Abstract
Large vessel occlusion stroke (LVO) represents a stroke subset associated with the highest morbidity and mortality. Multiple prospective randomized trials have shown that thrombectomy, alone or in conjunction with intravenous thrombolysis, is highly effective in reestablishing cerebral perfusion and improving clinical outcomes. In unselected patients and especially in patients with poor collaterals, the benefit of reperfusion therapy is exquisitely time sensitive; the earlier thrombectomy is started, the lower the likelihood of disability or death. Understanding both the pathophysiological underpinnings and the modifying factors of this strong time-to-treatment effect demonstrated in numerous randomized clinical trials is important for implementation of intrahospital workflow measures aiming to maximize time efficiency of thrombectomy. Reducing delays in reperfusion therapy initiation has become a priority in acute stroke care, and therefore, a thorough understanding of the main systems-based factors responsible for these delays is critical. Because the time spent evaluating the patient in the emergency department which typically includes neuroimaging studies performed in scanners remote from the angiography suite represents the main source of delays in thrombectomy initiation, the “Direct to Angiography (DTA)” model has emerged as a means to substantially reduce treatment times and is being instituted at an increasing number of thrombectomy centers across the world. This concept draws parallels to the cardiology model in which door-to-treatment initiation times are substantially shorter than those observed in thrombectomy for LVO stroke in large part due to the fact that at the vast majority of centers performing percutaneous interventions for STEMI, patients are either transferred directly to the cardiac catheterization lab or pass through the ED for the sole purpose of managing any immediately life-threatening condition that may be associated with the patient’s presentation. The aim of this chapter is to introduce DTA as an emerging stroke care paradigm for patients with suspicion of LVO, review results from studies evaluating its feasibility and impact on outcomes, describe current barriers to its more widespread adoption, and propose potential solutions to overcoming these barriers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Acute ischemic stroke (AIS) is a neurological emergency characterized by cessation of cerebral blood flow due to arterial occlusion, resulting in rapid loss of neurons in the territory supplied by the occluded vessel and consequent cerebral infarction. Large vessel occlusion (LVO) [1], defined as internal carotid artery (ICA), middle cerebral artery (MCA) M1 and M2 segments, and basilar artery (BA) occlusion, causes approximately one-third of ischemic stroke but accounts for more than 60% of morbidity and 90% of mortality due to AIS [2]. Thus, LVO stroke constitutes prognostically the most severe form of AIS with outcomes that to date have only been shown to be improved by timely reperfusion of the ischemic brain. This can be accomplished either with intravenous therapy (using lytic drugs such as tissue plasminogen activator [t-PA] or tenecteplase [TNK]) or with endovascular therapy (EVT), typically in the form of mechanical thrombectomy.
“Direct to Angiography” (DTA) is a recently coined term used to describe a time-efficient method of delivery of LVO stroke patients directly to the neuro-angiography suite without valuable time spent on additional evaluations including neuroimaging studies in the emergency department. This new approach may be employed both in transferred cases from a non-thrombectomy-capable stroke center (nTSC) to the thrombectomy-capable stroke center (TSC) and in directly presenting cases to the TSC. The primary rationale for DTA is to significantly reduce the time elapsed between hospital arrival and achievement of successful brain reperfusion. To better understand the rationale for this emerging approach, it is useful to review the importance of time, the role of imaging, the utility of the emergency room evaluation, and existing models of triage and transfer of LVO strokes.
Why Is Time Important?
Time and Outcomes
The time-dependent benefit demonstrated by the National Institute of Neurological Disorders and Stroke (NINDS) IV tissue plasminogen activator (t-PA) trial brought about a paradigm shift in the treatment of acute ischemic stroke [3]. Time became a fundamental patient selection criterion for reperfusion therapy as early reperfusion has long been considered a vital factor in achieving favorable outcomes as exemplified by the widespread use of the aphorism, “time is brain.” In 2015, five landmark randomized controlled trials demonstrated the superiority of EVT over medical therapy alone (which included intravenous thrombolysis in eligible patients) for LVO stroke [4,5,6,7,8], leading to a dramatic change in the landscape of acute reperfusion therapy for AIS. An individual-level meta-analysis of these five trials (HERMES) in which the vast majority of patients were treated in the 0–6-hour time window confirmed that thrombectomy is a highly effective treatment, with a number-needed-to-treat (NNT) of less than 5 to obtain an independent level of functioning at 3 months post-stroke [9]. Further knowledge gained from this meta-analysis revealed that similar to the benefit conferred by intravenous t-PA for stroke, the benefit of thrombectomy in non-selected patients is exquisitely time-dependent. The magnitude of the treatment effect is strongly correlated to the time from stroke onset to reperfusion and treatment benefit can no longer be demonstrated with high degree of certainty after 7.3 hours after stroke onset [10]. There are abundant data confirming the impact of time on outcomes post-thrombectomy [10,11,12,13]. On average, in the setting of an LVO stroke, 2 million neurons are lost per minute, and the earlier blood flow is restored to the brain, the higher the likelihood of a good clinical outcome [14]. The HERMES collaborators reported that for every 100 patients treated, every 15 min of earlier reperfusion results in a 2.5% higher chance of achieving independence in activities of daily living [10]. Retrospective data suggest that every minute of delay to recanalization leads to the reduction of 4.2 days of disability-free survival [15].
Heterogeneity in Stroke Pathophysiology
Two years after the publication of the early time window trials, the DAWN and DEFUSE-3 trials led to a dramatic expansion of the therapeutic time window for thrombectomy, with benefit demonstrated up to 24 hours from stroke onset [16, 17]. These trials, however, were highly selective with regard to inclusion criteria and only enrolled patients with “mismatch” (i.e., small infarct volumes in the presence of large areas at risk) presenting >6 hours after the patient was last seen well (TLSW).
