Abstract
Stem cells play critical roles in biological processes, such as tissue development and homeostasis; they also present great promise toward promoting breakthroughs in regenerative medicine. Stem cells may be used to explore disease modeling, for screening of new drugs, and for the treatment of intractable diseases. The transition of stem cell biology from basic research to clinical applications has involved both hope and hype. Indeed, premature application of stem cell therapy as a clinical “cure-all” without sufficient experimental, preclinical, or clinical research has led to shady practices and false promises. While this is, understandably, fueled by patients in need of cures for unmanageable chronic and degenerative diseases, hype-based practices have promoted the spread of clinically unproven therapies. These therapies may have no impact on the disease process or may result in devastating outcomes. Stem cells may ultimately have the capacity to treat intractable diseases, including diabetes, cardiovascular disorders, metabolic disorders, hematopoietic disorders, and immunodeficiency disorders. However, and despite significant promise, there remains a need to elucidate numerous misunderstandings associated with stem cell therapy and to define the current barriers and obstacles faced by those involved in stem cell research and its therapeutic applications. As such, the main goal of this chapter was to provide the reader with an overview of basic concepts in stem cell research and review the facts and the unfortunate hype with respect to current clinical applications and disease treatments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Stem cells
- Basic concepts
- Stem cell therapy
- Hematopoietic disorders
- Chronic diseases
- Bone marrow transplantation
This chapter provides the introduction and overview of stem cells, their definition, origin, and applications. It illustrates the unique properties of stem cells, such as potency, multilineage differentiation potential, self-renewal, and resistance to senescence and apoptosis. It provides a brief description of stem cell research, and its current applications in cell therapy, bone marrow transplantation, tissue engineering and its modern and diverse applications. These will cover approved human stem cell products, and therapies based on cells or their derivatives. Finally, the chapter will cover the gap between research and clinical applications, and concludes with the facts, hope, and hype in stem cell research and development.
1.1 What Is a Stem Cell?
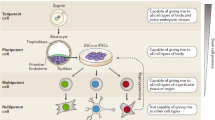
A stem cell is an unspecialized and undifferentiated cell that has a remarkable capacity for self-renewal and the ability to undergo prolonged periods of cell division, both in vitro and in vivo. Stem cells are also capable of asymmetrical division into two non-identical daughter cells with distinctive and different fates. Among the earliest evidence of the existence of stem cells were the breakthrough studies conducted in the early 1960s, when the radiation physicist, James Till, joined with the hematologist, Ernest McCulloch, to study the effects of radiotherapy on hematological cancers in the bone marrow. Among their findings, Till and McCulloch identified a self-renewing population of hematopoietic cells originating in the bone marrow that were capable of generating all blood cell lineages; they named these progenitors “stem cells” [1,2,3].
Unlike other types of cells, stem cells have the capacity to differentiate into various specialized cells and cell lineages under defined physiological, pathological, and/or experimental conditions. The regenerative capacities are high among younger individuals; aging is associated with lower regenerative potential [4,5,6]. Moreover, in a mature organism, some organs, such as the blood and intestinal epithelium, maintain a higher rate of regeneration throughout life, whereas other organs, including the heart and pancreas, have limited potential for repair [7]. Stem cells can be classified based on their differentiation capacity into totipotent, pluripotent, multipotent, oligopotent, and unipotent cells, as shown in Fig. 1.1. Totipotent stem cells exhibit the highest capacity for differentiation of any cell in an entire organism, the notable example of this phenomenon is the zygote (i.e., a fertilized egg) which has the capacity to give rise to all embryonic and extraembryonic structures [8, 9]. Pluripotent stem cells , such as embryonic stem cells (ESCs), are somewhat less potent and are capable of generating embryonic tissues only (i.e., the three germ layers, mesoderm, endoderm, and ectoderm [10]). Lineage specific multipotent stem cells such as mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs) have a more restricted capacity for differentiation and give rise to their specific tissues and cell types [11]. Oligopotent stem cells are even more restricted but maintain the capacity to differentiate into specific cells within specific tissues. A good example of an oligopotent stem cell is the common lymphoid progenitor (CLP) , which can give rise to T lymphocytes, B lymphocytes and natural killer cells [12, 13]. Unipotent stem cells are the most restricted, as they are capable of generating cells of a single lineage; examples of unipotent stem cells include epidermal stem cells of the skin [14, 15], myogenic precursors [16], and spermatogonial stem cells [17].
It is generally understood that the capacities for self-renewal and differentiation diminish as cells become more specialized. However, this dogma was recently challenged by the successful reprogramming of fully differentiated somatic cells into a pluripotent-like state in the form of somatic cell nuclear transfer (SCNT) [18] and likewise via the induction of pluripotent stem cells (iPSCs), first described in 2006 [19].
1.2 Origin and Types of Stem Cells
Stem cells are classified as embryonic or adult stem cells based on their source of origin (as shown in Fig. 1.1). Tissues associated with pregnancy, including the placenta, amniotic fluid, umbilical cord, and Wharton’s jelly, among others, are all rich in stem cells. Likewise, iPSCs are cells produced by the direct reprogramming of somatic cells into pluripotent stem cells. A comparison of the properties of embryonic, adult, and iPSCs is presented in Table 1.1.
1.2.1 Embryonic Stem Cells (ESCs)
ESCs can be collected from the inner cell mass of pre-implantation embryos 3–5 days following fertilization. ESCs are pluripotent cells that have the capability to divide for extended periods of time and to differentiate into cells of each of the three germ layers [10, 20]. This robust differentiation potential qualifies ESCs as the best-known source of cells that can be used to generate fully differentiated cells for cell therapy applications [21, 22]. Ethical concerns related to the destruction of human embryos have hampered the full application of ESCs, which are isolated from spare/discarded embryos that were generated to support in vitro fertilization (IVF) procedures and not from healthy in utero-implanted ones [23,24,25].
1.2.2 Adult Stem Cells
Somatic or adult stem cells are rare populations of undifferentiated cells that are found among their differentiated counterparts throughout the adult body. These cells contribute to tissue homeostasis, as they serve as a source of raw material for repair and/or replacement of injured or dead cells [5]. Adult stem cells have only a limited range of differentiation potential when compared with ESCs. Examples of adult stem cells include the following:
-
Mesenchymal Stem Cells (MSCs)
MSCs are adherent fibroblast-like cells when cultured in vitro. They were first isolated from the bone marrow [26, 27], where they are most abundant. They produce colony forming-unit fibroblast (CFU-F), when cultured in vitro and are distinguished by the capacity to differentiate into osteocytes, chondrocytes, and adipocytes. There are numerous sources of MSCs including bone marrow [28], adipose tissue [29], dental pulp [30], and synovial membranes [31].
-
Hematopoietic Stem Cells (HSCs)
HSCs have been isolated from the bone marrow; they have the capacity for self-renewal as well as the ability to differentiate into all blood cell lineages [3]. They are widely used clinically in HSC transplantaion for treating various blood disorders and malignancies.
-
Neural Stem Cells (NSCs)
NSCs are found in the central nervous system; they have the potential to differentiate into both neuronal and non-neuronal glial cells [32]. As such, they have been used clinically in efforts to repair injuries sustained by the nervous system [33, 34]. Currently, the use of NSCs for treating neurodegenerative diseases is under investigation [35].
1.2.3 Other Stem Cells
The discovery of stem cells in the human umbilical cord blood (UCB) paved a new and useful source of progenitors; notably umbilical cord blood hematopiotic stem cells (UCB-HSCs) have become a viable source of autologous bone marrow stem cells. UCB-HSCs are capable of differentiating into multiple hematopoietic lineages, in addition to their capacity for long-term self-renewal [36, 37]. Clinically, UCB stem cells have been employed successfully as HSC transplants in 1988 [38]. As such, parents in some countries now routinely bank the UCB of newborns so as to have a source of HSCs in the advent of any childhood hematological disorders or malignancies. Likewise, as noted earlier, MSCs have been identified in extraembryonic tissues, including Wharton’s jelly [39], amniotic membrane and placenta [39, 40], and amniotic fluid [41].
1.2.4 Induced Pluripotent Stem Cells (iPSCs)
iPSCs are generated in vitro in an effort to imitate the potential of ESCs by effectively reversing the differentiation of somatic cells (e.g., skin fibroblasts) in order to become pluripotent [19, 42]. The discovery of iPSCs was driven at least in part by the need to identify ESC-like pluripotent stem cells for clinical use which could be generated without raising strong ethical concerns. Many ongoing efforts are aimed at improving current reprogramming approaches so as to enhance the current clinical applicability of iPSCs.
1.3 Stem Cell Therapies: The Present and the Future
The remarkable potential of stem cells, including their capacities for self-renewal and differentiation, has led to their use in numerous clinical applications, including cell-based therapies [59], drug discovery [60], and tissue engineering [61]. The ultimate goal of stem cell-based therapies is to treat, repair, or replace diseased tissues or organs with ones that are new, healthy, and functional [62, 63]; numerous applications of this type are presented in Fig. 1.2. Therefore, stem cells are currently featured in several thousand ongoing clinical trials focused on disease treatment.
Most of these protocols focus on the use of stem cells for treating hematological disorders, including myeloid leukemia; lymphoma; sickle cell anemia; immune deficiencies; β-thalassemia [64,65,66,67]; wound healing and skin injuries [68]; neurological disorders, such as Parkinson’s diseases and spinal cord injury [69, 70]; autoimmune disorders, such as multiple sclerosis, rheumatoid arthritis, Crohn’s disease, and type-1 diabetes [71,72,73,74]; and cardiac diseases, including ischemic heart disease [75]. Promising trials, which focus on the use of stem cells to treat ocular disorders, including macular degeneration and retinitis pigmentosa [76, 77], and bone diseases, including osteosarcoma, osteoporosis, and osteoarthritis, are also in progress [78, 79]. So far, only a handful of the U.S. Food and Drug Administration (FDA) approved stem cell products are available for clinical use, including allogeneic cord blood hematopoietic stem/progenitor cells for treating hematological and immunological disorders (https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products). Currently approved stem cell-based therapies are listed in Table 1.2.
1.3.1 Routine Stem Cell Therapy for Hematopoietic Disorders
1.3.1.1 Hematological Malignancies
Transplantation of unmodified or genetically modified HSCs derived from different sources offers a promising approach to the reconstitution or replacement of diseased cells. Cell therapies for hematological disorders, such as hemoglobinopathies (e.g., sickle cell anemia) and blood malignancies (e.g., leukemia and lymphoma), have undergone substantial development over the past few decades, as in the examples discussed below [80].
