Abstract
A large body of analytical data is available on the inorganic composition of many thousands of plant species, for which typical concentration ranges have been tabulated for major, minor, and trace elements. These elements include those that have been shown essential for plant growth, as well as others that lack this status, at least universally. Metalliferous soils, having abnormally high concentrations of some of the elements that are generally present only at minor (e.g. 200–2000 μg g−1) or trace (e.g. 0.1–200 μg g−1) levels, have attracted increasing attention during the last 50 years. The effects vary widely, depending on the species, the relevant elements, and soil characteristics that collectively influence the availability of metals to plants. Some of these soils are toxic to all or most higher plants. Others have hosted the development of specialized plant communities consisting of a restricted and locally characteristic range of metal-tolerant species. These typically show a slightly elevated concentration of the elements with which the soil is enriched, but in places a species may exhibit extreme accumulation of one or more of these elements, to a concentration level that can be hundreds or even thousands of times greater than that usually found in plants on the most common soils. These plants, now widely referred to as hyperaccumulators, are a remarkable resource for many types of fundamental scientific investigation (plant systematics, ecophysiology, biochemistry, genetics and molecular biology) and for applications such as phytoremediation and agromining. Systematic analysis of herbarium specimens by X-ray Fluorescence, combined with auxiliary collection data, can provide insights into phylogenetic patterns of hyperaccumulation, and has the potential to complement and add insights to biogeographical and phylogenetic studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
It has been known since the 1850s and 1860s that certain plant species then found on the zinc (Zn)-rich ‘calamine’ soils near Aachen in Germany accumulated Zn to very high concentrations. Although the first record referred to Viola calaminaria (Violaceae) (Fig. 1), a later report presented data showing that Thlaspi alpestre var. calaminare, now classified as Noccaea caerulescens (Brassicaceae) (Fig. 2), contained at least 1 wt% Zn in the dry leaf tissue, or 10 wt% in the inorganic ash (Sachs 1865). During the last century, unusual accumulation of other metals and metalloids has been found, including for Pb in the 1920s; Se in the 1930s; Ni in the 1940s; Co, Cu and As in the 1960s; and Cd and Mn in the 1970s.
2 Hyperaccumulation
Normal concentration ranges in plants have been tabulated for major, minor, and trace elements in many reviews (e.g. Reeves and Baker 2000). The term ‘hyperaccumulation’, describing a highly abnormal level of metal accumulation, was first applied by Jaffré et al. (1976) in the title of their paper on Ni concentrations in the New Caledonian tree Sebertia acuminata (Sapotaceae), now classified as Pycnandra acuminata. In discussing Ni concentrations in species of Homalium (Salicaceae) and Hybanthus (Violaceae) from various parts of the world, Brooks et al. (1977a) used the term to indicate a defined concentration threshold (>1000 μg g−1) for Ni. A similar concept was used earlier by Jaffré and Schmid (1974), who referred to certain Ni-rich plants from the ultramafic soils of New Caledonia as ‘hypernickelophores’, i.e. ‘extreme nickel-bearers’.
Choice of the 1000 μg g−1 criterion was not entirely arbitrary. In many reports on Ni-rich soils, plant Ni concentrations are generally 5–100 μg g−1; levels of 100–1000 μg g−1 are quite rare. The local cases of accumulation to >1000 μg g−1 seem to represent a distinct form of plant response, implying some characteristic and unusual physiological behaviour. Greater precision in the definition of hyperaccumulation was provided by Reeves (1992) for Ni: “a hyperaccumulator of Ni is a plant in which a Ni concentration of at least 1000 mg kg−1 has been recorded in the dry matter of any above-ground tissue in at least one specimen growing in its natural habitat.” The criteria defining hyperaccumulation should therefore not be based on analyses of whole plants or subterranean plant parts, mainly because of the difficulty of ensuring that the samples are free of soil contamination, and also because plants that immobilize metals in the root system, and fail to translocate them further (Baker 1981), are of less interest for many purposes than those that actively accumulate metals into all tissues.
Definitions of hyperaccumulation have been extended to elements other than Ni. Malaisse et al. (1978) used the 1000 μg g−1 criterion for Cu accumulation, and Brooks et al. (1980) applied this to Co. Reeves and Brooks (1983b) used the same criterion in discussing Pb, but for Mn and Zn, which are normally present at higher and more widely varying concentrations (~20–400 μg g−1), a 10 000 μg g−1 threshold was suggested by Baker and Brooks (1989), following use of the term ‘hypermanganèsophore’ for plants having this level of Mn accumulation (Jaffré 1980).
Extensive recent discussions of appropriate criteria for defining hyperaccumulation of many elements are those of Baker and Whiting (2002) and van der Ent et al. (2013) who summarized the history of development of this topic. These papers also pay attention to the limitations of hydroponic experiments in relation to hyperaccumulation, because such experiments have often involved the use of unrealistic concentrations of free metal ions that are not relevant to the continuing life cycle of naturally occurring metallophyte populations living on metalliferous soils.
3 A Note on Nomenclature
As is often the case with botanical discussions, complexities arise from continual changes in botanical nomenclature and the differing views on the importance of various criteria in circumscribing species, genera, and even families. Many recent changes have resulted from studies of partial or complete plant genomes, bringing new information on the degree of interspecies and interpopulation relationships, and clarifying genotypic and phenotypic effects. In following the history of studies of hyperaccumulator species, there is a need to be aware of any nomenclatural changes that have occurred since the first hyperaccumulation reports. In some instances, the changes are not completely new, but involve simple intergeneric transfers or the resurrection of earlier generic names. In the context of the present account, the following are some of the more important nomenclatural changes:
Sebertia acuminata to Pycnandra (New Caledonia); Austromyrtus species to Gossia (Australia and New Caledonia); Peltaria emarginata to Leptoplax and then Bornmuellera (Greece); Cochlearia aucheri and C. sempervivum to Pseudosempervivum (Turkey); Ariadne shaferi to Mazaea and many species of Pentacalia to Antillanthus (Cuba); species of Alyssum sect. Odontarrhena to the resurrected genus Odontarrhena (Mediterranean Europe, Turkey and further east); species of Thlaspi to the resurrected genus Noccaea (many temperate regions worldwide).
The major problems arise where a great deal of research has been done and many publications have appeared under the names that were first used in the hyperaccumulation literature. Future work and literature searches should include synonyms. Further changes are inevitable. The following account includes, in particular, reference to species of Odontarrhena under their earlier Alyssum classification, and to some species of Noccaea previously discussed under Thlaspi as this genus was earlier broadly understood in major Floras.
4 Ecology and Conservational Status of Hyperaccumulator Plants
The soils produced from the weathering of surficial ore deposits or naturally enriched metalliferous country rocks (e.g. ultramafics, Cu-Co mineralization, calamine deposits) can be regarded as primary habitats for most hyperaccumulator plants. In certain cases, as in some of the ultramafic terranes of Cuba and New Caledonia, such soils are believed to have been continuously available for plant life and evolution for tens of millions of years (Reeves et al. 1996, 1999; Pelletier 2006; Cluzel et al. 2012). Other naturally occurring metalliferous soils are much younger, having been subjected to more recent geological processes such as erosion and re-deposition, hydrothermal alteration, or glaciation. Secondary habitats (on the scale of decades to a few thousand years) have resulted from the exploitation of mineral deposits via metalliferous mining and ore processing activities. A tertiary category of distribution results from the superficial deposition of dusts and particles derived from smelting operations and the beneficiation of processed ores, where effluents are discharged into river systems leading to metal enrichment of alluvial floodplains (Baker et al. 2010).
Present-day plant species that show metal tolerance through occurrence on metalliferous soils (i.e. metallophytes) may therefore have experienced any of this wide variety of soil histories. In relation to species that appear to be endemic to metalliferous soils, there has been extensive discussion of the concepts of palaeoendemism and neo-endemism (Stebbins 1942; Kruckeberg 1954; Antonovics et al. 1971; Brooks 1987). Palaeoendemic metallophytes are species formerly widespread that have survived in the metalliferous environment, restricted by competitive pressures and often having no or few closely related surviving species. Neoendemic metallophytes are species that have evolved in the metalliferous environment, leading to morphological characteristics now recognized as distinctive. The concept as applied generally to metallophytes can also be used in discussion of the particular case of hyperaccumulator species and their putative origins. Some Ni hyperaccumulators, for example, in genera consisting of only one or two species growing on ancient soils and without close relatives, may be termed palaeoendemics. Examples include Shafera platyphylla (Asteraceae) and Phyllomelia coronata (Rubiaceae) from Cuba and Oncotheca balansae (Oncothecaceae) from New Caledonia. These phylogenetically isolated hyperaccumulators contrast with the intense diversification in some genera, as shown by the large numbers of Ni hyperaccumulating endemics present in genera such as Odontarrhena (synonym Alyssum, Brassicaceae) in Mediterranean Europe, Turkey, and nearby parts of Asia; Buxus (Buxaceae) and Leucocroton (Euphorbiaceae) in Cuba; and Phyllanthus (Phyllanthaceae) in several tropical parts of the world (Reeves et al. 2017).
Mineral wastes have locally enabled the growth of endemic species that are both hypertolerant and hyperaccumulating to extend their distributions regionally, such that the current distributions of some hyperaccumulator plants are well beyond their primary habitats. Additionally, some species are known from both non-metalliferous and metalliferous locations, exhibiting hyperaccumulation solely from the latter. This situation described as ‘facultative hyperaccumulation’ has been discussed in detail by Pollard et al. (2014). Further, some species are reported to show extreme accumulation of certain elements (e.g. Zn, Mn, Se) from normal soils or those having only modest concentrations of the element concerned. Examples include: Noccaea caerulescens (Brassicaceae) that hyperaccumulates Zn from both metalliferous and non-metalliferous soils in France and elsewhere in Europe (Reeves et al. 2001); Gossia (formerly Austromyrtus) bidwillii (Myrtaceae) from eastern Australia (Bidwell et al. 2002; Fernando et al. 2009) and Alyxia rubricaulis (Apocynaceae) from New Caledonia (Jaffré 1977), that hyperaccumulate Mn from soils having only a slightly elevated Mn content; and species of Astragalus (Fabaceae) in the USA (Rosenfeld and Beath 1964) that hyperaccumulate Se from soils in which the elevated Se content is commonly up to 50 μg g−1.
