Abstract
Since the first speculations about organ preservation over 200 years ago, there has been enormous progress in this field. Progressin solid-organ transplantation from the level of experimental studies to becoming the criterion standard is undoubtedly due to advances in organ retrieval from deceased donors and their preservation. The objective of the preservation process is to preserve organ function and cellular integrity until the time of transplant. Because the number of patients on wait lists has increased and because of the limited organ and tissue pool, marginal grafts from deceased donors have become more frequently used. In this regard, organ and tissue preservation techniques are of even greater importance for outcomes attained with marginal grafts. In this chapter, we will review and discuss injury mechanisms during the organ preservation process, the history of organ preservation techniques and solutions and currently used practices, and future perspectives. These techniques have initiated in-depth studies about advanced graft preservation, viability assessment, and most importantly repair and regeneration. Studies on organ preservation continue intensively in many organ transplant centers worldwide, with the basic aim of prolonging preservation time without injuring the organ.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Organ transplantation has been one of the most significant advances in modern medicine in the second half of the twentieth century. It is regarded as the most effective treatment for end-stage organ failure. Progressin solid-organ transplantation from the level of experimental studies to becoming the criterion standard is undoubtedly due to advances in organ retrieval from deceased donors and their preservation. Organ preservation starts with making the diagnosis of brain death and continues until the time of the completion of vascular anastomoses and the return of proper organ function in the recipient. The objective of the preservation process is to preserve organ function and cellular integrity until the time of transplant [1, 2]. Because the number of patients on wait lists has increased and because of the limited organ and tissue pool, marginal grafts from deceased donors have become more frequently used. In this regard, organ and tissue preservation techniques are of even greater importance for outcomes attained with marginal grafts [3]. In this chapter, we will review and discuss injury mechanisms during the organ preservation process, the history of organ preservation techniques and solutions and currently used practices, and future perspectives.
6.2 History of Organ Preservation
First mentioned in the biography of Cesar Julien and Jean Le Gallois in 1812 and developed from the primitive concept of extracorporeal circulation, organ preservation has created a speculation that “if the heart’s function can be mimicked via injection and if that injection can be regularly sustained, then life can be maintained infinitely” [4]. In 1938, Carrel and Lindbergh conducted a study that described the first isolated organ perfusion in which organs of cats, dogs, and chickens could be preserved under normothermic conditions [5]. Carrel perfused isolated cat thyroids in the Lindbergh apparatus with a thyroid solution composed of glucose, ions, and 40% to 50% homologous serum. He discovered that organs could survive for 3 to 21 days [6]. With the discovery of heparin, blood-containing substances necessary for continued survival of organs has been used as the perfusion fluid [7]. However, many studies conducted under normothermic conditions have shown that organs could only survive for an hour. This negative outcome has directed researchers from normothermic studies to hypothermic ones, making it clear that tissues may preserve their viability 10 times longer when their temperature is lowered to 4 °C compared with normothermic conditions [8, 9].
The discovery of hypothermic preservation methods led to a flood of organ preservation studies. Belzer et al. reported in 1967 that kidneys could be preserved for 72 h using a pulsatile perfusion device [10]. After it was understood how cell integrity and function are impaired in ischemia and that hypothermia augments ischemic injury by inducing cellular swelling, new irrigation and preservation liquids were developed. For this purpose, plasma, Perfadex, and Ringer lactate solutions were initially used. However, because kidney irrigation with these solutions did not yield superior outcomes versus embedding them in ice, researchers discovered that kidneys could be preserved for 30 h at 4 °C using solutions having intracellular properties and higher potassium and lower sodium content [11]. As greater understanding developed regarding the pathophysiology of ischemia-reperfusion injury and the differing resistances of organs to ischemia, focus on the importance of the contents of preservation solutions increased. Pharmacological agents added to preservation solutions can prolong organ preservation times. In 1988, Belzer and colleagues developed the University of Wisconsin (UW) solution [12]. Thanks to other subsequently developed solutions, the hypothermia-induced cellular dilemma was mitigated, allowing a longer preservation time for retrieved organs.
6.3 Pathophysiology of Ischemia-Reperfusion
There are three important points to remember for organ preservation: hypothermia, cellular swelling, and reperfusion injury induced by free radicals. With ischemia, structural alterations occur in the mitochondria, cell nucleus, endoplasmic reticulum, lysosomes, and cell membrane [1]. Although it is difficult to ascertain whether these alterations are reversible or irreversible, it is already known that injuries to mitochondria and cell membrane can cause permanent injury; that is, they can lead to irreversible impairments in organ function. Ischemic changes begin at different times in each tissue of the human body, and each organ’s ischemia endurance time is different. For example, ischemic changes start 5 min after ischemia in the heart and 30 min in the proximal tubules of the kidneys [13].
There are 2 main mechanisms of transplant tissue injury: ischemia/hypothermia and reperfusion.
6.3.1 Ischemia
Ischemia is the inability of the circulation to supply a tissue’s need for oxygen and other metabolites and to remove waste products. Depending on ischemia time, reversible or irreversible changes can occur in the mitochondria, cell nucleus, endoplasmic reticulum, lysosomes, and cell membrane. During transplant, the two types of ischemia (cold and warm) follow each other [14].