1.3.1.1.1 Leukemia
Leukemias are a group of white blood cell malignancies classified by the World Health Organization (WHO) based on genetics, morphology, immunophenotype, and clinical features [81, 82]. Interestingly, one of the earliest known cases of leukemia was identified based on the findings from an Egyptian skeleton in dating back to 2160–2000 BCE [83]. Leukemias are classified into several major subtypes, including acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), and chronic lymphoblastic leukemia (CLL) [84]. Chemotherapy was an initially effective treatment for childhood ALL when first attempted in 1948; unfortunately, disease typically relapsed ultimately leading to death [85, 86]. Currently, the standard treatment includes combination chemotherapy to destroy the defective hematopoietic system followed by hematopoietic stem cell transplantation (HSCT ) [87, 88]. This approach is particularly indicated for recurrent disease, and can be introduced shortly after first-line treatment with chemotherapy [89, 90]. HSCs can be derived from the bone marrow (BM), umbilical cord blood (UCB), or peripheral blood (PB) [91]. The first successful allogeneic human bone marrow transplantation (BMT) performed in patients with leukemia following optimized radiation and chemotherapy doses resulted in a Nobel Prize in Medicine for Dr. E. Donnall in 1990 [92]. However, histocompatibility mismatching and graft rejection resulted in high relapse rates ; as such, the disease relapsed and the success rate was low [93]. Among the efforts made to improve these outcomes, donor leukocyte infusions (DLI) were introduced, by providing immune cells pre-collected from the anticipated HSC donor following myeloablation in patients undergoing leukemia treatment; the goal was to establish donor chimerism and thereby preventing graft rejection [94]. Although, DLI was effective in managing disease relapse, it was related to the development of graft versus host disease (GvHD) in treated patients, resulting from the activity of effector donor T-cells [95]. Reduced-intensity conditioning (RIC) was also applied in an effort to control graft versus host disease (GvHD), while enhancing the graft versus leukemia effect (GVL), thereby maintaining engraftment and eradicating malignancy [96]. The use of less aggressive RIC and non-myeloablative conditioning reduces the overall toxicity and mortality associated with conditioning prior to transplantation, especially in older patients [96].
The relatively recent inclusion of UCB as a source for HSCs overcame the challenges associated with an attempt to locate an HLA-matched allogeneic donor [97]. UCB cells were also less immunogenic and also easy to collect; UCB cells cryopreserved for decades still support the efficient recovery of HSCs [98]. However, UCB maintains comparatively fewer HSCs with respect to adult weight; as such, two bags of cord blood are typically required in order to obtain a sufficient yield of HSCs for transplantation into a single patient [99,100,101]. Nonetheless, a long-term follow-up of the Eurocord–European Group for Blood and Marrow Transplantation study revealed encouraging results. The study evaluated the outcome of UCB transplantation for 147 children, among whom 74% had been diagnosed with acute leukemia. In these patients, the cumulative incidence of neutrophil recovery was 90% at 2 years post-transplantation, the incidences of acute and chronic GvHD were reported to be 12% and 10%, respectively. At 5 years post-transplantation, the cumulative incidences of relapse and non-relapse mortality were 47% and 9%, respectively; the probability of disease-free survival was 44%. These results stand in strong support of UCB banking and the use of cord blood units to facilitate HLA-identical cord blood transplantation (CBT) [102].
PB-HSCs can be collected by noninvasive means; this provides a safe procedure for both the donor and recipient who can then undergo more rapid engraftment [103]. Administration of recombinant granulocyte colony-stimulating factor (G-CSF) stimulates the release of endogenous HSCs from the BM and into the blood. Currently, about 80% of all allogeneic transplantations are performed using stem cells derived from the PB of adult patients [104]. Similarly, recent developments in targeted therapy approaches have resulted in improved outcomes and can eliminate the negative sequelae associated with indiscriminate cytotoxic myeloablation. Genetically modified T-cells that express antigen-specific chimeric antigen receptor (CAR) will target leukemic cells while sparing those that are otherwise normal [105]. The FDA has approved the use of autologous genetically modified CD19-lymphocyte cells (CAR T-cells) for the treatment of relapsed ALL and diffuse large B-cell lymphoma [106].
1.3.1.1.2 Sickle Cell Anemia
In addition to traditional HSC transplantation, it is now possible to manipulate the diseased cells by removal, addition, or alteration of specific DNA sequences in order to correct defective or mutated genes. High efficiency and precise genetic manipulation or gene editing of the human genome has recently become possible with the use of the method known as clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 [107]; this procedure is outlined in Fig. 1.3. CRISPR/Cas9 was used to restore the normal blood cell phenotype by repairing CD34+ hematopoietic stem/progenitor cells (HSPCs) from patients diagnosed with sickle cell anemia, a disorder that typically results from a single nucleotide substitution within a β-globin gene [108]. The gene-edited HSPCs were transplanted back into the patient’s BM to function as a source of healthy autologous red blood progenitors; using this method the disease undergoes genetic correction, and graft rejection is evaded [108].
1.3.2 Stem Cell Therapy in Clinical Trials
1.3.2.1 Skin Injuries and Wound Healing
Skin is the largest organ in the body and a major part of the integumentary system that covers and protects the human body [109]. Physical, chemical, and biological factors can all disrupt skin integrity. Depending on the depth of injury, skin wounds can be epidermal, or they can involve either partial or full skin thickness [110]. The natural healing mechanisms are compromised by third- and fourth-degree burn injuries; this presents a significant challenge for both the surgeons and patients. Over the past century, the gold standard for treating burns has been grafting of healthy skin. Skin grafting can include split-thickness skin graft (STSG) and full-thickness skin grafts (FTSG) [111, 112]. Skin grafting involves the transfer of healthy skin (autograft or allograft) comprised of the epidermis and a portion of the dermis to the site of injury; problems arise when there is not enough healthy skin, a failure to treat deep wounds, a poor cosmetic outcome, and limited strength of grafted skin when compared with the original skin at the affected site [113]. Skin engineering thus represents an attractive alternative. Autologous keratinocytes or fibroblasts are cultured on a scaffold, in some cases, a scaffold alone is implanted into the wound to improve healing [114]. This technique results in the regeneration of both the epidermal and dermal layers; however, this method did not facilitate the regeneration of skin appendages, including hair, nails and skin glands. Of note, traditional skin grafting also failed to regenerate skin appendages; however, pigmented melanocytes and neural and vascular tissues were recovered using this method, an outcome that was not achieved using the engineered skin [114]. Skin replacements can be generated using cellular or acellular scaffolds; based on the composition of the skin-substitute [115]. Acellular skin-substitutes are biodegradable scaffolds (e.g., collagen, elastin, and silicon, among others) that facilitate wound healing by recruiting fibrocytes and vascular cells in vivo and by inhibiting granulation and scar formation. The most common acellular skin-substitutes currently approved by the FDA and undergoing review in clinical trials include Integra® [116], Alloderm™ [117], and NovoSorb™ BTM (Biodegradable Temporizing Matrix) [118]. Cellular skin-substitutes that contain epidermal cell sheets include Dermagraft® and Apligraf®; these products were approved by the FDA for the treatment of diabetic foot ulcer [118, 119]. ReCell® is an FDA-approved commercial cell spray device that provides autologous keratinocytes designed to heal second-degree burns. ReCell® works by facilitating enzymatic digestion of the patient’s healthy skin in order to harvest keratinocytes, which are then sprayed over the wound [120, 121]. Commercially available skin-substitutes are still far from perfect. The cells frequently fail to integrate; show poor vascularization, weak mechanical integrity, and scar formation; and are subjected to immune-mediated rejection [109]. Indeed, there are no completely functional skin-substitutes available at this time; of particular note, there is a great need for a functional skin-substitute that can undergo rapid vascularization. Recent advances in stem cell therapy, nanotechnology, tissue engineering, and microfluidics paved the way for improved skin tissue engineering focused on deep wound healing [122]. Bioscaffolds for skin engineering must all be biocompatible, nontoxic, non-immunogenic, biodegradable, and sufficiently porous so that free exchange of gases and nutrients can occur through a neo-vascularized functional skin-substitute [123]. The cell source for the engineered skin also has a significant impact on the outcome. For example, ESCs can be differentiated into both keratinocytes [124] and fibroblasts [125], but direct clinical applications of these cells are hampered by instability and concerns with respect to the functionality of the resultant tissues. Adipose-derived stem cells (ADSCs) can also differentiate into keratinocytes, fibroblasts, and other skin components; ADSCs also produce extracellular matrix (ECM) which is rich in growth factors and cytokines that enhance healing [126,127,128]. The ADSC secretome contains vascular endothelial growth factor (VEGF), growth differentiation factor (GDF-11), and transforming growth factor (TGF-β); all of these act on macrophages, fibroblasts, and endothelial cells and lead to limiting the immune responses, enhancing cell proliferation, and promoting angiogenesis at the transplantation site [129]. Clinical applications of autologous ADSCs are still under investigation for healing diabetic foot ulcers (NCT02092870, see https://www.clinicaltrials.gov) [130]. Furthermore, methods used to generate three-dimensional skin grafts using iPSC-derived keratinocytes and fibroblasts remain promising [131].
1.3.2.2 Osteoarthritis
Osteoarthritis (OA) is a chronic degenerative disease characterized by deterioration of joint articular cartilages; this results in exposed subcondylar bones and leads to friction, pain, and synovitis [132]. Globally, OA is currently estimated as the 11th highest contributor to adult disability; this results largely from pain, stiffness, and impaired mobility due to disease affecting the knees, feet, hands, and spine joints [133]. Non-surgical approaches for treating OA include intra-articular injections of corticosteroids, hyaluronic acid “viscosupplementation,” or autologous platelet-rich plasma into the deteriorating joints [134,135,136]. These approaches are designed to alleviate pain, but they do not treat the underlying cause of mechanisms associated with OA [137]. Joint surgery for OA varies from whole knee replacement (arthroplasty) to minimally invasive arthroscopic techniques such as microfracture or microdrilling [138,139,140]. The aforementioned arthroscopic techniques involve the generation of multiple small fractures within the affected joint, promoting the recruitment of progenitor cells from the underlying BM which then undergo differentiation into chondrocytes [139]. The drawbacks of these approaches include the formation of an inferior form of cartilage that lacks mechanical durability [138].
Alternative cell-based approaches have been applied, including osteochondral transplantation and soft tissue grafting [141]. Among the problems associated with these approaches, outcomes have included poor grafting and integration, calcification of the grafts, and limited number of available donor tissues [142, 143]. Accordingly, more effort has been directed toward autologous/allogeneic chondrocyte implantation (ACI) [144]. Currently, there are numerous phase III clinical trials involving ACI that include the expansion of autologous or allogeneic chondrocytes, followed by grafting into the deformed lesion [145]. As an example, a phase III clinical product that is now commercialized with the brand name Chondrosphere® utilizes scaffold-free spheroids of chondrocytes obtained from autologous articular cartilage that are introduced for use to treat cartilage defects associated with hip injuries (NCT01222559) [146]. The challenges currently encountered include increased susceptibility of the donor to OA after tissue sampling in normal joints and an overall insufficient number of harvested chondrocytes. Likewise, expanded chondrocytes may undergo dedifferentiation and lose their ability to generate cartilage matrix [147].
