Abstract
Water resource is the most precious resource for a human being that it is necessary to make the water resource to be clean and also non-toxic. Recently, water pollution becomes one of the most serious global issues, especially, water pollutions that contaminate various types of organic compounds, including pharmaceuticals and personal care products (PPCPs), persistent organic pollutants (POPs), and organic dyes. Photocatalysis as one of the advanced oxidation processes using reactive oxidative radicals or species to remediate the organic pollutants has drawn much attention recently.
Photocatalysis is a process which a chemical reaction is accelerated in the presence of a catalyst on exposure to light. The possibility to utilize solar energy as a free energy from nature to solve the environmental problems is the key significance of photocatalysis. Homogeneous photocatalysis has many advantages, e.g., high oxidation properties. It is, however, not popular in various photocatalytic applications, because it is difficult to separate the photocatalysts from the solution, the photocatalysts have low potential to reuse, purification of products is necessary, and almost homogeneous photocatalysts absorb narrowly light within the solar spectrum. It has been proven that heterogeneous photocatalysis is one of the most potential methods for treatment of organic pollutants in water. Anyhow, relatively large band gap energy causes some limitations of metal oxide-based heterogeneous photocatalysts. Modifications of the electronic band can be achieved by doping and composites of semiconductors. Another modification technique in heterogeneous photocatalysts is the utilization of electrical potential in photocatalysis. The coated semiconductor photocatalysts are used as photoelectrodes in photo-electrocatalytic applications. In addition, the photocatalytic reactor configuration for wastewater treatment by heterogeneous photocatalysis can be classified as two main groups, including fixed bed reactor and slurry type reactor. Apart from the conventional photocatalytic reactors, the combination of photocatalysis with another treatment process has also been developed to overcome the specific obstacles in each case, such as a photocatalytic membrane reactor.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Photocatalysis
- Photocatalysts
- Organic pollutants
- Personal care products
- Persistent organic pollutants
- Organic dyes
- Remediation
- Ozonation
- Fenton
- Metal oxide
1.1 Introduction
Water resource is the most precious resource for a human being that it is necessary to make the water resource to be clean and also non-toxic. Anyway, due to urbanization, industrialization, and lack of people awareness to consider water as a crucial commodity, people in many countries are now facing problems related to water supply and security (Jayaswal et. al 2018; Meenakshisundaram 2019). Presently, water pollution becomes one of the most serious global issues, especially, water pollutions that contaminate various types of organic compounds (Meenakshisundaram 2019), including pharmaceuticals and personal care products, persistent organic pollutants, and organic dyes.
In recent years, many researchers have developed the remediation techniques of organic pollutants in water. Photocatalysis, as one of the advanced oxidation processes (AOPs) using reactive oxidative radicals or species, particularly hydroxyl radicals, to remediate the organic pollutants, has drawn much attention recently. Various types of photocatalysis can be considered to be a green and effective strategy for solving global environmental and energy problems. The possibility to utilize solar energy as a free energy from nature to solve the environmental problems is the key significance of photocatalysis. In this chapter, the basic concept of photocatalysis, various organic pollutants in water, photocatalytic remediation of organic pollutants in water, and modifications of heterogeneous photocatalysts are discussed. Recent developments of photocatalytic reactors for remediation of organic pollutants are presented briefly.
1.2 Photocatalysis
Photocatalysis is a type of catalysis which a chemical reaction is accelerated in the presence of a catalyst (so-called photocatalyst) on exposure to light which is mostly described in term of photon (hν) – an elementary particle of light, where the photocatalyst participates in the chemical reaction without being consumed. Photocatalysis can be also defined as the acceleration of a photoreaction (e.g., photolysis) in the presence of a catalyst.
1.2.1 Type of Photocatalysis
Photocatalysis could be classified to be two types, i.e., homogeneous and heterogeneous photocatalysis, on the basis of appearances of the physical state of reactants.
1.2.1.1 Homogenous Photocatalysis
Homogeneous photocatalysis is the process that the photocatalyst is in the same phase (i.e., gas, solid, or liquid) with the reactant. The process of homogenous photocatalysis is driven under exposure to light which a molecular photocatalyst is promoted to the excited state (strong reductant and oxidant). Almost homogeneous photocatalysts can drive full redox reactions which most researchers use in water splitting to hydrogen and oxygen.
In homogeneous photocatalysis, the free radicals are produced by illumination of light over the homogeneous molecules of oxidizing agents such as hydrogen peroxide (H2O2) and ozone (O3), which are dissolved in water or another medium (Stan et al. 2012). The commonly known processes are ozonation (UV/O3), photo-Fenton processes (Fe2+ and Fe2+/H2O2), UV/H2O2, and UV/H2O2/O3.
1.2.1.2 Ozonation
Ozone, an unstable gas composed of three oxygen atoms (O3) that is a strong greenhouse gas and variable in the troposphere, becomes one of the most powerful oxidants with an oxidation potential of 2.07 V (North 2015). Ozone is often used in water and wastewater treatments, municipal and industrial treatments, agriculture, chemical synthesis, drinking water disinfection, and food and beverage (Ikehata and Li 2018; Loeb et al. 2012). Ozone can be generated by promoting potential energy, e.g., ultraviolet irradiation or electric discharge, to gaseous oxygen molecules. In terms of the process of ozone, it can react and decompose into various oxidative species, e.g., hydroxyl radical (HO•) and hydrogen peroxide (H2O2), leading to the ozonation process.
Ozonation is the oxidation method which ozone involves in the process. It is extremely used for water treatment that enormous contaminants (e.g., color substances and heavy metals) contained in the water sources. Furthermore, outgrowths of ozonation are bacteria disinfection, odorous removal, taste generation, inorganic component conversions, and cutting of hardly biodegradable organic compounds (Arvanitoyannis and Kassaveti 2008). Ozonation can be more effective with UV radiation and oxidizing agents that increase radical formations.
UV/Ozone (UV/O3) is one of the well-studied ozonation. Dissolved ozone molecules can absorb UV light (wavelength ~260 nm) by photolysis reaction, leading to the occurrence of hydrogen peroxide molecules (Eq. 1.1). Afterward, each mole of H2O2 will turn to absorb UV or react with O3, resulting in the generation of HO• as expressed in Eqs. (1.2) and (1.3) (Gong et al. 2008; Ikehata and Li 2018).
Ozonation has various advantages, such as the short half-life (~10 min) leading to the rapid reaction for degradation of organic molecules (Table 1.1). Anyway, unless at pH 10, the half-life of ozone in solution is less than 1 min that makes ozonation extensively consumes energy. The efficiency of this process depends on many variables such as UV light intensity, reactant constituent, the presence of scavenger species, pH, temperature, and type of organic target pollutants.
1.2.1.2.1 Photo-Fenton Process
H2O2 is usually used as oxidizing agents because of its environmentally benign and uncomplicated characters. Various metal ions and their oxidative forms, such as Fe2+, Fe3+, Cu+, Cu2+, Ti3+, Ti4+, Cr2+, and Cr3+, can be used as a catalyst in H2O2-based processes. Notwithstanding, Fe2+ and Fe3+ are most frequently used, because other metal ions are toxic and relatively unavailable. Fenton is one of several processes that can enhance the oxidative potential of H2O2 which can be used for the degradation of organic compounds. Fenton uses Fe2+ [ferrous ions or iron (II)] as a catalyst under acidic conditions according to Eqs. (1.4)–(1.9) (Ameta et al. 2018a).
In Fenton reactions, hydroxyl radicals (HO•) and hydroxide anions (HO−), the oxidizing and extremely powerful species, can be generated to discharge one electron from an electron-rich organic substrates or any other species present in the medium to form hydroxide anions. HO• produced from the reactions can also attack and degrade a wide range of organic compounds. The efficiency of Fenton oxidation depends on pH, concentrations of the pollutants and hydrogen peroxide, amount of ferrous ions, and temperature (Zheng et al. 2013; Ameta et al. 2018a).
In term of photocatalysis, photo-Fenton process is a combination of Fenton reactions and irradiation with light of suitable wavelength (180–400 nm) which can accelerate the formation of hydroxyl radicals and also increases the rate of degradation of organic pollutants. The continuous cycles of photo-Fenton process are shown in Eqs. (1.10)–(1.12). Fe2+ is generated through photoreduction of ferric ions (Fe3+). The generated Fe2+ will turn to react with H2O2 resulting in more HO• formation.
The efficiency of photo-Fenton process depends on pH, especially at pH 3, due to the soluble of hydroxy-Fe3+ complexes and Fe(OH)2+ leading to high catalytic activity (Ameta et al. 2018b).
1.2.1.2.2 UV/H2O2
Normally, UV radiation can work simultaneously as a disinfectant, by physical inactivation of microorganisms (Mierzwa et al. 2018). UV radiation can be also used in UV/H2O2 system for hemolytic cleavage of O-O bonds of H2O2 molecules, resulting in the production of hydroxyl radicals (HO•). The most application of UV/H2O2 is uses for water and wastewater treatments.
UV/H2O2 has three main reaction mechanisms of HO• production and recombination, which are initiation, propagation, and termination as shown in Eqs. (1.13)–(1.18). One mole of H2O2 theoretically produces two moles of HO•. The rate of HO• production strongly depends on the amount of H2O2 added, UV absorptivity of H2O2, and characteristics of wastewater (Jamil et al. 2017; Mierzwa et al. 2018).
H2O2 molecules can act as a scavenger to consume HO• and subsequently produces oxygen and water molecules according to Eq. (1.19), so the demand concentration of H2O2 must be high to generate a sufficiently high concentration of HO• for decomposition and mineralization of organic target pollutants.
1.2.1.2.3 UV/H2O2/O3
From ozonation discussed above, although UV absorptivity of O3 is much higher than H2O2, the self-decay rate of O3 is approximately 1000 times higher than that of H2O2. This limitation can be overcome by the addition of H2O2 into UV/O3 process for enhancement of the decomposition of O3, which is called “UV/H2O2/O3” process.
Even though homogeneous photocatalysis has many advantages, e.g., high oxidation properties, it is, however, not popular in various photocatalytic applications. This is because it is difficult to separate the photocatalysts from the solution; the photocatalysts have a low potential to reuse; purification of products is necessary; and almost homogeneous photocatalysts absorb narrowly light within the solar spectrum (Karimian et al. 2015; Zhu and Wang 2017). In addition, the photocatalytic activity and stability of homogeneous photocatalysts are limited due to the instability inherent to the molecular nature of their structures (Limburg et al. 2016; Ye et al. 2016).
1.2.1.3 Heterogeneous Photocatalysis
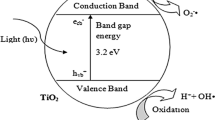
Many years ago, heterogeneous photocatalysis was found by Fujishima and Honda due to an electrochemical photocatalysis of water at a semiconductor electrode. In heterogeneous photocatalysis, the catalyst is totally separate from the reactants. It occurs by emerging materials with supremacy properties (Klavarioti et al. 2009). On the basis of band gap energy – the differential energy between the valence band (the highest occupied molecular orbital, HOMO) and the conduction band (the lowest unoccupied molecular orbital, LUMO) – the materials are classified into three basic categories (Fig. 1.1). Normally, heterogeneous photocatalysts are semiconductor materials (i.e., metal oxides), because semiconductor can absorb light to activate the movement of electrons, which causes the generation of the reactive species. The reactive species in heterogeneous photocatalysis is used in a different way compared with those in heterogeneous photocatalysts (Wu and Chang 2006). Heterogeneous photocatalysis occurs with several reactions, e.g., oxidation, dehydrogenation, metal deposition, water detoxification, and gaseous pollutant removals. Figure 1.2 shows an example of heterogeneous photocatalysis for hydrogen production from water.
Three basic categories of materials on the basis of band gap energy. In an insulator, there exists a large forbidden gap or band gap between the conduction band and valence band, so electrons cannot jump from the valence band to the conduction band. While the band gap in a semiconductor is narrower, so the energy provided at room temperature is sufficient to lift the electrons to the conduction band. In a metal or a conductor, there is no band gap, so the electrons can easily move in the space between the atoms
Hydrogen production from water using heterogeneous powder photocatalysts. The photocatalysts are dispersed in a reactor with water. Sun or light shines at the dispersed photocatalysts, and then hydrogen is obtained (modified after (Kudo and Miseki 2009))
Heterogeneous photocatalysis is generally carried out by utilizations of metal oxides as photocatalysts in the form of suspended phase or immobilized state (on other solid substrates). The illumination of light over the heterogeneous photocatalyst by photons with energy at least equal to its band gap energy can generate the electron–hole pairs. The photo-activated electrons are transferred from the valence band to the conduction band, leaving the positive holes in the valence band. Subsequently, the photo-activated electrons and holes can migrate from bulk to the surface of photocatalyst and react with some adsorbed substances on the surface to generate the free radicals (Srisasiwimon et al. 2018). Table 1.2 shows typical photocatalysts which are normally nanosized semiconductor materials with wide band gap energies (e.g., TiO2, ZnO, and SnO2,) (Bensebaa 2013; Yemmireddy and Hung 2017).
1.2.1.3.1 Oxidative Reactions
Typical photocatalysts, i.e., metal oxides (MO), such as oxides of titanium, zinc, tungsten, vanadium, chromium, and vanadium, can absorb photons to generate the photo-excited electrons and positive holes as expressed in Eq. (1.20). In the presence of water molecules, hydroxyl radicals (HO•) are produced by a reaction between positive holes and H2O according to Eq. (1.21) (Fig. 1.3). Furthermore, H2O2 is possibly formed through the oxidative pathway, leading to the HO• generation from the cleavage of H2O2 under photolysis as shown in Eqs. (1.22)–(1.23) (Li et al. 2014).
Basic model of heterogeneous photocatalysis. The photocatalysts can absorb photons to generate the photo-excited electrons and positive holes. In the oxidation side, hydroxyl radicals (HO•) are produced by a reaction between the positive holes and water molecules. In the reduction side, the dissolved oxygen molecules can generate the short-lived superoxide anion radicals (O2•−). HO• can be more generated from the other subsequently oxidation and reduction pathways. The radicals generated in photocatalysis are the key species to react with organic molecules in the photocatalytic applications
1.2.1.3.2 Reductive Reaction
The monovalent reduction of dissolved oxygen molecules which are adsorbed on the surface of photocatalyst can generate the short-lived free radicals in the form of superoxide anion radicals (O2•−). Subsequently, the uncharged hydroperoxyl radicals (HO2•) can be produced through protonation of O2•−. Hydrogen peroxide (H2O2) is feasibly formed by protonation and reduction of HO2•. Ultimately, the homolytic cleavage of H2O2 is also able to form more hydroxyl radicals (HO•) according to Eqs. (1.24–1.27) (Nosaka et al. 2002).
1.2.2 Photocatalytic Process
Photocatalytic process is normally described by heterogeneous photocatalysis. The process could be divided into four steps (Fig. 1.4): (I) light absorption for generation of electron–hole pair; (II) charge separation and migration of photogenerated carriers; (III) formation of hydroxyl radicals and superoxide ions via redox reactions; and (IV) photodecomposition of organic compounds via reaction with active species on the catalyst surface (Bensebaa 2013; Kudo and Miseki 2009).
