Abstract
Novel photocatalytic materials are currently required for advanced wastewater treatment or for self-cleaning applications. For these applications, (super)hydrophilic surfaces must be developed and UV photo-induced hydrophilicity can be a viable path to obtain such materials. This paper reports on the hydrophilicity variation of the novel TiO2/TiO2 GO composite thin films obtained by spray pyrolysis deposition (SPD) followed by the deposition of a diluted sol by spraying at 100℃. The thin films were kept in dark (in normal atmospheric conditions) and under UV irradiation, at different exposure periods (up to 72 h) and the variation in the contact angle of water were analysed along with the morphology, surface composition and roughness (RMS) to correlate the hydrophilicity with the materials properties. The results show an increase in the contact angle values over time for the samples kept in dark and a decrease of it when the films were UV irradiated mainly as results of the roughness variation, due to slight changes in the thin film (surface) composition. The thin films were further tested in methylene blue decomposition and the effect of the hydrophilicity on the photocatalytic efficiency was investigated. The results show that, in the experimental conditions, a pre-conditioning treatment of the thin films can lead to more efficient processes.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The photocatalytic processes represent potential routes for the removal, from wastewater, of organic pollutants at low concentrations, targeting water re-use [1]. For up scalable processes the photocatalytic materials and the equipment need to be affordable. In terms of materials, one of the most widely used photocatalyst is titanium dioxide, TiO2, because it has a high chemical stability in aqueous media, it is non-toxic, has high capacity to produce oxidative species when irradiated with the adequate radiation and has not too high production costs. However, as a wide band gap semiconductor, TiO2 can be activated only by UV radiation (λ < 380 nm) and this raises the costs of the entire process. This is why, VIS-active or solar-active photocatalysts are required to mitigate the process cost [2]. To reduce the TiO2 band gap, various techniques are used as doping or coupling the n-type semiconductor with a p-type one in a diode—type structure. Recently, graphene derivatives as graphene oxide (GO) or reduced graphene oxide (rGO) were used as fillers embedded in a metal oxide matrix [3]. These types of composites are reported to be Vis(solar)-active because of the n-p diode structure developed when using these materials [4], but the development of continuous, stable interfaces between the matrix and the graphene derivatives remains an important challenge. This is the reason why not TiO2—graphene composites are the targeted materials, as the non-polar graphene has little compatibility with the ionic TiO2.
The Vis-activation problem is solved when using TiO2-graphene derivatives composites and further on the photocatalytic properties of this type of samples have to be optimized to reach high performance materials. One important property of any photocatalysts is its surface hydrophilicity. This property is important particularly for applications as self-cleaning coatings, but it is also important in the pollutant adsorption step in the wastewater treatment mechanisms. It is reported that the (super)hydrophilicity of the metal oxide surfaces can be photo-induced. Wang et al. [5], were the first ones to analyse the behaviour of TiO2 when exposed to UV radiation and they concluded that the surface wettability can be controlled by irradiation to obtain surfaces with antifogging and self-cleaning properties. Different mechanisms are involved in the photo-induced hydrophilicity of the TiO2 surface, such as photo-induced oxygen vacancy generation [6] along with the adsorption of water molecules at the defects’ sites, forming hydrophilic domains [7], along with the possible photo-oxidation of the traces of hydrophobic carbonaceous species from the TiO2 structure [8]. Most of these mechanisms are reversible and the atmospheric oxygen can replace the adsorbed hydroxyl groups changing the surface wettability of TiO2 back, from hydrophilic to hydrophobic [9].
It was reported [6], that the hydrophilicity of the TiO2-rGO composites was increased by exposure to solar radiation and the water contact angle (WCA) decreased from 50° to <5° after 30 min of exposure under simulated solar irradiation. After that, the photo-induced self-cleaning properties were evaluated by measuring the degradation of methylene blue (MB) under simulated solar conditions and the material proved to be solar-active concluding that the graphene derivative is not affected by the irradiation. A similar conclusion is outlined by another study that also proved that the concentration of the filler in the composite can significantly change the wettability properties: a higher concentration of the filler can lead to a super-hydrophilic surface when irradiated only with VIS radiation [10]. Most of the hydrophilicity studies followed the development of materials for self-cleaning coatings and not for advanced wastewater treatment while studies on the behavior of these composites kept in dark (without any irradiation) are scarce.
