Abstract
In order to correct the genetic defects that are fundamental reasons for many pathologies, gene therapy uses exogenous nucleic acids for intentional modulation of gene expression in specific cells. Due to the large size and the negative charge of exogenous nucleic acids, the delivery of these macromolecules is typically mediated by carriers or vectors. Viral carriers are known to be very efficient however, they have a severe drawbacks such as toxicity and immunogenicity. In this regard, gene-based therapy using non-viral approaches has drawn increasing attention, and has become an important field of research. The diversity of materials used as of non-viral vectors known today highlights the recent progress of gene-based therapy using non-viral approaches. Herein, we describe the progress made by our group in the development of hybrid vectors that combine key features of classical carriers design rationally or formed by combinatorial approach using dynamic chemistry which are remarkable strategies to address the current challenges in gene delivery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Non-viral vectors
- Gene delivery
- Polymers
- Self-assembly
- Poly(ethyleneimine)
- Poly(ethylene glycol)
- Polyplex
- Transfection
- Cytotoxicity
1 Introduction
Nucleic acids (gene therapy) have the unique potential to provide cures for many targets that are currently unavailable and to do so with higher success rates in the clinic. In present, gene therapy, conceptualized since 1972 (Somia and Verma 2000; Yin et al. 2014), has been already successfully applied to treat genetic as well as acquired diseases (Dunbar et al. 2018; Ginn et al. 2018; Hanna et al. 2017; Thapa and Narain 2016), including Parkinson’s disease (Olanow 2014), cardiovascular (Hedman et al. 2011) or retinal (Bainbridge et al. 2006; Smith et al. 2012) and various types of cancer (Egger et al. 2004; Semenza 2003). Yet, so far, as restricted by many factors, the gene therapy using either plasmid DNA (pDNA) or small interfering RNA (siRNA) did not achieve ideal effects due to the lack of the targeting ability, efficient drug carrier and an appropriate drug delivery technique. It is still a difficult problem in gene therapy to address how the transfection efficiency of genes could be considerably improved and the gene be delivered to the targeted sites of the organism efficiently without toxic and side effects. To overcome these drawbacks, (Clima et al. 2015) the development of efficient nucleic acid targeted delivery systems (vectors) is critically required. The general requirements for an efficient vector include: (i) strong enough binding to DNA, but on the other hand to allow the release of the DNA into the cell nucleus; (Papadopoulos et al. 2016; Wu et al. 2018) (ii) to be nontoxic and biodegradable; (iii) to be stable towards enzymatic degradation and (iv) to facilitate endocytosis. Moreover, for an efficient transfection the size, shape and surface characteristics of the vectors are crucial (Gordon et al. 2012).
Vectors of the gene therapy mainly include two categories—viral vectors and non-viral vectors. Despite the fact that viral gene vectors have high transfection efficiency and already found their successful in vivo applications (Dunbar et al. 2018; Ginn et al. 2018; Hanna et al. 2017; Muramatsu 2018; Papadopoulos et al. 2016; Thapa and Narain 2016), the preparation of these vectors is complex and they present serious potential risks and failure of repeated applications in human body (Yang et al. 2014). Alternatively, all other approaches are based on non-viral gene delivery systems, which try to mimic the efficiency of viral vectors by artificial means (Neu et al. 2005; Wu et al. 2018; Zakeri et al. 2018). Non-viral vectors, are artificially synthesized non-bioactive materials, offering advantages such as low toxicity, low immunoreactions and excellent ability of being chemically modified (Neu et al. 2005; Wu et al. 2018). So far, the main candidates used in gene therapy for the nonviral approach are the cationic polymers such as chitosan (Gordon et al. 2012), cationic lipids (Gordon et al. 2012), polypropyleneimines (Gordon et al. 2012) or polyamidoamines (Ailincai et al. 2018, 2019) and poly(ethyenimine) (PEI) (Wang et al. 2011; Zakeri et al. 2018). Preparation of such vectors often involves multistep rational design approach, though a simple design of the vectors towards efficient carrier is in high demand. In the current mini-review we summarized recent results on different approaches and examples for design and properties of non-viral vectors reported by our group.
2 Functional Cyclodextrins and Polyrotaxanes for Gene Delivery
While designing a non-viral vector, the precise control of the size and functionality of the resulting macromolecular or supramolecular construct, can lead to a replication of the characteristics and function of biological entities involved in transport or protective storing of nucleic acids. Despite the numerous synthetic and structural design possibilities for these platforms, their development and use are far more complex than it appears since there are numerous variables to be balanced for a successful transfection including: (i) optimal charge value, (ii) charge distribution, (iii) flexibility, (iv) size, (v) cell internalization and processing. One viable strategy would be the design of a carrier using building blocks with known desired properties and biological effects. One notable example of this concept is the construction of a polycationic vector comprised of functional β-cyclodextrin (β-CD) units as coupling points for branched poly(ethyleneimine) (bPEI) and poly(ethylene glycol) (PEG) chains. The polycationic dendrons would ensure the nucleic acids complexing ability of the vector while the linear PEG chains were included to disrupt the charge density of the edifice and delay the unwanted interactions with biomolecules in the biological fluids during circulation (Dascalu et al. 2017). The synthetic strategy involved an esterification reaction at the primary hydroxyl groups (minor rim of β-CD) in the presence of an excess amount of acryloyl chloride at room temperature. The newly introduced activated double bonds ensured the subsequent coupling of a 750 Da mono-functional PEG derivative bearing an amino group and 2000 Da bPEI, through a Michael addition reaction resulting in a tri-dimensional polycationic carrier.
