Abstract
Biochanin A and biochanin B (Formononetin) are the principal dietary isoflavones extensively found in herbal formulations of red clover extract (Trifolium pratense). The red clover-derived isoflavonoids have therapeutic application for mitigating the postmenopausal symptoms in women and are commercially available and marketed as tablets, capsules, and tea preparations. These are also consumed widely with the allopathic medicines without any prescription. However, the phytopharmaceuticals have less or minimum adverse effects and side effects, but the multidrug therapy is also one of the crucial aspects responsible for many serious clinical drug-drug and drug-herb interactions. Further, the main mechanism underlying the pharmacokinetic interactions is the potency to modulate the cytochrome P450 enzymes that are dynamically involved in the metabolic conversion of the drugs into their metabolites and alteration in membrane ATP-binding cassette transporters activity. In the present chapter, the phytochemistry and pharmacological importance of biochanin A and formononetin, along with their pharmacokinetic characteristics will be discussed. Furthermore, alteration in functional efficacy of cytochromes, transporters and mechanistic incrimination of their interaction potential with other prescribed drugs will also be exhaustively reviewed after considering the past ten years literatures based on available pre-clinical and clinical data.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Isoflavonoids gained popularity due to their resemblance with the endogenous estrogen agonists structurally as well as pharmacologically and the potential of being used as safe alternatives in estrogen replacement therapy. They are widely distributed in the leguminous and non-leguminous families of plant kingdom (Veitch 2013). The present chapter particularly deals with the phytoestrogenic isoflavones, that is, biochanin B also known as formononetin (FMN) and biochanin A (BCA). Chemically, the BCA is written as 5,7-dihydroxy-40-methoxyisoflavone and FMN is 7-hydroxy-4′-methoxyisoflavone. The chemical structure of FMN and BCA is represented in Fig. 14.1. Although both isoflavones are extracted at high amount from the same plant, but the precursor of both FMN and BCA is different, that is, liquiritigenin (7,40-dihydroxyflavanone) for FMN and naringenin for BCA (Fig. 14.2). In the last two decades, there has been an increasing interest in these compounds as beneficial biochemical and pharmacological properties have been reported for a number of isoflavonoids. Their basic chemical structure consists of benzene ring that is linked by a heterocyclic pyran or pyrone ring and a phenyl ring (Wang 2011). The majorly distributed isoflavonoids in soy are daidzein, genistein, and glycitein and their glycoside conjugates, including 7-O-glucosides, i.e., daidzin, genistin, and glycitin (Barnes et al. 1994). In red clover, the principal isoflavonoids are FMN and BCA and their 7-O-glucosides, ononin and sissotrin (Raju et al. 2019).
Isoflavonoids are regarded as important nutraceuticals mainly due to their antioxidant effects, which give them a potential role in prevention of the various diseases associated with oxidative stress (Dixon and Pasinetti 2010). Understanding the basis of the health benefits derived from isoflavonoids requires a detailed knowledge on the absorption, distribution, metabolism, elimination (ADME), and bioavailability of these phytoestrogens. After ingestion, soy isoflavonoids are biotransformed in the intestinal tract, a process that is highly dependent on intestinal bacterial metabolism. Several major groups of colonic bacteria possess β-glucosidase activity, including Lactobacillus spp., Bacteroides spp., and Bifidobacterium spp. The respective isoflavone glucosides are hydrolyzed by both intestinal mucosal and bacterial β-glucosidases releasing the aglycones, which are then either absorbed directly or further metabolized by intestinal microflora in the large intestine into other metabolites, including equol (Setchell et al. 2001; Zhang et al. 2014b). Differences in the absorption rates between the glucosylated and aglycone forms of isoflavones were reported suggesting that not all isoflavonoids can be considered in the same form if present in different types of foods (Almeida et al. 2015). The absorption of these isoflavonoids would thus seem to be controlled by enzyme specificity and distribution. After initial absorption, FMN and BCA (aglycones) undergo extensive first-pass metabolism. The resulting glucuronide and sulfate conjugates could be transported through the systemic circulation to the tissue from where they can be excreted from the kidneys or secreted into bile and return to the intestine (Spencer and Crozier 2012). In the large intestine, the microbiota further degrade isoflavones; both daidzein and genistein can be further metabolized to secondary metabolites with significant interest in the potential health effects of equol, which is a reduced metabolite of daidzein. The plasma Tmax of daidzein and genistein metabolites typically reached 6–8 h after isoflavonoid intake (Spencer and Crozier 2012).
Cytochrome P450 enzymes (P450s or CYPs) are involved in a wide variety of biotransformations including endogenous substrates (e.g., steroids, fatty acids, prostaglandins, leukotrienes) as well as exogenous compounds (xenobiotics, as drugs, environmental toxins, food preservatives) (De Montellano 2005). P450s are primarily responsible for most of drug metabolism reactions in tissues such as the liver and the gastrointestinal tract, brain, lung, kidney, and heart (Anzenbacher and Anzenbacherová 2001). Liver microsomal P450s take part in most of the reactions involving drug biotransformations and interaction of other drugs or bioactive compounds as isoflavonoids with these enzymes may significantly affect drug action and efficacy (Zanger and Schwab 2013). There were scattered reports available in the literature on the P450 induction or inhibition, however, often with extracts and with some P450 activities only – reviewed by Taneja et al. (2015).
14.2 Pharmacological Importance of FMN and BCA
Isoflavonoids gained popularity due to their resemblance with the endogenous estrogen agonists structurally as well as pharmacologically and the potential of being used as safe alternatives in estrogen replacement therapy. They have also been found to be neuroprotective and promote neuronal survival both in vivo and in vitro. They have been tried extensively for their therapeutic activity in so many diseases, and an abundance of publications is reported in the last decade.
