Abstract
In 2050, the population of the world is expected to be 9 billion, which means we have to produce six times more food. With this population explosion and increase in food demand, the agricultural land is depleting at an alarming rate, jeopardizing future progress. In order to overcome this problem, the soil organic matter plays a dynamic role in the maintenance and improvement of soil properties. Organic matter determines larger part of soil and has tremendous ecological significance; it influences ecosystem productivity, soil health, and climate quality. The soil organic matter maintains and improves many physical, chemical, and biological properties. This chapter explicates the effect of organic matter on physical, chemical, and biological properties, including soil structure, water retention, available water capacity, thermal conductivity, erodibility, infiltration, soil aggregate formation, soil color, soil compaction, soil aeration, pH, buffering capacity, CEC, base saturation, zeta potential, exchangeable cations, soil fertility and nutrient release, microbial population, soil microbial biomass carbon, nitrogen transformation, mycorrhizal population, root length and root growth, and soil enzymes. It was concluded that increase in the organic matter enhanced these properties, while reduction in organic matter had a detrimental impact on these properties.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Soil organic matter plays a dynamic role in the improvement and maintenance of physical, chemical, and biological properties of soil. Agriculture is most concerned about these relationships, including the critical limits of soil organic matter with the key soil properties. There has been direct and indirect impact of soil organic matter on the soil properties, including its physical, chemical, and biological properties (Bezuglova et al. 2019; Sachkova et al. 2019; Orlova et al. 2019; Garratt et al. 2018; Zhang et al. 2017; Bhat et al. 2017b). In the physical properties, the soil organic matter affect soil structure, water retention, available water capacity, thermal conductivity, erodibility, infiltration, soil aggregate formation, soil color, soil compaction, soil aeration, saturated and unsaturated hydraulic conductivity (Beck-Broichsitter et al. 2018; Ajayi and Horn 2017; Blanchet et al. 2016; Angin et al. 2013). In the chemical properties, the SOM effect pH, buffering capacity, CEC, base saturation, zeta potential, exchangeable cations, soil fertility, and nutrient release (Garratt et al. 2018; Kwiatkowska-Malina 2018; Sulman et al. 2018). The biological properties include soil microbial population, soil microbial biomass carbon, nitrogen transformation, mycorrhizal population, root length and root growth, dehydrogenase, phosphatase, and urease (Fedoseeva et al. 2019; Orlova et al. 2019; Tikhova et al. 2019; Kallenbach et al. 2019; Wurzburger and Clemmensen 2018; Hazard and Johnson 2018; Parker et al. 2018).
Soil organic matter is a complex system of substance ranging from metabolic products of microbes, products of secondary synthesis components of organic residues undergoing decomposition, and humic substance. Soil organic matter is the plant and animal residues, microbial biomass, partly decomposed biomass fragments, stabilized organic matter, and soluble organic fraction (Zuber et al. 2015; Balesdent et al. 2000; Qadri and Bhat 2020). The soil organic matter generally describes the non-living product of plant and animal origin, but in broader term, it includes the total soil biomass, including the meso and macrofauna (Jiang et al. 2018; Laossi et al. 2008) and the biomass decomposition product. The soil organic matter is the heterogeneous product resulting from the chemical and microbial transformation of organic debris (Sidhu et al. 2016; Streubel et al. 2011; Liang et al. 2006; Mikutta et al. 2006; Dervash et al. 2020; Mushtaq et al. 2018). The soil organic matter has two important constituents: the non-living part, including the decomposed and un-decomposed products, and the living fraction of soil organic matter which is composed mainly of bacteria (109 organisms per gram of soil), fungi (107 organisms per gram of soil), actinomycetes (108 organisms per gram of soil), protozoa (106 organisms per gram of soil), algae (103 organisms per gram of soil), and nematodes (50 organisms per gram of soil). In the soil organic matter, these microbial populations play an important role in fermentation, mineralization, and humification process (Yuan et al. 2018; Gougoulias et al. 2014; Ahmad et al. 2007; Tiquia 2005; Alfreider et al. 2002; Zaman et al. 1999; Khanday et al. 2016; Singh et al. 2018a, b). The major constituents of the organic inputs are polysaccharides (hemicelluloses, cellulose) and lignin (Hsu et al. 2018; Liu 2014; Torres 2014; Zhu 2010; Kiem and Kögel-Knabner 2003), the others being biopolymers, such as (polyester, protein, tannins, suberin, cutin, and chlorophyll pigments) (Rui et al. 2016; Liu 2010; Bhat et al. 2017a, b). Soil organic matter serves as a substrate for microbial activity, nutrient source, soil conditioner, and major factor for sustaining agricultural productivity (Oldfield et al. 2018; Singh et al. 2020).
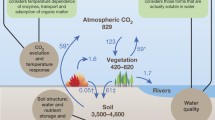
Soil organic matter decomposition is directly related to emission of atmospheric carbon, leading to global climate change. Environmental variables (soil moisture and soil temperature), microbial activity, soil chemical properties, and soil organic matter inputs (e.g., root exudates, plant litter, dead fine roots) usually impact decomposition dynamics (Dar et al. 2013, 2016; Genardzielinski et al. 2018; Yang et al. 2018; Dar and Bhat 2020). Apart from the organic inputs, the most influential are microbial activity and soil climate. In the soils, organic matter influences its output through priming effect (Fig. 7.1). The priming effect is small period transition in soil organic carbon turnover caused by the external organic matter input. A positive priming effect accelerates the decomposition rate, while as negative priming effect retards decomposition. The decline in the soil organic matter has a deleterious effect on the soil properties (Matos et al. 2019; Johnson et al. 2017; Huo et al. 2017; Kumar et al. 2016; Pausch et al. 2013; Zimmerman et al. 2011). As predicted in (European Environment Agency 2010) EU Soil Thematic Strategy, a decline of SOM contents is considered among eight main threats for soils. About 108–188 Pg C have been lost since the mid-nineteenth century, mostly from terrestrial ecosystem. However, several mitigating practices in agriculture, such as use of crop residues and residue incorporation into soil, maintain and build up soil organic matter. The mineralization and decomposition of soil organic matter is prejudiced by the land management, especially agricultural lands. Soils easily and quickly lose organic matter when natural soils are converted into agricultural soils. About 20–25% of the soil organic carbon is lost into atmosphere during its conversion and the possible reason is the destruction of soil aggregates. The sustainability in agriculture can be achieved now by the supply of exogenous organic matter. The organic matter having rich carbon pool and poor nitrogen content (e.g., straw, brown coal, wood and tree coniferous) act as substrate for microorganisms and relatively stable energy source (Singh et al. 2018a, b; Wei et al. 2017; Gogoi et al. 2017; Xiang et al. 2015) The current paper reviews the importance of soil organic matter in the enhancement and amelioration of physical, chemical, biological, and fertility aspects of the soil (Table 7.1).
7.2 Effect of Soil Organic Matter on Soil Physical Properties
7.2.1 Soil Structure and Aggregate Stability
Soil structure stability indicates the resistance of soil to structural arrangement of particles and to different stresses (compaction/trampling, cultivation, and irrigation). Soil structure is the most important property influencing biological, chemical, and physical processes within soil, such as determining water and nutrient movement, air accessibility, seedling emergence, root penetration, resistance to erosion, and soil drainage (Sandin et al. 2017; Toosi et al. 2017; Jozefaciuk et al. 2015; Mehmood et al. 2019). The soil structure is a property which can be greatly influenced and manipulated by organic agriculture management practice. The organic matter has been associated with improved soil structure through the production of organic acids, biodiversity improvement, chelates, and increased earthworm population (Pylak et al. 2019; Bongiornoa et al. 2019; Moos et al. 2016; Kravchenko et al. 2015; Peng et al. 2015; Regelink et al. 2015). The amount of water stable aggregate is connected to macro-aggregate stability and positively related with labile organic carbon. A minimum of 2% soil organic carbon is necessary to maintain soil structure stability (Liu et al. 2013; Celik et al. 2010; Huang et al. 2010). The concept of soil aggregation and soil structure involving different binding agents have been revealed by the work of many researchers. Fine network of roots and hyphae in soil with high soil organic matter held together large aggregates (200 micron), while as aggregates above this size are held by organic cementing agents. Water stable aggregates of 2–20 micron are bound by living and dead bacterial cells. The idea of aggregate hierarchy reveals that organic matter controls aggregate stability and destruction of large aggregates creating smaller and more stable aggregates (Keller and Håkansson 2010; Moni et al. 2010; Kaiser and Guggenberger 2003; Christensen 2001; Oades 1984). Particulate organic matter acts as a substrate for microbial activity, enhancing the production of microbial bonding material. Fresh and active part of soil organic matter (mono, polysaccharides, exudates, fungal hyphae, and roots) are largely responsible for soil aggregation (De Curtis et al. 2019; Frąc et al. 2018; Plaza-Bonilla et al. 2013; Miao et al. 2009). There has been seen a positive relationship between organic farming systems using FYM, compost, vermicompost, sewage, and sludge with the aggregate stability.
7.2.2 Soil Compaction
Bulk density is an indicator of soil compaction and affects infiltration, soil porosity, plant nutrient availability, available water capacity, and soil microbial activity (Karlen et al. 2019; Nunes et al. 2019; Laiho et al. 2004; Aşkın and Özdemir 2003). The soil compaction is regarded as the most serious problem caused by conventional agriculture and the most difficult type to determine, as it shows no evident marks on the surface (Meurer et al. 2018; Masto et al. 2007; Håkansson and Lipiec 2000). The effects of soil compaction on soil properties are very complex and the state of soil compactness is evident soil attribute and determined mostly by the bulk density and soil strength (Singh et al. 2014; Hati et al. 2007). The bulk density and soil strength gives the direct comparable value of soil compactness. Soil organic matter retains soil water, thus helping the soil to overcome the problem of soil compaction. The adequate amount of organic matter in the soil stabilizes soil structure and makes it more resistant to soil degradation and also decreases the bulk density and increases soil strength (Das et al. 2018; Gharahi-Gheni et al. 2012; Heuscher et al. 2005; Calhoun et al. 2001; Han et al. 2012). The various mechanisms by which the organic matter influences the soil compaction are as follows: (a) binding of mineral particles to soil; (b) aggregate wet ability reduction; and (c) influencing the strength of soil aggregates, which is a measure of inter-particle bond. There has been a varying result between the soil organic matter and soil compactness and different researchers have reported different behavior for the different types of organic matter (Pravin et al. 2013). These differences seem to be due to different types of organic manure, C/N ratio of manure, and degree of resistance to degradation. Soil compactness is more influenced by readily oxidizable soil organic matter than the total organic matter. The organic farming system increases the organic matter in the soil and thus improves the bulk density of soil. Furthermore, using high quantity of organic manure or wastes decreases the bulk density of the soil due to a dilution effect caused by the incorporation of the added organic material with the denser mineral fraction of the soil (Almajmaie et al. 2017; Boizard et al. 2017; Guimarães et al. 2017). The plant residues are the common source of organic matter, but the farmers also use animal manures to reduce the soil compaction in the different organic farming systems (Avnimelech et al. 2001; Curtis and Post 1964; Huntington et al. 1989; Bhat et al. 2018a, b). The elasticity of the manure reduces the transmission of stress toward subsoil thus acting as a buffer to subsoil compaction. Incorporation of 50–100 t/ha of cattle manure in the silty clay loam top soils significantly reduces the effects of load 1–2 passes of 48.5 kW tractor (Mosaddeghi et al. 2000). Green or brown manure may not be an efficient source of nutrient for high-yielding environment, but acts as a beneficial practice in improving soil physical properties in compacted soils. Reddy (1991) observed a decrease in bulk density and soil strength of 0.02 Mg/m3 and 11.8 kpa in the sandy loam soil due to the incorporation of 10 t/ha of green leaf manure. Incorporation of various organic matters in subsoils may prove a better alternative for stable retention in tackling soil compaction.