The seemingly difficult-to-reconcile discrepancy between the finding of a strong relationship between timing of reperfusion and outcomes in the early time window and the finding of strong benefit from thrombectomy in the late time window can be explained by patient selection with respect to speed of infarct progression. Whereas the early time window trials enrolled largely unselected patients, the late time window trials restricted enrollment to “slow progressors,” a term derived from the recognition of two distinct phenotypes with respect to infarct growth rate following proximal large vessel occlusion, the “slow” and “fast progressors” [18]. At a cellular level, the corresponding rate of neuronal loss following vessel occlusion has been described to be highly variable, ranging from <35,000 to >27 million neurons per minute [19]. The variability in the rate of infarct growth in the setting of LVO is highly dependent on the individual degree of leptomeningeal collateral blood flow [20,21,22]. Not surprisingly, leptomeningeal collaterals have also been shown to be an independent predictor of good outcome in LVO stroke [23]. Factors influencing the presence and extent of collaterals and consequent susceptibility to ischemic damage include genetic factors; physiologic parameters such as blood pressure, body position, CO2, temperature, and glucose; and demographics [18]. Long-standing intracranial stenosis can lead to the development of more extensive collaterals but comorbidities promoting its development (e.g., hypertension, diabetes) are associated with impaired collateral blood flow during acute stroke [24, 25]. Cerebral edema has also been shown to impair blood flow through collateral vessels, and this may explain the decreased final infarct size seen in studies evaluating sulfonylurea use for the reduction of cerebral edema [26].
When patients with M1 MCA occlusion are defined as fast progressors if harboring an infarct in the MCA territory greater than 70 ml within 6 hours of TLSW and slow progressors if their infarct is less than 30 ml beyond 6 hours of TLSW, about 50% of LVO strokes can be described as “slow progressors” and 25% can be described as “fast progressors” [27]. This latter category of individuals with acute stroke due to LVO experiences rapid infarct growth and represents the most sensitive group with respect to delays in time to recanalization [28]. The identification of predictive biomarkers for the rate of stroke progression is an active area of research. One recent study which utilized sequential brain MRI studies in patients with LVO strokes showed that an initial infarct growth rate of <4.1 ml/hr and core volume of <19.9 ml detected on MRI at a median of 5 hours post-TLSW had high accuracy in predicting an infarct core of <50 ml at 24 hours post-stroke onset and thus having a higher likelihood of good outcome with thrombectomy [29].
Time and Neuroimaging
Current AHA/ASA guidelines recommend the use of neuroimaging to select patients for thrombectomy according to time window. In the 0–6-hour window, head CT and CT angiography alone are deemed sufficient, but in the 6–24-hour window, advanced imaging with CT perfusion or MRI is recommended to select patients with small infarct volume and large tissue at risk (mismatch) [30].
What Is the Role of the Emergency Department?
Evaluation and Emergency Care
Acute ischemic stroke patients, either presenting directly to the thrombectomy center or transferred from another hospital, are routinely admitted to the emergency department (ED). There, they undergo a focused evaluation to rule out immediately life-threatening conditions that may be associated with acute stroke such as acute cardiac or respiratory failure, severe hyper- or hypotension, etc. Another critical element of the initial stroke patient evaluation is the National Institutes of Health Stroke Scale (NIHSS) score which can determine the likelihood of stroke as presenting diagnosis and, by measuring the degree of stroke severity, also determine the likelihood of large vessel occlusion given that higher NIHSS scores reflect a higher likelihood of large vessel occlusion [31].
Neuroimaging for IV t-PA and Thrombectomy Eligibility
Acute clinical evaluation is typically followed by relevant neuroimaging, which is comprised at most centers of head CT, with or without CT angiography. Alternatively, MRI-based imaging (MRI and MR angiography) is used instead at some centers although its use even at the most efficient centers is associated with significant additional delays compared to CT. This, along with implanted devices or other metallic structures that constitute absolute contraindications to MRI use, represents the main disadvantage of MRI use in acute stroke. Originally, the main goal of acute imaging was to rule out intracerebral hemorrhage for the purpose of administering IV t-PA. However, since the advent of thrombectomy as standard of care in LVO stroke, imaging has been increasingly utilized to assess for thrombectomy eligibility by confirming the presence of an LVO, by ruling out a large infarction and by confirming the presence of reversible ischemic tissue. While the exclusion of intracerebral hemorrhage is the only prerequisite with respect to IV t-PA administration, all other neuroimaging studies are essentially performed for screening purposes with the ultimate goal of accurately identifying patients considered ineligible for thrombectomy for the purpose of excluding them from angiography. Furthermore, stroke patients transferred to thrombectomy-capable stroke center (TSC) from a non-thrombectomy-capable stroke center (nTSC) routinely undergo repeat evaluation and neuroimaging upon arrival in the TSC emergency department prior to activation of the thrombectomy team. Oftentimes, repeat imaging occurs despite already having established thrombectomy eligibility at the referring hospital with the justification that the imaging study obtained at the referring hospital no longer accurately reflects tissue viability status [32].
What Are the Pitfalls of the Current Approach?
