Abstract
Craniotomy has generally been the standard of care for removing skull base meningiomas. Over the last 15 years, endoscopic and minimally invasive neurosurgery has become a popular option as a means of achieving ideal neurosurgical results by either using natural corridors or creating corridors to access the pathology. Skull base meningiomas, in selected cases, are ideal pathologies for these techniques, which can offer an alternative approach without compromising oncologic results. All three techniques discussed, EEA (endoscopic endonasal approach), SKM (supraorbital keyhole minicraniotomy), and TOA (transorbital approach), follow these foundational principles of minimally invasive neurosurgery and also utilize the endoscope either as the main source of visualization or as a valuable adjunct for improved visualization of surgical field. The key to successful application is selecting the right technique for each tumor and to have the necessary experience, technical skill, equipment, and the anatomical knowledge to execute the procedure to achieve an ideal surgical outcome.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Rationale and Background
In the last 15 years, several minimally invasive approaches have been developed that can be used in the removal of skull base meningiomas. Meningiomas of the anterior skull base have been the target for most endoscopic and minimally invasive approaches. We will focus on these lesions as we discuss how these approaches can be utilized in meningioma surgery. The minimally invasive techniques discussed will include EEA (endoscopic endonasal approach), SKM (supraorbital keyhole minicraniotomy) which involves an eyebrow incision and utilizes endoscopic visualization, and TOA (transorbital approach) which uses the four quadrants of the orbit as a natural access corridor.
Meningiomas of the anterior skull base most frequently cause symptoms by exerting mass effect on the frontal lobes, optic nerves, chiasm, and olfactory nerves. They may cause hydrocephalus and sometimes extensively involve the bony skull base . These tumors have been traditionally approached through transcranial route including pterional, bifrontal, transbasal, and orbitozygomatic approaches. These approaches can sometimes involve retraction of normal brain, require manipulation of neurovascular structures, and can involve air sinuses that can potentially create postoperative sequelae related to wound healing and unwanted cosmetic outcomes [1,2,3,4].
Endoscopic and minimally invasive surgery follows the concepts of keyhole approach in neurosurgery. This concept can be summarized by reducing the size of the craniotomy, making smaller dural openings, limiting cortical exposure, and minimizing normal brain manipulation. These key principles result in shorter hospital stay and minimize the risk of complications. This requires a conceptual change of predefined surgical corridors to individually tailored approaches [5].
Endoscopic, especially endonasal, approaches provide a direct access to skull base meningiomas that obviates the need for brain retraction. Also, it can take the neurosurgeon directly to pathology, without placing the optic nerves or carotid arteries between the surgeon and the tumor. EEA can provide complete, bilateral optic canal decompression without manipulation of a compressed optic nerve. Additionally, approaching these tumors from below enables the surgeon to remove the bone at the base of the tumor, which is a common site for meningioma recurrence and can interrupt the dural vascular supply early in the operation, minimizing blood loss [6].
The surgical treatment goals for meningiomas are not different when minimally invasive techniques are utilized. These goals include removal of the tumor, infiltrated dura, and bone. The skull base has a complex neurovascular anatomy that usually is intimately involved in the pathology. This will limit achieving these goals, and individual strategies have to be tailored based on the pathological anatomy, age of the patient, and availability of alternative treatment modalities [7] (Fig. 7.1).
Decision-making algorithm for the resection of anterior skull base meningiomas [7]. (Reprinted from Ottenhausen et al. [7], https://thejns.org/focus/view/journals/neurosurg-focus/44/4/article-pE1.xml, © 2018, with permission from Journal of Neurosurgery and AANS)
Endoscopic Endonasal Approach to Skull Base Meningiomas
EEA through the ethmoids and fovea ethmoidalis , cribriform plate, planum sphenoidale, tuberculum sella, sella, and clivus have developed as a useful alternative to conventional open cranial approaches [1]. The endoscope, by carrying the lens and light source closer to the focus of the operation, creates a panoramic, high-resolution image that surpasses the capability of the microscope. The anatomic and pathologic detail offered to the surgeon by the endoscope is even more enhanced by used of angled scopes [8].
