Abstract
In the United States, a patient suffers from a myocardial infarction (MI) every 40 seconds, and heart disease remains the leading cause of death worldwide. Despite optimal medical and surgical care, one in every two patients who develops heart failure will die within 5 years. A series of pathological remodeling changes occur to compensate for the myocardium lost during an MI that lead to fibrosis, hypertrophy, and ventricular dilation, with the end-stage pathology often culminating in decreased ejection fraction (EF) and heart failure (HF). Post-MI myocardial recovery is worsened by the adult myocardium’s limited regenerative ability, particularly in an ischemic environment; accordingly, there is great interest in stem cell therapy to treat post-MI injury, and the field is rapidly evolving. Several endogenous and exogenous cellular sources have already been evaluated in clinical trials, with varied results and modest efficacy. Limitations surrounding stem cell treatment include lack of standardization across clinical trials, poor cellular retention and differentiation rates, suboptimal delivery methods, and limited source of candidate cells. In order to optimize stem cell efficacy, future work should focus on efficient generation of the optimal cellular source, development of delivery carriers capable of noninvasive delivery methods that improve cellular survival, and standardization of clinical trials to allow for meaningful comparisons.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
14.1.1 General Considerations for Myocardial Infarction
Ischemic heart disease remains the leading cause of death worldwide [1]. According to the American Heart Association, 720,000 Americans experienced a new coronary artery event in 2018, with the median survival after a first myocardial infarction (MI) being 8.4, 5.6, 7, and 5.5 years, respectively, for white males, white females, Black males, and Black females [2]. The burden of cardiovascular disease (CVD) and MI affects low- and middle-income countries disproportionately, where 80% of CVD-related deaths occur [3]. While a large majority of the risk factors associated with ischemic heart disease such as high serum cholesterol, hypertension, diabetes, obesity, and smoking are modifiable, family history, age, male sex, and female sex associated with postmenopausal status cannot be altered [4].
Myocardial infarction is broadly defined as myocardial death secondary to prolonged ischemia and can result from multiple etiologies including coronary artery occlusion, supply/demand imbalance, MI related to percutaneous coronary intervention (PCI), stent thrombosis, MI associated with coronary artery bypass grafting (CABG) , and others [5]. Rupture or erosion of an atherosclerotic coronary plaque, with resultant exposure of highly thrombogenic material, is the most common inciting factor for coronary occlusion [6]. While a completely occlusive thrombus in the coronary circulation results in an ST-segment elevation MI (STEMI), incomplete thrombosis, or occlusion in the presence of well-established collaterals, results in a non-ST elevation MI (NSTEMI) or unstable angina [7, 8].
14.1.2 Current Myocardial Infarction Standard of Care
Patients with a suspected acute coronary syndrome should be immediately evaluated with an electrocardiogram and cardiac troponin testing. These diagnostic tests, along with history of symptoms, group patients into those suffering from STEMI, NSTEMI, or nonischemic chest pain, distinctions that dictate further care [7, 8]. Initial medical care of patients with STEMI and NSTEMI includes oxygen, analgesics, nitrates, beta-blockers, antiplatelet, and anticoagulation therapy [7, 8]. Urgent reperfusion of ischemic myocardium is the primary therapeutic goal in both groups. All patients with a STEMI should undergo percutaneous coronary intervention within 90 minutes of presentation, while those suffering from NSTEMI undergo immediate, early, and elective PCI depending on time of symptom onset [7, 8]. Diagnostic angiography delineates the extent of disease and dictates reperfusion strategies including stenting, fibrinolysis, or CABG.
Despite urgent reperfusion, life-threatening post-MI complications arise depending on the amount and location of lost myocardium. These complications can be grouped into five subtypes including ischemic, mechanical, arrhythmic, embolic, and inflammatory [9]. Coronary artery disease, including MI, is the number one cause for development of heart failure (HF) in the United States [10]. With improved medical and interventional care, patients are living longer post MI, resulting in a projected increase of HF from six to over eight million by 2030 [11].
14.1.3 Post-Myocardial Infarction Cardiac Remodeling
Following an MI, the injured myocardium and surrounding tissue undergo a series of early and late remodeling changes in an attempt to compensate for the ischemia-induced damage [12]. The early remodeling phase occurs hours to days post MI and includes myonecrosis-induced inflammation, matrix metalloproteinase (MMP) -driven collagen matrix breakdown, thinning and dilation of ventricular walls, as well as fibroblast-induced scar formation [13, 14]. Over the subsequent weeks to months, uninjured myocardium hypertrophies eccentrically overcompensate for increased stress, further contributing to ventricular dilation. As preload increases without resultant change in ventricular contractility, the ejection fraction (EF) decreases, and dilated cardiomyopathy and resultant HF ensue, worsened by the adult myocardium’s limited ability to recover after ischemia [15].
Unfortunately, current therapies aimed at decreasing pathologic post-MI remodeling and HF are limited and include pharmacological treatments to decrease scarring and tissue ischemia, devices and implants aimed at restoring heart function, as well as transplantation [16]. Mammalian myocardium has traditionally been viewed as a non-regenerative organ, and although resident cardiac stem cells (CSC) contribute to cardiac regeneration and some evidence of mammalian heart regeneration exists in animal models, resident stem cells lack the capacity to regenerate all of the myocardium lost after an MI [17, 18]. Furthermore, the contribution of CSC to cardiac regeneration remains highly controversial. Reports of cardiomyocyte exchange in humans range from 50 to 100% during a normal life span, with many such reports having been retracted secondary to lack of reproducibility [19, 20]. Delivery of endogenous and exogenous cardiac progenitor cells to increase myocardial regeneration post MI and in ischemic cardiomyopathy has recently been explored in animal and human studies, with promising results [21].
14.2 Stem Cells in Cardiac Regeneration
14.2.1 Exogenous Cellular Sources
Although cellular transfer for treatment of ischemic heart disease is a relatively new field, with the first clinical trial occurring in 2000, a multitude of cellular sources have been trialed to date in preclinical and clinical models of MI and HF, with relatively few cellular types remaining unexplored [22].
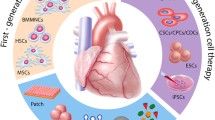
Exogenous cardiac progenitor and stem cells of clinical interest include skeletal myoblast cells (SMC), bone marrow-derived mononuclear cells (BM-MNC), bone marrow-derived populations including lin-c-kit+, CD133+, CD133-/CD34+, c-kit+, and Sca1+, mesenchymal stem cells (MSC), adipose-derived stem cells (ADSC), endothelial progenitor cells (EPC), induced pluripotent stem cells (iPSC), as well as embryonic stem cells (ESC) including early cardiovascular (Isl1+/SSEA1+) cells. Although exogenous stem cell therapy demonstrates some improvement of cardiac function post MI, direct cardiomyogenic differentiation from these cells is rare [23].
Non-satellite CD34-/CD45-/Sca1- stem cells isolated from skeletal muscle have demonstrated rhythmic beating similar to cardiomyocytes and when transplanted into adult mice differentiate into cardiac tissue, while C-kit+Sca1- cells improved survival, enhanced cardiac function, reduced regional strain, and attenuated remodeling in mice [24].
In rodent studies, embryonic stem cell-derived cardiomyocytes attenuated progression of HF after acute myocardial infarction (AMI) by reducing ventricular dilation and improving global left ventricular (LV) function . Subsequent studies established that human embryonic cell cardiomyocytes can limit AMI size and preserve LV contractility [25]. Further, xenotransplantation of cardiac-committed mouse embryonic cells into ovine models has shown that ESC are immune privileged and cardiomyocytes from human ESC are capable of repopulating rat hearts [24].
MSC can be derived from adult peripheral blood, adipose tissue, bone marrow, and neonatal umbilical cord, amnion, cord blood, and placenta [26]. They are potent stimulators of angiogenesis and cardiac regeneration and have been shown to be superior than hematopoietic stem cells in rodent post-MI models [27, 28]. Although they improve tissue regeneration predominantly via paracrine mechanisms, some porcine studies have shown that MSC injected intramyocardially can differentiate into vascular smooth muscle or endothelial cells. Furthermore, human umbilical cord blood-derived MSC preconditioned with 5-aza transdifferentiated into cardiomyocytes, when transplanted into mouse models of MI, preventing infarct expansion and improving heart function [24].