MSCs have also emerged as a promising source of cells for this application owing to their robust capacity for expansion and chondrogenic differentiation [148, 149]. In addition, MSCs secrete a variety of cytokines and growth factors with anti-inflammatory effects [150]; these cytokines may function to counteract the inflammatory processes associated with OA. Autologous bone marrow-derived MSCs have been used to repair full-thickness cartilage defects in two cases [151]. In this study, BM was aspirated from the iliac crests and cultured until adherent MSCs had undergone several expansion passages. Cultured MSCs were then collected, embedded in a collagen-gel scaffold, and transplanted onto the surface of the defective articular in the knee joint. Symptoms were relieved at 6 months, and both male and female patients were satisfied with the outcomes during the 4 years following transplantation [151]. MSCs derived from the umbilical cord, placenta, Wharton’s jelly or amniotic membrane all have shown promise with respect to novel treatments for patients diagnosed with OA [152,153,154]. In particular, UC-MSCs exhibited higher proliferative, clonogenic, anti-inflammatory, and chondrogenic potential compared with MSCs from maternal-derived decidua or BM [155]. CARTISTEM® is a commercialized product that utilizes UC-MSCs for the treatment of cartilage deterioration in patients with OA; it is currently approved for a phase III clinical trial with the goals of evaluating safety and expanding its indications for use (NCT01041001, NCT01626677). Recently, phase II clinical trials have been initiated to assess the role of ADSCs for the treatment of patients with OA (NCT02838069) [78].
1.3.3 From Bench to Bedside
1.3.3.1 Diabetes Mellitus (DM)
Diabetes mellitus (DM) is a chronic inflammatory metabolic disorder that results in sustained hyperglycemia due to defects in insulin production (Type I), insulin utilization (Type II), or a combination of both [156]. Type I DM (T1DM) is an autoimmune disease, wherein activated immune cells attack insulin-secreting β-cells in the pancreas, resulting in insulin deficiency [157]; contrarily, type II DM (T2DM) is characterized as a chronic inflammation state that ultimately leads to insulin resistance, reduced insulin secretion, β-cells exhaustion, and apoptosis [158,159,160]. Untreated DM leads to severe complications that can be life-threatening and have significant impact on numerous major organs including the kidneys [161], heart [162, 163], eyes [164, 165], and nervous system [166].
Patients with diabetes attempt to regulate their blood glucose levels and to maintain values at or near normal limits with dietary control [167], hypoglycemic drugs [168], and lifestyle changes [169]. However, these traditional methods often fail to maintain normoglycemia in the long run [170]. Islet transplantation (also known as Edmonton protocol) was developed in 1999 to provide more β-cells and thus increase insulin production for patients diagnosed with T1DM [171,172,173]. However, the use of this approach was limited due to the risks associated with the surgical procedure [174], the need for long-term immunosuppressive therapy [175], a shortage of organ donors [176], and only limited impact with respect to achieving insulin independence [177].
Stem cell-based therapy provides a new approach for the management and treatment of DM . First, this approach can create a virtually unlimited supply of insulin-producing cells [178,179,180,181]; other applications focus on restoring β-cell function [182], modifying immune dysregulations, and reversing the associated metabolic complications [183]. Pluripotent ESCs were successfully differentiated into β-cells in vitro [184, 185]; results in vivo revealed that insulin production and normal blood glucose level were sustained at 3 months post-transplantation [186]. Despite these promising results, there are few clinical trials addressing this approach, and there is currently no reliable information on its safety or efficacy (https://www.clinicaltrials.gov/).
Considering the different embryological origins of MSCs and pancreas, MSCs showed variable responses to the efforts made toward differentiating them into pancreatic β-cells. For instance, BM-MSCs failed to adopt functional characteristics of β-cells when cultured in vitro [187]; contrarily, ADSCs revealed some genetic and morphological similarities to pancreatic cells [188, 189]. However, MSC-mediated immunomodulation and inhibition of autoimmune progression may be achieved by educating autoreactive T lymphocytes, an approach in which the autoreactive T-cells are being regulated to be less reactive to the patient’s own islet cells, thereby reducing the extent of β-cell destruction in patients diagnosed with T1DM [181, 190, 191]. Moreover, for T2DM patients, the transplantation of autologous MSCs would reduce the associated inflammatory reactions and promote pancreatic healing [181, 192, 193]. Several clinical trials (NCT03343782, NCT01068951 and NCT01759823) demonstrated that autologous BM-MSC transplantation was a promising approach , as it coupled long-term efficacy and safety vis à vis the diabetic microenvironment [194,195,196]. Results from a limited number of trials for T1DM patients revealed improved clinical outcomes in patients treated with UC-MSCs than in those treated with BM-MSCs, although BM-HSCs were more effective than UCB-HSCs [197]. Despite the fact that stem cell therapy may ultimately overcome many of the well-known limitations of traditional DM therapy, more clinical trials are still required. At this time, short follow-up periods, small number of patients, missing control groups, and lack of standardization of the transplantation protocols were major setbacks for some of the clinical trials [196, 198].
1.3.3.2 Multiple Sclerosis (MS)
Multiple sclerosis (MS) is a chronic, autoimmune, inflammatory, and neurodegenerative disorder of the central nervous system [199]. MS is characterized by demyelination with axonal loss and long-term progressive disability due to disease exacerbation with the inflammatory microenvironment that enhances local oxidative stress and hypoxia [200, 201]. Several pharmacological and non-pharmacological therapies are currently approved for the treatment of MS; however, these treatments may only delay disease progression and reduce the severity of its symptoms [202]. Consequently, therapies that promote remyelination of injured axons remain among the challenges.
HSCT has been used to treat MS following high dose chemotherapy for immunosuppression [203]; this modality aims to reboot the immune system and eliminates autoreactive T- and B-cells, thereby facilitating the generation of a new and tolerant immune system [203]. HSCT has since become an alternative option for the treatment of other autoimmune-related diseases as well [204,205,206,207]. Despite the improvements observed in some MS patients, the high risk of chemotoxicity and immune deficiency in this patient cohort remains an important drawback to widespread implementation [208, 209].
MSCs have unique immunomodulatory and anti-fibrotic properties [210, 211] and are thus attractive choices for the development of targeted treatments for MS. Autologous BM-MSC transplantation resulted in diminished production of pro-inflammatory cytokines in association with improved vision and movement in patients diagnosed with MS [212, 213]. In another trial, UC-MSC transplantation resulted in improvements in physical movement with fewer side effects [214]. However, the potential therapeutic effects and mechanism of action of these cells require further investigation.
1.3.3.3 Parkinson’s Disease (PD)
PD is the second most prevalent neurodegenerative disease worldwide with an incidence that increases with age [215]. Characterized by gradual death of the dopaminergic neurons in the substantia nigra of the brain, PD leads to motor nerve impairment and reduction in the capacity for voluntary movements [216]. The exact cause of PD remains under investigation, however, the gene encoding α-synuclein (SNCA) was found to be involved with the abnormal accumulation of Lewy bodies inside neurons [217, 218]. There is currently no cure for PD; however, specific drugs are reasonably effective in restoring dopamine concentrations, as well as improving motor neuron function and relieving symptoms characteristic of PD . Nevertheless, these medications are often associated with off-target adverse events in long-term use [219, 220]; this limits their overall efficacy.
Pluripotent stem cells have the capacity to differentiate into dopaminergic neurons in vitro [221,222,223]. ESCs underwent efficient differentiation into midbrain dopaminergic neurons. When grafted into the striatum, these cells promote motor improvement, improved graft survival, and reduced levels of teratoma formation in mice [224]. A phase I/II clinical trial is currently underway, which aimed to investigate the safety and efficacy of neural precursor cells generated from human ESCs (NCT03119636) [225]. In addition, iPSCs are also promising candidates, in terms of the possible generation of dopaminergic neurons for transplantation to treat PD [226]. A personalized medicine approach revealed that differentiated dopaminergic neurons generated from autologous iPSCs could limit the progression of PD for 18–24 months [227]. A clinical trial designed to evaluate the efficacy of this approach in PD patients is currently ongoing (NCT00874783) [47].
Administration of MSCs that differentiated into dopaminergic neurons resulted in improved movement after transplantation using PD mouse models [228, 229]. Interestingly, MSCs were also found to exert a neuroprotective effect via their capacity to regulate both autophagy and α-SNCA expression, thereby rectifying PD brain-microenvironment [230]. In addition, the introduction of MSC-associated secretory factors and exosomes was associated with outstanding results in PD animal models [231,232,233]. BM-MSCs are the most commonly used cells in clinical trials; administration of autologous and allogeneic BM-MSC transplantation resulted in improved movement in three of seven patients; another two patients tolerated a reduction in PD drugs following BM-MSC transplantation [234]. No serious health concerns were reported during the 12–36-month trial; these findings encourage further testing of the BM-MSC transplantation in a larger number of patient cases [234]. Recently, administration of UC-MSCs resulted in promising outcomes in experiments conducted using PD animal models [235,236,237]; two clinical trials exploring both the efficacy and safety of this approach are ongoing (NCT03684122 and NCT03550183).
Administration of NSCs also resulted in positive outcomes with respect to treatment of PD ; these cells released neurotrophic factors that enhance neural functions and promote their migration to the site of the lesion, thereby facilitating repair of damaged tissue [238]. One clinical trial (NCT03815071) is currently testing the efficacy of administration of autologous NSCs to patients diagnosed with PD; more trials are required in order to evaluate the long-term efficacy and safety of the use of NSCs under these conditions.
1.3.3.4 Age-related Macular Degeneration (AMD)
Age-related macular degeneration (AMD) is an incurable disease resulting in the gradual loss of vision in one or both eyes [239, 240]. The macula, which is the central part of the retina, contains the photoreceptors (rods and cones) and is essential for central vision, perception of details, and differentiation among colors within a field of vision [241, 242]. Retinal pigment epithelial (RPE) cells are supportive cells that provide nutrition to retinal photoreceptors. In macular degeneration, RPE cells degenerate and fail to support the retina, resulting in the loss of central vision, blurred visual fields, and diminished capacity for color discrimination [240]. Macular degeneration exists in both wet exudative and dry non-exudative forms [239]. The dry type is associated with thinning and death of the RPE cells and is associated with yellow deposits (drusen), whereas the wet type involves the formation of new blood vessels and bleeding beneath the retina [240].
The current treatment for AMD focuses on delaying its progression, via the administration of antioxidants or anti-VEGF for patients diagnosed with dry or wet AMD, respectively [243,244,245,246]. While these therapies result in slight improvements in retinal function, they do not restore degenerating RPE cells. As such, preclinical studies have focused on transplantation of retinal progenitor sheets in an effort to replenish RPE cells in the injured area of the eye; this approach has shown promising results by improving vision in mice [247,248,249,250].