The in vitro cytotoxicity tests performed on the vector/DNA complexes with precise nitrogen/phosphorous compositional ratios (N/P) in comparison with 2000 Da bPEI revealed that at higher N/P ratios the cell viability exceeded 90% while at lower compositional ratios cellular proliferation was induced. The presence of the PEG segments did not hinder the complex formation between the proposed vector and plasmidic DNA (pLuc) by means of ionic interactions. Moreover, the performed molecular dynamic simulation revealed that in the presence of the double stranded nucleic acid the carrier molecules adapted their conformation in order to maximize the number of ionic interactions between the DNA negatively charged phosphate residues and the positively charged amino groups in the vector. The in vitro transfection efficiency on HeLa cells tests revealed a maximum efficiency at N/P = 80 indicating the need for a larger number of carrier molecules to effectively protect and transfect the nucleic acid into the tested cells. In comparison, the tested bPEI/pLuc polyplexes transfected poorly which clearly confirmed the proposed carrier design for the cooperative binding, transport and release of the nucleic acid in the cell. This behavior was supported by the TEM images which revealed that the polyplex particle size decreased as the N/P compositional ratio increased.
In a more ambitious attempt (Ardeleanu et al. 2018), cyclodextrins were used in the development of gene delivery vectors based on polyrotaxanes. This type of assembly consist in physically interlocked structures containing a linear molecular axle (guest) threaded through a macrocycle (host) and locked in place by large terminal bulky stopper groups (Jiří et al. 2012). The mechanically linked components are typically characterized by high freedom and mobility. As expected, with such complex structures, the preparation of the supramolecular carriers was achieved in multiple steps starting with β-CD functionalization. The adequate functionality of cyclic oligosaccharide was introduced by the partial esterification of the primary hydroxyl groups with acryloyl chloride. The number and position of the attached activated double bonds were controlled through the reagents compositional ratio, temperature and solvent mixture. In the next step the partially acrylated β-CD molecules where threaded onto a 1 kDa PEG derivative with terminal triple bonds. Although between β-CD and PEG no inclusion complex is usually formed (Harada et al. 1999) the presence of the acryloyl residues on the minor rim of the host molecule and the rigid propargyl residues on the linear guest ensured the formation of the poly(pseudorotaxane) assembly. This transient structure was stabilized through a Copper(I)-catalyzed Azide-Alkyne Cycloaddition reaction with silatrane azide resulting in the base polyrotaxane. The final step in the construction of the supramolecular carriers consisted in the addition of the functional “segments” on the base polyrotaxane through the previously mentioned Michael addition: 2 kDa bPEI braches or a mixture of 2 kDa bPEI and 0.75 kDa methoxypolyethylene glycol chains. A supplementary step was also described for two distinct post-functionalization reactions namely the decoration of polyrotaxane with bPEI with guanidine residues at the primary amino groups in bPEI or the addition of arginine residues. The described synthesis pathway relied on precise and highly reproducible reactions in order to precisely tailor the structure and functionality of the carriers.
In terms of transfection efficiency (pLuc or pGFP plasmids on HeLa cells) all four described vectors demonstrated their ability to transfect. The polyrotaxane carrier decorated solely with bPEI (ROT-PEI) exhibited the highest transfection efficiency, with a maximum yield at N/P 20 (Fig. 1) while the post-modified version with guanidine residues (ROT-PEI-G) was close, with a maximum yield at N/P 30.
The arginine post-decorated vector (ROT-PEI-Arg) vector showed a maximum transfection yield at an N/P ratio of 60. This observation indicated that for this particular study the simple one step guanidinylation is more useful than the arduous attachment of an amino acid with the purpose of mimicking the function of a arginine rich histone (Müller and Muir 2015). Lastly, the polyrotaxane decorated with PEG and bPEI (ROT-PEI-PEG750) showed a maximum transfection yield at a higher N/P ratio of 70 which is most likely due to the presence of the PEG chains. The cytotoxicity assay demonstrated that the presence of these chains achieved their purpose of increasing the biocompatibility of the carrier since the test indicated a cell proliferation at lower N/P ratios and a cell viability of approximately 100% throughout the tested range for this particular carrier.
3 Dynamic Combinatorial Systems as Non-viral Vectors
Constitutional dynamic chemistry can be considered a new evolutional approach to produce chemical diversity. A specific advantage with constitutionally generated systems addresses the possibility to self-adjust to biological target species at a given time, in a certain environment at nanoscale dimensions (Lehn 2012; Zhang and Barboiu 2016). The key concept is exploring the multivalent molecular recognition and self-assembly by using adaptive platforms interacting with biological targets. The use of reversible interactions as dynamic interfaces between the target and Dynamical Constitutional Frameworks (DCF) components allows one to self-adjust the system’s tridimensional geometry and functional properties.
