Abstract
This chapter deals with the systematic aspects of the molluscan and barnacle assemblages found in the Breiðavík Group in North Iceland. They are primarily based on collections and field work carried out more or less each year since 1972. They are also based on the collections of the Institute of Natural History (Museum) in Reykjavík, the Geological Museum in Copenhagen, the collection of the farmer Jóhannes Björnsson in Ytri-Tunga on Tjörnes, and the collection of Már Vilhjálmsson in Icelandic Institute of Natural History. We report 14 species of prosobranch gastropods, 2 opisthobranch gastropod species, 29 species of bivalves, and 2 species of barnacles have been identified from the Breiðavík Group. In total, it is a question of 45 species of molluscs and 2 barnacle species. Two of the mollusc species are new for the Breiðavík Group. Each species is illustrated on plates, and variation in shape is demonstrated where the material makes it possible. The distribution, recent or fossil, of the various species is recorded, and pertinent ecological and biological features are discussed. The larval development of the species is also recorded as their ability to migrate is significantly depending on the pelagic larval development. The Early Pleistocene Breiðavík fauna marks the transition of the warm temperate Tjörnes faunas to the subarctic-arctic faunas at the onset of the Pleistocene.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- North Iceland
- Tjörnes
- Marine molluscs
- Barnacles
- Zoogeography
- Quaternary biostratigraphy
- Marine invertebrate systematics
11.1 Paleontology
We mainly adhere to nomenclature used in, e.g., Molluscabase, which is a taxonomically oriented database, but partly to that of “Treatise on Invertebrate Paleontology” (Moore, 1960, 1969a, 1969b), with additions from Lemche (1948), Ockelmann (1958), Warén (1974, 1989, 1996a, 1996b), Bernard (1979), Lubinsky (1980), Høisæter (1986), Graham (1988), Bogdanov (1990), Golikov (1995), Sneli et al. (2005), Wesselingh and Pouwer (2011), Pouwer and Wesselingh (2012), and several other malacologists. When dealing with conispiral gastropods, the term “diameter” always means the maximal outer diameter of the shell. The following abbreviations are used: d diameter, h height, hlw height of last whorl (body whorl), ha height of aperture, h/d height/diameter ratio, h/l height/length ratio, and b/l breath/length ratio. The terms length and breadth are used in the case of patellate gastropods (cf. Cox, 1960).
When dealing with bivalves, the term “paired” means that the valves occur articulated (united) in the sediment, whereas “single” means that they were disarticulated when collected. The following abbreviations are used: l length, h height (from the beak to the ventral margin), and b breadth (from the surface of the left valve to the surface of the right valve).
The ecological preferences are discussed for each species with reference to the present-day zoogeographical division of the North Atlantic as shown on Fig. 11.1, and the stratigraphical range is given for each entry. The stratigraphical terms are, as far as possible, adjusted to the Pliocene-Pleistocene boundary in the Northern Hemisphere at 2.5–2.6 Ma (cf. Eiríksson, 2008; Gibbard et al., 1991; Símonarson et al., 1998; Zagwijn, 1992). The distribution of the fossils in units of the Breiðavík Group is based on Eiríksson (1981) and Eiríksson et al. (2020a), who divided the Breiðavík Group into 6 lithological formations and 13 members (Fig. 11.2), containing at least 14 lithological cycles.
Lithostratigraphic subdivision of the Breiðavík Group (cf. Eiríksson et al., 2020a)
11.2 Gastropoda
-
Class Gastropoda Cuvier, 1797
-
Subclass Prosobrancia Milne Edwards, 1848
-
Order Archaeogastropoda Thiele, 1925
-
Family Fissurellidae Fleming, 1822
-
Genus Erginus Jeffreys, 1877
Erginus cf. rubellus (Fabricius, 1780)
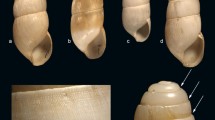
Plate 11.1, fig. 1
-
1780 Patella rubella – Fabricius: p. 386.
-
1878 Tectura rubella, Fabr. – Sars: p. 121, pl. 8, fig. 5a–b.
-
1923 Acmæa rubella (Fabricius) – Harmer: p. 792–793, pl. 62, fig. 30.
-
1962 Acmaea rubella (Fabr.) – Óskarsson: p. 170, fig. 11.
-
1980 Acmaea rubella (Fabricius, 1780) – Gladenkov et al.: p. 63–64, pl. 13, figs. 5–6a.
-
1985 Acmaea cf. Acmaea (Tectura) virginea Müller (1776) – Vilhjálmsson: p. 32–33, pl. 1, figs. 1–2.
Material: Five specimens from the Hörgi Formation. All the shells were found in conglomerate tongues interfingering with fossiliferous siltstone, containing molluscs of the Portlandia arctica assemblage.
Remarks: The largest specimen measures (l × b × h): 16.3 × 12.0 × 6.7 mm. The b/l ratios are 0.68–0.74. The shell material is more or less dissolved and therefore the surface ornamentation is badly preserved. According to Poppe and Goto (1991), early authors considered Erginus rubellus a valid species, but now it is found in the literature as a synonym of Acmaea virginea (Müller). We are among those “early authors” in considering E. rubellus as a distinct species as did Sars (1878), Harmer (1923, 1925), Thorson (1941), Óskarsson (1962), Høisæter (1986), Golikov (1995), and Funder et al. (2002). E. rubellus is more arctic in its distribution than A. virginea, and has been found in the Breiðavík Group together with molluscs in the Portlandia arctica assemblage.
Recent distribution, ecology, and biology: Erginus rubellus is an arctic-boreal almost circumpolar species. It extends from West and East Greenland, Svalbard, Novaya Zemlya, the Kara Sea, the Laptev Sea, Arctic Canada, and northern Alaska in the north southward to Iceland and along the Norwegian west coast to 69.5° and Newfoundland in the North Atlantic and the Bering Sea and Kurile Islands in the Pacific (Golikov, 1995; Høisæter, 1986; Macpherson, 1971; Thorson, 1941). Golikov (1995) found the species living at temperatures from −0.4 ° to +5 °C. Bathymetrical range: From the foreshore/tidal zone or 4 m in East Greenland to 565 m off northwestern Norway (Golikov, 1995; Thorson, 1941). The species is polyhaline or even euhaline with salinity tolerance above 25‰ (Funder et al., 2002) or 30‰ (Golikov, 1995). The larval development has no pelagic stage (Thorson, 1944).
In East Greenland, the species is associated with the red algae epifauna (Thorson, 1933, 1944), but in the Arctic, it also lives on silty and sandy substrates with scattered stones (Golikov, 1995).
Fossil occurrence: Lower Pleistocene: Hörgi Formation in Breiðavík (Eiríksson, 1981). Middle Pleistocene: Waltonian Crag of the Red Crag Formation (Harmer, 1923). Upper Pleistocene: Baltic Eemian (Funder et al., 2002), Billefiorden in Svalbard (Feyling-Hanssen, 1955). Stratigraphical range: Lower Pleistocene to Recent. The species is rare in Pleistocene sediments and the occurrence in the Hörgi Formation seems to be the earliest.
-
Family Patellidae Rafinesque, 1815
-
Genus Patella Linné, 1758
Patella pellucida Linné, 1758
Plate 11.1, fig. 2
-
1758 Patella pellucida – Linné: p. 783.
-
1878 Nacella pellucida, Lin. – Sars: p. 119.
-
1923 Helcion pellucidum (Linné) – Harmer: p. 793–794, pl. 62, fig. 31.
-
1925 Nacella pellucida, L. – Bárðarson: p.101.
-
1980 Helcion pellucidum (Linné, 1758) – Gladenkov et al.: p. 63, pl. 13, fig. 4.
-
1985 Helcion (Ansates) pellucidus (Linné, 1758) – Vilhjálmsson: p. 33–34, pl. 1, fig. 5.
Material: Four specimens from the Svarthamar Member of the Þrengingar Formation and one specimen from the Torfhóll Member of the Máná Formation.
Remarks: The largest specimen measures (l × b × h): 7.7 × 5.5 × 2.3 mm. The cap-shaped shell is thin and fragile, but generally broken in the sediments and partly dissolved. The apex is anteriorly placed and the apical area with radiating blue line or rays.
Recent distribution, ecology, and biology: Patella pellucida is a boreal-lusitanian species extending in the North Atlantic from the Murman Coast and Iceland in the north to Portugal and Morocco in the south, and makes its way into the Mediterranean (Fretter & Graham, 1976; Sneli et al., 2005; Thorson, 1941). It occurs on the west coasts of Denmark and Sweden south to Øresund, but it is absent from the Baltic, east coast of Denmark and those of the Netherlands and Belgium (Fretter & Graham, 1976; Sneli et al., 2005). Bathymetrical range: From high tide water mark in several places to ?1521 m north of Scotland (Thorson, 1941). The recent distribution indicates that the species prefers polyhaline waters and its salinity tolerance is probably well above 25‰ (cf. Funder et al., 2002). The larval development is with a short pelagic stage (Thorson, 1946).
In Norway it is invariably found living epifaunally on seaweeds, especially Laminaria and Fucus serratus Linné, 1753 (Vahl, 1971). Therefore, it is intertidal only close to the low-water stand, but prefers more sublittoral areas. Normally it is not found living where there is much sediment or freshwater inflow (Fretter & Graham, 1976).
Fossil occurrence: Pliocene: Estepona in southern Spain (Landau et al., 2004). Lower Pleistocene: Svarthamar Member (Eiríksson, 1981). Middle Pleistocene: Bridlington Crag in England (Harmer, 1923). Upper Pleistocene: Selsey beds in England (Harmer, 1923). Stratigraphical range: Pliocene to Recent.
-
Family Lepetidae Dall, 1869
-
Genus Lepeta Gray, 1847
Lepeta caeca (Müller, 1776)
Plate 11.1, fig. 3
-
1776 Patella caeca – Müller: p. 237.
-
1878 Lepeta cæca, Müll. – Sars: p. 123, pl. 20, fig. 17a–b.
-
1962 Lepeta coeca (Müller) – Óskarsson: p. 42, fig. 12.
-
1985 Lepeta (Lepeta) caeca (Müller, 1776) – Vilhjálmsson: p. 34–35, pl. 1, figs. 3–4.
-
1988 Lepeta caeca (Müller, 1776) – Graham: p. 84–85, fig. 23.
Material: Eleven specimens from the Svarthamar Member and one specimen from the Stapavík Member of the Máná Formation.
Remarks: The largest specimen measures (l × b × h): 6.2 × 4.6 × 2.8 mm.
The surface sculpture is rather badly preserved because the shells are partly dissolved. The apex is slightly anterior in the conical shell. It has fine radial ridges crossing concentric lines.
Recent distribution, ecology, and biology: Lepeta caeca is a circumpolar, arctic-boreal species extending from West and East Greenland, Franz Josef Land, Svalbard, Novaya Zemlya, the Kara Sea, the Siberian Arctic Sea, Parry Islands, and Ellesmere Island in the north to Shetland, West Scotland, Kattegat, and Cape Cod in the south (Fretter & Graham, 1976; Sneli et al., 2005; Thorson, 1941). In deeper waters, it is distributed in the North Atlantic southward to the Azores and the West Indies (Fretter & Graham, 1976). In the Pacific, it extends south to the Sea of Okhotsk and Sea of Japan (Sneli et al., 2005). It is not known from the Baltic. Bathymetrical range: From 0 m in Iceland to about 1300 m near the Azores (Óskarsson, 1962; Thorson, 1941). The marine species has salinity tolerance down to 15‰ (Funder et al., 2002). The larval development is unknown, but Thorson (1946) concluded that the development is most probably direct as he did not find any larvae in the East Greenland plankton, where this is a dominant prosobranch species.
Lepeta caeca is not a littoral species, and is generally found living with the epifauna on a hard bottom, but has also been collected from gravel and firm clays (Fretter & Graham, 1976). In Iceland, it prefers rocky bottom and occurs mainly at depths below 30 m (Thorson, 1941). In East Greenland, it is mainly attached to red algae at depths exceeding 20 m (Thorson, 1944).
Fossil occurrence: Pliocene: Serripes Zone of the Barmur Group (Bárðarson, 1925). Lower Pleistocene: Pattorfik beds in West Greenland (Símonarson, 1981). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
-
Family Trochidae Rafinesque, 1815
-
Genus Margarites Gray, 1847
Margarites groenlandicus (Gmelin, 1791)
Plate 11.1, fig. 4
-
1791 Trochus groenlandicus – Gmelin: p. 3574.
-
1872 Margarita Groenlandica, Chemnitz – Wood: p. 83, pl. 5, fig. 11a–b.
-
1878 Margarita grönlandica, Chemn. – Sars: p. 133–134.
-
1923 Eumargarita grænlandica (Chemnitz) – Harmer: p. 749–750, pl. 60, fig. 9.
-
1962 Margarites groenlandicus (Chemn.) – Óskarsson: p. 49–50, fig. 21.
-
1985 Margarites (Margarites) groenlandicus (Chemnitz, 1781) – Vilhjálmsson: p. 35–36, pl. 1, fig. 6A–B.
Material: One specimen from the Hörgi Formation.
Remarks: The shell consists of 4.5 whorls and measures (h × d): 4.2 × 5.8 mm, but the body whorl is damaged especially the outer lip. The helicoid shell has large last whorl and moderately high spire with well-developed spiral ridges.
Recent distribution, ecology, and biology: Margarites groenlandicus is an arctic-subarctic-boreal, circumpolar species distributed from West and East Greenland, Svalbard, Franz Josef Land, Novaya Zemlya, the Kara Sea, the Siberian Arctic Sea, and the Canadian Arctic in the north southward to Scotland, and Massachusetts Bay in the North Atlantic, and the Bering Sea and Kurile Islands in the Pacific (Fretter & Graham, 1977; Golikov, 1995; Thorson, 1941). It has not been found in Skagerrak, Kattegat, the Baltic, or the North Sea (Fretter & Graham, 1977). According to Golikov (1995), it has been found living at winter temperatures as low as −1.7 °C and summer temperatures up to 18 °C. Bathymetrical range: From 0 m (Iceland) to 859 m off the Faroe Islands (Sneli et al., 2005; Thorson, 1941). The species is mesohaline with salinity tolerance down to about 15‰ (Funder et al., 2002). Golikov (1995) recorded it from water with a salinity range 24–35‰. The larval development is non-pelagic (Thorson, 1941).
In East Greenland, this epifaunal species is mainly attached to Fucus, Laminaria, and Desmarestia, as well as Delessaria and other red algae (Thorson, 1944). In Iceland, it is also associated with the algal zone, but in the Faroe Islands, it has been found living on algae, stones, sand, shell-gravel, and sponges (Sneli et al., 2005; Thorson, 1941).
Fossil occurrence: Pliocene: Mactra Zone of the Barmur Group (Símonarson & Eiríksson, 2020). Lower Pleistocene: Pattorfik beds (Símonarson, 1981). Middle Pleistocene: Bridlington Crag (Harmer, 1923). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Lower Pleistocene to Recent.
-
Order Mesogastropda Thiele, 1925
-
Family Littorinidae Gray, 1840
-
Genus Littorina Férussac, 1822
Littorina obtusata (Linné, 1758)
Plate 11.1, fig. 5
-
1758 Turbo obtusatus – Linné: p. 761.
-
1878 Littorina obtusata, Lin. – Sars: p. 167–168.
-
1962 Littorina obtusta (L.) – Óskarsson: p. 180, figs. 31–32.
-
1985 Littorina obtusata (Linné, 1758) – Vilhjálmsson: p. 41–42, pl. 3, fig. 3A–B.
-
1996 Littorina (Neritrema) obtusata (Linnaeus, 1758) – Reid: p. 197–227, figs. 3G, 4, 5, 76–81.
Material: Two specimens from the Svarthamar Member.
Remarks: The larger specimen measures (h × d): 6.8 × 6.9 mm. The height of the other shell is 3.2 mm, but it is too fragmentary for further measurements.
Recent distribution, ecology, and biology: Littorina obtusata is subarctic-boreal-lusitanian in the North Atlantic. It extends from Southwest Greenland, Jan Mayen, ?Novaya Zemlya, the White Sea, and Labrador in the north to Portugal and New Jersey (Cape May) in the south (Golikov, 1995; Graham, 1988; Reid, 1996; Thorson, 1941). It has not been reported from East Greenland or Svalbard, but seems to extend into the western part of the Baltic (Graham, 1988; Thorson, 1941). According to Reid (1996), its absence in the Mediterranean may be explained by the almost total lack of Fucus and Ascophyllum together with high sea temperatures. Bathymetrical range: From the littoral zone in Denmark and Iceland to 3 m in the Faroe Islands (Sneli et al., 2005; Thorson, 1941) or 5 m (Poppe & Goto, 1991). The marine species has a lower salinity tolerance of 5‰ (Funder et al., 2002). Golikov (1995) reported it living at salinities between 10‰ and 35‰. The larval development is non-pelagic (Thorson, 1941).
The epifaunal species prefers stony and rocky substrates in the littoral zone. In the British Isles, it lives associated with fucoids, especially growing along the edge of tidal pools and centers on the lower part of the beach avoiding most exposed shores (Graham, 1988). In Lower Pleistocene, it probably lived on rather sandy substrates in Breiðavík.
Fossil occurrence: Lower Pleistocene: Svarthamar Member (Vilhjálmsson, 1985). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Lower Pleistocene to Recent. The species is not known from Pliocene deposits and only rarely found in Pleistocene sediments (Reid, 1996). The occurrence in the Svarthamar Member seems to be the earliest for the species.
-
Family Rissoidae Gray, 1847
-
Genus Alvania Risso, 1826
Alvania patorfikensis Laursen, 1944
Plate 11.1, fig. 6
-
1944 Alvania wyville-thomsoni (Friele) var. pátorfikensis – Laursen: p. 70, pl. 6, figs. 7a–b, 8a–b.
-
1981 Alvania (Alvania) patorfikensis Laursen, 1944 – Símonarson: p. 29–32, pl. 1, figs. 9, 10a–b.
-
1985 Alvania (Alvania) simonarsoni n. sp. – Vilhjálmsson: p. 36–39, pl. 2, figs. 1–5.
-
2010 Alvania patorfikensis Laursen, 1944 – Bennike et al.: p. 606–607, fig. 4B.
Material: Nine specimens from the Hörgi Formation collected by Vilhjálmsson 1982–1983. This species has not been mentioned before from the Breiðavík Group.
Remarks: The two largest specimens measure (l × d): 4.72 × 2.84 and 4.20 × 2.80 mm, respectively. The d/h ratios for the seven measurable shells are 0.64–0.70. The largest shell consists of 5.4 whorls and the shells are rather well preserved.
According to Vilhjálmsson (1985), Andérs Warén examined five of the specimens from Breiðavík and came to the conclusion that none of them belong to known recent species. Vilhjálmsson (1985) compared the shells with Alvania wyvillethomsoni (Friele) as well as A. patorfikensis and came to the conclusion that the specimens from Breiðavík belong to a distinct species closely related to these species. However, he stressed closer relationship between the shells from Breiðavík and A. patorfikensis than A. wyvillethomsoni. He proposed to name this species Alvania simonarsoni.
The specimens from Breiðavík have the same shell ratios as Alvania patorfikensis (cf. Símonarson, 1981). The protoconch of A. patorfikensis consists of 1.75 whorls, while it consists of 2.1–2.2 whorls in Alvania wyvillethomsoni. The protoconch seems smooth without any ornamentation as in A. wyvillethomsoni (see Warén, 1996b). The shell form and aperture are very similar and it is difficult to see differences in the surface features. The shells from Breiðavík as well as A. patorfikensis have distinct tubercles on the crossing point of the collabral ribs and the spiral lines. As we are not convinced that the differences are exceeding the intraspecific variation of A. patorfikensis, we refer the Breiðavík shells to that species.
Distribution, ecology, and biology: Alvania patorfikenis is extinct and only recorded as fossil from Lower Pleistocene sediments. Its distribution in West and East Greenland and northern Iceland strongly indicates that the species preferred ecological conditions as now prevailing in the arctic and subarctic regions of the North Atlantic. Generally, the species preferred rather cold water as we have no records from more southerly localities. It obviously lived in the Breiðavík area together with arctic species as Portlandia arctica (Gray). Bathymetrical range: Alvania patorfikensis was obviously living in the same area as the recent A. wyvillethomsoni, except for West Greenland (cf. Thorson, 1944). However, while A. patorfikensis presumably lived sublittorally in the nearshore environments, A. wyvillethomsoni has quite another bathymetrical range: from 95 m in East Greenland to 2814 m between Norway and Iceland (Thorson, 1944). The two species are similar in many aspects. However, the ribs are relatively finer in A. wyvillethomsoni, there are no tubercles on the crossing point of the longitudinal costae and the spiral lines, the spiral lines are finer, and the reticulate pattern is not so striking. We could expect to find changes like these when a species living in nearshore environments migrates to deeper water, where environmental energy as well as temperature is decreasing. It is possible that further studies may reveal that A. wyvillethomsoni evolved from A. patorfikensis already in the Lower Pleistocene when the latter migrated out to greater depths?.
The specimens in the Hörgi area in Breiðavík probably lived epifaunally on the kame conglomerates with lateral downslope contact to marine siltstone in the nearshore environments. The species was probably mesohaline or even oligohaline living so close to the Portlandia assemblage in Breiðavík. The larval development is unknown.
Fossil occurrence: Lower Pleistocene: Hörgi Formation (Vilhjálmsson, 1985: as Alvania simonarsoni), Pattorfik beds (Símonarson, 1981), and Store Koldeway Island (Bennike et al., 2010). We have no records from Pliocene deposits or younger Pleistocene sediments. Stratigraphical range: ?Lower Pleistocene.
-
Genus Onoba H. & A. Adams, 1854
Onoba aculeus (Gould, 1841)
Plate 11.1, fig. 7
-
1841 Cíngula acúleus – Gould: p. 266, fig. 172.
-
1878 Onoba aculeus, Gould – Sars: p. 172–173, pl. 9, fig. 12a–b.
-
1920 Onoba aculeus (Gould) – Harmer: p. 643, pl. 51, fig. 43.
-
1985 Onoba aculeus (Gould, 1841) – Vilhjálmsson: p. 39–40, pl. 3, figs. 1–2.
Material: Ten specimens from the Svarthamar Member.
Remarks: The specimens are fairly well preserved and the largest one measures (h × d × ha): 3.6 × 1.7 × 1.5 mm. The d/h ratios are 0.45–0.61.
Recent distribution, ecology, and biology: Onoba aculeus is an arctic-boreal species in the North Atlantic distributed from West and East Greenland, Svalbard, the Murman Coast, the whole Norwegian west coast, and Nova Scotia southward to northwestern Spain and New Jersey (Sneli et al., 2005). The species is common in Iceland, the Faroe Islands, Skagerrak, Kattegat, and the southernmost parts of the Baltic (Warén, 1996b). Bathymetrical range: From 0 m in Iceland to about 200 m in East Greenland (Thorson, 1941). In Iceland, the species lives preferably in the tidal zone and only very few specimens have been found at greater depths than 4 m (Thorson, 1941). The species has lower salinity tolerance down to 5‰ (Funder et al., 2002). Thorson (1941, 1946) stressed that apparently the species has not a pelagic larval development.
The species prefers sheltered rocky shores and is often found in very large numbers under stones or on algae, avoiding silty areas (Fretter & Graham, 1978; Warén, 1996b).
Fossil occurrence: Pliocene: Mactra Zone of the Barmur Group (Símonarson & Eiríksson, 2020). Lower Pleistocene: Svarthamar Member (Vilhjálmsson, 1985). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
-
Family Naticidae Forbes, 1838
-
Genus Cryptonatica Dall, 1892
Cryptonatica affinis (Gmelin, 1791)
Plate 11.1, figs. 8–9
-
1791 Nerita affinis – Gmelin: p. 3675.
-
1848 Natica clausa. Broderip and Sowerby – Wood: p. 147, pl. 16, fig. 2a–b.
-
1863 Natica clausa, Brodery u. Sowerby – Winkler; p. 209.
-
1878 Natica clausa, Brod. & Sow. – Sars: p. 159–160, pl. 21, figs. 12a–b, 13.
-
1923 Natica clausa, Broderip and Sowerby – Harmer: p. 672–674, pl. 61, figs. 1–5.
-
1923 Natica affinis (Gmelin) – Harmer: p. 674–675, pl. 61, figs. 6–7.
-
1980 Natica clausa Broderip et Sowerby, 1829 – Gladenkov et al.: p. 68–69, pl. 13, figs. 13–15.
-
1985 Natica (Tectonatica) affinis (Gmelin, 1790) – Vilhjálmsson: p. 42–43, pl. 3, fig. 4A–B, pl. 4, fig. 1.
Material: Twelve specimens from the Hörgi Formation, one from the Fossgil Member of the Threngingar Formation, three from the Svarthamar Member, and ten from the Stapavík Member as well as seven specimens from the Torfhóll Member. The last two members are assigned to the Máná Formation (Eiríksson, 1981).
Remarks: The largest specimen measures (h × d): 32.1 × ?28.5 mm. Generally the shells from Breiðavík are deformed, mainly compressed and difficult to measure exactly. Some of them are partly dissolved, especially in the lower and older parts of the deposits.
Recent distribution, ecology, and biology: Cryptonatica affinis is circumpolar, being distributed from the Arctic and south into the lusitanian region. It occurs from West and East Greenland, Svalbard, Franz Josef Land, Novaya Zemlya, the Kara Sea, the Siberian Arctic Sea, Alaska (off Point Barrow), and Ellesmere Island southward to the Mediterranean and Cape Hatteras in the North Atlantic and to Vancouver and Japan in the Pacific (MacGinitie, 1959; Macpherson, 1971; Thorson, 1944). It has not been recorded from the Baltic and only a few specimens have been found in the British Isles, especially in northern British waters (Funder et al., 2002; Graham, 1988). Bathymetrical range: From 0 m in Norway to 2660 m in Algeria (Thorson, 1944). The species extends south in the North Atlantic at ever increasing depths (Graham, 1988). The species is mesohaline with salinity tolerance down to 15‰ (Funder et al., 2002). According to Golikov (1995), it lives at salinity of 23–35‰. The larval development is non-pelagic (Thorson, 1935).
The species belongs to the infauna, and in Iceland, it is commonly found in the Arctic Macoma community in shallow water on mud or silt bottom, as well as in the Yoldia hyperborea community at depths of 45–162 m (Spärck, 1937). Cryptonatica affinis is carnivorous, which is a very common feeding habit among the boring Naticidae species (Thorson, 1941, 1944).
Fossil occurrence: Miocene: Narrow Cape Formation (Grant & Gale, 1931), Yakataka Formation, Alaska (Addicott et al., 1971), Arnum Formation in Denmark (Sorgenfrei, 1958). Pliocene: Oosterhout Formation in the Netherlands (Spaink, 1975). Pliocene/Pleistocene: Red Crag Formation (Harmer, 1921; Wood, 1848). Lower Pleistocene: Pattorfik beds (Símonarson, 1981), Santernian in Calabria (Malatesta & Zarlenga, 1986), Olkov and Tusatuva-Yamsk Suites (Petrov, 1982). Middle Pleistocene: Kresta, Kolvin, and Padymeiskii Suites (Petrov, 1966, 1982; Merklin, Zarkhidze, & Ilina, 1979), Kotzebuan (Hopkins et al., 1972), Anvilian (Hopkins et al., 1974). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Miocene to Recent.
-
Genus Euspira Agassiz, 1837
Euspira cf. pallida (Broderip & Sowerby, 1829)
Plate 11.1, fig. 10
-
1829 Natica pallida – Broderip & Sowerby: p. 372.
-
1848 Natica Grænlandica. Beck – Wood: p. 146–147, pl. 12, fig. 5a–b.
-
1878 Lunatia grönlandica, Beck – Sars: p. 158, pl. 21, fig. 15.
-
1921 Natica (Lunatia) pallida, Broderip and Sowerby – Harmer: p. 693–695, pl. 56, figs. 8–11.
-
1980 Polinices aff. pallidus (Broderip et Sowerby) – Gladenkov et al.: p. 72, pl. 22, fig. 22–22a.
-
1985 Natica (Lunatia) sp. – Vilhjálmsson: p. 43, pl. 4, fig. 2A–B.
Material: Three specimens from the Hörgi Formation and one specimen from the Svarthamar Member.
Remarks: The largest specimen measures 15.9 mm in diameter (d), but the height cannot be measured exactly as the first whorls are missing. The specimens are badly preserved, only internal cores are preserved as casts. Apparently the sutures were rather deep and the umbilicus seems wide. Therefore these shells are referred to the genus Euspira and most probably they belong to the species pallida. Exact identification is hardly possible.
Recent distribution, ecology, and biology: Euspira pallida is a circumpolar species extending from the Arctic and south into the boreal region. It is distributed from West and East Greenland, Svalbard, Franz Josef Land, Novaya Zemlya, the Barents Sea, the Kara Sea, the Siberian Arctic Sea, Arctic Canada, and Ellesmere Island southward to Skagerrak, the Swedish west coast, the North Sea, Isles of Man, Durham on the British east coast, and North Carolina in the North Atlantic and in the Pacific to Monterey, the Sea of Okhotsk, and Japan (Sneli et al., 2005; Thorson, 1941). It has been found in Øresund but not farther east into the Baltic (Sneli et al., 2005; Thorson, 1941). Bathymetrical range: From 0 m in Norway to 2430 m off Cape Hatteras (Thorson, 1941). It is by no means a littoral species, and in the Faroe Islands, it has been found from a depth of 65 m down to 1319 m (Sneli et al., 2005). The species is mesohaline, living at salinity from 23‰ to 34.5‰, with salinity tolerance down to about 15‰ (Funder et al., 2002; Golikov, 1995). The larval development is non-pelagic (Thorson, 1941).
The species belongs to the infauna, and in Iceland, it is commonly found in the Arctic Macoma community in shallow sublittoral water on mud, silt, or sand bottom, as well as in the Yoldia hyperborea community at depths of 45–162 m (Spärck, 1937).
Fossil occurrence: Pliocene/Lower Pleistocene: San Joaquin Formation (Durham & MacNeil, 1967), Red Crag Formation (Harmer, 1921). Lower Pleistocene: Pattorfik beds (Símonarson, 1981), Baventian (West et al., 1980), Olkov Suite (Petrov, 1982). Middle Pleistocene: Kresta, Kolvin, and Padymeiskii Suites (Merklin et al., 1979; Petrov, 1966, 1982), Kotzebuan (Hopkins et al., 1972), and Anvilian (Hopkins et al., 1974). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
-
Order Neogastropoda Wenz, 1838
-
Family Muricidae, Fleming, 1828
-
Genus Boreotrophon Fischer, 1884
Boreotrophon truncatus (Strøm, 1768)
Plate 11.2, fig. 1
-
1768 Buccinum Truncatum – Strøm: p. 369–370, pl. 16, fig. 26.
-
1878 Trophon truncatus, Strøm – Sars: p. 246–247, pl. 15, fig. 9.
-
1914 Trophon truncatus (Ström) – Harmer: p. 129–130, pl. 12, figs. 23–24.
-
1962 Boreotrophon truncatus (Ström) – Óskarsson: p. 236, fig. 88.
-
1985 Trophon (Boreotrophon) truncatus (Ström, 1768) – Vilhjálmsson: p. 44, pl. 4, fig. 4A–C.
Material: Three specimens from the Svarthamar Member collected by Vilhjálmsson (1985).
Remarks: The largest specimen measures (h × d): 5.5 × 4.3 mm. The d/h ratios are 0.51–0.58. Two of the specimens have fairly well-preserved shell, but the third one is only a cast (inner core) without shell material. Recent Boretrophon truncatus in Iceland attains a height of more than 160 mm and has up to 8 whorls. This indicates that the specimens from the Svarthamar Member are not fully grown. However, the costae or collabral ribs on the fourth whorl are 13, whereas B. clathratus (Linné) has fewer but taller ribs. The surface sculpture of the specimens from Breiðavík is somewhat eroded probably because of postmortal transport before they became buried in the sediment.