Cold ischemia encompasses the cold preservation time after hypothermic perfusion has started with the cessation of the deceased donor’s circulation. It ends when the organ is removed from the storage box.
Warm ischemia encompasses the time period between taking the organ out of the cold storage box and reperfusion. However, tissue injury caused by this process increases with increasing temperature. Hence, injury is reduced by taking measures such as shortening the warm ischemia time, reducing direct manual contact with the organ to limit an increase in temperature, and intermittent irrigation of the organ with isotonic saline [15].
6.3.2 Hypothermia-Induced Cellular Swelling
The main objective in organ preservation is to establish hypothermia, prevent cellular swelling, and minimize free radical-induced organ injury. Primary nonfunction of a transplanted organ is mainly due to preservation injury, where the death of the organ is inevitable [7, 16]. There are many complex physiological interactions during organ transplant. A thorough understanding of organ preservation, organ ischemia, and reperfusion pathophysiology is imperative [17].
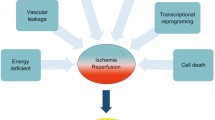
The basic factor for slowing cellular metabolism is hypothermia. When an organ’s temperature is kept at low levels, its metabolism is slowed, its need for oxygen and nutrients is reduced, the activity of hydratic enzymes is stopped, and the growth of microorganisms is arrested. With every 10 °C temperature decrease, organ metabolism reduces by 1.5 to 2.5 times, hence lowering approximately 10 times from 37 °C to 0 °C [1, 18]. In contrast to these favorable effects, hypothermia also has a detrimental effect, namely, cellular swelling. In this setting, ion pumps located at the cell membrane, particularly the sodium-potassium adenosine triphosphate (ATP) pump, are impaired. Normally, this mechanism pumps out intracellular sodium ions and pumps in extracellular potassium ions. Ischemia and hypothermia reduce the amount of ATP and hence impair the function of this pump. The most notable mechanism to preserve cellular integrity is the cell wall sodium-potassium pump, which maintains a cell’s ionic composition. When the activity of the sodium-potassium pump is impaired, sodium ions leak into the cell and potassium ions leak out. However, cells continue to function but at the expense of an anaerobic metabolic condition. This causes increased hydrogen ion and lactic acid production and hence acidosis. In addition, as a result of alterations in cell membrane permeability, calcium enters the cell and alters the intracellular acid buffering ability. Intracellular calcium triggers enzyme activation and induces cellular injury. At the same time, chlorine is transported outside the cell, intracellular oncotic pressure is increased, water is drawn into the cell, the cell swells, and cell death occurs (Fig. 6.1) [2, 19].
6.3.3 Reperfusion Injury
Another subject that is important for preservation, which has been recently intensely studied, is reperfusion injury caused by free oxygen radicals. Free oxygen radicals (FORs) and cytotoxins that are released when anastomoses are completed and organ reperfusion is established in the recipient are responsible for this type of injury. FORs are also responsible for augmented immunogenic properties of the graft. As ischemia time is prolonged, purine metabolites like xanthine and hypoxanthine, which are formed by ATP breakdown, are accumulated in the cell. An increase in calcium concentration causes the conversion of xanthine dehydrogenase into xanthine oxidase. A rapid and sudden oxygen supply to the tissue by reperfusion causes purine oxidation by xanthine oxidase and thus FOR formation. Lipid peroxidation, protein oxidative modification, and, by generating DNA chain breakdown, tissue injury form FORs. Cytokines are intercellular messenger molecules. Secretion of cytokines, including tumor necrosis factor-alpha, interferon-gamma, interleukin-1, and interleukin 8, is increased as a result of ischemia-reperfusion injury. Increased expression of these cytokines may cause leukocyte accumulation and formation of thrombocyte plugs, thus impairing graft function following revascularization.
With this understanding of the pathophysiology of reperfusion injury, some pharmacological agents that may prevent injury have been added to preservation solutions. Allopurinol is a xanthine oxidase inhibitor that has been shown to alleviate reperfusion injury when used before ischemia. Adenosine is used for ATP production during reperfusion. Glutathione, vitamin E, tryptophan, and histidine are used in perfusion solutions as FOR sweepers [20].
6.4 Organ Preservation Solutions and Techniques
Organ preservation is the most important step for all transplanted tissues and organs, including heart, lungs, liver, kidneys, cornea, pancreas, and small intestines. If metabolism can be slowed down during the ischemic process, which develops as a result of the cessation of an organ’s vascular supply, then the resulting tissue injury will be proportionately reduced. Preservation solutions should protect organ viability and metabolism while the organ is transplanted to the recipient [1, 9]. An appropriate and effective preservation solution should consider the following:
-
Reduce hypothermia-induced cellular swelling
-
Prevent intracellular acidosis
-
Not spread into the interstitial space during irrigation
-
Protect the organ from cellular injury caused by FORs formed during reperfusion
-
Provide necessary materials to reproduce ATP during reperfusion
6.4.1 Overview of Organ Preservation Solutions
Today, various solutions with different contents, but with a similar goal, are used for organ preservation. In general, preservation solutions contain electrolytes (sodium, potassium, chloride, gluconate, magnesium), acidity regulators (sulphate, bicarbonate, phosphate, lactobionate), sugars (glucose, trehalose, raffinose), colloids (HES, dextran), free oxygen radical scavengers (N-acetylcysteine, allopurinol, glutathione), and some other substances, albeit at variable proportions. The substances used in preservation solutions and their function are presented in Table 6.1 [21].