Four steps of photocatalytic process: (I) light absorption for the generation of electron–hole pair; (II) charge separation and migration of photogenerated carriers; (III) formation of hydroxyl radicals and superoxide ions via redox reactions; and (IV) photodecomposition of organic compounds via reaction with active species on the catalyst surface
For the first step (generation of electron–hole pair) as written above, the energy for photocatalysis reaction must be equal or exceed the band gap of photocatalysts (Nakata and Fujishima 2012). An electron (e−) is activated to conduction band after the light absorption, so holes (h+) are generated in the valence band.
For charge separation and migration of photogenerated carriers, this step strongly depends on the crystal structure, crystallinity, and particle size of photocatalysts. Low crystallinity leads to the increase of the amount of defects which operates as a trapping and a recombination center between photogenerated electrons and holes, causing a decrease in the photocatalytic activity. In addition, a small particle size creates the distance between photogenerated electrons (e−) and holes (h+) that migrate to reaction sites on the surface, leading to decrease in the recombination probability.
For formation of hydroxyl radicals and superoxide ions via redox reactions, the oxidative reaction between holes and water molecules or other organic compounds leads to generation of HO• and H+ (which can further form H2O2), while the reductive reaction between electron and O2 molecules leads to generation of superoxide ions (O2•-) (which can further form HO2•, H2O2, and HO•) (Sirelkhatim et al. 2015). However, this step is the surface chemical reactions, where the photogenerated electrons and holes can recombine with each other if the active sites for redox reactions do not exist on the surface, which depends on the surface character and surface area.
For the last step (photodecomposition of organic compounds via reaction with active species on the catalyst surface), radicals, ions, or molecules (HO•, O2•-, OH−, and H2O2) obtained from the reactions are key reactive oxygen species (ROS) in the initiation of other photocatalytic reactions which can react with the target organic compounds to degrade or otherwise convert them into harmless by-products or value-added chemicals or fuels (Payormhorm et al. 2017a; Payormhorm et al. 2017b). Recently, several catalysts have been progressed to produce good quality of photocatalysts like photoconversion processes such as solar to electricity, light to hydrogen, and light to mechanical works.
1.3 Organic Pollutants in Water
There are various types of pollutants in wastewater (e.g., organic pollutants, inorganic pollutants, pathogens, and radioactive pollutants). Organic pollutants are a main part of environmental pollution, which may cause an adverse effect on aquatic organisms even at low levels of exposure (Mao et al. 2017; Ahmad et al. 2018; Yu et al. 2019). Organic pollutants are found in various wastewater sources, e.g., domestic, industrial, and agricultural sectors.
Many kinds of organic pollutants, such as pharmaceuticals and personal care products (PPCPs), textile, food, beverage, persistent organic pollutants (POPs), insecticide, pesticide, oil, fertilizers, and chemical, are included in wastewater.
1.3.1 Sources of Organic Pollutants in Wastewater
Organic pollutants are found in three main wastewater sources which are domestic, industrial, and agricultural sectors. The examples of organic pollutants in wastewater from these three sectors are shown in Table 1.3.
1.3.1.1 Domestic Wastewater
Domestic wastewater is water derived from daily human activities in the residences, institutions, office buildings, commercial buildings, as well as healthcare and personal care facilities. Wastewater quantities from individual residences commonly depend on the water consumption rate per capita and population density. On the other hand, wastewater quantities from commercial sources are typically based on the land-use area or the number of guests (Metcalf and Eddy 1981). The domestic wastewater can be characterized by constituents of wastewater into four classes, which are grey water, yellow water, brown water, and black water.
Grey water is wastewater with small amounts of nutrients, pathogens, and suspended solids contamination, excluding toilet wastewater. It was called “grey water” because the color of wastewater will be gradually changed to grey during storage. The grey water is discharged from daily activities such as showering, hand washing, clothes washing, and dishwashing (Wang et al. 2010). General composition of grey water depends on lifestyles, personal hygiene of human, as well as climatic conditions. Showering wastewater is usually composed of soaps, dental cares, shampoos, cosmetics, hair color, and other personal care products. Clothes washing wastewater contains a group of nutrients (sodium, phosphorus, and nitrogen), surfactants, foams, suspended solids, oil and greases, bacteria, and many others. Dishwashing and cooking wastewater generally consist of discarded food, nutrients, cooking oil, dishwashing soaps or liquids, and bacteria.
Yellow water contains human urine with or without flush water, which is presented in domestic wastewater approximately 1% by volume. Urine is a natural source of macronutrients. The presence of nitrogen, phosphorus, and potassium in conventional domestic wastewater mostly originates from urine.
Brown water is human feces, which may be included flush water and toilet papers. The significant constituents of brown water are organic matters, phosphorus, and infectious agents (Balkaya and Guneysu 2019). Furthermore, human feces and urine are also important sources of both metabolized and non-metabolized pharmaceutical residues after absorption and metabolization from human bodies.
Black water is a combination of yellow and brown waters; thus it is composed of human feces, urine, toilet papers, and flush water.
1.3.1.2 Industrial Wastewater
Apart from domestic wastewater, one of the important organic pollutant sources is industrial wastewater, such as textile, chemical, food, and beverage, which is a high concentration of various organic pollutants. Presently, a huge amount of industrial wastewater from several industries was released into rivers, lakes, and coastal areas. The results of this problem lead to be a serious pollution problem in water with negative effects to the ecosystem.
Nowadays, there are many types of organic pollutants in industrial wastewater based on different industries, such as leather, textile, metal processing, brewery and fermentation, food, pharmaceuticals, oil refining, cosmetics, soaps, pesticides, herbicides, cellulose and paper manufacturing, glue, and adhesives industries. The main source of organic pollutants in industrial wastewaters is produced from the chemical industry using organic substances for chemical reactions.
1.3.1.3 Agricultural Wastewater
Agricultural sector is also identified as one of the important sources for organic pollutants in wastewater that can affect ecology and the environment. Agricultural wastewater principally comes from by-products of anthropogenic activities in the agricultural area such as farmland, fertilizer, animal manure, and agrichemicals. Agricultural wastewater has been recognized as non-point source pollution, which is released from different agricultural activities. All types of agricultural activities produce a large number of organic pollutants (e.g., pesticides or herbicides), which subsequently discharge into surface water and penetrate to groundwater. Most agricultural wastewater is composed of sediment, nutrients, microorganism, and chemical, which is difficult to control because these substances usually discharge into surrounding natural water bodies during rainfall (Neumann et al. 2002). Moreover, agricultural wastewater with a high content of nutrients, especially nitrogen and phosphorus, can lead to eutrophication of water bodies.
Finally, organic pollutants from various wastewater sources can contaminate into the environment by both direct and indirect pathways. Figure 1.5 shows general pathways of organic contamination in the environment.
1.3.2 Major Groups of Organic Pollutants in Wastewater
1.3.2.1 Pharmaceuticals and Personal Care Products
Pharmaceuticals and personal care products are intentionally synthetic compounds with specific properties for human or animal healthcare and medical purposes. The molecular weight of pharmaceuticals and personal care products are typically ranging from 150 to 1000 Daltons (Awfa et al. 2018). Pharmaceuticals are the chemicals used in human and veterinary medicine, including antibiotics, hormones, stimulant drugs, beta-blockers, anti-inflammatories, antiarrhythmic agents, blood–lipid lowering agents, cancer therapeutics, diuretics, and many others (Chen et al. 2016; Fent et al. 2006). Pharmaceuticals and their metabolites can be released into the environment after incomplete absorption and excretion from the body of consumer, which are mainly presented in the dissolved phase (Prasad et al. 2019). Personal care products are products used for beauty and hygiene to enhance the quality of daily life, such as sunscreen, skincare products, body lotion, moisturizers, soaps, shampoo, dental care, lipstick, perfume, as well as mosquito repellent lotion and spray (Cizmas et al. 2015). The daily washing activities of human appear to be the main pathway to release the personal care products from the human body into sewerage systems and aquatic environments. Furthermore, recreation activities such as swimming and other water sports can also contribute the personal care products’ contamination in water (Yang et al. 2017). Example list of pharmaceuticals and personal care products with their physical and chemical properties is shown in Table 1.4.
The environmental contamination of pharmaceuticals and personal care products is caused by both intentional and unintentional discharge from many sources, including households, industries, hospitals, sewage treatment plants, livestock farms, and landfill leachate. The effluent discharge from sewage treatment plants and industries is identified to be the predominant sources (Awfa et al. 2018). Pharmaceuticals and personal care products are usually synthesized to be persistent, high chemical stability, and low biodegradability, which cannot be completely removed by conventional treatment processes. Although the occurrence of pharmaceuticals and personal care products in surface water, groundwater, tap water, as well as drinking water is frequently detected at a trace concentration (ranging from ng/L up to μg/L), the continuous exposure to these compounds can significantly lead to adverse effects on aquatic living organisms, terrestrial organisms, and balance of ecosystem (Jamil et al. 2017).
Many pharmaceuticals and personal care products behave as antimicrobial agents; thus the biological degradation processes that use microorganisms to break down organic pollutants in water seem to be ineffective to remove them. Physical treatment processes such as adsorption and membrane filtration can only transfer pharmaceuticals and personal care products from one medium to another medium without the destruction of them, leading to the formation of secondary contaminants in the form of spent adsorbents and concentrated water, respectively (Awfa et al. 2018). Nowadays, the chemical oxidation processes especially advanced oxidation processes, involving photo-Fenton, ozonation, UV/H2O2, and semiconductor photocatalysis, are accepted as the most promising potential treatment method for removals of pharmaceuticals and personal care products. Unfortunately, degradation of pharmaceuticals and personal care products by photo-Fenton and ozonation can form toxic by-products (Wang and Wang 2016). The low UV absorbance of hydrogen peroxide and scavenging effects of •OH by H2O2 are the main drawbacks, leading to high operating costs in the UV/H2O2 process (Guo et al. 2018). Semiconductor photocatalysis has been accepted as a cost-effective process for degradation and mineralization of pharmaceuticals and personal care products in water because this process can be operated at ambient condition by using low-cost available semiconductor photocatalysts and their modified forms, which can be activated by UV light, visible light, as well as natural sunlight. Furthermore, the immobilization of semiconductor photocatalysts on support material has been proved as a promising way to enhance the recycling ability of them, resulting in effective operating and maintenance cost (Klavarioti et al. 2009).
1.3.2.2 Persistent Organic Pollutants
Persistent organic pollutants are a group of toxic organic chemicals with long half-lives and persistence in the environment. Persistent organic pollutants have been mentioned to toxic and harmful to human health and the environment. The commonly encountered persistent organic pollutants are organochlorine pesticides from agricultural discharge such as DDT, industrial chemicals such as polychlorinated biphenyls (PCBs), and industrial by-products, especially polychlorinated dibenzodioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) as known as dioxins. These pollutants are classified into three categories based on Stockholm Convention (Stockholm Convention 2019) as shown in Table 1.5.
Nowadays, the scientists, governments, and non-governmental organizations (NGOs) are very concerned about these pollutants because of their long persistence in the environment, long range transportability, high toxicity even at a low level of concentration, and accumulation in fatty tissues due to their high lipophilicity. Persistent organic pollutants are widely contaminated in air, water, soil, and migratory species which move across international regions. Moreover, persistent organic pollutants are found in even non-chemical areas such as the arctic regions (Teran et al. 2012). The resistance property of persistent organic pollutants under biological degradation is the main reason, causing bioaccumulation into animal bodies through food chains. The exposure of persistent organic pollutants creates various serious health problems such as cancer, allergies and hypersensitivity, hormone disruption, cardiovascular diseases, reproductive disorders, learning disabilities, and disruption of the immune system.
Therefore, it is very important to develop methods for mitigation and elimination of persistent organic pollutants. The existing remediation techniques include coagulation–flocculation–sedimentation, adsorption, membrane filtration, ozonation, and advanced oxidation processes (Kumari et al. 2020). Photocatalytic process is one solution to reduce these pollutants in the environment because of the high efficiency and fast degradation. However, a challenge of the elimination of persistent organic pollutants by-products should be concerned.
1.3.2.3 Organic Dyes
The industries in this century, whether they are the textile, paper, rubber, printing, plastics, cosmetics, dye intermediates, and so forth. All industries used dye as a component in the production to the desired color product. Nowadays, the organic dyes could be classified by two types which are natural and synthetic dyes. Firstly, the natural organic dyes are dyes extracted from organic compounds (contain carbon) which form the animal, minerals, and vegetable resources such as annatto (yellow to orange colors), henna plant (brown color), and tomatoes (orange or reddish colors). All of these dyes occur by natural which means they have no side effect from dyes and also can degrade by itself. Notwithstanding, natural dyes are not very in demand because of unendurability. Therefore, another type of dye which is synthetic organic dye became popular because of its lasting color pay-off and wide range of colors. Table 1.6 shows the examples of synthetic organic dyes. Synthetic organic dyes are manufactured from organic molecules. The resources for the synthesis of these dyes are chemicals, by-products of petroleum, and earth minerals (Ziarani et al. 2018).
Nevertheless, the disadvantage of dyes is not all of the dyes are eco-friendly, especially, synthetic organic dyes. Most of these dyes are very complex molecules, extremely toxic, chemical stability, and slow degradation (Reddy and Mohan 2016). Therefore, the discharge of organic dye containing in water is troublesome the environment, not only bad vision because of their color but also reduction of sunlight transmission (Crini and Badot 2008; Dinçer et al. 2007; Zhou et al. 2019). Moreover, these organic dyes also come with a risk substance, for instance, heavy metal (Zn, Pb, Cu, Cd, Co), amines, and aromatic compound (Zollinger 2003; Zhou et al. 2019). Therefore, these organic dyes are not only harmful to aquatic life but also mutagenic to humans. The health problems related to organic dyes are skin irritation, sneezing, sore eyes, carcinogenicity, dysfunction, and mutagenicity, including the brain, liver, kidney, central nervous and reproductive system, and others (Zhou et al. 2019).
Presently, many researchers have been found and develop the solutions to treat organic dyes in water, for example, advanced oxidation processes (Andreozzi et al. 1999; Ikehata et al. 2008), adsorption (Wu et al. 2001; Noll 1991), electro-oxidation (Särkkä et al. 2015; Recio et al. 2011), and reverse osmosis (Bodalo-Santoyo et al. 2003; Agenson et al. 2003). The solutions to treat these dyes are a challenging issue due to their high solubility and high persistence in the environment. Photocatalytic process is one of the interesting solutions for wastewater treatment because this process is non-toxic and it does not affect human life. Homogeneous or heterogeneous catalysts can be used as photocatalysts for the treatment of organic dyes in water (Mao et al. 2017; Bodson et al. 2016; Zhang et al. 2017; Mudassir et al. 2018; Qu et al. 2017).
1.4 Photocatalytic Remediation of Organic Pollutants in Water
In this section, photocatalytic organic pollutant treatments in water are categorized into two groups based on types of photocatalysis, i.e., homogenous and heterogeneous ones.
1.4.1 Homogeneous Photocatalysis
Homogeneous photocatalysis has attracted numerous attentions as a promising technology for remediation of organic pollutants in water.