Recent distribution, ecology, and biology: Boreotrophon truncatus is an arctic-subarctic-boreal species, with a discontinuous circumpolar range. It extends from southwest Greenland, Svalbard, Novaya Zemlya, the Laptev Sea, the Siberian Arctic Sea, northern Alaska (Point Barrow), and Baffin Island in the north to Kattegat, east and west of Scotland, Ireland, the Bay of Biscay, and Cape Cod on the east coast of North America in the south (Golikov, 1995; Graham, 1988; Macpherson, 1971; Sneli et al., 2005). It has not been found living in East Greenland or the Baltic, and there are no records from the Pacific. Bathymetrical range: From 3 m in Iceland to 964 m in the northern part of the Greenland Sea (Golikov, 1995; Thorson, 1941). The species is mesohaline with salinity tolerance down to 15‰ (Funder et al., 2002) or even polyhaline with salinity tolerance above 26‰ (Golikov, 1995). The larval development is unknown, but the breeding is probably direct (cf. Graham, 1988).
In the British Isles, the species is presumably a carnivore living on muddy or gravelly substrates from the laminarian zones down to a considerable depth (Graham, 1988). In the Faroe Islands, it prefers shelly sand and shelly gravel (Sneli et al., 2005).
Fossil occurrence: Lower Pleistocene: Pattorfik beds (Símonarson, 1981), Svarthamar Member in Breiðavík (Eiríksson et al., 1992). Pliocene/Lower Pleistocene: Red Crag Formation (Harmer, 1914). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Lower Pleistocene to Recent.
The occurrence in Svarthamar Member of the Threngingar Formation is apparently among the earliest for this species.
-
Genus Nucella Röding, 1798
Nucella lapillus (Linné, 1758)
Plate 11.2, fig. 2
-
1758 Buccinum lapillus – Linné: p. 739.
-
1848 Purpura lapillus. Linn. – Wood: p. 36–38, pl. 4, fig. 6a–h.
-
1878 Polytropa lapillus, Lin. – Sars: p. 250–251, pl. 23, fig. 15.
-
1914 Purpura lapillus (Linné) – Harmer: p. 117–119, pl. 11, figs. 1–5, 7–12, 14–17, 19–23.
-
1925 Purpura lapillus, L. – Bárðarson: p. 101.
-
1960 ?Purpura lapillus Linné – Áskelsson: p. 22.
-
1962 Nucella lapillus (L.) – Óskarsson: p. 233–234, fig. 85.
-
1985 Nucella (Nucella) lapillus (Linné, 1758) – Vilhjálmsson: p. 45–46, pl. 4, fig. 3A–B.
Material: Two specimens from the Svarthamar Member and two from the Torfhóll Member.
Remarks: The largest and best preserved specimen measures (h × d): 21.8 × 14.3 mm. The largest recent specimen from Iceland is about twice this size.
Recent distribution, ecology, and biology: Nucella lapillus is a boreal-lusitanian species with subarctic outposts (Fig. 11.3). In the North Atlantic, it extends from Southwest Greenland, southern Labrador, Iceland, and northeastern Kola Peninsula in the north to Vila Nova de Portimao in Portugal and New York in the south (Golikov, 1995; Sneli et al., 2005; Thorson, 1941). The species was living in northern Iceland in mid-Holocene, but then it disappeared and first returned in the beginning of last century after the sea temperatures rose at about 1920 (Áskelsson, 1935). It extends into the northwestern Kattegat but is neither known living in the Baltic nor the Mediterranean (Funder et al., 2002; Poppe & Goto, 1991). According to Thorson (1941), the species is also found in the northern Pacific, from the Bering Sea southward to Japan and Mexico, but these occurrences have not been verified. Bathymetrical range: From 0 m in the Faroe Islands and Iceland to 55 m in Iceland (Sneli et al., 2005; Thorson, 1941). Actually the species is spread throughout the littoral zones of the North Atlantic. In the British Isles, it occurs abundantly on the lower and middle parts of all rocky shores, extending sublittorally down to about 30 m water depth (Graham, 1988). It is apparently mesohaline or even polyhaline with salinity tolerance above 25‰ (Funder et al., 2002) or 12‰ (Golikov, 1995). The larval development is without a pelagic stage (Thorson, 1941).
The species prefers rocky shores where it feeds mainly upon mussels and barnacles (Graham, 1988; Poppe & Goto, 1991). In Reykjavík, two individuals were observed boring in shells of living mussels (Thorson, 1941). Generally it takes about 3 days for the snail to bore its way through a shell of an adult mussel (cf. Graham, 1988).
Fossil occurrence: ?Pliocene/Lower Pleistocene: Red Crag Formation (Wood, 1848). Lower Pleistocene: Emilian at Gallina, Scalea, and Grammichele in Italy (Malatesta & Zarlenga, 1986), Svarthamar Member (Eiríksson et al., 1992). Middle Pleistocene: ?Crag at Little Oakley (Harmer, 1914), Makulin horizon (Merklin et al., 1979). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: ?Pliocene to Recent.
Grant and Gale (1931) and Durham and MacNeil (1967) considered the genus Nucella of North Pacific origin and the latter authors listed N. lapillus among species that migrated into the North Atlantic and reached England during the Pliocene. However, the oldest occurrence in Iceland seems to be the one from the Svarthamar Member in Breiðavík, deposited during the Lower Pleistocene (cf. Bárðarson, 1925).
Malatesta and Zarlenga (1986) referred to Schlesch (1924) when reporting N. lapillus from the Pliocene Barmur Group. Actually, Schlesch (1924) mentioned one specimen of Purpura tetragona intermedia Wood as the only Nucella species found in the deposits, but Poulsen (1884) had referred the same specimen to the genus Neptunea (?cordifer). Unfortunately we could not revise these identifications as the specimen in question was not found in the collections of the Geological Museum in Copenhagen. Therefore, we can only conclude that the occurrence of a Nucella species has not been verified in the Pliocene Barmur Group (cf. Áskelsson, 1960; Bárðarson, 1925; Gladenkov et al., 1980; Mörch, 1871; Norton, 1975). This leads us to conclude that N. lapillus first reached Iceland in Lower Pleistocene after the deposition of the Barmur Group.
Further studies might reveal if N. lapillus ever lived in the Pacific and if so when it disappeared from the Pacific arena. It does not live there today, and we have no records of fossil occurrence in the Pacific area either. In the Lower Pleistocene, it also penetrated the Mediterranean, but the species does not live there at present (Malatesta & Zarlenga, 1986). This thermophilic prosobranch has obviously changed its distribution from time to time during the Pleistocene as well as the Holocene.
-
Family Buccinidae Latreille, 1825
-
Genus Buccinum Linné, 1758
Buccinum undatum Linné, 1758
Plate 11.2, figs. 3
-
1758 Buccinum undatum – Linné: p. 740.
-
1848 Buccinum undatum. Linn. – Wood: p. 35–36, Pl.3, fig. 12a–b.
-
1863 Buccinum undatum, Linn. – Winkler: p. 210.
-
1878 Buccinum undatum, Lin. – Sars: p. 254–256, pl. 13, fig. 12, pl. 24, figs. 2–4.
-
1914 Buccinum undatum, Linné, 1758 – Harmer: p. 90–97, pl. 6, figs. 1–10, pl. 7, figs. 1–6, pl. 8, figs. 1–2, pl. 10. fig. 13.
-
1980 Buccinum undatum Linné, 1758 – Gladenkov et al.: p. 73–74, pl. 14, figs. 1–5.
-
1985 Buccinum undatum Linné, 1758 – Vilhjálmsson: p. 46–47, pl. 5, figs. 2–3, 6A–B.
Material: Three specimens from the Hörgi Formation, three from the Svarthamar Member, and six specimens from the Stapavík Member.
Remarks: The specimens are poorly preserved. They are more or less fragmented, compressed, and dissolved. A juvenile measureable shell measures (h × d): 9.3 × 5.7 mm (Vilhjálmsson, 1985).
Recent distribution, ecology, and biology: Buccinum undatum is a subarctic-boreal species in the North Atlantic, with arctic as well as lusitanian outposts. It extends from Jones Sound, West Greenland (between Disko Island and Nuuk), Svalbard, Novaya Zemlya, the White Sea, and the Barents Sea in the north to the Bay of Biscay and New Jersey in the south (Sneli et al., 2005; Thorson, 1941, 1951). It does not occur off northern Alaska nor in the Pacific (Macpherson, 1971), and Thorson (1944) has excluded it from the East Greenland fauna. According to Golikov (1995), it is not living in the Kara Sea, Laptev Sea, or the Siberian Arctic Sea. The species has been found in Wadden Sea, Skagerrak and Kattegat south to Øresund but is not known from the Baltic (cf. Thorson, 1941). Bathymetrical range: From 0 m in Iceland to 1319 m in the Faroe Islands (Óskarsson, 1962; Sneli et al., 2005) or 0–2000 m (Nordsieck, 1968). The species lives in shallower water in more northern localities. It has salinity tolerance down to 15‰ (Funder et al., 2002). The larval development is non-pelagic (Thorson, 1941).
In the Faroe Islands, it lives at the northern as well as the southern islands, equally frequent in the fiords and off the islands, on a substrate of sandy mud, gravel, and stones (Sneli et al., 2005).
Fossil occurrence: Pliocene: Coralline Crag Formation (Wood, 1848), Lillo Formation (Marquet & Landau, 2006), Oousterhout Formation (Spaink, 1975). Pliocene/Lower Pleistocene: Red Crag Formation (Harmer, 1914; Wood, 1848). Lower Pleistocene: Pattorfik beds (Símonarson, 1981), Emilian and Sicilian (Malatesta & Zarlenga, 1986). Middle Pleistocene, Padymeiskii Suite (Merklin et al., 1979; Petrov, 1982). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
Buccinum cyaneum Bruguière, 1792
Plate 11.2, fig. 4
-
1792 Buccinum cyaneum – Bruguière: p. 266.
-
1878 Buccinum grönlandicum, Chemn. – Sars: p. 259–261, pl. 13, fig. 9a–b, pl. 25, figs. 1–2.
-
1914 Buccinum grænlandicum, Chemnitz – Harmer: p. 97–99, pl. 8, figs. 4–6, pl. 9, figs. 10.
-
1980 Buccinum groenlandicum Chemnitz, 1788 – Gladenkov et al.: p. 74, pl. 14, figs. 7–8.
-
1985 Buccinum groenlandicum Chemnitz, 1788 – Vilhjálmsson: p. 47–48, pl. 5, fig. 4.
-
2005 Buccinum cyaneum Bruguière, 1792 – Sneli et al.: p. 66.
Material: Four specimens from the Svarthamar Member.
Remarks: The thin shells are rather badly preserved, fragmentary, and partly compressed. The height of the most complete specimen is 54 mm, while the largest one is about 70 mm. This large shell is compressed and deformed and apparently the shell was not quite so high when the animal lived in Breiðavík. The height of the largest specimen found living in Iceland today is about 47 mm (Óskarsson, 1962). However, Posselt and Jensen (1898) found specimens as high as 66 mm in West Greenland and Macpherson (1971) recorded a 58 mm high shell from Arctic Canada. We have no records of larger shell which indicates that the few specimens of B. cyaneum that lived in Breiðavík in Lower Pleistocene had favorable ecological conditions.
We agree with Sneli et al. (2005) that the name Chemnitz gave this species in 1788 is not in accordance with the binominal nomenclature and unacceptable. Therefore we have referred the specimens from the Breiðavík Group to Buccinum cyaneum. Some authors obviously regard both B. cyaneum and B. glaciale Linné as synonyms of B. groenlandicum. However, we follow the opinions of Macpherson (1971) and Golikov (1995) that we are dealing with two different species. The difference is especially visible when regarding the spiral ridges on the body whorl. In B. cyaneum, those spiral ribs are rather low, almost equal, and progressively more widely separated, while B. glaciale has up to eight spiral carinae on the last whorl, usually forming knobs where they meet the axial folds (cf. Macpherson, 1971: pl. 6, figs. 2 and 9).
Recent distribution, ecology, and biology: Buccinum cyaneum is an arctic-subarctic-boreal species with circumpolar distribution. It extends from West and Southeast Greenland, Svalbard, Franz Josef Land, Novaya Zemlya, and the Siberian Arctic Sea in the north to Lofoten at the Norwegian west coast and the Faroe Islands in the south (Sneli et al., 2005; Thorson, 1941). In eastern North America, it is distributed from Ellesmere Island southward to Cape Cod and in the Pacific from Alaska to British Columbia (Golikov, 1995; Sneli et al., 2005). Bathymetrical range: From the littoral zone in several localities to 992 m in ?the Russian Arctic (Golikov, 1995). In the Faroe Islands, it lives at depths from 99 to 728 m (Sneli et al., 2005). The species is polyhaline with salinity tolerance above 25‰ (Funder et al., 2002) or 29–35‰ (Golikov, 1995). The larval development is without a pelagic stage (Thorson, 1941).
The species prefers coarse sand, gravel, or stony and rocky substrates (Golikov, 1995; Sneli et al., 2005).
Fossil occurrence: ?Miocene: Quyllayute Formation of Washington (Grant & Gale, 1931). Pliocene: Serripes Zone of the Barmur Group (Símonarson & Eiríksson, 2020). Pliocene/Lower Pleistocene: Red Crag Formation (Harmer, 1914). Lower Pleistocene: Pattorfik beds (Símonarson, 1981), Sicilian of Monte Pellegrino (Malatesta & Zarlenga, 1986). Middle Pleistocene: ?Little Oakley (Harmer, 1914), Kotzebuan (Hopkins et al., 1972). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: ?Miocene to Recent.
-
Genus Colus Röding, 1798
Colus sp.
Plate 11.2, fig. 5
Material: Two specimens from the Hörgi Formation.
Remarks: The specimens are fragmented and the top of the spire is lacking. The height of the larger specimen is 10.6 mm and the diameter is 6.0 mm. The suture is rather shallow and there is no sculpture visible. Most probably these fragmentary specimens belong to the Genus Colus Röding, 1798, but further identification is not possible.
-
Family Turridae Melvill, 1917
-
Genus Curtitoma Bartsch, 1941
Curtitoma cf. trevelliana (Turton, 1834)
Plate 11.2, fig. 6
-
1834 Pleurotoma Trevellianum –Turton: p. 351.
-
1848 Clavatula Trevelliana. Turt. – Wood: p. 63, pl. 7, fig. 14.
-
1878 Bela Trevelyana, Turt. – Sars: p. 235, pl. 16, fig. 13.
-
1915 Bela Trevelyana (Turton) – Harmer: p. 294–295, pl. 32, figs. 30–33.
-
1980 Lora trevelyana (Turton, 1834) – Gladenkov et al.: p. 84–85, pl. 15, fig. 29.
-
1985 Oenopota sp. A. – Vilhjálmsson: p. 49, pl. 5, fig. 7.
-
1988 Oenopota trevelliana (Turton, 1834) – Graham: p. 430, fig. 178.
-
1995 Curtitoma trevelliana (Turton, 1834) – Golikov: p. 49, fig. 137A-B.
Material: One specimen from the Svarthamar Member collected by Vilhjálmsson (1985).
Remarks: The shell is partly dissolved and fragmentary. The basal part of the aperture is lacking. The fragment consists of 4.6 whorls and the height is 3.8 mm. The spire is 2.2 mm and the diameter is 2.4 mm.
The whorls of the fusiform shell are slightly keeled and ornamented by longitudinal ribs crossed by finer spiral ribs, forming a reticulate pattern on the surface, but these surface structures are clearly somewhat eroded. The sutures are rather deep, but the apex is obtuse and only the uppermost part of the aperture is preserved. However, in our opinion, this fragmentary shell is most similar to those of Curtitoma trevelliana (cf. Graham, 1988: p. 430) and therefore it is tentatively referred to that species.
Recent distribution, ecology, and biology: Curtitoma trevelliana is an arctic-subarctic-boreal, circumpolar species with some lusitanian occurrences. It extends from West and East Greenland, the Barents Sea, the Kara Sea, the Laptev Sea, and the Siberian Arctic Sea in the north to the British Isles and Maine in the south (Golikov, 1995; Graham, 1988; Sneli et al., 2005; Thorson, 1941). In Scandinavia, it has been recorded from Kattegat, Bohuslän, and southeast to Øresund (Thorson, 1941). Furthermore, it is distributed from northern Alaska to California in eastern Pacific (Graham, 1988; Sneli et al., 2005). Bathymetrical range: From 5 m in the White Sea to 1447 m in Svalbard (Golikov, 1995). In the British Isles, it is especially recorded from depths of 25–30 m, and in the Faroe Islands, it seems to prefer depths more than 65 m (Graham, 1988; Sneli et al., 2005). The species is probably mesohaline with lower salinity limit of about 15‰ (Funder et al., 2002). In the Arctic, Golikov (1995) found it living at salinity between 23.4‰ and 34.5‰. The larval development seems to be non-pelagic (cf. Graham, 1988).
In the British Isles, the species is mainly recorded from sandy bottoms where it feeds on small annelid worms (Graham, 1988). In the Faroe Islands, it is most frequently found on muddy and sandy substrates (Sneli et al., 2005), and in the Arctic, Golikov (1995) found it especially on silty bottom mixed with sand, gravel, and pebbles.
Fossil occurrence: Pliocene: Serripes Zone of the Barmur Group (Símonarson & Eiríksson, 2020). Pliocene/Lower Pleistocene: Red Crag Formation (Harmer, 1915; Wood, 1848). Lower Pleistocene: Pattorfik beds (Símonarson, 1981). Middle Pleistocene: Padymeiskii Suite (Merklin et al., 1979). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
-
Subclass Opisthobranchia Milne Edwards, 1848
-
Family Retusiidae Thiele, 1931
-
Genus Retusa Brown, 1827
Retusa obtusa pertenuis (Mighels, 1843)
Plate 11.2, fig. 7
-
1843 Bulla pertenuis – Mighels: p. 346, pl. 16, fig. 3.
-
1878 Utriculus pertenuis, Migh. – Sars: p. 287–288, pl. 17, fig. 19a–b.
-
1923 Utriculus pertenuis (Mighels) – Harmer: p. 798–799, pl. 63, fig. 6.
-
1948 Retusa obtusa (Montagu, 1803) forma pertenuis Mighels, 1843 – Lemche: p. 51–54, figs. 43–45.
-
1962 Retusa pertenuis – Óskarsson: p. 293, fig. 149.
-
1985 Retusa (Retusa) obtusa (Montagu, 1803) var. pertenuis (Mighels, 1843) – Vilhjálmsson: p. 50–51, fig. 8A–B.
Material: One specimen from the Svarthamar Member and one from the Torfhóll Member collected by Vilhjálmsson (1985).
Remarks: The thin-shelled specimens are well preserved and measure (h × d): 2.0 × 1.2 and 1.6 × 1.0 mm, respectively.
Lemche (1948) thoroughly examined the genus Retusa from the North Atlantic and came to the conclusion that R. pertenuis is only an ecological variety of R. obtusa and not a distinct species. Quoting Lemche: p. 51: “The southern, larger and stronger obtusa is replaced by the smaller pertenuis in northern latitudes and in brackish waters, etc.” However, here the dwarf variety pertenuis (cf. Lemche, 1948) will be treated as a subspecies.
Recent distribution, ecology, and biology: Retusa obtusa pertenuis is a circumpolar subspecies, distributed in the arctic, subarctic, and the boreal regions of the North Atlantic. It extends from West and East Greenland, Svalbard, the arctic coasts of Europe and Asia, and Parry Islands in Arctic Canada in the north to southwestern Norway, ?the Danish Wadden Sea, Shetland, Nova Scotia, and the Aleutians in the south (Golikov, 1995; Harmer, 1923; Lemche, 1938, 1941a, 1941b, 1948). On the other hand, typical R. obtusa has been reported from more southerly localities as the Mediterranean (Lemche, 1941a). Bathymetrical range: From 5 m in the White Sea to 299 off Svalbard (Golikov, 1995). In Greenland, the subspecies is generally found in shallow water, but if currents cause warmer water inflow in areas where it is living, it may penetrate to greater depths than usual (Lemche, 1941a). Golikov (1995) found it living at water temperatures from negative to 6 °C. The subspecies seems to be polyhaline with salinity tolerance above 25‰ (Golikov, 1995). In most opisthobranch species, the eggs hatch as swimming shelled veliger larvae spending a relatively short time in the plankton (Thompson & Brown, 1976).
In East Greenland, the subspecies is apparently exclusively bound to the Macoma calcarea community, especially the lower Ophiocten Zone (Lemche, 1941b). In the Arctic, it prefers silty and sandy substrates (Golikov, 1995), and in the British Isles, Retusa obtusa burrows shallow in mud or muddy sand feeding upon small prosobranchs (Thompson & Brown, 1976).
Fossil occurrence: Lower Pleistocene: Pattorfik beds (Símonarson, 1981), Svarthamar and Torfhóll Members in Breiðavík (Eiríksson et al., 1992; Vilhjálmsson, 1985). Middle Pleistocene: Bridlington Crag (Harmer, 1923), ?Kotzebuan (Hopkins et al., 1972). Upper Pleistocene: ?Baltic Eemian (Funder et al., 2002). Stratigraphical range: Lower Pleistocene to Recent.
-
Family Scaphandridae Sars, 1878
-
Genus Cylichnoides Mighels & Adams, 1842
Cylichnoides occultus (Mighels, 1841)
Plate 11.2, fig. 8
-
1841 Bulla occulta – Mighels and Adams: p. 50.
-
1855 Bulla scalpta – Reeve: p. 392, pl. 32, fig. 3.
-
1878 Cylichna propinqva, M. Sars – Sars: p. 284–285, pl. 18, fig. 5a–d.
-
1923 Cylichna scalpta (Reeve) – Harmer: p. 803, pl. 63, fig. 11.
-
1924 Cylichna (Cylichna) reinhardti (Holböll) Mörch = C. propinqua M. Sars – Schlesch: p. 325 (from Poulsen’s manuscript 1884).
-
1938 Cylichna insculpta (Totten) – Lemche: p. 9–10.
-
1948 Cylichna occulta (Mighels, 1841) – Lemche: p. 78–79.
-
1985 Cylichna occulta occulta (Mighels, 1841) var. scalpta (Reeve, 1855) – Vilhjálmsson: p. 51–52, pl. 5, fig. 8A–B.
Material: Seven specimens from the Hörgi Formation and one from the Svarthamar Member.
Remarks: The two largest specimens measure (h × d): 8.0 × 5.4 and 7.5 × 4.9 mm, respectively. The d/h ratios are 0.46–0.60, considerably lower than in a typical Cylichna occulta (cf. Lemche, 1948; Óskarsson, 1962).
In addition to the lower d/h ratios and broader shell, the shells from Breiðavík are ornamented with more distinct and close set spiral striae, and therefore they can be referred to the form or variety scalpta (Reeve, 1855).
Recent distribution, ecology, and biology: Lemche (1948) pointed out that typical Cylichnoides occultus and the broader scalpta form perhaps are ecologically slightly different. Apparently the scalpta form has a stronger preference for colder water, and it is more frequently found in arctic and subarctic areas. It seems to be an arctic-subarctic-high boreal form with circumpolar distribution, as well as the typical C. occultus. Thus, it probably extends from West and East Greenland, Svalbard, Franz Josef Land, Novaya Zemlya, the Kara Sea, the Siberian Arctic Sea, northern Alaska, and the northeast coast of North America in the north and southward to Scotland and Cape Cod in the North Atlantic (Golikov, 1995; Lemche, 1938, 1941a, 1941b; MacGinitie, 1959). In the Pacific, it may occur in the southern part of the Bering Sea (Golikov, 1995). It has not been recorded from the Baltic, or the Faroe Islands where the closely related C. magna Lemche has been found (Funder et al., 2002; Sneli et al., 2005). Bathymetrical range: From 2 m in Franz Josef Land to 329 m in the Norwegian Sea (Golikov, 1995). Empty shells have been found at Greenland down to a depth of 900 m (Lemche, 1938). The species is probably mesohaline with salinity tolerance down to about 23‰ (Golikov, 1995). The larval development probably has a very short pelagic stage, if any.
The scalpta form seems to prefer silty and sandy substrates, and in Iceland, it is mainly found in the Arctic Macoma bottom infaunal community, often in great numbers (Lemche, 1938).
Fossil occurrence: Pliocene: Tapes and Mactra Zones of the Barmur Group (Símonarson & Eiríksson, 2020). Lower Pleistocene: Olkov Suite (Petrov, 1982), Pattorfik beds (Símonarson, 1981), Kap København Formation (Símonarson et al., 1998). Middle Pleistocene: Bridlington Crag (Harmer, 1923), Kotzebuan (Hopkins et al., 1972). Upper Pleistocene: Portlandia arctica Zone in the Skærumhede sequence in Denmark (Jessen et al., 1910). Stratigraphical range: Pliocene to Recent.
11.3 Bivalvia
-
Class Bivalvia Linné, 1758
-
Subclass Palaeotaxodonta Korobkov, 1954
-
Order Nuculoidea Dall, 1889
-
Family Nuculidae Gray, 1824
-
Genus Ennucula Iredal, 1931
Ennucula tenuis (Montagu, 1808)
Plate 11.3, fig. 1
-
1808 Arca tenuis – Montagu: p. 56–57, pl. 29, fig. 1.
-
1851 Nucula tenuis, Montague – Wood: p. 84–85 , pl. 10, fig. 5a–b.
-
1878 Nucula tenuis, Mont. – Sars: p. 33–34, pl. 4, fig. 6a–b.
-
1950 Nucula tenuis (Montagu, 1808) – Heering: p. 14–15, pl. 11, fig. 28.
-
1958 Nucula tenuis expansa Reeve – Ockelmann: p. 13–15.
-
1960 Nucula tenuis Montagu – Áskelsson: p. 16.
-
1980 Nucula (Ennucula) belloti Adams, 1856 – Lubinsky: p. 9–11, pl. 1, figs. 1–5.
-
1980 Nucula tenuis (Montagu, 1808) – Gladenkov et al.: p. 25–28, pl. 1, figs. 3–4 & 4a.
-
1985 Nucula (Leionucula) tenuis expansa Reeve, 1855 – Vilhjálmsson: p. 53–55, pl. 6, figs. 1A–B, 2–3.
Material: A total of 104 specimens with united valves and 23 disarticulated valves from the Svarthamar Member, 1 specimen with paired valves from the Stapavík Member, and 5 specimens with united valves and 8 valves from the Torfhóll Member, all collected by Vilhjálmsson (1985). Usually the specimens were found with articulated valves, even though some of them were partly dissolved and recrystallized or compressed.
Remarks: The five largest specimens with paired valves measure (l × h): 17.6 × 14.8, 16.8 × 13.3, 14.3 × 11.3, 12.5 × 9.8, and 12.3 × 10.0 mm. The h/l ratios are 0.78–0.86, and the b/l ratios are 0.47–0.55. The specimens from Breiðavík are more convex than the typical species and comparable to the expansa form of Bronn (1848), recorded from East Greenland by Ockelmann (1958). It is also the dominating form in the Lower Pleistocene Pattorfik beds in West Greenland (Símonarson, 1981).
Schenck (1939) revised the nomenclature of the nuculids and came to the conclusion that the northern forms known as Nucula expansa Reeve and N. inflata Hancock are conspecific with N. belloti Adams. Therefore Lubinsky (1980) considered that the only available name for these northern specimens is N. belloti. However, we will follow Ockelmann (1958), as well as Coan et al. (2000) and several other authors in using the species name of Montagu from 1808, even though Sneli et al. (2005) stressed that in the Canadian Arctic and Laptev Sea N. belloti “is a good species.”
Recent distribution, ecology, and biology: Ennucula tenuis is an arctic-subarctic-boreal, circumpolar species. It extends from West and East Greenland, Svalbard, Novaya Zemlya, the Kara Sea, the Laptev Sea, the Siberian Arctic Sea, northern Alaska, and Ellesmere Island in the north and south to the British Isles, Øresund and the Belt Sea in Denmark, the Mediterranean, Morocco, along the east coast of North America to the Florida Strait, and North Japan and South California in the Pacific (Ockelmann, 1958; Sneli et al., 2005; Tebble, 1966). Bathymetrical range: From 2 m in Svalbard to 2290 m off Ireland (Massy, 1930; Ockelmann, 1958). The species prefers offshore areas, in the Faroe Islands between 35 and 75 m (Sneli et al., 2005) and in Iceland between 10 and 200 m (Óskarsson, 1982). The species is mesohaline with salinity tolerance down to 15‰ (Funder et al., 2002). The larval development is apparently with a very short pelagic stage or it is entirely lacking (Ockelmann, 1958; Thorson 1936).
In the British Isles, Ennnucula tenuis prefers substrates of mud or muddy sand or gravel (Tebble, 1966). The species is an infaunal deposit feeder, burrowing only a few cm under the surface in search for food. The species is a rather slow burrower and the northern form or varieties are even slower than the typical form because of greater convexity (e.g., the expansa form). In East Greenland and Iceland, it is a common member of the Arctic Macoma community and the Yoldia hyperborea community (Ockelmann, 1958; Spärck, 1937).
Fossil occurrence: Pliocene: Coralline Crag Formation (Heering, 1950; Wood, 1851), San Joaquin beds (Durham & MacNeil, 1967). Pliocene/Lower Pleistocene: Red Crag Formation (Wood, 1851). Lower Pleistocene: Norwich Crag Formation (West et al., 1980), Maassluis Formation (Spaink, 1975), Pattorfik beds (Símonarson, 1981), Olkov Suite (Petrov, 1982). Middle Pleistocene: Pinakul, Kresta, and Padymeiskii Suites (Merklin et al., 1962; Merklin et al., 1979; Petrov, 1982). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
-
Family Nuculanidae Adams & Adams, 1858
-
Genus Nuculana Link, 1807
Nuculana minuta (Müller, 1776)
Plate 11.3, fig. 2
-
1776 Arca minuta – Müller: p. 247.
-
1851 Leda caudata, Donovan – Wood: p. 92–93, pl. 10, fig. 12a–b.
-
1878 Leda minuta, Müll. – Sars: p. 36, pl. 5, fig. 2a–b.
-
1934 Léda minúta (Müller) – Jensen & Spärck: p. 29, fig. 15.
-
1950 Leda (L.) minuta (Müller, 1779) – Heering: p. 19–20, pl. 9, figs. 5–6.
-
1980 Nuculana minuta (Fabricius, 1776) – Lubinsky: p. 14, pl. 2, fig. 4.
-
1980 Nuculana minuta (Müller, 1779) – Gladenkov et al.: p. 27, pl. 1, fig. 7.
-
1985 Nuculana (Nuculana) minuta (Müller, 1779) – Vilhjálmsson: p. 59–60, pl. 6, fig. 6.
-
2005 Jupiteria minuta (O.F. Müller, 1779) – Sneli et al.: p. 118, fig. 42.
Material: Two disarticulated left valves from the Svarthamar Member collected by Vilhjálmsson (1985).
Remarks: Both are left valves that are fragmented and only one of them is measurable. It measures (l × h): 8.7 × 4.7 mm.
Recent distribution, ecology, and biology: Nuculana minuta is distributed in the eastern North Atlantic in the arctic, subarctic, and boreal regions. It is discontinuously circumpolar, lacking in the more arctic seas. The species extends from East Greenland, Svalbard, the White Sea, the Beaufort Sea, Grinnell Land, and Baffinland south to the British Isles, Ireland, Maine, and North Japan (Bernard, 1979; Ockelmann, 1958; Sneli et al., 2005). In the Baltic, it has been found along the west coast of Sweden south to Øresund (Sneli et al., 2005). Bathymetrical range: 4–1900 m (Ockelmann, 1958). It seems to prefer depths between 10 and 190 m (Poppe & Goto, 1993; Tebble, 1966). The species is mesohaline with salinity tolerance down to 15‰ (Funder et al., 2002). The pelagic larval stage is probably very short or entirely lacking (Jørgensen, 1946).