6.4.2 Overview of Organ Preservation and Transplant
An organ must be preserved without being harmed during transport to the immunologically suitable recipient’s hospital. With suitable preservation solutions and methods, a heart can currently be preserved for more than 6 h, a liver 24 h, a pancreas 48 h, and a kidney 100 h. The two basic techniques used for organ preservation are the continuous perfusion technique and the simple hypothermic preservation using preservative solutions [16, 22].
Organ preservation begins before donor surgery. Allowing a donor’s hemodynamics to be maintained until organ retrieval allows healthier organs and longer graft survival to be achieved. Measures to be taken for this purpose include the following:
-
Donor’s blood pressure should be kept between 90 and 120 mmHg
-
Donor should have a urinary output of 100 mL/h
-
Hematocrit should be kept at 35%
-
Arterial blood-gas Spo2 value should exceed 95%
-
Body temperature should be kept above 35 °C
All necessary information about the organ should be provided to the transplant team. In particular, information about the organ’s anatomic structure and vascular anomalies should be provided. In addition, the exact time of harvest, how in situ perfusion was achieved, and the perfusion solution and its amount should be specified. It should be remembered that transplantation is a race against time [23].
6.5 The Simple Hypothermic Preservation Technique
6.5.1 Overview
Since the 1960s, static hypothermic preservation solutions have become the criterion standard for organ preservation. Static hypothermic preservation solutions include the irrigation of a retrieved organ with the preservation solution at 0 °C to 4 °C, with preservation in the solution at the same temperature until the time of transplant. The hypothermic medium is responsible for slowing cellular metabolism; a preservation solution reduces cellular metabolism and provides cytoprotection [1]. With the understanding of the cellular injury process during transplant, many preservation solutions have been developed to aid healing of injury. In 1969, Collins et al. developed a solution that could successfully protect kidneys for 30 h after organ retrieval until transplant, provided that the kidneys were perfused immediately after retrieval and preserved at 4 °C. Collins solution was the first preservation solution introduced to the commercial market [7]. It has been used to preserve kidney, heart, liver, and lung grafts. In 1980, the Collins solution was modified with an impermeant composition and an improved chemical stability. The renewed solution was called Euro-Collins solution, and it provided better preservation during prolonged cold ischemia [24]. In the mid-1980s. the University of Wisconsin (UW) solution was introduced. This solution is still used today for the preservation of intraabdominal organs [25].
In 1996, Haberal et al. were able to extend cold ischemia time to 111 h by using the simple hypothermic preservation technique. They reported that the organ functioned properly for 24 years [22]. The preservation solution is an intracellular-type solution characterized by a lower sodium ion concentration and a higher potassium ion concentration. Intracellular liquid-type solutions aim to prevent cellular edema by preserving intracellular ions over hypothermia-induced sodium/potassium ion pump dysfunction [26]. The simple hypothermic preservation technique is preferred by most transplant centers around the world due to its favorable properties, such as requiring no experienced team and complex devices, having low cost, allowing transport of organs to other centers, and having the advantage of not causing vascular damage seen in transplanted organs with the continuous perfusion technique [27].
During organ recovery with the simple hypothermic preservation method, organs are irrigated in situ with 2000 cm3 (4 °C) of preservation solution at a pressure of approximately 60 to 100 mmHg through a catheter placed inside the donor’s aorta. The recovered organs are perfused separately with the same solution prepared in a sterile fashion at 4 °C. The solution volume varies by the type of organ recovered. Kidneys are irrigated with 200 to 400 cm3, livers with 1000 to 1500 cm3 (hepatic artery 150 cm3, portal vein 1000 cm3, common bile duct 100 cm3), and pancreas with 200 to 500 cm3. Recovered organs are then placed into bags containing the same solution at 4 °C. To prevent warming, crushing, and infection of the organ during transport, a second bag containing Ringer lactate and ice and a third bag as preservative are used. To maintain the temperature at 4 °C, the recovered organ is placed into a box filled with crushed ice, after which it is rapidly transported. During the period from placement of the organ into preservation solution to transplant, ice cubes inside the cold storage box are checked intermittently to ensure effective cooling is maintained [28].
6.5.2 Hypothermic Preservation Solutions Used Today
Over the past quarter century, 160 to 170 different solutions have been introduced. Among them are histidine-tryptophan-ketoglutarate (HTK), UW, Collins, Euro-Collins, UW-polyethylene glycol, polysol, Kyoto, and New Kyoto. However, HTK and UW solutions are the most widely used and studied solutions worldwide [29, 30].
6.5.2.1 Euro-Collins Solution
The aim of this solution is to reduce substance traffic between the intracellular and extracellular spaces during ischemia. For this purpose, high concentrations of potassium, magnesium, phosphate, and glucose and lower concentrations of sodium and bicarbonate are added to the solution prepared with intracellular properties. It has been shown to reduce delayed graft function when used for kidney preservation. This solution is used for both living-donor and deceased-donor transplant procedures [31, 32].
6.5.2.2 Ross-Marshall Citrate Solution
This solution has been developed as an alternative to Collins solution. Although its electrolyte content is similar to that of Collins solution, this solution uses citrate instead of phosphate and mannitol instead of glucose. Citrate forms a chelate with magnesium and helps to stabilize the extracellular space [33].