In temperate regions, the plant assemblages on metalliferous soils generally consist of a limited range of obligate and facultative metallophytes (Baker 1987) that may or may not include hyperaccumulators. On ultramafic soils, in particular in Mediterranean Europe, almost monospecific communities of a Ni hyperaccumulator, e.g. Odontarrhena spp. can be found in Greece, Turkey and Albania. By contrast, in the tropics, ultramafic soils regularly show a high density of woody species where hyperaccumulators and non-hyperaccumulators may grow side by side. Often the most ancient and undisturbed metalliferous environments support the richest assemblages of hyperaccumulator plants (e.g. Reeves et al. 1996, 1999).
In spite of the rapidly increasing number of hyperaccumulator plants being discovered (especially for Ni), the overall rarity of this resource must be stressed because it represents only a few tenths of 1% of the known flora. Furthermore, anthropogenic and environmental factors threaten the habitats of many hyperaccumulator plants. These include ongoing mineral exploration and mining activities, reworking of ancient mine spoils, land reclamation and improvement for agricultural production, urbanization and development of brownfield sites, natural fire events, and probably climate change (Whiting et al. 2004; Baker et al. 2010; Wulff et al. 2013; Ibanez et al. 2018). Urgent conservation and management steps are clearly needed in areas under threat, in order to ensure persistence of the valuable phytotechnological resource. Appropriate options are the maintenance of living materials in botanical gardens and seed in germplasm banks, and regeneration in situ using ‘seed orchards’ on mining lands. Exploitation of the hyperaccumulator resource base for agromining must be considered with due caution and with appropriate management practices in place. An unfortunate incident has been reported in southwestern Oregon, USA, where poor crop management led to the extension of the distribution of O. chalcidica (under the name Alyssum murale) well outside the operational area, to the extent that this species is now regarded as a noxious weed in Oregon and future use has been banned (Strawn 2013). Invasions such as this may also affect the status of other local endemics native to the area that have been selected for agromining. Issues of the CITES convention may also apply when attempts are made to introduce an ‘alien’ species to a new country.
5 Instances of Hyperaccumulation
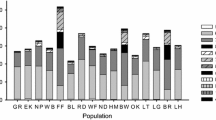
The following discussion outlines instances of hyperaccumulation of selected trace elements (Ni, Zn, Cd, Pb, Co, Cr, Cu, Mn, Se, As and Tl) for which a substantial body of reliable plant analysis data exists. Further exploration of various types of metalliferous environments, both natural and man-modified, will certainly uncover more examples. The exact enumeration of metal hyperaccumulator species is made difficult by the lack of recent and complete Floras for many tropical regions, in particular. The exact identification of some specimens of interest is still in doubt. In addition, since the first hyperaccumulator species were identified, numerous name changes have occurred, some species have been grouped into synonymy, whereas others have been split into several taxa (species, subspecies, and varieties). Some of the earlier information was published in periodicals that are difficult to access, and much useful detail has been omitted because of the space limitations of most journals. All of these difficulties have justified the initiative to create a Global Hyperaccumulator Database (www.hyperaccumulators.org), an ongoing project to encompass as much of the knowledge as possible on identified hyperaccumulator species, including synonymies and other taxonomic changes. A recent summary listed 721 hyperaccumulator species (532 Ni, 53 Cu, 42 Co, one Cr, 42 Mn, 20 Zn, two REEs, 41 Se, two Tl, seven Cs, five As and eight Pb) with some species showing hyperaccumulation of more than one element (Reeves et al. 2017). These numbers will change as more discoveries are made, or if earlier claims are shown to be spurious. The 721 hyperaccumulator species are from 52 families and ca. 130 genera; the families most strongly represented are the Brassicaceae (83 species) and the Phyllanthaceae (59 species). The countries with the greatest numbers of published hyperaccumulator plant species (including some subspecific taxa) are Cuba with 128 (Reeves et al. 1999), New Caledonia with 99 (Jaffré et al. 2013; Gei et al. 2020), Turkey with 59 (Reeves and Adıgüzel 2008) and Brazil with at least 30 (Reeves et al. 2007).
5.1 Nickel
Unprecedented Ni concentrations (up to about 10 000 μg g−1 or 1 wt%) were discovered in the Italian ultramafic Alyssum, now regarded as Odontarrhena bertolonii (Brassicaceae) (Minguzzi and Vergnano 1948). In the 1960s, two additional Ni-accumulating Alyssum species, now Odontarrhena muralis from Armenia (Doksopulo 1961) and O. serpyllifolia s.l. from Portugal (Menezes de Sequeira 1969), were reported to behave similarly. These observations were followed by studies in Zimbabwe (Wild 1970) and two independent discoveries of high Ni concentrations (3000–9800 μg g−1) in Hybanthus floribundus from Western Australia (Severne and Brooks 1972; Cole 1973).
Beginning in 1974, concerted attempts were made to discover the extent of Ni hyperaccumulation, both geographically and in terms of distribution in the plant kingdom. Detailed studies of the flora of ultramafic soils were carried out in New Caledonia (Jaffré and Schmid 1974; Jaffré et al. 1976, 1979a, b; Jaffré 1980). Particularly notable was the discovery that the latex of the New Caledonian tree Pycnandra (formerly Sebertia) acuminata contained about 10% Ni, yielding a dried solid with 20–25% Ni (Jaffré et al. 1976), in which citrate was a major organic constituent (Lee et al. 1977) (Figs. 3 and 4).
During the next 25 years, R.R. Brooks, R.D. Reeves, A.J.M. Baker, and co-workers in many other parts of the world collected and analyzed plant material from ultramafic areas in the search for further examples of Ni hyperaccumulation. Extensive use was made initially of leaf fragments from herbarium collections, but later this approach gave way to field studies. Brooks et al. (1977a) identified several species of Homalium and Hybanthus in New Caledonia as hyperaccumulators. A comprehensive survey of nearly all of the 170 then known species of Alyssum (Brooks and Radford 1978; Brooks et al. 1979) established the existence of 48 Ni hyperaccumulators, all in one section of the genus (now the genus Odontarrhena), distributed from Portugal across Mediterranean Europe to Turkey, Armenia, Iraq, Iran and Russia. Most are ultramafic-endemic species, and many have a very restricted geographical distribution. Several additions to the list of Ni hyperaccumulators in this genus have been made subsequently.
Further work by various groups has focused on other genera of the Mediterranean region, on species of ultramafic outcrops in the European Alps, southern Africa, Newfoundland (Canada), and the Pacific Northwest of the United States, and on plants of tropical ultramafic soils of Brazil, Cuba and other Caribbean islands, Queensland (Australia), Costa Rica, Sri Lanka, and Southeast Asia (especially certain islands of Indonesia and the Philippines). Hyperaccumulators discovered in temperate-zone areas include Leptoplax (formerly Peltaria and now Bornmuellera) emarginata from Greece (Reeves et al. 1980), species of Bornmuellera and Cochlearia (Pseudosempervivum) from Turkey and the Balkans (Reeves et al. 1983b; Reeves and Adıgüzel 2008), Streptanthus polygaloides from California (Reeves et al. 1981), and species of Thlaspi (Noccaea) from Europe (Reeves and Brooks 1983a), Turkey, and Japan (Reeves 1988; Reeves and Adıgüzel 2008), and California (Reeves et al. 1983b). Discoveries in tropical areas include several species from Palawan (Baker et al. 1992) and other parts of Southeast Asia (Wither and Brooks 1977), Stackhousia tryonii from Queensland (Batianoff et al. 1990), and numerous species from Brazil (Reeves et al. 2007).
The ultramafic soils of Cuba host the largest number of Ni hyperaccumulators reported from any one country. Following initial observations by Berazaín (1981), a survey of much of the Caribbean ultramafic flora revealed 128 such species in Cuba, as well as Phyllanthus nummularioides in the Dominican Republic (Reeves et al. 1996, 1999). Psychotria grandis is a Ni hyperaccumulator where it occurs on ultramafic soils in Puerto Rico (Reeves 2003; Campbell et al. 2013; McAlister et al. 2015). Other major sources of Ni hyperaccumulator plants, with more than 50 species identified in each country, are New Caledonia and Turkey. Substantial additions to the list are being made from ongoing work in New Caledonia (Jaffré et al. 2013; Gei et al. 2020), Brazil, Indonesia (Sulawesi and some of the smaller islands), Sabah (Malaysia) (van der Ent et al. 2015), and the Philippines (Fernando et al. 2013).
The most recent information brings the worldwide total of known Ni hyperaccumulator plant species to more than 500. Developments can be followed through earlier summaries, some of which deal with hyperaccumulators of other elements (Brooks 1987; Baker and Brooks 1989; Brooks 1998; Reeves et al. 1996, 1999; Reeves and Baker 2000; Reeves 2003, 2005); more recent results can be found in reports on Brazil by Reeves et al. (2007), and in Turkey by Reeves and Adıgüzel (2004, 2008). Ongoing investigations in Sabah (Malaysia) and New Caledonia continue to reveal numerous hyperaccumulator plants new to science (Gei et al. 2020).
Most Ni hyperaccumulators belong to two groups, geographically: (i) the Mediterranean region, extending from Portugal through Italy and the Balkans to Turkey and adjacent countries; and (ii) tropical and subtropical areas worldwide, particularly Cuba, New Caledonia and various islands of Indonesia and the Philippines. The plant family most strongly represented in the first group is Brassicaceae, whereas in tropical areas there is strong representation from Euphorbiaceae, Phyllanthaceae, Salicaceae, Buxaceae and Rubiaceae. Within Violaceae, species of Hybanthus (Severne and Brooks 1972; Brooks et al. 1974; Jaffré 1980; Paul et al. 2020) and Rinorea (Brooks and Wither 1977; Brooks et al. 1977b; Proctor et al. 1994) are notable as having potentially suitable biomass for agromining purposes. Hyperaccumulators in the Asteraceae appear in South Africa (Berkheya and Senecio; Morrey et al. 1989, 1992), in the Mediterranean-Turkey region (Centaurea; Reeves and Adıgüzel 2004), and in the neotropics (e.g. Pentacalia and Senecio in Cuba, Reeves et al. 1999; species in several genera in Brazil, Reeves et al. 2007). The Ni hyperaccumulator plants reported to date belong to about 40 different families, distributed widely throughout the plant kingdom; this syndrome is therefore presumed to have evolved independently many times (Boyd 2014; Cappa and Pilon-Smits 2014). It is certain that many more examples of Ni hyperaccumulation remain to be discovered. These will include species not yet discovered or described and known species that have never been analyzed. Further studies of plants growing on ultramafic areas in several islands of the Philippines and Indonesia, in Central America, mainland Asia, and possibly West Africa, are particularly likely to be fruitful.