Multi-step Approach and Time Delay
Current intrahospital stroke systems at most hospitals continue to reflect a workflow that is designed around IV t-PA administration. Emergency department evaluation and treatment by ED physicians and nurses, along with head CT performed in the ED CT scanner and (if available) emergent evaluation by a neurologist, represent the typical steps preceding IV t-PA administration, followed at some centers by additional imaging and admission to a specialized care unit. Despite the widespread adoption of thrombectomy, stroke systems of care have lagged behind in the evolution of workflow paradigms best suited to the particular aspects of endovascular therapy and continue to use approaches that lend themselves to delays in thrombectomy initiation including a serial rather than parallel workflow typically pursued at most centers. First, the patient is evaluated in the ED, head CT is obtained, IV t-PA is administered if appropriate, and, in many instances, only subsequently the patient undergoes further imaging (CTA,CTP,MRI, MRA) to assess for thrombectomy eligibility which is primarily determined by the presence of LVO and by exclusion of a large infarct. If the patient is then deemed eligible, transfer to the angio-suite for thrombectomy ensues. This serial, multi-step approach often results in significant time delays [33].
Large Ischemic Core Volume in the Early Time Window
A long-held belief among stroke experts has been that reperfusion of large volumes of infarcted brain tissue is detrimental to patient’s outcomes due to the development of symptomatic intracerebral hemorrhage, malignant edema, or other reperfusion-related deleterious effects. Important unresolved questions related to this concept include the definition of large infarct and the accuracy of available imaging-based tools for infarct measurement. While definitive evidence against a net detrimental effect of reperfusion is still lacking, the results of recent studies have shed considerable doubt on its validity as to date no category of patients defined by imaging or otherwise has been found to be harmed by thrombectomy.
Most commonly used imaging-based methods to estimate the ischemic core in LVO stroke patients are non-contrast CT, CT perfusion, and MRI. The score of 7 on the Alberta Stroke Program Early CT Score (ASPECTS) [34] as baseline infarct volume threshold associated with benefit from reperfusion was initially described in the first-generation endovascular trials [35, 36]. However, advances in technology and workflow have shifted this cut-off to lower values. Indeed, in the initial HERMES collaborator’s publication, a statistically significant benefit from mechanical thrombectomy could be demonstrated using an ASPECTS cut-off of 6 and above. Subsequent to this publication, in the prospective study ETIS [37], Panni et al. reported a rate of good outcome, defined as a modified Rankin Scale (mRS) score of 0–2 at 3 months, of 32% in the subgroup of patients with ASPECTS of 4–5. Similarly, analysis of patients with ASPECTS 0–5, who underwent thrombectomy in the BEYOND-SWIFT registry, showed that 40% had favorable outcome (mRS 0–3) at 90 days [38]. These outcome rates are superior to what would be expected in non-treated LVO stroke patients with similar baseline ASPECTS scores.
CT perfusion is considered superior to non-contrast CT in its ability to estimate the core although some studies have shown that with respect to its ability to estimate a large infarct (> 70 ml), a non-contrast CT cut-off of <7 is equivalent to CT perfusion when compared to the gold standard of DWI MRI [39]. Furthermore, in the era of modern thrombectomy, with fast and complete reperfusion achieved in an increasingly higher proportion of patients, CT perfusion-derived thresholds defining infarct are being redefined and seem to be a “moving target.” Indeed, as early as 2015, d’Esterre et al. showed that the cerebral blood flow (CBF) thresholds defining infarct were dependent on both the timing of reperfusion relative to the CT perfusion study and on the timing of reperfusion relative to the stroke symptom onset [40]. These findings were confirmed by Qiu et al. based on patients from the HERMES database [41]. Earlier reperfusion necessitates lower CBF thresholds in order to estimate infarct volume with same accuracy as those used in patients who achieve reperfusion later. However, even with these adjustments, thresholds used for infarct measurement on CTP are far from being 100% reliable.
Improvements in stroke systems of care, coupled with advances in thrombectomy technology, make it likely that high-quality reperfusion will be achieved earlier and earlier in the future. As such, it is not clear that the thresholds defining infarct on CT perfusion are ever going to have sufficient accuracy. Indeed, the final infarct volumes in patients who undergo successful reperfusion are not uncommonly shown to be smaller than predicted by CT perfusion imaging on the pre-thrombectomy CTP.
DWI MRI is widely considered as the most precise method for core estimation in clinical practice but it is associated with significant delays. Furthermore, even though superior to all other imaging modalities in clinical use, MRI is also prone to inaccurate characterization of the infarcted brain especially in the context of reperfusion. The earlier the pre-reperfusion MRI scan is performed relative to ischemia onset and the faster reperfusion occurs following completion of the MRI scan, the higher the likelihood that the DWI lesion noted on the initial MRI scan will no longer be demonstrated on a follow-up MRI scan. This phenomenon, termed DWI reversibility, has been noted to occur in up to a quarter of patients with acute stroke [42].
Data from HERMES collaboration indicate strong signals of benefit in favor of thrombectomy in patients with large (>70 ml) and even very large (>100 ml) baseline infarct volumes [43]. Thus, both the limitations of CTP or MRI in accurately measuring the core and the lack of convincing evidence of lack of benefit (or harm) with thrombectomy in patients with large core measured by these imaging modalities call into question the utility of advanced imaging (CTP or MRI) in determining thrombectomy eligibility in the early time window.
