Abstract
There are three major sub-processes in memory production – encoding, consolidation, and retrieval. While encoding and retrieval occur frequently during wakefulness, sleep likely plays a major role in consolidation, the process by which newly acquired (and generally labile) memories encoded during wakefulness are reprocessed and converted into a more stable form and then integrated into preexisting memory networks (long-term storage). This process depends on two types of consolidation mechanisms; the first is referred to as “synaptic consolidation” which leads to remodeling and more effective synapses, and the second is “system consolidation,” which redistributes newly encoded representations to other neuronal circuitries for long-term storage. Long-term memory is divided into two main types: declarative and non-declarative. Declarative memories require the involvement of medial temporal regions, specifically the hippocampus, with episodic memories rooted in temporal regions. Non-declarative memories, such as perceptual skills, originate from sensory cortices and procedural memories from the cerebellum, striatum, and motor areas. Slow wave sleep, sleep spindles, and REM sleep appear to be the three main neurophysiological counterparts of the memory consolidation processes taking place during sleep.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Encoding
- Consolidation
- Retrieval
- Declarative memory
- Non-declarative memory
- Interference
- Potentiation: memory and sleep
- Procedural memory
- Memory consolidation
Introduction
It is well known that sleep is comprised of two distinct phases: non-REM (NREM) vs REM. NREM is further divided into lighter stages (stage 1 and stage 2) and a deeper stage (stage 3). Stage 3, also known as slow wave sleep, is predominantly seen in the earlier portion of the night, while REM sleep is more predominant in the latter portion. The role of sleep has been reviewed extensively and has been postulated to allow for repair of cell tissue and thermoregulation, facilitate immune function, and preserve energy; however, other roles have been written about the function of sleep through the ages.
In the late sixteenth century and early seventeenth century, Dr. Christopher Wirtzung, writer of A General Practise of Physicke, described sleep as the “warming” of the body’s spirits, the vehicle of all the processes of life emanating from the soul [1], while John Jones discussed the need for sleep to refresh ones’ senses and improve digestion [2]. This was echoed by none other than Shakespeare in his works of Macbeth and Henry IV. Within the last few centuries, psychologists, philosophers, and playwrights alike spoke of new roles of sleep. British psychologist David Hartley proposed in 1801 the link between dreaming and memory [3]. However, it was not until 1924 when the first systematic studies of sleep and memory were performed by Jenkins and Dallenbach to test Ebbinghaus’s theory of memory decay [4]. The knowledge elucidating sleep’s impact in memory processing and brain plasticity has grown over the last half century and has become a topic of interest of many researchers. It is now assumed that sleep facilitates consolidation of memory by strengthening new memories and acclimating them into preexisting long-term memories. This chapter will outline what is known, while investigating perceived relationships of various sleep stages and their impact on the production, restructuring, consolidation, storage, and strengthening of memories, which is imperative to performing our daily activities/functions, while reviewing the neurophysiologic basis for this. This information, which continues to grow with time, has been gathered from various physiological and behavioral studies performed over the last several decades. Initially, we will review underlying memory processes and the notion of memory consolidation.
Memory Processes
There are three major sub-processes in memory production – encoding, consolidation, and retrieval. Encoding is the process of forming a new memory trace in response to the perception of a stimulus. This process is highly susceptible to forgetting. Therefore, consolidation occurs to stabilize the memory trace by strengthening and integrating the memory into preexisting knowledge networks. As explained by Dudai [5], consolidation is the process by which newly acquired and generally labile memories encoded during wakefulness are believed to be reprocessed and converted into a more stable form and then integrated into preexisting memory networks during sleep. Finally, the stored memory may then be accessed and recalled during retrieval. While encoding and retrieval occur frequently during wakefulness, it is believed that sleep plays a major role in the consolidation of newly encoded memory into long-term storage. It is believed that this process depends on multiple variables, including different processes of neuronal plasticity. There are believed to be two types of consolidation processes: the first is coined “synaptic consolidation” which leads to remodeling and subsequent more effective synapses [5,6,7,8], and the second is referred to as “system consolidation,” which redistribute newly encoded representations to other neuronal circuitries for long-term storage [5, 9].