Recently, the use of pluripotent stem cells for the repair of macular damage gained much attention. ESCs can differentiate in vitro into photoreceptor cells [251] that can then be transplanted into the eyes of an individual diagnosed with AMD; through this method, human ESC-derived RPE cells were injected directly into the injured eye. The results of preliminary studies revealed that this method is safe and that there is little immune rejection of the transplanted cells; the ESC-derived RPE cells were genetically stable, did not generate tumors, and maintained strong differentiation to >99% pure RPE cells (NCT01345006 and NCT01344993) [77, 252]. However, concerns regarding genetic instability and the potential for tumorigenesis when administering pluripotent stem cells for the treatment of AMD were recently addressed [253, 254]; a recent study aimed to validate the safety of ESC-derived RPE cells through genomic analysis [255]. Furthermore, iPSC cell lines were recently differentiated into three-dimensional retinal organoids which may be useful for replacing damaged photoreceptors [256]. Reprogramming of autologous skin fibroblasts into iPSCs, then their differentiation into RPE cells, has also been investigated (Clinical trial UMIN000011929) [257].
Although MSCs were tested repeatedly for their capacity to differentiate into neuronal cells or photoreceptors [258, 259], recent studies revealed that these cells should not be used to treat AMD . Despite the absence of appropriate preclinical studies, some physicians rushed forward and use MSCs in AMD treatment protocols; this unfortunately led to several incidents of complete blindness. As but one example, a 2017 report described the case of a 77-year-old woman who received autologous adipose MSC injections into both eyes, at a clinic in Georgia; she experienced bilateral retinal detachment and complete blindness at 3 months following the procedure [260].
1.4 Stem Cell Therapies: Facts, Hope and Hype
Stem cell therapies are among the most exciting and revolutionary medical advances of the twenty-first century. They are frequently described in the media as a “wonder-cure” or “cure-all.” Indeed, clinical applications of stem cells are increasing in number worldwide as its research progresses and matures. It remains important, however, to balance patients’ needs and desires with the fact that there are currently no well-established clinical outcomes from any stem cell-based protocol. Unfortunately, several clinicians have undertaken a “rogue” approach by misusing stem cell therapy and providing services to patients that go beyond currently approved applications [261]. Moreover, false marketing and unsubstantiated advertising in almost all media outlets feature unapproved stem cell therapies for conditions ranging from mild cosmetic enhancements to cure for intractable organ failure.
By 2018, more than 430 established enterprises in the USA were promoting numerous variants of stem cell therapy (all types of stem cells for so many diseases) in more than 710 clinics distributed in various states [262]; these numbers indicate a profound increase over those reported only 2 years earlier (i.e., during 2016 [263]). Taking together, these findings indicate an increasing trend toward embracing uncontrolled and unproven stem cell therapies. Moreover, in a study conducted in 2017, researchers found that only 43.6% of a total of 408 funding campaigns focused on stem cell therapy reported true and verifiable information in terms of efficacy, and only 8.8% mentioned the risks associated with their use [264]. Most of these businesses asserted scientific legitimacy by referring to published articles in journals with little or no scientific peer-review, and provided false claims regarding their involvement and relationship with preclinical research conducted at reputable research centers [265].
Warnings are issued constantly by the FDA, the U.S. Centers for Disease Control (CDC), Euro Stem Cell, the International Society for Stem Cell Research (ISSCR) as well as other international stem cell consortiums regarding the premature use of stem cells in clinical sittings. These cautions are fully justifiable, since claims of efficacy and safety of several uncontrolled and improperly identified stem cell therapies are portrayed with optimistic messages; that often ignore the associated risks and/or potential for adverse reactions [266, 267]. As such, there is a compelling need to increase patients’ awareness of what therapies are actually clinically approved as opposed to what is currently advertised inappropriately.
Some forms of stem cell therapy, particularly the use of HSCs for hematopoietic disorders, have been the subject of extensive research, are clinically proven, and have been established as routine standard of care. The skin stem cells used for treating severe burns have shown considerable promise as well as treating immune deficiencies and solid cancers. However, other modalities featuring stem cells are still under experimental investigation and have not yet been approved for clinical use.
Validated clinical trials are required in order to provide the utmost guarantee of safety and efficacy prior to the approval of any new drug, or therapy; stem cells are certainly no exception. Despite the enormous number of research articles published each day regarding the potential of stem cells and stem cell therapy, the absence of clear, verifiable information can lead to tragedy. For example, various incidences were reported in macular degeneration patients who developed blindness, retinal detachment and intraocular bleeding, following adult stem cell-based therapy [260, 268]. Moreover, we do not yet have clear information documenting the genetic stability of ESCs, nor do we have a handle on their capacity for sustained reproducible differentiation. The use of iPSCs may overcome some of these limitations; yet, we have a long journey of research is still required to prove its safety and efficacy range. Indeed, in 2008, Yamanaka advised against the “hype” associated with iPSCs and declared that it would be quite dangerous to predict the safety of this technology with respect to clinical trials and applications [269].
Numerous factors should be considered when designing stem cell therapies. For example, an important obstacle when considering the use of umbilical cord derived stem cells is the cost of cord blood banking; these must meet the international standard regulations for the collecting, storage, and use of UC blood for transplantation [270] as well as any and all associated legal regulations [271]. At this time, the UC blood banking industry has begun to decline due to the high costs associated with its implementation. This will certainly have an impact on the future availability and therefore the use of UC derived stem cells [272].
In conclusion, the hope place in stem cells remain strong; this is certainly warranted given the opportunity to use their powerful potential to develop new cures for acute and chronic diseases. With more clinical data and improved standardization, stem cells may be safely used for treating an ever-expanding list of diseases. However, the public needs to be aware that this will take some time and that they need to be wary regarding the advertised “hype” associated with this exciting cutting-edge field. Patients are encouraged to be cautious and to look for validated and credible information before deciding to undergo an unapproved and unproven stem cell-based therapy.
Abbreviations
- (ACI):
-
Autologous Chondrocyte Implantation
- (ADSCs):
-
Adipose-derived stem cells
- (ALL):
-
Acute Lymphoblastic Leukemia
- (AMD):
-
Age-related Macular Degeneration
- (AML):
-
Acute Myeloid Leukemia
- (BM):
-
Bone Marrow
- (BM-HSCs):
-
Bone Marrow Hematopoietic Stem Cells
- (BM-MSCs):
-
Bone Marrow Mesenchymal Stem Cells
- (CAR):
-
Chimeric Antigen Receptor
- (CBT):
-
Cord Blood Transplantation
- (CFU-F):
-
Colony Forming-Unit Fibroblast
- (CLL):
-
Chronic Lymphoblastic Leukemia
- (CLP):
-
Common Lymphoid Progenitor
- (CML):
-
Chronic Myeloid Leukemia
- (DLI):
-
Donor Leukocyte Infusion
- (DM):
-
Diabetes Mellitus
- (DMT1):
-
Type 1 Diabetes Mellitus
- (DMT2):
-
Type 2 Diabetes Mellitus
- (ECM):
-
Extracellular Matrix
- (ESCs):
-
Embryonic stem cells
- (FTSG):
-
Full-thickness Skin Graft
- (G-CSF):
-
Granulocyte Colony-stimulating Factor
- (GvHD):
-
Graft versus Host Disease
- (GVL):
-
Graft Versus Leukemia
- (HSCs):
-
Hematopoietic Stem Cells
- (HSCT):
-
Hematopoietic Stem Cell Transplantation
- (HSPCs):
-
Hematopoietic Stem/Progenitor Cells
- (iPSCs):
-
Induced Pluripotent Stem Cells
- (ISSCR):
-
International Society for Stem Cell Research
- (MS):
-
Multiple Sclerosis
- (MSCs):
-
Mesenchymal Stem Cells
- (NSCs):
-
Neural Stem Cells
- (OA):
-
Osteoarthritis
- (PB):
-
Peripheral Blood
- (PD):
-
Parkinson’s Disease
- (PRP):
-
Platelet-rich Plasma
- (RIC):
-
Reduced-intensity Conditioning
- (RPE):
-
Retinal Pigment Epithelial
- (SCNT):
-
Somatic Cell Nuclear Transfer
- (STSG):
-
Split-thickness Skin Graft
- (UCB):
-
Umbilical Cord Blood
- (UC-HSCs):
-
Umbilical Cord Hematopoietic Stem Cells
- (UC-MSCs):
-
Umbilical Cord Mesenchymal Stem Cells
References
Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197(4866):452–4.
McCulloch EA, Till JE. The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res. 1960;13(1):115–25.
Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14(2):213–22.
Khanh VC, Zulkifli AF, Tokunaga C, Yamashita T, Hiramatsu Y, Ohneda O. Aging impairs beige adipocyte differentiation of mesenchymal stem cells via the reduced expression of Sirtuin 1. Biochem Biophys Res Commun. 2018;500(3):682–90.
Cui H, Tang D, Garside GB, Zeng T, Wang Y, Tao Z, et al. Wnt signaling mediates the aging-induced differentiation impairment of intestinal stem cells. Stem Cell Rev Rep. 2019;15(3):448–55.
Huang T, Liu R, Fu X, Yao D, Yang M, Liu Q, et al. Aging reduces an ERRalpha-directed mitochondrial glutaminase expression suppressing glutamine anaplerosis and osteogenic differentiation of mesenchymal stem cells. Stem Cells. 2017;35(2):411–24.
Iismaa SE, Kaidonis X, Nicks AM, Bogush N, Kikuchi K, Naqvi N, et al. Comparative regenerative mechanisms across different mammalian tissues. npj Regenerative Med. 2018;3(1):6.
Tarkowski AK, Wróblewska J. Development of blastomeres of mouse eggs isolated at the 4- and 8-cell stage. J Embryol Exp Morpholog. 1967;18(1):155–80.
Tarkowski AK. Experiments on the development of isolated blastomeres of mouse eggs. Nature. 1959;184(4695):1286–7.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7.
Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–50.
Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169(2):338–46.
Warner K, Luther C, Takei F. Lymphoid progenitors in normal mouse lymph nodes develop into NK cells and T cells in vitro and in vivo. Exp Hematol. 2012;40(5):401–6.
Alonso L, Fuchs E. Stem cells of the skin epithelium. Proc Natl Acad Sci. 2003;100(Suppl 1):11830–5.
Xie JL, Li TZ, Qi SH, Huang B, Chen XG, Chen JD. A study of using tissue-engineered skin reconstructed by candidate epidermal stem cells to cover the nude mice with full-thickness skin defect. J Plast Reconstr Aesthet Surg. 2007;60(9):983–90.