Making a decision to utilize EEA in order to resect a meningioma of the anterior skull base requires a thorough understanding of the pathological anatomy of the lesion and the structures around the lesion, assessment of the size of the potential surgical corridor and its ability to provide bimanual dissection, and exposure adequate to remove the lesion. Reconstruction of the dura and skull base is an integral part of this thought process as well and should be planned ahead before the operation. The pathological anatomy of lesion and its surroundings, which include tumor location and its extent, degree of bony invasion and hyperostosis, vascularity of the tumor, brain edema and/or invasion and encasement of blood vessels, must be taken into consideration . Meningiomas usually present with a broad dural base, an enhancing dural tail, and hyperostosis and/or involvement of the neighboring bone. The involvement of the bone in meningiomas makes the endoscopic endonasal approach ideal for these lesions because it has the capability of removing all the involved bone in the initial phase of the approach. Tumor vasculature which is supplied by the dural base of the meningioma is again addressed by the endonasal approach earlier in the operation decreasing the amount of bleeding throughout the case. Tumors that do not extend laterally to the lamina papyracea or anteriorly to the frontal sinus are generally considered accessible for endonasal approaches [1].
In our experience, planum sphenoidale and tuberculum sella meningiomas are the best candidates for endonasal surgery [7]. Olfactory groove meningiomas can also be removed safely through an endonasal route; however, we prefer an eyebrow incision with a supraorbital craniotomy for larger tumors or those that abut the posterior wall of the frontal sinus [9] (Fig. 7.2). If a Simpson grade I resection (gross total resection with the associated tumor-infiltrated dura) is the goal of surgery , the endonasal approach must be paired with a close inspection of the relevant anatomy. Invasion of the medial optic canals should be appreciated before surgery, and, if necessary, the optic canals can be removed within the sphenoid sinus to ensure complete tumor removal. Nevertheless, in certain patients, aggressive debulking to alleviate symptoms followed by radiosurgery and fractionated radiation may be the preferred strategy [4].
Endoscopic endonasal approach also makes possible a less morbid decompression of the optic apparatus in frail or elderly patients who are losing vision in preparation for postresection radiation or observation. This approach also offers the advantage below which is the ability to attack the dural vascular supply to the tumor early in the operation. Since not only the dura but also the bone at the base of the tumor is removed during the approach , the transtuberculum transplanum approach potentially offers a higher likelihood of curing these meningiomas. Endoscopic opening of the optic canals bilaterally can help in accessing all of the tumor remnants that are located inferior to the optic nerve [4, 10]. A detailed neuro-ophthalmologic examination with visual field testing is recommended for patients with visual symptoms or optic apparatus compression on imaging. If the lesion is in vicinity of the pituitary gland, full endocrinological workup is usually indicated.
MRI of the brain and specifically a pituitary protocol MRI with fine coronal cuts is required for tumors of the sella. The location of the septations within the sphenoid sinus and the location of carotid arteries should be determined by CT or CTA as needed for all anterior skull base cases.
The pneumatization level of the sphenoid sinus, septal perforations, and spurs should be identified to establish quality and side of the nasoseptal flap [6].
Indications, Contraindications, and Considerations
Any anterior skull base meningioma exerting mass effect, causing neurologic, endocrinologic, or ophthalmologic symptoms, or any lesion which requires a tissue diagnosis is an indication for EEA to either resect or biopsy these lesions as necessary. Large tumors may not be completely resected using an endonasal approach . Depending on the age, comorbidities of the patient and the surgical goals, internal decompression, or staged resection with additional approaches may be utilized [11].
Lateral extension of the tumor relative to the orbits and the carotid artery creates a relative contraindication. Pathology which extends laterally over the orbits or lateral to or behind the carotid arteries is difficult to remove using EEA. The width of the planum sphenoidale, between the laminae papyracea, defines the preferred corridor by the EEA [6]. Visualization, with use of angled scopes, can be achievable, but resection of a lesion around a corner may not be technically feasible [12]. Encasement of neurovascular structures and cavernous sinus invasion is not a contraindication to EEA [13], but the possibility of radiosurgery and fractionated radiation to control the growth of residual unresectable meningioma should always be considered before tackling dissection of a tumor off a vital structure [12].