7.2.3 Soil Porosity
The architecture of soil refers to arrangement of soil particles and soil pores. The soil organic matter directly influences plant nutrition, penetration, and seedbed preparation, ease of cultivation, improved bulk density, greater aggregate stability, increased water holding capacity, and enhanced porosity (Xu et al. 2016; Ibrahim et al. 2013; Jack et al. 2011; Jarvis et al. 2007; Malkawi et al. 1999; Haynes and Naidu 1998). The organic carbon is mostly located in these pores between mineral grains as discrete particles or adsorbed on these particles. The soil porosity can influence the organic material stability through its effect on aggregate stability, isolation, and entrapment of decomposers and water and oxygen availability (Li et al. 2014; Masri and Ryan 2006). The changes in the pore size distribution are accompanied by the higher rates of organic matter mineralization at equivalent values of air-filled porosity. Rose (1991) studied that FYM not only changes aggregate stability but also affects the inter- and intramicro porosity. By the application of FYM and compost, both the micro porosity and macro porosity has increased (Pagliai and Vittori Antisari 1993). The increase in micro porosity occurs as a result of increase in elongated macropores of the newly formed aggregates. The effect of organic farming system can indirectly enhance soil porosity through the influence of soil fauna whose burrowing and feeding activity modify porosity (Park and Smucker 2005; McCallum et al. 2004). The various organic farming systems can also enhance the soil porosity by reducing soil crusting, clay dispersion, tillage, and compaction (Fig. 7.2).
7.2.4 Soil Color
A strong relationship exists between soil color and many other important soil properties, including soil fertility mineral composition, soil organic matter soil drainage class, soil moisture, and land suitability (Ben-Dor et al. 2008; Barron and Torrent 1986; Alexander 1971; Brown and O’Neal 1923). The soil color is used to characterize, classify, and differentiate soils. The most convenient way to determine soil color is by the Munsell color chart. The soil color determines the pedogenic process in soil and the most important soil pigmenting agents are organic matter, iron, and manganese (Erskine 2013; He et al. 2003; Ketterings and Bigham 2000). The application of soil organic matter darkens the soil. The concept of Russian chernozem and Mollisol shaving the high organic matter are mostly defined by relative thick, dark surface horizons. The dark brown soils having high amount of soil organic matter are generally considered as an ideal soil. Within similar soil textural class and landscape, the soil color and soil organic matter has a good linear correlation (Ertlen et al. 2015; Kirillova et al. 2015; Kweon et al. 2013; Li et al. 2012; Ertlen et al. 2010; Gao and Xia 2009). The dark color soil having high amount of the organic matter applied by various organic farming systems holds a large amount of water, absorbs more radiation, and affects heat transfer (Sánchez-Marañón et al. 2011; Ketterings and Bigham 2000). The relationship between burned soil color, soil fertility, and fire severity found that color and chroma decreased with increasing heat severity due to decrease of soil organic matter and the soils appeared red, while the light heated soils appeared black due to incomplete burning of soil organic matter (Valeeva et al. 2016).
7.2.5 Water-Holding Capacity
An important soil physical characteristic is the capacity of the soil to supply and store water and air for plant growth. Soil’s water holding capacity is the ability of the soil to store water. The soil organic matter increases the water holding capacity of the soil. The effect of organic matter on the water holding capacity is generally assumed to be positive (Ankenbauer and Loheide 2017; Basche et al. 2016; Jordán et al. 2010; Franzluebbers 2002; Bauer 1974; Jamison and Kroth 1958). An increase of 50% water content with per gram addition of organic carbon at −10 kpa suction (Emerson and McGarry 2003; Haynes and Naidu 1998).
The soil organic matter can enhance the hydraulic conductivity by improving the aggregate stability and porosity of the soil (Yang et al. 2014; Xu 2014). The organic manures reduce the compactness of soil, therefore improving water penetration. The effect of organic matter was more pronounced in the coarse-textured soil, followed by medium textured soil, and then the fine soils (Haynes and Naidu 1998). Several researchers have reported that, with an increase of soil organic matter, there is an increase of water holding capacity at both field capacity and wilting point (Sohail-Ur-Raza et al. 2015; Wang et al. 2015; Evrendilek et al. 2004; Matsi et al. 2003). The water holding capacity of soils is mainly dependent on the number of pores and specific surface area of soil. Both these pores and the specific surface area are increased by the application of organic matter. The increased water holding capacity is basically the result of an increase in the number of smaller pores at lower tension (Gülser et al. 2015; Hati et al. 2007; Khaleel et al. 1981). At higher tensions, all the pore space in the soil is filled by air and the water is retained mainly due to specific surface area and the thickness of water film on these surfaces.
7.2.6 Soil Thermal Properties
Soil thermal properties are considered a function of soil organic matter and soil carbon pool. Soil organic matter alters the thermal properties of soil because of its black dark nature. The albedo of soil gets reduced by the potential increase of dark color and more heat gets absorbed. The soils with least organic matter have albedo value of 0.6, while soils with 5% organic matter have albedo value of 0.08. At least, a soil organic matter of 2.5–3.0% is considered significant (Bi et al. 2018; Di Sipio and Bertermann 2018; Cai et al. 2017; Hortensia et al. 2018; Dębska et al. 2016). The soil organic matter also affects the actual thermal properties of soil, including both its storage and flow of heat (Table 7.2).
The soils with good amount of organic matter have ample germination and higher crop growth because of the favorable temperature. The soil organic matter has substantially different physical characters in view of other soil constituents and with the increase in the soil organic matter the potential change in the soil thermal properties occurs (Bi et al. 2018; Wardani and Purqon 2016; Mondal et al. 2015; Usowicz et al. 2013; Tarnawski et al. 2009). Soil organic matter has the direct effect on the bulk density, which later affects heat conductivity and capacity of the soil. Mostly increase in the soil organic matter decreases the thermal conductivity and the wet soils have higher heat capacity and require lot of heat to raise its temperature. The wet soils are considered to have higher thermal conductivity than the organic and other soils.
7.2.7 Soil Infiltration and Percolation
The soil organic matter influences the infiltration or the admittance of water into a soil, and percolation, or the descendent movement of water, in a soil. The rate of infiltration depends on soil structure, developed soil horizon, soil slope, soil texture, depth of water table, chemical content of water, rate of water applied, and the amount of organic matter. Initially, in any soil, the infiltration rate is higher and then decreases with time (Basche and DeLonge 2019; Korucu et al. 2018; Loecke et al. 2017; Haghnazari et al. 2015). The important characteristic of soil affecting the soil infiltration is porosity of soil. The organic matter has a direct impact on the soil porosity and as the soil organic matter increases the porosity of the soil is also enhanced. The rate of infiltration reduces with time due to deflocculation and breakdown of the peds. The soil organic matter enhances soil aggregate stability and thus allows the greater rate of infiltration. Initial infiltration is higher, but the later infiltration is sometimes superior if there are no macropores on the surface and the soils have good aggregate stability and no surface crusting (Zeng et al. 2019; Acharya et al. 2018; Chavarria et al. 2018; Rodrigo-Comino et al. 2018; Diadin et al. 2018; Balázs et al. 2018). In the tropics and semi tropics, the infiltration rate is more dependent on hydrous oxides and clay minerals because of having mineral origin of aggregates. In the humid and temperate zones, the infiltration depends on the soil organic matter because the stability of aggregates largely depends on the soil organic matter. The rate of infiltration varies from 0.1 to 5 inches per hour and the general infiltration rate varies from 0.3 to 1.0 inch per hour. The large amount of crop residues, mulches, and soil surfactants can regulate good infiltration rate by breaking droplet size of the rain water.
Percolation or downward movement of water is reliable on uninterrupted pore space in soil. For every doubling of pore size diameter, the rate of percolation in the soil increases by four times. The other factors affecting percolation rate are soil texture, soil structure, soil compaction, and amount of organic matter present. The soil temperature, soil moisture, and depth of soil horizons also affect the percolation rate (Issaka et al. 2018; Bullard et al. 2018; Jakab et al. 2017; Di Prima et al. 2017). The percolation of soil is restricted by the insufficient pans, clay pan, fragipans, plow pans, hard pans, and the presence of higher water table (Gómez et al. 2017; López-Vicente et al. 2016). In general, the percolation rate is considered higher in sandy soils, but the light soils having well stable soil aggregates, good soil structure, and high amount of organic matter have higher percolation rates than the fine sands. Mulches, crop residues, compost, FYM, and other organic soil amendments increase the soil macropores, thus increasing the percolation rate in the soil (Hlavčová et al. 2019; Gómez et al. 2018; Ben-Salem et al. 2018; Lucas-Borja et al. 2018; Fortugno et al. 2017).
7.3 Soil Chemical Properties
7.3.1 Buffering Capacity and Soil pH
Buffering capacity of soil is an important aspect as it assures the stability of soil pH. The buffering capacity of soil is the resistance to change in pH when an acid or base is added. At the pH value between 5 and 7.5, soil organic matter and clay acts as a sink for H and OH and the buffering capacity is governed by exchangeable reaction (Yuan et al. 2011; Nelson and Su 2010; Zhang et al. 2008; Herre et al. 2007). The presence of various functional groups (amine carboxylic, alchoal, phenolic, and amide) in soil organic matter allows it to act as a buffer over a wide range of soil pH (Sohi et al. 2010; Larney et al. 2008). The organic rich surface soils have higher buffering capacity than the mineral soils. James and Riha (1986) reported the buffering capacity of 18–36 Cmolc/kg in the forest soils and 1.5–3.5 Cmolc/kg in the mineral soils. Bloom (1999) studied that soil organic matter has a buffering capacity of 200 Cmolc/kg, while buffering capacity of soil organic carbon was reported 300 times in comparison to kaolonite. Cayley et al. (2002) recorded a good relationship between the buffering capacity and soil organic matter and the importance of soil organic matter to maintain soil pH despite certain acidifying factors. A strong correlation existed between initial soil pH value and the buffering capacity of subsurface horizon, and the highly acidic soils were better buffered than less acidic soils (Curtin and Trolove 2013; Lieb et al. 2011; Bowman et al. 2008; De Vries et al. 1989; Bhat et al. 2018a, b). The soils are poorly buffered between 4.5 and 6.5 and well buffered below 4 and above 7. A number of researchers have studied the relationship between soil organic matter and soil pH. The decomposition of young shoots of trees in Ultisols and Oxisols increased soil pH and decrease in exchangeable aluminum content and the acid neutralization was due to aluminum and proton complexation by the organic anions (Wong et al. 2000; Tian et al. 1992; Hue 1992; Vallis and Jones 1973). The under saturation of aluminum adsorption would result in 3 mol of protons consumed for each mol of aluminum dissolved. The aluminum dissolution by the organic anions would result in proton consumption and the pH increase. The pH increase is not the primary cause for the decrease of soluble and exchangeable aluminum, but also due to adsorption of these substances on soil organic matter (Luo et al. 2015) The soil organic matter and the exchangeable aluminum has a negative correlation at greater depths and the effect of soil organic matter was greater at lower pH values. The relationship of soil organic matter and pH was studied when different type of plant material was incubated in top soil and there occurred increase in pHkcl in a few days and the magnitude of which depends on type of soil organic matter and rate of application. The organic anions (malate, citrate, oxalate) balance the plant derived cations and the oxidation of anions consumes H+ ion and the release of OH− ion (Najafi and Jalali 2016). When the decomposed organic matter is added to the soil there is rise in pH due to complexation of proton by organic anion. If the undecomposed organic matter is added, the rise in soil pH is due to decarboxylation of organic anions during microbial decomposition. The decomposition of organic matter will transfer organic nitrogen to ammonia and releases OH− and results in increase in soil pH (Zhang et al. 2013; Qin et al. 2013; Galloway et al. 2008; Ju et al. 2004). The specific adsorption of soil organic matter onto the aluminum and iron hydroxides results in release of OH− ion. The long-term effect of organic matter increases soil pH as there occurs accumulation of humic material which complexes with aluminum compounds and decreases their solubility and protects soil from toxicity.