The groundbreaking discovery of iPSC generation via in vitro reprogramming of adult cells into a pluripotent state, and subsequent differentiation into any lineage, transformed the field of regenerative medicine [29]. Although their use in humans remains limited, cardiomyocytes, endothelial cells, and smooth muscle cells derived from iPSC have been tested on porcine infarct models with resultant reduction in infarct size, ventricular wall stress, and apoptosis [30].
14.2.2 Endogenous Cellular Sources
The concept of resident cardiac stem cells is not very well established, with reports quoting vastly different cardiomyocyte renewal capacity [19, 20]. At best, CSC account for approximately 1/30,000 cells in the human heart, although this number increases post injury, likely secondary to migration from bone marrow [31]. The hallmark of CSC is their ability to differentiate into every cardiac lineage including myocytes, fibroblasts, smooth muscle, and endothelial cells. Multiple previous studies have shown their contribution to cardiac regeneration [18, 32]. Specific subtypes of CSC implicated in cardiac regeneration include cardiosphere-derived cells, c-Kit+ cells, insulin gene enhancer protein 1 (Isl1+) progenitor cells, fetal liver kinase 1 (Flk1+) progenitor cells, glycolytic cardiac progenitors (GCP), stage-specific embryonic antigen1 (SSEA1+) progenitors, side population (SP) progenitors, as well as stem cell antigen-1 (Sca1+) progenitors [21]. CSC are localized mainly in the atria of the heart, including the right atrial appendage, and are more numerous in the subepicardium compared to the myocardium [33].
CSC have greater potential to differentiate into cardiomyocytes compared to MSC and in animal studies show potential to reduce post-MI scar size and vascular overload [18, 34]. Other studies have shown their ability to engraft in the myocardium, recruit endogenous stem cells, and attenuate myocyte apoptosis, via release of growth factors and promotion of angiogenesis [25]. Lastly, administration of human W8B2+ CSC into rat hearts 1 week post MI improved cardiac function and reduced scar tissue formation [35].
SP progenitor cells differentiate into cells expressing sarcomeric proteins including troponin and cardiac α-actinin [36]. Flk-1+ cells can give rise to myocardial, endothelial, and smooth muscle lineages, and their concentration in the circulation increases in humans during an MI [37, 38]. Although Isl1+ cells are capable of differentiating into mature cardiomyocytes, they can only be extracted from neonatal tissue [39]. C-kit+ cells migrate through infarcted myocardium, give rise to cardiomyocytes, and reduce oxidative stress and apoptosis in cardiac and noncardiac cell populations [40, 41]. Sca1+ progenitors are found in myocardial stromal tissue, can be differentiated into myocardium, smooth muscle, and endothelial cells, and their absence leads to myocardial contractile dysfunction in rodents [42,43,44]. Rodent SSEA+ cells express surface markers which signal cardiomyogenic differentiation potential, form beating colonies when co-cultured with primary cardiomyocytes, and induce myocardial regeneration and functional improvement post MI in animal studies [45]. GCP are isolated from the epicardial/subepicardial hypoxic environment; they express all cardiac stem cells markers and differentiate into endothelial, smooth muscle, and cardiac lineages [46].
In the clinics, skeletal myoblasts were the first cell type to be transferred to human hearts. Early clinical trials focused mainly on bone marrow-derived cells including unselected progenitor/stromal/hematopoietic cells, with a gradual transition to more specific cellular populations including hematopoietic stem and progenitor (CD34+, CD133+) cells and later MSC. More recently, the focus of trials has shifted to various cardiac-committed cell types, including C-kit+ and cardiosphere-derived cells, especially given their increased preclinical success. Embryonic-derived early cardiovascular cells are the most recent cellular type to be examined, and the first trial using iPSC-derived cardiomyocytes is in the early planning phase [22, 47]. Treatment and delivery models for post-acute MI myocardial salvage and ischemic cardiomyopathy regeneration overlap, mononuclear cells have seen a larger success in post-MI studies, while CSC and embryonic-derived early myocardial progenitors have been more extensively studied in ischemic cardiomyopathy trials. This chapter will focus on the specific results of clinical trials in early post-MI patients.
14.2.3 Paracrine Factors, Exosomes, and Direct Cellular Reprogramming
Despite showing some clinical efficacy, stem cell therapy is associated with several important limitations including immune rejection, tumorigenicity, and arrhythmogenicity. In addition, few cells survive after transplantation despite improved myocardial function, suggesting that the mechanism of their action is predominantly paracrine in nature [48]. A recent study of ischemia/reperfusion injury in rodents showed that intracardiac injection of two separate adult-derived stem cells improved cardiac function without altering the number of new cardiomyocytes. The proposed mechanism for this improvement was selective induction of CCR2+ and CX3CR1+ macrophages, resulting in altered fibroblast activity, extracellular matrix (ECM) content, and enhanced mechanical properties [49]. In order to circumvent these obstacles, researchers have begun to study stem cell-derived paracrine factors, exosomes, and cells directly reprogrammed into cardiac progenitors, although no such studies have yet entered clinical trials, despite animal studies showing promising results.
MSC-derived growth factors and cytokines derived from cell culture supernatants have been shown to decrease inflammation, decrease myocyte apoptosis, recruit endogenous stem cells, and decrease infarct size [25]. In further animal and in vitro studies, MSC-conditioned medium increased neovascularization and fibrosis while improving cardiomyocyte contractility [50].
Exosomes are 40–100-micron vesicles released from cells by fusion with cellular membranes and carry mRNA, miRNA, as well as antiapoptotic and proangiogenic proteins. Exosomes are involved in cell signaling, mediate stem cell paracrine effects, and improve resident cardiac stem cell function without the downsides associated with direct cellular use [48]. In murine models, ESC-derived exosomes increased cardiomyocyte proliferation, upregulated the number of cardiac progenitor cells, and increased cardiac repair following ischemic injury. MSC-derived exosomes also reduced the size of postischemic infarcts, in animal models, via increased cardiac progenitor cell proliferation and decreased fibroblast proliferation [51, 52]. In addition, MSC-derived exosomal miRNA upregulated angiogenesis in post-infarct ischemia [48].
One of the major challenges associated with iPSC use in clinical trials include their tumorigenic potential in an undifferentiated state. Accordingly, new protocols have been designed to directly reprogram cells via induction of lineage-specific factors, without passage through a pluripotent and tumorigenic state [53]. For example, three factors including Gata4, Mef2c, and Tbx5 reprogram cardiac fibroblasts into induced cardiomyocytes. Further addition or modification of reprogramming factors microRNAs has been shown to promote reprogramming efficiency and maturation [54]. In vivo reprogramming of cells following acute MI in animal models has been reported and resulted in improved cardiac function and reduced fibrosis [55]. Most recently, direct in vivo reprogramming has been achieved without genomic integration of viral DNA with the use of a Sendai virus vector, which remains outside of the nucleus [56].
14.2.4 Lineage-Specific Considerations
The advantages and drawbacks of specific cellular subtypes in post-MI regenerative therapy are summarized in Table 14.1. Skeletal muscle cells are easier to obtain although likely only provide structural benefits, as opposed to forming new cardiac tissue, secondary to lack of transdifferentiation. In addition, 90% of injected cells die within a few days, and higher cellular counts are arrhythmogenic [24].
Stem cell sources can be divided into three main groups: embryonic, induced, and adult. Embryonic stem cells are pluripotent and can differentiate into all three germ layers and have genomic stability and good differentiation and proliferative capacity [57]. They are, however, derived from human blastocysts and require the destruction of embryos to attain, raising ethical dilemmas, in addition to having tumorigenic and immunogenic potential [58, 59]. In human and rodent studies, it was noted that transplanted embryonic stem cells generated small numbers of cardiomyocytes [60]. As they are reprogrammed in vitro from adult cells, iPSC avoid the ethical dilemmas associated with embryonic stem cells. Differentiation of iPSC into adult cells is at times inefficient, and they are teratogenic in their undifferentiated states. Furthermore, cells that are derived via viral transfection suffer from genomic instability [61]. Adult-derived cardiac stem cells also avoid ethical dilemmas associated with embryonic stem cells, and they carry a lower risk of immune rejection. However, they are obtained via invasive techniques and have a limited regeneration potential [62].