Perhaps the most compelling data showing the benefit of EVT, even in patients with large baseline infarcts, come from the expanded dataset making up the HERMES collaboration which included seven randomized endovascular stroke trials showing superiority of EVT plus best medical therapy (that included IV lysis in eligible patients) over best medical therapy alone [4,5,6,7,8, 44, 45]. Although most of the participating trial’s protocols largely excluded patients with large baseline infarctions, some of these patients were inadvertently enrolled due to lack of recognition of large infarcts on initial imaging by local investigators. In a meta-analysis of these trials, of the 1764 patients enrolled, 126 underwent thrombectomy with core lab-adjudicated baseline ASPECTS <5 on CT (61 patients) or MRI (65 patients) [46]. Both in the MRI and CT groups there was a trend in favor of benefit with EVT, while in the combined analysis of CT- or MRI–imaged patients the benefit from EVT reached statistical significance (OR 2.15, 95% CI 1.06–4.37). Additionally, in 228 patients with evidence of hypodensity involving greater than one-third of the MCA territory, another measure of large baseline infarct, benefit from EVT was also demonstrated (OR 1.70, 95%CI 1.04–2.78) [46]. Using the same expanded HERMES dataset, similar results were seen on a CT perfusion-based analysis of patients with large core infarcts (>70 ml and even >100 ml) with no significant reduction in absolute treatment effect with EVT and no net signals of harm despite a higher incidence of sICH in the thrombectomy group compared to controls [43]. Findings from these studies suggest that patients with LVO who have large baseline infarcts are likely to benefit from EVT compared to medical treatment alone and that although a large baseline infarct represents a prognostic factor, it does not represent a treatment effect modifier.
Efforts to identify patients with large baseline infarcts for the purpose of exclusion from thrombectomy may lack justification not only due to the observed strong trends of benefit but also because in patients presenting in the early time window, the prevalence of large infarctions without significant salvageable tissue is exceedingly low, as demonstrated by the 95% of patients who had mismatch by SWIFT PRIME criteria and by the 78% of patients who had large penumbral volumes (>60 ml) in the HERMES collaboration-derived CT perfusion-based study [43]. In fact, the prevalence of large infarcts (ASPECTS 0–5) has been estimated to be no more than about 15% of patients with M1 MCA occlusion presenting within 6 hours after last seen well [38] while within the first 3 hours after symptom onset the prevalence of ASPECTS 0–5 in patients with MCA occlusion is 4% [47]. As such, not only there is no evidence to suggest a lack of benefit of EVT for LVO strokes with large baseline core infarcts, but also the prevalence of large infarcts in the early time window is low.
The Tradeoff Between Advanced Imaging and Delays in Time to Reperfusion
Advanced imaging requires not an insignificant time for completion. In SWIFT PRIME, the median time from start of CT to post-processing of CT perfusion imaging was 24 min while MRI-based selection was associated with a delay from door to groin puncture of a median of 16 min compared to CT-based selection [48]. Considering that based on data derived from HERMES every 15 min of delay in reperfusion results in a 3.9% lower chance of reduction in disability, if the delay in reperfusion caused by CTP in SWIFT PRIME was a conservative 15 min, this delay translates into 3.9% higher chance of disability in those patients who achieved reperfusion in the interventional arm of the trial. The delays incurred by advanced imaging can be inferred from the fact that in DAWN and DEFUSE-3, studies with protocol requirements of advanced imaging (CT perfusion or MRI), median ED arrival-to-groin puncture times were 109 and 112 min, respectively, compared to 89 min in the ESCAPE trial, 60 min in the ESCAPE NA-1 trial, and 60 min in the ARISE II study [5, 16, 17, 49, 50], none of which required advanced imaging. The limited usefulness of advanced imaging in the early time window is reflected in the AHA guidelines which endorse thrombectomy as level I evidence only for patients harboring an LVO and a limited core volume defined as an ASPECT score >5 [30] . However, based on data published after the publication of the AHA guidelines, in the early time window (0–6 hours), following exclusion of intracerebral hemorrhage (typically accomplished with a non-contrast head CT), it is justified to question the necessity for patient selection for thrombectomy based on any baseline infarct size.
Is Vessel Imaging Always Necessary?
Currently, the vast majority of stroke centers still perform a CT angiogram in a conventional CT scanner to confirm the presence of large vessel occlusion prior to the decision to proceed with transport to the angiography suite. Studies examining workflow efficiency in intrahospital systems of care have shown that when only a plain CT (showing arterial thrombus) is utilized in lieu of the standard CTA for the purposes of large vessel occlusion diagnosis, the time required for imaging decreases by an average of 28 min and the time elapsed from hospital presentation to access site puncture times decreased by 36 min [51]. Thus, in situations when there is an a priori high likelihood of large vessel occlusion, forgoing of the CTA and transport directly to the angiography suite after the initial head CT may save substantial time to brain reperfusion. Although a combination of findings on a plain CT combined with clinical information is likely to result in highest yield for prediction of LVO, an NIHSS cut-off of 10 or above has been shown to predict the presence of LVO with 80% accuracy in the early time window [52].
In the cardiology literature, the well-known entity of myocardial infarction with non-obstructive coronary arteries (MINOCA) is characterized by patent coronary arteries during emergent cardiac catheterization despite clinical, electrocardiographic, and biomarker features consistent with myocardial infarction. The finding of MINOCA is encountered with a frequency of 5–25% and typically results in no coronary intervention at the time of the emergent cardiac catheterization procedure [53]. However, despite this non-insignificant frequency of instances where an emergent cardiac catheterization in a patient with acute myocardial infarction results in no intervention, it is the standard of care in virtually all cardiac catheterization laboratories across the world to assess coronary patency via emergent coronary angiography without prior non-invasive studies (coronary CTA or MRI). The widely accepted fact among cardiologists that substantial savings in time to reperfusion for the entire population of reperfusion candidates are likely to offset any negative repercussions of invasive angiography in the minority of patients of reperfusion candidates who have patent arteries in the setting of myocardial infarction should equally apply in the setting of acute stroke. Therefore, it is reasonable to question the need for non-invasive vessel imaging prior to transfer to the neuro-angiography suite when the pre-test probability of large vessel occlusion is high and thus the likelihood of no intervention is low. The threshold for what constitutes an acceptable likelihood of patent vessel on angiography is likely to vary depending on resources available at each individual stroke center. However, based on cardiology data, a 15–20% such likelihood may represent a reasonable trade-off between the increase in necessary cath lab resources and the clinical benefit derived from timelier reperfusion associated with this approach. Furthermore, recent advances in flat panel angiography technology have made possible identification of both intracranial hemorrhage and presence of large vessel occlusion on the angiography table which represents a non-invasive way of answering the questions relevant for triage of LVO patients in the most time-efficient manner [54]. Flat panel CT and CTA, obtained by low-dose contrast injection to create CTA-like images, demonstrated high accuracy in their respective ability to rule out hemorrhage and detect LVO in the neuro-angiography suite [55, 56].