6.5.2.3 Histidine-Tryptophan-Ketoglutarate Solution
Histidine-tryptophan-ketoglutarate solution (CUSTODİOL®, Odyssey Pharmaceutical, Hanover, Germany) was developed in the 1980s for cardioplegia. In this solution, mannitol and histidine create both an antioxidant and an osmotic effect. Ketoglutarate and tryptophan, on the other hand, have membrane-protective effects and act as a substrate of the Krebs cycle in an oxygen-deprived cell. Because potassium and sodium ion concentrations are low, it was first widely used for heart transplants. It offers a more rapid cooling effect and is characterized by a threetimes greater flow rate. Because its viscosity is lower (2.0 centipoise), it is used in high volumes and at a lower flow rate (approximately 10–12 lt and 100–175 mL/kg). Compared with UW solution, its cost is lower, and it forms no hyperpotassemia when used in flushing fashion [34]. In experimental studies, HTK was shown to be more effective than Euro-Collins and UW solutions at clearing blood cells in a microvascular bed. It was also as effective as UW solution in liver transplant [35]. In general, HTK solution lowers leukocyte adhesion rate, reduces capillary permeability, increases tissue oxygenation, reduces ATP consumption, and increases lactate dehydrogenase levels.
6.5.2.4 University of Wisconsin Solution
The UW solution (Via Span Belzer®, DuPont Pharmaceutical, Dublin, Ireland) is a solution developed by Belzer and colleagues at the University of Wisconsin. It is currently used in all organ transplant procedures. This solution was first formulated to reduce cellular swelling caused by hypothermia. Therefore, it has an intracellular character (contains low sodium and high potassium).
Glutathione and allopurinol as free-radical scavengers and xanthineoxidase as an inhibitor have been added to UW solution [36]. Both lactobionate and glutathione have been shown to be strong matrix metalloproteinase inhibitors. It also contains adenosine for ATP need, hydroxyethyl starch as an interstitial edema preventer, and phosphate as a pH stabilizer. Because it contains the same concentration of electrolytes as the intracellular space, it is cardiotoxic and its use in flushing fashion carries the risk of hyperkalemia [37]. Compared with HTK, UW has a higher viscosity; however, its endothelial protective effects are greater, and thus the incidence of ischemic biliary complications has been shown to be higher [38]. This solution also increases hepatic artery resistance and leukocyte aggregation [39].
6.5.2.5 Phosphate-Buffered Sucrose Solution
This solution contains 140 mmol/L sucrose, sodium hydrogen, and dihydrogen phosphate as a buffer. It is not widely used [40].
6.5.2.6 Celsior Solution
Celsior solution was originally introduced as a heart preservation solution in the 1990s and has been used as both a thoracic and abdominal organ preservation solution thereafter [28]. It has an extracellular character. Its content is very similar to that of UW solution. Because it does not contain HES, its viscosity is lower. Similar to the HTK solution, it uses histidine buffer and a lower potassium ion concentration. However, its sodium ion concentration is much higher. It has equal properties as UW but with lower cost. Unlike UW solution, it contains glutamate as an antioxidant and an energy substrate. Other antioxidants like reduced glutathione, histidine, and mannitol have been added to the solution [41].
6.5.2.7 Kyoto Extracellular-Type Solution
Developed by Kyoto University, this solution contains higher concentrations of sodium, low concentrations of potassium, a disaccharide trehalose, and gluconate [41]. This extracellular-type solution is mostly used for experimental purposes.
Euro-Collins, HTK, and UW solutions have been in the commercial market since the 1980s; small modifications have been made to each of their compositions for marketing purposes. Table 6.2 provides a comparison of widely used preservation solutions [2].
6.6 The Continuous Hypothermic Perfusion Technique
The continuous perfusion technique was first used by Belzer et al. in 1967 [42]. Machine perfusion is a method that involves organ perfusion using a controlled perfusate flow. The main aims of this method is to provide oxygen and other nutrients through a perfusion device after organ retrieval, maintain the organ’s temperature at between 8 °C and 12 °C, and remove metabolic products from the medium in a continuous manner. The cold ischemia time is the main bottleneck for transplanted organs, particularly those coming from marginal donors. Use of pumps to perfuse organs with an oxygenated fluid during cold ischemia time would allow cells to maintain their metabolic functions with less hypoxic injury [43, 44]. In a randomized study, kidneys from deceased donors stored with the machine perfusion method showed better graft survival than simple cooling [7].
The metabolic rate of cells is proportional to the medium’s temperature. For example, at 0 °C to 4 °C, static cold preservation solutions reduce the metabolic rate by 5%. With hypothermic machine perfusion, the rate can be reduced by 10% at 0 °C to 8 °C.Normothermic machine perfusion can reduce at 35 °C to 38 °C by 70% to 80%, subnormothermic machine perfusion can reduce at 20 °C to 34 °C by 50%, and controlled oxygenated reheating can reduce at 8° to 20 °C by 20% to 25% (Fig. 6.2) [20].