7.3.2 Cation Exchange Capacity
Cation exchange capacity of soil is the measure of exchangeable cations that soil can hold and represents negative charge per unit mass of soil. Soils with high cation exchange capacity is favorable as it holds many plant nutrients and this cation exchange capacity is expressed as centimol of positive charge per kilogram of soil (Soares and Alleoni 2008; Wiseman and Puttmann 2006; Adams and Evans 1979). The soils are having two charges: permanent charge CECP and pH-dependent charge CECv which depends on pH and the soil organic matter (Bache 1976; Bascomb 1964). The addition of organic matter generally causes increase in CECv and this increase is due to the presence of certain functional groups in the soil organic matter. The organic compounds with high molecular weight contribute less to cation exchange capacity compared to low molecular weight compounds (Alburquerque et al. 2014; Asai et al. 2009; Bonfante et al. 2010; Das and Varma 2011; McClellan et al. 2007; Lehmann and Rondon 2006). The contribution of soil organic matter to CEC is in the range 25–90% and the variation is mainly due to presence of functional group in soil organic matter and the soil type. There is a greater increase due to addition of soil organic matter to the cation exchange capacity in the coarse-textured soils than the medium- and fine-textured soils. Due to the addition of soil organic matter to mineral soil, there is greater increase of cation exchange capacity than in the surface soil (Schulz and Glaser 2012; Kammann et al. 2011; Peng et al. 2011). The soil organic matter has a cation exchange capacity of 150–250 Cmol(p+)kg−1 and in the arable calcareous soil with high organic matter, the cation exchange capacity ranges from 230 + _ 47 (Wolf and Snyder 2003). In sandy forest soils, soil organic matter contribution to cation exchange capacity is very high in comparison to Vertisols derived from basalt. Stevenson (1982) worked on several laboratory methods to determine the relationship between soil organic matter and cation exchange capacity. The regression equation was developed for predicting the relationship between soil organic matter and cation exchange capacity (Hallsworth and Wilkinson 1958). The cation exchange capacity of soil organic matter varies and it depends on type of functional group present in soil organic matter and the pH of soil. The measure source of negative charge that contributes to cation exchange capacity is the carboxylic groups. The cation exchange capacity of soil organic matter in acid soils was estimated to be 134 Cmol(p+) kg−1 and in chernozem it ranged upto 297 Cmol(p+) kg−1. The potential sites for cation exchange in soil organic matter are more than the measured as the many sites become unavailable due to the association with polyvalent cations (Table 7.3).
7.3.3 Adsorption and Complexation
Adsorption reaction due to soil organic matter are dependent mostly on pH as well as cation exchange capacity because similar types of organic carbon species are involved in adsorption reaction (Ahmed et al. 2015; Figueroa-diva et al. 2010; Frossard et al. 2002; Jones and Edwards 1998). The presence of functional groups (COOR, NH2, OH, NHR, CONH2) are very important for adsorption of ions on humus particles (Wu et al. 2012). The important mechanism for protection of soil organic matter from decomposition is its adsorption with clay particles. The positive relationship between soil organic carbon, clay content and soil surface area illustrates the significance of adsorption of soil organic matter on to the clay particles (Schaumann 2006; Schulten 2002). The interaction of soil organic matter with clay is governed by nature of soil organic matter and type of clay present. The interaction between soil organic matter and positively charged ions is through cation exchange reaction (between positively charged cation and negatively charged carbon group) (Cheng et al. 2014; Schwarz et al. 2012; Ghosh et al. 2000). The complexation of soil organic matter with inorganic material enhances the soil fertility as it increases the availability of soil phosphorus by blocking iron, aluminum, and calcium adsorption sites. Soil organic matter decreases the phosphorus adsorption in oxisols and the greater phosphorus adsorption was observed in cultivated soils (800 mgP/kg) than forest soils (560 mgP/kg) and it is attributed to greater amount of soil organic carbon in the forest soils (Bai et al. 2012). With the exception of few non-crystalline minerals, soil organic matter has the greatest capacity to form bonds with metals and it is associated by a positive association between solubility of metals with soil organic matter content as well as to high amounts of trace metals in organic rich soils compared with the non-organic soils (Sparks 2003). The increased concentration of soil organic matter decreases the concentration of cupric, zinc, and manganese in soil solution and also decreases the extraction of calcium by calcium chloride and acetic acid. The adsorption of copper was much stronger than zinc and manganese in the peat, solid humic acid, and acid washed peat (Klucakova 2012; Barancikova et al. 2003). The organic manures reduce the aluminum toxicity and increases the phosphorus availability and the important organic carbon groups in this complex reaction were low molecular weight aliphatic organic acids and soluble humic molecules as it complexes with the monomeric aluminum. The formation of complexes between polyvalent metal ions and humic substance is due to O-containing functional groups (enolic, phenolic, alcoholic, and carboxylic) as hydroxyl there is also the decreases the sorption of chlorinated aliphatic hydrocarbons and low molecular weight organic acids (fumaric, lactic, oxalic, citric, tartaric, propionic, butyric, acetic and formic acid) which are derived from leaves and from microbial biomass and also forms complex with AL3+. Hydroxyl acids form stronger complexes than carbon groups. The presence of these functional groups in soil organic matter does not only determine sorption capacity but also have certain synergistic effects, such as aromaticity, polarity, and hydrophobicity.
7.4 Soil Biological Properties
7.4.1 Soil Organic Matter as a Driver of Biological Activity
The fundamental function of organic farming system is to supply high amounts of organic matter which provides metabolic energy and drives soil biological process. In the organic farming systems, there is basically the transformation of carbon compounds by macro and microorganisms and plants that provide energy and connects above and below surface energy by the formation of a cycle (Rossiter and Bouma 2018; Wade et al. 2018; Roper et al. 2017; Bonfante and Bouma 2015; Tiquia 2005). The plants assimilate carbon from atmosphere and form glucose and other complex plant biomolecules which, upon plant senescence, enter the soil through roots, litter, and root exudates (Zhang 2013; Zuber et al. 2018; Bashir et al. 2016). The plants supply energy to heterotrophs and, to a less extent, to chemotrophs (microbes, fungi, and earthworms) by the formation of recalcitrant organic matter (Adeniji and Babalola 2019) The carbon source acts as a source of energy and as long as net primary production exceeds respiration the organic carbon will accumulate in the soil (Miranda et al. 2019; Oburger and Jones 2018; Conrad et al. 2018; Mahanty et al. 2014; Dijkstra et al. 2013). The different organic farming systems provide various amounts of soil organic matter in soil, which reflects the balance between carbon produced and carbon leached (Gopalakrishnan et al. 2015). This balance occurs due to energy requirement of biota and is governed by certain factors (temperature, clay content, moisture, humidity, and rainfall). The transformation of labile soil organic matter into more complex form, that is, humus stabilizes this soil organic matter and can be used as a source of energy for longer period in an edaphic environment. The energy released from the soil organic matter decomposition is in the form of heat and the heat losses from 1 hectare is nearly equal to heat value of 1 metric ton coal and the highly productive organic matter soil releases heat of about 12 megagram of coal annually. The soil microorganisms play an immense role in the transformation of organic matter as these microbes carry 80–90% of total soil metabolism (Fernández-Gómez et al. 2019; Cui et al. 2019; Lamprecht et al. 2018; Pascual et al. 2018; Shen et al. 2014). The 1–5% of nitrogen and carbon are being preserved in the microbial tissue. A concept of microbial catabolic evenness (CE) was introduced by Degens et al. (2000) to measure soil microbial diversity by short term respiration response of soil over a certain range of organic compounds. They found a direct relationship between microbial catabolic evenness and soil organic pools and reported higher (CE) in pastures followed by agriculture and horticultural crops and the least was reported in the arable soils (Yuan et al. 2014). It was also found that, with the depletion of soil organic matter or carbon stock, there was greater decline in the microbial catabolic evenness.
7.4.2 Soil Organic Matter and Soil Microbial Population
Soil is species rich habitat on earth having diverse and abundant species which help in the formation and development of soil. The soil biodiversity is indicator of soil health, as greater biodiversity means greater soil stability in terms of certain functions, such as maintenance of soil structure, assimilation of organic wastes, and nutrient cycling (Miranda et al. 2019; Oburger and Jones 2018; Conrad et al. 2018; Mahanty et al. 2014; Bashir et al. 2016; Shen et al. 2014; Dijkstra et al. 2013; Wang et al. 2012; Bhatti et al. 2017). Soil organic matter, soil organic carbon, and soil biodiversity are closely related but distinct. Biodiversity means residing of certain organisms, such as bacteria, fungi, actinomycetes, protozoa, worms, vertebrates, and invertebrates (Oburger and Jones 2018; Conrad et al. 2018). All these organisms depend on soil organic matter for their energy, nutrients, and habitat. The topmost soil of earth, where concentration of organic matter and roots are higher, forms the largest habitat for these organisms (Bashir et al. 2016). A vast diversity of the organisms is present in the soil but only limited microorganism has been explored (Table 7.4).
7.4.3 Soil Enzyme Activity and Soil Organic Matter
Soil enzymes play a key role in organic matter decomposition and its recycling, with their activities being closely related to microbial activity, microbial biomass, soil physical property, and soil organic matter (Oburger and Jones 2018; Mahanty et al. 2014; Bashir et al. 2016; Dijkstra et al. 2013). These enzymes are either intracellular or extracellular, with intracellular being inside the cell in the cytoplasm bound by the cell wall. The extracellular enzymes are permanently immobilized and are being released into the soil on humic and clay colloids through hydrogen bonds, ionic bonds, covalent bonds, and other mechanism. These soil enzymes act as catalyst for decomposition of organic matter and effect agronomic production, environmental quality, and energy transformation (Wade et al. 2018; Rossiter and Bouma 2018; Roper et al. 2017; Bonfante and Bouma 2015). Soil enzymes are considered best soil detectors because they respond to the soil sooner than physical and chemical parameters.
7.4.4 Soil Organic Matter as Important Nutrient Source
In considering the importance of organic matter as source of nutrients, it is to be mentioned that soil formation is closely related to diverse forms of organic substance on parent material. Soil organic matter provides all the essential nutrients, including primary nutrients such as nitrogen, phosphorus, and sulfur and micronutrients such as iron, manganese, zinc, copper, boron, molybdenum, and chlorine (Nurhidayati et al. 2018; Pravin et al. 2013; Masto et al. 2007; Laiho et al. 2004; Katyal et al. 2001). These nutrients are being made available during mineralization of organic matter during their growing season and the important fraction of soil organic matter fraction which supplies nutrients is particulate organic matter. Ninety percent of soil organic matter is made of carbon, hydrogen, and oxygen, while 50% of remaining elements is made of nitrogen, potassium, and silicon (Gwenzi et al. 2016; Haynes and Naidu 1998; Mahanty et al. 2014; Moco et al. 2009; Reeves 1997; Steller et al. 2008; Stevenson 1994; Von Lutzow et al. 2005). However, the application of fertilizers supplies a major nutrient available to plants, but the organic matter along with the soil microbes, store and cycle large amounts of nutrients required for growth (Liu et al. 2009). Most of the nutrients held in the soil organic matter are not easily assessable to plants and are resistant to decomposition. Only 1–5% of the soil organic matter is decomposed annually and it takes almost a decade for its complete decomposition (Molina-Herrera and Romanya 2015; Haynes and Naidu 1998). Soil organic matter acts as larger reservoir of macronutrients with 90–95% nitrogen and sulfur and 20–75% phosphorus. The 90–95% nitrogen is held in both available and fixed form. The 40–45% of organic nitrogen is quantified and identifiable as amino-sugars and amino acids and the remaining portion consists of unidentifiable structure. The soil Sulfur in organic form is mainly in the form of amino-acids, such as cysteine, cystine, and methionine. Phosphorus is mainly present in ester form and nitrogen is covalently bonded to C–S or C–O–S. The process of net phosphorus mineralization occurs if ratio of C:P is less than 100, whereas ratio of greater than 300 indicate net immobilization. The soil organic matter has impact on phosphorus availability through specific adsorption reaction because the humic fraction shows competitive character on oxide surfaces (Zhao et al. 2019; Liu et al. 2009; ErdalSakin 2012; Gama-Rodrigues et al. 2008; Geeves et al. 1995; Lal 2011; Zhang et al. 2006; Madejón et al. 2003; Powlson et al. 2001). Mineralization and transformation of organic source of phosphorus occur through extracellular hydrolysis or by the oxidation of organic carbon. Decrease in the soil organic carbon pertains to reduced nutrient supply and less than 1% organic matter is considered a threshold value below which there is no nutrient supply. The release of NPS from organic matter depends upon ratio of these elements to carbon. A narrow ratio of these elements to carbon usually allows fast nutrient release and a wide ratio reduces their availability (Guan et al. 2019; Hu et al. 2019; Ma et al. 2019; Cai et al. 2018). There are various schools of thought pertaining to nutrient status of soil organic matter, including its C:N:P:S with ratio of 100:10:1:1, 155:10:0.68:1.4, and 140:10:1.4:1.4. C:N:S ratio in the agricultural soils vary from other soils due to higher carbon mineralization of carbon and greater fertilizer input.