The relatively large number of Ni hyperaccumulators discovered (compared with those of other elements) may be partly the result of the concerted attention to analytical work on ultramafic floras and partly to the ability to detect high Ni concentrations (>1000 μg g−1) in leaf tissue by a simple test with dimethylglyoxime. Among various types of metalliferous soils, the Ni-enriched ultramafics are the most widespread on a global scale, and in places continuous ultramafic areas of tens or even hundreds of km2 can be found (e.g. New Caledonia, Cuba, Turkey). Where such areas have been continuously available for plant colonization for millions of years, as appears to be the case in New Caledonia and eastern Cuba, a long-term opportunity has existed for the evolution of a characteristic flora with numerous endemic species, including some that have developed Ni accumulation as a particular response to growth on high-Ni soils (Isnard et al. 2016; Reeves et al. 1996).
Most of the known Ni hyperaccumulator species are endemic to ultramafic rocks, but some occur on a wider variety of soils and exhibit facultative hyperaccumulation, i.e. high Ni concentrations are found only in those specimens from Ni-rich soils. A tabulation of facultative hyperaccumulators, covering Ni and other elements, has been given by Pollard et al. (2014). In a few cases, ultramafic-endemic species may show a wide variation in Ni uptake, apparently being sensitive to parameters other than total soil Ni concentration, such as soil pH; this ‘erratic’ Ni hyperaccumulation occurs, for example, in the Queensland ultramafic endemic Pimelea leptospermoides (Thymelaeaceae) (Reeves et al. 2015).
When the focus is specifically on agromining potential, the interest logically moves towards those species that contain consistently >1 wt% Ni in their leaves (and ideally >1 wt% in total harvestable biomass). This property needs to be considered in conjunction with the rate of biomass production, and with other agronomic features considered elsewhere in this book by Nkrumah et al. (Chapter “Agronomy of ‘Metal Crops’ Used in Agromining”). The observation that the Californian Streptanthus polygaloides (Brassicaceae) could accumulate Ni to 1.5% of the dry plant matter (Reeves et al. 1981) stimulated studies by Nicks and Chambers (1995, 1998) on the use of this plant for phytomining. These included investigations of various fertilization regimes and the optimization of harvest time. They estimated that a crop of nearly 5 t ha−1 could be obtained with unfertilized plants in a small-scale trial in the native environment and predicted that fertilization could double that yield. Work elsewhere has been carried out with species capable of producing a larger biomass. The discovery of Ni hyperaccumulation by the South African Berkheya coddii (Morrey et al. 1989, 1992; Howes 1991) has been followed by extensive work on its cultivation and extraction of the accumulated Ni (Robinson et al. 1997a; Brooks and Robinson 1998); yields in excess of 20 t ha−1 were calculated, again by extrapolation from studies involving small plots.
Several Odontarrhena hyperaccumulators have attracted attention for their phytoextraction potential. Although some work has been done on O. bertolonii (Robinson et al. 1997b), more investigations have centred on species that have higher biomass such as O. corsica and O. muralis (Li et al. 2003; Bani et al. 2015a, b). Other species of the Brassicaceae in the Mediterranean region, such as Bornmuellera emarginata and B. tymphaea, have also been studied (Chardot et al. 2005). These authors concluded that B. emarginata compared favourably with O. chalcidica and Noccaea caerulescens in its phytoextraction performance.
About 70 tropical hyperaccumulator taxa with >1 wt% Ni have been listed by Reeves (2003). These include the facultative hyperaccumulator Rinorea bengalensis (Violaceae) of Southeast Asia, a large number of Cuban species in the Phyllanthaceae, Buxaceae, and Rubiaceae, and several New Caledonian species. Many of these are shrubs or small trees, probably with good rates of biomass production, although in many cases no data are available on this aspect. Some of these species are rare, and in most cases agronomic studies are lacking or are only in early stages.
5.2 Zinc, Lead and Cadmium
Since the early discovery of Zn accumulation by certain Viola and Noccaea species (noted above), further work, particularly on Noccaea from German and Belgian calamine soils and from British mine wastes, has been reported frequently, as discussed with detailed references by Baker et al. (1994), Reeves and Baker (2000), and Reeves et al. (2001). This species, often referred to as Thlaspi calaminare or T. alpestre in earlier work, and later as T. caerulescens, is now classified as N. caerulescens after a taxonomic revision by Meyer (1973) and subsequent DNA work (Koch and Mummenhoff 2001; Al-Shehbaz 2014).
Following the observation of Rascio (1977) that T. rotundifolium subsp. cepaeifolium (now N. rotundifolia) from Zn-polluted soils near the border of Italy and Austria was also a hyperaccumulator of Zn, surveys of the genus Thlaspi s.l. (including those species now belonging to Noccaea) (Reeves and Brooks 1983a, b; Reeves 1988) revealed that many species of this genus are hyperaccumulators of Ni from ultramafic soils and often have Zn levels above 1000 μg g−1, even from soils of background Zn content. Reeves and Baker (1984) showed that the ability of the Austrian species N. goesingensis to accumulate Ni and Zn was an innate or ‘constitutional’ property, i.e. not dependent on the geochemistry of the area from which the seed originated. Baker et al. (1994) showed that N. caerulescens grown in amended nutrient solutions had the ability to accumulate to high concentrations a wide variety of elements (Zn, Cd, Co, Mn and Ni throughout the plant; Al, Cr, Cu, Fe and Pb largely in the root system).
There are several other examples of Zn accumulation to a concentration of 10 000 μg g−1 set as the criterion for Zn hyperaccumulation by Baker and Brooks (1989), and later supported by van der Ent et al. (2013). The most notable is probably Arabidopsis (formerly Cardaminopsis) halleri (Brassicaceae) (Ernst 1968). Other occurrences, mainly from Zn-rich soils around mine sites or from the vicinity of smelters, are listed elsewhere (e.g. Reeves and Baker 2000).
Lead is usually present in vegetation at levels below <10 μg g−1. Even where concentrations of 1–10 μg g−1 are measured in above-ground plant parts, it is likely that much of this metal comes from various forms of environmental and/or laboratory contamination. Plant root systems restrict severely the uptake of Pb and significant translocation to the upper parts is uncommon in plants in natural environments. There have been several reports of very high Pb concentrations in plants from areas of Zn-Pb mineralization, and from mine or smelter wastes; notably, these have not generally been subjected to rigorous scrutiny in relation to washing procedures and contamination possibilities. Increased uptake of Pb can be achieved in hydroponic experiments or by various treatments of soil with complexing agents (Raskin and Ensley 2000). However, such soil treatments designed to mobilize relatively insoluble elements such as Pb and Au into harvestable plants, as promoted by several groups, are now regarded as being both economically and environmentally unfavourable.
Elevated levels of Cd (10–200 μg g−1, locally higher) can be found in soils containing waste materials from the mining of Zn ores but may also occur in soils treated with industrial wastes or Cd-rich phosphate fertilizers. Plant Cd is generally <3 μg g−1 but may reach 20 μg g−1 or more in the flora of Cd-rich soils. A plant concentration of >100 μg g−1 has been proposed as the threshold for hyperaccumulation of this element (van der Ent et al. 2013); such a level is exceptional, even on a Cd-contaminated site. However, on some Zn-Pb mine waste sites in the south of France and in Slovenia, Noccaea species such as N. caerulescens and N. praecox have been found to typically contain >100 μg g−1 Cd, and >1000 μg g−1 locally, with very large variations existing among sites and populations, and considerable intra-site variability (Robinson et al. 1998; Escarré et al. 2000; Lombi et al. 2000; Reeves et al. 2001; Schwartz et al. 2006). Similar observations have been made for A. halleri in Europe (Bert et al. 2002) and for Sedum alfredii (Crassulaceae) and Viola baoshanensis (Violaceae) in China (Liu et al. 2004; Deng et al. 2008). As stressed by van der Ent et al. (2013), further claims of hyperaccumulation of Cd (and other elements) should be restricted to the behaviour of self-sustaining natural populations. Extensive investigations of the behaviour of selected N. caerulescens populations have generally been carried out with a focus on phytoremediation rather than agromining (e.g. Chaney et al. 2005).
5.3 Cobalt and Copper
An earlier threshold of 1000 μg g−1 for plants to be considered as hyperaccumulators of Cu and Co (Baker and Brooks 1989) has been lowered to 300 μg g−1 (Krämer 2010; van der Ent et al. 2013) in the light of the apparent rarity of genuine accumulations of these elements in flora. Most reports of Co and Cu exceeding 1000 μg g−1 are derived from studies of the metalliferous soils of the Democratic Republic of the Congo, where the two metals occur together at elevated levels, although in widely varying proportions. Elsewhere, there are local early records of plants having >1000 μg g−1 Cu from Cu-mineralized areas (Blissett 1966; Dykeman and De Sousa 1966; Ernst 1966). These reports, and the plant species involved, need more detailed investigation, particularly in view of the potential for soil and dust contamination and the difficulty of its removal from many plant surfaces prior to analysis. The problem is exacerbated in the case of Cu mineral exposures by the common occurrence of more or less pure Cu compounds occurring as secondary mineralization products: a very small amount of such contamination remaining on the plant material can elevate the analytical result considerably (van der Ent et al. 2013; Lange et al. 2017). A similar problem arises in the case of plants sampled from the vicinity of smelters.
