus fixed with paraformaldehyde, cryoprotected in sucrose, snap-frozen, and sectioned using a cryostat is the preferred substrate for the detection of autoantibodies against cell-surface or synaptic neural antigens.
A major advantage of TBAs is that a large number of neural antigens are available and accessible in the tissue sections. Thus, TBAs can be used to screen for a wide variety of neural autoantibodies at the same time and to discover new autoantibodies. Indeed, many neural autoantibodies have been discovered using this methodology. A major disadvantage is that it takes significant training to become proficient at identifying all of the possible patterns [5]. Additional disadvantages include the fact that autoantibodies against different antigens can produce similar patterns of staining, so additional testing must be performed to confirm the specificity of the autoantibodies. It can also be difficult to identify coexisting autoantibodies using this method. Many of these autoantibodies are very rare making it difficult to obtain positive specimens for validating assays, functioning as controls for the assay, training new staff, and maintaining competency and proficiency. TBAs are also time consuming, labor intensive, lack standardization, and can be subjective [4].
Detection of autoantibodies using TBAs can be performed individually or using mosaics of biochips containing various brain or other tissue sections [6,7,8]. This technology consolidates the ability to screen for multiple neural autoantibodies and identification/confirmation of some of their specific targets into a single assay. An important consideration when using this approach is whether positive controls for all autoantibodies to be reported are tested on every run [4].
Western Blot or Line Immunoblot Assays
Western blot (WB) or line immunoblot assays (LIAs) are the preferred method for confirming the presence of autoantibodies against intracellular targets. These methods are performed using lysates or proteins purified from extracts of brain tissue or cells expressing the proteins of interest, which are either run on a polyacrylamide gel and transferred to a membrane in the case of WBs or printed directly on a membrane in the case of LIAs. The membranes are cut into strips, incubated with patient serum or CSF and bound autoantibodies are detected using an enzyme-conjugated antibody against human IgG, which after addition of the substrate are visualized as a change in color at a specific position on the strip (Fig. 2.1b). Advantages of this methodology are that multiple autoantibodies can be tested for simultaneously, the testing can be automated and the results are more specific than those obtained by TBAs because specific antigens are present at particular locations on the membrane. Disadvantages of this method are that purification of the proteins often affects their conformation and/or interactions with other proteins, which can lead to false-negative results if the autoantibodies in the patient serum recognize conformational epitopes. This method also suffers from the same problem as TBAs with regard to difficulty in obtaining samples containing rare autoantibodies in order to validate the assay, serve as controls for performance of the assay, train laboratory staff, and maintain competency and proficiency. In addition, clinical significance of an immunoblot positive but TBA negative result is uncertain.
WB of brain tissue extracts allows for the detection of multiple autoantibodies. However, this advantage is off-set by the possibility of more than one antigen occupying the same location on the membrane. This problem is solved using LIAs where antigens are placed at specific locations. Thus, LIA does not offer the advantage of examining the entire repertoire of proteins observed by WB, as it is limited to the number of proteins selected for inclusion on the membrane.
Enzyme-Linked Immunosorbent Assays
ELISAs can be used to identify autoantibodies against intracellular antigens as well as select cell-surface or synaptic receptors. Similar to immunoblots, this method is performed using protein extracts but instead of using a membrane, the antigens are coated on the wells of a 96-well plate, and bound autoantibodies are detected using spectrophotometry. Several ELISAs used to detect neural autoantibodies use a variation of the technique, where autoantibodies present in patient serum or CSF form a bridge between antigen coated on the plate and biotinylated-antigen, which after addition of streptavidin peroxidase and substrate are detected using a spectrophotometer (Fig. 2.1c). In bridge ELISA testing, the detection method is not a secondary antigen against human IgG. Therefore, these assays are not antibody isotype specific. This can result in detection of autoantibodies of the IgA and/or IgM isotypes in addition to IgG autoantibodies. The clinical relevance of IgA or IgM autoantibodies is currently uncertain [9, 10]. In contrast to an immunoblot, ELISAs typically only test for autoantibodies against one target at a time, which can be considered a disadvantage of this method. Advantages of ELISA include increased sensitivity and specificity, decreased subjectivity compared to TBAs, and it is a high-throughput method that can be performed in many laboratories and can be automated. ELISAs can suffer a similar disadvantage to immunoblots in that the antigens may not be in their native conformation as a result of the purification process, which can lead to false-negative results. ELISAs can also yield false-positive results as a result of nonspecific binding due to the antibodies themselves binding to the plate or to the presence of heterophile antibodies [3]. In addition, some ELISAs, such as those used for the detection of autoantibodies against aquaporin 4 (AQP4) and glutamic acid decarboxylase (GAD65) antibodies, evaluate serum directly, whereas most methodologies dilute serum prior to testing in order to reduce background signal. Taken together, the lack of isotype specificity and the use of undiluted serum may explain differences in correlation with disease and/or other methodologies, especially for sera found to have low positive results by ELISA.