Cost Considerations
In the era of increased cost awareness related to healthcare utilization, economic considerations should also be taken into account when assessing the overall value of a DTA approach. At a first glance, the DTA approach requires a higher level of resources compared to the conventional approach. However, there is a decrease in cost associated with bypassing the CTA and other advanced imaging for all patients and a decrease in healthcare cost associated with earlier brain reperfusion. Because earlier reperfusion leads to lower infarct volumes and infarct volume is the main driver of hospitalization costs in LVO stroke patients [57], earlier reperfusion leads to lower hospitalization costs. In addition the economical benefit of reperfusion therapy has been shown to translate beyond the acute hospitalization [58]. Therefore, the cost reduction associated with a DTA approach which will lead to intervention and earlier reperfusion in a majority of patients without additional CTA or CTP must be weighed against the increase in cost associated with the performance of cerebral angiography with findings of no occlusion in a minority of patients.
Thus, if assumed conservatively that in the 0–6-hour time window an NIHSS cut-off of 10 and above predicts the presence of treatable large vessel occlusion 80% of the time; that patients with largest baseline infarcts, which constitute a minority, are not harmed by thrombectomy; and that forgoing of any type of CT or MRI imaging prior to transfer to the angiography suite (because intracerebral hemorrhage has been already performed at the transferring hospital or will be performed with flat panel detector in the angiography suite), then per hundred evaluated patients, a direct to angio approach saves on average 45 min to reperfusion for 80 patients and saves 100 CTAs or CTPs at the expense of 20 negative angiograms (conventional or flat panel CTA).
How to Address Time Delays in the Emergency Department?
Existing Transfer Paradigms
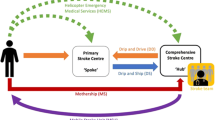
Most transfer paradigms currently in use include transfer of patients from site of stroke onset (home or field) to the emergency room at the non-thrombectomy center or at the thrombectomy center (either directly or via the non-thrombectomy center or via the mobile stroke unit, an increasingly utilized transfer modality). These are portrayed in Fig. 6.1 in black arrows and are indirect ways of patient transfer to the angio-suite.
Bypassing the Emergency Department: Direct to Angiography
Given that emergency department admission leads to time delays, a novel way of transferring patients directly to the angio-suite involves bypassing the emergency department. Broadly, three types of Direct to Angiography pathway may occur—Route ADTA, ED at non-thrombectomy-capable center to angio-suite at TSC; Route BDTA, home or field direct to angio-suite at TSC; or Route CDTA, home or field to mobile stroke unit (MSU) to thrombectomy center angio-suite at TSC (red arrows in Fig. 6.1).
Initial Experience with Direct to Angiography (Table 6.1)
Several single-center series have reported their experiences with Direct to Angiography. All studies have reported significant reductions in door-to-groin puncture time using the Direct to Angiography approach [32, 59,60,61,62,63]. Jadhav et al. reported a time saving of 1 hour using the Direct to Angiography approach [32]. Across studies, median door-to-groin puncture times in the DTA paradigm were between 16 and 33 min and clinical benefit compared to historical controls was observed in three of these studies [60, 61, 63] (Table 6.1). Other studies did not report significant improvement in outcomes via the DTA approach compared to historical controls highlighting the importance of trials utilizing active controls in this area. Nonetheless, these data uniformly point toward substantial gains in door to access site puncture while demonstrating that the DTA approach is feasible and safe. Furthermore, recent data suggest that patients who stand most to benefit from a DTA approach are those who present to the thrombectomy center in the earliest time window (0–3 hours) [63].
Practical Considerations of Bypassing the Emergency Department
Evaluation and Emergency Care
Evaluation and treatment of stroke-related acute medical complications begin in the ambulance, the MSC, or the ED of the nTSC. To decrease time delays, ED care can be bypassed and initial in-hospital care can be provided in the neuro-angiography suite at the thrombectomy center. For judicious use of resources, only patients with high likelihood of LVO and those deemed to be most sensitive to time delays (NIHSS >9 presenting within 6 hours of TLSW) will be evaluated under the DTA model. Ideally, the mandatory (according to US law—EMTALA) [64] ED evaluation for patients presenting directly to a thrombectomy-capable center should occur very rapidly either by the patient passing through (without stopping) in the ED or by the patient being evaluated by ED staff in the angiography suite in those cases where the angiography suite is in close proximity to the ED.
IV t-PA
A majority of LVO stroke patients present beyond the 4.5-hour time window for t-PA administration. Of the LVO strokes presenting within this window, a large percentage are transferred from an NTSC [65] and can receive IV t-PA there while transfer is being coordinated. The same holds true for patients being transferred from home or the field using a mobile stroke unit. Hence, Direct to Angiography from an NTSC or MSU (Fig. 6.1, Route ADTA and CDTA) is feasible for most patients who are treated with IV t-PA.