Isoflavones have been found to cross the blood-brain barrier due to their highly lipophilic nature. This characteristic property of FMN and BCA has been applied by many researchers for their protective activity against neurodegenerative disorders. Both have been found to improve learning, logical thinking, and planning ability. Both FMN and BCA have been suggested to be a lead molecule for treating neurodegenerative disorders such as Alzheimer’s and Parkinson’s. It has been shown to provide neuroprotection against cerebral ischemia through modulation of concentration of antioxidants and inflammatory agents in the cells through Nrf2 signaling cascade (Guo et al. 2019). BCA and FMN also improve the cognitive neurobehavioral alteration through increasing the viable cells and ameliorating the degenerative cell count in cognitive deficit mice that further prove its potency towards treatment of Alzheimer’s disease (Biradar et al. 2014). It has been reported to inhibit lipopolysaccharide (LPS)-induced activation of microglia and the production of TNF-α, nitric oxide, and superoxide in mesencephalic neuroglia and microglia-enriched culture (Wang et al. 2016). Furthermore, both the isoflavones had also been proved to be an antihyperglycemic molecule when tested in streptozotocin-induced diabetic rats. BCA successfully reduces the glucose and glycosylated hemoglobin levels in plasma of diabetic rats. It had also normalized the amount of plasma insulin and various enzymes involved in glucose metabolism (Harini et al. 2012). BCA also indirectly enhances the autoimmunity of host involved in protection against many fungi and bacteria by enhancing the retinoic acid receptor-related orphan receptors (ROR) α and γ that play the major role in IL-17 cascade pathway (Takahashi et al. 2017). BCA might also be a useful alternative estrogen therapy as suggested by Galal et al. for the management of renal and cutaneous changes observed in postmenopausal women (Galal et al. 2018). Despite of all the abovementioned therapeutic activity, BCA and FMN have also been proclaimed as an anticancer and anti-invasion by modulating the cell growth and migration of MDA-MB-231, MCF-7 (breast cancer cell line), and HUVEC cells (Zakłos-Szyda and Budryn 2020), hepatoprotective alone or in combination with any other hepatoprotective drug (Chaturvedi et al. 2018; Liu et al. 2016; Youssef et al. 2016) and renoprotective agent (Suliman et al. 2018) through different translational and molecular signaling cascades (Sarfraz et al. 2020).
These isoflavones possessing antioxidant activity lower the risk of certain cancers like breast cancer, prostate cancer, etc. They have also been used as expectorants in asthma (T. Li et al., 2018a) by inducing the vasorelaxation in thoracic aorta through regulating the PI3K/PTEN/Akt signaling pathway. These isoflavones have also been utilized as an alternative therapy to treat psoriasis, eczema and other skin conditions, help in reducing the blood pressure, in cardiac ischemia by inhibition of inflammasome pathway (D.-S. Wang et al., 2020) and lowering the cholesterol levels in blood. Recently, hepatoprotective activity of FMN has also been investigated against acetaminophen-induced hepatotoxicity by enhancing the Nrf2 binding (Jin et al. 2017).
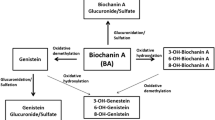
Apart from innumerable human health benefits and pharmacological activity, these isoflavonoids could not be directly administered due to their low oral bioavailability resulting to less systemic exposure. This role is played by pharmacokinetics of all the compounds in which the compound is absorbed, distributed throughout the organs, and metabolized mainly by liver cytochromes (microsomal enzymes) and sometimes other enzymes present in different organs. Further, the chapter will be discussing the pharmacokinetics of both the isoflavones and their modulation by various cytochrome enzymes and membrane transporters. The multimechanistic role of BCA and FMN has been diagrammatically represented in Fig. 14.3 and Table 14.1.
14.3 Pharmacokinetics of FMN and BCA
The isoflavonoids exist as biologically inactive glucoconjugates in their natural form (Setchell et al. 2001). They could be only absorbed in their active aglycone form which is the result of a biotransformation reaction through an intestinal bacteria undergoing a deglycosylation pathway. They are inactive and could not be absorbed post administration in their natural form. There are literatures suggesting the conversion of inactive isoflavonoids into their active metabolite starts from the mouth itself (Allred et al. 2001). The pharmacokinetics and bioavailability information are assessed on the basis of the isoflavone’s absorption, distribution, and metabolism and excretion data obtained from preclinical and clinical trials. Considering pharmacokinetics of FMN and BCA, they are reported to be metabolized into their demethoxylated isoforms, daidzein, and genistein. The schematic representation of metabolic pathway for both FMN and BCA is being detailed in Fig. 14.2. Many researchers have investigated about the pharmacokinetic characteristics of FMN and BCA under both in vitro and in vivo experimental conditions. Recently, pharmacokinetics, bioavailability, and permeation through Caco-2 cells of FMN were studied after oral and IV administration to rats at a dose of 20 mg/kg and 4 mg/kg. The investigators found that FMN showed absolute bioavailability of 21.8% after oral exposure with sufficient systemic exposure. Upon scrutinizing through Caco-2 cells, the efflux ratio of FMN was found to be within the range of 1.0–1.5, suggesting that there was no significant difference between permeability in either direction of bilayer, and transportation is basically across intestinal epithelial cells and was mainly through passive diffusion (Luo et al. 2018). The results from the latter mentioned research also manifest that FMN is largely absorbed from the large intestine of the gastrointestinal segment followed by the small intestine. This indicates the site-specific distribution of FMN and other related isoflavonoids (Luo et al. 2018). Similar pattern was also followed by BCA in another experiment performed by Jia and colleagues (Jia et al. 2004). The same was also justified by earlier studies through single-pass intestinal perfusion model (Liu et al. 2013). Zhang et al. have determined the pharmacokinetic behavior of FMN along with other isoflavones after oral administration of Buyang Huanwu Decoction to rats using tandem mass spectrometric technique (Zhang et al. 2011). Furthermore, upon oral administration of a cardio-cerebral vascular protective Chinese traditional medicine, Buyang Huanwu Tang, FMN was found to be at the second largest isoflavonoids which have the highest area under the curve concentration (Jia et al. 2004). A recent report has suggested the single pass intestinal perfusion technique to be the applicable agent for physiological based pharmacokinetic model to predict assess the human absorption of these aglycones (Liu et al. 2019).