Cell-Based Assays
CBAs are the preferred method for detecting autoantibodies against cell-surface antigens and some synaptic receptors. They are performed using cells transfected with the antigen and/or receptor of interest. Transfected cells are incubated with patient serum or CSF, and bound autoantibodies are detected using a fluorescently conjugated antibody against human IgG and evaluated either by microscopy or flow cytometry (Fig. 2.1d). Advantages of this method include that the antigens are in their native conformation and that the results are more specific than TBAs because the cells are transfected with a single antigen of interest. Thus, interpretation is less subjective than TBAs and requires less training to become proficient. Disadvantages include that only autoantibodies against the antigen expressed by the cells are detected. Thus, this method cannot be used for the discovery of new autoantibodies.
Both live and fixed CBAs have been used for the detection of autoantibodies against cell-surface antigens. Commercially available CBAs use fixed cells out of necessity. Use of live cells requires continuous culturing of cells and the generation and maintenance of transfected cell lines. An important difference between assays using live cells instead of fixed cells is that antibodies only have access to targets on the surface of the cells. Fixation of cells can lead to permeabilization of the cell membrane, which can allow antibodies access to antigens inside the cell in addition to those on the cell surface. Fixation can also alter the presentation or accessibility of antigens, so the antigens present on live cells may be present in a more native form than those of fixed cells. Difference in performance between fixed and live CBAs varies among antigens, with live cells showing slightly better sensitivity for some autoantibodies, whereas fixed cells demonstrate higher sensitivity for others such as N-methyl-D-aspartate glutamate receptor (NMDAR) [11]. However, it is important to consider the number of clinically defined patient specimens used to make these comparisons. Differences in the results for a single specimen can appear to have a considerable effect on sensitivity or specificity when few clinically defined specimens are included in the analysis, as is frequently the case for these rare autoantibodies.
Detection of autoantibodies using fixed CBAs evaluated by IFA can be performed individually or using mosaics of biochips containing various transfected cells expressing different neural antigens, brain and/or other tissue sections [6,7,8]. This technology consolidates the ability to screen for multiple neural autoantibodies and identification/confirmation of some of their specific targets into a single assay. An important consideration when using this approach is whether positive controls for all autoantibodies to be reported are tested on every run. Multiplexing of CBA using mosaics can present a challenge with regard to manual reading and interpretation. As the number of biochips included in the mosaic increases so does the risk of confusing which biochip is being observed. Automation can aid in this process, but additional process controls should be incorporated to ensure the accuracy of results.
Detection of autoantibodies using live cell CBAs evaluated by flow cytometry decreases the subjectivity in visual interpretation commonly observed in CBA/IFA assays [5]. This method is gaining in popularity, but it is currently only available for diagnostic testing of a few neural autoantibodies (Table 2.1).