In view of the macronutrients, soil organic matter forms a number of chelates that make metal nutrient elements available over a wide range of pH. The micronutrient chelation has greater significance because of the nature of these elements to become fixed in high pH soils. The most chelates formed in the soil are of iron, copper, and zinc. In the plant’s heme group forms the most common chelates, including chlorophyll and iron porphyrin. Oxalic and malic acids are reported to have high chelating properties. The root exudates and complex organic matter form the chelates that remain available to plants for a longer period. Ketogluconic acid has been reported as a highly chelating agent, but probably there are more chelating agents formed by organic matter (Shi et al. 2018; Du et al. 2018; Zhou et al. 2018; Liu et al. 2017; Hu et al. 2016; Guo et al. 2015; Wiatrowska et al. 2013). The organic matter supplied to high pH soils forms chelates and corrects lime-induced chlorosis. The carbon dioxide favors bicarbonate formation, which decreases iron uptake and translocation within plants.
7.5 Conclusion
The diverse nature of soil organic matter plays a defining role in determining the dimensions of various soil processes and properties, including its physical, chemical, and biological properties. This chapter revealed that interaction of soil organic matter with soil properties is very complex. The soil organic matter is considered as an important soil health indicator, as most of the soil properties depend on it. Almost all the soil properties were strengthened with the increase in the soil organic matter. The increase in soil organic matter had clear impact on the soil structure, water retention, thermal conductivity, available water capacity, zeta potential, exchangeable cations, soil fertility, erodibility, infiltration, soil aggregate formation, soil color, soil compaction, soil aeration, pH, buffering capacity, CEC, base saturation, and microbial population. The soil organic matter is an important factor in nutrient cycling, nutrient supply, especially nitrogen, phosphorus, sulfur, and micronutrients. The soil organic matter was more dominant where clay content was low. The important conclusion was that, with the addition of soil organic matter, we can improve many soil properties simultaneously. The certain issues that need to be readdressed in future are as follows: (a) application of soil organic matter to enhance soil health, (b) integrated nutrient management for sustainable agriculture, (c) carbon sequestration for mitigating climate change, and (d) organic residues management concerning recycling and environmental protection.
References
Acharya BS, Kharel G, Zou BP, Wilcox CB, Halihan T (2018) Woody plant encroachment impacts on groundwater recharge: a review. Water 10:1466
Adams JM, Evans S (1979) Determination of the cation-exchange capacity (layer charge) of small quantities of clay minerals by nephelometry. Clay Clay Miner 27:137–139
Adeniji AA, Babalola OO (2019) Phylogeny, useful applications, and avenues for exploitation. Appl Microbiol Biotechnol 103:3669–3682
Ahmad R, Jilani G, Arshad M, Zahir ZA, Khalid A (2007) Bio-conversion of organic wastes for their recycling in agriculture: an overview of perspectives and prospects. Ann Microbiol 57(4):471–479
Ahmed AA, Thiele-Bruhn S, Aziz SG, Hilal RH, Elroby SA, Al-Youbi AO, Leinweber P, Kühn O (2015) Interaction of polar and nonpolar organic pollutants with soil organic matter: sorption experiments and molecular dynamics simulation. Environ Sci Technol 508:276–287
Ajayi AE, Horn R (2017) Biochar-induced changes in soil resilience: effects of soil texture and biochar dosage. Pedosphere 27:236–247
Alburquerque JA, Calero JM, Barrón V, Torrent J, del Campillo MC, Gallardo A, Villar R (2014) Effects of biochars produced from different feedstocks on soil properties and sunflower growth. J Plant Nutr Soil Sci 177(1):16–25
Alexander JD (1971) Color chart for estimating organic carbon in mineral soils in Illinois. Bull. AG-1941. Univ. of Ill. Coop. Ext. Serv., Champaign
Alfreider A, Peters S, Tebbe CC (2002) Microbial community dynamics during composting of organic matter as determined by 16S ribosomal DNA analysis. Compost Sci Util 10:303–312
Almajmaie A, Hardie M, Acuna T, Birch C (2017) Evaluation of methods for determining soil aggregate stability. Soil Tillage Res 167:39–45
Angin I, Aksakal EL, Oztas T, Hanay A (2013) Effects of municipal solid waste compost (MSWC) application on certain physical properties of soils subjected to freeze- thaw. Soil Tillage Res 130:58–61
Ankenbauer K, Loheide SP (2017) The effects of soil organic matter on soil water retention and plant water use in a meadow of the Sierra Nevada, CA. Hydrol Process 31:891–901
Asai H, Samson BK, Stephan HM, Songyikhangsuthor K, Homma K, Kiyono Y, Horie T (2009) Biochar amendment techniques for upland rice production in Northern Laos: soil physical properties, leaf SPAD and grain yield. Field Crop Res 111(1):81–84
Aşkın T, Özdemir N (2003) Soil bulk density as related to soil particle size distribution and organic matter content. Agriculture 9(2):52–55
Avnimelech Y, Ritvo G, Meijer LE, Kochba M (2001) Water content, organic carbon and dry bulk density in flooded sediments. Aquac Eng 25:25–33
Bache BW (1976) The measurement of cation exchange capacity of soils. J Sci Food Agric 27:273–280
Bai E, Boutton TW, Liu F, Wu XB, Hallmark CT, Archer SR (2012) Spatial variation of soil δ13C and its relation to carbon input and soil texture in a subtropical lowland woodland. Soil Biol Biochem 44:102–112
Balázs B, Bíró T, Dyke GJ, Singh SK, Szabó SZ (2018) Extracting water-related features using reflectance data and principal component analysis of Landsat images. Hydrol Sci J 63:269–284
Balesdent J, Chenu C, Balabane M (2000) Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillage Res 53:215–230
Barancikova G, Klucakova M, Madaras M, Makovnikova J, Pekar M (2003) Chemical structure of humic acids isolated from various soil types and lignite. Humic Subst Environ 3:3–8
Barron V, Torrent J (1986) Use of the Kubelka-Munk theory to study the influence of iron oxides on soil color. Eur J Soil Sci 37:499–510
Basche AD, DeLonge MS (2019) Comparing infiltration rates in soils managed with conventional and alternative farming methods: a meta-analysis. PLoS One 14(9):0215702
Basche AD, Kaspar TC, Archontoulis SV, Jaynes DB, Sauer TJ, Parkin TB (2016) Soil water improvements with the long-term use of a winter rye cover crop. Agric Water Manag 172:40–50
Bascomb L (1964) Rapid method for the determination of the cation exchange capacity of calcareous and non-calcareous soils. J Sci Food Agric 15:821–823
Bashir O, Kamran K, Khalid RH, Naseer AM, Rather GH, Mohiuddin R (2016) Soil microbe diversity and root exudates as important aspects of rhizosphere ecosystem. In: Plant, soil and microbes. Springer International Publishing, Cham, pp 337–357
Bauer A (1974) Influence of soil organic matter on bulk density and available water capacity of soils North Dakota Agricultural Experimental Station. Farm Res 31:44–52
Beck-Broichsitter S, Fleige H, Horn R (2018) Compost quality and its function as a soil conditioner of recultivation layers – a critical review. Int Agrophys 32:11–18
Ben-Dor E, Heller D, Chudnovsky A (2008) A novel method for classifying soil profiles in the field using optical means. Soil Sci Soc Am J 72:1113–1123
Ben-Salem N, Álvarez S, López-Vicente M (2018) Soil and water conservation in rainfed vineyards with common sainfoin and spontaneous vegetation under different ground conditions. Water 10:1058
Bezuglova OS, Gorovtsov AV, Polienko EA, Zinchenko VE, Grinko AV, Lykhman VA, Dubinina MN, Demidov A (2019) Effect of humic preparation on winter wheat productivity and rhizosphere microbial community under herbicide-induced stress. J Soils Sediments 19:2665–2675
Bhat RA, Dervash MA, Mehmood MA, Bhat MS, Rashid A, Bhat JIA, Singh DV, Lone R (2017a) Mycorrhizae: a sustainable industry for plant and soil environment. In: Varma A et al (eds) Mycorrhiza-nutrient uptake, biocontrol, ecorestoration. Springer International Publishing, Cham, pp 473–502
Bhat RA, Shafiq-ur-Rehman, Mehmood MA, Dervash MA, Mushtaq N, Bhat JIA, Dar GH (2017b) Current status of nutrient load in Dal Lake of Kashmir Himalaya. J Pharmacogn Phytother 6(6):165–169
Bhat RA, Beigh BA, Mir SA, Dar SA, Dervash MA, Rashid A, Lone R (2018a) Biopesticide techniques to remediate pesticides in polluted ecosystems. In: Wani KA, Mamta (eds) Handbook of research on the adverse effects of pesticide pollution in aquatic ecosystems. IGI Global, Hershey, pp 387–407
Bhat RA, Dervash MA, Qadri H, Mushtaq N, Dar GH (2018b) Macrophytes, the natural cleaners of toxic heavy metal (THM) pollution from aquatic ecosystems. In: Environmental contamination and remediation. Cambridge Scholars Publishing, Newcastle upon Tyne, pp 189–209
Bhatti AA, Haq S, Bhat RA (2017) Actinomycetes benefaction role in soil and plant health. Microb Pathog 111:458–467
Bi J, Zhang M, Wu CW, Lu J, Lai Y (2018) A new model to determine the thermal conductivity of fine-grained soils. Int J Heat Mass Transf 123:407–417
Blanchet G, Gavazov K, Bragazza L, Sinaj S (2016) Responses of soil properties and crop yields to different inorganic and organic amendments in a Swiss conventional farming system. Agric Ecosyst Environ 230:116–126
Bloom PR (1999) Soil pH and pH buffering. In: Sumner ME (ed) Handbook of soil science. CRC Press, Boca Raton, pp B333–B352
Boizard H, Peigné J, Sasal MC, de Fátima Guimarães M, Piron D, Tomis V, Vian JF, Cadoux S, Ralisch R, Tavares Filho J, Heddadj D, De Battista J, Duparque A, Franchini JC, Roger-Estrade J (2017) Developments in the “profile cultural” method for an improved assessment of soil structure under no-till. Soil Tillage Res 173:93–103
Bonfante A, Bouma J (2015) The role of soil series in quantitative land evaluation when expressing effects of climate change and crop breeding on future land use. Geoderma 259–260:187–195
Bonfante A, Basile A, Acutis M, De Mascellis R, Manna P, Perego A, Terribile F (2010) SWAP, CropSyst and MACRO comparison in two contrasting soils cropped with maize in Northern Italy. Agric Water Manag 97:1051–1062
Bongiornoa G, Postma J, Bünemann EK, Brussaard L, de Goede RG, Mäder P, Tamm L, Thuerig B (2019) Soil suppressiveness to Pythium ultimum in ten European long-term field experiments and its relation with soil parameters. Soil Biol Biochem 133:174–187
Bowman WD, Cleveland CC, Halada Ĺ (2008) Negative impact of nitrogen deposition on soil buffering capacity. Nat Geosci 1:767–770
Brown PE, O’Neal AM (1923) The color of soils in relation to organic carbon content. Res Bull No. 75. Iowa State College of Agric. and Mechanic Arts, Ames
Bullard JE, Ockelford A, Strong CL, Aubault H (2018) Impact of multi-day rainfall events on surface roughness and physical crusting of very fine soils. Geoderma 313:181–192
Cai G, Liu S, Puppala AJ (2017) Investigation on thermal characteristics and prediction models of soils. Int J Heat Mass Transf 106:1074–1086
Cai X, Lin ZW, Penttinen P, Li YF, Li YC, Luo Y (2018) Effects of conversion from a natural evergreen broadleaf forest to a Moso bamboo plantation on the soil nutrient pools, microbial biomass and enzyme activities in a subtropical area. For Ecol Manag 422:161–171
