Abstract
Anaerobic digestion is being considered as a sustainable technology to treat organic wastes to reduce contamination and emission of greenhouse gasses and at the same time produce energy in the form of methane. The microbiological process of AD represents the most challenged step during biogas production due to microbial complexity. At the time, at least 11 microbial groups have been described. These populations have been shown unique metabolism and an interspecies interaction because of the limited amount of energy available for growth. The microbial community structure is considered as the core in the success of AD method. Furthermore, to expand AD technology in order to approach an economically feasible process under the concept of biorefinery and not only the advances on engineering processes, the design of new biogas digesters and tools for real-time monitoring for AD are the keys for a successful implementation of this process. In addition, the classification of the microbial community structure and the understanding of the metabolic networks play a crucial role for its development. In this chapter, different aspects of the microbiology of AD of full-scale biogas digesters are discussed with specific focus on the presence of different microbial groups, their activity, and interactions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Anaerobic digestion (AD) represents the most prominent worldwide technology to convert organic wastes, such as livestock manures, municipal solid waste, municipal and industrial wastewaters, and agro-industrial residues, into biogas due to an engineered and biochemical process, which involves a series of operational parameters, such as organic loading rate, and the interactions of at least eleven microbial groups (Alvarado et al. 2014). The importance of AD is not only because of its significance in waste management but also because AD offers carbon recovery in the form of methane, which demonstrates to be a sustainable manner to produce clean energy as electricity and heat and as vehicle fuel. Notwithstanding the advances on the engineering processes, the design of new biogas digesters, and tools for real-time monitoring for AD, the microbiology aspect always poses challenges. Microbial community composition analyses, in biogas digesters of several substrates, have been widely reported. However, due to their complexity, these populations have been shown unique metabolism and an interspecies interaction which have not been yet precisely characterized (García-Lozano et al. 2019). This process is still contemplated as the core in the success of AD method. In addition, the classification of the microbial community structure and the understanding of the metabolic networks are crucial to expand the implementation of AD technology in order to achieve an economically feasible process. Moreover, the purpose of this process is presently exploring to include the generation of value-added products, under the concept of biorefinery, not only the energy generation and nutrient recovery (Schnürer 2016). This chapter describes several aspects of the microbiology of AD, including the presence of different microbial groups, their activity and interactions, and the consequent response of the different operational parameters in a full-scale biogas digesters are discussed.
2 Metabolism of Anaerobic Digestion Process
2.1 Anaerobic Digestion: Functional Role
Anaerobic digestion is a chain of interconnected biological reactions in which at least 11 groups of microorganisms, belonging to domain bacteria and archaea, interact in numerous associations where the organic matter, as carbohydrates, proteins, lipids, or more complex compounds, is transformed into biogas (containing ~65% CH4, 35% CO2, and trace amounts of H2S, NH3, and H2) and anaerobic biomass. Besides bioenergy production in the form of methane, AD presents several advantages, such as lesser biomass sludge production in comparison to aerobic treatment technologies, elimination of pathogens, the digestate produced is an improved fertilizer, and the reduction of greenhouses gasses (GHG) emissions.
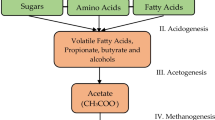
Usually AD is conceptually divided into three or four stages, hydrolysis and/or fermentation, acetogenesis, and methanogenesis. The performance of these processes is carried out by the combined action of hydrolytic-fermentative bacteria, syntrophic acetogenic bacteria, and methanogenic archaea. During the first stage, insoluble and complex polymers (carbohydrates, lipids, proteins, etc.) are hydrolyzed and converted into simple and soluble products (sugars, long-chain fatty acids, glycerol, amino acids, etc.), which are catabolized by fermentative bacteria into alcohol, fatty acids, hydrogen, and carbon dioxide. Subsequent steps involve the oxidation of such alcohols and fatty acids by syntrophic acetogens, forming acetate, H2, and CO2. Finally, during methanogenesis, acetate and other methyl-containing C1 compounds are reduced to methane by aceticlastic and methylotrophic methanogens, and CO2 is reduced by H2-oxidizing methanogens (Nagamani and Ramasamy 1999).
2.2 Hydrolysis
This first step of AD is considered as the rate limiting performed by the microbial decomposition of organic matter (proteins, lipids, and polysaccharides) into soluble small molecules by extracellular enzymes of facultative and obligate anaerobic bacteria (Cazier et al. 2015; Boontian 2014). Substrates are cleaved enzymatically, mainly by the amylases, cellulases, proteases, and lipases excreted by microorganisms (Bajpai 2017). Interaction networks from domains help us to understand the substrate conversion process (Shaw et al. 2017). Usually AD is greater than 1016 cells/mL which involves saccharolytic bacteria (~108 cells/mL), proteolytic bacteria (~106 cells/mL), and lipolytic bacteria (~105 cells/mL) (Amani et al. 2010). Proportions of the enzymes excreted from these bacteria and the optimum operation of a biogas plant will depend on the substrate and its degradation characteristics (Weinrich and Nelles 2015). Mostly, substrates employed to start up this stage are wastes like animal waste and lipid-rich wastes from oil industry, pulp-paper processing, wastewaters, animal fat, agricultural waste, or energy crops, which show different microbial communities according to degradation demand (Montañez-Hernández et al. 2018; Tabatabaei et al. 2010; Appels et al. 2011).
2.2.1 Polysaccharide Hydrolysis
Lignocellulosic biomass is mainly found in biodigesters and consists of cellulose (30–56%), hemicellulose (10–27%), and lignin (3–30%). It is worth to mention that lignin is a recalcitrant compound that can limit the hydrolysis rate for biogas production (Sawatdeenarunat et al. 2016; Venkiteshwaran et al. 2016). At present, two types of polysaccharide hydrolysis systems are known: multienzymatic complex systems, called cellulosomes, and free enzymatic systems (Felix and Ljungdahl, 1993). Anaerobic microorganisms produce cellulosomes, fixed on the bacterial cell wall, which bind to the substrate for its hydrolysis. Aerobic microorganisms degrade cellulose, by secreting a set of enzymes, viz., endoglucanase, exoglucanase (cellobiohydrolase), and cellobiases (Fig. 1). Meanwhile, the hydrolysis of starch is performed by a mixture of amylases as α-amylase, α-xylosidase, and β-xylosidase able to hydrolyze amylose and amylopectin (Zhu et al. 2016).
2.2.2 Protein Hydrolysis
As well as carbohydrates and lipids, protein constitutes a major percentage of the organic load in anaerobic sludges and wastewaters. Wastewater and sewage from food processing industries as abattoir, dairy, fish, and vegetables comprise around 40% of protein (Barnett et al. 1994; Ramsay and Pullammanappallil 2001). Proteins are natural nitrogen-rich polymers that are mainly composed of amino acids linked by peptide bonds. Nitrogen provides an essential element for the synthesis of amino acids, protein, and nucleic acids and acts as a strong base when it is converted to ammonia. Physiologically, proteases are released to the extracellular media to cleavage proteins into its constituents, peptides and free amino acids, which are subsequently metabolized to VFAs, CO2, H2, NH4+, and S2−. Proteases are classified principally based on their site of action in two major groups: exoproteases (carboxipeptidases or aminopeptidases) and endoproteases. Further classification is based on the functional group (serine, cysteine, aspartate, or metallo) and optimal pH (acidic, neutral, or alkaline) (Schaechter 2009).
2.2.3 Lipid Hydrolysis
Lipid is the term used to describe fat, oil, and grease contained mostly in wastewater stream and other sources. Lipids are considered as excellent substrates for anaerobic digestion and co-digestion due to the higher methane yield obtained when compared to proteins or carbohydrates (Yang et al. 2016). Most of lipids in wastes are present as triacylglycerides, a glycerol ester with three long-chain fatty acids (LCFA). During hydrolysis of triacylglycerides, glycerol and LCFA (typically 14 to 24 carbon atoms) are produced by extracellular lipases in order to increase lipid solubility. These enzymes are excreted by acidogenic bacteria, and the further conversion of the hydrolysis products takes place inside the bacterial cells.
2.3 Acidogenesis
The second stage from AD is fermentation, also called acidogenesis, where monomers will be further decomposed by fermentative bacteria into short-chain fatty acids or volatile fatty acids (VFAs). Generally, acetate, butyrate, and propionate (most prevalent VFAs), lactate, valerate, pyruvate, formic acids, CO2, and/or hydrogen are present as by-products of this stage (Chen et al. 2017; Mani et al. 2016; Ren et al. 2018). During AD, acidogenesis is the quickest step producing precursors of methane. Three main types of fermentation are known: ethanol/acetic acid-type, butyric acid-type, and propionic acid-type. These pathways are determinant to achieve a high performance of methane production, where the major products are butyric and acetic acid (70–90%) (Chen et al. 2015). The performance of the fermentation stage is one of the most attractive strategies for biogas production enhancement in AD process goals, especially on organic wastes (Lu et al. 2018).