In the British Isles, the species lives in muddy sand and gravel (Tebble, 1966). In Iceland, it occurs as infaunal deposit feeder mainly in the Arctic Macoma community together with N. pernula (Spärck, 1937).
Fossil occurrence: Pliocene: Serripes Zone of the Barmur Group (Gladenkov et al., 1980). Pliocene/Lower Pleistocene: Red Crag Formation (Wood, 1851). Lower Pleistocene: Icenian (Heering, 1950), Olkov Suite (Petrov, 1982). Middle Pleistocene: Karagin Suite (Petrov, 1982). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
Nuculana pernula (Müller, 1779)
Plate 11.3, fig. 3
-
1779 Arca pernula – Müller: p. 57.
-
1851 Leda pernula, Müller – Wood: p. 93, pl. 10, fig. 13a–c.
-
1878 Leda pernula, Müll. – Sars: p. 35 pl. 5, fig. 1a–d.
-
1950 Leda (L.) pernula (Müller, 1779) – Heering: p. 20–21, pl. 9, figs. 3–4.
-
1958 Leda pernula costigera Leche – Ockelmann: p. 15–18, pl. 1, fig. 9.
-
1980 Nuculana pernula (Müller, 1779) – Gladenkov et al.: p. 28, pl. 1, figs. 5–6a.
-
1985 Nuculana (Nuculana) pernula (Müller, 1779) – Vilhjálmsson: p. 55–59, pl. 6, figs. 4–5.
Material: Two specimens with paired valves from the Hörgi Formation, nine specimens with united valves and six single valves from the Svarthamar Member, and one specimen with paired valves and two disarticulated valves from the Torfhóll Member. Most of the shells were collected by Vilhjálmsson (1985).
Remarks: Generally, the shells are more or less dissolved and in several cases only internal casts are preserved. Therefore the measurements are not very accurate, but the largest measureable specimens measure (l × h): 27.3 × ?15.6, 21.0 × 12.5, 20.0 × 10.8, 19.3 × 9.5, and 19.2 × 9.5 mm. The h/l ratios are 0.49–0.59, and the b/l ratios are 0.28–0.33.
Recent distribution, ecology, and biology: Nuculana pernula is widely distributed in the arctic, subarctic, and boreal regions in the North Atlantic. This circumpolar species extends from Franz Josef Land, Novaya Zemlya, the Kara Sea, the Siberian Arctic Sea, Ellesmere Island, Beaufort Sea, and the Bering Strait southward to the Sea of Okhotsk, Queen Charlotte Islands, northern Japan, Cape Cod, the English Channel, and Denmark (Bernard, 1979; Ockelmann, 1958) (Sneli et al., 2005). In the Baltic, it is living in the Belt Sea and along the Swedish west coast to Øresund (Sneli et al., 2005). Only empty valves have been found in the Bay of Biscay (Ockelmann, 1958). Bathymetrical range: From 3–9 m in East Greenland or 4 m in Svalbard to 1275 m near Jan Mayen (Ockelmann, 1958) or 1400 m in North Japan (Bernard, 1979). In Kattegat (Denmark), it seems to prefer offshore areas with depths exceeding 20 m (Jensen & Spärck, 1934). The species is mesohaline with salinity tolerance down to 15‰ (Funder et al., 2002). The pelagic larval stage is probably very short or entirely lacking (Ockelmann, 1958).
In East Greenland, N. pernula lives mainly infaunally as deposit feeder in mud or clay bottom, often mixed with sand or gravel (Ockelmann, 1958). It attains greatest abundance in the Arctic Macoma community (Thorson, 1934, 1936).
Experiments have shown that in water with normal salinity and temperatures between 7 and 8 °C and in silty sand substrate it takes the species from 30 s to 1 min to attain life position in the sediment with the posterior end upwards (Vilhjálmsson, 1985).
Fossil occurrence: Pliocene: Serripes Zone of the Barmur Group (Gladenkov et al., 1980), Diemerbrug Well in the Netherlands (Heering, 1950). Lower Pleistocene: Pattorfik beds (Símonarson, 1981), Maassluis Formation (Heering, 1950), Olkov Suite (Petrov, 1967, 1982). Middle Pleistocene: Karagin and Padymeiskii Suites (Merklin et al., 1979; Petrov, 1982). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
-
Genus Portlandia Mörch, 1857
Portlandia arctica (Gray, 1824)
Plate 11.3, figs. 4–5
-
1824 Nucula arctica – Gray: p. 240.
-
1878 Portlandia arctica, Gray – Sars: p. 37, pl. 4, fig. 7a–b.
-
1934 Portlándia árctica (Gray) – Jensen & Spärck: p. 32–33, fig. 19.
-
1958 Portlandia arctica (Gray) – Ockelmann: p. 23–26.
-
1979 Portlandia (Portlandia) arctica (Gray, 1824) – Bernard: p. 16–17, figs. 15–17.
-
1980 Portlandia arctica (Gray, 1824) – Gladenkov et al.: p. 29–30, pl. 1, figs. 12–17.
-
1985 Portlandia (Portlandia) arctica (Gray, 1824) – Vilhjálmsson: p. 61–62, pl. 7, figs. 1–2.
-
2002 Portlandia arctica (Gray, 1824) – Símonarson and Leifsdóttir: p. 72–78, fig. 1.
Material: A total of 76 specimens with paired valves and 45 disarticulated valves from the Hörgi Formation, 3 specimens with paired valves from the Fossgil Member, and 6 specimens with paired valves from the Svarthamar Member. Most of the material was collected by Vilhjálmsson (1985).
Portlandia arctica has also been found in Iceland in Lower Pleistocene sediments on Snæfellsnes Peninsula, West Iceland, and in sediments from Late Weichselian in Northwest, West, and North Iceland (Fig. 11.4). The occurrence on Tjörnes in Late Weichselian sediments resting on the Pliocene Barmur Group was recorded by Gladenkov (1974; see also Eiríksson, 1981), but we have never found this locality.
The most important localities with faunal assemblages compared with the Lower Pleistocene Breiðavík Group fauna. TJ Breiðavík Group, Tjörnes, BH Búlandshöfði Formation on Snæfellsnes, western Iceland, EA East Anglia, NO North Sea Basin, BS Barents Sea, European Russia, LE Lodin Elv, East Greenland, SK Store Koldewey, East Greenland, IF Île de France, East Greenland, KK Kap København, North Greenland, PA Pattorfik, central West Greenland, HL Hvitland, North Canada, AP Gubik Formation, Alaska, YF Yakataga Formation, Alaska, CH Chukotka, East Siberia, KA Kamchatka
Remarks: The five largest specimens measure (l × h × b): 20.5 × 11.7 × 7.4, 19.6 × 10.1 × 7.1?, 19.2 × 11.6 × 7.1, 19.2 × 11.2 × 7.0, and 16.8 × 9.2 × 6.2 mm. The h/l ratios are 0.52–0.60, and the b/l ratios are 0.36–0.37. Generally the shells are rather poorly preserved, often as internal casts without shell material, even though the shells were articulated.
Size-frequency distribution of 25 measurable pairs from one sample in the lower part of the Hörgi Formation indicates a low death rate among juveniles (Vilhjálmsson, 1985). The fossiliferous siltstone, with lateral contacts to thick bodies of conglomerate of supraglacial outwash origin, was probably deposited when the sea transgressed glacial landscape in front of retreating glacier. The fine-grained and well-stratified sediment with Portlandia arctica, Similipecten greenlandicus (Sowerby), and some other cold water species was probably laid down in sheltered and quiet environment where the juvenile had rather good possibilities to survive, without a harsh competition. Low mortality among juvenile animals can certainly help the larvae to settle successfully and to form a life assemblages (biocoenosis) preserved in the sediment (Fig. 11.5).
1. Erginus cf. rubellus (Fabricius), two specimens from the Hörgi Formation. 2. Patella pellucida Linné, shell from the Torfhóll Member. 3. Lepeta caeca (Müller), specimen from the Svarthamar Member. 4. Margarites groenlandicus (Gmelin), fragmentary specimen from the Hörgi Formation. 5. Littorina obtusata (Linné), shell from the Svarthamar Member. 6. Alvania patorfikensis Laursen, specimens from the Hörgi Formation. 7. Onoba aculeus (Gould), shell from the Svarthamar Member. 8. Cryptonatica affinis (Gmelin), specimen from the Hörgi Formation. 9. Cryptonatica affinis (Gmelin), shell from the Svarthamar Member. 10. Euspira cf. pallida (Broderip & Sowerby), two internal casts from the Hörgi Formation
Recent distribution, ecology, and biology: Portlandia arctica is an arctic, circumpolar species with a few southern outposts, especially into colder and deeper waters of the subarctic region (Fig. 11.6). It is not living in Iceland at present. The species is known from West Greenland north of 77°N and East Greenland south to Mikis Fjord (68°10´N), Svalbard, the White Sea, the Barent Sea, Novaya Zemlya, the Kara Sea, the Laptev Sea, the Siberian Arctic Sea, the Beaufort Sea, Canadian Archipelago, Foxe Basin, Hudson Bay, and Newfoundland (Bernard, 1979; Jensen, 1942; Lubinsky, 1980; Ockelmann, 1958; Scarlato, 1981). The isolated occurrence in the White Sea is considered a relict in deeper water (Brøgger, 1900; Ockelmann, 1958). Bathymetrical range: From 2 m in East Greenland to 339 m in East Greenland (Ockelmann, 1958). Bernard (1979) reported the species from depths between 0 and 2560 m in the Beaufort Sea, although he considered the species as a frequent member of the shallow water bivalve fauna. In East Greenland, it lives mainly associated with the Polar Current water at depths between 10 and 50 m and sea temperatures from 0 to –1.7 °C, but sometimes it is also associated with the Fjord water with slightly higher temperatures (Ockelmann, 1958; Ussing, 1934). It is adapted to reduced salinities, and in East Greenland and Arctic Canada, it is mainly found off the mouth of rivers and glacial fronts where large quantities of mud and silt are deposited (Lubinsky, 1980; Ockelmann, 1958). Generally, the species is mesohaline with salinity tolerance down to 25‰, but locally it can possibly live in water with still lower salinity (Jensen, 1942; Ockelmann, 1958) (Funder et al., 2002). The larval development is with a short pelagic stage (Ockelmann, 1958).
1. Boreotrophon truncatus (Strøm), specimen from the Svarthamar Member. 2. Nucella lapillus (Linné), fragmentary shell from the Svarthamar Member. 3. Buccinum undatum Linné, halfgrown specimen from the Svarthamar Member. 4. Buccinum cyaneum Bruguière, fragmentary shell from the Svarthamar Member. 5. Colus sp., damaged specimen from the Hörgi Formation. The top of the spire is broken off. 6. Curtitoma cf. trevelliana (Turton), fragmentary specimen from the Svarhamar Member. 7. Retusa obtusa pertenuis (Mighels), fragmentary shell from the Svarthamar Member. 8. Cylichnoides occultus (Mighels), specimen from the Hörgi Formation
Portlandia arctica is almost exclusively an infaunal deposit feeder (Bernard, 1979; Ockelmann, 1958). In East Greenland, it forms almost monospecific community in clayey or muddy bottom in front of calving glaciers and meltwater outlets (Ockelmann, 1958). The optimal habitat of P. arctica is in the inner parts of fiords with cold water and lowered salinity off the mouth of rivers with melt water and off glacial fronts, where large quantities of clay and mud are in suspension in the water (Ockelmann, 1958). The sedimentation rate is high and environmental energy rather low under these conditions attractive only to a few species, and certainly not a carnivorous epifauna.
Fossil occurrence: Lower Pleistocene: Maassluis Formation of the Netherlands (Spaink, 1975), Icenian of the Netherlands (Heering, 1950), Île de France Formation, East Greenland (Bennike et al., 2002). Yakataka Formation, Middleton Island (Allison, 1978). Middle Pleistocene: Karagin, Pinakul, Kolvin, and Padymeiskii Suites (Merklin et al., 1962; Merklin et al., 1979; Petrov, 1982), Kotzebuan (Hopkins et al., 1972), Anvilian (Hopkins et al., 1974), Pre-Cape Christian sediments (Andrews et al., 1981). Upper Pleistocene: Baltic Eemian (Funder et al., 2002), Portlandia arctica Zone in the Skærumhede sequence in Vendsyssel, Denmark (Nordmann in Jessen et al., 1910). Stratigraphical range: Lower Pleistocene to Recent.
The fossil occurrence of Portlandia arctica demonstrates the southward expansion of cold water as much as 2000 km south of its present southern boundary. The species is considered to have originated in the Atlantic (Durham & MacNeil, 1967).
-
Genus Yoldiella Verrill & Bush, 1897
Yoldiella lenticula (Møller, 1842)
Plate 11.3, fig. 6
-
1842 Nucula lenticula – Møller: p. 90.
-
1878 Portlandia lenticula, Møll. – Sars: p. 39, pl. 4, fig. 10a–b.
-
1934 Portlándia lentícula (Møller) – Jensen & Spärck: p. 35, fig. 23.
-
1958 Portlandia lenticula (Møller) – Ockelmann: p. 30–32, pl. 1, fig. 13.
-
1979 Portlandia (Yoldiella) lenticula (Møller, 1842) – Bernard: p. 19, fig. 23.
-
1980 Portlandia lenticula (Möller, 1842) – Gladenkov et al.: p. 31, pl. 1, figs. 23–25.
-
1985 Portlandia (Yoldiella) lenticula (Møller, 1842) – Vilhjálmsson: p. 63–64, pl. 7, fig. 3A–B.
-
1989 Yoldiella lenticula (Möller, 1842) – Warén: p. 239, figs. 8C–D, 10E–F.
Material: Fifty-three specimens with united valves and four disarticulated valves from the Hörgi Formation, one left valve from the Fossgil Member.
Remarks: The specimens are rather well preserved and the majority of valves are paired in the sediment. Taxodont bivalves are often found with articulated valves in sediments where other bivalves are more or less disarticulated. The taxodont hinge seems stronger and is keeping the valves together even when they become fragmented.
The five largest specimens with paired valves measure: (l × h × b): 5.9 × 4.4 × 3.0, 5.9 × 4.2 × ?, 5.8 × 4.2 × 2.9, 5.7 × 3.6 × 2.7, and 5.3 × 3.8 × 2.6 mm. The h/l ratios are 0.63–0.74, and the b/l ratios are 0.47–0.51. The b/l ratios are slightly lower than those recorded by Ockelmann (1958) for recent specimens from East Greenland.
Size-frequency distribution of 26 specimens with paired valves from a sample in the lowermost part of the Hörgi Formation is very similar to that observed for Portlandia arctica from a sample slightly higher up in the silty sediments (Vilhjálmsson, 1985). The left-skewed diagram representing low death rate among the juveniles, which may indicate quiet environments and a life assemblage (biocoenosis) as in the case of P. arctica.
Recent distribution, ecology, and biology: Yoldiella lenticula is an arctic-subarctic-boreal species. It extends from West and East Greenland, Svalbard, Novaya Zemlya, the Kara Sea, the Laptev Sea, the Siberian Arctic Sea, the Beaufort Sea, and the Canadian Arctic in the north to the west coast of Norway at Bodø (67°N), the Shetland Islands, and Cape Cod (only empty shells) in the south (Bernard, 1979; Høisæter, 1986; Lubinsky, 1980; Ockelmann, 1958). There are sporadic records from the northernmost Bering Sea, but otherwise none from the Pacific (Bernard, 1979). Bathymetrical range: From 0–13 m in East Greenland to about 1400 m north of Shetland (Ockelmann, 1958). The recent species seems to be polyhaline with salinity tolerance above 30‰ (Funder et al., 2002), but apparently the salinity was considerably lower in Breiðavík when the species lived there together with Portlandia arctica. The larval development is non-pelagic or with a very short pelagic stage (Ockelmann, 1958).
In East Greenland, this infaunal species prefers substrates of mud or clay, sometimes mixed with sand and even gravel, and there it is most frequently found in the Astarte crenata community (Ockelmann, 1958; Thorson, 1934). It also occurs in the Portlandia arctica community (Thorson, 1934).
Fossil occurrence: Lower Pleistocene: Hörgi Formation (Gladenkov et al., 1980), Kap København Formation, North Greenland (Símonarson et al., 1998), Lodin Elv Formation, Île de France Formation, and Store Koldewy Formation in East Greenland (Feyling-Hanssen et al., 1983; Bennike et al., 2002; Bennike et al., 2010). Middle Pleistocene: Kolvin and Padymeiskii Suites (Merklin et al., 1979), Kresta Suite (Petrov, 1966), Ossor Suite (Petrov, 1982). Upper Pleistocene: Baltic Eemian (Funder et al., 2002), Portlandia arctica Zone in the Skærumhede sequence in Vendsyssel, Denmark (Nordmann, in Jessen et al., 1910; Petersen, in Bahnson et al., 1974). Stratigraphical range: Lower Pleistocene to Recent.
Yoldiella intermedia (Sars, 1865)
Plate 11.3, fig. 7
-
1865 Yoldia intermedia – Sars: p. 38, pl. 3, figs. 92–96 .
-
1878 Portlandia intermedia, M. Sars – Sars: p. 38, pl. 4, fig. 9a–b.
-
1934 Portlándia intermédia (Sars) – Jensen & Spärck: p. 35–36, fig. 24.
-
1958 Portlandia intermedia (M. Sars) – Ockelmann: p. 27–29, pl. 1, fig. 12.
-
1979 Portlandia (Yoldiella) intermedia (M. Sars, 1865) – Bernard: p. 18–19, fig. 22.
-
1980 Portlandia intermedia (Sars, 1865) – Gladenkov et al.: p. 30–31, pl. 1, figs. 18–22.
-
1985 Portlandia (Yoldiella) intermedia (Sars, 1865) – Vilhjálmsson: p. 65–66, pl. 7, fig. 4A–B.
-
1989 Yoldiella intermedia (M. Sars, 1865) – Warén: p. 243–244, fig. 12B–C.
Material: Thirty-seven specimens with articulated valves and one single valve from the Hörgi Formation, one specimen with united valves from the Fossgil Member, and one specimen with paired valves from the Svarthamar Member.
Remarks: Some of the specimens are somewhat dissolved and slightly compressed. The three largest specimens measure (l × h × b): 14.1 × 7.8 × 5.2, 10.9 × 5.8 × 3.9, and 10.3 × 5.6 × 3.7 mm. The h/l ratios are 0.53–0.57, and the b/l ratios are 0.30–0.38. The h/l ratios are distinctly lower than in the slightly more convex Yoldiella lenticula.
Recent distribution, ecology, and biology: Yoldiella intermedia is circumpolar living in the arctic, subarctic, and the northernmost boreal regions of the North Atlantic. It extends from West and East Greenland, Svalbard, Novaya Zemlya, the Barents Sea, the Kara Sea, the Laptev Sea, the Siberian Arctic Sea, the Beaufort Sea, and the Canadian Arctic Archipelago in the north to northern and eastern Iceland, East Finnmark in northern Norway, the Shetlands, and Hudson Bay in the south (Bernard, 1979; Høisæter, 1986; Lubinsky, 1980; Ockelmann, 1958; Warén, 1989). Warén (1989) considered the records from southern Scandinavia based on fossil material. Bathymetrical range: From 7–9 m in the Siberian Arctic Sea to about 1150 m off the Shetlands (Ockelmann, 1958). Empty shells have been recorded from depths down to 2330 m in the North Atlantic (Ockelmann, 1958). The recent species seems to be polyhaline with salinity tolerance above 30‰ (Funder et al., 2002), but apparently the salinity was considerably lower in Breiðavík when the species lived there together with Portlandia arctica. The larval development is unknown.
The species is infaunal deposit feeder, and in East Greenland, it is almost always found to live on substrates of mud or clay, occasionally mixed with sand and gravel (Ockelmann, 1958). In the fiords of East Greenland, it often replaces Portlandia arctica at greater depths (Ockelmann, 1958).
Fossil occurrence: Lower Pleistocene: Hörgi Formation (Gladenkov et al., 1980), Kap København Formation (Símonarson et al., 1998), Île de France Formation (Bennike et al., 2002). Middle Pleistocene: Ossor and Kresta Suites (Merklin et al., 1962; Petrov, 1966, 1982), Kotzebuan (Hopkins et al., 1972). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Lower Pleistocene to Recent.
Yoldiella intermedia is considered to be of Atlantic origin (Bernard, 1979) and the specimens from Breiðavík and the Kap København Formation seems to be the earliest records of this species.
Yoldiella frigida (Torell, 1859)
Plate 11.3, fig. 8
-
1859 Yoldia Frigida – Torell: p. 148–149, pl. 1, fig. 3.
-
1878 Portlandia frigida, Torell – Sars: p. 39–40, pl. 4, fig. 11a–b.
-
1934 Portlándia frígida (Torell) – Jensen & Spärck: p. 34, fig. 21.
-
1958 Portlandia frigida (Torell) – Ockelmann: p. 34–37, pl. 1, fig. 14.
-
1979 Portlandia (Yoldiella) frigida (Torell, 1859) – Bernard: p. 17–18, figs. 20–21.
-
1985 Portlandia cf. Portlandia (Yoldiella) fraterna (Verrill & Bush, 1898) – Vilhjálmsson: p. 66–67, pl. 8, fig. 1A–B, 2.
-
1989 Yoldiella frigida (Torell, 1859) – Warén: p. 239, figs. 7E–F, 10G–H.
Material: Two specimens with united valves from the Hörgi Formation. This is the first record of the species in the Breiðavík Group as well as in the Icelandic deposits.
Remarks: The two specimens are well preserved and measure (l × h × b): 4.6 × 3.8 × 2.4 and 3.9 × 3.0 × 1.8 mm, respectively. The h/l ratios are 0.82 and 0.77, and the b/l ratios are 0.52 and 0.46. These few measurements and the shell ratios are closer to those Ockelmann (1958) recorded for Yoldiella frigida than Y. fraterna Verrill & Bush (=Y. nana M. Sars) from East Greenland. The species is never as convex as P. fraterna. Further characteristic feature is the radial flexure in the posterior region of the valves.
Recent distribution, ecology, and biology: Yoldiella frigida is first and foremost distributed in the arctic and subarctic regions of the North Atlantic. It has been recorded from Northwest and East Greenland, Jan Mayen, Svalbard, Novaya Zemlya, the Kara Sea, the western part of the Siberian Arctic Sea, and the Canadian Arctic Archipelago (Lubinsky, 1980; Ockelmann, 1958; Warén, 1989). Warén (1989) could not confirm the recent Icelandic records, and the specimens in question are most probably referable to Y. nana (= Y. fraterna). According to Ockelmann (1958), the records from the New England area need confirmation and the species is not known from the North Pacific (Bernard, 1979). Bathymetrical range: From 5–24 m in East Greenland to about 400 m in West Greenland (Ockelmann, 1958). The recent species seems to be polyhaline with lower salinity tolerance above 30‰ (Funder et al., 2002), but apparently the salinity was considerably lower in Breiðavík when the species lived there together with Portlandia arctica. The larval development is unknown.
In East Greenland, the species prefers substrates of mud, clay, and clay mixed with sand and even gravel (Ockelmann, 1958).
Fossil occurrence: Lower Pleistocene: Hörgi Formation (this chapter), Lodin Elv Formation (Feyling-Hanssen et al., 1983). Upper Pleistocene: Baltic Eemian (Funder et al., 2002), Portlandia arctica Zone in the Skærumhede sequence in Vendsyssel, Denmark (Nordmann in Jessen et al., 1910). Stratigraphical range: Lower Pleistocene to Recent.
-
Subclass Pteriomorphia Beurlen, 1944
-
Order Arcoida Stoliczka, 1871
-
Family Arcidae Lamarck, 1809
-
Genus Bathyarca Kobelt, 1891
Bathyarca cf. glacialis (Gray, 1824)
Plate 11.3, fig. 9
-
1824 Arca glacialis – Gray: p. 240.
-
1878 Arca glacialis, Gray – Sars: p. 43–44, pl. 4, fig. 1a–c.
-
1934 Árca glaciális Gray – Jensen & Spärck: p. 38–39, fig. 27.
-
1958 Arca (Bathyarca) glacialis Gray – Ockelmann: p. 44–48, pl. 1, fig. 18.
-
1979 Bathyarca glacialis (Gray, 1824) – Bernard: p. 22–23, fig. 30.
-
1985 Arca cf. Arca (Bathyarca) glacialis Gray, 1847 – Vilhjálmsson: p. 67–68, pl. 8, fig. 3.
Material: One left valve from the Hörgi Formation, collected by Vilhjálmsson (1985).
Remarks: The valve is fragmented, slightly compressed, and somewhat dissolved, but the radial ribs are visible except on the umbonal part. The valve has nine teeth in a straight dental line on the dorsal posterior part. The fragmentary valve is about 1.9 mm long.
Recent distribution, ecology, and biology: Bathyarca glacialis is an arctic-subarctic species with boreal occurrences in the North Atlantic. It extends from West and East Greenland, Svalbard, the Barent Sea, Novaya Zemlya, the Kara Sea, the Siberian Arctic Sea, the Beaufort Sea, and the Canadian Arctic Archipelago in the north to North and East Iceland, East Finnmark, and Hudson Bay (in deep water) in the south (Lubinsky, 1980; Ockelmann, 1958; Óskarsson, 1952). It has not been recorded from the Pacific (Bernard, 1979). Bathymetrical range: From 5 m in Svalbard and East Greenland (Ockelmann, 1958) to 916 m in the Baffin Bay (Clarke, 1974). In Iceland, it has been found living at depths between 80 and about 400 m (Óskarsson, 1952, 1982). The species is mesohaline with salinity tolerance down to 15‰ (Funder et al., 2002). The larval development is with a very short or suppressed pelagic stage (Ockelmann, 1958; Thorson, 1936).
The species is an epifaunal suspension feeder attached to the substratum with a byssus. In Iceland and East Greenland, it mainly occurs in the Astarte crenata community on a bottom of mud or clay which may be mixed up with sand, gravel, and stones (Ockelmann, 1958; Spärck, 1933; Thorson, 1933).
Fossil occurrence: Lower Pleistocene: Kap København Formation (Símonarson et al., 1998), Store Koldewy Formation (Bennike et al., 2010). Middle Pleistocene: Kresta, Kolvin, and Padymeiskii Suites (Merklin et al., 1962; Merklin et al., 1979; Petrov, 1966), Pre-Cape Christian beds (Andrews et al., 1981). Upper Pleistocene: Baltic Eemian (Funder et al., 2002), Early/Middle Weichselian beds in Vendsyssel, Denmark (Petersen, 2004). Stratigraphical range: Lower Pleistocene to Recent.
The species is considered to have originated in the Atlantic, and if it first reached the Pacific during the Middle (or ?Late) Pleistocene, it did not live there to recent time (cf. Durham & MacNeil, 1967).
-
Order Mytiloidea Férussac, 1822
-
Family Mytilidae Rafinesque, 1815
-
Genus Mytilus Linné, 1758
Mytilus edulis Linné, 1758
Plate 11.3, fig. 10
-
1758 Mytilus edulis – Linné: p. 705.
-
1851 Mytilus edulis, Linnæus – Wood: p. 52–54, pl. 8, fig. 9a–e.
-
1878 Mytilus edulis – Sars: p. 27.
-
1950 Mytilus (M.) edulis Linné, 1758 – Heering: p. 38–40.
-
1980 Mytilus edulis Linné, 1758 – Gladenkov et al.: p. 32–33, pl. 3, figs. 10–13.
-
1985 Mytilus (Mytilus) edulis Linné, 1758 – Vilhjálmsson: p. 69–70, pl. 8, figs. 4–5.
-
1985 ?Ostrea sp. – Vilhjálmsson: p. 112–113, pl. 15, fig. 5A–B.
-
2005 Mytilus edulis Linnaeus, 1758 – Sneli et al.: 131.
Material: A few fragments from the Hörgi Formation, six disarticulated right valves and eight left valves from the Svarthamar Member, one right valve and one left valve from the Stapavík Member, and five right valves and four left valves from the Torfhóll Member.
Remarks: All valves are disarticulated, generally fragmented and partly dissolved. The largest valves is about 53 mm in length.
A single right valve found in Svarthamar Member was temporarily referred to ?Ostrea sp. by Vilhjálmsson (1985; pl. 15, fig. 5). The shell is clearly mytiliform, but secondary calcite has more or less filled up the shell cavity and also cover more or less the exterior of the shell. This calcite is obviously formed by thin foliated layers. On the other hand, the shell seems to consist of fibrous calcite characteristic for the genus Mytilus. The shell seems dysodont, with a few small teeth near the umbo. They seems to be exaggerated of the secondary calcite. There are no sign of isodont hinge as in Ostrea. As far as we can see, the shell was probably monomyar or heteromyar with rather large adductor muscle scar in the posterior end. The shell is clearly deformed of the aforementioned secondary calcite, but from this short description it seems more plausible to refer it to Mytilus edulis. It is not very likely that Ostrea lived in Breiðavík during the Lower Pleistocene since it did not live in the area when the Pliocene Barmur Group sediments were deposited, while the sea temperatures were considerably higher.
Recent distribution, ecology, and biology: Mytilus edulis is now widely distributed in the subarctic, boreal, and lusitanian regions of the North Atlantic and in the Pacific from Alaska in the north to California and Japan in the south (cf. Símonarson & Eiríksson, 2020: Fig. 16). In the eastern North Atlantic, it extends from Jan Mayen, Novaya Zemlya, and the western Kara Sea and Chuckchi Sea (where it is rare) to the Bay of Biscay (Filatova, 1957; Malatesta & Zarlenga, 1986; Scarlato, 1981). It is not known living in Svalbard, Franz Josef Land, and the Siberian Arctic Sea, but apparently there is an isolated outpost at Bjørnøya (Símonarson et al., 1998). In North America, it lives in the Beaufort Sea, Victoria Island, Hudson Bay, Baffin Bay, and Padloping Island south to Cape Hatteras, California, and Japan in the Pacific (Ellis, 1960; La Rocque, 1953; Lubinsky, 1980; Sneli et al., 2005). In West Greenland, it is known from Dundas and Siorapaluk in the north (Theisen, 1973) to Qaqortoq/Julianehåb in the south (Hjort & Funder, 1974; Madsen, 1940). In East Greenland, it is known from the Ammassalik district and further south near 61°N (Ockelmann, 1958). Bathymetrical range: From 0 m (several localities) to 180 m in Jan Mayen (Ockelmann, 1958). The species is mainly littoral-intertidal and most frequently found at depths less than 10 m. The species is apparently mesohaline with tolerance down to 5‰ (Funder et al., 2002). Its reproduction is by rather long pelagic larval stage which explains the large distribution (Thorson, 1936).
In Iceland and East Greenland, the young animals most probably belong to the algal epifauna, while the adults belong to the epifauna associated with gravel, stones, and rocks (Madsen, 1949; Ockelmann, 1958). It is attached to the substratum with byssus-threads (Jensen & Spärck, 1934), but belongs to the suspension feeders (Ockelmann, 1958).
Fossil occurrence: ?Miocene: Blakeley Formation (Grant & Gale, 1931). Pliocene: Kallo and Doel sections (Marquet, 2002), Nassarius reticosus-Chlamys opercularis Subzone of the Netherlands (Spaink, 1975), Diemerbrug Well, Vreeburg-Utrecht Well, and Goes Well in the Netherlands (Heering, 1950). Pliocene/Lower Pleistocene: Red Crag Formation (Wood, 1872). Lower Pleistocene: Ludhamnian (Norton, 1967), Baventian (West et al., 1980), Emilian and Sicilian (Malatesta & Zarlenga, 1986), Pattorfik beds (Símonarson, 1981), Kap København Formation (Símonarson et al., 1998), Olkov Suite (Petrov, 1967, 1982). Middle Pleistocene: Karagin, Kolvin, and Padymeiskii Suites (Merklin et al., 1979; Petrov, 1982), Anvilian (Hopkins et al., 1974). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: ?Miocene to Recent.