Undeniably, EEA has the disadvantage of a narrow working corridor reducing the measurability of surgical instruments. Careful analysis of the anatomy of the lesion and the skull base, combined with knowledge of the restrictions, can overcome these limitations.
Operating through the nose carries the theoretical risk of intracranial infection and CSF leak, given the challenge sterilizing the nose and reconstructing the dura and skull base from below, respectively. Surgeon’s expertise with endoscopic techniques and knowledge of the limitations of the endonasal corridor affects operative quality and time which may significantly impact patient outcomes [1, 14].
Large tumors or tumors that are located laterally may not be resectable in a gross total fashion. But this should not be a contraindication. Endoscopic approaches may still have value, in these circumstanses, when age and condition of the patient may require other strategies such as internal decompression and/or staged resections [12].
Surgical Technique
Instruments
Endoscopic instruments are designed differently than microsurgical instruments. Bayoneted instruments are not usually preferred . Specially designed bipolar coagulation is used for intradural hemostasis. 18′ and 30′ endoscopes with 0-, 30-, and 45-degree lenses provide extended visualization, and scopes can be held by stationary holders or held by a second surgeon for a dynamic visualization. Dynamic visualization can help the neurosurgeon overcome the two- dimensionality of the visual output of the endoscope since the movement of the endoscope helps depth orientation. By bringing the lens and light source directly to the target, the endoscope provides a major advantage when compared to the microscope [6, 14].
The Approach
The head is placed in rigid fixation and registered with frameless neuronavigation, with the neck slightly extended to facilitate visualization of the cribriform plate and fovea ethmoidalis. The abdomen or thigh is prepared for harvesting autologous fat and fascia lata for multilayered skull base reconstruction. A long nasoseptal flap is harvested. A bilateral transethmoidal, transsellar, transtuberculum transplanum, transcribriform approach is performed depending on the location of the meningioma. This exposes the skull base from the back of the frontal sinus to the sella between the fovea ethmoidalis bilaterally to expose the meningioma. The anterior and posterior ethmoidal arteries are identified, cauterized, and transected at the junction between the fovea ethmoidalis and lamina papyracea. The skull base is drilled down to the dura circumferentially around the base of the tumor which is removed en bloc, exposing the attachment of the tumor. The dura is cauterized and internal decompression is performed. Once radical decompression is completed, the margins of the tumor can be mobilized into the cavity created in the center of the tumor. We generally remove the top of the tumor first, then open the canals and remove the lateral components, and then open below the superior intercavernous sinus and remove the bottom of the tumor. The closure is multilayer and has evolved at our institutions. We prefer a gasket seal closure in which an onlay of dural substitute or fascia lata is held in place with a rigid buttress of Medpore wedged into the bone defect [15, 16]. This construct is then covered with a nasoseptal flap and tissue sealant [17].
The Supraorbital Keyhole Craniotomy for Meningiomas
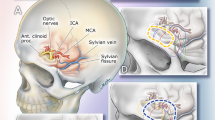
SKM is one of the minimally invasive techniques that can be utilized in the treatment of meningiomas. It has become increasingly popular due to its cosmetically appealing eyebrow (or eyelid) incisions and the ability to access the skull base through an incision on the forehead. It offers a corridor that can be used to treat most of the anterior cranial fossa meningiomas as well as parasellar ones [7]. It is usually used as a microscopic approach, but an endoscope can always be used to visualize and access areas which are otherwise hard to access with the microscope [5] (Fig. 7.3).
Indications, Contraindications, and Considerations
SKM offers the advantage of accessing neurovascular structures like the optic nerves and the chiasm and the ICAs from both a superior and an inferior perspective and is ideal for meningiomas that extend to superior or lateral to these structures. It can be performed with significantly reduced brain retraction when compared to traditional counterparts like the bicoronal or pterional approach [5, 7, 17].