Normal concentrations of Co and Cu in plants are in the ranges of 0.03–2 μg g−1 and 5–25 μg g−1, respectively. Even on Co-rich soils, such as those derived from ultramafic rocks, Co in plants rarely exceeds 20 μg g−1. Plant Cu concentrations are also controlled within a remarkably narrow range, even in the presence of high soil Cu; plant Cu concentrations above 100 μg g−1 are rare. However, the black gum of the southeastern United States (Nyssa sylvatica var. biflora and var. sylvatica) (Nyssaceae) shows exceptional Co accumulation (as much as 845 μg g−1) from normal soils (Beeson et al. 1955; Kubota et al. 1960; Brooks et al. 1977c). Duvigneaud (1959) found accumulation of Co to 354 μg g−1 by Crotalaria cobalticola (Fabaceae) on Co-rich soils in the Democratic Republic of the Congo; Brooks et al. (1980) reported even higher concentrations in this species. In the extensive survey of the Cuban ultramafic flora, using herbarium specimens and others collected directly from the field, notably elevated Co levels were measured in some of the Ni hyperaccumulators. Mention was made of Co attaining 1140 μg g−1 in Phyllanthus williamioides (Reeves et al. 1996) and of values in the range 100–800 μg g−1 in a number of other species (Reeves et al 1999). Details of the latter were not published, as the concentrations did not reach the threshold for Co hyperaccumulation that was being applied at that time. With the lowering of the threshold to 300 μg g−1 it can be noted that the following maximum Co concentrations were found (always accompanying Ni hyperaccumulation): Buxus historica (Buxaceae) 667 μg g−1; Euphorbia helenae subsp. grandifolia (Euphorbiaceae) 357 μg g−1 and 392 μg g−1 in latex; Phyllanthus myrtilloides subsp. erythrinus (Phyllanthaceae) 378 μg g−1; Heterosavia maculata var. clementis (Phyllanthaceae) 336 μg g−1.
Extensive studies of the vegetation of many sites of mining and smelting activity throughout the Democratic Republic of Congo by F. Malaisse, R. R. Brooks, A.J.M. Baker, and co-workers identified 30 hyperaccumulator plants of Co and 32 of Cu, with 12 species being common to the two lists. The species involved have been summarized and updated in several papers and chapters (Brooks 1977; Malaisse et al. 1979; Brooks et al. 1978, 1980, 1987, 1995; Brooks and Malaisse 1985; Reeves and Baker 2000). Assessment of these data is difficult for several reasons: (i) numerous changes have been made to the classification and nomenclature of the species involved; (ii) uncertainties exist surrounding pre-treatment of the samples prior to analysis, and in particular the efficacy of the washing regimes; (iii) few of the Co- and Cu-accumulating species appear to be absolutely restricted to metalliferous soils, although some have had local or regional uses as indicator plants; (iv) there are wide variations in the apparent metal concentrations occurring within many species, even from the same area; (v) a lack of reproducibility exists in cases where the plants from a given location have been re-examined later; and (vi) difficulties have been reported in attempting to reproduce the metal accumulating behaviour in plants in cultivation. A detailed re-assessment of several putative hyperaccumulators was presented by Faucon et al. (2007), who concluded that at least part of the previously reported elevated metal levels could be ascribed to inefficient washing of sample materials prior to analysis. However, in spite of the suspicion that the last of these possibilities is sometimes relevant, many records of Cu and Co hyperaccumulation represent some degree of abnormal uptake by the plant from the soil: Malaisse et al. (1994), for example, presented iron data that indicate little likelihood of soil contamination (e.g. Anisopappus davyi (Asteraceae) having 3504 μg g−1 Cu, 3 μg g−1 Co and 67 μg g−1 iron). A re-examination of putative Cu hyperaccumulation by Millotia myosotidifolia (Asteraceae) from a Cu mine site in South Australia (R.D. Reeves, unpublished data), has not supported the earlier finding of 4 wt% Cu in the plant ash or 2400 μg g−1 in the leaves (Blissett 1966), but instead showed Cu levels averaging 516 μg g−1. This concentration is still abnormally high, and much higher than found in other species from the same site, apart from Arctotheca calendula (Asteraceae) that averaged 779 μg g−1 Cu. Extensive analyses of plants from some unusually Cu-rich ultramafic soils in Malaysia and Brazil have not shown any instance of Cu concentrations reaching 300 μg g−1 (van der Ent and Reeves 2015).
Even with the adoption of a 300 μg g−1 threshold in defining hyperaccumulation of Cu and Co, and with the addition of reports of Cu accumulation from Sri Lanka, China, and Indonesia, we conclude that Cu and Co hyperaccumulation in plants is very rare. From the point of view of agromining applications, it is scarcely relevant whether the threshold is set at 300 or 1000 μg g−1, because the levels of 5000 to 10 000 μg g−1 of interest for agromining of these elements have never been observed. The high specificity of Ni hyperaccumulation, relative to uptake of Co by Ni accumulator plants on ultramafic soils, also implies that extracting Co as a by-product of Ni agromining will rarely be economically feasible.
5.4 Manganese
Jaffré (1977, 1979, 1980) found that 98 out of 445 species (22%) growing on ultramafic soils of New Caledonia had mean Mn concentrations above 1000 μg g−1; six species had means exceeding 10 000 μg g−1, and nine had at least one specimen above this level. The total Mn concentrations within these soils ranged from about 4000–6000 μg g−1 (Isnard et al. 2016), only a little above the range determined for many types of soils worldwide. High Mn concentration in leaves can be attributed to: (i) Mn bioavailability, which depends on soil pH, independently from total or exchangeable soil [Mn] (Fernando et al. 2008; Jaffré 1980); and (ii) the release of carboxylates by specialized cluster roots, such as in many Mn hyperaccumulators belonging to Proteaceae (Lambers et al. 2015).
Recognizing that normal levels of Mn in plant dry matter fall within the rather wide range of 20–500 μg g−1, Baker and Brooks (1989) chose a level of 10 000 μg g−1 to define Mn hyperaccumulation. This criterion has been maintained in the review by van der Ent et al. (2013). After accounting for synonymies and changes of nomenclature for several species, data are now available for 42 species that have been found to meet this threshold in at least one specimen (Reeves et al. 2017). These include single species of Alyxia (Apocynaceae), Beaupreopsis and Grevillea (Proteaceae) (Jaffré 1977, 1979; Losfeld et al. 2015), all from New Caledonia; Chengiopanax and Polyscias (Araliaceae) from Japan and New Caledonia, respectively (Mizuno et al 2008; Losfeld et al. 2015); Garcinia amplexicaulis (Clusiaceae) from New Caledonia (Jaffré 1980); two species each of Phytolacca (Phytolaccaceae) from China (Xue et al. 2004); Polygonum (Polygonaceae) from China (Deng et al. 2010), three species of Denhamia (formerly in Maytenus—Celastraceae) from New Caledonia and Australia (Jaffré 1977); two of Virotia (formerly in Macadamia—Proteaceae) from New Caledonia (Jaffré 1979); and nine of Gossia (formerly in Austromyrtus and Eugenia—Myrtaceae) from eastern Australia (Jaffré 1980; Bidwell et al. 2002; Fernando et al. 2008, 2009; Losfeld et al. 2015). Because of the extreme levels of Mn, locally reaching 2–5 wt% in dry matter in some of these species, the plant ash may contain 10–25 wt% Mn, which should make agromining for Mn worthy of further study and field trials.
5.5 Chromium
Even on ultramafic soils having high Cr concentrations (500–5000 μg g−1) it is normal to find Cr in plant material in the range of 1–30 μg g−1. Occasional reports of much higher concentrations are believed to reflect contamination by wind-blown dusts or smelter fallout, or analytical problems. High Cr concentrations in plants from ultramafic soils (up to 1000 μg g−1) have been used as an indicator of soil contamination (see e.g. Jaffré et al. 1979b; Brooks and Yang 1984). In the absence to date of evidence for consistently high Cr concentrations existing in any species, there seems to be little hope of finding a future for agromining of this element.
5.6 Selenium
Selenium is essential for animal and human health and exhibits a narrow range between the levels required to prevent deficiency diseases and those that produce symptoms of toxicity. The Se content of soils is typically below 2 μg g−1 but can reach several hundred μg g−1 in soils derived from certain Cretaceous shales. In plant dry matter, Se concentrations are generally below 1 μg g−1 and may even be <0.01 μg g−1 in areas of Se-poor soils. However, the accumulation of Se to high levels (locally >1000 μg g−1) by legumes in the genus Astragalus (Fabaceae) from seleniferous soils in the western United States was found to be responsible for the poisoning of livestock (Byers et al. 1938). A detailed account of the discovery of Se-accumulating plants in the western USA can be found elsewhere (Rosenfeld and Beath 1964). Reeves and Baker (2000) tabulated values and references for 20 species that have shown maximum Se concentrations above 1000 μg g−1. Because of the very low levels of Se that normally occur in plants, a case can be made (Reeves 2005; van der Ent et al. 2013) for taking 100 μg g−1 as the threshold for Se hyperaccumulators. The use of plants showing some degree of Se accumulation for economic extraction of elemental Se has not been proposed. However, there are potential applications in: (i) phytoremediation of soils that have become Se-contaminated through extensive use of Se-rich irrigation waters (Parker et al. 2003), (ii) harvesting crop plants suitable for stock feed from high-Se areas and transport of this material to areas of Se deficiency (Bañuelos and Mayland 2000), and (iii) Se biofortification for improving human health (Bañuelos et al. 2014).
5.7 Arsenic
Normal As concentrations in igneous rocks and soils are in the range of 1–10 μg g−1. Higher soil As concentrations can be found in areas of polymetallic sulfide mineralization and of some pyritic black shales, in places contaminated through the smelting of chalcophile element or gold ores, in areas of geothermal activity, and where As compounds have been used as horticultural sprays (e.g. blueberry fields in Maine, USA) or timber preservation agents. Plant As concentrations are normally on the order of 1 μg g−1, but higher values can be found in contaminated areas. Arsenic hyperaccumulation (based on a 1000 μg g−1 dry matter criterion) has been known for more than 50 years. Warren et al. (1964) found As in the ash of growing tips of Pseudotsuga menziesii to be 2500–10 000 μg g−1 over soils containing 1000–5000 μg g−1 As. The highest of these values almost certainly corresponds to >1000 μg g−1 As on a dry weight basis.