-
Family: Pectinidae Rafinesque, 1815
-
Genus Chlamys Röding, 1798
Chlamys breidavikensis MacNeil, 1967
Plate 11.3, figs. 11–12
-
1967 Chlamys (“Chlamys”) breidavikensis – MacNeil: p. 16, pl. 25, figs. 4–8.
-
1925 Pecten islandicus, Müll. – Bárðarson: p. 100, 104.
-
1980 Chlamys (Chlamys) breidavikensis MacNeil, 1967 – Gladenkov et al.: p. 35, pl. 3, figs. 1–7.
-
1985 Chlamys (Chlamys) breidavikensis MacNeil, 1967 – Vilhjálmsson: p. 70–73, pl. 9, figs. 1–5.
Material: Seven fragmentary pairs, 18 disarticulated right valves, 21 left valves, and about 50 plate fragments (without umbo) of variable size from the Stapavík Member.
Remarks: Almost all the shell are fragmentary, compressed and even recrystallized. The largest and best measured valve is 71.1 mm in length, and the height was close to 75 mm.
MacNeil (1967) came to the conclusion that the larger pectinid from the Breiðavík deposits belongs to a new species he named after the Breiðavík Bay. It differs from Chlamys islandica (Müller) in having more even and lower radiating ribs, shorter anterior ears, and shallower V-shaped byssal notch. Furthermore, MacNeil (1967) stated that C. islandica has up to 30 ribs fewer than C. breidavikensis on half-grown individuals. However, Vilhjálmsson (1985) has noticed that many of the radial ribs in C. islandica bifurcate more often than in C. breidavikensis, producing even higher number of ribs in the ventral part of the shell in C. islandica. Apparently this species is closely related to C. islandica, but we agree with MacNeil (1967), Gladenkov et al. (1980), and Vilhjálmsson (1985) in considering it as a distinct species. On the other hand, MacNeil (1967) emphasized that C. breidavikensis is more related to the group of Talochlamys multistriata (Poli) and C. tauroperstriata (Sacco) known from the Burdigalian of Europe. In his opinion, C. islandica shows closer relationship to early Pleistocene forms in the northern Pacific.
Distribution, ecology, and biology. Chlamys breidavikensis most likely had similar distribution as the recent C. islandica. Obviously, it avoided the most arctic (high-arctic) areas and we have no records of the species south off Iceland. C. islandica is a subarctic-boreal species with arctic outposts, and it is discontinuously circumpolar. From Foxe Channel and Cumberland Peninsula in the Canadian eastern Arctic, Baffinland, West and Southeast Greenland, Svalbard, Novaya Zemlya, the Barents Sea, the Kara Sea in the north, it extend southwards to Stavanger at the Norwegian west coast, the Faroe Islands, and Cape Cod (Lubinsky, 1980; Ockelmann, 1958; Sneli et al., 2005). Empty (dead) shells have been found in Bohuslän (Sweden), the North Sea, west of Scotland, Ireland, France, the Mediterranean, and the Azores (Ockelmann, 1958). It has been recorded from the Pacific south to South Korea and Puget Sound (Sneli et al., 2005). However, MacNeil (1967) has stated that typical C. islandica does not live in the Pacific at present. Bathymetrical range: From 2 m in Iceland to 356 m in West Greenland (Ockelmann, 1958; Óskarsson, 1952). The species is polyhaline with salinity tolerance above 25‰ (Funder et al., 2002). Apparently, the species has a pelagic larval development (Ockelmann, 1958).
In East Greenland, this epifaunal suspension feeder (Chlamys islandica) is associated with the algal epifauna. The young animals are mainly living with Fucus in shallower water, but the adults migrate out to greater depths to the zone with red algae (Ockelmann, 1958; Thorson, 1933). In Iceland, it also prefers substrates of mud, sand, and shells (Madsen, 1949). Unfortunately, we have no further information about the ecological preferences of C. breidavikensis.
Fossil occurrence: Lower Pleistocene: Stapavík Member (MacNeil, 1967; Vilhjálmsson, 1985). As we have no records of this species from other deposits, its stratigraphical range is supposed to be limited to Lower Pleistocene, at least until its distribution has been studied further.
-
Genus Similipecten Winckworth, 1932
Similipecten greenlandicus (Sowerby, 1842)
Plate 11.3, fig. 13
-
1842 Pecten Greenlandicus – Sowerby: p. 57, pl. 13, fig. 40.
-
1878 Pecten grønlandicus, Sow. – Sars: p. 23, pl. 2, fig. 4a–c.
-
1934 Pécten groenlándicus Sowerby – Jensen & Spärck: p. 57–58, fig. 37.
-
1958 Propeamussium (Arctinula) groenlandicum (Sowerby) – Ockelmann: p. 68–72, pl. 2, fig. 2.
-
1967 Arctinula groenlandica (Sowerby) – MacNeil: p. 8, pl. 4, fig. 6.
-
1979 Arctinula greenlandica (Sowerby, 1842) – Bernard: p. 29, fig. 42.
-
1980 Delectopecten greenlandicus (Sowerby, 1842) – Lubinsky: p. 28, pl. 5, fig. 7.
-
1980 Propeamussium (Parvamussium) cf. groenlandicus (Sowerby, 1878) – Gladenkov et al.: p. 36, pl. 1, figs. 28–32.
-
1985 Palliolum (Delectopecten) greenlandicum (Sowerby, 1842) – Vilhjálmsson: p. 74–75, pl. 9, figs. 6–7.
Material: Five specimens with united valves, 13 disarticulated right valves, and 23 left valves from the Hörgi Formation.
Remarks: The thin shells are rather poorly preserved, often more or less dissolved and only preserved as internal casts. The three largest specimens measure (l × h): 17.7 × 17.5, 17.5 × 16.7, and 13.3 × 12.6 mm. The h/l ratios are 0.95–0.99. The largest specimens are recorded from East Greenland where they can exceed 32 mm in length (Ockelmann, 1958), whereas it only reaches 12 mm in Iceland (Madsen, 1949; Óskarsson, 1982). The size of the specimens may be indicative about the sea temperature as the largest shells are generally living in the coldest waters.
Recent distribution, ecology, and biology: Similipecten greenlandicus is arctic-subarctic species, but also well known from the northern part of the boreal region in the North Atlantic. It is discontinuously circumpolar extending from Northwest and East Greenland, Svalbard, Franz Josef Land, the Barents Sea, Novaya Zemlya, the Kara Sea, the Laptev Sea, the Siberian Arctic Sea, the Beaufort Sea, and the Canadian Arctic in the north and southward to western Norway at 69.5°N, the Faroe Islands, and the Gulf of St. Lawrence, and in the deep waters to Cape Cod (Bernard, 1979; Høisæter, 1986; Lubinsky, 1980; Ockelmann, 1958; Sneli et al., 2005). It has not been found in the Bering Sea or the Pacific (Bernard, 1979). Bathymetrical range: From 1 m in North Greenland (Schiøtte, 1989) to 2560 m in the Beaufort Sea (Bernard, 1979). It is common along the East Greenland coast especially at depths between 20 and 60–70 m (Ockelmann, 1958). The species is apparently mesohaline with salinity tolerance down to 15‰ (Funder et al., 2002). The pelagic larval stage is very short or lacking (Thorson, 1936).
The species is a suspension feeder, and in East Greenland, it prefers clayey or muddy substrates with gravel, stones, and shells mainly in the lower Ophiocten Zone of the Arctic Macoma community (Ockelmann, 1958; Thorson, 1933).
Fossil occurrence: Miocene/Pliocene: Sagavanirktok Formation, Nuwok Member (MacNeil, 1957; Repenning et al., 1987). Lower Pleistocene: Hyalina baltica beds of the Emilian (Malatesta & Zarlenga, 1986), Pattorfik beds (Símonarson, 1981), Kap København Formation (Símonarson et al., 1998), Île de France Formation (Bennike et al., 2002). Middle Pleistocene: Kolvin and Padymeiskii Suites (Merklin et al., 1979; Petrov, 1982), Pre-Cape Christian beds (Andrews et al., 1981). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: ?Miocene to Holocene.
Taking into consideration the early appearance in Alaska, as recorded at least by two authors, it may be questioned if the species originated in the Atlantic as suggested by Durham and MacNeil (1967).
-
Family Anomiidae Rafinesque, 1815
-
Genus Heteranomia Winckworth, 1922
Heteranomia squamula (Linné, 1758)
Plate 11.4, fig. 1
-
1758 Anomia squamula – Linné: p. 701.
-
1851 Anomia ephippium, Linnæus – Wood: p. 8–9, pl. 1, fig. 3a–d.
-
1878 Anomia ephippium, Lin. var. squamata – Sars: p. 14.
-
1934 Anómia (Heteranomia) squámula L. – Jensen & Spärck: p. 51–52, fig. 32.
-
1950 Anomia (Heteranomia) squamula (Linné, 1758) – Heering: p. 54–55.
-
2005 Heteranomia squamula (Linnaeus, 1758) – Sneli et al.: p. 137.
1. Ennucula tenuis (Montagu), right valve from the Stapavík Member. 2. Nuculana minuta (Müller), left valve from the Svarthamar Member. 3. Nuculana pernula (Müller), left valve from the Hörgi Formation. 4. Portlandia arctica (Gray), paired valves seen from the right side from the Hörgi Formation. 5. Portlandia arctica (Gray), paired valves showing the taxodont hinge in a cast from the Hörgi Formation. 6. Yoldiella lenticula (Møller), specimen with paired valves seen from the right side from the Hörgi Formation. 7. Yoldiella intermedia (Sars), shell with articulated valves from the Hörgi Formation. 8. Yoldiella frigida (Torell), specimen with paired valves from the Hörgi Formation. 9. Bathyarca cf. glacialis (Gray), fragmentary left valve from Hörgi Formation. 10. Mytilus edulis Linné, right valve from the Hörgi Formation. 11–12. Chlamys breidavikensis MacNeil, a dorsal part of a right valve from the Stapavík Member (from MacNeil, 1967) and a dorsal part of a left valve from Stapavík Member (from MacNeil, 1967). 13. Similipecten greenlandicus (Sowerby), damaged left valve from the Hörgi Formation
Material: Two disarticulated right valves and ?five left valves from the Fossgil Member and two single right valves and three left valves from the Svarthamar Member. Most of the shells collected by Vilhjálmsson (1985).
Remarks: No specimen with paired valves was found, only rather well-preserved disarticulated valves. The largest valves measure (l × h × b): 12.1 × 11.7 × 2.5 and 11.5 × 11.9 × 2.4 mm.
Recent distribution, ecology, and biology: Heteranomia squamula is mostly boreal species with lusitanian and possibly subarctic outposts. It has been found in the North Atlantic from the White Sea and Iceland south along the European coasts to the Bay of Biscay and the Mediterranean (Sneli et al., 2005; Tebble, 1966). In East North America, it extends from Labrador in the north to North Carolina in the south (Madsen, 1949). In the Baltic, it extends south to ?Øresund (Jensen & Spärck, 1934). Bathymetrical range: From 0 m in several places to almost 2000 m south of Vestmannaeyjar in South Iceland (Madsen, 1949). In the Faroe Islands, it has been found at depths from 66 m to 1006 m where the temperatures range from 6.0 to 8.6 °C (Sneli et al., 2005). The species is mesohaline with salinity tolerance down to about 15‰ (Funder et al., 2002). The larval development includes a pelagic stage (Jørgensen, 1946).
The species is epifaunal, and in the British Isles, it is widely distributed on stones, shells, and seaweeds, and in the Faroe Islands, it has been found on sand, gravel, and shells (Sneli et al., 2005; Tebble, 1966).
Fossil occurrence: Miocene: Gram Formation in Denmark (Schnetler, 2005). Pliocene: Coralline Crag Formation (Wood, 1851). Pliocene/Lower Pleistocene: Oosterhout Formation (Heering, 1950), Kattendijk and Lillo Formations (Marquet, 2002). Middle Pleistocene: Mikulin horizon (Merklin et al., 1979). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Miocene to Recent.
Heteranomia squamula aculeata (Müller, 1776)
Plate 11.4, fig. 2
-
1776 Anomia aculeata – Müller: p. 249.
-
1851 Anomia aculeata, Müller – Wood: p. 9–10, pl. 1, fig. 2a–b.
-
1878 Anomia aculeata – Sars: p. 15, pl. 19, Fig. 1a–c.
-
1949 Anomia squamula Linné forma aculeata O.F. Müller – F.J. Madsen: p. 41–42.
-
1952 Anomia squamula (L.) var. aculeata (Möller) – Óskarsson: p. 60–61, fig. 43.
-
1985 Pododesmus (Heteranomia) squamula (Linné, 1758) var. aculeata (Müller, 1776) – Vilhjálmsson: p. 76–77, pl. 10, figs. 1–3.
Material: Two disarticulated right valves and four left valves from the Fossgil Member and one single right valve and three left valves from the Svarthamar Member. All the shells were collected by Vilhjálmsson (1985).
Remarks: The largest three valves measure (l × h): 9.9 × 8.8, 8.2 × 8.2, and 7.8 × 8.7 mm. The h/l ratios are 0.89–1.17. The left valve is more solid and convex than the right one. This may explain why the left valves are considerably more numerous than the right valves in the sediments in Breiðavík. Furthermore, it indicates strongly a winnowed assemblage where the lighter valves were easier transported out of the community.
Recent distribution, ecology, and biology: As Heteranomia squamula aculeata has almost always been treated as a form belonging to H. squamula, we are not having separate informations on the distribution, ecology, and biology of this subspecies. However, in Iceland, it has mainly been found off the northern and eastern parts of the island (Óskarsson, 1952). The subspecies is clearly more arctic-subarctic than Heteranomia squamula, which is first and foremost boreal species with lusitanian and possibly subarctic outposts. H. squamula has been found in the North Atlantic from the White Sea and Iceland south along the European coasts to the Bay of Biscay and the Mediterranean (Sneli et al., 2005; Tebble, 1966). In East North America, it extends from Labrador in the north and to North Carolina in the south (Madsen, 1949). It extends into the Baltic south to ?Øresund (Jensen & Spärck, 1934). Bathymetrical range: From 0 in several places to almost 2000 m south of Vestmannaeyjar in South Iceland (Madsen, 1949). In the Faroe Islands, it has been found at depths from 66 m to 1006 m where the temperatures are from 6.0 ° to 8.6 °C (Sneli et al., 2005). The species is mesohaline with salinity tolerance down to about 15‰ (Funder et al., 2002). The larval development is with a pelagic stage (Jørgensen, 1946).
The species (Heteranomia squamula) belongs to the epifauna, and in the British Isles, it is widely distributed on stones, shells, and seaweeds, and in the Faroe Islands, it has been found on sand, gravel, and shells (Sneli et al., 2005; Tebble, 1966).
Fossil occurrence: Pliocene: Coralline Crag Formation (Wood, 1851), Mactra Zone in the Barmur Group (Símonarson & Eiríksson, 2020). Lower Pleistocene: Fossgil and Svarthamar Members (Eiríksson et al., 1992). Stratigraphical range: Pliocene to Recent.
On the other hand, the fossil occurrence of Heteranomia squamula is: Miocene to Recent.
-
Subclass Heterodonta Neumayr, 1884
-
Order Veneroida Adams & Adams, 1856
-
Family Astartidae d’Orbigny, 1844
-
Genus Tridonta Schumacher, 1817
Tridonta borealis Schumacher, 1817
Plate 11.4, fig. 3
-
1817 Tridonta borealis – Schumacher: p. 147, pl. 17, fig. 1.
-
1853 Astarte borealis, Chemnitz – Wood: p. 175–177, pl. 16, fig. 3a–b.
-
1878 Astarte borealis, Chemn. – Sars: p. 50, pl. 5, fig. 8.
-
1950 ?Astarte semisulcata (Leach, 1819) – Heering: p. 76–77, pl. 2, figs. 3–8.
-
1958 Astarte borealis (Chemnitz) – Ockelmann: p. 74–79.
-
1980 Tridonta borealis (Schumacher), 1817 – Gladenkov et al.: p. 40, pl. 4, figs. 24–27.
-
1985 Tridonta (Tridonta) borealis (Chemnitz, 1784) – Vilhjálmsson: p. 84–86, pl. 11, figs. 1–3.
Material: One external cast of a single right valve from the Furugerði Member, seven specimens with articulated valves, two single right valves and two left valves from the Hörgi Formation, three specimens with paired valves, three right valves and eight left valves from the Svarthamar Member, one single right valve from the Stapavík Member, and three valves from the Torfhóll Member of the Máná Formation.
Remarks: The three largest specimens with paired valves measure (l × h × b): 35.5 × 30.0 × 15.0, 31.0 × 27.4 × 14.4, and 29.5 × 26.0 × ?13.9 mm. The h/l ratios are 0.85–0.88, and the b/l ratios are 0.41–0.47. The specimens from the Hörgi Formation are partly dissolved and most of them somewhat compressed.
Recent distribution, ecology, and biology: Tridonta borealis is widespread in the arctic, subarctic, and the boreal regions of the North Atlantic. It is distributed from Franz Josef Land, Novaya Zemlya, the Kara Sea, the Siberian Arctic Sea, the Beaufort Sea, the Bering Strait, Parry Islands, and Ellesmere Island southward to the Baltic, Massachusetts, the Gulf of Alaska, and northern Japan (Ockelmann, 1958). It is very common in Iceland, but not known living from the Faroe Islands or the British Isles (Madsen, 1949; Sneli et al., 2005). In Norway, it has been found to Bergen in the south and the occurrence in the Baltic seems therefore isolated and may be a relic from colder times (Jensen & Spärck, 1934; Madsen, 1949). Bathymetrical range: From 0 m in East Finnmark to 463 m north of Svalbard, but empty shells have been found in the North Atlantic down to a depth of 2710 m (Ockelmann, 1958). In North Greenland, it was frequently found in Jørgen Brønlunds Fjord at depths between 6 and 16 m (Schiøtte, 1989). The species is mesohaline, and the salinity tolerance goes down to about 5‰ (Funder et al., 2002). This explains how frequently it has been found in the Baltic. The larval development is with a very short or lacking pelagic stage (Thorson, 1936).
The species is an infaunal suspension feeder living on a bottom varying from mud to rocks in the Arctic Macoma community, the Gomphina fluctuosa community, or even the Astarte crenata community in East Greenland (Ockelmann, 1958; Thorson, 1933; Thorsson, 1934). In Iceland, it has been recorded on a bottom of clay, sand, gravel, shells, or mixed substrates (Madsen, 1949).
Fossil occurrence: Pliocene: Serripes Zone of the Barmur Group (Gladenkov et al., 1980). Pliocene/Lower Pleistocene: Lodin Elv Formation (Feyling-Hanssen et al., 1983). Lower Pleistocene: Maassluis Formation in the Netherlands (Heering, 1950), Norwich Crag Formation, Pre-Pastonian (Funnell et al., 1979), Pattorfik beds (Símonarson, 1981), Kap København Formation (Símonarson et al., 1998), Lodin Elv Formation (Feyling-Hanssen et al., 1983), Île de France Formation (Bennike et al., 2002), Store Koldewey Formation (Bennike et al., 2010), Olkov and Tusatuva-Yamsk Suites (Petrov, 1982, 1986), Gubik Formation, Fishcreekian (Repenning et al., 1987). Middle Pleistocene: Pinakul, Kresta, Karagin, Kolvin, and Padymeiskii Suites (Merklin et al., 1962; Merklin et al., 1979; Petrov, 1982), Kotzebuan (Hopkins et al., 1972), Anvilian (Hopkins et al., 1974). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
Tridonta montagui (Dillwyn, 1817)
Plate 11.4, fig. 4
-
1817 Venus montagui – Dillwyn: p. 167.
-
1853 Astarte compressa, Montague – Wood: p. 183–184, pl. 16, fig. 8a–c.
-
1878 Nicania Banksii, Leach – Sars: p. 51–52, pl. 6, fig. 1a–b.
-
1950 Astarte montagui (Dillwyn, 1817) – Heering: p. 77–79, pl. 2, figs. 9–10, pl. 6, figs. 19–20.
-
1958 Astarte montagui (Dillwyn) – Ockelmann: p. 80–85.
-
1980 ?Astarte montagui (Dillwyn, 1817) – Gladenkov et al.: p. 37–38, pl. 4, figs. 18–23.
-
1985 Tridonta (Nicania) montagui (Dillwyn, 1817) – Vilhjálmsson: p. 87–88, pl. 11, fig. 5A–B.
Material: Six specimens with paired valves, four right and four left valves from the Hörgi Formation, one single valve from the Svarthamar Member, and one specimen with articulated valves from the Torfhóll Member.
Remarks: The specimens from the Hörgi Formation are rather badly preserved, fragmented, and compressed, and difficult to measure. The three largest specimens with paired valves measure (l × h): 14.4 × 13.1, 13.8 × 12.8, and 12.5 × 11.9 mm. The h/l ratios seem to be 0.88–0.95.
Recent distribution, ecology, and biology: Tridonta montagui is a circumpolar species distributed in the arctic, subarctic, and boreal regions of the North Atlantic. It occurs from Franz Josef Land, Novaya Zemlya, the Kara Sea, the Siberian Arctic Sea, the Beaufort Sea, and Ellesmere Island southward to the British Isles, Denmark, the western Baltic, Massachusetts, the Aleutians, and northern Japan (Bernard, 1979; Ockelmann, 1958). Bathymetrical range: The species prefers shoreface areas and has been found from 0 m in the western Baltic (Ockelmann, 1958) to 455 m in the Beaufort Sea (Bernard, 1979). In North Greenland, it is rather common in the Jørgen Brønlund Fjord at depths between 6 and 16 m (Schiøtte, 1989). It is a mesohaline species with a salinity tolerance down to 15‰ (Funder et al., 2002). The larval development is with a very short pelagic stage or it is entirely lacking (Thorson, 1936).
The species is a suspension feeder, and in East Greenland, it lives on a bottom varying from mud to gravel and rocks in the Arctic Macoma community as well as in the Gomphina fluctuosa and Astarte crenata communities (Ockelmann, 1958; Thorson, 1933, 1934).
Fossil occurrence: Pliocene: Serripes Zone of the Barmur Group (Gladenkov et al., 1980). Pliocene/Lower Pleistocene: Red Crag Formation (Wood, 1853), Tugidak Formation (Allison, 1978). Lower Pleistocene: Maassluis Formation (Heering, 1950), Yakataga Formation (Allison, 1978), Pre-Pastonian (Funnell et al., 1979), Norwich Crag Formation (West et al., 1980), Pattorfik beds (Símonarson, 1981), Kap København Formation (Símonarson et al., 1998), Île de France Formation (Bennike et al., 2002), Store Koldewey Formation (Bennike et al., 2010), Olkov and Tusatuva-Yamsk Suites (Petrov, 1982). Middle Pleistocene: Pinakul, Kresta, Karagin, Kolvin, and Padymeiskii Suites (Merklin et al., 1962; Merklin et al., 1979; Petrov, 1982), Kotzebuan (Hopkins et al., 1972). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
Tridonta elliptica (Brown, 1827)
Plate 11.4, fig. 5.
-
1827 Crassina elliptica – Brown: pl. 18, fig. 3.
-
1853 Astarte elliptica, Brown – Wood: p. 181–182, pl. 16, fig. 7.
-
1878 Astarte compressa, Lin. – Sars: p. 53–54.
-
1950 Astarte sulcata (da Costa) var. elliptica (Brown, 1827) – Heering: p. 71–72, pl. 4, figs. 11–13, pl. 5, fig. 1.
-
1958 Astarte elliptica (Brown) – Ockelmann: p. 86–89.
-
1980 Tridonta cf. elliptica (Brown, 1827) – Gladenkov et al.: p. 41, pl. 12, fig. 26.
-
1980 Astarte aff. sulcata (da Costa, 1778) – Gladenkov et al.: p. 39–40, pl. 4, figs. 13–17a.
-
1985 Tridonta (Tridonta) elliptica (Brown, 1827) – Vilhjálmsson: p. 86–87, pl. 11, fig. 4A–B.
Material: Eighteen specimens with united valves, 7 right valves and 15 left valves from the Hörgi Formation, and 1 single left valve from the Svarthamar Member.
Remarks: The specimens from the Hörgi Formation are rather poorly preserved, fragmented, and compressed, and difficult to measure. The three largest specimens with articulated valves measure (l × h): 20.2 × 14.8, 18.3 × 13.5, and 16.5 × 12.8 mm. The h/l ratios seem to be 0.72–0.78, which is considerably lower than for the measured specimens in the Barmur Group (0.87–0.93).
Recent distribution, ecology, and biology: Tridonta elliptica is distributed in the arctic, subarctic, and boreal regions of the North Atlantic. It extends from Franz Josef Land, Svalbard, Novaya Zemlya, the Kara Sea, the Smith Sund, and Baffin Bay southward to the British Isles and Massachusetts Bay (Lubinsky, 1980; Ockelmann, 1958; Sneli et al., 2005). It is known from Kattegat and Øresund and the western Baltic southward to Bornholm (Jensen & Spärck, 1934; Sneli et al., 2005). It is very common all around Iceland (Madsen, 1949; Óskarsson, 1952). Bathymetrical range: The species lives offshore from 2 m in East Greenland to 442 m in West Greenland (Ockelmann, 1958). It is a mesohaline species with salinity tolerance down to about 15% (Funder et al., 2002). The pelagic larval stage is very short or lacking (Thorson, 1936).
In East Greenland, T. elliptica belongs to the infaunal Arctic Macoma community where it lives as suspension feeder on substrates of mud, silt, and sand (Ockelmann, 1958). In Iceland, it is also common on a bottom of mixed substrates (Madsen, 1949).
Fossil occurrence: Pliocene: Serripes Zone of the Barmur Group (Símonarson & Eiríksson, 2020) Pliocene/Lower Pleistocene: Tugidak Formation (Allison, 1978). Lower Pleistocene: Norwich Crag Formation (Funnell et al., 1979), Yakataga Formation, Middleton Island (Allison, 1978). Pattorfik beds (Símonarson, 1981), Kap København Formation (Símonarson et al., 1998). Middle Pleistocene: Kolvin and Padymeiskii Suites (Merklin et al., 1979; Petrov, 1982), Pre-Cape Christian beds (Andrews et al., 1981). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
-
Family Arcticidae Newton, 1891
-
Genus Arctica Schumacher, 1817
Arctica islandica (Linné, 1767)
Plate 11.6, fig. 6
-
1767 Venus islandica – Linné: p. 1131.
-
1853 Cyprina Islandica. Linn. – Wood: p. 196–197, pl. 18, fig. 2a–e.
-
1878 Cyprina islandica, Lin. – Sars: p. 50.
-
1950 Cyprina islandica (Linné, 1767) – Heering: p. 91–93, pl. 11, figs. 23–26.
-
1980 Arctica islandica (Linné, 1767) – Gladenkov et al.: p. 52, pl. 6, figs. 1–10.
-
1985 Arctica islandica (Linné, 1767) – Vilhjálmsson: p. 97–99, pl. 13, figs. 4–5.
Material: There are several hundreds, even thousands of specimens with articulated valves in the Svarthamar Member, as well as the Torfhóll Member. Furthermore, there are many disarticulated right and left valves in both members. Apparently, there is no significant difference between the number of united and single valves or between single right and left valves. One left valve has been found in the Stapavík Member.
Remarks: The five largest specimens with united valves measure (l × h): 67.7 × 58.9, 42.0 × 36.0, 26.0 × 23.0, 21.0 × 18.7, and 18.0 × 15.2 mm. The h/l ratios are 0.84–0.89 (10 specimens were measured). The shells are generally fragmentary, but often with the fragments in situ, which indicates low degree of transport. This is further supported by the similar numbers of right and left valves as well as the high number of articulated valves in the sediments of the Svarthamar and Torfhóll Members.
Recent distribution, ecology, and biology: Arctica islandica has been found living in the subarctic, boreal, and lusitanian regions of the North Atlantic (cf. Símonarson & Eiríksson, 2020: Fig. 17). It is amphiatlantic and extending from Newfoundland, the southeastern Barents Sea, and the Spitsbergen Bank in the north to North Carolina and the Gulf of Cádiz in the south (Funder & Weidick, 1991; Madsen, 1949; Zenkevitsch, 1963). The warm North Atlantic Current seems to control its occurrence on the Spitsbergen Bank, and in the Barents Sea, the species inhabits areas with summer temperatures above 6 °C (Funder & Weidick, 1991; Zenkevitsch, 1963). The species is absent from Greenland waters today, but it has lived in West Greenland during milder Holocene time from c. 7500 to slightly after 5000 BP (Funder, 1989; Kelly, 1986). Bathymetrical range: From 0 m in the British Isles (Tebble, 1966) to 2000 m west of Ireland (Madsen, 1949). It prefers foreshore areas, and in Iceland, it is most abundant at depths between 10 and 90 m (Eiríksson, 1988). It is a mesohaline species with salinity tolerance down to about 5‰ (Funder et al., 2002). The planktotrophic larvae have a rather long pelagic stage of great significance for its distribution (Jørgensen, 1946).
In the British Isles, the species lives on a rather firm bottom of sand and muddy sand from the lower parts of the intertidal zone to considerable depths (Tebble, 1966). In Iceland, it lives mainly on a sandy bottom from a depth of 3–5 m to about 100 m within the Spisula elliptica community (Madsen, 1949; Spärck, 1937). In Iceland, this suspension feeder also prefers depths just below the low-water marks; in the winter, it burrows shallow in the bottom, but in the summer, it is lying in abundance on the surface of the bottom (Eiríksson, 1988; Madsen, 1949). Experiment has shown that under normal salinity conditions and at temperatures 7–8 °C, it takes the species 8–10 min to reach an upright position in a muddy sand and further 15–20 min to attain life position (in situ) (cf. Vilhjálmsson, 1985).
Fossil occurrence: ?Oligocene: Borszony in Hungary (Malatesta & Zarlenga, 1986). Miocene: Lenham beds (Harmer, 1900), Edegem, Kiel, Atwerpen, Zonderschot, and Breda Formation (Herman & Marquet, 2007). Miocene/Pliocene: Acroperna sericea-Chlamys tigerinus Zone of the Netherlands (Spaink, 1975). Pliocene: Coralline Crag Formation (Harmer, 1900; Wood, 1853), Kallo and Doel sections (Marquet, 2002). Pliocene/Lower Pleistocene: Red Crag Formation (Wood, 1853). Lower Pleistocene: Ludhamnian (Norton, 1967), Serripes groenlandicus-Yoldia lanceolata Zone of the Netherlands (Spaink, 1975), Baventian (West et al., 1980), Santernian, Emilian, and Sicilian (Malatesta & Zarlenga, 1986), Yagataga Formation on Middleton Island (Allison, 1978), Kap København Formation (Símonarson et al., 1998), Île de France Formation (Bennike et al., 2002). Middle Pleistocene: Kolvin and Padymeiskii Suites (Merklin et al., 1979; Petrov, 1982). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: ?Oligocene to Recent.
-
Family Thyasiridae Dall, 1901
-
Genus Axinopsida Keen & Chavan, 1951
Axinopsida orbiculata (Sars, 1878)
Plate 11.4, fig. 7
-
1878 Axinopsis orbiculata – Sars: p. 63–64, pl. 19, fig. 11a–d.
-
1934 Axinópsis orbiculáta G.O. Sars – Jensen & Spärck: p. 88–89, fig. 69.
-
1949 Axinopsis orbiculata G.G. Sars – F.J. Madsen: p. 55–56.
-
1958 Axinopsis orbiculata G.O. Sars – Ockelmann: p. 111–113, pl. 2, figs. 7–8.
-
1979 Axinopsida orbiculata (G. Sars, 1878) – Bernard: p. 32–33., fig. 49.
-
1985 Axinopsida orbiculata (Sars, 1878) – Vilhjálmsson: p. 77–78, pl. 110, fig. 4A–B.
Material: Two specimens with articulated valves from the Hörgi Formation, one specimen with paired valves and three left valves from the Svarthamar Member, and one right valve from the Stapavík Member.