In cases of t-PA-eligible thrombectomy candidates who present directly to a TSC (the minority of patients in the USA [65], bypassing the emergency department is dependent on the feasibility of obtaining acute brain imaging to rule out intracranial hemorrhage without stopping in the ED. Neuro-angiography suites that are equipped with multi-detector flat panel CT imaging can serve as a single location where imaging, t-PA administration, and endovascular therapy for LVO can all be performed without intrahospital time delays [59]. This would enable Route BDTA on Fig. 6.1 (home or field to angio-suite). It has been shown that administration of IV t-PA based on flat panel CT technology has a similar safety profile to IV t-PA treatment when hemorrhage is excluded with conventional CT scanners [62]. Furthermore, the need for IV thrombolytic administration in IV thrombolysis-eligible thrombectomy candidates presenting directly to a TSC has been called into question, and recent randomized trials showed no difference in outcomes when IV t-PA is administered prior to thrombectomy compared to thrombectomy without prior IV t-PA [66, 67]. If this finding is confirmed by other ongoing randomized trials, in the future IV thrombolysis may no longer be indicated for thrombectomy candidates presenting directly to TSC, and thus, the issue of ruling out intracerebral hemorrhage for the purpose of IV thrombolysis administration may become moot.
Conclusions
Acute stroke due to large vessel occlusion is a prognostically ominous stroke subset with clinical outcomes that to date have only been shown to be improved by reperfusion therapy in the form of IV thrombolysis, endovascular thrombectomy, or a combination of both. Multiple randomized trials have shown that thrombectomy with or without IV thrombolysis is associated with the strongest clinical response in LVO stroke and that the benefit of this approach is exquisitely time dependent especially in the early time window (0–6 hours). Therefore, system-based approaches aiming at reducing times from stroke onset to reperfusion have been a priority in the quest to improve acute stroke care. The Direct to Angiography (DTA) suite represents a new paradigm which aims to dramatically reduce reperfusion times through bypass of conventional pathways for early-presenting LVO stroke patients and consists of clinical and imaging evaluation on the angiography table using either no additional imaging (in the case of transferred patients) or flat panel technology (in case of patients presenting directly to a thrombectomy-capable center) (Table 6.2). This model relies on emerging data suggesting that knowledge of baseline infarct volume may no longer be necessary for patient selection in the early time window and that the presence of LVO can be estimated with high likelihood either based on the NIHSS or even more accurately with a flat panel CTA performed after patient arrival in the angiography suite. DTA has been shown to be feasible and safe and is associated with significant decreases in time to treatment initiation. There are logistical challenges in adopting this model, but preliminary experience has shown that most if not all of them can be overcome. Because of these challenges, widespread implementation of this concept akin to the cardiology model for STEMI is unlikely to occur without level I evidence. Therefore, planned randomized trials will need to clarify whether the substantial time savings associated with this approach will ultimately translate into clinical benefits.
References
González RG, Furie KL, Goldmacher GV, Smith WS, Kamalian S, Payabvash S, et al. Good outcome rate of 35% in IV-tPA-treated patients with computed tomography angiography confirmed severe anterior circulation occlusive stroke. Stroke. 2013;44(11):3109–13.
Malhotra K, Gornbein J, Saver JL. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: a review. Front Neurol. 2017;8:651.
National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995 14;333(24):1581–7.
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–306.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30.
Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20.
Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18.
Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–95.
Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31.
Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CBLM, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279–88.
He AH, Churilov L, Mitchell PJ, Dowling RJ, Yan B. Every 15-min delay in recanalization by intra-arterial therapy in acute ischemic stroke increases risk of poor outcome. Int J Stroke. 2015;10(7):1062–7.
Alawieh A, Vargas J, Fargen KM, Langley EF, Starke RM, De Leacy R, et al. Impact of procedure time on outcomes of thrombectomy for stroke. J Am Coll Cardiol. 2019;73(8):879–90.
Jahan R, Saver JL, Schwamm LH, Fonarow GC, Liang L, Matsouaka RA, et al. Association between time to treatment with endovascular reperfusion therapy and outcomes in patients with acute ischemic stroke treated in clinical practice. JAMA. 2019;322(3):252–63.
Saver JL. Time is brain--quantified. Stroke. 2006;37(1):263–6.
Meretoja A, Keshtkaran M, Tatlisumak T, Donnan GA, Churilov L. Endovascular therapy for ischemic stroke: save a minute-save a week. Neurology. 2017;88(22):2123–7.
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21.
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–18.
Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke. 2017;48(9):2621–7.
Desai SM, Rocha M, Jovin TG, Jadhav AP. High variability in neuronal loss. Stroke. 2019;50(1):34–7.
Jung S, Gilgen M, Slotboom J, El-Koussy M, Zubler C, Kiefer C, et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain. 2013;136(Pt 12):3554–60.
Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30(5):923–34.
Campbell BCV, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. 2013;33(8):1168–72.
Lima FO, Furie KL, Silva GS, Lev MH, Camargo ECS, Singhal AB, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010;41(10):2316–22.
Chan S-L, Sweet JG, Bishop N, Cipolla MJ. Pial collateral reactivity during hypertension and aging: understanding the function of collaterals for stroke therapy. Stroke. 2016;47(6):1618–25.
Letourneur A, Roussel S, Toutain J, Bernaudin M, Touzani O. Impact of genetic and renovascular chronic arterial hypertension on the acute spatiotemporal evolution of the ischemic penumbra: a sequential study with MRI in the rat. J Cereb Blood Flow Metab. 2011;31(2):504–13.