SKM may not always provide the necessary maneuverability for midline meningiomas, especially if Simpson grade I resection is the surgical goal since it technically is hard to remove the tumor invading into the skull base at the midline especially at the level of the cribriform plate and crista galli. In addition , if a defect is created into the aerated nasal sinuses, it can be difficult to appreciate or repair [18]. Another limitation of SKM is limited visibility of the ipsilateral medial optic canal, although the contralateral medial optic canal is well-exposed [19].
Approach
The patient is placed in rigid head fixation and navigation is employed. Slight rotation and extension of the head is performed to facilitate the frontal lobe falling away from the skull base. An incision is made in the eyebrow in the direction of the follicles, and dissection is carried out through the orbicularis oculi muscle. A pericranial flap is raised. A single-piece craniotomy is performed, ideally including the orbital rim, from the keyhole to just medial to the supraorbital nerve. We prefer to remove the orbital rim to enlarge the working corridor and maximize upward visualization and facilitate exposure of the cribriform plate. Bony prominences on the roof of the orbit are flattened with high-speed drill. The dura is opened in a C-shape and reflected toward the orbit. CSF is drained either via a previously placed lumbar drain or through the subarachnoid opening until the brain is relaxed. The tumor is internally decompressed and sharply dissected off surrounding structures in a standard microsurgical fashion. Given the small opening, the most difficult aspect of the operation is the removal of the tumor deep in the cribriform plate and anterior along the crista galli in cases of olfactory groove meningiomas. To obtain adequate visualization of these areas, we may use endoscope assistance with a 45° endoscope. The base of the tumor has to be removed down to the bone. If a defect is anticipated, or if the tumor extends into the ethmoids, we prefer to combine the supraorbital keyhole approach with the endoscopic endonasal approach, so the defect can be patched from below, and a nasoseptal flap can be used [17].
The Transorbital Endoscopic Eyelid Approach for Meningiomas
The EEA together with SKA has achieved in proving a minimally invasive alternative to a variety of anterior skull base meningiomas except orbital and lateral sphenoid wing meningiomas that extended to anterior skull base, namely, spheno-orbital meningiomas, due to their lateral location [20] (Fig. 7.4). The lateral limit of the endoscopic endonasal approach has been the orbits, and the conventional open craniotomy techniques have been utilized to access these areas despite their cosmetic and functional disadvantages [21]. Gross total resection, with removal of the affected dura and drilling of the hyperostotic bone, might not be safely achieved in more than 50% of patients [22,23,24]. Progressive enlargement of those tumors is followed by compression of surrounding structures, such as the orbit, superior orbital fissure, optic canal, and cavernous sinus, which may lead to visual deterioration, disturbance of ocular movements, proptosis, and cosmetic alterations [22] (Fig. 7.5).
Transorbital approach to spheno-orbital meningiomas [20]. ((C) Weill Cornell Medicine)
A variety of skull base approaches may be used for excision of those lesions, including the pterional, orbitozygomatic, and subfrontal approaches. Recently, a multiportal transorbital neuroendoscopic surgery (TONES) approach has been proposed as a new minimally invasive option to reach the lateral orbit and middle fossa [25, 26].
With the development of endoscopic techniques, the transorbital endoscopic approaches may replace a lateral craniotomy in selected cases. This selection should be based on symptom relief since gross total resection is often not achieved with ease with any of the approaches. Debulking of the hyperostosis and orbital decompression would be the primary goals of surgery, rather than complete tumor removal [20].
Indications, Contraindications, and Considerations
Spheno-orbital meningiomas that involve the lateral orbit are the typical indications in this tumor group. Like in other skull base approaches, concern for CSF exists as well as possibility of orbital damage, but so far there is not enough data for comparative analysis of these risks [20, 26, 27]. Like in other minimally invasive approaches, patient selection and balancing the surgical goals with the approach is of paramount importance.