Studies by several groups on the behaviour of aquatic plants in the Waikato River in the North Island of New Zealand showed that three aquatic plants act as As hyperaccumulators. Natural geothermal activity, together with borefield drainage and wastewater from the Wairakei geothermal power plant that opened in 1953, combined to raise the As concentration in the river from ca. 0.01 mg L−1 to as much as 0.08–0.09 mg L−1 before dilution and sedimentation processes lower the concentrations downstream. The adventive aquatic weeds Ceratophyllum demersum (Ceratophyllaceae), Egeria densa, and Lagarosiphon major (Hydrocharitaceae) act as As hyperaccumulators (Lancaster et al. 1971; Aggett and Aspell 1980; Liddle 1982; Reeves and Liddle 1986), yielding As concentrations in the plant dry matter from ca. 100 μg g−1 to 1000–1500 μg g−1. The bioaccumulation factor, taken as the plant/substrate concentration ratio, can be as high as 30 000, e.g. where the plants contain 1500 μg g−1 in water with 0.05 mg L−1 As.
More recent attention has been paid to As accumulation by fern species, particularly those growing in areas of As contamination from waste disposal related to timber preservation processes or mining. Ma et al. (2001) reported As at 3280–4980 μg g−1 in Pteris vittata (Pteridaceae) plants from soils containing 19 to 1603 μg g−1 As. Arsenic hyperaccumulation was also found by Vittoottiviseth et al. (2002) in the fern Pityrogramma calomelanos (Pteridaceae). A number of fern species may possess this capability of As accumulation as a constitutive property (Meharg 2002). However, applications of As hyperaccumulators seem likely to lie more in the area of remediation of As-contaminated waters and land, rather than in economic extraction of the As itself.
5.8 Thallium
Currently only a small number of Tl hyperaccumulator plants have been reported, mainly from France: Biscutella laevigata (Brassicaceae) with up to 15 200 μg g−1 Tl (Anderson et al. 1999), and Iberis intermedia (Brassicaceae) (now regarded as a synonym of I. linifolia) with up to 2810 μg g−1 Tl (LaCoste et al. 1999; Leblanc et al. 1999). Van der Ent et al. (2013) proposed a threshold value of 100 μg g−1 to define Tl hyperaccumulation. The substantial value of Tl might justify Tl agromining, but the locations at which this could take place appear to be rather limited (Zn-Pb mine tailings mainly).
6 X-ray Fluorescence for Discovery of Hyperaccumulator Plants: Case Studies
The discovery of hyperaccumulator plants has largely been based on analytical methods (e.g. AAS, ICP-AES) after acid digestion or dry ashing of dried leaf material obtained from herbarium collections or field sampling. New technical advances now permit massive screening of herbarium specimens using non-destructive and rapid portable X-ray Fluorescence Spectroscopy (XRF), an approach that has already led to the discovery of numerous hyperaccumulator species new to science. A full assessment of the advantages and limitations of this method is given in Chapter “Tools for the Discovery of Hyperaccumulator Plant Species in the Field and in the Herbarium” of this book.
6.1 Herbarium XRF Discoveries in Papua New Guinea
The flora of Papua New Guinea is one of the richest worldwide (estimated at 25 000 plant species). However, no plant species from this country were reported as hyperaccumulators until a herbarium XRF scanning of native Papua New Guinean specimens in Queensland Herbarium (Australia) was performed by Do et al. (2020). During the scanning campaign, 3164 plant specimens were measured and a total of 19 hyperaccumulators were identified: one Ni hyperaccumulator (>1000 µg g−1 Ni), eight Mn accumulators (>5000 µg g−1 Mn) and 10 Zn hyperaccumulators (>3000 µg g−1 Zn). These hyperaccumulators were previously unknown, thus adding to the global inventory of hyperaccumulator plants.
6.2 Herbarium XRF Discoveries in Central America
In Central America, the first successful endeavour to discover hyperaccumulators was performed using a portable XRF instrument (McCartha et al. 2019). This study aimed to confirm the status of three species from the genus Psychotria (P. grandis, P. costivenia and P. viridis) that were reported to be hyperaccumulators in its neighbouring region, Greater Antilles. Also, it evaluated whether four species (P. clivorum, P. flava, P. lorenciana and P. papantlensis) that are close relatives of the three known hyperaccumulators do indeed hyperaccumulate. Results show that P. costivenia, P. grandis, P. lorenciana and P. papantlensis were identified as valid hyperaccumulators, the two latter being obligate. The study also found that the geographic distribution of these Ni hyperaccumulators does not correspond to that of Ni-laterite soils or more widely to ultramafic outcrops. Such a finding is another benefit offered by herbarium XRF scanning that can be used in an initial stage of exploration. Recently, when sampling these species of Psychotria in the field in southeastern Mexico and Central America, two other interesting groups of Ni hyperaccumulators were identified. The first belongs also to the Rubiaceae and includes the monospecific genus Blepharidium (B. guatemalense) and the closely related Arachnothryx longiflorum. Blepharidium guatemalense exhibits the same extraordinary green Ni-rich phloem tissues as some of the Ni hyperaccumulators from Southeast Asia and the Pacific region. Its leaves can contain Ni concentrations above 2 wt%. The second group belongs to the Violaceae and includes two very closely related genera (Orthion and the mono-specific genus Mayanaea). Brooks et al. (1977a) found from herbarium sampling that a species of Hybanthus from Mexico, H. malpighiifolius, had unusually high Ni concentrations in leaves (638 μg g−1) and therefore qualified as a ‘strong’ accumulator. Since then, several species of Hybanthus from the New World were grouped in the genus Orthion (Wahlert et al. 2014), in which nearly all species are Ni hyperaccumulators (facultative and obligate). Unsurprisingly, Mayanaea and Orthion belong to the same phylogenetic clade as the hyperaccumulating Hybanthus from Australia and New Caledonia (Wahlert et al. 2014). Among Orthion species, O. subsessile is an obligate hyperaccumulator that can have Ni concentrations in leaves that exceed 2 wt%. Further XRF herbarium scanning campaigns revealed that at least four of the six species of Orthion and Mayanaea caudata are Ni hyperaccumulators, and that these species are not endemic to ultramafic soils although some are obligate Ni hyperaccumulators (Navarrete-Gutiérrez, to be submitted). In total, 14 Ni hyperaccumulator taxa are now identified in Central America, most of which are not endemic to ultramafic areas (McCartha et al. 2019) because local soils have notably high Ni concentrations independent of the presence of ultramafic substrates.
6.3 Herbarium XRF Discoveries in New Caledonia
Pioneer geobotanical studies were carried out in the 1970s in New Caledonia, where many hyperaccumulator plants have been discovered (Jaffré et al. 1976; 1979a; 1979b; Kersten et al. 1979). Current knowledge of the flora and availability of plant specimens in herbaria have provided a unique opportunity to carry out a systematic assessment of the incidence of hyperaccumulation in the regional flora. XRF herbarium screening was undertaken at the Herbarium of New Caledonia (NOU) on ca. 11 200 herbarium specimens. The selection of herbarium specimens to scan was based on families that were already known to contain numerous hyperaccumulator species (Jaffré et al. 1976, 1979a, 2013). All available specimens were scanned in the Cunoniaceae, Phyllanthaceae, Salicaceae, Sapotaceae, Oncothecaceae, and Violaceae, as well as a systematic screening of one to four specimens (depending on availability) of species known to occur on ultramafic soils in New Caledonia (Isnard et al. 2016). This screening included 1484 species (1620 taxa) covering 35 orders, 96 families, 281 genera, and ~89% of the ultramafic-related dicotyledonous flora. The study led to the recording of numerous hyperaccumulator plant species: 99 taxa for Ni (65 known previously), 74 taxa for Mn (11 known previously), eight taxa for Co (two known previously), and four taxa for Zn (none previously recorded). This work demonstrated that XRF screening of herbarium specimens has the potential to discover vast numbers of new hyperaccumulator species, even in well-studied flora such that of New Caledonia. New hyperaccumulator species are also expected to be discovered in the field, as demonstrated by the recently described new species Pycnandra caeruleilatex (Swenson and Munzinger 2010) and Pycnandra kouakouensis (Swenson and Munzinger 2016), both of which have a bluish or greenish latex. These species were confirmed to be strong hyperaccumulators (Gei et al. 2020). This approach points to further opportunities to study the ecology and biogeography of hyperaccumulation.
6.4 Herbarium XRF Discoveries in Sabah, Malaysia
In Sabah, Malaysia, a recent herbarium XRF scanning campaign scanned a total of ~7300 plant species (van der Ent et al. 2019). This campaign recorded 91 hyperaccumulators: 28 Ni hyperaccumulators, 12 Co hyperaccumulators and 51 Mn hyperaccumulators. Among 51 Mn hyperaccumulators, 14 Mn hyperaccumulators were previously known from Sabah. Interestingly, most Mn (hyper) accumulative plants encountered in this campaign did not occur in ultramafic soils. Cobalt hyperaccumulation is rare, even with a Co hyperaccumulation threshold of >300 μg g−1. Nevertheless, this campaign discovered a species, Ashtonia excelsa that accumulates 1500 μg g−1 Co. The study further demonstrates the usefulness of the herbarium XRF scanning technique for identifying hyperaccumulators.
7 Knowledge Gaps: Priority Regions for Exploration and Discovery
Currently it is estimated that hyperaccumulation occurs in 0.2% of angiosperms and 1–2% of known ultramafic global flora (Baker et al. 2000; Baker and Brooks 1989; Cappa and Pilon-Smits 2014). It is certain that more unidentified hyperaccumulator species remain to be discovered. Systematic herbarium specimen XRF scanning, combined with auxiliary collection data, can provide insights into phylogenetic patterns of hyperaccumulation, and has the potential to complement and add insights to biogeographical and evolutionary studies.
There is also a need for further field exploration of ultramafic and other metalliferous areas that have not so far been subjected to extensive exploration and collection of herbarium material. Furthermore, because herbarium analysis most often consists of a single sample taken from a population, in the most striking cases of hyperaccumulation (where there is agromining potential), there is a need for more detailed field investigation of various occurrences of the species. This needs to be done (i) to obtain reliable statistical information on the distribution of metal concentration within each population, and their relation to local soil, (ii) to examine interpopulation variability, and (iii) to obtain information about the natural reproduction of the species and any significant interactions with other biota in the immediate environment of the specimens.