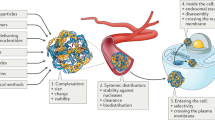
Despite the existing progress, the rational design is sometimes limited to the choice of a low number of components. Within this context, the dynamic combinatorial and constitutional screening approaches appeared to be an attractive strategy for the rapid identification of the active delivery vectors from dynamically exchanging libraries of complex mixtures of components. The construction of dynamic covalent polymers via the incorporation of reversible covalent bonds is therefore a promising strategy for generating effective vectors that allow multivalent interactions for dsDNA binding in biological media (Fig. 2) (Catana et al. 2015; Clima et al. 2019; Clima et al. 2015; Craciun et al. 2018; Marin et al. 2016; Pricope et al. 2018,; Turin-Moleavin et al. 2015; Zhang and Barboiu 2016). DCFs approach is relevant in biological and medicinal research, especially when the biotargets like nucleic acids contain a large number of available reaction units.
The simplicity of the synthetic constitutional strategy using accessible and simple building blocks for easily self-generation DCFs presenting synergistic DNA and cell membrane affinities might be considered as a valuable path toward the systematic discovery of active delivery systems (Fig. 3). In order to enhance transfection efficiency of the DCF-based vectors, several crucially important components like: cationic heads, biocompatible linkers, self-assembly triggers and multifunctional core connectors have to be taken into account to generate specific DCFs for DNA binding (Fig. 3).
PEIs units utilized as cationic heads, demonstrated safer alternative to viral vectors and has shown good transfection efficiency (TE) in different types of cells (Ailincai et al. 2018; Funhoff et al. 2004; Neu et al. 2005; Neuberg and Kichler 2014; Wang et al. 2011; Wu et al. 2018). PEI is able to bind DNA by an electrostatic interaction to form small complexes (polyplexes) that are internalized into cells by endocytosis and can be localized to the nucleus as distinct structures (Clima et al. 2015; Neuberg and Kichler 2014; Olden et al. 2018). Commonly, the molecular weight of PEI for the most effective transfection ranges between 5 and 25 kDa (Paul et al. 2014; Zakeri et al. 2018), higher molecular weights lead to increased cytotoxicity in polyplexes, while low molecular weight PEI demonstrate low toxicity in cell culture studies (Godbey et al. 2000; Neu et al. 2005; Yao et al. 2018). A strategy to increase the transfection efficiency with simultaneous decrease in cytotoxicity could be the coupling of the low molecular weight PEIs (maximum 2000 Da) to a core connector, forming conjugates of 8–54 kDa PEI (Ardeleanu et al. 2018; Uritu et al. 2015a, b). Among the biocompatible linkers PEGs are considered a powerful tool due to their surface charge shielding ability especially in nucleic acid delivery systems. It forms hydrophilic coating to minimize the interactions of the formed polyplexes with blood components and reduces the polyplex uptake by macrophages, thus increasing the overall blood circulation time (Suk et al. 2016). Since the conformation, hydrophobicity and electrostatic binding properties change through PEGylation, identifying the degree of the vector PEGylation and the molecular weight is crucial. Excessive PEGylation may lead to a risk of reduction of the DNA-binding capacity leading to considerable drop of in vitro transfection efficiency. Besides biocompatible linkers, some non-viral vectors incorporate a self-assembly triggers as hydrophobic or lipid component able to form amphiphile in aqueous medium (Ailincai et al.). These important units contribute to the interaction of the vector with the cell membrane, facilitating cell internalization and increasing gene transfer efficiency.
3.1 Dynamic Constitutional Frameworks (DCF) for DNA Biomimetic Recognition
Catana et al. (2015) first suggested the idea of dynamic constitutional frameworks (DCF) for DNA biomimetic recognition when multifunctional core centers, linear PEG macromonomers and cationic heads were used to produce functional DCFs for DNA binding. The building constituents were: poly(ethylene glycol)-bis(3-aminopropyl) (6), Girard's reagent T, monoprotonated N,N-dimethylene amine or aminoguanidine hydrochloride, all linked to 1,3,5-Benzenetrialdehyde (1) via the amino - carbonyl/imine reversible bonds. As the result of the series of components combinations, it was established that compact guanidinium with a cross-linked structure strongly influenced the DNA binding, whereas the frameworks containing Girard's reagent T or monoprotonated N,N-dimethylene amine having linear structure, presented no binding properties. This behavior highlighted dominant coiling versus linear DNA binding mechanism behaviors.
Turin-Moleavin's study (Turin-Moleavin et al. 2015) defining the synthesis and characterization of a class of DNA nanovectors based on unique component and connector center frameworks, connected by reversible covalent bonds, continued further development of the systems. In the presence of interacting DNA biotargets, the dynamic self-assembly of PEG unit (6) with bPEI (2) cationic binding groups around the 1,3,5-Benzenetrialdehyde core connector (1) has led to adaptive spatial distributions. By forming stable polyplexes with dsDNA, the obtained DCF polyplexes were able to perform as gene nanovectors. Polyplexes possessed sizes ranging between 40 and 125 nm depending on the type and quantity of associated DNA and on the molar ratio of bPEI/PEG. All tested vectors presented DNA transfection ability on HeLa cell line and showed low cytotoxicity, even at high N/P ratio of 200, the viability of cells being over 90% compared to untreated cells as control. This led to the conclusion that both the presence of the PEG component and a moderate amount of bPEI in DCFs were significant in the production of highly transfecting and cyto-friendly polyplexes.
3.2 Dynamers Based on Hydrophob-Hydrophil Assembly for DNA Transfection
Another strategy in the development of dynamic vectors was to use hydrophobic/hydrophilic connectors as core-centers (Ailincai et al. 2019; Clima et al. 2019,2015; Craciun et al. 2019; Marin et al. 2016; Pricope et al. 2018) and positively charged molecular heads in the design of 3D Dynameric Frameworks (DFs) as advanced nanomaterials for DNA recognition and transfection. They form modular networks/platforms that self-adapt to the DNA targets, depending on their variable composition due to reversible communication between the constituents of DFs.