Furthermore, FMN and BCA are also known to be metabolized by some demethylating enzymes of Eubacterium limosum (Hur and Rafii 2000). The bioconversion of FMN and BCA by glucosyl and malonyltransferase enzymes produces the most important bioactive isoflavones known as daidzein and genistein (Nielsen et al. 2000). Both FMN and BCA are extensively and majorly known to be metabolized in liver undergoing the glucuronidation and sulfation reaction. Moreover, inter-individual and genetic variability due to ethnic and seasonal differences in food phytoestrogen are also the paramount factors to affect the metabolism of these isoflavonoids (Křížová et al. 2019). Further, the metabolite daidzein gets converted to dihydrodaidzein followed by major metabolism in to desmethylangolensin and minorly to equol (reported in one-third of the human population). Moreover the bioactive molecule genistein also gets further metabolized in to dihydrogenistein followed by formation of p-ethylphenol. Microbial culture studies gave evidence of conversion of genistein into 5-hydroxyequol, but there is no investigation performed regarding this conversion at clinical level (Matthies et al. 2012). Enzymes like lactase-phlorizin hydrolase, enterocytic/microbial β-glucosidase also take part in the intestinal gut wall metabolism of these phytoestrogens. Despite the contribution of intestinal enzymes, their enzymatic conversion to their active metabolite also took place through hepatic phase I and phase II microsomal enzymes. The major pathway of metabolism is the demethylation at 4′methoxy group followed by hydroxylation by liver enzymes. The extent of metabolism through gut microsomes is far lesser than the liver microsomes. The rat liver microsomes could metabolize the BCA to genistein more rapidly as compared to the human liver microsomes (Křížová et al. 2019). Metabolism of isoflavonoids including BCA and FMN by phase II enzymes (uridine 5′-diphospho-glucuronosyl transferase and sulfotransferase) has also been reported instead of lower glucuronidation rate after oral administration of red clover extract. In some leguminous plants such as Medicago truncatula, FMN is known to get metabolized to a phytoalexin medicarpin, a pathogenic-resistant compound (Liu et al. 2017) and recently found to be an osteoprotective agent (Taneja et al. 2020). In vitro metabolic conversion of metabolite daidzein to 6-hydroxy daidzein by catechol-O-methyltransferase enzymes has been explored by one of the researchers. Same as other isoflavonoids, they are also reabsorbed again and undergo the enterohepatic circulation. Much like any other endogenous substance or xenobiotic, enterohepatic circulation and biliary elimination complicate the pharmacokinetic profiles of the majority of isoflavonoids. Extensive first-pass metabolism and biliary excretion are thought to be the causative agents for their lower bioavailability which is a major bottleneck in advancement of isoflavonoids as therapeutic agents. The metabolism of the isoflavones seems to be complicated and has not been studied extensively. However, the significant metabolites reported are daidzein and genistein in most of the cases. The newer metabolites need to be identified and evaluated for their biological activity and therapeutic efficacy if any.
These FMN and BCA are also present in the daily dietary intake of animals in high concentration. In earlier studies, the data indicates that unlike humans, these isoflavonoids are directly metabolized in to equol (Blair et al. 2003) followed by conversion to an inactive p-ethylphenol (Wocławek-Potocka et al. 2013). When investigated for the metabolism of FMN in sheeps, it is firstly demethylated to daidzein followed by further formation of equol through hydrogenation. The in vitro studies that described the incubation of FMN and BCA in the bovine rumen fluid showed that the half-lives were 4.3 h for FMN and 3.9 h for BCA (Křížová et al. 2019). The concentration of BCA and FMN was found to be only 0–9% of ingested BCA and 7–16% of FMN in the omasum of dairy cows pattern similar to sheep (Njåstad et al. 2014).
The lower and limited bioavailability of isoflavonoids could also be due to the extensive first-pass metabolism of isoflavonoids and their rapid elimination from the body. The maximum time to reach the maximum plasma concentration is within 4–8 h. In a study, the systemic levels of BCA after dietary intake were found to be minimal as compared to the administration of prepared herbal formulation. However, in some studies, it has been seen that exposure of BCA as a pure compound shows a level of saturation during absorption. The bioavailability of BCA when administered orally at a dose of 5 mg/kg is 2.6% and 1.2% at 50 mg/kg, suggesting the rapid absorption, extensive first-pass metabolism, and biliary elimination by the authors for its low bioavailability (Moon et al. 2006). There are also investigations revealing the higher bioavailability of BCA that could be the result of competitive inhibition and modulation of metabolic enzymes and transporters due to some other structurally similar compounds. This low bioavailability could be enhanced by administration of mixture of isoflavonoids that would also aid in increasing the therapeutic efficacy of isoflavonoids. However, there are reports that refuse the fact of accumulation of isoflavonoids upon long-term administration. The apparent isoflavone bioavailability in healthy children is found to be 30–40% higher relative to healthy adults. The differences in gender do not affect the bioavailability of isoflavonoids to much higher extent. The mode of excretion of these isoflavonoids is through urine and bile and sometimes through feces. The excretion of these isoflavonoids by urine is determined by the type of the dietary product consumed and its composition regarding isoflavones and also on the nature of the isoflavone. Foods rich in isoflavone glucosides have poor urinary isoflavone excretion. However, a few studies report that the rates of urinary isoflavone excretion are not affected significantly by the nature of the food. Daidzein excretion was found to be markedly lower in equol producers as compared to equol non-producers. The primary metabolites found are glucuronide conjugates (70–90% of the total urinary isoflavones), sulfate conjugates (10–25%), and aglycone forms (1–10%). A multiethnic population study reported that Japanese, Chinese, or Native Hawaiian ancestry women excreted more isoflavones in urine than Caucasian and Filipino women. Fecal elimination as mentioned above is a minor route of elimination. Only up to 4% of the dietary isoflavones are eliminated by this route. Aglycone of the isoflavones forms the principal (>80%) part of the fecal isoflavone content.
14.4 Interaction with Cytochrome P450 Enzymes
Cytochrome P450 enzymes are the metabolic enzymes constituting the hemeprotein involved in the enzymatic conversion of many drugs, steroids, carcinogens, xenobiotics, hormones, fat-soluble vitamins, and other chemicals into their inactive and active forms. These enzymes have been the responsible facet for number of reactions inside the body which indirectly implicates their role in drug-drug interaction, drug-herb interaction, side effects, adverse effects, and unwanted increase and decrease in plasma concentration of therapeutically active drugs that lead to their toxicity and less response towards the targeted disease. Furthermore, the herbal drugs are widely used throughout the world along with the prescribed allopathic medicines; hence, the maximum possibility of herb drug interactions occurs at this stage. Various IC50 values of both the isoflavonoids are mentioned in Tables 14.2 and 14.3. Moreover, FMN and BCA are among the extensively administered isoflavonoids owing to their therapeutic beneficial properties. Taking into consideration, their pharmacokinetic and pharmacodynamic interactions with other drugs are summarized in Table 14.4.