References
Aggett J, Aspell AC (1980) Arsenic from geothermal sources in the Waikato catchment. NZ J Sci 23:77–82
Al-Shehbaz IA (2014) A synopsis of the genus Noccaea (Coluteocarpeae, Brassicaceae). Harv Papers Bot 19:25–51
Anderson C, Brooks R, Chiarucci A, LaCoste C, Leblanc M, Robinson B, Simcock R, Stewart R (1999) Phytomining for nickel, thallium and gold. J Geochem Explor 67:407–415
Antonovics J, Bradshaw AD, Turner AG (1971) Heavy metal tolerance in plants. Adv Ecol Res 7:1–85
Baker AJM (1981) Accumulators and excluders—strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654
Baker AJM (1987) Metal tolerance. New Phytol 106:93–111
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Baker AJM, Whiting SN (2002) In search of the Holy Grail—a further step in understanding metal hyperaccumulation? New Phytol 155:1–4
Baker AJM, Proctor J, van Balgooy MMJ, Reeves RD (1992) Hyperaccumulation of nickel by the ultramafic flora of Palawan, Republic of the Philippines. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils, Intercept Ltd. Andover, UK, pp 291–304
Baker AJM, Reeves RD, Hajar ASM (1994) Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl (Brassicaceae). New Phytol 127:61–68
Baker AJM, McGrath SP, Reeves RD, Smith JAC (2000) Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Terry N, Bañuelos GS (eds) Phytoremediation of contaminated soil and water. CRC Press Inc, Boca Raton, FL, USA, pp 85–107
Baker AJM, Ernst WHO, van der Ent A, Malaisse F, Ginocchio R (2010) Metallophytes: the unique biological resource, its ecology and conservational status in Europe, central Africa and Latin America. In: Batty LC, Hallberg KB (eds) Ecology of industrial pollution. Cambridge University Press, UK, pp 7–40
Bani A, Echevarria G, Sulce S, Morel JL (2015a) Improving the agronomy of Alyssum murale for extensive phytomining: a five-year field study. Int J Phytoremediation 17:117–127
Bani A, Echevarria G, Zhang X, Benizri A, Laubie E, Morel JL, Simonnot M-O (2015b) The effect of plant density in nickel-phytomining field experiments with Alyssum murale in Albania. Aust J Bot 63:72–77
Bañuelos GS, Mayland HF (2000) Absorption and distribution of selenium in animals consuming canola grown for selenium phytoremediation. Ecotoxicol Environ Safety 46:322–328
Bañuelos GS, Lin Z-Q, Yin X (2014) Selenium in the environment and human health. CRC Press, Boca Raton, FL, USA
Batianoff GN, Reeves RD, Specht RL (1990) Stackhousia tryonii Bailey: a nickel-accumulating serpentinite-endemic species of central Queensland. Aust J Bot 38:121–130
Beeson KC, Lazar VA, Boyce SG (1955) Some plant accumulators of the micronutrient elements. Ecology 36:155–156
Berazaín Iturralde R (1981) Sobre el endemismo de la florula serpentinicola de Lomas de Galindo, Canasi, Habana. Rev Jard Bot Nacional (Cuba) 2:29–59
Bert V, Bonnin I, Saumitou-Laprade P, De Laguérie P, Petit D (2002) Do Arabidopsis halleri from nonmetallicolous populations accumulate zinc and cadmium more effectively than those from metallicolous populations? New Phytol 155:47–57
Bidwell SD, Woodrow IE, Batianoff GN, Sommer-Knudsen J (2002) Hyperaccumulation of manganese in the rainforest tree Austromyrtus bidwillii (Myrtaceae) from Queensland, Australia. Funct Plant Biol 29:899–905
Blissett AH (1966) Copper tolerant plants from the Ukaparinga copper mine, Williamstown. Quart Geol Notes Geol Surv S Australia 18:1–3
Boyd RS (2014) Ecology and evolution of metal-hyperaccumulating plants. In: Rajakaruna N, Boyd RS, Harris TB (eds) Plant ecology and evolution in harsh environments. Novinka, New York, USA, pp 227–241
Brooks RR (1977) Copper and cobalt uptake by Haumaniastrum species. Plant Soil 48:541–544
Brooks RR (1987) Serpentine and its vegetation: a multidisciplinary approach. Dioscorides Press, Portland, Oregon, USA
Brooks RR (1998) Geobotany and hyperaccumulators. In: Brooks RR (ed) Plants that hyperaccumulate heavy metals. CAB International, Wallingford, UK, pp 55–94
Brooks RR, Malaisse F (1985) The heavy metal-tolerant flora of Southcentral Africa. Balkema, Rotterdam. The Netherlands
Brooks RR, Radford CC (1978) Nickel accumulation by European species of the genus Alyssum. Proc Roy Soc Lond B 200:217–222
Brooks RR, Robinson BH (1998) The potential use of hyperaccumulators and other plants for phytomining. In: Brooks RR (ed) Plants that hyperaccumulate heavy metals. CAB International, Wallingford, UK, pp 327–356
Brooks RR, Wither ED (1977) Nickel accumulation by Rinorea bengalensis (Wall.) O.K. J Geochem Explor 7:295–300
Brooks RR, Yang XH (1984) Elemental levels and relationships in the endemic serpentine flora of the Great Dyke, Zimbabwe, and their significance as controlling factors for the flora. Taxon 33:392–399
Brooks RR, Lee J, Jaffré T (1974) Some New Zealand and New Caledonian plant accumulators of nickel. J Ecol 62:493–499
Brooks RR, Lee J, Reeves RD, Jaffré T (1977a) Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J Geochem Explor 7:49–57
Brooks RR, Wither ED, Zepernick B (1977b) Cobalt and nickel in Rinorea species. Plant Soil 47:707–712
Brooks RR, McCleave JA, Schofield EK (1977c) Cobalt and nickel uptake by the Nyssaceae. Taxon 26:197–201
Brooks RR, Morrison RS, Reeves RD, Malaisse F (1978) Copper and cobalt in African species of Aeolanthus Mart. (Plectranthinae, Labiatae). Plant Soil 50:503–507
Brooks RR, Morrison RS, Reeves RD, Dudley TR, Akman Y (1979) Hyperaccumulation of nickel by Alyssum Linnaeus (Cruciferae). Proc Roy Soc Lond B 203:387–403
Brooks RR, Reeves RD, Morrison RS, Malaisse F (1980) Hyperaccumulation of copper and cobalt—a review. Bull Soc roy Bot Belg 113:166–172
Brooks RR, Naidu SD, Malaisse F, Lee J (1987) The elemental content of metallophytes from the copper/cobalt deposits of central Africa. Bull Soc roy Bot Belg 119:179–191
Brooks RR, Dunn CE, Hall GEM (1995) Biological systems in mineral exploration and processing. Ellis Horwood, Hemel Hempstead, UK
Byers HG, Miller JT, Williams KT, Lakin HW (1938) Selenium occurrence in certain soils in the United States, with a discussion of related topics. III. US Dept Agriculture Tech Bull 601:1–74
Campbell LR, Stone CO, Shamsedin NM, Kolterman DA, Pollard AJ (2013) Facultative hyperaccumulation of nickel in Psychotria grandis (Rubiaceae). Carib Nat 1:1–8
Cappa JJ, Pilon-Smits EAH (2014) Evolutionary aspects of elemental hyperaccumulation. Planta 239:267–275
Chardot V, Massoura S, Echevarria G, Reeves RD, Morel JL (2005) Phytoextraction potential of the nickel hyperaccumulators Leptoplax emarginata and Bornmuellera tymphaea. Int J Phytoremediation 7:323–335
Chaney RL, Angle JS, McIntosh MS, Reeves RD, Li Y-M, Brewer EP, Chen K-Y, Roseberg RJ, Perner H, Synkowski EC, Broadhurst CL, Wang A, Baker AJM (2005) Using hyperaccumulator plants to phytoextract soil Ni and Cd. Z Naturforsch C 60c:190–198
Cole MM (1973) Geobotanical and biogeochemical investigations in the sclerophyllous woodland and scrub associations of the eastern goldfields area of Western Australia, with particular reference to the role of Hybanthus floribundus (Lindl.) F. Muell. as nickel indicator and accumulator plant. J Appl Ecol 10:269–320
Cluzel D, Maurizot P, Collot J, Sevin B (2012) An outline of the geology of New Caledonia; from Permian-Mesozoic Southeast Gondwanaland active margin to Cenozoic obduction and supergene evolution. Episodes 35:72–86
Deng D-M, Deng J-C, Li J-T, Zhang J, Hu M, Lin Z, Liao B (2008) Accumulation of zinc, cadmium, and lead in four populations of Sedum alfredii growing on lead/zinc mine spoils. J Integr Plant Biol 50:691–698
Deng H, Li MS, Chen YX, Luo YP, Yu FM (2010) A new discovered manganese hyperaccumulator—Polygonum pubescens Blume. Fresenius Environ Bull 19:94–99
Do C, Abubakari F, Brown G, Casey LW, Burtet-Sarramegna V, Gei V, Erskine PD, van der Ent A (2020) A preliminary survey of hyperaccumulation in the Papua New Guinean flora from herbarium XRF scanning. Chemoecology 30:1–13
Doksopulo EP (1961) Nickel in rocks, soils, water and plants adjacent to the talc deposits of the Chorchanskaya group. Izdat Tbilisk Univ, Tbilisi
Duvigneaud P (1959) Plantes cobaltophytes dans le Haut Katanga. Bull Soc Roy Bot Belg 91:111–134
Dykeman WR, De Sousa AS (1966) Natural mechanisms of copper tolerance in a copper swamp. Can J Bot 44:871–878
Ernst WHO (1966) Ökologisch-soziologische Untersuchungen an Schwermetall-pflanzengesellschaften Südfrankreichs und des östlichen Harzvorlandes. Flora (Jena) B156:301–318
Ernst WHO (1968) Das Violetum calaminariae westfalicum, eine Schwermetall-pflanzengesellschaften Südfrankreichs und des östlichen Harzvorlandes. Mitteil Floristisch Arbeit 13:263–268