1.4.1.1 Treatments of Pharmaceuticals and Personal Care Products
The combination of UV and hydrogen peroxide (UV/H2O2), ozonation (UV/O3), and photo-Fenton has demonstrated their effectiveness in the degradation of pharmaceuticals and personal care products with initial concentration ranging from μg/L to ng/L as low as the commonly found levels in the environment. The completed removal of pharmaceuticals and personal care products, such as sulfamethoxazole, trimethoprim, bezafibrate, diclofenac, naproxen, ketoprofen, atenolol, metoprolol, propranolol, diazepam, carbamazepine, primidone (Kim et al. 2009), ofloxacin, ciprofloxacin, gemfibrozil, ibuprofen, sotalol, triclosan (De la Cruz et al. 2012), tylosin (He and Hua 2013), and enoxacin (Santoke et al. 2015) from water by UV/H2O2, has been reported by many researchers.
In the recent decade, the removal of some pharmaceuticals and personal care products, such as ketoprofen (Illés et al. 2014), caffeine, diethyltoluamide, cyclophosphamide (Kim and Tanaka 2010), mefenamic acid (Chang et al. 2012), and berberine (Qin et al. 2015), by UV/O3 process, was performed. The degradation of pharmaceuticals and personal care products by UV/O3 process has not been widely applied in the large scale compared with other treatment methods in homogeneous photocatalysis, because of its high-energy requirement for both ozone generator and UV lamps (Kim and Tanaka 2011), resulting in higher operating costs. The removal efficiencies of pharmaceuticals and personal care products by different conditions of photo-Fenton systems have been reviewed (Wang and Wang 2016). Tetracycline with the initial concentration as high as 24 mg/L can be completely removed by photo-Fenton process. Moreover, bezafibrate, ibuprofen, and diclofenac with the trace initial concentration of millimolar level can also be completely removed by this process (Wang and Wang 2016).
1.4.1.2 Treatments of Persistent Organic Pollutants
Persistent organic pollutants, a group of hazardous pollutants, such as pesticides that are often found in agricultural wastewater, also continuously increased the environmental risks, so it is a challenge to solve this problem. A number of physical and chemical methods have been developed to treat persistent organic pollutants.
Degradation of persistent organic pollutants can be carried out by ozonation process. Atrazine has been reported to be degraded by catalytic ozonation with iron scraps (Li and Zhou 2019). Pesticide wastewater (Solís et al. 2019) and organophosphorus pesticide in water (Aimer et al. 2019) can be treated by ozonation.
Fenton or photo-Fenton is one of the alternative ways for remediation of persistent organic pollutants. FeIII(OH)2+ under UV irradiation has been revealed to generate OH radicals which can further degrade 4-cholorophenol and other Cr(VI) phenolic compounds (e.g., 4-bromophenol, 4-nitrophenol, and phenol) (Kim et al. 2019). Among the typical iron salts, iron (III) nitrate can generate iron aquo complexes in aqueous and organic solutions, which are highly efficient and selective homogenous photocatalyst for degradation of cyclohexane into cyclohexanol and cyclohexanone up to 80 and nearly 100%, respectively (Iqbal et al. 2018). Fipronil, a pesticide, can be degraded via photo-Fenton catalysis (Singh et al. 2019). It has been found that the catalysis exhibited the highest degradation efficiency of 88.71% at pH 3 with an H2O2 concentration of 10 mM and the amount of catalyst of 1.5 g/L for 120 min reaction time (Singh et al. 2019). These show photo-Fenton is a promising technique due to the fast regeneration of Fe2+ and the less formation of iron sludge compared with the conventional Fenton process. Carbendazim can be degraded with a degradation efficiency of 96% within 15 min by Fenton process (da Costa et al. 2019). Moreover, modified Fenton processes (e.g., electro-Fenton) have been introduced (Méndez-Torres et al. 2019) with potential uses for degradations or removals of pesticide mixtures (Rosa Barbosa et al. 2018), organochlorine pesticide lindane (Dominguez et al. 2018), chlordimeform insecticide (Rezgui et al. 2018), and methoxychlor (Huang et al. 2018). It should be noted that the modified Fenton processes that use solid-state materials as the ferrous sources may also be considered as heterogeneous photocatalysis.
Normally, heterogeneous photocatalytic degradation of pesticides is a promising method because of the short time of treatment. However, it also needs more technical and economic developments, because the main source of the system (UV irradiation) often requires a large amount of the electrical energy and the use of UV with ozone or hydrogen peroxide causes relatively high costs of the processes.
1.4.1.3 Treatments of Organic Dyes
Homogeneous photocatalysis has proved to be sufficiently effective alternatives for remediation of organic dyes in water, even though it has some limitations.
A few works have recently focused on the degradation of organic dyes by ozonation. For example, degradation of reactive red 24 from aqueous solution (Van et al. 2019), removal of methylene blue in wastewater (Ikhlaq et al. 2019), organic dye removals with acid-treated clay catalysts (Boudissa et al. 2019), and degradation of azo dyes (El Hassani et al. 2019; Pérez et al. 2019; Pandian et al. 2018) have been reported with ozonation relations.
Azo dyes (Wang et al. 2019b; Innocenzi et al. 2019), acid orange 7 (AO7) (Wang et al. 2019b), reactive black 5 (RB5) (Wang et al. 2019b), reactive red 24 (Van et al. 2019), rhodamine B (Wang et al. 2019a), and methylene blue (Anantharaman et al. 2019; Ikhlaq et al. 2019) have been studied to be decolorized by Fenton or photo-Fenton processes.
UV/H2O2 is an alternative way that is often found for photocatalytic remediation of organic dyes (e.g., methylene blue (Malvestiti et al. 2019), brilliant green (Rehman et al. 2018), and rhodamine B (Changchao et al. 2018)) in water.
In a comparison of Fenton and UV/H2O2 for dye degradation, increasing concentration of ferrous ions (that catalyze the Fenton reaction) can increase the generation of OH radicals from hydrogen peroxide up to an optimum level, while in UV/H2O2, the hydroxyl radicals generated are less due to the presence of photo-stable organic UV absorbers (ultimately, it reduces the efficiency of UV/H2O2 oxidation).
1.4.2 Heterogeneous Photocatalysis
Photocatalysts used in heterogeneous photocatalysis can be used as (1) powder and suspension forms and (2) coated forms on supporters or substrates.
1.4.2.1 Powder and Suspension Forms of Photocatalysts
Suspension of the small size photocatalysts, such as microparticles, nanoparticles, and other nanomaterials in other forms, in wastewater, is the traditional way to remove the organic contaminants via heterogeneous photocatalysis.
1.4.2.1.1 Treatments of Pharmaceuticals and Personal Care Products
Among various photocatalysts, photocatalytic removal of pharmaceuticals and personal care products in water using titanium dioxide (TiO2) powder or nanoparticles, especially Degussa P25 – a commercially available TiO2 – has been extensively investigated. Effects of photocatalyst amount, light source, and pH of the solution have also been studied to determine the optimum conditions for pharmaceuticals and personal care products removal over powder suspension in water. The examples are photocatalytic removal of crotamiton, clofibric acid, and sulfamethoxazole using TiO2 under UV irradiation. Crotamiton is an antipruritic frequently detected in Japanese rivers. The removal efficiency of crotamiton is not affected by the initial pH of the solution in the range of 3–9, whereas the removal efficiencies of clofibric acid and sulfamethoxazole are significantly decreased when the initial pH is adjusted higher than 6.5, because of repulsive force between TiO2 particle and these pollutants (Fukahori et al. 2012). Photocatalytic removal of ibuprofen, which is the common nonsteroidal anti-inflammatory drugs (NSAID) in the presence of TiO2 powder suspended in the wastewater under UV/visible light irradiation is another example. Ibuprofen can be rapidly mineralized over TiO2. However, small amounts of intermediates in the form of oligomeric species can be detected during the photocatalytic reaction, leading to catalyst deactivation (Choina et al. 2013). The utilization of solar light, replacing an expensive and bio-hazardous UV light, as a light source is receiving considerable attention for photocatalytic removals of pharmaceuticals and personal care products, such as photocatalytic removals of amoxicillin over tungsten trioxide (WO3) (Nguyen et al. 2019) and photocatalytic removals of caffeine over titanium dioxide (TiO2) and zinc oxide (ZnO) nanoparticles (Ghosh et al. 2019).
1.4.2.1.2 Treatments of Persistent Organic Pollutants
In the case of elimination of persistent organic pollutants in water, photocatalysis using powder photocatalysts can be successfully applied for various target pollutants, such as diuron, alachlor, isoproturon, atrazine (Cruz et al. 2017), chlorpyrifos, cypermethrin, chlorothalonil (Affam and Chaudhuri 2013), rhodamine B, aldicarb, norfloxacin (Li et al. 2013), and perfluorooctanoic acid (Zhao et al. 2012). Photocatalytic mineralization of the representatives of aqueous persistent organic pollutants was performed, e.g., rhodamine B, aldicarb, and norfloxacin as representatives of color substances, pesticides, and antibiotics, respectively (Li et al. 2013). Under simulated sunlight irradiation, rhodamine B and norfloxacin can be decomposed, while aldicarb is difficult to be decomposed (Li et al. 2013). Perfluorooctanoic acid is a recent-found hazardous persistent organic pollutant. The shorter chain compounds of perfluorooctanoic acid are less bioaccumulative and produce a low level of environmental pollution; therefore photocatalytic degradation of perfluorooctanoic acid is increasingly interested as one of the alternative treatment processes. Photocatalytic degradation of perfluorooctanoic acid using β-Ga2O3 photocatalyst powder suspended in perfluorooctanoic acid aqueous solution exhibited the degradation efficiency as high as 98.8% (Zhao et al. 2012).
1.4.2.1.3 Treatments of Organic Dyes
Photocatalytic degradation of dyes is the typical way to investigate the photocatalytic activity of synthesized photocatalysts. Before utilizations of the catalysts in other applications, the photocatalytic degradations of model dyes, e.g., methylene blue or methyl orange, are commonly done. Therefore, there are a large number of publications reported on this issue.
Powder suspension of titanium dioxide (TiO2), zinc oxide (ZnO), and others has been extensively performed for photocatalytic removal of industrial organic pollutants in wastewater, especially dye-containing wastewater. Photocatalytic activity of ZnO nanoparticles for the decomposition of rhodamine B dye under UV illumination has been investigated. It was found that ZnO nanoparticles could degrade rhodamine B dye to 95% within 70 min. Photocatalytic degradation of azo dye in aqueous solutions under UV irradiation using nanostructured strontium titanate (Sr2TiO3) showed high photocatalytic activity compared with that of TiO2 (Karimi et al. 2014). In addition, in photocatalytic degradation of dimethyl phthalate (DMP) (Jing et al. 2018) by TiO2 particles, it was found that the photodegradation occurs at the surface of the photocatalysts more than in the homogenous phase.
Graphene–oxide hydrogel (zeolitic imidazolate framework) shows photocatalytic dye degradation ability with multiple cycles of uses because of its hydrophobic properties and high specific surface area (Mao et al. 2017). Amorphous photocatalysts of Zn-Al layered double hydroxide can be used for photocatalytic decoloration of methyl orange (Qu et al. 2017). SrSn(OH)6 can be used for the degradation of rhodamine B (Luo et al. 2016). At low pH, SrSn(OH)6 shows a good photocatalytic activity compared with commercial TiO2 (P25), because the hexagonal phase of hydroxide stannate can create hydroxyl groups for degradation of rhodamine B. Many modifications of metal oxides, e.g., Ag/TiO2 nanoparticles (Abdel Messih et al. 2017) and Nd2Sn2O7 (Zinatloo-Ajabshir et al. 2019), have been developed for degradation various organic dyes. Some details of the modifications will be discussed in another session.
Even though the suspension of photocatalysts in wastewater is the efficient form for photocatalytic treatments of organic pollutants in water because of high surface contacts between the surface of heterogeneous photocatalysts and organic pollutants in water, the suspension form has major concerns about the recovery and reuse of the suspended materials and also the leak possibility to the environment of the photocatalysts. Therefore, the reuse strategies and leakage protections of the catalysts are ones of the challenges.
1.4.2.2 Coated Photocatalysts on Supporters or Substrates
Coating of a photocatalyst as a thin layer on supporting materials is an effective strategy to overcome the limitation of nanostructured photocatalyst powders involving the post-separation of slurry catalysts from the treated wastewater. Recent approaches for treatments of organic pollutants are as follows.
1.4.2.2.1 Treatments of Pharmaceuticals and Personal Care Products
Photocatalytic removal of pharmaceuticals and personal care products in water using photocatalysts coated on various support materials has been investigated, e.g., removal of salicylic acid, naproxen, diclofenac, and ibuprofen by TiO2 (P25)/tetraethyl orthosilicate coated on glazed ceramics (Zhang et al. 2015); removal of ibuprofen by micro-TiO2 on coated glass rings (Czech and Tyszczuk-Rotko 2018); and removal of a wide variety of pharmaceuticals and personal care products and their metabolites [i.e., pharmaceuticals (carbamazepine, venlafaxine, fluoxetine, atenolol, sulfamethoxazole, ibuprofen, atorvastatin, and naproxen) and personal care products (triclosan and triclocarban)] by TiO2 coated on quartz fiber filters (Arlos et al. 2016). Dip-coating technique is mostly used for the photocatalyst coatings in wide areas.
1.4.2.2.2 Treatments of Persistent Organic Pollutants
Photocatalytic removal of persistent organic pollutants in water is widely carried out using photocatalysts coated on solid substrates, especially glass substrates. The commercial TiO2-coated glass microrods were applied to degrade phenol in water. The adherence of TiO2 to glass microrods was proved to be good. The powder suspension of TiO2 in bulk solution was not observed after experimental runs (Medina-Valtierra et al. 2006). Glass tubes and glass beads were used as supporting materials of TiO2 thin film for degradation of paraquat in water. In the case of glass tubes, the photocatalytic activity of three different types of TiO2 was compared, including commercial TiO2 (P25), TiO2 synthesized by hydrothermal method, and TiO2 synthesized by sol–gel method. It was found that TiO2 synthesized by hydrothermal method exhibited the highest paraquat herbicide removal efficiency (99%), followed by commercial TiO2 (75%) and TiO2 synthesized by sol–gel method (65%), respectively. The reason was that anatase phase of TiO2 transformed to rutile phase during sol–gel preparation method with heat treatment above 400 °C (Lee et al. 2002). In the case of glass beads, paraquat can be efficiently degraded by N, S codoped TiO2-coated glass beads under sunlight and visible light irradiation. The paraquat removal efficiencies could maintain after ten consecutive runs (Zahedi et al. 2015). Furthermore, photocatalytic removal of mixed pesticides (methyl parathion, dichlorvos, and lindane) in water using TiO2-coated glass plates is presented in the literature. All of the pesticides were completely removed, when the TiO2 coated glass plates were used as a baffle wall of the reactor under solar light irradiation (Senthilnathan and Philip 2012).