Remarks: The three largest specimens measure (l × h × b): 3.3 × 3.5 × 1.4, 3.1 × 3.1 × 1.4, and 2.9 × 2.9 × ?1.3 mm. The h/l ratios are close to 1.0, and the b/l ratios are 0.42–0.50. The specimens are rather fragmented and partially dissolved.
Recent distribution, ecology, and biology: Axinopsida orbiculata is known from the arctic, subarctic, and the northern part of the boreal region in the North Atlantic. It extends from West and East Greenland, Svalbard, Novaya Zemlya, the Kara Sea, the Siberian Arctic Sea, the Beaufort Sea, and the Canadian Arctic Archipelago in the north, southward to the west coast of Norway at 67.5°N, north of the Hebrides, and north of Cape Cod (Bernard, 1979; Høisæter, 1986; Lubinsky, 1980; Ockelmann, 1958). The species has not been found south of the Bering Strait or in the Pacific (Bernard, 1979). Bathymetrical range: From 2–3 m several places in the Arctic to 944 m north off the Hebrides (Ockelmann, 1958). It seems to prefer shoreface areas and is polyhaline with salinity tolerance above 25‰ (Funder et al., 2002). The larval development is with a very short or entirely lacking pelagic stage (Ockelmann, 1958).
In East Greenland, this suspension feeder prefers substrates of clay or mud mixed with sand or even pure sand in the Arctic Macoma community, the Gomphina fluctuosa community, as well as the Yoldia hyperborea community (Ockelmann, 1958).
Fossil occurrence: ?Pliocene/Pleistocene: Tugidak Formation (Allison, 1978: as A. cf. A. orbiculata). Lower Pleistocene: Pattorfik beds (Símonarson, 1981), Kap København Formation (Símonarson et al., 1998), Hörgi Formation, Svarthamar Member, and Stapavík Member (Vilhjálmsson, 1985). Upper Pleistocene: Baltic Eemian (Funder et al., 2002), Portlandia arctica Zone in the Skærumhede sequence in Vendsyssel, Denmark (Nordmann in Jessen et al., 1910). Stratigraphical range: ?Pliocene to Recent.
There are records from Lower Pleistocene, as well as Middle Pleistocene sediments in the Pacific area, but according to Bernard (1979), they should probably be referred to Axinopsida viridis (Dall).
-
Genus Thyasira Leach, 1818
Thyasira cf. sarsii (Philippi, 1845)
Plate 11.4, fig. 8
-
1845 Axinus Sarsii – Philippi: p. 91.
-
1878 Axinus Sarsii, Phil. – Sars: p. 60, pl. 19, fig. 5a–b.
-
1934 Thyasíra sársi Philippi – Jensen & Spärck: p. 91, fig. 71.
-
1953 Thyasira sarsii Philippi, 1845 – La Rocque: p. 57.
-
1959 Thyasira flexuosa (Montagu, 1803) Var. sarsi Philippi – MacGinitie: p. 171–172, pl. 4, fig. 12.
-
1985 Thyasira sarsi (Philippi, 1836) – Vilhjálmsson: p. 79–81, pl. 10, fig. 5.
Material: One disarticulated right valve and one left valve from the Fossgil Member, one left valve from the Svarthamar Member. The valves were all collected by Vilhjálmsson (1985).
Remarks: The only measurable valve measures (l × h): 4.0 × 4.1 mm. It was not possible to measure the breadth of the specimen. The valves are rather badly preserved, fragmented, and partially dissolved.
The shell is rather circular with an indistinct radial groove in the posterior end, but the hinge is not visible in any of the valves. Therefore the species identification is difficult, but with some hesitation we refer to it as Thyasira sarsii, which is generally regarded as a variety of Thyasira flexuosa (Montagu).
Recent distribution, ecology, and biology: Thyasira sarsii is mainly living in the subarctic and boreal regions of the North Atlantic, probably with some arctic outposts. The distributions of Thyasira sarsii, T. gouldii (Philippi), and T. equalis (Verrill & Bush) are not well known because they have generally been regarded as varieties of T. flexuosa (Montagu). The latter seems distributed from northern Norway at 62°N and south to the Mediterranean and Morocco (Høisæter, 1986; Sneli et al., 2005). According to Ockelmann (1958), the Icelandic records are doubtful, and the species is not known from the Baltic. It prefers offshore areas in the British Isles and depths between 11 and 183 m (Tebble, 1966). It is mesohaline with salinity tolerance down to 15‰ (Funder et al., 2002). The larval development has a very short pelagic stage, or it is entirely lacking (Ockelmann, 1958).
Thyasira sarsii is apparently slightly more northern in distribution than T. flexuosa. In the North Atlantic, it most probably extends from Novaya Zemlya and Iceland in the north and south along the entire Norwegian west coast to Kattegat (Høisæter, 1986; Vilhjálmsson, 1985). Sneli et al. (2005) did not record it from the Faroe Islands where T. flexuosa as well as T. gouldii are rather common. In Iceland, T. sarsii is most frequently found off the west and northwest coasts (Óskarsson, 1952). Bernard (1979) did not mention it from the Beaufort Sea, whereas MacGinitie (1959) found some specimens off Point Barrow in northern Alaska. Lubinsky (1980) did not include it in the fauna of Arctic Canada, but LaRocque (1953) listed it from the Cape Cod area.
In East Greenland, the Thyasira species are infaunal suspension feeders burrowing rather deep into soft sediments as mud, clay, sand, and even gravel (Ockelmann, 1958).
Fossil occurrence: Lower Pleistocene: Fossgil and Svarthamar Members (Eiríksson et al., 1992), but Thyasira gouldii has also been found in the Lower Pleistocene Kap København Formation (Símonarson et al., 1998). Stratigraphical range: Lower Pleistocene to Recent.
Schnetler (2005) has recorded Thyasira flexuosa from the Miocene Gram Formation in Denmark and Wood (1851) from the Pliocene Coralline Crag in England (as Cryptodon sinuosum (Donovan)). It has obviously longer-lasting geological history than T. sarsii, but it cannot be excluded that some fossil specimens referred to as T. flexuosa actually belong to T. sarsii.
-
Genus Mysella Angas, 1877
Mysella bidentata (Montagu, 1803)
Plate 11.4, figs. 9–10
-
1803 Mya bidentata – Montagu: p. 44–45.
-
1878 Montacuta bidentata, Mont. – Sars: p. 69, pl. 19, fig. 17a–b.
-
1934 Montacúta (Mysélla) bidentáta (Mtg.) – Jensen & Spärck: p. 100–101, fig. 81.
-
1966 Mysella bidentata (Montagu) – Tebble: p. 91–92, fig. 44A–C.
-
1985 Mysella bidentata (Montagu, 1803) – Vilhjálmsson: p. 82–84, pl. 10, figs. 6–7.
Material: Thirty-seven specimens with united valves, and 42 disarticulated right valves and 41 left valves from the Fossgil Member, 46 specimens with articulated valves, 17 single right valves and 20 left valves from the Svarthamar Member, and 44 specimens with paired valves, 5 right valves and 6 left valves from the Torfhóll Member. The specimens were all collected by Vilhjálmsson (1985).
Remarks: The three largest specimens with articulated valves measure (l × h × b): 2.2 × 1.8 × 1.0, 2.1 × 1.8 × 1.0, and 2.1 × 1.8 × 1.0 mm. The h/l ratios are 0.80–0.89, and the b/h ratios are 0.45–0.53 (based on 10 measurements).
We are aware of that Gofas and Salas (2008) referred Mysella bidentata to a new genus Kurtiella. However, we have not seen the necessity, consequently we use the name Mysella as have most other malacologists done for years.
Recent distribution, ecology, and biology. Mysella bidentata is well known in the a subarctic, boreal, and lusitanian regions of the North Atlantic. It extends from Iceland and western Norway at 70°N in the north, and south to Kattegat, Øresund and the Belt Sea, the Mediterranean and the Black Sea, Madeira, the Azores, and Portuguese Guinea on the northwestern African coast (Høisæter, 1986; Ockelmann, 1958; Ockelmann & Muus, 1978; Sneli et al., 2005; Tebble, 1966). The species is not known from the Russian or Siberian Seas, the Pacific, or the Canadian Arctic. Bathymetrical range: From the intertidal zone in the British Isles to 600 m in Skagerrak (Jensen & Spärck, 1934; Tebble, 1966) or 2500 m (Poppe & Goto, 1993). The species is mesohaline with salinity tolerance down to about 15‰ (Funder et al., 2002). The larvae are pelagic for about 4 weeks (Ockelmann & Muus, 1978).
In the British Isles, the species lives epifaunal as a suspension feeder generally attached to other epifaunal animals on muddy sand and gravel, even in the burrows in older oysters shells, and in association with ophiuroids (Tebble, 1966).
Fossil occurrence: Pliocene: Coralline Crag Formation (Wood, 1851), Kallo and Doel sections in Belgium (Marquet, 2005). Pliocene/Lower Pleistocene: Red Crag Formation (Wood, 1851). Lower Pleistocene: Fossgil and Svarthamar Member, Torfhóll Member (Eiríksson et al., 1992). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
-
Family Carditidae Lamarck, 1809
-
Genus Ciliatocardium Kafanov, 1974
Ciliatocardium ciliatum (Fabricius, 1780)
Plate 11.4, fig. 11
-
1780 Cardium ciliatum – Fabricius: p. 410.
-
1878 Cardium ciliatum, Fabr. – Sars: p. 46, pl. 5, fig. 4a–b.
-
1958 Cardium (Clinocardium) ciliatum Fabricius – Ockelmann: p. 118–121.
-
1980 Clinocardium ciliatum (Fabricius, 1780) – Lubinsky: p. 38–39, pl. 8, fig. 1.
-
1980 Clinocardium ciliatum (Fabricius, 1780) – Gladenkov et al.: p. 43, pl. 8, figs. 1–3.
-
1985 Clinocardium ciliatum (Fabricius, 1780) – Vilhjálmsson: p. 89–91, pl. 12, fig. 1–2.
Material: Nine specimens with united valves, five disarticulated right valves, and six left valves from the Stapavík Member, and five specimens with articulated valves, five single right valves, and seven left valves from the Torfhóll Member.
Remarks: The three largest specimens with paired valves measure (l × h): 49.7 × 46.8, 35.0 × 32.1, and 10.0 × 9.2 mm. The h/l ratios are 0.92–0.94. The specimens are rather badly preserved, somewhat dissolved, and fragmented.
Recent distribution, ecology, and biology: Ciliatocardium ciliatum is probably circumpolar, living in the arctic and subarctic regions of the North Atlantic and extends into the northern part of the boreal region. From Northeast Greenland, Svalbard, Franz Josef Land, the Barent Sea, Novaya Zemlya, the Kara Sea, and the Siberian Arctic Sea, it is distributed southward to South Iceland, East Finnmark, and northern Japan (Ockelmann, 1958). In North America, it is known from the Beaufort Sea, the Canadian Arctic Archipelago, and Baffin Bay south to Cape Cod and the Gulf of Alaska (Bernard, 1979; Lubinsky, 1980). Bathymetrical range: From 0 m in Iceland (Óskarsson, 1952) to 677 m in West Greenland (Ockelmann, 1958). In Iceland where it attains largest size at the north and east coasts, it is distributed all around the country at moderate depths (Madsen, 1949). The species is mesohaline with salinity tolerance down to about 15‰ (Funder et al., 2002). The larval development is with a very short or lacking pelagic stage (Thorson, 1936).
In Iceland, C. ciliatum is mainly found on muddy bottom in the infaunal Yoldia hyperborea community in the fiords of East Iceland at depths between 45 and 160 m (Spärck, 1937). The species is a suspension feeder, and in East Greenland, it prefers more clayey bottom than Serripes groenlandicus (cf. Ockelmann, 1958).
Fossil occurrence: Pliocene: Serripes Zone of the Barmur Group (Gladenkov et al., 1980). Lower Pleistocene: Pattorfik beds (Símonarson, 1981), Kap København Formation (Símonarson et al., 1998), Lodin Elv Formation (Feyling-Hanssen et al., 1983), Île de France Formation (Bennike et al., 2002), Store Koldewey Formation (Bennike et al., 2010), Olkov and Tusatuva-Yamsk Suites (Petrov, 1982), Gubik Formation, Fishcreekian (Repenning et al., 1987). Middle Pleistocene: Pinakul, Kolvin, Kresta, Karagin, and Padymeiskii Suites (Merklin et al., 1962; Merklin et al., 1979; Petrov, 1982), Kotzebuan (Hopkins et al., 1972), Pre-Cape Christian beds (Andrews et al., 1981). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
-
Genus Serripes Gould, 1841
Serripes groenlandicus (Mohr, 1786)
Plate 11.4, fig. 12
-
1786 Cardium grönlandicum – Mohr: p. 129.
-
1853 Cardium groenlandicum, Chemnitz – Wood: p. 160–161, pl. 13, fig. 1a–d.
-
1878 Aphrodite grønlandica, Chemn. – Sars: p. 49, pl. 5, fig. 3a–b.
-
1950 Serripes groenlandicus (Bruguière, 1758) – Heering: p. 108–109, pl. 11, figs. 13–16.
-
1958 Serripes groenlandicus (Bruguière) – Ockelmann: p. 113–118.
-
1980 Serripes groenlandicus (Bruguière, 1789) – Lubinsky: p. 39, pl. 8, fig. 2.
-
1980 Serripes groenlandicus (Bruguiere, 1789) – Gladenkov et al.: p. 44–45, pl. 8, figs. 4–8.
-
1985 Serripes groenlandicus (Chemnitz, 1782) – Vilhjálmsson: p. 91–93, pl. 12, figs. 3–4.
Material: Two specimens with united valves from the Hörgi Formation, 10 specimens with paired valves, 11 single right valves, and 9 left valves from the Fossgil Member, 3 specimens with articulated valves, 9 right valves, and 4 left valves from the Svarthamar Member. Five specimens with united valves, 13 single right valves, and 11 left valves from the Stapavík Member, and 4 specimens with paired valves, 8 disarticulated right valves, and 5 left valves from the Torfhóll Member. Several umbonal fragments were also found especially in the Svarthamar Member.
Remarks: The three largest specimens with paired valves measure (l × h): 66.0 × 54.0, 33.0 × 28.3, and 25.0 × 20.5 mm. The h/l ratios are 0.82–0.87 or slightly higher than those found for the same species in the Barmur Group. The specimens are generally rather poorly preserved, and the thin shells are often fragmentary.
Recent distribution, ecology, and biology: Serripes groenlandicus is apparently circumpolar and well known in the arctic and subarctic regions of the North Atlantic, and it extends into the northern part of the boreal region slightly farther to the south than Ciliatocardium ciliatum. It is widespread from Northeast Greenland, Svalbard, Franz Josef Land, the Barent Sea, Novaya Zemlya, the Kara Sea, and the Siberian Arctic Sea southward to South Iceland, Finnmark, and Japan (Ockelmann, 1958). In North America, it is known from the Beaufort Sea, the Canadian Arctic Archipelago, and Baffin Bay south to Cape Cod and Oregon (Bernard, 1979; Lubinsky, 1980). Bathymetrical range: From 0 m in Iceland (Madsen, 1949) to 303 m in West Greenland (Ockelmann, 1958). In Iceland, it is mainly found living in West, North, and East Iceland at depths from 0 to about 120 m (Madsen, 1949). The species is mesohaline with salinity tolerance down to about 15‰ (Funder et al., 2002). The larval development is supposed to be planktotrophic with a pelagic stage (Ockelmann, 1958; Thorson, 1936).
In Iceland, this suspension feeder has been found on a bottom of mud, sand, and gravel in the infaunal Arctic Macoma community (Madsen, 1949; Spärck, 1937). In East Greenland, it prefers more sandy and less clayey substrates than Ciliatocardium ciliatum (cf. Ockelmann, 1958).
Fossil occurrence: Pliocene: Diemerbrug Well, Vreeburg-Utrecht Well, and Goes Well in the Netherlands (Heering, 1950). Pliocene/Lower Pleistocene: Red Crag Formation (Harmer, 1925; Wood, 1853), Omma-Manganji Fauna (Chinzei, 1978). Lower Pleistocene: Maassluis Formation of the Netherlands (Spaink, 1975), Baventian (West et al., 1980), Pattorfik beds (Símonarson, 1981), Kap København Formation (Símonarson et al., 1998), Île de France Formation (Bennike et al., 2002), Store Koldewey Formation (Bennike et al., 2010), Olkov and Tusatuva-Yamsk Suites (Petrov, 1982), Gubik Formation, Fishcreekian (Repenning et al., 1987). Middle Pleistocene: Pinakul, Kolvin, Kresta, Karagin, and Padymeiskii Suites (Merklin et al., 1962; Merklin et al., 1979; Petrov, 1982), Kotzebuan (Hopkins et al., 1972), Anvilian (Hopkins et al., 1974), Pre-Cape Christian beds (Andrews et al., 1981). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
-
Family Mactridae Lamarck, 1809
-
Genus Spisula Gray, 1837
Spisula elliptica ( Brown, 1827 )
Plate 11.5, fig. 1
-
1827 Mactra elliptica – Brown: pl. 15, fig. 6.
-
1857 Mactra ovalis, J. Sowerby – Wood: p. 246–247, pl. 23, fig. 1a–d.
-
1878 Mactra elliptica, Brown – Sars: p. 72.
-
1934 Spísula sólida (L.) var. ellíptica Brown – Jensen & Spärck: p. 160–161, fig. 146.
-
1949 Spisula solida (Linné) f. elliptica Brown – F.J. Madsen: p. 68–69.
-
1950 Spisula elliptica (Brown, 1827) – Heering: p. 144–145, pl. 17, figs. 5–8.
-
1985 Spisula (Spisula) elliptica (Brown, 1827) – Vilhjálmsson: p. 93–94, pl. 12, fig. 5A–B.
-
1986 Spisula (Spisula) elliptica (Brown, 1827) – Malatesta & Zarlenga: p. 105–106, fig. 17.
1. Heteranomia squamula (Linné), left valve from the Fossgil Member 2. Heteranomia squamula aculeata (Müller), left valve from the Fossgil Member. 3. Tridonta borealis Schumacher, left valve from the Svarthamar Member. 4. Tridonta montagui (Dillwyn), left valve from the Svarthamar Member. 5. Tridonta elliptica (Brown), left valve from the Hörgi Formation. 6. Arctica islandica (Linné), right valve from the Svarthamar Member. 7. Axinopsida orbiculata (Sars), right valve from the Svarthamar Member. 8. Thyasira cf. sarsii (Philippi), right valve from the Svarthamar Member. 9. Mysella bidentata (Montagu), left valve from the Svarthamar Member. 10. Mysella bidentata (Montagu), fragmented valve with heterodont hinge from the Torfhóll Member. 11. Ciliatocardium ciliatum (Fabricius), ?left fragmented valve from the Stapavík Member. 12. Serripes groenlandicus (Mohr), left valve from the Svarthamar Member
Material: One specimen with articulated valves, two right valves, and six left valves from the Svarthamar Member. All specimens were collected by Vilhjálmsson (1985).
Remarks: The three largest valves measure (l × h): 19.7 × 13.0, 15.3 × 10.0, and 15.0 × 10.0 mm. The h/l ratios are 0.65–0.68, and therefore the valves are slightly more elongated than those of Spisula solida (Linné, 1758), which has h/l ratios almost always over 0.75 and a thicker shell.
Recent distribution, ecology, and biology: Spisula elliptica is mainly a boreal species. In the North Atlantic, it extends from the Barents Sea and all around Iceland in the north southward to Kattegat and the Belt Sea, the British Isles, the English Channel, and Ireland south to the Biscaye Bay (Tebble, 1966; Sneli et al., 2005). A single valves has been recorded from Jan Mayen, but according to Ockelmann (1958), it may be subfossil or has been subject to transport. Obviously it did not penetrate in the Baltic, but the occurrence in the Bay of Biscay indicates lusitanian outposts (Malatesta & Zarlenga, 1986). The records from the Atlantic coasts of the Iberian Peninsula as well as the Mediterranean are most probably based on fossil or subfossil specimens leading back to the last glaciation (Malatesta & Zarlenga, 1986). The species is unknown from the Pacific. Bathymetrical range: From the upper zones of the littoral off the Norwegian coasts or 10–872 m in the Faroe Islands (Malatesta & Zarlenga, 1986; Sneli et al., 2005). The species has salinity tolerance down to about 15‰ (Funder et al., 2002). Probably it has a pelagic larval development (Malatesta & Zarlenga, 1986).
In the British Isles, this suspension feeder prefers substrates of sand and muddy gravel (Tebble, 1966). In Iceland, it has often been found in stomachs of fishes, especially the haddock (Madsen, 1949).
Fossil occurrence: Pliocene: Coralline Crag Formation (Wood, 1857). Pliocene-Pleistocene: Red Crag Formation (Wood, 1857). Lower Pleistocene: Norwich Crag Formation and Maassaluis Formation of the Netherlands (Heering, 1950), the Emilian at Barcelona and the Sicilian at Montescaglioso in Sicily (Malatesta & Zarlenga, 1986), Svarthamar Member (Eiríksson et al., 1992). Middle Pleistocene: Padymeiskii Suite and Makulin Horizon (Merklin et al., 1979). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
-
Family Tellinidae de Blainville, 1814
-
Genus Macoma Leach, 1819
Macoma calcarea (Gmelin, 1791)
Plate 11.5, fig. 2
-
1791 Tellina calcarea – Gmelin in Linné: p. 3236.
-
1857 Tellina lata, Gmelin – Wood: p. 228–229, pl. 21, fig. 6a–d.
-
1878 Macoma calcaria, Chemn. – Sars: p. 76, pl. 6, fig. 2a–b.
-
1950 Macoma (M.) calcarea (Gmelin, 1790) – Heering: p. 170–171, pl. 15, figs. 15–16.
-
1958 Macoma calcaria (Chemnitz) – Ockelmann: p. 125–128, pl. 2, fig. 10.
-
1980 Macoma calcarea (Gmelin, 1790) – Gladenkov et al.: p. 47–48, pl. 9, figs. 1–2 and 5–10.
-
1985 Macoma (Macoma) calcarea (Chemnitz, 1782) – Vilhjálmsson: p. 94–97, pl. 13, figs. 1–3.
Material: Twenty-five specimens with articulated valves, 1 single right valve, and 2 left valves from the Hörgi Formation, 303 specimens with paired valves, 31 disarticulated right valves, and 29 left valves from the Fossgil Member, 103 specimens with united valves, 80 single right valves, and 84 left valves from the Svarthamar Member, 45 specimens with paired valves, 17 disarticulated right valves, and 10 left valves from the Stapavík Member, and 76 specimens with articulated valves, 22 single right valves, and 36 left valves from the Torfhóll Member. All those specimens were collected and counted by Vilhjálmsson (1985). However, it should be borne in mind that especially in the sediments in the Fossgil and Svarthamar Members, there are probably thousands of specimens of Macoma calcarea. Disarticulated valves are rare in the Hörgi sediments, probably deposited in more quiet environments than the sediments in the younger formations.
Remarks: The largest valve measures (l × h × b): 40.0 × 30.1 × 6.2 mm. The h/l ratios were calculated for 30 specimens, and they are 0.68–0.75. The b/l ratios are difficult to measure exactly because both specimens with paired valves as well as single valves are generally somewhat compressed, but apparently they are close to 0.15 for valves or 0.30 for specimens with paired valve. In spite of thin and fragile valves, the shells are not particularly fragmented.
Recent distribution, ecology, and biology: Macoma calcarea is circumpolar and widely distributed in the arctic, subarctic, and boreal regions of the North Atlantic and also well known from the North Pacific. It extends from Northeast Greenland, Svalbard, Franz Josef Land, the Barent Sea, Novaya Zemlya, the Kara Sea, and the Siberian Arctic Sea southward to the Faroe Islands, New York, Oregon, and ?northern Japan (Heering, 1950; Madsen, 1949; Ockelmann, 1958; Petersen, 1968). The occurrence in the Oslofiord, Kattegat, and the Baltic is probably isolated and has been considered a relic by C.G.J. Petersen (1888) and Jensen (1905). In North America, it is known living in the Beaufort Sea, the Canadian Arctic Archipelago, Baffin Bay, Hudson Bay, and the Labrador Sea to New York or even Washington D.C. area in the south (Bernard, 1979; Lubinsky, 1980). In West Greenland, it has been found living to Iita in the north (Thorson, 1951), and in North Greenland it is common in Jørgen Brønlund Fjord at depths between 2 and 16 m (Schiøtte, 1989). Bathymetrical range: From 0 m in Iceland (Madsen, 1949) to 677 m in West Greenland (Ockelmann, 1958). M. calcarea is a mesohaline species with salinity tolerance down to 5‰ (Funder et al., 2002). The larval development is with a pelagic stage (Ockelmann, 1958; Thorson, 1936).
The species is one of the most characteristic animals of the infaunal Arctic Macoma community (Thorson, 1957). In Iceland and East Greenland, this deposit feeder lives mainly in the fiords in rather shallow water on a bottom of mud and fine sand sometimes mixed up with gravel and stones (Ockelmann, 1958; Spärck, 1937).
Fossil occurrence: Pliocene: Sagavannirktok Formation, Middle Nuwok beds, and Gubik Formation (MacNeil, 1957). Pliocene/Lower Pleistocene: Red Crag Formation (Wood, 1857). Lower Pleistocene: Norwich Crag Formation (Norton, 1967), Maassluis Formation of the Netherlands (Spaink, 1975), Norwich Crag Formation (West et al., 1980), Pattorfik beds (Símonarson, 1981), Kap København Formation (Símonarson et al., 1998), Store Koldewey Formation (Bennike et al., 2010), Olkhov and Tusatuva-Yamsk Suites (Petrov, 1982). Middle Pleistocene: Kotzebuan (Hopkins et al., 1972), Pinakul, Karagin, Kolvin, Kresta, and Padymeiskii Suites (Merklin et al., 1979; Petrov, 1982), Pre-Cape Christian beds (Andrews et al., 1981). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
-
Order Myoida Stoliczka, 1879
-
Family Myidae Lamarck, 1809, 1814
-
Genus Mya Linné, 1758
Mya truncata Linné, 1758
Plate 11.5, fig. 3
-
1758 Mya truncata – Linné: p. 670.
-
1857 Mya truncata, Linnæus – Wood: p. 277–279, pl. 23, fig. 1a–f.
-
1878 Mya truncata, Lin. – Sars: p. 92.
-
1958 Mya truncata Linné – Ockelmann: p. 144–149.
-
1965 Mya (Mya) truncata Linné – MacNeil: p. G38–G40, pl. 8, figs. 1–12, pl. 9, figs. ?1–3, 5–20.
-
1972 Mya (Mya) truncata truncata Linné, 1758 – Strauch: p. 140–141, pl. 10, figs. 5, 7–8, pl. 11, fig. 7.
-
1980 Mya truncata Linné, 1758 – Gladenkov et al.: p. 54, pl. 11, figs. 10–11.
-
1985 Mya (Mya) truncata Linné, 1758 – Vilhjálmsson: p. 100–103, pl. 14, figs. 1, 6, pl. 16, fig. 2.
Material: One internal cast without shell material from the Furugerði Member, 4 specimens with articulated valves and 1 single left valve from the Hörgi Formation, 35 specimens with united valves, 10 disarticulated right valves, and 7 left valves from the Fossgil Member, 44 specimens with paired valves, 27 single right valves, and 21 left valves from the Svarthamar Member, 3 specimens with paired valves, 4 disarticulated right valves, and 7 left valves from the Stapavík Member and 11 specimens with articulated valves from the Torfhóll Member.
Remarks: The three largest specimens measure (l × h × b): 54.1 × 41.9 × 27.2, 49.0 × 38.2 × 22.9, and 45.1 × 33.5 × 22.2 mm. The h/l ratios are 0.63–0.78, and the b/l ratios are 0.44–0.50. Generally the specimens are partly dissolved and fragmented, but often with the fragments in situ, indicative of postdepositional compaction.
No juvenile specimens have been found in the Breiðavík deposits, but they have an ovate posterior end like Mya arenaria Linné, 1758. It is not until the animal begins to burrow into the bottom sediments that the posterior end becomes truncate. Generally this starts when the shell has reached a length of about 20 mm.
Recent distribution, ecology, and biology: Mya truncata is a well-known circumpolar species occurring in the arctic, subarctic, and boreal regions in the North Atlantic, but with some isolated lusitanian occurrences. It extends from West and East Greenland, Svalbard, Franz Josef Land, Novaya Zemlya, the Barents Sea, the Kara Sea, the Laptev Sea, the Siberian Arctic Sea, the Beaufort Sea, and the Canadian Arctic Archipelago in the north to the western Baltic (to Øresund), the English Channel, the Bay of Biscay, Massachusetts, Hakodadi in Japan, and Port Orchard in Washington in the south (Bernard, 1979; Lubinsky, 1980; Ockelmann, 1958; Sneli et al., 2005). In North Greenland, it has been found living in Jørgen Brønlund Fjord at depths between 2 and 40 m (Schiøtte, 1989). Bathymetrical range: It prefers shoreface areas, but range from 0 m at several localities down to 625 m in West Greenland (Ockelmann, 1958). It is mesohaline with salinity tolerance down to 5‰ (Funder et al., 2002). The larval development is planktotrophic with a long-lasting pelagic stage (Jørgensen, 1946; Thorson, 1936).
The species is a suspension feeder, and in East Greenland, the young animals are found attached to the algal epifauna in large numbers, while the adults burrows in the sediments in the infaunal Arctic Macoma community (Ockelmann, 1958). In the British Isles, it prefers substrates of clay, muddy sand, or sand (Tebble, 1966).
Fossil occurrence: Miocene: Takinoue Formation, Yakataga Formation (MacNeil, 1965). Pliocene: Serripes Zone of the Barmur Group (Símonarson & Eiríksson, 2020), Coos Conglomerate, Towsley Formation, Quillayute Formation, Beaufort Formation, Meighen Island (Fyles et al., 1991; MacNeil, 1965). Lower Pleistocene: Emilian and Sicilian (Malatesta & Zarlenga, 1986), Norwich Crag Formation (West et al., 1980), Furuvík, Hörgi, Threngingar, and Máná Formations (Eiríksson, 1981), Pattorfik beds (Símonarson, 1981), Kap København Formation (Símonarson et al., 1998), Lodin Elv Formation (Feyling-Hanssen et al., 1983), Île de France Formation (Bennike et al., 2002), Store Koldewey Formation (Bennike et al., 2010), Olkov and Tusatuva-Yamsk Suites (Petrov, 1982), Pico Formation (MacNeil, 1965), Gubik Formation, Fischcreekian (Repenning et al., 1987). Middle Pleistocene: Kotzebuan (Hopkins et al., 1972), Anvilian (Hopkins et al., 1974), Karagin, Pinakul, Kresta, Kolvin, and Padymeiskii Suites (Merklin et al., 1979; Petrov, 1982). Upper Pleistocene: Baltic Eemian (Funder et al., 2002), Portlandia arctica Zone in the Skærumhede sequence in Vendsyssel, Denmark (Nordmann, in Jessen et al., 1910). Stratigraphical range: Miocene to Recent.
Mya truncata is very frequently found in Pleistocene faunal assemblages in the North Atlantic area and is one of the dominating species in the Breiðavik Group sediments. On the other hand, we have only found one single valve in the Barmur Group, which seems to be the only known occurrence known so far in Pliocene deposits in the North Atlantic area.