Zapletalova and colleagues have unveiled the isoflavonoids safety efficacy by systematically investigating the interaction potential of 12 isoflavonoids with nine isoforms of cytochromes (Kopečná-Zapletalová et al. 2017). He found the genistein and daidzein and the metabolites of BCA and FMN to be the most potent inhibitors of cytochrome enzymes that non-competitively inhibit the CYP2C9 and CYP3A4. CYP3A4 was also efficiently inhibited by the parent isoflavonoid BCA, but FMN inhibited the same only about 20–30% of the total concentration. Hence, he concluded the maximal possible pharmacokinetic interaction of other drugs with these isoflavonoids. Moreover, Arora et al. have predicted the in vivo potential of CYP metabolic interaction of FMN and BCA using human and rat liver microsomal data obtained from in vitro studies (Arora et al. 2015). In the aforementioned investigation, they concluded that both FMN and BCA showed concentration-dependent competitive inhibition of CYP1A2 activity in human and rat liver microsomes, respectively. CYP2D6 inhibition was also perceived by FMN as concluded by the researchers. The in vivo prediction data showed the significant level of inhibition of both the isoflavonoids at intestinal level but non-significant at the hepatic level. Thereby, they have suggested for the special care to be considered during the administration of these isoflavonoids along with any prescribed drug which is metabolized by the enzyme CYP1A2. In an another study, researchers have evinced the harmful effect of red clover extract containing FMN and BCA administered to breast cancer patients (Dunlap et al. 2017). They investigated the red clover effect on metabolic CYP enzymes using the non-malignant ER-negative breast epithelial cells (MCF-10A) and malignant ER-positive breast epithelial cancer cell line, and the quantification of methoxy estrogen metabolites was performed using LC-MS/MS technique. They found that there was no effect of red clover in MCF-10A cells, while the expression of CYP1A1 was downregulated in MCF-7 cell line. These data suggest that the isoflavonoid containing red clover extract has distinctive effect on both the cells. Therefore, it is necessary to avoid red clover extract and formulations composed of these isoflavonoids to the breast cancer patients.
In addition to the pharmacokinetic assessment, these CYP450 enzymes are also being targeted by isoflavonoids for therapeutic benefits. Taking an example, FMN has been supposed to be mechanistically involved in suppression of colorectal cancer by modulation of CYP 1A1 isoform of CYP450 (Zhang et al. 2019). Furthermore, BCA has also been reported to follow similar pattern as it is found to be an anti-fibrotic agent against carbon tetrachloride-induced hepatotoxicity in rats (Breikaa et al. 2013a). The BCA acts through multimechanistic pathway among which one of the targeted molecules is the CYP450 enzymes (CYP4502E1 and CYP4501A1) in conjugation with pro-inflammatory and pro-fibrotic mediators (Breikaa et al. 2013b). Moreover, after searching the database, there are very few findings in the last 10 years that could be found reporting the interaction of both FMN and BCA with the microsomal enzyme cytochromes (CYP450).
Furthermore, despite inhibition metabolic enzymes, few investigations also reported the induction of some cytochrome enzymes by FMN listed in Table 14.4. This contradictory research needs more data to confirm the exact role of bioflavonoids upon CYP enzymes. Hence, still the studies are required to explore the further action of FMN and BCA upon various microsomal enzymes.
14.5 Interaction with ABC Transporters
The most widely distributed and largest integral protein family known is the ABC (ATP-binding cassette) transporter present in the body. Presence of two nucleotide-binding domains and two transmembrane domains is the characteristic feature of ABC transporter. Binding of any exogenous and endogenous ligand to the transporter leads to the conformational changes in the nuclear-binding domain that further alters the transmembrane domains of the receptor. These rearrangements of the domains outturn in to the modulation of internal cytosolic and nuclear molecular messengers for initiation of signaling pathways. Further, an untoward activation or inhibition in this feature of ABC transport could lead to various types of side effects or adverse events and drug toxicity. Moreover, the potential involvement of ABC transporters against the drug-drug, herb-drug, and herb-herb interaction had been already established by many researchers.
Furthermore, FMN and BCA are two major isoflavonoids found in red clover extract. They are also highly recommended for their anti-osteoporotic activity. Hence, it could be possible that they are being administered with other co-prescribed allopathic medicines that are ABC transporter activators or inhibitors or the substrates. Therefore, it is important to know the effect of FMN and BCA towards ABC transporters. There are literatures published regarding the modulation of ABC transporter by the administration of isoflavonoids (Wongrattanakamon et al. 2017) in which the authors have performed the molecular docking, pharmacophore modeling, and molecular dynamic simulation studies for an ABC transporter, P-gp (P-glycoprotein), of mouse origin with some of the mostly utilized bioflavonoids. They have discerned the certainty of plausible interaction of these isoflavonoids with other co-prescribed drugs as they have the potential to inhibit the P-gp efflux mechanism. Therefore, it is a major concern when the P-gp substrate drug is co-administered with these isoflavonoids which might lead to the intracellular increase in concentration of the substrate drug, thereby altering its therapeutic index and safety profile. In a research, FMN has been investigated as a nephroprotective agent against cisplatin-induced renal toxicity by altering the expression of organic cation transporter 2 (OCT2) and multidrug resistance-associated proteins (MRPs). FMN is reported to enhance the expression of MRP gene whilst alleviating the OCT2 expression, hence decreasing the intratubular accumulation of cisplatin in kidney resulting in to the reduced nephrotoxicity of the drug (Huang et al. 2017). In a recent investigation, a group of researchers evaluated the inhibitory effect of 99 major flavonoids upon BCRP (breast cancer resistance protein) under both in vitro and in vivo experimental conditions using BCRP-associated MDCK II cells and rat as an animal model (Fan et al. 2019). Their findings linked to molecular docking analysis along with structural activity relationship could further assist in predicting the potential risk in interaction between the isoflavonoids and other co-administered drugs.
Further, it has also been observed that BCA along with ciprofloxacin could also inhibit the efflux pump of methicillin-resistant Staphylococcus aureus in a synergistic manner (Zou et al. 2014). The concentration of ciprofloxacin was found to be significantly increased by 83% at 15 min after combining with BCA which was similar to the effect of positive control drug, reserpine. BCA was also examined as a P-gp inhibitor after being formulated into a solid dispersion. When investigated under in vitro experimental conditions, it remarkably augmented the cellular uptake of a P-gp substrate, rhodamine123 in NCI/ADR-RES cells by 2-3 folds. They have also examined the BCA for its inhibitory efficacy after oral and intravenous administration of BCA with diltiazem (a P-gp substrate) and its metabolite desacetyldiltiazem to rats. Upon pharmacokinetic analysis, the AUC (area under the curve) of desacetyldiltiazem was found to be threefold without affecting the concentration of diltiazem. Therefore, when BCA is developed into a new formulation as solid dispersion, its inhibitory potency is enhanced. Moreover, in a study, Singh and his colleagues have utilized BCA as a P-gp and CYP inhibitor to investigate its effect on bioavailability of an anticancer P-gp substrate tamoxifen and its metabolite. The concentration of tamoxifen and its metabolite was found to be decreased suggesting the low bioavailability of tamoxifen owing to its characteristic of being the P-gp substrate (Singh et al. 2012).