2.3.1 Carbohydrates Fermentation
In the absence of methanogens, the major products of sugar fermentation by anaerobic bacteria are acetate, ethanol, H2, and CO2. When H2-utilizing bacteria are active, acetate production is increased. Formerly, for most of microorganisms, fermentation of glucose occurs by the glycolytic pathway, producing pyruvate, or by-products of pyruvate (Fig. 2). Glucose can be fermented to lactate by homofermentative bacteria to lactate or to multiple end products as acetate, formate, butyrate, propionate, valerate, and CO2, by heterofermentative bacteria. Usually, these microorganisms produce CO2 and H2 with the concomitant production of formate, acetate, lactate, and succinate. Commonly, heterofermentative bacteria include Lactobacillus, Microbacterium, and Leuconostoc. The main product of clostridia, eubacteria, fusobacteria, and butivibrios is butyrate, acetate, CO2, and H2, while Clostridium species can ferment those end products plus others, as acetone. Other anaerobic bacteria, as Propionibacterium species, ferment glucose to form CO2, propionate, acetate, and succinate. Propionate is produced by the partial reversal of Krebs cycle reactions and implies a CO2 fixation by pyruvate (the Wood-Werkman reaction) which forms oxaloacetate. Subsequently, oxaloacetate is reduced in three steps and then decarboxylated to propionate. In another three-carbon pathway, propionate is formed by a lactyl-SCoA intermediate.
2.3.2 Amino Acid Fermentation
Amino acid can be fermented anaerobically by two principal ways: a pair of amino acids can be decomposed through the Stickland reaction, or one single amino acid can be degraded by H2-utilizing bacteria. The end products of fermentation include short-chain and branched-chain organic acids, NH3, CO2, and small amounts of H2 and sulfur-containing compounds (Ramsay and Pullammanappallil 2001). The Stickland reaction implies one amino acid, used as electron donor, while another amino acid acts as an electron acceptor. This reaction produces 0.5 mole of ATP per mole of amino acid transformed, and their utilization may be linked to the oxidative deamination step and/or the decarboxylation step (Andreesen et al. 1989). The alternative pathway to the Stickland reaction proceeds when hydrogen partial pressure is sufficient low, releasing hydrogen as electrons (Schnürer 2016). It is worth to mention that the oxidative deamination reactions are endergonic under standard conditions. Thus, the reaction cannot proceed unless the reducing equivalents produced are taken up via interspecies hydrogen transfer by methanogens, sulfate reducers, or acetogens and by another amino acid in the Stickland reaction or in the reduction of acetate to butyrate (Örlygsson et al. 1995).
2.3.3 Glycerol Fermentation
As mentioned earlier, most of glycerol present in biodigesters is a product of lipid hydrolysis plus LCFA. Glycerol is a source of carbon and energy, and its uptake can occur by active or passive transport (Holst et al. 2000). Anaerobic fermentation of glycerol can be carried out by a reductive or an oxidative pathway (Biebl et al. 1999).
The reductive pathway leads to 1,3-propanediol production by means of glycerol dehydration. The oxidative pathway leads to glycerol dehydrogenation to produce phosphoenolpyruvate which can in turn be converted to propionate by several decarboxylations, or it can be converted to pyruvate. Thus, pyruvate can be then be fermented in simpler compounds, depending of the microorganism and the environmental conditions, such as 2,3-butanediol, lactate, butyrate, n-butanol, ethanol, acetate, formate, hydrogen, and carbon dioxide (Siles et al. 2009).
2.4 Acetogenesis and Syntrophy
Obligate anaerobic bacteria that synthesize acetyl-CoA by the reductive acetyl-CoA or Wood-Ljungdahl pathway, for energy and cell carbon obtaining from CO2, are called acetogens; acetogenic bacteria that produce acetate as sole end product are called homoacetogen (Drake 1994). The pathway consists in the reduction of 2 moles of CO2 to 1 mole of acetate by 8 protons (Hattori 2008). In addition, the participant key enzyme for the pathway is the acetyl-CoA synthase (ACS) (Müller and Frerichs 2013). Thereby, the aforementioned statements separate acetogens from those microbial groups that produce acetate as an end product of fermentation (Schuchmann and Müller 2016). Acetogens versatility is demonstrated by their wide variety of useful substrates, i.e., sugars, CO2 + H2, C1 compounds, dicarboxylic acids, and alcohols (Müller and Frerichs 2013). In addition, electron acceptors such as nitrate, nitrite, thiosulfate, and fumarate can be used by acetogens. However, repression of acetyl-CoA pathway takes place (Müller and Frerichs 2013). In anaerobic digestion, acetogenic bacteria contribute in the formation of acetate as a precursor for CH4 production by aceticlastic methanogenesis. Therefore, their presence in anaerobic digesters benefits the process.
There are other bacteria that are in the presence of hydrogen-scavenger microorganisms, such as hydrogenotrophic methanogens, which act in syntrophic relationship to obtain energy. Syntrophic acetate oxidation (SAO) process consists in the oxidation of acetate, by syntrophic acetate-oxidizing bacteria (SAOB), to produce H2 and CO2, available substrates for hydrogenotrophic methanogens to form CH4 (Sun et al. 2014). It is believed that acetate oxidation is carried out by the reversible reactions of the Wood-Ljungdahl pathway (Müller and Frerichs 2013). The process of transferring reducing equivalents (such as H2) from bacteria to archaea is called interspecies electron transfer (Stams and Plugge 2009). Oxidation of acetate is a highly endergonic reaction under standard conditions (̕ΔG0’ = +104.6 kJ/mol) (Hattori 2008). Therefore, it requires low H2 partial pressure (<10 Pa) that can be obtained by the activity of hydrogenotrophic methanogens (Schink 1997). Coupling of both pathways results in an overall exergonic reaction (̕ΔG0’ = −31.0 kJ/mol). However, this small energy released is shared by both microorganisms, explaining their slow growth rate (Hattori 2008).
2.5 Methanogenesis
Methanogenesis is the final stage of anaerobic digestion, where the biological formation of methane is performed by methanogens, an obligate anaerobic archaeon. Methanogens use three main substrates to obtain energy. The first type of substrate is CO2; most of the methanogens are capable to reduce CO2 to methane by electrons from H2, but also other electron donors such as formate, secondary alcohols such as 2-propanol, 2-butanol, and even ethanol might be used by methanogens. The oxidation of these last compounds occurs partially generating ketones and acetate. The second substrate is compounds that contain methyl groups, such as methanol and amines. The last group corresponds to acetate.
Methane biosynthesis occurs through two main pathways known as hydrogenotrophic or CO2-reduction and aceticlastic. In the CO2-reduction pathway, formate (Hungate et al. 1970; Archer and Harris 1986) or H2 is oxidized, and CO2 is reduced to CH4, whereas in the aceticlastic pathway, acetate is cleaved with the carbonyl group oxidized to CO2 and the methyl group reduced to CH4 (Ferry 2011). Although both routes differ in terms of reactions and enzymes, the last step that corresponds to the production of methane and the formation of heterodisulfide is common in both pathways. The reduction of CO2 to CH4 reaction sequence starts with a two-electron reduction of CO2 and methanofuran (MFR) to formyl-MFR where the formyl group is bound to the amino group of the coenzyme. The formyl group is then transferred to the N5 of tetrahydromethanopterin (H4MPT); the formyl-H4MPT thus generated cyclizes to the methenyl-H4MPT, which is reduced in two steps to the methyl-H4MPT. Finally, the methyl group is transferred to the thiol group of coenzyme M. The methyl thioether formed is reduced to CH4 in the final step of the pathway (Hedderich and Whitman 2013).
In methanogenesis from methanol, the methyl group enters the C1 pathway at the level of coenzyme M and is reduced to methane. The electrons for this reduction are obtained from the oxidation of an additional methyl group to CO2 using the reverse of the steps of the reductive C1 pathway (Hedderich and Whitman 2013). During growth on acetate, the methyl (C-2) carbon of acetate is reduced to methane using electrons obtained from the oxidation of the carboxyl (C-1) carbon of acetate. In this metabolism, the methyl group enters the C1 pathway at the level of methyl-H4MPT (Hedderich and Whitman 2013).