Naldaiz-Gastesi N, Goicoechea M, Aragón IM, Pérez-López V, Fuertes-Alvarez S, Herrera-Imbroda B, et al. Isolation and characterization of myogenic precursor cells from human cremaster muscle. Sci Rep. 2019;9(1):3454.
de Rooij DG. The nature and dynamics of spermatogonial stem cells. Development. 2017;144(17):3022–30.
Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morpholog. 1962;10(4):622–40.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–6.
Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–80.
Vazin T, Freed WJ. Human embryonic stem cells: derivation, culture, and differentiation: a review. Restor Neurol Neurosci. 2010;28(4):589–603.
Mehta RH. Sourcing human embryos for embryonic stem cell lines: problems & perspectives. Indian J Med Res. 2014;140(Suppl 1):S106–11.
Council NR. Final Report of the National Academies’ Human Embryonic Stem Cell Research Advisory Committee and 2010 Amendments to the National Academies’ Guidelines for Human Embryonic Stem Cell Research. National Academies Press (US); 2010.
de Wert G, Mummery C. Human embryonic stem cells: research, ethics and policy. Human Reproduct (Oxford, England). 2003;18(4):672–82.
Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98(14):7841–5.
Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of Guinea-Pig bone marrow and spleen cells. Cell Prolif. 1970;3(4):393–403.
Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4(1):102–6.
Schneider S, Unger M, van Griensven M, Balmayor ER. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur J Med Res. 2017;22(1):17.
Di Scipio F, Sprio AE, Carere ME, Yang Z, Berta GN. A simple protocol to isolate, characterize, and expand dental pulp stem cells. In: Di Nardo P, Dhingra S, Singla DK, editors. Adult stem cells: methods and protocols. New York: Springer; 2017. p. 1–13.
Hatakeyama A, Uchida S, Utsunomiya H, Tsukamoto M, Nakashima H, Nakamura E, et al. Isolation and characterization of synovial Mesenchymal stem cell derived from hip joints: a comparative analysis with a matched control knee group. Stem Cells Int. 2017;2017:9312329.
Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96(1):25–34.
Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–8.
Lien BV, Tuszynski MH, Lu P. Astrocytes migrate from human neural stem cell grafts and functionally integrate into the injured rat spinal cord. Exp Neurol. 2019;314:46–57.
McLauchlan D, Robertson NP. Stem cells in the treatment of central nervous system disease. J Neurol. 2018;265(4):984–6.
Ueno Y, Koizumi S, Yamagami M, Miura M, Taniguchi N. Characterization of hemopoietic stem cells (CFUc) in cord blood. Exp Hematol. 1981;9(7):716–22.
Till J, McCulloch E. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 2012;178(2):AV3–7.
Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86(10):3828–32.
Beeravolu N, McKee C, Alamri A, Mikhael S, Brown C, Perez-Cruet M, et al. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J Vis Exp. 2017;122:55224.
Wu M, Zhang R, Zou Q, Chen Y, Zhou M, Li X, et al. Comparison of the biological characteristics of mesenchymal stem cells derived from the human placenta and umbilical cord. Sci Rep. 2018;8(1):1–9.
Wouters G, Grossi S, Mesoraca A, Bizzoco D, Mobili L, Cignini P, et al. Isolation of amniotic fluid-derived mesenchymal stem cells. J Prenat Med. 2007;1(3):39–40.
Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9(9):725–9.
Reubinoff BE, Pera MF, Fong C-Y, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18(4):399–404.
Deng Z-L, Sharff KA, Tang N, Song W-X, Luo J, Luo X, et al. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13(1):2001–21.
Dai R, Wang Z, Samanipour R, Koo K-I, Kim K. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016;2016:6737345.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. the international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–7.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20.
Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6):947–56.
Horwitz E, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: the international society for cellular therapy position statement. Cytotherapy. 2005;7(5):393–5.
Ahmed TA, Shousha WG, Abdo SM, Mohamed I, El-Badri N. Human adipose-derived pericytes: biological characterization and reprogramming into induced pluripotent stem cells. Cell Physiol Biochem. 2020;54:271–86.
Ilic D, Ogilvie C. Concise review: human embryonic stem cells—what have we done? What are we doing? Where are we going? Stem Cells. 2017;35(1):17–25.
Perez-Cunningham J, Ames E, Smith RC, Peter AK, Naidu R, Nolta JA, et al. Natural killer cell subsets differentially reject embryonic stem cells based on licensing. Transplantation. 2014;97(10):992–8.
Ng AP, Alexander WS. Haematopoietic stem cells: past, present and future. Cell Death Dis. 2017;3(1):1–4.
Mosaad YM. Immunology of hematopoietic stem cell transplant. Immunol Investig. 2014;43(8):858–87.
Morandi F, Raffaghello L, Bianchi G, Meloni F, Salis A, Millo E, et al. Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells. 2008;26(5):1275–87.
Kruse V, Hamann C, Monecke S, Cyganek L, Elsner L, Hübscher D, et al. Human induced pluripotent stem cells are targets for allogeneic and autologous natural killer (NK) cells and killing is partly mediated by the activating NK receptor DNAM-1. PLoS One. 2015;10(5):e0125544.
Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15(1):36.
Zheng YL. Some ethical concerns about human induced pluripotent stem cells. Sci Eng Ethics. 2016;22(5):1277–84.
Kimbrel EA, Lanza R. Next-generation stem cells — ushering in a new era of cell-based therapies. Nat Rev Drug Discov. 2020;
Rubin LL, Haston KM. Stem cell biology and drug discovery. BMC Biol. 2011;9:42.
Wang Y, Yin P, Bian G-L, Huang H-Y, Shen H, Yang J-J, et al. The combination of stem cells and tissue engineering: an advanced strategy for blood vessels regeneration and vascular disease treatment. Stem Cell Res Ther. 2017;8(1):194.
Trounson A. New perspectives in human stem cell therapeutic research. BMC Med. 2009;7:29.
Zhang C-L, Huang T, Wu B-L, He W-X, Liu D. Stem cells in cancer therapy: opportunities and challenges. Oncotarget. 2017;8(43):75756–66.
Persons DA. The challenge of obtaining therapeutic levels of genetically modified hematopoietic stem cells in beta-thalassemia patients. Ann N Y Acad Sci. 2010;1202:69–74.
Yannaki E, Stamatoyannopoulos G. Hematopoietic stem cell mobilization strategies for gene therapy of beta thalassemia and sickle cell disease. Ann N Y Acad Sci. 2010;1202:59–63.
Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Villasboas JC, et al. Autograft immune content and survival in non-Hodgkin’s lymphoma: a post hoc analysis. Leuk Res. 2019;81:1–9.
Platzbecker U, Thiede C, Freiberg-Richter J, Röllig C, Helwig A, Schäkel U, et al. Early allogeneic blood stem cell transplantation after modified conditioning therapy during marrow aplasia: stable remission in high-risk acute myeloid leukemia. Bone Marrow Transplant. 2001;27(5):543–6.
Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13(1):49.
Cristante AF, Barros-Filho TEP, Tatsui N, Mendrone A, Caldas JG, Camargo A, et al. Stem cells in the treatment of chronic spinal cord injury: evaluation of somatosensitive evoked potentials in 39 patients. Spinal Cord. 2009;47(10):733–8.
Lévesque M, Neuman T, Rezak M. Therapeutic microinjection of autologous adult human neural stem cells and differentiated neurons for Parkinson’s disease: five-year post-operative outcome. The Open Stem Cell Journal. 2009;1:20–9.
Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–94.
Álvaro-Gracia JM, Jover JA, García-Vicuña R, Carreño L, Alonso A, Marsal S, et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 2017;76(1):196–202.
García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48(7):1416–23.
Haller MJ, Wasserfall CH, McGrail KM, Cintron M, Brusko TM, Wingard JR, et al. Autologous umbilical cord blood transfusion in very young children with type 1 diabetes. Diab Care. 2009;32(11):2041–6.
Patel AN, Henry TD, Quyyumi AA, Schaer GL, Anderson RD, Toma C, et al. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet (London, England). 2016;387(10036):2412–21.
Siqueira RC, Messias A, Messias K, Arcieri RS, Ruiz MA, Souza NF, et al. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell -clinical trial). Stem Cell Res Ther. 2015;6(1):29.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet (London, England). 2015;385(9967):509–16.
Maumus M, Manferdini C, Toupet K, Peyrafitte JA, Ferreira R, Facchini A, et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013;11(2):834–44.
Loeb DM, Hobbs RF, Okoli A, Chen AR, Cho S, Srinivasan S, et al. Tandem dosing of samarium-153 ethylenediamine tetramethylene phosphoric acid with stem cell support for patients with high-risk osteosarcoma. Cancer. 2010;116(23):5470–8.
Bordignon C. Stem-cell therapies for blood diseases. Nature. 2006;441(7097):1100–2.
Jaffe ES, Harris NL, Diebold J, Muller-Hermelink HK. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. A progress report. Am J Clin Pathol. 1999;111(1 Suppl 1):S8–12.
Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361–92.
Isidro A, Seiler R, Seco M. Leukemia in Ancient Egypt: earliest case and state-of-the-art techniques for diagnosing generalized osteolytic lesions. Int J Osteoarchaeol. 2019;29
Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997–2002. Cancer Causes Control: CCC. 2008;19(4):379–90.
Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238(23):787–93.
Miller DR. A tribute to Sidney Farber-- the father of modern chemotherapy. Br J Haematol. 2006;134(1):20–6.
Kharfan-Dabaja MA, Kumar A, Hamadani M, Stilgenbauer S, Ghia P, Anasetti C, et al. Clinical practice recommendations for use of allogeneic hematopoietic cell transplantation in Chronic Lymphocytic Leukemia on Behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2016;22(12):2117–25.
Dreger P, Schetelig J, Andersen N, Corradini P, van Gelder M, Gribben J, et al. Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood. 2014;124(26):3841–9.
Caballero D, García-Marco JA, Martino R, Mateos V, Ribera JM, Sarrá J, et al. Allogeneic transplant with reduced intensity conditioning regimens may overcome the poor prognosis of B-cell chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene and chromosomal abnormalities (11q- and 17p-). Clin Cancer Res. 2005;11(21):7757–63.
Moreno C, Villamor N, Colomer D, Esteve J, Martino R, Nomdedéu J, et al. Allogeneic stem-cell transplantation may overcome the adverse prognosis of unmutated VH gene in patients with chronic lymphocytic leukemia. J Clin Oncol. 2005;23(15):3433–8.
Henig I, Zuckerman T. Hematopoietic stem cell transplantation-50 years of evolution and future perspectives. Rambam Maimonides Med J. 2014;5(4):e0028-e.
E Donnall Thomas (1920–2012). Bone marrow transplantation. 2013;48(1):1.
Savani BN, Mielke S, Reddy N, Goodman S, Jagasia M, Rezvani K. Management of relapse after allo-SCT for AML and the role of second transplantation. Bone Marrow Transplant. 2009;44(12):769–77.
Kolb H, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462–5.
Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106(3):1113–22.
Nagler A, Slavin S, Varadi G, Naparstek E, Samuel S, Or R. Allogeneic peripheral blood stem cell transplantation using a fludarabine-based low intensity conditioning regimen for malignant lymphoma. Bone Marrow Transplant. 2000;25(10):1021–8.
Marks DI, Woo KA, Zhong X, Appelbaum FR, Bachanova V, Barker JN, et al. Unrelated umbilical cord blood transplant for adult acute lymphoblastic leukemia in first and second complete remission: a comparison with allografts from adult unrelated donors. Haematologica. 2014;99(2):322–8.
Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337(6):373–81.
Scaradavou A, Brunstein CG, Eapen M, Le-Rademacher J, Barker JN, Chao N, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121(5):752–8.
Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122(4):491–8.
Rocha V, Gluckman E. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft- and transplantation-related factors. Br J Haematol. 2009;147(2):262–74.
Herr AL, Kabbara N, Bonfim CM, Teira P, Locatelli F, Tiedemann K, et al. Long-term follow-up and factors influencing outcomes after related HLA-identical cord blood transplantation for patients with malignancies: an analysis on behalf of Eurocord-EBMT. Blood. 2010;116(11):1849–56.
Visani G, Lemoli R, Tosi P, Martinelli G, Testoni N, Ricci P, et al. Use of peripheral blood stem cells for autologous transplantation in acute myeloid leukemia patients allows faster engraftment and equivalent disease-free survival compared with bone marrow cells. Bone Marrow Transplant. 1999;24(5):467–72.
D’Souza A, Lee S, Zhu X, Pasquini M. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2017;23(9):1417–21.
Wang X, Xiao Q, Wang Z, Feng WL. CAR-T therapy for leukemia: progress and challenges. Transl Res. 2017;182:135–44.
Ali S, Kjeken R, Niederlaender C, Markey G, Saunders TS, Opsata M, et al. The European medicines agency review of Kymriah (Tisagenlecleucel) for the treatment of acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Oncologist. 2020;25(2):e321–e7.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21.
Patmanathan SN, Gnanasegaran N, Lim MN, Husaini R, Fakiruddin KS, Zakaria Z. CRISPR/Cas9 in stem cell research: current application and future perspective. Curr Stem Cell Res Therapy. 2018;13(8):632–44.
MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445(7130):874–80.
Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K. Skin tissue engineering--in vivo and in vitro applications. Adv Drug Deliv Rev. 2011;63(4–5):352–66.
Ragnell A. The secondary contracting tendency of free skin grafts; an experimental investigation on animals. Br J Plast Surg. 1952;5(1):6–24.
Blair VP, Brown JB. The use and uses of large split skin grafts of intermediate thickness. Plast Reconstr Surg. 1968;42(1):65–75.
Johnson TM, Ratner D, Nelson BR. Soft tissue reconstruction with skin grafting. J Am Acad Dermatol. 1992;27(2):151–65.
Boyce ST, Lalley AL. Tissue engineering of skin and regenerative medicine for wound care. Burns & Trauma. 2018;6
Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, et al. Advances in skin regeneration using tissue engineering. Int J Mol Sci. 2017;18(4):789.
Heimbach D, Luterman A, Burke J, Cram A, Herndon D, Hunt J, et al. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann Surg. 1988;208(3):313–20.
Jansen LA, De Caigny P, Guay NA, Lineaweaver WC, Shokrollahi K. The evidence base for the acellular dermal matrix AlloDerm: a systematic review. Ann Plast Surg. 2013;70(5):587–94.
Larson KW, Austin CL, Thompson SJ. Treatment of a full-thickness burn injury with NovoSorb biodegradable temporizing matrix and RECELL autologous skin cell suspension: a case series. J Burn Care Res. 2020;41(1):215–9.
Zaulyanov L, Kirsner RS. A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin Interv Aging. 2007;2(1):93–8.
Gerlach JC, Johnen C, Ottomann C, Bräutigam K, Plettig J, Belfekroun C, et al. Method for autologous single skin cell isolation for regenerative cell spray transplantation with non-cultured cells. Int J Artificial Organs. 2011;34(3):271–9.
Peirce SC, Carolan-Rees G. ReCell(®) spray-on skin system for treating skin loss, scarring and depigmentation after burn injury: a NICE medical technology guidance. Appl Health Econ Health Policy. 2019;17(2):131–41.
Ng WL, Wang S, Yeong WY, Naing MW. Skin bioprinting: impending reality or fantasy? Trends Biotechnol. 2016;34(9):689–99.
Pereira RF, Barrias CC, Granja PL, Bartolo PJ. Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine (London, England). 2013;8(4):603–21.
Guenou H, Nissan X, Larcher F, Feteira J, Lemaitre G, Saidani M, et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study. Lancet (London, England). 2009;374(9703):1745–53.
Shamis Y, Hewitt KJ, Carlson MW, Margvelashvilli M, Dong S, Kuo CK, et al. Fibroblasts derived from human embryonic stem cells direct development and repair of 3D human skin equivalents. Stem Cell Res Ther. 2011;2(1):10.
Tang KC, Yang KC, Lin CW, Chen YK, Lu TY, Chen HY, et al. Human adipose-derived stem cell secreted extracellular matrix incorporated into electrospun Poly(Lactic-co-Glycolic Acid) nanofibrous dressing for enhancing wound healing. Polymers. 2019;11(10).
Petry L, Kippenberger S, Meissner M, Kleemann J, Kaufmann R, Rieger UM, et al. Directing adipose-derived stem cells into keratinocyte-like cells: impact of medium composition and culture condition. J Eur Acad Dermatol Venereol JEADV. 2018;32(11):2010–9.
Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol (Baltimore, Md: 1950). 2008;180(4):2581–7.
Luo H, Guo Y, Liu Y, Wang Y, Zheng R, Ban Y, et al. Growth differentiation factor 11 inhibits adipogenic differentiation by activating TGF-beta/Smad signalling pathway. Cell Prolif. 2019;52(4):e12631.
Hanft JR, Surprenant MS. Healing of chronic foot ulcers in diabetic patients treated with a human fibroblast-derived dermis. J Foot Ankle Surg. 2002;41(5):291–9.
Itoh M, Umegaki-Arao N, Guo Z, Liu L, Higgins CA, Christiano AM. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8(10):e77673-e.
Fang H, Huang L, Welch I, Norley C, Holdsworth DW, Beier F, et al. Early changes of articular cartilage and subchondral bone in the DMM mouse model of osteoarthritis. Sci Rep. 2018;8(1):2855.
Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–30.
McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, et al. Effect of Intra-articular Triamcinolone vs Saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967–75.
Montañez-Heredia E, Irízar S, Huertas PJ, Otero E, Del Valle M, Prat I, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritic knee pain: a randomized clinical trial in the context of the Spanish National Health Care System. Int J Mol Sci. 2016;17(7)
Estades-Rubio FJ, Reyes-Martín A, Morales-Marcos V, García-Piriz M, García-Vera JJ, Perán M, et al. Knee viscosupplementation: cost-effectiveness analysis between stabilized hyaluronic acid in a single injection versus five injections of standard hyaluronic acid. Int J Mol Sci. 2017;18(3)
Gallagher B, Tjoumakaris FP, Harwood MI, Good RP, Ciccotti MG, Freedman KB. Chondroprotection and the prevention of osteoarthritis progression of the knee: a systematic review of treatment agents. Am J Sports Med. 2015;43(3):734–44.
Orth P, Gao L, Madry H. Microfracture for cartilage repair in the knee: a systematic review of the contemporary literature. Knee Surg Sports Traumatol Arthrosc. 2020;28(3):670–706.
Broyles JE, O'Brien MA, Stagg MP. Microdrilling surgery augmented with intra-articular bone marrow aspirate concentrate, platelet-rich plasma, and hyaluronic acid: a technique for cartilage repair in the knee. Arthrosc Tech. 2017;6(1):e201–e6.
Sanna M, Sanna C, Caputo F, Piu G, Salvi M. Surgical approaches in total knee arthroplasty. Joints. 2013;1(2):34–44.
Karataglis D, Green MA, Learmonth DJ. Autologous osteochondral transplantation for the treatment of chondral defects of the knee. Knee. 2006;13(1):32–5.
Kizaki K, El-Khechen HA, Yamashita F, Duong A, Simunovic N, Musahl V, et al. Arthroscopic versus open osteochondral autograft transplantation (Mosaicplasty) for cartilage damage of the knee: a systematic review. J Knee Surg. 2019.
Angermann P, Riegels-Nielsen P, Pedersen H. Osteochondritis dissecans of the femoral condyle treated with periosteal transplantation. Poor outcome in 14 patients followed for 6-9 years. Acta Orthop Scand. 1998;69(6):595–7.
Negoro T, Takagaki Y, Okura H, Matsuyama A. Trends in clinical trials for articular cartilage repair by cell therapy. NPJ Regen Med. 2018;3:17.
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–95.
Fickert S, Schattenberg T, Niks M, Weiss C, Thier S. Feasibility of arthroscopic 3-dimensional, purely autologous chondrocyte transplantation for chondral defects of the hip: a case series. Arch Orthop Trauma Surg. 2014;134(7):971–8.
Davies RL, Kuiper NJ. Regenerative medicine: a review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioengineering (Basel). 2019;6(1):22.
Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;54(4):1222–32.
Narakornsak S, Poovachiranon N, Peerapapong L, Pothacharoen P, Aungsuchawan S. Mesenchymal stem cells differentiated into chondrocyte-Like cells. Acta Histochem. 2016;118(4):418–29.
Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22.
Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13(5):595–600.
Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized Phase I/II trial. Stem Cells Transl Med. 2019;8(3):215–24.
Castellanos R, Tighe S. Injectable amniotic membrane/umbilical cord particulate for knee osteoarthritis: a prospective, single-center pilot study. Pain Med. 2019;20(11):2283–91.
Khalifeh Soltani S, Forogh B, Ahmadbeigi N, Hadizadeh Kharazi H, Fallahzadeh K, Kashani L, et al. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: a pilot study. Cytotherapy. 2019;21(1):54–63.
González PL, Carvajal C, Cuenca J, Alcayaga-Miranda F, Figueroa FE, Bartolucci J, et al. Chorion mesenchymal stem cells show superior differentiation, immunosuppressive, and angiogenic potentials in comparison with haploidentical maternal placental cells. Stem Cells Transl Med. 2015;4(10):1109–21.
Organization WH. Classification of diabetes mellitus. 2019.
Farooq T, Rehman K, Hameed A, Akash MSH. Stem cell therapy and type 1 diabetes mellitus: treatment strategies and future perspectives. Tissue Eng Regen Med. 2019: Springer.
Alicka M, Marycz K. The effect of chronic inflammation and oxidative and endoplasmic reticulum stress in the course of metabolic syndrome and its therapy. Stem Cells Int. 2018;2018:4274361.