Approach
After induction of general anesthesia, the patient is placed supine, and head is fixed after gentle elevation with a Mayfield head holder. A sterile protective corneal shield lubricated with ophthalmic ointment is placed in the eye. We employ oculoplastic to help with the initial aspects of the approach as well as the closure, but this is not required.
The orbicularis layer is opened after a superior eyelid approach along the length of the incision to expose the periosteum of the lateral orbital rim. The periorbita is elevated along the inner aspect of the lateral orbital rim using a freer elevator. The orbit may be protected by a silastic sheet. The hyperostotic bone of the orbit is exposed along the lateral orbit posteriorly to the level of the superior orbital fissure and superiorly under the anterior cranial fossa. CT image guidance is used to aid in identifying the orbital landmarks. Once adequate exposure is obtained, decompression of the hyperostotic bone and tumor removal are done using high-speed drills and Kerrison rongeurs. The medial landmarks were the superior and inferior orbital fissures. Any CSF leak can be closed with inlay and onlay of dural substitute. The relaxation of the orbit generally provides adequate pressure to prevent a CSF leak. The eyelid incision is closed using interrupted 6–0 nylon sutures [20].
Conclusion
Several minimally invasive approaches can be employed for removal of meningiomas, particularly those occurring along the anterior skull base. Patient selection is crucial in the successful application of these methods as well as surgeon’s experience and proper instrumentation. When coupled with the right indication, an experienced minimally invasive neurosurgeon can achieve the expected oncologic results with minimal morbidity using one or a combination of these techniques.
References
Woodworth G, McCoul E, Anand V, Greenfiled J, Schwartz T. Endoscopic management of anterior cranial fossa meningiomas. Oper Tech Otolaryngol Head Neck Surg. 2011;22(4):254–62.
Patel SG, Singh B, Polluri A, Bridger PG, Cantu G, Cheesman AD, et al. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003;98(6):1179–87.
Origitano TC, Petruzzelli GJ, Leonetti JP, Vandevender D. Combined anterior and anterolateral approaches to the cranial base: complication analysis, avoidance, and management. Neurosurgery. 2006;58(4 Suppl 2):ONS-327-36; discussion ONS-36–7.
Schwartz TH, Fraser JF, Brown S, Tabaee A, Kacker A, Anand VK. Endoscopic cranial base surgery: classification of operative approaches. Neurosurgery. 2008;62(5):991–1002; discussion -5.
Reisch R, Perneczky A, Filippi R. Surgical technique of the supraorbital key-hole craniotomy. Surg Neurol. 2003;59(3):223–7.
Khan O, Anand V, Raithatha R, Schwartz TH. Endoscopic surgery of the Sella and Suprasellar region. In: Sataloff RT, editor. Sataloff’s comprehensive textbook of otolaryngology: head and neck surgery. New Delhi: Jaypee Brothers Medical Pub; 2016.
Ottenhausen M, Rumalla K, Alalade AF, Nair P, La Corte E, Younus I, et al. Decision-making algorithm for minimally invasive approaches to anterior skull base meningiomas. Neurosurg Focus. 2018;44(4):E7.
Schaberg MR, Anand VK, Schwartz TH, Cobb W. Microscopic versus endoscopic transnasal pituitary surgery. Curr Opin Otolaryngol Head Neck Surg. 2010;18(1):8–14.
Shetty SR, Ruiz-Treviño AS, Omay SB, Almeida JP, Liang B, Chen YN, et al. Limitations of the endonasal endoscopic approach in treating olfactory groove meningiomas. A systematic review. Acta Neurochir. 2017;159(10):1875–85.
Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal versus open transcranial resection of anterior midline skull base meningiomas. World Neurosurg. 2012;77(5–6):713–24.
Silva D, Attia M, Kandasamy J, Alimi M, Anand VK, Schwartz TH. Endoscopic endonasal transsphenoidal “above and below” approach to the retroinfundibular area and interpeduncular cistern--cadaveric study and case illustrations. World Neurosurg. 2014;81(2):374–84.