Mesenchymal stem cells are adult fibroblast-like cells and can differentiate into osteoblasts, adipocytes, and cardiomyocytes, among others [63]. Mesenchymal stem cells can be extracted from peripheral blood, bone marrow, dental pulp, placenta, umbilical cord, or adipose tissue with minimally invasive biopsy. They can self-renew, proliferate, and differentiate, as well as promote growth of adjacent tissue via strong paracrine signaling pathways [64]. MSC have immunosuppressive properties; they decrease the immune response by inhibiting T-cell proliferation and cytotoxicity while increasing the production of regulatory T cells. Drawbacks of MSC include small number in bone marrow and blood, as well as source-dependent variation [25].
Hematopoietic stem cells are multipotent cells, with capacity to differentiate into multiple lineages including cardiomyocytes and endothelial cells [65]. Although they can be harvested from peripheral blood and bone marrow, bone marrow yields are higher [66]. Hematopoietic stem cells are perfect regenerative candidates as they can achieve myogenesis and angiogenesis concomitantly, although their low numbers, difficult in vitro maintenance, and unknown signaling pathways need to be improved [66]. Endothelial progenitor cells are also found in the bone marrow and peripheral blood although in very low concentrations. They can differentiate into endothelial cells and participate in angiogenesis [67]. The number of circulating EPC increases with myocardial ischemia and cytokine release, infiltrating the injured myocardium and possibly differentiating into myocytes [68, 69].
14.3 Stem Cell Delivery Methods
14.3.1 Delivery Methods in Humans
The ideal delivery platform for stem cell therapy in cardiac regeneration should use a noninvasive technique that directly delivers cells to the site of infarct, to prevent cellular loss via aberrant homing. The carrier vehicle for cells should promote survival in the ischemic environment, facilitate retention and promote stem cell differentiation, augment paracrine effects, and protect native myocardium from scarring and arrhythmias [70]. Despite continued studies predominantly in animal studies, no such vehicle exists for use in clinical trials. At present, stem cell delivery can be accomplished via intravenous injection, intracoronary infusion, direct epicardial and endocardial injection, as well as topical application at the time of surgery [24]. Peripheral intravenous (IV) injection is by far the least invasive, though studies examining homing of radioactively labeled bone marrow cells into infarcted myocardium did not reveal any signal in the heart [71]. Intracoronary and intramyocardial delivery is by far the most commonly used methods reported in clinical trials [72]. Intracoronary (IC) infusion is less invasive than intramyocardial injection and can be achieved in an antegrade or retrograde fashion. IC delivery is also less arrhythmogenic and has been associated with a modest improvement in EF and infarcted area size [72]. Despite these benefits, IC delivery results in delivery of only 1.3–2.6% of cells into the infarcted myocardium, with the majority of cells circulating to the liver or spleen [71]. In addition, IC injection depends on patency of coronary arteries and is associated with a small risk of embolization [73, 74]. Intramyocardial delivery (IM) of cells facilitates their delivery to target tissues and can be accomplished via transepicardial, transendocardial, or transcoronary routes [57]. Transepicardial injection requires direct exposure of the heart, and all intramuscular injections are associated with ventricular arrhythmias [75].
14.3.2 Implantable and Injectable Systems
Cellular scaffolds and hydrogels enhance stem cell survival, and while hydrogels can retain cells at desired locations, scaffolds provide mechanical support to adjacent structures; unfortunately, both require invasive topical application [76, 77]. In rodent studies, human bone marrow CD133+ cells delivered in collagen patches increased local angiogenesis, though the cells themselves failed to differentiate into cardiomyocytes [78]. In addition to collagen, multiple other substrates mimic the ECM of the heart, including polyurethane (PU), poly(ester urethane) (PEU), polyester urethane urea (PEUU), and poly(glycerol sebacate) (PGS) [24]. Animal studies of biodegradable PU patches promoted the contractile phenotype of smooth muscle cells and improved cardiac remodeling [79].
Hydrogels composed of materials such as fibrin, Matrigel, alginate, and polyethylene glycol can all be modified to resemble the physical properties of cardiac tissue [24]. ECM and collagen containing hydrogels allowed for differentiation of human ESC into functional cardiomyocytes in vitro, and cell-impregnated alginate hydrogels delivered to murine hearts reduced left ventricular remodeling [80, 81]. Engineered heart tissue (EHT) has been developed from type I collagen and neonatal heart tissue. When sutured onto rat hearts in vivo, this tissue becomes electrically integrated and perfused [82, 83]. Finally, engineered heart muscle has been developed by ESC-derived cardiomyocytes onto EHT [84].
To overcome the invasive methods required for scaffold and hydrogel delivery, gelling systems based on materials including fibrin glue, collagen, Matrigel, hyaluronic acid, and alginate have been developed that undergo a fluid-to-solid transition when in vivo, allowing catheter-based delivery [24]. Self-assembling RAD16-II scaffolds induced angiogenesis, retained myocytes, and promoted ESC differentiation into MHC-positive cells [85]. Catheter-delivered, collagen-encapsulated bone marrow cells showed improved LV function, and vascularization and self-assembling peptides loaded with insulin-like growth factor (IGF) allowed for sustained release of paracrine factors [86, 87]. Acellular alginate is undergoing clinical trials to prevent ventricular remodeling [24].
14.4 Clinical Trials of Post-MI Regeneration
14.4.1 Trial Design
A query of completed clinical trials in post-acute MI stem cell therapy shows that approximately 28 studies have been completed thus far (Table 14.2) [88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119]. The number of patients randomized varied from 20 to 250 in each trial. The intracoronary route of cell delivery after initial diagnostic and therapeutic PCI, used in 25 out of 29 trials, was the most widely used method of cell delivery. One study delivered cells both via the intracoronary and intramyocardial routes concomitantly, one study injected cells intramyocardially at the time of CABG, and two studies delivered cells peripherally via intravenous injection.
Autologous, rather than allogenic, cells were most commonly used, with just five studies employing allogenic sources (Table 14.2). Autologous bone marrow (BM)-derived mononuclear cells were the most commonly studied, followed by autologous bone marrow-derived unselected progenitor cells. Several studies further sorted out autologous bone marrow-derived hematopoietic, endothelial, endothelial/cardiac, and early progenitor cells based on differential expression of various combinations of cell surface markers including CD34, CD45, CD133, CXCR4, among others . Less common cell sources included autologous bone marrow-derived MSC from commercially available products, allogenic Wharton’s jelly-derived MSC, and autologous peripheral blood stem cells. Although more extensively studied in the context of heart failure, as compared with acute MI, autologous cardiosphere-derived stem cells and allogenic cardiac stem cells have also been examined.
The timing of cell delivery and number of cells varied widely across studies. Despite all being acute post-MI models, therapy was delivered anywhere from less than 24 hours to several months post-initial therapeutic PCI. Although the ideal timing of cell delivery has not yet been standardized, the majority of studies implemented the therapeutic intervention within 10 days of PCI. Comparison of early (3–6 weeks) versus late (3–4 months) delivery did not change the primary outcome, increased left ventricular ejection fraction (LVEF), and decreased infarct size [94]. Final cell count delivered differed significantly between and often within studies; all studies used a magnitude of cells on the order of millions, in the range of 1.9–1300 million cells. Although the majority of studies used a fixed number across participants, several studies used weight-based dosing of 0.5–five million cells/kg. In preparations containing mixed cell subtypes, such as nucleated and mononuclear cells, the percentage of cells between subjects varied to a small degree.