Cersosimo E, Triplitt C, Solis-Herrera C, Mandarino LJ, DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Endotext [Internet]: MDText. com, Inc.; 2018.
Mahmoud M, Abu-Shahba N, Azmy O, El-Badri N. Impact of diabetes mellitus on human mesenchymal stromal cell biology and functionality: implications for autologous transplantation. Stem Cell Rev Rep. 2019;15(2):194–217.
Mahaffey KW, Jardine MJ, Bompoint S, Cannon CP, Neal B, Heerspink HJ, et al. Canagliflozin and cardiovascular and renal outcomes in Type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups: results from the randomized CREDENCE trial. Circulation. 2019;140(9):739–50.
Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis. 2019;62(4):298–302.
Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 diabetes mellitus and heart failure: a scientific statement From the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7):e294–324.
Han SB, Yang HK, Hyon JY. Influence of diabetes mellitus on anterior segment of the eye. Clin Interv Aging. 2019;14:53.
Huang X, Zhang P, Zou X, Xu Y, Zhu J, He J, et al. Two-year incidence and associated factors of dry eye among residents in Shanghai communities with type 2 diabetes mellitus. Eye Contact Lens. 2020;46:S42–S9.
Xiao Y, Chen M-J, Shen X, Lin L-R, Liu L-L, Yang T-C, et al. Metabolic disorders in patients with central nervous system infections: associations with neurosyphilis. Eur Neurol. 2019;81(5-6):270–7.
Ewers B, Trolle E, Jacobsen SS, Vististen D, Almdal TP, Vilsbøll T, et al. Dietary habits and adherence to dietary recommendations in patients with type 1 and type 2 diabetes compared with the general population in Denmark. Nutrition. 2019;61:49–55.
Montvida O, Green J, Atherton J, Paul S. Treatment with incretins does not increase the risk of pancreatic diseases compared to older anti-hyperglycaemic drugs, when added to metformin: real world evidence in people with Type 2 diabetes. Diabet Med. 2019;36(4):491–8.
Chong S, Ding D, Byun R, Comino E, Bauman A, Jalaludin B. Lifestyle changes after a diagnosis of type 2 diabetes. Diabetes Spectr. 2017;30(1):43–50.
Fanelli CG, Porcellati F, Pampanelli S, Bolli GB. Insulin therapy and hypoglycaemia: the size of the problem. Diabetes Metab Res Rev. 2004;20(S2):S32–42.
Street CN, Lakey JR, Shapiro AM, Imes S, Rajotte RV, Ryan EA, et al. Islet graft assessment in the Edmonton protocol: implications for predicting long-term clinical outcome. Diabetes. 2004;53(12):3107–14.
Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–30.
Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8.
Maffi P, Secchi A. Islet transplantation alone versus solitary pancreas transplantation: an outcome-driven choice? Curr Diab Rep. 2019;19(5):26.
Oberholzer J, Triponez F, Mage R, Andereggen E, Bühler L, Crétin N, et al. Human islet transplantation: lessons from 13 autologous and 13 allogeneic transplantations. Transplantation. 2000;69(6):1115–23.
Chang CA, Lawrence MC, Naziruddin B. Current issues in allogeneic islet transplantation. Curr Opin Organ Transplant. 2017;22(5):437–43.
Badet L, Benhamou PY, Wojtusciszyn A, Baertschiger R, Milliat-Guittard L, Kessler L, et al. Expectations and strategies regarding islet transplantation: metabolic data from the GRAGIL 2 trial. Transplantation. 2007;84(1):89–96.
Pavathuparambil Abdul Manaph N, Sivanathan KN, Nitschke J, Zhou X-F, Coates PT, Drogemuller CJ. An overview on small molecule-induced differentiation of mesenchymal stem cells into beta cells for diabetic therapy. Stem Cell Res Ther. 2019;10(1):293.
Mitutsova V, Yeo WWY, Davaze R, Franckhauser C, Hani E-H, Abdullah S, et al. Adult muscle-derived stem cells engraft and differentiate into insulin-expressing cells in pancreatic islets of diabetic mice. Stem Cell Res Ther. 2017;8(1):86.
Kieffer TJ. Closing in on mass production of mature human beta cells. Cell Stem Cell. 2016;18(6):699–702.
El-Badri N, Ghoneim MA. Mesenchymal stem cell therapy in diabetes mellitus: progress and challenges. J Nucleic Acids. 2013;2013:194858.
Ardestani A, Maedler K. MST1: a promising therapeutic target to restore functional beta cell mass in diabetes. Diabetologia. 2016;59(9):1843–9.
Balaji S, Keswani SG, Crombleholme TM. The role of mesenchymal stem cells in the regenerative wound healing phenotype. Adv Wound Care (New Rochelle). 2012;1(4):159–65.
Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–39.
Candiello J, Grandhi TSP, Goh SK, Vaidya V, Lemmon-Kishi M, Eliato KR, et al. 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials. 2018;177:27–39.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52.
Taneera J, Rosengren A, Renstrom E, Nygren JM, Serup P, Rorsman P, et al. Failure of transplanted bone marrow cells to adopt a pancreatic beta-cell fate. Diabetes. 2006;55(2):290–6.
Okura H, Komoda H, Fumimoto Y, Lee CM, Nishida T, Sawa Y, et al. Transdifferentiation of human adipose tissue-derived stromal cells into insulin-producing clusters. Journal of Artificial Organs. 2009;12(2):123–30.
Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, et al. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341(4):1135–40.
Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Yin Z, et al. Reversal of type 1 diabetes via islet β cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012;10:3.
Negi N, Griffin MD. Effects of mesenchymal stromal cells on regulatory T cells: current understanding and clinical relevance. Stem Cells. 2020;38(5):596–605.
Qi Y, Ma J, Li S, Liu W. Applicability of adipose-derived mesenchymal stem cells in treatment of patients with type 2 diabetes. Stem Cell Res Ther. 2019;10(1):274.
Zang L, Hao H, Liu J, Li Y, Han W, Mu Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol Metab Syndr. 2017;9:36.
Bhansali S, Dutta P, Yadav MK, Jain A, Mudaliar S, Hawkins M, et al. Autologous bone marrow-derived mononuclear cells transplantation in type 2 diabetes mellitus: effect on β-cell function and insulin sensitivity. Diabetol Metab Syndr. 2017;9:50.
Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587–92.
Bhansali A, Asokumar P, Walia R, Bhansali S, Gupta V, Jain A, et al. Efficacy and safety of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transplant. 2014;23(9):1075–85.
El-Badawy A, El-Badri N. Clinical efficacy of stem cell therapy for diabetes mellitus: a meta-analysis. PLoS One. 2016;11(4):e0151938.
Cheng SK, Park EY, Pehar A, Rooney AC, Gallicano GI. Current progress of human trials using stem cell therapy as a treatment for diabetes mellitus. Am J Stem Cells. 2016;5(3):74–86.
Prat A, Antel J. Pathogenesis of multiple sclerosis. Curr Opin Neurol. 2005;18(3):225–30.
Siotto M, Filippi MM, Simonelli I, Landi D, Ghazaryan A, Vollaro S, et al. Oxidative stress related to iron metabolism in relapsing remitting multiple sclerosis patients with low disability. Front Neurosci. 2019;13:86.
Padureanu R, Albu CV, Mititelu RR, Bacanoiu MV, Docea AO, Calina D, et al. Oxidative stress and inflammation interdependence in multiple sclerosis. J Clin Med. 2019;8(11):1815.
Klotz L, Havla J, Schwab N, Hohlfeld R, Barnett M, Reddel S, et al. Risks and risk management in modern multiple sclerosis immunotherapeutic treatment. Ther Adv Neurol Disord. 2019;12:1756286419836571.
Swart JF, Delemarre EM, van Wijk F, Boelens J-J, Kuball J, van Laar JM, et al. Haematopoietic stem cell transplantation for autoimmune diseases. Nat Rev Rheumatol. 2017;13(4):244–56.
Lowenthal RM, Cohen ML, Atkinson K, Biggs JC. Apparent cure of rheumatoid arthritis by bone marrow transplantation. J Rheumatol. 1993;20(1):137–40.
Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112(9):3543–53.
Fassas AS, Passweg JR, Anagnostopoulos A, Kazis A, Kozak T, Havrdova E, et al. Hematopoietic stem cell transplantation for multiple sclerosis. J Neurol. 2002;249(8):1088–97.
Burt RK, Traynor AE, Cohen B, Karlin KH, Davis FA, Stefoski D, et al. T cell-depleted autologous hematopoietic stem cell transplantation for multiple sclerosis: report on the first three patients. Bone Marrow Transplant. 1998;21(6):537–41.
Saccardi R, Tyndall A, Coghlan G, Denton C, Edan G, Emdin M, et al. Consensus statement concerning cardiotoxicity occurring during haematopoietic stem cell transplantation in the treatment of autoimmune diseases, with special reference to systemic sclerosis and multiple sclerosis. Bone Marrow Transplant. 2004;34(10):877–81.
Daikeler T, Tichelli A, Passweg J. Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases. Pediatr Res. 2012. https://doi.org/10.1038/pr.2011.57
Gharibi T, Ahmadi M, Seyfizadeh N, Jadidi-Niaragh F, Yousefi M. Immunomodulatory characteristics of mesenchymal stem cells and their role in the treatment of Multiple Sclerosis. Cell Immunol. 2015;293(2):113–21.
Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262(5):509–25.
Dahbour S, Jamali F, Alhattab D, Al-Radaideh A, Ababneh O, Al-Ryalat N, et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci Ther. 2017;23(11):866–74.
Cohen JA. Mesenchymal stem cell transplantation in multiple sclerosis. J Neurol Sci. 2013;333(1):43–9.
Riordan NH, Morales I, Fernández G, Allen N, Fearnot NE, Leckrone ME, et al. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J Transl Med. 2018;16(1):57.
Pringsheim T, Jette N, Frolkis A, Steeves TDL. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1583–90.
Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–9.
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–7.
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. [Alpha]-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841.
Jankovic J. Complications and limitations of drug therapy for Parkinson’s disease. Neurology. 2000;55(12 Suppl 6):S2–6.
Stocchi F, Tagliati M, Olanow CW. Treatment of levodopa-induced motor complications. Mov Disord. 2008;23(S3):S599–612.
Jiang H, Ren Y, Yuen EY, Zhong P, Ghaedi M, Hu Z, et al. Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun. 2012;3(1):668.
Schulz TC, Noggle SA, Palmarini GM, Weiler DA, Lyons IG, Pensa KA, et al. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22(7):1218–38.
Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L, et al. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells. 2010;28(10):1893–904.
Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: effect of in vitro differentiation on graft survival and Teratoma formation. Stem Cells. 2006;24(6):1433–40.