Simard JM, Yurovsky V, Tsymbalyuk N, Melnichenko L, Ivanova S, Gerzanich V. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke. 2009;40(2):604–9.
Rocha M, Desai SM, Jadhav AP, Jovin TG. Prevalence and temporal distribution of fast and slow progressors of infarct growth in large vessel occlusion stroke. Stroke. 2019;50(8):2238–40.
Ribo M, Molina CA, Cobo E, Cerdà N, Tomasello A, Quesada H, et al. Association between time to reperfusion and outcome is primarily driven by the time from imaging to reperfusion. Stroke. 2016;47(4):999–1004.
González RG, Silva GS, He J, Sadaghiani S, Wu O, Singhal AB. Identifying severe stroke patients likely to benefit from thrombectomy despite delays of up to a day. Sci Rep. 2020;10(1):4008.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–418.
Heldner MR, Zubler C, Mattle HP, Schroth G, Weck A, Mono M-L, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153–7.
Jadhav AP, Kenmuir CL, Aghaebrahim A, Limaye K, Wechsler LR, Hammer MD, et al. Interfacility transfer directly to the neuroangiography suite in acute ischemic stroke patients undergoing thrombectomy. Stroke. 2017;48(7):1884–9.
Aghaebrahim A, Streib C, Rangaraju S, Kenmuir CL, Giurgiutiu D-V, Horev A, et al. Streamlining door to recanalization processes in endovascular stroke therapy. J Neurointerv Surg. 2017;9(4):340–5.
Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of Hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta stroke Programme early CT score [Internet]. Lancet (London, England). 2000; [cited 2020 May 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/10905241/?from_term=ASPECTS+score+2000&from_pos=1.
Kimura K, Iguchi Y, Shibazaki K, Terasawa Y, Inoue T, Uemura J, et al. Large ischemic lesions on diffusion-weighted imaging done before intravenous tissue plasminogen activator thrombolysis predicts a poor outcome in patients with acute stroke. Stroke. 2008;39(8):2388–91.
Hill MD, Rowley HA, Adler F, Eliasziw M, Furlan A, Higashida RT, et al. Selection of acute ischemic stroke patients for intra-arterial thrombolysis with pro-Urokinase by using ASPECTS [Internet]. Stroke. 2003; [cited 2020 May 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/12843342/.
Panni P, Gory B, Xie Y, Consoli A, Desilles J-P, Mazighi M, et al. Acute stroke with large ischemic core treated by thrombectomy. Stroke. 2019;50(5):1164–71.
Kaesmacher J, Chaloulos-Iakovidis P, Panos L, Mordasini P, Michel P, Hajdu SD, et al. Mechanical thrombectomy in ischemic stroke patients with Alberta stroke program early computed tomography score 0-5. Stroke. 2019;50(4):880–8.
Demeestere J, Garcia-Esperon C, Garcia-Bermejo P, Ombelet F, McElduff P, Bivard A, et al. Evaluation of hyperacute infarct volume using ASPECTS and brain CT perfusion core volume. Neurology. 2017;88(24):2248–53.
d’Esterre CD, Boesen ME, Ahn SH, Pordeli P, Najm M, Minhas P, et al. Time-dependent computed tomographic perfusion thresholds for patients with acute ischemic stroke. Stroke. 2015;46(12):3390–7.
Qiu W, Kuang H, Lee TY, Boers AM, Brown S, Muir K, et al. Confirmatory study of time-dependent computed tomographic perfusion thresholds for use in acute ischemic stroke. Stroke. 2019;50(11):3269–73.
Nagaraja N, Forder JR, Warach S, Merino JG. Reversible diffusion-weighted imaging lesions in acute ischemic stroke: a systematic review. Neurology. 2020;94(13):571–87.
Campbell BCV, Majoie CBLM, Albers GW, Menon BK, Yassi N, Sharma G, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019;18(1):46–55.
Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138–47.
Muir KW, Ford GA, Messow C-M, Ford I, Murray A, Clifton A, et al. Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017;88(1):38–44.
Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CBLM, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018;17(10):895–904.
Tsivgoulis G, Saqqur M, Sharma VK, Lao AY, Hoover SL, Alexandrov AV. Association of Pretreatment ASPECTS scores with tPA-induced arterial recanalization in acute middle cerebral artery occlusion. J Neuroimaging. 2008;18(1):56–61.
Goyal M, Jadhav AP, Bonafe A, Diener H, Mendes Pereira V, Levy E, et al. Analysis of workflow and time to treatment and the effects on outcome in endovascular treatment of acute ischemic stroke: results from the SWIFT PRIME randomized controlled trial. Radiology. 2016;279(3):888–97.
Zaidat Osama O, Hormozd B, Marc R, Saver Jeffrey L, Mattle Heinrich P, René C, et al. Primary results of the multicenter ARISE II study (analysis of revascularization in ischemic stroke with EmboTrap). Stroke. 2018;49(5):1107–15.
Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet [Internet]. 2020. [cited 2020 Feb 24];0(0). Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30258-0/abstract.
Atchaneeyasakul K, Tipirneni A, Khandelwal P, Saini V, Ronca R, Lord S, et al. Utilizing CT with maximum intensity projection reconstruction bypassing CTA improves time to groin puncture in large vessel occlusion stroke thrombectomy. Interv Neurol. 2017;6(3–4):147–52.
Martins-Filho RK do V, Dias FA, Alves FFA, Camilo MR, Barreira CMA, Libardi MC, et al. Large vessel occlusion score: a screening tool to detect large vessel occlusion in the acute stroke setting. J Stroke Cerebrovasc Dis. 2019;28(4):869–75.