Around 70% of the methane synthesized by methanogens in a full-scale biogas plant comes from the acetoclastic pathway, while the remaining percentage comes from the CO2-reduction. However, phylogenetic studies recognize the CO2-reduction pathway as the oldest since some of the specific enzymes for this pathway are not distributed in other microorganisms. In contrast, the enzymes required for the acetoclastic pathway are also found in some acetogenic and fermentative bacteria, suggesting that the appearance of this pathway occurred much later than hydrogenotrophic pathway (Bapteste et al. 2005).
3 Microbial Composition in Full-Scale Biogas Digesters
3.1 Hydrolytic Bacteria
The implementation of polysaccharides, as substrate for carbon source, is generally used on biogas plants. Mostly, lignocellulosic-rich substrates are feedstock in high-capacity bioreactor and present as energy crops, agricultural residues, animal manure, and food waste as a sustainable source (Koch et al. 2010; Ziganshin et al. 2013). Microorganism presence found in biogas plants for the hydrolytic anaerobic process varies on the type of reactor as is shown in Table 1. However, mostly the phyla of Proteobacteria (within Deltaproteobacteria, Gammaproteobacteria, Betaproteobacteria, Alphaproteobacteria classes) are present in the initial phase where Clostridiales (Clostridium, Ruminococcus, Butyrivibrio, Acetivibrio, and Eubacterium), Thermoanaerobacterales (Caldicellulosiruptor), Fibrobacteres (Fibrobacter), Spirochaetales (Spirochaeta), Tissierellia (Anaerococcus) orders are involved and some archaea started to appear, as well as in the hydrolytic stage (Cirne et al. 2007; Manyi-Loh et al. 2013; Narihiro and Sekiguchi 2007). A study reported by Tian et al. (2017) observed the order of Bacteroidales accounted for the 30% of the total prokaryote population; in this order of microorganisms, the family of Marinilabiaceae accounted the 85% of the order. Thus, Bacteroidales were predicted as the microorganisms able to degrade biopolymers, including xylan, and also reported to degrade chitin in anaerobic conditions. Nonetheless, Bacteroidales was identified by their abundance and its role in anaerobic digestion of cellulose and hemicellulose.
Generally, the main microorganisms in anaerobic digesters involved in protein hydrolysis are from the order Bacteroidales, Clostridiales, Fusobacteriales, Selenomonadales, and Lactobacillales (Amani et al. 2010). Clostridiales and Bacteroidales are recognized as the main contributors in polymer hydrolysis and fermentation steps. In biogas plants (BGPs), these versatile orders are capable of hydrolyzing a wide range of substrates, including carbohydrates, lipids, and proteins. Previous metagenomic studies in BGPs have demonstrated its dominance ranging from 15 to 84% of the total microorganisms (Schlüter et al. 2008; Sundberg et al. 2013). More recently, its prevalence is shown in mesophilic and thermophilic biogas plants fed with lignocellulosic wastes (agricultural) and animal manure (Table 2).
Nonetheless, other works have acknowledged groups as Spirochaetales and Bacillales participating in protein degradation specifically of maize silage either with pig or chicken manure (Ortseifen et al. 2016; Stolze et al. 2015) and Candidatus Cloacamonas as the main protein degrader phylum from a BGP treating dairy manure (Li et al. 2014). Particularly wastes from food or ethanol fermentation have shown an increased abundance of specific groups of microorganisms. Thermotogales (10.4%) dominated a BGP treating food waste wastewater, while Coprothermobacterales (68.2%) showed a marked dominance in a mesophilic farm-scale digester treating brewery and swine wastes (Cho et al. 2017; Lee et al. 2016).
As seen in Table 2, serine and metalloproteases seem to have an important role generally in anaerobic bacteria. Clostridiales, Bacteroidales, and Coprothermobacterales encode for both enzymes, while other groups of microorganisms synthetize mostly for serine proteases. Both proteases are ubiquitously found in prokaryotes, and its mechanism depends on the active site which include a nucleophilic serine amino acid (serine proteases) or generally requires zinc or cobalt (metalloproteases) (Hedstrom 2002; Rawlings and Barrett 1995). However, little is known about the role of proteases in anaerobic digestion. Hence, a deeper insight is still necessary in order to known which specific proteases are participating in AD and to understand the dynamic changes within the community treating specific kind of substrates.
On the other hand, when treating lipids, Firmicutes and Bacteroidetes are crucial for the performance of the biodigester (Salama et al. 2019). Syntrophic β-oxidizing bacteria from microbial consortium as Proteobacteria and Syntrophomonas sp. from Clostridiales order have been reported as FOG degraders. Moreover, within the Proteobacteria phylum, Rheinheimera sp. and Bacillus sp. can digest FOG under anaerobic conditions and decrease LCFA deposition (Klaucans and Sams 2018). Studies on reactor treating palmitate and oleate revealed a predominance of Clostridiaceae and Syntrophomonadaceae from Clostridiales (Alves et al. 2009).
It is well-known that long-chain fatty acid (LCFA) oxidation on AD is performed through the path of β-oxidation, where coenzyme A is utilized for LCFAs conversion into acetate and hydrogen (Rasit et al. 2015). Studies have reported that FOG biodegradability has high potential biogas production on methane yielding (~1200 L CH4/kg VS) on full-scale wastewater treatment plants (WWTPs) (Shen et al. 2015). Lipid degradation is critical for the effective degradation of food waste to produce biogas; also lipids are considered as a good substrate to produce renewable energy at an industrial level (Ziels et al. 2016). A study by He et al. (2018) presented an organic loading rate for stable biogas production of 0.5–1.5 g VS−1 days−1 using cooking oil skimmed from food waste as the only carbon source, where Anaerovibrio (lipid hydrolysis bacteria) hydrolyze triglycerides to produce glycerol and fatty acids. This increased from 9.3 to 40% in a relative high concentration of lipids with the highest value of 2.0 g VS L−1 days−1, while the genus of Syntrophomonas increased to ~29%, playing significant roles in the mesophilic anaerobic digestion.
3.2 Fermentative Bacteria
Biogas reactors have been tested in different manners during monosacharides fermentation, sucha as ADM1 model, with lactate suggesting that Clostridiales is a butyrate-producing bacterium predominantly, and other microorganisms were found Propionibacteriales synthetizing propionate, Lactobacillales (Carnobacterium sp.), a lactic acid bacteria, and Synergistales (Lactivibrio alcoholicus) a lactate-degrading bacteria (Satpathy et al. 2016). On thermophilic biogas plants, the order of Petrogales, Defluviitoga tunisiensis, and Desulfotomaculum australicum are described as lactic acid degraders, also contained acidogenic/acetogenic bacteria belonging to the Clostridiales, Tissierellales, and Bacillales orders (Table 3) (Maus et al. 2016).
When a high rate of amino acid fermentation occurs, high amounts of NH3 and ammonium (NH4+) are produced, mostly when treating a proteinaceous-rich feedstock as animal wastes as slaughterhouse waste, dairy manure, animal manure, and aquaculture sludge and wastes from food industry and households. In AD, high concentrations of NH3 are toxic to some microorganisms inhibiting cytosolic enzymes, as well as NH4+ which can be intracellular accumulated modifying the pH and K+ concentration causing process instability. Hence, an overproduction of ammonia can inhibit the whole process of AD due to that protein hydrolysis is faster than carbohydrate or lipid hydrolysis (Andreesen et al. 1989).
Although several studies have demonstrated ammonia-tolerant bacteria population by high methane yields, the fraction of NH3 relative to the total (NH3 + NH4+)-nitrogen (TAN) should be monitored (Hansen et al. 1993). TAN concentration of 0.68 g L−1 does not affect the methanogenic activity at mesophilic conditions. However, a range between 1.5 and 3 g L−1 of TAN is inhibitory, and a TAN concentration > 4 g L−1 fully inhibits AD (Angelidaki and Ahring 1993; Hansen et al. 1993).
Carbohydrate-fermenting bacteria usually degrade proteins in a process energetically favorable. Many studies have shown proteolytic bacteria from the genus Clostridia, which also play an important role in amino acid fermentation (de Vladar 2012). In fact, Clostridia species only carry out Stickland reaction using all amino acids and producing δ-aminovalerate, α-aminobutyrate, or γ-aminobutyrate as intermediates in the fermentation (Mead 1971). As shown in Table 4 several orders have been grouped as including the order Clostridiales. However, other groups as Synergistales, Thermotogales, and Thermoanaerobacterales have been found in biogas plant treating agricultural wastes, food waste wastewater, and sewage sludge, and they have been recognized to degrade several amino acids to produce propionate and/or acetate (Lee et al. 2016; Maus et al. 2016; Świątczak et al. 2017).