This paper reports on the hydrophilicity variations of the novel TiO2/TiO2—grapehene oxide (GO) two-layered composite thin films obtained by spray pyrolysis deposition (SPD) coupled with spraying (at lower temperature) a sol of the composite material. The surface morphology, composition and roughness (RMS) were discussed to correlate the hydrophilicity variation with the material’s properties. The methylene blue (MB) decomposition, under simulated solar radiation at low irradiance values, was investigated to outline the influence of the WCA values on the photocatalytic efficiencies for advanced wastewater treatment applications.
2 Materials and Methods
Glass plates (1.5 cm × 1.5 cm) covered with a thin film of fluorine doped tin oxide (FTO) were used as substrates. These substrates were cleaned with water and detergent by ultra-sonication, followed by rinsing in ethanol and drying in air.
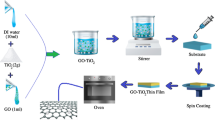
The composite thin films were deposited in a two-layered system in order to get a performance material in photocatalytic processes with controlled hydrophilicity. The first TiO2 layer was obtained using a precursor system containing titanium tetra-isopropoxide (TTIP, Sigma Aldrich, 97%) mixed with acetylacetone (AcAc, Scharlau, 99%) and ethanol (EtOH, Chemical Company, 99.3%) in a 1:1:15 volume ratio. This layer was deposited by Spray Pyrolysis Deposition (SPD) using an ABB/IRB5400 robot. The deposition temperature was 400℃ and 30 spraying sequences were used, with 60 s break between consecutive pulses. After deposition, this first layer was annealed for 3 h, at 450℃, to increase its crystallinity degree.
The second TiO2-GO layer was sprayed on the top of the first one using a diluted sol obtained based on TTIP as precursor, ethanol as continuous medium, acetylacetone as complexing agent and acetic acid (HAc 99.8%, Scharlau) to control the pH (pH ~5). These were mixed, under stirring, in an overall volume ratio: TTIP:EtOH:AcAc:HAc = 1:0.8:0.04:0.009.
An aqueous GO dispersion (30 mg/mL) was obtained using the Hummers method: 12 g of graphite powder (99.99% purity, Elektrokarbon) were mixed with 247 mL H2SO4 (96%, VLSI grade, BASF) and continuously stirred for 24 h. Afterwards, 6 g NaNO3 (99%, Sigma Aldrich) were added and the dispersion was cooled down to 5 ℃, then 36 g of KMnO4 (99%, Merck) were slowly added for a good control of the exothermic reaction. Further on, the graphite powder was kept in the oxidation environment for 4 days; after that the excess KMnO4 and the manganese oxide precipitate were removed using 200 mL H2O2 5% and 150 mL HCl 5% respectively. The dispersion was then filtered and the graphite oxide powder was washed several times with deionized water. This powder was further dispersed in deionized water and placed in an ultrasonic bath to support the exfoliation of the graphene oxide sheets.
The GO dispersion, prepared as previously discussed, was added in the TiO2 precursor system, to obtain the composite sol with stable interfaces between TiO2 and the graphene derivative. Further on, the sol was sonicated for 1.5 h and after sonication, the sol was diluted with ethanol in a 1:5 sol: EtOH volume ratio. The GO weight ratio in the precursor system was 1.4%w.
This diluted sol was deposited, by robotic spraying, over the first TiO2 layer, using 15 spraying sequences, with 60 s break between pulses. The deposition temperature was selected at 100℃ to support the ethanol evaporation while preventing the GO oxidation. The composite thin film was further thermally treated for one hour at 150℃ to decompose/remove the carbon containing by-products, without degrading the GO filler [11].
This two-layered composite structure was selected to increase the crystallinity degree of the composite upper layer by using a first layer that has a predominant crystalline structure, considering the low temperature of the thermal treatment applied to the final thin films. Further on, previous results outlined that the adherence between the composite and the substrate is increased by using an intermediate matrix layer.
The thin film composites obtained as above discussed were kept under various conditions: in dark (in regular atmospheric conditions) and under UV irradiation (irradiance value, G = 8.3 W/m2, measured using a Delta-T, type BF3 pyranometer), at different exposure periods (up to 72 h) and the WCA variations were analyzed using a DataPhysics OCA20 EC instrument. The volume of the water drop was 10 µL. The surface elemental composition was analyzed by Energy Dispersive X-ray spectrometry (EDS, Thermo) while the surface morphology and roughness (RMS) were investigated using Scanning Electron Microscopy (SEM, Hitachi model S-3400 N type II) and Atomic Force Microscopy (AFM, NT-MDT model BL222RNTE). The results were correlated with the photocatalytic efficiencies recorded in the photo-degradation of the methylene blue (MB) pollutant, as indicated by the ISO 10678:2010 [12], standard.