Immunoprecipitation Assays
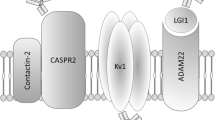
RIAs are the preferred method for detecting many of the autoantibodies against synaptic targets. RIA is performed using either iodine-125 radioactively labeled recombinant proteins or lysates of brain, muscle or cells expressing the antigen of interest that have been incubated with radioactively labeled toxins with high affinity for specific synaptic receptors. Patient serum or CSF is incubated with the radioactively labeled proteins or lysates containing the radioactively labeled receptors, unbound antibodies are washed away, and then anti-human IgG or protein A or G sepharose is added to form immune complexes and facilitate precipitation of the antigen/autoantibody complexes. Presence of autoantibodies is measured by the detection of radioactivity using a gamma counter, and quantitation is based on comparison to a standard curve or directly based on the specific activity of the radioactive ligand (Fig. 2.1e). Advantages of this method include that the antigens are in their native conformation and that the method is very sensitive due to the use of radioactivity. However, use of radioactivity is also a disadvantage because it poses a health hazard to laboratory personnel and requires a license for use and proper disposal [4]. Another disadvantage of this method is that it is possible to precipitate entire immune complexes, which then requires additional testing to confirm the specificity of the autoantibody [5]. This is the case for immunoprecipitation of the voltage-gated potassium channel (VGKC) complex, which is then followed by CBA to evaluate whether the reactivity is specific for leucine-rich glioma inactivated 1 protein (LGI1), contactin-associated protein 2 (CASPR2), or other proteins in the complex.
Primary Neuronal Cell Cultures
Primary cell culture-based IFA is performed by isolating cells from specific parts of the brain and culturing them for 2–3 weeks before using them to detect autoantibodies. Thus, this method is primarily used on a research basis. Patient serum or CSF is incubated with live neurons, and bound autoantibodies are detected using a fluorescently conjugated antibody against human IgG and visualized by microscopy. Autoantibodies to a variety of extracellular antigens can be detected using this method, but the staining patterns produced are often indistinguishable requiring the use of additional methods to determine the specific targets. Important considerations when interpreting the results are the presence and level of expression of the antigen of interest at the time of testing and that the binding of antibodies against some cell-surface proteins can alter their localization. This second point is relevant for both autoantibodies that may be present in patient serum or CSF and purified antigen-specific antibodies used as controls or in co-localization studies performed to evaluate the specificity of the autoantibodies [4, 5].
Specimen Type (Serum or CSF)
In addition to considering which methodology to use when testing for neural autoantibodies, another important consideration is specimen type. Some autoantibodies may only be present in one body fluid due to the location of their antigenic target, for example, autoantibodies against the muscle acetylcholine receptor (mAChR) are primarily only detected in serum. Alternatively, intrathecally synthesized autoantibodies may only be detectable in CSF (e.g., NMDAR autoantibodies) [4, 12]. Advantages of using serum include that its collection is less invasive making it more suitable for serial testing in monitoring response to treatment, and autoantibodies are often present at higher titers in serum. However, serum contains other proteins and non-neural autoantibodies that can cause high background or nonspecific binding resulting in false-positive results. Advantages of testing CSF include that it contains less extraneous proteins, so fewer false positives are observed due to nonspecific binding. CSF can be more sensitive and specific for the detection of neural cell-surface autoantibodies, and if the neural autoantibodies are being produced intrathecally, serum may be negative. Disadvantages to testing CSF include that its collection is more invasive and that autoantibodies are often present at low titers, if at all, which can lead to false-negative results. For example, CSF has been reported to be less sensitive than serum for the detection of AQP4 and LGI1 [4, 7]. Thus in some cases it may be important to test both serum and CSF, specifically in the setting of suspected LGI1 autoimmune encephalitis [13].
Sensitivity and Specificity
Widely divergent figures of the combined sensitivity for the known neural autoantibodies have been published [14, 15]. Discussion of this large group of heterogeneous surrogate biomarkers of disease and/or pathogenic autoantibodies is complicated by autoantibody presence being the defining characteristic in certain ANDs. Inconsistent laboratory findings in a setting of multiple autoantibody-associated disorders, with similar clinical presentation, add to the confusion. In addition, autoantibodies are not detected in all patients with clinically defined encephalitis suggestive of an autoimmune etiology [3]. This is in part due to the ongoing identification of additional antigenic targets and differing composition of autoantibody panels performed at different reference laboratories. In one single-center 1-year retrospective cohort, a combined sensitivity for paraneoplastic autoantibodies of 34% was estimated [14]. Whereas, in another multiyear retrospective study an estimated combined sensitivity between 60% and 80% was reported for clinically defined autoimmune encephalitis patients [15]. An important consideration when evaluating sensitivity of specific methods are the species and/or the region(s) of the brain from which the tissues or proteins are derived. Lack of detection of neural autoantibodies may be due to absence of epitopes/antigenic targets due interspecies differences [5].