1.4.2.2.3 Treatments of Organic Dyes
Photocatalyst-coated substrates can also be applied for degradation the organic dyes in water, which can form reactive oxygen species to individual or combination treatments. These coated substrates can be prepared by traditional coating techniques, such as pulsed laser deposition, spin coating, electron beam evaporation, spray pyrolysis, chemical bath deposition, sol–gel, dip-coating, and doctor blade. For instance, titanium dioxide-coated glass, ceramic tile, and stainless steel sheets can decolorize methylene blue and industrial dye wastewater up to 93% and can reuse up to 20 times with the same efficiencies (Sirirerkratana et al. 2019). TiO2 layers immobilized on glass substrates by dip-coating technique for degradation of methyl orange were reported (Bouarioua and Zerdaoui 2017). It was found that three layers of TiO2 are the best condition for the test with good adhesion and reproducibility. Besides, they claimed that immobilized TiO2 can replace the suspension mode and eliminate the costly separation process of the catalysts (Bouarioua and Zerdaoui 2017). Other examples of photocatalyst-coated substrates for organic dye degradation are carbon-coated tungsten oxide (Tong et al. 2019), nebulizer spray-coated BiVO4 thin films (Dhas et al. 2019), Fe ion-doped polyaniline film on tin-doped indium oxide (ITO)-coated glass substrate (Haspulat et al. 2013), and P-doped TiO2 nanoparticles film coated on a ground glass substrate (Lv et al. 2011).
1.5 Modifications of Heterogeneous Photocatalysts
It has been proven that heterogeneous photocatalysis is one of the most potential methods for the treatment of organic pollutants in water. Relatively large band gap energy is a limitation of metal oxide-based heterogeneous photocatalysts, causing the requirement of UV light for activation. In addition, electron–hole recombination can also occur after the charge separation and migration of photogenerated carriers, resulting in the unsatisfactory photocatalytic activity to treat the target pollutants. The electronic band structure modifications and charge separation improvements of metal oxide-based photocatalysts have attracted significant attentions in the field of environmental treatments. Modifications of electronic band can be achieved by doping and composites of semiconductors. These enhance the photocatalytic activity of photocatalysts and shift the light absorption range toward visible region.
Another modification technique in heterogeneous photocatalysts is the utilization of electrical potential in photocatalysis. The coated semiconductor photocatalysts are used as the photoelectrodes in photo-electrocatalytic applications.
Tables 1.7, 1.8 and 1.9 conclude several modification strategies for remediation of pharmaceuticals and personal care products, persistent organic pollutants, and organic dyes in water.
1.5.1 Doping
Doping of semiconductor photocatalysts with one or more foreign ions is one of the promising modification strategies to enhance the photocatalytic activity of photocatalyst under UV irradiation and shift the absorption wavelength to visible light. Metal doping displays a successful approach for modifications of photocatalysts with improved photonic efficiencies (Coronado et al. 2013). Metal-doped photocatalysts, such as Fe-doped TiO2, Bi-doped TiO2, Ni-doped TiO2, Pt-doped ZnO, Ag-doped ZnO, and Au-doped ZnO, were published in the recent years (Lin et al. 2017; Bhatia and Dhir 2016; Vaiano et al. 2019). The nonmetal-doped photocatalysts are also available in the literature such as N-doped TiO2 (Shetty et al. 2017) and S-doped TiO2 (Yi et al. 2019). Doping can be done by several methods, such as impregnation, coprecipitation, ion implantation, and in situ synthesis methods (e.g., sol–gel, hydrothermal, and solvothermal).
1.5.1.1 Treatments of Pharmaceuticals and Personal Care Products
The concentration of dopants is an important factor affecting removal efficiencies of pharmaceuticals and personal care products. One example is ibuprofen removal over transition metal-doped ZnO under solar light irradiation (Bhatia and Dhir 2016). The concentration of transition metal dopant was varied from 0.25% to 1% by weight. The 0.25 wt% Bi-doped TiO2 exhibited the maximum removal efficiency, and the removal efficiencies decreased with increasing Bi content. On the other hand, the maximum removal efficiency over Ni-doped TiO2 was observed at Ni content of 0.5 wt%, and the removal efficiencies decreased when Ni content was higher or lower than 0.5 wt%. Consequently, the optimum content of dopants should be considered case by case.
1.5.1.2 Treatments of Persistent Organic Pollutants
P-doped and Ag-doped TiO2 photocatalysts for photocatalytic degradation of p-nitrophenol under UV light (Bodson et al. 2016), copper-doped anatase/brookite TiO2 nanohybrids (Manga Raju et al. (2019), Fe-TiO2/Bent-Fe photocatalyst for removal of diazinon pesticide (Phuong et al. 2019), degradation of paraoxon and parathion pesticides on carbon-doped TiO2 nanorod thin films (Rasoulnezhad et al. 2017), degradation of diazinon by iron-doped TiO2 nanoparticles (Tabasideh et al. 2017), Pd-doped In2O3 nanocomposites to degradation of atrazine (Aazam et al. 2018), degradation of methomyl pesticide boron-doped diamond electrode (Costa et al. 2017), and degradation of the insecticide propoxur by boron-doped diamond/air-diffusion cell (Guelfi et al. 2017) have been reported.
1.5.1.3 Treatments of Organic Dyes
Cu-doped and Zn-doped TiO2 nanoparticles synthesized by sol–gel method were applied for methyl orange degradation (Khairy and Zakaria 2014). The small crystallite size and doping were found to cause an increase in the adsorption edge wavelength with decrease in band gap energy. Cu-doped TiO2 showed the optimum photocatalytic activity for methyl orange degradation.
Magnesium- and iron-doped ZnO nanoparticles were fabricated to use for degradation of methyl orange and/or methylene blue under UV irradiation (Paula et al. 2019; Saleh and Djaja 2014). It was found that the various parameters, i.e., pH, dopant concentrations, and photocatalytic dosage, affected the photocatalytic activity, especially dopant concentration is the most important factor.
NaTaO3 photocatalysts were synthesized with doping of Sr cations through crystallization in molten NaCl flux, resulting in an increase in the population of excited electrons. However, the reaction rate of the obtained photocatalysts showed less enhancement compared with the increase in electron population, which ascribed to a limited fraction of electrons overriding the energy gradient and returning back to the surface (An et al. 2018).
Doping of graphitic carbon nitride (g-C3N4) by various types of metals (Na, K, transition metals, and rare earth metals) and nonmetal materials (phosphorus, sulfur, oxygen, nitrogen, carbon, boron, and halogen) for photocatalytic remediation of organic dyes in water was reviewed (Hasija et al. 2019). It was shown that the photocatalytic activity for degradation of organic pollutants (rhodamine B and methylene blue) of the doped materials was successfully enhanced up to 50% compared with the bare ones, because of changes of band gaps of the materials.
Co-doing of two metal dopants or a metal ion with a nonmetal dopant for synergistic photocatalytic effects, i.e., the working together of two things to produce an effect greater than the sum of their individual effects, of the dopants is an alternative way to modify metal oxide photocatalysts (Sanitnon et al. 2019). For an example, ferroelectric Fe3+Cr3+ codoped BaTiO3 nanopowders for the photocatalytic oxidation of azo dyes were fabricated (Amaechi et al. 2019). The photocatalytic activity of the powders was found to be maintained after three cycle uses, and the powders could be reused without generating any secondary residue.
1.5.2 Composite of Semiconductors
Composite (also called coupling) of two or more semiconductors is considered as an effective method for modification of photocatalysts, because the separation of photo-excited electrons and holes was accelerated, resulting in improved photocatalytic performances.
1.5.2.1 Treatments of Pharmaceuticals and Personal Care Products
A large number of semiconductor composites have been developed as photocatalyst for the removal of pharmaceuticals and personal care products in water, such as Mg-ZnO-Al2O3 (Elhalil et al. 2018), TiO2/reduced graphene oxide (Lin et al. 2017), MWCNT-TiO2-SiO2 (Czech and Tyszczuk-Rotko 2018), and ZnO-zeolite (Jagannatha et al. 2019).
Presently, the visible light-responsive photocatalytic removal of pharmaceuticals and personal care products by carbon–oxygen–titanium linkages in the composite system has attracted significant attention. Photocatalytic removal of 29 different pharmaceuticals and personal care products over carbonaceous TiO2 composites was summarized (Awfa et al. 2018). The main carbonaceous materials included activated carbon, carbon nanotubes, and graphene. These materials can enhance the photocatalytic removal efficiency of pharmaceuticals and personal care products due to their high specific surface area and large electron storage capacity. Moreover, the carbonaceous materials can behave as a sensitizer to provide electrons for TiO2 which can subsequently be activated by photons with suitable energy leading to higher photocatalytic performance (Awfa et al. 2018).
1.5.2.2 Treatments of Persistent Organic Pollutants
Highly photoactive metal oxides could be achieved by composite with two or more different materials of TiO2, ZnO, SnO2, SrTiO2, WO3, Cu2O, and Fe2O3 with nonmetal elements such as N, S, C, and F for photocatalytic remediation of persistent organic pollutants. For example, a development of polyaniline/FeZSM-5 composites for the degradation of herbicide glyphosate was reported (Milojević-Rakić et al. 2018). The composites showed efficient green catalytic degradation of pesticide/herbicide pollutants in environmental remediation systems.
MIL(Fe)/Fe-doped nanospongy porous biocarbon (MIL(Fe)/Fe-SPC) composites were used for the degradation of thiamethoxam, pesticides, and other environmental pollutants (Wei et al. 2018).
Semiconductor composites with unique selective adsorption properties, such as Pd/ZnWO4 nanocomposite (Chen et al. 2019a), TiO2/Fe2O3 nanocomposite (Mirmasoomi et al. 2017), zinc oxide nanorod-incorporated carboxylic graphene/polyaniline composite (Anirudhan et al. 2018), Fe3O4/metal–organic framework nanocomposite (Sajjadi et al. 2019), TiO2/ZrO2 nanocomposite (Mbiri et al. 2018), Ag-ZnO composite (Kanwal et al. 2018), and In-S-TiO2/reduced graphene oxide nanocomposite (Khavar et al. 2018) for degradation and detoxification of pesticides, were reported. High crystallinity, small particle size, high surface area, and well-defined porosity are important parameters to provide active sites for adsorption of pollutants and facilitate the diffusions of pollutants and products away from the photoactive centers that assisted the effective performances of the photocatalysts.
1.5.2.3 Treatments of Organic Dyes
Many researchers have studied the photocatalytic remediation of organic dyes in water by composite materials. Degradation of methylene blue, methyl orange, acid blue 25 (AB-25), crystal violet dye, orange II azo dye, rhodamine B by NiO-ZnO-Ag nanocomposites (Aydoghmish et al. 2019), tetraphenylporphyrin/WO3/exfoliated graphite nanocomposite (Malefane et al. 2019), reduced graphene oxide-ZrO2 composite (Ali et al. 2019), WO3/TiO2/carbon fiber composite (Balta et al. 2019), Zn3(PO4)2/BiPO4 composite (Naciri et al. 2019), BiFeWO6/α-AgVO3 composite (Senthil et al. 2019), graphene/ZnO composite (Wang et al. 2019c), Nb/TiO2 composite (Ravishankar et al. 2019), BiOBr/BiOI/cellulose composite (Du et al. 2019), CdS/g-C3N4/metal–organic framework composite (Chen et al. 2019b), g-C3N4/TiO2 composite (Monga and Basu 2019), CuS-CdS composite (Mahanthappa et al. 2019), MFe2O4-Ag2O composite (M = Zn, Co, & Ni) (Sun et al. 2019), Cds/reduced graphene oxide composite (Chen et al. 2019a), and CeO2/sugarcane bagasse composite (Channei et al. 2017) was developed and reported.
Carbon–metal oxide composites are another interesting photocatalysts. For example, graphene oxide-doped mesoporous TiO2 photocatalysts for rhodamine B degradation were reported (Zhang et al. 2017). The obtained photocatalysts showed photodegradation efficiency up to 81% under visible light irradiation. In addition, the well dispersion of graphene oxide and mesoporous TiO2 nanoparticles leads the good influences on the photocatalytic performance of the photocatalysts.
Additionally, semiconductors composited with polymers were also developed. For example, modification of niobium oxides and different polymer matrices, e.g., polypropylene (PP), poly(3-hydroxibutyrate) (PHB), and polyurethane (WPU), were proposed (Heitmann et al. 2019). Nano-/micro-scaled TiO2/polyacrylamide beads for efficient photodegradation of organic dyes were reported (Mudassir et al. 2018). Nanoscale feature and high surface area of the TiO2/polyacrylamide beads showed the superior degradation of organic dyes and enhanced rate constant of the reactions. The composite beads also showed interesting properties of efficient disinfections of E.coli and S. aureus under photocatalytic applications (Mudassir et al. 2018). Other several semiconductor/polymer composites for photocatalytic dye degradations are BiVO4-GO-PTFE (Dowla et al. 2017), ZnO/PMMA (Di Mauro et al. 2017), poly(methyl methacrylate)/TiO2 (Mirhoseini and Salabat 2015), and Fe2O3-loaded activated carbon fiber/polymer [polyester fiber or polyethylene pulp) (Kadirova et al. 2017).
1.5.3 Photoelectrodes in Photo-Electrocatalytic Process
Photo-electrocatalysis has become an attractive way to increase the catalytic efficiency of photocatalysis. Photo-electrocatalytic degradation of organic pollutants in water using photocatalyst-coated substrates as photoelectrodes has been developed.
1.5.3.1 Treatments of Pharmaceuticals and Personal Care Products
TiO2 nanopore array for photo-electrocatalytic removal of tetracycline was reported (Liu et al. 2009). A comparative removal of diclofenac by magnetically attached TiO2/SiO2/Fe3O4 coated on graphite under UV irradiation with and without electric potential was performed by Hu et al. (2011). In the presence of +0.8 V, the removal efficiency of diclofenac was significantly higher than that of the conventional photocatalysis (Hu et al. 2011).
1.5.3.2 Treatments of Persistent Organic Pollutants
Photo-electrocatalytic remediation of persistent organic pollutants using TiO2/Ni photoelectrode showed the reduction of chemical oxygen demand (COD) of water up to 82.6% (Fang et al. 2012). This method is more efficient than typical photocatalysis and typical electrochemical oxidations. A study of coral-like porous WO3/W photoelectrode for degradation of perfluorooctanoic acid, a highly toxic persistent organic pollutant, was reported (Pan et al. 2019). The uniqueness of this research is the porous coral-like structure that has a suitable energy band position and strong oxidation ability, leading to a strong ability for photo-electrocatalytic degradation of perfluorooctanoic acid. Another example is the use of fluorine-doped tin oxide/WO3/BiVO4 photoelectrodes that showed that mixing of metal oxides on the BiVO4 photocatalysts could enhance the charge separation (Chatchai et al. 2009). In the study of efficient photocatalytic degradation of phenol as a persistent organic pollutant substrate over Co3O4/BiVO4 composite, the key factor for the high photocatalytic activity is the sequence of WO3 and BiVO4 layers (Long et al. 2006).
1.5.3.3 Treatments of Organic Dyes
Photoelectrodes made of Ag2Mn8O16 nanocrystals/TiO2 nanotubes were fabricated via anodization and annihilation methods and used for photocatalytic degradation of rhodamine B under solar-simulated light irradiation (Thabit et al. 2018). RuO2/TiO2 photoelectrodes for degradation of reactive brilliant red (X-3B) were also reported (Fang et al. 2013).
1.6 Photocatalytic Reactors for Remediation of Organic Pollutants
Photocatalytic reactor design is the major challenge in photocatalytic remediation of organic pollutants in water. The important key in photocatalytic reactor design consideration is that the large area of photocatalysts has to be illuminated efficiently. In general, the photocatalytic reactor configuration for wastewater treatment can be classified as two main groups, including fixed bed reactor and slurry type reactor (Ibhadon and Fitzpatrick 2013). Apart from the conventional photocatalytic reactor, the combination of photocatalysis with another treatment process has also been developed to overcome the specific obstacles in each case, such as a photocatalytic membrane reactor.