The photo-degradation experiments were developed in static regime using a 10 ppm (3.125 × 10–5 M) MB solution and a photo-reactor equipped with 2 UV light tubes (Philips, TL-D BLB 18 W/108) and 5 Vis light tubes (Philips, TL-D Super 80 18 W/865). This mix simulates solar radiation conditions but at much lower irradiance value; the total irradiance was G = 55 W/m2, out of which 3 W/m2 corresponds to the UV radiation. Before the photocatalytic tests, the films were kept in dark for a shorter time (24 h) and for a longer duration (7 days) and were subsequently pre-conditioned under UV radiation for 24 h. These films were immersed in 20 mL MB solution and left one hour in dark to reach the adsorption/desorption equilibrium, as previously optimized [13]. After one hour in dark the thin film samples were continuously irradiated for 8 h. The MB removal efficiency, η was calculated using Eq. (26.1), based on the initial absorbance of the MB solution (A0) and the absorbance after up to 8 h of irradiation (A8), measured at the maximum absorbance wavelength for MB (λ = 664 nm), using a UV–Vis–NIR spectrophotometer (Perkin Elmer Lambda 950):
3 Results and Discussions
The investigations on the hydrophilic character show an increase in the WCA values over time, for the samples stored in dark (Fig. 26.1) and, respectively, the decrease of the WCA value when exposing the films to UV radiation (Fig. 26.2). The optimum irradiation duration was found to be of 24 h. After this period of time the values of the contact angles remained constant.
It can be also observed that for the sample containing only TiO2 SPD kept in dark the WCA variation is rather low, indicating that this type of coatings can be stored without any significant consequences on the hydrophilic properties. For the composite sample, the storage in dark leads to a significant increase in the WCA values, thus a reduction in the hydrophilic character.
To understand these changes in hydrophilicity in different conditions, characterization analyzes were performed. According to the results in Tables 26.1 and 26.2, it can be noticed that the hydrophilicity increase over time when irradiated with UV radiation is due to the variation of the film roughness correlated with the decrease in the carbon content from the surface of the samples. For the films kept in the dark, the hydrophilicity decreases and the RMS values have a constant increase over time while under UV irradiation the RMS values decrease in time. These results are attributed to the possible densification of the thin films and less to the oxidation of the carbon existing in the sample from the sol precursors, under UV irradiation. It is also possible a continuation of the reactions in the composite for the samples kept in dark and for the ones irradiated during the first 24 h, when UV radiation can accelerate the processes. Previous results of the photocatalysis tests performed under UV + VIS irradiation compared with those under UV irradiation, prove the Vis-activation of the composites and also that the graphene oxide is not affected by the exposure to UV irradiation (under the experimental conditions).
The SEM images of the composite films kept in dark, Fig. 26.3, outline large aggregates on the surface of the composite films, compared to the initial structure concluding that the increase in the RMS values can be due to these aggregates and hence the decrease of the sample hydrophilicity.
In good agreement with the SEM images, the AFM results, Fig. 26.4, outline surface changes of the composite thin film under UV irradiation and in dark.
To correlate the variation of the hydrophilic character with the performances of the films during the photocatalytic processes, photo-degradation tests of the MB pollutant were performed, under UV + VIS irradiation, in static regime, on the multilayered composite films and, for comparison, on the TiO2 SPD films. The films were kept, as previously discussed, in dark for 24 h and for a long duration (7 days) and were further pre-conditioned under UV irradiation for 24 h.
The photocatalytic results included in Table 26.3 show that the samples stored for long-term in dark lead to lower photo-degradation efficiencies compared to the more recently deposited and UV pre-conditioned films. The results can be correlated with the higher WCA values for the samples kept 7 days in dark, in agreement with the results already discussed. From Table 26.3 it can also be observed that the adsorption (1 h dark) is lower for the samples kept in dark, and the results show that this step depends on the hydrophilicity of the films. It may be noticed that the composite thin films have a small variation in the MB removal efficiency (smaller than 4.5%) and that UV conditioning is important.
4 Conclusions
The hydrophilicity of the FTO/TiO2 SPD/TiO2—GO SG composite thin films varies depending on the conditions under which the samples are exposed, namely: the WCA increases over time for the samples kept in dark and decreases after irradiation of these samples with UV radiation. This is mainly the consequence of the variation in the roughness values of the films, in correlation with the morphology and the surface composition. The variation of the hydrophilicity mainly affects the pollutant adsorption step and hence the photocatalytic efficiencies are lower for the samples with a higher contact angle. However conditioning the thin film through UV irradiation allows reaching similar efficiencies as in the case of recently deposited samples and for the improvement of the photocatalytic efficiency, a first step of pre-conditioning the films by UV irradiation is required followed by the photocatalysis process, especially when the films were stored for longer periods before use.