Neurobiology of Memory
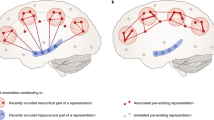
Long-term memory is generally divided into two main types: declarative (explicit) and procedural (implicit). Declarative memory (“knowing what”) is memory that is consciously recalled, i.e., declared, and includes facts and events. It is further divided into episodic and semantic memory . Procedural or non-declarative memory (“knowing how”) relates to unconscious memory such as skills and “how to do things.” Examples of this include riding a bike or playing piano. These memories are generally acquired through repetition and are composed of automatic sensorimotor behaviors. Figure. 6.1 illustrates memory types and the stages of memory production (Fig. 6.1).
Memory systems and memory stages. (a) Memory systems. Human memory is most commonly divided into declarative forms, with further subdivisions into episodic and semantic, and non-declarative forms, subdivided into an array of different types including procedural skill memory. (b) Developing stages of memory. Following initial encoding of a memory, several ensuing stages are proposed, beginning with consolidation, as well as integration of the memory representation, and translocation of the representation or erasure of the memory. Also, following later recall, the memory representation is believed to become unstable once again, requiring periods of reconsolidation. (Reprinted by permission from Springer: Spencer et al. [10])
The locations in the brain where formation of long-term memory is processed were illustrated following a procedure in 1953 which included removal of a patient’s medial temporal lobe, hippocampus, and amygdala to treat intractable seizures. After the surgery, the patient was still able to form procedural memories and short-term memories but demonstrated failure in converting immediate memory into stable long-term memory. As well, studies have shown that lesions of the hippocampus abolish the ability to acquire new declarative memory and produce retrograde amnesia where only older memories remain intact [9, 11].
Declarative memories require the involvement of medial temporal regions, specifically the hippocampus, with episodic memories rooted in temporal regions. Non-declarative memories such as perceptual skills originate from sensory cortices and procedural memories from the cerebellum, striatum, and motor areas. However, there is interaction between these systems, specifically seen in the early stages of new knowledge acquisition [12] and the transition from fast learning, seen in the hippocampus, to slow learning for long-term storage in the neocortex.
Role of Sleep in Memory
There have been several studies through the years reviewing barriers to memory retention and how sleep affects this process. Ebbinghaus performed memory research in 1885 and noticed that forgetting rapidly occurs in the first hours after learning, known as the “forgetting curve,” and is reduced when sleep occurred in the retention period [13]. As well, others have reported impairment in the ability to remember following sleep deprivation [14]. There were two main beliefs that were initially considered when it came to forgetting, one related to “decay” which acknowledges the potential role time plays in the forgetting of memory [15] and another relating to “interference,” an idea whereby new information interferes with the ability to retain older memories [16]. This is felt to have an even stronger effect when the new memories are quite similar to the old memories [17]. It was later demonstrated that sleep after learning reduced the amount of forgetting [4]. This was replicated on subsequent studies, strengthening this positive effect of sleep on memory and protecting memories from interference [18]. However, there appears to be a time dependency to how well memories may be protected from interference, such that, sleep occurring soon after learning has a stronger impact on inhibiting interference versus sleep occurring at a more remote time. One such example was seen in a study by Gais et al. who demonstrated that sleep within 3 hours of learning vocabulary had a more beneficial effect compared to sleep occurring 10 hours after [19]. Thus, not only does sleep have a positive effect on memory, but the timing when sleep occurs also plays an important role. However, it has additionally been illustrated that the stage of sleep also has an impact on how memory is protected from interference and how memory consolidation occurs.
Role of Specific Sleep Stages in Memory
Sleep stages are identified by characteristic brain activity rhythms: delta waves in slow wave sleep and spindle activity in stage 2 sleep.