6.6.1 Hypothermic Machine Perfusion
Hypothermic machine perfusion (HMP) (0–8 °C) is based on the concept of maintaining oxidative energy production at hypothermic temperatures via mitochondrial electron transport. Hypothermic machine perfusion constantly provides metabolic substrates for ATP production to replenish the graft tissue’s energy. The first clinical HMP device was developed by Belzer in 1960; it was first used by Belzer et al. in 1968 to perform a preserved human kidney transplant. They performed kidney perfusion with hypothermic, diluted plasma or blood for 3 days [45, 46]. The newer HMP technology was shown to improve late graft function in marginal donors compared with simple cold preservation solutions. In 2015, approximately 25% to 35% of all transplanted kidneys in the United States were preserved with HMP [47]. However, because the liver is perfused via both the portal vein and hepatic artery, use of HMP for liver transplant is challenging. Nevertheless, the first clinical study of liver grafts preserved with HMP showed shorter hospitalization times and reduced vascular and biliary complications. A few studies reported use of HMP for heart and lung transplants. Nakajima et al. reported that short-lived HMP (1–2 h) may increase energy levels in lung tissue and that it may improve ischemia-reperfusion injury by reducing the rate of reactive oxygen species production in rat lungs [48, 49]. Michel et al. showed that HMP protected the cellular structure of donor hearts better than simple cooling during prolonged ischemia times in pigs (Fig. 6.3) [50].
6.6.2 Normothermic Machine Perfusion
Normothermic machine perfusion (NMP) (35–38 °C) is a method that perfuses donor organs under physiological conditions to maintain activity and viability. Normothermic machine perfusion provides oxygen and basic substrates and keeps donor organs at body temperature. In 2001, Steen et al. reintroduced the exvivo lung perfusion (EVLP) technique to assess lungs after cardiac death [51]. In 2007, the group performed the first human lung transplant using a donor lung rejected after being assessed by EVLP [52, 53]. The first clinical study on NMP in liver transplant was published in 2006; it assessed the outcomes of 16 transplant patients operated after NMP. The results suggested that 30-day graft survival was similar between the NMP and simple cooling techniques and that the median peak aspartate aminotransferase level was significantly lower in the NMP group compared with the simple cooling technique [54].
6.6.3 Subnormothermic Machine Perfusion
Subnormothermic machine perfusion (SNMP) (20–34 °C) is an intermediary approach between HMP and NMP. Although better preservation times have been provided with NMP compared with HMP, it has been considered that the cytoprotective benefits of reduced cellular metabolism under hypothermic temperatures would further improve organ preservation. In addition, adequate metabolism would be provided for viability assessment and organ repair/replenishment [55]. Although some studies have shown that livers and kidneys perfused with SNMP were superior to those preserved by the simple cooling technique, a recent study reported that swine kidneys preserved with SNMP had a higher rate of graft dysfunction than those preserved with the simple cooling technique [56, 57].
6.6.4 Controlled Oxygen Reheating
After cold ischemic protection, a sudden change of temperature from hypothermia to normothermia upon reperfusion may result in reperfusion-induced organ injury, affecting mitochondria and proapoptotic signal conduction. Hypothermic protection serves to help the ischemic organ by lowering metabolism. However, ischemic redox dyshomeostasis may disrupt mitochondrial membrane potential via mitochondrial passage opening. Mitochondrial injury may be worsened after reperfusion [58]. Controlled oxygen reheating (COR) (8–20 °C) is an alternative organ perfusion technique that involves a slow, gradual increase in perfusate temperature. Controlled oxygen reheating aims to minimize graft injury and prevent mitochondrial membrane potential disruption. Clinical studies have shown that COR could be used safely during liver transplant [59]. In 2016, COR was used effectively during 15 human liver transplant procedures [60].
6.6.5 Pulsatile Perfusion
Continuous perfusion can be accomplished by pulsatile or continuous techniques. Pulsatile flow can reduce capillary pressures, augment the expression of vasodilatory molecules, and provide a better solution flow, thereby improving organ function [44]. In addition, other agents like vasodilators and free oxygen radical scavengers can also be added to the solution. The use of a pump has dramatically improved organ use and its outcomes, particularly from marginal donors. In addition to improved graft function, it allows longer cold ischemia time. Several pumps are being developed for hepatic, cardiac, and pulmonary allografts. These pumps are portable and allow organ transport during pumping. The pump properties can also serve as a tool for diagnosis to decide whether a graft is suitable for use. Poor pump properties have been linked to a higher rate of graft dysfunction and thus higher rejection rates [1, 44]. In pulsatile perfusion, the solution used to perfuse the organ is usually given at a rate of 60 beats/min. The initial systolic pressure is 60 mmHg and diastolic pressure is 8 to 14 mmHg. Within hours after starting perfusion, the systolic pressure drops to 35 to 40 mmHg. The main superiority of pulsatile perfusion over continuous perfusion is that the former does not expose the organ’s vascular structures to continuous pressure [40].