Escarré J, Lefèbvre C, Gruber W, Leblanc M, Lepart J, Rivière Y, Delay B (2000) Zinc and cadmium accumulation by Thlaspi caerulescens from metalliferous and nonmetalliferous sites in the Mediterranean area: implications for phytoremediation. New Phytol 145:429–437
Faucon M-P, Shutcha MN, Meerts P (2007) Revisiting copper and cobalt concentrations in supposed hyperaccumulators from SC Africa: influence of washing and metal concentrations in soil. Plant Soil 301:29–36
Fernando DR, Woodrow IE, Jaffré T, Dumontet V, Marshall AT, Baker AJM (2008) Foliar manganese accumulation by Maytenus fournieri (Celastraceae) in its native New Caledonian habitats: populational variation and localization by X-ray microanalysis. New Phytol 177:178–185
Fernando DR, Guymer G, Reeves RD, Woodrow IE, Baker AJM, Batianoff GN (2009) Foliar Mn accumulation in eastern Australian herbarium specimens: prospecting for ‘new’ Mn hyperaccumulators and potential applications in taxonomy. Ann Bot 103:931–939
Fernando ES, Quimado MO, Trinidad LC, Doronila AL (2013) The potential use of indigenous nickel hyperaccumulators for small-scale mining in The Philippines. J Degraded Mining Lands Manage 1:21–26
Gei V, Erskine PD, Echevarria G, Isnard S, Fogliani B, Jaffré T, van der Ent A (2020) A systematic assessment of the occurrence of trace element hyperaccumulation in the flora of New Caledonia. Bot J Linn Soc 194(1):1–22
Howes A (1991) Investigations into nickel hyperaccumulation by the plant Berkheya coddii. MSc thesis, University of Natal, Pietermaritzburg, South Africa
Ibanez T, Birnbaum P, Gâteblé G, Hequet V, Isnard S, Munzinger J, Pillon Y, Pouteau R, Vandrot H, Jaffré T (2018) Twenty years after Jaffré et al (1998) is the system of protected areas now adequate in New Caledonia? Biodivers Conserv 28:245–254
Isnard S, L’Huillier L, Rigault F, Jaffré T (2016) How did the ultramafic soils shape the flora of the New Caledonian hotspot? Plant Soil 403:53–76
Jaffré T (1977) Accumulation du manganèse par les espèces associées aux terrains ultrabasiques de Nouvelle Calédonie. Compt Rend Acad Sci Paris Sér D 284:1573–1575
Jaffré T (1979) Accumulation du manganèse par les Proteacées de Nouvelle Calédonie. Compt Rend Acad Sci Paris Sér D 289:425–428
Jaffré T (1980) Etude écologique du peuplement végétal des sols dérivés de roches ultrabasiques en Nouvelle Calédonie. Trav et Documents de l’ORSTOM 124, Paris, France
Jaffré T, Schmid M (1974) Accumulation du nickel par une Rubiacée de Nouvelle Calédonie, Psychotria douarrei (G. Beauvisage) Däniker. Compt Rend Acad Sci Paris Sér D 278:1727–1730
Jaffré T, Brooks RR, Lee J, Reeves RD (1976) Sebertia acuminata: a hyperaccumulator of nickel from New Caledonia. Science 193:579–580
Jaffré T, Brooks RR, Trow JM (1979a) Hyperaccumulation of nickel by Geissois species. Plant Soil 51:157–162
Jaffré T, Kersten WJ, Brooks RR, Reeves RD (1979b) Nickel uptake by the Flacourtiaceae of New Caledonia. Proc Roy Soc Lond B205:385–394
Jaffré T, Pillon Y, Thomine S, Merlot S (2013) The metal hyperaccumulators from New Caledonia can broaden our understanding of nickel accumulation in plants. Front Plant Sci 4(279):1–7
Kersten WJ, Brooks RR, Reeves RD, Jaffré T (1979) Nickel uptake by New Caledonian species of Phyllanthus. Taxon 28:529–534
Koch M, Mummenhoff K (2001) Thlaspi s.str. (Brassicaceae) versus Thlaspi s.l. morphological and anatomical characters in the light of ITS and nrDNA sequence data. Plant Syst Evol 227:209–225
Krämer U (2010) Metal hyperaccumulation in plants. Ann Rev Plant Biol 61:517–534
Kruckeberg AR (1954) The ecology of serpentine soils. III. Plant species in relation to serpentine soils. Ecology 35:267–274
Kubota J, Lazar VA, Beeson KC (1960) The study of cobalt status of soils in Arkansas and Louisiana using the black gum as the indicator plant. Soil Sci Soc Amer Proc 24:527–528
LaCoste C, Robinson BH, Brooks RR, Anderson CWN, Chiarucci A, Leblanc M (1999) The phytoremediation potential of thallium-contaminated soils using Iberis and Biscutella species. Int J Phytoremediation 1:327–338
Lambers H, Hayes PE, Laliberte E, Oliveira RS, Turner BL (2015) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90
Lange B, van der Ent A, Baker AJM, Echevarria G, Mahy G, Malaisse F, Meerts P, Pourret O, Verbruggen N, Faucon MP (2017) Copper and cobalt hyperaccumulation in plants: a critical assessment of the current status of knowledge. New Phytol 213(2):537–551
Leblanc M, Petit D, Deram A, Robinson BH, Brooks RR (1999) The phytomining and environmental significance of hyperaccumulation of thallium by Iberis intermedia from southern France. Econ Geol 94:109–113
Lancaster RJ, Coup MR, Hughes JW (1971) Toxicity of arsenic present in lakeweed. NZ Vet J 19:141–145
Lee J, Reeves RD, Brooks RR, Jaffré T (1977) Isolation and identification of a citrato-complex of nickel from nickel-accumulating plants. Phytochem 16:1503–1505
Li Y-M, Chaney RL, Brewer E, Roseberg R, Angle JS, Baker AJM, Reeves RD, Nelkin J (2003) Development of a technology for commercial phytoextraction of nickel: economic and technical considerations. Plant Soil 249:107–115
Liddle JR (1982) Arsenic and other elements of geothermal origin in the Taupo volcanic zone. PhD thesis, Massey University, New Zealand
Liu W, Shu W, Lan C (2004) Viola baoshanensis, a plant that hyperaccumulates cadmium. Chin Sci Bull 49:29–32
Lombi E, Zhao FJ, Dunham SJ, McGrath SP (2000) Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytol 145:11–20
Losfeld G, L’Huillier L, Fogliani B, McCoy S, Grison C, Jaffré T (2015) Leaf-age and soil-plant relationships: key factors for reporting trace-elements hyperaccumulation by plants and design applications. Environ Sci Pollut Res 22:5620–5632
Ma LQ, Komar KM, Tu C, Zhang WH, Cai Y, Kennelley ED (2001) A fern that hyperaccumulates arsenic. Nature 409:579
Malaisse F, Grégoire J, Brooks RR, Morrison RS, Reeves RD (1978) Aeolanthus biformifolius: a hyperaccumulator of copper from Zaïre. Science 199:887–888
Malaisse F, Grégoire J, Brooks RR, Morrison RS, Reeves RD (1979) Copper and cobalt in vegetation of Fungurume, Shaba Province, Zaïre. Oikos 33:472–478
Malaisse F, Brooks RR, Baker AJM (1994) Diversity of vegetation communities in relation to soil heavy metal content at the Shinkolobwe copper/cobalt/uranium mineralization, Upper Shaba, Zaïre. Belg J Bot 127:3–16
McAlister RL, Kolterman DA, Pollard AJ (2015) Nickel hyperaccumulation in populations of Psychotria grandis (Rubiaceae) from serpentine and non-serpentine soils of Puerto Rico. Aust J Bot 63:85–91
McCartha GL, Taylor CM, van der Ent A, Echevarria G, Navarrete Gutiérrez DM, Pollard AJ (2019) Phylogenetic and geographic distribution of nickel hyperaccumulation in neotropical Psychotria (Rubiaceae). Am J Bot 106(10):1377–1385
Meharg A (2002) Arsenic and old plants. New Phytol 156:1–4
Menezes de Sequeira E (1969) Toxicity and movement of heavy metals in serpentinitic rocks (north-eastern Portugal). Agron Lusit 30:115–154
Meyer FK (1973) Conspectus der “Thlaspi”-Arten Europas, Afrikas und Vorderasiens. Feddes Rep 84:449–470
Minguzzi C, Vergnano O (1948) Il contenuto di nichel nelle ceneri di Alyssum bertolonii Desv. Atti Soc Tosc Sci Nat Mem Ser A 55:49–77
Mizuno T, Asahina R, Hosono A, Tanaka A, Senoo K, Obata H (2008) Age-dependent manganese hyperaccumulation in Chengiopanax sciadophylloides (Araliaceae). J Plant Nutr 31:1811–1819
Morrey DR, Balkwill K, Balkwill M-J (1989) Studies on serpentine flora: preliminary analyses of soils and vegetation associated with serpentine rock formations in the southeastern Transvaal. S Afr J Bot 55:171–177
Morrey DR, Balkwill K, Balkwill M-J, Williamson S (1992) A review of some studies of the serpentine flora of southern Africa. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils, Intercept Ltd. Andover, UK, pp 147–157
Nicks LJ, Chambers MF (1995) Farming for metals. Mining Environ Manage 3:15–18
Nicks LJ, Chambers MF (1998) A pioneering study of the potential of phytomining for nickel. In: Brooks RR (ed) Plants that hyperaccumulate heavy metals. CAB International, Wallingford, UK, pp 313–325
Pelletier B (2006) Geology of the New Caledonia region and its implications for the study of the New Caledonian biodiversity. In: Payri C, Richer de Forges B (eds) Forum Biodiversité des Ecosystèmes Coralliens. Vol. Doc. Sci. Tech. IRD, II 7, Nouméa, Nouvelle-Calédonie, pp 17–30
Paul ALD, Gei V, Isnard S, Fogliani B, Echevarria G, Erskine PD, Jaffré T, Munzinger J, van der Ent A (2020) Nickel hyperaccumulation in New Caledonian Hybanthus (Violaceae) and occurrence of nickel-rich phloem in Hybanthus austrocaledonicus. Ann Bot 126:905–914
Parker DR, Feist LJ, Varvel TW, Thomason DN, Zhang Y (2003) Selenium phytoremediation potential of Stanleya pinnata. Plant Soil 249:157–165