Nonetheless, a phylum lately recognized as protein degrader and amino acid fermenter is Candidatus Cloacamonas acidaminovorans belonging to WWE1 candidate division, which encode all the machinery for protein degradation and derive most of the carbon and energy from amino acid fermentation (Pelletier et al. 2008). C. Cloacamonas has been found in great abundance in mesophilic BGPs, mainly digesting agricultural wastes and animal manure (Stolze et al. 2015, 2015; Sun et al. 2016). However, this phylum was more abundant (28.6%) in a mesophilic-thermophilic lagoon-type reactor treating pig manure and several wastes (Pampillón-González et al. 2017). In spite of proteinaceous feedstock are usually no recommended for biogas production considering the increased risk of inhibition by ammonium (Kragl and Aivasidis 2005), several studies had led to reach an adaptation of the microbial community to protein-rich biomass which can be appropriate to sustainable biogas production (Kovács et al. 2015, 2013).
Anaerobic digestion of glycerol as sole source or in co-digestion with other organic materials has been widely explored (Viana et al. 2012). However, both ways showed clear limitations mainly associated (1) to the presence of toxic compounds as LCFAs and inorganic salts of chloride and sulfates and (2) to the high chemical oxygen demand of glycerol. Despite of such disadvantages, microbial communities are able to adapt to high salinity, achieving promising methane potentials in anaerobic reactors treating only glycerol. Various works have shown that methane potential values are near to the theoretical methane production potential for glycerol (0.426m3 CH4/kg glycerol), making glycerol a challenge (Kolesárová et al. 2011; Siles et al. 2009; Yang et al. 2008).
Many microorganisms are able to metabolize glycerol in aerobic conditions; nevertheless, few are able to do it anaerobically. Species from the order Enterobacteriales, Clostridiales, Lactobacillales, Bacillales, and Burkholderiales have been reported to ferment glycerol in 1,3-propanediol and ethanol (Varrone et al. 2013; Yazdani and Gonzalez 2007; Zhou et al. 2017). More recently, sludge from brewery and glycerol used to methane production in a shock loading consortia acclimation showed that species from order Thermotogales, Lactobacillales, and Clostridiales were strongly dependent on the glycerol feeding system (Vásquez and Nakasaki 2016). Microbial dynamicity on glycerol fermentation has been also evaluated in anaerobic reactors overloaded with lipids, demonstrating a predominant order of Selenomodales, Lactobacillales, Clostridiales, and Bacteroidales (De Francisci et al. 2015). Lately, only one work has analyzed the enrichment of ammonia oxidation bacteria as Candidatus Brocadia caroliniensis from a full-scale process treating anaerobic digester effluent with the addition of glycerol. This worked attributed greatly the order Brocadiales, a partial transformation capability of glycerol (Park et al. 2017).
3.3 Acetogens and Syntrophic Acetate Oxidizers
Acetogens are mainly found in three phyla Firmicutes, Acidobacteria, and Spirochaetes. Nevertheless, most of them are inside of the first phylum and belong to Clostridia class (Scherer et al. 2018), as it can be observed in Table 5. In the study of St-Pierre and Wright (2014), the mentioned phyla were found in three full-scale digesters fed with cow manure as the main substrate. Firmicutes phylum was the most diverse and predominant in all digesters, the same occurred with Clostridia class. In contrast, the presence of Negativicutes, another class were acetogens can be found, was almost null (0.1% from Firmicutes reads). Interestingly, the dominant pathway for methane production affected Clostridia presence, showing less abundance when hydrogenotrophic methanogenesis prevail.
In an anaerobic digester fed with excess activated sludge, Clostridium, Eubacterium, Thermoanaerobacter, Moorella (all from Clostridia class) and Treponema (from Spirochaetia class) were the dominant acetogenic genus, but just the first two were among the top 50 in abundance. Furthermore, the prevalence of genes involved in the Wood-Ljungdahl pathway (i.e., acetate kinase and phosphate acetyltransferase) confirmed the constant formation of acetate and its role as precursor for CH4 production; this latter was observed by the higher abundance of Methanosaeta (26.2% from total reads of methanogens) and Methanosarcina (12.8%) genera over hydrogenotrophic methanogens (Methanospirillum, 13.1%; Methanoculleus, 11.1%; Methanoregula, 7.6%) (Guo et al. 2015). A similar outcome was reported by Zhang et al. (2009) after the implementation of a Focused-Pulse treatment in a WWTP for biosolids removal enhancement. Here, microbial populations suffered a shift that caused the loss of hydrogenotrophic methanogens dominance against aceticlastic methanogenesis. In addition, an acetogenic group called Treponema primitia was favored by the shift and increased its abundance from 7.1 to 11.5% (from Spirochaetes reads) even it is phylum reads decreased (18.8 to 13.2%), supporting acetate production.
As we have seen in digesters with cow manure as substrate, acetogens can also be found in reactors treating poultry or pig manure. Furthermore, their presence is not limited by awkward conditions such as the predominance of hydrogenotrophic methanogens. For example, in a pilot-scale digester exclusively fed with poultry manure, Firmicutes dominated bacterial abundance with 76%. Within it, Clostridia was composed of Clostridiales (64%) and Thermoanaerobacterales (11%). Two OTUs in Clostridia probably belonged to the last-mentioned order because they had a close similarity to Moorella glycerini and Moorella thermoacetica. Furthermore, 1.6% of total OTUs abundance belonged to Negativicutes class and possibly to acetogenic microorganisms (Smith et al. 2014). However, aceticlastic methanogenesis did not prevail in the reactor, explaining the limited abundance of acetogens. The same prevalence of hydrogenotrophic methanogens was observed in another pilot-scale digester reported by Liu et al. (2009); pig manure was managed in this case. From microbial analysis, the abundant presence of phylum Firmicutes and Spirochaetes was observed with 47.2 and 13.2%, respectively. The former contained Clostridia class and most of its OTUs belonged to Clostridiaceae family, a striking source of acetogens, and the latter contained Treponema genus but not T. primitia species. More species related to homoacetogens were found, including M. glycerini and Sporobacter termitidis, but just comprised the 0.5 and 1% of total OTUs abundance.
Due to the prevalence of acetogens in Clostridia class, a common taxonomic classification for microorganisms participating in hydrolysis and acidogenesis phase, it is complicated to ensure which of them are present in biodigesters. Furthermore, direct production of acetate by fermentation can produce more confusion. Therefore, combination of metagenomic studies with the analysis of specific genes present in Wood-Ljungdahl pathway can be a useful method to present a clearer image of microbial species involved in this phase of the process. In addition, metatranscriptomic and metaproteomic studies can enhance this purpose.
Species belonging to SAOB are Clostridium ultunense, Thermacetogenium phaeum, and Thermotoga lettingae, the only mesophilic microorganism of the group is C. ultunense, and the rest are thermophilic. In addition, it has demonstrated ammonium resistance. Furthermore, the first two microorganisms have shown the ability to produce acetate with H2 and CO2 as substrates (Hattori 2008). Therefore, SAOB strains can belong to acetogenic bacteria. More examples of these microorganisms are given in Table 6.
The predominance of SAO for CH4 production in biodigesters requires to overcome acetogenic bacteria and aceticlastic methanogens; this can be reached by their inhibition. It is known that both groups are susceptible to high ammonia concentrations (Chen et al. 2014); on the other hand, SAOB and hydrogenotrophic methanogens are more resistant to this compound (Ruiz-Sánchez et al. 2018). Therefore, it is feasible that in anaerobic digestion of nitrogen-rich compounds, which present constant release of large ammonia-nitrogen amounts, such as animal manures, slaughterhouse, and food wastes, supremacy of CH4 production by SAO will be observed (Chen et al. 2014; Ruiz-Sánchez et al. 2018). Hence, according to ammonia content, microbial populations in biodigesters will differ (Ruiz-Sánchez et al. 2018).
A scenario that favors SAO occurs in thermophilic digesters, which are present in high metabolic rates, large OLR management, greater CH4 production, and lower HRT (Zinder 1990) compared with mesophilic digesters. In a thermophilic biogas plant, Tissierella class (Firmicutes phylum) and a species confirmed as SAOB, Tepidanaerobacter and Syntrophaceticus (Thermoanaerobacteraceae family), were found. As it was expected, hydrogenotrophic methanogens (Methanomicrobiales and Methanobacteriales orders) dominate the digester (Maus et al. 2016). Due to that the identified SAOB species are very scarce, this grants the possibility that unknown taxonomic groups belong to them. In the mentioned biogas plant, Defluviitoga tunisiensis abundance overpassed by far other microorganisms. Albeit, this genus is not identified as a SAOB; it probably participates in syntrophy with hydrogenotrophic methanogens in the digestion of biomass (Maus et al. 2016). Stolze et al. (2016) suggested the same idea and confirmed strain’s ability to produce H2 in a thermophilic digester dominated by hydrogenotrophic methanogens.