A core-shell hydrophobic/hydrophilic structure with a high number of low molecular weight PEI units was expected to create a vector with a transfection efficiency similar to that of high molecular weight PEI, but with much lower toxicity, according to the proposed idea. Nano-nonviral vectors with a linear siloxane and aldehyde as hydrophobic core and a hydrophilic shell determined by presence of bPEI 800(2) or bPEI 2000(3) have been designed and synthesized.
The following components were proposed for the DFs according to Marin et al. (2016):
-
(i)
1,3,5-Benzenetrialdehyde (1) as tri-carbonyl functionalized core;
-
(ii)
bis-poly-(propylene glycol), amine-terminated (6), Jeffamine-400 and Jeffamine-2000 (10 and 11) as cross-linkers contribute to the formation of framework connecting DNA-binding sites by the reversible bond of amino-carbonyl/imine. Additionally 10 and 11 are known for low toxicity and high cellular DNA uptake and are suitable for building of nonviral vectors (Ailincai et al.);
-
(iii)
Poly(ethylene glycol-3-aminopropyl) terminated (9) as specific PEG units, in order to enhance the solubility in aqueous media, increase biocompatibility and diminish the immunogenicity of the vectors;
-
(iv)
Spermine (5), linear-poly(ethylenimine), (Mn ≈ 2500 gmol−1), bPEI (2, 3) as cationic moieties capable of DNA complexation.
Combination of JD1-bPEI800, JD1-bPEI2000 and PEG-ylated JD1-PEG-bPEI800, JD1-PEG-bPEI2000, and JD1-PEG-spermine led to water-soluble DFs (Fig. 4), while formation of DFs from Jeffamine-2000 (11) and cationic moieties of linear PEI or Spermine (5) in aqueous media led to insoluble precipitate and this system was no longer investigated. The soluble DFs spontaneously self-assembled in aqueous solution as predicted, resulting in spherical nanoparticles. Transmission electron microscopy (TEM) showed that JD1-bPEI800 had a mean diameter of 6 nm, while JD1-bPEI2000 had a mean diameter of 23 nm and mean diameter of 50 nm for the combined JD1-PEG-bPEI800. BPEI-based DFs (PEGylated or non PEGylated) were able to bind DNA effectively as shown in agarose gels by delay of the electrophoretic mobility of formed polyplexes, whereas JD1-PEG-spermine showed a low binding capacity even under acidic conditions due to the reduced positive binding sites associated to bPEI DFs.
Formed polyplexes exhibited great tolerance by human embryonic kidney 293 T. JD1-bPEI2000/pEYFP, among the studied polyplexes, confirmed higher gene transfection efficacy on HEK 293T cells, ∼9% at N/P 100. This value is 1.2 fold higher than that obtained with SuperFect commercial reagent. Such high performance was explained by the narrow polydispersity observed in AFM for the obtained polyplex, having an average diameter of 100 nm, whereas the higher polydispersity and polyplex diameter resulted in inferior transfection efficiency.
Siloxane structure, viewed as strong hydrophobic units and enhancing high transfection efficiency in non-viral vectors, was investigated as an alternative hydrophobic moiety in a related strategy (Bainbridge et al. 2006).
The diamine-bearing siloxane unit (12) has therefore been proposed as suitable hydrophobic core for a library of compounds (Ailincai et al. 2019). In order to build an oligomeric chain of aldehyde functionalities capable of binding the hydrophilic shell in several positions, siloxane (12) was reacted with trialdehyde (1) in a 1/1 molar ratio (Scheme 1). In the vector architecture, hyperbranched bPEI 800 (2) and bPEI 2000 (3) were used as hydrophilic moieties (Fig. 5).
UV-VIS spectroscopy has underlined the dynamic aspects of synthesized A1 and A2 amphiphiles.
Absorption maxium at 300 nm corresponded to benzenetrialdehyde (1) and assigned to electronic transitions π-π* of the CHO group, on the other hand, A1 and A2 did not display any absorption maximum in the UV-VIS spectrum since the presence of hyperbranched PEI induces shielding effect of the chromophoric groups, these being hindered. The peak corresponding to the aldehyde occurred however, when the pH reached the value of 6.1, showing that the imine linkages that held the hydrophobic core and the hydrophilic shell together were cleaved by the acid by successive addition of small amounts of HCl. Furthermore, the stability of the two compounds at neutral pH was additionally proven by the NMR spectra of the two amphiphiles recorded in deuterium oxide at different time periods showing no degradation or changes, even after 24 h. The peak corresponding to the carbonyl functionality from trialdehyde occurred by adding a small quantity of HCl to the NMR tubes (pH = 5.8), indicating the cleavage of the imine bond. A1 and A2 were self-assembled in a spherical particles with mean size ranging from 20 to 95 nm. Regardless of the small particle sizes of the vectors, the polyplexes formed with pEGFP tended to have a larger particle size from the vector A1, compared to polyplexes formed from A2. Thus, this behavior of polyplex formed from A2 can be attributed to the presence of bPEI 2000 (3) in its composition, due to multiple protonated amine groups on the surface, these were sufficient to compensate the negative charge of DNA. On the other site, compound A1 with bPEI 800(2) in its composition, formed larger polyplxes, due to the possible aggregation of the A1 entities in order to be able to balance negative charge of DNA.