Pollard AJ, Reeves RD, Baker AJM (2014) Facultative hyperaccumulation of metals and metalloids. Plant Sci 217–218:8–17
Proctor J, van Balgooy MMJ, Fairweather GM, Nagy L, Reeves RD (1994) A preliminary re-investigation of a plant geographical “El Dorado.”. Trop Biodiversity 2:303–316
Rascio N (1977) Metal accumulation by some plants growing on zinc-mine deposits. Oikos 29:250–253
Raskin I, Ensley BD (eds) (2000) Phytoremediation of toxic metals: using plants to clean up the environment. Wiley, New York
Reeves RD (1988) Nickel and zinc accumulation by species of Thlaspi L., Cochlearia L., and other genera of the Brassicaceae. Taxon 37:309–318
Reeves RD (1992) Hyperaccumulation of nickel by serpentine plants. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils, Intercept Ltd. Andover, UK, pp 253–277
Reeves RD (2003) Tropical hyperaccumulators of metals and their potential for phytoextraction. Plant Soil 249:57–65
Reeves RD (2005) Hyperaccumulation of trace elements by plants. In: Morel JL, Echevarria G, Goncharova N (eds) NATO Science series: IV: Earth and Environmental Sciences, vol 68, 360 pp. Springer, Berlin (2005), pp 25–52; online as pp 1–25 in Phytoremediation of Metal-Contaminated Soils, NATO Advanced Study Institute, Třešť Castle, Czech Republic, 18–30 Aug 2002, at www.pravo.by/UNESCOChairs/eng/kefedra.asp?idf=4andidt=64
Reeves RD, Adıgüzel N (2004) Rare plants and nickel accumulators from Turkish serpentine soils, with special reference to Centaurea species. Turk J Bot 28:147–153
Reeves RD, Adıgüzel N (2008) The nickel hyperaccumulating plants of Turkey and adjacent areas: a review with new data. Turk J Biol 32:143–153
Reeves RD, Baker AJM (1984) Studies on metal uptake by plants from serpentine and non-serpentine populations of Thlaspi goesingense Halácsy (Cruciferae). New Phytol 98:191–204
Reeves RD, Baker AJM (2000) Metal accumulating plants. In: Raskin I, Ensley B (eds) Phytoremediation of toxic metals: using plants to clean up the environment. Wiley, New York, pp 193–229
Reeves RD, Brooks RR (1983a) European species of Thlaspi L. (Cruciferae) as indicators of nickel and zinc. J Geochem Explor 18:275–283
Reeves RD, Brooks RR (1983b) Hyperaccumulation of lead and zinc by two metallophytes from a mining area in central Europe. Environ Pollut 31:277–287
Reeves RD, Liddle JR (1986) Dispersal of arsenic from geothermal sources of the central North Island. In: Baker MJ (ed) Trace Elements in the Eighties. NZ Trace Element Group, Palmerston North, NZ, pp 31–34
Reeves RD, Baker AJM, Jaffré T, Erskine PD, Echevarria G, van der Ent A (2017) A global database for hyperaccumulator plants of metal and metalloid trace elements. New Phytol 218:407–411
Reeves RD, Brooks RR, Press JR (1980) Nickel accumulation by species of Peltaria Jacq. (Cruciferae). Taxon 29:629–633
Reeves RD, Brooks RR, Macfarlane RM (1981) Nickel uptake by Californian Streptanthus and Caulanthus with particular reference to the hyperaccumulator S. polygaloides Gray (Brassicaceae). Amer J Bot 68:708–712
Reeves RD, Brooks RR, Dudley TR (1983a) Uptake of nickel by species of Alyssum, Bornmuellera and other genera of Old World Tribus Alysseae. Taxon 32:184–192
Reeves RD, Macfarlane RM, Brooks RR (1983b) Accumulation of nickel by western North American genera containing serpentine-tolerant species. Amer J Bot 70:1297–1303
Reeves RD, Baker AJM, Borhidi A, Berazaín R (1996) Nickel-accumulating plants from the ancient serpentine soils of Cuba. New Phytol 133:217–224
Reeves RD, Baker AJM, Borhidi A, Berazaín R (1999) Nickel hyperaccumulation in the serpentine flora of Cuba. Ann Bot 83:29–38
Reeves RD, Schwartz C, Morel JL, Edmondson J (2001) Distribution and metal-accumulating behaviour of Thlaspi caerulescens and associated metallophytes in France. Int J Phytoremediation 3:145–172
Reeves RD, Baker AJM, Becquer T, Echevarria G, Miranda ZJG (2007) The flora and biogeochemistry of the ultramafic soils of Goiás State, Brazil. Plant Soil 293:107–119
Reeves RD, Laidlaw WS, Doronila A, Baker AJM, Batianoff GN (2015) Erratic hyperaccumulation of nickel, with particular reference to the Queensland serpentine endemic Pimelea leptospermoides F. Mueller. Aust J Bot 63:119–127
Robinson BH, Brooks RR, Howes AW, Kirkman JH, Gregg PEH (1997a) The potential of the high-biomass nickel hyperaccumulator Berkheya coddii for phytoremediation and phytomining. J Geochem Explor 60:115–126
Robinson BH, Chiarucci A, Brooks RR, Petit D, Kirkman JH, Gregg PEH, De Dominicis V (1997b) The nickel hyperaccumulator plant Alyssum bertolonii as a potential agent for phytoremediation and phytomining of nickel. J Geochem Explor 59:75–86
Robinson BH, Leblanc M, Petit D, Brooks RR, Kirkman JH, Gregg PEH (1998) The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant Soil 203:47–56
Rosenfeld I, Beath OA (1964) Selenium—geobotany, biochemistry, toxicity and nutrition. Academic Press, New York, USA
Sachs J (1865) In: Hofmeister W (ed) Handbuch der Experimental-Physiologie der Pflanzen. Handbuch der Physiologischen Botanik Vol IV. Engelmann, Leipzig, Germany pp 153–154
Schwartz C, Sirguey C, Peronny S, Reeves RD, Bourgaud F, Morel JL (2006) Testing of outstanding individuals of Thlaspi caerulescens for cadmium phytoextraction. Int J Phytoremediation 8:339–357
Swenson U, Munzinger J (2010) Revision of Pycnandra subgenus Achradotypus (Sapotaceae), with five new species from New Caledonia. Aust Syst Bot 23:185–216
Swenson U, Munzinger J (2016) Five new species and a systematic synopsis of Pycnandra (Sapotaceae), the largest endemic genus in New Caledonia. Aust Syst Bot 29:1–40
Severne BC, Brooks RR (1972) A nickel accumulating plant from Western Australia. Planta 103:91–94
Stebbins GL (1942) The genetic approach to rare and endemic species. Madroño 6:241–272
Strawn KE (2013) Unearthing the habitat of a hyperaccumulator: case study of the invasive plant yellowtuft (Alyssum; Brassicaceae) in southwest Oregon, USA. Manage Biol Invasions 4:249–259
van der Ent A, Reeves RD (2015) Foliar metal accumulation in plants from copper-rich ultramafic outcrops: case studies from Malaysia and Brazil. Plant Soil 389:401–418
van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334
van der Ent A, Erskine P, Sunmail S (2015) Ecology of nickel hyperaccumulator plants from ultramafic soils in Sabah (Malaysia). Chemoecol 25:243–259
van der Ent A, Ocenar A, Tisserand R, Sugau JB, Erskine PD, Echevarria G (2019) Herbarium X-ray Fluorescence Screening for nickel, cobalt and manganese hyperaccumulation in the flora of Sabah (Malaysia, Borneo Island). J Geochem Explor 202:49–58
Vittoottiviseth P, Francesconi K, Sridokchan W (2002) The potential of Thai indigenous plant species for the phytoremediation of arsenic contaminated land. Environ Pollut 118:453–461
Wahlert GA, Marcussen T, Paula-Souza J, Ming F, Ballard HE Jr (2014) A phylogeny of the Violaceae (Malpighiales) inferred from plastid DNA sequences: implications for generic diversity and intrafamilial classification. Systematic Bot 39:239–252
Warren HV, Delavault RE, Barakso J (1964) The role of arsenic as a pathfinder in biogeochemical prospecting. Econ Geol 59:1381–1389
Wild H (1970) The vegetation of nickel-bearing soils. Kirkia 7 (suppl):1–62
Whiting SN, Reeves RD, Richards D, Johnson MS, Cooke JA, Malaisse F, Paton A, Smith JAC, Angle JS, Chaney RL, Ginocchio R, Jaffré T, Johns R, McIntyre T, Purvis OW, Salt DE, Schat H, Zhao FJ, Baker AJM (2004) Research priorities for conservation of metallophytes and their potential for restoration and site remediation. Restor Ecol 12:106–116
Wither ED, Brooks RR (1977) Hyperaccumulation of nickel by some plants of South-East Asia. J Geochem Explor 8:579–583
Wulff A, Hollingsworth PM, Ahrends A, Jaffré T, Veillon JM, L’Huillier L, Fogliani B (2013) Conservation priorities in a biodiversity hotspot; analysis of narrow endemic plant species in New Caledonia. PLoS ONE 8(9):e73371
Xue SG, Chen YX, Reeves RD, Baker AJM, Lin Q, Fernando DR (2004) Manganese uptake and accumulation by the hyperaccumulator plant Phytolacca acinosa Roxb. (Phytolaccaceae). Environ Pollut 131:393–399
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Reeves, R.D., van der Ent, A., Echevarria, G., Isnard, S., Baker, A.J.M. (2021). Global Distribution and Ecology of Hyperaccumulator Plants. In: van der Ent, A., Baker, A.J., Echevarria, G., Simonnot, MO., Morel, J.L. (eds) Agromining: Farming for Metals. Mineral Resource Reviews. Springer, Cham. https://doi.org/10.1007/978-3-030-58904-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-58904-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-58903-5
Online ISBN: 978-3-030-58904-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)