Calhoun FG, Smeck NE, Slater BL, Bigham JM, Hall GF (2001) Predicting bulk density of Ohio soils from morphology, genetic principles, and laboratory characterization data. Soil Sci Soc Am J 65:811–819
Cayley JWD, McCaskill MR, Kearney GA (2002) Changes in pH and organic carbon were minimal in a long-term field study in the Western District of Victoria. Aust J Agric Res 53:115–126
Celik I, Gunal H, Budak M, Akpinar C (2010) Effects of long-term organic and mineral fertilizers on bulk density and penetration resistance in semi-arid Mediterranean soil conditions. Geoderma 160:236–243
Chavarria DN, Pérez-Brandan C, Serri DL, Meriles JM, Restovich SB, Andriulo AE, Jacquelin L, Vargas-Gil S (2018) Response of soil microbial communities to agroecological versus conventional systems of extensive agriculture. Agric Ecosyst Environ 264:1–8
Cheng G, Zhu L, Sun M, Deng J, Chen H, Xu X, Lou L, Chen Y (2014) Desorption and distribution of pentachlorophenol (PCP) on aged black carbon containing sediment. J Soils Sediments 14(2):344–352
Christensen BT (2001) Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur J Soil Sci 52:345–353
Conrad KA, Dalal RC, Dalzell SA, Allen DE, Fujinuma R, Menzies NW (2018) Soil nitrogen status and turnover in subtropical leucaena-grass pastures as quantified by δ15N natural abundance. Geoderma 313:126–134
Cui Y, Bing H, Fang L, Wu Y, Yu J, Shen G et al (2019) Diversity patterns of the rhizosphere and bulk soil microbial communities along an altitudinal gradient in an alpine ecosystem of the eastern Tibetan Plateau. Geoderma 338:118–127
Curtin D, Trolove S (2013) Predicting pH buffering capacity of New Zealand soils from organic matter content and mineral characteristics. Soil Res 51:494–502
Curtis RO, Post BW (1964) Estimating bulk density from organic matter content in some Vermont forest soils. Soil Sci Soc Am Proc 28:285–286
Dar S, Bhat RA (2020) Aquatic pollution stress and role of biofilms as environment cleanup technology. In: Qadri H, Bhat RA, Dar GH, Mehmood MA (eds) Freshwater pollution dynamics and remediation. Springer Nature, Singapore, pp 293–318
Dar GH, Bandh SA, Kamili AN, Nazir R, Bhat RA (2013) Comparative analysis of different types of bacterial colonies from the soils of Yusmarg Forest, Kashmir valley India. Ecol Balkanica 5(1):31–35
Dar GH, Kamili AN, Chishti MZ, Dar SA, Tantry TA, Ahmad F (2016) Characterization of Aeromonas sobria isolated from Fish Rohu (Labeo rohita) collected from polluted pond. J Bacteriol Parasitol 7(3):1–5. https://doi.org/10.4172/2155-9597.1000273
Das SK, Varma A (2011) Role of enzymes in maintaining soil health. In: Shukla G, Varma A (eds) Soil enzymology, Soil biology, vol 22. Springer-Verlag, Berlin Heidelberg
Das A, Lyngdoh D, Ghosh PK, Lal R, Layek J, Idapuganti RG (2018) Tillage and cropping sequence effect on physico-chemical and biological properties of soil in Eastern Himalayas, India. Soil Tillage Res 180:182–193
De Curtis F, Ianiri G, Raiola A, Ritieni A, Succi M, Tremonte P, Castoria R (2019) Integration of biological and chemical control of brown rot of stone fruits to reduce disease incidence on fruits and minimize fungicide residues in juice. Crop Prot 119:158–165
De Vries W, Posch M, Kämäri J (1989) Simulation of the long-term soil response to acid deposition in various buffer ranges. Water Air Soil Pollut 48:349–390
Dębska B, Długosz J, Piotrowska-Długosz A, Banach-Szott M (2016) The impact of a bio-fertilizer on the soil organic matter status and carbon sequestration—results from a field-scale study. J Soils Sediments 16:2335–2343
Degens BP, Schipper LA, Sparling GP, Vojvodic-Vukovic M (2000) Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol Biochem 32:189–196
Dervash MA, Bhat RA, Shafiq S, Singh DV, Mushtaq N (2020) Biotechnological intervention as an aquatic clean up tool. In: Qadri H, Bhat RA, Mehmood MA, Dar GH (eds) Freshwater pollution dynamics and remediation. Springer Nature, Singapore, pp 183–196
Di Prima S, Bagarello V, Lassabatere L, Angulo-Jaramillo R, Bautista I, Burguet M, Cerda A, Iovino M, Prosdocimi M (2017) Comparing Beerkan infiltration tests with rainfall simulation experiments for hydraulic characterization of a sandy-loam soil. Hydrol Process 31:3520–3532
Di Sipio E, Bertermann D (2018) Thermal properties variations in unconsolidated material for very shallow geothermal application (ITER project). Int Agrophys 32:149–164
Diadin D, Vystavna Y, Vergeles Y (2018) Quantification of nitrate fluxes to groundwater and rivers from different land use types. Hung Geogr Bull 67:333–341
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA (2013) Rhizosphere priming: a nutrient perspective. Front Microbiol 4:216
Du H, Huang Q, Peacock C, Tie B, Lei M, Liu X, Wei X (2018) Competitive binding of Cd, Ni and Cu on goethite organo-mineral composite made with soil bacteria. Environ Pollut 243:444–452
Emerson WW, McGarry D (2003) Organic carbon and soil porosity. Aust J Soil Res 41:107–118
ErdalSakin (2012) Organic carbon organic matter and bulk density relationships in arid-semi arid soils in Southeast Anatolia region. Afr J Biotechnol 11(6):1373–1377
Erskine WD (2013) Soil colour as a tracer of sediment dispersion from erosion of forest roads in Chichester State Forest, NSW, Australia. Hydrol Process 27:933–942
Ertlen D, Schwartz D, Trautmann M, Webster R, Brunet D (2010) Discriminating between organic matter in soil from grass and forest by near-infrared spectroscopy. Eur J Soil Sci 61:207–216
Ertlen D, Schwartz D, Brunet D, Trendel JM, Adam P, Schaeffer P (2015) Qualitative near infrared spectroscopy, a new tool to recognize past vegetation signature in soil organic matter. Soil Biol Biochem 82:127–134
European Environment Agency (2010). Annual report 2010 and Environmental statement 2011. European Environment Agency Kongens Nytorv 6 1050 Copenhagen K Denmark. file:///C:/Users/User/Downloads/Annual-Report-2010.pdf
Evrendilek F, Celik I, Kilic S (2004) Changes in soil organic carbon and other physical soil properties along adjacent Mediterranean forest, grassland, and cropland ecosystems in Turkey. J Arid Environ 59(4):743–752
Fedoseeva E, Stepanov A, Yakimenko O, Patsaeva S, Freidkin M, Khundzhua D, Terekhova V (2019) Biodegradation of humic substances by microscopic filamentous fungi: chromatographic and spectroscopic proxies. J Soils Sediments 19:2676–2687
Fernández-Gómez B, Maldonado J, Mandakovic D, Gaete A, Gutiérrez RA, Maass A (2019) Bacterial communities associated to Chilean altiplanic native plants from the Andean grassland’s soils. Sci Rep 9:1042
Figueroa-diva RA, Vasudevan D, Mackay AA (2010) Trends in soil sorption coefficients within common antimicrobial families. Chemosphere 79:786–793
Fortugno D, Boix-Fayos C, Bombino G, Denisi P, Quiñonero Rubio JM, Tamburino V, Zema DA (2017) Adjustments in channel morphology due to land-use changes and check dam installation in mountain torrents of Calabria (southern Italy). Earth Surf Process Landf 42:2469–2483
Frąc M, Hannula SE, Bełka M, Jędryczka M (2018) Fungal biodiversity and their role in soil health. Front Microbiol 9:707
Franzluebbers A (2002) Water infiltration and soil structure related to organic matter and its stratification with depth. Soil Tillage Res 66:197–205
Frossard E, Skrabul P, Sinaj S, Bangerter F, Traore O (2002) Forms and exchangeability of inorganic phosphate in composted solid organic wastes. Nutr Cycl Agroecosyst 62:103–113
Galloway JN, Townsend AR, Erisman JW (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Gama-Rodrigues EF, Barros NF, Viana AP, Santos GA (2008) Microbial biomass and activity in soil and forest litter of eucalyptus plantations and native vegetation in southeastern Brazil. Rev Bras Cienc Solo 32:1489–1499
Gao F, Xia J (2009) Soil organic-mineral complex status and distribution of organic carbon in tea plantations of different planting year. J Soil Water Conserv 23:50–53
Garratt MPD, Bommarco R, Kleijn D, Martin E, Mortimer SR, Redlich S, Senapathi D, Steffan-Dewenter I, Świtek S, Takacs V, van Gils S, van der Putten WH, Potts SG (2018) Enhancing soil organic matter as a route to the ecological intensification of European arable systems. Ecosystems 21:1404–1415
Geeves GW, Cresswell HP, Murphy BW, Gessler PE, Chartres CJ, Little IP, Bowman GM (1995) The physical, chemical and morphological properties in the wheat-belt of south eastern New South Wales and north eastern Victoria. CSIRO Division of Soils, Canberra and NSW Department of Conservation and Land Management
Genardzielinski AC, Boissard C, Ormeño E, Lathière J, Reiter IM, Wortham H (2018) Seasonal variations of Quercus pubescens isoprene emissions from an in natura forest under drought stress and sensitivity to future climate change in the Mediterranean area. Biogeosciences 15:4711–4730
Gharahi-Gheni N, Nemes A, Verdoodt A, van Ranst E, Cornelis WM, Boeckx P (2012) Nonparametric techniques for predicting soil bulk density of tropical rainforest topsoils in Rwanda. Soil Sci Soc Am J 76:1172–1183
Ghosh U, Gillette JS, Luthy RG, Zare RN (2000) Microscale location, characterization, and association of polycyclic aromatic hydrocarbons on harbor sediment particles. Environ Sci Technol 34:1729–1736
Gogoi A, Sahoo UK, Singh SL (2017) Assessment of biomass and total carbon stock in a tropical wet evergreen rainforest of eastern Himalaya along a disturbance gradient. J Plant Biol Soil Health 4(1):8
Gómez JA, Francia JR, Guzmán G, Vanwalleghem T, Durán Zuazo VH, Castillo C, Barrón V (2017) Lateral transfer of organic carbon and phosphorus by water erosion at hillslope scale in southern Spain olive orchards. Vadose Zone J 16:1–15
Gómez JA, Campos M, Guzmán G, Castillo-Llanque F, Van walleghem T, Lora A, Giráldez JV (2018) Soil erosion control, plant diversity, and arthropod communities under heterogeneous cover crops in an olive orchard. Environ Sci Pollut Res 25:977–989
Gopalakrishnan S, Sathya A, Vijayabharathi R, Varshney RK, Gowda CL, Krishnamurthy L (2015) Plant growth promoting rhizobia: challenges and opportunities. 3 Biotech 5:355–377
Gougoulias C, Clark MJ, Shaw LS (2014) The role of soil microbes in the global carbon cycle: tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J Sci Food Agric 94:2362–2237
Guan S, Liu SJ, Liu RY, Zhang JJ, Ren J, Cai HG, Li XX (2019) Soil organic carbon associated with aggregate-size and density fractions in a Mollisol amended with charred and uncharred maize straw. J Integr Agric 18(7):1496–1507
Guimarães RML, Lamandé M, Munkholm LJ, Ball BC, Keller T (2017) Opportunities and future directions for visual soil evaluation methods in soil structure research. Soil Tillage Res 173:104–113
Gülser C, Candemir F, Kanel Y, Demirkaya S (2015) Effect of manure on organic carbon content and fractal dimensions of aggregates. Eur J Soil Sci 4(1):1–5
Guo XJ, Zhu NM, Chen L, Yuan DH, He LS (2015) Characterizing the fluorescent properties and copper complexation of dissolved organic matter in saline-alkali soils using fluorescence excitation-emission matrix and parallel factor analysis. J Soils Sediments 15:1473–1482