Wang Y-K, Zhu W-W, Wu M-H, Wu Y-H, Liu Z-X, Liang L-M, et al. Human clinical-grade parthenogenetic ESC-derived dopaminergic neurons recover locomotive defects of nonhuman primate models of Parkinson’s disease. Stem Cell Reports. 2018;11(1):171–82.
Mahajani S, Raina A, Fokken C, Kügler S, Bähr M. Homogenous generation of dopaminergic neurons from multiple hiPSC lines by transient expression of transcription factors. Cell Death Dis. 2019;10(12):898.
Schweitzer JS, Song B, Herrington TM, Park T-Y, Lee N, Ko S, et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N Engl J Med. 2020;382(20):1926–32.
Kang EJ, Lee YH, Kim MJ, Lee YM, Kumar BM, Jeon BG, et al. Transplantation of porcine umbilical cord matrix mesenchymal stem cells in a mouse model of Parkinson’s disease. J Tissue Eng Regen Med. 2013;7(3):169–82.
Park H-J, Shin JY, Lee BR, Kim HO, Lee PH. Mesenchymal stem cells augment neurogenesis in the subventricular zone and enhance differentiation of neural precursor cells into dopaminergic neurons in the Substantia Nigra of a Parkinsonian model. Cell Transplant. 2012;21(8):1629–40.
Park HJ, Shin JY, Kim HN, Oh SH, Lee PH. Neuroprotective effects of mesenchymal stem cells through autophagy modulation in a parkinsonian model. Neurobiol Aging. 2014;35(8):1920–8.
Chen H-X, Liang F-C, Gu P, Xu B-L, Xu H-J, Wang W-T, et al. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020;11(4):288.
Vilaça-Faria H, Salgado AJ, Teixeira FG. Mesenchymal stem cells-derived exosomes: a new possible therapeutic strategy for Parkinson’s disease? Cell. 2019;8(2).
Teixeira FG, Carvalho MM, Panchalingam KM, Rodrigues AJ, Mendes-Pinheiro B, Anjo S, et al. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of Parkinson’s disease. Stem Cells Transl Med. 2017;6(2):634–46.
Venkataramana NK, Kumar SKV, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155(2):62–70.
Liu X-S, Li J-F, Wang S-S, Wang Y-T, Zhang Y-Z, Yin H-L, et al. Human umbilical cord mesenchymal stem cells infected with adenovirus expressing <i>HGF</i> promote regeneration of damaged neuron cells in a Parkinson’s Disease Model. Biomed Res Int. 2014;2014:909657.
Yan M, Sun M, Zhou Y, Wang W, He Z, Tang D, et al. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s Jelly to Dopamine Neurons Mediated by the Lmx1a and Neurturin In Vitro: Potential Therapeutic Application for Parkinson’s Disease in a Rhesus Monkey Model. PLoS One. 2013;8(5):e64000.
Shetty P, Ravindran G, Sarang S, Thakur AM, Rao HS, Viswanathan C. Clinical grade mesenchymal stem cells transdifferentiated under xenofree conditions alleviates motor deficiencies in a rat model of Parkinson’s disease. Cell Biol Int. 2009;33(8):830–8.
Bjugstad KB, Teng YD, Redmond DE, Elsworth JD, Roth RH, Cornelius SK, et al. Human neural stem cells migrate along the nigrostriatal pathway in a primate model of Parkinson’s disease. Exp Neurol. 2008;211(2):362–9.
Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006;58(3):353.
Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48(3):257–93.
Storchi R, Rodgers J, Gracey M, Martial FP, Wynne J, Ryan S, et al. Measuring vision using innate behaviours in mice with intact and impaired retina function. Sci Rep. 2019;9(1):1–16.
Procyk CA, Eleftheriou CG, Storchi R, Allen AE, Milosavljevic N, Brown TM, et al. Spatial receptive fields in the retina and dorsal lateral geniculate nucleus of mice lacking rods and cones. J Neurophysiol. 2015;114(2):1321–30.
Swanson MW, McGwin G Jr. Anti-inflammatory drug use and age-related macular degeneration. Optom Vis Sci. 2008;85(10):947–50.
Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv Ophthalmol. 2000;45(3):195–214.
Spaide RF, Laud K, Fine HF, James M, Klancnik J, Meyerle CB, Yannuzzi LA, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26(4):383–90.
Martin DF, Maguire MG, Fine SL, G-s Y, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–98.
Li LX, Turner JE. Inherited retinal dystrophy in the RCS rat: prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp Eye Res. 1988;47(6):911–7.
Klassen HJ, Ng TF, Kurimoto Y, Kirov I, Shatos M, Coffey P, et al. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest Ophthalmol Vis Sci. 2004;45(11):4167–73.
Luo J, Baranov P, Patel S, Ouyang H, Quach J, Wu F, et al. Human retinal progenitor cell transplantation preserves vision. J Biol Chem. 2014;289(10):6362–71.
Lin B, McLelland BT, Mathur A, Aramant RB, Seiler MJ. Sheets of human retinal progenitor transplants improve vision in rats with severe retinal degeneration. Exp Eye Res. 2018;174:13–28.
Yanai A, Laver CR, Joe AW, Viringipurampeer IA, Wang X, Gregory-Evans CY, et al. Differentiation of human embryonic stem cells using size-controlled embryoid bodies and negative cell selection in the production of photoreceptor precursor cells. Tissue Eng Part C: Methods. 2013;19(10):755–64.
Schwartz SD, Hubschman J-P, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713–20.
Chakradhar S. An eye to the future: researchers debate best path for stem cell–derived therapies. Nature Publishing Group; 2016.
Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nature Publishing Group; 2015.
Petrus-Reurer S, Kumar P, Padrell Sánchez S, Aronsson M, André H, Bartuma H, et al. Preclinical safety studies of human embryonic stem cell-derived retinal pigment epithelial cells for the treatment of age-related macular degeneration. Stem Cells Transl Med. 2020.
Capowski EE, Samimi K, Mayerl SJ, Phillips MJ, Pinilla I, Howden SE, et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development. 2019;146(1):dev171686.
Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, et al. Autologous induced stem-cell–derived retinal cells for macular degeneration. N Engl J Med. 2017;376(11):1038–46.
Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2007;245(3):414–22.
Kicic A, Shen WY, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci. 2003;23(21):7742–9.
Saraf SS, Cunningham MA, Kuriyan AE, Read SP, Rosenfeld PJ, Flynn HW Jr, et al. Bilateral retinal detachments after intravitreal injection of adipose-derived ‘stem cells’ in a patient with exudative macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2017;48(9):772–5.
Pean CA, Kingery MT, Strauss E, Bosco JA, Halbrecht J. Direct-to-consumer advertising of stem cell clinics: ethical considerations and recommendations for the health-care community. J Bone Joint Surg Am. 2019;101(19):e103.
Turner L, The US. Direct-to-consumer marketplace for autologous stem cell interventions. Perspect Biol Med. 2018;61(1):7–24.
Turner L, Knoepfler P. Selling Stem cells in the USA: assessing the direct-to-consumer industry. Cell Stem Cell. 2016;19(2):154–7.
Snyder J, Turner L, Crooks VA. Crowdfunding for unproven stem cell–based interventions. JAMA. 2018;319(18):1935–6.
Lysaght T, Munsie M, Hendl T, Tan L, Kerridge I, Stewart C. Selling stem cells with tokens of legitimacy: an analysis of websites in Japan and Australia. Cytotherapy. 2018;20(5):S77–S8.
Lau D, Ogbogu U, Taylor B, Stafinski T, Menon D, Caulfield T. Stem cell clinics online: the direct-to-consumer portrayal of stem cell medicine. Cell Stem Cell. 2008;3(6):591–4.
Piuzzi NS, Dominici M, Long M, Pascual-Garrido C, Rodeo S, Huard J, et al. Proceedings of the signature series symposium “cellular therapies for orthopaedics and musculoskeletal disease proven and unproven therapies-promise, facts and fantasy,” international society for cellular therapies, Montreal, Canada, May 2, 2018. Cytotherapy. 2018;20(11):1381–400.
Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, Leonard RE, et al. Vision loss after intravitreal injection of autologous “Stem Cells” for AMD. N Engl J Med. 2017;376(11):1047–53.
Belmonte JCI, Ellis J, Hochedlinger K, Yamanaka S. Induced pluripotent stem cells and reprogramming: seeing the science through the hype. Nat Rev Genet. 2009;10(12):878–83.
Petrini C. Umbilical cord blood collection, storage and use: ethical issues. Blood Transfus. 2010;8(3):139.
Stewart CL, Aparicio LC, Kerridge IH. Ethical and legal issues raised by cord blood banking - the challenges of the new bioeconomy. Med J Aust. 2013;199(4):290–2.
Dessels C, Alessandrini M, Pepper MS. Factors influencing the umbilical cord blood stem cell industry: an evolving treatment landscape. Stem Cells Transl Med. 2018;7(9):643–50.
Acknowledgments
This work was supported by grant # 5300 from the Egyptian Science and Technology Development Fund (STDF), and by internal funding from Zewail City of Science and Technology (ZC 003-2019).
Take Home Message
-
The biology of stem cells in tissue homeostasis and development has made it the prospect for the field of regenerative medicine.
-
Stem cell potency is more pronounced in embryonic tissues compared to adult cells. In the adult tissues, stem cells are widely distributed throughout the body including, but not limited to, the bone marrow, adipose tissue, intestine, skin, synovial membrane, and dental pulp.
-
Reprogramming somatic cells by induced pluripotent stem cell (iPSC) technology, gene editing, and applying modern techniques of nanotechnology and bioprinting have all made it possible for extensive applications of adult stem cells in regenerative medicine.
-
Hematopoietic stem cells transplantation (HSCT) is already a routine practice, and has secured FDA approval for its cellular products to treat hematological diseases.
-
Research is still in progress for wound healing and osteoarthritis treatment using stem cells.
-
Preclinical and clinical studies showed new hope in treating incurable chronic diseases like multiple sclerosis, macular degeneration, Parkinson’s Disease, and diabetes mellitus with stem cells.
-
FDA, CDC, ISSCR and other stem cell societies and institutes are regularly warning about the misused stem cell therapy away from their approved applications to minimize patients’ risks.
-
Various types of stem cells need more clinical investigations to test their safety and efficacy before being clinically translated.
-
Patients have to be cautious about the credibility of any cell-based medical application; and especially before undergoing stem cell therapy.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Essawy, M., Shouman, S., Magdy, S., Abdelfattah-Hassan, A., El-Badri, N. (2020). Introduction and Basic Concepts in Stem Cell Research and Therapy: The Facts and the Hype. In: El-Badri, N. (eds) Regenerative Medicine and Stem Cell Biology . Learning Materials in Biosciences. Springer, Cham. https://doi.org/10.1007/978-3-030-55359-3_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-55359-3_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-55358-6
Online ISBN: 978-3-030-55359-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)