Neural autoantibodies are rarely detected in serum from non-encephalitis disease control or healthy individuals. False-positive rates vary by methodology, isotype of secondary detection antibody used (polyclonal, IgG only, IgG1), and autoantibody of interest (NMDAR IgA, IgM, and IgG polyclonal detection by CBA has approximately a 10% false-positive rate) [6, 14, 16]. Interestingly, autoantibodies associated with classical PNS can be detected at an elevated frequency in patients with particular malignancies (approximately 20% of small cell lung cancer patients have Hu autoantibodies), yet very few of these patients develop neurologic symptoms (<0.01%) [3, 17].
Important considerations when evaluating sensitivity and specificity include the cut-offs used, how they were generated, and whether the results reported are qualitative or quantitative. Interpretation of low-titer antibody results can be challenging since some autoantibodies such as those against the VGKC complex and GAD65 have been found at low levels in patients without neurologic disease, but these have also been shown to be clinically relevant as is the case for patients with low level VGKC complex results but high LGI1 or CASPR2 results [1]. Low titer results can also be seen in the setting of immunotherapy as some antibody levels may decrease in response to therapy. It is important to note that the majority of information regarding sensitivity and specificity of assays to detect neural autoantibodies is based on testing of serum. Data are lacking for sensitivity and specificity for detection of neural autoantibodies in CSF.
Challenges Related to Detection of Neural Autoantibodies
Current challenges for the detection of autoantibodies associated with ANDs include that testing is very segmented in some countries with only certain labs able to perform testing for certain autoantibodies due to intellectual property restrictions. Many neural autoantibodies are very rare creating difficulty in obtaining positive samples for validation, training, competency, proficiency, and to function as controls when performing the assays. This is complicated by the fact that detection of staining patterns associated with neural autoantibodies requires significant training to become proficient and that overlap of symptoms between patients with multiple autoantibodies makes it difficult to determine the sensitivity of an assay since the diseases are defined by the presence of the autoantibody. Another challenge is that the number of autoantibodies associated with ANDs is continuing to grow, as is the availability of commercial assays to detect them, but the thorough characterization required to establish clinical context and prevalence often lags due to the rarity of positive patients.
Introduction to the Role of Neural Autoantibodies in Pathogenesis
Although clear associations between autoantibodies and ANDs have been demonstrated, it is less clear whether these autoantibodies play a role in the pathogenesis of the diseases or are simply markers of the disease process, since only a few have actually been shown to cause disease. Distinction between neural autoantibodies based on the subcellular location of their antigenic targets is also relevant in discussions on the pathogenic role of these autoantibodies. The three main groups include autoantibodies that target intracellular nuclear and cytoplasmic antigens, autoantibodies that target intracellular synaptic antigens, and autoantibodies that target cell-surface and synaptic antigens (Fig. 2.2). Evidence for the pathogenicity of the autoantibodies is based on data from in vitro studies, animal models, and biopsy and autopsy tissue studies, as well as the responsiveness of patients positive for these autoantibodies to immunotherapy [18].