The synaptic homeostasis theory describes the importance of not only upscaling synapses, which leads to their strengthening, but a system where downscaling may occur as well to simplify encoding of new information. This is believed to occur during slow wave sleep (SWS). One example is seen when the ability to encode pictures following a nap without slow wave activity was significantly diminished [20, 21]. With this impairment, there was a noted reduction in hippocampus activity during learning. However, neocortical regions were not impacted, and the ability to learn motor skill tasks was not hindered. Additionally, slow wave activity [22], slow wave amplitude, and the length of the up-state have been positively related to overnight memory consolidation [23]. SWS was also demonstrated to play a role in declarative memory consolidation by Rasch et al. [24]. Subjects were taught a visuospatial task of recognizing the location of images within a matrix. The task was performed while participants were exposed to an odor (the scent of roses), and then the odor was reintroduced during SWS for some participants (Fig. 6.2a). Those who were exposed to the odor during learning the task and subsequent SWS sleep had greater recall following sleep. This response was not seen with other sleep stages tested such as REM sleep. When the odor was presented during REM sleep, there was no benefit on subsequent recall compared to the no-odor (vehicle) control condition (Fig. 6.2b). This demonstrates the unique role of SWS on declarative memory consolidation.
Declarative memory reactivation during slow wave sleep. (a) Subjects performed a visuospatial learning task in the presence of an experimental odor (scent of roses). During subsequent slow wave sleep, this odor or a non-odor vehicle control was presented. Memory for the visuospatial task was tested following sleep. (b) Participants recalled more locations following sleep when the experimental odor was re-presented during slow wave sleep compared to vehicle (first panel). Such an effect was not observed if the odor present during slow wave sleep was not also present during learning (second panel), if the odor cue was re-presented during REM (third panel), or if the odor was re-presented during waking. (Reprinted by permission from Springer: Spencer et al. [10])
Sleep spindles, generated in the thalamus and a component of stage 2 sleep, have been postulated to be associated with multiple forms of memory formation, consolidation, and storage. Spindle-associated discharges effectively triggered long-term potentiation (LTP) in neocortical synapses in in vitro models [25, 26]. It has been reported by several studies that there is a positive correlation between both time spent in stage 2 sleep and amount of spindle activity and motor skill improvement [27,28,29,30,31]. Thus, it is hypothesized that spindle activity induces synaptic potentiation [25] and plays a role in procedural memory consolidation [27, 32,33,34]. As well, motor memory consolidation is thought to be impacted by spindle activity [28] with changes in striatal [35, 36] and hippocampal activity [37,38,39]. As an example, motor sequence learning tasks and the degree of overnight performance improvements positively correlate with increased spindle activity [29, 30, 33]. Sleep spindle promotion of skill consolidation is achieved by locally reactivating and functionally binding task-relevant cortical-cortical and subcortical regions, such as the hippocampus, putamen, thalamus, and motor-related cortical regions. Additionally, spindles are positively associated with declarative learning [40], declarative overnight performance changes [41], and intelligence [42].
Evidence shows that REM sleep also plays an important role in memory consolidation, specifically in regard to emotional memory [43,44,45]. Healthy students selectively deprived of REM sleep were found to exhibit impaired next-day recognition performance for negative, but not neutral, images encoded pre-sleep when compared to students deprived of NREM slow wave sleep [46]. Furthermore, 3 hours of late night REM-dominant sleep facilitate the consolidation of negative images [47]. This was reiterated by Payne, Chambers, and Kensinger in 2012 [48] who reported that greater negative memory consolidation is predicted by longer duration of REM sleep during retention intervals. As well, Gilson et al. [49] found in 2015 that greater REM density predicts greater selective consolidation of emotionally negative stories. With this in mind, both Walker and van der Helm in 2009 [43] and Harrington, Pennington, and Durant in 2017 [50] suggested that changes in REM sleep may increase vulnerability to the onset and recurrence of major depressive disorder by promoting the development of negative memory bias. Aside from emotional memory, there is also evidence that REM sleep plays a role in gross motor learning [51].
Role of Alternative Sleep Periods in Memory
Many studies have focused on full night sleep to investigate sleep and memory consolidation. However, it is also important to note that alternative sleep periods may play a role as well. This was achieved by van Schalkwijk et al. who, in 2017, studied 76 participants who were randomly assigned to a nap or wake group and performed a declarative word-pair association or procedural mirror tracing task. Performance changes from before compared to after a 90-minute retention interval filled with sleep or quiet wakefulness were evaluated between groups. Associations between performance changes, sleep architecture, spindles, and slow oscillations were investigated. They found a trend toward stronger forgetting in the declarative task across a wake period compared with a nap period while improved procedural task accuracy with a daytime nap compared to a period following daytime wakefulness [52]. This demonstrates that daytime napping prevented deterioration of performance for procedural mirror tracing, with a similar trend observed for declarative word-pair learning.