A wide variety of reactor configurations for removals of pharmaceuticals and personal care products have been reported in the literature. For example, removal of amoxicillin in water by a conventional slurry photocatalytic reactor under simulated solar light irradiation was performed (Nguyen et al. 2019). The optimal conditions for that study are an initial amoxicillin concentration of 1.0 μM, a photocatalyst amount of 0.104 g/L, and a pH of 4 (Nguyen et al. 2019). The continuous fixed bed photocatalytic reactor for the removal of paracetamol was developed (Borges et al. 2015). The reactor consists of TiO2-coated glass spheres placed in the glass tube. The synthetic wastewater was recirculated along with the system by a peristaltic pump during the irradiation of the simulated solar light. A submerged ceramic membrane photocatalytic reactor for amoxicillin removal was designed and reported (Li et al. 2019). The system is composed of two stainless steel rectangular tanks with the ceramic membrane fixed inside the tanks. The air compressor was connected to the top of the membrane for backwashing. The aeration pipe was installed at the bottom of the tanks to prevent the accumulating of photocatalyst powder on the membrane surface (Li et al. 2019).
A combination of conventional slurry or fixed bed photocatalytic reactors and membrane filtration for remediation of persistent organic pollutants in water has attracted considerable attention from researchers since the last decade. For example, a slurry bed photocatalytic membrane reactor for the removal of 32 different persistent organic pollutants was developed. The system is composed of a pre-filter unit, an irradiation unit with 32 UV lamps, and a photocatalyst recovery unit. A ceramic microfiltration membrane was used to separate photocatalysts (Benotti et al. 2009).
For organic dyes, a number of reactors (apart from conventional slurry-type ones) for remediation of organics dye in water have been created. A slurry-type reactor combined with an air sparging unit for degradation of methylene blue in water was reported (Abdellah et al. 2018). A fixed bed photocatalytic membrane reactor for degradation of 4BS dye was designed. N-doped TiO2 was immobilized on a ceramic membrane, and then the membrane was installed between a reaction chamber and a separation chamber. A xenon lamp was used as a light source. The dye-containing aqueous solution was fed by a diaphragm pump (Wang et al. 2016). A photocatalytic reactor consisted of a UVA or UVC light source installed on the top of a chamber was designed to decolorization of a synthetic (methylene blue-contained) wastewater and an actual (reactive purple-contained) dye wastewater. A pump was used to circulate the water on the 15° tiled TiO2-coated substrates, i.e., glass, ceramic tile, and stainless steel sheets (Sirirerkratana et al. 2019). Two reactors – a batch reactor and a continuous reactor – were designed for degradation of acid violet 7 dye (AV7) using ZnO/polypyrrole powder photocatalysts. In the batch reactor, the powder photocatalysts were fixed on a rectangular glass plate, and the fixed glass was immersed in the dye solution. In a continuous annular reactor, the powders were supported on the inner wall of an external quartz ring that covered a UV lamp quartz tube (González-Casamachin et al. 2019).
1.7 Conclusions
Photocatalysis is a process which a chemical reaction is accelerated in the presence of a catalyst on exposure to light. Photocatalysis could be classified to be two types, i.e., homogeneous and heterogeneous photocatalysis, on the basis of appearances of the physical state of reactants. Ozonation (UV/O3), photo-Fenton processes (Fe2+ and Fe2+/H2O2), UV/H2O2, and UV/H2O2/O3 are examples of homogeneous photocatalysis, while photocatalysts in heterogeneous photocatalysis are typically semiconductor materials (i.e., metal oxides) which can be used in powder and suspension forms or coated forms on other substrates. Both homogeneous and heterogeneous photocatalytic processes have been utilized as alternative technologies for remediation of organic pollutants in water, including (i) pharmaceuticals and personal care products (synthetic compounds with specific properties for human or animal healthcare and medical purposes); (ii) persistent organic pollutants (organochlorine pesticides and industrial chemicals with long half-lives and persistence in the environment); and (iii) organic dyes (synthetic organic substances for colorants). Homogeneous photocatalysis has many advantages, e.g., high oxidation properties. It is, however, not popular in various photocatalytic applications, because it is difficult to separate the photocatalysts from the solution, the photocatalysts have low potential to reuse, purification of products is necessary, and almost homogeneous photocatalysts absorb narrowly light within the solar spectrum. It has been proven that heterogeneous photocatalysis is one of the most potential methods for the treatment of organic pollutants in water. Anyhow, relatively large band gap energy causes some limitations of metal oxide-based heterogeneous photocatalysts. Modifications of the electronic band can be achieved by doping and composites of semiconductors. Another modification technique in heterogeneous photocatalysts is the utilization of electrical potential in photocatalysis. The coated semiconductor photocatalysts are used as photoelectrodes in photo-electrocatalytic applications. In addition, the photocatalytic reactor configuration for wastewater treatment by heterogeneous photocatalysis can be classified as two main groups, including fixed bed reactor and slurry-type reactor. Apart from the conventional photocatalytic reactors, the combination of photocatalysis with another treatment process has also been developed to overcome the specific obstacles in each case, such as a photocatalytic membrane reactor.
References
Aazam E, Mohamed R, Hassan T (2018) Pd-doped In2O3 nanocomposites for the photocatalytic degradation of atrazine. Desalin Water Treat 101:216–222. https://doi.org/10.5004/dwt.2018.21804
Abdel Messih MF, Ahmed MA, Soltan A, Anis SS (2017) Facile approach for homogeneous dispersion of metallic silver nanoparticles on the surface of mesoporous titania for photocatalytic degradation of methylene blue and indigo carmine dyes. J Photochem Photobiol A Chem 335:40–51. https://doi.org/10.1016/j.jphotochem.2016.11.001
Abdellah MH, Nosier SA, El-Shazly AH, Mubarak AA (2018) Photocatalytic decolorization of methylene blue using TiO2/UV system enhanced by air sparging. Alex Eng J 57(4):3727–3735. https://doi.org/10.1016/j.aej.2018.07.018
Affam AC, Chaudhuri M (2013) Degradation of pesticides chlorpyrifos, cypermethrin and chlorothalonil in aqueous solution by TiO2 photocatalysis. J Environ Manag 130:160–165. https://doi.org/10.1016/j.jenvman.2013.08.058
Agenson KO, Oh J-I, Urase T (2003) Retention of a wide variety of organic pollutants by different nanofiltration/reverse osmosis membranes: controlling parameters of process. J Membr Sci 225(1–2):91–103. https://doi.org/10.1016/j.memsci.2003.08.006
Ahmad YH, Mohamed AT, Sliem MH, Abdullah AM, Al-Qaradawi SY (2018) Enhanced photocatalytic performance of WON@porous TiO2 nanofibers towards sunlight-assisted degradation of organic contaminants. RSC Adv 8(57):32747–32755. https://doi.org/10.1039/C8RA06477F
Aimer Y, Benali O, Groenen Serrano K (2019) Study of the degradation of an organophosphorus pesticide using electrogenerated hydroxyl radicals or heat-activated persulfate. Sep Purif Technol 208:27–33. https://doi.org/10.1016/j.seppur.2018.05.066
Ali TT, Narasimharao K, Basahel SN, Mokhtar M, Alsharaeh EH, Mahmoud HA (2019) Template assisted microwave synthesis of rGO-ZrO2 composites: efficient photocatalysts under visible light. J Nanosci Nanotechnol 19(8):5177–5188. https://doi.org/10.1166/jnn.2019.16827
Amaechi IC, Hadj Youssef A, Rawach D, Claverie JP, Sun S, Ruediger A (2019) Ferroelectric Fe–Cr codoped BaTiO3 nanoparticles for the photocatalytic oxidation of azo dyes. ACS Appl Nano Materials 2(5):2890–2901. https://doi.org/10.1021/acsanm.9b00336
Ameta RK, Chohadia A, Jain A, Punjabi PB (2018a) Chapter 3: Fenton and Photo-Fenton processes. In: Ameta SC, Ameta R (eds) Advanced oxidation processes for waste water treatment. Academic, pp 49–87. https://doi.org/10.1016/B978-0-12-810499-6.00003-6
Ameta R, Solanki MS, Benjamin S, Ameta SC (2018b) Chapter 6: Photocatalysis. In: Ameta SC, Ameta R (eds) Advanced oxidation processes for waste water treatment. Academic, pp 135–175. https://doi.org/10.1016/B978-0-12-810499-6.00006-1
An L, Kitta M, Iwase A, Kudo A, Ichikuni N, Onishi H (2018) Photoexcited electrons driven by doping concentration gradient: flux-prepared NaTaO3 photocatalysts doped with strontium cations. ACS Catal 8(10):9334–9341. https://doi.org/10.1021/acscatal.8b02437
Anantharaman A, Josephine BA, Teresita VM, Ajeesha T, George M (2019) Photo-Fenton activity of magnesium substituted cerium ferrite perovskites for degradation of methylene blue via sol–gel method. J Nanosci Nanotechnol 19(8):5116–5129. https://doi.org/10.1166/jnn.2019.16819
Andreozzi R, Caprio V, Insola A, Marotta R (1999) Advanced oxidation processes (AOP) for water purification and recovery. Catal Today 53(1):51–59. https://doi.org/10.1016/S0920-5861(99)00102-9
Anirudhan TS, Shainy F, Manasa Mohan A (2018) Fabrication of zinc oxide nanorod incorporated carboxylic graphene/polyaniline composite and its photocatalytic activity for the effective degradation of diuron from aqueous solutions. Sol Energy 171:534–546. https://doi.org/10.1016/j.solener.2018.06.111
Arlos MJ, Hatat-Fraile MM, Liang R, Bragg LM, Zhou NY, Andrews SA, Servos MR (2016) Photocatalytic decomposition of organic micropollutants using immobilized TiO2 having different isoelectric points. Water Res 101:351–361. https://doi.org/10.1016/j.watres.2016.05.073
Arvanitoyannis IS, Kassaveti A (2008) 8 – Olive oil waste management: treatment methods and potential uses of treated waste. In: Arvanitoyannis IS (ed) Waste management for the food industries. Academic, Amsterdam, pp 453–568. https://doi.org/10.1016/B978-012373654-3.50011-0
Awfa D, Ateia M, Fujii M, Johnson MS, Yoshimura C (2018) Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: a critical review of recent literature. Water Res 142:26–45. https://doi.org/10.1016/j.watres.2018.05.036
Aydoghmish SM, Hassanzadeh-Tabrizi SA, Saffar-Teluri A (2019) Facile synthesis and investigation of NiO–ZnO–Ag nanocomposites as efficient photocatalysts for degradation of methylene blue dye. Ceram Int 45(12):14934–14942. https://doi.org/10.1016/j.ceramint.2019.04.229
Ayekoe PY, Robert D, Gone DL (2016) Preparation of effective TiO2/Bi2O3 photocatalysts for water treatment. Environ Chem Lett 14(3):387–393. https://doi.org/10.1007/s10311-016-0565-3
Balkaya N, Guneysu S (2019) Recycling and reuse approaches for better sustainability. Springer. ISBN 978-3-319-95888-0
Balta Z, Bilgin Simsek E, Berek D (2019) Solvothermal synthesis of WO3/TiO2/carbon fiber composite photocatalysts for enhanced performance under sunlight illumination. Photochem Photobiol. https://doi.org/10.1111/php.13117
Benotti MJ, Stanford BD, Wert EC, Snyder SA (2009) Evaluation of a photocatalytic reactor membrane pilot system for the removal of pharmaceuticals and endocrine disrupting compounds from water. Water Res 43(6):1513–1522. https://doi.org/10.1016/j.watres.2008.12.049
Bensebaa F (2013) Clean energy. In: Interface science and technology, vol 19. Elsevier, pp 279–383. https://doi.org/10.1016/B978-0-12-369550-5.00005-7
Bhatia V, Dhir A (2016) Transition metal doped TiO2 mediated photocatalytic degradation of anti-inflammatory drug under solar irradiations. J Environ Chem Eng 4(1):1267–1273. https://doi.org/10.1016/j.jece.2016.01.032
Bodalo-Santoyo A, Gómez-Carrasco J, Gomez-Gomez E, Maximo-Martin F, Hidalgo-Montesinos A (2003) Application of reverse osmosis to reduce pollutants present in industrial wastewater. Desalination 155(2):101–108. https://doi.org/10.1016/S0011-9164(03)00287-X
Bodson CJ, Heinrichs B, Tasseroul L, Bied C, Mahy JG, Man MWC, Lambert SD (2016) Efficient P-and Ag-doped titania for the photocatalytic degradation of waste water organic pollutants. J Alloys Compd 682:144–153. https://doi.org/10.1016/j.jallcom.2016.04.295
Borges ME, Garcia DM, Hernandez T, Ruiz-Morales JC, Esparza P (2015) Supported photocatalyst for removal of emerging contaminants from wastewater in a continuous packed-bed photoreactor configuration. Catalysts 5(1):77–87. https://doi.org/10.3390/catal5010077
Bouarioua A, Zerdaoui M (2017) Photocatalytic activities of TiO2 layers immobilized on glass substrates by dip-coating technique toward the decolorization of methyl orange as a model organic pollutant. J Environ Chem Eng 5(2):1565–1574. https://doi.org/10.1016/j.jece.2017.02.025
Boudissa F, Mirilà D, Arus V-A, Terkmani T, Semaan S, Proulx M, Nistor I-D, Roy R, Azzouz A (2019) Acid-treated clay catalysts for organic dye ozonation – thorough mineralization through optimum catalyst basicity and hydrophilic character. J Hazard Mater 364:356–366. https://doi.org/10.1016/j.jhazmat.2018.09.070