Another investigation that strengths the taxonomic diversity of SAOB was the one done by Ruiz-Sánchez et al. (2018). Their investigation of microbial diversity in mesophilic full-scale CSTR fed with pig manure and agricultural wastes at high ammonia levels (6–7 g TAN/L) did not find known SAOB species. Instead, genera Longilinea and Alloprevotella from Chloroflexi and Bacteroidetes phylum, respectively, predominated along with hydrogenotrophic methanogens (Methanoculleus and Methanomassiliicoccus). Furthermore, reactors developed well, CH4 content in biogas ranged between 66 and 74%, and it was positively correlated with TAN concentration (Ruiz-Sánchez et al. 2018).
Sun et al. (2014) investigated specifically the presence of SAOB and the dominant methanogenic pathway in 13 well-functioning biogas plants and three thermophilic, and the rest were mesophilic. All thermophilic and seven mesophilic SAO coupled with hydrogenotrophic methanogens prevailed. In contrast, the rest three mesophilic digesters were dominated by aceticlastic methanogenesis. Interestingly, SAO process was observed in co-digestion plants, while one substrate biogas plant was managed by aceticlastic methanogenesis. In addition, higher free ammonia levels were present in co-digestion plants compared with single substrate plants; the former ranged values between 0.16 and 0.82 g/L and the latter 0.03–0.09 g/L of free ammonia. The results from metagenomic reads demonstrated that in all digesters, Syntrophaceticus schinkii was present; clearly, in digesters dominated by SAO, their abundance was higher. Another representative microorganism was Tepidanobacter acetotoxidans; however, it wasn’t found where aceticlastic methanogenesis predominated. The mesophilic Clostridium ultunense was limited to the digesters with high ammonia content, and Thermacetogenium phaeum showed the same behavior but in thermophilic conditions. Dominant methanogenic orders that accompanied SAOB were Methanomicrobiales and Methanobacteriales with more than 80% of methanogenic reads, overpassing the percent reached by aceticlastic methanogens in their digesters. The first order demonstrated high abundance in all digesters, and the second was preferentially found in thermophilic digesters (Sun et al. 2014).
It is generally thought that hydrogenotrophic methanogens contributes for a little fraction of the total methane produced. However, in digesters dominated by SAO, these methanogens dominate and can work under stressful conditions in a stable way. Therefore, the development of knowledge about the network involving this syntrophic relationship is an outstanding topic for a better understanding in process efficiency. Finally, Table 7 shows the microorganisms that have been identified in anaerobic full-scale reactors involved in acetate oxidation.
3.4 Methanogens
Methanogenesis is an antique process carried by methanogenic archaea which belongs to the phylum Euryarchaeota. These microorganisms are distributed around the planet, and they are the main source of methane emissions to the atmosphere. Up this date, seven taxonomic orders are acknowledged, each one grouping members with unique features. The methanogens’ division included the orders Methanobacteriales, Methanomicrobiales, Methanocellales, Methanopyrales, Methanococcales, Methanosarcinales, and Methanoplasmatales (Alvarado et al. 2014). Albeit, in anaerobic biogas digesters, only Methanobacteriales, Methanomicrobiales, and Methanosarcinales group members were recognized (Alvarado et al. 2014). In biogas reactors, the amount of profitable methanogenesis is perhaps the most prominent indicator of a good performance and efficient.
It is well documented that 70% of methane production in biodigesters is carried out by the acetoclastic pathway, meanwhile the other 30% corresponding to the CO2-reduction pathway. Members of Methanobacteriales and Methanomicrobiales utilize the hydrogenotrophic pathway. Hydrogen is commonly used as electron donor in this case, but some species also use formate and alcohols. On the other hand, Methanosarcinales are the most diverse in terms of metabolism. Acetate, hydrogen, format, ethanol, isopropanol, and methylated compounds can be metabolized by members from this order (Kendall and Boone 2006). Microbial community structure of archaeal communities has been evaluated in recent publications on full-scale biodigesters (Cheon et al. 2008; Werner et al. 2011; Regueiro et al. 2012; Sundberg et al. 2013; Li et al. 2015; Abendroth et al. 2015). Studies that describe the archaeal population in full-scale mesophilic biodigesters feed with dairy manure indicates a major prevalence of Methanosarcina thermophila with an abundance of 98.5% (St-Pierre and Wright 2013). In addition, Li et al. (2014) evaluated a mixed plug-flow loop reactor; the results indicate a high proportion (86%) assigned to the genus Methanosaeta sp. Apart from that, a lagoon-type biodigester feed with pig waste showed a relative abundance of 52% and 42% of hydrogenotrophic Methanospirillum and acetoclastic Methanosaeta, respectively (Pampillón-González et al. 2017). Sundeberg et al. (2013) carried out a study in which 21 full-scale biogas digesters were evaluated. The microbial diversity indicates a prevalence of acetoclastic Methanosaeta sp. across all sewage sludge digesters. Meanwhile co-digestion at mesophilic conditions reactors was dominated by hydrogenotrophic Methanoculleus sp. and Methanobrevibacter sp. In addition, reactors operated under thermophilic conditions were dominated by Methanobacterium sp. Kirkegaard et al. (2017) evaluated 32 full-scale anaerobic digester systems fed with activated sludge. The report found that Methanosaeta sp. genus dominates the mesophilic reactors, and genus Methanothermobacter sp. was more abundant in thermophilic conditions over the 6-year period of the study.
4 Conclusions
The complexity of microbial diversity, their functional role, and its community interactions in a specialized environment such as biogas digester denote how particular is the phenomena behind AD process. Several microbial groups belonged to the phylum of Firmicutes, Proteobacteria, and Bacteroidetes indicate that AD have stronger relationship between community structure and its function rather than its environment. On the other hand, methanogens seem to have more heterogeneity across full-scale biogas digesters and might substrate and operational conditions be the main factors that affect methanogen populations. In this regard, hydrogenotrophic methanogens show a high relative abundance in more biodigesters.
The multifunctionally of this process and the recent advances in next-generation sequencing technology will allow a best understanding of the microbial populations and their responses to environmental gradients during the digestion course. Furthermore, a comprehensive analysis of the microbial populations in full-scale anaerobic digesters allows to create economical strategies to improve bioenergy production in form of methane.