Mya truncata pseudoarenaria Schlesch, 1931
Plate 11.5, fig. 4
-
1931 Mya pseudoarenaria – Schlesch: p. 136, pl. 13, figs. 10–12.
-
1900 Mya truncata L. forma ovata – Jensen: p. 142, figs. 3–7.
-
1958 Mya truncata Linné f. ovata Jensen – Ockelmann: p. 148.
-
1965 Mya (Mya) pseudoarenaria Schlesch – MacNeil: p. G37–G38, pl. 7, figs. 9–11, 13–14, pl. 9, fig. ?4.
-
1980 Mya pseudoarenaria Schlesch, 1931 – Lubinsky: p. 45. 1980.
-
1980 Mya pseudoarenaria Schlesch, 1931 – Gladenkov et al.: p. 55–56, pl. 11, figs. 6–9 (in part, the specimens from the Breiðavík deposits).
-
1985 Mya (Mya) pseudoarenaria Schlesch, 1931 – Vilhjálmsson: p. 104, pl. 14, figs. 2–5.
Material: Two specimens with articulated valves, one disarticulated right valve, and five left valves from the Svarthamar Member, one specimen with paired valves, one disarticulated right valve, and one left valve from the Stapavík Member, and one single right and left valve from the Torfhóll Member. All the specimens were collected by Vilhjálmsson (1985).
Remarks: The largest specimen is about 81 mm long, but the shells are more or less fragmented and partly dissolved. One ?measurable specimen is 10 mm in length and the height seems to be 5.8 mm, and therefore the h/l ratio appears to be about 0.58, which is distinctly lower than found in the specimens of Mya truncata from the Breiðavík sediments.
Jensen (1900) was the first to realize the existence of an arctic form of Mya distinct from both Mya truncata and M. arenaria Linné. Externally, this form most closely resembles M. arenaria as it has similar ovate posterior end. Therefore, he named this form ovata, but unfortunately the name is preoccupied by Donovan (1802) and therefore Schlesch (1931) proposed the name pseudoarenaria to denote the Mya arenaria-like outline of the shell. Internally, this form is closer to M. truncata with similar trigonal chondrophore although it is in outline more right angled (rather than equilateral) and also the pallial sinus seems proportional deeper (larger) in the pseudoarenaria form. Furthermore, MacGinitie (1959) stressed that in living pseudoarenaria specimens, collected of Point Barrow (Alaska), the sheath that extends onto the siphon is more like that of M. arenaria than M. truncata.
The synonym list shows clearly that many authors have regarded the pseudoarenaria form as a distinct species, but Jensen (1900), Laursen (1944, 1950, 1966), Madsen (1949), Feyling-Hanssen (1955), and Ockelmann (1958) did not regard it to be more than an infraspecies. Ockelmann (1958) pointed out that all grades of transitional stages between the pseudoarenaria form and M. truncata exist. Therefore, until further evidence is given, we will take the middle course and regard this ovate form as a subspecies. Obviously this form has kept the juvenile form of the predecessor when it evolved from the M. truncata probably by proterogenese during the transition of the Pliocene and Pleistocene, while the latter migrated from the Pacific to the North Atlantic.
Recent distribution, ecology, and biology: Mya truncata pseudoarenaria is known from the arctic and subarctic regions of the North Atlantic, with a few documented boreal occurrences. It extends from West and East Greenland, Svalbard, Franz Josef Land, Novaya Zemlya, the Kara Sea, the Siberian Arctic Sea, the Beaufort Sea, and the Canadian Arctic Archipelago in the north to western Norway at 67°N, Iceland, Hudson Bay, and the northern Bering Sea in the south (Bernard, 1979; Høisæter, 1986; Lubinsky, 1980; Strauch, 1972). Schlesch (1931), MacNeil (1965), and Strauch (1972) pointed out that the subspecies has most probably retreated from the entire northwest Atlantic, but it has clearly more northern occurrence than typical M. truncata. Bathymetrical range: Soot-Ryan (1951) reported it from northern Norway at depths between 25 and 50 m and Coan et al. (2000) from 2 to 30 or 50 m in the Arctic (cf. Bernard, 1983). The subspecies is probably more mesohaline than the typical M. truncata, and in western Norway, it is often found in localities with low salinity (Soot-Ryen, 1951). The larval development is probably with pelagic stage as in M. truncata.
The subspecies seems to live in a similar way as Mya truncata, i.e., juvenile specimens have byssus threads and lives as epifaunal suspension feeder attached to algae, sediment grains, and other objects on the bottom (Soot-Ryen, 1951). The adults are infaunal suspension feeders burrowing in the sediments in the Arctic Macoma community (Ockelmann, 1958).
Fossil occurrence: Lower Pleistocene: Pattorfik beds (Símonarson, 1981), Svarthamar Member (Eiríksson, 1981). Middle Pleistocene: Olkov Suite (Petrov, 1982), Padymeiskii Suite and Mikulin Horizon (Merklin et al., 1979). Stratigraphical range: Lower Pleistocene to Recent.
Mya truncata pseudoarenaria reached Iceland later than M. truncata, when the sediments in the Svarthamar Member were deposited about 1.5 Ma ago (Símonarson & Eiríksson, 2020: Fig. 7.19). The other subspecies, Mya truncata uddevalensis Forbes, 1846, is not known for certain in the Breiðavík sediments as suggested by Símonarson and Leifsdóttir (2009). They compared the outline of M. truncata uddevalensis with the specimen in the Breiðavík Group in the paper of Gladenkov et al. (1980: pl. 11, fig. 11). Even though they are somewhat similar, the uddevalensis form most probably reached Iceland after the deposition of the Lower Pleistocene part of the Breiðavík Group (cf. Símonarson & Eiríksson, 2020: fig. 7.19), as already mentioned by MacNeil.
-
Family Hiatellidae Gray, 1824
-
Genus Hiatella Bosc, 1801
Hiatella rugosa (Linné, 1767)
Plate 11.5, fig. 5
-
1767 Mytilus rugosus – Linné: p. 1156 .
-
1857 Saxicava rugosa, Pennant – Wood: p. 285–287, pl. 29, fig. 3a–g.
-
1878 Saxicava pholadis, Lin. – Sars: p. 95, pl. 20, fig. 7a–c.
-
1958 Hiatella arctica (Linné) – Ockelmann: p. 135–142 (in part).
-
1980 Hiatella arctica (Linné, 1767) – Gladenkov et al.: p. 57, pl. 12, figs. 14–18 (in part).
-
1985 Hiatella (Hiatella) arctica (Linné, 1767) – Vilhjálmsson: p. 108–110, pl. 15, fig. 2.
-
1986 Hiatella gallicana (Lamarck, 1818) – Høisæter: p. 126.
-
2005 Hiatella rugosa Linnaeus, 1767 – Sneli et al.: p. 154.
Material: One internal cast from the Furugerði Member, one specimen with articulated valves from the Hörgi Formation, and six specimens with paired valves, four disarticulated right valves, and four left valves from the Svarthamar Member.
Remarks: The two largest specimens measure (l × h): 37.1 × ?19.3 and 35.0 × 11.6 mm, respectively. The h/l ratios are rather variable from 0.50 to 0.68. The shells from the Breiðavík deposits are considerably larger than the Hiatella shells from the Pliocene Barmur Group.
Studies of bivalve larvae in the North Atlantic have shown that at least two different larval forms of Hiatella do exist here (cf. Símonarson & Eiríksson, 2020: Fig. 7.23). The two forms are a more rounded larva referred to as H. rugosa and a more triangular one referred to as H. arctica (Thorson, 1951). They have both been observed from the Mediterranean and northward to Iceland, but only the rounded H. rugosa type is with certainty known from the arctic region. Thus, Thorson (1936) found the rounded type as the only Hiatella larva in the East Greenland plankton, and Sullivan (1948) found it also as the only Hiatella larva in Malpeque Bay, East Canada. Regarding the existence of these different larval forms as evidence of different species of Hiatella, their distribution indicates strongly that H. arctica is a more southerly form in spite of the species name (cf. Thorson, 1951). Some authors have stressed that only H. rugosa is a borer, but Hunter (1949) has shown that the character of the larvae does not determine the boring or non-boring habit of the adult because that is determined by the nature of the substratum upon which settlement takes place. Some malacologists (cf. Poppe & Goto, 1993) have pointed out that H. arctica is, at least as juvenile, attached by byssal threads to all kinds of substrates, while H. rugosa has no byssus at all. The shell of H. arctica is more equivalve, thinner, and less chalky, and generally smaller than the shell of H. rugosa. The spines on the surface of the juvenile shells disappear when the animal burrows into the substrates. However, it should be borne in mind that it is far from easy to separate the two species when they mature because of an extreme convergence in form, and it is impossible to identify the larval forms in fossil material.
Specimens of Hiatella from Greenland were referred to H. byssifera by Petersen (1978). However, as he based his arguments on different parasites and life strategy, it is hardly possible to use his methods on a fossil material.
Recent distribution, ecology, and biology: Hiatella rugosa extends far into the arctic region in the North Atlantic where it has been found in Northeast Greenland, Svalbard, Franz Josef Land, the Barent Sea, Novaya Zemlya, the Kara Sea, and the Siberian Arctic Sea, the Beaufort Sea, and the Canadian Arctic Archipelago, while H. arctica has its northern limit in northern Iceland, northern Norway, and ?Alaska (MacGinitie, 1959; Símonarson, 2004; Thorson, 1951). The latter is distributed farther south in the Atlantic to North Angola and the West Indies and in the Pacific to northern Japan, Panama, and Hawaii, whereas H. rugosa reaches Morocco, but the southern limit on the east coast of North America is unknown, and apparently it is not living in the Pacific (Bernard, 1979; Poppe & Goto, 1993; Strauch, 1968). It occurs in the Mediterranean (Poppe & Goto, 1993), and it extends into the Baltic southward to Øresund and the Kiel area (Jensen & Spärck, 1934). The geographical distribution indicates strongly that H. rugosa is circumpolar, but the occurrences of H. arctica seems too discontinuous for a circumpolar species. However, it must be pointed out that it is difficult to give the exact geographical distribution, as well as bathymetrical range for each species because they are treated together as one species in most papers.
The geographical distribution indicates that Hiatella arctica generally prefers higher sea temperature than H. rugosa, which is more arctic in spite of the specific name. In the Faroe Islands, H. arctica prefers water temperatures above 7.0 °C, whereas H. rugosa has been found living at 6.8 °C (Sneli et al., 2005). However, Strauch (1968) has pointed out that there is a close relationship between sea temperatures and the shell length of H. arctica and suggested that it can be used to estimate paleotemperatures. According to him, there is only one species of Hiatella in the Atlantic as well as the Pacific and he did not accept that we are dealing with two species with different geographical distribution and preferring different sea temperatures. His method has been discussed, and Rowland and Hopkins (1971) stressed that in the Pacific, the length is governed by mode of life of each population and not only the sea temperature. Therefore, this method should be used with care as an exact paleothermometer although it is often a good indicator (Símonarson, 2004).
The bathymetrical range may be similar as in the case of Hiatella arctica, from the littoral zone or 0 m in several localities down to 2190 m west of Ireland, ?alive (Ockelmann, 1958). However, it is difficult to give the exact bathymetrical range for H. rugosa as the two species are treated together as one species in most papers. Petersen (1968) did not separate the two species in the Faroe Islands and found them in fiords as well as offshore in shallow water to a depth of 300 m or even 1006 m (Sneli et al., 2005). H. rugosa is probably mesohaline as H. arctica with salinity tolerance down to 5‰ (Funder et al., 2002). The larval development has most probably a prolonged pelagic stage as described by Thorson (1936, 1951). That explains the almost cosmopolitan distribution of the species (Barash & Danin, 1987; Poppe & Goto, 1993).
In the British Isles, Hiatella arctica is living as a nestler, attached by its byssus in a hole or rock crevice or it is living as a borer in rather soft sediments or rocks (Tebble, 1966). Probably H. rugosa lives in similar way. Some authors have stated that H. rugosa is a borer, but H. arctica never bores. As mentioned before, this is most probably not the case.
Fossil occurrence: Pliocene: Coralline Crag Formation (Wood, 1857). Pliocene/Lower Pleistocene: Red Crag Formation (Wood, 1857). Lower Pleistocene: Furuvík and Hörgi Formations (Eiríksson, 1981; Vilhjálmsson, 1985), Pattorfik beds (Símonarson, 1981), Kap København Formation (Símonarson et al., 1998), Lodin Elv Formation (Feyling-Hanssen et al., 1983), Île de France Formation (Bennike et al., 2002), Store Koldewey Formation (Bennike et al., 2010). Middle Pleistocene: Kotzebuan (Hopkins et al., 1972), Kresta Suite (Petrov, 1966). Upper Pleistocene: Baltic Eemian (Funder et al., 2002), Portlandia arctica Zone in the Skærumhede sequence in Vendsyssel, Denmark (Nordmann in Jessen et al., 1910). Stratigraphical range: Pliocene to Holocene.
According to Gordillo (2001) Hiatella rugosa might have evolved from H. vera (Deshayes), which characterized the Eocene Anglo-Paris Basin. Following this interpretation, the species must be considered of Atlantic origin, and apparently it never reached the Pacific (cf. Grant & Gale, 1931).
-
Genus Cyrtodaria Reuss, 1801
Cyrtodaria angusta (Nyst & Westendorp, 1893)
Plate 11.5, fig. 6
-
1893 Glycimeris angusta – Nyst & Westendorp: p. 396, pl. 4, fig. 1.
-
1857 Glycimeris angusta, Nyst & Westendorp – Wood: p. 291, pl. 29, fig. 2a–d.
-
1925 Cyrtodaria siliqua, Spengler – Bárðarson: p. 101–102, 105.
-
1972 Cyrtodaria angusta (Nyst & Westerndorph, 1839) – Strauch: p. 93–95, pl. 8, figs. 1–20.
-
1972 Cyrtodaria jenisseae Sachs 1953 – Strauch: p: 92–93, pl. 7, fig. 9.
-
1980 Cyrtodaria angusta (Nyst et Westendorp, 1839) – Gladenkov et al.: p. 58, pl. 10, figs. 17–21, pl. 21, figs. 1–5.
-
1985 Cyrtodaria angusta (Nyst & Westendorp, 1893) – Vilhjálmsson: p. 106–107, pl. 14, fig. 7, pl. 15, fig. 1.
Material: Twenty-nine specimens with articulated valves, 12 disarticulated right valves, and 8 left valves from the Svarthamar Member and 4 specimens with paired valves, 4 single right valves, and 2 left valves from the Torfhóll Member.
Remarks: The two largest specimens with united valves measure (l × h): 60.1 × 30.7 and 57.0 × 27.9 mm. The h/l ratios are 0.40–0.58 for 18 measurable specimens. Vilhjálmsson (1985) has shown that the valves from the Breiðavík deposits have lowest h/l ratios when the shell is about 30 mm long, after that it becomes less elongated.
The majority of valves is articulated in the sediment, but only a few are in the position of life. Often, the left and the right valves are lying side by side after disarticulation. This indicates a restricted postmortal transport and reworking before sedimentation and parautochthonous type of a death assemblage rather than biocoenosis. Burrowing species as Cyrtodaria angusta are more often found with articulated valves and occasionally in life position.
Strauch (1972) referred Cyrtodaria from the Breiðavík sediments to Cyrtodaria jenisseae Sachs, a species considered to be intermediate in shell form between the extinct C. angusta (Nyst & Westendorp, 1893) and the recent C. siliqua (Spengler). We are of the opinion, as many authors today, that the differences between C. angusta and C. jenisseae are intraspecific and therefore we refer these specimens to C. angusta in accordance with Gladenkov et al. (1980). It is worth mentioning that Strauch (1972: p. 107) was of the opinion that the youngest specimens of C. angusta from the Barmur Group are so similar to C. jenesseae, and it is almost impossible to see any differences (“die sich nicht mehr scharf von jenisseae trennen lassen”).
Distribution, ecology, and biology: The distribution of sediments with Cyrtodaria angusta indicates strongly that the species preferred conditions as now prevailing in the boreal region of the North Atlantic. It was apparently distributed from Iceland in the north to South England in the south. The bathymetrical range is not known but almost everywhere it has been found in sediments deposited in rather shallow water close to the coasts. The co-occurrence with Lentidium complanatum here and there in the Barmur Group indicates a mesohaline species, but the salinity tolerance is unknown, possibly it was down to 15‰. The species may have tolerated depressed salinities, but its presence in full-marine faunas in the southern North Sea implies that it was essentially a marine species. Apparently, the larval development had a pelagic stage as frequently found in boreal bivalves today.
The species was most probably a suspension feeder that lived infaunal in sandy bottom. The burrowing depth of C. angusta is unknown, whereas the recent and closely related infaunal species C. siliqua prefers a bottom of fine-grained sand, but its burrowing depth is only a few centimeters as the siphons are rather short (Nesis, 1965). Perhaps it could descend several tens of centimeters from the bottom surface if disturbed as has been observed in the sword razor (Ensis ensis) of similar elongated form.
Fossil occurrence: Miocene: Edegem, Kiel, Atwerpen, Zonderschot, and Deume Units in Belgium (Herman & Marquet, 2007). Pliocene: Coralline Crag Formation (Wood, 1857), Vreeburg-Utrect Well in the Netherlands (Heering, 1950). Pliocene/Lower Pleistocene: Red Crag Formation (Wood, 1857). Lower Pleistocene: Svarthamar Member (Eiríksson, 1981; Vilhjálmsson, 1985). Middle Pleistocene: Russian Arctic, from Kolgujew Island to Khatanga River (Nesis, 1965). Stratigraphical range: Miocene to Middle Pleistocene.
-
Subclass Anomalodesmata Dall, 1889
-
Order Pholadomyoida Newell, 1965
-
Family Thraciidae Stoliczka, 1870
-
Genus Thracia Sowerby, 1823
Thracia cf. septentrionalis Jeffreys, 1872
Plate 11.5, fig. 7
-
1872 Thracia septentrionalis – Jeffreys: p. 238.
-
1941 Thracia (Crassithracia) septentrionalis Jeffreys – Soot-Ryen: p. 19–22, pl. 1, figs. 9–10.
-
1949 Thracia septentrionalis Jeffreys – F.J. Madsen: p. 83.
-
1958 Thracia (Crassithracia) septentrionalis Jeffreys – Ockelmann: p. 144–149.
-
1980 Thracia septentrionalis Jeffreys, 1872 – Lubinsky: p. 49.
-
1985 Thracia cf. Thracia (Crassithracia) septentrionalis Jeffreys, 1872 – Vilhjálmsson: p. 111–112, pl. 15, fig. 4.
Material: One specimen with articulated valves from the Hörgi Formation.
Remarks: The specimen is slightly compressed, but it seems to measure (l × h): 3.0 × 2.6 mm.
The posterior end of the shell is slightly truncate, while the anterior end is oval and the umbo is situated just behind the midline. The shell is ornamented by fine growth lines. Because of the imperfect state of preservation, a safe identification is difficult, but the specimen can best be referred to Thracia septentrionalis.
Recent distribution, ecology, and biology: Thracia septentrionalis is an arctic-subarctic species in the Northwest Atlantic, but with boreal occurrence. It extends from West and East Greenland, Svalbard, Smith Sound, and Jones Sound in the north to Iceland and New England in the south (Lubinsky, 1980; Ockelmann, 1958). Neither Sars (1878) nor Høisæter (1986) did mention the species from Norway, and Ockelmann (1958) stated that it does not occur in Eurasian seas. Bathymetrical range: From 9 m in East Greenland to 113 m in West Greenland (Ockelmann, 1958). The species is probably polyhaline or even euhaline with salinity tolerance above 25‰ or even 30‰ as most of the recent species of Thracia (cf. Funder et al., 2002). That is in a good agreement with the distribution in East Greenland, where it seems restricted to the outer coast (Ockelmann, 1958). The larval development is with a very short pelagic stage or it is entirely suppressed (Ockelmann, 1958).
In East Greenland, the species is scattered along the outer coast where it prefers sandy substrates at depths between 1–9.5 m and 20 m (Ockelmann, 1958).
Fossil occurrence: Lower Pleistocene: Hörgi Formation (Vilhjálmsson, 1985). We have not found any records from Middle or Upper Pleistocene deposits of this rare species. Stratigraphical range: Lower Pleistocene to Recent.
-
Family Lynonsiidae Fisher, 1887
-
Genus Lyonsia Turton, 1822
Lyonsia cf. arenosa (Møller, 1842)
Plate 11.5, fig. 8
-
1842 Pandorina arenosa – Møller: p. 93.
-
1878 Lyonsia arenosa, Möll. – Sars: p. 81.
-
1958 Lyonsia arenosa (Møller) – Ockelmann: p. 149–151.
-
1979 Lyonsia (Lyonsia) arenosa (Møller, 1842) – Bernard: p. 58–59, figs. 98–100.
-
1985 Lyonsia sp. – Vilhjálmsson: p. 110–111, pl. 15, fig. 3.
-
2000 Lyonsia arenosa (Möller, 1842) – Coan et al.: p. 525, pl. 113.
Material: One fragmentary specimen with paired valves from the Hörgi Formation.
Remarks: The fragment is about 31 mm long and 22 mm in height. Apparently the intact shell was considerably larger than the recent Icelandic specimens of Lyonsia, i.e., L. arenosa and L. norwegica (Gmelin, 1791). The shell is somewhat dissolved and slightly compressed.
Only the posterior part of the specimen is preserved. The posterior dorsal margin is straight and so is the truncate posterior edge, while the ventral margin is curved. The sculpture consists of irregular concentric growth lines or striae and fine almost invisible radial threads. Exact species identification is hardly possible because of the badly preserved material. However, from this brief description of the posterior end of the specimen, it is most tempting to refer it to Lyonsia arenosa.
Recent distribution, ecology, and biology: Lyonsia arenosa is circumpolar and known from the arctic and the subarctic regions of the North Atlantic, but with several boreal occurrence records as well. It extends from West and East Greenland, Svalbard, Novaya Zemlya, the Kara Sea, the Laptev Sea, the Siberian Arctic Sea, the Beaufort Sea, and the Canadian Arctic Archipelago in the north to North and East Iceland, western Norway at 69.5°N, Gulf of St. Lawrence, Nova Scotia, Cape Ann (Massachusetts), northern Japan, and Kodiak Island in the south (Bernard, 1979; Ockelmann, 1958). Bernard (1979) has not seen any specimens south of the northern part of the Gulf of Alaska. Bathymetrical range: From 3 m in Novaya Zemlya to 200 m in West Greenland (Ockelmann, 1958). It is rather common in eastern Iceland, where it lives at depths between 10 and 100 m (Óskarsson, 1964). In East Greenland, the species is common in the inner parts of the large fiords as well as in places near the open sea. The fiord water layer in East Greenland is a mixture of Polar Current water and fresh water from rivers and melting ice and therefore the salinity is somewhat reduced, generally between 25‰ and 30‰, but it may be must lower in the uppermost parts of the fiords (Ockelmann, 1958). The larvae have no pelagic stage or it is very short (Ockelmann, 1958; Thorson, 1936).
In East Greenland, this suspension feeder has been found on various substrates, i.e., mud, clay, sand, gravel, and even on stony bottom (Ockelmann, 1958).
Fossil occurrence: Lower Pleistocene: Hörgi Formation (this chapter), Pattorfik beds (Símonarson, 1981). Middle Pleistocene: Kresta Suite (Petrov, 1966). Upper Pleistocene: Portlandia arctica Zone in the Skærumhede sequence in Vendsyssel (Nordmann, in Jessen et al., 1910). Stratigraphical range: Lower Pleistocene to Recent.
11.4 Arthropoda
-
Class Crustacea Pennant, 1777
-
Subclass Cirripedia Burmeister, 1834
-
Order Thoracica Darwin, 1854
-
Family Verrucidae Darwin, 1854
-
Genus Verruca Schumacher, 1817
Verruca stroemia (Müller, 1776)
Plate 11.5, fig. 9
-
1776 Lepas stroemia – Müller: p. 251.
-
1933 Vérruca stroemia (O. Fr. Müller) Schumacher – Stephensen: p. 115, fig. 40,1.
-
1938 Verruca stroemia (O. Fr. Müller) Schumacher – Stephensen: p. 3–4.
-
1955 Verruca stroemia (Müller, 1776) – Feyling-Hanssen: p. 171, pl. 26, figs. 15–16.
-
1985 Verruca (Verruca) stroemia (Müller, 1776) – Vilhjálmsson: p. 114, pl. 15, fig. 6.
Material: Three parietal plates from the Hörgi Formation.
Remarks: We have no intact wall rings to measure, but in Denmark, the shell is up to about 10 mm in diameter and as the height is lower it has a rather flat appearance.
Recent distribution, ecology, and biology: Verruca stroemi has been found from the more southern parts of the arctic faunal region in the North Atlantic and south into the lusitanian region. It extends from Svalbard, the Murman Coast, and the White Sea in the north to the Mediterranean in the south (Stephensen, 1933, 1938). In Danish water, it seems to live eastward to Bohuslän and most likely it does not occur in Greenland today (Stephensen, 1933, 1938). Bathymetrical range: It prefers shoreface areas, but extends from the littoral zone and down to a depth of about 200 m (Funder et al., 2002; Stephensen, 1933). It is a mesohaline species with salinity tolerance down to 15‰ (Funder et al., 2002). The larval development is not well known, but most probably it is similar as in Balanus balanus.
The species is a sessil suspension feeder, generally attached to living or death shells on the bottom. In Iceland, it has also been found on the roots of colonies of red algae (Stephensen, 1938).
Fossil occurrence: Pliocene: Coralline Crag Formation (Collins et al., 2014). Pliocene/Lower Pleistocene: Red Crag Formation (Collins et al., 2014). Lower Pleistocene: Hörgi Formation (Vilhjálmsson, 1985). Upper Pleistocene: Baltic Eemian (Funder et al., 2002). Stratigraphical range: Pliocene to Recent.
The species is rather rare and there seems to be very few (?if any) records from Middle Pleistocene deposits.
-
Family Balanidae Leach, 1817
-
Genus Balanus da Costa 1778
Balanus balanus (Linné, 1758)
Plate 11.5, figs. 10–11
-
1758 Lepas balanus – Linné: p. 667.
-
1933 Bálanus (Eubálanus) bálanus (Linné) Da Costa – Stephensen: p. 119–120, fig. 41.
-
1938 Balanus balanus (Linné) da Costa (=B. porcatus aut.) – Stephensen: p. 4–5.
-
1968 Balanus (Balanus) balanus (Linnaeus) – Zullo: p. 6–9, figs. 11–12.
-
1981 Balanus (Balanus) balanus (Linné, 1758) – Símonarson: p. 86–87, pl. 7, figs. 1–3.
-
1985 Balanus (Balanus) balanus (Linné, 1758) – Vilhjálmsson: p. 115–116, pl. 15, fig. 7, pl. 16, fig. 1A–B.
Material: A few fragments of parietal plates from the Hörgi Formation, and 68 complete wall rings (shells) and 73 separated parietal plates from the Svarthamar Member, 18 wall rings from the Stapavík Member, and 133 wall rings, 62 parietal plates, 4 scuta, and 6 terga from the Torfhóll Member. Most of this material was collected by Vilhjálmsson (1985).
Remarks: The occurrence in the Svarthamar Member is more or less concentrated on the upper part of a 20 cm large drop stone. The specimens from the Torfhóll and Stapavík Members were almost exclusively found attached to larger shells or shell fragments.
Recent distribution, ecology, and biology: Balanus balanus is an arctic-subarctic-boreal species with circumpolar distribution. It extends from about 82°N in West and East Greenland, the Canadian Arctic, Svalbard, Franz Josef Land, and the seas north of Russia and Siberia in the north to the English Channel, Long Island Sound, Kurile Islands, and Puget Sound in the south (Feyling-Hanssen, 1955; Stephensen, 1933, 1936, 1938, 1943; Wagner, 1970; Zullo, 1968). Bathymetrical range: From the littoral zone in Iceland or ?1 m in the Faroe Islands to about 300 m in the southernmost part of its area of distribution (Pilsbry, 1916; Stephensen, 1933; Vilhjálmsson, 1985). In Denmark, it occurs from the coast and down to a depth of 37 m (Stephensen, 1933). Poulsen (1935) pointed out that salinity above 14–15‰ seems to determine the occurrence in inner Danish waters, and according to Funder et al. (2002), it seems mesohaline with salinity tolerance down to 5‰. The cirripeds have internal fertilization and does not shed sperms or eggs into the water for subsequent fertilization (Schäfer, 1972). Then the larvae have a pelagic stage lasting up to about 1 month before settlement, which generally takes place near the adults (cf. Kaestner, 1967).
The species is a sessil epifaunal suspension feeder and seems to prefer places with high water turbulence (Rasmussen, 1973). Generally, it lives in clusters, cemented to stones, rocks or shells, where it is elevated above the bottom to avoid to be buried in sediment.
Fossil occurrence: Pliocene/Lower Pleistocene: Red Crag Formation (Collins et al., 2014). Lower Pleistocene: Norwich Crag Formation (Collins et al., 2014), Pattorfik beds (Símonarson, 1981). Middle Pleistocene: Cromer Forest Bed (Collins et al., 2014). Upper Pleistocene: Baltic Eemian (Funder et al., 2002), Lateglacial beds on Seltjarnarnes, Southwest Iceland (Eiríksson et al., 2004). Stratigraphical range: ?Pliocene to Recent.
There are numerous Tertiary reports of Balanus balanus from Miocene and Pliocene sediments in Europe, but according to Zullo (1968), almost all of these identifications are incorrect.
11.5 Review of the Breiðavík Fauna
In the present chapter, 45 species of marine molluscs from the Breiðavík Group sediments have been treated, i.e., 14 species of prosobranch gastropods, 2 opisthobranch gastropod species, and 29 species of bivalves. Furthermore, two species of barnacle are dealt with. Other groups as annelids, foraminifera, ostracods, bryozoans, echinoderms, dinoflagellates, trace fossils, as well as birds, are not treated in this publication. However, it should be mentioned that Knudsen, Eiríksson and Símonarson (2020) has studied the foraminifera, Cronin (1991) studied the ostracods, and Verhoeven et al. (2011) dealt with the dinoflagellates from the Breiðavík Group. A compilation of their results and the outcome of the present macrofossil study, as well as lithological results by Eiríksson et al. (2020a) from the Breiðavík Group is presented by Eiríksson et al., 2020b.
Two species of molluscs have not been recorded previously from the Breiðavík Group, i.e., the prosobranch gastropod Alvania patorfikensis Laursen and the bivalve Yoldiella frigida (Møller). Three molluscan species are extinct, i.e., A. patorfikensis, and the bivalves Chlamys breidavikensis MacNeil and Cyrtodaria angusta Nyst & Westendorp, which have their last appearance (LAD) in the beds. This only comprises 6.7% of the fauna compared to 26% of the extinct molluscan species in the Pliocene Barmur Group. Only four (?five) species are not living now in Icelandic waters. Apparently, the prosobranch gastropods Erginus rubellus (Fabricius) and Littorina obtusata (Linné) have their first appearance (FAD) in the Breiðavík Group, as well as the bivalve species C. breidavikensis, Thyasira sarsii (Philippi), and Thracia septentrionalis (Jeffreys). Two species of barnacles were found, but Balanus hopkinsi Zullo, well known in the Barmur Group, has never been reported from the Breiðavik beds.
The molluscan assemblages found in the Breiðavík Group are quite different from those in the Pliocene Barmur Group. Arctic and subarctic taxa are more frequent, and the fauna has more species in common with Pleistocene faunas than with Pliocene assemblages. Numerous thermophilic species found in the Barmur Group have disappeared, and the faunal diversity is considerably lower in the Breiðavík Group. Extinct species are not as numerous, and the fauna has a more modern appearance and does not deviate significantly from other Pleistocene faunal assemblages in the North Atlantic area.
11.6 Correlation with Pleistocene Faunas
The faunal succession in the Breiðavík Group sediments reflects a change from arctic to subarctic or even boreal conditions. This is quite different from the succession in the older Barmur Group sediments. Furthermore, most of the more thermophilic species found in the Barmur Group have disappeared, and only three extinct species have been recorded in the Breiðavík Group, Alvania patorfikensis Laursen, Chlamys breidavikensis MacNeil, and Cyrtodaria angusta Nyst & Westendorp. The latter is the only one of those three known from the Pliocene Barmur Group. This indicates strongly that the Breiðavík fauna can be correlated with Pleistocene faunal assemblages rather than Pliocene.