Furthermore, after searching the database, there are only few publications regarding the pharmacokinetic and pharmacodynamic interactions of both the isoflavonoids with either CYP450 microsomal enzymes or ABC transporters that have been summarized in the aforementioned paragraphs and their respective tables.
14.6 Conclusion
A large number of literatures have been published regarding the pharmacological importance of both the isoflavones. Newer drugs and conventional therapies involving the use of plants and/their constituents have been continuously scrutinized against many disorders. Most of the plant-derived compounds constituting flavonoids are polyphenolic in nature. A large number of papers have been published confirming the health-related benefits of dietary flavonoids. Large-scale clinical trials are required to be conducted in order to establish the potential usefulness of flavonoids in the treatment of various disease conditions. This requires the development of rapid and validated assays for the characterization and quantification of the phytol-constituents and their metabolites in biological matrices. Plant extracts though contain a mixture of chemical constituents which complicates the process of bioanalysis required in the drug development process. The present chapter highlights the pharmacokinetic interaction of FMN and BCA involving the microsomal CYP enzymes, multi-mechanistic membrane ABC transporters resulting in to the altered bioavailability of other co-administered drugs. They are increasingly being examined for their beneficial effect against many diseases. Apart from their therapeutic importance, large scale of research is required to investigate their interaction and binding capacity towards metabolic enzymes and ABC transporters at preclinical and clinical level. This necessitates to study the pharmacokinetic effect of these phytoestrogenic compounds in order to overcome the adverse events and synergizing the therapeutic potency of isoflavonoids.
References
Allred CD, Ju YH, Allred KF, Chang J, Helferich WG (2001) Dietary genistin stimulates growth of estrogen-dependent breast cancer tumors similar to that observed with genistein. Carcinogenesis 22(10):1667–1673
Almeida I, Rodrigues F, Sarmento B, Alves R, Oliveira M (2015) Isoflavones in food supplements: chemical profile, label accordance and permeability study in Caco-2 cells. Food Funct 6(3):938–946
Anzenbacher P, Anzenbacherová E (2001) Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci 58(5–6):737–47
Arora S, Taneja I, Challagundla M, Raju KSR, Singh SP, Wahajuddin M (2015) In vivo prediction of CYP-mediated metabolic interaction potential of formononetin and biochanin A using in vitro human and rat CYP450 inhibition data. Toxicol Lett 239(1):1–8
Auyeung KK-W, Ko JK-S (2010) Novel herbal flavonoids promote apoptosis but differentially induce cell cycle arrest in human colon cancer cell. Investig New Drugs 28:1–13
Auyeung KK-W, Law P-C, Ko JK-S (2012) Novel anti-angiogenic effects of formononetin in human colon cancer cells and tumor xenograft. Oncol Rep 28:2188–2194
Barnes S, Kirk M, Coward L (1994) Isoflavones and their conjugates in soy foods: extraction conditions and analysis by HPLC-mass spectrometry. J Agric Food Chem 42(11):2466–2474
Bhardwaj V, Tadinada SM, Jain A, Sehdev V, Daniels CK, Lai JC, Bhushan A (2014) Biochanin A reduces pancreatic cancer survival and progression. Anti-Cancer Drugs 25(3):296–302
Biradar S, Joshi H, Chheda T (2014) Biochanin-A ameliorates behavioural and neurochemical derangements in cognitive-deficit mice for the betterment of Alzheimer’s disease. Hum Exp Toxicol 33(4):369–382
Blair RM, Appt SE, Franke AA, Clarkson TB (2003) Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis). J Nutr 133(7):2262–2267
Breikaa RM, Algandaby MM, El-Demerdash E, Abdel-Naim AB (2013a) Biochanin A protects against acute carbon tetrachloride-induced hepatotoxicity in rats. Biosci Biotechnol Biochem 77(5):909–916
Breikaa RM, Algandaby MM, El-Demerdash E, Abdel-Naim AB (2013b) Multimechanistic antifibrotic effect of biochanin a in rats: implications of proinflammatory and profibrogenic mediators. PLoS One 8(7):e69276
Chaturvedi S, Malik MY, Azmi L, Shukla I, Naseem Z et al (2018) Formononetin and biochanin A protects against ritonavir induced hepatotoxicity via modulation of NfκB/pAkt signaling molecules. Life Sci 213:174–182
Chen J, Sun L (2012) Formononetin-induced apoptosis by activation of Ras/p38 mitogen-activated protein kinase in estrogen receptor-positive human breast cancer cells. Horm Metab Res 44:943–948
Chen J, Zeng J, Xin M, Huang W, Chen X (2011) Formononetin induces cell cycle arrest of human breast cancer cells via IGF1/PI3K/Akt pathways in vitro and in vivo. Horm Metab Res 43:681–686
Chen J, Zhao X, Ye Y, Wang Y, Tian J (2013) Estrogen receptor beta-mediated proliferative inhibition and apoptosis in human breast cancer by calycosin and formononetin. Cell Physiol Biochem 32:1790–1797
Chen J, Ge B, Wang Y, Ye Y, Zeng S et al (2015) Biochanin A promotes proliferation that involves a feedback loop of microRNA-375 and estrogen receptor alpha in breast cancer cells. Cell Physiol Biochem 35(2):639–646
Cho I-A, You S-J, Kang K-R, Kim S-G, Oh J-S, You J-S et al (2017) Biochanin-A induces apoptosis and suppresses migration in FaDu human pharynx squamous carcinoma cells. Oncol Rep 38(5):2985–2992
Chung MJ, Sohng JK, Choi DJ, Park YI (2013) Inhibitory effect of phloretin and biochanin A on IgE-mediated allergic responses in rat basophilic leukemia RBL-2H3 cells. Life Sci 93(9–11):401–408
Dash TK, Konkimalla VB (2017) Selection of P-glycoprotein inhibitor and formulation of combinational nanoformulation containing selected agent curcumin and DOX for reversal of resistance in K562 cells. Pharm Res 34(8):1741–1750