6.7 Summary of Continuous Perfusion Versus Simple Hypothermic Preservation and the Available Preservation Solutions
6.7.1 Advantages and Disadvantages of Continuous Perfusion Techniques Versus Simple Hypothermic Preservation Techniques
6.7.1.1 Advantages
The advantages of the continuous perfusion technique versus the simple hypothermic preservation technique are as follows:
-
Preservation of organs with a long cold ischemia time
-
Supporting organ metabolism with various factors (nutrient factors, osmotic and oncotic factors, oxygen, pH regulation, removal of metabolic wastes)
-
Prevention of vasoconstriction
-
Better removal of blood and its elements from the organ
-
Ability to monitor the organ’s physiological parameters
-
Ability to determine levels of enzymes released from the organ, indicative of cellular injury
-
Has been shown to have a reduced rate of primary graft organ dysfunction
6.7.1.2 Disadvantages
The advantages of the continuous perfusion technique versus the simple hypothermic preservation technique are as follows:
-
Experienced personnel and perfusion devices may not be available in every center (thus, limiting transfer of organ to other centers)
-
Organ injury may occur due to possible device malfunction
-
May result in alterations induced by continuous pressure perfusion in the vascular system
6.7.1.3 Other Considerations
Ultrastructural examination of continuously perfused kidneys have shown that the vascular endothelium is affected [44]. Electron microscopic examination of biopsies taken 1 h after organ transplant detected endothelial cell and basal membrane injury. These injuries may be caused by high perfusion pressures and colloid concentrations found in perfusion solutions [2]. In kidneys treated with the simple hypothermic preservation technique, on the other hand, these injuries are far less common, which is the reason why the simple hypothermic preservation technique is widely utilized. Perfusion machines developed for organ preservation in clinical practice include the Belzer machine, GAmbro machine, and Waters machine. These machines are single-use devices that are similar in many aspects. Today, only the Waters machine is in active use [2].
Widely used perfusates, such as Steen solution and the Organ Care System perfusate, use glucose as the sole energy source. However, organs are perfused at body temperature during NMP. Glucose alone is not sufficient for organ metabolism. Adding more nutrients such as aminoacids, vitamins, lipids, and others should be considered to prolong NMP for organ repair. Aminoacids are the basic structural components of proteins and are essential for cell survival and proliferation. Vitamins may aid in utilizing chemical energy supplied for processing proteins, carbohydrates, and fats, which are necessary for cell metabolism [61].
In studies in liver and kidney transplants, aminoacids and extra glucose were added to the perfusate during NMP; this approach yielded promising results in dogs [62].
Although only a few prospective studies are available about continuous perfusion devices, several studies have indicated that it was effective for organ preservation. Nevertheless, the technique and its higher cost limit its use.
6.7.2 Which Is the Best Solution?
The answer is debatable, and there is no comprehensive study to answer this question. The UW solution and its derivatives are the most widely used solutions throughout the United States. There is some evidence that the HTK solution is less viscous than the UW solution, flows more smoothly through small vessels, and causes less vascular complications after transplant, especially in donors with prolonged cold ischemia time [1, 3, 43]. The Euro-Collins solution has been recently used mostly for kidney transplants. Haberal et al. reported that, with this solution, kidneys with a cold ischemia time of upto 111 h could be successfully transplanted [22]. In liver and pancreas transplants, the UW solution is widely used. With the use of this solution and the simple cooling technique, liver preservation can be successfully applied for 48 h under experimental conditions and for 36 h under clinical conditions, whereas pancreas preservation can extend to 48 to 72 h under experimental conditions and to 24 h in clinical practice [1, 2, 63].
6.7.3 Conclusions
Since the first speculations about organ preservation over 200 years ago, there has been enormous progress in this field. The introduction of the simple organ cooling technique in the 1960s caused a revolution. It has become a standard procedure for preserving organs in a static manner at hypothermic temperatures. With more demands to expand the organ donor pool, the current status of organ preservation has evolved from a simple cooling procedure to a continuous perfusion technique. These techniques have initiated in-depth studies about advanced graft preservation, viability assessment, and most importantly repair and regeneration. Studies on organ preservation continue intensively in many organ transplant centers worldwide, with the basic aim of prolonging preservation time without injuring the organ. Current techniques allow slowing, but not complete cessation, of organ metabolism. It is thought that complete, reversible cessation of metabolism and organ function is possible with the use of “cryopreservation.” However, this technique has yet been developed for clinical practice.
References
Demirbaş A. Organ prezervasyonu. In: Haberal M, editor. Doku ve Organ Transplantasyonları. Ankara: Haberal Eğitim Vakfı; 1993. p. 69–75.
Haberal M, Emiroğlu R. Karaciğer transplantasyonu. In: Gulay H, editor. Temel ve Sistematik Cerrahi. İzmir: Güven Kitapevi; 2005. p. 711–38.
Brodie T. The perfusion of surviving organs. J Physiol. 1903;29:266–75.
Le Gallois M. Experiments on the principle of life, and particularly on the principle of the motions of the heart, and on the seat of this principle: including the report made to the first class of the Institute, upon the experiments relative to the motions of the heart. Philadelphia, PA: M. Thomas; 1813.
Carrel A, Lindbergh CA. The culture of whole organs. Science. 1935;81:621–3.
Carrel A. The culture of whole organs: I. technique of the culture of the thyroid gland. J Exp Med. 1937;65:515–26.
Collins GM, Bravo-Shugarman M, Terasaki PI. Kidney preservation for transportation. Initial perfusion and 30 hours’ icestorage. Lancet. 1969;2:1219–22.
Ueda Y, Todo S, Imventarza O, Furukawa H, Oks A, Wu YM, et al. The UW solution for canine kidney preservation. Its specific effect on renal hemodynamics and microvasculature. Transplantation. 1989;48:913–8.