Depiction of the subcellular location of the antigenic targets of neural autoantibodies and the relationship between location and the pathogenic role of these autoantibodies. Three main groups of neural autoantibodies include autoantibodies that target intracellular nuclear and cytoplasmic antigens, autoantibodies that target intracellular synaptic antigens, and autoantibodies that target cell-surface and synaptic antigens. Autoantibodies to intracellular nuclear or cytoplasmic targets have limited access to their target antigens and are therefore not considered directly pathogenic. In contrast, autoantibodies to intracellular synaptic targets are proposed to have access to their target antigens during fusion and reuptake of synaptic vesicles, and thus maybe directly pathogenic. Autoantibodies against cell surface and neuromuscular junction have direct access to their antigenic targets and are considered to be directly pathogenic. The majority of the targets of neural autoantibodies are expressed in neurons, but AQP4 and MOG are expressed on the cell surface of astrocytes and oligodendrocytes, respectively. * = antigens expressed both on the neuronal cell surface and in the neuromuscular junction. AGNA-1 anti-glial nuclear antibody, AMPAR alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor, ANNA anti-neuronal nuclear antibody, AQP4 aquaporin 4, CASPR2 contactin-associated protein 2, CRMP5 collapsing response mediator protein 5, DPPX dipeptidyl aminopeptidase-like protein, GABABR gamma-aminobutyric acid receptor, type B, GAD glutamic acid decarboxylase, gAChR ganglionic acetylcholine receptor, LGI1 leucine-rich glioma inactivated 1 protein, mGluR metabotropic glutamate receptor, MOG myelin oligodendrocyte glycoprotein, mAChR muscle acetylcholine receptor, MuSK muscle-specific tyrosine kinase, NMDAR N-methyl-D-aspartate glutamate receptor, PCCA Purkinje cell cytoplasmic antibody, VGCC voltage-gated calcium channel, VGKC voltage-gated potassium channel
Autoantibodies to intracellular nuclear or cytoplasmic targets have limited access to their target antigens and are therefore considered not to be directly pathogenic. Instead, they are thought to be biomarkers of a T-cell-mediated response against their corresponding neuronal target antigen [2]. Evidence exists that autoantibodies to intracellular cytoplasmic or nuclear targets present in the CNS, may be synthesized intrathecally, can be taken up by neurons and in some cases lead to neuronal cell death in vitro [19,20,21]. However, animal models involving passive transfer of these autoantibodies or immunization with their corresponding target antigens have failed to confirm a pathogenic role for these autoantibodies in vivo [22, 23]. Evidence for a T-cell-mediated response against intracellular nuclear or cytoplasmic neuronal antigens includes the detection of neuronal antigen-specific T-cell responses in patients with paraneoplastic encephalomyelitis, the presence of more T cells than B cells in their brain and peripheral nerve tissues, expression of a marker of cytotoxic effector T-cell function, granzyme B, in close proximity to neurons in areas with evidence of neuronal cell loss [19, 24, 25].
Intracellular synaptic targets such as GAD65 and amphiphysin may be targeted by both T cells and autoantibodies. In contrast to the antigenic targets discussed in the previous section, autoantibodies have access to these antigens during fusion and reuptake of synaptic vesicles [16]. A direct pathogenic role for this group of autoantibodies was demonstrated by intrathecal injection of anti-amphiphysin into rats resulting in stiff person syndrome-like symptoms [26]. Evidence for a T-cell-mediated response against intracellular synaptic antigens includes development of encephalomyelitis in immunized mice producing GAD65-specific T cells and development of neurologic symptoms upon transfer of these GAD65-specific T cells to naïve mice or mice lacking B cells [2, 27].
Autoantibodies against cell-surface receptors are thought to play a more direct role in pathogenesis through agonistic or antagonistic effects on the receptors, disrupting the function of the receptors either by causing them to be internalized or preventing their ligands from binding to them, or potentially leading to cytotoxicity due to antibody- and/or complement-mediated mechanisms [18, 25]. These pathogenic effects of cell-surface neural autoantibodies have been demonstrated both in vitro and in vivo using passive transfer of patient IgG into mice [28, 29]. Reports that this neural dysfunction is frequently reversible upon removal of the autoantibodies and many patients often experience complete recovery in response to immunotherapy suggests that autoantibodies play a direct pathogenic role [18]. Additional support for a pathogenic role is that autoantibodies against some neural cell-surface receptors produce effects similar to genetic or pharmacologic disruption of the receptors [2].