Gwenzi W, Muzava M, Mapanda F, Tauro TP (2016) Comparative short-term effects of sewage sludge and its biochar on soil properties, maize growth and uptake of nutrients on a tropical clay soil in Zimbabwe. J Integr Agric 15:1395–1406
Haghnazari F, Shahgholi H, Feizi M (2015) Factors affecting the infiltration of agricultural soils: review. Int J Agron Agric Res 6(5):21–35
Håkansson I, Lipiec J (2000) A review of the usefulness of relative bulk density values in studies of soil structure and compaction. Soil Tillage Res 53:71–85
Hallsworth EG, Wilkinson GK (1958) Contribution of clay and organic matter to cation exchange capacity of soil. J Agric Sci 51:1–3
Han GZ, Zang GL, Gong ZT, Wang GF (2012) Pedotransfer functions for estimating soil bulk density in China. Soil Sci 177:158–164
Hati KM, Swarup A, Dwivedi AK, Misra AK, Bandyopadhyay KK (2007) Changes in soil physical properties and organic carbon status at the topsoil horizon of a vertisol of central India after 28 years of continuous cropping, fertilization and manuring. Agric Ecosyst Environ 119:127–134
Haynes RJ, Naidu R (1998) Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: a review. Nutr Cycl Agroecosyst 51(2):123–137
Hazard C, Johnson D (2018) Does genotypic and species diversity of mycorrhizal plants and fungi affect ecosystem function? New Phytol 220:1122–1128
He X, Vepraskas MJ, Lindbo DL, Skaggs RW (2003) A method to predict soil saturation frequency and duration from soil color. Soil Sci Soc Am J 67:961–969
Herre A, Lang F, Siebe CH, Dohrmann R, Kaupenjohann M (2007) Mechanisms of acid buffering and formation of secondary minerals in vitric Andosols. Eur J Soil Sci 58:431–444
Heuscher SA, Craig C, Brandt CC, Jardine PM (2005) Using soil physical and chemical properties to estimate bulk density. Soil Sci Soc Am J 69:1–7
Hlavčová K, Danáčová M, Kohnová S, Szolgay J, Valent P, Výleta R (2019) Estimating the effectiveness of crop management on reducing flood risk and sediment transport on hilly agricultural land—a Myjava case study, Slovakia. Catena 172:678–690
Hortensia BV, Jose MSD, Edmundo GM, Armando GV, Bernardo JAG (2018) Physico-chemical properties of soil and pods (Theobroma cacao L.) in cocoa agroforestry systems. J Agron 17:48–55
Hsu TH, Lawrence CR, Winnick MJ, Bargar JR, Maher K (2018) A molecular investigation of soil organic carbon composition across a subalpine catchment. Soil Syst 2:6
Hu S, Lu C, Zhnag C, Hang Y, Yau M, Wu Y (2016) Effects of fresh and degraded dissolved organic matter derived from maize straw on copper sorption onto farmland loess. J Soils Sediments 16:327–338
Hu J, Wu J, Sharaf A, Sun J, Qu X (2019) Effects of organic wastes on structural characterizations of fulvic acid in semiarid soil under plastic mulched drip irrigation. Chemosphere 234:830–838
Huang S, Peng XX, Huang QR, Zhang WJ (2010) Soil aggregation and organic carbon fractions affected by long-term fertilization in a red soil of subtropical China. Geoderma 154:364–369
Hue NV (1992) Correcting soil acidity of a highly weathered Ultisol with chicken manure and sewage sludge. Commun Soil Sci Plant Anal 23:241–264
Huntington TG, Johnson CE, Jonhson AH, Sicama TG, Ryan DF (1989) Carbon, organic matter and bulk density relationships in a forested spodosol. Soil Sci 148:380–386
Huo C, Luo Y, Cheng W (2017) Rhizosphere priming effect: a meta-analysis. Soil Biol Biochem 111:78–84
Ibrahim H, Hatira A, Pansu M (2013) Modelling the functional role of microorganisms in the daily exchanges of carbon between atmosphere, plants and soil. Procedia Environ Sci 19:96–105
Issaka Z, Li H, Yue J, Tang P, Darko RO (2018) Water-smart sprinkler irrigation, prerequisite to climate change adaptation: a review. J Water Clim Chang 9:383–398
Jack ALH, Rangarajan A, Culman SW, Sooksa-Nguan T, Thies JE (2011) Choice of organic amendments in tomato transplants has lasting effects on bacterial rhizosphere communities and crop performance in the field. Appl Soil Ecol 48:94–101
Jakab G, Madarász B, Szabó JA, Tóth A, Zacháry D, Szalai Z, Kertész Á, Dyson J (2017) Infiltration and soil loss changes during the growing season under ploughing and conservation tillage. Sustainability 9:1726
James BR, Riha SJ (1986) pH buffering in forest soil organic horizons: relevance to acid precipitation. J Environ Qual 15:229–234
Jamison V, Kroth E (1958) Available moisture storage capacity in relation to textural composition and organic matter content of several Missouri soils. Soil Sci Soc Am J 22:189–192
Jarvis N, Larsbo M, Roulier S, Lindahl A, Persson L (2007) The role of soil properties in regulating non-equilibrium macropore flow and solute transport in agricultural topsoils. Eur J Soil Sci 58:282–292
Jiang T, Chen XS, Wang DY, Liang J, Bai WY, Zhang C, Wang QL, Wei SQ (2018) Dynamics of dissolved organic matter (DOM) in a typical inland lake of the Three Gorges Reservoir area: fluorescent properties and their implications for dissolved mercury species. J Environ Manag 206:418–429
Johnson SP, Miller ZJ, Lehnhoff EA, Miller PR, Menalled FD (2017) Cropping systems modify soil biota effects on wheat (Triticum aestivum) growth and competitive ability. Weed Res 57:6–15
Jones DL, Edwards AC (1998) Influence of sorption on the biological utilisation of two simple carbon substrates. Soil Biol Biochem 30:1895–1902
Jordán A, Zavala LM, Gil J (2010) Effects of mulching on soil physical properties and runoff under semi-arid conditions in southern Spain. Catena 81:77–85
Jozefaciuk G, Czachor H, Lamorski K, Hajnos M, Swieboda R, Franus W (2015) Effect of humic acids, sesquioxides and silica on the pore system of silt aggregates measured by water vapour desorption, mercury intrusion and microtomography. Eur J Soil Sci 66:992–1001
Ju X, Liu X, Zhang F, Roelcke M (2004) Nitrogen fertilization, soil nitrate accumulation, and policy recommendations in several agricultural regions of China. Ambio 33:300–305
Kaiser K, Guggenberger G (2003) Mineral surfaces and soil organic matter. Eur J Soil Sci 54:219–236
Kallenbach CM, Wallenstein MD, Schipanksi ME, Stuart Grandy A (2019) Managing agroecosystems for soil microbial carbon use efficiency: ecological unknowns, potential outcomes, and a path forward. Front Microbiol 10:1146
Kammann CI, Linsel S, Gößling JW, Koyro HW (2011) Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil–plant relations. Plant Soil 345(1–2):195–210
Karlen DL, Veum KS, Sudduth KA, Obrycki JF, Nunes MR (2019) Soil health assessment: past accomplishments, current activities and future opportunities. Soil Tillage Res 195:104365
Katyal JC, Rao NH, Reddy MN (2001) Critical aspects of organic matter management in the tropics: the example of India. Nutr Cycl Agroecosyst 61(1–2):77–88
Keller T, Håkansson I (2010) Estimation of reference bulk density from soil particle size distribution and soil organic matter content. Geoderma 154:398–406
Ketterings QM, Bigham JM (2000) Soil color as an indicator of slash-and-burn fire severity and soil fertility in Sumatra, Indonesia. Soil Sci Soc Am J 64:1826–1833
Khaleel R, Reddy KR, Overcash MR (1981) Changes in soil physical properties due to organic waste applications: a review. J Environ Qual 10(2):133–141
Khanday M, Bhat RA, Haq S, Dervash MA, Bhatti AA, Nissa M, Mir MR (2016) Arbuscular mycorrhizal fungi boon for plant nutrition and soil health. In: Hakeem KR, Akhtar J, Sabir M (eds) Soil science: agricultural and environmental prospectives. Springer International Publishing, Cham, pp 317–332
Kiem R, Kögel-Knabner I (2003) Contribution of lignin and polysaccharides to the refractory carbon pool in C-depleted arable soils. Soil Biol Biochem 35:101–118
Kirillova NP, Vodyanitskii YN, Sileva TM (2015) Conversion of soil color parameters from the Munsell system to the CIE-L*a*b* system. Eurasian Soil Sci 48:468–475
Klucakova M (2012) Comparative study of binding behaviour of Cu(II) with humic acid and simple organic compounds by ultrasound spectrometry. Open Colloid Sci J 5:5–12
Korucu T, Shipitalo MJ, Kaspar TC (2018) Rye cover crop increases earthworm populations and reduces losses of broadcast, fall-applied, fertilizers in surface runoff. Soil Tillage Res 180:99–106
Kravchenko AN, Negassa WC, Guber AK, Rivers ML (2015) Protection of soil carbon within macro-aggregates depends on intra-aggregate pore characteristics. Sci Rep 5:16261
Kumar A, Kuzyakov Y, Pausch J (2016) Maize rhizosphere priming: field estimates using 13C natural abundance. Plant Soil 409:1–11
Kweon G, Lund E, Maxton C (2013) Soil organic matter and cation-exchange capacity sensing with on-the-go electrical conductivity and optical sensors. Geoderma 199:80–89
Kwiatkowska-Malina J (2018) Qualitative and quantitative soil organic matter estimation for sustainable soil management. J Soils Sediments 18:2801–2812
Laiho R, Penttilä T, Laine J (2004) Variation in soil nutrient concentrations and bulk density within peatland forest sites. Silva Fenn 38(1):29–41
Lal R (2011) Organic matter, effects on soil physical properties and processes. In: Gliński J, Horabik J, Lipiec J (eds) Encyclopedia of agrophysics, Encyclopedia of earth sciences series. Springer, Dordrecht
Lamprecht A, Semenchuk PR, Steinbauer K, Winkler M, Pauli H (2018) Climate change leads to accelerated transformation of high-elevation vegetation in the central Alps. New Phytol 220:447–459
Laossi KR, Barot S, Carvalho D, Desjardins T, Lavelle P, Martins M, Mitja D, Rendeiro AC, Rousseau G, Sarrazin M, Velasquez E, Grimaldi M (2008) Effects of plant diversity on plant biomass production and soil macrofauna in Amazonian pastures. Pedobiologia 51:397–407
Larney FJ, Olson AF, Miller JJ, DeMaere PR, Zvomuya F, McAllister TA (2008) Physical and chemical changes during composting of wood chip-bedded and straw-bedded beef cattle feedlot manure. J Environ Qual 37(2):725–735
Lehmann J, Rondon M (2006) Bio-char soil management on highly weathered soils in the humid tropics. In: Biological approaches to sustainable soil systems. CRC Press, Boca Raton, pp 517–530
Li D, Chen XZ, Peng ZP, Chen SS, Chen WQ, Han LS, Li YJ (2012) Prediction of soil organic matter content in a litchi orchard of South China using spectral indices. Soil Tillage Res 123:78–86
Li J, Pu L, Zhu M, Zhang J, Li P, Dai X, Xu Y, Liu L (2014) Evolution of soil properties following reclamation in coastal areas: a review. Geoderma 226–227:130–139
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O’Neill B, Skjemstad JO, Thies J, Luizão FJ, Petersen J, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730
Lieb AM, Darrouzet-Nardi A, Bowman WD (2011) Nitrogen deposition decreases acid buffering capacity of alpine soils in the southern Rocky Mountains. Geoderma 164:220–224
Liu E (2010) Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 158:173–180
Liu S (2014) Differential responses of crop yields and soil organic carbon stock to fertilization and rice straw incorporation in three cropping systems in the subtropics. Agric Ecosyst Environ 184:51–58
Liu M, Hu F, Chen X, Huang Q, Jiao J, Zhang B, Li H (2009) Organic amendments with reduced chemical fertilizer promote soil microbial development and nutrient availability in a subtropical paddy field: the influence of quantity, type and application time of organic amendments. Appl Soil Ecol 42:166–175
Liu YR, Li X, Shen QR, Xu YC (2013) Enzyme activity in water-stable soil aggregates as affected by long-term application of organic manure and chemical fertilizer. Pedosphere 23:111–119