The cytotoxicity performed on HeLa cell line of the A1/pEGFP and A2/pEGFP polyplexes, showed excellent cell viability (above 90%) even at high N/P ratios (above 400), making them ideal candidates for non-viral vectors. Compared to the free bPEI 800 (2) and bPEI 2000 (3), both compounds had a superior capacity to transfect HeLa cells used as references. This was the product of various synthesized vector properties, such as: (i) high hydrophobicity (Ailincai et al.), (ii) formation of nano-sized particles and spherical morphology (iii) Zeta potential of positive values for the polyplexes (Gordon et al. 2012) and (iv) solubility of the vectors and poliplexes in aqueous milieu.
3.3 Dynamic Combinatorial Frameworks Based on Lipid Hydrophob-Hydrophil Assembly for Nuclei Acid Delivery
Drug carriers with a lipophilic component have long been considered to have a high capacity to cross biological membranes (Banks 2009; Finbloom et al. 2020), including brain barriers (Banks 2009). And because of their hydrophobicity, these molecules are in high demand as non-viral vector components. Direct membrane permeabilization may be caused by specific lipophilic units that interfere with the cell membrane. Due to its propensity to self-assemble in aqueous media, creating amphiphilic compounds, squalene (13), a natural triterpene lipid and precursor to cholesterol biosynthesis, has gained attention as a biocompatible material for drug deliver (Couvreur 2009; Craciun et al. 2018; Desmaele et al. 2012; Lepeltier et al. 2013).
Squalene was chemically functionalized and PEGylated before being used as a component in dynamic combinatorial frameworks (DCF). Its DCF composition ratio was designed and optimized to ensure the best possible rapport between self-assembly capacity and nanoassembly shape (Pricope et al. 2018). Constitutional strategy was applied to build DCFs as multivalent DyNAvectors for DNA transfection. The 1,3,5-benzenetrialdehyde (1), PEG-ylated squalene (SQ-PEG), H2N-PEG-NH2 (6) and low molecular weight bPEI 800 (2) were combined in different molar ratios to form a library of DCFs (Fig. 6) (Clima et al. 2015).
The DCFs self-assembled in spherical particles with the hydrophobic squalene core and the PEG/PEI hydrophilic shell. Although the shaped particles were in the µm range and could be deemed unsuitable as non-viral vectors due to their large size, their polyplex with plasmid pEYFP showed more compact structures than the noncomplexed DCFs. The sizes of polyplexes were ranged between 20–100 nm, depending on N/P ratio. The improved performance of both P6 and P8 polyplexes at the N/P ratio of 50 compared to bPEI 800 (Fig. 6) depicted the multivalent as in the case of P6 and P8 versus monomeric presentation of the bPEI 800 (2).
The polyplex P6, on the other hand, showed low toxicity and higher transfection values than P8, owing to the presence of H2N-PEG-NH2 (6) not only in the SQ-moiety, but also as an external constitutive component that stabilized P6 in a serum-rich environment (Fig. 6).
The particle size of P6 at N/P 50 were about 100 nm, which is in line with the criteria for gene delivery nanoparticles. Shielding the vectors with hydrophilic polymers, especially PEGs, has become a common strategy (Kumar et al. 2014), demonstrating that PEGylation of PEIs resulted in increased solubility of the complexes as well as a reduction in the overall surface charge of the polyplexes (Kursa et al. 2003). Yet, PEGylation has the disadvantage of lowering the cationic polymer's DNA-binding ability, primarily due to sterical hindrance of interactions between polyplexes and targeted cells (Elouahabi and Ruysschaert 2005), lowering in vitro transfection performance. For conjugating to PEI, PEG units of different chain lengths were used, and the results showed that the degree of PEGylation and the molecular weight of PEG have a significant impact on the properties of the resulting PEG-PEI-based non-viral vector.
A systematic investigation of a sequence of DCFs (Fig. 7, NV library) composed from PEGylated squalene (14) (Clima et al. 2015; Craciun et al. 2018), H2N-PEG-NH2 of certain length (1500 Da, 2000 Da and 3000 Da), and hiperbranched bPEI 800 of low molecular weight (2), all coupled in a hyperbranched structure, revealed some key findings. First, the sterical interactions of PEG units with the framework's components led to the development of smaller size particles as the molecular weight of H2N-PEG-NH2 increased from 1500 to 3000 Da, according to TEM and DLS results. Since H2N-PEG-NH2 is a linear polymer, it causes larger DCFs to form, resulting in more complex cross-linking and eventual self-assembly of the DCFs in larger particles (Craciun et al. 2019). Likewise, due to the PEG shielding effect over the bPEI 800 (2), raising the length of H2N-PEG-NH2 in the formulation of NVs resulted in a poorer binding ability of plasmid DNA (pCS2 + MT-Luc). Moreover, we have shown that increasing the ratio of H2N-PEG-NH2 from 0.1 to 1 equivalent improved significantly the transfection performance of NV1/pDNA-NV30/pCS2 + MT-Luc polyplexes on the HeLa cell line. In general, transfection efficiency was higher at N/P ratio 100 than at N/P ratio 50 for all studied polyplexes. As a result, using H2N-PEG-NH2 with a comparatively high molecular weight within the complex structure improved the vector’s biocompatibility, thus highlighting that the molecular weight of H2N-PEG-NH2 had an important impact on both transfection performance and cell viability in general.