Scalone G, Niccoli G, Crea F. Editor’s choice- pathophysiology, diagnosis and management of MINOCA: an update. Eur Heart J Acute Cardiovasc Care. 2019;8(1):54–62.
Leyhe JR, Tsogkas I, Hesse AC, Behme D, Schregel K, Papageorgiou I, et al. Latest generation of flat detector CT as a peri-interventional diagnostic tool: a comparative study with multidetector CT. J Neurointerv Surg. 2017;9(12):1253–7.
Heran NS, Song JK, Namba K, Smith W, Niimi Y, Berenstein A. The utility of DynaCT in neuroendovascular procedures. Am J Neuroradiol. 2006;27(2):330–2.
Namba K, Niimi Y, Song JK, Berenstein A. Use of Dyna-CT angiography in neuroendovascular decision-making. Interv Neuroradiol. 2009;15(1):67–72.
Streib CD, Rangaraju S, Campbell DT, Winger DG, Paolini SL, Zhang AJ, et al. Infarct volume predicts hospitalization costs in anterior circulation large-vessel occlusion stroke. AJNR Am J Neuroradiol. 2019;40(1):51–8.
Shireman TI, Wang K, Saver JL, Goyal M, Bonafé A, Diener H-C, et al. Cost-effectiveness of solitaire stent retriever thrombectomy for acute ischemic stroke: results from the SWIFT-PRIME trial (solitaire with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke) [Internet]. Stroke. 2017; [cited 2020 May 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/28028150/.
Psychogios M-N, Behme D, Schregel K, Tsogkas I, Maier IL, Leyhe JR, et al. One-stop management of acute stroke patients: minimizing door-to-reperfusion times. Stroke. 2017;48(11):3152–5.
Ribo M, Boned S, Rubiera M, Tomasello A, Coscojuela P, Hernández D, et al. Direct transfer to angiosuite to reduce door-to-puncture time in thrombectomy for acute stroke. J Neurointerv Surg. 2018;10(3):221–4.
Mendez B, Requena M, Aires A, Martins N, Boned S, Rubiera M, et al. Direct transfer to Angio-suite to reduce workflow times and increase favorable clinical outcome: a case-control study. Stroke. 2018;49(11):2723–7.
Brehm A, Tsogkas I, Maier IL, Eisenberger HJ, Yang P, Liu J-M, et al. One-stop management with perfusion for transfer patients with stroke due to a large-vessel occlusion: feasibility and effects on in-hospital times. AJNR Am J Neuroradiol. 2019;40(8):1330–4.
Manuel R, Marta O, Álvaro G-T, Noelia R-V, Matías D, Jesús J, et al. Time matters. Stroke. 0(0):STROKEAHA.119.028586.
Zibulewsky J. The emergency medical treatment and active labor act (EMTALA): what it is and what it means for physicians. Proc (Bayl Univ Med Cent). 2001;14(4):339–46.
Rinaldo L, Brinjikji W, McCutcheon BA, Bydon M, Cloft H, Kallmes DF, et al. Hospital transfer associated with increased mortality after endovascular revascularization for acute ischemic stroke. J Neurointerv Surg. 2017;9(12):1166–72.
Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, et al. Endovascular thrombectomy with or without intravenous Alteplase in acute stroke. N Engl J Med. 2020;382(21):1981–93.
Suzuki K, Kimura K, Takeuchi M, Morimoto M, Kanazawa R, Kamiya Y, et al. The randomized study of endovascular therapy with versus without intravenous tissue plasminogen activator in acute stroke with ICA and M1 occlusion (SKIP study). Int J Stroke. 2019;14(7):752–5.
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–492.
Lakomkin N, Dhamoon M, Carroll K, Singh IP, Tuhrim S, Lee J, et al. Prevalence of large vessel occlusion in patients presenting with acute ischemic stroke: a 10-year systematic review of the literature. J Neurointerv Surg. 2019;11(3):241–5.
Smith Eric E, Kent David M, Bulsara Ketan R, Leung Lester Y, Lichtman Judith H, Reeves Mathew J, et al. Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49(3):e111–22.
de la Ossa NP, Carrera D, Gorchs M, Querol M, Millán M, Gomis M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale [Internet]. Stroke. 2014; [cited 2020 May 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/24281224/.
EEG controlled triage in the ambulance for acute ischemic stroke - full text view - ClinicalTrials.gov [Internet]. [cited 2020 May 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT03699397.
Antipova D, Eadie L, Macaden AS, Wilson P. Diagnostic value of transcranial ultrasonography for selecting subjects with large vessel occlusion: a systematic review. Ultrasound J. 2019;11(1):29.
Giacalone G, Zanoletti M, Re R, Germinario B, Contini D, Spinelli L, et al. Time-domain near-infrared spectroscopy in acute ischemic stroke patients. Neurophotonics [Internet]. 2019 [cited 2020 May 20];6(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6365799/.
UCSF. UCSF ischemic stroke trial: head pulse for ischemic stroke detection [Internet]. [cited 2020 May 20]. Available from: https://clinicaltrials.ucsf.edu/trial/NCT03824496.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jovin, T.G. (2021). Direct to Angiography—An Emerging Paradigm in Large Vessel Occlusion Stroke: Rationale, Feasibility, and Preliminary Results. In: Hui, F.K., Spiotta, A.M., Alexander, M.J., Hanel, R.A., Baxter, B.W. (eds) 12 Strokes. Springer, Cham. https://doi.org/10.1007/978-3-030-56857-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-56857-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-56856-6
Online ISBN: 978-3-030-56857-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)