Taylor MJ, Baicu SC. Current state of hypothermic machine perfusion preservation of organs: the clinical perspective. Cryobiology. 2010;60(Suppl 1):20–35.
Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45(4):673–6.
Calne RY, Pegg DE, Pryse-Davies J, Brown FL. Renal preservation by ice-cooling: an experimental study relating to kidney transplantation from cadavers. Br Med J. 1963;2:651–5.
Vreugdenhil PK, Belzer FO, Southard JH. Effect of cold storage on tissue and cellular glutathione. Cryobiology. 1991;28:143–9.
Mühlbacher F, Langer F, Mittermayer C. Preservation solutions for transplantation. Transplant Proc. 1999;31(5):2069–70.
Collins GM, Hartley LC, Clunie GJ. Kidney preservation for transportation. Experimental analysis of optimal perfusate composition. Br J Surg. 1972;59:187–9.
Starzl TE, Hakala TR, Shaw BW Jr, Hardesty RL, Rosenthal TJ, Griffith BP, et al. Flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–30.
Arnault I, Bao YM, Dimicoli JL, et al. Combinedeffect of fasting and alanine on liver function recovery after cold ischemia. Transplant Int. 2002;15:89–95.
Pienaar BH, Lindell SL, Van Gulik T, Southard JH, Belzer FO. Seventy-two hour preservation of the canine liver by machine perfusion. Transplantation. 1990;49(2):258–60.
Guibert EE, Petrenko AY, Balaban CL, Somov AY, Rodriguez JV, Fuller BJ. Organ preservation: current concepts and new strategies for the next decade. Transfus Med Hemother. 2011;38(2):125–42.
Moray G, Sevmis S, Karakayali FY, Gorur SK, Haberal M. Comparison of histidine-tryptophan-ketoglutarate and University of Wisconsin in living-donor liver transplantation. Transplant Proc. 2006;38:3572–5.
Jing L, Yao L, Zhao M, Peng LP, Liu M. Organ preservation: from the past to the future. Acta Pharmacol Sin. 2018;39(5):845–57.
O’Callaghan JM, Morgan RD, Knight SR, Morris PJ. The effect of preservation solutions for storage of liver allografts on transplant outcomes. Ann Surg. 2014;260:46–55.
Haberal M, Moray G, Bilgin N, Karakayali H, Arslan G, Büyükpamukçu N. Ten-year survival after a cold-ischemia time of 111 hours in the transplanted kidney. Transplant Proc. 1996;28(4):2333.
ANZOD Registry. Australia and New Zealand Organ Donation Registry Report 2015. http://www.anzdata.org.au/anzod/v1/ar-2015.html. Accessed 4 Jun 2016.
Aydin G, Okiye SE, Zincke H. Successful 24-hour preservation of the ischemic canine kidney with Euro-Collins solution. J Urol. 1982;128:1401–3.
Jamieson RW, Friend PJ. Organ reperfusion and preservation. Front Biosci. 2008;13:221–35.
Okada Y, Kondo T. Preservation solution for lung transplantation. Gen Thorac Cardiovasc Surg. 2009;57:635–9.
Voigt MR, DeLario GT. Perspectives on abdominal organ preservation solutions: a comparative literature review. Prog Transplant. 2013;23:383–91.
Karam G, Compagnon P, Hourmant M, Despins P, Duveau D, Noury D, et al. A single solution for multiple organ procurement and preservation. Transpl Int. 2005;18:657–63.
Stewart ZA, Cameron AM, Singer AL, Montgomery RA, Segev DL. Histidine-tryptophan-ketoglutarate (HTK) is associated with reduced graft survival in deceased donor livers, especially those donated after cardiac death. Am J Transplant. 2009;9(2):286–93.
El-Wahsh M. Liver graft preservation: an overview. Hepatobiliary Pancreat Dis Int. 2007;6(1):12–6.
Groenewoud AF, Thorogood J. Current status of the Eurotransplant randomized multicenter study comparing kidney graft preservation with histidine tryptophan-ketogluterate, University of Wisconsin, and Euro-Collins solutions. The HTK Study Group. Transplant Proc. 1993;25:1582–5.
Latchana N, Peck JR, Whitson BA, Henry ML, Elkhammas EA, Black SM. Preservation solutions used during abdominal transplantation: current status and outcomes. World J Transplant. 2015;5(4):154–64.
Catena F, Coccolini F, Montori G, Vallicelli C, Amaduzzi A, Ercolani G, et al. Kidney preservation: review of present and future perspective. Transplant Proc. 2013;45:3170–7.
Feng L, Zhao N, Yao X, et al. Histidine-tryptophan-ketoglutarate solution vs. University of Wisconsin solution for liver transplantation: a systematic review. Liver Transpl. 2007;13(8):1125–36.
Ringe B, Braun F, Moritz M, et al. Safety and efficacy of living donor liver preservation with HTK solution. Transplant Proc. 2005;37(1):316–9.
Rentsch M, Post S, Palma P, Lang G, Menger MD, Messmer K. Anti-ICAM–1 blockade reduces postsinusoidal WBC adherence following cold ischemia and reperfusion, but does not improve early graft function in rat liver transplantation. J Hepatol. 2000;32:821–8.
Lynch RJ, Kubus J, Chenault RH, Pelletier SJ, Campbell DA, Englesbe MJ. Comparison of histidine-tryptophan-ketoglutarate and University of Wisconsin preservation in renal transplantation. Am J Transplant. 2008;8(3):567–73.
Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK, Tector AJ. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in extended criteria liver donors. Liver Transpl. 2008;14(3):365–73.
Rayya F, Harms J, Martin AP, Bartels M, Hauss J, Fangmann J. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in adult liver transplantation. Transplant Proc. 2008;40(4):891–4.
Terasaki PI, McClelland JD, Yuge J, Cecka JM, Gjertson DW, Takemoto S, Cho YW.Advances in kidney transplantation: 1985–1995. Clin Transpl. 1995:487–501.
Montiel-Casado MC, Pérez-Daga JA, Blanco-Elena JA, Aranda-Narváez JM, Sánchez-Pérez B, Cabello-Díaz M, Ruiz-Esteban P, León-Díaz FJ, Gutiérrez-de la Fuente C, Santoyo-Santoyo J. Pancreas preservation with viaspan, celsior, and custodiol solutions: an initial experience. Transplant Proc. 2016;48(9):3040–2.
Belzer FO, May R, Berry M, Lee JC. Short term preservation of porcine livers. J Surg Res. 1970;10:55–61.
Fontes P, Lopez R, van der Plaats A, Vodovotz Y, Minervini M, Scott V, et al. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant. 2015;15:381–94.
Guarrera JV, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10:372–81.
Humphries AL, Russell R, Stoddard LD, Moretz WH. Successful five-day kidney preservation. Perfusion with hypothermic, diluted plasma. Investig Urol. 1968;5:609–18.
Belzer FO, Ashby BS, Dunphy JE. 24-hour and 72-hour preservation of canine kidneys. Lancet. 1967;2:536–8.
Henry SD, Guarrera JV. Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev. 2012;26:163–75.
Nakajima D, Chen F, Yamada T, Sakamoto J, Osumi A, Fujinaga T, et al. Hypothermic machine perfusion ameliorates ischemia-reperfusion injury in rat lungs from non-heart-beating donors. Transplantation. 2011;92:858–63.
Nakajima D, Chen F, Okita K, Motoyama H, Hijiya K, Ohsumi A, et al. Reconditioning lungs donated after cardiac death using short-term hypothermic machine perfusion. Transp J. 2012;94:999–1004.
Michel SG, LaMuraglia GM II, Madariaga MLL, Titus JS, Selig MK, Farkash EA, et al. Twelve-hour hypothermic machine perfusion for donor heart preservation leads to improved ultrastructural characteristics compared to conventional cold storage. Ann Transplant. 2015;20:461–8.
Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjöberg T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg 2003;76:244–52; discussion 252.
Erasmus ME, Fernhout MH, Elstrodt JM, Rakhorst G. Normothermic ex vivo lung perfusion of non-heart-beating donor lungs in pigs: from pretransplant function analysis towards a 6-h machine preservation. Transpl Int. 2006;19:589–93.
Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Hear Lung Transpl. 2008;27:1319–25.
Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MTPR, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant. 2016;16:1779–87.
Bejaoui M, Pantazi E, Folch-Puy E, Baptista PM, García-Gil A, Adam R, et al. Emerging concepts in liver graft preservation. World J Gastroenterol. 2015;21:396–407.
Hoyer DP, Gallinat A, Swoboda S, Wohlschläger J, Rauen U, Paul A, et al. Subnormothermic machine perfusion for preservation of porcine kidneys in a donation after circulatory death model. Transpl Int. 2014;27:1097–106.
Adams TD, Patel M, Hosgood SA, Nicholson ML. Lowering perfusate temperature from 37 °C to 32 °C diminishes function in a porcine model of ex vivo kidney perfusion. Transplant Direct. 2017;3:e140.
Schopp I, Reissberg E, Lüer B, Efferz P, Minor T. Controlled rewarming after hypothermia: adding a new principle to renal preservation. Clin Transl Sci. 2015;8:475–8.
Hoyer DP, Mathé Z, Gallinat A, Canbay AC, Treckmann JW, Rauen U, et al. Controlled oxygenated rewarming of cold stored livers prior to transplantation. Transplantation. 2016;100:147–52.
Hoyer DP, Paul A, Minor T. Prediction of hepatocellular preservation injury immediately before human liver transplantation by controlled oxygenated rewarming. Transplant Direct. 2017;3:e122.
Bender DA. Nutritional biochemistry of the vitamins. Cambridge: Cambridge University Press; 2003.
Kaths JM, Echeverri J, Goldaracena N, Louis KS, Chun YM, Linares I, et al. Eight-hour continuous normothermic ex vivo kidney perfusion is a safe preservation technique for kidney transplantation: a new opportunity for the storage, assessment, and repair of kidney grafts. Transplantation. 2016;100:1862–70.
Jing L, Yao L, Zhao M, Peng L-p, Liu M. Organ preservation: from the past to the future. Acta Pharmacol Sin. 2018;39:845–57.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kirnap, M., Haberal, M. (2021). Organ Preservation. In: Hakim, N., Haberal, M., Maluf, D. (eds) Transplantation Surgery. Springer Specialist Surgery Series. Springer, Cham. https://doi.org/10.1007/978-3-030-55244-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-55244-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-55243-5
Online ISBN: 978-3-030-55244-2
eBook Packages: MedicineMedicine (R0)