In PNS, pathogenesis is thought to be due to an immune response against a neural protein that is aberrantly expressed on a tumor, leading to activation and expansion of autoreactive T and B cells, and production of autoantibodies [17]. When these immune agents gain access to the nervous system, they can cause damage leading to neurologic symptoms. However, tumors are detected in less than a third of patients with ANDs at the onset of neurologic symptoms and autoantibody detection, which begs the question of what triggers autoantibody production in these patients. Current hypotheses include an infectious trigger and multiple recent publications have drawn a link between herpes simplex encephalitis and NMDA receptor encephalitis (discussed further in Chap. 25) [9]. Another possibility is that a tumor is not detected because the immune response is effective in fighting the malignancy [17]. It also remains to be determined, how chronicity is established or how relapses are triggered in the case of idiopathic AND [29].
Additional questions about the role of autoantibodies in the pathogenesis of ANDs include the following: why there is diversity in clinical presentations associated with a particular autoantibody and how specificity of symptoms occurs despite widespread expression? Potential explanations include heterogeneity in the antibody response with respect to subtype of IgG and epitope(s), post-translational modifications or conformational changes specific to particular regions of the brain, and the presence of co-existing autoantibodies [2, 17, 18].
Conclusion
The number of autoantibodies associated with ANDs continues to grow, as does the number of specimens being tested. Clinical laboratories are faced with the challenge of determining how many and which assays to offer, by which methods and in which combinations. Clinicians are faced with deciding which tests or panels of tests to use in the assessment for neural autoantibodies in their patients. Despite uncertainty about the role of autoantibodies in the pathogenesis of ANDs, they can play a significant role in diagnosis and treatment. Thus, it is important for clinicians to be aware of the limitations of the various methods for detecting autoantibodies and to note that failure to detect a neural autoantibody does not rule out diagnosis of an AND.
References
Linnoila J, Pittock SJ. Autoantibody-associated central nervous system neurologic disorders. Semin Neurol. 2016;36:382–96.
Lancaster E, Dalmau J. Neuronal autoantigens – pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–90.
Tebo AE, Haven TR, Jackson BR. Autoantibody diversity in paraneoplastic syndromes and related disorders: the need for a more guided screening approach. Clin Chim Acta. 2016;459:162–9.
Waters P, Pettingill P, Lang B. Detection methods for neural autoantibodies. Handb Clin Neurol. 2016;133:147–63. https://doi.org/10.1016/B978-0-444-63432-0.00009-8. PMID: 27112676.
van Coevorden-Hameete MH, Titulaer MJ, Schreurs MW, de Graaff E, Sillevis Smitt PA, Hoogenraad CC. Detection and characterization of autoantibodies to neuronal cell-surface antigens in the central nervous system. Front Mol Neurosci. 2016;9:37. https://doi.org/10.3389/fnmol.2016.00037.
Dahm L, Ott C, Steiner J, Stepniak B, Teegen B, Saschenbrecker S, et al. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann Neurol. 2014;76:82–94.
McCracken L, Zhang J, Greene M, Crivaro A, Gonzalez J, Kamoun M, et al. Improving the antibody-based evaluation of autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. 2017;4:e404.
Naides SJ. The role of the laboratory in the expanding field of neuroimmunology: autoantibodies to neural targets. J Immunol Methods. 2018;463:1–20.
Prüss H, Höltje M, Maier N, Gomez A, Buchert R, Harms L, et al. IgA NMDA receptor antibodies are markers of synaptic immunity in slow cognitive impairment. Neurology. 2012;78:1743–53.
Hara M, Martinez-Hernandez E, Ariño H, Armangue T, Spatola M, Petit-Pedrol M, et al. Clinical and pathogenic significance of IgG, IgA, and IgM antibodies against the NMDA receptor. Neurology. 2018;90:e1386–94.
Ramberger M, Peschl P, Schanda K, Irschick R, Höftberger R, Deisenhammer F, et al. Comparison of diagnostic accuracy of microscopy and flow cytometry in evaluating N-methyl-D-aspartate receptor antibodies in serum using a live cell-based assay. PLoS One. 2015;10:e0122037.
Suh-Lailam BB, Haven TR, Copple SS, Knapp D, Jaskowski TD, Tebo AE. Anti-NMDA-receptor antibody encephalitis: performance evaluation and laboratory experience with the anti-NMDA-receptor IgG assay. Clin Chim Acta. 2013;421:1–6.