Schwartz TH, Anand VK. The endoscopic endonasal transsphenoidal approach to the suprasellar cistern. Clin Neurosurg. 2007;54:226–35.
Khan OH, Anand VK, Schwartz TH. Endoscopic endonasal resection of skull base meningiomas: the significance of a “cortical cuff” and brain edema compared with careful case selection and surgical experience in predicting morbidity and extent of resection. Neurosurg Focus. 2014;37(4):E7.
Schwartz TH, Anand VJ. Endonasal transplanum approach to the anterior cranial fossa. In: Snyderman C, Gardner P, editors. Master techniques in otolaryngology-head and neck surgery. Philadelphia: LWW; 2014.
Leng LZ, Brown S, Anand VK, Schwartz TH. “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery. 2008;62(5 Suppl 2):ONSE342–3; discussion ONSE3.
Garcia-Navarro V, Anand VK, Schwartz TH. Gasket seal closure for extended endonasal endoscopic skull base surgery: efficacy in a large case series. World Neurosurg. 2013;80(5):563–8.
Banu MA, Mehta A, Ottenhausen M, Fraser JF, Patel KS, Szentirmai O, et al. Endoscope-assisted endonasal versus supraorbital keyhole resection of olfactory groove meningiomas: comparison and combination of 2 minimally invasive approaches. J Neurosurg. 2016;124(3):605–20.
Borghei-Razavi H, Truong HQ, Fernandes-Cabral DT, Celtikci E, Chabot JD, Stefko ST, et al. Minimally invasive approaches for anterior skull base meningiomas: supraorbital eyebrow, endoscopic endonasal, or a combination of both? Anatomic study, limitations, and surgical application. World Neurosurg. 2018;112:e666–e74.
Singh H, Essayed WI, Jada A, Moussazadeh N, Dhandapani S, Rote S, et al. Contralateral supraorbital keyhole approach to medial optic nerve lesions: an anatomoclinical study. J Neurosurg. 2017;126(3):940–4.
Almeida JP, Omay SB, Shetty SR, Chen YN, Ruiz-Treviño AS, Liang B, et al. Transorbital endoscopic eyelid approach for resection of sphenoorbital meningiomas with predominant hyperostosis: report of 2 cases. J Neurosurg. 2018;128(6):1885–95.
Altay T, Patel BC, Couldwell WT. Lateral orbital wall approach to the cavernous sinus. J Neurosurg. 2012;116(4):755–63.
Oya S, Sade B, Lee JH. Sphenoorbital meningioma: surgical technique and outcome. J Neurosurg. 2011;114(5):1241–9.
Ringel F, Cedzich C, Schramm J. Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery. 2007;60(4 Suppl 2):214–21; discussion 21–2.
Moe KS, Bergeron CM, Ellenbogen RG. Transorbital neuroendoscopic surgery. Neurosurgery. 2010;67(3 Suppl Operative):ons16–28.
Ramakrishna R, Kim LJ, Bly RA, Moe K, Ferreira M Jr. Transorbital neuroendoscopic surgery for the treatment of skull base lesions. J Clin Neurosci. 2016;24:99–104.
Dallan I, Castelnuovo P, Locatelli D, Turri-Zanoni M, AlQahtani A, Battaglia P, et al. Multiportal combined transorbital transnasal endoscopic approach for the management of selected skull base lesions: preliminary experience. World Neurosurg. 2015;84(1):97–107.
Bly RA, Morton RP, Kim LJ, Moe KS. Tension pneumocephalus after endoscopic sinus surgery: a technical report of multiportal endoscopic skull base repair. Otolaryngol Head Neck Surg. 2014;151(6):1081–3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Omay, S.B., Schwartz, T.H. (2020). Endoscopic and Minimally Invasive Meningioma Surgery. In: Moliterno, J., Omuro, A. (eds) Meningiomas. Springer, Cham. https://doi.org/10.1007/978-3-030-59558-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-59558-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59557-9
Online ISBN: 978-3-030-59558-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)