Desai V, Jain A, Shaghaghi H, Summer R, Lai JC, Bhushan A (2019) Combination of biochanin A and temozolomide impairs tumor growth by modulating cell metabolism in glioblastoma multiforme. Anticancer Res 39(1):57–66
De Montellano PRO (2005) Cytochrome P450: structure, mechanism, and biochemistry: Springer Science & Business Media. 183–245
Dixon RA, Pasinetti GM (2010) Flavonoids and isoflavonoids: from plant biology to agriculture and neuroscience. Plant Physiol 154(2):453–457
Dunlap TL, Howell CE, Mukand N, Chen S-N, Pauli GF, Dietz BM, Bolton JL (2017) Red clover aryl hydrocarbon receptor (AhR) and estrogen receptor (ER) agonists enhance genotoxic estrogen metabolism. Chem Res Toxicol 30(11):2084–2092
Fan X, Bai J, Zhao S, Hu M, Sun Y, Wang B et al (2019) Evaluation of inhibitory effects of flavonoids on breast cancer resistance protein (BCRP): from library screening to biological evaluation to structure-activity relationship. Toxicol In Vitro 61:104642
Fan X, Bai J, Hu M, Xu Y, Zhao S, Sun Y et al (2020) Drug interaction study of flavonoids toward OATP1B1 and their 3D structure activity relationship analysis for predicting hepatoprotective effects. Toxicology 437:152445
Galal AA, Mohamed AA-R, Khater SI, Metwally MM (2018) Beneficial role of biochanin A on cutaneous and renal tissues of ovariectomized rats treated with anastrozole. Life Sci 201:9–16
Guo M, Lu H, Qin J, Qu S, Wang W, Guo Y et al (2019) Biochanin a provides neuroprotection against cerebral ischemia/reperfusion injury by Nrf2-mediated inhibition of oxidative stress and inflammation signaling pathway in rats. Med Sci Monit 25:8975
Harini R, Ezhumalai M, Pugalendi KV (2012) Antihyperglycemic effect of biochanin A, a soy isoflavone, on streptozotocin-diabetic rats. Eur J Pharmacol 676(1–3):89–94
Hsu Y-N, Shyu H-W, Hu T-W, Yeh J-P, Lin Y-W, Lee L-Y et al (2018) Anti-proliferative activity of biochanin A in human osteosarcoma cells via mitochondrial-involved apoptosis. Food Chem Toxicol 112:194–204
Huang W-J, Bi L-Y, Li Z-Z, Zhang X, Ye Y (2014) Formononetin induces the mitochondrial apoptosis pathway in prostate cancer cells via downregulation of the IGF-1/IGF-1R signaling pathway. Pharm Biol 52:466–470
Huang J, Xie M, Gao P, Ye Y, Liu Y et al (2015) Antiproliferative effects of formononetin on human colorectal cancer via suppressing cell growth in vitro and in vivo. Process Biochem 50(6):912–917
Huang D, Wang C, Duan Y, Meng Q, Liu Z, Huo X et al (2017) Targeting Oct2 and P53: formononetin prevents cisplatin-induced acute kidney injury. Toxicol Appl Pharmacol 326:15–24
Hur H-G, Rafii F (2000) Biotransformation of the isoflavonoids biochanin A, formononetin, and glycitein by Eubacterium limosum. FEMS Microbiol Lett 192(1):21–25
Jain A, Lai JC, Bhushan A (2015) Biochanin A inhibits endothelial cell functions and proangiogenic pathways: implications in glioma therapy. Anti-Cancer Drugs 26(3):323–330
Jia X, Chen J, Lin H, Hu M (2004) Disposition of flavonoids via enteric recycling: enzyme-transporter coupling affects metabolism of biochanin A and formononetin and excretion of their phase II conjugates. J Pharmacol Exp Ther 310(3):1103–1113
Jin Y-M, Xu T-M, Zhao Y-H, Wang Y-C, Cui M-H (2014) In vitro and in vivo anti-cancer activity of formononetin on human cervical cancer cell line HeLa. Tumor Biol 35:2279–2284
Jin F, Wan C, Li W, Yao L, Zhao H, Zou Y et al (2017) Formononetin protects against acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. PLoS One 12(2):e0170900
Kopečná-Zapletalová M, Krasulová K, Anzenbacher P, Hodek P, Anzenbacherová E (2017) Interaction of isoflavonoids with human liver microsomal cytochromes P450: inhibition of CYP enzyme activities. Xenobiotica 47(4):324–331
Kosaka K, Sakai N, Endo Y, Fukuhara Y, Tsuda-Tsukimoto M, Ohtsuka T et al (2011) Impact of intestinal glucuronidation on the pharmacokinetics of raloxifene. Drug Metab Dispos 39(9):1495–1502
Křížová L, Dadáková K, Kašparovská J, Kašparovský T (2019) Isoflavones. Molecules 24(6):1076
Lai X, Li Y, Gao M (2020) Biochanin A regulates the growth and migration of NSCLC through suppressing the VEGF/VEGFR2 signaling pathway. Oncol Res. https://doi.org/10.3727/096504018x15321979274728
Li T, Zhao X, Mo Z, Huang W, Yan H et al (2014) Formononetin promotes cell cycle arrest via downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer cells. Cell Physiol Biochem 34(4):1351–1358
Li T, Zhong Y, Tang T, Luo J, Cui H, Fan R et al (2018a) Formononetin induces vasorelaxation in rat thoracic aorta via regulation of the Pi3K/PTen/akt signaling pathway. Drug Des Devel Ther 12:3675
Li Y, Yu H, Han F, Wang M, Luo Y, Guo X (2018b) Biochanin a induces S phase arrest and apoptosis in lung cancer cells. Biomed Res Int 2018:3545376
Liu Y, Xiong X, Su D, Song Y, Zhang L, Yang S (2013) Study on intestinal absorption of formononetin in Millettia nitita var. hirsutissima in rats. China J Chin Mater Med 38(20):3571–3575
Liu X-J, Li Y-Q, Chen Q-Y, Xiao S-J, Zeng S-E (2014) Up-regulating of RASD1 and apoptosis of DU-145 human prostate cancer cells induced by formononetin in vitro. Asian Pac J Cancer Prev 15:2835–2839
Liu Q, Sun Y, Zheng J-M, Yan X-L, Chen H-M et al (2015) Formononetin sensitizes glioma cells to doxorubicin through preventing EMT via inhibition of histone deacetylase 5. Int J Clin Exp Pathol 8(6):6434
Liu X, Wang T, Liu X, Cai L, Qi J, Zhang P, Li Y (2016) Biochanin A protects lipopolysaccharide/D-galactosamine-induced acute liver injury in mice by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Int Immunopharmacol 38:324–331
Liu Y, Hassan S, Kidd BN, Garg G, Mathesius U, Singh KB, Anderson JP (2017) Ethylene signaling is important for isoflavonoid-mediated resistance to Rhizoctonia solani in roots of Medicago truncatula. Mol Plant-Microbe Interact 30(9):691–700