References
Abendroth C, Vilanova C, Günther T, Luschnig O, Porcar M (2015) Eubacteria and archaea communities in seven mesophile anaerobic digester plants in Germany. Biotechnol Biofuels 8:87. https://doi.org/10.1186/s13068-015-0271-6
Alvarado A, Montañez-Hernández LE, Palacio-Molina SL, Oropeza Navarro R, Luévanos-Escareño MP, Balagurusamy N (2014) Microbial trophic interactions and mcrA gene expression in monitoring of anaerobic digesters. Front Microbiol 5:1–14. https://doi.org/10.3389/fmicb.2014.00597
Alves MM, Pereira MA, Sousa DZ, Cavaleiro AJ, Picavet M, Smidt H, Stams AJM (2009) Waste lipids to energy: how to optimize methane production from long-chain fatty acids (LCFA). Microb Biotechnol 2(5):538–550. https://doi.org/10.1111/j.1751-7915.2009.00100.x
Amani T, Nosrati M, Sreekrishnan TR (2010) Anaerobic digestion from the viewpoint of microbiological, chemical, and operational aspects — a review. Environ Rev 18(NA):255–278. https://doi.org/10.1139/A10-011
Andreesen J R, Bahl H, Gottschalk G (1989) Introduction to the Physiology and Biochemistry of the Genus Clostridium. En N. P. Minton & D. J. Clarke (Eds.), Clostridia (pp. 27-62). https://doi.org/10.1007/978-1-4757-9718-3_2
Angelidaki I, Ahring BK (1993) Thermophilic anaerobic digestion of livestock waste: the effect of ammonia. Appl Microbiol Biotechnol 38(4):560–564
Appels L, Lauwers J, Degrève J, Helsen L, Lievens H, Willems K, Van Impe J, Dewil R (2011) Anaerobic digestion in global bio-energy production: potential and research challenges. Renew Sust Energ Rev
Archer DB, Harris JE (1986) Methanogenic bacteria and methane production in various habitats. Soc Appl Bacteriol Symp Ser 13:185–223
Bajpai P (2017) “Basics of anaerobic digestion process”. SpringerBriefs in applied sciences and technology, 7-12. https://doi.org/10.1007/978-981-10-4130-3_2
Bapteste É, Brochier C, Boucher Y (2005) Higher-level classification of the Archaea: evolution of methanogenesis and methanogens. Archaea 1:353–363
Barnett JW, Kerridge GJ, Russell JM (1994) Effluent treatment systems for the dairy industry. Australas Biotechnol 4:26–26
Biebl H, Menzel K, Zeng AP, Deckwer WD (1999) Microbial production of 1,3-propanediol. Appl Microbiol Biotechnol 52(3):289–297
Bombardiere J, Espinosa-Solares T, Domaschko M, Chatfield M (2007) Thermophilic anaerobic digester performance under different feed-loading frequency. Appl Biochem Biotechnol 137–140(1–12):765–775
Boontian N (2014) Conditions of the anaerobic digestion of biomass. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering 8(9):1036–1114
Buettner C, Noll M (2018) Differences in microbial key players in anaerobic degradation between biogas and sewage treatment plants. Int Biodeterior Biodegradation 133:124–132
Cazier EA, Trably E, Steyer JP, Escudie R (2015) Biomass hydrolysis inhibition at high partial pressure in solid-state anaerobic digestion. Bioresour Technol 190:106–113. https://doi.org/10.1016/j.biortech.2015.04.055
Chen Y, Liu H, Zheng X, Wang X, Wu J (2017) New method for enhancement of bioenergy production from municipal organic wastes via regulation of anaerobic fermentation process. Appl Energy 196:190–198. https://doi.org/10.1016/j.apenergy.2017.01.100
Chen JL, Ortiz R, Steele TWJ, Stuckey DC (2014) Toxicants inhibiting anaerobic digestion: a review. Biotechnol Adv 32(8):1523–1534
Chen X, Yuan H, Zou D, Liu Y, Zhu B, Chufo A, Jaffar M, Li X (2015) Improving biomethane yield by controlling fermentation type of acidogenic phase in two-phase anaerobic co-digestion of food waste and rice straw. Chem Eng J 273:254–260. https://doi.org/10.1016/j.cej.2015.03.067
Cheon J, Hidaka T, Mori S, Koshikawa H, Tsuno H (2008) Applicability of random cloning method to analyze microbial community in full-scale anaerobic digesters. J Biosci Bioeng 106:134–140. https://doi.org/10.1263/jbb.106.134
Cho K, Shin SG, Kim W, Lee J, Lee C, Hwang S (2017) Microbial community shifts in a farm-scale anaerobic digester treating swine waste: correlations between bacteria communities associated with hydrogenotrophic methanogens and environmental conditions. Sci Total Environ 601:167–176
Ciccoli R, Sperandei M, Petrazzuolo F, Broglia M, Chiarini L, Correnti A et al (2018) Anaerobic digestion of the above ground biomass of Jerusalem artichoke in a pilot plant: impact of the preservation method on the biogas yield and microbial community. Biomass Bioenergy 108:190–197
Cirne DG, Lehtomäki A, Björnsson L, Blackall LL (2007) Hydrolysis and microbial community analyses in two-stage anaerobic digestion of energy crops. J Appl Microbiol 103(3):516–527. https://doi.org/10.1111/j.1365-2672.2006.03270.x
De Francisci D, Kougias PG, Treu L, Campanaro S, Angelidaki I (2015) Microbial diversity and dynamicity of biogas reactors due to radical changes of feedstock composition. Bioresour Technol 176:56–64
de Vladar HP (2012) Amino acid fermentation at the origin of the genetic code. Biol Direct 7:6. https://doi.org/10.1186/1745-6150-7-6
Doloman A, Soboh Y, Walters AJ, Sims RC, Miller CD (2017) Qualitative analysis of microbial dynamics during anaerobic digestion of microalgal biomass in a UASB reactor. Int J Microbiol 2017:1–12. https://doi.org/10.1155/2017/5291283
Drake HL (1994) Acetogenesis. (H. L. Drake, Ed.), Acetogenesis. Boston, MA: Springer US
Felix CR, Ljungdahl LG (1993) The cellulosome: the exocellular organelle of clostridium. Annu Rev Microbiol 47(1):791–819. https://doi.org/10.1146/annurev.mi.47.100193.004043
Ferry JG (2011) Fundamentals of methanogenic pathways that are key to the biomethanation of complex biomass. Curr Opin Biotechnol 22:351–357. https://doi.org/10.1016/j.copbio.2011.04.011
García-Lozano M, Hernández-De Lira IO, Huber DH, Balagurusamy N (2019) Spatial variations of bacterial communities of an anaerobic lagoon-type biodigester fed with dairy manure. PRO 7:408. https://doi.org/10.3390/pr7070408
Guo J, Peng Y, Ni B-J, Han X, Fan L, Yuan Z (2015) Dissecting microbial community structure and methane-producing pathways of a full-scale anaerobic reactor digesting activated sludge from wastewater treatment by metagenomic sequencing. Microb Cell Factories 14(1):33
Hansen RC, Keener HM, Marugg C, Dick WA, Hoitink HAJ (1993) Composting of poultry manure. Design, Environmental, Microbiological and Utilization Aspects. Renaissance Publications, Ohio, Science and Engineering of Composting, pp 131–153
Hattori S (2008) Syntrophic acetate-oxidizing microbes in Methanogenic environments. Microbes Environ 23(2):118–127
He J, Wang X, Yin X, Li Q, Li X, Zhang Y, Deng Y (2018) Insights into biomethane production and microbial community succession during semi-continuous anaerobic digestion of waste cooking oil under different organic loading rates. AMB Expr 8:92. https://doi.org/10.1186/s13568-018-0623-2
Hedderich R, Whitman WB (2013) Physiology and biochemistry of the methane-producing Archaea. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The prokaryotes: prokaryotic physiology and biochemistry (pp. 635–662). Springer. https://doi.org/10.1007/978-3-642-30141-4_81
Hedstrom L (2002) Serine protease mechanism and specificity. Chem Rev 102(12):4501–4524
Holst B, Lunde C, Lages F, Oliveira R, Lucas C, Kielland-Brandt MC (2000) GUP1 and its close homologue GUP2, encoding multimembrane-spanning proteins involved in active glycerol uptake in Saccharomyces cerevisiae. Mol Microbiol 37(1):108–124
Hungate RE, Smith W, Bauchop T, Yu I, Rabinowitz JC (1970) Formate as an intermediate in the bovine rumen fermentation. J Bacteriol 102:389–397
Kendall MM, Boone DR (2006) The order Methanosarcinales. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes: volume 3: Archaea. Bacteria: Firmicutes, Actinomycetes (pp. 244-256). Springer. https://doi.org/10.1007/0-387-30743-5_12
Kirkegaard RH, McIlroy SJ, Kristensen JM, Nierychlo M, Karst SM, Dueholm MS, Albertsen M, Nielsen PH (2017) The impact of immigration on microbial community composition in full-scale anaerobic digesters. Sci Rep 7:1–11. https://doi.org/10.1038/s41598-017-09303-0
Klaucans E, Sams K (2018) Problems with fat, oil, and grease (FOG) in food industry wastewaters and recovered FOG recycling methods using anaerobic co-digestion: a short review. Key Eng Mater 762:61–68. https://doi.org/10.4028/www.scientific.net/kem.762.6
Koch K, Lübken M, Gehring T, Wichern M, Horn H (2010) Biogas from grass silage – measurements and modeling with ADM1. Bioresour Technol 101:8158–8165. https://doi.org/10.1016/j.biortech.2010.06.009