Liu B, Ai S, Zhang W, Huang D, Zhang Y (2017) Assessment of the bioavailability, bioaccessibility and transfer of heavy metals in the soil-grain-human systems near a mining and smelting area in NW. Sci Total Environ 609:822–829
Loecke TD, Burgin AJ, Riveros-Iregui DA, Ward AS, Thomas SA, Davis CA (2017) Weather whiplash in agricultural regions drives deterioration of water quality. Biogeochemistry 133:7–15
López-Vicente M, García-Ruiz RG, Guzmán G, Vicente-Vicente JL, Van Wesemael B, Gómez JA (2016) Temporal stability and patterns of runoff and runon with different cover crops in an olive orchard (SW Andalusia, Spain). Catena 147:125–137
Lucas-Borja ME, Zema DA, Carrà BG, Cerdà A, Plaza-Alvarez PA, Cózar JS, de las Heras J (2018) Short-term changes in infiltration between straw mulched and non-mulched soils after wildfire in Mediterranean forest ecosystems. Ecol Eng 122:27–31
Luo WT, Nelson PN, Li MH (2015) Contrasting pH buffering patterns in neutral-alkaline soils along a 3600 km transect in northern China. Biogeosciences 12:7047–7056
Ma RT, Hu FN, Liu YF, Xu CY, Yang ZH, Wang ZL, Zhao SW (2019) Evolution characteristics of soil surface electrochemical properties during vegetation restoration in the Loess Plateau. Acta Pedol Sin:1–12
Madejón E, Burgos P, López R, Cabrera F (2003) Agricultural use of three organic residues: effect on orange production and on properties of a soil of the “Comarca Costade Huelva” (SWSpain). Nutr Cycl Agroecosyst 65(3):281–288
Mahanty T, Bhattacharjee S, Goswami M, Bhattacharyya P, Das B, Ghosh A, Tribedi P (2014) Biofertilizers: a potential approach for sustainable agriculture development. Environ Sci Pollut Res 24:3315–3335
Malkawi AIH, Alawneh AS, Abu-Safaqah OT (1999) Effects of organic matter on the physical and the physicochemical properties of an illitic soil. Appl Clay Sci 14:257–278
Masri Z, Ryan J (2006) Soil organic matter and related physical properties in a Mediterranean wheat-based rotation trial. Soil Tillage Res 87:146–154
Masto RE, Chhonkar PK, Singh D, Patra AK (2007) Soil quality response to long-term nutrient and crop management on a semi-arid Inceptisol. Agric Ecosyst Environ 118:130–142
Matos CC, Costa MD, Silva IR, Silva AA (2019) Competitive capacity and rhizosphere mineralization of organic matter during weed-soil microbiota interactions. Planta Daninha 37:e019182676
Matsi T, Lithourgidis AS, Gagianas AA (2003) Effects of injected liquid cattle manure on growth and yield of winter wheat and soil characteristics. Agron J 95(3):592–596
McCallum MH, Kirkegaard JA, Green TW, Cresswell HP, Davies SL, Angus JF, Peoples MB (2004) Improved subsoil macroporosity following perennial pastures. Aust J Exp Agric 44:299–307
McClellan AT, Deenik J, Uehara G, Antal M (2007) Effects of flash carbonized macadamia nutshell charcoal on plant growth and soil chemical properties. In: Proceedings American Society of Agronomy Conference. Abstracts. 3–7 November, New Orleans, LA. ASA, Madison, WI
Mehmood MA, Qadri H, Bhat RA, Rashid A, Ganie SA, Dar GH, Shafiq-ur-Rehman (2019) Heavy metal contamination in two commercial fish species of a trans-Himalayan freshwater ecosystem. Environ Monit Assess 191:104. https://doi.org/10.1007/s10661-019-7245-2
Meurer KHE, Haddaway NR, Bolinder MA, Kätter T (2018) Tillage intensity affects total SOC stocks in boreo-temperate regions only in the topsoil—a systematic review using an ESM approach. Earth Sci Rev 177:613–622
Miao SJ, Qiao YF, Zhou LR (2009) Aggregation stability and microbial activity of China’s black soils under different long-term fertilisation regimes. N Z J Agric Res 52:57–67
Mikutta R, Kleber M, Torn MS, Jahn R (2006) Stabilization of soil organic matter: association with minerals or chemical recalcitrance? Biogeochemistry 77:25–56
Miranda V, Rothen C, Yela N, Aranda-Rickert A, Barros J, Calcagno J, Fracchia S (2019) Subterranean desert rodents (genus Ctenomys) create soil patches enriched in root endophytic fungal propagules. Microb Ecol 77:451–459
Moco MKS, Gama-Rodrigues EF, Gama-Rodrigues AC, Machado RCR, Baligar VC (2009) Soil and litter fauna of cacao agroforestry systems in Bahia, Brazil. Agrofor Syst 76:127–138
Molina-Herrera S, Romanya J (2015) Synergistic and antagonistic interactions among organic amendments of contrasted stability, nutrient availability and soil organic matter in the regulation of C mineralisation. Eur J Soil Biol 70:118–125
Mondal S, Padmakumar GP, Sharma V, Singh DN, Baghini MS (2015) A methodology to determine thermal conductivity of soils from flux measurement. Geomech Geoeng 11:73–85
Moni C, Rumpel C, Virto I, Chabbi A, Chenu C (2010) Relative importance of sorption versus aggregation for organic matter storage in subsoil horizons of two contrasting soils. J Soil Sci 61:958–969
Moos JH, Schrader S, Paulse HM, Rahmann G (2016) Occasional reduced tillage in organic farming can promote earthworm performance and resource efficiency. Appl Soil Ecol 103:22–30
Mosaddeghi MR, Hajabbasi MA, Hemmat A, Afyuni M (2000) Soil compactibility as affected by soil moisture content and farmyard manure in central Iran. Soil Tillage Res 55:87–97
Mushtaq N, Bhat RA, Dervash MA, Qadri H, Dar GH (2018) Biopesticides: the key component to remediate pesticide contamination in an ecosystem. In: Environmental contamination and remediation. Cambridge Scholars Publishing, Newcastle upon Tyne, pp 152–178
Najafi S, Jalali M (2016) Effect of heavy metals on pH buffering capacity and solubility of Ca, Mg, K, and P in non-spiked and heavy metal-spiked soils. Environ Monit Assess 188:1–11
Nelson PN, Su N (2010) Soil pH buffering capacity: a descriptive function and its application to some acidic tropical soils. Aust J Soil Res 48:210–207
Nunes MR, Pauletto EA, Denardin JE, Suzuki LEAS, van-Es HM (2019) Dynamic changes in compressive properties and crop response after chisel tillage in a highly weathered soil. Soil Tillage Res 186:183–190
Nurhidayati N, Machfudz M, Murwani I (2018) Direct and residual effect of various vermicompost on soil nutrient and nutrient uptake dynamics and productivity of four mustard Pak-Coi (Brassica rapa L.) sequences in organic farming system. Int J Recycl Org Waste Agric 7(2):173–181
Oades JM (1984) Soil organic matter and structural stability: mechanisms and implications for management. Plant Soil 76:319–337
Oburger E, Jones DL (2018) Sampling root exudates–mission impossible? Rhizosphere 6:116–133
Oldfield EE, Wood SA, Bradford MA (2018) Direct effects of soil organic matter on productivity mirror those observed with organic amendments. Plant Soil 423:63–373
Orlova N, Abakumov E, Orlova E, Yakkonen K, Shahnazarova V (2019) Soil organic matter alteration under biochar amendment: study in the incubation experiment on the Podzol soils of the Leningrad region (Russia). J Soils Sediments 19:2708–2716
Pagliai M, Vittori Antisari L (1993) Influence of waste organic matter on soil micro and macrostructure. Bioresour Technol 43:205–213
Park EJ, Smucker A (2005) Saturated hydraulic conductivity and porosity within macroaggregates modified by tillage. Soil Sci Soc Am J 69:38–45
Parker TC, Sanderman J, Holden RD, Blume-Werry G, Sjögersten S, Large D, Castro-Díaz M, Street LE, Subke JA, Wookey PA (2018) Exploring drivers of litter decomposition in a greening Arctic: results from a transplant experiment across a treeline. Ecology 99:2284–2294
Pascual J, Blanco S, Ramos JL, van Dillewijn P (2018) Responses of bulk and rhizosphere soil microbial communities to thermoclimatic changes in a Mediterranean ecosystem. Soil Biol Biochem 118:130–144
Pausch J, Zhu B, Kuzyakov Y, Cheng W (2013) Plant inter-species effects on rhizosphere priming of soil organic matter decomposition. Soil Biol Biochem 57:91–99
Peng X, Ye LL, Wang CH, Zhou H, Sun B (2011) Temperature- and duration dependent rice straw-derived biochar: characteristics and its effects on soil properties of an Ultisol in southern China. Soil Tillage Res 112(2):159–166
Peng X, Horn RF, Hallett PD (2015) Soil structure and its functions in ecosystems: phase matter & scale matter. Soil Tillage Res 146:1–3
Plaza-Bonilla D, Alvaro-Fuentes J, Cantero-Martinez C (2013) Soil aggregate stability as affected by fertilization type under semiarid no-tillage conditions. Soil Sci Soc Am J 77:284–292
Powlson DS, Hirsch PR, Brookes PC (2001) The role of soil microorganisms in soil organic matter conservation in the tropics. Nutr Cycl Agroecosyst 61(1/2):41–51
Pravin RC, Dodha VA, Vidya DA, Manab C, Saroj M (2013) Soil bulk density as related to soil texture, organic matter content and available total nutrients of Coimbatore soil. Int J Sci Res Publ 3:2250–3153
Pylak M, Oszust K, Frąc M (2019) Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev Environ Sci Biotechnol 18:597–616
Qadri H, Bhat RA (2020) The concerns for global sustainability of freshwater ecosystems (2019). In: Qadri H, Bhat RA, Dar GH, Mehmood MA (eds) Freshwater pollution dynamics and remediation. Springer Nature, Singapore, pp 1–15
Qin Z, Zhang Y, Zhou Z, Shi X, Guo T (2013) Characteristics of mineralization and nitrification in neutral purple paddy soil from a long-term fertilization experiment. Sci Agric Sin 46:3392–3400
Reddy MS (1991) Effects of soil amendments on the hardening of red sandy loams (chalka soils) of Andhra Pradesh. Ann Agric Res 12:174–176
Reeves DW (1997) The role of soil organic matter in maintaining soil quality in continuous cropping systems. Soil Tillage Res 43:131–167
Regelink IC, Stoof CR, Rousseva S, Weng L, Lair GJ, Kram P, Nikolaidis NP, Kercheva M, Banwart S, Comans RNJ (2015) Linkages between aggregate formation, porosity and soil chemical properties. Geoderma 247–248:24–37
Rodrigo-Comino J, Neumann M, Remke A, Ries JB (2018) Assessing environmental changes in abandoned German vineyards. Understanding key issues for restoration management plans. Hung Geogr Bull 67:319–332
Roper WR, Osmond DL, Heitman JL, Wagger MG, Reberg-Horton SC (2017) Soil health indicators do not differentiate among agronomic management systems in North Carolina soils. Soil Sci Soc Am J 81:828–843
Rose DA (1991) The effects of long-continued manuring on some physical properties of soils. In: Wilson WS (ed) Advances in soil organic matter research: the impact on agriculture and the environment. Royal Society of Chemistry, Cambridge, pp 197–205
Rossiter DG, Bouma J (2018) A new look at soil phenoforms–definition, identification, mapping. Geoderma 314:113–121
Rui Y, Murphy DV, Wang X, Hoyle FC (2016) Microbial respiration, but not biomass, responded linearly to increasing light fraction organic matter input: consequences for carbon sequestration. Sci Rep 6:354–396
Sachkova AS, Kovel ES, Churilov GN, Stom DI, Kudryasheva NS (2019) Biological activity of carbonic nano-structures-comparison via enzymatic bioassay. J Soils Sediments 19:2689–2696
Sánchez-Marañón M, Martín-García JM, Delgado R (2011) Effects of the fabric on the relationship between aggregate stability and color in a Regosol-Umbrisol soils cape. Geoderma 162:86–95
Sandin M, Koestel J, Jarvis N, Larsbo M (2017) Post-tillage evolution of structural pore space and saturated and near-saturated hydraulic conductivity in a clay loam soil. Soil Tillage Res 165:161–168