Conclusion
The knowledge surrounding the role of sleep and its impact on the body has grown over the centuries. However, only in recent history has the significance of sleep on memory been postulated and studied. Within just the last half century, we have learned how important sleep can be on multiple types of memory, including memory formation, consolidation, and storage. As well, it has been shown how various sleep stages and neural systems aid in this process. Yet, further work is needed to better understand this complex interaction and to determine how this information can be translated to patient care.
References
Wirtzung C. The general Practise of Physicke: Conteyning all inward and outward parts of the body, with all the accidents and infirmities that are incident unto them, Euen from the Crowne of the head to the sole of the Foote. London: Thomas Adams; 1617.
Jones J. The mysteries of opium Reveal’d: by Dr. John Jones. London: Richard Smith; 1701.
Hartley D. Observations on man, his frame, his duty, and his expectations. Warrington: J. Johnson; 1801.
Jenkins J, Dallenbach K. Obliviscence during sleep and waking. Am J Psychol. 1924;35:605–12.
Dudai Y. The neurobiology of consolidations, or how stable is the engram? Annu Rev Psychol. 2004;55:51–86.
Hofer SB. Structural traces of past experience in the cerebral cortex. J Mol Med. 2010;88:235–9.
Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–8.
Redondo RL, Morris RGM. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12:17–30.
Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–30.
Spencer R, Walker M, Stickgold R. Sleep and memory consolidation. In: Sleep disorders medicine: basic science, technical considerations and clinical aspects. 4th ed. New York: Springer; 2017. p. 205–23.
Corkin S. What’s new with the amnesic patient HM? Nat Rev Neurosci. 2002;3:153–60.
Peigneux P, Laureys S, Delbeuck X, Maquet P. Sleeping brain, learning brain. The role of sleep for memory systems. Neruoreport. 2001;12:A111–24.
Van Ormer EB. Sleep and retention. Psychol Bull. 1933;30:415–39.
Patrick G. On the effects of loss of sleep. Psychol Rev. 1896;3:468–83.
Thorndike EL. Educational psychology, The psychology of learning, vol. 2. New York: Teachers College Press; 1913.
McGeoch JA. Forgetting and the law of disuse. Psychol Rev. 1932;39:352–70.
Keppel G. Consolidation and forgetting. In: Weingartner H, Parker ES, editors. Consolidation and forgetting. Hillsdale, NJ: Lawrence Erlbaum Associates; 1984.
Ellenbogen J, Payne J, Stickgold R. The role of sleep in declarative memory consolidation: passive, permissive, active or none? Curr Opin Neurobiol. 2006;16:716–22.
Gais S, Lucas B, Born J. Sleep after learning aids memory recall. Learn Mem. 2006;13:259–62.
Van der Werf Y, Altena E, Schoonheim M, Sanz-Arigita E, Vis J, de Rijke W, van Someren EJW. Sleep benefits subsequent hippocampal functioning. Nat Neurosci. 2009;12:122–3.
Van der Werf Y, Altena E, Vis J, Koene T, van Someren EJW. Reduction of nocturnal slow-wave activity affects daytime vigilance lapses and memory encoding but not reaction time or implicit learning. Prog Brain Res. 2011;193:245–55.
Holz J, Piosczyk H, Feige B, et al. EEG sigma and slow-wave activity during NREM sleep correlate with overnight declarative and procedural memory consolidation. J Sleep Res. 2012;21:612–9.
Heib DP, Hoedlmoser K, Anderer P, et al. Slow oscillation amplitudes and up-state lengths relate to memory improvement. PLoS One. 2013;8:e82049.
Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315(5817):1426–9.
Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25:9398–405.
Timofeev I, Grenier F, Bazhenov M, Houweling AR, Sejnowski TJ, Steriade M. Short-and medium-term plasticity associated with augmenting responses in cortical slabs and spindles in intact cortex of cats in vivo. J Physiol. 2002;542:583–98.
Fogel SM, Smith CT. Learning-dependent changes in sleep spindles and Stage 2 sleep. J Sleep Res. 2006;15(3):250–5.
Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35(5):1154–65.
Laventure S, Fogel S, Lungu O, Albouy G, Sevigny-Dupont P, Vien C, et al. NREM2 and sleep spindles are instrumental to the consolidation of motor sequence memories. PLoS Biol. 2016;14(3):e1002429.
Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2(4):e341.
Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35(1):205–11.
Ackermann S, Rasch B. Differential effects of non-REM and REM sleep on memory consolidation? Curr Neurol Neurosci Rep. 2014;14(2):430.
Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, et al. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res. 2011;217(1):117–21.
Barakat M, Carrier J, Debas K, Lungu O, Fogel S, Vandewalle G, et al. Sleep spindles predict neural and behavioral changes in motor sequence consolidation. Hum Brain Mapp. 2013;34(11):2918–28.
Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, et al. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci U S A. 2010;107(41):17839–44.
Debas K, Carrier J, Barakat M, Marrelec G, Bellec P, Hadj Tahar A, et al. Off-line consolidation of motor sequence learning results in greater integration within a cortico-striatal functional network. NeuroImage. 2014;99:50–8.
Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang-Vu T, et al. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron. 2008;58(2):261–72.
Albouy G, Sterpenich V, Vandewalle G, Darsaud A, Gais S, Rauchs G, et al. Interaction between hippocampal and striatal systems predicts subsequent consolidation of motor sequence memory. PLoS One. 2013;8(3):e59490.
Albouy G, Fogel S, King BR, Laventure S, Benali H, Karni A, et al. Maintaining vs. enhancing motor sequence memories: respective roles of striatal and hippocampal systems. NeuroImage. 2015;108:423–34.
Gais S, Molle M, Helms K, Born J. Learning-dependent € increases in sleep spindle density. J Neurosci. 2002;22:6830–4.
Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–35.
Bodizs R, Gombos F, Ujma PP, Kovacs I. Sleep spindling and fluid intelligence across adolescent development: sex matters. Front Hum Neurosci. 2014;8:952.
Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135(5):731e748.
Bennion KA, Payne JD, Kensinger EA. Selective effects of sleep on emotional memory: what mechanisms are responsible? Trans Issu Psychol Sci. 2015;1(1):79e88.
Genzel L, Spoormaker V, Konrad BN, Dresler M. The role of rapid eye movement sleep for amygdala-related memory processing. Neurobiol Learn Mem. 2015;122:110e121.
Wiesner CD, Pulst J, Krause F, Elsner M, Baving L, Pedersen A, et al. The effect of selective REM-sleep deprivation on the consolidation and affective evaluation of emotional memories. Neurobiol Learn Mem. 2015;122:131e141.
Groch S, Zinke K, Wilhelm I, Born J. Dissociating the contributions of slow-wave sleep and rapid eye movement sleep to emotional item and source memory. Neurobiol Learn Mem. 2015;122:122e130.
Payne JD, Chambers AM, Kensinger EA. Sleep promotes lasting changes in selective memory for emotional scenes. Front Integr Neuros. 2012;6:108.
Gilson M, Deliens G, Leproult R, Bodart A, Nonclercq A, Ercek R, et al. REM-enriched naps are associated with memory consolidation for sad stories and enhance mood-related reactivity. Brain Sci. 2015;6(1):1e18.
Harrington MO, Pennington K, Durrant SJ. The “affect tagging and consolidation” (ATaC) model of depression vulnerability. Neurobiol Learn Mem. 2017;140:43e51.
Smith CT, Nixon MR, Nader RS. Posttraining increases in REM sleep intensity implicate REM sleep in memory processing and provide a biological marker of learning potential. Learn Mem. 2004;11(6):714e719.
Van Schalkwijk F, Sauter C, Hoedlmoser K, Heib D, et al. The effect of daytime napping and full-night sleep on the consolidation of declarative and procedural information. J Sleep Res. 2007;28(1).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Blechner, M. (2021). Neurobiology of Memory and Sleep. In: DelRosso, L.M., Ferri, R. (eds) Sleep Neurology. Springer, Cham. https://doi.org/10.1007/978-3-030-54359-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-54359-4_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-54358-7
Online ISBN: 978-3-030-54359-4
eBook Packages: MedicineMedicine (R0)