Another goal of this particular research was understanding the effect of the PEI ratio in the frameworks composition on self-assembling properties, DNA binding affinity, biocompatibility, and transfection properties since these are critical issue for developing a successful gene therapy vector. It is known that the presence of light exes of PEI in composition of non-viral vectors generally facilitate the endosome escape phenomenon. However, since toxicity increases with increasing PEI concentrations, finding the maximum amount of PEI in the composition of DCFs, is critical. Thus, in order to better understand this behavior, a library of frameworks was prepared (Craciun et al. 2019). The 1,3,5-benzenetrialdehyde (1), PEGylated squalene derivative (14), H2N-PEG-NH2 (6), and bPEI 2000 were chosen for building the library of DCFs (3). To define the toxicity limit, the ratio of bPEI 2000 (3) in the investigated DCFs was steadily increased from 1.5 eq to 3.5 eq. As the ratio of PEI in the composition of DCFs approached 3.5 eq, the tested DCFs/DNA polyplexes showed a high cytotoxic behavior at N/P 50. The noticeable cytotoxicity was observed while rising the N/P ratio to 100, regardless of the presence of NH2-PEG-NH2.
Unrelatedly to the PEI ratio in DCF composition, the studied polyplexes showed significantly higher transfection rate at N/P ratios of 50 as compared to bPEI 2000 (3). At N/P ratio 100, polyplexes showed a poor transfection due to high ratio of PEI in composition of DCFs.
Notably, at N/P ratios of 30 and 50, the presence of H2N-PEG-NH2 caused a subtle improvement in transfection efficiency. As the result of the investigation, it was found that the optimum DCF composition ratio of PEI:H2N-PEG-NH2 to be 1.5:1 for highest efficiency in transfection of pDNA in HeLa cells.
This study contributed to understanding the correlation and synergy between the constituent elements in dynamic combinatorial frameworks, as well as tuning the structure of DCFs, that are critical issues in the production of an effective nucleic acid carrier.
4 Rational Design Approach for Amphiphilic Squalene-Polyethylenimine Based Conjugates
Novel gene delivery systems based on squalene (Sq) (13) and bPEI of 1.8 kDa were synthesized using Michael addition to 1,1′,2-trisnorsqualenaldehyde with the goal of developing small controlled structures with good stability and low toxicity. The Sq/bPEI conjugates (Sq-BPEI-NH2) as well as their guanidinylated derivatives (Sq-BPEI-G) were found to have effective DNA complexation. When used in transfection of HeLa cells, the conjugates achieved highest transfection efficiency at N/P ratios of 20 for the polyplex Sq-BPEI-NH2/DNA and N/P 15 for the polyplex Sq-BPEI-G/DNA, both being more effective than the reference bPEI/DNA polyplex. A new strategy to release in a controlled manner of encapsulated vectors for improvement of gene delivery yield was explored. Knowing that using a matrix-mediated gene transfer technique enhance gene delivery, increase extent and duration of transgene expression, and ensure a stable gene therapy profile (Cam C and Segura T 2013; Tierney et al. 2013). Thus, the polyplexes fomed from the vector and pEYFP included in a hybrid cryogel (containing natural/synthetic polymers (Atelocollagen, hyaluronic acid derivative, and poly(ε-caprolactone)), and PEI functionalized nano-hydroxyapatite (CH10P10/HAp25-15) (Simionescu et al. 2017) yielded a constant release of genetic material for approximately 26 days with a maximal expression on day 5 without any apparent toxic impact on HEK 293T cells.
Tunable material for genetic release, long-term bioavailability, and a relatively simple synthesis process are all features of the evolved systems.
Final Remarks
It is certain that the next generation of non-viral vectors relies on multi-purpose personalized materials with high degree of biocompatibility and biodegradability, which deliver cargo targeted at specific sites in tissues, organs or cells, with no harm to the body, in formulations that are easy to use and convenient for patients. In this context, the concept of simple,combinatoriar modular aproach that yield materials with tunable properties is of a great interest. The simplicity of the synthetic strategy and combinatorial approach using accessible and simple building blocks for facile assembly of carriers, can be considered as a valuable path toward the systematic discovery of active delivery systems that aims to translate the scientific progress into clinical benefits for patients.