Chang E, Liu T-Y, Huang C-P, Liang C-H, Chiang P-C (2012) Degradation of mefenamic acid from aqueous solutions by the ozonation and O3/UV processes. Sep Purif Technol 98:123–129. https://doi.org/10.1016/j.seppur.2012.02.020
Changchao Z, Feng C, Jintao Y, Mingqiang Z, Jianping S, Xiaoping J (2018) Enhanced UV/H2O2 process by expanded graphite: an effective method for rhodamine B dye decolorization. Res Chem Intermed 44(4):2425–2437. https://doi.org/10.1007/s11164-017-3238-3
Channei D, Nakaruk A, Phanichphant S (2017) Photocatalytic degradation of dye using CeO2/SCB composite catalysts. Spectrochim Acta A Mol Biomol Spectrosc 183:218–224. https://doi.org/10.1016/j.saa.2017.04.063
Chatchai P, Murakami Y, Kishioka S-y, Nosaka AY, Nosaka Y (2009) Efficient photocatalytic activity of water oxidation over WO3/BiVO4 composite under visible light irradiation. Electrochim Acta 54(3):1147–1152. https://doi.org/10.1016/j.electacta.2008.08.058
Chen Y, Vymazal J, Březinová T, Koželuh M, Kule L, Huang J, Chen Z (2016) Occurrence, removal and environmental risk assessment of pharmaceuticals and personal care products in rural wastewater treatment wetlands. Sci Total Environ 566:1660–1669. https://doi.org/10.1016/j.scitotenv.2016.06.069
Chen F, Zou X, Chen C, Hu Q, Wei Y, Wang Y, Xiang B, Zhang J (2019a) Surfactant-free synthesis of homogeneous nano-grade cadmium sulfide grafted reduced graphene oxide composite as a high-activity photocatalyst in visible light. Ceram Int 45(11):14376–14383. https://doi.org/10.1016/j.ceramint.2019.04.153
Chen Y, Zhai B, Liang Y, Li Y, Li J (2019b) Preparation of CdS/g-C3N4/MOF composite with enhanced visible-light photocatalytic activity for dye degradation. J Solid State Chem 274:32–39. https://doi.org/10.1016/j.jssc.2019.01.038
Choina J, Kosslick H, Fischer C, Flechsig GU, Frunza L, Schulz A (2013) Photocatalytic decomposition of pharmaceutical ibuprofen pollutions in water over titania catalyst. Appl Catal B Environ 129:589–598. https://doi.org/10.1016/j.apcatb.2012.09.053
Cizmas L, Sharma VK, Gray CM, McDonald TJ (2015) Pharmaceuticals and personal care products in waters: occurrence, toxicity, and risk. Environ Chem Lett 13(4):381–394. https://doi.org/10.1007/s10311-015-0524-4
Coronado JM, Fresno F, Hernández-Alonso MD, Portela R (2013) Design of advanced photocatalytic materials for energy and environmental applications. Springer. https://doi.org/10.1007/978-1-4471-5061-9
Costa DJ, Santos JC, Sanches-Brandao FA, Ribeiro WF, Salazar-Banda GR, Araujo MC (2017) Boron-doped diamond electrode acting as a voltammetric sensor for the detection of methomyl pesticide. J Electroanal Chem 789:100–107. https://doi.org/10.1016/j.jelechem.2017.02.036
Crini G, Badot P-M (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33(4):399–447. https://doi.org/10.1016/j.progpolymsci.2007.11.001
Cruz M, Gomez C, Duran-Valle CJ, Pastrana-Martínez LM, Faria JL, Silva AM, Faraldos M, Bahamonde A (2017) Bare TiO2 and graphene oxide TiO2 photocatalysts on the degradation of selected pesticides and influence of the water matrix. Appl Surf Sci 416:1013–1021. https://doi.org/10.1016/j.apsusc.2015.09.268
Czech B, Tyszczuk-Rotko K (2018) Visible-light-driven photocatalytic removal of acetaminophen from water using a novel MWCNT-TiO2-SiO2 photocatalysts. Sep Purif Technol 206:343–355. https://doi.org/10.1016/j.seppur.2018.06.025
da Costa EP, Bottrel SEC, Starling MCV, Leão MM, Amorim CC (2019) Degradation of carbendazim in water via photo-Fenton in Raceway Pond Reactor: assessment of acute toxicity and transformation products. Environ Sci Pollut Res 26(5):4324–4336. https://doi.org/10.1007/s11356-018-2130-z
De la Cruz N, Gimenez J, Esplugas S, Grandjean D, de Alencastro LF, Pulgarin C (2012) Degradation of 32 emergent contaminants by UV and neutral photo-Fenton in domestic wastewater effluent previously treated by activated sludge. Water Res 46(6):1947–1957. https://doi.org/10.1016/j.watres.2012.01.014
Dhas CR, Arivukarasan D, Venkatesh R, Josephine AJ, Gnana Malar KCM, Santhoshi Monica SE, Subramanian B (2019) Influence of precursor aging time period on physical and photocatalytic properties of nebulizer spray coated BiVO4 thin films. Solid State Sci 92:36–45. https://doi.org/10.1016/j.solidstatesciences.2019.04.006
Di Mauro A, Cantarella M, Nicotra G, Pellegrino G, Gulino A, Brundo MV, Privitera V, Impellizzeri G (2017) Novel synthesis of ZnO/PMMA nanocomposites for photocatalytic applications. Sci Rep 7:40895. https://doi.org/10.1038/srep40895
Dinçer AR, Güneş Y, Karakaya N, Güneş E (2007) Comparison of activated carbon and bottom ash for removal of reactive dye from aqueous solution. Bioresour Technol 98(4):834–839. https://doi.org/10.1016/j.biortech.2006.03.009
Dominguez CM, Oturan N, Romero A, Santos A, Oturan MA (2018) Optimization of electro-Fenton process for effective degradation of organochlorine pesticide lindane. Catal Today 313:196–202. https://doi.org/10.1016/j.cattod.2017.10.028
Dowla BMRU, Cho JY, Jang WK, Oh W-C (2017) Synthesis of BiVO 4-GO-PTFE nanocomposite photocatalysts for high efficient visible-light-induced photocatalytic performance for dyes. J Mater Sci Mater Electron 28(20):15106–15117. https://doi.org/10.1007/s10854-017-7386-4
Du M, Du Y, Feng Y, Li Z, Wang J, Jiang N, Liu Y (2019) Advanced photocatalytic performance of novel BiOBr/BiOI/cellulose composites for the removal of organic pollutant. Cellulose 26(9):5543–5557. https://doi.org/10.1007/s10570-019-02474-1
El Hassani K, Kalnina D, Turks M, Beakou BH, Anouar A (2019) Enhanced degradation of an azo dye by catalytic ozonation over Ni-containing layered double hydroxide nanocatalyst. Sep Purif Technol 210:764–774. https://doi.org/10.1016/j.seppur.2018.08.074
Elhalil A, Elmoubarki R, Farnane M, Machrouhi A, Sadiq M, Mahjoubi F, Qourzal S, Barka N (2018) Photocatalytic degradation of caffeine as a model pharmaceutical pollutant on Mg doped ZnO-Al2O3 heterostructure. Environ Nanotechnol Monit Manag 10:63–72. https://doi.org/10.1016/j.enmm.2018.02.002
Fang T, Yang C, Liao L (2012) Photoelectrocatalytic degradation of high COD dipterex pesticide by using TiO2/Ni photo electrode. J Environ Sci 24(6):1149–1156. https://doi.org/10.1016/S1001-0742(11)60882-6
Fang T, Liao L, Zhan S, Wu X (2013) Photoelectrocatalytic decolorization of reactive brilliant red X-3B. Asian J Chem 25(2):807–810. https://doi.org/10.14233/ajchem.2013.12920
Fent K, Weston AA, Caminada D (2006) Ecotoxicology of human pharmaceuticals. Aquat Toxicol 76(2):122–159. https://doi.org/10.1016/j.aquatox.2005.09.009
Fukahori S, Fujiwara T, Ito R, Funamizu N (2012) Photocatalytic decomposition of crotamiton over aqueous TiO2 suspensions: determination of intermediates and the reaction pathway. Chemosphere 89(3):213–220. https://doi.org/10.1016/j.chemosphere.2012.04.018
Ghosh M, Manoli K, Shen X, Wang JH, Ray AK (2019) Solar photocatalytic degradation of caffeine with titanium dioxide and zinc oxide nanoparticles. J Photochem Photobiol A Chem 377:1–7. https://doi.org/10.1016/j.jphotochem.2019.03.029
Gong J, Liu Y, Sun X (2008) O3 and UV/O3 oxidation of organic constituents of biotreated municipal wastewater. Water Res 42(4–5):1238–1244. https://doi.org/10.1016/j.watres.2007.09.020
González-Casamachin DA, De la Rosa JR, Lucio-Ortiz CJ, De Rio DADH, Martínez-Vargas DX, Flores-Escamilla GA, Guzman NED, Ovando-Medina VM, Moctezuma-Velazquez E (2019) Visible-light photocatalytic degradation of acid violet 7 dye in a continuous annular reactor using ZnO/PPy photocatalyst: synthesis, characterization, mass transfer effect evaluation and kinetic analysis. Chem Eng J 373:325–337. https://doi.org/10.1016/j.cej.2019.05.032
Guelfi DRV, Gozzi F, Sirés I, Brillas E, Machulek A, de Oliveira SC (2017) Degradation of the insecticide propoxur by electrochemical advanced oxidation processes using a boron-doped diamond/air-diffusion cell. Environ Sci Pollut Res 24(7):6083–6095. https://doi.org/10.1007/s11356-016-6416-8
Guo KH, Wu ZH, Yan SW, Yao B, Song WH, Hua ZC, Zhang XW, Kong XJ, Li XC, Fang JY (2018) Comparison of the UV/chlorine and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater: kinetics, radical mechanism and energy requirements. Water Res 147:184–194. https://doi.org/10.1016/j.watres.2018.08.048
Hasija V, Raizada P, Sudhaik A, Sharma K, Kumar A, Singh P, Jonnalagadda SB, Thakur VK (2019) Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: a review. Appl Mater Today 15:494–524. https://doi.org/10.1016/j.apmt.2019.04.003
Haspulat B, Gülce A, Gülce H (2013) Efficient photocatalytic decolorization of some textile dyes using Fe ions doped polyaniline film on ITO coated glass substrate. J Hazard Mater 260:518–526. https://doi.org/10.1016/j.jhazmat.2013.06.011
He Y, Hua I (2013) Photochemical reactions of ibuprofen, naproxen, and tylosin. ACS National Meetings 350
Heitmann AP, Rocha IC, Pereira IM, Oliveira LCA, de Oliveira Patrício PS (2019) Nanoparticles of niobium oxyhydroxide incorporated in different polymers for photocatalytic degradation of dye. J Polym Res 26(7):159. https://doi.org/10.1007/s10965-019-1824-3
Hu XY, Yang J, Zhang JD (2011) Magnetic loading of TiO2/SiO2/Fe3O4 nanoparticles on electrode surface for photoelectrocatalytic degradation of diclofenac. J Hazard Mater 196:220–227. https://doi.org/10.1016/j.jhazmat.2011.09.009
Huang Y, Yang Y, Wang X, Yuan X, Pi N, Yuan H, Liu X, Ni C (2018) Heterogeneous Fenton-like degradation of methoxychlor in water using two different FeS@hydrotalcites (LHDs) and Fe3O4@LHDs catalysts prepared via an in situ growth method. Chem Eng J 342:142–154. https://doi.org/10.1016/j.cej.2018.02.056
Ibhadon AO, Fitzpatrick P (2013) Heterogeneous photocatalysis: recent advances and applications. Catalysts 3(1):189–218. https://doi.org/10.3390/catal3010189
Ikehata K, Li Y (2018) Chapter 5: Ozone-based processes. In: Ameta SC, Ameta R (eds) . Academic, Advanced oxidation processes for waste water treatment, pp 115–134. https://doi.org/10.1016/B978-0-12-810499-6.00005-X
Ikehata K, Gamal El-Din M, Snyder SA (2008) Ozonation and advanced oxidation treatment of emerging organic pollutants in water and wastewater. Ozone Sci Eng 30(1):21–26. https://doi.org/10.1080/01919510701728970
Ikhlaq A, Munir HMS, Khan A, Javed F, Joya KS (2019) Comparative study of catalytic ozonation and Fenton-like processes using iron-loaded rice husk ash as catalyst for the removal of methylene blue in wastewater. Ozone Sci Eng 41(3):250–260. https://doi.org/10.1080/01919512.2018.1525276
Illés E, Szabó E, Takács E, Wojnárovits L, Dombi A, Gajda-Schrantz K (2014) Ketoprofen removal by O3 and O3/UV processes: kinetics, transformation products and ecotoxicity. Sci Total Environ 472:178–184. https://doi.org/10.1016/j.scitotenv.2013.10.119
Innocenzi V, Prisciandaro M, Centofanti M, Vegliò F (2019) Comparison of performances of hydrodynamic cavitation in combined treatments based on hybrid induced advanced Fenton process for degradation of azo-dyes. J Environ Chem Eng 7(3):103171. https://doi.org/10.1016/j.jece.2019.103171
Iqbal MF, Tominaka S, Peng W, Takei T, Tsunoji N, Sano T, Ide Y (2018) Iron aquo complex as an efficient and selective homogeneous photocatalyst for organic synthetic reactions. ChemCatChem 10(20):4509–4513. https://doi.org/10.1002/cctc.201801360
Jagannatha RB, Rani RS, Padaki M (2019) ZnO zeolite nanocomposite for photocatalytic elimination of benzophenone and caffeine. ChemistrySelect 4(6):1989–1993. https://doi.org/10.1002/slct.201804006
Jamil TS, Roland H, Michael H, Jens-Uwe R (2017) Homogeneous photocatalytic processes for degradation of some endocrine disturbing chemicals under UV irradiation. J Water Process Eng 18:159–168. https://doi.org/10.1016/j.jwpe.2017.04.005
Jayaswal K, Sahu V, Gurjar BR (2018) Chapter 2: Water pollution, human health and remediation. In: Bhattacharya S, Gupta A, Gupta A, Pandey A (eds) Water remediation. Energy, environment, and sustainability. Springer, Singapore, pp 11–27. https://doi.org/10.1007/978-981-10-7551-3_2
Jing W, Li D, Li J, Li X, Wu Z, Liu Y (2018) Photodegradation of dimethyl phthalate (DMP) by UV–TiO2 in aqueous solution: operational parameters and kinetic analysis. Int J Environ Sci Technol 15(5):969–976. https://doi.org/10.1007/s13762-017-1471-3
Kadirova ZC, Hojamberdiev M, Katsumata K-I, Isobe T, Matsushita N, Nakajima A, Okada K (2017) Fe2O3-loaded activated carbon fiber/polymer materials and their photocatalytic activity for methylene blue mineralization by combined heterogeneous-homogeneous photocatalytic processes. Appl Surf Sci 402:444–455. https://doi.org/10.1016/j.apsusc.2017.01.131
Kanwal M, Tariq SR, Chotana GA (2018) Photocatalytic degradation of imidacloprid by Ag-ZnO composite. Environ Sci Pollut Res 25(27):27307–27320. https://doi.org/10.1007/s11356-018-2693-8
Karimi L, Zohoori S, Yazdanshenas ME (2014) Photocatalytic degradation of azo dyes in aqueous solutions under UV irradiation using nano-strontium titanate as the nanophotocatalyst. J Saudi Chem Soc 18(5):581–588. https://doi.org/10.1016/j.jscs.2011.11.010
Karimian D, Yadollahi B, Mirkhani V (2015) Harvesting visible light for aerobic oxidation of alcohols by a novel and efficient hybrid polyoxometalate. Dalton Trans 44(4):1709–1715. https://doi.org/10.1039/C4DT03299C
Khairy M, Zakaria W (2014) Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt J Pet 23(4):419–426. https://doi.org/10.1016/j.ejpe.2014.09.010