Kolesárová N, Hutňan M, Špalková V, Kuffa R, Bodík I (2011) Anaerobic treatment of biodiesel by-products in a pilot scale reactor. Chem Pap 65(4):447–453
Kovács E, Wirth R, Maróti G, Bagi Z, Nagy K, Minárovits J et al (2015) Augmented biogas production from protein-rich substrates and associated metagenomic changes. Bioresour Technol 178:254–261
Kovács E, Wirth R, Maróti G, Bagi Z, Rákhely G, Kovács KL (2013) Biogas production from protein-rich biomass: fed-batch anaerobic fermentation of casein and of pig blood and associated changes in microbial community composition. PLoS One 8(10). https://doi.org/10.1371/journal.pone.0077265
Kragl U, Aivasidis A (eds) (2005) Technology transfer in biotechnology: from lab to industry to production. Springer, Berlin
Lee J, Han G, Shin SG, Koo T, Cho K, Kim W, Hwang S (2016) Seasonal monitoring of bacteria and archaea in a full-scale thermophilic anaerobic digester treating food waste-recycling wastewater: correlations between microbial community characteristics and process variables. Chem Eng J 300:291–299
Lee S-H, Kang H-J, Lee YH, Lee TJ, Han K, Choi Y, Park H-D (2012) Monitoring bacterial community structure and variability in time scale in full-scale anaerobic digesters. J Environ Monit 14(7):1893. https://doi.org/10.1039/c2em10958a
Li Y-F, Chen P-H, Yu Z (2014) Spatial and temporal variations of microbial community in a mixed plug-flow loop reactor fed with dairy manure. Microb Biotechnol 7:332–346. https://doi.org/10.1111/1751-7915.12125
Li J, Rui J, Yao M, Zhang S, Yan X, Wang Y, Yan Z, Li X (2015) Substrate type and free ammonia determine bacterial community structure in full-scale Mesophilic anaerobic digesters treating cattle or swine manure. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.01337
Liu FH, Wang SB, Zhang JS, Zhang J, Yan X, Zhou HK et al (2009) The structure of the bacterial and archaeal community in a biogas digester as revealed by denaturing gradient gel electrophoresis and 16S rDNA sequencing analysis. J Appl Microbiol 106(3):952–966
Lu X, Wang H, Ma F, Zhao G, Wang S (2018) Improved process performance of the acidification phase in a two-stage anaerobic digestion of complex organic waste: effects of an iron oxide-zeolite additive. Bioresour Technol 262:169–176. https://doi.org/10.1016/j.biortech.2018.04.052
Mani S, Sundaram J, Das KC (2016) Process simulation and modeling: Anerobic digestion of complex organic matter. Biomass Bioenergy 93:158–167. https://doi.org/10.1016/j.biombioe.2016.07.018
Manyi-Loh CE, Mamphweli S, Meyer EL, Okoh AI, Makaka G, Simon M (2013) Microbial anaerobic digestion (bio-digesters) as an approach to the decontamination of animal wastes in pollution control and the generation of renewable energy. Int J Environ Res Public Health 10(9):4390–4417. https://doi.org/10.3390/ijerph10094390
Maus I, Koeck DE, Cibis KG, Hahnke S et al (2016) Unraveling the microbiome of a thermophilic biogas plant by metagenome and metatranscriptome analysis complemented by characterization of bacterial and archaeal isolates. Biotechnol Biofuels 9(1):171. https://doi.org/10.1186/s13068-016-0581-3
Mead GC (1971) The amino acid-fermenting clostridia. J Gen Microbiol 67(1):47–56. https://doi.org/10.1099/00221287-67-1-47
Montañez-Hernández LE, Lira H-D, Rafael-Galindo G, Froto Madariaga L, Balagurusamy N (2018) Sustainable production of biogas from renewable sources: global overview. Scale Up Opportunities and Potential Market Trends Sustainable Biotechnology-Enzymatic Resources of Renewable Energy:325–354. https://doi.org/10.1007/978-3-319-95480-6_13
Müller V, Frerichs J (2013) Acetogenic Bacteria. In eLS (pp. 1–9). Wiley, Chichester, UK
Nagamani B, Ramasamy K (1999) Biogas production technology: an Indian perspective. Curr Sci 77:44–55
Narihiro T, Sekiguchi Y (2007) Microbial communities in anaerobic digestion processes for waste and wastewater treatment: a microbiological update. Curr Opin Biotechnol 18(3):273–278. https://doi.org/10.1016/j.copbio.2007.04.003
Örlygsson J, Houwen FP, Svensson BH (1995) Thermophilic anaerobic amino acid degradation: deamination rates and end-product formation. Appl Microbiol Biotechnol 43(2):235–241. https://doi.org/10.1007/BF00172818
Ortseifen V, Stolze Y, Maus I, Sczyrba A, Bremges A, Albaum SP et al (2016) An integrated metagenome and-proteome analysis of the microbial community residing in a biogas production plant. J Biotechnol 231:268–279
Pampillón-González L, Ortiz-Cornejo NL, Luna-Guido M, Dendooven L, Navarro-Noya YE (2017) Archaeal and bacterial community structure in an anaerobic digestion reactor (lagoon type) used for biogas production at a pig farm. Microbiol Physiol 27(5):306–317. https://doi.org/10.1159/000479108
Park H, Brotto AC, van Loosdrecht MC, Chandran K (2017) Discovery and metagenomic analysis of an anammox bacterial enrichment related to Candidatus “Brocadia caroliniensis” in a full-scale glycerol-fed nitritation-denitritation separate centrate treatment process. Water Res 111:265–273
Pelletier E, Kreimeyer A, Bocs S, Rouy Z, Gyapay G, Chouari R et al (2008) “Candidatus Cloacamonas Acidaminovorans”: genome sequence reconstruction provides a first glimpse of a new bacterial division. J Bacteriol 190(7):2572–2579. https://doi.org/10.1128/JB.01248-07
Ragsdale SW, Pierce E (2008) Acetogenesis and the wood–Ljungdahl pathway of CO2 fixation. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 1784(12):1873–1898
Ramsay IR, Pullammanappallil PC (2001) Protein degradation during anaerobic wastewater treatment: derivation of stoichiometry. Biodegradation 12(4):247–256. https://doi.org/10.1023/A:1013116728817
Rasit N, Idris A, Harun R, Wan Ab Karim Ghani WA (2015) Effects of lipids inhibition on biogas production of anaerobic digestion from oily effluents and sludges: an overview. Renew Sust Energ Rev 45:351–358. https://doi.org/10.1016/j.rser.2015.01.066
Rawlings ND, Barrett AJ (1995) Evolutionary families of metallopeptidases. En Proteolytic Enzymes: Aspartic and Metallo Peptidases: Vol. 248. Methods in Enzymology (pp. 183-228). https://doi.org/10.1016/0076-6879(95)48015-3
Regueiro L, Veiga P, Figueroa M, Alonso-Gutierrez J, Stams AJM, Lema JM, Carballa M (2012) Relationship between microbial activity and microbial community structure in six full-scale anaerobic digesters. Microbiol Res 167:581–589. https://doi.org/10.1016/j.micres.2012.06.002
Ren Y, Yu M, Wu C, Wang Q, Gao M, Huang Q, Liu Y (2018) A comprehensive review on food waste anaerobic digestion: research updates and tendencies. Bioresour Technol 247:1069–1076. https://doi.org/10.1016/j.biortech.2017.09.109
Rittmann BE, Lee HS, Zhang H, Alder J, Banaszak JE, Lopez R (2008) Full-scale application of focused-pulsed pre-treatment for improving biosolids digestion and conversion to methane. Water Sci Technol 58(10):1895–1901
Ruiz-Sánchez J, Campanaro S, Guivernau M, Fernández B, Prenafeta-Boldú FX (2018) Effect of ammonia on the active microbiome and metagenome from stable full-scale digesters. Bioresour Technol 250(October 2017):513–522
Salama E, Saha S, Kurade MB, Dev S, Chang SW, Jeon B-H (2019) Recent trends in anaerobic co-digestion: fat, oil, and grease (FOG) for enhanced biomethanation. Prog Energy Combust Sci 70:22–42. https://doi.org/10.1016/j.pecs.2018.08.002
Satpathy P, Biernacki P, Uhlenhut F, Cypionka H, Steinigeweg S (2016) Modelling anaerobic digestion in a biogas reactor: ADM1 model development with lactate as an intermediate (part I). J Environ Sci Health A 51(14):1216–1225. https://doi.org/10.1080/10934529.2016.1212558
Sawatdeenarunat C, Nguyen D, Surendra KC, Shrestha S, Rajendran K, Oechsner H et al (2016) Anaerobic biorefinery: current status, challenges, and opportunities. Bioresour Technol 215:304–313. https://doi.org/10.1016/j.biortech.2016.03.074
Schaechter M (2009) Encyclopedia of microbiology (third edition). Elsevier Science
Scherer P, Klocke M, Pühler A, Off S, Schlüter A, Maus I, Hassa J (2018) Metagenome, metatranscriptome, and metaproteome approaches unraveled compositions and functional relationships of microbial communities residing in biogas plants. Appl Microbiol Biotechnol 102(12):5045–5063
Schink B (1994) Diversity, ecology, and isolation of Acetogenic bacteria. In: Drake HL (ed) Acetogenesis. Chapman & Hall Microbiology Series (physiology / ecology / molecular biology / biotechnology). Springer, Boston, MA
Schink B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiology and Molecular Biology Reviews : MMBR 61(2):262–280