Abbreviations
- pDNA:
-
Plasmid DNA
- siRNA:
-
Small interfering RNA
- β-CD:
-
β-Cyclodextrin
- bPEI:
-
Branched poly(ethyleneimine)
- PEG:
-
Poly(ethylene glycol)
- pLuc:
-
Luciferase plasmid
- pGFP:
-
Green fluorescent protein plasmid
- CDC:
-
Constitutional dynamic chemistry
- DCF:
-
Dynamical constitutional frameworks
- dsDN:
-
Duble stranded DNA
- TE:
-
Transfection efficiency
- Girard’s reagent T:
-
(Carboxymethyl)trimethylammonium chloride hydrazide
- HeLa:
-
Human cervix adenocarcinoma
- DF:
-
Dynameric frameworks
- HEK 293T:
-
Human embryonic kidney 293T cells
- pEGFP:
-
Enhanced green fluorescent protein plasmid
- N/P:
-
Nitrogen/phosphorus
- AFM:
-
Atomic force microscopy
References
Ailincai D, Marin L, Morariu S et al (2016) Dual crosslinked iminoboronate-chitosan hydrogels with strong antifungal activity against Candida planktonic yeasts and biofilms Carbohyd Polym 152:306–316
Ailincai D, Tartau Mititelu L, Marin L (2018) Drug delivery systems based on biocompatible imino-chitosan hydrogels for local anticancer therapy. Drug Deliv. 25(1):1080–1090
Ailincai D, Peptanariu D, Pinteala M et al (2019) Dynamic constitutional chemistry towards efficient nonviral vectors Mater Sci Eng C 94:635–646
Ardeleanu R, Dascalu AI, Neamtu A et al (2018) Multivalent polyrotaxane vectors as adaptive cargo complexes for gene therapy. Polym Chem 9(7):845–859
Bainbridge JWB, Tan MH, Ali RR (2006) Gene therapy progress and prospects: the eye. Gene Ther 13(16):1191–1197
Banks WA (2009) Characteristics of compounds that cross the blood-brain barrier. BMC Neurol 9(1):S3
Cam C, Segura T (2013) Matrix-based gene delivery for tissue repair. Curr Opin Biotech 24(5):855–863
Catana R, Barboiu M, Moleavin I et al (2015) Dynamic constitutional frameworks for DNA biomimetic recognition. Chem Commun 51(11):2021–2024
Clima L, Peptanariu D, Pinteala M et al (2015) DyNAvectors: dynamic constitutional vectors for adaptive DNA transfection. Chem Commun 51(99):17529–17531
Clima L, Craciun BF, Gavril G et al (2019) Tunable composition of dynamic non-viral vectors over the DNA polyplex formation and nucleic acid transfection. Polymers 11(8):1313
Couvreur P (2009) “Squalenoylation”: a new approach to the design of anticancer and antiviral nanomedicines. B Acad Nat Med Paris 193(3):663–673
Craciun BF, Vasiliu T, Marangoci N et al (2018) Pegylated squalene: a biocomptible polymer as precursor for drug delivery. Rev Roum Chim 63(7–8):621–628
Craciun FB, Gavril G, Peptanariu D et al (2019) synergistic effect of low molecular weight polyethylenimine and polyethylene glycol components in dynamic nonviral vector structure, toxicity, and transfection efficiency. Molecules 24(8)
Dascalu AI, Ardeleanu R, Neamtu A et al (2017) Transfection-capable polycationic nanovectors which include PEGylated-cyclodextrin structural units: a new synthesis pathway. J Mater Chem B 5(34):7164–7174
Desmaele D, Gref R, Couvreur P (2012) Squalenoylation: a generic platform for nanoparticular drug delivery. J Control Release 161(2):609–618
Dunbar CE, High KA, Joung JK et al (2018) Gene therapy comes of age. Science 359(6372):eaan4672
Egger G, Liang G, Aparicio A et al (2004) Epigenetics in human disease and prospects for epigenetic therapy. Nature 429(6990):457–463
Elouahabi A, Ruysschaert J-M (2005) Formation and intracellular trafficking of lipoplexes and polyplexes. Mol Ther 11(3):336–347
Finbloom JA, Sousa F, Stevens MM et al (2020) Engineering the drug carrier biointerface to overcome biological barriers to drug delivery. Adv Drug Deliv Rev 167:89–108
Funhoff AM, van Nostrum CF, Koning GA et al (2004) Endosomal escape of polymeric gene delivery complexes is not always enhanced by polymers buffering at low pH. Biomacromol 5(1):32–39
Ginn SL, Amaya AK, Alexander IE et al (2018) Gene therapy clinical trials worldwide to 2017: an update. J Gene Med 20(5):e3015
Godbey WT, Barry MA, Saggau P et al (2000) Poly(ethylenimine)-mediated transfection: a new paradigm for gene delivery Journal of Biomedical Materials Research 51(3):321–328
Gordon R, Ranjith R, Leighann S et al (2012) Fungal Biofilm resistance. Int J Microbiol 2012
Hanna E, Rémuzat C, Auquier P et al (2017) Gene therapies development: slow progress and promising prospect. J Mark Access Health Policy 5(1):1265293
Harada A, Okada M, Kawaguchi Y et al (1999) Macromolecular recognition: new cyclodextrin polyrotaxanes and molecular tubes. Polym Adv Technol 10(1–2):3–12
Hedman M, Hartikainen J, Ylä-Herttuala S (2011) Progress and prospects: hurdles to cardiovascular gene therapy clinical trials. Gene Ther 18(8):743–749
Jiří V, Edward SW, Andrey Y et al (2012) Terminology and nomenclature for macromolecular rotaxanes and pseudorotaxanes (IUPAC Recommendations 2012). Pure Appl Chem 84(10):2135–2165
Kumar V, Qin J, Jiang Y et al (2014) Shielding of lipid nanoparticles for sirna delivery: impact on physicochemical properties, cytokine induction, and efficacy. Mol Ther Nucleic Acids 3(11):e210-e
Kursa M, Walker GF, Roessler V et al (2003) Novel shielded transferrin−polyethylene glycol−polyethylenimine/DNA complexes for systemic tumor-targeted gene transfer. Bioconjug Chem 14(1):222–231
Lehn J-M (2012) Constitutional dynamic chemistry. Barboiu M (ed.) Springer, Heidelberg, p 1–32
Lepeltier E, Bourgaux C, Rosilio V et al (2013) Self-assembly of squalene-based nucleolipids: relating the chemical structure of the bioconjugates to the architecture of the nanoparticles. Langmuir 29(48):14795–14803
Marin L, Ailincai D, Cahn M et al (2016) Dynameric frameworks for DNA transfection. ACS Biomater Sci Eng 2(1):104–111
Müller MM, Muir TW (2015) Histones: at the crossroads of peptide and protein chemistry. Chem Rev 115(6):2296–2349
Muramatsu S (2018) Gene therapy using adeno-associated virus vectors Cancer Sci 109(1200-.