11.6.1 Other Icelandic Sites
Other Icelandic assemblages comparable in age and faunal composition with the Breiðavík Group fauna have only been found in the Búlandshöfði Formation on Snæfellsnes, western Iceland (Fig. 11.7). The upper part of the Búlandshöfði Formation, the Stöð and Höfði Members, are at about 1.1–1.2 Ma old (Albertsson, 1976; Einarsson, 1977). The deposition of the Búlandshöfði Formation took place in a sedimentary basin that was gradually filled up with sediments and volcanics from the east and southeast (Símonarson & Leifsdóttir, 2007). The sediments of the Máná Formation were also deposited in a sedimentary basin, as part of the Breiðavík Group, and these two basins were contemporaneous for a period of time during the Lower Pleistocene, while the upper units of the Máná Formation were deposited. The lowermost marine assemblage in Búlandshöfði includes arctic molluscan species as Portlandia arctica (Gray), Tridonta placenta (Mørch), and Tachyrhynchus erosus (Couthouy). The assemblages in the upper part are subarctic, and in Höfði Member, we have found Macoma calcarea-Tridonta borealis infaunal assemblage and Nucella lapillus-Balanus epifaunal assemblage. The faunal successions in the two sedimentary basins were apparently very similar, reflecting changes from glacial to interglacial conditions.
1. Spisula elliptica (Brown), left valve from the Svarthamar Member 2. Macoma calcarea (Gmelin), right valve from the Svarthamar Member. The valve is probably bored by naticid gastropod (cf. Vilhjálmsson, 1985). 3. Mya truncata Linné, left valve from the Svarthamar Member. 4. Mya truncata pseudoarenaria Schlesch, left valve from the Svarthamar Member. 5. Hiatella rugosa (Linné), left valve from the Hörgi Formation. 6. Cyrtodaria angusta (Nyst & Westendorp), internal view of paired valves from the Svarthamar Member. External view also shown in specimens found in the Barmur Group (cf. Plate 7.18, Símonarson & Eiríksson, 2020). 7. Thracia cf. septentrionalis Jeffreys, left valve of articulated specimen from the Hörgi Formation. 8. Lyonsia cf. arenosa (Møller), fragmentary specimen with paired valves from the Hörgi Formation. 9. Verruca stroemia (Müller), a few parietal plates from the Hörgi Formation. At least two of them are connected. 10. Balanus balanus (Linné), cluster of four wall rings of specimens from the Torfhóll Member. 11. Balanus balanus (Linné), the wall rings have parietal tubes (see the single parietal plate) and calcareous basis
11.6.2 North Sea Sites
The assemblage in Covehite includes subarctic or even arctic species that reached the Rhine and Thames estuaries (EA, Fig. 11.7) at least as early as Upper Tiglian or Pre-Pastonian/Baventian about 1.9 Ma ago (Gibbard & Zalasiewicz, 1988; Gibbard et al., 1991). It is even possible that arctic bivalves such as Portlandia arctica reached the North Sea (Fig. 11.7) area as early as 2.0 Ma, during the deposition of the Lower Pleistocene (Tiglian) Serripes groenlandicus-Yoldia lanceolata Zone of the Netherlands (Heering, 1950; Spaink, 1975; Zagwijn, 1975, 1992). These deposits seem contemporaneous with the lower part of the Breiðavík Group, close to the Lower Pleistocene age of the Hörgi Formation. The faunal diversity was probably similar in the North Sea area and in Breiðavík at that time (cf. Spaink, 1975). However, the number of thermophilic species is higher in the North Sea Lower Pleistocene faunas than in the Breiðavík Group, with species such as Arctica islandica (Linné), Cerastoderma edule (Linné), and Mya arenaria Linné (cf. Spaink, 1975; West et al., 1980). The Breiðavík Group even contain species as Macoma praetenuis (Woodward) which is well known in the Pliocene Barmur Group, and furthermore, Macoma obliqua (Sowerby) which does not live any longer in the North Atlantic. Such species may be derived from older or distant sources. A. islandica first occurs higher up in the Breiðavík Group, i.e., in the Svarthamar Member deposited about 1.5 Ma ago (Eiríksson et al., 1992).
11.6.3 Greenlandic Sites
In the central East Greenland, the fauna in the Lodin Elv Formation (Fig. 11.7) seems of similar age as the Furuvík Formation, the oldest part of the Breiðavík Group. The foraminiferal and ostracod faunas, as well as amino acid analysis of mollusc shells indicate deposition either shortly after 2.4 Ma or at about 2.6 Ma (Penney, 1993). However, Feyling–Hanssen et al. (1983) considered the formation to be of Pliocene age after comparison with foraminiferal assemblages in deep borings in the central North Sea. The mollusc fauna in Member B of the formation is poor in species, and they are all living today and of arctic provenance, reflecting environmental conditions considerably harsher than during the deposition of the Barmur Group. This is stressed by the occurrence of the arctic Yoldiella frigida (Torell) and Similipecten greenlandicus (Sowerby) in the Lodin Elv Formation, and also by the absence of more thermophilic species as Nucula nucleus (Linné), Ensis ensis (Linné), and Zirfaea crispata (Linné), all found in the Serripes Zone of the Barmur Group. While a Pliocene age cannot be excluded for Member A, the fauna, as well as the age determination indicate strongly that Member B of the Lodin Elv Formation is of Lower Pleistocene age (cf. Bennike et al., 2010). Therefore, the molluscan assemblages can best be correlated with the oldest faunal assemblages of the Breidavík Group.
The Kap København Formation in Northeast Greenland (Fig. 11.7) is considered from the very beginning of the Pleistocene 2.5–2.6 Ma (Funder et al., 2001). The glaciation, which caused the formation of the lowermost diamicton of Member A, was correlated with the Praetiglian of northern Europe or isotope stages 100-96, and Member B of the Kap København Formation with the warm stages following the last Praetiglian glaciation or isotope stage 95-91 of the Tiglian (Símonarson et al., 1998). The mollusc fauna found in the Kap København Formation consists of at least 42 extant species and 9 of them are arctic or subarctic in their distribution. The arctic elements include Portlandia arctica (Gray), Pandora glacialis Leach, Macoma moesta (Deshayes), and Cyrtodaria kurriana Dunker (cf. Símonarson et al., 1998). Therefore, the fauna is considered of Lower Pleistocene age comparable with the oldest marine molluscan assemblages in the Breiðavík Group sediments.
The mollusc fauna in the Kap København Formation is considered to be the oldest arctic fauna from marine, shallow-water environments known so far. It has been suggested that the fauna evolved in response to the new and harsher environments following the Praetiglian glaciation, most probably during migration into shallow-water areas (Símonarson et al., 1998). When the Polar Basin became ice covered, the molluscs were attracted to the ice margin, where the food conditions were more favorable. One of these species was the well-known Portlandia arctica (Gray).
The mollusc fauna in the Pattorfik beds in Uummanaq Fjord (Fig. 11.7), central West Greenland, has many species in common with the Breiðavík Group sediments (Funder & Símonarson, 1984; Símonarson, 1981). The fauna consists of at least 51 species of gastropods and bivalves, and only one of them is extinct the prosobranch gastropod Alvania patorfikensis Laursen. This species was first described by Laursen (1944), who considered it a variety of Alvania wyvillethomsoni (Friele). It was raised to species level by Símonarson (1981). A. patorfikensis is now recorded from the Hörgi Formation in Breiðavik and has also been recorded from Lower Pleistocene sediments on Store Koldewey Island in central East Greenland. The species has never been found in the Barmur Group, but it was apparently distributed from West Greenland to northern Iceland in Lower Pleistocene. However, we do not know when it became extinct. The Pattorfik mollusc fauna has no arctic species and is considerably more thermophilic than the mollusc fauna in Hörgi Formation. However, the number of extinct species is much higher (26% of the fauna) in the Tjörnes bed fauna with much higher faunal diversity. The Pattorfik fauna is therefore considered of Lower Pleistocene age and probably slightly younger than the Kap København Formation (Funder & Símonarson, 1984).
Bennike et al. (2010) described Lower Pleistocene sediments with marine fauna and plant remains from Store Koldewey Island, northeast Greenland (Fig. 11.7). The presence of the extinct prosobranch gastropod Alvania paorfikensis Laursen, as well as the arctic bivalves Portlandia arctica (Gray), Bathyarca glacialis (Gray), and Cyrtodaria kurriana Dunker strongly indicates Pleistocene age. These species, except the last one, are known from the Breiðavík Group sediments on Tjörnes. Therefore, there is strong faunal similarity to the assemblages in Breiðavík, which is in a good agreement with the amino acid analysis, as well as the paleomagnetic results (Bennike et al., 2010). The sediments with marine faunal assemblages on Île de France, northeast Greenland (Fig. 11.7), were considered of Pliocene age by Bennike et al. (2002). However, the presence of the arctic bivalves P. arctica and Similipecten greenlandicus (Sowerby) indicates strongly a Pleistocene age. Bennike et al. (2010) suggested a correlation with the Store Koldewey Formation and concluded that both formations were possibly deposited during the Olduvai subchron about 1.8–1.9 Ma. Hence, they seem of similar age as the Hörgi Formation in the Breiðavík Group sediments and of Lower Pleistocene age.
11.6.4 North American Sites
The Yakataga Formation is a late Miocene to Pleistocene record of temperate glacial marine sediments in the Gulf of Alaska (Fig. 11.7) at least 5 km in thickness. There are some faunal similarities with the Lower Pleistocene part of the succession and the Breiðavík Group, but the formation has considerably more molluscs of Pacific origin as may be expected (Addicott et al., 1971; Allison, 1978). Hence, the formation on Middleton Island has about 75% of species of North Pacific origin (Allison, 1978). The assemblage includes the prosobranch gastropod Natica affinis (Gmelin) and the bivalves Portlandia arctica (Gray), Tridonta elliptica (Brown), T. montagui (Dillwyn), and Arctica islandica (Linné), all well known from the Breiðavík Group sediments. Allison (1978) recorded the arctic bivalve P. arctica from the deposits on Middleton Island, probably deposited during the Olduvai event (about 1.8 Ma). This is probably the first occurrence of the species in the Pacific arena. Another arctic bivalve, Cyrtodaria kurriana Dunker, well known in the Middle Pleistocene Kotzebuan sediments in western Alaska (Hopkins et al., 1972) has never been found in the Breiðavík Group.
There are also faunal correlation between the Lower Pleistocene parts of the Gubik Formation in northern Alaska (Fig. 11.7), but they also include considerably more Pacific molluscs than the Breiðavik beds (Durham & MacNeil, 1967; Gladenkov, 1981). The oldest Lower Pleistocene sediments of the Gubik Formation are the Nulavik unit of the Colvillian transgression, supposed to be 2.7–2.48 Ma, the Killi Creek unit of the Bigbendian transgression about 2.48 Ma, and the Tuapaktushak unit of the Fischreekian 2.48–2.14 Ma (Brigham-Grette & Carter, 1992). These sediments were apparently contemporaneous with the oldest parts of the Breiðavík Group units with the exception of the oldest beds of the Nulavik unit of the Bigbendian transgression. All the faunas in these sediments include taxa of Pacific origin and must postdate the tide of the migration which occurred while the Barmur Group sediments were deposited. The sea-surface temperatures during the Fishcreekian transgression were actually higher than at present, as indicated by the presence of the bivalve Clinocardium californiense (Deshayes) (cf. Brigham-Grette & Carter, 1992). Apparently, the sea ice preceded the major Caenozoic climatic deterioration in the Northern Hemisphere with successive glaciations. These faunas also include taxa as the prosobranch gastropod Littorina squalida Broderip & Sowerby and the bivalve species Serripes groenlandicus (Mohr), Macoma calcarea (Gmelin), Mya truncata Linné, and Hiatella arctica (Linné) (cf. Brigham-Grette & Carter, 1992; MacNeil, 1957). In contrast to the relatively warm marine conditions, the late Fishcreekian terrestrial climate was apparently harsh and dropstones, probably of Canadian shield provenance, observed in the Tuapaktushak unit suggest a moderate buildup of Laurentide ice sheet (Brigham-Grette & Carter, 1992). However, noteworthy is the presence of the arctic Cyrtodaria kurriana Dunker, as well as the subarctic-boreal Macoma balthica (Linné) in the Fish Creek beds (Repenning et al., 1987; cf. Bennike et al., 2010). These two species, with rather different recent distribution, have also been found together in unit B1, as well as unit B2 of Member B in the Kap København Formation (Símonarson et al., 1998). It may be questioned if C. kurriana is as “high-arctic” as stated by many authors (cf. Lubinsky, 1980).
In the Hvitland beds on the west coast of Ellesmere Island (Fig. 11.7), a molluscan fauna has been found with Hiatella arctica (Linné), Similipecten greenlandicus (Sowerby), Yoldiella cf. lenticula (Møller), Astarte sp., Dentalium sp., and Arctica sp., most probably A. islandica (Linné). The faunal assemblages lived most probably at depth of about 25–30 m, and the age is probably no younger than 2.4 Ma, and correlation with the Bigbendian unit of the Gubik Formation is suggested (Fyles et al., 1998). The fauna may be contemporaneous with the Breiðavík fauna, especially the assemblages in the Hörgi Formation.
Located in the Atlantic area, the Breiðavík fauna seems actually more related to Middle Pleistocene faunas than Lower Pleistocene faunas in Alaska and Siberia (cf. Hopkins et al., 1972; Hopkins et al., 1974). The same was the case with the mollusc fauna in the Kap København Formation in North Greenland (Símonarson et al., 1998).
11.6.5 Siberian and Russian Sites
Comparison with late Neogene Siberian deposits shows certain faunal similarities between the Breiðavík Group assemblages and the fauna in the Lower Pleistocene Olkov-Tusatuva Yamsk Suites (Fig. 11.7) in eastern Kamchatka (Petrov, 1982). These are about 2 Ma old, with the first appearance of Cyrtodaria kurriana Dunker in arctic assemblages, which indicates Lower Pleistocene age rather than Pliocene, even though the suites have nearly 20% extinct species (Gladenkov, 1981; Petrov, 1982, 1986). In the coastal plain area of Chukotka (Fig. 11.7), the extant arctic marine assemblages with Portlandia arctica (Gray) in the Pinakul Suite is even younger and considered to date from Middle Pleistocene (Petrov, 1967, 1982, 1986). The Pinakul Suite rests on the eroded preglacial sediments of the Pliocene Koynatkhun Suite both in the northern and southern parts of Chukotka (Petrov, 1967). Cainozoic shelf deposits are known from the European part of Russia (Fig. 11.7) in the Barents Sea (Zarkhidze & Samoilovich, 1989). The mollusc fauna in these deposits contains more or less arctic species such as P. arctica, Yoldiella lenticula (Møller), Tridonta borealis (Schumacher), Ciliatocardium ciliatum (Fabricius), and Serripes groenlandicus (Mohr), and Bennike et al. (2010) consider the fauna to be comparable to the Lower Pleistocene mollusc fauna on Store Koldewey, East Greenland. Furthermore, the faunal assemblages contain the more subarctic-boreal Macoma balthica (Linné), and the oldest known occurrence of that species in the North Atlantic area is in the Lower Pleistocene Kap København Formation in North Greenland (cf. Coan, 1971; Símonarson et al., 1998). Therefore, these deposits in the Barents Sea are considered of post-Pliocene age, younger than the Barmur Group, and a correlation with the Lower Pleistocene Breiðavík deposits seems appropriate.
11.7 Concluding Remarks
The molluscs and barnacles assemblages found in the Breiðavík Group in North Iceland consists of infaunal, as well as epifaunal species. Generally, they died at the place where they lived or only suffered a limited postmortal transport. The faunal succession in the Breiðavík Group sediments reflects a change from arctic to subarctic or even boreal conditions. This is quite different from the succession in the older Barmur Group sediments. The assemblages are primarily based on collections and field work carried out more or less each year since 1972.
Until now, 14 species of prosobranch gastropods, 2 opisthobranch gastropod species, 29 species of bivalves, and 2 species of barnacles have been identified from the Breiðavík Group. Totally, of the fauna comprises 45 species of molluscs and 2 barnacle species. Two of the mollusc species have not been recorded before from the Breiðavík Group; Alvania patorfikensis Laursen and Yoldiella frigida (Torell). Three extinct species have been recorded from the Breiðavík Group sediments; Alvania patorfikensis, Chlamys breidavikensis MacNeil, and Cyrtodaria angusta Nyst & Westendorp.
The larval development of the species is recorded as their ability to migrate is significantly depending on the pelagic larval development. At least two species of molluscs seems of Pacific origin; Nucella lapillus (Linné) and Ennucula tenuis (Montagu), and also the subspecies Mya truncata pseudoarenaria Schlesch. They have probably migrated to northern Iceland during the Early Pleistocene after the deposition of the Tjörnes Group sediments.
Two species of molluscs have not been recorded before from the Breiðavík Group, i.e., the prosobranch gastropod Alvania patorfikensis and the bivalve Yoldiella frigida. Three molluscan species are extinct; Alvania patorfikensis, and the bivalves Chlamys breidavikensis and Cyrtodaria angusta, which have their last appearance (LAD) in the Group. This is only 6.7% of the fauna compared to 26% of the extinct molluscan species in the Barmur Group. Only four (five) species are not living now in Icelandic waters. Apparently, the prosobranch gastropods Erginus rubellus and Littorina obtusata have their first appearance (FAD) in the Breiðavík Group, as well as the bivalve species Chlamys breidavikensis, Thyasira sarsii, and Thracia septentrionalis. Two species of barnacles were found but Balanus hopkinsi, well known in the Barmur Group, has never been reported from the Breiðavík Group.
References
Addicott, W. O., Kanno, S., & Sakamoto, K. (1971). Clark’s Tertiary molluscan types from the Yakataga District, Gulf of Alaska. Geological Survey Professional Paper, 750-C, C18–C33.
Albertsson, K. J. (1976). K/Ar ages of Pliocene-Pleistocene glaciations in Iceland with special reference to the Tjörnes sequence, Northern Iceland (268 pp). PhD thesis. University of Cambridge, Cambridge.
Allison, R. C. (1978). Late Oligocene through Pleistocene molluscan faunas in the Gulf of Alaska Region. The Veliger, 21(2), 171–188.
Andrews, J. T., Nelson, A. R., Mode, W. N., & Locke, W. W. I. I. I. (1981). Quaternary near-shore environments on eastern Baffin Island N.W.T. In W. C. Mahaney (Ed.), Quaternary paleoclimates (pp. 13–44). Norwich, UK: University of East Anglia.
Áskelsson, J. (1935). Some remarks on the distribution of the species Zirphaea crispata L. and Purpura lapillus L. on the North-Coast of Iceland. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening, 99, 65–72.
Áskelsson, J. (1960). Fossiliferous xenoliths in the Móberg Formation of South Iceland. Acta Naturalia Islandica, 2(2), 1–30.
Bahnson, H., Petersen, K. S., Konradi, P. B., & Knudsen, K. L. (1974). Stratigraphy of Quaternary deposits in the Skærumhede II boring: Lithology, molluscs and foraminifera. Danmarks Geologiske Undersøgelse Årbog, 1973, 27–62.
Barash, A., & Danin, Z. (1987). Red Sea malacology V. Notes on the antilessepsian migration of Mollusca into the Indo-Pacific region. Gloria Maris, 26(5–6), 81–100.
Bárðarson, G. G. (1925). A stratigraphical survey of the Pliocene deposits at Tjörnes, in northern Iceland. Det kongelige danske Videnskabernes Selskab, Biologiske Meddelelser, 4(5), 1–118.
Bennike, O., Abrahamsen, N., Bak, M., Israelson, C., Konradi, P., Matthiessen, J., et al. (2002). A multi-proxy study of Pliocene sediments from Île de France, North-East Greenland. Palaeogeography, Palaeoclimatology, Palaeoecology, 186, 1–23.
Bennike, O., Knudsen, K. L., Abrahamsen, N., Böcher, J., Cremer, H., & Wagner, B. (2010). Early Pleistocene sediments on Store Koldewey, northeast Greenland. Boreas, 39(3), 603–619.
Bernard, F. R. (1979). Bivalve mollusks of the western Beaufort Sea. Contributions in Sciences, Natural Museum of Los Angeles, 313, 1–80.
Bernard, F. R. (1983). Catalogue of the living Bivalvia of the eastern Pacific Ocean: Bering Strait to Cape Horn. Canadian Special Publication of Fisheries and Aquatic Sciences, 61, 1–102.
Bogdanov, I. P. (1990). Fauna of USSR. Molluscs. Molluscs of Oenopotinae subfamily (Gastropoda, Pectinibranchia, Turridae) in the seas of USSR. Zoologiyeskogo Instituta, Academiya Nauk SSSR, N142. Fauna SSSR. Mollyuski, 5(3), 1–224.
Brigham-Grette, J., & Carter, L. D. (1992). Pliocene marine transgressions of northern Alaska: Circumarctic correlations and paleoclimatic interpretations. Arctic, 45(1), 74–89.
Broderip, W. J., & Sowerby, G. B. (1829). Observations on new or interesting Mollusca contained, for the most part, in the Museum of the Zoological Society. Zoological Journal, 4, 359–379.
Brøgger, W. C. (1900). Om de senglaciale og postglaciale nivåforandringer i Kristianiafeltet (Molluskfaunaen). Norges Geologiske Undersøgelse, 31, 1–731.
Brown, T. (1827). Illustrations of the conchology of Great Britain and Ireland (144 pp). Edinburgh, Scotland/London: W.H. Lizars, D. Lizars & S. Highley.
Bruguière, J. G. (1792). Encyclopédie méthodique, ou par ordre de matières. Histoire naturelle des vers (1) (758 pp). Paris: Panckoucke.
Chinzei, K. (1978). Neogene molluscan faunas in the Japanese Islands: An ecologic and zoogeographic syntheses. The Veliger, 21(2), 155–170.
Clarke, A. H. J. (1974). Molluscs from Baffin Bay and the northern North Atlantic Ocean. Publications in Biological Oceanography, 7, 1–23.
Coan, E. V. (1971). The Northwest American Tellinidae. The Veliger, 14(Suppl), 63.
Coan, E. V., Scott, P. V., & Bernard, F. R. (2000). Bivalve seashells of western North America. Santa Barbara Museum of Natural History Monographs 2. Studies in Biodiversity, 2, 1–764.
Collins, J. S. H., Donovan, S. K., & Mellish, C. (2014). An illustrated guide to the fossil barnacles (Cirripedia) from the Crags (Plio-Pleistocene) of East Anglia. Proceedings of the Geologists Association, 125(2), 215–226.
Cox, L. R. (1960). Gastropoda – General characteristics of gastropods. In R. C. Moore (Ed.), Treatise on invertebrate paleontology I, Mollusca 1 (pp. 84–331). Lawrence, Kansas: The Geological Society of America and University of Kansas Press.
Cronin, T. M. (1991). Late Neogene marine ostracoda from Tjörnes, Iceland. Journal of Paleontology, 65, 767–794.
Dillwyn, L. W. (1817). A descriptive catalogue of recent shells, arranged according to the Linnæan method; with particular attention to the synonymy (580 pp). London: Arthur Arch.
Dinter, W. P. (2001). Biogeography of the OSPAR maritime area: Synopsis and synthesis of biogeographical distribution patterns described for the North-East Atlantic (167 pp). Bonn, Germany: Federal Agency for Nature Conservation.
Donovan, E. (1802). The natural history of British shells, including figures and descriptions of all the species hitherto discovered in Great Britain, systematically arranged in the Linnean manner, with scientific and general observations on each (4). London: Donovan and F. & C. Rivington, 8 pp and plates 109–144.
Durham, J. W., & MacNeil, F. S. (1967). Cenozoic migrations of marine invertebrates through the Bering Strait region. In D. M. Hopkins (Ed.), The Bering Land Bridge (pp. 326–349). Stanford, CA: Stanford University Press.
Einarsson, T. (1977). Um gróður á ísöld á Íslandi. In H. Guðmundsson, H. Ragnarsson, I. Þorsteinsson, J. Jónsson, S. Sigurðsson, & S. Blöndal (Eds.), Skógarmál (pp. 56–72). Reykjavík, Iceland: Sex vinir Hákonar Bjarnasonar.
Eiríksson, H. (1988). Um stofnstærð og veiðimöguleika á kúfskel í Breiðafirði, Faxaflóa og við SA-land. Ægir, 81(2), 58–68.
Eiríksson, J. (1981). Lithostratigraphy of the upper Tjörnes sequence, North Iceland: The Breidavík Group. Acta Naturalia Islandica, 29, 1–37.
Eiríksson, J. (2008). Glaciation events in the Pliocene – Pleistocene volcanic succession of Iceland. Jökull, 58, 315–329.
Eiríksson, J., Knudsen, K. L., & Vilhjálmsson, M. (1992). An Early Pleistocene glacial-interglacial cycle in the Breidavík Group on Tjörnes, Iceland: Sedimentary facies, foraminifera, and molluscs. Quaternary Science Reviews, 11, 733–757.
Eiríksson, J., Knudsen, K. L., & Símonarson, L. A. (2004). Lateglacial oceanographic conditions off Southwest Iceland inferred from shallow-marine deposits in Reykjavík and Seltjarnarnes Peninsula. Boreas, 33, 269–283.
Eiríksson, J., Guðmundsson, A. I., Símonarson, L. A., & Knudsen, K. L. (2020a). Lithostratigraphy of the upper part of the Tjörnes sequence in Furuvík, Breiðavík, Öxarfjörður, and Central Tjörnes Mountains, North Iceland. In J. Eiríksson & L. A. Símonarson (Eds.), Pacific – Atlantic Mollusc migration. Cham, Switzerland: Springer. This volume, Chapter 10.
Eiríksson, J., Knudsen, K. L. & Símonarson, L. A. (2020b). Reconstructing the Paleoenvironments of the Quaternary Tjörnes Basin, North Iceland. In J. Eiríksson & L. A. Símonarson (Eds.), Pacific – Atlantic Mollusc migration. Cham, Switzerland: Springer. This volume, Chapter 13.
Ellis, D. V. (1960). Marine infaunal benthos in Arctic North America. Arctic Institute of North America, Technical Paper, 5, 1–53.
Fabricius, O. (1780). Fauna Groenlandica (452 pp). Lipsiae, Germany: Rothe.
Feyling-Hanssen, R. W. (1955). Stratigraphy of the marine Late-Pleistocene of Billefiorden, Vestspitsbergen. Norsk Polarinstitutt. Skrifter, 107, 1–186.
Feyling-Hanssen, R. W., Funder, S., & Petersen, K. S. (1983). The Lodin Elv Formation; a Plio-Pleistocene occurrence in Greenland. Bulletin of the Geological Society of Denmark, 31(3–4), 81–106.
Filatova, Z. A. (1957). General survey of the bivalve mollusc fauna of the northern seas of the USSR. Trudy Instituta Okeanologiya, Academiya Nauk SSSR, 20, 3–59.
Fretter, V., & Graham, A. (1976). The prosobranch molluscs of Britain and Denmark 1. Pleurotomariacea, Fissurellacea and Patellacea. The Journal of Molluscan Studies, 1(Suppl), 1–37.
Fretter, V., & Graham, A. (1977). The prosobranch molluscs of Britain and Denmark 2. Trochacea. The Journal of Molluscan Studies, 3(Suppl), 39–100.
Fretter, V., & Graham, A. (1978). The prosobranch molluscs of Britain and Denmark 3. Neritacea, Viviparacea, Valvatacea, terrestrial and freshwater Littorinacea and Rissoacea. The Journal of Molluscan Studies, 5(Suppl), 101–152.
Funder, S. (1989). Quaternary geology of the ice-free areas and adjacent shelves of Greenland. In R. J. Fulton (Ed.), Quaternary geology of Canada and Greenland (pp. 741–792). Ottawa, Canada: Geological Survey of Canada.
Funder, S., Bennike, O., Böcher, J., Israelson, C., Petersen, K. S., & Símonarson, L. A. (2001). Late Pliocene Greenland – The Kap København Formation in North Greenland. Bulletin of the Geological Society of Denmark, 48, 117–134.
Funder, S., Demidov, I., & Yelovicheva, Y. (2002). Hydrography and mollusc faunas of the Baltic and White Sea-North Sea seaway in the Eemian. Palaeogeography, Palaeoclimatology, Palaeoecology, 184, 275–304.
Funder, S., & Símonarson, L. A. (1984). Bio- and aminostratigraphy of some Quaternary marine deposits in West Greenland. Canadian Journal of Earth Sciences, 21(7), 843–842.
Funder, S., & Weidick, A. (1991). Holocene boreal molluscs in Greenland – Palaeoceanographic implicationes. Palaeogeography, Palaeoclimatology, Palaeoecology, 85(1–2), 123–135.
Funnell, B. M., Norton, P. E. P., & West, R. G. (1979). The Crag of Bramerton, near Norwich, Norfolk. Philosophical Transactions of the Royal Society of London, B287, 489–534.
Fyles, J. G., DH, M. N., JVJ, M., Barendregt, R. W., Marincovich, L. J., Brouwers, E., et al. (1998). Geology of the Hvitland beds (Late Pliocene), White Point lowland, Ellesmere Island, Northwest Terratories. Geological Survey of Canada Bulletin, 512, 35.
Fyles, J. G., Marincovich, L. J., Matthews, J. V. J., & Barendregt, R. (1991). Unique mollusc find in the Beaufort Formation (Pliocene) on Meighen Island, Arctic Canada. Geological Survey of Canada Paper, 91-B, 105–112.
Gibbard, P. L., West, R. G., Zagwijn, W. H., Balson, P. S., Burger, A. W., Funnell, B. M., et al. (1991). Early and Early Middle Pleistocene correlations in the southern North Sea Basin. Quaternary Science Reviews, 10, 23–52.
Gibbard, P. L., & Zalasiewicz, J. A. (1988). Pliocene-Middle Pleistocene of East Anglia. Field Guide (195 pp). Cambridge, UK: Quaternary Research Association.
Gladenkov, Y. B. (1974). The palaeontological character of the Plio-Pleistocene in the North Atlantic (Iceland). Academiya Nauk SSSR, Geologicheskoye Seriya, 7, 129–133.
Gladenkov, Y. B. (1981). Marine Plio-Pleistocene of Iceland and problems of its correlation. Quaternary Research, 15(1), 18–23.
Gladenkov, Y. B., Norton, P., & Spaink, G. (1980). Upper Cenozoic of Iceland. Trudy Geologischeskogo Instituta Academiya Nauk SSSR, 345, 1–116.
Gmelin, J. F. (1791). Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synoymis, locis. Editio decima tertia reformata (2(6)) (pp. 3011–3910). Lipsiae, Germany: Beer.
Gofas, S., & Salas, C. (2008). A review of European ‘Mysella’ species (Bivalvia, Montacutidae), with description of Kurtiella new genus. Journal of Molluscan Studies, 74(2), 119–135.
Golikov, A. N. (1995). Shell-bearing gastropods of the Arctic (108 pp). Moscow: Colus.
Gordillo, S. (2001). Puzzling distribution of the fossil and living genus Hiatella (Bivalvia). Palaeogeography, Palaeoclimatology, Palaeoecology, 165, 231–249.
Gould, A. A. (1841). Report on the Invertebrata of Massachusetts, comprising the Mollusca, Crustacea, Annelida, and Radiata (371 pp). Cambridge, MA: Folson, Wells & Thurston.
Graham, A. (1988). Molluscs: Prosobranch and pyramidellid gastropods. Synopses of the British Fauna (New Series), 2(2nd edition), 1–662.
Grant IV, U. S., & Gale, H. R. (1931). Catalogue of the marine Pliocene and Pleistocene Mollusca of California and adjacent regions (1) (1036 pp). San Diego, CA: San Diego Society of Natural History.