Schlüter, A., Bekel, T., Diaz, N. N., Dondrup, M., Eichenlaub, R., Gartemann, K.-H., … Goesmann, A. (2008). The metagenome of a biogas-producing microbial community of a production-scale biogas plant fermenter analysed by the 454-pyrosequencing technology
Schnürer A (2016) Biogas production: microbiology and technology. In: Hatti-Kaul R, Mamo G, Mattiasson B (eds) Anaerobes in biotechnology (pp. 195-234). Springer International Publishing. https://doi.org/10.1007/10_2016_5
Schuchmann K, Müller V (2016) Energetics and application of heterotrophy in Acetogenic bacteria. Appl Environ Microbiol 82(14):4056–4069
Shaw GT-W, Liu A-C, Weng C-Y, Chou C-Y, Wang D (2017) Inferring microbial interactions in thermophilic and mesophilic anaerobic digestion of hog waste. PLoS One 12(7):e0181395. https://doi.org/10.1371/journal.pone.0181395
Shen Y, Linville JL, Urgun-Demirtas M, Mintz MM, Synder SW (2015) An overview of biogas production and utilization at full-scale wastewater treatment plants (WWTPs) in the United States: challenges and opportunities towars energy-neutral WWTPs. Renew Sust Energ Rev 50:346–362. https://doi.org/10.1016/j.rser.2015.04.129
Siles JA, Martín M, Chica AF, Martín A (2009) Anaerobic digestion of glycerol derived from biodiesel manufacturing. Bioresour Technol 100(23):5609–5615. https://doi.org/10.1016/j.biortech.2009.06.017
Smith AM, Sharma D, Lappin-Scott H, Burton S, Huber DH (2014) Microbial community structure of a pilot-scale thermophilic anaerobic digester treating poultry litter. Appl Microbiol Biotechnol 98(5):2321–2334
Stams AJM, Plugge CM (2009) Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat Rev Microbiol 7(8):568–577
Stolze Y, Bremges A, Rumming M, Henke C, Maus I, Pühler A et al (2016) Identification and genome reconstruction of abundant distinct taxa in microbiomes from one thermophilic and three mesophilic production-scale biogas plants. Biotechnol Biofuels 9(1):1–18
Stolze Y, Zakrzewski M, Maus I, Eikmeyer F, Jaenicke S et al (2015) Comparative metagenomics of biogas producing microbial communities from production-scale biogas plants operating under wet or dry fermentation conditions. Biotechnol Biofuels 8(1):14. https://doi.org/10.1186/s13068-014-0193-8
St-Pierre B, Wright A-DG (2013) Metagenomic analysis of methanogen populations in three full-scale mesophilic anaerobic manure digesters operated on dairy farms in Vermont, USA. Bioresour Technol 138:277–284. https://doi.org/10.1016/j.biortech.2013.03.188
St-Pierre B, Wright ADG (2014) Comparative metagenomic analysis of bacterial populations in three full-scale mesophilic anaerobic manure digesters. Appl Microbiol Biotechnol 98(6):2709–2717
Sun L, Liu T, Müller B, Schnürer A (2016) The microbial community structure in industrial biogas plants influences the degradation rate of straw and cellulose in batch tests. Biotechnol Biofuels 9(1):128
Sun L, Müller B, Westerholm M, Schnürer A (2014) Syntrophic acetate oxidation in industrial CSTR biogas digesters. J Biotechnol 171(1):39–44
Sun L, Pope PB, Ejisink VGH, Schnürer A (2015) Characterization of microbial community structure during continuous anaerobic digestion of straw and cow manure. Microb Biotechnol 8(5):815–827. https://doi.org/10.1111/1751-7915.12298
Sundberg C, Al-Soud WA, Larsson M, Alm E, Yekta SS, Svensson BH, Sørensen SJ, Karlsson A (2013) 454 pyrosequencing analyses of bacterial and archaeal richness in 21 full-scale biogas digesters. FEMS Microbiol Ecol 85(3):612–626. https://doi.org/10.1111/1574-6941.12148
Świątczak P, Cydzik A, Rusanowska P (2017) Microbiota of anaerobic digesters in a full-scale wastewater treatment plant. Archives of Environmental Protection 43(3):53–60
Tabatabaei M, Rahim RA, Abdullah N, Wright A-DG, Shirai Y, Sakai K, Sylaiman A, Hassan MA (2010) Importance of the methanogenic archaea populations in anaerobic wastewater treatments. Process Biochem 45(8):1214–1225. https://doi.org/10.1016/j.procbio.2010.05.017
Tian J-H, Pourcher A-M, Bureau C, Peu P (2017) Cellulose accessibility and microbial community in solid state anaerobic digestion of rape straw. Bioresour Technol 223:192–201. https://doi.org/10.1016/j.biortech.2016.10.009
Varrone C, Rosa S, Fiocchetti F, Giussani B, Izzo G, Massini G et al (2013) Enrichment of activated sludge for enhanced hydrogen production from crude glycerol. Int J Hydrog Energy 38(3):1319–1331
Vásquez J, Nakasaki K (2016) Effects of shock loading versus stepwise acclimation on microbial consortia during the anaerobic digestion of glycerol. Biomass Bioenergy 86:129–135
Venkiteshwaran K, Bocher B, Maki J, Zitomer D (2016) Relating anaerobic digestion microbial community and process function. Microbiology Insights 8(S2):37–44. https://doi.org/10.4137/MBI.S33593
Viana M, Freitas A, Leitão R, Pinto G, Santaella S (2012) Anaerobic digestion of crude glycerol: a review. Environ Technol 1:81–92. https://doi.org/10.1080/09593330.2012.692723
Weinrich S, Nelles M (2015) Critical comparison of different model structures for the applied simulation of the anaerobic digestion of agricultural energy crops. Bioresour Technol 178:306–312. https://doi.org/10.1016/j.biortech.2014.10.138
Werner JJ, Knights D, Garcia ML, Scalfone NB, Smith S, Yarasheski K, Cummings TA, Beers AR, Knight R, Angenent LT (2011) "bacterial community structures are unique and resilient in full-scale bioenergy systems". Proceedings of the National Academy of Sciences of the United States of America, 108, 4158-4163. https://doi.org/10.1073/pnas.1015676108
Westerholm M, Moestedt J, Schnürer A (2016) Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Appl Energy 179:124–135. https://doi.org/10.1016/j.apenergy.2016.06.061
Yang Y, Tsukahara K, Sawayama S (2008) Biodegradation and methane production from glycerol-containing synthetic wastes with fixed-bed bioreactor under mesophilic and thermophilic anaerobic conditions. Process Biochem 43(4):362–367
Yang Z-H, Xu R, Zheng Y, Chen T, Zhao L-J, Li M (2016) Characterization of extracellular polymeric substances and microbial diversity in anaerobic co-digestion reactor treated sewage sludge with fat, oil, grease. Bio/Technology 212:164–173. https://doi.org/10.1016/j.biortech.2016.04.046
Yazdani SS, Gonzalez R (2007) Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr Opin Biotechnol 18(3):213–219
Zhang H, Banaszak JE, Parameswaran P, Alder J, Krajmalnik-Brown R, Rittmann BE (2009) Focused-pulsed sludge pre-treatment increases the bacterial diversity and relative abundance of acetoclastic methanogens in a full-scale anaerobic digester. Water Res 43(18):4517–4526
Zhou J-J, Shen J-T, Jiang L-L, Sun Y-Q, Mu Y, Xiu Z-L (2017) Selection and characterization of an anaerobic microbial consortium with high adaptation to crude glycerol for 1, 3-propanediol production. Appl Microbiol Biotechnol 101(15):5985–5996
Zhu N, Yang J, Ji L, Liu J, Yang Y, Yuan H (2016) Metagenomic and metaproteomic analyses of a corn Stover-adapted microbial consortium EMSD5 reveal its taxonomic and enzymatic basis for degrading lignocellulose. Biotechnol Biofuels 9(243):1–23. https://doi.org/10.1186/s13068-016-0658-z
Ziels RM, Karlsson A, Beck DAC, Ejlertsson J, Yekta SS, Bjorn A, Stensel HD, Svensson BH (2016) Microbial community adaptation influences long chain fatty acid conversion during anaerobic co digestion of fats, oils, and grease with municipal sludge. Water Res 103:372–382. https://doi.org/10.1016/j.watres.2016.07.043
Ziganshin AM, Liebetrau J, Pröter J, Kleinsteuber S (2013) Microbial community structure and dynamics during anaerobic digestion of various agricultural waste materials. Appl Microbiol Biotechnol 97(11):5161–5174. https://doi.org/10.1007/s00253-013-4867-0
Zinder SH (1990) Conversion of acetic acid to methane by thermophiles. FEMS Microbiol Lett 75(2):125–137
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Talavera-Caro, A.G., Lira, I.O.HD., Cruz, E.R., Sánchez-Muñoz, M.A., Balagurusamy, N. (2020). The Realm of Microorganisms in Biogas Production: Microbial Diversity, Functional Role, Community Interactions, and Monitoring the Status of Biogas Plant. In: Balagurusamy, N., Chandel, A.K. (eds) Biogas Production. Springer, Cham. https://doi.org/10.1007/978-3-030-58827-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-58827-4_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-58826-7
Online ISBN: 978-3-030-58827-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)