Neu M, Fischer D, Kissel T (2005) Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J Gene Med 7(8):992–1009
Neuberg P, Kichler A (2014) Advances in genetics. Huang L et al (eds) Academic Press, vol 88, pp 263–288
Olanow CW (2014) Gene therapy for Parkinson disease—a hope, or a dream? Nat Rev Neurol 10(4):186–187
Olden BR, Cheng YL, Yu JL et al (2018) Cationic polymers for non-viral gene delivery to human T cells. J Control Release 282:140–147
Papadopoulos KI, Wattanaarsakit P, Prasongchean W et al (2016) Polymers and nanomaterials for gene therapy. Narain R (ed) Woodhead Publishing, p 231–56
Paul A, Eun CJ, Song JM (2014) Cytotoxicity mechanism of non-viral carriers polyethylenimine and poly-L-lysine using real time high-content cellular assay. Polymer 55(20):5178–5188
Pricope G, Pinteala M, Clima L (2018) Dynamic self-organazing systems for DNA delivery. Rev Roum Chim 63(7–8):613–619
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3(10):721–732
Simionescu BC, Drobota M, Timpu D et al (2017) Biopolymers/poly(epsilon-caprolactone)/polyethylenimine functionalized nano-hydroxyapatite hybrid cryogel: Synthesis, characterization and application in gene delivery. Mat Sci Eng C-Mater 81:167–176
Smith AJ, Bainbridge JWB, Ali RR (2012) Gene supplementation therapy for recessive forms of inherited retinal dystrophies. Gene Ther 19(2):154–161
Somia N, Verma IM (2000) Gene therapy: trials and tribulations. Nat Rev. Genet 1(2):91–99
Suk JS, Xu Q, Kim N et al. (2016) PEGylation as a strategy for improving nanoparticle-based drug and gene delivery Adv Drug Deliver Rev 99:28–51
Thapa B, Narain R (2016) Polymers and nanomaterials for gene therapy. Narain R (ed) Woodhead Publishing, p 1–27
Tierney EG, Duffy GP, Cryan SA et al (2013) Non-viral gene-activated matrices next generation constructs for bone repair. Organogenesis 9(1):22–28
Turin-Moleavin IA, Doroftei F, Coroaba A et al (2015) Dynamic constitutional frameworks (DCFs) as nanovectors for cellular delivery of DNA. Org Biomol Chem 13(34):9005–9011
Uritu CM, Varganici CD, Ursu L et al (2015) Hybrid fullerene conjugates as vectors for DNA cell-delivery. J Mater Chem B 3(12):2433–2446
Uritu CM, Calin M, Maier SS et al (2015) Flexible cyclic siloxane core enhances the transfection efficiency of polyethylenimine-based non-viral gene vectors. J Mater Chem B 3(42):8250–8267
Wang Y, Zheng M, Meng F et al (2011) Branched polyethylenimine derivatives with reductively cleavable periphery for safe and efficient in vitro gene transfer. Biomacromol 12(4):1032–1040
Wu P, Chen H, Jin R et al (2018) Non-viral gene delivery systems for tissue repair and regeneration. J Transl Med 16(1):29
Yang J, Liu H, Zhang X (2014) Design, preparation and application of nucleic acid delivery carriers. Biotechnol Adv 32(4):804–817
Yao WJ, Cheng X, Fu SX et al (2018) Low molecular weight polyethylenimine-grafted soybean protein gene carriers with low cytotoxicity and greatly improved transfection invitro. J Biomater Appl 32(7):957–966
Yin H, Kanasty RL, Eltoukhy AA et al (2014) Non-viral vectors for gene-based therapy. Nat Rev. Genet 15(8):541–555
Zakeri A, Kouhbanani MAJ, Beheshtkhoo N et al (2018) Polyethylenimine-based nanocarriers in co-delivery of drug and gene: a developing horizon. Nano Rev Exp 9(1):1488497
Zhang Y, Barboiu M (2016) Constitutional dynamic materials-toward natural selection of function. Chem Rev 116(3):809–834
Acknowledgements
This publication is part of a project that has received funding from the H2020 ERA Chairs Project no 667387: SupraChem Lab Laboratory of Supramolecular Chemistry for Adaptive Delivery Systems ERA Chair initiative.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Clima, L., Dascalu, A.I., Craciun, B.F., Pinteala, M. (2021). Polymeric Carriers for Transporting Nucleic Acids—Contributions to the Field. In: J.M. Abadie, M., Pinteala, M., Rotaru, A. (eds) New Trends in Macromolecular and Supramolecular Chemistry for Biological Applications. Springer, Cham. https://doi.org/10.1007/978-3-030-57456-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-57456-7_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57455-0
Online ISBN: 978-3-030-57456-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)