14.4.2 Trial Outcomes
Comparison of outcomes across studies is difficult due to the lack of standardization of timing, inclusion criteria, cell number, and type. Despite these limitations, a generalization can be made that stem cell treatment is associated with only a modest improvement in outcome , as only 64% of the studies examined showed efficacy. Autologous bone marrow-derived mononuclear cells, although most studied, were associated with the least favorable outcomes. Six out of 12 patient cohorts treated with mononuclear cells did not have any significant improvement in any outcome. Three studies showed improvement in LVEF. One study showed decreased left ventricular end-diastolic volume (LVEDV), and one study showed reduced infarct size. Mononuclear cell treatment was also associated with decreased infarct size regardless of treatment timing (3–6 weeks versus 3–4 months) in one study and decreased systolic wall thickening in another. LVEF improved in one study only in a subset of patients with initial EF < 37%.
Autologous BM-derived stem cells, including hematopoietic, endothelial, cardiac/hematopoietic, and early progenitor cells, showed by far the highest efficacy rates with 90% of studies showing a significant increase in various outcome parameters. Six studies reported increased LVEF, up to as much as 18 months after treatment. Perhaps most notably, treatment with a combination of CD34+/CD45+ and CD34+/CD133+/CD45+ cells reduced combined death, recurrent MI, and any revascularization procedures at 1 year. Other outcomes associated with BM-derived stem cell treatment included reduced myocardial infarct size, recovery of regional systolic function and myocardial deformation, improved perfusion, decrease in end-systolic volume (ESV), improved myocardial salvage index, decreased systolic wall thickening and nonviable segments, as well as increased LVEF in patients with baseline EF < 37%.
Autologous BM-MSC improved LVEF in two studies, while allogenic BM-MSC were only efficacious 50% of the time, though they were only used in two studies. They increased LVEF and global symptom score in patients at 6 months in one cohort, although no change in LVEF or perfusion was observed in another study at the same time point. Wharton’s jelly-derived MSC were associated with a higher absolute increase in myocardial viability and perfusion at 4 months, as well as increased LVEF and decreased end-systolic and diastolic volumes at 18 months.
Autologous cardiosphere-derived stem cells reduced scar mass and increased viable heart mass, regional contractility, and regional systolic wall thickening, though there was no appreciable change in LVEF at 12 months. Interestingly, allogenic cardiac stem cells were not associated with a change in infarct size or LV remodeling. Peripheral blood stem cells mobilized with granulocyte colony-stimulating factor (GCSF) increased exercise capacity, myocardial perfusion, and systolic function, although the use of GCSF was associated with a higher rate of in-stent stenosis at 6 months. Beneficial effects based on cellular type are summarized in Table 14.3 and Fig. 14.1.
Stem cell types and benefits in treatment after MI. SC, stem cell; MNC, mononuclear cell; MSC, mesenchymal stem cell; LVEF, left ventricular ejection fraction; MI, myocardial infarction; ESV, end-systolic volume; LVEDV, left ventricular end-diastolic volume; EDV, end diastolic volume; CDSC, cardiosphere-derived stem cell
14.4.3 Stem Cell Therapy in Ischemic Cardiomyopathy
Although the primary focus of stem cell therapy remains to prevent myocardial loss and allow for regeneration of tissue immediately after an MI, clinical trials are also underway to evaluate the ability of stem cells to remuscularize and reactivate innate cardiac regeneration pathways in models of heart failure secondary to chronic cardiomyopathy [22]. Similar to studies targeting treatment of acute MI, cells evaluated in ischemic cardiomyopathy include skeletal myoblasts , bone marrow-derived unselected and selected stem cells, MSC, embryonic stem cells, and cardiac-committed progenitor cells [22].
Skeletal myoblasts do not appear to improve LVEF [120]. Unselected bone marrow stem cells are less commonly used in HF models although appear to have as little efficacy as when used in acute MI trials [121,122,123,124,125]. Bone marrow-derived hematopoietic stem and progenitor cells appear to improve unstable angina, but their efficacy in HF is less established [126,127,128,129]. Similar to post-MI studies, MSC appear to be among the most efficacious in HF models [130,131,132,133]. Cardiac stem cells including KIT+ and cardiosphere-derived cells (CDC) both appear to show some efficacy in clinical trials [134, 135]. Transplantation of embryonic stem cell-derived cardiac progenitor cells appears to confer a symptomatic benefit, although a very small number of patients have been evaluated thus far, necessitating further trials [136]. Analysis of completed clinical trials in chronic HF suggests MSC and CSC as the most promising cell types, and although some efficacy has been established, many more clinical trials and optimal delivery vehicles are needed before stem cell therapy becomes standard of care.
14.5 Limitations of Stem Cells Therapy
A substantial limitation of stem cell therapy post-MI is the low homing, retention, and differentiation rate of cells in the ischemic microenvironment of the infarcted heart [137]. Human studies have shown a 39% cellular retention rate just 1 hour following transplantation that is attributable to high rates of apoptosis [71]. The high rate of cell death after transplantation can be attributed to inflammation, mechanical injury, hypoxia, and reperfusion injury [138]. Furthermore, loss of matrix attachment during cell preparation and following injection contributes to programmed cell death [139]. Ischemia is a major hurdle for stem cell populations to differentiate into cardiomyocytes, particularly ones that become electromechanically integrated [24].
Although most clinical trials demonstrate safety following stem cell transfer, with only a few complications reported, animal studies have shown increased risk of ventricular arrhythmias following human cardiomyocyte transfer into guinea pigs and nonhuman primates [140, 141]. In addition, isolation of adequate quantity of stem cells, expansion, and optimal delivery methods that allow for cell retention and differentiation are lacking [28]. Although peripheral and bone marrow stem cells are easier to harvest, attaining adequate number of organ-derived cells, such as cardiac stem cells, is invasive and often low yield [28]. Several clinical trials have also shown that transplanted cells may not be capable of integration and electrochemical coupling, suggesting that their effects are predominantly paracrine in nature and may not add directly to myocyte mass [142].
Meaningful decisions and meta-analyses of clinical trial data are difficult to interpret and synthesize in light of heterogeneity of trial design and reporting. Clinical trials completed thus far have varying, though usually low, number of participants. Primary outcomes measured vary from study to study , and some lack diverse clinical assessment tools. Inclusion criteria, stem cell type and number, delivery methods, and timing vary greatly across trials. Some studies lack placebo groups, making them prone to observation bias, while others evaluate safety only without efficacy. Variable outcomes across studies can easily be attributed to the heterogeneous number and quality of cells used [143].
14.5.1 Modifications to Enhance Cell Function
Multiple strategies including in vitro cellular preconditioning or reprogramming via environmental, pharmacological, and genetic means have been explored in order to increase in vivo cell survival [137]. These strategies include culturing cells under ischemic conditions, supplementing culture medium with growth factors, as well as transfecting cells with proangiogenic and anti-apoptotic factors [57]. Culturing MSC in low oxygen conditions prior to transplant activates survival pathways, upregulates pro-survival genes, increases anti-apoptotic genes including Akt and eNOS, and upregulates pro-angiogenic cytokines including vascular endothelial growth factor (VEGF) [144]. Additionally, hypoxia allows cells to preserve stemness and promote differentiation and proliferation in vivo [145]. In vitro burst exposure of cells to low levels of oxidative stress and thermal shock treatment also improves cell viability and functional outcomes [146, 147].
Preconditioning of cells with several therapeutic drugs increased secretion of growth factors, including vascular endothelial growth factor (VEGF), angiopoietin-1α (Ang-1α), stromal cell-derived factor-1 (SDF-1), hepatocyte growth factor (HGF), and IGF [148]. Several mitochondrial potassium channel opening drugs, including pinacidil and diazoxide, suppress apoptosis and increase cell survival in ischemic conditions [149, 150]. In one study, treatment of cardiac stem cells with hydrogen peroxide increased endothelial and vascular smooth muscle gene expression and angiogenesis [25]. In vivo treatment with statins increased cell survival and differentiation, while in vitro treatment improved function of endothelial progenitor cells [151, 152]. Pre-treatment of several cell lines with oxytocin improves their response to oxidative stress and differentiation into cardiomyocytes and vascular cells [153, 154]. Multiple other drug classes including trimetazine, β-mercaptoethanol, caspase inhibitors, 5-Azacytidine, and the kinase inhibitor Imatinib have been used in vitro to increase cell viability, confer resistance to oxidative injury, increase cellular engraftment, and prime cellular differentiation toward a cardiac fate, respectively [155,156,157].