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical diagnostic approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404.
Albadareen R, Gronseth G, Goeden G, Sharrock M, Lechtenberg C, Yang Y. Paraneoplastic autoantibody panrels: sensitivity and specificity, a retrospective cohort. Int J Neurosci. 2017;127:531–8.
Wandinger KP, Leypold F, Jumker R. Autoantibody-mediated encephalitis - differential diagnosis in patients with impaired consciousness of unclear origin. Dtsch Arztebl Int. 2018;115:666–73.
Lang K, Prüss H. Frequencies of neuronal autoantibodies in healthy controls: estimation of disease specificity. Neurol Neuroimmunol Neuroinflamm. 2017;4:e386.
Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–54.
van Coevorden-Hameete MH, de Graaff E, Titulaer MJ, Hoogenraad CC, Sillevis Smitt PA. Molecular and cellular mechanisms underlying anti-neuronal antibody mediated disorders of the central nervous system. Autoimmun Rev. 2014;13:299–312.
Dalmau J, Furneaux HM, Rosenblum MK, Graus F, Posner JB. Detection of the anti-Hu antibody in specific regions of the nervous system and tumor from patients with paraneoplastic encephalomyelitis/sensory neuronopathy. Neurology. 1991;41:1757–64.
Greenlee JE, Clawson SA, Hill KE, Wood B, Clardy SL, Tsunoda I, et al. Neuronal uptake of anti-Hu antibody, but not anti-Ri antibody, leads to cell death in brain slice cultures. J Neuroinflammation. 2014;11:160.
Greenlee JE, Clawson SA, Hill KE, Wood B, Clardy SL, Tsunoda I, et al. Anti-Yo antibody uptake and interaction with its intracellular target antigen causes Purkinje cell death in rat cerebellar slice cultures: a possible mechanism for paraneoplastic cerebellar degeneration in humans with gynecological or breast cancers. PLoS One. 2015;10:e0123446.
Sillevis Smitt PA, Manley GT, Posner JB. Immunization with the paraneoplastic encephalomyelitis antigen HuD does not cause neurologic disease in mice. Neurology. 1995;45:1873–8.
Graus F, Illa I, Agusti M, Ribalta T, Cruz-Sanchez F, Juarez C. Effect of intraventricular injection of an anti-Purkinje cell antibody (anti-Yo) in a guinea pig model. J Neurol Sci. 1991;106:82–7.
Rousseau A, Benyahia B, Dalmau J, Connan F, Guillet JG, Delattre JY, et al. T cell response to Hu-D peptides in patients with anti-Hu syndrome. J Neuro-Oncol. 2005;71:231–6.
Bien CG, Vincent A, Barnett MH, Becker AJ, Blümcke I, Graus F, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135(Pt 5):1622–38.
Geis C, Weishaupt A, Hallermann S, Grünewald B, Wessig C, Wultsch T, et al. Stiff person syndrome-associated autoantibodies to amphiphysin mediate reduced GABAergic inhibition. Brain. 2010;133:3166–80.
Burton AR, Baquet Z, Eisenbarth GS, Tisch R, Smeyne R, Workman CJ, et al. Central nervous system destruction mediated by glutamic acid decarboxylase-specific CD4+ T cells. J Immunol. 2010;184:4863–70.
Williams JP, Carlson NG, Greenlee JE. Antibodies in autoimmune human neurological disease: pathogenesis and immunopathology. Semin Neurol. 2018;38:267–77.
Bradl M, Lassmann H. Neurologic autoimmunity: mechanisms revealed by animal models. Handb Clin Neurol. 2016;133:121–43.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Haven, T.R., Peterson, L.K. (2021). Antibody Detection Methods for Neural Autoantibodies and Introduction to Antibody Pathogenesis. In: Piquet, A.L., Alvarez, E. (eds) Neuroimmunology. Springer, Cham. https://doi.org/10.1007/978-3-030-61883-4_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-61883-4_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-61882-7
Online ISBN: 978-3-030-61883-4
eBook Packages: MedicineMedicine (R0)