Khavar AHC, Moussavi G, Mahjoub AR, Satari M, Abdolmaleki P (2018) Synthesis and visible-light photocatalytic activity of In, S-TiO2@rGO nanocomposite for degradation and detoxification of pesticide atrazine in water. Chem Eng J 345:300–311. https://doi.org/10.1016/j.cej.2018.03.095
Kim I, Tanaka H (2010) Use of ozone-based processes for the removal of pharmaceuticals detected in a wastewater treatment plant. Water Environ Res 82(4):294–301. https://doi.org/10.2175/106143009x12487095236630
Kim I, Tanaka H (2011) Energy consumption for PPCPs removal by O3 and O3/UV. Ozone Sci Eng 33(2):150–157. https://doi.org/10.1080/01919512.2011.549427
Kim I, Yamashita N, Tanaka H (2009) Photodegradation of pharmaceuticals and personal care products during UV and UV/H2O2 treatments. Chemosphere 77(4):518–525. https://doi.org/10.1016/j.chemosphere.2009.07.041
Kim D-h, Lee D, Monllor-Satoca D, Kim K, Lee W, Choi W (2019) Homogeneous photocatalytic Fe3+/Fe2+ redox cycle for simultaneous Cr(VI) reduction and organic pollutant oxidation: roles of hydroxyl radical and degradation intermediates. J Hazard Mater 372:121–128. https://doi.org/10.1016/j.jhazmat.2018.03.055
Klavarioti M, Mantzavinos D, Kassinos D (2009) Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ Int 35(2):402–417. https://doi.org/10.1016/j.envint.2008.07.009
Kudo A, Miseki Y (2009) Heterogeneous photocatalyst materials for water splitting. Chem Soc Rev 38(1):253–278. https://doi.org/10.1039/B800489G
Kumari P, Bahadur N, Duméea LF (2020) Photo-catalytic membrane reactors for the remediation of persistent organic pollutants – a review. Sep Purif Technol 230:115878. https://doi.org/10.1016/j.seppur.2019.115878
Lee J-C, Kim M-S, Kim B-W (2002) Removal of paraquat dissolved in a photoreactor with TiO2 immobilized on the glass-tubes of UV lamps. Water Res 36(7):1776–1782. https://doi.org/10.1016/S0043-1354(01)00378-5
Lewis K, Archer R (1979) pKa values of estrone, 17β-estradiol and 2-methoxyestrone. Steroids 34(5):485–499. https://doi.org/10.1016/s0039-128x(79)80011-2
Li H, Zhou B (2019) Degradation of atrazine by catalytic ozonation in the presence of iron scraps: performance, transformation pathway, and acute toxicity. J Environ Sci Health B 54(5):432–440. https://doi.org/10.1080/03601234.2019.1574175
Li K, Xiong J, Chen T, Yan L, Dai Y, Song D, Lv Y, Zeng Z (2013) Preparation of graphene/TiO2 composites by nonionic surfactant strategy and their simulated sunlight and visible light photocatalytic activity towards representative aqueous POPs degradation. J Hazard Mater 250:19–28. https://doi.org/10.1016/j.jhazmat.2013.01.069
Li M, Yin JJ, Wamer WG, Lo YM (2014) Mechanistic characterization of titanium dioxide nanoparticle-induced toxicity using electron spin resonance. J Food Drug Anal 22(1):76–85. https://doi.org/10.1016/j.jfda.2014.01.006
Li Q, Jia R, Shao J, He Y (2019) Photocatalytic degradation of amoxicillin via TiO2 nanoparticle coupling with a novel submerged porous ceramic membrane reactor. J Clean Prod 209:755–761. https://doi.org/10.1016/j.jclepro.2018.10.183
Limburg B, Bouwman E, Bonnet S (2016) Rate and stability of photocatalytic water oxidation using [Ru(bpy)3]2+ as photosensitizer. ACS Catal 6(8):5273–5284. https://doi.org/10.1021/acscatal.6b00107
Lin L, Wang HY, Jiang WB, Mkaouar AR, Xu P (2017) Comparison study on photocatalytic oxidation of pharmaceuticals by TiO2-Fe and TiO2-reduced graphene oxide nanocomposites immobilized on optical fibers. J Hazard Mater 333:162–168. https://doi.org/10.1016/j.jhazmat.2017.02.044
Liu Y, Gan X, Zhou B, Xiong B, Li J, Dong C, Bai J, Cai W (2009) Photoelectrocatalytic degradation of tetracycline by highly effective TiO2 nanopore arrays electrode. J Hazard Mater 171(1–3):678–683. https://doi.org/10.1016/j.jhazmat.2009.06.054
Loeb BL, Thompson CM, Drago J, Takahara H, Baig S (2012) Worldwide ozone capacity for treatment of drinking water and wastewater: a review. Ozone Sci Eng 34(1):64–77. https://doi.org/10.1080/01919512.2012.640251
Long M, Cai W, Cai J, Zhou B, Chai X, Wu Y (2006) Efficient photocatalytic degradation of phenol over Co3O4/BiVO4 composite under visible light irradiation. J Phys Chem B 110(41):20211–20216. https://doi.org/10.1021/jp063441z
Louangsouphom B, Wang X, Song J, Wang X (2019) Low-temperature preparation of a N-TiO2/macroporous resin photocatalyst to degrade organic pollutants. Environ Chem Lett 17(2):1061–1066. https://doi.org/10.1007/s10311-018-00827-z
Luo Y, Chen J, Liu J, Shao Y, Li X, Li D (2016) Hydroxide SrSn(OH)6: a new photocatalyst for degradation of benzene and rhodamine B. Appl Catal B Environ 182:533–540. https://doi.org/10.1016/j.apcatb.2015.09.051
Lv Y, Yu L, Zhang X, Yao J, Zou R, Dai Z (2011) P-doped TiO2 nanoparticles film coated on ground glass substrate and the repeated photodegradation of dye under solar light irradiation. Appl Surf Sci 257(13):5715–5719. https://doi.org/10.1016/j.apsusc.2011.01.082
Mahanthappa M, Kottam N, Yellappa S (2019) Enhanced photocatalytic degradation of methylene blue dye using CuSCdS nanocomposite under visible light irradiation. Appl Surf Sci 475:828–838. https://doi.org/10.1016/j.apsusc.2018.12.178
Malefane ME, Feleni U, Kuvarega AT (2019) Tetraphenylporphyrin/WO3/exfoliated graphite nanocomposite for photocatalytic degradation of an acid dye under visible light irradiation. New J Chem 43:11348–11362. https://doi.org/10.1039/C9NJ02747E
Malvestiti JA, Fagnani E, Simão D, Dantas RF (2019) Optimization of UV/H2O2 and ozone wastewater treatment by the experimental design methodology. Environ Technol 40(15):1910–1922. https://doi.org/10.1080/09593330.2018.1432698
Manga Raju I, Rao S, KV DL, Divya G (2019) Poly 3-Thenoic acid sensitized, Copper doped anatase/brookite TiO2 nanohybrids for enhanced photocatalytic degradation of an organophosphorus pesticide. J Environ Chem Eng 7(4):103211. https://doi.org/10.1016/j.jece.2019.103211
Mao J, Ge M, Huang J, Lai Y, Lin C, Zhang K, Meng K, Tang Y (2017) Constructing multifunctional MOF@rGO hydro−/aerogels by the self-assembly process for customized water remediation. J Mater Chem A 5(23):11873–11881. https://doi.org/10.1039/C7TA01343D
Mbiri A, Wittstock G, Taffa DH, Gatebe E, Baya J, Wark M (2018) Photocatalytic degradation of the herbicide chloridazon on mesoporous titania/zirconia nanopowders. Environ Sci Pollut Res 25(35):34873–34883. https://doi.org/10.1007/s11356-017-1023-x
Medina-Valtierra J, García-Servín J, Frausto-Reyes C, Calixto S (2006) The photocatalytic application and regeneration of anatase thin films with embedded commercial TiO2 particles deposited on glass microrods. Appl Surf Sci 252(10):3600–3608. https://doi.org/10.1016/j.apsusc.2005.05.045
Meenakshisundaram S (2019) Environmental photocatalysis/photocatalytic decontamination. In: Martínez L, Kharissova O, Kharisov B (eds) Handbook of ecomaterials. Springer, Cham, pp 1625–1640. https://doi.org/10.1007/978-3-319-68255-6_65
Méndez-Torres AM, Castro J, Fernández F, Garrido-Ramírez E, Escalona N, Gutiérrez C, Marco JF, Ureta-Zañartu MS (2019) Electrodes based on zeolites modified with cobalt and/or molybdenum for pesticide degradation. Part I: physicochemical characterization and efficiency of the electrodes for O2 reduction and H2O2 production. Electrocatalysis 10(1):95–111. https://doi.org/10.1007/s12678-018-0500-4
Metcalf, Eddy (1981) Wastewater engineering: Collection and pumping of wastewater. McGraw-Hill. ISBN: 007041680X/9780070416802
Mierzwa JC, Rodrigues R, Teixeira AC (2018) UV-hydrogen peroxide processes. In: Advanced oxidation processes for waste water treatment. Elsevier, pp 13–48. https://doi.org/10.1016/B978-0-12-810499-6.00002-4
Milojević-Rakić M, Bajuk-Bogdanović D, Nedić Vasiljević B, Rakić A, Škrivanj S, Ignjatović L, Dondur V, Mentus S, Ćirić-Marjanović G (2018) Polyaniline/FeZSM-5 composites – synthesis, characterization and their high catalytic activity for the oxidative degradation of herbicide glyphosate. Microporous Mesoporous Mater 267:68–79. https://doi.org/10.1016/j.micromeso.2018.03.019
Mirhoseini F, Salabat A (2015) Ionic liquid based microemulsion method for the fabrication of poly (methyl methacrylate)–TiO2 nanocomposite as a highly efficient visible light photocatalyst. RSC Adv 5(17):12536–12545. https://doi.org/10.1039/C4RA14612C
Mirmasoomi SR, Ghazi MM, Galedari M (2017) Photocatalytic degradation of diazinon under visible light using TiO2/Fe2O3 nanocomposite synthesized by ultrasonic-assisted impregnation method. Sep Purif Technol 175:418–427. https://doi.org/10.1016/j.seppur.2016.11.021
Monga D, Basu S (2019) Enhanced photocatalytic degradation of industrial dye by g-C3N4/TiO2 nanocomposite: role of shape of TiO2. Adv Powder Technol 30(5):1089–1098. https://doi.org/10.1016/j.apt.2019.03.004
Mudassir MA, Hussain SZ, Khan M, Asma ST, Iqbal Z, Huma Z, Ullah N, Zhang H, Ansari TM, Hussain I (2018) Polyacrylamide exotemplate-assisted synthesis of hierarchically porous nanostructured TiO2 macrobeads for efficient photodegradation of organic dyes and microbes. RSC Adv 8(52):29628–29636. https://doi.org/10.1039/C8RA06197A
Naciri Y, Chennah A, Jaramillo-Páez C, Navío JA, Bakiz B, Taoufyq A, Ezahri M, Villain S, Guinneton F, Benlhachemi A (2019) Preparation, characterization and photocatalytic degradation of Rhodamine B dye over a novel Zn3(PO4)2/BiPO4 catalyst. J Environ Chem Eng 7(3):103075. https://doi.org/10.1016/j.jece.2019.103075
Nakata K, Fujishima A (2012) TiO2 photocatalysis: design and applications. J Photochem Photobiol C: Photochem Rev 13(3):169–189. https://doi.org/10.1016/j.jphotochemrev.2012.06.001
Neumann M, Schulz R, Schafer K, Muller W, Mannheller W, Liess M (2002) The significance of entry routes as point and non-point sources of pesticides in small streams. Water Res 36(4):835–842. https://doi.org/10.1016/s0043-1354(01)00310-4
Nguyen TT, Nam SN, Son J, Oh J (2019) Tungsten trioxide (WO3)-assisted photocatalytic degradation of amoxicillin by simulated solar irradiation. Sci Rep 9:18. https://doi.org/10.1038/s41598-019-45644-8
Noll KE (1991) Adsorption technology for air and water pollution control. CRC Press. ISBN 9780873713405
North GR (2015) Climate and climate change | greenhouse effect. In: North GR, Pyle J, Zhang F (eds) Encyclopedia of atmospheric sciences, 2nd edn. Academic, Oxford, pp 80–86. https://doi.org/10.1016/B978-0-12-382225-3.00470-9
Nosaka Y, Nakamura M, Hirakawa T (2002) Behavior of superoxide radicals formed on TiO2 powder photocatalysts studied by a chemiluminescent probe method. Phys Chem Chem Phys 4(6):1088–1092. https://doi.org/10.1039/B108441K
Pan D, Xiao S, Chen X, Li R, Cao Y, Zhang D, Pu S, Li Z, Li G, Li H (2019) Efficient photocatalytic fuel cell via simultaneous visible-photoelectrocatalytic degradation and electricity generation on a porous coral-like WO3/W photoelectrode. Environ Sci Technol 53(7):3697–3706. https://doi.org/10.1021/acs.est.8b05685
Pandian L, Rajasekaran R, Govindan P (2018) Synthesis, characterization and application of Cu doped ZnO nanocatalyst for photocatalytic ozonation of textile dye and study of its reusability. Mater Res Express 5(11):115505. https://doi.org/10.1088/2053-1591/aadcdf
Paula CHR, Neto NFA, Garcia LMP, Nascimento RM, Paskocimas CA, Bomio MRD, Motta FV (2019) Increased degradation capacity of methylene blue dye using Mg-doped ZnO nanoparticles decorated by Ag0 nanoparticles. J Electron Mater 48(5):3017–3025. https://doi.org/10.1007/s11664-019-07059-z
Payormhorm J, Chuangchote S, Laosiripojana N (2017a) CTAB-assisted sol-microwave method for fast synthesis of mesoporous TiO2 photocatalysts for photocatalytic conversion of glucose to value-added sugars. Mater Res Bull 95:546–555. https://doi.org/10.1016/j.materresbull.2017.08.016
Payormhorm J, Chuangchote S, Kiatkittipong K, Chiarakorn S, Laosiripojana N (2017b) Xylitol and gluconic acid productions via photocatalytic-glucose conversion using TiO2 fabricated by surfactant-assisted techniques: effects of structural and textural properties. Mater Chem Phys 196:29–36. https://doi.org/10.1016/j.matchemphys.2017.03.058
Pérez A, Rodríguez JL, Galicia A, Chairez I, Poznyak T (2019) Recycling strategy for water contaminated with Reactive Black 5 in the presence of additives treated by simple ozonation. Ozone Sci Eng 41(1):46–59. https://doi.org/10.1080/01919512.2018.1483816
Phuong NM, Chu NC, Van Thuan D, Ha MN, Hanh NT, Viet HDT, Thu M, Thi N, Van Quan P, Truc T (2019) Novel removal of diazinon pesticide by adsorption and photocatalytic degradation of visible light-driven Fe-TiO2/Bent-Fe photocatalyst. J Chem 2019:2678927. https://doi.org/10.1155/2019/2678927
Prasad MNV, Vithanage M, Kapley A (2019) Pharmaceuticals and personal care products: waste management and treatment technology: emerging contaminants and micro pollutants. Butterworth-Heinemann. https://doi.org/10.1016/C2017-0-03544-9
Qin W, Song Y, Dai Y, Qiu G, Ren M, Zeng P (2015) Treatment of berberine hydrochloride pharmaceutical wastewater by O3/UV/H2O2 advanced oxidation process. Environ Earth Sci 73(9):4939–4946. https://doi.org/10.1007/s12665-015-4192-2
Qu J, He X, Li X, Ai Z, Li Y, Zhang Q, Liu X (2017) Precursor preparation of Zn–Al layered double hydroxide by ball milling for enhancing adsorption and photocatalytic decoloration of methyl orange. RSC Adv 7(50):31466–31474. https://doi.org/10.1039/C7RA05316A