Liu Y, Zhang X, Shi X-J, Wen Y-X, Yang L, Dong L (2019) Applicability analysis and evaluation of aglycones in single-pass intestinal perfusion technique based on PBPK model. China J Chin Mater Med 44(17):3645–3652
Lou Y, Guo Z, Zhu Y, Zhang G, Wang Y, Qi X et al (2019) Astragali radix and its main bioactive compounds activate the Nrf2-mediated signaling pathway to induce P-glycoprotein and breast cancer resistance protein. J Ethnopharmacol 228:82–91
Luo L-Y, Fan M-X, Zhao H-Y, Li M-X, Wu X, Gao W-Y (2018) Pharmacokinetics and bioavailability of the isoflavones formononetin and ononin and their in vitro absorption in using chamber and Caco-2 cell models. J Agric Food Chem 66(11):2917–2924
Luo Q, Shi X, Ding J, Ma Z, Chen X, Leng Y et al (2019) Network pharmacology integrated molecular docking reveals the antiosteosarcoma mechanism of biochanin A. Evid Based Complement Alternat Med 2019:1410495
Matthies A, Loh G, Blaut M, Braune A (2012) Daidzein and genistein are converted to equol and 5-hydroxy-equol by human intestinal Slackia isoflavoniconvertens in gnotobiotic rats. J Nutr 142(1):40–46
Michaelis M, Sithisarn P, Cinatl J Jr (2014) Effects of flavonoid-induced oxidative stress on anti-H5N1 influenza a virus activity exerted by baicalein and biochanin A. BMC Res Notes 7(1):384
Moon YJ, Sagawa K, Frederick K, Zhang S, Morris ME (2006) Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. AAPS J 8(3):E433–E442
Navrátilová L, Applová L, Horký P, Mladěnka P, Pávek P, Trejtnar F (2018) Interaction of soy isoflavones and their main metabolites with hOATP2B1 transporter. Naunyn Schmiedeberg’s Arch Pharmacol 391(10):1063–1071
Nielsen S, Breinholt V, Cornett C, Dragsted L (2000) Biotransformation of the citrus flavone tangeretin in rats. Identification of metabolites with intact flavan nucleus. Food Chem Toxicol 38(9):739–746
Njåstad KM, Adler SA, Hansen-Møller J, Thuen E, Gustavsson A-M, Steinshamn H (2014) Gastrointestinal metabolism of phytoestrogens in lactating dairy cows fed silages with different botanical composition. J Dairy Sci 97(12):7735–7750
Oza MJ, Kulkarni YA (2020) Formononetin ameliorates diabetic neuropathy by increasing expression of SIRT1 and NGF. Chem Biodivers 17(6):e2000162
Park S, Bazer FW, Lim W, Song G (2018) The O-methylated isoflavone, formononetin, inhibits human ovarian cancer cell proliferation by sub G0/G1 cell phase arrest through PI3K/AKT and ERK1/2 inactivation. J Cell Biochem 119:7377–7387
Qi C, Xie M, Liang J, Li H, Li Z et al (2016) Formononetin targets the MAPK and PI3K/Akt pathways to induce apoptosis in human nasopharyngeal carcinoma cells in vitro and in vivo. Int J Clin Exp Med 9(2):1180–1189
Qiu L, Lin B, Lin Z, Lin Y, Lin M et al (2012) Biochanin A ameliorates the cytokine secretion profile of lipopolysaccharide-stimulated macrophages by a PPARγ-dependent pathway. Mol Med Rep 5(1):217–222
Raju KSR, Rashid M, Gundeti M, Taneja I, Malik MY, Singh SK, Gayen J (2019) LC-ESI-MS/MS method for the simultaneous determination of isoformononetin, daidzein, and equol in rat plasma: application to a preclinical pharmacokinetic study. J Chromatogr B 1129:121776
Sarfraz A, Javeed M, Shah MA, Hussain G, Shafiq N, Sarfraz I et al (2020) Biochanin A: a novel bioactive multifunctional compound from nature. Sci Total Environ 722:137907
Sayed AA, Elfiky AA (2018) In silico estrogen-like activity and in vivo osteoclastogenesis inhibitory effect of Cicer arietinum extract. Cell Mol Biol 64:29–39
Sehm T, Fan Z, Weiss R, Schwarz M, Engelhorn T, Hore N et al (2014) The impact of dietary isoflavonoids on malignant brain tumors. Cancer Med 3(4):865–877
Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT et al (2001) Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr 131(4):1362S–1375S
Singh SP, Raju K, Ali MM, Kohli K, Jain GK (2012) Reduced bioavailability of tamoxifen and its metabolite 4-hydroxytamoxifen after oral administration with biochanin A (an isoflavone) in rats. Phytother Res 26(2):303–307
Spencer J, Crozier A (2012) Bioavailability and function of flavonoids. CRC Press, Boca Raton
Suliman FA, Khodeer DM, Ibrahiem A, Mehanna ET, El-Kherbetawy MK, Mohammad HM et al (2018) Renoprotective effect of the isoflavonoid biochanin A against cisplatin induced acute kidney injury in mice: effect on inflammatory burden and p53 apoptosis. Int Immunopharmacol 61:8–19
Szliszka E, Czuba ZP, Mertas A, Paradysz A, Krol W (2013) The dietary isoflavone biochanin-A sensitizes prostate cancer cells to TRAIL-induced apoptosis. Paper presented at the Urologic Oncology: Seminars and Original Investigations
Takahashi M, Muromoto R, Kojima H, Takeuchi S, Kitai Y, Kashiwakura J-I, Matsuda T (2017) Biochanin A enhances RORγ activity through STAT3-mediated recruitment of NCOA1. Biochem Biophys Res Commun 489(4):503–508
Taneja I, Raju KSR, Wahajuddin M (2015) Dietary isoflavones as modulators of drug metabolizing enzymes and transporters: effect on prescription medicines. Critical reviews in food science and nutrition 56(sup1), S95-S109
Taneja I, Raghuvanshi A, Raju KSR, Awasthi P, Rashid M, Singh S et al (2020) Bioavailability, tissue distribution and excretion studies of a potential anti-osteoporotic agent, medicarpin, in female rats using validated LC–MS/MS method. J Pharm Biomed Anal 180:112978
Veitch NC (2013) Isoflavonoids of the Leguminosae. Nat Prod Rep 30(7):988–1027
Wang X (2011) Structure, function, and engineering of enzymes in isoflavonoid biosynthesis. Funct Integr Genomics 11(1):13–22
Wang J, Wu W-Y, Huang H, Li W-Z, Chen H-Q, Yin Y-Y (2016) Biochanin A protects against lipopolysaccharide-induced damage of dopaminergic neurons both in vivo and in vitro via inhibition of microglial activation. Neurotox Res 30(3):486–498