Genetic manipulation of stem cells prior to transfer is another strategy used to improve efficacy, as transgenes can be targeted to release pro-angiogenic and chemoattractant factors, as well as anti-apoptotic proteins. For example, insertion of the pro-survival gene Pim-1 kinase into cardiac stem cells decreased infarct scar mass in a pig model [25]. Transformation of stem cells with IGF-1, which induces expression of survival genes, enhanced survival, engraftment, and differentiation [158]. IGF-1-transformed MSC showed efficacy in improving ejection fraction in animal studies. Overexpression of Ang-1, HGF, VEGF, and MyoD in post-MI studies have consistently shown improved cellular retention, likely secondary to increased angiogenic potential of pre-treated cells [159,160,161]. Akt-modified bone marrow-derived MSC survival is upregulated via secretion of numerous growth factors, including bFGF, HGF, IFG-1, and VEGF [162].
Because adhesion to an extracellular matrix is important for the survival of several stem cells, notably MSC, injection of cells and lack of healthy ECM in infarcted hearts potentiate apoptosis. To address this, overexpression of tissue transglutaminase in MSC increased survival leading to improved restoration of cardiac function [163]. Transfection of integrin-linked kinase (ILK), which contributed to cell adhesion and ECM assembly, improves cellular survival in hypoxic conditions and reduces infarct size in animal studies [164].
Resident stem cells become senescent and lose their regenerative capacity with age, resulting in reduced proliferation, differentiation, and metabolic activity [165]. These changes are driven by telomere shortening and upregulation of p53 genes [166]. For example, MSC derived from older patients are not as efficacious in post-MI models as those derived from younger patients [167]. Strategies to combat senescence have been examined and include modification of human cardiac progenitor cells with Pim-1 and upregulation of the WNT/β-catenin signaling pathway, both of which result in improved cellular function [168, 169].
14.6 Future Directions
Although stem cell therapy after MI is gaining momentum with promising initial results, multiple limitations must be overcome to realize the full potential that cellular therapy has to offer. The optimal cell source for use in clinical trials must be determined. Although embryonic stem cells confer immune privilege, they are associated with ethical dilemmas and are teratogenic in undifferentiated forms. While embryonic stem cell-derived cardiac precursors eliminate teratogenic potential, their differentiation protocols currently produce low yields and must be improved. Resident cardiac stem cells are difficult to harvest and are low in number. Bioreactors and devices to standardize and improve differentiation yields are on the horizon, although further research needs to be accomplished [170].
iPSC are an ideal cell candidate for clinical translation since they are derived from adult somatic cells via noninvasive techniques and can repopulate any cardiac lineage. Although the first iPSC clinical trial is currently being planned, nonviral transfection protocols to derive iPSC cells must be optimized to prevent genomic instability. Furthermore, differentiation protocols and elimination of undifferentiated cells via induced cell apoptosis must ensure patient safety. Paracrine effects of cell therapy must be defined more clearly, and the potential of exosomes must be studied, as use of exosomes alone without cellular transfer could realize the full potential of iPSC cells.
Cell survival and homing, particularly with intravenous and intracoronary routes, are extremely low, with cell loss being exacerbated by the ischemic post-infarct environment. Preconditioning of cells prior to transfer via genetic modifications and drug treatments, as well as improved homing mechanisms, must be developed to improve the number of cells participating in repair. In addition, methods of preventing resident stem cell senescence and improve mobilization must be elucidated.
Optimal delivery methods for stem cell treatment must be redesigned. Although intramyocardial injections deliver cells directly to infarcted areas, they are invasive and associated with generating pro-arrhythmogenic foci. Intracoronary and intravenous injections suffer from poor cellular homing. While patches and scaffolds afford the added benefit of maintaining an optimal scaffold, they can only be delivered at the time of surgery. Gelling systems loaded with cytokines and pro-survival proteins must be refined to allow for noninvasive delivery. In addition, three-dimensional (3D) and bioprinted cellularized vascular constructs are currently being developed.
Currently, protocols for clinical trials of stem cell therapy vary greatly and lack standardization. In order to make meaningful comparisons and interpretations across trials cell type, delivery methods and timing, as well as measured outcomes, must be standardized.
14.7 Conclusion
Myocardial infarction and ischemic cardiomyopathy confer significant morbidity and mortality, yet despite best medical care, many patients who suffer from an MI go on to develop heart failure, secondary to myocardial necrosis and pathologic myocardial remodeling. The population of resident cardiac stem cells available to replenish lost cells is low and easily overwhelmed by ischemia. Although the design of clinical trials is not uniform, and comparisons cannot be easily made, delivery of both endogenous and exogenous stem cells to ischemic myocardium has shown some efficacy at reducing infarct size and improving long-term function.
Several issues are currently being addressed in order to optimize stem cell efficacy. In addition to standardizing cellular type, delivery method, and timing, clinical trials must focus on similar outcomes. The optimal cell type and differentiation methods are being determined, with iPSC and exosomes holding great promise. The most direct, least invasive delivery method and improvement of cell homing and survival are yet to be overcome. Despite all of these obstacles, stem cell therapy holds great promise in post-MI regeneration.
Abbreviations
- 3D:
-
Three dimensional
- ADSC:
-
Adipose-derived stem cell
- AMI:
-
Acute myocardial infarction
- Ang-1α:
-
Angiopoietin-1α
- BM:
-
Bone marrow
- BM-MNC:
-
Bone marrow-derived mononuclear cell
- BM-SC:
-
Bone marrow stem cells
- CABG:
-
Coronary artery bypass grafting
- CDC:
-
Cardiosphere-derived cells
- CSC:
-
Cardiac stem cell
- CVD:
-
Cardiovascular disease
- ECM:
-
Extracellular matrix
- EDV:
-
End-diastolic volume
- EF:
-
Ejection fraction
- EHT:
-
Engineered heart tissue
- EPC:
-
Endothelial progenitor cell
- ESC:
-
Embryonic stem cell
- ESV:
-
End-systolic volume
- FGF:
-
Fibroblast growth factor
- Flk1:
-
Fetal liver kinase 1
- GCP:
-
Glycolytic cardiac progenitor
- GCSF:
-
Granulocyte colony-stimulating factor
- HF:
-
Heart failure
- HGF:
-
Hepatocyte growth factor
- IC:
-
Intracoronary
- IGF:
-
Insulin-like growth factor
- ILK:
-
Integrin-linked kinase
- IM:
-
Intramyocardial
- iPSC:
-
Induced pluripotent stem cell
- Isl1:
-
Insulin gene enhancer protein-1
- IV:
-
Intravenous injection
- LV:
-
Left ventricular
- LVEDV:
-
Left ventricular end-diastolic volume
- LVEF:
-
Left ventricular ejection fraction
- LVESV:
-
Left ventricular end-systolic volume
- MI:
-
Myocardial infarction
- MMP:
-
Matrix metalloproteinase
- MSC:
-
Mesenchymal stem cell
- NSTEMI:
-
Non-ST segment elevation myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- PEU:
-
Polyester urethane
- PEUU:
-
Polyester urethane urea
- PGS:
-
Polyglycerol sebacate
- PU:
-
Polyurethane
- SC:
-
Stem cell
- Sca1:
-
Stem cell antigen-1
- SDF1:
-
Stromal cell-derived factor 1
- SMC:
-
Skeletal myoblast cell
- SP:
-
Side population
- SSEA:
-
Stage-specific embryonic antigen 1
- STEMI:
-
ST-segment elevation myocardial infarction
- VEGF:
-
Vascular endothelial growth factor
References
Smit M, Coetzee AR, Lochner A. The pathophysiology of myocardial ischemia and perioperative myocardial infarction. J Cardiothorac Vasc Anesth. 2019. https://doi.org/10.1053/j.jvca.2019.10.005.
Benjamin Emelia J, Virani Salim S, Callaway Clifton W, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492.
Dalys GBD, Collaborators H, Murray CJL, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet (London, England). 2015;386(10009):2145–91.
Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham heart study and the epidemiology of cardiovascular disease: a historical perspective. Lancet (London, England). 2014;383(9921):999–1008.
White H, Thygesen K, Alpert JS, Jaffe A. Universal MI definition update for cardiovascular disease. Curr Cardiol Rep. 2014;16(6):492.
Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368(21):2004–13.
2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. Executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Catheter Cardiovasc Interv. 2013;82(1):E1–E27.
Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the Management of Patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;64(24):e139–228.
Stephens NR, Restrepo CS, Saboo SS, Baxi AJ. Overview of complications of acute and chronic myocardial infarctions: revisiting pathogenesis and cross-sectional imaging. Postgrad Med J. 2019;95(1126):439.
Mozaffarian D, Benjamin Emelia J, Go Alan S, et al. Executive summary: heart disease and stroke statistics—2015 update. Circulation. 2015;131(4):434–41.
Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–19.
Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81(4):1161–72.
Cleutjens JPM, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995;27(6):1281–92.
Warren SE, Royal HD, Markis JE, Grossman W, Mckay RG. Time course of left ventricular dilation after myocardial infarction: influence of infarct-related artery and success of coronary thrombolysis. J Am Coll Cardiol. 1988;11(1):12–9.
Mckay RG, Pfeffer MA, Pasternak RC, et al. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation. 1986;74(4):693–702.
Qasim M, Arunkumar P, Powell HM, Khan M. Current research trends and challenges in tissue engineering for mending broken hearts. Life Sci. 2019;229:233–50.
Kajstura J, Urbanek K, Perl S, et al. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107(2):305–15.
Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–76.
Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98.
Kajstura J, Rota M, Cappetta D, et al. Cardiomyogenesis in the aging and failing human heart. Circulation. 2012;126(15):1869–81.
Arbatlı S, Aslan GS, Kocabaş F. Stem cells in regenerative cardiology. In: Turksen K, editor. Cell biology and translational medicine, Volume 1: Stem cells in regenerative medicine: advances and challenges. Cham: Springer International Publishing; 2018. p. 37–53.
Menasché P. Cell therapy trials for heart regeneration — lessons learned and future directions. Nat Rev Cardiol. 2018;15(11):659–71.
Wang WE, Chen X, Houser SR, Zeng C. Potential of cardiac stem/progenitor cells and induced pluripotent stem cells for cardiac repair in ischaemic heart disease. Clinical science (London, England : 1979). 2013;125(7):319–27.
Carvalho E, Verma P, Hourigan K, Banerjee R. Myocardial infarction: stem cell transplantation for cardiac regeneration. Regen Med. 2015;10(8):1025–43.
Henning RJ. Current status of stem cells in cardiac repair. Futur Cardiol. 2018;14(2):181–92.
Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12.
Armiñán A, Gandía C, García-Verdugo JM, et al. Mesenchymal stem cells provide better results than hematopoietic precursors for the treatment of myocardial infarction. J Am Coll Cardiol. 2010;55(20):2244–53.
Sun Q, Zhang Z, Sun Z. The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes Dis. 2014;1(1):113–9.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Ye L, Chang Y-H, Xiong Q, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15(6):750–61.
Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci. 2007;104(35):14068.
Hosoda T, D’amario D, Cabral-Da-Silva MC, et al. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci U S A. 2009;106(40):17169–74.
Castaldo C, Di Meglio F, Nurzynska D, et al. CD117-positive cells in adult human heart are localized in the subepicardium, and their activation is associated with Laminin-1 and α6 integrin expression. Stem Cells. 2008;26(7):1723–31.
Oskouei BN, Lamirault G, Joseph C, et al. Increased potency of cardiac stem cells compared with bone marrow mesenchymal stem cells in cardiac repair. Stem Cells Transl Med. 2012;1(2):116–24.
Zhang Y, Sivakumaran P, Newcomb AE, et al. Cardiac repair with a novel population of mesenchymal stem cells resident in the human heart. Stem Cells. 2015;33(10):3100–13.
Liang SX, Tan TYL, Gaudry L, Chong B. Differentiation and migration of Sca1+/CD31− cardiac side population cells in a murine myocardial ischemic model. Int J Cardiol. 2010;138(1):40–9.
Kattman SJ, Huber TL, Gordon m K. Multipotent Flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11(5):723–32.
Suresh R, Chiriac A, Goel K, et al. CXCR4+ and FLK-1+ identify circulating cells associated with improved cardiac function in patients following myocardial infarction. J Cardiovasc Transl Res. 2013;6(5):787–97.
Laugwitz K-L, Moretti A, Lam J, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433(7026):647–53.
Kazakov A, Meier T, Werner C, et al. C-kit+ resident cardiac stem cells improve left ventricular fibrosis in pressure overload. Stem Cell Res. 2015;15(3):700–11.
Sullivan KE, Burns LJ, Black LD. An in vitro model for the assessment of stem cell fate following implantation within the infarct microenvironment identifies ISL-1 expression as the strongest predictor of c-kit+ cardiac progenitor cells’ therapeutic potential. J Mol Cell Cardiol. 2015;88:91–100.
Bailey B, Fransioli J, Gude NA, et al. Sca-1 knockout impairs myocardial and cardiac progenitor cell function. Circ Res. 2012;111(6):750–60.
Linke A, Müller P, Nurzynska D, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102(25):8966–71.
Uchida S, De Gaspari P, Kostin S, et al. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Rep. 2013;1(5):397–410.
Ott HC, Matthiesen TS, Brechtken J, et al. The adult human heart as a source for stem cells: repair strategies with embryonic-like progenitor cells. Nat Clin Pract Cardiovasc Med. 2007;4(1):S27–39.
Kocabas F, Mahmoud AI, Sosic D, et al. The hypoxic Epicardial and Subepicardial microenvironment. J Cardiovasc Transl Res. 2012;5(5):654–65.
Sadahiro T. Cardiac regeneration with pluripotent stem cell-derived cardiomyocytes and direct cardiac reprogramming. Regenerat Therap. 2019;11:95–100.
Moghaddam AS, Afshari JT, Esmaeili S-A, Saburi E, Joneidi Z, Momtazi-Borojeni AA. Cardioprotective microRNAs: lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis. 2019;285:1–9.
Vagnozzi RJ, Maillet M, Sargent MA, et al. An acute immune response underlies the benefit of cardiac stem-cell therapy. Nature. 2019; https://doi.org/10.1038/s41586-019-1802-2.
Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair. 2008;1(1):4–4.
Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–22.
Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301–12.
Fu X, Khalil H, Kanisicak O, et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest. 2018;128(5):2127–43.
Ieda M, Fu J-D, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–86.
Jayawardena TM, Egemnazarov B, Finch EA, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110(11):1465–73.
Miyamoto K, Akiyama M, Tamura F, et al. Direct in vivo reprogramming with sendai virus vectors improves cardiac function after myocardial infarction. Cell Stem Cell. 2018;22(1):91–103.e105.
Parizadeh SM, Jafarzadeh-Esfehani R, Ghandehari M, et al. Stem cell therapy: a novel approach for myocardial infarction. J Cell Physiol. 2019;234(10):16904–12.
Lev S, Kehat I, Gepstein L. Differentiation pathways in human embryonic stem cell-derived cardiomyocytes. Ann N Y Acad Sci. 2005;1047(1):50–65.
Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–6.
Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227(2):271–8.
Gorecka J, Kostiuk V, Fereydooni A, et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res Ther. 2019;10(1):87.
Abbott JD, Giordano FJ. Stem cells and cardiovascular disease. J Nucl Cardiol. 2003;10(4):403–12.
Thakker R, Yang P. Mesenchymal stem cell therapy for cardiac repair. Curr Treat Options Cardiovasc Med. 2014;16(7):323.
Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217(2):318–24.
Krause K, Schneider C, Kuck K-H, Jaquet K. REVIEW: stem cell therapy in cardiovascular disorders. Cardiovasc Ther. 2010;28(5):e101–10.
Asahara T, Kalka C, Isner JM. Stem cell therapy and gene transfer for regeneration. Gene Ther. 2000;7(6):451–7.
Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–53.
Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103(23):2776–9.
Badorff C, Brandes RP, Popp R, et al. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107(7):1024–32.
Perin EC, López J. Methods of stem cell delivery in cardiac diseases. Nat Clin Pract Cardiovasc Med. 2006;3(1):S110–3.
Hofmann M, Wollert Kai C, Meyer Gerd P, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111(17):2198–202.
Campbell NG, Suzuki K. Cell delivery routes for stem cell therapy to the heart: current and future approaches. J Cardiovasc Transl Res. 2012;5(5):713–26.
Moreira RDC, Haddad AF, Silva SA, et al. Injeção intracoronariana de células tronco após infarto do miocárdio: subestudo da microcirculação. Arq Bras Cardiol. 2011;97:420–6.
Vulliet PR, Greeley M, Halloran SM, Macdonald KA, Kittleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363(9411):783–4.
Fukushima S, Varela-Carver A, Coppen Steven R, et al. Direct Intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation. 2007;115(17):2254–61.
Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces Neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44(3):654–60.
Suuronen Erik J, Veinot John P, Wong S, et al. Tissue-engineered injectable collagen-based matrices for improved cell delivery and vascularization of ischemic tissue using CD133+ progenitors expanded from the peripheral blood. Circulation. 2006;114(1_supplement):I-138-I-144.
Pozzobon M, Bollini S, Iop L, et al. Human bone marrow-derived CD133+ cells delivered to a collagen patch on Cryoinjured rat heart promote angiogenesis and Arteriogenesis. Cell Transplant. 2010;19(10):1247–60.
Fujimoto KL, Tobita K, Merryman WD, et al. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol. 2007;49(23):2292–300.
Duan Y, Liu Z, O'neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J Cardiovasc Transl Res. 2011;4(5):605–15.
Leor J, Aboulafia-Etzion S, Dar A, et al. Bioengineered cardiac grafts. Circulation. 2000;102(suppl_3):Iii-56-Iii-61.
Naito H, Melnychenko I, Didié M, et al. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114(1_supplement):I-72-I-78.
Zimmermann W-H, Didié M, Wasmeier Gerald H, et al. Cardiac grafting of engineered heart tissue in Syngenic rats. Circulation. 2002;106(12_suppl_1):I-151-I-157.
Soong PL, Tiburcy M, Zimmermann W-H. Cardiac differentiation of human embryonic stem cells and their assembly into engineered heart muscle. Curr Protoc Cell Biol. 2012;55(1):–23.28.21.
Davis ME, Motion JPM, Narmoneva DA, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111(4):442–50.
Huang NF, Yu J, Sievers R, Li S, Lee RJ. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng. 2005;11(11–12):1860–6.
Davis ME, Hsieh PCH, Takahashi T, et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103(21):8155–60.
Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet (London, England). 2012;379(9819):895–904.
Traverse JH, Henry TD, Pepine CJ, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308(22):2380–9.
Traverse JH, Mckenna DH, Harvey K, et al. Results of a phase 1, randomized, double-blind, placebo-controlled trial of bone marrow mononuclear stem cell administration in patients following ST-elevation myocardial infarction. Am Heart J. 2010;160(3):428–34.
Sürder D, Manka R, Lo Cicero V, et al. Intracoronary injection of bone marrow–derived mononuclear cells early or late after acute myocardial infarction. Circulation. 2013;127(19):1968–79.
Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–86.
Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–21.
Gyöngyösi M, Lang I, Dettke M, et al. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: the MYSTAR prospective, randomized study. Nat Clin Pract Cardiovasc Med. 2009;6(1):70–81.
Schächinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI trial. J Am Coll Cardiol. 2004;44(8):1690–9.
Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow–derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–21.
Schächinger V, Erbs S, Elsässer A, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27(23):2775–83.
Lee J-W, Lee S-H, Youn Y-J, et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014;29(1):23–31.
Gao LR, Chen Y, Zhang NK, et al. Intracoronary infusion of Wharton's jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015;13:162.
Wöhrle J, Von Scheidt F, Schauwecker P, et al. Impact of cell number and microvascular obstruction in patients with bone-marrow derived cell therapy: final results from the randomized, double-blind, placebo controlled intracoronary stem cell therapy in patients with acute myocardial infarction (SCAMI) trial. Clin Res Cardiol. 2013;102(10):765–70.
Choudry F, Hamshere S, Saunders N, et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE-AMI clinical trial. Eur Heart J. 2016;37(3):256–63.
Naseri MH, Madani H, Ahmadi Tafti SH, et al. COMPARE CPM-RMI trial: Intramyocardial transplantation of autologous bone marrow-derived CD133+ cells and MNCs during CABG in patients with recent MI: a phase II/III, multicenter, placebo-controlled, randomized, double-blind clinical trial. Cell J. 2018;20(2):267–77.
Nair V, Madan H, Sofat S, et al. Efficacy of stem cell in improvement of left ventricular function in acute myocardial infarction--MI3 trial. Indian J Med Res. 2015;142(2):165–74.
San Roman JA, Sánchez PL, Villa A, et al. Comparison of different bone marrow–derived stem cell approaches in Reperfused STEMI: a multicenter, prospective, randomized, open-labeled TECAM trial. J Am Coll Cardiol. 2015;65(22):2372–82.
Quyyumi AA, Waller EK, Murrow J, et al. CD34+ cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161(1):98–105.
Huikuri HV, Kervinen K, Niemelä M, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008;29(22):2723–32.
Chullikana A, Majumdar AS, Gottipamula S, et al. Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy. 2015;17(3):250–61.
Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–8.
Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–209.
Kang H-J, Kim H-S, Zhang S-Y, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363(9411):751–6.
Quyyumi AA, Vasquez A, Kereiakes DJ, et al. PreSERVE-AMI: a randomized, double-blind, placebo-controlled clinical trial of intracoronary administration of autologous CD34+ cells in patients with left ventricular dysfunction post STEMI. Circ Res. 2017;120(2):324–31.
Miettinen JA, Ylitalo K, Hedberg P, et al. Determinants of functional recovery after myocardial infarction of patients treated with bone marrow-derived stem cells after thrombolytic therapy. Heart. 2010;96(5):362.
Miettinen JA, Salonen RJ, Niemelä M, et al. Effects of intracoronary infusion of bone marrow-derived stem cells on pulmonary artery pressure and diastolic function after myocardial infarction. Int J Cardiol. 2010;145(3):631–3.
Roncalli J, Mouquet F, Piot C, et al. Intracoronary autologous mononucleated bone marrow cell infusion for acute myocardial infarction: results of the randomized multicenter BONAMI trial. Eur Heart J. 2011;32(14):1748–57.
Fernández-Avilés F, Sanz-Ruiz R, Bogaert J, et al. Safety and efficacy of intracoronary infusion of allogeneic human cardiac stem cells in patients with ST-segment elevation myocardial infarction and left ventricular dysfunction. Circ Res. 2018;123(5):579–89.
Kim SH, Cho JH, Lee YH, et al. Improvement in left ventricular function with intracoronary mesenchymal stem cell therapy in a patient with Anterior Wall ST-segment elevation myocardial infarction. Cardiovasc Drugs Ther. 2018;32(4):329–38.
Herbots L, D’hooge J, Eroglu E, et al. Improved regional function after autologous bone marrow-derived stem cell transfer in patients with acute myocardial infarction: a randomized, double-blind strain rate imaging study. Eur Heart J. 2008;30(6):662–70.
Tendera M, Wojakowski W, Rużyłło W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre myocardial regeneration by intracoronary infusion of selected population of stem cells in acute myocardial infarction (REGENT) trial. Eur Heart J. 2009;30(11):1313–21.
Traverse JH, Henry TD, Vaughan DE, et al. LateTIME: a phase-II, randomized, double-blinded, placebo-controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Tex Heart Inst J. 2010;37(4):412–20.
Menasché P, Alfieri O, Janssens S, et al. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial. Circulation. 2008;117(9):1189–200.
Hendrikx M, Hensen K, Clijsters C, et al. Recovery of regional but not global contractile function by the direct Intramyocardial autologous bone marrow transplantation. Circulation. 2006;114(1_supplement):I-101-I-107.