Rasoulnezhad H, Kavei G, Ahmadi K, Rahimipour MR (2017) Visible light photocatalytic degradation of paraoxon and parathion pesticides on carbon-doped TiO2 nanorod thin films. J Mater Sci Mater Electron 28(24):18337–18347. https://doi.org/10.1007/s10854-017-7780-y
Ravishankar TN, de O Vaz M, Ramakrishnappa T, Teixeira SR, Dupont J (2019) Ionic liquid–assisted hydrothermal synthesis of Nb/TiO2 nanocomposites for efficient photocatalytic hydrogen production and photodecolorization of Rhodamine B under UV-visible and visible light illuminations. Mater Today Chem 12:373–385. https://doi.org/10.1016/j.mtchem.2019.04.001
Recio F, Herrasti P, Sirés I, Kulak A, Bavykin D, Ponce-de-León C, Walsh F (2011) The preparation of PbO2 coatings on reticulated vitreous carbon for the electro-oxidation of organic pollutants. Electrochim Acta 56(14):5158–5165. https://doi.org/10.1016/j.electacta.2011.03.054
Reddy CN, Mohan SV (2016) Integrated bio-electrogenic process for bioelectricity production and cathodic nutrient recovery from azo dye wastewater. Renew Energy 98:188–196. https://doi.org/10.1016/j.renene.2016.03.047
Rehman F, Sayed M, Khan JA, Shah NS, Khan HM, Dionysiou DD (2018) Oxidative removal of brilliant green by UV/S2O82−, UV/HSO5− and UV/H2O2 processes in aqueous media: a comparative study. J Hazard Mater 357:506–514. https://doi.org/10.1016/j.jhazmat.2018.06.012
Rezgui S, Amrane A, Fourcade F, Assadi A, Monser L, Adhoum N (2018) Electro-Fenton catalyzed with magnetic chitosan beads for the removal of Chlordimeform insecticide. Appl Catal B Environ 226:346–359. https://doi.org/10.1016/j.apcatb.2017.12.061
Rosa Barbosa MP, Lima NS, de Matos DB, Alves Felisardo RJ, Santos GN, Salazar-Banda GR, Cavalcanti EB (2018) Degradation of pesticide mixture by electro-Fenton in filter-press reactor. J Water Process Eng 25:222–235. https://doi.org/10.1016/j.jwpe.2018.08.008
Sajjadi S, Khataee A, Bagheri N, Kobya M, Şenocak A, Demirbas E, Karaoğlu AG (2019) Degradation of diazinon pesticide using catalyzed persulfate with Fe3O4@MOF-2 nanocomposite under ultrasound irradiation. J Ind Eng Chem 77:280–290. https://doi.org/10.1016/j.jiec.2019.04.049
Saleh R, Djaja NF (2014) UV light photocatalytic degradation of organic dyes with Fe-doped ZnO nanoparticles. Superlattice Microst 74:217–233. https://doi.org/10.1016/j.spmi.2014.06.013
Sanitnon P, Chiarakorn S, Chawengkijwanich C, Chuangchote S, Pongprayoon T (2019) Synergistic effects of zirconium and silver co-dopants in TiO2 nanoparticles for photocatalytic degradation of an organic dye and antibacterial activity. J Aust Ceram Soc. https://doi.org/10.1007/s41779-019-00368-w
Santoke H, Tong AYC, Mezyk SP, Johnston KM, Braund R, Cooper WJ, Peake BM (2015) UV Photodegradation of Enoxacin in water: kinetics and degradation pathways. J Environ Eng ASCE 141(10):7. https://doi.org/10.1061/(asce)ee.1943-7870.0000954
Särkkä H, Bhatnagar A, Sillanpää M (2015) Recent developments of electro-oxidation in water treatment—a review. J Electroanal Chem 754:46–56. https://doi.org/10.1016/j.jelechem.2015.06.016
Senthil RA, Sun M, Pan J, Osman S, Khan A, Sun Y (2019) Facile fabrication of a new BiFeWO6/α-AgVO3 composite with efficient visible-light photocatalytic activity for dye-degradation. Opt Mater 92:284–293. https://doi.org/10.1016/j.optmat.2019.04.046
Senthilnathan J, Philip L (2012) Elimination of pesticides and their formulation products from drinking water using thin film continuous photoreactor under solar radiation. Sol Energy 86(9):2735–2745. https://doi.org/10.1016/j.solener.2012.06.011
Shetty R, Chavan VB, Kulkarni PS, Kulkarni BD, Kamble SP (2017) Photocatalytic degradation of pharmaceuticals pollutants using N-doped TiO2 photocatalyst: identification of CFX degradation intermediates. Indian Chem Eng 59(3):177–199. https://doi.org/10.1080/00194506.2016.1150794
Singh J, Sharma S, Aanchal, Basu S (2019) Synthesis of Fe2O3/TiO2 monoliths for the enhanced degradation of industrial dye and pesticide via photo-Fenton catalysis. J Photochem Photobiol A Chem 376:32–42. https://doi.org/10.1016/j.jphotochem.2019.03.004
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano Micro Lett 7(3):219–242. https://doi.org/10.1007/s40820-015-0040-x
Sirirerkratana K, Kemacheevakul P, Chuangchote S (2019) Color removal from wastewater by photocatalytic process using titanium dioxide-coated glass, ceramic tile, and stainless steel sheets. J Clean Prod 215:123–130. https://doi.org/10.1016/j.jclepro.2019.01.037
Solís RR, Gimeno O, Rivas FJ, Beltrán FJ (2019) Simulated solar driven photolytic ozonation for the oxidation of aqueous recalcitrant-to-ozone tritosulfuron. Transformation products and toxicity. J Environ Manag 233:513–522. https://doi.org/10.1016/j.jenvman.2018.12.068
Srisasiwimon N, Chuangchote S, Laosiripojana N, Sagawa T (2018) TiO2/lignin-based carbon composited photocatalysts for enhanced photocatalytic conversion of lignin to high value chemicals. ACS Sustain Chem Eng 6(11):13968–13976. https://doi.org/10.1021/acssuschemeng.8b02353
Stan CD, Cretescu I, Pastravanu C, Poulios I, Drăgan M (2012) Treatment of pesticides in wastewater by heterogeneous and homogeneous photocatalysis. Int J Photoenergy 2012:194823. https://doi.org/10.1155/2012/194823
Stockholm Convention. Available at: http://www.pops.int/ (access: August 2019)
Sun F, Zeng Q, Tian W, Zhu Y, Jiang W (2019) Magnetic MFe2O4-Ag2O (M = Zn, Co, & Ni) composite photocatalysts and their application for dye wastewater treatment. J Environ Chem Eng 7(2):103011. https://doi.org/10.1016/j.jece.2019.103011
Tabasideh S, Maleki A, Shahmoradi B, Ghahremani E, McKay G (2017) Sonophotocatalytic degradation of diazinon in aqueous solution using iron-doped TiO2 nanoparticles. Sep Purif Technol 189:186–192. https://doi.org/10.1016/j.seppur.2017.07.065
Teran T, Lamon L, Marcomini A (2012) Climate change effects on POPs’ environmental behaviour: a scientific perspective for future regulatory actions. Atmos Pollut Res 3(4):466–476. https://doi.org/10.5094/APR.2012.054
Thabit M, Liu H, Zhang J, Wang B (2018) Synthesis, characterization of Hollandite Ag2Mn8O16 on TiO2 nanotubes and their photocatalytic properties for Rhodamine B degradation. Pol J Chem Technol 20(2):85–91. https://doi.org/10.2478/pjct-2018-0027
Tong M, Yang J, Jin Q, Zhang X, Gao J, Li G (2019) Facile preparation of amorphous carbon-coated tungsten trioxide containing oxygen vacancies as photocatalysts for dye degradation. J Mater Sci 54(15):10656–10669. https://doi.org/10.1007/s10853-019-3645-y
Vaiano V, Jaramillo-Paez CA, Matarangolo M, Navio JA, Hidalgo MD (2019) UV and visible-light driven photocatalytic removal of caffeine using ZnO modified with different noble metals (Pt, Ag and Au). Mater Res Bull 112:251–260. https://doi.org/10.1016/j.materresbull.2018.12.034
Van HT, Nguyen LH, Hoang TK, Tran TP, Vo AT, Pham T, Nguyen X (2019) Using FeO-constituted iron slag wastes as heterogeneous catalyst for Fenton and ozonation processes to degrade Reactive Red 24 from aqueous solution. Sep Purif Technol 224:431–442. https://doi.org/10.1016/j.seppur.2019.05.048
Wang JL, Wang SZ (2016) Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: a review. J Environ Manag 182:620–640. https://doi.org/10.1016/j.jenvman.2016.07.049
Wang LK, Tay J-H, Tay STL, Hung Y-T (2010) Environmental bioengineering, vol 11. Springer. ISBN 978-1-60327-031-1
Wang Z-b, Guan Y-j, Chen B, Bai S-l (2016) Retention and separation of 4BS dye from wastewater by the N-TiO2 ceramic membrane. Desalin Water Treat 57(36):16963–16969. https://doi.org/10.1080/19443994.2015.1082940
Wang C, Cao Y, Wang H (2019a) Copper-based catalyst from waste printed circuit boards for effective Fenton-like discoloration of Rhodamine B at neutral pH. Chemosphere 230:278–285. https://doi.org/10.1016/j.chemosphere.2019.05.068
Wang D, Qiu S, Wang M, Pan S, Ma H, Zou J (2019b) Spectrophotometric determination of hydrogen peroxide in water by oxidative decolorization of azo dyes using Fenton system. Spectrochim Acta A Mol Biomol Spectrosc 221:117138. https://doi.org/10.1016/j.saa.2019.117138
Wang L, Li Z, Chen J, Huang Y, Zhang H, Qiu H (2019c) Enhanced photocatalytic degradation of methyl orange by porous graphene/ZnO nanocomposite. Environ Pollut 249:801–811. https://doi.org/10.1016/j.envpol.2019.03.071
Wei Y, Wang B, Cui X, Muhammad Y, Zhang Y, Huang Z, Li X, Zhao Z, Zhao Z (2018) Highly advanced degradation of thiamethoxam by synergistic chemisorption-catalysis strategy using MIL (Fe)/Fe-SPC composites with ultrasonic irradiation. ACS Appl Mater Interfaces 10(41):35260–35272. https://doi.org/10.1021/acsami.8b12908
Wu C-H, Chang C-L (2006) Decolorization of Reactive Red 2 by advanced oxidation processes: comparative studies of homogeneous and heterogeneous systems. J Hazard Mater 128(2–3):265–272. https://doi.org/10.1016/j.jhazmat.2005.08.013
Wu F-C, Tseng R-L, Juang R-S (2001) Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res 35(3):613–618. https://doi.org/10.1016/S0043-1354(00)00307-9
Yang Y, Ok YS, Kim KH, Kwon EE, Tsang YF (2017) Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Sci Total Environ 596:303–320. https://doi.org/10.1016/j.scitotenv.2017.04.102
Ye S, Chen R, Xu Y, Fan F, Du P, Zhang F, Zong X, Chen T, Qi Y, Chen P (2016) An artificial photosynthetic system containing an inorganic semiconductor and a molecular catalyst for photocatalytic water oxidation. J Catal 338:168–173. https://doi.org/10.1016/j.jcat.2016.02.024
Yemmireddy VK, Hung YC (2017) Using photocatalyst metal oxides as antimicrobial surface coatings to ensure food safety—opportunities and challenges. Compr Rev Food Sci Food Saf 16(4):617–631. https://doi.org/10.1111/1541-4337.12267
Yi C, Liao Q, Deng W, Huang YW, Mao J, Zhang BP, Wu GP (2019) The preparation of amorphous TiO2 doped with cationic S and its application to the degradation of DCFs under visible light irradiation. Sci Total Environ 684:527–536. https://doi.org/10.1016/j.scitotenv.2019.05.338
Yu C, He H, Liu X, Zeng J, Liu Z (2019) Novel SiO2 nanoparticle-decorated BiOCl nanosheets exhibiting high photocatalytic performances for the removal of organic pollutants. Chin J Catal 40:1212–1221. https://doi.org/10.1016/S1872-2067(19)63359-0
Zahedi F, Behpour M, Ghoreishi SM, Khalilian H (2015) Photocatalytic degradation of paraquat herbicide in the presence TiO2 nanostructure thin films under visible and sun light irradiation using continuous flow photoreactor. Sol Energy 120:287–295. https://doi.org/10.1016/j.solener.2015.07.010
Zhang H, Zhang P, Ji Y, Tian J, Du Z (2015) Photocatalytic degradation of four non-steroidal anti-inflammatory drugs in water under visible light by P25-TiO2/tetraethyl orthosilicate film and determination via ultra performance liquid chromatography electrospray tandem mass spectrometry. Chem Eng J 262:1108–1115. https://doi.org/10.1016/j.cej.2014.10.019
Zhang J-J, Liu X, Ye T, Zheng G-P, Zheng X-C, Liu P, Guan X-X (2017) Novel assembly of homogeneous reduced graphene oxide-doped mesoporous TiO2 hybrids for elimination of Rhodamine-B dye under visible light irradiation. J Alloys Compd 698:819–827. https://doi.org/10.1016/j.jallcom.2016.12.279
Zhao B, Lv M, Zhou L (2012) Photocatalytic degradation of perfluorooctanoic acid with β-Ga2O3 in anoxic aqueous solution. J Environ Sci 24(4):774–780. https://doi.org/10.1016/S1001-0742(11)60818-8
Zheng C, Zhao L, Zhou X, Fu Z, Li A (2013) Treatment technologies for organic wastewater. In: Elshorbagy W, Chowdhury RK (eds) Water treatment. IntechOpen, pp 249–286. https://doi.org/10.5772/52665
Zhou Y, Lu J, Zhou Y, Liu Y (2019) Recent advances for dyes removal using novel adsorbents: a review. Environ Pollut 252(Pt A):352–365. https://doi.org/10.1016/j.envpol.2019.05.072
Zhu S, Wang D (2017) Photocatalysis: basic principles, diverse forms of implementations and emerging scientific opportunities. Adv Energy Mater 7(23):1700841. https://doi.org/10.1002/aenm.201700841
Ziarani GM, Moradi R, Lashgari N, Kruger HG (2018) Chapter 1: Introduction and importance of synthetic organic dyes. In: Ziarani GM, Moradi R, Lashgari N, Kruger HG (eds) Metal-free synthetic organic dyes. Elsevier, pp 1–7. https://doi.org/10.1016/B978-0-12-815647-6.00001-7
Zinatloo-Ajabshir S, Morassaei MS, Salavati-Niasari M (2019) Eco-friendly synthesis of Nd2Sn2O7–based nanostructure materials using grape juice as green fuel as photocatalyst for the degradation of erythrosine. Compos Part B 167:643–653. https://doi.org/10.1016/j.compositesb.2019.03.045
Zollinger H (2003) Color chemistry: syntheses, properties, and applications of organic dyes and pigments. Wiley. https://doi.org/10.1002/anie.200385122
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kemacheevakul, P., Chuangchote, S. (2021). Photocatalytic Remediation of Organic Pollutants in Water. In: Inamuddin, Ahamed, M.I., Lichtfouse, E. (eds) Water Pollution and Remediation: Photocatalysis. Environmental Chemistry for a Sustainable World, vol 57. Springer, Cham. https://doi.org/10.1007/978-3-030-54723-3_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-54723-3_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-54722-6
Online ISBN: 978-3-030-54723-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)