Wang AL, Li Y, Zhao Q, Fan LQ (2018a) Formononetin inhibits colon carcinoma cell growth and invasion by microRNA-149-mediated EphB3 downregulation and inhibition of PI3K/AKT and STAT3 signaling pathways. Mol Med Rep 17(6):7721–7729
Wang Y, Li JJ, Chen YM (2018b) Biochanin A extirpates the epithelial-mesenchymal transition in a human lung cancer. Exp Ther Med 15(3):2830–2836
Wang D-S, Yan L-Y, Yang D-Z, Lyu Y, Fang L-H et al (2020) Formononetin ameliorates myocardial ischemia/reperfusion injury in rats by suppressing the ROS-TXNIP-NLRP3 pathway. Biochem Biophys Res Commun 525(3):759–766
Wocławek-Potocka I, Mannelli C, Boruszewska D, Kowalczyk-Zieba I, Waśniewski T, Skarżyński DJ (2013) Diverse effects of phytoestrogens on the reproductive performance: cow as a model. Int J Endocrinol 2013:1–15
Wongrattanakamon P, Lee VS, Nimmanpipug P, Sirithunyalug B, Chansakaow S, Jiranusornkul S (2017) Insight into the molecular mechanism of p-glycoprotein mediated drug toxicity induced by bioflavonoids: an integrated computational approach. Toxicol Mech Methods 27(4):253–271
Wu XY, Xu H, Wu ZF, Chen C, Liu JY et al (2015a) Formononetin, a novel FGFR2 inhibitor, potently inhibits angiogenesis and tumor growth in preclinical models. Oncotarget 6(42):44563
Wu W-Y, Wu Y-Y, Huang H, He C, Li WZ, Wang H-L et al (2015b) Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells. Int J Mol Med 35(2):391–398
Wu Y, Zhang X, Li Z, Yan H, Qin J et al (2017) Formononetin inhibits human bladder cancer cell proliferation and invasiveness via regulation of miR-21 and PTEN. Food Funct 8(3):1061–1066
Xiao P, Zheng B, Sun J, Yang J (2017) Biochanin A induces anticancer effects in SK-Mel-28 human malignant melanoma cells via induction of apoptosis, inhibition of cell invasion and modulation of NF-κB and MAPK signaling pathways. Oncol Lett 14(5):5989–5993
Yang Y, Zhao Y, Ai X, Cheng B, Lu S (2014) Formononetin suppresses the proliferation of human non-small cell lung cancer through induction of cell cycle arrest and apoptosis. Int J Clin Exp Pathol 7:8453
Ye Y, Hou R, Chen J, Mo L, Zhang J et al (2012) Formononetin-induced apoptosis of human prostate cancer cells through ERK1/2 mitogen-activated protein kinase inactivation. Horm Metab Res 44(4):263–267
Youssef MM, Tolba MF, Badawy NN, Liu AW, El-Ahwany E, Khalifa AE et al (2016) Novel combination of sorafenib and biochanin-A synergistically enhances the anti-proliferative and pro-apoptotic effects on hepatocellular carcinoma cells. Sci Rep 6(1):1–12
Yu X, Gao F, Li W, Zhou L, Liu W et al (2020) Formononetin inhibits tumor growth by suppression of EGFR-Akt-Mcl-1 axis in non-small cell lung cancer. J Exp Clin Cancer Res 39:1–17
Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138(1):103–41
Zakłos-Szyda M, Budryn G (2020) The effects of Trifolium pratense L. sprouts’ phenolic compounds on cell growth and migration of MDA-MB-231, MCF-7 and HUVEC cells. Nutrients 12(1):257
Zakłos-Szyda M, Budryn G, Grzelczyk J, Pérez-Sánchez H, Żyżelewicz D (2020) Evaluation of Isoflavones as bone resorption inhibitors upon interactions with receptor activator of nuclear factor-κB ligand (RANKL). Molecules 25:206
Zhang J, Yang G, Lin R, Hu Z (2011) Determination of paeoniflorin, calycosin-7-O-β-d-glucoside, ononin, calycosin and formononetin in rat plasma after oral administration of Buyang Huanwu decoction for their pharmacokinetic study by liquid chromatography–mass spectrometry. Biomed Chromatogr 25(4):450–457
Zhang X, Bi L, Ye Y, Chen J (2014a) Formononetin induces apoptosis in PC-3 prostate cancer cells through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt pathway. Nutr Cancer 66:656–661
Zhang W, Jiang S, Qian D, Shang E-X, Duan J-A (2014b) Analysis of interaction property of calycosin-7-O-β-D-glucoside with human gut microbiota. J Chromatogr B 963:16–23
Zhang G, Ou R, Li F, Wu J, Zheng L, Tong Y et al (2016) Regulation of drug-metabolizing enzymes and efflux transporters by Astragali radix decoction and its main bioactive compounds: implication for clinical drug–drug interactions. J Ethnopharmacol 180:104–113
Zhang X, Ni Q, Wang Y, Fan H-W, Li Y (2018) Synergistic anticancer effects of formononetin and temozolomide on glioma C6 cells. Biol Pharm Bull 41(8):1194–1202
Zhang L, Gong Y, Wang S, Gao F (2019) Anti-colorectal cancer mechanisms of formononetin identified by network pharmacological approach. Med Sci Monit 25:7709
Zhao Y, Wang L, Zhai X, Cui T, Wang G, Pang Q (2018) The effect of biochanin A on cell growth, apoptosis, and migration in osteosarcoma cells. Pharmazie 73(6):335–341
Zhou R, Xu L, Ye M, Liao M, Du H et al (2014) Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm Metab Res 46:753–760
Zou D, Xie K, Wang H, Chen Y, Xie M (2014) Inhibitory effects of biochanin A on the efflux pump of methicillin-resistant Staphylococcus aureus (MRSA). Acta Microbiol Sin 54(10):1204–1211
Acknowledgments
The authors are thankful to Director, CSIR-CDRI, for continuous encouragement and support. Swati Chaturvedi acknowledges CSIR, India, for providing the research fellowship.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chaturvedi, S., Sultana, N., Rashid, M., Naseem, Z., Singh, S.K., Wahajuddin, M. (2021). Phytochemistry, Pharmacology, and Pharmacokinetics of Phytoestrogens from Red Clover Extract: An Exhaustive Overview. In: Aftab, T., Hakeem, K.R. (eds) Medicinal and Aromatic Plants. Springer, Cham. https://doi.org/10.1007/978-3-030-58975-2_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-58975-2_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-58974-5
Online ISBN: 978-3-030-58975-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)