Ang K-L, Chin D, Leyva F, et al. Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG alone. Nat Clin Pract Cardiovasc Med. 2008;5(10):663–70.
Pätilä T, Lehtinen M, Vento A, et al. Autologous bone marrow mononuclear cell transplantation in ischemic heart failure: a prospective, controlled, randomized, double-blind study of cell transplantation combined with coronary bypass. J Heart Lung Transplant. 2014;33(6):567–74.
Perin EC, Willerson JT, Pepine CJ, et al. Effect of Transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307(16)
Heldman AW, Difede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311(1):62–73.
Povsic TJ, Henry TD, Traverse JH, et al. The RENEW trial: efficacy and safety of Intramyocardial autologous CD34+ cell administration in patients with refractory angina. J Am Coll Cardiol Intv. 2016;9(15):1576–85.
Stamm C, Kleine H-D, Choi Y-H, et al. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133(3):717–25.e715.
Nasseri BA, Ebell W, Dandel M, et al. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J. 2014;35(19):1263–74.
Noiseux N, Mansour S, Weisel R et al., The IMPACT-CABG trial: a multicenter, randomized clinical trial of CD133+ stem cell therapy during coronary artery bypass grafting for ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2016;152(6):1582–8.e1582.
Mathiasen AB, Qayyum AA, Jørgensen E, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J. 2015;36(27):1744–53.
Bartunek J, Terzic A, Davison BA, et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J. 2017;38(9):648–60.
Butler J, Epstein Stephen E, Greene Stephen J, et al. Intravenous allogeneic mesenchymal stem cells for nonischemic cardiomyopathy. Circ Res. 2017;120(2):332–40.
Perin Emerson C, Borow Kenneth M, Silva Guilherme V, et al. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res. 2015;117(6):576–84.
Chugh AR, Beache GM, Loughran JH, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126(11 Suppl 1):S54–64.
Malliaras K, Makkar RR, Smith RR, et al. Intracoronary Cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol. 2014;63(2):110–22.
Menasché P, Vanneaux V, Hagège A, et al. Transplantation of human embryonic stem cell–derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol. 2018;71(4):429–38.
Der Sarkissian S, Lévesque T, Noiseux N. Optimizing stem cells for cardiac repair: current status and new frontiers in regenerative cardiology. World J Stem Cells. 2017;9(1):9–25.
Hodgetts SI, Beilharz MW, Scalzo AA, Grounds MD. Why do cultured transplanted myoblasts die in vivo? DNA quantification shows enhanced survival of donor male myoblasts in host mice depleted of CD4+ and CD8+ cells or NK1.1+ cells. Cell Transplant. 2000;9(4):489–502.
Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76(11):1352–64.
Chong JJH, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–7.
Shiba Y, Filice D, Fernandes S, et al. Electrical integration of human embryonic stem cell-derived cardiomyocytes in a Guinea pig chronic infarct model. J Cardiovasc Pharmacol Ther. 2014;19(4):368–81.
Leri A, Kajstura J, Anversa P, Frishman WH. Myocardial regeneration and stem cell repair. Curr Probl Cardiol. 2008;33(3):91–153.
Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010-2015). Stem Cell Res Ther. 2016;7(1):82.
Tang YL, Zhu W, Cheng M, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104(10):1209–16.
Theus MH, Wei L, Cui L, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210(2):656–70.
Zhang J, Chen G-H, Wang Y-W, et al. Hydrogen peroxide preconditioning enhances the therapeutic efficacy of Wharton’s Jelly mesenchymal stem cells after myocardial infarction. Chin Med J. 2012;125(19):3472–8.
Su C-Y, Chong K-Y, Chen J, Ryter S, Khardori R, Lai C-C. A physiologically relevant hyperthermia selectively activates constitutive hsp70 in H9c2 cardiac myoblasts and confers oxidative protection. J Mol Cell Cardiol. 1999;31(4):845–55.
Haider KH, Ashraf M. Chapter 15 – preconditioning approach in stem cell therapy for the treatment of infarcted heart. In: Tang Y, editor. Progress in molecular biology and translational science. Cambridge, MA: Academic Press. 2012. p. 323–56.
Sato T, Li Y, Saito T, Nakaya H. Minoxidil opens mitochondrial K(ATP) channels and confers cardioprotection. Br J Pharmacol. 2004;141(2):360–6.
Niagara Muhammad I, Haider Husnain K, Jiang S, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote Angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100(4):545–55.
Yang Y-J, Qian H-Y, Huang J, et al. Atorvastatin treatment improves survival and effects of implanted mesenchymal stem cells in post-infarct swine hearts. Eur Heart J. 2008;29(12):1578–90.
Assmus B, Urbich C, Aicher A, et al. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res. 2003;92(9):1049–55.
Szeto A, Nation DA, Mendez AJ, et al. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab. 2008;295(6):E1495–501.
Cattaneo MG, Lucci G, Vicentini LM. Oxytocin stimulates in vitro angiogenesis via a Pyk-2/Src-dependent mechanism. Exp Cell Res. 2009;315(18):3210–9.
Wisel S, Khan M, Kuppusamy ML, et al. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharmacol Exp Ther. 2009;329(2):543–50.
Čížková D, Rosocha J, Vanický I, Radonák J, Gálik J, Čížek M. Induction of mesenchymal stem cells leads to HSP72 synthesis and higher resistance to oxidative stress. Neurochem Res. 2006;31(8):1011–20.
Imai Y, Adachi Y, Shi M, et al. Caspase inhibitor ZVAD-fmk facilitates engraftment of donor hematopoietic stem cells in intra–bone marrow–bone marrow transplantation. Stem Cells Dev. 2009;19(4):461–8.
Kanemitsu N, Tambara K, Premaratne GU, et al. Insulin-like growth Factor-1 enhances the efficacy of myoblast transplantation with its multiple functions in the chronic myocardial infarction rat model. J Heart Lung Transplant. 2006;25(10):1253–62.
Duan H-F, Wu C-T, Wu D-L, et al. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther. 2003;8(3):467–74.
Murry CE, Kay MA, Bartosek T, Hauschka SD, Schwartz SM. Muscle differentiation during repair of myocardial necrosis in rats via gene transfer with MyoD. J Clin Invest. 1996;98(10):2209–17.
Payne TR, Oshima H, Okada M, et al. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50(17):1677–84.
Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20(6):661–9.
Song H, Chang W, Lim S, et al. Tissue transglutaminase is essential for integrin-mediated survival of bone marrow-derived mesenchymal stem cells. Stem Cells. 2007;25(6):1431–8.
Cho H-J, Youn S-W, Cheon S-I, et al. Regulation of endothelial cell and endothelial progenitor cell survival and vasculogenesis by integrin-linked kinase. Arterioscler Thromb Vasc Biol. 2005;25(6):1154–60.
Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–4.
Chimenti C, Kajstura J, Torella D, et al. Senescence and death of primitive cells and myocytes Lead to premature cardiac aging and heart failure. Circ Res. 2003;93(7):604–13.
Fan M, Chen W, Liu W, et al. The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction. Rejuvenation Res. 2010;13(4):429–38.
Muraski JA, Rota M, Misao Y, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13(12):1467–75.
Brunt KR, Zhang Y, Mihic A, et al. Role of WNT/β-catenin signaling in rejuvenating myogenic differentiation of aged mesenchymal stem cells from cardiac patients. Am J Pathol. 2012;181(6):2067–78.
Cameron CM, Hu W-S, Kaufman DS. Improved development of human embryonic stem cell-derived embryoid bodies by stirred vessel cultivation. Biotechnol Bioeng. 2006;94(5):938–48.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gorecka, J., Dardik, A. (2021). Stem Cell Therapy to Improve Acute Myocardial Infarction Remodeling. In: Navarro, T.P., Minchillo Lopes, L.L.N., Dardik, A. (eds) Stem Cell Therapy for Vascular Diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-56954-9_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-56954-9_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-56953-2
Online ISBN: 978-3-030-56954-9
eBook Packages: MedicineMedicine (R0)