Schaumann GE (2006) Soil organic matter beyond molecular structure part I: macromolecular and supramolecular characteristics. J Plant Nutr Soil Sci 169:145–156
Schulten HR (2002) New approaches to the molecular structure and properties of soil organic matter: humic-, xenobiotic-, biological-, and mineral bonds. Dev Soil Sci 28:351–381
Schulz H, Glaser B (2012) Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J Plant Nutr Soil Sci 175(3):410–422
Schwarz J, Thiele-Bruhn S, Eckhardt KU, Schulten HR (2012) Sorption of sulfonamide antibiotics to soil organic sorbents: batch experiments with model compounds and computational chemistry. ISRN Soil Sci 2012:10
Shen C, Liang W, Shi Y, Lin X, Zhang H, Wu X (2014) Contrasting elevational diversity patterns between eukaryotic soil microbes and plants. Ecology 95:3190–3202
Shi W, Lu C, He J, En H, Gao M, Zhao B (2018) Nature differences of humic acids fractions induced by extracted sequence as explanatory factors for binding characteristics of heavy metals. Ecotoxicol Environ Saf 15:59–68
Sidhu V, Sarkar D, Datta R (2016) Effects of biosolids and compost amendment on chemistry of soils contaminated with copper from mining activities. Environ Monit Assess 188(3):176
Singh J, Salaria A, Kaul A (2014) Impact of soil compaction on soil physical properties and root growth: a review. Int J Food Agric Vet Sci 5(1):22–32
Singh SL, Sahoo UK, Kenye A, Gogoi A (2018a) Assessment of growth, carbon stock and sequestration potential of oil palm plantations in Mizoram, Northeast India. J Environ Prot 9:912–931
Singh DV, Bhat JIA, Bhat RA, Dervash MA, Ganei SA (2018b) Vehicular stress a cause for heavy metal accumulation and change in physico-chemical characteristics of road side soils in Pahalgam. Environ Monit Assess 190:353. https://doi.org/10.1007/s10661-018-6731-2
Singh DV, Bhat RA, Dervash MA, Qadri H, Mehmood MA, Dar GH, Hameed M, Rashid N (2020) Wonders of nanotechnology for remediation of polluted aquatic environs. In: Qadri H, Bhat RA, Dar GH, Mehmood MA (eds) Freshwater pollution dynamics and remediation. Springer Nature, Singapore, pp 319–339
Soares MR, Alleoni LRF (2008) Contribution of soil organic carbon to the ion exchange capacity of tropical soils. J Sustain Agric 32:439–462
Sohail-Ur-Raza M, Ahmed ZI, Malik MA, Ijaz SS (2015) Effective soil additives for improved soil water retention. Pak J Agric Sci 52(1):463–468
Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agron 105:47–82
Sparks DL (2003) Kinetics of Soil Chemical Processes. In. Environmental Soil Chemistry (Second Edition) 2003, Pages 207-244. https://doi.org/10.1016/B978-012656446-4/50007-4
Steller RM, Jelinsk NA, Kucharik CJ (2008) Developing models to predict soil bulk density in southern Wisconsin using soil chemical properties. J Int Biosci 6:53–63
Stevenson FJ (1982) Humus Chemistry. New York, Wiley
Stevenson FJ (1994) Humus chemistry. Genesis, composition, reactions. Wiley, New York
Streubel JD, Collins HP, Garcia-Perez M, Tarara J, Granatstein D, Kruger CE (2011) Influence of contrasting biochar types on five soils at increasing rates of application. Soil Sci Soc Am J 75(4):1402–1413
Sulman BN, Moore JAM, Abramoff R, Averill C, Kivlin S, Georgiou K, Sridhar B, Hartman MD, Wang G, Wieder WR, Bradford MA, Luo Y, Mayes MA, Morrison E, Riley WJ, Salazar A, Schimel JP, Tang J, Classen AT (2018) Multiple models and experiments underscore large uncertainty in soil carbon dynamics. Biogeochemistry 141:109–123
Tarnawski VR, Momose T, Leong WH (2009) Assessing the impact of quartz content on the prediction of soil thermal conductivity. Géotechnique 59:331–338
Tian G, Kang BT, Brussaard L (1992) Biological effects of plant residues with contrasting chemical compositions under humid tropical conditions-decomposition and nutrient release. Soil Biol Biochem 24:1051–1060
Tikhova VD, Deryabina YM, Vasilevich RS, Lodygin ED (2019) Structural features of tundra and taiga soil humic acids according to IR EXPERT analytical system data. J Soils Sediments 19:2697–2707
Tiquia SM (2005) Microbiological parameters as indicators of compost maturity. J Appl Microbiol 99:816–828
Toosi ER, Kravchenko AN, Mao J, Quigley MY, Rivers ML (2017) Effects of management and pore characteristics on organic matter composition of macroaggregates: evidence from characterization of organic matter and imaging. Eur J Soil Sci 68:200–211
Torres IF (2014) The role of lignin and cellulose in the carbon-cycling of degraded soils under semiarid climate and their relation to microbial biomass. Soil Biol Biochem 75:152–160
Usowicz B, Lipiec J, Usowicz JB, Marczewski W (2013) Effects of aggregate size on soil thermal conductivity: comparison of measured and model-predicted data. Int J Heat Mass Transf 57:536–541
Valeeva AA, Aleksandrova AB, Koposov GF (2016) Color estimation of forest-steppe soils by digital photography under laboratory conditions. Eurasian Soil Sci 49:1033–1037
Vallis I, Jones RJ (1973) Net mineralisation of nitrogen in leaves and leaf litter of Desmodium intortum and Phaseolus atropurpureus mixed with soil. Soil Biol Biochem 5:391–398
Von Lutzow M, Kogel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H (2005) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Eur J Soil Sci 57:426–445
Wade J, Culman SW, Hurisso TT, Miller RO, Baker L, Horwath WR (2018) Sources of variability that compromise mineralizable carbon as a soil health indicator. Soil Sci Soc Am J 82:243–252
Wang J, Soininen J, He J, Shen J (2012) Phylogenetic clustering increases with elevation for microbes. Environ Microbiol Rep 4:217–226
Wang X, Ciu H, Shi J, Zhao Y, Wei Z (2015) Relationship between bacterial diversity and environmental parameters during composting of different raw materials. Bioresour Technol 198:395–402
Wardani AK, Purqon A (2016) Thermal conductivity prediction of soil in complex plant soil system using artificial neural networks. J Phys Conf Ser 739:012–017
Wei Z, Zhongmin HU, Shenggong LI, Guo Q, Yang H, Zang T (2017) Impact of land use conversion on soil organic carbon stock in an agro-pastoral ecotone of Inner Mongolia. J Geogr Sci 27:999–1010
Wiatrowska K, Komisarek J, Dłużewski P (2013) Metal enrichment of particulate organic matter in soil contaminated by metallurgical fallout in Poland. Fresenius Environ Bull 11:3424–3432
Wiseman CLS, Puttmann W (2006) Interactions between mineral phases in the preservation of soil organic matter. Geoderma 134:109–118
Wolf B, Snyder GH (2003) Sustainable soils: the place of organic matter in sustaining soils and their productivity. Food Products Press of the Haworth Press, New York
Wong MTF, Gibbs P, Nortcliff S, Swift RS (2000) Measurement of the acid neutralizing capacity of agroforestry tree prunings added to tropical soils. J Agric Sci 134:269–276
Wu LM, Zhou CH, Keeling J, Tong DS, Yu WH (2012) Towards an understanding of the role of clay minerals in crude oil formation, migration and accumulation. Earth Sci Rev 115:373–386
Wurzburger N, Clemmensen KE (2018) From mycorrhizal fungal traits to ecosystem properties – and back again. J Ecol 106:463–467
Xiang H, Zhang L, Wen D (2015) Change of soil carbon fractions and water-stable aggregates in a forest ecosystem succession in South China. Forests 6(8):2703–2718
Xu Z (2014) Study on ecological function evaluation and biodiversity of typical forest stands in Jinxi of the Loess Plateau. Master’s thesis, Beijing Forestry University, Beijing, China
Xu X, Shi Z, Li D, Rey A, Ruan H, Craine JM, Liang J, Zhou J, Luo Y (2016) Soil properties control decomposition of soil organic carbon: results from data-assimilation analysis. Geoderma 262:235–242
Yang F, Zhang GL, Yang JL, Li DC, Zhao YG, Liu F (2014) Organic matter controls of soil water retention in an alpine grassland and its significance for hydrological processes. J Hydrol 519:3086–3093
Yang H, Zhao X, Liu C, Bai L, Zhao M, Li L (2018) Diversity and characteristics of colonization of root-associated fungi of Vaccinium uliginosum. Sci Rep 8:15283
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Yuan Y, Si G, Wang J, Luo T, Zhang G (2014) Bacterial community in alpine grasslands along an altitudinal gradient on the Tibetan Plateau. FEMS Microbiol Ecol 87:121–132
Yuan DH, An YC, He XS, Yan CL, Jia YP, Wang HT, He LS (2018) Fluorescent characteristic and compositional change of dissolved organic matter and its effect on heavy metal distribution in composting leachates. Environ Sci Pollut Res 25:18866–18878
Zaman MD, Di HJ, Cameron KC, Frampton CM (1999) Gross nitrogen mineralization and nitrification rates and their relationships to enzyme activities and the soil microbial biomass in soils treated with dairy shed effluent and ammonium fertilizer at different water potentials. Biol Fertil Soils 29(2):178–186
Zeng J, Yue FJ, Wang ZJ, Wu Q, Qin CQ, Li SL (2019) Quantifying depression trapping effect on rainwater chemical composition during the rainy season in karst agricultural area, southwestern China. Atmos Environ 218:116998
Zhang J (2013) Agricultural land use affects nitrate production and conservation in humid subtropical soils in China. Soil Biol Biochem 62:107–114
Zhang M, Heaney D, Henriquez B, Solberg E, Bittner E (2006) A four-year study on influence of biosolids/MSW co-compost application in less productive soils in Alberta: nutrient dynamics. Compost Sci Util 14:68–80
Zhang HM, Wang BR, Xu MG (2008) Effects of inorganic fertilizer inputs on grain yields and soil properties in a long-term wheatcorn cropping system in South China. Commun Soil Sci Plant Anal 39:1583–1599
Zhang N, Yu X, Pradhan A, Puppala AJ (2017) A new generalized soil thermal conductivity model for sand–kaolin clay mixtures using thermo-time domain reflectometry probe test. Acta Geotech 12:739–752
Zhao ZJ, Chang E, Lai P, Dong Y, Xu RK, Fang DM, Jiang J (2019) Evolution of soil surface charge in a chronosequence of paddy soil derived from Alfisol. Soil Tillage Res 192:144–150
Zhou T, Wu L, Luo Y, Christie P (2018) Effects of organic matter fraction and compositional changes on distribution of cadmium and zinc in long-term polluted paddy soil. Environ Pollut 232:514–522
Zhu H (2010) Improving fertility and productivity of a highly-weathered upland soil in subtropical China by incorporating rice straw. Plant Soil 331:427–437
Zimmerman A, Gao B, Ahn M-Y (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179
Zuber SM, Behnke GD, Nafziger ED, Villamil MB (2015) Crop rotation and tillage effects on soil physical and chemical properties in Illinois. Agron J 107:971–978
Zuber SM, Behnke GD, Nafziger ED, Villamil MB (2018) Carbon and nitrogen content of soil organic matter and microbial biomass under long-term crop rotation and tillage in Illinois, USA. J Agric 8:37
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bashir, O. et al. (2021). Soil Organic Matter and Its Impact on Soil Properties and Nutrient Status. In: Dar, G.H., Bhat, R.A., Mehmood, M.A., Hakeem, K.R. (eds) Microbiota and Biofertilizers, Vol 2. Springer, Cham. https://doi.org/10.1007/978-3-030-61010-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-61010-4_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-61009-8
Online ISBN: 978-3-030-61010-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)