Gray, J. E. (1824). A supplement to the appendix of Captain Parry’s voyage for the discovery of a North-West Passage, in the years 1819-1820. Shells. Appendix 10, Natural History (pp. 240–246). London: John Murray.
Harmer, F. W. (1900). The Pliocene deposits of the east of England 1. The Lenham Beds and the Coralline Crag. Quarterly Journal of the Geological Society of London, 56, 705–738.
Harmer, F. W. (1914). The Pliocene Mollusca of Great Britain, being supplementary to S.V. Wood’s Monograph of the Crag Mollusca (1(1)) (pp. 1–200). London: The Palæontographical Society.
Harmer, F. W. (1915). The Pliocene Mollusca of Great Britain, being supplementary to S.V. Wood’s Monograph of the Crag Mollusca (1(2)) (pp. 201–302). London: The Palæontographical Society.
Harmer, F. W. (1920). The Pliocene Mollusca of Great Britain, being supplementary to S.V. Wood’s Monograph of the Crag Mollusca (2(1)) (pp. 485–652). London: The Palæontographical Society.
Harmer, F. W. (1921). The Pliocene Mollusca of Great Britain, being supplementary to S.V. Wood’s Monograph of the Crag Mollusca (2(2)) (pp. 653–705). London: The Palæontographical Society.
Harmer, F. W. (1923). The Pliocene Mollusca of Great Britain, being supplementary to S.V. Wood’s Monograph of the Crag Mollusca (2(3)) (pp. 705–856). London: The Palæontographical Society.
Harmer, F. W. (1925). The Pliocene Mollusca of Great Britain, being supplementary to S.V. Wood’s Monograph of the Crag Mollusca (2(4)) (pp. 857–900). London: The Palæontographical Society.
Heering, J. (1950). Pelecypoda (and Scapopoda) of the Pliocene and Older-Plistocene deposits of the Netherlands. Mededeelingen van de Geologische Stichting C(IV), 1(9), 1–225.
Herman, J., & Marquet, R. (2007). Observations paléontologiquès réalisées dans les terrains Néogènes Belges de 1971 à 2004 entre Kallo et Doel, port d’Anvers, rive Gauche (Flandre-Orientale, Belgique). La Miocène du Deurganckdok à Doel. Memoirs of the Geological Survey of Belgium, 54, 1–48.
Hjort, C., & Funder, S. (1974). The subfossil occurrence of Mytilus edulis L. in central East Greenland. Boreas, 3(1), 23–33.
Høisæter, T. (1986). An annoted check-list of marine molluscs of the Norwegian coast and adjacent waters. Sarsia, 71, 73–145.
Hopkins, D. M., Rowland, R. W., Echols, R. E., & Valentine, P. C. (1974). An Anvilian (Early Pleistocene) marine fauna from western Seward Peninsula, Alaska. Quaternary Research, 4(4), 441–470.
Hopkins, D. M., Rowland, R. W., & Patton, J. W. W. (1972). Middel Pleistocene molluscs from St. Lawrence Island and their significance for the paleo-oceanography of the Bering Sea. Quaternary Research, 4(4), 119–134.
Hunter, W. R. (1949). The structure and behaviour of Hiatella gallicana (Lamarck) and H. arctica (L.), with special reference to the boring habit. Proceedings of the Royal Society of Edinburgh, 63B(3), 271–289.
Jeffreys, J. G. (1872). The Mollusca of Europe compared with those of eastern North America. The Annals and Magazine of Natural History, 4th ser, 10, 237–247.
Jensen, A. S. (1900). Studier over nordiske mollusker. I. Mya. Videnskabelige Meddelelser fra den naturhistoriske Forening i Kjøbenhavn for Aaret, 1900(62), 133–158.
Jensen, A. S. (1905). Studier over nordiske mollusker 3. Tellina (Macoma). Videnskabelige Meddelelser fra den naturhistoriske Forening i Kjøbenhavn for Aaret, 67, 21–51.
Jensen, A. S. (1942). Two West Greenland localities for deposits from the Ice Age and the Post-Glacial Warm Period. Det kongelige danske Videnskabernes Selskab, Biologiske Meddelelser, 17(4), 1–35.
Jensen, A. S., & Spärck, R. (1934). Saltvandsmuslinger. Danmarks Fauna, 40, 208.
Jessen, A., Milthers, V., Nordmann, V., Hartz, N., & Hesselbo, A. (1910). En Boring gennem de kvartære Lag ved Skærumhede. Undersøgelse af en Forekomst af naturlig Gas i Vendsyssel. Danmarks Geologiske Undersøgelse, 2(25), 1–175.
Jørgensen, C. B. (1946). Reproduction and larval development of Danish marine bottom invertebrates. Lamellibrancia. Meddelelser fra Kommissionen for Danmarks Fiskeri- og Havundersøgelser. Plankton, 4, 277–311.
Kaestner, A. (1967). Lehrbuch der Speziellen Zoologie, Zweite Auflage (1(2)) (pp. 449–1282). Stuttgart, Germany: Gustav Fischer.
Kelly, M. (1986). Quaternary pre-Holocene marine events of western Greenland. Rapport. Grønlands Geologiske Undersøgelse, 131, 23.
Knudsen, K. L., Eiríksson, J., & Símonarson, L. A. (2020). Foraminifera in the Early Pleistocene part of the Breiðavík Group on Tjörnes, North Iceland. In J. Eiríksson & L. A. Símonarson (Eds.), Pacific – Atlantic Mollusc migration. Cham, Switzerland: Springer. This volume, Chapter 12.
La Rocque, A. (1953). Catalogue of the recent Mollusca of Canada. National Museum of Canada Bulletin, 129, 1–406.
Landau, B., Marquet, R., & Grigis, M. (2004). The Early Pliocene Gastropoda (Mollusca) of Estepona, southern Spain. Palaeontos, 3, 87.
Laursen, D. (1944). Contributions to the Quaternary geology of northern West Greenland especially the raised marine deposits. Meddelelser om Grønland, 135(8), 1–125.
Laursen, D. (1950). The stratigraphy of the marine Quaternary deposits in West Greenland. Meddelelser om Grønland, 151(1), 1–152.
Laursen, D. (1966). The genus Mya in the arctic region. Malacologia, 3, 399–418.
Lemche, H. (1938). Gastropoda opisthobranciata (The zoology of Iceland 4(61)) (54 pp). Copenhagen, Denmark/Reykjavík, Iceland: Munksgaard.
Lemche, H. (1941a). The Godthaab Expedition 1928. Gastropoda opisthobranciata (excl. Pteropoda). Meddelelser om Grønland, 80(7), 1–65.
Lemche, H. (1941b). The zoology of East Greenland. Gastropoda opisthobranciata. Meddelelser om Grønland, 121(7), 1–50.
Lemche, H. (1948). Northern and Arctic tectibranch gastropods. I. The larval shells, II. A revision of the cephalaspid species. Det kongelige danske Videnskabernes Selskab, Biologiske Skrifter, 5(3), 1–136.
Linné, C. (1758). Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synoymis, locis. Editio decima, reformata (1) (824 pp). Holmiæ, Germany: Laurentius Salvius.
Linné, C. (1767). Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synoymis, locis. Editio duodecima, reformata (1(2)). Holmiæ, Germany: Laurentius Salvius, pp. 533–1327.
Lubinsky, I. (1980). Marine bivalve molluscs of the Canadian central and eastern Arctic: Faunal composition and zoogeography. Canadian Bulletin of Fisheries and Aquatic Sciences, 207, 111.
MacGinitie, N. (1959). Marine Mollusca of Point Barrow, Alaska. Proceedings of the United States National Museum, 109(3412), 59–208.
MacNeil, F. S. (1957). Cenozoic megafossils of northern America. Geological Survey Professional Paper, 294-C, 99–126.
MacNeil, F. S. (1965). Evolution and distribution of the genus Mya and Tertiary migrations of molluscs. United States Geological Survey Professional Paper, 483-G, 1–51.
MacNeil, F. S. (1967). Cenozoic pectinids of Alaska, Iceland, and other northern regions. United States Geological Survey Professional Paper, 553, 57.
Macpherson, E. (1971). The marine molluscs of Arctic Canada. Prosobranch gastropods, chitons and scaphopods. National Museum of Canada, Publications in Biological Oceanography, 3, 149.
Madsen, F. J. (1949). Marine Bivalvia (The zoology of Iceland 4(63)) (116 pp). Copenhagen, Denmark/Reykjavík, Iceland: Munksgaard.
Madsen, H. (1940). A study of the littoral fauna of Northwest Greenland. Meddelelser om Grønland, 124(3), 1–24.
Malatesta, A., & Zarlenga, F. (1986). Northern guests in the Pleistocene Mediterranean Sea. Geologica Romana, 25, 91–154.
Marquet, R. (2002). The Neogene Amphineura and Bivalvia (Protobranchia and Pteromorphia) from Kallo and Doel (Oost-Vlaanderen, Belgium). Palaeontos, 2, 1–99.
Marquet, R. (2005). The Neogene Bivalvia (Heterodonta and Anomalodesmata) and Scapopoda from Kallo and Doel (Oost-Vlaanderen, Belgium). Palaeontos, 6, 1–142.
Marquet, R., & Landau, B. (2006). The gastropod fauna of the Luchtbal Sand Member (Lillo Formation, Zanclean, Early Pliocene) of the Antwerp region (Belgium). Cainozoic Research, 5(1-2), 13–49.
Massy, A. L. (1930). Mollusca (Pelecypoda, Scaphopoda, Gastropoda, Opistobranchia) of the Irish Atlantic slope. Proceedings of the Royal Irish Academy, 39(B13), 232–342.
Merklin, R. L., Petrov, O. M., & Amitrov, O. V. (1962). Atlas-guide to the molluscs of the Quaternary deposits of the Chukotsk Peninsula (83 pp). Moscow: Academyia Nauk SSSR, Komissa po Izucheniyu Chetvertichogo Perioda.
Merklin, R. L., Zarkhidze, V. S., & Ilina, L. B. (1979). A descriptive catalogue of Pliocene-Pleistocene molluscs of the north-east region of European USSR. Trudy Paleontologieskogo Instituta, Academiya Nauk SSSR, 173, 1–96.
Mighels, J. W. (1843). Descriptions of six species of shells regarded as new. Boston Journal of Natural History, 4, 345–350.
Mighels, J. W., & Adams, C. B. (1841). Descriptions of twenty-five new species of New England shells. Proceedings of the Boston Society of Natural History, 1, 48–50.
Mohr, N. (1786). Forsøg til en Islandsk Naturhistorie, med adskillige oekonomiske samt andre Anmærkninger (413 pp). Kiøbenhavn, Denmark: Holm.
Møller, H. P. C. (1842). Index molluscorum Groenlandiae. Naturhistorisk Tidsskrift, 4, 76–97.
Montagu, G. (1803). Testacea Britannica or natural history of British shells, marine, land and fresh-water including the most minute: systematically arranged and embellished with figures (1-2) (183 pp). London: J. White.
Montagu, G. (1808). Supplement to Testacea Britannica with additional plates. London: J. White.
Moore, R. C. (Ed.). (1960). Treatise on invertebrate paleontology I, Mollusca 1 (374 pp). Lawrence, Kansas: The Geological Society of America and University of Kansas Press.
Moore, R. C. (Ed.). (1969a). Treatise on invertebrate paleontology N, Mollusca 6. Bivalvia 1-2 (462 pp). Lawrence, Kansas: The Geological Society of America and the University of Kansas.
Moore, R. C. (Ed.). (1969b). Treatise on invertebrate paleontology R, Arthropoda 4 (1) (651 pp). Lawrence, Kansas: The Geological Society of America and the University of Kansas.
Mörch, O. A. L. (1871). On the Mollusca of the crag-formation of Iceland. The Geological Magazine, or, Monthly Journal of Geology, 8(9), 391–400.
Müller, O. F. (1776). Zoologiae danicae prodromus, seu animalium Daniae et Norwegiae indigenarium characteres, nomina et synonyma imprimis popularium (282 pp). Havniae, Germany: Hallageriis.
Müller, O. F. (1779). Von zwoen wenig bekannten Muscheln, der Schinkenarche und der gerunzelten Mahlermuscheln. Beschaftigungen der Berlinischen Gesellschaft Naturforschender Freunde, 4, 55–59.
Nesis, K. N. (1965). Ecology of Cyrtodaria siliqua and history of the genus Cyrtodaria (Bivalvia: Hiatellidae). Malacologia, 3(2), 197–210.
Nordsieck, F. (1968). Die europäischen Meeres-Gehäuseschnecken (Prosobranchia). Vom Eismeer bis Kapverden und Mittelmeer (273 pp). Stuttgart, Germany: Gustav Fischer.
Norton, P. E. P. (1967). Marine molluscan assemblages in the Early Pleistocene of Sidestrand, Bramerton and the Royal Society Borehole at Ludham, Norfolk. Philosophical Transactions of the Royal Society of London, B253, 161–200.
Norton, P. E. P. (1975). Paleoecology of the Mollusca of the Tjörnes sequence, Iceland. Boreas, 4, 97–110.
Nyst, P. H. J., & Westendorp, G. D. (1839). Nouvelles recherches sur les coquilles fossiles de la province d’Anvers. Bulletins de l’Académie Royale des Sciences et Belles-Lettres de Bruxelles, 6(2), 393–414.
Ockelmann, K. W., & Muus, K. (1978). The biology, ecology and behavior of the bivalve Mysella bidentata (Montagu). Ophelia, 17(1), 1–93.
Ockelmann, W. K. (1958). The zoology of East Greenland. Marine lamellibranchiata. Meddelelser om Grønland, 122(4), 1–256.
Óskarsson, I. (1952). Skeldýrafána Íslands 1. Samlokur í sjó (119 pp). Reykjavík, Iceland: Atvinnudeild Háskólans, fiskideild.
Óskarsson, I. (1962). Skeldýrafána Íslands 2. Sæsniglar með skel (167 pp). Reykjavík, Iceland: Leiftur.
Óskarsson, I. (1964). Skeldýrafána Íslands 1. Samlokur í sjó. 2. útgáfa aukin (123 pp). Reykjavík, Iceland: Asór.
Óskarsson, I. (1982). Skeldýrafána Íslands 1-2. Samlokur í sjó. Sæsniglar með skel (351 pp). Reykjavík, Iceland: Leiftur.
Penney, D. N. (1993). Late Pliocene to Early Pleistocene ostracod stratigraphy and palaeoclimate of the Lodin Elv and Kap København formations, East Greenland. Palaeogeography, Palaeoclimatology, Palaeoecology, 101(1-2), 49–66.
Petersen, C. G. J. (1888). Om de skalbærende Molluskers Udbredningsforhold i de danske Have indenfor Skagen (162 pp). Kjøbenhavn, Denmark: Andr. Høst & Søn.
Petersen, G. H. (1968). Marine Lamellibranchiata. The Zoology of the Faroes, 3(55), 1–80.
Petersen, G. H. (1978). Life cycles and population dynamics of marine benthic bivalves from the Disko Bugt area of West Greenland. Ophelia, 17(1), 95–120.
Petersen, K. S. (2004). Late Quaternary environmental changes recorded in the Danish marine molluscan faunas. Geological Survey of Denmark and Greenland Bulletin, 3, 1–268.
Petrov, O. M. (1966). Stratigraphy and fauna of marine molluscs in the Quaternary depositrs of the Chukotsk Peninsula. Trudy Geologischeskogo Instituta, Academiya Nauk SSSR, 135, 1–288.
Petrov, O. M. (1967). Paleogeography of Chukotka during Late Neogene and Quaternary time. In D. M. Hopkins (Ed.), The Bering Land Bridge (pp. 144–171). Stanford, CA: Stanford University Press.
Petrov, O. M. (1982). Marine molluscs of the Anthropogene from the northern region of the Pacific. Academiya Nauk SSSR, 357, 1–142.
Petrov, O. M. (1986). Geological history of the Bering Strait in the Late Cenozoic. In V. L. Kontrimavichus (Ed.), Beringia in the Cenozoic Era (pp. 30–45). Rotterdam, the Netherlands: Balkema.
Philippi, R. A. (1845). Kritische Bemerkungen über einige Trochus-Arten und die Gattung Axinus. Zeitschrift für Malacozoologie, 2(6), 87–91.
Pilsbry, H. A. (1916). The sessil barnacles (Cirripedia) contained in the collection of the U. S. National Museum; including a monograph of the American species. United States National Museum, Bulletin, 93, 1–366.
Poppe, G. T., & Goto, Y. (1991). European seashells (1) (352 pp). Wiesbaden, Germany: Christa Hemmen.
Poppe, G. T., & Goto, Y. (1993). European seashells (2) (221 pp). Wiesbaden, Germany: Christa Hemmen.
Posselt, H. J., & Jensen, A. S. (1898). Grønlands Brachiopoder og Bløddyr. Meddelelser om Grønland, 23, 1–298.
Poulsen, C. M. (1884). On the fossil Crag molluscs of the Tjörnes. Unpublished manuscript. University of Copenhagen, Copenhagen, Denmark.
Poulsen, E. M. (1935). De danske farvandes rurer (Balanomorpha og Verrucomorpha). Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening, 99, 5–27.
Pouwer, R., & Wesselingh, F. P. (2012). De fossiele schelpen van de Nederlandske kust II. (deel 3). Trochidae, Solariellidae en Calliostomatidae. Spirula, 389, 151–164.
Rasmussen, E. (1973). Systematics and ecology of the Isefiord marine fauna (Denmark). Ophelia, 11, 1–507.
Reeve, L. A. (1855). Notes on the natural history. Account of shells collected by Captain Sir Edward Belcher, C. B., north of Beechey Island. In E. Belcher (Ed.), The last of the Arctic Voyages 2 (pp. 392–399). London: Reeve.
Reid, D. G. (1996). Systematics and evolution of Littorina (463 pp). London: The Ray Society.
Repenning, C. A., Brouwers, E. M., Carter, L. D., Marincovich, L. J., & Ager, T. A. (1987). The Beringian ancestry of Phenacomys (Rodentia: Cricetidae) and the beginning of the modern Arctic Ocean borderland biota. Bulletin. United States Geological Survey, 1687, 1–31.
Rowland, R. W., & Hopkins, D. M. (1971). Comments on the use of Hiatella arctica for determining Cenozoic sea temperatures. Palaeogeography, Palaeoclimatology, Palaeoecology, 9, 59–64.
Sars, G. O. (1878). Bidrag til kundskaben om Norges arktiske fauna I. Mollusca regionis arcticæ Norvegiæ (466 pp). Christiania, Norway: Brøgger.
Sars, M. (1865). Om de i Norge forekommende dyrelevninger fra Qvartærperioden, bidrag til vor faunas historie (134 pp). Christiania, Norway: Brøgger & Christie.
Scarlato, O. A. (1981). Bivalve molluscs of the temperate latitudes of the western part of the Pacific Ocean. Academyia Nauk SSSR, Zoologischeskii Institut, Opredeliteli po Fauna SSSR, 126, 480.
Schäfer, W. (1972). Ecology and palaeoecology of marine environments (568 pp). Edinburgh, Scotland: Oliver and Boyd.
Schenck, H. G. (1939). Revised nomenclature for some nuculid pelecypods. Journal of Paleontology, 13(1), 21–41.
Schiøtte, T. (1989). Marine Mollusca from Jørgen Brønlund Fjord, North Greenland, including the description of Diaphana vedelsbyae n. sp. Meddelelser om Grønland, Bioscience, 28, 1–24.
Schlesch, H. (1924). Zur Kenntnis der pliocänen Cragformation von Hallbjarnarstadur, Tjörnes, Nord-Island und ihrer Molluskenfauna. Archiv für Molluskenkunde, 1(3), 309–370.
Schlesch, H. (1931). Kleine Mittteilungen VII. Archiv für Molluskenkunde, 63(4-5), 133–155.
Schnetler, K. I. (2005). The Mollusca from the stratotype of the Gram Formation (Late Miocene, Denmark). Palaeobios, 7, 62–189.
Schumacher, C. F. (1817). Essai d’un nouveau système des habitations des vers testacés (287 pp). Copenhague, Denmark: Schultz.
Símonarson, L. A. (1981). Upper Pleistocene and Holocene marine deposits and faunas on the north coast of Nûgssuaq, West Greenland. Bulletin. Grønlands Geologiske Undersøgelse, 140, 1–107.
Símonarson, L. A. (2004). Rataskel og forn sjávarhiti. Náttúrufræðingurinn, 72(1-2), 29–34.
Símonarson, L. A., & Eiríksson, J. (2020). Systematic overview of the Molluscs and Barnacles of the Pliocene Barmur Group, North Iceland. In J. Eiríksson & L. A. Símonarson (Eds.), Pacific – Atlantic Mollusc migration. Cham, Switzerland: Springer. This volume, Chapter 7.
Símonarson, L. A., & Leifsdóttir, Ó. E. (2002). Jökultodda á Íslandi. Náttúrufræðingurinn, 71(1-2), 72–78.
Símonarson, L. A., & Leifsdóttir, Ó. E. (2007). Early Pleistocene molluscan migration to Iceland – Palaeoceanographic implication. Jökull, 57, 1–20.
Símonarson, L. A., & Leifsdóttir, Ó. E. (2009). Miguskeljar á Íslandi. Náttúrufræðingurinn, 78, 57–65.
Símonarson, L. A., Petersen, K. S., & Funder, S. (1998). Molluscan palaeontology of the Pliocene-Pleistocene Kap København Formation, North Greenland. Meddelelser om Grønland, Geoscience, 36, 1–104.
Sneli, J. A., Schiøtte, T., Jensen, K. R., Wikander, P. B., Stokland, Ø., & Sørensen, J. (2005). The marine Mollusca of the Faroes. Annales Societatis Scientarium Færoensis Supplementum, 42, 1–190.
Soot-Ryen, T. (1941). Northern Pelecypods in the collection of Tromsø Museum 1. Order Anomalodesmacea, families Pholadomyidae, Thraciidae and Periplomatidae. Tromsø Museums Årshefter, 61(1), 1–41.
Soot-Ryen, T. (1951). New records on the distribution of marine Mollusca in northern Norway. Astarte, 1, 1–11.
Sorgenfrei, T. (1958). Molluscan assemblages from the marine Middle Miocene of South Jutland and their environments. Geological Survey of Denmark. 2. Series, 1–2, 79, 1–503.
Sowerby, C. B. (1842). Monograph of the genus Pecten. In C. B. Sowerby (Ed.), Thesaurus conchyliorum, or figures and descriptions of shells 1(2) (pp. 45–82). London: Sowerby.
Spaink, G. (1975). Zonering van het mariene Onder-Pleistoceen en Plioceen op grond van mollusken-fauna’s. In W. H. Zagwijn & C. V. van Staalduinen (Eds.), Toelichting bij geologische Overzichtskaarten van Nederland (pp. 118–122). Haarlem, The Netherlands: Rikjs geologische Dienst.
Spärck, R. (1933). Contributions to the animal ecology of the Franz Joseph Fjord and adjacent East Greenland waters. I-II. Meddelelser om Grønland, 100(1), 1–38.
Spärck, R. (1937). The benthonic animal communities of the coastal waters (The zoology of Iceland 1(6)) (45 pp). Copenhagen, Denmark/Reykjavík, Iceland: Munksgaard.
Stephensen, K. (1933). Havedderkopper og rankefødder. Danmarks Fauna, 38, 1–158.
Stephensen, K. (1936). The Godthaab Expedition 1928. Crustacea varia. Meddelelser om Grønland, 80(2), 1–38.
Stephensen, K. (1938). Cirripedia (incl. Rhizocephala) (The zoology of Iceland 3(30-31)) (11 pp). Copenhagen, Demark/Reykjavík, Iceland: Munksgaard.
Stephensen, K. (1943). The zoology of East Greenland. Marine Ostracoda, parasitic and semi-parasitic Copepoda and Cirripedia. Meddelelser om Grønland, 121(9), 1–24.
Strauch, F. (1968). Determination of Cenozoic sea-temperatures using Hiatella arctica (Linné). Palaeogeography, Palaeoclimatology, Palaeoecology, 5, 213–233.
Strauch, F. (1972). Phylogenese, Adaptation und Migration einiger nordischer mariner Molluskengenera (Neptunea, Panomya, Cyrtodaria und Mya). Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 531, 1–210.
Strøm, H. (1768). Beskrivelse over norske insecter, 2. Det Kongelige Norske Videnskabers Selskabs Skrifter, 4, 313–371.
Sullivan, C. M. (1948). Bivalve larvae of Malpeque Bay, P. E. I. Bulletin of the Fisheries Research Board of Canada, 77, 1–36.
Tebble, N. (1966). British bivalve seashells. London: British Museum (Natural History).
Theisen, B. I. (1973). The growth of Mytilus edulis L. (Bivalvia) from Disko and Thule district, Greenland. Ophelia, 12(1), 59–77.
Thompson, T. E., & Brown, G. H. (1976). British opisthobranch molluscs. Synopses of the British Fauna (New Series), 8, 1–203.
Thorson, G. (1933). Investigations on shallow water animal communities in the Franz Joseph Fjord (East Greenland) and adjacent waters. Meddelelser om Grønland, 100(2), 1–70.
Thorson, G. (1934). Contributions to the animal ecology of the Scoresby Sound fiord complex (East Greenland). Meddelelser om Grønland, 100(3), 1–68.
Thorson, G. (1935). Studies on the egg-capsules and development of arctic marine prosobranchs. Meddelelser om Grønland, 100(5), 1–71.
Thorson, G. (1936). The larval development, growth, and metabolism of arctic marine bottom invertebrates. Meddelelser om Grønland, 100(6), 1–155.
Thorson, G. (1941). Marine Gastropoda Prosobranhiata (The zoology of Iceland 4(60)) (150 pp). Copenhagen, Denmark/Reykjavík, Iceland: Munksgaard.
Thorson, G. (1944). The zoology of East Greenland. Marine Gastropoda Prosobranchiata. Meddelelser om Grønland, 121(13), 181.
Thorson, G. (1946). Reproduction and larval development of Danish marine bottom invertebrates. Meddelelser fra Kommissionen for Danmarks Fiskeri- og Havundersøgelser. Plankton, 4, 1–523.
Thorson, G. (1951). The Godthaab Expedition 1928. Scaphopoda, Placophora, Solenogastres, Gastropoda Prosobranciata, Lamellibaranchiata. Meddelelser om Grønland, 81(2), 1–117.
Thorson, G. (1957). Bottom communities (sublittoral or shallow shelf). In J. Hedgpeth (Ed.), Treatise on marine ecology and paleoecology 1 (pp. 461–534). Boulder, CO: Geological Society of America.
Torell, O. M. (1859). Bidrag till Spitsbergens molluskfauna. Jemte en allmän öfversigt af arktiska regionens naturförhållanden och forntida utbredning. I (152 pp). Stockholm: Typografiska föreningens bogtryckeri.
Turton, W. (1834). Description of some nondescript and rare British species of shells. The Magazine of Natural History, and Journal of Zoology, Botany, Mineralogy, Geology, and Meteorology, 7, 350–353.
Ussing, H. (1934). Contribution to the animal ecology of the Scoresby Sound Fjord complex (East Greenland). III. The hydrographical conditions in the Scoresby Sound Fjord complex. Meddelelser om Grønland, 100(3), 10–17.
Vahl, O. (1971). Growth and density of Patina pellucida (L.) (Gastropoda: Prosobranchia) on Laminaria hyperborea (Gunnerus) from western Norway. Ophelia, 9, 31–50.
Verhoeven, K., Louwye, S., Eiríksson, J., & De Schepper, S. (2011). A new age model for the Pliocene-Pleistocene Tjörnes section on Iceland: Its implication for the timing of North Atlantic-Pacific palaeoceanographic pathways. Palaeogeography, Palaeoclimatology, Palaeoecology, 309, 33–52.
Vilhjálmsson, M. (1985). The Lower Pleistocene mollusc fauna of the Breidavík Beds, Tjörnes, North Iceland. Cand. scient. thesis. University of Copenhagen, Copenhagen, Denmark, 207 pp.
Wagner, F. J. E. (1970). Faunas of the Pleistocene Champlain Sea. Geological Survey of Canada, Bulletin, 181, 1–104.
Warén, A. (1974). Revision of the Arctic-Atlantic Rissoidae (Gastropoda, Prosobranchiata). Zoologica Scripta, 3, 121–135.
Warén, A. (1989). Taxonomic comments on some protobranch bivalves from the northeastern Atlantic. Sarsia, 74, 223–259.
Warén, A. (1996a). Ecology and systematics of the northern European species of Rissoa and Pusillina (Prosobranchia: Rissoidae). Journal of the Marine Biological Association of the United Kingdom, 76, 1013–1059.
Warén, A. (1996b). New and little known Mollusca from Iceland and Scandinavia. Part 3. Sarsia, 81, 197–245.
Wesselingh, F. P., & Pouwer, R. (2011). De fossile schelpen van de Nederlandske kust II. Patellogastropoda en Vestigastropoda. Spirula, 383, 129–142.
West, R. G., Funnell, B. M., & Norton, P. E. P. (1980). An Early Pleistocene cold marine episode in the North Sea: Pollen and faunal assemblages at Covehithe, Suffolk, England. Boreas, 9(1), 1–10.
Winkler, G. G. (1863). Island, der Bau seiner Gebirge und dessen geologische Bedeutung: nach eigenen, dort ausgeführten Untersuchungen (303 pp). München, Germany: E. H. Gummi.
Wood, S. V. (1848). A monograph of the Crag Mollusca, or, descriptions of shells from the Middle and Upper Tertiaries of the east of England 1. Univalves (208 pp). London: The Palæontographical Society.
Wood, S. V. (1851). A monograph of the Crag Mollusca, or, descriptions of shells from the Middle and Upper Tertiaries of the east of England 2. Bivalves (150 pp). London: The Palæontographical Society.
Wood, S. V. (1853). A monograph of the Crag Mollusca, or, descriptions of shells from the Middle and Upper Tertiaries of the east of England 2. Bivalves., 151–216.
Wood, S. V. (1857). A monograph of the Crag Mollusca with description of shells from the Upper Tertiaries of the British Isles 2. Bivalves. Monographs of the Palaeontographical Society, 9(33), 217–342.
Wood, S. V. (1872). Supplement to the Crag Mollusca comprising Testacea from the Upper Tertiaries of the east of England 1. Univalves (98 pp). London: The Palæontographical Society.
Zagwijn, W. H. (1975). Correlatives van laat-tertiaire en kwartaire afzettingen in de gebieden rond de Noordzee. In W. H. Zagwijn & C. V. van Staalduinen (Eds.), Toelichting bij geologische Overzichtkaarten van Nederland (pp. 122–123). Haarlem, The Netherlands: Rikjs geologische Dienst.
Zagwijn, W. H. (1992). The beginning of the Ice Age in Europe and its major subdivisions. Quaternary Science Reviews, 11, 583–591.
Zarkhidze, V. S., & Samoilovich, Y. G. (1989). Late Cenozoic stratigraphy and paleoceanography of the Barents Sea. In Y. Herman (Ed.), The Arctic Seas (pp. 721–727). New York: Van Norstrand Reinhold Company.
Zenkevitsch, L. (1963). Biology of the seas of the U.S.S.R (955 pp). London: George Allen & Unwin.
Zullo, V. A. (1968). Balanus hopkinsi, new species, and Balanus balanus (Linnaeus, 1758) (Cirripedia, Thoracia) from Plio-Pleistocene sediments on Tjörnes, northern Iceland. Occasional Papers of the California Academy of Sciences, 69, 1–11.
Plates
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s)
About this chapter
Cite this chapter
Símonarson, L.A., Eiríksson, J. (2021). Systematic Overview of the Molluscs and Barnacles of the Quaternary Breiðavík Group North Iceland. In: Eiríksson, J., Símonarson, L.A. (eds) Pacific - Atlantic Mollusc Migration . Topics in Geobiology, vol 52. Springer, Cham. https://doi.org/10.1007/978-3-030-59663-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-59663-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59662-0
Online ISBN: 978-3-030-59663-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)