References
M.N. Chong, B. Jin, C.W.K. Chow, C. Saint, Recent developments in photocatalytic water treatment technology: a review. Water Res. 44(10), 2997–3027 (2010)
J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo, D.W. Bahnemann, Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev. 114(19), 9919–9986 (2014)
S.K. Fanourakis, J. Peña-Bahamonde, P.C. Bandara, D.F. Rodrigues, Nano-based adsorbent and photocatalyst use for pharmaceutical contaminant removal during indirect potable water reuse. Npj Clean Water 3(1) (2020)
C. Chen, W. Cai, M. Long, B. Zhou, Y. Wu, D. Wu, Y. Feng, Synthesis of visible-light responsive graphene oxide/TiO2 composites with p/n heterojunction. ACS Nano 4(11), 6425–6432 (2010)
R. Wang, K. Hashimoto, A. Fujishima, M. Chikuni, E. Kojima, A. Kitamura, M. Shimohigoshi, T. Watanabe, Light-induced amphiphilic surfaces. Nature 388, 431–432 (1997)
S. Prabhu, L. Cindrella, O. Joong Kwon, K. Mohanraju, Superhydrophilic and self-cleaning rGO-TiO2 composite coatings for indoor and outdoor photovoltaic applications. Sol. Energy Mater. Sol. Cells 169, 304–312 (2017)
M. Janczarek, J. Hupka, H. Kisch, Hidrophilicity of TiO2 exposed to UV and VIS radiation. Physicochemical Probl. Mineral Process. 40, 287–292 (2006)
T. Zubkov, D. Stahl, T.L. Thompson, D. Panayotov, O. Diwald, J.T. Yates, Ultraviolet light-induced hydrophilicity effect on TiO2(110)(1×1). Dominant role of the photooxidation of adsorbed hydrocarbons causing wetting by water droplets. J. Phys. Chem. B 109(32), 15454–15462 (2005)
M. Miyauchi, N. Kieda, S. Hishita, T. Mitsuhashi, A. Nakajima, T. Watanabe, K. Hashimoto, Reversible wettability control of TiO2 surface by light irradiation. Surf. Sci. 511(1–3), 401–407 (2002)
M.K. Kavitha, L. Rolland, L. Johnson, H. John, M.K. Jayaraj, Visible light responsive superhydrophilic TiO2/reduced graphene oxide coating by vacuum-assisted filtration and transfer method for self-cleaning application. Mater. Sci. Semicond. Process. 113, 105011 (2020)
X.Z. Tang, W. Li, Z.-Z. Yu, M.A. Raffie, J. Raffie, F. Yavari, N. Koratkar, Enhanced thermal stability in graphene oxide covalently functionalized with 2-amino-4,6-didodecylamino-1,3,5- triazine. Carbon 49, 1258–1265 (2011)
* * * INTERNATIONAL STANDARD ISO 10678:2010, Fine ceramics (advanced ceramics, advanced technical ceramics)—Determination of photocatalytic activity of surfaces in an aqueous medium by degradation of methylene blue
A. Enesca, M. Baneto, D. Perniu, L. Isac, C. Bogatu, A. Duta, Solar-activated tandem thin films based on CuInS2, TiO2 and SnO2 in optimized wastewater treatment processes. Appl. Catal. B 186, 69–76 (2016)
Acknowledgements
This work was supported by the grant of the Romanian Ministry of Research and Innovation, CCCDI-UEFISCDI, project number PN-III-P1-1.2-PCCDI-2017-0619, contract no. 42 PCCDI/2018 within PNCDI.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Tismanar, I., Obreja, A.C., Buiu, O., Duta, A. (2020). Hydrophilicity Variation of TiO2—Graphene Oxide Composite Thin Films for Photocatalytic Applications. In: Visa, I., Duta, A. (eds) Solar Energy Conversion in Communities. Springer Proceedings in Energy. Springer, Cham. https://doi.org/10.1007/978-3-030-55757-7_26
Download citation
DOI: https://doi.org/10.1007/978-3-030-55757-7_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-55756-0
Online ISBN: 978-3-030-55757-7
eBook Packages: EnergyEnergy (R0)